Abstract
Humans express several orthologs of yeast Atg8, in the LC3 and GABARAP families, which play crucial roles in autophagy through their covalent ligation to lipids, typically phosphatidylethanolamine (PE), in a process known as lipidation. Lipidation of LC3 and GABARAP regulates numerous facets of the autophagy process, including regulating expansion of the phagophore membrane, recruiting selected cargoes for degradation, and providing an autophagosome membrane-bound platform mediating dynamic interactions with other regulatory proteins. LC3 and GABARAP are families of related ubiquitin-like proteins (UBLs) (referred to here collectively as LC3/GABARAP), and their lipidation involves a divergent UBL conjugation cascade including ATG7, ATG3, and ATG12~ATG5-ATG16L1 acting as E1, E2, and E3 enzymes, respectively. ATG7 initiates LC3/GABARAP conjugation by catalyzing their C-terminal adenylation and conjugation to the catalytic cysteine of ATG3. Ultimately, the ATG12~ATG5-ATG16L1 complex catalyzes LC3/GABARAP ligation to a primary amino group on PE or other acceptor lipids. This chapter describes methods for expressing and purifying human LC3 or GABARAP, ATG7, ATG3, and the ATG12~ATG5-ATG16L1 complex for in vitro studies of LC3/GABARAP lipidation.
1. INTRODUCTION
In eukaryotic cells, autophagy is a tightly regulated process responsible for degrading and recycling cellular components, and eliminating toxic assemblies and pathogens by sequestering cargo within an autophagosome, which fuses with a lysosome (or vacuole in yeast) harboring hydrolytic enzymes (Klionsky & Emr, 2000; Kobayashi, 2015; Wileman, 2013). Several key aspects of this regulation involve ubiquitin-like proteins (UBLs) in the Atg8 family, which form LC3 and GABARAP subfamilies in higher eukaryotes. LC3/GABARAP proteins have molecular weights in solution of approximately 14–17 kDa, but their electrophoretic migration is relatively accelerated upon ligation to lipids such as in autophagic membranes. LC3/GABARAP ligation to lipids (lipidation) plays numerous roles in regulation, including influencing the formation, maturation, and size of autophagosomes, as well as recruiting selected cargoes and regulatory proteins (Ichimura et al., 2000; Ohsumi & Mizushima, 2004; Nakatogawa, Ichimura, & Ohsumi, 2007; Tanida, Ueno, & Kominami, 2008).
LC3 and GABARAP lipidation is orchestrated by a UBL ligation system, specifically conjugating the carboxyl group of the C-terminal glycine of LC3/GABARAP to the amine group of phosphatidylethanolamine (PE) (or potentially other lipids such as phosphatidyl serine) in autophagic membranes (Hanada et al., 2007; Ichimura et al., 2000; Klionsky & Emr, 2000; Sou, Tanida, Komatsu, Ueno, & Kominami, 2006; Tanida, Ueno, & Kominami, 2004a, 2004b). This involves a specialized enzyme cascade, where ATG7 acts as the E1 and ATG3 acting as the E2 to produce covalent, reactive thioester-linked ATG3~LC3/GABARAP (“~” denotes a covalent protein interaction and “-” denotes a noncovalent complex) intermediates. Efficient lipidation requires an ATG12~ATG5-ATG16L1 multiprotein complex acting as the E3. Notably, formation of this E3-like assembly first requires a related conjugation cascade, wherein the UBL ATG12 is covalently conjugated to ATG5 by ATG7 acting as the E1 and ATG10 acting as the E2 (Hanada et al., 2007; Noda, Fujioka, Hanada, Ohsumi, & Inagaki, 2013), and then the ATG12~ATG5 binds ATG16L1 to achieve full E3-like activity toward ATG3~LC3/GABARAP intermediates. Mutations or dysfunction of proteins in the LC3/GABARAP lipidation cascade results in severe diseases and poor prognosis in various eukaryotic organisms including humans (Cadwell, Patel, Komatsu, & Virgin, 2009; Dezelak, Repnik, Koder, Ferkolj, & Potocnik, 2016; Kim et al., 2016; Lenz, Vierstra, Nurnberger, & Gust, 2011; Moloughney et al., 2011; Reed, Morris, Owczarczyk, & Lukacs, 2015; Tindwa et al., 2015; Wu et al., 2014; Xi et al., 2016; Xue, Chiu, & Oleinick, 2010).
Given the importance of LC3 and GABARAP lipidation, and potential interest in targeting their conjugation for therapeutic purposes (Cheng, Ren, Hait, & Yang, 2013; Puri & Chandra, 2014), purified proteins for assaying LC3/GABARAP lipidation and performing structural analyses would be useful (Bae & Park, 2010; Kaiser et al., 2013; Metlagel, Otomo, Ohashi, Takaesu, & Otomo, 2014; Metlagel, Otomo, Takaesu, & Otomo, 2013; Nath et al., 2014; Otomo, Metlagel, Takaesu, & Otomo, 2013; Sakoh-Nakatogawa et al., 2013; Taherbhoy et al., 2011). However, our optimal expression and purification procedures differ between the human LC3/GABARAP lipidation components and their yeast counterparts (Qiu, Hofmann, Coats, Schulman, & Kaiser, 2013). Thus, in this chapter, we describe detailed methods for expression and purification of human ATG proteins involved in LC3/GABARAP lipidation, including ATG7, ATG3, ATG12~ATG5-ATG16L1, and a method for expressing LC3B as exemplary for the human Atg8 orthologs.
2. EXPRESSION OF HUMAN LC3B, ATG7, ATG3, ATG12~ATG5, AND ATG16L1
2.1 General Overview of Protein Expression and Purification
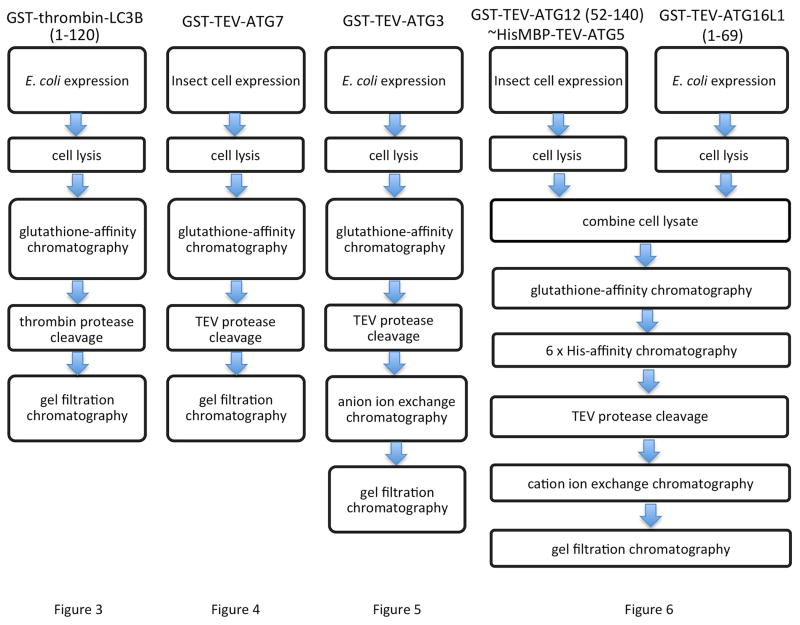
LC3B (emblematic of LC3/GABARAP family members), ATG3, and ATG16L1 were expressed as GST-fusion proteins in Escherichia coli, whereas ATG7 and the ATG12~ATG5 conjugate were expressed through distinct methods in insect cells (Berger, Fitzgerald, & Richmond, 2004). An overview of the subsequent purification strategies is shown in Fig. 1.
Fig. 1.
Flow-chart indicating the expression system and purification strategies for production of human ATG proteins for lipidation assays.
2.2 Expression Constructs
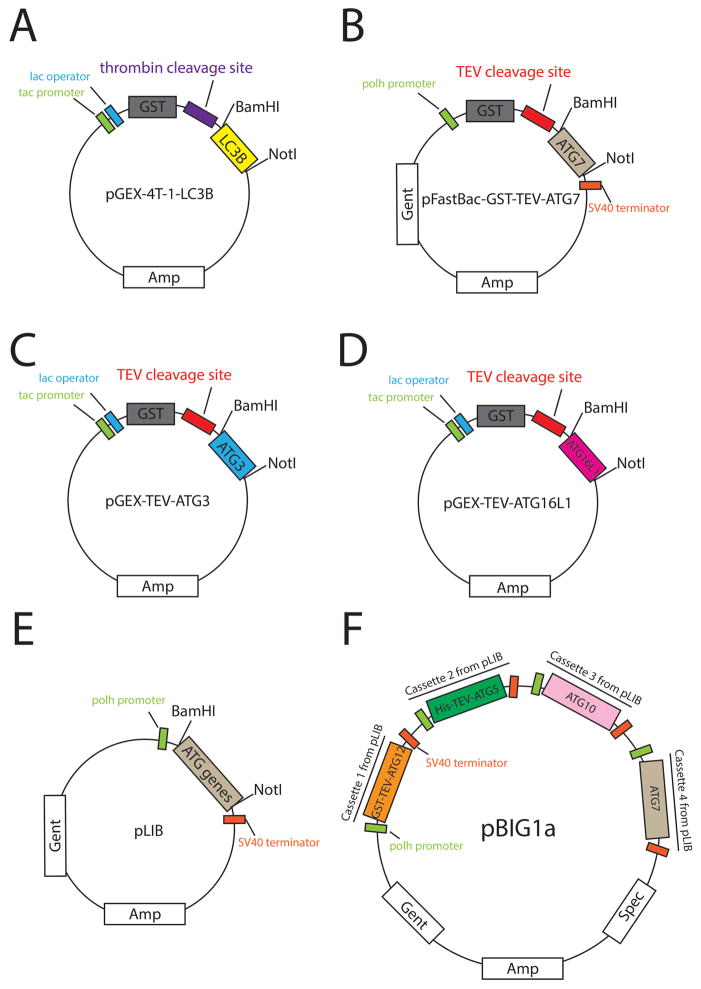
A truncated version of LC3B, corresponding to residues 1–120 that form the activated form with a C-terminal glycine that is the site of chemical reactions (Tanida et al., 2004a, 2004b), is expressed from pGEX-4T-1 (Table 1, Fig. 2A) in E. coli.
Full-length ATG7 was expressed from a modified version of pFastBac, with TEV-cleavable N-terminal GST tag (pFastBac-GST) (Table 1, Fig. 2B) for expression in insect cells.
Full-length ATG3 was expressed from a modified version of pGEX-4T-1 harboring a cleavage site for TEV protease instead of thrombin between GST and ATG3 (pGEX-TEV) (Table 1, Fig. 2C), for expression in E. coli.
A truncated version of ATG16L1 corresponding to residues 1–69 was expressed from pGEX-TEV (Table 1, Fig. 2D) in E. coli.
The ATG12~ATG5 subcomplex corresponds to a truncated version of ATG12 (residues 52–140) that comprises the ubiquitin-like domain (Hanada & Ohsumi, 2005) linked via an isopeptide bond between its C-terminus to Lys130 on full-length ATG5 (Hanada et al., 2007; Otomo et al., 2013). The linkage between ATG12 and ATG5 is achieved by their coexpression with ATG7 and ATG10 in insect cells using the biGBac system (Weissmann et al., 2016). First, four separate plasmids were generated. The coding sequences were inserted into pLIB vectors, with ATG7, and ATG10 untagged, ATG5 harboring a N-terminal TEV cleavable His-tag, and ATG12 (52–140) with a N-terminal TEV cleavable GST-tag (Table 1, Fig. 2E). Next, GST-TEV-ATG12 (52–140), His-TEV-ATG5, ATG10, and ATG7 along with the promoter and terminator from the pLIB vectors were sequentially amplified using primers described in Weissmann et al. (2016), and coassembled into the pBIG1a vector (Table 1, Fig. 2F) using the method of Gibson Assembly (Gibson, 2011; Gibson et al., 2009; Weissmann et al., 2016).
All constructs were generated through transformation into DH5alpha (NEB), bacterial growth, minipreps, and verified by automated sequencing. The recommended antibiotic concentrations are: 100 μg/mL for ampicillin, 50 μg/mL for kanamycin and spectinomycin, 25 μg/mL for chloramphenicol, 10 μg/mL for tetracyclin, and 7 μg/mL for gentamycin.
Table 1.
Plasmids Used in This Chapter
| Proteins Expressed | Plasmids Generated | Functions |
|---|---|---|
| LC3B (1–120) | pGEX-4T-1-LC3B | Protein expression in E. coli |
| ATG7 | pFastBac-GST-ATG7 | To generate bacmid for protein expression in insect cells |
| ATG3 | pGEX-TEV-ATG3 | Protein expression in E. coli |
| ATG12 (52–140)~ATG5 | pLIB-GST-TEV-ATG12 | To generate pBIG1a plasmid |
| pLIB-His-TEV-ATG5 | To generate pBIG1a plasmid | |
| pLIB-ATG10 | To generate pBIG1a plasmid | |
| pLIB-ATG7 | To generate pBIG1a plasmid | |
| pBIG1a-ATG12-ATG5-ATG10-ATG7 | To generate bacmid for protein expression in insect cells | |
| ATG16L1 (1–69) | pGEX-TEV-ATG16L1 | Protein expression in E. coli |
Fig. 2.
Graphical representation of plasmids used in this chapter. (A) pGEX-4T-1-LC3B for LC3B (1–120) expression in E. coli. (B) pFastBac-GST-ATG7 to construct bacmid for ATG7 expression in insect cells. (C) pGEX-TEV-ATG3 for ATG3 expression in E. coli. (D) pGEXTEV-ATG16L1 for ATG16L1 (1–69) expression in E. coli. (E) pLIB plasmid containing a GSTTEV-ATG12 (52–140), His-TEV-ATG5, ATG10, or ATG7 expression cassette to generate pBIG1a plasmid. (F) pBIG1a-ATG12-ATG5-ATG10-ATG7 plasmid to construct bacmid for ATG12 (52–140)~ATG5 expression in insect cells.
2.3 Protein Expression
2.3.1 Expression in E. coli
Transform plasmid into BL21-CodonPlus (DE3)-RIL E. coli competent cells (Agilent Technologies), plate on LB-agar with appropriate antibiotics. Incubate at 37 °C for 12–18 h.
Prepare a starter culture from a single colony in 5 mL LB medium with antibiotics. Shake culture at 37 °C, 200 rpm for 10–16 h.
Dilute the 5 mL starter culture into 1 L LB medium with appropriate antibiotics. Grow cells at 37 °C, 200 rpm, and monitor closely by measuring the absorbance at 600 nm. When OD600 reaches 0.8–1.0, add IPTG at a final concentration of 0.6 mM, change shaker temperature to 18°C, and grow with shaking at 200 rpm for 16–20 h.
2.3.2 Expression in Insect Cells
Transform pFastBac or pBIG1a vector containing expression cassettes into DH10EMBacY E. coli competent cells (Geneva Biotech). This enables transposase-mediated recombination resulting in a bacmid containing expression cassettes (Bac-to-Bac Baculovirus Expression System, Thermo Fisher Scientific).
Screen for colonies harboring bacmids that have undergone recombination using the blue/white identification method described in the Bac-to-Bac Baculovirus Expression System manual. Briefly, a LB plate containing ampicillin, kanamycin, tetracycline, and gentamycin, is spread with 40 μL 40 mg/mL X-Gal (dissolved in dimethylformamide) and 40 μL 0.1 M IPTG is incubated at 37 °C for 4 h to let the dimethylformamide evaporate before plating the transformation product.
Incubate at 37 °C for at least 24 h until colonies grow and background colonies with a clear blue color appear. White colonies are candidates for harboring a correctly recombined bacmid. PCR screen for candidates that contains all the target genes. At the same time, restreak white colonies on a fresh plate with X-gal, IPTG, and the four antibiotics. Include a blue colony as a control for color development. Perform bacmid extraction from culture of a single white colony with positive PCR screening result.
All insect cell medium used in this chapter must be prewarmed at 27 °C. Transfect to obtain baculovirus in five steps. (a) Coat 6 well flat bottom culture plate (CoStar) with 5 X106 Sf9 insect cells (Thermo Scientific) per well and incubate at 27 °C for 30 min for cell attachment. (b) Dilute 200 ng bacmid with 100 μL SFX serum-free insect cell medium (GE Healthcare Life Sciences) in a 15 mL polystyrene tube (Falcon) (do not use polypropylene tube). Mix 10 μL FuGENE (Promega) with diluted bacmid. After incubation at 27 °C for 30 min, add 900 μL medium to achieve a 1 mL volume. (c) Aspirate medium from Sf9 cells. Evenly apply 1 mL bacmid-FuGENE mixture onto cells and incubate for 4 h. (d) Add another 1 mL medium and incubate for 4 days. (e) Collect supernantant that contains P1 virus by syringe and pass through a 0.2 μm PES syringe filter (Thermo Fisher Scientific). Virus can be stored at 4 °C.
Amplify virus to generate a P2 stock: Plate 3 X107 Sf9 cells on a 150 mm25 mm dish (Corning) and incubate 30 min at 27 °C to allow cell adherence (add more SFX serum-free insect cell medium if liquid volume is below 12 mL or fails to cover the whole dish surface). Apply 0.5 mL P1 virus dropwise to adhered cells, gently rock to spread virus evenly, and incubate plate at 27 °C for 2 h. Then add more medium to a total volume of 25 mL per dish and incubate at 27 °C for another 3 days. Collect supernatant that contains virus into a 50 mL conical tube followed by centrifugation at 500 X g for 10 min to remove floating cells. Save the clarified supernatant as P2 amplified virus.
Amplify virus to generate a P3 stock. Use 1 mL P2 virus for infection using the same procedure for P2 amplification, except may scale up depending on the ultimate culture size for protein production. The volume of P3 amplified virus produced by each 150 mm cell culture dish supports approximately 2 L Hi5 cells during infection to produce protein. Repeat if further virus amplification is needed.
Infection for protein production. Pellet Hi5 insect cell (Thermo Fisher Scientific) culture at log phase by centrifugation at 500 X g for 10 min. Then resuspend the cell pellet by medium to reach a cell density of 1.25 X 107/mL in PC Erlenmeyer flasks (VWR). Add 5 mL P3 amplified virus per 100 mL resuspended cells, then incubate cells in shaker at 27 °C, 75 rpm for 2 h with ambient CO2.
Diluted cell culture by adding medium to a cell density of 2.5 X 106/mL (make sure the culture volume is less than 50% of the flask volume). Let cells grow and express target protein in shaker at 27 °C, 155 rpm for 3 more days with ambient CO2.
3. PROTEIN PURIFICATION
3.1 Preparation of Cell Lysate
Harvest bacteria cells by centrifugation at 5900 X g for 12 min at 4 °C, or insect cells at 500 X g for 15 min at 4 °C.
Resuspend cell pellet in 15 mL lysis buffer per liter bacteria culture, or 50 mL per liter insect cell culture. Lysis buffer for all proteins except the ATG12~ATG5 subcomplex is: 25 mM Tris–HCl pH 7.6, 200 mM NaCl, 2.5 mM phenylmethanesulfonyl fluoride (PMSF), 5 mM 2-Mercaptoethanol (BME). Lysis buffer for ATG12~ATG5 complex is: 1 PBS, 400 mM NaCl, 2.5 mM PMSF, 5 mM BME. Also add 20 mg/L aprotinin, 10 mg/L leupeptin, or a protease inhibitor cocktail of choice to insect cell lysis buffer.
Lyse bacteria cells by sonicating 7 times, or insect cells 3 times, 10 s each time with intermittent cooling on ice.
Clear lysate by centrifugation at 32,000 X g for 1 h at 4 °C. Collect the supernatant and keep it on ice.
3.2 Glutathione-Affinity Chromatography for LC3B, ATG7, ATG3, and ATG12~ATG5-ATG16L1
For GST-thrombin-LC3B, GST-TEV-ATG7, and GST-TEV-ATG3, glutathione-affinity chromatography is directly applied to their corresponding cell lysates. To obtain GST-TEV-ATG12 (52–140)~HisTEV-ATG5-GST-TEV-ATG16L1 (1–69) complex, cell lysates from insect cells and bacteria are mixed at 1:1 volume ratio and incubated at 4 °C for 10 min before processing.
Wash Glutathione Sepharose 4B (GS4B, GE Healthcare Life Sciences) 3 times with 10 column volumes of wash buffer in a chromatography column at 4 °C. Alternatively, wash the beads with 3 volumes of wash buffer by centrifugation at 500 X g for 5 min at 4 °C, decanting the wash buffer, and repeating twice. Suggested bead volumes: 0.75 mL per 1 L bacterial culture, or 2 mL per 1 L Hi5 insect cell culture, which is appropriate for both GST and nickel beads and all species of ATG proteins described in this chapter. Wash buffer is the same as lysis buffer but without PMSF, aprotinin, leupeptin, or any other proteasome inhibitor.
Incubate lysate with washed beads. Gently rock the mixture at 4 °C for 2 h for GS4B beads or 1 h for nickel beads to allow full binding.
Transfer lysate with beads into a Poly-Prep chromatography column (Bio-Rad) at 4 °C. Allow lysate to flow through by gravity to generate an even resin surface in column. Although the flow-through is unneeded in principle, it is a good practice to save the flow-through for troubleshooting in the event of problems.
If the lysate-bead solution is too thick to pass through the column, centrifugate the solution in 50 mL tubes at 500 X g for 5 min at 4 °C. Collect the supernatant as flow through. Gently resuspend pelleted beads in 10 column volumes of wash buffer and follow step 3.
Wash resin in column with 10 column volumes of wash buffer without disturbing the flat resin surface. Repeat 3 times.
3.3 Protease-Mediated Elution From Beads for Purification of LC3B, ATG3, and ATG7
Measure rough protein concentration of bead turbid solution using BioRad protein assay with a standard curve. Bovine serum albumin is a suitable standard.
Store 10 μL of uncleaved sample as a negative control for cleavage efficiency check before adding protease. Add thrombin protease to LC3B (1 mg thrombin per 100 mg of total protein), or TEV protease to ATG7 and ATG3 (2 mg TEV per 100 mg total protein). Keep gently rotating on a rocking shaker overnight to allow full cleavage.
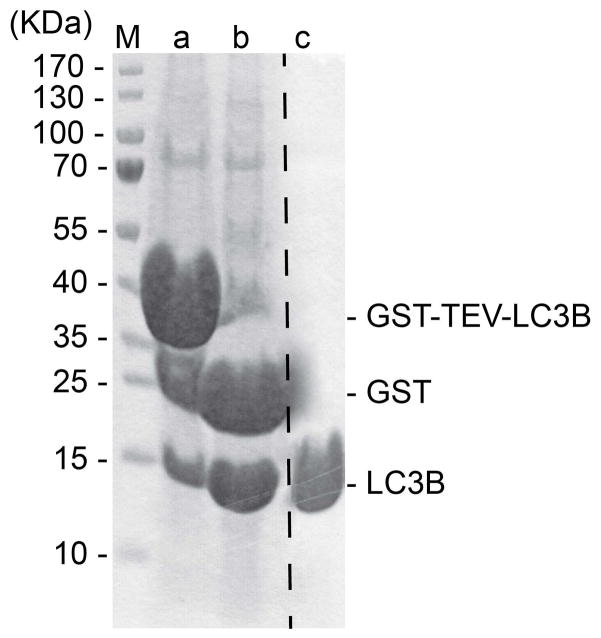
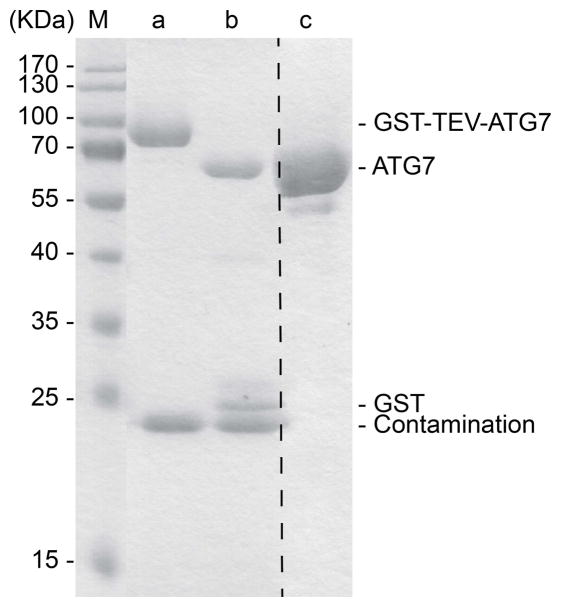
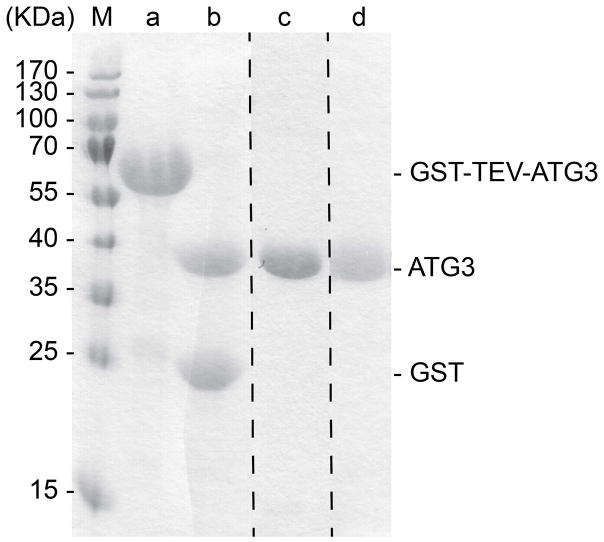
To be certain that all desired protein is cleaved from the GST-tag, examine the bead slurry by SDS-PAGE, using a 15% acrylamide gel and Coomassie-staining, comparing cleaved samples to uncleaved samples (LC3B: Fig. 3, Lanes a and b; ATG7: Fig. 4, Lanes a and b; ATG3: Fig. 5, Lanes a and b).
Transfer slurry into an empty Poly-Prep chromatography column pre-rinsed with wash buffer, and collect flow through. When liquid completely passes through, apply another 1 column volume of wash buffer on the top of resin, and collect this second fraction containing residual protein.
Fig. 3.
15% SDS-PAGE showing LC3B (1–120) at different steps of purification. Lane M shows the molecular weight markers. Lane a shows constituents of bead slurry after binding GS4B resin. Lane b shows constituents of bead slurry after thrombin treatment. Lane c shows final purified LC3B (1–120) after gel filtration chromatography.
Fig. 4.
15% SDS-PAGE showing ATG7 at different steps of purification. Lane M shows the molecular weight markers. Lane a shows constituents of bead slurry after binding GS4B resin. Lane b shows constituents of bead slurry after TEV treatment. Lane c shows final purified ATG7 after gel filtration chromatography.
Fig. 5.
15% SDS-PAGE showing ATG3 at different steps of purification. Lane M shows the molecular weight markers. Lane a shows constituents of bead slurry after binding GS4B resin. Lane b shows constituents of bead slurry after TEV treatment. Lane c shows purified ATG3 after anion ion exchange chromatography. Lane d shows final purified ATG3 after gel filtration chromatography.
3.4 Glutathione-Mediated ATG12~ATG5-ATG16L1 Elution From GS4B
Gently apply 0.7 resin volume of GS4B elution buffer on the top of resin surface while keeping stopcock closed. After a 10-min incubation, open the stopcock and collect the eluate in a tube. Repeat 6 times and keep elution fractions in separate tubes. GS4B elution buffer: wash buffer supplemented with 20 mM glutathione (reduced, free acid) (EMD Millipore), adjust pH by NaOH solution to the same as wash buffer.
Run each eluted fraction on a 15% SDS-PAGE gel to determine which fractions contain the majority of target protein. Pool desired fractions.
3.5 Nickel-Affinity Chromatography, Imidazole-Mediated Elution From Nickel Beads, and TEV Cleavage to Obtain ATG12 (52–140)~ATG5-ATG16L1 (1–69) Complex
After glutathione chromatography and glutathione mediated elution, the excessive GST-TEV-ATG16L1 (1–69) is further removed by nickel affinity chromatography.
Wash HIS-Select Nickel Affinity Gel (Sigma-Aldrich) 3 times with 10 column volumes of wash buffer in a chromatography column at 4 °C. Then perform nickel-affinity chromatography to enrich the ATG5-bound complex. Apply the glutathione elution of GST-TEV-ATG12 (52–140)~His-TEV-ATG5-GST-TEV-ATG16L1 (1–69) to the HIS-Select Nickel Affinity Gel beads and incubate following the same procedure described above for glutathione-affinity chromatography.
Perform an imidazole elution following steps described in glutathione elution using nickel bead elution buffer (wash buffer supplemented with 100 mM imidazole pH 7.5).
Store 10 μL uncleaved sample as a negative control to check cleavage efficiency. Add TEV protease (2 mg TEV per 100 mg total protein) to imidazole eluate, then dialyze in wash buffer while performing cleavage overnight at 4 °C.
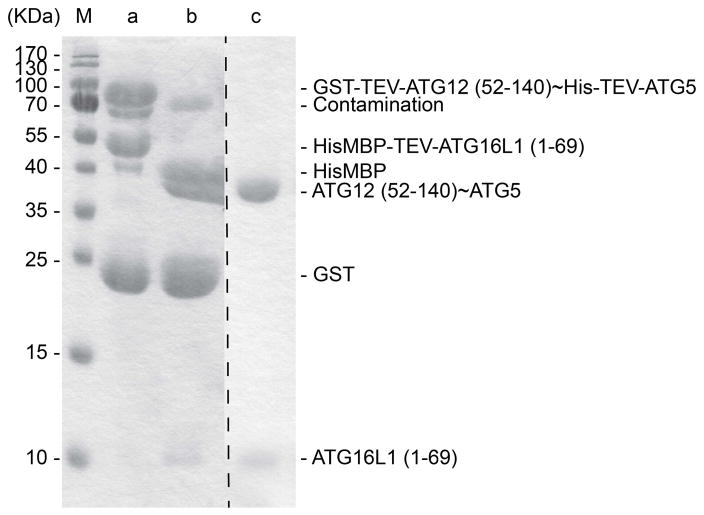
Examine cleavage completeness by SDS-PAGE, using a 15% acrylamide gel and Coomassie-staining (Fig. 6, Lanes a and b).
The cleaved GST and HisMBP tags are removed from ATG12 (52–140)~ATG5-ATG16L1 (1–69) complex by further cation ion exchange purification described below.
Fig. 6.
15% SDS-PAGE showing ATG12 (52–140)~ATG5-ATG16L1 (1–69) complex at different stages of purification. Lane M shows the molecular weight markers. Lane a shows elution from nickel-affinity chromatography, capturing His- and His-MBP tags on ATG5 and ATG16L1 (1–69), respectively. This is the second step in the purification, following an initial purification by glutathione affinity chromatography to capture GST-tag on ATG12. Lane b shows product of TEV cleavage reaction preformed during dialysis. Lane c shows final purified ATG12 (52–140)~ATG5-ATG16L1 (1–69) complex after subsequent cation ion exchange and gel filtration chromatography.
3.6 Ion Exchange Chromatography on ATG3 and ATG12 (52–140)~ATG5-ATG16L1 (1–69)
Perform anion ion exchange to tag-free ATG3 and cation ion exchange to tag-free ATG12 (52–140)~ATG5-ATG16L1 (1–69) accordingly to their isoelectric points, before ultimately purifying by gel filtration chromatography. This step is optional for LC3B and ATG7.
Equilibrate a 5 mL HiTrap SP (cation exchange) or a Q (anion exchange) HP column (GE Healthcare Life Sciences) on an AKTA FPLC (GE Healthcare Life Sciences) in 95% buffer A with 5% buffer B. Cation exchange buffer A: 25 mM HEPES pH 7.0, 5 mM DTT. Anion exchange buffer A: 25 mM Tris–HCl pH 8.0, 5 mM DTT. Buffer B corresponds to buffer A with 1 M NaCl.
Dilute protein samples with buffer A to a final NaCl concentration of 50 mM.
Clarify samples by centrifugation at 4000 X g for 5 min at 4 °C before loading onto a FPLC system. If loading is through a sample pump, equilibrate the sample pump in buffer A. The ATG proteins described in this chapter can be concentrated to 10 mg/mL if desired to reduce volume prior to loading onto FPLC.
Load sample onto column with a suggested loading rate of 2 mL/min. After loading, wash column by 5 column volumes of 95% buffer A with 5% buffer B.
After all unbound sample is washed out, initiate gradient, raising buffer B proportion from 5% to 50% over 25 column volumes at 2 mL/min flow rate, and collecting fractions with a volume of 2 mL.
Examine peak fractions by SDS-PAGE with Coomassie-stained 15% acrylamide gels to determine which fractions contain the majority of the ATG proteins (the combined fractions of ATG3 is shown in Fig. 5, Lane c; ATG12~ATG5-ATG16L1 is not shown).
3.7 Gel Filtration Chromatography for LC3B, ATG7, ATG3, and ATG12~ATG5-ATG16L1
Superdex 200 10/300 GL columns (GE Healthcare Life Sciences) are used for all the ATG proteins described here, except for LC3B using a Superdex 75 10/300 GL column (GE Healthcare Life Sciences). Protein samples of LC3B and ATG7 used in this step are directly collected from protease cleavage, while ATG3 and ATG12~ATG5-ATG16L1 are prepurified by ion exchange.
Equilibrate a Superdex 75/200 column on an AKTA FPLC system with storage buffer (25 mM Tris–HCl pH 7.6, 200 mM NaCl, 5 mM DTT). Increase NaCl concentration to 500 mM in the buffer used for LC3B, or to 300 mM in the buffer used for ATG12 (52–140)~ATG5-ATG16L1 (1–69).
Concentrate protein samples by Amicon Ultra Centrifugal Filter Units (EMD Millipore) with appropriate molecular weight cutoff. Transfer concentrated protein samples to one or multiple 1.7 mL microcentrifuge tubes. Perform centrifugation at 16,000 X g for 10 min at 4 °C to clarify samples before loading onto a FPLC system.
Load 1–2 mL clarified protein sample onto a Superdex 200 column, run gel filtration in storage buffer, and collect fractions with a fraction volume of 0.5 mL.
Examine peak fractions by SDS-PAGE with Coomassie-stained 15% acrylamide gels to determine which fractions contain the majority of the ATG proteins. Pool desired fractions (the pooled fractions of LC3B, ATG7, ATG3, and ATG12~ATG5-ATG16L1 are shown in Fig. 3, Lane c; Fig. 4, Lane c; Fig. 5, Lane d; Fig. 6, Lane c, respectively).
4. STORING PURIFIED PROTEINS
Purified proteins can be concentrated to approximately 10 mg/mL and stored in 20 μL aliquots at −80 °C after flash-freezing in liquid nitrogen. Thaw on ice for use and refreeze for storage up to twice to ensure protein activity.
5. CONCLUDING REMARKS
Proteins prepared as described earlier are eligible for multiple biochemical assays, including LC3B lipidation. In our experience, aliquots of purified ATG proteins stored at −80 °C are stable for up to a year, giving consistent results in assays for LC3B lipidation, formation of thioesterbonded ATG7~LC3B and ATG3~LC3B intermediates, binding using BioLayer Interferometry (Octet Red, ForteBio) or isothermal titration calorimetry (ITC), and for structural studies using NMR and crystallography.
Acknowledgments
This work was supported by ALSAC, the NIH R01GM077053, the Howard Hughes Medical Institute, and AHA 14POST19890021. B.A.S. is an Investigator of the Howard Hughes Medical Institute.
References
- Bae JY, Park HH. Purification and characterization of a ubiquitin-like system for autophagosome formation. Journal of Microbiology and Biotechnology. 2010;20(12):1647–1652. [PubMed] [Google Scholar]
- Berger I, Fitzgerald DJ, Richmond TJ. Baculovirus expression system for heterologous multiprotein complexes. Nature Biotechnology. 2004;22(12):1583–1587. doi: 10.1038/nbt1036. [DOI] [PubMed] [Google Scholar]
- Cadwell K, Patel KK, Komatsu M, Virgin HWT, Stappenbeck TS. A common role for Atg16L1, Atg5 and Atg7 in small intestinal Paneth cells and Crohn disease. Autophagy. 2009;5(2):250–252. doi: 10.4161/auto.5.2.7560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Ren X, Hait WN, Yang JM. Therapeutic targeting of autophagy in disease: Biology and pharmacology. Pharmacological Reviews. 2013;65(4):1162–1197. doi: 10.1124/pr.112.007120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dezelak M, Repnik K, Koder S, Ferkolj I, Potocnik U. A prospective pharmacogenomic study of Crohn’s disease patients during routine therapy with antiTNF-alpha drug adalimumab: Contribution of ATG5, NFKB1, and CRP genes to pharmacodynamic variability. Omics: A Journal of Integrative Biology. 2016;20(5):296–309. doi: 10.1089/omi.2016.0005. [DOI] [PubMed] [Google Scholar]
- Gibson DG. Enzymatic assembly of overlapping DNA fragments. Methods in Enzymology. 2011;498:349–361. doi: 10.1016/B978-0-12-385120-8.00015-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson DG, Young L, Chuang RY, Venter JC, Hutchison CA, 3rd, Smith HO. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nature Methods. 2009;6(5):343–345. doi: 10.1038/nmeth.1318. [DOI] [PubMed] [Google Scholar]
- Hanada T, Noda NN, Satomi Y, Ichimura Y, Fujioka Y, Takao T, et al. The Atg12–Atg5 conjugate has a novel E3-like activity for protein lipidation in autophagy. The Journal of Biological Chemistry. 2007;282(52):37298–37302. doi: 10.1074/jbc.C700195200. [DOI] [PubMed] [Google Scholar]
- Hanada T, Ohsumi Y. Structure–function relationship of Atg12, a ubiquitin-like modifier essential for autophagy. Autophagy. 2005;1(2):110–118. doi: 10.4161/auto.1.2.1858. [DOI] [PubMed] [Google Scholar]
- Ichimura Y, Kirisako T, Takao T, Satomi Y, Shimonishi Y, Ishihara N, et al. A ubiquitin-like system mediates protein lipidation. Nature. 2000;408(6811):488–492. doi: 10.1038/35044114. [DOI] [PubMed] [Google Scholar]
- Kaiser SE, Qiu Y, Coats JE, Mao K, Klionsky DJ, Schulman BA. Structures of Atg7–Atg3 and Atg7–Atg10 reveal noncanonical mechanisms of E2 recruitment by the autophagy E1. Autophagy. 2013;9(5):778–780. doi: 10.4161/auto.23644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Sandford E, Gatica D, Qiu Y, Liu X, Zheng Y, et al. Mutation in ATG5 reduces autophagy and leads to ataxia with developmental delay. eLife. 2016;5:e12245. doi: 10.7554/eLife.12245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klionsky DJ, Emr SD. Autophagy as a regulated pathway of cellular degradation. Science. 2000;290(5497):1717–1721. doi: 10.1126/science.290.5497.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S. Choose delicately and reuse adequately: The newly revealed process of autophagy. Biological & Pharmaceutical Bulletin. 2015;38(8):1098–1103. doi: 10.1248/bpb.b15-00096. [DOI] [PubMed] [Google Scholar]
- Lenz HD, Vierstra RD, Nurnberger T, Gust AA. ATG7 contributes to plant basal immunity towards fungal infection. Plant Signaling & Behavior. 2011;6(7):1040–1042. doi: 10.4161/psb.6.7.15605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metlagel Z, Otomo C, Ohashi K, Takaesu G, Otomo T. Structural insights into E2–E3 interaction for LC3 lipidation. Autophagy. 2014;10(3):522–523. doi: 10.4161/auto.27594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metlagel Z, Otomo C, Takaesu G, Otomo T. Structural basis of ATG3 recognition by the autophagic ubiquitin-like protein ATG12. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(47):18844–18849. doi: 10.1073/pnas.1314755110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moloughney JG, Monken CE, Tao H, Zhang H, Thomas JD, Lattime EC, et al. Vaccinia virus leads to ATG12–ATG3 conjugation and deficiency in autophagosome formation. Autophagy. 2011;7(12):1434–1447. doi: 10.4161/auto.7.12.17793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatogawa H, Ichimura Y, Ohsumi Y. Atg8, a ubiquitin-like protein required for autophagosome formation, mediates membrane tethering and hemifusion. Cell. 2007;130(1):165–178. doi: 10.1016/j.cell.2007.05.021. [DOI] [PubMed] [Google Scholar]
- Nath S, Dancourt J, Shteyn V, Puente G, Fong WM, Nag S, et al. Lipidation of the LC3/GABARAP family of autophagy proteins relies on a membrane-curvaturesensing domain in Atg3. Nature Cell Biology. 2014;16(5):415–424. doi: 10.1038/ncb2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda NN, Fujioka Y, Hanada T, Ohsumi Y, Inagaki F. Structure of the Atg12–Atg5 conjugate reveals a platform for stimulating Atg8-PE conjugation. EMBO Reports. 2013;14(2):206–211. doi: 10.1038/embor.2012.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohsumi Y, Mizushima N. Two ubiquitin-like conjugation systems essential for autophagy. Seminars in Cell & Developmental Biology. 2004;15(2):231–236. doi: 10.1016/j.semcdb.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Otomo C, Metlagel Z, Takaesu G, Otomo T. Structure of the human ATG12-ATG5 conjugate required for LC3 lipidation in autophagy. Nature Structural & Molecular Biology. 2013;20(1):59–66. doi: 10.1038/nsmb.2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puri P, Chandra A. Autophagy modulation as a potential therapeutic target for liver diseases. Journal of Clinical and Experimental Hepatology. 2014;4(1):51–59. doi: 10.1016/j.jceh.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Y, Hofmann K, Coats JE, Schulman BA, Kaiser SE. Binding to E1 and E3 is mutually exclusive for the human autophagy E2 Atg3. Protein Science. 2013;22(12):1691–1697. doi: 10.1002/pro.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed M, Morris SH, Owczarczyk AB, Lukacs NW. Deficiency of autophagy protein Map1-LC3b mediates IL-17-dependent lung pathology during respiratory viral infection via ER stress-associated IL-1. Mucosal Immunology. 2015;8(5):1118–1130. doi: 10.1038/mi.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakoh-Nakatogawa M, Matoba K, Asai E, Kirisako H, Ishii J, Noda NN, et al. Atg12–Atg5 conjugate enhances E2 activity of Atg3 by rearranging its catalytic site. Nature Structural & Molecular Biology. 2013;20(4):433–439. doi: 10.1038/nsmb.2527. [DOI] [PubMed] [Google Scholar]
- Sou YS, Tanida I, Komatsu M, Ueno T, Kominami E. Phosphatidylserine in addition to phosphatidylethanolamine is an in vitro target of the mammalian Atg8 modifiers, LC3, GABARAP, and GATE-16. The Journal of Biological Chemistry. 2006;281(6):3017–3024. doi: 10.1074/jbc.M505888200. [DOI] [PubMed] [Google Scholar]
- Taherbhoy AM, Tait SW, Kaiser SE, Williams AH, Deng A, Nourse A, et al. Atg8 transfer from Atg7 to Atg3: A distinctive E1–E2 architecture and mechanism in the autophagy pathway. Molecular Cell. 2011;44(3):451–461. doi: 10.1016/j.molcel.2011.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanida I, Ueno T, Kominami E. LC3 conjugation system in mammalian autophagy. The International Journal of Biochemistry & Cell Biology. 2004a;36(12):2503–2518. doi: 10.1016/j.biocel.2004.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanida I, Ueno T, Kominami E. Human light chain 3/MAP1LC3B is cleaved at its carboxyl-terminal Met121 to expose Gly120 for lipidation and targeting to autophagosomal membranes. The Journal of Biological Chemistry. 2004b;279(46):47704–47710. doi: 10.1074/jbc.M407016200. [DOI] [PubMed] [Google Scholar]
- Tanida I, Ueno T, Kominami E. LC3 and autophagy. Methods in Molecular Biology. 2008;445:77–88. doi: 10.1007/978-1-59745-157-4_4. [DOI] [PubMed] [Google Scholar]
- Tindwa H, Jo YH, Patnaik BB, Noh MY, Kim DH, Kim I, et al. Depletion of autophagy-related genes ATG3 and ATG5 in Tenebrio molitor leads to decreased survivability against an intracellular pathogen, Listeria monocytogenes. Archives of Insect Biochemistry and Physiology. 2015;88(1):85–99. doi: 10.1002/arch.21212. [DOI] [PubMed] [Google Scholar]
- Weissmann F, Petzold G, VanderLinden R, Huis In ‘t Veld PJ, Brown NG, Lampert F, et al. biGBac enables rapid gene assembly for the expression of large multisubunit protein complexes. Proceedings of the National Academy of Sciences of the United States of America. 2016;113(19):E2564–E2569. doi: 10.1073/pnas.1604935113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wileman T. Autophagy as a defence against intracellular pathogens. Essays in Biochemistry. 2013;55:153–163. doi: 10.1042/bse0550153. [DOI] [PubMed] [Google Scholar]
- Wu DH, Jia CC, Chen J, Lin ZX, Ruan DY, Li X, et al. Autophagic LC3B overexpression correlates with malignant progression and predicts a poor prognosis in hepatocellular carcinoma. Tumour Biology: The Journal of the International Society for Oncodevelopmental Biology and Medicine. 2014;35(12):12225–12233. doi: 10.1007/s13277-014-2531-7. [DOI] [PubMed] [Google Scholar]
- Xi Y, Dhaliwal JS, Ceizar M, Vaculik M, Kumar KL, Lagace DC. Knockout of Atg5 delays the maturation and reduces the survival of adult-generated neurons in the hippocampus. Cell Death & Disease. 2016;7:e2127. doi: 10.1038/cddis.2015.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue LY, Chiu SM, Oleinick NL. Atg7 deficiency increases resistance of MCF-7 human breast cancer cells to photodynamic therapy. Autophagy. 2010;6(2):248–255. doi: 10.4161/auto.6.2.11077. [DOI] [PMC free article] [PubMed] [Google Scholar]