Abstract
BACKGROUND
Birth trauma to pelvic floor muscles is a major risk factor for pelvic floor disorders. Intramuscular extracellular matrix determines muscle stiffness, supports contractile component, and shields myofibers from mechanical strain.
OBJECTIVE
Our goal was to determine whether pregnancy alters extracellular matrix mechanical and biochemical properties in a rat model, which may provide insights into the pathogenesis of pelvic floor muscle birth injury. To examine whether pregnancy effects were unique to pelvic floor muscles, we also studied a hind limb muscle.
STUDY DESIGN
Passive mechanical properties of coccygeus, iliocaudalis, pubocaudalis, and tibialis anterior were compared among 3-month old Sprague–Dawley virgin, late-pregnant, and postpartum rats. Muscle tangent stiffness was calculated as the slope of the stress–sarcomere length curve between 2.5 and 4.0 μm, obtained from a stress-relaxation protocol at a bundle level. Elastin and collagen isoform concentrations were quantified by the use of enzyme-linked immunosorbent assay. Enzymatic and glycosylated collagen crosslinks were determined by high-performance liquid chromatography. Data were compared by the use of repeated-measures, 2-way analysis of variance with Tukey post-hoc testing. Correlations between mechanical and biochemical parameters were assessed by linear regressions. Significance was set to P < .05. Results are reported as mean ± SEM.
RESULTS
Pregnancy significantly increased stiffness in coccygeus (P < .05) and pubocaudalis (P < .0001) relative to virgin controls, with no change in iliocaudalis. Postpartum, pelvic floor muscle stiffness did not differ from virgins (P > .3). A substantial increase in collagen V in coccygeus and pubocaudalis was observed in late-pregnant, compared with virgin, animals, (P < .001). Enzymatic crosslinks decreased in coccygeus (P < .0001) and pubocaudalis (P < .02) in pregnancy, whereas glycosylated crosslinks were significantly elevated in late-pregnant rats in all pelvic floor muscles (P < .05). Correlations between muscle stiffness and biochemical parameters were inconsistent. In contrast to the changes observed in pelvic floor muscles, the tibialis anterior was unaltered by pregnancy.
CONCLUSIONS
In contrast to other pelvic tissues, pelvic floor muscle stiffness increased in pregnancy, returning to prepregnancy state post-partum. This adaptation may shield myofibers from excessive mechanical strain during parturition. Biochemical alterations in pelvic floor muscle extracellular matrix due to pregnancy include increase in collagen V and a differential response in enzymatic vs glycosylated collagen crosslinks. The relationships between pelvic floor muscle biochemical and mechanical parameters remain unclear.
Keywords: passive mechanics, pelvic floor muscles, pregnancy, rat
Pelvic floor disorders include pelvic organ prolapse, urinary, and fecal incontinence. Collectively, they represent a major public health problem, given their high prevalence, negative impact on quality of life, lack of ability to predict who is at risk, lack of preventive measures, high failure rate of available treatments, and the associated economic burden.1–3 Clinical studies provide ample evidence that pelvic floor skeletal muscles (PFMs) are major contributors to proper female pelvic floor function. Radiologically detected defects and dysfunction of the PFMs are associated with a significantly increased risk of pelvic floor disorders, as well as recurrence of pelvic organ prolapse after surgical treatment.4 PFMs are composed of coccygeus (C) and the levator ani muscle complex; however, most published investigations use PFMs and levator ani muscle interchangeably and do not include C.
Childbirth is identified as the leading cause of PFM trauma. The reason for the trauma, suggested by modeling and imaging studies, is excessive PFM strain that occurs during vaginal delivery.5–7 Surprisingly, despite these predictions, many parous women do not sustain PFM injury.8 It is therefore likely that, to achieve extraphysiologic strain without injury, pregnancy-induced adaptations take place within these muscles. Previously we showed that PFMs adjust their contractile architecture to increase excursion, or range of motion, in preparation for delivery.9 Another major skeletal muscle component is the extracellular matrix (ECM) network, which consists of the endomysium that envelops individual muscle fibers, the perimysium, which connects adjacent fibers and surrounds muscle bundles and fascicles, and the epimysium, which ensheathes the whole muscle.10 Intramuscular ECM bears the majority of passive load, provides support to the myofibers, and is the main determinant of muscle stiffness.10,11 Human and animal studies of ligaments, symphysis pubis, cervix, and vaginal connective tissue demonstrate dramatic changes in the mechanical properties of these structures during pregnancy.12–17 Biochemical alterations and remodeling of ECM are thought to account for the decreased stiffness and increased distensibility of these tissues, which facilitates delivery of the fetus and protects against maternal birth injury.18,19
Taken together, these findings motivate our thesis that pregnancy-induced protective adaptations also take place in the PFM ECM. One of the main functions of intramuscular ECM is to shield myofibers from mechanical stresses and strains by increasing passive tension, which is tension generated when muscle is stretched independently of muscle electrical activation.11 Thus, we hypothesized that, in contrast to other pelvic tissues, PFM stiffness would increase in pregnancy. We postulated that such adaptation will protect the contractile component of the PFMs from injury caused by excessive mechanical strain during parturition.
Given the obvious ethical constraints associated with procurement of sufficient human PFM tissue from pregnant women to perform such a study, we used a rat model to determine PFM ECM alterations during gestation. Comparison of PFM structural parameters between human and rat demonstrated architectural similarity, suggesting that they have comparable functional design.20 The rat model also has proven valuable in many studies of pregnancy-related changes in other pelvic structures.17,21,22 Furthermore, we recently reported that adaptations during pregnancy occur in the myofibers of rat PFMs, which increase their length by adding sarcomeres in series.9 As a complement, the current investigation primarily focuses on the biomechanical properties of PFM ECM to provide further insight into potential pregnancy-induced functional changes of human PFMs and pathogenesis of PFMs maternal birth injury.
The secondary objectives of this study were to explore pregnancy-induced biochemical alterations in the PFM ECM that could potentially account for the changes in muscle mechanical properties. Pregnancy induces marked alterations in collagen isoform ratios and increases elastin in vaginal connective tissue.23 In this study, we analyzed the content of pertinent to skeletal muscle collagen isoforms and elastin, as well as collagen crosslinks, because previous research has shown that increased collagen crosslinks are associated with greater skeletal and cardiac muscle stiffness.24,25 Consistent with our hypothesis that PFM stiffness will increase in pregnancy, we speculated that pregnancy does not lead to a decrease in collagen I content or an increase in elastin of the intramuscular ECM. We further proposed that PFM collagen crosslinks would increase in the pregnant group. To determine whether the results were specific to PFMs and not a general skeletal muscle response to an altered hormonal environment, we examined the tibialis anterior (TA) hind limb muscle as well.
Materials and Methods
The University of California Institutional Animal Care Committee approved all procedures performed. Seven virgin and 7 late-pregnant (19–21 days) 3-month-old Sprague–Dawley rats (Rattus norvegicus) were euthanized, and samples of C, iliocaudalis (IC), pubocaudalis (PC), and TA were obtained immediately and placed in storage solution at −20°C, as previously described, to prevent hyperpolarization and destruction of muscle tissue.26 PFMs from 7 four-week postpartum rats were used to determine recovery of the passive mechanical properties after delivery. Virgin and postpartum animals were in similar parts of the estrus cycle, as determined by vaginal smears.
Passive mechanics
All muscles were tested within 2 weeks of harvest in a relaxing solution to ensure muscle tissue integrity. Muscle stiffness was determined at the bundle level, which reflects mechanical behavior predominantly due to intramuscular ECM.11 To avoid compromising the structural proteins, which can affect the elastic modulus, tissue digestion, often used to facilitate dissection, was not performed.11 Three fiber bundles, composed of ~30 individual muscle cells or fibers and the associated endo-and perimysium, were tested from each muscle via the use of a custom apparatus, as previously described.27 To summarize in brief, muscle bundles were placed into a testing chamber and secured between a force transducer (405 A; Aurora Scientific Aurora, ON, Canada) and a fixed pin connected to a rotational bearing (MT-RS; Newport Irvine, CA). Sarcomere length (Ls) provided objective quantification of muscle strain and myofibrillar array quality control and was measured throughout the experiments by laser diffraction.28 Bundle length was set to the minimum length that produced measurable forces, and baseline force and Ls were determined. Baseline sample diameter was measured optically with a cross-hair reticle mounted on a dissecting microscope and micromanipulators on an x–y mobile stage to determine cross-sectional area.
Force–Ls data were generated for each bundle subjected to a stress-relaxation protocol. Bundles were elongated until failure or to a Ls of 4.0 μm in 10% strain increments followed by 3-minute stress–relaxation periods after which further stress decline is insignificant.11 Force was converted to stress by dividing force by baseline cross-sectional area, assuming isovolumetric cylindrical shape of fiber bundles.29 Muscle passive tension varies depending on starting Ls, with increasing stiffness occurring at longer Ls. To assure proper comparison of passive mechanical properties among PFMs, we used tangent stiffness, which quantifies stress at a given Ls, instead of tangent modulus, which defines stress required to achieve a particular percent strain and loses Ls as a relevant parameter. Tangent stiffness was quantified as the slope of the stress-Ls curve between 2.5 and 4.0 μm.
Biochemical studies
To associate biochemical with mechanical results, samples were derived from adjacent portions of the same muscle used for mechanical testing. Tissue samples were immediately placed in complete mini protease inhibitor/ phosphate-buffered saline solution (Roche, Indianapolis, IN) to prevent collagen degradation, snap frozen in liquid nitrogen, and stored at −80°C. Previously, we found that total collagen content of PFM ECM substantially increases in pregnancy9; however, quantitative changes in total collagen have not been found to correlate well with passive mechanical properties of skeletal muscles.30,31 In this study, we analyzed the content of collagens I, III, V, and VI (intramuscular ECM) and IV (basement membrane), and elastin by using specific monoclonal antibodies and a sandwich enzyme-linked immunosorbent assay (Antibodies-Online Inc, Atlanta, GA).
To solubilize collagen fibrils and digest intra- and intermolecular crosslinkages, samples were placed in buffers containing pepsin (10 mg/mL) in 0.5 M acetic acid, pH 3.0, and pancreatic elastase (1 mg/mL) for 6 hours and 24 hours, respectively, at 4°C. Solubilized tissue homogenates and standards were pipetted into 96-well microplates precoated with antibodies specific to the proteins of interest after blocking nonspecific binding with bovine serum albumin, followed by addition of a biotin-conjugated specific primary antibodies and avidin conjugated horseradish peroxidase. After removal of unbound avidin-enzyme reagent, a substrate solution was added, followed by a colorimetric quantification by spectrophotometry at 450 nm, normalized to tissue sample mass.
We also compared the amount of enzymatic (hydroxylysyl pyridinoline [HPy], lysyl pyridinoline [LPy]), and glycosylated (pentosidine [PE]) collagen crosslinks in PFMs and TA of nonpregnant and pregnant animals. Collagen crosslinks were determined by high-performance liquid chromatography (HPLC), using previously reported methods.32,33 To summarize, tissue was hydrolyzed in 6 M hydrochloric acid at 110°C for 20 hours. After hydrolysis, samples were dried in a vacuum desiccator, redissolved, and then purified with 0.22-mm spin-X centrifuge tube filters (Costar, Corning, NY). The HPLC column (TSK gel ODS-80 Tm, 4.6 mm I.D. × 15 cm packed with 5-mm particles; TOSOH Bioscience, Tokyo, Japan) was equilibrated with 0.15% (v/v) heptafluorobutyric acid in 24% (v/v) methanol. Samples were then injected into the HPLC system. Elution of crosslinks and a pyridoxine internal standard was achieved at 40°C at a flow rate of 1.0 mL/min in 2 steps. Fluorescence was monitored at 0–22 minutes, 295/400 nm; 22–45 minutes, 328/378 nm (gain 100; band width 18 mm). Elution of HPy was achieved at 9.8 minutes, LPy at 12.1 minutes, and PE at 21.5 minutes.
Statistical analyses
Comparisons between muscles and conditions were performed by repeated measures 2-way analysis of variance. Comparisons between individual groups at each Ls were made with multiple comparisons with Tukey’s range test, as appropriate. Significance level (α) was set to 5%. Sample size of 7/group was calculated to achieve 80% statistical power based on the variability of previous data obtained from rodent limb muscles from our laboratory. Correlations between biochemical and biomechanical properties were determined by linear regression. All data were screened for normality and skew to satisfy the assumptions of the parametric tests used. Results are presented as mean ± SEM. All statistical analyses were performed with GraphPad Prism version 6.00 (GraphPad Software, San Diego, CA).
Results
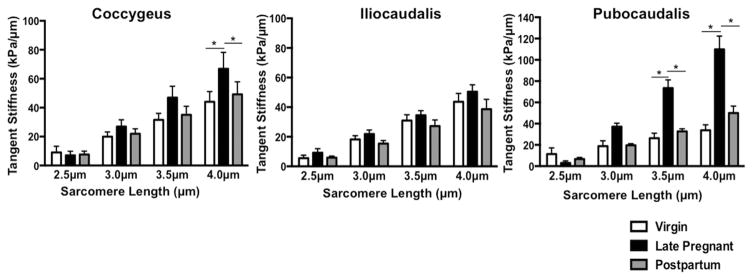
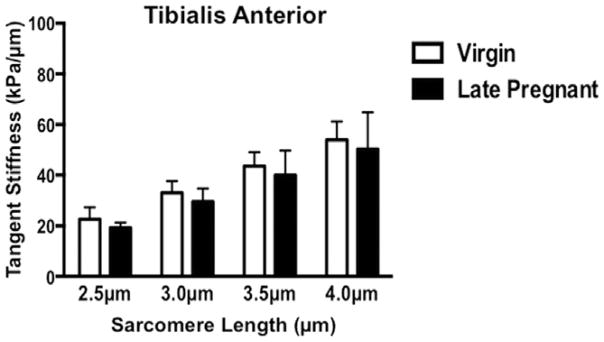
The most notable finding of our study was that, unlike other pelvic tissues, PFM stiffness did not decrease in pregnancy. On the contrary, PFM from pregnant animals were significantly stiffer. At larger strains, pregnant rats demonstrated a 52% increase in tangent stiffness of C and more than 200% increase in stiffness of PC, compared with virgin controls (Figure 1). At Ls of 4 μm, tangent stiffness of C was 66.9 ± 11.3 kPa/μm in pregnant rats vs 44.0 ± 7.1 kPa/μm in virgin animals (P < 0.05; Figure 1). In PC, significantly greater tangent stiffness was observed in the late-pregnant group starting at Ls of 3.5 μm, with 73.4 ± 7.8 kPa/μm vs 26.3 ± 4.7 kPa/μm in virgin animals (P <.0001, Figure 1). Further strain from 3.5 μm to 4 μm resulted in a 50% increase in tangent stiffness in pregnant rats, compared with only a 27% increase in virgins. At Ls of 4 μm, tangent stiffness of PC in late-pregnant rats reached 109.8 ± 12.5 kPa/μm vs 33.8 ± 5.1 kPa/μm in virgin controls (P < .0001; Figure 1). Passive tension of IC remained unchanged in pregnancy (P > .4 at Ls = 4μm). Tangent stiffness of C and PC decreased 4 weeks after vaginal delivery, with no differences observed between postpartum and virgin animals (Figure 1). To determine whether the results were specific to PFM and not a general skeletal muscle response to an altered hormonal environment, we also measured tangent stiffness of TA in virgin and late-pregnant groups. The stiffness of this muscle did not change during pregnancy (P >.9 at Ls 2.5–4 μm) (Figure 2).
FIGURE 1. Tangent stiffness of coccygeus, iliocaudalis, and pubocaudalis in virgin, late-pregnant, and postpartum groups (n = 7/group), represented as mean ± SEM.
*Significantly different P values derived from Tukey pairwise comparisons, after repeated measures 2-way with significance level set to 5%.
ANOVA, analysis of variance.
FIGURE 2.
Tangent stiffness of tibialis anterior in virgin and late-pregnant groups (n = 7/group), represented as mean ± SEM
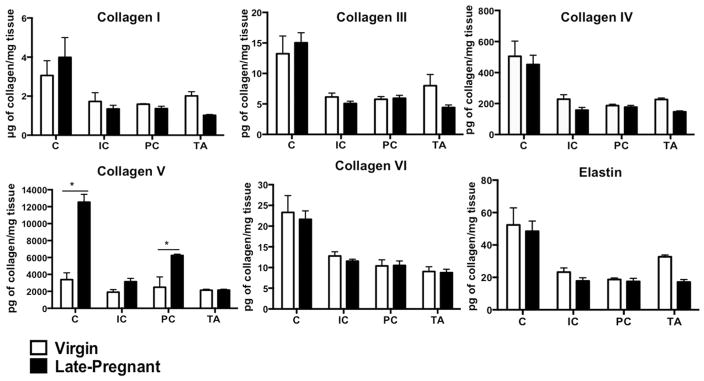
One goal of this investigation was to explore potential sources of pregnancy-induced alterations in the PFM passive mechanical properties. In our previous study, performed on fixed tissue, we found that collagen content rose dramatically in the PFM ECM by the end of gestation.9 In the current study, we quantified and compared elastin, collagen isoforms, and collagen cross-links in PFMs and the TA of virgin and pregnant rats. Elastin content did not differ between groups in either the pelvic or the hind limb muscles (Figure 3). As expected, collagen I was the predominant isoform in the ECM of all muscles examined (Figure 3). The ratio of collagen I to collagen III did not decrease in pregnancy in the intramuscular ECM of the PFMs, as reported for vaginal connective tissue.23 Collagen V increased significantly in C and PC in late-pregnant rats relative to virgin controls (P < .001; Figure 3). Analyses of enzymatic and glycosylated crosslinks in the PFM ECM revealed a differential response to pregnancy. Enzymatic crosslinks were reduced in pregnant rat PFMs, with significant decrease of HPy in PC (P < .02, Table 1) and LPy in C (P < .0001), relative to virgin controls (Table 1). The decrease in enzymatic crosslinks suggests that collagen turnover is lower in these muscles during pregnancy. In contrast, PE levels were significantly elevated in all 3 PFMs in pregnancy (Table 1). Nonetheless, when muscle stiffness was plotted against biochemical parameters examined, there were no significant correlations. Importantly, none of the biochemical changes observed in PFMs were present in the TA (Figure 3, Table 1).
FIGURE 3. Content of intramuscular collagen isoforms and elastin of C, IC, PC, and TA in virgin and late-pregnant groups (n = 7/group), represented as mean ± SEM.
Data presented as mass per milligram tissue wet weight. *Significantly different P values derived from Tukey pairwise comparisons, after repeated measures 2-way ANOVA with significance level set to 5%.
ANOVA, analysis of variance; C, coccygeus; IC, iliocaudalis; PC, pubocaudalis; TA, tibialis anterior.
TABLE 1.
Comparison of collagen crosslinks in the intramuscular extracellular matrix of pelvic floor and hind limb muscles between virgin and late-pregnant rats, reported as mean ± SEM
| Collagen crosslinks | Virgin | Late-pregnant | P-value* |
|---|---|---|---|
| Hydroxylysyl pyridinoline (HPy), pmole/mg tissue | |||
| Coccygeus | 0.080 ± 0.004 | 0.111 ± 0.011 | .24 |
| Iliocaudalis | 0.150 ± 0.012 | 0.116 ± 0.013 | .17 |
| Pubocaudalis | 0.182 ± 0.011 | 0.127 ± 0.014 | .01 |
| Tibialis anterior | 0.156 ± 0.011 | 0.153 ± 0.014 | .99 |
| Lysyl pyridinoline (LPy), pmole/mg tissue | |||
| Coccygeus | 0.091 ± 0.009 | 0.036 ± 0.002 | <.0001 |
| Iliocaudalis | 0.057 ± 0.013 | 0.050 ± 0.004 | .88 |
| Pubocaudalis | 0.053 ± 0.005 | 0.045 ± 0.004 | .84 |
| Tibialis anterior | 0.042 ± 0.002 | 0.037 ± 0.002 | .97 |
| Pentosidine (PE), pmole/mg tissue | |||
| Coccygeus | 0.083 ± 0.007 | 0.122 ± 0.007 | .0002 |
| Iliocaudalis | 0.057 ± 0.002 | 0.081 ± 0.003 | .02 |
| Pubocaudalis | 0.037 ± 0.002 | 0.081 ± 0.010 | <.0001 |
| Tibialis anterior | 0.079 ± 0.004 | 0.086 ± 0.006 | .86 |
P values derived from Tukey’s pairwise comparisons, following 2-way analysis of variance with significance level set to 5%.
Comment
Much effort has been devoted to studying biomechanical and biochemical changes in the vagina and its supportive structures in response to pregnancy. To our knowledge, however, this is the first investigation of the impact of pregnancy on the PFM intramuscular ECM. Increased distensibility of cervix, vagina, and its supportive structures during pregnancy is thought to facilitate delivery of the fetus while minimizing maternal birth injury.12,17,19,22,23,34 In skeletal muscles, the primary mechanism of injury is overstretching the sarcomeres.35–38 Passive tension borne by ECM dominates skeletal muscle load bearing capacity and shields myofibers from mechanical stress and strain.39 With this in mind, it quickly becomes apparent that decreased ECM stiffness would undermine PFM function, which are subjected to increased loads in pregnancy and are strained dramatically during vaginal delivery. The most important finding of this study is that, contrary to changes observed in the connective tissue of other structures, pregnancy induced a significant increase in the PFM passive tension at longer Ls. These functional ECM changes would likely decrease susceptibility of the PFM contractile compartment to injury due to large deformations associated with parturition. In the absence of maternal birth injury, PFM passive mechanical properties recovered, returning to pre-pregnancy state 4 weeks postpartum.
Although a significant increase in stiffness occurred in C and PC, muscle stiffness of IC did not increase in pregnancy. At this time, it remains unclear which biochemical parameters dictate the increase in stiffness of the intramuscular ECM. Thus, we cannot definitively say what caused the differential response in the PFM passive mechanics. It is possible that the substantial increase in collagen V, observed in C and PC but not in IC, is partially responsible. Even though collagen V is a minor fibrillar collagen, this isoform is critical for maintaining the structure of collagen I that dominates intramuscular ECM.40 Furthermore, the protruding amino terminal domains of collagen V inhibit slippage of collagen molecules during mechanical deformation, resulting in increased ECM stiffness.41
With respect to collagen crosslinks, our initial investigations yielded intriguing results. Pregnancy induced a decrease in enzymatic crosslinks whereas it significantly escalated glycosylated crosslinks in the PFMs. Enzymatic crosslinks, hydroxylysyl pyridinoline, and lysyl pyridinoline are formed in fibrillar collagens by lysyl oxidase, which reacts with hydroxylysyl and lysyl side chains. The aforementioned posttranslational modification leads to the formation of stable and insoluble collagen fibers.42 Glycosylated crosslinks, such as pentosidine, are formed via nonenzymatic bonds between reducing sugar and collagen protein residues.43 Pentosidine, which is one of the advanced glycation end-products, has been shown to increase in the intramuscular ECM with aging, diabetes, obesity, and increased oxidative stress.43 Greater glycosylated crosslinks in the PFM ECM in the pregnant group may result from the increased oxidative stress, measured in pregnancy.44 Previous reports showed an association between enzymatic crosslinks and muscle stiffness. Therefore, we were somewhat surprised to find decreased HPy and LPy in C and PC that demonstrated increased passive tension in pregnancy, but not in IC, where stiffness remained unchanged. Consistent with this finding is a recent report from our laboratory, which also found no significant correlation between collagen crosslinks and limb muscle stiffness.33 The precise source(s) of increased PFM stiffness in pregnancy remain elusive at this time.
Once we confirmed our initial hypothesis that pregnancy does indeed induce adaptations in the PFM ECM, we assessed whether such changes occurred in the muscles outside of pelvis. None of the biomechanical or biochemical differences that occurred in the PFMs were observed in the TA of pregnant animals, compared with virgin controls. This result suggests that PFMs are uniquely exposed to the transformed mechanical and/or biochemical environment associated with pregnancy. It is also possible that muscles outside of pelvis are subjected to similar mechanical and biochemical changes during pregnancy but do not mount the same response as the PFMs due to intrinsic differences. This is a topic of future studies.
Muscle passive mechanical properties are important for active and passive muscle function, as well as protection from mechanical injury. Despite the limitations inherent to the use of a rodent model for the studies of muscle properties, the rat provides a valuable model for directly determining changes in PFM stiffness that occur in pregnancy and elucidating mechanisms that govern these alterations. Maternal birth injury of the PFMs occurs in thousands of women every year; however, currently we do not have effective approach to prevent such injuries in women delivering vaginally. One impediment to developing preventative strategies stems from the key unanswered question: what differentiates women who sustain PFM birth injury from those who do not? Our studies describe the pregnancy-induced alterations in the PFM contractile and intramuscular ECM components. Delineation of protective alterations in the PFM in our animal model provides specific parameters to be targeted by noninvasive imaging to investigate the extent of such adaptations in women. Furthermore, understanding tissue-level changes is necessary to enable future investigations focused on the identification of novel targets for interventions designed to promote protective adaptations in the PFMs that can potentially reduce PFM injury, and in turn, decrease the incidence of postpartum pelvic floor disorders.
Acknowledgments
The authors gratefully acknowledge funding by National Institutes of Health grants K12 HD001259 and R24 HD050837 for the conduct of this research.
Footnotes
Presented as a podium presentation at the annual meeting of the American Urogynecologic Society, Seattle, WA, Oct. 13–17, 2015.
References
- 1.Subak LL, Waetjen LE, van den Eeden S, Thom DH, Vittinghoff E, Brown JS. Cost of pelvic organ prolapse surgery in the United States. Obstet Gynecol. 2001:98646–51. doi: 10.1016/s0029-7844(01)01472-7. [DOI] [PubMed] [Google Scholar]
- 2.Wilson L, Brown JS, Shin GP, Luc K-O, Subak LL. Annual direct cost of urinary incontinence. Obstet Gynecol. 2001;98:398–406. doi: 10.1016/s0029-7844(01)01464-8. [DOI] [PubMed] [Google Scholar]
- 3.DeLancey JO. Anatomy and biomechanics of genital prolapse. Clin Obstet Gynecol. 1993;36:897–909. doi: 10.1097/00003081-199312000-00015. [DOI] [PubMed] [Google Scholar]
- 4.Morgan DM, Larson K, Lewicky-Gaupp C, Fenner DE, DeLancey JO. Vaginal support as determined by levator ani defect status 6 weeks after primary surgery for pelvic organ prolapse. Int J Gynaecol Obstet. 2011;114:141–4. doi: 10.1016/j.ijgo.2011.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lien KC, Mooney B, DeLancey JO, Ashton-Miller JA. Levator ani muscle stretch induced by simulated vaginal birth. Obstet Gynecol. 2004;103:31–40. doi: 10.1097/01.AOG.0000109207.22354.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoyte L, Damaser MS, Warfield SK, et al. Quantity and distribution of levator ani stretch during simulated vaginal childbirth. Am J Obstet Gynecol. 2008;199(198):e1–5. doi: 10.1016/j.ajog.2008.04.027. [DOI] [PubMed] [Google Scholar]
- 7.Shek KL, Dietz HP. Intrapartum risk factors for levator trauma. BJOG. 2010;117:1485–92. doi: 10.1111/j.1471-0528.2010.02704.x. [DOI] [PubMed] [Google Scholar]
- 8.Dietz HP, Lanzarone V. Levator trauma after vaginaldelivery. Obstet Gynecol. 2005;106:707–12. doi: 10.1097/01.AOG.0000178779.62181.01. [DOI] [PubMed] [Google Scholar]
- 9.Alperin M, Lawley DM, Esparza MC, Lieber RL. Pregnancy-induced adaptations in the intrinsic structure of rat pelvic floor muscles. Am J Obstet Gynecol. 2015;213:191.e1–7. doi: 10.1016/j.ajog.2015.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gillies AR, Lieber RL. Structure and function of the skeletal muscle extracellular matrix. Muscle Nerve. 2011;44:318–31. doi: 10.1002/mus.22094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meyer GA, Lieber RL. Elucidation of extracellular matrix mechanics from muscle fibers and fiber bundles. J Biomech. 2011;44:771–3. doi: 10.1016/j.jbiomech.2010.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sherwood OD, Crnekovic VE, Gordon WL, Rutherford JE. Radioimmunoassay of relaxin throughout pregnancy and during parturition in the rat. Endocrinology. 1980;107:691–8. doi: 10.1210/endo-107-3-691. [DOI] [PubMed] [Google Scholar]
- 13.Samuel CS, Coghlan JP, Bateman JF. Effects of relaxin, pregnancy and parturition on collagen metabolism in the rat pubic symphysis. J Endocrinol. 1998;159:117–25. doi: 10.1677/joe.0.1590117. [DOI] [PubMed] [Google Scholar]
- 14.Soh YM, Tiwari A, Mahendroo M, Conrad KP, Parry LJ. Relaxin regulates hyaluronan synthesis and aquaporins in the cervix of late pregnant mice. Endocrinology. 2012;153:6054–64. doi: 10.1210/en.2012-1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sherwood OD. Relaxin’s physiological roles and other diverse actions. Endocr Rev. 2004;25:205–34. doi: 10.1210/er.2003-0013. [DOI] [PubMed] [Google Scholar]
- 16.Harvey MA, Johnston SL, Davies GA. Mid-trimester serum relaxin concentrations and postpartum pelvic floor dysfunction. Acta Obstet Gynecol Scand. 2008;87:1315–21. doi: 10.1080/00016340802460321. [DOI] [PubMed] [Google Scholar]
- 17.Alperin M, Feola A, Duerr R, Moalli P, Abramowitch S. Pregnancy- and delivery-induced biomechanical changes in rat vagina persist postpartum. Int Urogynecol J. 2010;21:1169–74. doi: 10.1007/s00192-010-1149-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feola A, Moalli P, Alperin M, Duerr R, Gandley RE, Abramowitch S. Impact of pregnancy and vaginal delivery on the passive and active mechanics of the rat vagina. Ann Biomed Eng. 2011;39:549–58. doi: 10.1007/s10439-010-0153-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oliphant SS, Nygaard IE, Zong W, Canavan TP, Moalli PA. Maternal adaptations in preparation for parturition predict uncomplicated spontaneous delivery outcome. Am J Obstet Gynecol. 2014;211(630):e1–7. doi: 10.1016/j.ajog.2014.06.021. [DOI] [PubMed] [Google Scholar]
- 20.Alperin M, Tuttle LJ, Conner BR, et al. Comparison of pelvic muscle architecture between humans and commonly used laboratory species. Int Urogynecol J. 2014;25:1507–15. doi: 10.1007/s00192-014-2423-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kokenyesi R, Woessner JF., Jr Effects of hormonal perturbations on the small dermatan sulfate proteoglycan and mechanical properties of the uterine cervix of late pregnant rats. Connective Tissue Res. 1991;26:199–205. doi: 10.3109/03008209109152438. [DOI] [PubMed] [Google Scholar]
- 22.Lowder JL, Debes KM, Moon DK, Howden N, Abramowitch SD, Moalli PA. Biomechanical adaptations of the rat vagina and supportive tissues in pregnancy to accommodate delivery. Obstet Gynecol. 2007;109:136–43. doi: 10.1097/01.AOG.0000250472.96672.6c. [DOI] [PubMed] [Google Scholar]
- 23.Ulrich D, Edwards SL, Su K, et al. Influence of reproductive status on tissue composition and biomechanical properties of ovine vagina. PloS One. 2014;9:e93172. doi: 10.1371/journal.pone.0093172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lopez B, Querejeta R, Gonzalez A, Larman M, Diez J. Collagen cross-linking but not collagen amount associates with elevated filling pressures in hypertensive patients with stage C heart failure: potential role of lysyl oxidase. Hypertension. 2012;60:677–83. doi: 10.1161/HYPERTENSIONAHA.112.196113. [DOI] [PubMed] [Google Scholar]
- 25.Ducomps C, Mauriege P, Darche B, Combes S, Lebas F, Doutreloux JP. Effects of jump training on passive mechanical stress and stiffness in rabbit skeletal muscle: role of collagen. Acta Physiol Scand. 2003;178:215–24. doi: 10.1046/j.1365-201X.2003.01109.x. [DOI] [PubMed] [Google Scholar]
- 26.Brown SH, Carr JA, Ward SR, Lieber RL. Passive mechanical properties of rat abdominal wall muscles suggest an important role of the extracellular connective tissue matrix. J Orthop Res. 2012;30:1321–6. doi: 10.1002/jor.22068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Friden J, Lieber RL. Spastic muscle cells are shorter and stiffer than normal cells. Muscle Nerve. 2003;27:157–64. doi: 10.1002/mus.10247. [DOI] [PubMed] [Google Scholar]
- 28.Lieber RL, Yeh Y, Baskin RJ. Sarcomere length determination using laser diffraction. Effect of beam and fiber diameter. Biophys J. 1984;45:1007–16. doi: 10.1016/S0006-3495(84)84246-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith LR, Lee KS, Ward SR, Chambers HG, Lieber RL. Hamstring contractures in children with spastic cerebral palsy result from a stiffer extracellular matrix and increased in vivo sarcomere length. J Physiol. 2011;589:2625–39. doi: 10.1113/jphysiol.2010.203364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith LR, Barton ER. Collagen content does not alter the passive mechanical properties of fibrotic skeletal muscle in mdx mice. Am J Physiol Cell Physiol. 2014;306:C889–98. doi: 10.1152/ajpcell.00383.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lieber RL, Ward SR. Cellular mechanisms of tissue fibrosis. 4. Structural and functional consequences of skeletal muscle fibrosis. Am J Physiol Cell Physiol. 2013;305:C241–52. doi: 10.1152/ajpcell.00173.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bank RA, Beekman B, Verzijl N, de Roos JA, Sakkee AN, TeKoppele JM. Sensitive fluorimetric quantitation of pyridinium and pentosidine crosslinks in biological samples in a single high-performance liquid chromatographic run. J Chromatogr B Biomed Sci Appl. 1997;703:37–44. doi: 10.1016/s0378-4347(97)00391-5. [DOI] [PubMed] [Google Scholar]
- 33.Chapman MA, Pichika R, Lieber RL. Collagen crosslinking does not dictate stiffness in a transgenic mouse model of skeletal muscle fibrosis. J Biomech. 2015;48:375–8. doi: 10.1016/j.jbiomech.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao S, Sherwood OD. Monoclonal antibodies specific for rat relaxin. X. Endogenous relaxin induces changes in the histological characteristics of the rat vagina during the second half of pregnancy. Endocrinology. 1998;139:4726–34. doi: 10.1210/endo.139.11.6327. [DOI] [PubMed] [Google Scholar]
- 35.Lieber RL, Friden J. Muscle damage is not a function of muscle force but active muscle strain. J Appl Physiol. 1993;74:520–6. doi: 10.1152/jappl.1993.74.2.520. [DOI] [PubMed] [Google Scholar]
- 36.Lieber RL, Friden J. Mechanisms of muscle injury gleaned from animal models. Am J Phys Med Rehabil. 2002;81(11 Suppl):S70–9. doi: 10.1097/00002060-200211001-00008. [DOI] [PubMed] [Google Scholar]
- 37.Patel TJ, Das R, Friden J, Lutz GJ, Lieber RL. Sarcomere strain and heterogeneity correlate with injury to frog skeletal muscle fiber bundles. J Appl Physiol. 2004;97:1803–13. doi: 10.1152/japplphysiol.00505.2003. [DOI] [PubMed] [Google Scholar]
- 38.Morgan DL. New insights into the behavior of muscle during active lengthening. Biophys J. 1990;57:209–21. doi: 10.1016/S0006-3495(90)82524-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Purslow PP. Muscle fascia and force transmission. J Bodyw Mov Ther. 2010;14:411–7. doi: 10.1016/j.jbmt.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 40.Collins M, Posthumus M. Type V collagen genotype and exercise-related phenotype relationships: a novel hypothesis. Exerc Sport Sci Rev. 2011;39:191–8. doi: 10.1097/JES.0b013e318224e853. [DOI] [PubMed] [Google Scholar]
- 41.Silver FH, Freeman JW, Seehra GP. Collagen self-assembly and the development of tendon mechanical properties. J Biomech. 2003;36:1529–53. doi: 10.1016/s0021-9290(03)00135-0. [DOI] [PubMed] [Google Scholar]
- 42.Lucero HA, Kagan HM. Lysyl oxidase: an oxidative enzyme and effector of cell function. Cell Mol Life Sci. 2006;63:2304–16. doi: 10.1007/s00018-006-6149-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haus JM, Carrithers JA, Trappe SW, Trappe TA. Collagen, cross-linking, and advanced glycation end products in aging human skeletal muscle. J Appl Physiol. 2007;103:2068–76. doi: 10.1152/japplphysiol.00670.2007. [DOI] [PubMed] [Google Scholar]
- 44.Toescu V, Nuttall SL, Martin U, Kendall MJ, Dunne F. Oxidative stress and normal pregnancy. Clin Endocrinol. 2002;57:609–13. doi: 10.1046/j.1365-2265.2002.01638.x. [DOI] [PubMed] [Google Scholar]