Abstract
Endothelium, a thin monolayer of specialized cells lining the lumen of blood vessels is the key regulatory interface between blood and tissues. Endothelial abnormalities are implicated in many diseases, including common acute conditions with high morbidity and mortality lacking therapy, in part because drugs and drug carriers have no natural endothelial affinity. Precise endothelial drug delivery may improve management of these conditions. Using ligands of molecules exposed to the bloodstream on the endothelial surface enables design of diverse targeted endothelial nanomedicine agents. Target molecules and binding epitopes must be accessible to drug carriers, carriers must be free of harmful effects, and targeting should provide desirable sub-cellular addressing of the drug cargo. The roster of current candidate target molecules for endothelial nanomedicine includes peptidases and other enzymes, cell adhesion molecules and integrins, localized in different domains of the endothelial plasmalemma and differentially distributed throughout the vasculature. Endowing carriers with an affinity to specific endothelial epitopes enables an unprecedented level of precision of control of drug delivery: binding to selected endothelial cell phenotypes, cellular addressing and duration of therapeutic effects. Features of nanocarrier design such as choice of epitope and ligand control delivery and effect of targeted endothelial nanomedicine agents. Pathological factors modulate endothelial targeting and uptake of nanocarriers. Selection of optimal binding sites and design features of nanocarriers are key controllable factors that can be iteratively engineered based on their performance from in vitro to pre-clinical in vivo experimental models. Targeted endothelial nanomedicine agents provide antioxidant, anti-inflammatory and other therapeutic effects unattainable by non-targeted counterparts in animal models of common acute severe human disease conditions. The results of animal studies provide the basis for the challenging translation endothelial nanomedicine into the clinical domain.
Keywords: Drug delivery, Vascular immunotargeting
1. Introduction: drug delivery systems for specific interventions in endothelial cells
Targeted delivery to intended sites enhances the mechanistic precision, effficacy and safety of action of drugs [1–5]. Drug delivery systems (DDSs) devised for this goal use carriers including multi-molecular structures (e.g., liposomes, polymersomes, dendrimers, nanoparticles, micelles and others) assembled from elements – both distinct and modular – providing drug loading/unloading, masking, targeting and other functions of DDS [6–16].
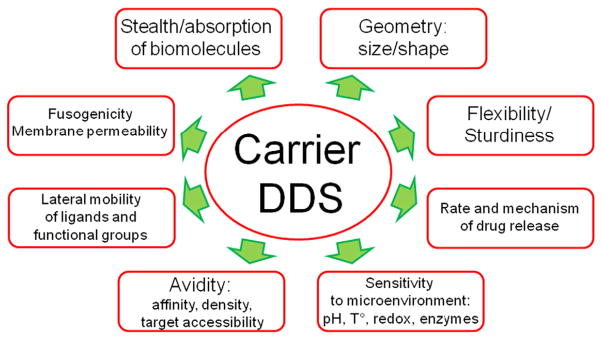
Permutations of these elements of DDS's design yield a diversity of size, shape, morphologies, chemical structures, stability, physical and surface properties, ability to change in response to microenvironment, elude undesirable interactions in the body, etc. [17–20]. These parameters of DDS's design govern its characteristic behavior in the body and its interaction with its target - binding, uptake, sub-cellular addressing and release of drug (Fig. 1).
Fig. 1.
Parameters of design of drug delivery systems and carriers that define their functional characteristics. These DDS features influence diverse aspects and phases of drug delivery: circulation, pharmacokinetics and biodistribution; target recognition and anchoring; subsequent intracellular delivery and drug release; duration, beneficial and adverse effects of the DDS. Some design parameters influence distinct aspects of DDSs performance, whereas other parameters influence many aspects. For example, lateral mobility of ligands predominantly inf;uences anchoring on the target, whereas carrier's geometry inf;uences available routes of administration, circulation, targeting and uptake by target and non-target cells.
On the other hand, biological factors, both systemic and local, modulate and in many cases govern characteristics of DDS behavior and interaction with the target. For example, the heart rate and status of defense systems modulate the PK of carriers, while perfusion of the target tissue, accessibility of binding sites and their function all affect targeting. Furthermore, pathological factors typical of the condition that a DDS is devised to treat often cardinally alter these biological factors, in turn affecting targeting.
Therefore, no single DDS can optimally serve diverse therapeutic goals. Each disease condition, therapeutic goal and characteristics of drugs ask for a unique set of DDS's parameters optimally serving every phase of drug delivery — administration, circulation, targeting, sub-cellular addressing and effect in the given disease, and, in fact, in each individual patient.
The endothelium represents an important target for therapeutic interventions [21–24]. A thin monolayer of endothelial cells lining the vascular lumen forms a regulatory interface between blood and tissues. Via strategic location and its numerous functions, the endothelium is involved in the pathogenesis of many human maladies. It is a key therapeutic site in cardiovascular, pulmonary, neurological, oncologic, metabolic, rheumatologic, and many other conditions, including acute dangerous conditions that have no current therapeutic options.
Design of DDS targeted to the endothelium (“endothelial nanomedicine”) is a field of biomedicine in which many challenges and opportunities are distinct from the various other realms of nanomedicine. In this context it is illuminating to draw comparisons between non-oncological endothelial nanomedicine and the dominant field of drug delivery — oncological nanomedicine, or drug delivery to cancer cells (Table 1) [25–27]. The top priorities for oncological drug delivery are maximal selectivity for tumor cells and effectiveness of tumor cell killing. Thus, if the DDS itself has incidental toxicity to the target cell (including endothelium in the tumor vasculature), that is a bonus. In contrast, the typical cargo drugs for endothelial nanomedicine are relatively benign. Therefore, the criteria for targeting selectivity are not as stringent as in oncology. Systemic effects (e.g., anti-inflammatory) may be a bonus in many conditions in the realms of “endothelial nanomedicine”. However, in contrast with oncology, target cell toxicity is not at all tolerable in endothelial nanomedicine. Another contrast between endothelial and oncological nanomedicine is the targeting challenges. Targeting tumors is impeded by the major problems of accessibility of DDS to the internal portions of tumors and the heterogeneity of malignant cells. These targeting challenges may ultimately necessitate the use of cocktails of anti-tumor drugs targeted to multiple markers. In contrast, the endothelium is readily accessible to blood, with far less heterogeneity than tumors [28]. Thus, the fields of endothelial and oncological nanomedicine have markedly different constraints, and thus will likely necessitate quite divergent solutions. Drug targeting to endothelium in tumor vasculature in some aspects shares similarities with oncological nanomedicine and in some aspects –with endothelial nanomedicine, thereby forming a distinct area of oncological drug delivery.
Table 1.
Drug targeting outlook in different fields.
| Oncology | Heart, lung and blood diseases |
|---|---|
| Challenges: | Challenges: |
| Heterogeneity of targets and diseases | High benefit/risk bar and low ROI |
| Poor target accessibility | Limited investments and research |
| Specific paradigms: | Specific paradigms: |
| Toxic drugs, collateral damage | More benign drugs vs. oncology |
| Maximal specificity of targeting needed | Systemic effects often welcome |
| Side effects to target are welcome | Side effects to target unacceptable |
| Advantages: | Advantages: |
| Dominant field of drug targeting | Huge medical need (I/R, ARDS) |
| Side effects are often beneficial | Common mechanisms and targets |
| Low benefit/risk bar and high ROI | Target accessibility to blood |
The endothelium plays a major role in numerous impactful diseases. Among these conditions are adult respiratory distress syndrome (ARDS), sepsis, thrombosis, disseminated intravascular coagulation (DIC) and bleeding disorders, ischemia–reperfusion (I/R, including myocardial infarction, stroke, transplantation, cardiopulmonary bypass and other “tourniquet” syndromes), hypertension, restenosis, atherosclerosis, diabetes, arthritis, and many others [29–34]. These maladies involve, to varying degrees, endothelial-regulated stresses such as inflammation, clotting, hypoxia, abnormal blood floow, oxidative stress, local abnormal metabolism, drug processing, and more [35–37].
This review focuses on dangerous and common acute pathological conditions lacking therapy. We will mention only in passing chronic conditions involving the endothelium. The treatment of acute diseases with endothelial nanomedicine is well positioned for translation into the clinical domain, and our hope is this review of the basic and pre-clinical literature will help focus the drive to clinic use.
2. Vascular endothelium
Endothelium is an integral vascular tissue formed by highly specialized “epithelial-like” cells. Similar to epithelia, endothelia line extended tissue interfaces. Endothelial cells line the lumen of blood and lymphatic circulatory systems, heart chambers and valves, and cavities in the central nervous system. This article focuses on endothelium in blood vessels
Endothelial cells form a continuous thin monolayer lining the vascular lumen. In blood vessels — arteries, capillaries and veins — endothelium and its basal membrane form the tunica intima, or inner layer, surrounded in arteries and veins by the tunica media (containing smooth muscle and other vascular cells) and the external tunica adventitia (loosely organized components of extracellular matrix containing nerves and blood vessels feeding the vessel wall, called the vasa vasorum). Endothelial cells can be found only in the intima. In capillaries they represent the only continuous cellular elements, reinforced by external pericytes and other cell types [38].
Endothelial cell dimensions vary from roughly square ~30–50 × 30–50 μm in capillaries to elongated shape ~10–20 × 40–50 μm in the arteries, where endothelial cells align with flow direction due to hydrodynamic forces. Endothelial cells are flat, with thickness varying from a few microns in the nuclear region to less than one micron in the part of cellular body lacking organelles, through which gas exchange occurs.
The blood vessel endothelium in most organs is presented in the form of a continuous cellular monolayer with tight yet dynamic inter-cellular junctions. In the liver and spleen, the endothelial monolayer is discontinuous, with an average size of 145 nm intercellular openings into the hepatic sinuses and micron-sized gaps in the splenic pulp, through which large macromolecules including medium-size chylomicrons and blood cells, respectively, are able to migrate from the blood to these organs of the reticulo-endothelial system (RES) [39–41]. In the renal glomeruli and some other specialized vascular areas, endothelial cells have fenestrae, with and without diaphragms, allowing trans-cellular transport [42].
For a long time after its discovery in the 19th century, the endothelium was viewed only as a lubricating inner vascular layer preventing adhesion of blood elements to the vessel wall. Studies of the last few decades revealed that, in addition to this important role, endothelial cells exert many sensory and executive functions. These functions include control of vascular permeability, adhesiveness, contractility and formation of new vessels (angiogenesis), sensing of mechanical forces related to blood flow and vessel contraction, interaction of blood components with vessels and underlying tissues, blood metabolism, clotting and fluidity, and gas exchange. Via control of these multifaceted features, the endothelium helps to execute the main functions of cardiovascular and pulmonary systems; namely, delivery of needed substances to tissues, waste removal and immune surveillance. Therefore, the endothelium is the key regulatory interface between the blood and extravascular tissues.
2.1. Blood fluidity and clotting
The endothelium regulates blood fluidity by a variety of mechanisms [43]. First, the endothelium forms a non-adhesive surface coated with a layer of negatively charged glycocalyx that mechanically prevents collisions of blood elements with the vascular wall and keeps away blood cellular elements via this mechanical barrier and electrostatic repelling. Second, endothelial cells express surface glycoproteins that inhibit blood clotting. Examples include, endothelial thrombomodulin, which binds thrombin and converts it from a pro-thrombotic to an anti-thrombotic agent, and endothelial CD39/NTPDase-1, which degrades the platelet agonist ADP. Third, the endothelium secrets and facilitates the enzymatic activity of plasminogen activators urokinase and tPA, which form plasmin that in turn cleaves fibrin. Fourth, small molecules released from endothelial cells, namely, nitric oxide (NO, a gas molecule formed from arginine by nitric oxide synthase, NOS) and prostacyclin (a prostaglandin enzymatically produced from arachidonic acid) both inhibit platelets. Under normal circumstances these mechanisms provide uninterrupted blood flow in the vascular system
In contrast to this flow-facilitating role of normal endothelium, in sites of vascular damage, endothelial cells instead support clotting, with the teleological goal of preventing bleeding [44,45]. In this role, endothelial cells expose pro-coagulant molecules phosphatidyl serine (PS), inhibitors of plasmin and its activators, and release glycoproteins that stimulate blood clotting and platelet activation, such as von Willebrand factor and P-selectin from intracellular stores (Weibel-Palade bodies). Further, the endothelium ceases its anti-clotting activities listed above, for example, via inactivation and/or shedding of regulatory proteins such as thrombomodulin.
2.2. Transport across the vessel wall
Endothelial cells control transport to and from parenchyma the compounds circulating in blood, including natural carrier molecules, nutrients, and cells [46–48]. Both transcellular and pericellular (inter-cellular) mechanisms are involved in endothelial transport. In the former case, endothelial cells bind natural carrier molecules (albumin, lipoproteins, transferrin, etc.) via cognate receptors, which enter vesicles formed from the plasmalemma in the ensuing endocytic pathways, some of which transfer these cargo molecules to the parenchyma. Leukocytes (white blood cells, WBC) also can use these vacuolar–vesicular organelles (VVO)m forming dynamic channels through endothelial cells for extravasation in response to some agonists. However, the majority of leukocyte transport occurs via reversibly widening inter-endothelial junctions, i.e., via pericellular pathway
The endothelium delivers to tissues blood plasma components constitutively using the transcellular fluid uptake pathways of pinocytosis. Transient widening of intercellular junctions provides qualitative increases in plasma exudation in response to pathological agents. Some pathological mediators, for example, VEGF, predominantly stimulate transcellular fluid transport via formation in the endothelial luminal surface of fenestrae directing fluids to VVO-type channels. Some mediators, e.g., thrombin, predominantly open intercellular junctions. Understanding these mechanisms is vital for vascular nanomedicine, because of their key role in inflammation and edema, as well as the potential utility for drug delivery from blood to tissues.
2.3. Regulation of vascular tone and blood pressure
Endothelial cells produce vasoactive molecules that diffuse to the tunica media and regulate contraction of vascular smooth muscle cells (SMC) by regulation of actin–myosin interaction and signal transduction via SMC receptors. Nitric oxide and prostacyclin enzymatically produced by endothelial cells inhibit SMC contraction, thereby causing vasodilation and reduction of blood pressure [49]
A contrasting role is provided by several endothelium-produced vasoconstrictors. Angiotensin-converting enzyme (ACE), a transmembrane ecto-peptidase anchored on the luminal endothelial surface, cleaves two amino acids from an inactive precursor in plasma, angiotensin I, producing a potent pro-contractile, pro-inflammatory and pro-proliferative peptide, angiotensin-II. In addition, ACE cleaves and thus inactivates a vasodilating peptide, bradykinin. Furthermore, endothelial cells produce a short peptide, endothelin, the most potent vasoconstricting agent (on the molar basis). The balance between vasodilating and vasoconstricting mediators produced by the endothelium may either suppress or stimulate vascular contraction [45].
2.4. Control of host defense and inflammation
Endothelial cells provide important regulatory functions in many cellular and humoral mechanisms of innate and adaptive immune defense. For example, endothelial surface glycoproteins such as Decay Acceleration Factor (DAF) and CD59 inhibit activation of complement and thus prevent vascular injury. Another key immune role of endothelial cells is their binding and recycling of immunoglobulins via endothelial FcRn receptors supports prolonged circulation of IgG, needed for continuous immunological surveillance. Perhaps the greatest immune role of the endothelium is its interaction with WBCs. In inflammation, endothelial cells release cytokines and chemokines that activate and attract WBC and guide their transmigration from blood to tissues via exposure of cell adhesion molecules [45]
A notable role of the endothelium in the immune system is binding of WBCs to endothelial cells via cell adhesion molecules. This process is particularly interesting for endothelial nanomedicine, as many DDSs recapitulate such endothelial binding to effect drug delivery. Adhesion molecules involved early in WBC-endothelium interaction include the P- and E-selectins, glycoproteins transiently exposed on the endothelial lumen via mobilization from intracellular stores (Weibel-Palade bodies) and also produced by de novo in response to pathological stimuli. Inducible selectins mediate low-affinity binding and deceleration of WBC rolling. Firm adhesion is provided by leukocyte binding via β1 and β2 integrins to Inter-Cellular Adhesion Molecule-1 (ICAM-1) and Vascular Cell Adhesion Molecule-1 (VCAM-1), which are constitutively expressed by quiescent endothelial cells and up-regulated in sites of inflammation and other vascular pathologies. Stably expressed platelet-endothelial cell adhesion molecule 1 (PECAM-1), which interacts with its leukocyte homolog at the endothelial cell-cell borders supports leukocyte diapedesis [50].
2.5. Reception and transmission of chemical and mechanical signals
Via an extremely rich repertoire of receptors, endothelial cells receive myriad chemical and physical signals from blood and tissues, and transmit these signals to the blood, tunica media, and tissue parenchyma. Chemical signals include oxygen and CO2, vasoactive agents (e.g., peptides and prostaglandins), transporting molecules (e.g., albumin, lipoproteins, and transferrin), pathological mediators (cytokines, reactive oxygen species, and proteases including thrombin), nutrients, and hundreds of other natural and injected compounds. In addition to sensing chemicals, endothelial cells sense and transmit the physical forces of flow shear stress via sensor systems including glycocalyx, caveolae, cytoskeleton and integrins interconnected with components of extracellular matrix and inter-cellular junctions (in particular, PECAM-1).
3. Drug delivery to endothelial cells: general principles
3.1. Passive vascular targeting
Carriers may accumulate in sites of interest via diverse mechanisms, some of which do not involve target affinity. Mechanical retention underlies the mechanism of several types of such non-affinity or “passive targeting”. For example, nanocarriers are mechanically retained in stagnant blood in the vasculature upstream of sites of ischemia or thrombotic occlusion. Rigid particles larger than a few microns such as albumin microspheres are mechanically entrapped in the microvasculature downstream of the injection site via first-pass uptake. Even small (<50 nm in diameter) particles, if hydrophobic, charged, or possessing non-specific affinity for proteins, tend to agglomerate in blood and resultant aggregates behave as large particles. Cationic particles bind to the negatively charged glycocalyx on the surface of endothelial and other accessible cells. Finally, circulating particles may accumulate in tissues that exhibit elevated vascular permeability due to inflammation, angiogenesis, or tumor growth.
Additional approaches not involving ligand-mediated binding to defined targets are evolving. For example, a high-throughput variable synthesis of lipid-based nanocarriers yielded numerous DDSs varying in shape and surface features, showing distinct patterns of biodistribution in animals [51]. The mechanisms underlying such particles' homing to given organs remain to be understood. Most likely it involves interaction with endothelial cells directly or indirectly, via intermediary blood components. Another strategy for “passive” endothelial DDS is hitchhiking on the surface of red blood cells (RBCs) [52]. RBCs may carry some types of nanoparticles in circulation, diverting their clearance by the liver and spleen to transfer to the endothelium predominantly in the pulmonary vasculature. The mechanism, selectivity, cellular targets and effects of chemical combinatorial and RBC-hitchhiking approaches need to be better understood to gauge their potential clinical utility.
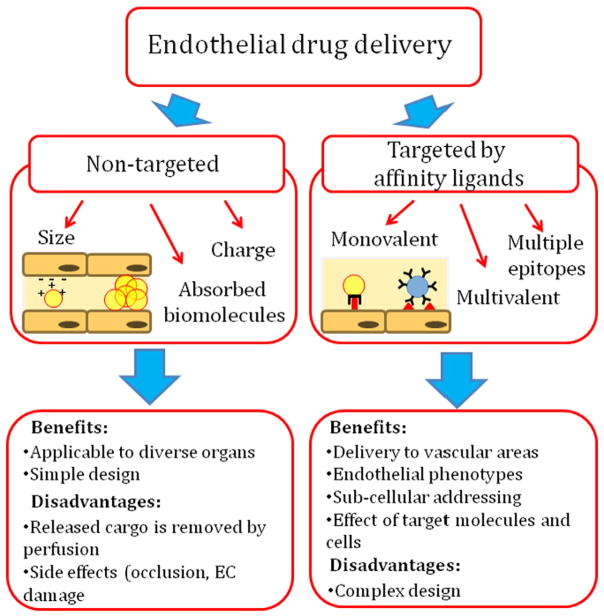
“Passively targeted” DDS may deliver cargoes to vascular areas downstream of the injection site and may have translational advantage vs. more complex DDS containing affinity ligands (Fig. 2). However, mechanisms that do not involve specific affinity to a target provide very little, if any, control of the intracellular localization of drug cargoes [53]. Blood perfusion will eliminate a major fraction of drugs released from carriers that are not guided for delivery into the target cells. Further, vascular drug delivery relying on the carrier's mechanical retention may hinder vessel's patency and affect vascular cells, causing adverse effects typical of embolism.
Fig. 2.
‘Passive’ vs. ‘active’ delivery of nanomedicine agents to vascular endothelium. Passive delivery relies on mechanisms bypassing affinity interaction with defined molecules. They include adhesion and uptake in the vessels due to size, charge, rigidity, or amphiphilic features of the carrier itself, carrier agglomerates, or carrier-adsorbing intermediary molecules in blood. Affinity targeting is provided by ligands that can be conjugated to the DDS surface in molecular configurations permitting diverse modes of specific anchoring of DDS onto the target surface and subsequent cellular uptake.
3.2. Active targeting mediated by affinity ligands
“Active targeting” offers arguably a more precise and safer approach. Active targeting is typically achieved by conjugating DDSs to ligand molecules that bind to the endothelial [23,24,54–57]. Antibodies and their derivatives including single chain antigen binding fragments (scFv), nutrients, hormones, receptor ligands, peptides, aptamers, and nucleic acids have been explored as targeting ligands for vascular DDSs [21,58–61]
DDSs using this approach can use monovalent and multivalent binding, as well as binding to multiple endothelial determinants and epitopes, providing versatile mechanisms for target recognition. Furthermore, the mode of DDS anchoring and the nature of the endothelial “target determinant” (binding epitope on the endothelium) dictate drug delivery to specific vascular areas and endothelial sub-cellular compartments, and mediate additional effects. Numerous studies in animal models and a few clinical studies [23,62–70] showed that affinity-guided DDSs enable targeting of diverse agents to normal and pathological endothelium [71–74].
The intravenous routes bypassing the liver direct the first pass of blood to the lungs [75]. The pulmonary vasculature represents ~25% of the total endothelial surface in the body and receives the entire cardiac output (through the lower pressure pulmonary arterial system), whereas all other organs share a fraction of cardiac output through the high pressure systemic arterial system. This privileged perfusion pattern and enormous surface area favors pulmonary accumulation of pan-endothelial ligands. An infusion via the conduit vessel favors uptake in the downstream microvasculature [75,76].
4. Endothelial target determinants
4.1. Approaches to define endothelial determinants
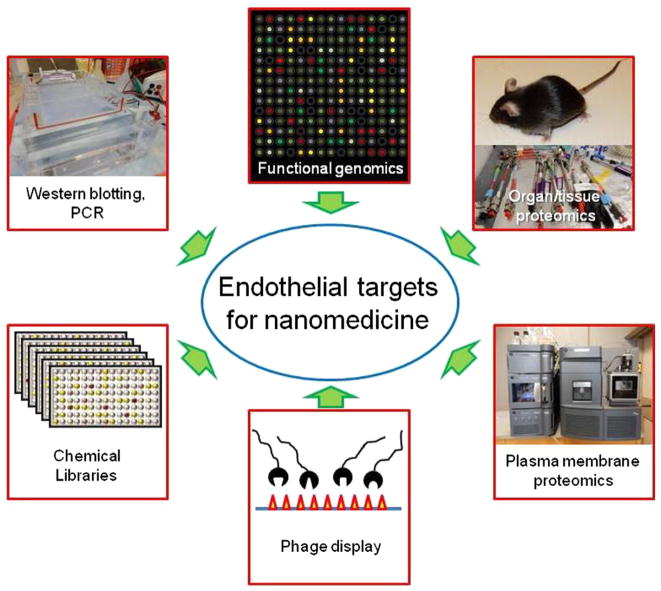
Immunostaining, FACS, Western blotting, PCR and high-throughput approaches of functional genomics and proteomics help to detect and localize proteins enriched in target tissue (Fig. 3). However, target determinants must meet several criteria that cannot be characterized by these standard methods. Proteomics of the plasmalemma separated from the vasculature of organs of interest [77] and selection of homing ligands using phage display libraries in animals and in cadavers provide additional important insights in target accessibility on the cell surface [78–80]
Fig. 3.
Approaches endothelial determinants search. A variety of high throughput ‘omics’ approaches allow detection and localization of target determinants. Ultimately, such target determinants must be assessed by creating DDSs that bind the determinants, and then assessing the resulting biodistribution and therapeutic efficacy in vivo.
The endothelium is accessible to blood, but accessibility to particles depends on binding site localization and vascular conditions [81]. For example, target determinants are less suitable for binding to DDSs if the epitopes are located in close proximity to the plasmalemma, masked by the glycocalyx, or buried in intercellular junctions or in plasmalemma invaginations [82]. Shedding of glycocalyx in pathology exposes en-dothelial ICAM-1 [83]. On the other hand, adherent leukocytes and thrombi may mask parts of the endothelial surface [84]. Accessibility is especially important for large multivalent carriers.
Anchoring of a carrier may affect functions of the target determinant either in an adverse or beneficial fashion. For instance, targeting to thrombomodulin is likely to increase the risk of thrombotic and inflammatory side effects. For other molecules, the benefit/risk ratio depends on the clinical context. For example, inhibition of ACE may be a bonus in treatment of hypertension and inflammation. On the other hand, ACE and APP are peptidases that cleave mediators including bradykinin [85]; unintended elevation of bradykinin may aggravate vascular edema. Cross-linking of target molecules may cause their shedding, uptake, changes in their functionality adverse endothelial activation, or other vascular disturbances.
4.2. Endothelial uniformity and heterogeneity
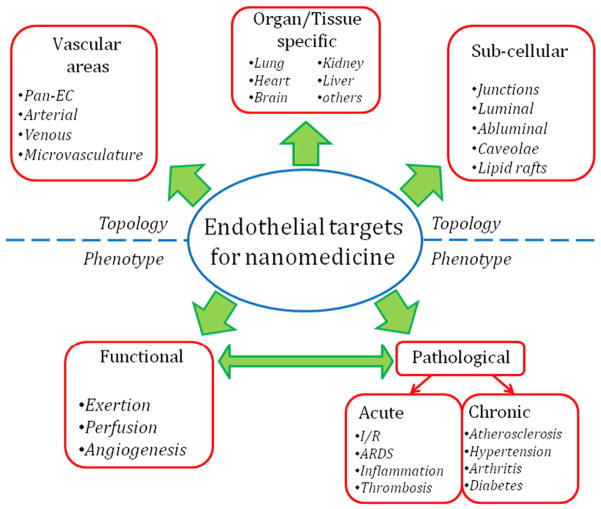
Endothelial cells throughout the body share a few characteristics. These include luminal location, flat shape, and pan-endothelial molecular markers enriched in or unique to endothelium (e.g., thrombomodulin, E-selectin, VE-cadherin, PECAM-1, etc.). However, endothelial cells in distinct organs and types of vessels each have unique morphologic and functional differences [86,87]. Distinctive features and markers of endothelial cells typical of certain organs, types of endothelia, and vascular areas provide the mechanistic basis for drug targeting (Fig. 4)
Fig. 4.
Topological and phenotypic parameters of endothelial targeting determinants. “Topological” characteristics of the target molecule include the type of blood vessel the target molecule is found on, the tissue types in which it is expressed, and its subcellular localization. “Phenotypic” parameters are the dynamic states in which the target molecule's expression or localization changes, such as normal physiological processes like angiogenesis or during pathological processes like inflammation.
Arterial endothelial cells are elongated and contain extensive long bundles of actin stress fibers, whereas short cortical actin fibers are more typical of the microvascular endothelial cells which have a “cobble stone” appearance. In large arteries, endothelial cells in the branching points, exposed to turbulent blood flow, have different gene expression profiles from their counterparts localized in less disturbed vascular areas [88]. Pulmonary and cardiac endothelial cells contain numerous caveolae, while cerebral endothelium is lacking these organelles and fenestrae. Distribution of endothelial markers varies in the vasculature. For example, ACE and thrombomodulin are greatly enriched in the capillaries, especially in the lungs, whereas the endothelial-specific glycoprotein EPCR is enriched in large vessels.
4.3. Constitutive vs. inducible endothelial determinants
Constitutively expressed endothelial surface molecules (e.g., PECAM, ACE, and APP) seem ideal for prophylactic drug delivery. Some, including ACE [89], disappear from the endothelium in pathological conditions, which inhibits targeting [90,91]. Constitutive molecules stably exposed in the lumen, such as PECAM, can be used for prophylactic and/or therapeutic delivery [84]
Endothelial phenotype changes under pathological conditions. Pathologically activated endothelial cells contract, open intercellular gaps, form or lose caveolae and fenestrae (depending on types of insult), shed their glycocalyx and endothelial-specific glycoproteins, and expose abnormal molecules including inducible adhesion molecules. Pathological endothelia tend to lose specific constitutive markers such as ACE and commonly exhibit features such as leakiness and expression of common pathological markers (e.g., VCAM-1 and selectins). In contrast to constitutive molecules, inducible counterparts expressed or exposed to the lumen in pathological sites (e.g., APN, TEM-1, VCAM-1, and selectins) are less likely to find prophylactic utility but seem preferable for diagnostic imaging and therapeutic interventions [23,55,64,92,93].
4.4. Candidate endothelial targets for nanomedicine
Table 2 introduces an incomplete list of endothelial surface determinants that in theory may be used for drug targeting. They are localized in diverse plasmalemma domains. For example, PECAM and VE-cadherin are localized in cell-cell borders [94,95] whereas VCAM-1 and ICAM-1 are found in micro-domains of the cellular apical surface [96–98], special types of membrane ‘rafts’ [99]. Glycoprotein GP85 localizes to the luminal surface of the plasmalemma that belongs to a thin organelle-free part of the endothelial cell separating alveoli from blood [100, 101]. APP and PV-1 are located in caveolae [102]
Table 2.
Selected endothelial target determinants for drug delivery.
| Target | Vascular localization | Cellular localization | Functions | Regulation |
|---|---|---|---|---|
| Constitutive | ||||
| ACE (CD143) | Ubiquitous, enriched in the lung capillaries | Cell surface, single pass type I membrane protein | Plays a key role in rennin-angiotensin system, regulates blood pressure. Converts Ang I into Ang II and degrades bradykinin. |
Catalytic activity is increased by chloride. |
| TM (CD141) | Ubiquitous, specific for endothelium | Cell surface, single pass type I membrane protein | Receptor for thrombin, participates in the generation of activated protein C. | Pathological factors affect TM expression. |
| Cell adhesion molecules | ||||
| PECAM-1 (CD31) | Ubiquitous | Cell junction, lateral border recycling compartment | Leukocyte trans-endothelial migration; anti-apoptotic repulsion signaling from phagocyte | Stable expression |
| ICAM-1 (CD54) | Ubiquitous | Tetraspanin microdomains on cell surface, single pass type I membrane protein | Leukocyte firm arrest and trans-endothelial migration; immunological synapse formation. Ligand of LFA-1, MAC-1 |
Up-regulated in inflammation |
| VCAM-1 (CD106) | Inflamed vascular endothelium | Tetraspanin microdomains on cell surface, single pass type I membrane protein | Leukocyte firm arrest and trans-endothelial migration. Ligand of VLA-4 | Up-regulated in inflammation |
| E-selectin (CD62E) | Inflamed vascular endothelium | Cell surface, single pass type I membrane protein | Leukocyte rolling | Up-regulated in inflammation |
| P-selectin (CD62P) | Inflamed vascular endothelium | Cytosol: Weibel-Palade bodies of endothelial cells, alpha-granules of platelets | Leukocyte rolling | Upon activation by agonists transported rapidly to cell surface |
| Caveolar | ||||
| APP | Enriched in pulmonary endothelium. Expressed in heart, liver, kidney | Enriched in caveolae on the luminal surface of endothelial cells; lipid-anchored to cell membrane | Metalloprotase that plays a role in inflammation; cleaves and inactivates circulating polypeptides such as bradykinin | Unknown |
| PV1 (PLVAP) | Expressed in many tissues | Endothelial fenestrae and caveolae | Forms aperture in fenestrae and caveolae, regulation of microvasculature permeability | Up-regulated by VEGF |
| Angiogenesis and tumor-related | ||||
| APN (CD13) | Vasculature of tissues that undergo angiogenesis and in multiple tumor types | Cell surface, single pass type II membrane protein; cytosol | Metabolism of regulatory peptides, processing of peptide hormones (angiotensin III and IV, | Estradiol and interleukin-8 decrease APN activity in vitro |
| Integrins αvβ3, αvβ5, α5β1 | Enriched in tumor vessels and other types of angiogenesis | Cell surface | Angiogenesis | αvβ3 is up-regulated by bFGF, TNF αvβ5 is up-regulated by VEGF, TGF-a |
Abbreviations: EC — endothelial cells, ACE — angiotensin-converting enzyme, TM — thrombomodulin, PECAM — platelet-endothelial cell adhesion molecule, ICAM — intercellular adhesion molecule; VCAM — vascular cell adhesion molecule; APN —aminopeptidase N; APP — membrane-bound aminopeptidase P; TEM-1: tumor endothelial marker-1; PV-1 — plasmalemma vesicle-associated protein.
4.4.1. ACE and thrombomodulin
Initial efforts in endothelial drug targeting focused on two constitutive targets, ACE [67,73,91,103] and thrombomodulin (TM) [56,104]. ACE, a luminal transmembrane endothelial glycoprotein, produces Ang II, a vasoconstricting, pro-oxidant, pro-thrombotic and pro-inflammatory peptide [105,106]. Some ACE antibodies do not affect its activity, while others inhibit ACE and cause its shedding [103,107]. This “side effect” may be beneficial in treatment conditions associated with hypertension, oxidative stress and inflammation [24,108,109]. The pulmonary vasculature is enriched in ACE: ~100% vs. <15% of endothelial cells are ACE-positive in the alveolar as compared with the extra-pulmonary capillaries [91]. Oxidants, cytokines and other pathological agents suppress ACE expression and thus inhibit targeting to ACE [110,111]
The pulmonary accumulation of isotope-labeled ACE antibodies has been visualized in real time after IV injection in diverse animal species including primates and humans [62,91,112]. Reduction of the pulmonary uptake of anti-ACE is an indicator of endothelial disturbance in models of endotoxemia, edema, and ischemia–reperfusion [84,111, 113]. Limited clinical studies using thoracic imaging revealed that pulmonary uptake of isotope-labeled anti-ACE is reduced in the patients with sarcoidosis, in comparison with healthy volunteers [62].
Endothelial cells internalize ACE antibodies [108,114]. Antibodies to rat, mouse, cat, primate and human ACE showed effective and selective accumulation in the pulmonary vasculature after IV injection in these species [73,91,112,115]. Pilot safety tests did not reveal overt harmful effects of injection of anti-ACE in animals and humans [62,103]. ACE targeting holds promise for endothelial delivery of imaging, antioxidant, genetic materials and other agents. Further, hetero-conjugates that consist of antibodies to both ACE and a viral surface protein attenuate the virus's natural tropism to non-endothelial cells and redirect it towards endothelial cells [116]. Such anti-ACE/anti-virus conjugates have been used for re-targeting of viral gene therapy to endothelial cells in culture [112] and the pulmonary endothelium in rats [70,116]. Combining this targeted delivery with insertion of an endothelium-specific promoter augments the pulmonary specificity of transgene expression by several orders of magnitude [70]. Using this approach for transfection of pulmonary endothelium by viral gene delivery of genes encoding NO-synthase and bone marrow morphogenetic protein type 2 receptor showed a reduction of spontaneous pulmonary hypertension [117] and hypoxic pulmonary hypertension [118].
Thrombomodulin (TM), a transmembrane glycoprotein expressed on the luminal surface of the endothelium, converts thrombin into an anti-thrombotic and anti-inflammatory enzyme [119]. TM antibodies accumulate in the lungs after IV injection and have been employed in animals for targeting of liposomes, genetic materials, model enzymes and isotopes to the pulmonary endothelium [120]. Pulmonary accumulation of 125I-anti-TM was reduced to 50% of basal level in mice with acute lung injury [90], consistent with loss of thrombomodulin in the pathologically altered pulmonary endothelium [121]. However, targeting may inhibit TM activity and compromise important endothelial functions and provoke thrombosis [122,123].
4.4.2. Caveolae
Caveolae are flask-shaped (~50 nm in diameter) invaginations in the plasmalemma involved in endothelial intracellular trafficking and signaling. Caveolae, similarly to lipid rafts, are enriched in cholesterol and sphingolipids, but unlike the rafts also contain the protein caveolin-1. Caveolae are dynamic structures forming caveolar endosomes and transporting ligands via this endocytic vesicular pathway into and across the endothelium [124]
Ligands binding to some caveolar determinants accumulate in the lungs after systemic vascular administration, quickly pass the endothelial barrier, and get into the pulmonary interstitium. Antibodies of determinants localized to caveoli, including amino peptidase P (APP) and the glycoproteins GP60 and GP90 accumulate in the pulmonary vasculature in rats after intravenous injection, enter endothelial intracellular vesicles and traverse endothelial barriers [23]. Caveoli-mediated endocytosis and transcytosis are involved in endothelial transport functions [125–130]. Interaction of a protein ligand (e.g., antibodies) leading to receptor clustering in caveoli further activates endocytosis and transcytosis [131,132]. Caveoli seem to be involved in trans-endothelial transport of albumin [133,134], a process further stimulated by albumin nitration that may take place during oxidant stress [135]. In addition, antibodies to the glycoprotein PV-1, which forms diaphragm-like structures in caveolae and fenestrae, also accumulate in the pulmonary vasculature with exceptionally high affinity, yet conjugation to 100 nm particles obliterates targeting, most likely due to inaccessibility of PV1 to even relatively small carriers [136]. Caveolae have been reported to be able to merge into “caveolosomes” engulfing large particles, but it is unclear to what extent data obtained in static cell cultures reflect this aspect of endothelial physiology in vivo [102,137,138].
4.4.3. Inducible cell adhesion molecules: selectins and VCAM-1
These molecules are normally absent on the vascular lumen, but become exposed on pathologically activated endothelium and facilitate adhesion of leukocytes [139]. Pathological mediators cause mobilization of intracellular P-selectin to the endothelial surface within 10–30 min [140] and induce de novo synthesis and surface expression of E-selectin [141] and VCAM-1 [142] within several hours. E-selectin and VCAM-1 seem to be more readily expressed in activated endothelia of non-pulmonary vasculature, e.g., in the dermal microvasculature [143]
These determinants are good targets for delivery of agents to activated endothelium [57,144]. Endothelial cells constitutively internalize selectins via clathrin-coated pits [145–147], permitting entry into endothelial cells of anti-E-selectin targeted liposomes [148], anti-inflammatory drugs [148,149] and genetic materials [150]. P-selectin targeted compounds also bind to activated platelets [151]. Selectins and VCAM-1 are exposed transiently and at relatively low surface density. Hence, robustness of the targeting may be sub-optimal for therapies requiring delivery of large doses of drugs. These determinants might be optimal for diagnostic visualization of activated endothelium in inflammation using isotopes [92] or ultrasound contrasts [151,152].
4.4.4. Stable and up-regulated cell adhesion molecules (CAMs)
CAMs are also good targets for drug delivery [37]. ICAM-1 or PECAM-1 antibodies and antibody-targeted drugs and drug-loaded carriers bind to endothelial cells in vasculature and exert biodistribution and effects typical of pan-endothelial targeting in models of thrombotic, oxidative, inflammatory, cancer, and genetic conditions [37]. Inducible and stable CAMs are attractive targets for therapeutic and prophylactic drug targeting, respectively [37,153]. Inflammation enhances targeting of nanocarriers to ICAM-1
Endothelial cells do not internalize PECAM-1 antibodies, and rapidly recycle ICAM-1 antibodies to the surface [154]. This enables intravascular retention of monovalent fusion proteins targeted to CAMs, including fibrinolytic urokinase [155]. However, multivalent conjugates and carriers enter endothelial cells via inducible CAM-endocytosis [156]. Therefore, ICAM-1 and PECAM-1 offer flexibility for drug delivery to different endothelial compartments.
5. Control of DDS targeting
Specifics of design and molecular configuration of ligand-coated carriers dictate the targeting outcome. Here we consider several examples of such regulatory influences
5.1. Multi-ligand coated nanocarriers
Nanomedicine agents may utilize more than one target recognition mechanism. Attractive intellectually, this sophisticated approach may further complicate the translational aspects, but, at least in theory, may provide unique new functional features to the DDSs. Combining on the carrier ligands that bind to different determinants may boost targeting to pathological endothelium. Carriers coated by antibodies to inducible adhesion molecules providing the selectivity (selectin, VCAM-1, ELAM) and antibodies supporting anchoring via less specific high-density molecules (ICAM, PECAM), have been tested in vitro in models of co-immobilized antigens [157] or cytokine-activated cells [158]. In studies with particles targeted to inflamed vasculature using ICAM-1 and P-selectin, greater binding was achieved with dually targeted particles relative to particles targeted to P-selectin or ICAM-1 alone [157,159,160]. Targeting liposomes to E-selectin and either VCAM-1 or ICAM-1 on cultured endothelial cells has also been reported [158]. Maximal binding was observed with equimolar ratios of both ligands [158,161,162]
The dual targeting has also shown promising results in imaging inflammation in mice using contrast particles targeted to P-selectin/ VCAM [163] or P-selectin/ICAM-1 [164]. Of note, in imaging the target/non-target ratio is more important than the absolute level of delivery, which may favor better outcome with dual-targeting even with relatively low absolute levels of targeting. A dual targeting strategy employing carriers carrying antibodies to both ICAM and transferrin receptors has recently been tested in vivo and showed promising results: each of the ligands apparently promoted targeting to the vascular area of its destination, i.e., nanocarriers could be directed to the inflamed pulmonary vasculature via ICAM and to cerebral vasculature via the transferrin receptor [165].
5.2. Collaborative endothelial targeting
Ligands binding to distinct epitopes on the same target molecule may influence each other, for example, inhibiting binding to adjacent epitopes [107,166,167]. Recently, it has been found, however, that distinct monoclonal antibodies directed to adjacent epitopes in the extra-cellular moiety of PECAM, stimulate binding of each other [168]. This unusual finding can be explained by an increase in accessibility of an epitope to its ligand due to conformational changes in the PECAM molecule induced by binding of a paired “stimulatory” ligand. Utilizing this strategy, an improved therapeutic effect was realized with increased activity of an scFv targeted to PECAM on endothelial cells [168].
5.3. Ligand surface density
Targeting of a carrier depends on the surface density of the ligand. In turn, the optimal surface density of a ligand depends on features of the carrier and the ligand itself (size, orientation, affinity, density, etc.) and of the target determinant (membrane localization, glycocalyx, environment, clustering, etc.). Ligand-coated carriers with higher avidity do not necessarily provide the best targeting. Unlike free ligands, they require congruency with target molecules for multivalent binding, which does not necessarily correspond to the maximal ligand density. In some cases, an excessive ligand density may inhibit the binding via “ligand overcrowding” that may inhibit individual ligand molecules from achieving the optimal orientation or congruency with target molecules [169,170]. Beyond a certain optimal level, further elevation of ligand density may result in decreased in targeting [169,171].
Varying ligand surface density may help enhance the signal-to-noise ratio of the target tissue, i.e., boost the selectivity of detection of pathological endothelium. For example, controlled reduction of a carrier's avidity to ICAM-1 (achieved by reducing antibody surface density) enhances the selectivity of targeting to and PET imaging of the inflamed pulmonary vasculature in animal models [136,172]. In the case of ICAM-1 and other markers that are expressed in normal endothelial cells but upregulated in disease, it may be useful to use carriers with “marginal” avidity, sufficient to anchor on cells with pathologically elevated but not normal level of expression of the marker.
5.4. Carrier geometry
Geometry parameters - i.e., size, shape and plasticity of the DDS carrier - modulate all aspects of its function, including suitability to administration routes, behavior in circulation and interactions with target and non-target cells. Overall, spherical carriers smaller than tens of nanometers can be injected using diverse vascular, muscular and dermal routes, whereas carriers larger than few hundred nanometers can be administered via large vessels and airways [173–177]. The shape further modulates delivery: for example, non-spherical carriers circulate longer and avoid uptake by defense cells more effectively than spherical counterparts, whereas propeller-shape nanoparticles reach more deep deposition sites in the airways than spherical ones [81,178]
Effects of the geometry on active targeting mediated by carrier's anchoring on the cells of interest are beginning to emerge in the literature [179–182]. The interplay between size, geometry and plasticity of the carrier, ligand's affinity, spatial freedom and density, and nature of the target determinants - accessibility, clustering, surface density - is immensely complex. For example, larger carriers may have advantages of higher avidity attained by formation of multiple engagements, and disadvantages of limited access and enhanced detaching dragging force of blood [81,174,183].
Generally, the absolute level of endothelial targeting (e.g., number or mass of nanocarriers bound per cell or organ) increases with size into the micron range due to more effective multivalent anchoring. However, beyond a certain optimal size, targeting specificity (i.e., ratio of tissue uptake of targeted vs. non-targeted formulations) may deteriorate due to mechanical uptake and other non-specific interactions of large particles with endothelium. For example, within the size range from <100 nm to a few microns, maximal specificity of targeting to the pulmonary endothelium in mice has been observed with synthetic and protein-based nanocarriers with sizes of 100–300 nm, targeted to ICAM [184] or PECAM [185].
The carrier's shape is also an important factor. Elongated carriers display higher specificity of targeting to vascular endothelium vs. spherical counterparts [184]. Nanorods targeted to ICAM and the transferrin receptor showed more effective endothelial targeting than spherical counterparts in static culture of brain endothelial cells [186]. In vivo, ICAM targeted disks accumulated in the pulmonary vasculature in mice with higher specificity vs. spheres [184], whereas ICAM-targeted filomicelles accumulated in lungs less effectively than spheres, although with similar targeting specificity [187].
6. Intracellular endothelial delivery
Binding to the target should provide desirable sub-addressing of the drug. A conventional strategy is to direct targeting to surface molecules involved in natural endocytic pathways. For example, antibodies against gp90, a 90 kDa glycoprotein located in the caveoli, and compounds conjugated to these antibodies enter vesicular organelles from caveoli [23]. In contrast, liposomes targeted to E-selectin, a transmembrane glyco-protein that is taken up by clathrin-mediated endocytosis [146,147], enter cells via this pathway and traffic to lysosomes [148,149]. Similarly, antibodies to VCAM-1 are internalized by endothelial cells and addressed in the lysosomes via clathrin-mediated endocytosis [149,188] and anti-VCAM conjugated compounds generally follow this fate [189–191]. Antibodies to the transferrin receptor (TfR) and compounds conjugated with TfR ligands also enter cells via this pathway, similarly to the endogenous ligand transferrin [192]
Binding to molecules involved in endocytosis usually favors uptake, though not necessarily via the same endocytic pathway. Phagocytosis and pinocytosis take particles and fluid, respectively, into micron-size vesicles, typical of host defense cells. Clathrin- and caveolar endocytosis take particles smaller than 100 nm [93]. Static endothelial cells in vitro may engulf large carriers via engorged fused vesicles (e.g., “caveolosomes”) and may exert unconventional pathways (e.g., associated with GPI-anchored proteins, bound growth factors, etc.), but most of these findings remain to be confirmed and elucidated in vivo [124]. Most ligands and ligand-targeted carriers entering via vesicular pathways traffic to the lysosomes, whereas some ligands traffic to the Golgi apparatus or the endoplasmic reticulum, or across the endothelial cells via transcytosis. Several endothelial determinants have been identified including receptors and enzymes, elements of the glycocalyx and specific domains in the plasmalemma [68,109,193,194]. In particular, cell adhesion molecules are good determinants for delivery of drugs to selected endothelial compartments [28,37].
Ligands binding to distinct epitopes of the same molecules may enter cells differently. Selection of ligands facilitating cellular uptake is a mostly empirical task [195,196]. Using a phage-display library, a series of peptides binding to VCAM-1 epitopes was identified, some of which have shown enhanced uptake [191]. VCAM-1 binding peptides undergoing enhanced endocytosis provided improved imaging of vascular inflammation in animal models [190,191]. Phage display and other high-throughput methods facilitate selection of internalizable ligands [197–199].
6.1. Uptake of free ligands vs ligand-coated carriers
In many instances, carriers coated by molecules of a ligand enter cells similarly or even more effectively than free ligands [200]. An extensive clustering or re-clustering of receptors by multivalent carriers eliciting strong endocytic signaling seems as a plausible explanation. Generally, this high carrier avidity is viewed as favorable for intracellular delivery. However, coating a nanocarrier with internalizable molecules does not necessarily result in internalization. The uptake of too large a carrier may require prohibitively extensive mobilization of cell membrane and cytoskeleton. Coupling ligands to carriers impedes their interaction with receptors and epitopes inaccessible to the particles of such size. Conjugation of ligands of caveolar epitopes to carriers larger than the diameter of the neck of caveolae (50 nm) abolishes endothelial targeting [201]
On the other hand, endothelial cells do not internalize free antibodies to PECAM and ICAM, yet internalize multivalent conjugates targeted to these adhesion molecules [93]. CAM-endocytosis is mediated by series of unique cellular signaling pathways leading to formation of ceramide-rich domains at the plasmalemma and formation of actin stress fibers. Contrary to constitutive endocytosis that is continuously used by natural ligands, the CAM pathway is believed to be “dormant” until induced by certain pathogens, leukocytes, and drug targeting systems [156]. CAM-endocytosis delivers cargoes to the lysosomes markedly slower than other vesicular pathways [202]. Lysosomal delivery is ideal for treatment of the lysosomal storage diseases and in cases where the lysosomal acidic pH and hydrolytic environment induces release of membrane-permeable drugs or provides escape from this compartment [203]. Efficacy, rate and destination of carriers entering endothelial cells via CAM-endocytosis are modulated by selection of epitopes on CAMs, and carrier geometry.
CAM-endocytosis provides internalization of carriers of different sizes and shapes (50 nm–10 μm, spherical and elongated) [156,184]. Internalization is modulated by carrier geometry and selection of epitopes on the target determinant. ICAM targeted disks enter endothelial cells more slowly than spherical counterparts, whereas intracellular traffic is controlled by size: smaller particles arrive to the lysosomes faster [184].
Intracellular uptake of carriers varies depending on which specific epitope on the target determinant is engaged [82]. For example, nanocarriers directed to distinct PECAM epitopes are internalized and trafficked by endothelial cells differently. One out of four antibody-coated nanocarrier formulations failed to enter the endothelium despite high level of binding to cells [82]. Further, the kinetics of vesicular trafficking from early endosomes to lysosomes varied among different types of PECAM-targeted nanocarriers despite the fact that they all entered cells with a similar rate (T½ was close to 20–30 min and max uptake reached 80–90%) [82].
6.2. Biological modulation of carrier intracellular delivery
The functional status of target cells and their microenvironment modulate endocytosis. Thus, activated endothelium internalizes ICAM-targeted nanocarriers faster than quiescent endothelium both in vitro and in vivo [204]. A few studies addressed the role of flow in endothelial uptake of non-targeted nanoparticles [205,206]. Further, studies in flow chambers revealed that prolonged exposure to flow leads to inhibition of endocytosis of nanocarriers targeted to ICAM and PECAM [204,207]. The trend corroborates with in vivo data showing less effective internalization of ICAM-targeted nanocarriers in arterioles vs. capillaries, i.e., by endothelial cells adapted and not adapted to shear stress, respectively [204]. In contrast, exposure to acute shear stress (which happens in re-perfusion and in physiological hyper-perfusion in exertion) stimulated endocytosis of PECAM-targeted nanocarriers [207]
Findings in cell culture and in genetically modified mice revealed that binding of nanocarriers to ICAM-1 in endothelial and other cell types activates the enzyme(s) metabolizing sphingomyelin in the plasmalemma, stimulating endocytosis of coated carriers ranging in size from <100 to >1000 nm in diameter [208]. This does not happen in sphingomyelinase-deficient cells and animals; however, carriers coated with both anti-ICAM and the enzyme, devised to deliver enzyme replacement therapy in Neimann–Pick syndrome, do get internalized [209]. Co-immobilization of sphingomyelinase with ligands anchoring carriers to transferrin receptor permitted internalization of carriers larger than 200 nm [210].
6.3. Trans-endothelial delivery
Some ligands of receptors involved in endocytosis via clathrin-coated pits such as TfR [211] and caveoli, such as APP [132,212,213] are capable of crossing the endothelial barrier, providing pathways for trans-endothelial transport of carriers with sizes suitable for these endocytic vesicles (<100 nm). For example, antibodies to caveolar APP undergo fast transport across the endothelium, but particles >100 nm do not enter this pathway [102]. The effects of engaging these determinants must be more fully understood [65,129,214]. Some disease conditions, including inflammation, may affect this pathway [93, 201,215–218]
CAM-mediated endocytosis allows entrance of objects up to several microns. Gastrointestinal epithelial cells, which normally express ICAM, uptake ICAM-targeted carriers (~100 nm diameter spheres) via this pathway and transport the carriers across the cellular monolayer without cell damage or disruption of intercellular junctions in vitro [219] and in vivo thereby providing oral delivery of nanocarriers into the vascular compartment [220]. It is plausible that similar transcellular pathways operate in the vascular endothelium as well.
7. Endothelial targeting and effects of nanomedicine agents in pre-clinical studies
The literature reporting effects of various drugs targeted to endothelia is extended and diverse. As mentioned in the Introduction, we focus this review on acute diseases, which, in our opinion, are more favorable targets for nanomedicine. In this section we analyze endothelial nanomedicine for inflammation, oxidative stress, enzyme deficiency and thrombosis. We omit studies in cell culture unless they represent an important phase for studies in animal models of human pathology
7.1. Antioxidants
Vascular oxidative stress is a common mechanism of a plethora of human diseases [30–32,34]. Surplus reactive oxygen species (ROS), such as superoxide anion and H2O2, released from activated leukocytes and endothelial cells, induce tissue damage, vascular dysfunction [221,222], and pro-inflammatory signaling [223–227]. Detoxification of endothelial ROS is an important goal [109,228] that, in theory, can be achieved using antioxidants including superoxide dismutase (SOD) and catalase [109,193]. Alas, so far all antioxidant therapies have failed in clinical trials, likely due to lack of appropriate delivery
Diverse antioxidant DDSs have been designed. Antioxidant DDSs employing PEG, PEG-based pluronic, and PEG-nanocarriers display improved circulation, systemic bioavailability, and therapeutic effects in animal models of stroke, inflammation and radiation injury [229–237]. Antioxidant formulations that bind to the glycocalyx and plasmalemma have been devised and reported to alleviate oxidative stress in cell cultures [238–241] and, to a more limited extent, in animal models [242–246], including models of myocardial ischemia [236,247]. ROS act on the nanometer scale and precise delivery of antioxidants to desirable cells and compartments is required. For example, intra-tracheal delivery of antioxidants alleviated oxidative stress in the airways but not in the lung vasculature [248,249]. However, untargeted agents do not have specific endothelial affinity [227] and precision is needed for interception of cellular ROS [250].
Targeting antioxidants to determinants, including ACE and CAMs [251,252], achieves this goal in vitro [253,254] and in numerous studies in animal models of acute oxidative stress, providing immediate protective effects that last for several hours after a single dose delivery [255]. Antioxidants targeted to ACE, PECAM and ICAM provided protective effects superior to non-targeted formulations in models of acute pulmonary oxidative stress caused by infusion of ROS or ischemia–reperfusion [69,256–263].
CAM-targeted SOD or SOD mimetics alleviated ROS toxicity in endothelial cells [252,264,265], normalized vasoconstriction in mice [259], attenuated VEGF-induced endothelial leakage [266] and inhibited cytokine-induced endothelial ROS flux and inflammatory activation in cells and mice via quenching of signaling in endothelial endosomes [227]. Endosomal SOD delivery blocked NFκB signaling activation by both cytokines or TLR agonists and the protection was significantly more effective when SOD delivery was combined with NO donors [267]. CAM-targeted catalase alleviated endothelial leakage caused by H2O2 [266], alleviated vascular [114] oxidative stress [268] and pulmonary ischemia–reperfusion [258,259] including lung transplantation in rats and larger animal species in models including a warm ischemic period [262,263]. Thus, targeting antioxidants to the endothelial endosomes enables potent interception of pro-inflammatory signaling [227,266,267,269].
A number of techniques have been devised to further enhance the therapeutic effects of endothelial-targeted antioxidants. For example, adding pharmacological agents that modulate vesicular trafficking delays lysosomal degradation of CAM-targeted antioxidant enzymes [154,270]. Another technique to prolong antioxidant enzyme half-life employed polymer nanocarriers permeable for ROS but not to proteases. When such encapsulated antioxidant enzymes were targeted to endothelium, they provided immediate and prolonged protection in vitro and in vivo [271–274]. A final technique to improve antioxidant half-life is the use of CAM-targeted liposomes loaded with either non-enzymatic antioxidant catalysts or inhibitors of enzymes producing ROS. Such liposomes produced antioxidant effects and alleviate endotoxin-induced acute pulmonary inflammation in animals [275]. To further the gains made by the above techniques, the mechanism of anti-inflammatory effect of antioxidants targeted to endothelial endosomes must be further elucidated. Recent studies in animal models show that mechanisms include both interception of intracellular signaling of superoxide and enhancement of anti-inflammatory activity on nitric oxide-mediated pathways [227,267].
7.2. Anti-inflammatory agents (AIA)
In addition to the anti-inflammatory effects of endothelial targeting of antioxidants, this approach is useful for delivery of other anti-inflammatory agents. For example, thrombomodulin (TM) naturally exerts multifaceted direct and indirect anti-thrombotic and anti-inflammatory activities. Fusing TM with single chain fragments of antibodies to PECAM affords more effective TM replenishment therapy than using soluble TM, due to endothelial targeting and anchoring in the natural microenvironment [276]. Interestingly, targeting of a scFv/TM fusion to ICAM was even more effective than to PECAM, likely due to the fact that the former CAM is localized in the endothelial plasmalemma next to the natural molecular partner of TM, namely, endothelial protein C receptor, EPCR [277]. However, co-targeting of scFv/TM and scFv/EPCR to adjacent PECAM epitopes provided the maximal effect, due to a combination of the collaborative enhancement of targeting and juxtaposition of two enzymatically partnering proteins, TM and EPCR [278]. These results have been observed in a mouse model of severe acute lung injury caused by endotoxin challenge that reflects some key pathological features of human ARDS, a common morbid condition with >30% mortality that has no current pharmacological therapy
The effect of some classical AIAs either requires or can be improved by targeting. For example reducing the dose of steroids such as dexa-methasone (Dex) may help alleviate its side effects of hypertension, hyperglycemia, osteoporosis and adrenal insufficiency. Currently, steroids are used mainly as a bridging therapy for the acute phase of chronic conditions such as rheumatoid arthritis. The endothelium is a key regulatory tissue in inflammation and targeting it may enable more potent and specific anti-inflammatory therapy. In this vein, targeted liposomes and nanogels have been shown to deliver encapsulated dexamethasone to the pulmonary vasculature and alleviate edematous acute lung injury in a mouse model of endotoxin challenge [279, 280]. It is tempting to postulate that this approach is translatable to the treatment of human ARDS.
However, the majority of studies focus on chronic inflammation: atherosclerosis, arthritis, glomerulonephritis and alike. Inducible cell adhesion molecules are widely used in this domain. For example, VCAM-targeted liposomes loaded with an anti-inflammatory prostaglandin, PGE2, injected daily for two weeks in mutant mice genetically prone to “atherosclerosis” showed ~50% higher uptake in inflamed sites vs. untargeted formulations, and, quite astonishingly, reversed atherosclerotic lesions to the extent that mutant mice survived to old age despite being fed a high-fat diet [281]. In a rat model of renal inflammation, E-selectin-targeted Dex-liposomes showed greater uptake in inflamed kidneys and alleviated inflammatory markers by 60–70% vs non-targeted controls, with negligible side effects typical of Dex [282]. In a mouse model of ocular inflammation, selectin-targeted Dex-liposomes accumulated in the inflamed eye within five minutes of injection and suppressed expression of pro-inflammatory genes in the tissue, whereas non-targeted liposomes showed negligible accumulation and effect [283]. Targeting to E-selectin improved delivery of Dex-liposomes to activated dermal and renal endothelium in animal models of inflammation of skin [284] and kidneys [285]. In the latter model, E-selectin targeted Dex-liposomes reduced renal expression of pro-inflammatory genes and proteins and renal injury without affecting blood glucose level [285].
E-selectin- and ICAM-targeted nanoparticles carrying siRNA silencing inflammatory mediators suppressed their expression in cell culture [286]. Cationic lipid-based formulations of siRNA targeted to E-selectin silenced VE-cadherin in activated endothelial cells in vitro [287]. E-selectin-targeted adenovirus homed to the kidneys and down-regulated inflammatory molecules in a mouse model of glomerulonephritis [286]. Targeting to selectins favors endocytosis, whereas using membrane-permeating moieties and pH-dependent disruption of intra-cellular vacuoles may enhance the efficacy of siRNA transfer from endocytic vacuoles to the cytosol. However, it is important to note that such endosomal-disrupting features may produce adverse effects on the endothelial cells. Therefore, it will be important to learn from the burgeoning field nanocarriers designed for safe and effective delivery of nucleic acid agents [288,289].
Other targets are explored as well, including integrins. In a rat model of arthritis, Dex-loaded liposomes targeted by RGD peptide accumulated in inflammatory sites, providing protective effects superior to non-targeted Dex-liposomes [290]. RGD-targeted liposomal delivery of anti-inflammatory siRNA to the endothelium was also studied in mice [291].
7.3. Enzyme replacement therapies
Lysosomal delivery is problematic for many biotherapeutics, unless the cargo is protected from degradation, but a lysosomal destination is necessary for drugs that are supposed to act in this organelle, such as drugs for lysosomal storage diseases (LSD) [292]
The LSDs are morbid conditions caused by dysfunction of lysosomal enzymes, in most cases due to mutations, leading to accumulation of the enzyme substrate and cellular abnormalities throughout the body [293–295]. Enzyme replacement therapy (ERT) relies on repetitive injections of recombinant enzymes [296–298]. Cells take up lysosomal enzymes via mannose and/or mannose-6-phosphate receptors [299–301]. In the absence of gene therapy, ERT is the only treatment of LSD [298,302], such as type B Niemann–Pick disease (NPD), caused by a mutation of acid sphingomyelinase (ASM), leading to deposition of sphingomyelin and cholesterol [303].
The endothelium suffers damage in LSDs, which aggravates inflammation and the injury to other tissues [292]. Delivery of lysosomal enzymes to endothelium is not efficient and management of vascular abnormalities in LSDs is ineffective [302,304]. In order to overcome this hurdle, ICAM was used as a target for nanoparticles carrying lysosomal enzymes [203]. ICAM expression by endothelial cells is up-regulated in the inflammation typical of many LSDs [305–308]. Coupling ERT to ICAM-targeted nanocarriers (~100 nm) enhanced delivery and effects in animal models of LSD, and the results were similar for carriers made of polystyrene [203] and biodegradable PLGA [209]. Delivery by ICAM targeted carriers can be optimized by the carrier's geometry: spherical 100–200 nm carriers offered more effective lysosomal delivery and effects than discoid or micron-size spherical particles [184].
This approach was extended for endothelial delivery of ERTs for other LSDs, including a cell culture model of Fabry disease [309] and in models of Pompe disease in cell culture and in vivo in mice, where ICAM-targeted endothelial delivery of ERT markedly improved delivery of the deficient enzymes in the heart, spleen and brain, and provided an unprecedented ~600 fold increase in the lung delivery vs non-targeted formulations [310].
Lysosomal enzyme delivery by nanocarriers targeted to ICAM was more effective than targeted to transferrin receptor (TfR, entering cells via clathrin endocytosis) [311], whereas carriers targeted to both ICAM and TfR showed different organ distribution vs. non-targeted ERT and either mono-targeted carriers [165]. Interestingly, ICAM-targeted multivalent nanocarriers induced ASM activity in target cells, facilitating membrane turnover and endocytosis. The potential utility and significance of these findings are worth further investigations [292].
7.4. Anti-thrombotic agents (ATA)
Pathologically altered vessels are prone to thrombosis due to suppression of natural anti-thrombotic mechanisms in the endothelium [312]. Anchoring of recombinant anti-thrombotic proteins such as TM and plasminogen activators (tissue type, tPA, or urokinase, uPA) on the endothelial lumen may help to compensate for this dysfunction. Vascular gene transduction of these proteins in animal models supports this notion [313]. Targeting of anti-thrombotic proteins to the endothelial surface seems a more practical approach for short-term thromboprophylaxis in acute settings in patients with a high propensity for thrombosis, particularly in settings where the risk of bleeding prohibits the use of systemic anticoagulation
In early studies, anticoagulants cross-linked to E-selectin antibody bound to cytokine-activated endothelial cells and inhibited thrombin [144]. In animal studies, tPA and uPA conjugated with antibodies to endothelial determinants preferentially accumulated in the pulmonary vasculature after intravenous injection in rats [101,314]. However, endocytosis removed the drugs from the vascular lumen, where they need to exert their activity, thereby limiting therapeutic effect in vivo [57].
Endothelial cells do not internalize antibodies to PECAM and ICAM, hence tPA conjugated with these ligands accumulated and dissolved clots in the pulmonary vasculature after systemic injection [315]. Further, fusion with recombinant antibody scFv (fragments, comprising variable domains of heavy chain VH and light chain VL) yields monovalent, homogeneous and relatively small targeted biotherapeutics. PECAM scFv fused with urokinase (scFv/uPA) accumulated in the pulmonary vasculature after injection in mice, resided in the pulmonary lumen for hours in active form [251,258,316] and augmented local lysis of pulmonary emboli in a mouse pulmonary thrombotic model [108]. Further, scFv/uPA accumulated in the cerebral vasculature after injection via carotid atery, dissolved cerebral clots and improved reperfusion without hemorrhagic complications, mitigating post-thrombotic brain edema in a mouse model of cerebral embolism [76].
Urokinase is produced naturally as a pro-enzyme, scuPA [317]. In the presence of fibrin, endogenously formed plasmin cleaves the Lys158-Ile159 peptide bond in scuPA, converting it into active uPA. However, thrombin inactivates uPA by cleaving Arg156-Phe157, negating its effect at sites of active thrombosis [318]. Deleting Phe157 and Lys158 solves these problems yielding a plasmin-resistant mutant activated by thrombin (uPA-T) [319], used to produce thrombin-activated PECAM-targeted scFv/uPA-T [276]. This fusion protein accumulated in the lungs, provided potent thromboprophylaxis in mouse models of lung thrombosis and ischemia/reperfusion, attenuated pulmonary fibrin deposition and restored arterial oxygen tension, to a significantly greater extent than scFv/uPA [276].
The suppression of TM is a characteristic of many vascular pathologies including sepsis. Some success has been found with a replacement therapy using soluble recombinant TM and activated protein C (APC) [320]. TM fused with a tissue factor antibody has potent antithrombotic activity in a rat model [321]. Yet, utility of these biotherapeutics is limited by fast disappearance from the vascular lumen. In order to solve this problem, a PECAM-targeted scFv/TM fusion has been produced and shown to bind and reside on the endothelial surface, accumulate in the pulmonary vasculature, and attenuate thrombosis and tissue damage in mouse models of lung ischemia–reperfusion and endotoxin-induced acute inflammatory lung injury to a greater extent than non-targeted soluble TM, without causing bleeding (a known liability of APC treatment) [322].
Thus, endothelium-targeted thromboprophylaxis triggered by a pro-thrombotic enzyme illustrates a novel approach to time- and site-specific regulation of “on demand” reactions that can be modulated for therapeutic benefit. In clinical settings, this strategy of targeting anti-thrombotic drugs to the endothelial surface may provide local thromboprophylaxis in patients with an acute risk of thrombosis and it may also prevent clot extension.
8. Conclusion
The field of targeted drug delivery to the endothelium has shown steady growth and has become one of the key subfields of nanomedicine. From the standpoint of pharmaceutical sciences, the endothelium is an important target, barrier, and also victim of drugs. From the medical perspective, it is the site of intended pharmacological interventions in a plethora of human maladies. Three decades of research in endothelial nanomedicine have yielded both important general principles and specific details that can guide the design of endothelial drug delivery systems.
8.1. Advantages of targeting
Defined and mechanistically understood endothelial targeting of drugs is an intellectually exciting, practically challenging and translationally promising avenue. Recent advent of combinatorial chemical carrier libraries reiterated the fact that carriers may accumulate in vascular areas of interest via fortuitous mechanisms that do not require specific binding to identified target molecules. Yet, such an identification is vital for understanding the nature, localization, function, pathological tole, trafficking, and effects – both intended and unintended – of the endothelial nanomedicine agent. Not only is this necessary for analysis of the potential efficacy and benefit/risk ratio in a given medical context, but it also diversifies immensely delivery options: in fact, anchoring to adjacent epitopes of the same surface molecule may provide quite different delivery and effects of the same nanomedicine agent
The experience of the last two decades instructs us to pay more attention to mechanistic aspects of the drug delivery systems and the role of biological factors in DDS's performance. Most likely, nanocarriers showing “fortuitous” accumulation and delivery of cargoes to and into endothelia in any given organ have some unidentified mechanisms for recognition of endothelia in corresponding vascular areas — either direct, or mediated by natural ligands acquired from plasma. Their identification represents an intriguing and promising direction, which will likely yield new knowledge, similarly to other high-throughput approaches for defining potential vascular targets and ligands. No doubt, the more diversified the list of such targets, the more precise drug delivery is possible — both in spatiotemporal terms and in the context of biomedical applications.
8.2. Experimental models
Endothelial nanomedicine research has amassed a potent arsenal of methods and approaches developed for creating and studying drug delivery systems, including tools for imaging, chemical and recombinant conjugation, and immense synthetic abilities. Among these methods, the utility of experimental models for endothelial targeting deserves a specific analysis
Endothelial cell cultures, such as human umbilical vein endothelial cells (HUVECs, used in many thousands studies since seventies), offer relatively straightforward high-throughput experimental models with controlled conditions. However, deprivation of the natural microenvironment associated with transfer of endothelial cells from in vivo to in vitro leads to loss of phenotypic characteristics - both archetypical and the local individuality of endothelia in vascular areas of interest. For example, endothelial cells' content of ACE and caveolae drop to a fraction of their natural level within a single passage under static culture conditions. Thus, cell culture studies are prone to artifacts of cell “bastardization” and non-physiological treatments.
Studies in isolated blood vessels and organs allow us to address specific mechanistic questions, but are low-throughput and short-term. Advanced sophisticated cell models reflect some key features of the tissue microenvironment: flow and vascular geometry for endothelial cells, stretch for smooth muscle and epithelial cells. Both flow adaptation and exposure to abrupt changes of flow in endothelial cell culture profoundly alter both endothelial physiology and hydrodynamic parameters of DDSs interaction with cells. For example, exposure to chronic physiological shear stress and turbulent flow or ischemia–reperfusion changes endothelial signaling and redox status. Experimental models that address these aspects of endothelial nanomedicine represent important advances (yet usually at cost of lowering the throughput). Systems using microfluidic, permeable 3D matrices with controlled elasticity, co-culturing of different cell types and other elements of tissue engineering (organ-on-chip) are rapidly evolving and may find utility in the design of targeted therapeutics imitating corresponding biological aspects of the vascular system.
In vivo models provide the ultimate proving grounds for targeting, but they are confounded by uncontrolled systemic and local factors, high cost, and several other downsides. However, expensive and time-consuming animal studies are vital for the design of DDS. Studies in naive animals characterize pharmacokinetics and biodistribution (PK/ BD), metabolism, targeting, and toxicity. Studies in animal models of human disease reaffirm these parameters and test the benefit/risk ratio of drug-loaded and drug-free carriers. Yet, animal models do not always recapitulate human PK/BD, disease, and treatment. Mice provide high-throughput models including mutants lacking or expressing genes of interest, but interspecies differences may be large. For example, nano-particles are directly cleared from the body of rodents by hepatic excretion into the bile, which does not occur in large animals [323].
A major new model system for endothelial nanomedicine is the perfusion of isolated human organs that have been declined for human transplantation. Such a model allows for the testing of targeting at physiological conditions, with controlled content of the perfusion fluid, for testing carriers targeted by ligands binding to human molecules. The data obtained in such explanted human will be directly applicable to clinical use in organ transplantation. Using human organs not suitable for transplantation will allow us to measure and localize tissue uptake and test protective and adverse effects in organs morphologically, histo-logically, by biochemical analysis of tissue homogenate, and with analysis of the level of endothelial cell-injury markers shed in the perfusion solution. The human organ model thus represents the key translational phase of targeted endothelial nanomedicine.
8.3. Challenges, opportunities and perspectives
A little more than a quarter century ago, even the cohesive concept of drug delivery to endothelium did not exist, let alone specific approaches that would work in vivo. Since the first reports of endothelial drug targeting via angiotensin-converting enzyme and thrombomodulin in the late eighties, many candidate target molecules have been identified. Some left the race (ciao, thrombomodulin), but others still have good chances to enable precise delivery of certain agents to certain endothelial compartments in certain vascular areas for treatment of certain disease conditions
Endothelial nanomedicine is poised to enter into the clinical domain in the near future, likely within several years, yet translational challenges are paramount. We must better understand biological aspects required to achieve endothelial-targeted pharmaceuticals. To provide tangible benefits, they must be free of intolerable side effects. These concerns are especially serious in treatment of very ill patients with severe acute diseases such as ARDS and massive stroke. DDSs may alter the toxicity of the cargo and/or incite their own side effects: systemic (e.g., due to interactions with host defense or blood clotting), off-target (e.g., due to unintended toxicity in clearing organs), or in intended therapeutic sites. The latter is especially undesirable in the context of endothelial nanomedicine, but may occur due to enhanced local concentration or harmful interactions of delivery systems with target cells, including interference with functions of the target molecule determinants.
The perspectives are glowing. The challenges are immense. They encompass many disciplines and require seamless interdisciplinary collaboration. It seems safe to postulate that iterative engineering of endothelial nanomedicine agents will soon optimize their efficacy and safety to the point when the scientific aspects of the problem (such as permeation of biological barriers) will be replaced by the translational issues – production, cost, approval, and practical use.
References
- 1.Pagano RE, Weinstein JN. Interactions of liposomes with mammalian cells. Annu Rev Biophys Bioeng. 1978;7(1):435–468. doi: 10.1146/annurev.bb.07.060178.002251. [DOI] [PubMed] [Google Scholar]
- 2.Gregoriadis G. Liposomes in therapeutic and preventive medicine: the development of the drug-carrier concept. Ann N Y Acad Sci. 1978;308(1):343–370. doi: 10.1111/j.1749-6632.1978.tb22034.x. [DOI] [PubMed] [Google Scholar]
- 3.Ryman BE, Tyrrell DA. Liposomes — methodology and applications. Front Biol. 1979;48:549–574. [PubMed] [Google Scholar]
- 4.Juliano RL. Drug delivery systems: a brief review. Can J Physiol Pharmacol. 1978;56(5):683–690. doi: 10.1139/y78-112. [DOI] [PubMed] [Google Scholar]
- 5.Langer RS, Peppas NA. Present and future applications of biomaterials in controlled drug delivery systems. Biomaterials. 1981;2(4):201–214. doi: 10.1016/0142-9612(81)90059-4. [DOI] [PubMed] [Google Scholar]
- 6.Elsabahy M, Wooley KL. Design of polymeric nanoparticles for biomedical delivery applications. Chem Soc Rev. 2012;41(7):2545–2561. doi: 10.1039/c2cs15327k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perry JL, Herlihy KP, Napier ME, Desimone JM. PRINT: a novel platform toward shape and size specific nanoparticle theranostics. Acc Chem Res. 2011;44(10):990–998. doi: 10.1021/ar2000315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sawant RR, Torchilin VP. Multifunctionality of lipid-core micelles for drug delivery and tumour targeting. Mol Membr Biol. 2010;27(7):232–246. doi: 10.3109/09687688.2010.516276. [DOI] [PubMed] [Google Scholar]
- 9.Venkataraman S, Hedrick JL, Ong ZY, Yang C, Ee PL, Hammond PT, Yang YY. The effects of polymeric nanostructure shape on drug delivery. Adv Drug Deliv Rev. 2011;63(14–15):1228–1246. doi: 10.1016/j.addr.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 10.van der Poll DG, Kieler-Ferguson HM, Floyd WC, Guillaudeu SJ, Jerger K, Szoka FC, Frechet JM. Design, synthesis, and biological evaluation of a robust, biodegradable dendrimer. Bioconjug Chem. 2010;21(4):764–773. doi: 10.1021/bc900553n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Szoka F, Jr, Papahadjopoulos D. Comparative properties and methods of preparation of lipid vesicles (liposomes) Annu Rev Biophys Bioeng. 1980;9(1):467–508. doi: 10.1146/annurev.bb.09.060180.002343. [DOI] [PubMed] [Google Scholar]
- 12.Percec V, Wilson DA, Leowanawat P, Wilson CJ, Hughes AD, Kaucher MS, Hammer DA, Levine DH, Kim AJ, Bates FS, Davis KP, Lodge TP, Klein ML, DeVane RH, Aqad E, Rosen BM, Argintaru AO, Sienkowska MJ, Rissanen K, Nummelin S, Ropponen J. Self-assembly of Janus dendrimers into uniform dendrimersomes and other complex architectures. Science. 2010;328(5981):1009–1014. doi: 10.1126/science.1185547. [DOI] [PubMed] [Google Scholar]
- 13.Eniola AO, Hammer DA. Characterization of biodegradable drug delivery vehicles with the adhesive properties of leukocytes II: effect of degradation on targeting activity. Biomaterials. 2005;26(6):661–670. doi: 10.1016/j.biomaterials.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 14.Discher BM, Won YY, Ege DS, Lee JC, Bates FS, Discher DE, Hammer DA. Polymersomes: tough vesicles made from diblock copolymers. Science. 1999;284(5417):1143–1146. doi: 10.1126/science.284.5417.1143. [DOI] [PubMed] [Google Scholar]
- 15.Farokhzad OC, Langer R. Impact of nanotechnology on drug delivery. ACS Nano. 2009;3(1):16–20. doi: 10.1021/nn900002m. [DOI] [PubMed] [Google Scholar]
- 16.Liong M, Lu J, Kovochich M, Xia T, Ruehm SG, Nel AE, Tamanoi F, Zink JI. Multifunctional inorganic nanoparticles for imaging, targeting, and drug delivery. ACS Nano. 2008;2(5):889–896. doi: 10.1021/nn800072t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Y, Mirkin CA, Park SJ. Nanofabrication beyond electronics. ACS Nano. 2009;3(5):1049–1056. doi: 10.1021/nn900448g. [DOI] [PubMed] [Google Scholar]
- 18.Bayer CL, Peppas NA. Advances in recognitive, conductive and responsive delivery systems. J Control Release. 2008;132(3):216–221. doi: 10.1016/j.jconrel.2008.06.021. [DOI] [PubMed] [Google Scholar]
- 19.Kieler-Ferguson HM, Frechet JM, Szoka FC., Jr Clinical developments of chemotherapeutic nanomedicines: polymers and liposomes for delivery of camptothecins and platinum (II) drugs. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2013;5(2):130–138. doi: 10.1002/wnan.1209. [DOI] [PubMed] [Google Scholar]
- 20.Batrakova EV, Kabanov AV. Pluronic block copolymers: evolution of drug delivery concept from inert nanocarriers to biological response modifiers. J Control Release. 2008;130(2):98–106. doi: 10.1016/j.jconrel.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wickline SA, Neubauer AM, Winter PM, Caruthers SD, Lanza GM. Molecular imaging and therapy of atherosclerosis with targeted nanoparticles. J Magn Reson Imaging. 2007;25(4):667–680. doi: 10.1002/jmri.20866. [DOI] [PubMed] [Google Scholar]
- 22.Hamilton AJ, Huang SL, Warnick D, Rabbat M, Kane B, Nagaraj A, Klegerman M, McPherson DD. Intravascular ultrasound molecular imaging of atheroma components in vivo. J Am Coll Cardiol. 2004;43(3):453–460. doi: 10.1016/j.jacc.2003.07.048. [DOI] [PubMed] [Google Scholar]
- 23.McIntosh DP, Tan XY, Oh P, Schnitzer JE. Targeting endothelium and its dynamic caveolae for tissue-specific transcytosis in vivo: a pathway to overcome cell barriers to drug and gene delivery. Proc Natl Acad Sci U S A. 2002;99(4):1996–2001. doi: 10.1073/pnas.251662398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muzykantov VR. Immunotargeting of drugs to the pulmonary vascular endothelium as a therapeutic strategy. Pathophysiology. 1998;5:15–33. [Google Scholar]
- 25.Moses MA, Brem H, Langer R. Advancing the field of drug delivery: taking aim at cancer. Cancer Cell. 2003;4(5):337–341. doi: 10.1016/s1535-6108(03)00276-9. [DOI] [PubMed] [Google Scholar]
- 26.Kirby C, Gregoriadis G. Dehydration–rehydration vesicles: a simple method for high yield drug entrapment in liposomes. Nat Biotechnol. 1984;2(11):979–984. [Google Scholar]
- 27.Park JW, Hong K, Kirpotin DB, Colbern G, Shalaby R, Baselga J, Shao Y, Nielsen UB, Marks JD, Moore D, Papahadjopoulos D, Benz CC. Anti-HER2 immunoliposomes: enhanced efficacy attributable to targeted delivery. Clin Cancer Res. 2002;8(4):1172–1181. [PubMed] [Google Scholar]
- 28.Muzykantov VR. Biomedical aspects of targeted delivery of drugs to pulmonary endothelium. Expert Opin Drug Deliv. 2005;2(5):909–926. doi: 10.1517/17425247.2.5.909. [DOI] [PubMed] [Google Scholar]
- 29.Fisher AB. Redox signaling across cell membranes. Antioxid Redox Signal. 2009;11(6):1–8. doi: 10.1089/ars.2008.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Segal BH, Han W, Bushey JJ, Joo M, Bhatti Z, Feminella J, Dennis CG, Vethanayagam RR, Yull FE, Capitano M, Wallace PK, Minderman H, Christman JW, Sporn MB, Chan J, Vinh DC, Holland SM, Romani LR, Gaffen SL, Freeman ML, Blackwell TS. NADPH oxidase limits innate immune responses in the lungs in mice. PLoS One. 2010;5(3):e9631 9631–9614. doi: 10.1371/journal.pone.0009631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Janssen-Heininger YM, Mossman BT, Heintz NH, Forman HJ, Kalyanaraman B, Finkel T, Stamler JS, Rhee SG, van der Vliet A. Redox-based regulation of signal transduction: principles, pitfalls, and promises. Free Radic Biol Med. 2008;45(1):1–17. doi: 10.1016/j.freeradbiomed.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van der Vliet A. NADPH oxidases in lung biology and pathology: host defense enzymes and more. Free Radic Biol Med. 2008;44(6):938–955. doi: 10.1016/j.freeradbiomed.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rhee SG. Cell signaling H2O2, a necessary evil for cell signaling. Science. 2006;312(5782):1882–1883. doi: 10.1126/science.1130481. [DOI] [PubMed] [Google Scholar]
- 34.Forman HJ, Maiorino M, Ursini F. Signaling functions of reactive oxygen species. Biochemistry. 2010;49(5):835–842. doi: 10.1021/bi9020378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cybulsky MI, Gimbrone MA., Jr Endothelial expression of a mononuclear leukocyte adhesion molecule during atherogenesis. Science. 1991;251(4995):788–791. doi: 10.1126/science.1990440. [DOI] [PubMed] [Google Scholar]
- 36.Iiyama K, Hajra L, Iiyama M, Li H, DiChiara M, Medoff BD, Cybulsky MI. Patterns of vascular cell adhesion molecule-1 and intercellular adhesion molecule-1 expression in rabbit and mouse atherosclerotic lesions and at sites predisposed to lesion formation. Circ Res. 1999;85(2):199–207. doi: 10.1161/01.res.85.2.199. [DOI] [PubMed] [Google Scholar]
- 37.Muro S, Muzykantov VR. Targeting of antioxidant and anti-thrombotic drugs to endothelial cell adhesion molecules. Curr Pharm Des. 2005;11(18):2383–2401. doi: 10.2174/1381612054367274. [DOI] [PubMed] [Google Scholar]
- 38.Aird WC. Endothelium and haemostasis. Hamostaseologie. 2015;35(1):11–16. doi: 10.5482/HAMO-14-11-0075. [DOI] [PubMed] [Google Scholar]
- 39.Molema G, Aird WC. Vascular heterogeneity in the kidney. Semin Nephrol. 2012;32(2):145–155. doi: 10.1016/j.semnephrol.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 40.Braet F, Wisse E. Structural and functional aspects of liver sinusoidal endothelial cell fenestrae: a review. Comp Hepatol. 2002;1(1):1. doi: 10.1186/1476-5926-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reichen J. The role of the sinusoidal endothelium in liver function. News Physiol Sci. 1999;14:117–121. doi: 10.1152/physiologyonline.1999.14.3.117. [DOI] [PubMed] [Google Scholar]
- 42.Stan RV. Endothelial stomatal and fenestral diaphragms in normal vessels and an-giogenesis. J Cell Mol Med. 2007;11(4):621–643. doi: 10.1111/j.1582-4934.2007.00075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schafer AI. Vascular endothelium: in defense of blood fluidity. J Clin Invest. 1997;99(6):1143–1144. doi: 10.1172/JCI119266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Hinsbergh VW. Endothelium—role in regulation of coagulation and inflammation. Semin Immunopathol. 2012;34(1):93–106. doi: 10.1007/s00281-011-0285-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goldenberg NM, Kuebler WM. Endothelial cell regulation of pulmonary vascular tone, inflammation, and coagulation. Comp Physiol. 2015;5(2):531–559. doi: 10.1002/cphy.c140024. [DOI] [PubMed] [Google Scholar]
- 46.Vanhoutte PM. Endothelium-derived free radicals: for worse and for better. J Clin Invest. 2001;107(1):23–25. doi: 10.1172/JCI11832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Simionescu M, Gafencu A, Antohe F. Transcytosis of plasma macromolecules in endothelial cells: a cell biological survey. Microsc Res Tech. 2002;57(5):269–288. doi: 10.1002/jemt.10086. [DOI] [PubMed] [Google Scholar]
- 48.Gimbrone MA., Jr Vascular endothelium, hemodynamic forces, and atherogenesis. Am J Pathol. 1999;155(1):1–5. doi: 10.1016/S0002-9440(10)65090-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Feletou M, Kohler R, Vanhoutte PM. Nitric oxide: orchestrator of endothelium-dependent responses. Ann Med. 2012;44(7):694–716. doi: 10.3109/07853890.2011.585658. [DOI] [PubMed] [Google Scholar]
- 50.Sullivan DP, Muller WA. Neutrophil and monocyte recruitment by PECAM, CD99, and other molecules via the LBRC. Semin Immunopathol. 2014;36(2):193–209. doi: 10.1007/s00281-013-0412-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dong Y, Love KT, Dorkin JR, Sirirungruang S, Zhang Y, Chen D, Bogorad RL, Yin H, Chen Y, Vegas AJ, Alabi CA, Sahay G, Olejnik KT, Wang W, Schroeder A, Lytton-Jean AK, Siegwart DJ, Akinc A, Barnes C, Barros SA, Carioto M, Fitzgerald K, Hettinger J, Kumar V, Novobrantseva TI, Qin J, Querbes W, Koteliansky V, Langer R, Anderson DG. Lipopeptide nanoparticles for potent and selective siRNA delivery in rodents and nonhuman primates. Proc Natl Acad Sci U S A. 2014;111(11):3955–3960. doi: 10.1073/pnas.1322937111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Anselmo AC, Gupta V, Zern BJ, Pan D, Zakrewsky M, Muzykantov V, Mitragotri S. Delivering nanoparticles to lungs while avoiding liver and spleen through adsorption on red blood cells. ACS Nano. 2013;7(12):11129–11137. doi: 10.1021/nn404853z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Danhier F, Feron O, Preat V. To exploit the tumor microenvironment: passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J Control Release. 2010;148(2):135–146. doi: 10.1016/j.jconrel.2010.08.027. [DOI] [PubMed] [Google Scholar]
- 54.Oh P, Li Y, Yu J, Durr E, Krasinska KM, Carver LA, Testa JE, Schnitzer JE. Subtractive proteomic mapping of the endothelial surface in lung and solid tumours for tissue-specific therapy. Nature. 2004;429(6992):629–635. doi: 10.1038/nature02580. [DOI] [PubMed] [Google Scholar]
- 55.Schnitzer JE. Vascular targeting as a strategy for cancer therapy. N Engl J Med. 1998;339(7):472–474. doi: 10.1056/NEJM199808133390711. [DOI] [PubMed] [Google Scholar]
- 56.Kennel SJ, Lee R, Bultman S, Kabalka G. Rat monoclonal antibody distribution in mice: an epitope inside the lung vascular space mediates very efficient localization. Int J Rad Appl Instrum B. 1990;17(2):193–200. doi: 10.1016/0883-2897(90)90147-s. [DOI] [PubMed] [Google Scholar]
- 57.Spragg DD, Alford DR, Greferath R, Larsen CE, Lee KD, Gurtner GC, Cybulsky MI, Tosi PF, Nicolau C, Gimbrone MA., Jr Immunotargeting of liposomes to activated vascular endothelial cells: a strategy for site-selective delivery in the cardiovascular system. Proc Natl Acad Sci U S A. 1997;94(16):8795–8800. doi: 10.1073/pnas.94.16.8795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huang RB, Mocherla S, Heslinga MJ, Charoenphol P, Eniola-Adefeso O. Dynamic and cellular interactions of nanoparticles in vascular-targeted drug delivery. Mol Membr Biol. 2010;27(7):312–327. doi: 10.3109/09687688.2010.522117. [DOI] [PubMed] [Google Scholar]
- 59.Corti A, Pastorino F, Curnis F, Arap W, Ponzoni M, Pasqualini R. Targeted drug delivery and penetration into solid tumors. Med Res Rev. 2012;32(5):1078–1091. doi: 10.1002/med.20238. [DOI] [PubMed] [Google Scholar]
- 60.Noble CO, Kirpotin DB, Hayes ME, Mamot C, Hong K, Park JW, Benz CC, Marks JD, Drummond DC. Development of ligand-targeted liposomes for cancer therapy. Expert Opin Ther Targets. 2004;8(4):335–353. doi: 10.1517/14728222.8.4.335. [DOI] [PubMed] [Google Scholar]
- 61.Xia W, Low PS. Folate-targeted therapies for cancer. J Med Chem. 2010;53(19):6811–6824. doi: 10.1021/jm100509v. [DOI] [PubMed] [Google Scholar]
- 62.Muzykantov VR, Danilov SM. In: Targeted Delivery of Imaging Agents. Torchilin V, editor. CRC Press; Roca Baton, Florida: 1995. pp. 465–485. [Google Scholar]
- 63.Goetz DJ, El-sabban ME, Hammer DA, Pauli BU. Lu-ECAM-1-mediated adhesion of melanoma cells to endothelium under conditions of flow. Int J Cancer. 1996;65(2):192–199. doi: 10.1002/(SICI)1097-0215(19960117)65:2<192::AID-IJC11>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 64.Huang X, Molema G, King S, Watkins L, Edgington TS, Thorpe PE. Tumor infarction in mice by antibody-directed targeting of tissue factor to tumor vasculature. Science. 1997;275(5299):547–550. doi: 10.1126/science.275.5299.547. [DOI] [PubMed] [Google Scholar]
- 65.Stan RV, Ghitescu L, Jacobson BS, Palade GE. Isolation, cloning, and localization of rat PV-1, a novel endothelial caveolar protein. J Cell Biol. 1999;145(6):1189–1198. doi: 10.1083/jcb.145.6.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rajotte D, Arap W, Hagedorn M, Koivunen E, Pasqualini R, Ruoslahti E. Molecular heterogeneity of the vascular endothelium revealed by in vivo phage display. J Clin Invest. 1998;102(2):430–437. doi: 10.1172/JCI3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Danilov SM, Muzykantov VR, Martynov AV, Atochina EN, Sakharov I, Trakht IN, Smirnov VN. Lung is the target organ for a monoclonal antibody to angiotensin-converting enzyme. Lab Investig. 1991;64(1):118–124. [PubMed] [Google Scholar]
- 68.Pasqualini R, McDonald DM, Arap W. Vascular targeting and antigen presentation. Nat Immunol. 2001;2(7):567–568. doi: 10.1038/89704. [DOI] [PubMed] [Google Scholar]
- 69.Muzykantov VR, Atochina EN, Ischiropoulos H, Danilov SM, Fisher AB. Immunotargeting of antioxidant enzyme to the pulmonary endothelium. Proc Natl Acad Sci U S A. 1996;93(11):5213–5218. doi: 10.1073/pnas.93.11.5213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Reynolds PN, Nicklin SA, Kaliberova L, Boatman BG, Grizzle WE, Balyasnikova IV, Baker AH, Danilov SM, Curiel DT. Combined transductional and transcriptional targeting improves the specificity of transgene expression in vivo. Nat Biotechnol. 2001;19(9):838–842. doi: 10.1038/nbt0901-838. [DOI] [PubMed] [Google Scholar]
- 71.Langer R. Drug delivery and targeting. Nature. 1998;392(6679 Suppl):5–10. [PubMed] [Google Scholar]
- 72.Trubetskoy VS, Torchilin VP, Kennel S, Huang L. Cationic liposomes enhance targeted delivery and expression of exogenous DNA mediated by N-terminal modified poly(L-lysine)-antibody conjugate in mouse lung endothelial cells. Biochim Biophys Acta. 1992;1131(3):311–313. doi: 10.1016/0167-4781(92)90030-4. [DOI] [PubMed] [Google Scholar]
- 73.Danilov SM, Martynov AV, Klibanov AL, Slinkin MA, Sakharov I, Malov AG, Sergienko VB, Vedernikov A, Muzykantov VR, Torchilin VP. Radioimmunoimaging of lung vessels: an approach using indium-111-labeled monoclonal antibody to angiotensin-converting enzyme. J Nucl Med. 1989;30(10):1686–1692. [PubMed] [Google Scholar]
- 74.Maruyama K, Takizawa T, Yuda T, Kennel SJ, Huang L, Iwatsuru M. Targetability of novel immunoliposomes modified with amphipathic poly(ethylene glycol)s conjugated at their distal terminals to monoclonal antibodies. Biochim Biophys Acta. 1995;1234(1):74–80. doi: 10.1016/0005-2736(94)00263-o. [DOI] [PubMed] [Google Scholar]
- 75.Scherpereel A, Rome JJ, Wiewrodt R, Watkins SC, Harshaw DW, Alder S, Christofidou-Solomidou M, Haut E, Murciano JC, Nakada M, Albelda SM, Muzykantov VR. Platelet-endothelial cell adhesion molecule-1-directed immunotargeting to cardiopulmonary vasculature. J Pharmacol Exp Ther. 2002;300(3):777–786. doi: 10.1124/jpet.300.3.777. [DOI] [PubMed] [Google Scholar]
- 76.Danielyan K, Ding BS, Gottstein C, Cines DB, Muzykantov VR. Delivery of anti-platelet-endothelial cell adhesion molecule single-chain variable fragment-urokinase fusion protein to the cerebral vasculature lyses arterial clots and attenuates postischemic brain edema. J Pharmacol Exp Ther. 2007;321(3):947–952. doi: 10.1124/jpet.107.120535. [DOI] [PubMed] [Google Scholar]
- 77.Durr E, Yu J, Krasinska KM, Carver LA, Yates JR, Testa JE, Oh P, Schnitzer JE. Direct proteomic mapping of the lung microvascular endothelial cell surface in vivo and in cell culture. Nat Biotechnol. 2004;22(8):985–992. doi: 10.1038/nbt993. [DOI] [PubMed] [Google Scholar]
- 78.Tradonsky A, Rubin T, Beck R, Ring B, Seitz R, Mair S. A search for reliable molecular markers of prognosis in prostate cancer: a study of 240 cases. Am J Clin Pathol. 2012;137(6):918–930. doi: 10.1309/AJCPF3QWIG8FWXIH. [DOI] [PubMed] [Google Scholar]
- 79.Wood KC, Azarin SM, Arap W, Pasqualini R, Langer R, Hammond PT. Tumor-targeted gene delivery using molecularly engineered hybrid polymers functionalized with a tumor-homing peptide. Bioconjug Chem. 2008;19(2):403–405. doi: 10.1021/bc700408r. [DOI] [PubMed] [Google Scholar]
- 80.Valadon P, Garnett JD, Testa JE, Bauerle M, Oh P, Schnitzer JE. Screening phage display libraries for organ-specific vascular immunotargeting in vivo. Proc Natl Acad Sci U S A. 2006;103(2):407–412. doi: 10.1073/pnas.0506938103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Howard M, Zern BJ, Anselmo AC, Shuvaev VV, Mitragotri S, Muzykantov V. Vascular targeting of nanocarriers: perplexing aspects of the seemingly straightforward paradigm. ACS Nano. 2014;8(5):4100–4132. doi: 10.1021/nn500136z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Garnacho C, Albelda SM, Muzykantov VR, Muro S. Differential intra-endothelial delivery of polymer nanocarriers targeted to distinct PECAM-1 epitopes. J Control Release. 2008;130(3):226–233. doi: 10.1016/j.jconrel.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mulivor AW, Lipowsky HH. Role of glycocalyx in leukocyte-endothelial cell adhesion. Am J Physiol Heart Circ Physiol. 2002;283(4):H1282–H1291. doi: 10.1152/ajpheart.00117.2002. [DOI] [PubMed] [Google Scholar]
- 84.Muzykantov VR, Puchnina EA, Atochina EN, Hiemish H, Slinkin MA, Meertsuk FE, Danilov SM. Endotoxin reduces specific pulmonary uptake of radiolabeled monoclonal antibody to angiotensin-converting enzyme. J Nucl Med. 1991;32(3):453–460. [PubMed] [Google Scholar]
- 85.Skidgel RA. Bradykinin-degrading enzymes: structure, function, distribution, and potential roles in cardiovascular pharmacology. J Cardiovasc Pharmacol. 1992;20(Suppl 9):S4–S9. doi: 10.1097/00005344-199200209-00003. [DOI] [PubMed] [Google Scholar]
- 86.Aird WC, Edelberg JM, Weiler-Guettler H, Simmons WW, Smith TW, Rosenberg RD. Vascular bed-specific expression of an endothelial cell gene is programmed by the tissue microenvironment. J Cell Biol. 1997;138(5):1117–1124. doi: 10.1083/jcb.138.5.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Aird WC. Phenotypic heterogeneity of the endothelium: Structure, function I, and mechanisms. Circ Res. 2007;100(2):158–173. doi: 10.1161/01.RES.0000255691.76142.4a. [DOI] [PubMed] [Google Scholar]
- 88.Davies PF. Hemodynamic shear stress and the endothelium in cardiovascular pathophysiology. Nat Clin Pract Cardiovasc Med. 2009;6(1):16–26. doi: 10.1038/ncpcardio1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Beneteau-Burnat B, Baudin B. Angiotensin-converting enzyme: clinical applications and laboratory investigations on serum and other biological fluids. Crit Rev Clin Lab Sci. 1991;28(5–6):337–356. doi: 10.3109/10408369109106868. [DOI] [PubMed] [Google Scholar]
- 90.Shuvaev VV, Christofidou-Solomidou M, Scherpereel A, Simone E, Arguiri E, Tliba S, Pick J, Kennel S, Albelda SM, Muzykantov VR. Factors modulating the delivery and effect of enzymatic cargo conjugated with antibodies targeted to the pulmonary endothelium. J Control Release. 2007;118(2):235–244. doi: 10.1016/j.jconrel.2006.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Danilov SM, Gavrilyuk VD, Franke FE, Pauls K, Harshaw DW, McDonald TD, Miletich DJ, Muzykantov VR. Lung uptake of antibodies to endothelial antigens: key determinants of vascular immunotargeting. Am J Physiol Lung Cell Mol Physiol. 2001;280(6):L1335–L1347. doi: 10.1152/ajplung.2001.280.6.L1335. [DOI] [PubMed] [Google Scholar]
- 92.Keelan ET, Harrison AA, Chapman PT, Binns RM, Peters AM, Haskard DO. Imaging vascular endothelial activation: an approach using radiolabeled monoclonal antibodies against the endothelial cell adhesion molecule E-selectin. J Nucl Med. 1994;35(2):276–281. [PubMed] [Google Scholar]
- 93.Muro S, Koval M, Muzykantov V. Endothelial endocytic pathways: gates for vascular drug delivery. Curr Vasc Pharmacol. 2004;2(3):281–299. doi: 10.2174/1570161043385736. [DOI] [PubMed] [Google Scholar]
- 94.Newman PJ, Albelda SM. Cellular and molecular aspects of PECAM-1. Nouv Rev Fr Hematol. 1992;34(Suppl):S9–13. [PubMed] [Google Scholar]
- 95.Giannotta M, Trani M, Dejana E. VE-cadherin and endothelial adherens junctions: active guardians of vascular integrity. Dev Cell. 2013;26(5):441–454. doi: 10.1016/j.devcel.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 96.Barreiro O, Yanez-Mo M, Sala-Valdes M, Gutierrez-Lopez MD, Ovalle S, Higginbottom A, Monk PN, Cabanas C, Sanchez-Madrid F. Endothelial tetraspanin microdomains regulate leukocyte firm adhesion during extravasation. Blood. 2005;105(7):2852–2861. doi: 10.1182/blood-2004-09-3606. [DOI] [PubMed] [Google Scholar]
- 97.Cook-Mills JM, Marchese ME, Abdala-Valencia H. Vascular cell adhesion molecule-1 expression and signaling during disease: regulation by reactive oxygen species and antioxidants. Antioxid Redox Signal. 2011;15(6):1607–1638. doi: 10.1089/ars.2010.3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Almenar-Queralt A, Duperray A, Miles LA, Felez J, Altieri DC. Apical topography and modulation of ICAM-1 expression on activated endothelium. Am J Pathol. 1995;147(5):1278–1288. [PMC free article] [PubMed] [Google Scholar]
- 99.Wright MD, Moseley GW, van Spriel AB. Tetraspanin microdomains in immune cell signalling and malignant disease. Tissue Antigens. 2004;64(5):533–542. doi: 10.1111/j.1399-0039.2004.00321.x. [DOI] [PubMed] [Google Scholar]
- 100.Ghitescu L, Jacobson BS, Crine P. A novel, 85 kDa endothelial antigen differentiates plasma membrane macrodomains in lung alveolar capillaries. Endothelium. 1999;6(3):241–250. doi: 10.3109/10623329909053414. [DOI] [PubMed] [Google Scholar]
- 101.Murciano JC, Harshaw DW, Ghitescu L, Danilov SM, Muzykantov VR. Vascular immunotargeting to endothelial surface in a specific macrodomain in alveolar capillaries. Am J Respir Crit Care Med. 2001;164(7):1295–1302. doi: 10.1164/ajrccm.164.7.2010076. [DOI] [PubMed] [Google Scholar]
- 102.Oh P, Borgstrom P, Witkiewicz H, Li Y, Borgstrom BJ, Chrastina A, Iwata K, Zinn KR, Baldwin R, Testa JE, Schnitzer JE. Live dynamic imaging of caveolae pumping targeted antibody rapidly and specifically across endothelium in the lung. Nat Biotechnol. 2007;25(3):327–337. doi: 10.1038/nbt1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Danilov S, Atochina E, Hiemisch H, Churak-ova T, Moldobayeva A, Sakharov I, Deichman G, Ryan U, Muzykantov VR. Interaction of mAb to angiotensin-converting enzyme (ACE) with antigen in vitro and in vivo: antibody targeting to the lung induces ACE antigenic modulation. Int Immunol. 1994;6(8):1153–1160. doi: 10.1093/intimm/6.8.1153. [DOI] [PubMed] [Google Scholar]
- 104.Kennel SJ, Falcioni R, Wesley JW. Microdistribution of specific rat monoclonal antibodies to mouse tissues and human tumor xenografts. Cancer Res. 1991;51(5):1529–1536. [PubMed] [Google Scholar]
- 105.Erdos EG. Angiotensin I converting enzyme and the changes in our concepts through the years. Lewis K. Dahl memorial lecture. Hypertension. 1990;16(4):363–370. doi: 10.1161/01.hyp.16.4.363. [DOI] [PubMed] [Google Scholar]
- 106.Heitsch H, Brovkovych S, Malinski T, Wiemer G. Angiotensin-(1–7)-stimulated nitric oxide and superoxide release from endothelial cells. Hypertension. 2001;37(1):72–76. doi: 10.1161/01.hyp.37.1.72. [DOI] [PubMed] [Google Scholar]
- 107.Balyasnikova IV, Karran EH, Albrecht RF, 2nd, Danilov SM. Epitope-specific antibody-induced cleavage of angiotensin-converting enzyme from the cell surface. Biochem J. 2002;362(Pt 3):585–595. doi: 10.1042/0264-6021:3620585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Muzykantov VR. In: Biomedical Aspects of Drug Targeting. Muzykantov VR, Torchilin VP, editors. Kluwer Academic Publishers; Boston: 2002. pp. 129–148. [Google Scholar]
- 109.Muzykantov VR. Targeting of superoxide dismutase and catalase to vascular endothelium. J Control Release. 2001;71(1):1–21. doi: 10.1016/s0168-3659(01)00215-2. [DOI] [PubMed] [Google Scholar]
- 110.Watanabe K, Lam G, Keresztes RS, Jaffe EA. Lipopolysaccharides decrease angiotensin converting enzyme activity expressed by cultured human endothelial cells. J Cell Physiol. 1992;150(2):433–439. doi: 10.1002/jcp.1041500228. [DOI] [PubMed] [Google Scholar]
- 111.Atochina EN, Hiemisch HH, Muzykantov VR, Danilov SM. Systemic administration of platelet-activating factor in rat reduces specific pulmonary uptake of circulating monoclonal antibody to angiotensin-converting enzyme. Lung. 1992;170(6):349–358. doi: 10.1007/BF00177581. [DOI] [PubMed] [Google Scholar]
- 112.Balyasnikova IV, Sun ZL, Metzger R, Taylor PR, Vicini E, Muciaccia B, Visintine DJ, Berestetskaya YV, McDonald TD, Danilov SM. Monoclonal antibodies to native mouse angiotensin-converting enzyme (CD143): ACE expression quantification, lung endothelial cell targeting and gene delivery. Tissue Antigens. 2006;67(1):10–29. doi: 10.1111/j.1399-0039.2005.00516.x. [DOI] [PubMed] [Google Scholar]
- 113.Atochina EN, Muzykantov VR, Al-Mehdi AB, Danilov SM, Fisher AB. Normoxic lung ischemia/reperfusion accelerates shedding of angiotensin converting enzyme from the pulmonary endothelium. Am J Respir Crit Care Med. 1997;156(4 Pt 1):1114–1119. doi: 10.1164/ajrccm.156.4.96-12116. [DOI] [PubMed] [Google Scholar]
- 114.Muzykantov VR, Atochina EN, Kuo A, Barnathan ES, Notarfrancesco K, Shuman H, Dodia C, Fisher AB. Endothelial cells internalize monoclonal antibody to angiotensin-converting enzyme. Am J Phys. 1996;270(5 Pt 1):L704–L713. doi: 10.1152/ajplung.1996.270.5.L704. [DOI] [PubMed] [Google Scholar]
- 115.Balyasnikova IV, Metzger R, Visintine DJ, Dimasius V, Sun ZL, Berestetskaya YV, McDonald TD, Curiel DT, Minshall RD, Danilov SM. Selective rat lung endothe-lial targeting with a new set of monoclonal antibodies to angiotensin I-converting enzyme. Pulm Pharmacol Ther. 2005;18(4):251–267. doi: 10.1016/j.pupt.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 116.Reynolds PN, Zinn KR, Gavrilyuk VD, Balyasnikova IV, Rogers BE, Buchsbaum DJ, Wang MH, Miletich DJ, Grizzle WE, Douglas JT, Danilov SM, Curiel DT. A targetable, injectable adenoviral vector for selective gene delivery to pulmonary endothelium in vivo. Mol Ther. 2000;2(6):562–578. doi: 10.1006/mthe.2000.0205. [DOI] [PubMed] [Google Scholar]
- 117.Miller WH, Brosnan MJ, Graham D, Nicol CG, Morecroft I, Channon KM, Danilov SM, Reynolds PN, Baker AH, Dominiczak AF. Targeting endothelial cells with adenovirus expressing nitric oxide synthase prevents elevation of blood pressure in stroke-prone spontaneously hypertensive rats. Mol Ther. 2005;12(2):321–327. doi: 10.1016/j.ymthe.2005.02.025. [DOI] [PubMed] [Google Scholar]
- 118.Reynolds AM, Xia W, Holmes MD, Hodge SJ, Danilov S, Curiel DT, Morrell NW, Reynolds PN. Bone morphogenetic protein type 2 receptor gene therapy attenuates hypoxic pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2007;292(5):L1182–L1192. doi: 10.1152/ajplung.00020.2006. [DOI] [PubMed] [Google Scholar]
- 119.Martin FA, Murphy RP, Cummins PM. Thrombomodulin and the vascular endothelium: insights into functional, regulatory, and therapeutic aspects. Am J Phys-iol Heart Circ Physiol. 2013;304(12):H1585–H1597. doi: 10.1152/ajpheart.00096.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Christofidou-Solomidou M, Kennel S, Scherpereel A, Wiewrodt R, Solomides CC, Pietra GG, Murciano JC, Shah SA, Ischiropoulos H, Albelda SM, Muzykantov VR. Vascular immunotargeting of glucose oxidase to the endothelial antigens induces distinct forms of oxidant acute lung injury: targeting to thrombomodulin, but not to PECAM-1, causes pulmonary thrombosis and neutrophil transmigration. Am J Pathol. 2002;160(3):1155–1169. doi: 10.1016/S0002-9440(10)64935-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Perkowski S, Sun J, Singhal S, Santiago J, Leikauf GD, Albelda SM. Gene expression profiling of the early pulmonary response to hyperoxia in mice. Am J Respir Cell Mol Biol. 2003;28(6):682–696. doi: 10.1165/rcmb.4692. [DOI] [PubMed] [Google Scholar]
- 122.Brisson C, Archipoff G, Hartmann ML, Hanau D, Beretz A, Freyssinet JM, Cazenave JP. Antibodies to thrombomodulin induce receptor-mediated endocytosis in human saphenous vein endothelial cells. Thromb Haemost. 1992;68(6):737–743. [PubMed] [Google Scholar]
- 123.Guermazi S, Mellouli F, Trabelsi S, Bejaoui M, Dellagi K. Anti-thrombomodulin antibodies and venous thrombosis. Blood Coagul Fibrinolysis. 2004;15(7):553–558. doi: 10.1097/00001721-200410000-00004. [DOI] [PubMed] [Google Scholar]
- 124.Parton RG, Simons K. The multiple faces of caveolae. Nat Rev Mol Cell Biol. 2007;8(3):185–194. doi: 10.1038/nrm2122. [DOI] [PubMed] [Google Scholar]
- 125.Riezman H, Woodman PG, van Meer G, Marsh M. Molecular mechanisms of endocytosis. Cell. 1997;91(6):731–738. doi: 10.1016/s0092-8674(00)80461-4. [DOI] [PubMed] [Google Scholar]
- 126.Predescu D, Palade GE. Plasmalemmal vesicles represent the large pore system of continuous microvascular endothelium. Am J Phys. 1993;265(2 Pt 2):H725–H733. doi: 10.1152/ajpheart.1993.265.2.H725. [DOI] [PubMed] [Google Scholar]
- 127.Stan RV. Structure and function of endothelial caveolae. Microsc Res Tech. 2002;57(5):350–364. doi: 10.1002/jemt.10089. [DOI] [PubMed] [Google Scholar]
- 128.Minshall RD, Tiruppathi C, Vogel SM, Malik AB. Vesicle formation and trafficking in endothelial cells and regulation of endothelial barrier function. Histochem Cell Biol. 2002;117(2):105–112. doi: 10.1007/s00418-001-0367-x. [DOI] [PubMed] [Google Scholar]
- 129.Schnitzer JE. Caveolae: from basic trafficking mechanisms to targeting transcytosis for tissue-specific drug and gene delivery in vivo. Adv Drug Deliv Rev. 2001;49(3):265–280. doi: 10.1016/s0169-409x(01)00141-7. [DOI] [PubMed] [Google Scholar]
- 130.Schnitzer JE, McIntosh DP, Dvorak AM, Liu J, Oh P. Separation of caveolae from associated microdomains of GPI-anchored proteins. Science. 1995;269(5229):1435–1439. doi: 10.1126/science.7660128. [DOI] [PubMed] [Google Scholar]
- 131.Minshall RD, Tiruppathi C, Vogel SM, Niles WD, Gilchrist A, Hamm HE, Malik AB. Endothelial cell-surface gp60 activates vesicle formation and trafficking via G(i)-coupled Src kinase signaling pathway. J Cell Biol. 2000;150(5):1057–1070. doi: 10.1083/jcb.150.5.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Vogel SM, Easington CR, Minshall RD, Niles WD, Tiruppathi C, Hollenberg SM, Parrillo JE, Malik AB. Evidence of transcellular permeability pathway in microvessels. Microvasc Res. 2001;61(1):87–101. doi: 10.1006/mvre.2000.2274. [DOI] [PubMed] [Google Scholar]
- 133.John TA, Vogel SM, Tiruppathi C, Malik AB, Minshall RD. Quantitative analysis of albumin uptake and transport in the rat microvessel endothelial monolayer. Am J Physiol Lung Cell Mol Physiol. 2003;284(1):L187–L196. doi: 10.1152/ajplung.00152.2002. [DOI] [PubMed] [Google Scholar]
- 134.Tiruppathi C, Song W, Bergenfeldt M, Sass P, Malik AB. Gp60 activation mediates albumin transcytosis in endothelial cells by tyrosine kinase-dependent pathway. J Biol Chem. 1997;272(41):25968–25975. doi: 10.1074/jbc.272.41.25968. [DOI] [PubMed] [Google Scholar]
- 135.Predescu D, Predescu S, Malik AB. Transport of nitrated albumin across continuous vascular endothelium. Proc Natl Acad Sci U S A. 2002;99(21):13932–13937. doi: 10.1073/pnas.212253499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Simone EA, Zern BJ, Chacko AM, Mikitsh JL, Blankemeyer ER, Muro S, Stan RV, Muzykantov VR. Endothelial targeting of polymeric nanoparticles stably labeled with the PET imaging radioisotope iodine-124. Biomaterials. 2012;33(21):5406–5413. doi: 10.1016/j.biomaterials.2012.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Iversen TG, Frerker N, Sandvig K. Uptake of ricinB-quantum dot nanoparticles by a macropinocytosis-like mechanism. J Nanobiotechnol. 2012;10:33. doi: 10.1186/1477-3155-10-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wang Z, Tiruppathi C, Minshall RD, Malik AB. Size and dynamics of caveolae studied using nanoparticles in living endothelial cells. ACS Nano. 2009;3(12):4110–4116. doi: 10.1021/nn9012274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Springer TA. Adhesion receptors of the immune system. Nature. 1990;346(6283):425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- 140.Doerschuk CM, Quinlan WM, Doyle NA, Bullard DC, Vestweber D, Jones ML, Takei F, Ward PA, Beaudet AL. The role of P-selectin and ICAM-1 in acute lung injury as determined using blocking antibodies and mutant mice. J Immunol. 1996;157(10):4609–4614. [PubMed] [Google Scholar]
- 141.Kishimoto TK, Rothlein R. Integrins, ICAMs, and selectins: role and regulation of adhesion molecules in neutrophil recruitment to inflammatory sites. Adv Pharmacol. 1994;25:117–169. doi: 10.1016/s1054-3589(08)60431-7. [DOI] [PubMed] [Google Scholar]
- 142.Albelda SM. Endothelial and epithelial cell adhesion molecules. Am J Respir Cell Mol Biol. 1991;4(3):195–203. doi: 10.1165/ajrcmb/4.3.195. [DOI] [PubMed] [Google Scholar]
- 143.Tsourkas A, Shinde-Patil VR, Kelly K, Patel P, Wolley A, Allport JR, Weissleder R. In Vivo Imaging of Activated Endothelium Using an Anti-VCAM-1 Magneto-Optical Probe. 2005 doi: 10.1021/bc050002e. [DOI] [PubMed] [Google Scholar]
- 144.Kiely JM, Cybulsky MI, Luscinskas FW, Gimbrone MA., Jr Immunoselective targeting of an anti-thrombin agent to the surface of cytokine-activated vascular endothelial cells. Arterioscler Thromb Vasc Biol. 1995;15(8):1211–1218. doi: 10.1161/01.atv.15.8.1211. [DOI] [PubMed] [Google Scholar]
- 145.Kuijpers TW, Raleigh M, Kavanagh T, Janssen H, Calafat J, Roos D, Harlan JM. Cytokine-activated endothelial cells internalize E-selectin into a lysosomal compartment of vesiculotubular shape. A tubulin-driven process. J Immunol. 1994;152(10):5060–5069. [PubMed] [Google Scholar]
- 146.Straley KS, Green SA. Rapid transport of internalized P-selectin to late endosomes and the TGN: roles in regulating cell surface expression and recycling to secretory granules. J Cell Biol. 2000;151(1):107–116. doi: 10.1083/jcb.151.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.von Asmuth EJ, Smeets EF, Ginsel LA, Onderwater JJ, Leeuwenberg JF, Buurman WA. Evidence for endocytosis of E-selectin in human endothelial cells. Eur J Immunol. 1992;22(10):2519–2526. doi: 10.1002/eji.1830221009. [DOI] [PubMed] [Google Scholar]
- 148.Kessner S, Krause A, Rothe U, Bendas G. Investigation of the cellular uptake of E-selectin-targeted immunoliposomes by activated human endothelial cells. Biochim Biophys Acta. 2001;1514(2):177–190. doi: 10.1016/s0005-2736(01)00368-6. [DOI] [PubMed] [Google Scholar]
- 149.Everts M, Kok RJ, Asgeirsdottir SA, Melgert BN, Moolenaar TJ, Koning GA, van Luyn MJ, Meijer DK, Molema G. Selective intracellular delivery of dexamethasone into activated endothelial cells using an E-selectin-directed immunoconjugate. J Immunol. 2002;168(2):883–889. doi: 10.4049/jimmunol.168.2.883. [DOI] [PubMed] [Google Scholar]
- 150.Harari OA, Wickham TJ, Stocker CJ, Kovesdi I, Segal DM, Huehns TY, Sarraf C, Haskard DO. Targeting an adenoviral gene vector to cytokine-activated vascular endothelium via E-selectin. Gene Ther. 1999;6(5):801–807. doi: 10.1038/sj.gt.3300898. [DOI] [PubMed] [Google Scholar]
- 151.Lindner JR, Song J, Christiansen J, Klibanov AL, Xu F, Ley K. Ultrasound assessment of inflammation and renal tissue injury with microbubbles targeted to P-selectin. Circulation. 2001;104(17):2107–2112. doi: 10.1161/hc4201.097061. [DOI] [PubMed] [Google Scholar]
- 152.Lindner JR, Klibanov AL, Ley K. In: Biomedical Aspects of Drug Targeting. Muzykantov VR, Torchilin VP, editors. Kluwer Academic Pub; Boston: 2003. pp. 149–172. [Google Scholar]
- 153.Koning GA, Schiffelers RM, Storm G. Endothelial cells at inflammatory sites as target for therapeutic intervention. Endothelium. 2002;9(3):161–171. doi: 10.1080/10623320213631. [DOI] [PubMed] [Google Scholar]
- 154.Muro S, Gajewski C, Koval M, Muzykantov VR. ICAM-1 recycling in endothelial cells: a novel pathway for sustained intracellular delivery and prolonged effects of drugs. Blood. 2005;105(2):650–658. doi: 10.1182/blood-2004-05-1714. [DOI] [PubMed] [Google Scholar]
- 155.Ding BS, Dziubla T, Shuvaev VV, Muro S, Muzykantov VR. Advanced drug delivery systems that target the vascular endothelium. Mol Interv. 2006;6(2):98–112. doi: 10.1124/mi.6.2.7. [DOI] [PubMed] [Google Scholar]
- 156.Muro S, Wiewrodt R, Thomas A, Koniaris L, Albelda SM, Muzykantov VR, Koval M. A novel endocytic pathway induced by clustering endothelial ICAM-1 or PECAM-1. J Cell Sci. 2003;116(Pt 8):1599–1609. doi: 10.1242/jcs.00367. [DOI] [PubMed] [Google Scholar]
- 157.Omolola Eniola A, Hammer DA. In vitro characterization of leukocyte mimetic for targeting therapeutics to the endothelium using two receptors. Biomaterials. 2005;26(34):7136–7144. doi: 10.1016/j.biomaterials.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 158.Gunawan RC, Auguste DT. The role of antibody synergy and membrane fluidity in the vascular targeting of immunoliposomes. Biomaterials. 2010;31(5):900–907. doi: 10.1016/j.biomaterials.2009.09.107. [DOI] [PubMed] [Google Scholar]
- 159.Sun D, Nakao S, Xie F, Zandi S, Schering A, Hafezi-Moghadam A. Superior sensitivity of novel molecular imaging probe: simultaneously targeting two types of endothelial injury markers. FASEB J. 2010;24(5):1532–1540. doi: 10.1096/fj.09-148981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Eniola AO, Willcox PJ, Hammer DA. Interplay between rolling and firm adhesion elucidated with a cell-free system engineered with two distinct receptor-ligand pairs. Biophys J. 2003;85(4):2720–2731. doi: 10.1016/s0006-3495(03)74695-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Gunawan RC, Auguste DT. Immunoliposomes that target endothelium in vitro are dependent on lipid raft formation. Mol Pharm. 2010;7(5):1569–1575. doi: 10.1021/mp9003095. [DOI] [PubMed] [Google Scholar]
- 162.Gunawan RC, Almeda D, Auguste DT. Complementary targeting of liposomes to IL-1alpha and TNF-alpha activated endothelial cells via the transient expression of VCAM1 and E-selectin. Biomaterials. 2011;32(36):9848–9853. doi: 10.1016/j.biomaterials.2011.08.093. [DOI] [PubMed] [Google Scholar]
- 163.McAteer MA, Schneider JE, Ali ZA, Warrick N, Bursill CA, Von zur Muhlen C, Greaves DR, Neubauer S, Channon KM, Choudhury RP. Magnetic resonance imaging of endothelial adhesion molecules in mouse atherosclerosis using dual-targeted microparticles of iron oxide. Arterioscler Thromb Vasc Biol. 2008;28(1):77–83. doi: 10.1161/ATVBAHA.107.145466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Weller GE, Villanueva FS, Tom EM, Wagner WR. Targeted ultrasound contrast agents: in vitro assessment of endothelial dysfunction and multi-targeting to ICAM-1 and sialyl Lewisx. Biotechnol Bioeng. 2005;92(6):780–788. doi: 10.1002/bit.20625. [DOI] [PubMed] [Google Scholar]
- 165.Papademetriou IT, Garnacho C, Schuchman EH, Muro S. In vivo performance of polymer nanocarriers dually-targeted to epitopes of the same or different receptors. Biomaterials. 2013;34(13):3459–3466. doi: 10.1016/j.biomaterials.2013.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Danilov S, Jaspard E, Churakova T, Towbin H, Savoie F, Wei L, Alhenc-Gelas F. Structure-function analysis of angiotensin I-converting enzyme using monoclonal antibodies. Selective inhibition of the amino-terminal active site. J Biol Chem. 1994;269(43):26806–26814. [PubMed] [Google Scholar]
- 167.Gordon K, Balyasnikova IV, Nesterovitch AB, Schwartz DE, Sturrock ED, Danilov SM. Fine epitope mapping of monoclonal antibodies 9B9 and 3G8 to the N domain of angiotensin-converting enzyme (CD143) defines a region involved in regulating angiotensin-converting enzyme dimerization and shedding. Tissue Antigens. 2010;75(2):136–150. doi: 10.1111/j.1399-0039.2009.01416.x. [DOI] [PubMed] [Google Scholar]
- 168.Chacko AM, Nayak M, Greineder CF, Delisser HM, Muzykantov VR. Collaborative enhancement of antibody binding to distinct PECAM-1 epitopes modulates en-dothelial targeting. PLoS One. 2012;7(4):e34958. doi: 10.1371/journal.pone.0034958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Elias DR, Poloukhtine A, Popik V, Tsourkas A. Effect of ligand density, receptor density, and nanoparticle size on cell targeting. Nanomedicine. 2013;9(2):194–201. doi: 10.1016/j.nano.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Fakhari A, Baoum A, Siahaan TJ, Le KB, Berkland C. Controlling ligand surface density optimizes nanoparticle binding to ICAM-1. J Pharm Sci. 2011;100(3):1045–1056. doi: 10.1002/jps.22342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Gu F, Zhang L, Teply BA, Mann N, Wang A, Radovic-Moreno AF, Langer R, Farokhzad OC. Precise engineering of targeted nanoparticles by using self-assembled biointegrated block copolymers. Proc Natl Acad Sci U S A. 2008;105(7):2586–2591. doi: 10.1073/pnas.0711714105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zern BJ, Chacko A-M, Liu J, Greineder CF, Blankemeyer ER, Radhakrishnan R, Muzykantov V. Reduction of nanoparticle avidity enhances the selectivity of vascular targeting and PET detection of pulmonary inflammation. ACS Nano. 2013;7(3):2461–2469. doi: 10.1021/nn305773f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Perry JL, Kai MP, Reuter KG, Bowerman C, Christopher Luft J, DeSimone JM. Calibration-quality cancer nanotherapeutics. Cancer Treat Res. 2015;166:275–291. doi: 10.1007/978-3-319-16555-4_12. [DOI] [PubMed] [Google Scholar]
- 174.Pillai JD, Dunn SS, Napier ME, DeSimone JM. Novel platforms for vascular carriers with controlled geometry. IUBMB Life. 2011;63(8):596–606. doi: 10.1002/iub.497. [DOI] [PubMed] [Google Scholar]
- 175.Anselmo AC, Zhang M, Kumar S, Vogus DR, Menegatti S, Helgeson ME, Mitragotri S. Elasticity of nanoparticles influences their blood circulation, phagocytosis, endocytosis, and targeting. ACS Nano. 2015;9(3):3169–3177. doi: 10.1021/acsnano.5b00147. [DOI] [PubMed] [Google Scholar]
- 176.Oltra NS, Nair P, Discher DE. From stealthy polymersomes and filomicelles to “self” peptide-nanoparticles for cancer therapy. Annu Rev Chem Biomol Eng. 2014;5:281–299. doi: 10.1146/annurev-chembioeng-060713-040447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Mahmud A, Discher DE. Lung vascular targeting through inhalation delivery: insight from filamentous viruses and other shapes. IUBMB Life. 2011;63(8):607–612. doi: 10.1002/iub.481. [DOI] [PubMed] [Google Scholar]
- 178.Mitragotri S, Lahann J. Materials for drug delivery: innovative solutions to address complex biological hurdles. Adv Mater. 2012;24(28):3717–3723. doi: 10.1002/adma.201202080. [DOI] [PubMed] [Google Scholar]
- 179.Reuter KG, Perry J, Kim D, Luft JC, Liu R, DeSimone JM. Targeted PRINT hydrogels: the role of nanoparticle size and ligand density on cell association, biodistribution, and tumor accumulation. Nano Lett. 2015 doi: 10.1021/acs.nanolett.5b01362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Roberts RA, Eitas TK, Byrne JD, Johnson BM, Short PJ, McKinnon KP, Reisdorf S, Luft JC, DeSimone JM, Ting JP. Towards programming immune tolerance through geometric manipulation of phosphatidylserine. Biomaterials. 2015;72:1–10. doi: 10.1016/j.biomaterials.2015.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Ma D, Tian S, Baryza J, Luft JC, DeSimone JM. Reductively responsive hydrogel nanoparticles with uniform size, shape, and tunable composition for systemic siRNA delivery in vivo. Mol Pharm. 2015 doi: 10.1021/acs.molpharmaceut.5b00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Decuzzi P, Pasqualini R, Arap W, Ferrari M. Intravascular delivery of particulate systems: does geometry really matter? Pharm Res. 2009;26(1):235–243. doi: 10.1007/s11095-008-9697-x. [DOI] [PubMed] [Google Scholar]
- 183.Anselmo AC, Mitragotri S. Cell-mediated delivery of nanoparticles: taking advantage of circulatory cells to target nanoparticles. J Control Release. 2014;190:531–541. doi: 10.1016/j.jconrel.2014.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Muro S, Garnacho C, Champion JA, Leferovich J, Gajewski C, Schuchman EH, Mitragotri S, Muzykantov VR. Control of endothelial targeting and intracellular delivery of therapeutic enzymes by modulating the size and shape of ICAM-1-targeted carriers. Mol Ther. 2008;16(8):1450–1458. doi: 10.1038/mt.2008.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Shuvaev VV, Tliba S, Pick J, Arguiri E, Christofidou-Solomidou M, Albelda SM, Muzykantov VR. Modulation of endothelial targeting by size of antibody-antioxidant enzyme conjugates. J Control Release. 2011;149(3):236–241. doi: 10.1016/j.jconrel.2010.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kolhar P, Anselmo AC, Gupta V, Pant K, Prabhakarpandian B, Ruoslahti E, Mitragotri S. Using shape effects to target antibody-coated nanoparticles to lung and brain endothelium. Proc Natl Acad Sci U S A. 2013;110(26):10753–10758. doi: 10.1073/pnas.1308345110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Shuvaev VV, Ilies MA, Simone E, Zaitsev S, Kim Y, Cai S, Mahmud A, Dziubla T, Muro S, Discher DE, Muzykantov VR. Endothelial targeting of antibody-decorated polymeric filomicelles. ACS Nano. 2011;5(9):6991–6999. doi: 10.1021/nn2015453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Ricard I, Payet MD, Dupuis G. VCAM-1 is internalized by a clathrin-related pathway in human endothelial cells but its alpha 4 beta 1 integrin counter-receptor remains associated with the plasma membrane in human T lymphocytes. Eur J Immunol. 1998;28(5):1708–1718. doi: 10.1002/(SICI)1521-4141(199805)28:05<1708::AID-IMMU1708>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 189.Sakhalkar HS, Dalal MK, Salem AK, Ansari R, Fu J, Kiani MF, Kurjiaka DT, Hanes J, Shakesheff KM, Goetz DJ. Leukocyte-inspired biodegradable particles that selectively and avidly adhere to inflamed endothelium in vitro and in vivo. Proc Natl Acad Sci U S A. 2003;100(26):15895–15900. doi: 10.1073/pnas.2631433100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Tsourkas A, Shinde-Patil VR, Kelly KA, Patel P, Wolley A, Allport JR, Weissleder R. In vivo imaging of activated endothelium using an anti-VCAM-1 magnetooptical probe. Bioconjug Chem. 2005;16(3):576–581. doi: 10.1021/bc050002e. [DOI] [PubMed] [Google Scholar]
- 191.Kelly KA, Allport JR, Tsourkas A, Shinde-Patil VR, Josephson L, Weissleder R. Detection of vascular adhesion molecule-1 expression using a novel multimodal nanoparticle. Circ Res. 2005;96(3):327–336. doi: 10.1161/01.RES.0000155722.17881.dd. [DOI] [PubMed] [Google Scholar]
- 192.Pardridge WM. Blood–brain barrier drug targeting: the future of brain drug development. Mol Interv. 2003;3(2):90–105. 151. doi: 10.1124/mi.3.2.90. [DOI] [PubMed] [Google Scholar]
- 193.Christofidou-Solomidou M, Muzykantov VR. Antioxidant strategies in respiratory medicine. Treat Respir Med. 2006;5(1):47–78. doi: 10.2165/00151829-200605010-00004. [DOI] [PubMed] [Google Scholar]
- 194.Pasqualini R, Arap W, McDonald DM. Probing the structural and molecular diversity of tumor vasculature. Trends Mol Med. 2002;8(12):563–571. doi: 10.1016/s1471-4914(02)02429-2. [DOI] [PubMed] [Google Scholar]
- 195.Tagliabue E, Centis F, Campiglio M, Mastroianni A, Martignone S, Pellegrini R, Casalini P, Lanzi C, Menard S, Colnaghi MI. Selection of monoclonal antibodies which induce internalization and phosphorylation of p185HER2 and growth inhibition of cells with HER2/NEU gene amplification. Int J Cancer. 1991;47(6):933–937. doi: 10.1002/ijc.2910470625. [DOI] [PubMed] [Google Scholar]
- 196.Feero WG, Li S, Rosenblatt JD, Sirianni N, Morgan JE, Partridge TA, Huang L, Hoffman EP. Selection and use of ligands for receptor-mediated gene delivery to myogenic cells. Gene Ther. 1997;4(7):664–674. doi: 10.1038/sj.gt.3300453. [DOI] [PubMed] [Google Scholar]
- 197.Zhou Y, Zhao L, Marks JD. Selection and characterization of cell binding and internalizing phage antibodies. Arch Biochem Biophys. 2012;526(2):107–113. doi: 10.1016/j.abb.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Zhou Y, Zou H, Zhang S, Marks JD. Internalizing cancer antibodies from phage libraries selected on tumor cells and yeast-displayed tumor antigens. J Mol Biol. 2010;404(1):88–99. doi: 10.1016/j.jmb.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Heitner T, Moor A, Garrison JL, Marks C, Hasan T, Marks JD. Selection of cell binding and internalizing epidermal growth factor receptor antibodies from a phage display library. J Immunol Methods. 2001;248(1–2):17–30. doi: 10.1016/s0022-1759(00)00340-9. [DOI] [PubMed] [Google Scholar]
- 200.Muro S. Challenges in design and characterization of ligand-targeted drug delivery systems. J Control Release. 2012;164(2):125–137. doi: 10.1016/j.jconrel.2012.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Carver LA, Schnitzer JE. Caveolae: mining little caves for new cancer targets. Nat Rev Cancer. 2003;3(8):571–581. doi: 10.1038/nrc1146. [DOI] [PubMed] [Google Scholar]
- 202.Muro S, Cui X, Gajewski C, Murciano JC, Muzykantov VR, Koval M. Slow intra-cellular trafficking of catalase nanoparticles targeted to ICAM-1 protects endothe-lial cells from oxidative stress. Am J Physiol Cell Physiol. 2003;285(5):C1339–C1347. doi: 10.1152/ajpcell.00099.2003. [DOI] [PubMed] [Google Scholar]
- 203.Muro S, Schuchman EH, Muzykantov VR. Lysosomal enzyme delivery by ICAM-1-targeted nanocarriers bypassing glycosylation- and clathrin-dependent endocy-tosis. Mol Ther. 2006;13(1):135–141. doi: 10.1016/j.ymthe.2005.07.687. [DOI] [PubMed] [Google Scholar]
- 204.Bhowmick T, Berk E, Cui X, Muzykantov VR, Muro S. Effect of flow on endothelial endocytosis of nanocarriers targeted to ICAM-1. J Control Release. 2012;157(3):485–492. doi: 10.1016/j.jconrel.2011.09.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Samuel SP, Jain N, O'Dowd F, Paul T, Kashanin D, Gerard VA, Gun'ko YK, Prina-Mello A, Volkov Y. Multifactorial determinants that govern nanoparticle uptake by human endothelial cells under flow. Int J Nanomedicine. 2012;7:2943–2956. doi: 10.2147/IJN.S30624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Terrisse AD, Puech N, Allart S, Gourdy P, Xuereb JM, Payrastre B, Sie P. Internalization of microparticles by endothelial cells promotes platelet/endothelial cell interaction under flow. J Thromb Haemost. 2010;8(12):2810–2819. doi: 10.1111/j.1538-7836.2010.04088.x. [DOI] [PubMed] [Google Scholar]
- 207.Han J, Zern BJ, Shuvaev VV, Davies PF, Muro S, Muzykantov V. Acute and chronic shear stress differently regulate endothelial internalization of nanocarriers targeted to platelet-endothelial cell adhesion molecule-1. ACS Nano. 2012;6(10):8824–8836. doi: 10.1021/nn302687n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Serrano D, Bhowmick T, Chadha R, Garnacho C, Muro S. Intercellular adhesion molecule 1 engagement modulates sphingomyelinase and ceramide, supporting uptake of drug carriers by the vascular endothelium. Arterioscler Thromb Vasc Biol. 2012;32(5):1178–1185. doi: 10.1161/ATVBAHA.111.244186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Garnacho C, Dhami R, Simone E, Dziubla T, Leferovich J, Schuchman EH, Muzykantov V, Muro S. Delivery of acid sphingomyelinase in normal and Niemann–Pick disease mice using intercellular adhesion molecule-1-targeted polymer nanocarriers. J Pharmacol Exp Ther. 2008;325(2):400–408. doi: 10.1124/jpet.107.133298. [DOI] [PubMed] [Google Scholar]
- 210.Ansar M, Serrano D, Papademetriou I, Bhowmick TK, Muro S. Biological functionalization of drug delivery carriers to bypass size restrictions of receptor-mediated endocytosis independently from receptor targeting. ACS Nano. 2013;7(12):10597–10611. doi: 10.1021/nn404719c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Zhang Y, Schlachetzki F, Pardridge WM. Global non-viral gene transfer to the primate brain following intravenous administration. Mol Ther. 2003;7(1):11–18. doi: 10.1016/s1525-0016(02)00018-7. [DOI] [PubMed] [Google Scholar]
- 212.Schnitzer JE, Oh P, McIntosh DP. Role of GTP hydrolysis in fission of caveolae directly from plasma membranes. Science. 1996;274(5285):239–242. doi: 10.1126/science.274.5285.239. [DOI] [PubMed] [Google Scholar]
- 213.Dvorak AM, Feng D. The vesiculo-vacuolar organelle (VVO). A new endothelial cell permeability organelle. J Histochem Cytochem. 2001;49(4):419–432. doi: 10.1177/002215540104900401. [DOI] [PubMed] [Google Scholar]
- 214.Schnitzer JE, Liu J, Oh P. Endothelial caveolae have the molecular transport machinery for vesicle budding, docking, and fusion including VAMP, NSF, SNAP, annexins, and GTPases. J Biol Chem. 1995;270(24):14399–14404. doi: 10.1074/jbc.270.24.14399. [DOI] [PubMed] [Google Scholar]
- 215.Dejana E. Endothelial adherens junctions: implications in the control of vascular permeability and angiogenesis. J Clin Invest. 1996;98(9):1949–1953. doi: 10.1172/JCI118997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Mehta D, Bhattacharya J, Matthay MA, Malik AB. Integrated control of lung fluid balance. Am J Physiol Lung Cell Mol Physiol. 2004;287(6):L1081–L1090. doi: 10.1152/ajplung.00268.2004. [DOI] [PubMed] [Google Scholar]
- 217.Pardridge WM, Buciak J, Yang J, Wu D. Enhanced endocytosis in cultured human breast carcinoma cells and in vivo biodistribution in rats of a humanized monoclo-nal antibody after cationization of the protein. J Pharmacol Exp Ther. 1998;286(1):548–554. [PubMed] [Google Scholar]
- 218.Predescu D, Vogel SM, Malik AB. Functional and morphological studies of protein transcytosis in continuous endothelia. Am J Physiol Lung Cell Mol Physiol. 2004;287(5):L895–L901. doi: 10.1152/ajplung.00075.2004. [DOI] [PubMed] [Google Scholar]
- 219.Ghaffarian R, Bhowmick T, Muro S. Transport of nanocarriers across gastrointestinal epithelial cells by a new transcellular route induced by targeting ICAM-1. J Control Release. 2012;163(1):25–33. doi: 10.1016/j.jconrel.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Mane V, Muro S. Biodistribution and endocytosis of ICAM-1-targeting antibodies versus nanocarriers in the gastrointestinal tract in mice. Int J Nanomedicine. 2012;7:4223–4237. doi: 10.2147/IJN.S34105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Harrison DG, Gongora MC, Guzik TJ, Widder J. Oxidative stress and hypertension. J Am Soc Hypertens. 2007;1(1):30–44. doi: 10.1016/j.jash.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 222.Schulz E, Gori T, Munzel T. Oxidative stress and endothelial dysfunction in hypertension. Hypertens Res. 2011;34(6):665–673. doi: 10.1038/hr.2011.39. [DOI] [PubMed] [Google Scholar]
- 223.Li Q, Spencer NY, Oakley FD, Buettner GR, Engelhardt JF. Endosomal Nox2 facilitates redox-dependent induction of NF-kappaB by TNF-alpha. Antioxid Redox Signal. 2009;11(6):1249–1263. doi: 10.1089/ars.2008.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Lajoie P, Goetz JG, Dennis JW, Nabi IR. Lattices, rafts, and scaffolds: domain regulation of receptor signaling at the plasma membrane. J Cell Biol. 2009;185(3):381–385. doi: 10.1083/jcb.200811059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Ushio-Fukai M. Compartmentalization of redox signaling through NADPH oxidase-derived ROS. Antioxid Redox Signal. 2009;11(6):1289–1299. doi: 10.1089/ars.2008.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Oakley FD, Abbott D, Li Q, Engelhardt J. Signaling components of redox active endosomes: the redoxosomes. Antioxid Redox Signal. 2009;11(6):1313–1333. doi: 10.1089/ars.2008.2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Shuvaev VV, Han J, Yu KJ, Huang S, Hawkins BJ, Madesh M, Nakada M, Muzykantov VR. PECAM-targeted delivery of SOD inhibits endothelial inflammatory response. FASEB J. 2011;25(1):348–357. doi: 10.1096/fj.10-169789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Muzykantov VR. Delivery of antioxidant enzyme proteins to the lung. Antioxid Redox Signal. 2001;3(1):39–62. doi: 10.1089/152308601750100489. [DOI] [PubMed] [Google Scholar]
- 229.Machtay M, Scherpereel A, Santiago J, Lee J, McDonough J, Kinniry P, Arguiri E, Shuvaev VV, Sun J, Cengel K, Solomides CC, Christofidou-Solomidou M. Systemic polyethylene glycol-modified (PEGylated) superoxide dismutase and catalase mixture attenuates radiation pulmonary fibrosis in the C57/bl6 mouse. Radiother Oncol. 2006;81(2):196–205. doi: 10.1016/j.radonc.2006.09.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Yi X, Zimmerman MC, Yang R, Tong J, Vinogradov S, Kabanov AV. Pluronic-modified superoxide dismutase 1 attenuates angiotensin II-induced increase in intracellular superoxide in neurons. Free Radic Biol Med. 2010;49(4):548–558. doi: 10.1016/j.freeradbiomed.2010.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Lee S, Yang SC, Heffernan MJ, Taylor WR, Murthy N. Polyketal microparticles: a new delivery vehicle for superoxide dismutase. Bioconjug Chem. 2007;18(1):4–7. doi: 10.1021/bc060259s. [DOI] [PubMed] [Google Scholar]
- 232.Reddy MK, Labhasetwar V. Nanoparticle-mediated delivery of superoxide dis-mutase to the brain: an effective strategy to reduce ischemia–reperfusion injury. FASEB J. 2009;23(5):1384–1395. doi: 10.1096/fj.08-116947. [DOI] [PubMed] [Google Scholar]
- 233.Rosenbaugh EG, Roat JW, Gao L, Yang RF, Manickam DS, Yin JX, Schultz HD, Bronich TK, Batrakova EV, Kabanov AV, Zucker IH, Zimmerman MC. The attenuation of central angiotensin II-dependent pressor response and intra-neuronal signaling by intracarotid injection of nanoformulated copper/zinc superoxide dismutase. Biomaterials. 2010;31(19):5218–5226. doi: 10.1016/j.biomaterials.2010.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Wen J, Jiang X, Dai Y, Zhang Y, Tang Y, Sun H, Mi T, Kellems RE, Blackburn MR, Xia Y. Adenosine deaminase enzyme therapy prevents and reverses the heightened cavernosal relaxation in priapism. J Sex Med. 2009;7(9):3011–3022. doi: 10.1111/j.1743-6109.2009.01552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Epperly MW, Guo HL, Jefferson M, Nie S, Gretton J, Bernarding M, Bar-Sagi D, Archer H, Greenberger JS. Cell phenotype specific kinetics of expression of intratracheally injected manganese superoxide dismutase-plasmid/liposomes (MnSOD-PL) during lung radioprotective gene therapy. Gene Ther. 2003;10(2):163–171. doi: 10.1038/sj.gt.3301852. [DOI] [PubMed] [Google Scholar]
- 236.Gao B, Flores SC, Leff JA, Bose SK, McCord JM. Synthesis and anti-inflammatory activity of a chimeric recombinant superoxide dismutase: SOD2/3. Am J Physiol Lung Cell Mol Physiol. 2003;284(6):L917–L925. doi: 10.1152/ajplung.00374.2002. [DOI] [PubMed] [Google Scholar]
- 237.Matsui T, Yamagishi S, Nakamura K, Inoue H. Bay w 9798, a dihydropyridine structurally related to nifedipine with no calcium channel-blocking properties, inhibits tumour necrosis factor-alpha-induced vascular cell adhesion molecule-1 expression in endothelial cells by suppressing reactive oxygen species generation. J Int Med Res. 2007;35(6):886–891. doi: 10.1177/147323000703500617. [DOI] [PubMed] [Google Scholar]
- 238.Igarashi R, Hoshino J, Ochiai A, Morizawa Y, Mizushima Y. Lecithinized superox-ide dismutase enhances its pharmacologic potency by increasing its cell membrane affinity. J Pharmacol Exp Ther. 1994;271(3):1672–1677. [PubMed] [Google Scholar]
- 239.Koo DD, Welsh KI, West NE, Channon KM, Penington AJ, Roake JA, Morris PJ, Fuggle SV. Endothelial cell protection against ischemia/reperfusion injury by lecithinized superoxide dismutase. Kidney Int. 2001;60(2):786–796. doi: 10.1046/j.1523-1755.2001.060002786.x. [DOI] [PubMed] [Google Scholar]
- 240.Ishihara T, Tanaka K, Tasaka Y, Namba T, Suzuki J, Okamoto S, Hibi T, Takenaga M, Igarashi R, Sato K, Mizushima Y, Mizushima T. Therapeutic effect of lecithinized superoxide dismutase against colitis. J Pharmacol Exp Ther. 2009;328(1):152–164. doi: 10.1124/jpet.108.144451. [DOI] [PubMed] [Google Scholar]
- 241.Kagan VE, Wipf P, Stoyanovsky D, Greenberger JS, Borisenko G, Belikova NA, Yanamala N, Samhan Arias AK, Tungekar MA, Jiang J, Tyurina YY, Ji J, Klein-Seetharaman J, Pitt BR, Shvedova AA, Bayir H. Mitochondrial targeting of electron scavenging antioxidants: regulation of selective oxidation vs random chain reactions. Adv Drug Deliv Rev. 2009;61(14):1375–1385. doi: 10.1016/j.addr.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Epperly MW, Sikora CA, DeFilippi SJ, Gretton JE, Bar-Sagi D, Archer H, Carlos T, Guo H, Greenberger JS. Pulmonary irradiation-induced expression of VCAM-I and ICAM-I is decreased by manganese superoxide dismutase-plasmid/liposome (MnSOD-PL) gene therapy. Biol Blood Marrow Transplant. 2002;8(4):175–187. doi: 10.1053/bbmt.2002.v8.pm12014807. [DOI] [PubMed] [Google Scholar]
- 243.Supinski GS, Callahan LA. Polyethylene glycol-superoxide dismutase prevents endotoxin-induced cardiac dysfunction. Am J Respir Crit Care Med. 2006;173(11):1240–1247. doi: 10.1164/rccm.200410-1346OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Hernandez-Saavedra D, Zhou H, McCord JM. Anti-inflammatory properties of a chimeric recombinant superoxide dismutase: SOD2/3. Biomed Pharmacother. 2005;59(4):204–208. doi: 10.1016/j.biopha.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 245.Chang LY, Subramaniam M, Yoder BA, Day BJ, Ellison MC, Sunday ME, Crapo JD. A catalytic antioxidant attenuates alveolar structural remodeling in bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2003;167(1):57–64. doi: 10.1164/rccm.200203-232OC. [DOI] [PubMed] [Google Scholar]
- 246.Vujaskovic Z, Batinic-Haberle I, Rabbani ZN, Feng QF, Kang SK, Spasojevic I, Samulski TV, Fridovich I, Dewhirst MW, Anscher MS. A small molecular weight catalytic metalloporphyrin antioxidant with superoxide dismutase (SOD) mimetic properties protects lungs from radiation-induced injury. Free Radic Biol Med. 2002;33(6):857–863. doi: 10.1016/s0891-5849(02)00980-2. [DOI] [PubMed] [Google Scholar]
- 247.Bonder CS, Knight D, Hernandez-Saavedra D, McCord JM, Kubes P. Chimeric SOD2/3 inhibits at the endothelial-neutrophil interface to limit vascular dysfunction in ischemia–reperfusion. Am J Physiol Gastrointest Liver Physiol. 2004;287(3):G676–G684. doi: 10.1152/ajpgi.00049.2004. [DOI] [PubMed] [Google Scholar]
- 248.Danel C, Erzurum SC, Prayssac P, Eissa NT, Crystal RG, Herve P, Baudet B, Mazmanian M, Lemarchand P. Gene therapy for oxidant injury-related diseases: adenovirus-mediated transfer of superoxide dismutase and catalase cDNAs protects against hyperoxia but not against ischemia–reperfusion lung injury. Hum Gene Ther. 1998;9(10):1487–1496. doi: 10.1089/hum.1998.9.10-1487. [DOI] [PubMed] [Google Scholar]
- 249.Folz RJ, Abushamaa AM, Suliman HB. Extracellular superoxide dismutase in the airways of transgenic mice reduces inflammation and attenuates lung toxicity following hyperoxia. J Clin Invest. 1999;103(7):1055–1066. doi: 10.1172/JCI3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Jiang J, Kurnikov I, Belikova NA, Xiao J, Zhao Q, Amoscato AA, Braslau R, Studer A, Fink MP, Greenberger JS, Wipf P, Kagan VE. Structural requirements for optimized delivery, inhibition of oxidative stress, and antiapoptotic activity of targeted nitroxides. J Pharmacol Exp Ther. 2007;320(3):1050–1060. doi: 10.1124/jpet.106.114769. [DOI] [PubMed] [Google Scholar]
- 251.Muzykantov VR, Christofidou-Solomidou M, Balyasnikova I, Harshaw DW, Schultz L, Fisher AB, Albelda SM. Streptavidin facilitates internalization and pulmonary targeting of an anti-endothelial cell antibody (platelet-endothelial cell adhesion molecule 1): a strategy for vascular immunotargeting of drugs. Proc Natl Acad Sci U S A. 1999;96(5):2379–2384. doi: 10.1073/pnas.96.5.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Shuvaev VV, Tliba S, Nakada M, Albelda SM, Muzykantov VR. Platelet-endothelial cell adhesion molecule-1-directed endothelial targeting of superoxide dismutase alleviates oxidative stress caused by either extracellular or intracellular superoxide. J Pharmacol Exp Ther. 2007;323(2):450–457. doi: 10.1124/jpet.107.127126. [DOI] [PubMed] [Google Scholar]
- 253.Muzykantov VR, Sakharov DV, Domogatsky SP, Goncharov NV, Danilov SM. Directed targeting of immunoerythrocytes provides local protection of endothelial cells from damage by hydrogen peroxide. Am J Pathol. 1987;128(2):276–285. [PMC free article] [PubMed] [Google Scholar]
- 254.Sakharov DV, Muzykantov VR, Domogatsky SP, Danilov SM. Protection of cultured endothelial cells from hydrogen peroxide-induced injury by antibody-conjugated catalase. Biochim Biophys Acta. 1987;930(2):140–144. doi: 10.1016/0167-4889(87)90025-5. [DOI] [PubMed] [Google Scholar]
- 255.Sweitzer TD, Thomas AP, Wiewrodt R, Nakada MT, Branco F, Muzykantov VR. PECAM-directed immunotargeting of catalase: specific, rapid and transient protection against hydrogen peroxide. Free Radic Biol Med. 2003;34(8):1035–1046. doi: 10.1016/s0891-5849(03)00029-7. [DOI] [PubMed] [Google Scholar]
- 256.Atochina EN, Balyasnikova IV, Danilov SM, Granger DN, Fisher AB, Muzykantov VR. Immunotargeting of catalase to ACE or ICAM-1 protects perfused rat lungs against oxidative stress. Am J Phys. 1998;275(4 Pt 1):L806–L817. doi: 10.1152/ajplung.1998.275.4.L806. [DOI] [PubMed] [Google Scholar]
- 257.Scherpereel A, Wiewrodt R, Christofidou-Solomidou M, Gervais R, Murciano JC, Albelda SM, Muzykantov VR. Cell-selective intracellular delivery of a foreign enzyme to endothelium in vivo using vascular immunotargeting. FASEB J. 2001;15(2):416–426. doi: 10.1096/fj.00-0022com. [DOI] [PubMed] [Google Scholar]
- 258.Kozower BD, Christofidou-Solomidou M, Sweitzer TD, Muro S, Buerk DG, Solomides CC, Albelda SM, Patterson GA, Muzykantov VR. Immunotargeting of catalase to the pulmonary endothelium alleviates oxidative stress and reduces acute lung transplantation injury. Nat Biotechnol. 2003;21(4):392–398. doi: 10.1038/nbt806. [DOI] [PubMed] [Google Scholar]
- 259.Shuvaev VV, Christofidou-Solomidou M, Bhora F, Laude K, Cai H, Dikalov S, Arguiri E, Solomides CC, Albelda SM, Harrison DG, Muzykantov VR. Targeted de-toxification of selected reactive oxygen species in the vascular endothelium. J Pharmacol Exp Ther. 2009;331(2):404–411. doi: 10.1124/jpet.109.156877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Nowak K, Kolbel HC, Metzger RP, Hanusch C, Frohnmeyer M, Hohenberger P, Danilov SM. Immunotargeting of the pulmonary endothelium via angiotensin-converting-enzyme in isolated ventilated and perfused human lung. Adv Exp Med Biol. 2013;756:203–212. doi: 10.1007/978-94-007-4549-0_26. [DOI] [PubMed] [Google Scholar]
- 261.Morecroft I, White K, Caruso P, Nilsen M, Loughlin L, Alba R, Reynolds PN, Danilov SM, Baker AH, Maclean MR. Gene therapy by targeted adenovirus-mediated knockdown of pulmonary endothelial Tph1 attenuates hypoxia-induced pulmonary hypertension. Mol Ther. 2012;20(8):1516–1528. doi: 10.1038/mt.2012.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Nowak K, Hanusch C, Nicksch K, Metzger RP, Beck G, Gebhard MM, Hohenberger P, Danilov SM. Pre-ischaemic conditioning of the pulmonary endothelium by immunotargeting of catalase via angiotensin-converting-enzyme antibodies. Eur J Cardiothorac Surg. 2010;37(4):859–863. doi: 10.1016/j.ejcts.2009.10.029. [DOI] [PubMed] [Google Scholar]
- 263.Nowak K, Weih S, Metzger R, Albrecht RF, 2nd, Post S, Hohenberger P, Gebhard MM, Danilov SM. Immunotargeting of catalase to lung endothelium via anti-angiotensin-converting enzyme antibodies attenuates ischemia–reperfusion injury of the lung in vivo. Am J Physiol Lung Cell Mol Physiol. 2007;293(1):L162–L169. doi: 10.1152/ajplung.00001.2007. [DOI] [PubMed] [Google Scholar]
- 264.Shuvaev VV, Muzykantov VR. Targeted modulation of reactive oxygen species in the vascular endothelium. J Control Release. 2011;153(1):56–63. doi: 10.1016/j.jconrel.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Howard MD, Greineder CF, Hood ED, Muzykantov VR. Endothelial targeting of liposomes encapsulating SOD/catalase mimetic EUK-134 alleviates acute pulmonary inflammation. J Control Release. 2014;177C:34–41. doi: 10.1016/j.jconrel.2013.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Han J, Shuvaev VV, Muzykantov VR. Catalase and superoxide dismutase conjugated with platelet-endothelial cell adhesion molecule antibody distinctly alleviate abnormal endothelial permeability caused by exogenous reactive oxygen species and vascular endothelial growth factor. J Pharmacol Exp Ther. 2011;338(1):82–91. doi: 10.1124/jpet.111.180620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Shuvaev VV, Han J, Tliba S, Arguiri E, Christofidou-Solomidou M, Ramirez SH, Dykstra H, Persidsky Y, Atochin DN, Huang PL, Muzykantov VR. Anti-inflammatory effect of targeted delivery of SOD to endothelium: mechanism, synergism with NO donors and protective effects in vitro and in vivo. PLoS One. 2013;8(10):e77002. doi: 10.1371/journal.pone.0077002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Christofidou-Solomidou M, Scherpereel A, Wiewrodt R, Ng K, Sweitzer T, Arguiri E, Shuvaev V, Solomides CC, Albelda SM, Muzykantov VR. PECAM-directed delivery of catalase to endothelium protects against pulmonary vascular oxidative stress. Am J Physiol Lung Cell Mol Physiol. 2003;285(2):L283–L292. doi: 10.1152/ajplung.00021.2003. [DOI] [PubMed] [Google Scholar]
- 269.Han J, Shuvaev VV, Muzykantov VR. Targeted interception of signaling reactive oxygen species in the vascular endothelium. Ther Deliv. 2012;3(2):263–276. doi: 10.4155/tde.11.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Muro S, Mateescu M, Gajewski C, Robinson M, Muzykantov VR, Koval M. Control of intracellular trafficking of ICAM-1-targeted nanocarriers by endothelial Na+/H+ exchanger proteins. Am J Physiol Lung Cell Mol Physiol. 2006;290(5):L809–L817. doi: 10.1152/ajplung.00311.2005. [DOI] [PubMed] [Google Scholar]
- 271.Dziubla TD, Karim A, Muzykantov VR. Polymer nanocarriers protecting active enzyme cargo against proteolysis. J Control Release. 2005;102(2):427–439. doi: 10.1016/j.jconrel.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 272.Simone EA, Dziubla TD, Arguiri E, Vardon V, Shuvaev VV, Christofidou-Solomidou M, Muzykantov VR. Loading PEG-catalase into filamentous and spherical polymer nanocarriers. Pharm Res. 2009;26(1):250–260. doi: 10.1007/s11095-008-9744-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Simone EA, Dziubla TD, Discher DE, Muzykantov VR. Filamentous polymer nanocarriers of tunable stiffness that encapsulate the therapeutic enzyme catalase. Biomacromolecules. 2009 doi: 10.1021/bm900189x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Dziubla TD, Shuvaev VV, Hong NK, Hawkins BJ, Madesh M, Takano H, Simone E, Nakada MT, Fisher A, Albelda SM, Muzykantov VR. Endothelial targeting of semi-permeable polymer nanocarriers for enzyme therapies. Biomate-rials. 2008;29(2):215–227. doi: 10.1016/j.biomaterials.2007.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Hood ED, Greineder CF, Dodia C, Han J, Mesaros C, Shuvaev VV, Blair IA, Fisher AB, Muzykantov VR. Antioxidant protection by PECAM-targeted delivery of a novel NADPH-oxidase inhibitor to the endothelium in vitro and in vivo. J Control Release. 2012 doi: 10.1016/j.jconrel.2012.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Ding BS, Hong N, Murciano JC, Ganguly K, Gottstein C, Christofidou-Solomidou M, Albelda SM, Fisher AB, Cines DB, Muzykantov VR. Prophylactic thrombolysis by thrombin-activated latent prourokinase targeted to PECAM-1 in the pulmonary vasculature. Blood. 2008;111(4):1999–2006. doi: 10.1182/blood-2007-07-103002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Greineder CF, Chacko AM, Zaytsev S, Zern BJ, Carnemolla R, Hood ED, Han J, Ding BS, Esmon CT, Muzykantov VR. Vascular immunotargeting to endothelial determinant ICAM-1 enables optimal partnering of recombinant scFv-thrombomodulin fusion with endogenous cofactor. PLoS One. 2013;8(11):e80110. doi: 10.1371/journal.pone.0080110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Greineder CF, Brenza JB, Carnemolla R, Zaitsev S, Hood ED, Pan DC, Ding BS, Esmon CT, Chacko AM, Muzykantov VR. Dual targeting of therapeutics to endothelial cells: collaborative enhancement of delivery and effect. FASEB J. 2015 doi: 10.1096/fj.15-271213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Ferrer MC, Shuvaev VV, Zern BJ, Composto RJ, Muzykantov VR, Eckmann DM. Icam-1 targeted nanogels loaded with dexamethasone alleviate pulmonary inflammation. PLoS One. 2014;9(7):e102329. doi: 10.1371/journal.pone.0102329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Hegeman MA, Cobelens PM, Kamps J, Hennus MP, Jansen NJG, Schultz MJ, van Vught AJ, Molema G, Heijnen CJ. Liposome-encapsulated dexamethasone attenuates ventilator-induced lung inflammation. Br J Pharmacol. 2011;163(5):1048–1058. doi: 10.1111/j.1476-5381.2011.01314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Homem de Bittencourt PI, Jr, Lagranha DJ, Maslinkiewicz A, Senna SM, Tavares AM, Baldissera LP, Janner DR, Peralta JS, Bock PM, Gutierrez LL, Scola G, Heck TG, Krause MS, Cruz LA, Abdalla DS, Lagranha CJ, Lima T, Curi R. LipoCardium: endothelium-directed cyclopentenone prostaglandin-based liposome formulation that completely reverses atherosclerotic lesions. Atherosclerosis. 2007;193(2):245–258. doi: 10.1016/j.atherosclerosis.2006.08.049. [DOI] [PubMed] [Google Scholar]
- 282.Asgeirsdottir SA, Zwiers PJ, Morselt HW, Moorlag HE, Bakker HI, Heeringa P, Kok JW, Kallenberg CG, Molema G, Kamps JA. Inhibition of proinflammatory genes in anti-GBM glomerulonephritis by targeted dexamethasone-loaded AbEsel liposomes. Am J Physiol Ren Physiol. 2008;294(3):F554–F561. doi: 10.1152/ajprenal.00391.2007. [DOI] [PubMed] [Google Scholar]
- 283.Hashida N, Ohguro N, Yamazaki N, Arakawa Y, Oiki E, Mashimo H, Kurokawa N, Tano Y. High-efficacy site-directed drug delivery system using sialyl-lewis X conjugated liposome. Exp Eye Res. 2008;86(1):138–149. doi: 10.1016/j.exer.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 284.Everts M, Koning G, Kok R, Ásgeirsdóttir S, Vestweber D, Meijer DF, Storm G, Molema G. In vitro cellular handling and in vivo targeting of E-selectin-directed immunoconjugates and immunoliposomes used for drug delivery to inflamed endothelium. Pharm Res. 2003;20(1):64–72. doi: 10.1023/a:1022298725165. [DOI] [PubMed] [Google Scholar]
- 285.Asgeirsdottir SA, Kamps JA, Bakker HI, Zwiers PJ, Heeringa P, van der Weide K, van Goor H, Petersen AH, Morselt H, Moorlag HE, Steenbergen E, Kallenberg CG, Molema G. Site-specific inhibition of glomerulonephritis progression by targeted delivery of dexamethasone to glomerular endothelium. Mol Pharmacol. 2007;72(1):121–131. doi: 10.1124/mol.107.034140. [DOI] [PubMed] [Google Scholar]
- 286.Kuldo JM, Asgeirsdottir SA, Zwiers PJ, Bellu AR, Rots MG, Schalk JA, Ogawara KI, Trautwein C, Banas B, Haisma HJ, Molema G, Kamps JA. Targeted adenovirus mediated inhibition of NF-kappaB-dependent inflammatory gene expression in endothelial cells in vitro and in vivo. J Control Release. 2013;166(1):57–65. doi: 10.1016/j.jconrel.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 287.Asgeirsdottir SA, Talman EG, de Graaf IA, Kamps JA, Satchell SC, Mathieson PW, Ruiters MH, Molema G. Targeted transfection increases siRNA uptake and gene silencing of primary endothelial cells in vitro—a quantitative study. J Control Release. 2010;141(2):241–251. doi: 10.1016/j.jconrel.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 288.Zuckerman JE, Choi CHJ, Han H, Davis ME. Polycation-siRNA nanoparticles can disassemble at the kidney glomerular basement membrane. Proc Natl Acad Sci. 2012;109(8):3137–3142. doi: 10.1073/pnas.1200718109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Lee JB, Zhang K, Tam YYC, Tam YK, Belliveau NM, Sung VYC, Lin PJC, LeBlanc E, Ciufolini MA, Rennie PS, Cullis PR. Lipid nanoparticle siRNA systems for silencing the androgen receptor in human prostate cancer in vivo. Int J Cancer. 2012;131(5):E781–E790. doi: 10.1002/ijc.27361. [DOI] [PubMed] [Google Scholar]
- 290.Koning GA, Schiffelers RM, Wauben MHM, Kok RJ, Mastrobattista E, Molema G, ten Hagen TLM, Storm G. Targeting of angiogenic endothelial cells at sites of inflammation by dexamethasone phosphate–containing RGD peptide liposomes inhibits experimental arthritis. Arthritis Rheum. 2006;54(4):1198–1208. doi: 10.1002/art.21719. [DOI] [PubMed] [Google Scholar]
- 291.Vader P, Crielaard BJ, van Dommelen SM, van der Meel R, Storm G, Schiffelers RM. Targeted delivery of small interfering RNA to angiogenic endothelial cells with liposome-polycation-DNA particles. J Control Release. 2012;160(2):211–216. doi: 10.1016/j.jconrel.2011.09.080. [DOI] [PubMed] [Google Scholar]
- 292.Muro S. New biotechnological and nanomedicine strategies for treatment of lysosomal storage disorders. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2010;2(2):189–204. doi: 10.1002/wnan.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Beutler E. Lysosomal storage diseases: natural history and ethical and economic aspects. Mol Genet Metab. 2006;88(3):208–215. doi: 10.1016/j.ymgme.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 294.Futerman AH, van Meer G. The cell biology of lysosomal storage disorders. Nat Rev Mol Cell Biol. 2004;5(7):554–565. doi: 10.1038/nrm1423. [DOI] [PubMed] [Google Scholar]
- 295.Meikle PJ, Hopwood JJ, Clague AE, Carey WF. Prevalence of lysosomal storage disorders. JAMA. 1999;281(3):249–254. doi: 10.1001/jama.281.3.249. [DOI] [PubMed] [Google Scholar]
- 296.Grabowski GA. Delivery of lysosomal enzymes for therapeutic use: glucocerebrosidase as an example. Expert Opin Drug Deliv. 2006;3(6):771–782. doi: 10.1517/17425247.3.6.771. [DOI] [PubMed] [Google Scholar]
- 297.Brady RO, Schiffmann R. Enzyme-replacement therapy for metabolic storage disorders. Lancet Neurol. 2004;3(12):752–756. doi: 10.1016/S1474-4422(04)00938-X. [DOI] [PubMed] [Google Scholar]
- 298.Desnick RJ, Schuchman EH. Enzyme replacement and enhancement therapies: lessons from lysosomal disorders. Nat Rev Genet. 2002;3(12):954–966. doi: 10.1038/nrg963. [DOI] [PubMed] [Google Scholar]
- 299.Rosenfeld MG, Kreibich G, Popov D, Kato K, Sabatini DD. Biosynthesis of lysosomal hydrolases: their synthesis in bound polysomes and the role of co- and post-translational processing in determining their subcellular distribution. J Cell Biol. 1982;93(1):135–143. doi: 10.1083/jcb.93.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Du H, Levine M, Ganesa C, Witte DP, Cole ES, Grabowski GA. The role of mannosylated enzyme and the mannose receptor in enzyme replacement therapy. Am J Hum Genet. 2005;77(6):1061–1074. doi: 10.1086/498652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Neufeld EF. The uptake of enzymes into lysosomes: an overview. Birth Defects Orig Artic Ser. 1980;16(1):77–84. [PubMed] [Google Scholar]
- 302.Miranda SR, He X, Simonaro CM, Gatt S, Dagan A, Desnick RJ, Schuchman EH. Infusion of recombinant human acid sphingomyelinase into Niemann–Pick disease mice leads to visceral, but not neurological, correction of the pathophysiology. FASEB J. 2000;14(13):1988–1995. doi: 10.1096/fj.00-0014com. [DOI] [PubMed] [Google Scholar]
- 303.Schuchman EH, Desnick RJ. In: Lysosomal Disorders The Metabolic and Molecular Bases of Inherited Disease. 8. Scriver C, Beaudet A, Sly W, Valle D, Childs B, Kinzler K, Vogelstein B, editors. McGraw-Hill; 2000. XVI Chapter. [Google Scholar]
- 304.Bae JS, Jang KH, Schuchman TR, Jin HK. Comparative effects of recombinant acid sphingomyelinase administration by different routes in Niemann–Pick disease mice. Exp Anim. 2004;53(5):417–421. doi: 10.1538/expanim.53.417. [DOI] [PubMed] [Google Scholar]
- 305.Marlin SD, Springer TA. Purified intercellular adhesion molecule-1 (ICAM-1) is a ligand for lymphocyte function-associated antigen 1 (LFA-1) Cell. 1987;51(5):813–819. doi: 10.1016/0092-8674(87)90104-8. [DOI] [PubMed] [Google Scholar]
- 306.Bevilacqua MP. Endothelial-leukocyte adhesion molecules. Annu Rev Immunol. 1993;11:767–804. doi: 10.1146/annurev.iy.11.040193.004003. [DOI] [PubMed] [Google Scholar]
- 307.Muro S. In: Endothelial Biomedicine. Aird WC, editor. Cambridge University Press; 2007. pp. 1058–1070. [Google Scholar]
- 308.DeGraba T, Azhar S, Dignat-George F, Brown E, Boutiere B, Altarescu G, McCarron R, Schiffmann R. Profile of endothelial and leukocyte activation in Fabry patients. Ann Neurol. 2000;47(2):229–233. [PubMed] [Google Scholar]
- 309.Hsu J, Serrano D, Bhowmick T, Kumar K, Shen Y, Kuo YC, Garnacho C, Muro S. Enhanced endothelial delivery and biochemical effects of α-galactosidase by ICAM-1-targeted nanocarriers for Fabry disease. J Control Release. 2011;149(3):323–331. doi: 10.1016/j.jconrel.2010.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Hsu J, Northrup L, Bhowmick T, Muro S. Enhanced delivery of α-glucosidase for Pompe disease by ICAM-1-targeted nanocarriers: comparative performance of a strategy for three distinct lysosomal storage disorders. Nanomed Nanotechnol Biol Med. 2012;8(5):731–739. doi: 10.1016/j.nano.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Papademetriou J, Garnacho C, Serrano D, Bhowmick T, Schuchman EH, Muro S. Comparative binding, endocytosis, and biodistribution of antibodies and antibody-coated carriers for targeted delivery of lysosomal enzymes to ICAM-1 versus transferrin receptor. J Inherit Metab Dis. 2012 doi: 10.1007/s10545-012-9534-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Esmon CT. Inflammation and thrombosis. J Thromb Haemost. 2003;1(7):1343–1348. doi: 10.1046/j.1538-7836.2003.00261.x. [DOI] [PubMed] [Google Scholar]
- 313.Dichek DA, Anderson J, Kelly AB, Hanson SR, Harker LA. Enhanced in vivo antithrombotic effects of endothelial cells expressing recombinant plasminogen activators transduced with retroviral vectors. Circulation. 1996;93(2):301–309. doi: 10.1161/01.cir.93.2.301. [DOI] [PubMed] [Google Scholar]
- 314.Muzykantov VR, Barnathan ES, Atochina EN, Kuo A, Danilov SM, Fisher AB. Targeting of antibody-conjugated plasminogen activators to the pulmonary vasculature. J Pharmacol Exp Ther. 1996;279(2):1026–1034. [PubMed] [Google Scholar]
- 315.Murciano JC, Muro S, Koniaris L, Christofidou-Solomidou M, Harshaw DW, Albelda SM, Granger DN, Cines DB, Muzykantov VR. ICAM-directed vascular immunotargeting of antithrombotic agents to the endothelial luminal surface. Blood. 2003;101(10):3977–3984. doi: 10.1182/blood-2002-09-2853. [DOI] [PubMed] [Google Scholar]
- 316.Ding BS, Gottstein C, Grunow A, Kuo A, Ganguly K, Albelda SM, Cines DB, Muzykantov VR. Endothelial targeting of a recombinant construct fusing a PECAM-1 single-chain variable antibody fragment (scFv) with prourokinase facilitates prophylactic thrombolysis in the pulmonary vasculature. Blood. 2005;106(13):4191–4198. doi: 10.1182/blood-2005-05-2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Husain SS. Single-chain urokinase-type plasminogen activator does not possess measurable intrinsic amidolytic or plasminogen activator activities. Biochemistry. 1991;30(23):5797–5805. doi: 10.1021/bi00237a024. [DOI] [PubMed] [Google Scholar]
- 318.Ichinose A, Fujikawa K, Suyama T. The activation of pro-urokinase by plasma kal-likrein and its inactivation by thrombin. J Biol Chem. 1986;261(8):3486–3489. [PubMed] [Google Scholar]
- 319.Yang WP, Goldstein J, Procyk R, Matsueda GR, Shaw SY. Design and evaluation of a thrombin-activable plasminogen activator. Biochemistry. 1994;33(8):606–612. doi: 10.1021/bi00174a043. [DOI] [PubMed] [Google Scholar]
- 320.Esmon CT. Inflammation and the activated protein C anticoagulant pathway. Semin Thromb Hemost. 2006;32(Suppl 1):49–60. doi: 10.1055/s-2006-939554. [DOI] [PubMed] [Google Scholar]
- 321.Wang YX, Wu C, Vincelette J, Martin-McNulty B, Alexander S, Larsen B, Light DR, McLean K. Amplified anticoagulant activity of tissue factor-targeted thrombomodulin: in-vivo validation of a tissue factor-neutralizing antibody fused to soluble thrombomodulin. Thromb Haemost. 2006;96(3):317–324. doi: 10.1160/TH06-04-0219. [DOI] [PubMed] [Google Scholar]
- 322.Ding BS, Hong N, Christofidou-Solomidou M, Gottstein C, Albelda SM, Cines DB, Fisher AB, Muzykantov VR. Anchoring fusion thrombomodulin to the endothelial lumen protects against injury-induced lung thrombosis and inflammation. Am J Respir Crit Care Med. 2009;180(3):247–256. doi: 10.1164/rccm.200809-1433OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Bulte JW, Schmieder AH, Keupp J, Caruthers SD, Wickline SA, Lanza GM. MR cholangiography demonstrates unsuspected rapid biliary clearance of nanoparticles in rodents: implications for clinical translation. Nanomedicine. 2014 doi: 10.1016/j.nano.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]