Abstract
An infant’s vocal capacity develops significantly during the first year of life. Research suggests early measures of pre-speech development, such as canonical babbling and volubility, can differentiate typical versus disordered development. This study offers a new contribution by comparing early vocal development in 10 infants with Fragile X syndrome and 14 with typical development. Results suggest infants with Fragile X syndrome produce fewer syllables and have significantly lower canonical babbling ratios (i.e., canonical syllables/total syllables) compared to infants who are typically developing. Furthermore, the particular measures of babbling were strong predictors of group membership, adding evidence regarding the possible utility of these markers in early identification.
The first few years of life are a time of remarkable brain growth and behavior development (National Center for Infants, Toddlers, and Families, 2012). Throughout the first year, infants begin exploring and using sounds that will eventually take on the form of their native language. From the beginning, these sounds reveal emerging foundations for the development of spoken language. Research on early differences in these language foundations in children with neurodevelopmental disabilities is underway; however, many aspects of early language learning remain unexamined. Recent research suggests that early differences in the use of canonical syllables (as in [baba] or [dada]) and in volubility (i.e., quantity of vocalizations) have the potential to differentiate infants with atypical language development from those with typical development (Patten et al., 2014; Oller et al., 2010; Masataka, 2001). Moreover, these differences may serve as behavioral indicators in the first year of life that development is unfolding in an atypical fashion. Reliable and valid behavioral early indicators are critical for early identification that can lead to earlier intervention, which is necessary for maximizing a child’s long-term potential.
Fragile X Syndrome and Language Development
Fragile X syndrome (FXS) is a neurodevelopmental disorder resulting from a genetic mutation on the X chromosome, specifically the fragile X mental retardation 1 (FMR1) gene (Verkerk et al., 1991). Having a certain nucleotide repeated 200 or more times compared to the 10–40 repeat in a typically developing individual triggers the mutation. When the FMR1 gene is mutated, the production of the fragile X mental retardation protein (FMRP), a critical protein involved in brain development and functioning (Greenough, Klintsova, Irwin, Galvez, Bates & Weiler, 2001) is stopped. The absence of FMRP results in a specific profile of deficits across cognitive, motor, physical, and language domains. Language skills, in particular, are severely affected compared to such skills in chronological age peers. Individuals with FXS have deficits in all aspects of language, including comprehension, expression and pragmatics (Brady, Skinner, Roberts, & Hennon, 2006; Finestack & Abbeduto, 2010; Roberts, Mankowski, Sideris, Goldman, Hatton, Mirrett, et al., 2008), and the deficits persist throughout life. Earlier identification would provide an earlier avenue for entry into treatment thereby possibly lessening the significant and lifelong impact of FXS on the child’s development, especially their language development.
Early Signs and Early Identification
There is a substantial body of literature documenting language deficits in school-aged children and adolescents with FXS (Abbeduto et al., 2003; Finestack & Abbeduto, 2010; Maes, Fryns, Ghesquiere, Borghgraef, 2000 Martin, Losh, Estigarribia, Sideris, & Losh, 2013), but a dearth of information concerning deficits in infancy. The limited research about early development is surprising since early signs and symptoms have indeed been reported. In fact, some parents report noticing first symptoms of FXS before and around 12 months of age (Bailey, Raspa, Bishop, & Holiday, 2009; Zhang et al., 2016). One study with seven 9 – 12 month old infants with FXS (Marschik et al., 2014) suggests this population may exhibit limited forms of social-communication behaviors for their age based on the Inventory of Potential Communicative Acts (IPCA; Sigafoos, Arthur-Kelly, & Butterfield, 2006). Despite infants with FXS showing symptoms early, confirmation of a developmental delay is not typically provided until about 20 months for males and 26 months for females (Bailey, Raspa, Bishop, & Holiday, 2009), at the age when mothers report the majority of males and some females with FXS are nonverbal (Brady, Skinner, Roberts, & Hennon 2006; Hinton et al., 2013). More alarming is that about 16 additional months pass between confirmation of a child’s developmental delay and a diagnosis of FXS (Bailey, Raspa, Bishop, & Holiday, 2009).
Some argue that earlier diagnoses of FXS have benefits for children, such as early intervention, and for their families, including reduced financial and emotional burden, and the opportunity for family planning related to subsequent pregnancies (Center for Diseases Control and Prevention, 2015). One potential method of earlier diagnosis, universal genetic newborn screening, remains controversial for several reasons including issues of the cost-to-benefit ratio of such screening and more notably a lack of evidence for treatments that can be initiated in the newborn period to prevent and/or reverse behavioral symptoms of the disorder (Tassone, 2014). Nevertheless, earlier detection would make it possible to connect families with services, interventions and/or resources sooner. There is substantial evidence documenting the effectiveness of early intervention services in improving children’s language deficits in neurodevelopmental disorders like autism spectrum disorder (ASD; Dawson et al., 2010). A validated behavioral indicator that could lead to earlier genetic testing for infants with FXS would provide opportunities to evaluate whether early intervention has similar benefits for this population as for young children with ASD. Such an indicator would be especially useful in cases with no known family history and is particularly important given that the physical features of FXS are often not noticeable very early in infancy. Canonical babbling and volubility are two potential early indicators for problems with language development in neurodevelopmental disorders such as FXS.
Canonical babbling status
Early developmental stages unique to humans lay foundations for language development. One such stage occurs when infants begin to produce adult-like, or canonical syllables (Stark, 1980; Oller, 1980; Oller and Griebel, 2006). During the first few stages of vocal development, infants explore phonation as well as moving their articulators to manipulate resonance. Usually between five and ten months, these systems come to be coordinated so that canonical syllables can be produced. Canonical syllables are fully articulated sound sequences with full resonance of nuclei and rapid transitions between consonant-like closures of the vocal tract and vowel-like openings, resulting in syllables listeners perceive as adult-like consonant-vowel combinations (Oller, 2000). These syllables are often reduplicated (e.g., [dada] i.e., the same consonant-vowel syllable is repeated). Universally, infants produce canonical babbling before producing words. The vast majority of infants who are typically developing reach the “canonical babbling stage” between five and ten months of age, as indicated by parent report and laboratory judgments (Eilers & Oller, 1994). In addition, the propensity for canonical babbling appears to be robust in human infants such that no delay in onset of canonical babbling has been found in infants at-risk for communication deficits due to premature birth and/or low socioeconomic status (SES; Eilers et al., 1993; Oller, Eilers, Basinger, Steffens, & Urbano, 1995). Infants with Down syndrome show a delay in canonical babbling onset of about two months at the group level (Lynch et al., 1995). Infants tracheostomized at birth also produce age-appropriate canonical syllables shortly after decannulation (Bleile, Start, & McGowan, 1993; Locke & Pearson, 1990). Therefore, the evidence strongly suggests canonical babbling is part of the natural course for learning vocal language. The robustness of this developmental milestone suggests its importance to language learning.
Despite the robustness of canonical babbling for most human infants, some infants have been found to have difficulty with this developmental stage. Populations with substantial delays in canonical babbling onset include infants with profound hearing impairment (Eilers & Oller, 1994), Williams syndrome (Masataka, 2001), and infants later diagnosed with ASD (Patten et al., 2014). Moreover, infants without previously identified disorders who reach the canonical babbling stage after ten months are at-risk for later language delay or other developmental disabilities (Oller, Eilers, Neal, & Schwartz, 1999; Stark, Ansel, & Bond, 1988; Stoel-Gammon, 1989). The observation of differences in canonical babbling in infants with clinical disorders suggests that this milestone has the potential to serve as a reference point for identifying and possibly differentiating neurodevelopmental disorders. Two additional types of evidence support the potential utility of this behavioral indicator for early identification: (a) parents are reliably able to identify when their child reaches the canonical babbling stage (Lewedag, 1995; Oller, Eilers, Neal, & Schwartz, 1999), and (b) canonical babbling status at 9 – 12 months emerged as a strong predictor of diagnostic group in a study of 9- to 12-month-old infants with ASD versus typically developing infants (Patten et al., 2014).
Volubility
Another potentially clinically significant measure of vocal development is volubility, or the quantity of infant vocalizations (defined as the rate of speech-like vocalizations in utterances per minute regardless of whether the vocalizations include canonical syllables; Nathani, Oller, & Neal, 2007). Oller and colleagues suggest lower volubility is associated sometimes with environmental factors, given that children from low SES households vocalize less frequently than middle or high SES peers (Eilers et al., 1993; Oller et al., 1995). Lower volubility in infants from low SES homes may be attributed to a smaller amount of caregiver communication to infants (Hart & Risley, 1995; Snow, 1995). Patten et al. (2014) found infants with ASD had lower volubility than TD infants at 9 – 12 months, and proposed the idea that lower volubility may be due to less social motivation among infants with ASD. Lower volubility among infants with ASD may also result in these infants eliciting fewer adult responses and thus not setting the stage for infant-caregiver reciprocal vocalizations to the extent that these appear to occur with their TD peers (Warlaumont et al., 2014). Although infants later diagnosed with ASD have demonstrated low volubility (Patten et al., 2014; Warren et al., 2010), infants with severe or profound hearing loss and infants with cleft palate have not shown differences in volubility compared to peers with typical development (Iyer, Lynch, & Oller, 2008; Iyer, Oller, & Neal, 2007; Oller, Eilers, Basinger, Steffens, & Urbano, 1995; Chapman, Hardin-Jones, Schulte, & Halter, 2001). Also, in one study (Steffens, Oller, Lynch, & Urbano, 1992), the means for total vocalizations for 13 infants with Down syndrome at 12 months were similar to those of infants with typical development (TD = 1.41 per minute; Down syndrome = 1.25 per minute). The failure to find a difference may suggest there simply are no differences. Alternatively, the lack of significant difference might have resulted from small sample size. In addition, it could be that regardless of possible endogenous vocal limitations, infants with Down syndrome tend to be socially engaged to such an extent that their endogenous tendencies to vocalize are offset and volubility is not reduced compared with typically developing infants.
As reflected in these disparate findings, lowered volubility has not consistently been associated with problems with language development. Therefore, volubility may or may not be a strong indicator of potential problems with language development. However, given that low volubility has been reported in some clinical populations, it is important to understand to what extent it contributes to predicting differences in typical versus atypical development. The combination of lower canonical babbling and low volubility may also have potential to be a stronger indicator of problems with language development than either of these measures alone.
Aims and Approach
Further research on canonical babbling and volubility in the clinical population of FXS may contribute to the development of a cost-effective method for earlier diagnosis based on a valid and reliable indicator. Parents and healthcare providers could use such an indicator to help determine whether a child is developing similarly to his or her typically developing (TD) peers. The purpose of this study is to test these two measures of early vocal/language development, as potential behavioral indicators for FXS. To the authors’ knowledge, this is the first study comparing early vocal behaviors of infants with FXS to TD peers in the first year of life. This study is a step toward understanding possible differences in the early vocal language trajectory of children with FXS compared to their TD peers.
There are two aims of the study. First, it is important to determine if there are differences in the likelihood infants with FXS and infants with TD by 9 – 12 months will meet or exceed the conventional criterion of a .15 canonical babbling ratio (canonical syllables/all syllables). The .15 criterion has been utilized as the most common standard for indicating an infant has reached the canonical stage in laboratory studies since publication of Lynch et al. (1995). Previously, studies from the Oller laboratory in Miami (e.g., Eilers & Oller, 1994) had used a different criterion (.20) based on the ratio of all canonical syllables to all utterances in a sample. The ratio of all canonical syllables to all syllables has the advantage of being a proportion, which of course varies from 0 to 1, in contrast with the prior ratio which has no upper limit in principle. The change to the new ratio suggested by Lynch et al. that the criterion should be reduced to correspond to the different range of possible values on the measure.
The value selected (.15), just as in the case of the prior ratio (.20), was based on informal observations made in the Oller et al. studies that most infants in the canonical stage (as judged intuitively by parents and laboratory staff) tended to show recorded samples in the laboratory exceeding the criterion value. However, the value was never presumed to be more than a heuristic—we know that canonical babbling ratios vary substantially based on, for example, sample size (Molemans, Van den Berg, Van Severen, & Gillis, 2011), and there is good reason to believe, based on ongoing research, that it varies based on other factors, such as infant arousal level, and especially on the extent to which infants are engaged in vocal interaction at the time of the sample.
It is hypothesized in the present work that fewer infants with FXS than infants with TD will meet the .15 criterion for canonical babbling stage by 9 – 12 months, indicating a delay in the development of canonical babbling. Second, we will investigate whether there are differences in canonical babbling ratio and volubility between infants with FXS and infants with TD, and whether volubility and canonical babbling status predict group membership. It is hypothesized that infants with FXS will have a lower mean canonical babbling ratio and volubility compared to infants with TD and that these two variables together will significantly predict group membership.
It is challenging to study early communication behaviors in infants with neurodevelopmental disorders like FXS because diagnoses are not typically made until three or four years of age (Bailey, Raspa, Bishop, & Holiday, 2009). Retrospective interviews or surveys with parents are not ideal methods for studying early communication development, as there is likely bias and lack of detail in recall. Prospective designs similar to Paul et al. (2011) offer advantages of standardization of data collection procedures and elimination of the need to depend on parent recall of their children’s early behaviors, but are expensive and challenging to implement for studying infants with FXS due to the small numbers of children with FXS who are identified in infancy.
This study used an alternative method to study infant behaviors, a retrospective video analysis. Retrospective video analysis is a method in which researchers collect home movies previously recorded by caregivers during a child’s infancy. This method offers the opportunity to study videos of infants later diagnosed with a neurodevelopmental disability or later confirmed to have typical development. Retrospective video analysis is not only a cost-effective way to study the early behavioral manifestation of developmental phenomenon, but also a way to describe patterns of behaviors prior to diagnosis in the FXS population, as demonstrated by previous retrospective video analyses with this population (Baranek, 1999; Marschik et al., 2014). The current study used retrospective video analysis to compare canonical babbling and volubility of 9- to 12- month-old infants later diagnosed with FXS to age-matched infants later confirmed to be developing typically.
Methods
Participants
There were a total of 24 participants in this study. Ten participants had a diagnosis of FXS confirmed by medical records, and 14 met criteria for the TD group. Given the sample sizes and assuming a Type 1 error rate of 5%, we had 80% power to detect a very large effect size of 1.06 (Cohen, 1988). Previous research (Patten et. al., 2014) with 37 9 – 12 month old infants with ASD and TD demonstrated effect sizes ranging from 1.09 – 2.07. Thus, it was reasonable at the outset of the study to assume the current sample would be sufficient to detect effect sizes in a range comparable to those found for infants with ASD.
There was one female in the FXS group and three in the TD group. Parents of eight infants with FXS and 11 with TD identified the infants as Caucasian. One infant with FXS and one infant with TD were identified as Asian. Indications of races of three infants, one with FXS and two with TD, were missing. Exact tests revealed no significant sex or race/ethnicity differences between members of the FXS and TD groups (see Table 1 for means and standard deviations (SD). Participants with FXS included children enrolled in a previous study, Baranek, 1999, and one child newly recruited for the current study. Children with TD were recruited through research efforts spanning a 15-year time period. Recruitment criteria for children with TD in the previous studies (e.g., X) included: (1) child age at recruitment between two and seven years, (2) available home video footage of the child between birth and two years that the parents were willing to share; and (3) enough footage for at least one five-minute codeable segment of the infant at 9 – 12 months of age. Children in the TD group were excluded if they demonstrated one or more of the following: significant hearing, vision, or motor impairments; symptoms of ASD as measured by the Childhood Autism Rating Scales (CARS; Schopler, Reichler, & Renner, 1988); and/or positive test for FXS or other genetic syndrome per parent report. The group of children with TD also had no history of developmental or learning difficulties per parent report and received scores in the average range for overall developmental maturity on the Vineland Adaptive Behavior Scales, Interview Edition, Survey Form (VABS; Sparrow, Balla, & Cicchetti, 1984). The sample chosen for the current study was based on the availability of two five-minute edited videos at 9 – 12 months; using this selection criterion, 14 infants were chosen at random from all eligible infants with TD in the larger sample. All infants were from English-speaking households.
Table 1.
Participant demographics
| Characteristic | Fragile X Syndrome (FXS) M (SD) % (n/N) |
Typical Development (TD) M (SD) % (n/N) |
Group Comparison |
|---|---|---|---|
| Malea | 90% (9/10) | 79% (11/14) | – |
| Caucasianb | 80% (8/10) | 79% (11/14) | – |
| Chronological age (months)c | 11.18 (1.09) | 10.63 (.45) | – |
| Intelligence Quotientd | 52.40 (3.87) | 101.38 (7.25) | p ≤ .0025** |
| Maternal Educatione | 5.44 (1.13) | 5.83 (.72) | – |
| Vineland Adaptive Behavior Scales Composite Standard Scoref | 61.44 (10.11) | 105.15 (11.38) | p ≤ .0001*** |
| Childhood Autism Rating Scaleg | 24.16 (3.12) | 16.2 (1.21) | p ≤ .0001*** |
| FMRP Levelh | 6.94 (6.97) | NA | NA |
Note. n/N = number with characteristic / number nonmissing for the group
Male: Fisher’s Exact Test, p ≤ .61.
Caucasian: Fisher’s Exact Test, p ≤ 1.00.
Chronological Age (months): Exact Wilcoxon (Mann-Whitney) test S = 186, exact p ≤ .2262.
IQ: Missing 5 FXS & 7 TD; 0=Average/Above Average Intelligence (standard scores above 85); 1=Borderline (70-84); 2 = Mild Intellectual Disability (ID; 55-69); 3=Moderate ID (40-54); 4=Severe/Profound ID (<39); Exact Wilcoxon (Mann-Whitney) test S =15, exact p ≤ .0025.
Maternal Education: Missing 1 FXS & 2 TD; 1 = 6th grade or lower; 2 = 7th to 9th grade; 3 = partial high school; 4 = high school graduate/GED; 5 = associate of arts/associate of science or technical training or partial college training; 6 = bachelor of arts/science; 7 = master of arts/science or doctorate or other professional degree completed; Exact Wilcoxon (Mann-Whitney) test S = 87, exact p ≤ .4415.
VABS ABC SS: Missing 1 FXS & 1 TD; Exact Wilcoxon (Mann-Whitney) test S = 45, exact p ≤ .0001.
CARS: Missing 1 FXS and 4 TD; Exact Wilcoxon (Mann-Whitney) test S = 134.5, exact p ≤ .0001.
FMRP: Missing 2 FXS; TD not applicable.
p < .05
p < .01
p < .001
This study was specifically designed to examine babbling in infants with FXS who did not later meet criteria for ASD. Nine of the 10 participants with FXS were drawn from extant data collected in a longitudinal study of children with FXS (Bailey, Hatton, & Skinner, 1998), where participants with FXS were older than 12 months at the time of recruitment. The children with FXS needed to have a full-mutation (i.e., more than 200 CGG repeats of the FMR1 gene which turns off FMRP production causing FXS) confirmed by DNA analysis and not meet the cut-off score for ASD as measured by the CARS (Schopler, Reichler, & Renner, 1988). Available records for potential participants with FXS were screened for reports indicative of ASD (e.g., CARS scores >30 or ASD clinical diagnosis), and four potential infants with FXS were excluded due to documentation suggesting they later met criteria for an ASD diagnosis. Additional participants with FXS were recruited for this study via flyers posted on social media and distributed to Fragile X community groups, a mailing from the North Carolina Fragile X Registry, and by word of mouth. Recruitment criteria for new participants included being at least three years of age, full-mutation, below threshold for ASD as measured by CARS scores less than 30 or parent report that no ASD diagnosis had been received by age three years, and available video footage of the child at 9 – 12 months for two five-minute edited video segments. This recruitment effort yielded one more participant meeting inclusion criteria. Parents were asked to share other demographic information such as the child’s intelligent quotient (IQ), FMRP level, and adaptive behavior skills (descriptively or with VABS (Sparrow, Balla, & Cicchetti, 1984) scores if available). Table 1 provides descriptive information on the study participants.
Assessment Measures
Participants in the TD group were assessed at initial recruitment for descriptive purposes (Baranek, 1999; Poon, Watson, Baranek, & Poe, 2012; Watson, Crais, Baranek, Dykstra, & Wilson, 2013). Measures included the VABS for developmental/adaptive ability. The VABS scores for nine participants in the FXS group were available from the prior longitudinal study (Bailey, Hatton, & Skinner, 1998). The parents of the newly enrolled child did not provide VABS scores. Because the children’s chronological ages varied at the start of the study, the VABS composite standard score was used as an index of developmental/adaptive status at the time of recruitment to describe and compare the groups. An Exact Wilcoxon (Mann-Whitney) test was used to test for differences between the two groups on VABS. As expected, the group with TD had significantly higher scores than the group with FXS (Wilcoxon S = 45, exact p ≤ .0001; ; .
Since level of intellectual impairment is also of interest for descriptive and comparison purposes, standardized scores (overall IQ) on cognitive assessments were gathered from psychological reports/assessments. All children with FXS in the earlier longitudinal study were assessed with the Battelle Developmental Inventory (BDI; Newborg, Stock, Wnek, Guidubaldi, & Svinicki, 1988). For the newly recruited child with FXS, parents were asked to share any developmental assessment reports, but none were available. Children from the TD group either received the VABS (Sparrow, Balla, & Cicchetti, 1984) or Mullen Scales of Early Learning (Mullen, 1995). Consistent with previously published infant video studies (Baranek, 1999), the overall level of intellectual disability for both groups was coded as 0 = Average/Above Average Intelligence (standard scores above 85); 1 = Borderline (70-84); 2 = mild (55-69); 3 = moderate (40-54); 4 = Severe/profound (39-30). An Exact Wilcoxon (Mann-Whitney) test confirmed the expected statistically significant differences on level of cognitive scores between the two groups (S =15, exact p ≤ .0025) with the FXS group having lower scores.
Procedures
Procedures included those for videotape editing and behavioral coding. The Institutional Review Board of the university approved this study.
Videotape editing
The procedures for videotape editing were established by a previous retrospective video analysis (Baranek, 1999) and applied to the newly recruited participant to maintain consistency. Families provided home videos of their child between birth and two years. The videotapes included a variety of events such as family vacations, mealtimes, special events such as birthday parties, and play routines. The footage for each child varied in the recorded events, as expected in family video footage (see Table 2 for percentage of activity type). All videotapes were copied, transcoded into digital formats and then original videotapes were returned to families. The newly recruited participant shared digital footage using a password-protected flash drive. Only footage for which parents could confidently identify the child’s age was used.
Table 2.
Percentage of each event type
| Type | TD N = 14 |
FXS N = 10 |
|---|---|---|
| Mealtime | 11% | 16% |
| Active play | 61% | 56% |
| Special event | 17% | 8% |
| Bathtime | 6% | 12% |
| Passive activity | 4% | 6% |
| Other | 1% | 2% |
In the Baranek (1999) study, the investigators chose the 9 – 12 month age range for two reasons. First, it is the earliest age range at which most of the parents had enough footage for at least one five-minute codeable segment. Second, it represents a time period when a variety of important early social and communication behaviors typically emerge. The age range is appropriate for the current study because it represents a time period when infants with TD are expected to be in the canonical babbling stage. Research assistants, blind to study purpose and research questions, selected events from the raw footage of the home videos to compile five-minute segments for coding. Instructions for editing tapes included (a) to focus on the footage during which the child was consistently visible, and (b) to compile two five-minute video segments for each child if possible based on available video footage in which the child was visible. The assistants were further instructed to quasi-randomly select a cross-section of events and to include events from each one-month age interval for which video footage was available. To ensure videos were similar in contexts, research assistants coded each events included in the selected video segments for the following variables: social interaction (i.e., amount of intrusion from another person to engage the child) and amount of physical restriction (i.e., level of child’s freedom to move as rated on a three-point intensity scale (i.e., low, medium, high); age of infant; number of people present; and number of events (Baranek, 1999). These characteristics were compared between groups with Exact Wilcoxon (Mann-Whitney) tests (see Table 3 for means, standard deviations, and statistical test details for each variable), which yielded no significant group differences in mean numbers of people present, or mean numbers of social intrusions during interactions. The mean number of total events differed between groups (Exact Wilcoxon test S =112, exact p ≤ .0072) with TD children having more events in a video (MTD = 5.07) than children with FXS (MFXS = 4.17). The mean degree of physical restriction used during the interactions was marginally statistically significant (Exact Wilcoxon test S = 199, exact p ≤ .0596) with children with FXS tending to have more physical restriction (MFXS = 1.77) than TD children (MTD = 1.51). Thus, the FXS group had fewer total events and marginally more physical restrictions during the interactions than the TD group.
Table 3.
Content variables for videos
| Type | FXS M (SD) N = 10 |
TD M (SD) N = 14 |
Group Significant Difference |
|---|---|---|---|
| Number of people presenta | 3.04 (1.01) | 3.28 (1.03) | – |
| Amount of physical restrictionb | 1.77 (.49) | 1.51 (.27) | p ≤ .0596 |
| Amount of social intrusionc | 2.03 (.31) | 2.04 (.34) | – |
| Total number of different eventsd | 4.17 (.65) | 5.07 (.94) | p ≤ .0072** |
Note.
Number of People: Exact Wilcoxon (Mann-Whitney) test S = 153, exact p ≤ .6673.
Physical Restriction: Exact Wilcoxon (Mann-Whitney) test S = 199, exact p ≤ .0596.
Social Intrusion: Exact Wilcoxon (Mann-Whitney) test S = 160, exact p ≤ .9396.
Number Events: Exact Wilcoxon (Mann-Whitney) test S = 112, exact p ≤ .0072.
p < .05
p < .01
p < .001
Coding procedures
Each five-minute video segment was coded for infant-produced canonical and non-canonical syllables. In the coding scheme, syllables are defined as rhythmic prominences (excluding raspberries, effort sounds, ingressive sounds, sneezes, hiccups, crying and laughing) within one vocal breath group (Lynch, Oller, Steffens, & Buder, 1995). A canonical syllable is defined as having a consonant-like and a vowel-like sound, and a rapid transition between them. The transition in a canonical syllable is too rapid (nominally <120 ms) to be tracked by ear, which is to say that it is experienced auditorily as an integral part of the whole syllable and cannot be heard independently of the syllable (Oller, 1980; Buder, Warlaumont, & Oller, 2013). Examples of canonical syllables are [ga], [do], and [ma]. Words are composed predominantly of canonical syllables. No vocalizations were coded when an infant had an object or food in their oral cavity or on their lips. The reason for excluding these vocalizations is that an object can obstruct the vocal tract and create the illusion of supraglottal articulation.
A naturalistic listening approach was used to code the syllables. This procedure has been used in previous studies (Patten et al, 2014; Ramsdell, Oller, Buder, Ethington, & Chorna, 2012) that have shown it to be a reliable technique for identifying canonical versus non-canonical syllables when compared to phonetic transcription with repeated reviews of audio recordings. The naturalistic listening approach is designed to have laboratory coders listen in a way similar to the way the caregiver would listen to their child, hearing each utterance just once.
The videotapes were randomly ordered and coded by a certified speech-language pathologist, the first author of this study, blind to participants’ group status. The first author was experienced with this particular coding scheme, having been trained by Oller and having coded for Patten et al. (2014). The first author trained an undergraduate research assistant studying speech and hearing sciences and ASD to code vocalizations. This training was based on home videos separate from the FXS project videos. In the first phase of the training, the research assistant and first author achieved 100% agreement on three training videos regarding whether or not the infant was in the canonical babbling stage (i.e., exceeded the .15 criterion), and agreed at least 80% of the time on the occurrence of non-canonical syllables and canonical syllables. Then in a second phase, the research assistant coded a random sample of about 20% of the actual study video segments (i.e., 10 five-minute segments). The agreement of the reliability coder with the primary coder was checked after each reliability video was coded. The agreement for coded videos was computed as (a) the percentage of video segments for which the coders agreed that the infant was or was not in the canonical babbling stage (with the goal being an agreement of 90% or higher), and (b) the intraclass correlation coefficients (ICCs) for volubility and frequency of canonical syllables (with the goal of ICCs of .80 or higher). The percentage of video segments for which the coders agreed the infant was in the canonical babbling stage was 90% (9/10). ICCs were .94 and .89 for volubility and frequency of canonical syllables, respectively.
Data Analysis Strategy
Given the small sample sizes, unequal variances, and non-normally distributed data (i.e., skewness greater than 1.0) for canonical babbling ratio and volubility, exact tests of non-parametric statistics were warranted. Groups were compared on maternal education (Wilcoxon S = 87, exact p ≤ .4415), race (Fisher’s exact test p ≤ 1.00), and sex (Fisher’s Exact Test, p ≤ .61), with non-significant differences on these variables. Data were analyzed using SPSS 21 and SAS 9.4.
Results
Likelihood of Meeting .15 Criterion by 9 – 12 Months
For our participants, eight of 14 (57%) infants with TD met the criterion for being in the canonical babbling stage at 9 – 12 months, whereas 0 of 10 (0%) infants with FXS did so. According to an exact Pearson chi-square test, there was a significant between-group difference, Pearson χ2 (1) = 8.57, exact p < .0044, with infants with FXS less likely to be in the canonical babbling stage at 9 – 12 months than infants with TD. It is important to note that the data for both groups of infants on canonical syllables was coded in such a way as to include both words, which can be composed of either canonical or non-canonical syllables in infancy, and non-words (babbling), which can similarly be composed of canonical or non-canonical syllables. At this age, of course none of the infants in either group produced more than a very small number of words.
Between Group Test of Canonical Babbling Ratios
The median canonical babbling ratio was .03 for all infants with FXS (range = 0 – .13) and .16 for all infants with TD (range = 0 – .27). An exact Wilcoxon rank sum test was used to test for a between group canonical babbling ratio difference. The test was statistically significant (exact Wilcoxon test S = 86, exact p ≤ .0164) with infants with FXS producing lower canonical babbling ratios. See Figure 1a for between-group canonical babbling ratios by participant.
Figure 1a.
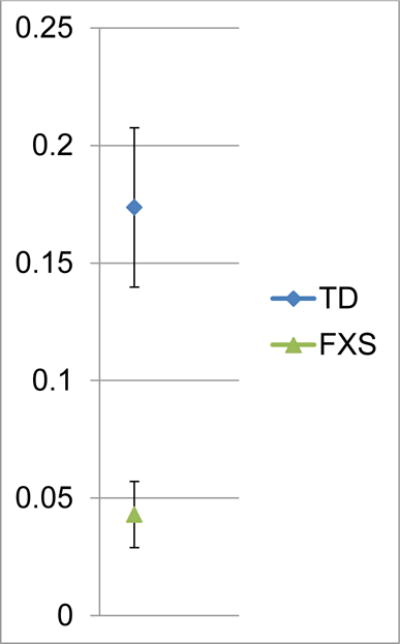
Group canonical babbling ratios by participant across 10-minute sample. Error bars represent standard error.
Between Group Differences in Volubility (total vocalizations)
The median volubility totals (i.e., number of syllables) across the 10-minute samples were 31.5 for all infants with FXS (range = 4 – 59) and 54.0 for all infants with TD (range = 24 – 82). An exact Wilcoxon rank sum test showed that infants with FXS had lower volubility than infants with TD (Wilcoxon S = 82, exact p ≤ .0102). See Figure 1b for participant volubility totals.
Figure 1b.
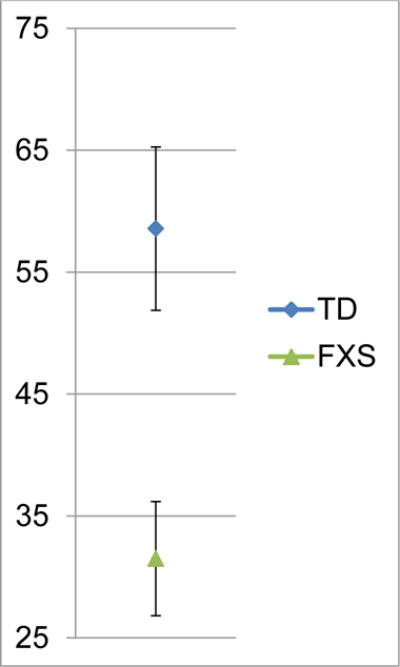
Group volubility across 10-minute samples. Error bars represent standard errors.
Predicting Group Membership With Volubility and Canonical Babbling Status
Because the overall sample for this project was small and unbalanced, an exact conditional logistic regression (Hirji, Mehta, & Patel, 1987; Mehta and Patel, 1995) was performed to determine whether the variables (canonical babbling ratio of .15 or higher and total volubility) could predict group membership. In the instances of small and unbalanced samples, where the asymptotic properties of maximum likelihood-based estimators and inferential methods are likely to fail, exact logistic regression can still provide valid parameter estimates and statistical tests (Stokes, Davis, and Koch, 1995). The canonical babbling data had a quasi-separation of data points since canonical babbling status perfectly predicted membership in the FXS group, but not the TD group (see Table 4). Results from the exact logistic regression tests suggest that (1) as total volubility increased, the predicted likelihood of FXS decreased by a factor of -.09 logits per unit increase in total volubility (conditional score test = 6.24; exact p ≤ .049); and (2) as infants went from not being in the canonical babbling stage to being in the canonical babbling stage at 9 – 12 months, the predicted likelihood of being in the FXS group decreased by a factor of -2.1 logits (conditional score test = 4.59; exact p ≤ .028). Total volubility was a stronger predictor of group than canonical babbling status.
Table 4.
Exact Logistic Regression predicting membership in the FXS group from canonical babbling status and total volubility.
| Parameter Estimate | 95% Confidence Intervals | Score Statistic | Two-sided p-value | ||
|---|---|---|---|---|---|
| Canonical babbling status | −2.12 | −Infinity | −.33 | 6.24 | .05 |
| Total volubility | −.09 | −.19 | −.01 | 4.59 | .03* |
Note.
p < .05.
p < .01.
p < .001.
Discussion
This study examined the potential of early vocalization measures, i.e., canonical babbling and volubility, to contribute to the set of behavioral indicators that could alert parents and physicians to the need for a neurodevelopmental assessment and earlier referral for services. The usefulness of canonical babbling as a behavioral indicator is of particular interest because previous research has demonstrated that parents readily recognize when their infant makes the transition to canonical babbling (Oller, Eilers, & Basinger, 2001), and thus most parents can reliably report to the child’s primary care provider whether the infant is using canonical syllables regularly.
Results from this study support the potential utility of canonical babbling status and total volubility at 9 – 12 months as predictors FXS, a possibility that has also been shown by Patten et al. (2014) for infants with ASD. Given that trained coders were used in the current study, further research will need to be conducted on the reliability of parent identification of canonical babbling absence of canonical babbling and low levels of volubility in infants to further support the use of these measures as early indicators of potential problems with language development. The fact that the canonical babbling and volubility measures can both be obtained with good coder agreement after real-time coding suggests the possibility of a relatively inexpensive clinical measure.
In the current study, infants with FXS were significantly less likely to be in the canonical babbling stage at 9 – 12 months than their TD peers; further, the magnitude of the differences at 9 – 12 months, and the fact that none of the 10 infants with FXS met criterion for being in the canonical babbling stage (i.e., none exceeded the .15 canonical babbling ratio criterion), suggest this could be an important surveillance question for physicians to ask parents when seeing infants in this age range.
However, six of the infants with TD also did not meet the criterion for the canonical babbling stage at 9 – 12 months. This failure to reach the criterion could be thought to indicate the infants were not well-selected as being typically developing since prior research has reported that typically developing infants reach this stage between 5 – 10 months (Eilers and Oller, 1994). But there appear to exist other reasons that the canonical babbling ratios were low in the present research. First, the samples were very different from those of most previous studies. They were considerably shorter than those used in prior research and they were not collected in a controlled laboratory environment, where parents are typically instructed to engage in vocal interaction or at least to remain near the infant during the recording. These laboratory circumstances appear to encourage the production of canonical syllables by infants. Consequently, it is not surprising that some of the TD infants failed to reach the criterion of .15 canonical babbling ratio. Indeed, several of the TD infants in the Patten et al. study (also based on home movies) similarly failed to reach the .15 criterion, even though there is every reason to expect they were in the canonical stage. Even prior research based on laboratory samples has illustrated that infants reported to be in the canonical stage by their parents often fail to meet the .15 criterion (Lewedag, 1995). Factors influencing the ratios that are actually obtained include infant age (infants near 12 months are more likely to meet the criterion than those at nine months for example) and length of sample (in recordings of more than an hour in duration one can target highest periods of volubility, and then the likelihood of exceeding the criterion is higher; Molemans, 2011; Molemans, Van den Berg, Van Severen, & Gillis, 2011).
It has been argued (Oller, 2000) that canonical babbling is a measure where parent opinion is particularly important because parents are with their infants much more than laboratory staff, and thus parents do not have to rely on short-term sampling in making a judgment of canonical babbling status. In fact, research has shown that parents are usually very accurate in indicating canonical babbling status (Oller, Wieman, Doyle, & Ross, 1975; Oller, Eilers, & Basinger, 2001), as indicated by agreement with laboratory-based judgments. Furthermore, Papoušek (1994) has reported that parents often begin intuitively instructing their infants on word production right at the onset of canonical babbling. Thus, parents appear to be aware of the onset of canonical babbling in their infants when it occurs because that is a point at which it becomes possible to begin instructing the infant about the possible meanings of words that must be pronounced with canonical syllables (e.g., we once heard a parent say to an infant who had just entered the canonical stage and had produced a long [bababa…] sequence, “baba, yes, baba, you mean bubble”). Given these facts, parents have been found to be extremely important informants about canonical babbling. We reason that the recognition of canonical babbling status by parents may enhance the potential for its use in clinical settings.
The results for our study suggest a robust difference between canonical babbling in FXS and TD infants, given that over 50% of TD infants met the .15 criterion for being in the canonical babbling stage in these 10-minute home video samples, whereas none of the infants with FXS did. In addition, the separation in median canonical babbling ratios between the two groups (.03 for infants with FXS compared to .16 for TD infants) further emphasizes the marked difference between the groups. This delay in canonical babbling for infants with FXS appears similar to that found in infants later diagnosed with ASD (Patten et al., 2014). Importantly, the current study explicitly excluded infants with FXS who later exhibited symptoms consistent with a diagnosis of ASD.
In our study, we also found infants with FXS had lower volubility than TD infants, again similar to findings for infants with ASD (Patten et al. 2014). The lower volubility may be the result of a lower endogenous tendency to vocalize than in typically developing infants, impaired social skills, reduced parent input, or depressed transactional communication processes operating between infant and adult. Both FXS and ASD are associated with pragmatic and language deficits, but with potentially different underlying reasons (among the possibilities are increased social anxiety in children with FXS without ASD versus reduced social motivation in children with ASD; Hagerman, 2002; Chevallier, Kohls, Troiani, Brodkin & Schultz, 2012). The extent to which such differences between the groups are present in infancy remains unknown. Of course, social impairment may affect the amount of infant babbling in a cyclic fashion. Since the frequency of infant vocalizations is strongly associated with frequency of parent responses (Goldstein et al., 2003; Gros-Louis et al., 2006; Gilkerson & Richards, 2009; Warlaumont et al., 2014), infants with low volubility may receive reduced language input. The reduced input may in turn decrease infant babbling. Thus, social impairments inherent in the infant and/or social transactions between infant and caregiver may negatively impact babbling. The current research did not address the interaction between parent and child, nor its role in infant volubility, but these factors will be important to understand to further assess the utility of volubility as an indicator of potential problems with early language development.
One important goal is for children with neurodevelopmental disorders to receive earlier intervention, especially since speech and language therapy has the potential to alter the extent of the communication delays later in life for individuals with disorders (Dawson et al., 2010). In order to receive earlier intervention, a child must exhibit a developmental delay, most often first identified by a parent or caregiver. Thus, it is critical to find a measure parents can use to identify potential delays without having to wait until a child fails to develop single words or phrases so they can report early concerns to their pediatrician and be directed to appropriate services. The present study’s results contribute to the evidence suggesting that a child’s canonical babbling and volubility may provide reliable and easy ways to identify children at-risk for communication disorders, including those associated with FXS and ASD. This information combined with previous research indicating parents are able to identify when their child is in the canonical babbling stage (Lewedag, 1995; Oller, Eilers, Neal, & Schwartz, 1999) suggests more parent and physician education and awareness of this stage are warranted. The hope is that with greater awareness, parents and physicians may be able to notice a potential delay earlier and seek clinical evaluation services to determine whether the child qualifies for early intervention.
Limitations
Our home videos offered multiple advantages, including a sample of children who had a confirmed diagnosis of FXS, ability to observe children as infants, well before they were diagnosed, and data from natural environments where infants presumably vocalized more representatively (Lewedag, Oller, & Lynch, 1994). However, home videos have drawbacks including potentially poor video/audio quality, limited availability of footage in a given age range, and lack of experimental control (Palomo, Belinchón, & Ozonoff, 2006; Ozonoff, Iosif, Young, Hepburn, Thomson & Colombi, 2011; Marschik & Einspieler, 2011). In the present study, all of these limitations were involved. The videos cannot be expected to be fully representative of the infants’ language development, given that we only examined 10 minutes of vocal behavior, and consequently, we expected greater variability in canonical babbling ratios compared to studies with longer sampling periods (Molemans, 2011).
There was a between-group difference in mean total events with the children with FXS having fewer total events. The lower number of total events was not a likely influential confound given the lack of differences on the other contextual variables (e.g., number of people present, amount of restriction) that characterized the nature of the events. The mean age of infants in the FXS group was higher, though not statically significant, compared to the infants with TD. While older age can be expected to have given children with FXS an advantage in the development of canonical babbling and volubility, no such advantage was evident in the data. Thus, it is unlikely these differences biased our findings related to canonical babbling or volubility.
Another limitation of this study was the small sample sizes. Although we could have included infants with comorbid FXS and ASD to increase sample size (and be more representative of the total population of infants with FXS), the role of low volubility and delayed transition to the canonical babbling stage has already been demonstrated for ASD (Patten et al., 2014). Thus, our primary interest here was in examining the vocal patterns in a sample of infants with FXS but without ASD. Given differences in FXS presentation in males and females, it may be important to assess early vocal development for both sexes. Although we included a small number of females in the samples, sex influences could not be usefully assessed given the small numbers.
Future Directions
In order to test the utility of canonical babbling and volubility as behavioral indicators, a logical next step is to conduct a larger sample retrospective video analysis or longitudinal study. Molemans (2011) has convincingly demonstrated the importance of larger samples to assess vocal development reliably. The challenges will be developing new ways to identify these children early in order to acquire data during the first and second year of life.
Given the evidence that canonical babbling and volubility are disrupted in ASD and FXS, it will be important to study vocal development in comorbid FXS and ASD, where there may be additive or multiplicative effects. FXS may be the most common genetic cause of ASD (Hagerman and Hagerman, 2002) and the communication deficits associated with the disorders are similar, although among older children with FXS, those with comorbid ASD have more severe communication deficits than those with FXS alone, especially in pragmatic language functioning (Estigarribia, Martin, Roberts, Spencer, Gucwa & Sideris, 2011; Martin, Losh, Estigarribia, Sideris & Roberts, 2013). Additionally, using a group of infants with Down syndrome as a comparison group would provide more information regarding whether findings on FXS reflect a profile associated with intellectual disability in general. To further control for whether the findings are associated with overall IQ, future studies may profit from matching on mental age or a mental age proxy to try to disentangle vocal and cognitive impairments.
While studies have addressed the utility of parent report on canonical babbling in both TD infants and infants deemed at-risk for disorders due to premature birth or low SES, there have not been specific studies on the value of parent report with infants who have neurodevelopmental disorders. A challenge for the future will be to identify infants with neurodevelopmental disorders in the first year so the value of parent report can be evaluated. Family studies on risk of FXS as well as direct genetic testing of infants at birth may provide the best methods of obtaining a first-year-of-life sample to follow-up in longitudinal vocal development research.
There is little known about early relations of vocal development and other motoric milestones in infants with FXS. Examining babbling in the context of important motor milestones, such as postural stability and onset of walking may also provide insight into the relationship between motor and language systems (Iverson, 2010; Walle & Campos, 2014).
Finally, it is a logical next step to examine the environment’s role in canonical babbling. Results from one previous study support the idea that parents of children with TD intuitively begin advancing their language input to foster word-learning when their infant begins canonical babbling (Papoušek, 1994). We can only speculate whether the same is true for parents of children with neurodevelopmental disabilities. Available literature on maternal responsivity with older children with FXS and ASD suggests frequency and contingency of maternal responses influences a child’s language development (Warren, Brady, Sterling, Fleming, & Marquis, 2010; Sterling, Warren, Brady, & Fleming, 2013; Yoder, Watson, & Lambert, 2015), supporting the potential importance of studying parental responses to canonical babbling in infants with neurodevelopmental disorders. Further, testing interventions designed to elicit more canonical babbling, similar to a treatment that has been used with a sample of children with intellectual disability (Woynaroski, Yoder, Fey, & Warren, 2014), could further inform early treatments to promote language learning in FXS.
Acknowledgments
This research was made possible by family participation and research funding. The grants supporting this research include: The National Institute for Child Health and Human Development (R01-HD42168), The Cure Autism Now Foundation (Sensory-Motor and Social-Communicative Symptoms of Autism in Infancy); The National Institute of Child Health and Human Development (P30-HD003110), The National Institute of Mental Health (R01-MH090194, F31-MH095318), and The Office of Special Education Programs, U.S. Department of Education (H324C990042). We are grateful for the family participation and funding.
Footnotes
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: Informed consent was obtained from all individual participants included in the study.
Contributor Information
Katie Belardi, Department of Allied Health Sciences, The University of North Carolina - Chapel Hill, Bondurant Hall, CB #7190, Chapel Hill, NC 27599-7190, USA.
Linda R. Watson, Department of Allied Health Sciences, The University of North Carolina - Chapel Hill, Bondurant Hall, CB #7190, Chapel Hill, NC 27599-7190, USA
Richard A. Faldowski, Richard A. Faldowski, Department of Allied Health Sciences, University of North Carolina - Chapel Hill, CB# 7120, Chapel Hill, NC 27599-7120, USA
Heather Hazlett, The Carolina Institute for Developmental Disabilities, The University of North Carolina - Chapel Hill, CB #7255, Chapel Hill, NC 27599-7255.
Elizabeth Crais, Department of Allied Health Sciences, The University of North Carolina - Chapel Hill, Bondurant Hall, CB #7190, Chapel Hill, NC 27599-7190, USA.
Grace T. Baranek, Department of Allied Health Sciences, The University of North Carolina - Chapel Hill, Bondurant Hall, CB #7122, Chapel Hill, NC 27599-7122, USA
Cara McComish, Department of Allied Health Sciences, The University of North Carolina - Chapel Hill, Bondurant Hall, CB #7190, Chapel Hill, NC 27599-7190, USA.
Elena Patten, Department of Audiology and Speech Pathology, University of Tennessee Health Science Center, 434 South Stadium Hall, Knoxville, TN 37996, USA.
D. Kimbrough Oller, The University of Memphis, 807 Jefferson Avenue, Memphis, TN 28105, USAKonrad Lorenz Institute for Evolution and Cognition Research, Klosterneuburg, Austria.
References
- Abbeduto L, Hagerman RJ. Language and communication in fragile X syndrome. Mental Retardation and Developmental Disabilities Research Reviews. 1997;3(4):313–322. [Google Scholar]
- Abbeduto L, Murphy MM, Cawthon SW, Richmond EK, Weissman MD, Karadottir S, O’Brien A. Receptive language skills of adolescents and young adults with Down or fragile X syndrome. American Journal on Mental Retardation. 2003;108(3):149–160. doi: 10.1352/0895-8017(2003)108<0149:RLSOAA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Bailey DB, Jr, Hatton DD, Skinner M. Early developmental trajectories of males with fragile X syndrome. American Journal on Mental Retardation. 1998;103(1):29–39. doi: 10.1352/0895-8017(1998)103<0029:EDTOMW>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Bailey DB, Raspa M, Bishop E, Holiday D. No change in the age of diagnosis for fragile X syndrome: Findings from a national parent survey. Pediatrics. 2009;124(2):527–533. doi: 10.1542/peds.2008-2992. [DOI] [PubMed] [Google Scholar]
- Baranek GT. Autism during infancy: A retrospective video analysis of sensory-motor and social behaviors at 9–12 months of age. Journal of Autism and Developmental Disorders. 1999;29(3):213–224. doi: 10.1023/a:1023080005650. [DOI] [PubMed] [Google Scholar]
- Bleile KM, Mcgowan JS, Bernthal JE. Professional judgments about the relationship between speech and intelligence in African American preschoolers. Journal of Communication Disorders. 1997;30(5):367–383. doi: 10.1016/s0021-9924(96)00109-8. [DOI] [PubMed] [Google Scholar]
- Brady N, Skinner D, Roberts J, Hennon E. Communication in young children with fragile X syndrome: A qualitative study of mothers’ perspectives. American Journal of Speech-Language Pathology. 2006;15(4):353–364. doi: 10.1044/1058-0360(2006/033). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buder EH, Warlaumont AS, Oller DK. An acoustic phonetic catalog of prespeech vocalizations from a developmental perspective. In: Peter Beate, MacLeod Andrea AN., editors. Comprehensive perspectives on child speech development and disorders: Pathways from linguistic theory to clinical practice. Hauppauge, NY: NOVA; 2013. [Google Scholar]
- Chapman K, Hardin-Jones M, Schulte J, Halter K. Vocal development of 9 months-old babies with cleft palate. Journal of Speech, Language and Hearing Research. 2001;44:1268–1283. doi: 10.1044/1092-4388(2001/099). [DOI] [PubMed] [Google Scholar]
- Chevallier C, Kohls G, Troiani V, Brodkin ES, Schultz RT. The social motivation theory of autism. Trends in Cognitive Sciences. 2012;16(4):231–239. doi: 10.1016/j.tics.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobo-Lewis AB, Oller DK, Lynch MP, Levine SL. Relations of motor and vocal milestones in typically developing infants and infants with Down syndrome. American Journal of Mental Retardation. 1996;100:456–467. [PubMed] [Google Scholar]
- Cohen J. A coefficient of agreement for nominal scales. Educational and Psychological Measurement. 1960;20:37–46. [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2nd. Hillsdale, NJ: Lawrence Earlbaum Associates; 1988. [Google Scholar]
- Dawson G, Rogers S, Munson J, Smith M, Winter J, Greenson J, Varley J. Randomized, controlled trial of an intervention for toddlers with autism: the Early Start Denver Model. Pediatrics. 2010;125(1):e17–e23. doi: 10.1542/peds.2009-0958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eilers RE, Oller DK. Infant vocalizations and the early diagnosis of severe hearing impairment. Journal of Pediatrics. 1994;124:199–203. doi: 10.1016/s0022-3476(94)70303-5. [DOI] [PubMed] [Google Scholar]
- Eilers RE, Oller DK, Levine S, Basinger D, Lynch MP, Urbano R. The role of prematurity and socioeconomic status in the onset of canonical babbling in infants. Infant Behavior and Development. 1993;16:297–315. [Google Scholar]
- Fenson L, Pethick S, Renda C, Cox JL, Dale PS, Reznick JS. Short-form versions of the MacArthur Communicative Development Inventories. Applied Psycholinguistics. 2000;21:95–116. [Google Scholar]
- Finestack LH, Abbeduto L. Expressive language profiles of verbally expressive adolescents and young adults with Down syndrome or fragile X syndrome. Journal of Speech, Language, and Hearing Research. 2010;53(5):1334–1348. doi: 10.1044/1092-4388(2010/09-0125). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girolametto LE. Improving the social conversational skills of developmentally delayed children: An intervention study. Journal of Speech and Hearing Disorders. 1988;53:156–167. doi: 10.1044/jshd.5302.156. [DOI] [PubMed] [Google Scholar]
- Greenough WT, Klintsova AY, Irwin SA, Galvez R, Bates KE, Weiler IJ. Synaptic regulation of protein synthesis and the fragile X protein. Proceedings of National Academy Science USA. 2001;98(13):7101–7106. doi: 10.1073/pnas.141145998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagerman RJ, Hagerman PJ. Fragile X syndrome: Diagnosis, treatment, and research. Taylor & Francis; US: 2002. [Google Scholar]
- Hanson DM, Jackson AW, Hagerman RJ, Opitz JM, Reynolds JF. Speech disturbances (cluttering) in mildly impaired males with the Martin-Bell/fragile X syndrome. American Journal of Medical Genetics. 1986;23(1–2):195–206. doi: 10.1002/ajmg.1320230114. [DOI] [PubMed] [Google Scholar]
- Hart B, Risley TR. Meaningful differences in the everyday experience of young American children. Paul H Brookes Publishing; 1995. [Google Scholar]
- Hinton R, Budimirovic DB, Marschik PB, Talisa VB, Einspieler C, Gipson T, Johnston MV. Parental reports on early language and motor milestones in fragile X syndrome with and without autism spectrum disorders. Developmental Neurorehabilitation. 2013;16(1):58–66. doi: 10.3109/17518423.2012.704414. [DOI] [PubMed] [Google Scholar]
- Hirji KF, Mehta CR, Patel NR. Computing distributions for exact logistic regression. Journal of the American Statistical Association. 1987;82(400):1110–1117. [Google Scholar]
- Iverson JM. Developing language in a developing body: The relationship between motor development and language development. Journal of Child Language. 2010;37(02):229–261. doi: 10.1017/S0305000909990432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer S, Lynch MP, Oller DK. Prelinguistic vocal development in infants with typical hearing and infants with severe-to-profound hearing loss. Volta Review. 2008;108:115–138. [PMC free article] [PubMed] [Google Scholar]
- Iyer S, Oller DK, Neal R. On the robustness of vocal development: an examination of infants with moderate-to-severe hearing loss and additional risk factors. Journal of Speech, Language, and Hearing Research. 2007;50:1425–1444. doi: 10.1044/1092-4388(2007/099). [DOI] [PubMed] [Google Scholar]
- Jacquemont S, Berry-Kravis E, Hagerman R, Von Raison F, Gasparini F, Apostol G, Gomez-Mancilla B. The challenges of clinical trials in fragile X syndrome. Psychopharmacology. 2014;231(6):1237–1250. doi: 10.1007/s00213-013-3289-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent RD, Osberger MJ, Netsell R, Hustedde CG. Phonetic development in identical twins differing in auditory function. Journal of Speech and Hearing Disorders. 1987;52(1):64–75. doi: 10.1044/jshd.5201.64. [DOI] [PubMed] [Google Scholar]
- Koopmans-van Beinum FJ, van der Stelt JM. Early stages in the development of speech movements. Precursors of Early Speech. 1986:37–50. [Google Scholar]
- Lewedag VL, Oller DK, Lynch MP. Infants’ vocalization patterns across home and laboratory environments. First Language. 1994;14(42–43):49–65. [Google Scholar]
- Lewedag VL. Doctoral dissertation. University of Miami; Coral Gables, FL: 1995. Patterns of onset of canonical babbling among typically developing infants. [Google Scholar]
- Locke JL. Parental selection of vocal behavior. Human Nature. 2006;17(2):155–168. doi: 10.1007/s12110-006-1015-x. [DOI] [PubMed] [Google Scholar]
- Locke JL, Pearson DM. Linguistic significance of babbling: evidence from a tracheostomized infant. Journal of Child Language. 1990;17(01):1–16. doi: 10.1017/s0305000900013076. [DOI] [PubMed] [Google Scholar]
- Loesch DZ, Huggins RM, Hagerman RJ. Phenotypic variation and FMRP levels in fragile X. Mental Retardation and Developmental Disabilities Research Reviews. 2004;10(1):31–41. doi: 10.1002/mrdd.20006. [DOI] [PubMed] [Google Scholar]
- Lynch MP, Oller DK, Steffens ML, Buder EH. Phrasing in prelinguistic vocalizations. Developmental Psychobiology. 1995;28(1):3–25. doi: 10.1002/dev.420280103. [DOI] [PubMed] [Google Scholar]
- Lynch MP, Oller DK, Steffens ML, Levine SL, Basinger DL, Umbel VM. Development of speech-like vocalizations in infants with Down syndrome. American Journal of Mental Retardation. 1995;100(1):68–86. [PubMed] [Google Scholar]
- Maes B, Fryns JP, Ghesquière P, Borghgraef M. Phenotypic checklist to screen for fragile X syndrome in people with mental retardation. Mental Retardation. 2000;38(3):207–215. doi: 10.1352/0047-6765(2000)038<0207:PCTSFF>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Marschik PB, Bartl-Pokorny KD, Sigafoos J, Urlesberger L, Pokorny F, Didden R, Kaufmann WE. Development of socio-communicative skills in 9- to 12-month-old individuals with fragile X syndrome. Research in Developmental Disabilities. 2014;35(3):597–602. doi: 10.1016/j.ridd.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marschik PB, Einspieler C. Methodological note: Video analysis of the early development of Rett syndrome—one method for many disciplines. Developmental Neurorehabilitation. 2011;14(6):355–357. doi: 10.3109/17518423.2011.604355. [DOI] [PubMed] [Google Scholar]
- Martin GE, Losh M, Estigarribia B, Sideris J, Roberts J. Longitudinal profiles of expressive vocabulary, syntax and pragmatic language in boys with fragile X syndrome or Down syndrome. International Journal of Language & Communication Disorders. 2013;48(4):432–443. doi: 10.1111/1460-6984.12019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masataka N. Why early linguistic milestones are delayed in children with Williams syndrome: late onset of hand banging as a possible rate-limiting constraint on the emergence of canonical babbling. Developmental Science. 2001;4(2):158–164. [Google Scholar]
- Mehta CR, Patel NR. Exact logistic regression: theory and examples. Statistics in Medicine. 1995;14(19):2143–2160. doi: 10.1002/sim.4780141908. [DOI] [PubMed] [Google Scholar]
- Molemans I. Sounds like babbling: A longitudinal investigation of aspects of the prelexical speech repertoire in young children acquiring Dutch: Normally hearing children and hearing impaired children with a cochlear implant (PhD) University of Antwerp; Antwerp, Belgium: 2011. [Google Scholar]
- Molemans I, Van den Berg R, Van Severen L, Gillis S. How to measure the onset of babbling reliably. Journal of Child Language. 2011;39(03):523–552. doi: 10.1017/S0305000911000171. [DOI] [PubMed] [Google Scholar]
- Nathani S, Ertmer DJ, Stark RE. Assessing vocal development in infants and toddlers. Clinical Linguistics and Phonetics. 2006;20(5):351–367. doi: 10.1080/02699200500211451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathani S, Oller DK, Neal AR. On the robustness of vocal development: an examination of infants with moderate-to-severe hearing loss and additional risk factors. Journal of Speech, Language, and Hearing Research. 2007;50:1425–44. doi: 10.1044/1092-4388(2007/099). [DOI] [PubMed] [Google Scholar]
- National Center for Infants, Toddlers, and Families: Zero to Three. Brain Development. 2012 Retrieved from: http://www.zerotothree.org/child-development/brain-development/
- Newborg J, Stock JR, Wnek L, Guidubaldi J, Svinicki J. Battelle developmental inventory. Allen, Tex: DLM; 1988. [Google Scholar]
- Oller DK. The emergence of the sounds of speech in infancy. In: Yeni-Komshian G, Kavanagh J, Ferguson C, editors. Child phonology, Vol. 1: Production. New York: Academic Press; 1980. pp. 93–112. [Google Scholar]
- Oller DK, Eilers RE, Basinger D, Steffens ML, Urbano R. Extreme poverty and the development of precursors to the speech capacity. First Language. 1995;15:167–188. [Google Scholar]
- Oller DK, Eilers RE, Basinger D. Intuitive identification of infant vocal sounds by parents. Developmental Science. 2001;4:49–60. [Google Scholar]
- Oller DK, Griebel U, editors. Integrative and Comparative Biology, icp087. 2008. Review of the book Evolution of Communicative Flexibility: Complexity, Creativity, and Adaptability in Human and Animal Communication by C.T. Miller & M. Osmanski. [Google Scholar]
- Oller DK, Niyogi P, Gray S, Richards JA, Gilkerson J, Xu D, Warren SF. Automated vocal analysis of naturalistic recordings from children with autism, language delay, and typical development. Proceedings of the National Academy of Sciences. 2010;107(30):13354–13359. doi: 10.1073/pnas.1003882107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oller K, Wieman L, Doyle W, Ross C. Infant babbling and speech. Journal of Child Language. 1975;3:1–11. [Google Scholar]
- Ozonoff S, Iosif AM, Young GS, Hepburn S, Thompson M, Colombi C, Rogers SJ. Onset patterns in autism: correspondence between home video and parent report. Journal of the American Academy of Child & Adolescent Psychiatry. 2011;50(8):796–806. doi: 10.1016/j.jaac.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palomo R, Belinchón M, Ozonoff S. Autism and family home movies: a comprehensive review. Journal of Developmental & Behavioral Pediatrics. 2006;27(2):S59–S68. doi: 10.1097/00004703-200604002-00003. [DOI] [PubMed] [Google Scholar]
- Papoušek M. Vom ersten Schrei zum ersten Wort: Anfänge der Sprachentwickelung in der vorsprachlichen Kommunikation. Bern: Verlag Hans Huber; 1994. [Google Scholar]
- Patten E, Belardi K, Baranek GT, Watson LR, Labban JD, Oller DK. Vocal patterns in infants with autism spectrum disorder: canonical babbling status and vocalization frequency. Journal of Autism and Developmental Disorders. 2014;44(10):2413–2428. doi: 10.1007/s10803-014-2047-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul R, Fuerst Y, Ramsay G, Chawarska K, Klin A. Out of the mouths of babes: Vocal production in infant siblings of children with ASD. Journal of Child Psychology and Psychiatry. 2011;52(5):588–598. doi: 10.1111/j.1469-7610.2010.02332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon KK, Watson LR, Baranek GT, Poe MD. To what extent do joint attention, imitation, and object play behaviors in infancy predict later communication and intellectual functioning in ASD? Journal of Autism and Developmental Disorders. 2012;42(6):1064–1074. doi: 10.1007/s10803-011-1349-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsdell HL, Oller DK, Buder EH, Ethington CA, Chorna L. Identification of prelinguistic phonological categories. Journal of Speech, Language, and Hearing Research. 2012;55(6):1626–1639. doi: 10.1044/1092-4388(2012/11-0250). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JE, Mankowski JB, Sideris J, Goldman BD, Hatton DD, Mirrett PL, Bailey DB. Trajectories and predictors of the development of very young boys with fragile X syndrome. Journal of Pediatric Psychology. 2009;34(8):827–836. doi: 10.1093/jpepsy/jsn129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schopler E, Reichler RJ, Renner BR. Child Autism Rating Scale. Western Psychological Services Corporation; 1988. [Google Scholar]
- Schopmeyer BB, Lowe F. Speech and language characteristics in fragile X syndrome. The Fragile X Child. 1992:71–90. [Google Scholar]
- Sigafoos J, Arthur-Kelly M, Butterfield N. Enhancing everyday communication with children with disabilities. Baltimore: Paul H Brookes Publishing Company; 2006. [Google Scholar]
- Snow CE. Issues in the study of input: Fine-tuning universality, individual and developmental differences and necessary causes. In: MacWhinney B, Fletcher P, editors. NETwerken: Bijdragen van het vijfde NET symposium. Antwerp: University of Antwerp; 1995. pp. 5–17. (Antwerp Papers in Linguistics 74). [Google Scholar]
- Sparrow SS, Balla DA, Cicchetti DV. Vineland adaptive behavior scales: Interview edition, survey form manual. Circle Pines, MN: American Guidance Service; 1984. [Google Scholar]
- Stark RE. Stages of speech development in the first year of life. In: Yeni-Komshian G, Kavanagh J, Ferguson C, editors. Child Phonology. Vol. 1. New York: Academic Press; 1980. pp. 73–90. [Google Scholar]
- Sterling AM, Warren SF, Brady N, Fleming K. Influences on maternal responsivity in mothers of children with fragile X syndrome. American Journal on Intellectual and Developmental Disabilities. 2013;118(4):310–326. doi: 10.1352/1944-7558-188.4.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoel-Gammon C. Prespeech and early speech development of two late talkers. First Language. 1989;9(6):207–223. [Google Scholar]
- Stoel-Gammon C. Prelinguistic vocal development: Measurement and prediction. In: Ferguson C, Menn L, Stoel-Gammon C, editors. Phonological development: Models, Research, Implications. Parkton, MD: York Press Inc; 1992. pp. 439–456. [Google Scholar]
- Stoel-Gammon C, Otomo K. Babbling development of hearing impaired and normally hearing subjects. Journal of Speech and Hearing Disorders. 1986;51:33–41. doi: 10.1044/jshd.5101.33. [DOI] [PubMed] [Google Scholar]
- Stokes ME, Davis CS, Koch GG. Categorical analysis using the SAS system. SAS Institute Inc; Cary, NC: 1995. [Google Scholar]
- Tassone F. Newborn screening for fragile X syndrome. Journal of the American Medical Association (JAMA) Neurology. 2014;71(3):355–359. doi: 10.1001/jamaneurol.2013.4808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Dikkenberg-Pot I, Koopmans-van Beinum F, Clement C. Influence of lack of auditory speech perception on sound productions of deaf infants. Proceedings of the Institute of Phonetic Sciences Amsterdam. 1998;22:47–60. [Google Scholar]
- Verkerk AJ, Pieretti M, Sutcliffe JS, Fu YH, Kuhl DP, Pizzuti A, Eussen BE. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell. 1991;65(5):905–914. doi: 10.1016/0092-8674(91)90397-h. [DOI] [PubMed] [Google Scholar]
- Walle EA, Campos JJ. Infant language development is related to the acquisition of walking. Developmental Psychology. 2014;50:336–348. doi: 10.1037/a0033238. [DOI] [PubMed] [Google Scholar]
- Warlaumont AS, Richards JA, Gilkerson J, Oller DK. A Social Feedback Loop for Speech Development and Its Reduction in Autism. Psychological Science. 2014;25(7):1314–1324. doi: 10.1177/0956797614531023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren SF, Brady N, Sterling A, Fleming K, Marquis J. Maternal responsivity predicts language development in young children with fragile X syndrome. American Association on Intellectual and Developmental Disabilities. 2010;115(1):54–75. doi: 10.1352/1944-7558-115.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren SF, Gilkerson J, Richards JA, Oller DK, Xu D, Yapanel U, Gray S. What automated vocal analysis reveals about the vocal production and language learning environment of young children with autism. Journal of Autism and Developmental Disorders. 2010;40(5):555–569. doi: 10.1007/s10803-009-0902-5. [DOI] [PubMed] [Google Scholar]
- Watson LR, Crais ER, Baranek GT, Dykstra JR, Wilson KP, Hammer CS, Woods J. Communicative gesture use in infants with and without autism: A retrospective home video study. American Journal of Speech-Language Pathology. 2013;22(1):25–39. doi: 10.1044/1058-0360(2012/11-0145). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiler IJ, Greenough WT. Synaptic synthesis of the Fragile X protein: possible involvement in synapse maturation and elimination. American Journal of Medical Genetics. 1999;83:248–252. doi: 10.1002/(sici)1096-8628(19990402)83:4<248::aid-ajmg3>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Yoder P, Watson LR, Lambert W. Value-added predictors of expressive and receptive language growth in initially nonverbal preschoolers with autism spectrum disorders. Journal of Autism and Developmental Disorders. 2015;45(5):1254–1270. doi: 10.1007/s10803-014-2286-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder P, Woynaroski T, Fey M, Warren S. Effects of dose frequency of early communication intervention in young children with and without Down syndrome. American Journal on Intellectual and Developmental Disabilities. 2014;119(1):17–32. doi: 10.1352/1944-7558-119.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Kaufmann WE, Sigafoos J, Bartl-Pokorny KD, Krieber M, Marschik PB, Einspieler C. Parents’ initial concerns about the development of their children later diagnosed with fragile X syndrome. Journal of Intellectual & Developmental Disability. 2016:1–9. doi: 10.3109/13668250.2016.1228858. [DOI] [PMC free article] [PubMed] [Google Scholar]


