Abstract
Constraint-induced movement therapy (CIMT) is used in stroke rehabilitation to promote recovery of upper limb motor function. However, its efficacy in improving functional outcomes in children with hemiplegic cerebral palsy has not been clearly determined in clinical or experimental research. The aim of our study was to assess the efficacy of a new experimental model of CIMT, evaluated in terms of mortality, stress, motor and cognitive function in rats having undergone a neonatal hypoxic-ischemic (HI) brain injury. Neonatal HI injury was induced at post-natal day 7 through unilateral ligation of the common carotid artery followed by exposure to hypoxia for 2 h. CIMT was implemented at 3 weeks, post-HI injury, using a pouch to constrain the unimpaired forelimb and forcing use of the affected forelimb using a motorized treadmill. After HI injury, animals demonstrated motor and cognitive deficits, as well as volumetric decreases in the ipsilateral hemisphere to arterial occlusion. CIMT yielded a modest recovery of motor and cognitive function, with no effect in reducing the size of the HI lesion or post-HI volumetric decreases in brain tissue. Therefore, although animal models of stroke have identified benefits of CIMT, CIMT was not sufficient to enhance brain tissue development and functional outcomes in an animal model of hemiplegic cerebral palsy. Based on our outcomes, we suggest that CIMT can be used as an adjunct treatment to further enhance the efficacy of a program of rehabilitation in children with hemiplegic cerebral palsy.
Keywords: constraint-induced movement therapy, rehabilitation, neonatal rat, hypoxic-ischemic brain injury, behavioral recovery
Introduction
The prevalence of neonatal encephalopathy, caused by perinatal hypoxic-ischemic (HI) brain injury, is 1–3 per 1,000 term births (1) and is associated with high mortality and chronic neurological disabilities that form the syndrome of cerebral palsy (CP) (2,3). Although rehabilitation is the only effective therapeutic option to ameliorate the functional outcome of CP, there is little to no evidence regarding which interventions can provide optimal therapeutic benefit. Animal models are useful to explore the biological basis of rehabilitation interventions and their mode of delivery.
Approximately one-third of children with CP have spastic hemiplegia, in which one side of the body is significantly more impaired than the other (1). As a result of the functional impairments of the hemiplegic upper limb, these children tend to rely on their unaffected upper limb for daily activities. This learned non-use of the hemiplegic arm often persists despite the capacity for movement of the hemiplegic arm.
Constraint-induced movement therapy (CIMT) promotes functional recovery by forcing intensive use of the hemiplegic arm through immobilization of the unaffected arm. In adult stroke survivors, CIMT promotes plasticity within the central nervous system and enhances recovery of functional organization (4,5) and reduces gray matter atrophy (6). In animal models of adult stroke, CIMT enhances behavioral recovery (7). Combining CIMT with other therapies, such as intensive task-oriented training, can further enhance functional recovery of the hemiplegic arm (8–11). Despite this evidence (12–14), the effect of CIMT on the developing brain remains limited. In a mice model of pediatric HI injury, the combination of CIMT and an enriched learning environment enhanced neurogenesis and functional recovery (15). However, the therapeutic benefit of CIMT is still debated and experimental models are needed to clarify the biological processes underlying the therapeutic effects of CIMT (16).
Experimental modeling of CIMT is challenging and remains fundamentally different from its clinical application where patients receive supervised, assisted, and highly motivating rehabilitation by trained experts. In contrast, the experimental rehabilitation is largely ‘hands-off’ and animals are more difficult to motivate. Therefore, decisions must be made as to how to best represent the validity of an experimental model with respect to a particular aspect of the therapy being evaluated.
Current animal models of CIMT (8,10,17,18) do not provide appropriate constraint or intensive functional training. To address this limitation, we developed a rat model of CIMT, which includes two novel elements. The first is the provision of constraint of the unaffected forelimb by wearing a pouch, combined with intensive, repetitive daily training of the impaired forelimb using a motorized treadmill. The second is inclusion of both functional and histological assessments of the effectiveness of CIMT in the immature brain after HI injury. The aim of our study was to evaluate the efficacy of our model.
Materials and methods
General surgical preparation
Pregnant Sprague-Dawley rats (E17; Doo Yeol Biotech, Seoul, Korea) were housed at 22°C, 12-h light-dark cycle, fed a commercial diet, and allowed tap water ad libitum throughout the study period. All animal procedures were conducted in accordance with our institutional guidelines for animal research, and were approved by the University Animal Care and Use Committee (Permit Number: PNU-2014-0651). Using a computer-generated randomization scheme, animals were allocated to the sham/control (n=14) and HI (n=56) groups. Two weeks after surgical induction of the HI injury, rats were randomly assigned, in blinded fashion, into four groups for analysis.
Hypoxic-ischemic model
HI injury was induced in 7-day old pups, of both sex, using a previously described method (19,20), with some modifications (Fig. 1). This age is approximately equivalent to a 32- to 34-week-old human fetus (21). Both sex were included as sex has not been reported to influence the appearance of reflexes nor the extent of HI-related brain damage (22). The ischemic injury was induced by ligation of the left common carotid artery, performed under microscopy, with animals under general anesthesia, provided using isoflurane delivered by facemask (2% induction and 1.5% maintenance, in 80% N2O and 20% O2). Rectal temperature was maintained at 36.5°C-37.5°C using a Panlab thermostatically controlled heating mat (Harvard Apparatus, Holliston, MA, USA). Sham animals underwent anesthesia and incision to expose the left-common carotid artery only. After surgery, pups were placed in a humidified hypoxic chamber (ProOx oxygen animal chamber, BioSpherix, Redfield, NY, USA) with an 8% O2/N2 mixture for 2 h in an incubator maintained at 37±0.5°C. Following induction of HI injury, pups were returned to their mother for recovery and feeding period up to 3 weeks. Body weight was monitored at 3 weeks post-injury, and measured up to 6-week post-injury using a CAS Digital scale (Gimpo, South Korea).
Figure 1.
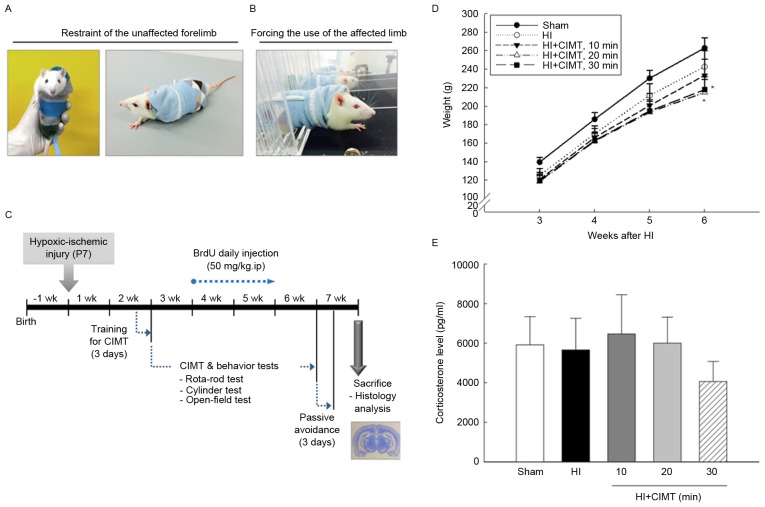
Effect of CIMT on body weight and plasma corticosterone levels. (A and B) A CIMT-treated rat, with restraint to the unaffected forelimb by wearing a pouch, forcing the use of the affected limb on a motorized treadmill. (C) Timeline of the experimental design. (D) Body weight was monitored from 3 weeks after HI injury, and measured up to 6 weeks after HI injury. Groups were as follows: HI, HI injury and non-treated group (HI/vehicle: n=12); 10, HI injury and CIMT for 10 min (n=12); 20, HI injury and CIMT for 20 min (n=12); 30, HI injury CIMT for 30 min (n=12). (E) Corticosterone concentration in plasma was determined by ELISA assay at 7 weeks after HI. *P<0.05 vs. sham group (unpaired t-test). HI, hypoxic-ischemic; CIMT, constraint-induced movement therapy.
Constraint-induced movement therapy
The unaffected forelimb in the CIMT group was restrained by wearing a pouch (Fig. 1A) and animals were forced to use their impaired forelimb on a motorized treadmill, set an inclination of 15° and a speed of 5 m/min (Fig. 1B). CIMT was implemented at 3 weeks after HI injury, with treatment sessions of 10, 20, or 30 min per day, depending on treatment group allocation, performed 4 times per week for 4 weeks (Fig. 1C).
Corticosterone level
Plasma concentration of corticosterone, a stress hormone, was quantified using an ELISA kit 7 weeks after HI, according to the manufacturer's instructions (Enzo Life Science, Farmingdale, NY, USA). Trunk blood samples were collected from the posterior vena cava immediately after decapitation, collected in heparinized plastic tubes and centrifuged at 3,000 rpm for 10 min to obtain the plasma.
Behavioral experiments
All behavioral tests were performed at 3, 4, 5 and 6 weeks after HI injury, by evaluators blinded to group allocation.
The cylinder test evaluates asymmetry in forelimb use (23). Briefly, rats were placed in a transparent Plexiglas cylinder (30 cm high, 20 cm diameter) and initial forepaw (left/right/both) preference for weight-bearing contacts during the rearing behavior was scored. The forelimb asymmetry score was calculated as: (left-right)/total of number of contacts.
The accelerated rota-rod test assessed motor coordination and balance (Panlab S.L.U., Barcelona, Spain). Following adaptation trials, animals were placed on the rotating rod for five trials per day, starting at a speed of 4 rpm and increasing to 40 rpm over 2 min. The latency time for the animal to fall from the rotating drum was recorded for each trial and the mean duration for five trials calculated.
The open field apparatus consisted of a black square box (60×60×30 cm) made of Plexiglas. After a 5-min adaptation in the black box, locomotor and exploratory behaviors were recorded for 15 min using a SMART 2.5.18 system (Panlab S.L.U., Barcelona, Spain).
The passive avoidance task was used to evaluate cognitive function and memory acquisition post-HI injury (Panlab S.L.U.). The apparatus consisted of an illuminated compartment (20×20×25 cm) and a dark compartment (20×20×25 cm) with a grid floor, separated by a sliding door. In the training trial, rats were placed in the illuminated compartment. For analysis, the latency for animals to step through the door and enter the dark compartment was measured, 24 h after the training trials. If an animal crossed the door into the dark compartment, the door closed automatically and a single inescapable scrambled foot shock (0.8 mA, 5 sec) was delivered through the grid floor. The procedure was repeated until the latency to enter the dark compartment of the box was ≥300 s. The retention trial was performed 24 h after the acquisition trial in the same manner and the time taken to re-enter the dark compartment was recorded. No foot-shock was applied in the retention trial. Animals that did not enter the dark chamber during the retention test were assigned a latency of 300 sec for analysis.
Histological assessment via cresyl violet staining
Following decapitation, brains were removed, fixed in 4% paraformaldehyde at 4°C for 24 h, cryoprotection in 30% sucrose for 72 h at 4°C, and mounted in a medium for frozen tissue specimens (Sakura Finetek, Torrance, CA, USA). The frozen brains were cut into 40 µm slices using a CM 3050 cryostat (Leica Microsystems GmbH, Wetzlar, Germany), and stored at −20°C in a freezing medium (50% glycerol in phosphate-buffered saline, pH 7.4) before analysis. Cresyl violet staining was used to prepare brain tissue for volumetric analysis. The contralateral and ipsilateral sizes of the cortex, striatum, hippocampus and midbrain were measured using the iSolution full image analysis software (Image & Microscope Technology, Vancouver, Canada).
5-bromo-2-deoxyuridine injection and immunofluorescence staining
Rats received an intraperitoneal injection of 5-bromo-2-deoxyuridine (BrdU; 50 mg/kg; Sigma-Aldrich) once daily for 10 days, beginning at 3 weeks post-HI injury. Brain sections (40 µm) were stained with anti-BrdU (1:400; Serotec, Oxford, UK) overnight at 4°C, followed by FITC-conjugated secondary antibodies (1:500; Vector Laboratories, Inc., Burlingame, CA, USA). The nuclei were stained with DAPI (molecular probe). The fluorescent images were obtained with a Zeiss LSM 700 laser scanning confocal device (Carl Zeiss, Jena, Germany). Quantification of immunofluorescene was conducted using the iSolution full image analysis software (Image & Microscope Technology).
Data analysis
All data are expressed as the mean ± standard error of the mean (SEM). Differences between the sham/control and HI/vehicle groups were evaluated using an unpaired t-test. Comparisons between the HI/vehicle and HI+CIMT treatment groups were evaluated by one-way or two-way ANOVA, as applicable, followed by the Holm-Sidak test to evaluate identified main effects of group and CIMT duration. P<0.05 was considered to indicate a statistically significant difference. All statistical analyses were performed using SigmaPlot 11.2 (Systat Software Inc.).
Results
Physiological analysis
Eight pups died over the first 24 h after HI injury and another five died during the CIMT intervention period (Table I). The overall survival rate was 85.71% at 24 h and 76.79% at 6 weeks. Among the 56 pups assigned to the HI injury group, 43 completed the 4-week CIMT intervention (Fig. 1A-C). Although birth weight was similar among all pups, CIMT was associated with a lower weight gain throughout the period of observation (Fig. 1D). Plasma concentration of corticosterone was comparable among all groups at 7 weeks, indicating that HI and CIMT did not increase the production of corticosterone (Fig. 1E).
Table I.
Mortality rates.
| Group | Before HI | 24 h after HI | 3 weeks after HI | 6 weeks after HI |
|---|---|---|---|---|
| Sham/control | 14 | 14 | 14 | 14 |
| HI/vehicle | 14 | 12 | 12 | 12 |
| HI+CIMT, 10 min | 14 | 12 | 12 | 9 |
| HI+CIMT, 20 min | 14 | 12 | 12 | 11 |
| HI+CIMT, 30 min | 14 | 12 | 12 | 11 |
HI rats received CIMT for 10, 20 or 30 min. The HI/vehicle gropu did not receive CIMT. HI, hypoxic-ischemic; CIMT, constraint-induced movement therapy.
CIMT and behavioral analysis
Preference for ipsilateral forelimb use for the rearing behavior in the cylinder test is shown in Fig. 2A. Although animals in the sham/control did not show a side preference for forepaw initiation of the rearing behavior, animals in the HI/vehicle group exhibited a marked asymmetry, with a preference to initiate the rearing behavior with the unaffected forepaw at all time-points of measurement. Animals in the 10- and 30-min CIMT groups recovered symmetrical forelimb use 3 weeks after HI injury. No differences were identified in performance on the cylinder-rearing test between the HI/vehicle and 20-min CIMT groups (Fig. 2A).
Figure 2.
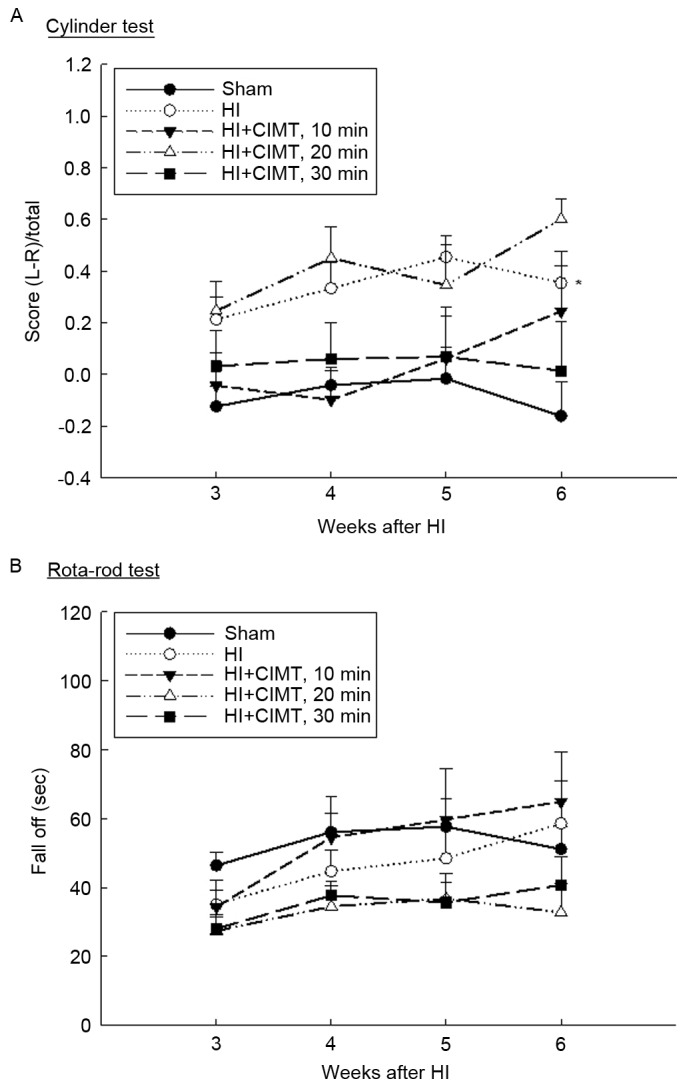
Effect of CIMT on cylinder test and rota-rod test. (A) The use of forelimb for support during weight bearing behavior was assessed through examination of preference for the ipsilateral forelimb use by the cylinder test. (B) Motor coordination and balance was assessed by the rota-rod test. *P<0.05 vs. sham group (unpaired t-test). HI, hypoxia-ischemia; CIMT, constraint-induced movement therapy.
Performance on the rota-rod test is shown in Fig. 2B. Performance for animals in the 10-min CIMT group recovered to the level of the sham/control group. By contrast, animals in the 20- and 30-min CIMT groups did not show improvement in their performance over time (Fig. 2B). Therefore, HI injury resulted in impairments in motor coordination and balance, which was ameliorated by a CIMT intervention of 10 min per day, 4 days per week.
Performance in the open-field test was comparable among all groups at all measurement time-points (Fig. 3A), although animals in the sham/control group did spend more time at rest, compared to animals in the HI injury groups. There were no difference between animals in the HI/vehicle group and those in the CIMT groups (Fig. 3B). On the passive avoidance task (Fig. 4), latency to enter the dark compartment from the training to the retention trial increased for the sham/control group from 15.47±2.91 sec to 285.48±8.90 sec. By comparison, latency time in the retention trial was significantly shorter for animals in the HI groups, with no significant effect of CIMT.
Figure 3.
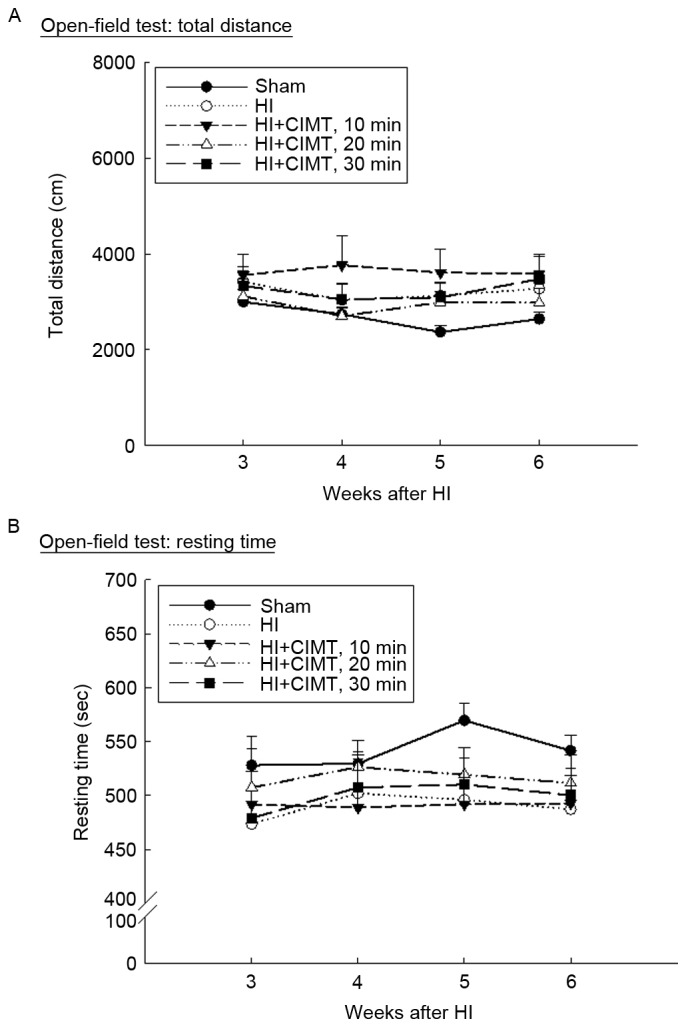
Effect of CIMT on open-field test. Locomotor and exploratory behaviors were evaluated using the open-field test. After 5 min of adaptation in a black box, (A) total distance traveled and (B) resting time were video-recorded for 15 min. HI, hypoxia-ischemia; CIMT, constraint-induced movement therapy.
Figure 4.
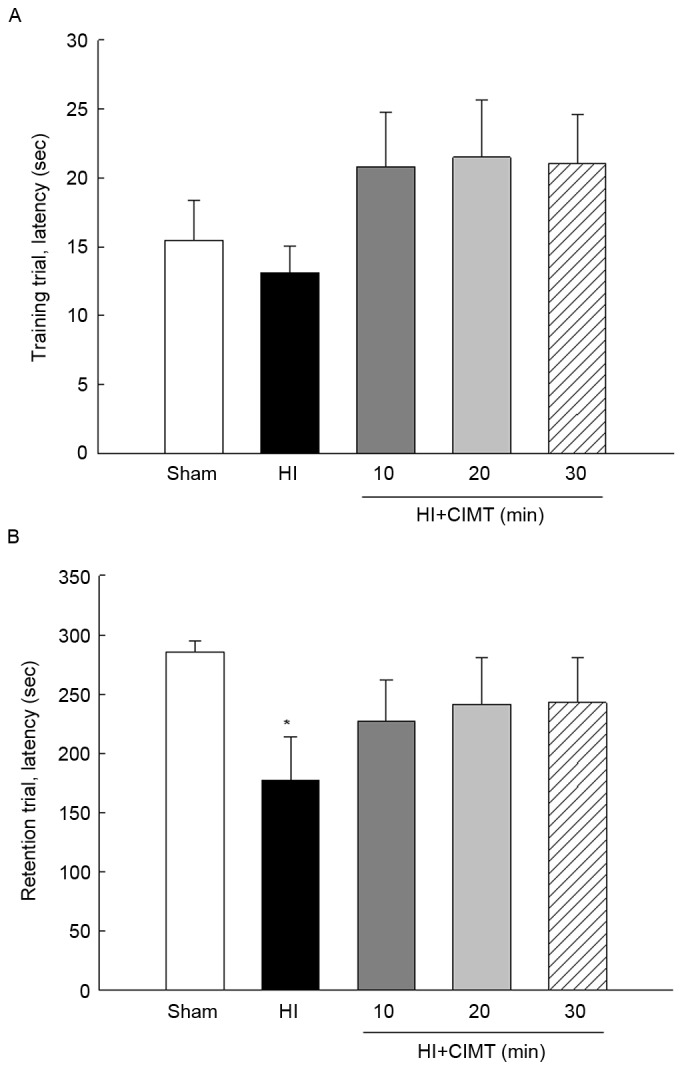
Effect of CIMT on passive avoidance task. The cognitive function and memory acquisition were assessed by passive avoidance task at 7 weeks after HI. (A) In the training trial, the rats were placed in the illuminated compartment, and the step-through latency for animals to enter the dark compartment was measured. (B) The retention trial was performed 24 h after the acquisition trial in the same manner and the time taken to re-enter the dark compartment was recorded. *P<0.05 vs. sham group (unpaired t-test). HI, hypoxia-ischemia; CIMT, constraint-induced movement therapy.
CIMT and histological analysis
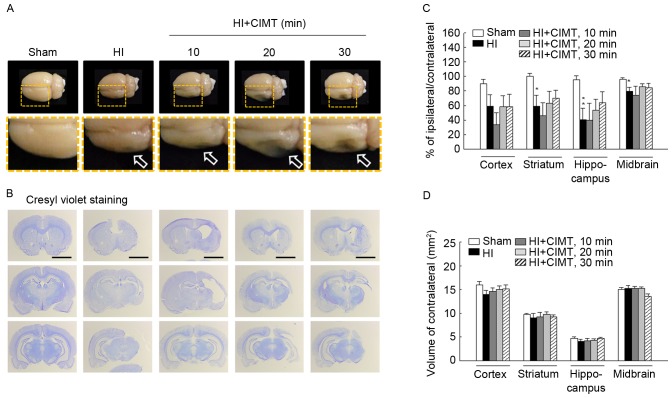
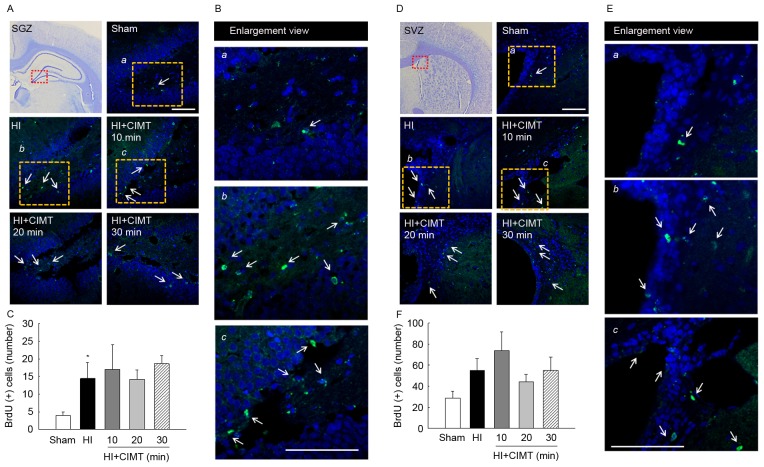
On volumetric analysis performed at 7-weeks (Fig. 5), remaining cortical volume in animals in the HI groups was substantially smaller than volumes in the sham/control group (Fig. 5A and B). HI injury was associated with severe atrophy, particularly in the hippocampus, with a 40% decrease in volume on the side ipsilateral to the ligation, compared to the contralateral side. Moreover, a 40% reduction in the volume of the cortex and the striatum and 20% of the midbrain was identified (Fig. 5C). Subarea brain volumes were comparable for the HI/vehicle and CIMT groups, indicative that CIMT did not offer neuroprotection. Measurement of the subareas of the contralateral brain, including the cortex, striatum, hippocampus and midbrain, were comparable to volumes for the sham/control group, indicative that CIMT did not influence neural development of the contralateral brain (Fig. 5D). With regards to cell proliferation (Fig. 6), BrdU(+) cell count in the subgranular zone was greater in the HI/vehicle rats than in the sham/control rats (14.45±4.53 and 3.89±1.07, respectively). Cell count was comparable for the HI/vehicle and CIMT groups in the subgranular and subventricular zones (Fig. 6A-C and D-F, respectively).
Figure 5.
Effect of CIMT on volume atrophy. (A) Gross assessment of the HI injury lesion. (B) Photomicrograph of cresyl violet staining. Each region was located at 1.28, −3.48 and −5.28 mm from the bregma. Scale bar, 4 mm. (C and D) Histogram of the cortex, striatum, hippocampus and midbrain for histological analysis. *P<0.05 and **P<0.01, vs. sham group (unpaired t-test). HI, hypoxia-ischemia; CIMT, constraint-induced movement therapy.
Figure 6.
Effect of CIMT on proliferation in the subgranular zone and subventricular zone. BrdU staining in the (A-C) SGZ and (D-F) SVZ at 7 weeks after HI. Quantification graphs of BrdU-positive cells (white arrows) in (C) SGZ and (F) SVZ. *P<0.05 vs. sham group (unpaired t-test). The red rectangle illustrates the imaging field. DAPI (blue) for nuclei. Scale bar, 20 µm. CIMT, constraint-induced movement therapy; SGZ, subgranular zone; SVZ, subventricular zone; HI, hypoxia-ischemia.
Discussion
In this study, we examined the efficacy of a new animal model of CIMT in terms of mortality, stress, motor and cognitive function in neonatal HI injury. In addition, we tested different durations of CIMT (10, 20 or 30 min per day, 4 days per week) to determine the optimal length for CIMT sessions which would yield long-term benefits on behavioral outcomes. We also evaluated the effects of CIMT on brain development using volumetric analysis of different subareas of the brain, both ipsilateral and contralateral of the HI injury. The CIMT intervention in our animal model of CP did not replicate the effects of CIMT previously reported in animal models of stroke, where CIMT was reported to improve histological and behavioral outcomes.
In order to accurately assess the effect of CIMT, outcome measures that are sensitive both to the nature of the damage caused by the HI injury and to possible improvements that could be attributed to CIMT are required. Although studies have reported that animals sustaining an HI injury early in development display long-term deficits in motor coordination, identifiable on the rota-rod test (24), we did not find the rota-rod test to be sufficiently sensitive to detect neuromotor deficits in all rats after HI injury. The poor sensitivity of the rota-rod test for measuring HI-related deficits has been previously reported (25,26). In our view, the cylinder test is a more reliable measure of asymmetry in forelimb use.
Although hyperactivity at 3 weeks post-HI injury has been previously reported (26), we did not identify any clear HI-related behavioral deficits, other than slightly longer travel distances in the open-field test compared to the sham/control group (Fig. 3). Moreover, although previous studies described significant impairments in both spatial and non-spatial learning/memory tasks after an HI injury (27,28), we identified significant reduction in retention, associated with a volume of the left hippocampus (Figs. 4, 5). Our findings are consistent with a previous study reporting a 60% reduction in hippocampal volume on the side of arterial occlusion (29). As the hippocampus mediates both spatial navigation and memory (30), disruptions in the hippocampus, and of its associated projections, could result in deficits in memory and spatial processing in children with CP (31).
There have been several attempts to develop animal models of CIMT (8,10,17,18), including the use of a sleeveless jacket wrapped around the upper torso and attached to a metal wrist bracelet and the use of a plaster cast to fix the position of the unaffected forelimb to the torso. These constraint methods, however, increase animal stress (11,32) and lack the behavioral pressure to rely on the impaired forelimb (8,32). Combining CIMT with an enriched environment reduces animal stress and encourages voluntary movement of the impaired forelimb (33). However, the effect of whole body physical exercises cannot be differentiated from the specific effects of CIMT in these models. Furthermore, motivating animals to participate in learning tasks is also a major challenge of current experimental models of CIMT. Combining CIMT with repetitive practice on a task can encourage animal motivation and reduce stress (32,34). However, a repetitive training paradigm is time intensive and requires too many efforts. Our novel model combining CIMT with a treadmill activity overcomes some of the challenges associated with animal motivation and stress, while reducing the time demand to train the animals. In our paradigm, the unaffected forelimb was restrained by wearing a pouch, which forced the animals to use their impaired forelimb on a motorized treadmill with an angle of 15° and a speed of 5 m/min (Fig. 1A and B). Use of the pouch also allowed us to control for the effects of whole body exercise on measured outcomes by restricting use of the impaired forelimb only during the designated training periods. One of our concerns was that our CIMT protocol, including use of the pouch, would stress to the animals. However, plasma concentrations of corticosterone were comparable between the sham/control and HI/vehicle and HI+CIMT groups, indicating that our protocol did not stress the animals (Fig. 1E).
Pursuing animal models to investigate CIMT is important. Although positive results of CIMT in children with hemiplegic CP have received considerable attention (12–14), in reality, the effects of CIMT are still a matter of debate as evidence of therapeutic effects are limited (16). Functional improvement with CIMT has only been reported in a mouse model of CP created using unilateral ligation (15), with improvement in functional outcome and neurogenesis observed at 4 weeks post-injury in animals receiving a combination of CIMT and enriched-environment training. We could not confirm the findings of Rha et al (15). The reasons for this discrepancy is unclear, but are likely to involve interactions between the animal strain used (mouse vs. rat), the duration of hypoxia (90 min vs. 120 min), the modeling of CIMT (CIMT+enriched-environment vs. CIMT+treadmill-induced forced limb use) and the duration of intervention (2 weeks vs. 4 weeks). Therefore, although CIMT is a safe therapy, it may not yield significant therapeutic benefits, although we did observe that CIMT produced a trend to a modest improvement in the symmetry of forelimb use, motor coordination and cognitive function in rats that received CIMT.
Previous studies have reported that CIMT increases gray matter volume in motor areas and the hippocampus in children with hemiplegic CP (35) and adults with chronic stroke (6). In addition, increased neurogenesis associated to CIMT contributes to behavioral recovery after stroke in adult rats (36). However, our CIMT protocol did not influence the volume of the cortex, striatum, hippocampus and midbrain. In addition, the animals treated with CIMT did not show a significant increase in BrdU-positive cells in either the subgranular and subventricular zones, compared to rats in the HI/vehicle group. Therefore, benefits of CIMT in enhancing recovery of brain tissue and upper limb function reported in animal models of adult stroke were not evident in our neonatal model of hemiplegic CP. Based on our findings, we suggest that CIMT provides an adjunct treatment to further enhance the efficacy of a program of rehabilitation in children with hemiplegic CP. Future studies are needed to investigate other therapeutic approaches, which, in combination with CIMT, would enhance functional recovery following HI injury.
Acknowledgements
This study was supported by Medical Research Institute Grant (2014–22), Pusan National University Hospital.
References
- 1.Kurinczuk JJ, White-Koning M, Badawi N. Epidemiology of neonatal encephalopathy and hypoxic-ischaemic encephalopathy. Early Hum Dev. 2010;86:329–338. doi: 10.1016/j.earlhumdev.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 2.Bennet L, Tan S, van den Heuij L, Derrick M, Groenendaal F, van Bel F, Juul S, Back SA, Northington F, Robertson NJ, et al. Cell therapy for neonatal hypoxia-ischemia and cerebral palsy. Ann Neurol. 2012;71:589–600. doi: 10.1002/ana.22670. [DOI] [PubMed] [Google Scholar]
- 3.Edwards AD, Brocklehurst P, Gunn AJ, Halliday H, Juszczak E, Levene M, Strohm B, Thoresen M, Whitelaw A, Azzopardi D. Neurological outcomes at 18 months of age after moderate hypothermia for perinatal hypoxic ischaemic encephalopathy: Synthesis and meta-analysis of trial data. BMJ. 2010;340:c363. doi: 10.1136/bmj.c363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolf SL, Winstein CJ, Miller JP, Taub E, Uswatte G, Morris D, Giuliani C, Light KE, Nichols-Larsen D. EXCITE Investigators: Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: The EXCITE randomized clinical trial. JAMA. 2006;296:2095–2104. doi: 10.1001/jama.296.17.2095. [DOI] [PubMed] [Google Scholar]
- 5.Taub E, Miller NE, Novack TA, Cook EW, III, Fleming WC, Nepomuceno CS, Connell JS, Crago JE. Technique to improve chronic motor deficit after stroke. Arch Phys Med Rehabil. 1993;74:347–354. [PubMed] [Google Scholar]
- 6.Gauthier LV, Taub E, Perkins C, Ortmann M, Mark VW, Uswatte G. Remodeling the brain: Plastic structural brain changes produced by different motor therapies after stroke. Stroke. 2008;39:1520–1525. doi: 10.1161/STROKEAHA.107.502229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qu HL, Zhao M, Zhao SS, Xiao T, Song CG, Cao YP, Jolkkonen J, Zhao CS. Forced limb-use enhanced neurogenesis and behavioral recovery after stroke in the aged rats. Neuroscience. 2015;286:316–324. doi: 10.1016/j.neuroscience.2014.11.040. [DOI] [PubMed] [Google Scholar]
- 8.DeBow SB, Davies ML, Clarke HL, Colbourne F. Constraint-induced movement therapy and rehabilitation exercises lessen motor deficits and volume of brain injury after striatal hemorrhagic stroke in rats. Stroke. 2003;34:1021–1026. doi: 10.1161/01.STR.0000063374.89732.9F. [DOI] [PubMed] [Google Scholar]
- 9.Biernaskie J, Corbett D. Enriched rehabilitative training promotes improved forelimb motor function and enhanced dendritic growth after focal ischemic injury. J Neurosci. 2001;21:5272–5280. doi: 10.1523/JNEUROSCI.21-14-05272.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schäbitz WR, Berger C, Kollmar R, Seitz M, Tanay E, Kiessling M, Schwab S, Sommer C. Effect of brain-derived neurotrophic factor treatment and forced arm use on functional motor recovery after small cortical ischemia. Stroke. 2004;35:992–997. doi: 10.1161/01.STR.0000119754.85848.0D. [DOI] [PubMed] [Google Scholar]
- 11.Ke Z, Yip SP, Li L, Zheng XX, Tong KY. The effects of voluntary, involuntary, and forced exercises on brain-derived neurotrophic factor and motor function recovery: A rat brain ischemia model. PLoS One. 2011;6:e16643. doi: 10.1371/journal.pone.0016643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taub E, Ramey SL, DeLuca S, Echols K. Efficacy of constraint-induced movement therapy for children with cerebral palsy with asymmetric motor impairment. Pediatrics. 2004;113:305–312. doi: 10.1542/peds.113.2.305. [DOI] [PubMed] [Google Scholar]
- 13.Taub E, Griffin A, Uswatte G, Gammons K, Nick J, Law CR. Treatment of congenital hemiparesis with pediatric constraint-induced movement therapy. J Child Neurol. 2011;26:1163–1173. doi: 10.1177/0883073811408423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eliasson AC, Krumlinde-sundholm L, Shaw K, Wang C. Effects of constraint-induced movement therapy in young children with hemiplegic cerebral palsy: An adapted model. Dev Med Child Neurol. 2005;47:266–275. doi: 10.1111/j.1469-8749.2005.tb01132.x. [DOI] [PubMed] [Google Scholar]
- 15.Rha DW, Kang SW, Park YG, Cho SR, Lee WT, Lee JE, Nam CM, Han KH, Park ES. Effects of constraint-induced movement therapy on neurogenesis and functional recovery after early hypoxic-ischemic injury in mice. Dev Med Child Neurol. 2011;53:327–333. doi: 10.1111/j.1469-8749.2010.03877.x. [DOI] [PubMed] [Google Scholar]
- 16.Chen YP, Pope S, Tyler D, Warren GL. Effectiveness of constraint-induced movement therapy on upper-extremity function in children with cerebral palsy: A systematic review and meta-analysis of randomized controlled trials. Clin Rehabil. 2014;28:939–953. doi: 10.1177/0269215514544982. [DOI] [PubMed] [Google Scholar]
- 17.Muller HD, Hanumanthiah KM, Diederich K, Schwab S, Schäbitz WR, Sommer C. Brain-derived neurotrophic factor but not forced arm use improves long-term outcome after photothrombotic stroke and transiently upregulates binding densities of excitatory glutamate receptors in the rat brain. Stroke. 2008;39:1012–1021. doi: 10.1161/STROKEAHA.107.495069. [DOI] [PubMed] [Google Scholar]
- 18.Bland ST, Schallert T, Strong R, Aronowski J, Grotta JC, Feeney DM. Early exclusive use of the affected forelimb after moderate transient focal ischemia in rats: Functional and anatomic outcome. Stroke. 2000;31:1144–1152. doi: 10.1161/01.STR.31.5.1144. [DOI] [PubMed] [Google Scholar]
- 19.Rice JE, III, Vannucci RC, Brierley JB. The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann Neurol. 1981;9:131–141. doi: 10.1002/ana.410090206. [DOI] [PubMed] [Google Scholar]
- 20.Vannucci RC, Connor JR, Mauger DT, Palmer C, Smith MB, Towfighi J, Vannucci SJ. Rat model of perinatal hypoxic-ischemic brain damage. J Neurosci Res. 1999;55:158–163. doi: 10.1002/(SICI)1097-4547(19990115)55:2<158::AID-JNR3>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 21.Yager JY. Animal models of hypoxic-ischemic brain damage in the newborn. Semin Pediatr Neurol. 2004;11:31–46. doi: 10.1016/j.spen.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 22.Vannucci RC, Rossini A, Towfighi J, Vannucci SJ. Measuring the accentuation of the brain damage that arises from perinatal cerebral hypoxia-ischemia. Biol Neonate. 1997;72:187–191. doi: 10.1159/000244483. [DOI] [PubMed] [Google Scholar]
- 23.Kim JH, Choi KH, Jang YJ, Kim HN, Bae SS, Choi BT, Shin HK. Electroacupuncture preconditioning reduces cerebral ischemic injury via BDNF and SDF-1a in mice. BMC Complement Altern Med. 2013;13:22. doi: 10.1186/1472-6882-13-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jansen EM, Low WC. Long-term effects of neonatal ischemic-hypoxic brain injury on sensorimotor and locomotor tasks in rats. Behav Brain Res. 1996;78:189–194. doi: 10.1016/0166-4328(95)00248-0. [DOI] [PubMed] [Google Scholar]
- 25.Lubics A, Reglodi D, Tamás A, Kiss P, Szalai M, Szalontay L, Lengvári I. Neurological reflexes and early motor behavior in rats subjected to neonatal hypoxic-ischemic injury. Behav Brain Res. 2005;157:157–165. doi: 10.1016/j.bbr.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 26.Balduini W, de Angelis V, Mazzoni E, Cimino M. Long-lasting behavioral alterations following a hypoxic/ischemic brain injury in neonatal rats. Brain Res. 2000;859:318–325. doi: 10.1016/S0006-8993(00)01997-1. [DOI] [PubMed] [Google Scholar]
- 27.Alexander M, Garbus H, Smith AL, Rosenkrantz TS, Fitch RH. Behavioral and histological outcomes following neonatal HI injury in a preterm (P3) and term (P7) rodent model. Behav Brain Res. 2014;259:85–96. doi: 10.1016/j.bbr.2013.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arteni NS, Salgueiro J, Torres I, Achaval M, Netto CA. Neonatal cerebral hypoxia-ischemia causes lateralized memory impairments in the adult rat. Brain Res. 2003;973:171–178. doi: 10.1016/S0006-8993(03)02436-3. [DOI] [PubMed] [Google Scholar]
- 29.Felt BT, Schallert T, Shao J, Liu Y, Li X, Barks JD. Early appearance of functional deficits after neonatal excitotoxic and hypoxic-ischemic injury: Fragile recovery after development and role of the NMDA receptor. Dev Neurosci. 2002;24:418–425. doi: 10.1159/000069053. [DOI] [PubMed] [Google Scholar]
- 30.White NM, McDonald RJ. Multiple parallel memory systems in the brain of the rat. Neurobiol Learn Mem. 2002;77:125–184. doi: 10.1006/nlme.2001.4008. [DOI] [PubMed] [Google Scholar]
- 31.Aylward GP. Neurodevelopmental outcomes of infants born prematurely. J Dev Behav Pediatr. 2005;26:427–440. doi: 10.1097/00004703-200512000-00008. [DOI] [PubMed] [Google Scholar]
- 32.Livingston-Thomas JM, Hume AW, Doucette TA, Tasker RA. A novel approach to induction and rehabilitation of deficits in forelimb function in a rat model of ischemic stroke. Acta Pharmacol Sin. 2013;34:104–112. doi: 10.1038/aps.2012.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Janssen H, Bernhardt J, Collier JM, Sena ES, McElduff P, Attia J, Pollack M, Howells DW, Nilsson M, Calford MB, Spratt NJ. An enriched environment improves sensorimotor function post-ischemic stroke. Neurorehabil Neural Repair. 2010;24:802–813. doi: 10.1177/1545968310372092. [DOI] [PubMed] [Google Scholar]
- 34.O'Bryant AJ, Adkins DL, Sitko AA, Combs HL, Nordquist SK, Jones TA. Enduring poststroke motor functional improvements by a well-timed combination of motor rehabilitative training and cortical stimulation in rats. Neurorehabil Neural Repair. 2016;30:143–154. doi: 10.1177/1545968314562112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sterling C, Taub E, Davis D, Rickards T, Gauthier LV, Griffin A, Uswatte G. Structural neuroplastic change after constraint-induced movement therapy in children with cerebral palsy. Pediatrics. 2013;131:e1664–e1669. doi: 10.1542/peds.2012-2051. [DOI] [PubMed] [Google Scholar]
- 36.Zhao SS, Zhao Y, Xiao T, Zhao M, Jolkkonen J, Zhao CS. Increased neurogenesis contributes to the promoted behavioral recovery by constraint-induced movement therapy after stroke in adult rats. CNS Neurosci Ther. 2013;19:194–196. doi: 10.1111/cns.12058. [DOI] [PMC free article] [PubMed] [Google Scholar]