Abstract
The present study investigated the hemodynamics, vascular and extravascular volume expansion induced by infusion of lactated Ringer's solution in children and adults before surgery. This was a prospective randomized double-blind study. A total of 28 patients (14 children and 14 adult patients; American Society of Anesthesiology status I) scheduled for similar minor pelvic, anal rectal or lower limb surgery were recruited for the present study. All patients were administered with 10 ml/kg of lactated Ringer's solution at a constant rate over 20 min. After fluid infusion, plasma dilutions were calculated based on the concentration of hemoglobin. Heart rate (HR), mean arterial pressure (MAP) and urine output were measured before anesthesia was administered for surgery. Results demonstrated that the plasma dilution within 90 min of infusion initiation of lactated Ringer's solution was less pronounced in children compared with adult patients (0.07 vs. 0.16; P<0.001). Children also excreted more of the infused fluid through the kidney within 90 min of infusion initiation than the adults (55% vs. 24%; P=0.01). Following completion of fluid infusion, the volume expansion efficiency was higher in adults [0.82 (0.52–1.00)] than in children [0.46 (0.26–0.68)]. The relative changes in HR were significantly greater in children than in adults 15–60 min after infusion initiation (P<0.01). After 60 min, HRs were comparable between the groups; however, MAP declined significantly from 25–90 min after infusion initiation in children (P<0.05), yet remained nearly constant in adults (P>0.05). Simple regression analysis revealed a positive relationship between the relative changes in MAP and the plasma dilution, and the reduction in MAP in children was able to explain 47% of the variation in plasma dilution (R2=0.47; P=0.007). In conclusion, different hemodynamics and dynamics of fluid shift of Ringer's solution prior to surgery in children and adults may provide anesthesiologists with new information of how to administer fluid treatment for each patient.
Keywords: child, adults, Ringer's solution, fluid shift
Introduction
The purpose of preoperative fluid therapy is to correct plasma volume loss or deficiency induced by fasting, water-deprivation and bowel preparation prior to elective surgery. The clinical data were collected from the First Affiliated Hospital and Children's Hospital, College of Medicine, Zhejiang University (Hangzhou China). Adults were fasted routinely for at least 10 h and children for at least 6 h according to the hospital guidelines for elective surgery. Ringer's solution is a widely used preoperative volume expander for patients in this hospital. Approximately 20% of crystalloid solution infused into the intravascular space will remain in the vasculature system, according to the ‘4:1 rule’ quoted in a surgical textbook (1). A previous study demonstrated that the induction of regional and general anesthesia resulted in an increase of infused fluid remaining in the vascular system, which was observed to be inversely correlated with mean arterial blood pressure (MAP) (2). Co-administration of different catecholamines has been demonstrated to induce different effects on the dynamics of volume expansion of crystalloid infusion (3,4). Research has also indicated that the particular illness in the patient may influence the dynamics of volume expansion (5). Additionally, different fluids present different fluid shift (6). Previous research has demonstrated that plasma clearance was four times higher and the renal clearance of lactated Ringer's solution was seven times higher in children compared with adults, by means of volume kinetics and simulated data (7). However, the fluid shift between vascular and extravascular space, as well as hemodynamics after fluid infusion prior to elective surgery in children and adults remain unexplored. The aim of the present study was to identify the difference between the children and adults in the fluid shift and hemodynamic changes following fluid infusion prior to surgery. Children and adults with American Society of Anesthesiologist (ASA) physical status I and scheduled for elective surgery were recruited. Hemodynamics (such as mean artery blood pressure and heart rate) and dynamics of fluid shift (such as volume expansion, volume expansion efficiency and extra volume expansion) were compared in children and adults, following the use of Ringer's solution prior to surgery. Furthermore, the correlation between the hemodynamics and dynamics of volume expansion and fluid infusion on cardiac oxygen consumption were investigated in the present study. These findings provide an experimental basis for developing effective individual fluid therapy.
Materials and methods
Ethics statement
The present investigation (reference no. 20090105) was approved by the Ethics Committee of The First Affiliated Hospital, College of Medicine, Zhejiang University (Hangzhou, China). All patients and the children's parents were familiar with the protocol of the research and provided signed informed consent. All procedures strictly complied with standard operating procedures and were conducted by an experienced anesthetist.
Subject selection
Between June 2010 and July 2012, a total of 28 ASA physical status I patients that were admitted to the First Affiliated Hospital and Children's Hospital, College of Medicine, Zhejiang University participated in the present study. Patients, including 14 pediatric patients aged between 3 and 6 years and 14 adults aged between 21 to 42 years with body mass index from 18 to 25, were allocated to one of two groups (children or adult) according to age. All participants were scheduled for inguinal hernia, urethral fissure repair or lower limb lump removal under regional anesthesia. Exclusion criteria included contraindication to regional anesthesia and patients with severe heart, lung, liver, kidney or neurological disorders.
Experimental design
The present study was a randomized, double-blind, prospective intervention study. Non-repeated random integers between 1 and 28 were produced using Excel 2007 (Microsoft Corporation, Redmond, WA, USA) and even numbers were assigned to adult participants and odd numbers were assigned to children. A researcher who did not know the protocol in detail recorded the data. Patients who experienced side effects were disregarded and the case was repeated.
Procedure
All children fasted for 6 h and adults fasted for 10 h prior to experiment initiation. All patients were administered with an intramuscular injection of midazolam (batch no. 20031037; Enhua Pharmaceutical Co., Ltd., Jiangsu, China) 0.25 mg.kg−1 and ketamine (batch no. H31022247; Shanghai Chinese and Western Pharmaceutical Co., Ltd., Shanghai, China) 2.5 mg.kg−1 (children) or intravenous (i.v.) injection of midazolam 0.05 mg.kg−1 (adults) before the experiment. After entering the operating theater at 7:30 a.m., a cannula was placed in the cubital vein of the left arm of the patients for infusion of fluid and drug. A radial artery catheter was inserted for taking blood pressure measurements and sampling with the patients in the supine position. After insertion of the urine tube under topical anesthesia and emptying the urine collection container, the i.v. fluid bolus of 10 ml.kg−1 of lactated Ringer's solution (LR; Shanghai Baxter Healthcare Co., Ltd., Shanghai, China), with ionic contents of Na+ 130 mmol/l, K+ 4 mmol/l, Cl− 109 mmol/l and lactate 28 mmol/l, was administered at a constant rate over 20 min by using a macro-infusion pump (ICE 601-1; Class 1; Abbott Laboratory, North Chicago, IL, USA).
Data collection and measurements
Arterial blood samples (1 ml) were collected in heparinized 2-ml syringes every 5 min during the first 50 min after infusion initiation, and every 10 min during the next 40 min to determine hemoglobin (Hb) concentration and hematocrit (Hct) using an i-STAT Portable Clinical Analyzer (i-STAT Corp., East Windsor, NJ, USA) with a coefficient of variation of 1%. The first sample for each patient was measured in duplicate. To prevent any mixture of rinsing solution, a small quantity of blood was drawn and discarded before the collection of each blood sample. Following collection of each sample, 2 ml saline in adults and 0.5–1.0 ml saline in children was injected into the patient to prevent clotting. Parameters of electrocardiography, saturation of pulse oxygen, invasive arterial blood pressure and heart rate (HR) were monitored and recorded using an anesthesia system (Datex-Ohmeda, Hoevelaken, The Netherlands) at the same time as sample collection. Fluid balance data, including the quantity of input fluid and cumulative urine output, were recorded at the end of the experiment. During the study, 100% oxygen (2 l/min) was delivered to all patients through an oxygen mask. When systolic blood pressure decreased by >30% of the pre-study value or systolic blood pressure was <90 mmHg, the patients were considered to be experiencing hypotension. Ephedrine (6 mg) was administered intravenously to treat hypotension and 0.5 mg atropine (i.v.) was administered against bradycardia (<50 beats per min). Regional anesthesia was started following completion of the study (Fig. 1).
Figure 1.
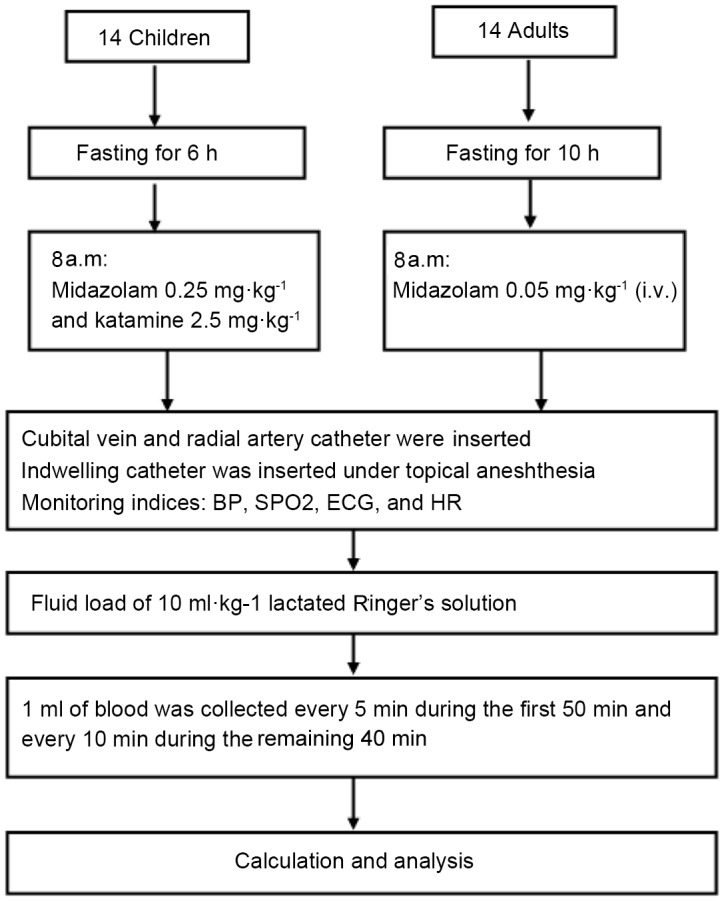
Flow chart of patients included in the study and methods used. BP, blood pressure; ECG, electrocardiogram; HR, heart rate; SPO2, saturation of pulse oxygen.
Calculations
Baseline plasma volume (PV0) was estimated from the height and weight of the patients according to previous research (8) (Formula 1). Hb, Hct, PV0, infused volume (IV) as an indicator of intravascular gain of fluid and urine volume (UV) as an indicator of intravascular loss of fluid were used to calculate plasma dilution (PD; Formula 2), the plasma volume changes at a specific time (PVt; Formula 3), the quantity of infused volume remaining in circulation (volume expansion; VE; Formula 4) and extravascular volume (EVV; Formula 5). Volume expansion efficiency (VEE; Formula 6) was calculated by the change in plasma volume divided by the volume of fluid infused. All volumes were expressed as ml/kg. The rate of urine output was expressed as the urine volume per min (ml/min), while urine output efficiency was calculated as UV divided by IV. Rate-pressure-product (RPP), as an indicator of cardiac oxygen consumption was calculated by systolic blood pressure and HR (Formula 7).
Statistical analysis
Excel 2007 and SPSS, version 11.5 (SPSS, Inc., Chicago, IL, USA) were used for statistical analysis. Quantitative variables were expressed as the mean ± standard deviation (SD) or median (25th-75th) where appropriate. Comparisons were performed using Student's t-tests or Mann-Whitney U tests. Changes in hemodynamic data were presented as ratios compared to the baseline data. Relationships between the parameters were evaluated by simple linear regression. According to preliminary experiments, sample size was estimated on the basis of setting the SD for 50% of the mean. Power was given at 0.85 to detect a growth of double of urine output efficiency between the two groups by using the G*Power 3.0.10 (University Kiel, Germany). It was estimated that a minimum of 13 patients in each group was required; however, 14 subjects were recruited in each group to allow for the possibility of patient withdrawals.
Results
Demographic data, fluid input and output
A total of 28 patients, including 14 children and 14 adults, were enrolled in the present study. Demographic data, fluid input and output for all patients are presented in Table I. There were significant differences in demographic data, such as age, weight, height, BMI, and sex ratio between the two groups (P<0.001). There was no significant difference noted in cumulative amount of urine (ml) and the rate of urine output (ml/min) between adults and children; however, there were significant differences between infused fluid, cumulative amount of urine (ml/kg) and urine output efficiency between children and adults (Table I).
Table I.
Demographic data of patients and infusion input and output.
| Parameters | Children (n=14) | Adults (n=14) | P-value |
|---|---|---|---|
| Age (years) | 4±1 | 32±6 | <0.001 |
| Weight (kg) | 15±1 | 58±9 | <0.001 |
| Height (cm) | 102.8±6.5 | 166.8±9.2 | <0.001 |
| Sex, male (%) | 100 | 50 | <0.001 |
| BMI | 14.6±1.5 | 20.8±1.5 | <0.001 |
| Infused fluid (ml) | 153±14 | 581±90 | <0.001 |
| Cumulative amount of urine (ml) | 86±58 | 138±96 | 0.102 |
| Cumulative amount of urine (ml/kg) | 5.5±3.6 | 2.4±1.9 | 0.010 |
| Rate of urine output (ml/min) | 1.0±0.6 | 1.5±1.1 | 0.102 |
| Urine output efficiency (%) | 55±36 | 24±19 | 0.010 |
Data are presented as the mean ± standard deviation or percentage.
Hemodilution, vascular and extravascular volume expansion
Plasma dilution degree calculated from the Hb and Hct was significantly higher (P<0.001) in adults [0.16 (0.12–0.18)] than in children [0.07 (0.03–0.09)] in the last 10 min of infusion and for 60 min following completion of infusion. Plasma dilution degree peaked at the end of infusion, reaching 0.33 (0.23–0.41) in adults and 0.16 (0.10–0.24) in children (P=0.004). Following this, the dilution decreased gradually in both groups. The plasma dilution in both groups at 5 and 90 min after infusion was comparable (P>0.05); however, the dilutions were significantly higher in adults than in children at 20, 30, and 60 min after infusion of fluid (P<0.001; Fig. 2).
Figure 2.
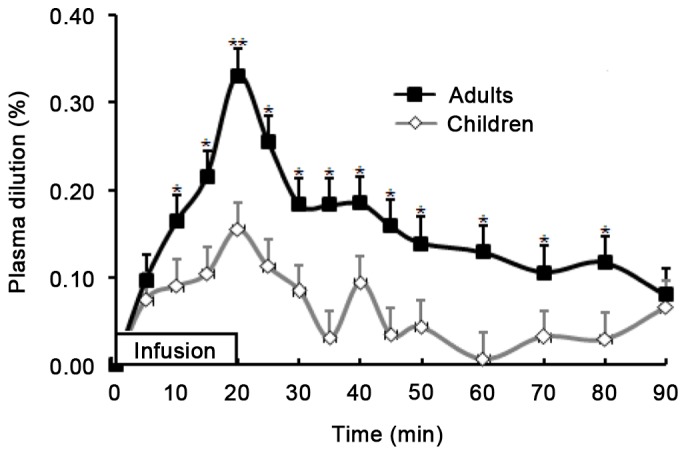
Relative changes in plasma dilution during and after 20-min infusion of 10 ml/kg lactated Ringer's solution in children and adults. There were significant differences between the plasma dilution of the children and adults at all time points except 5 and 90 min after infusion initiation. Data are expressed as the mean ± standard deviation. *P<0.001 and **P=0.004 vs. children.
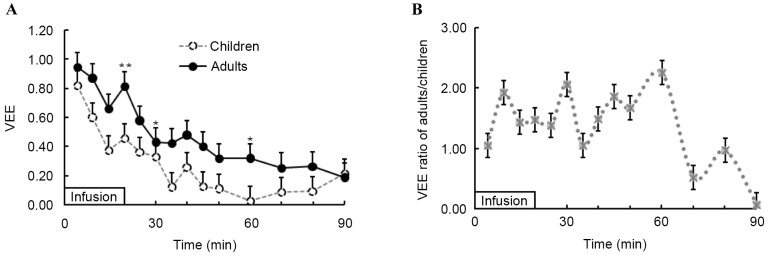
Fig. 3 demonstrates the time course of VE produced by LR solution in the two groups. During and after infusion of LR solution, the curves of VE (Fig. 3) and plasma dilution (Fig. 2) were similar and VE in the two groups presented the same change in trend during and after infusion of fluid. VE peaked at the end of infusion in both groups, at 8.18 (5.25–9.96) and 4.55 (2.64–6.97) ml/kg in adults and children, respectively (P=0.011). Additionally, at the time points of 20, 30 and 60 min after infusion initiation, the VE in adults was significantly higher than that in children (P<0.05); thereafter, the VE exhibited no significant difference between the adults and children (P>0.05). At the end of the study, the VE measurements were 1.84 (0.51–2.41) in adults and 2.13 (−0.18–4.49) in children (P=0.716).
Figure 3.
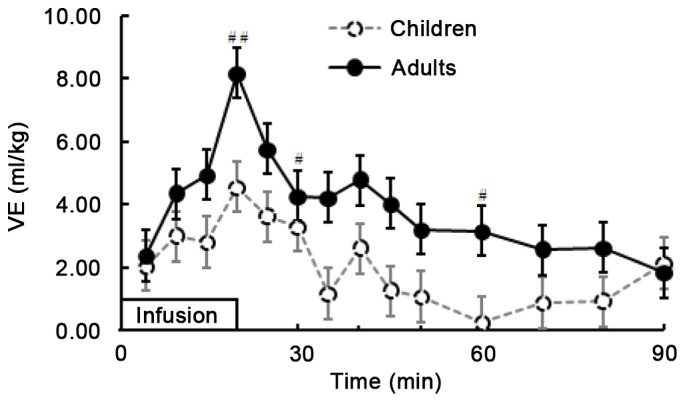
Comparison of blood VE during and after 20-min infusion of lactated Ringer's solution (10 ml/kg) in children and adults. Infusion began at time 0. Mann-Whitney U tests for comparison between two groups were performed at 20, 30, 60 and 90 min. Data are expressed as the median (25th-75th). #P<0.05 and ##P=0.011 vs. children. VE, volume expansion.
At all time points during and after infusion of fluid in the two groups, the median VE induced by the LR amounted to less than the volume infused. VEE was 0.95 (0.79–1.51) in adults and 0.82 (0.25–1.02) in children (P=0.152) during the first 5 min of LR infusion (Fig. 4A). VEE subsequently declined, following the same trend in both groups. When the infusion was complete, the VEE was significantly higher in adults [0.82 (0.52–1.00)] than in children [0.46 (0.26–0.68); P=0.005; Fig. 4A]. At the end of study (after 90 min), VEE in the two groups was similar [0.18 (0.05–0.24) in adults vs. 0.21 (−0.02–0.45) in children; P>0.05; Fig. 4A].
Figure 4.
(A) VEE, calculated as volume expansion (ml) divided by infused fluid (ml), during and after 20-min infusion of lactated Ringer's solution (10 ml/kg) in children and adults. (B) Relative VEE ratio during and after 20-min infusion of lactated Ringer's solution in adults vs. children, calculated by dividing the median of VEE in adults by the median of VEE in children. Infusion began at time 0. Mann-Whitney U tests for comparison between two groups were performed at times of 20, 30, 60 and 90 min. Data are expressed as the median (25th-75th). *P<0.05 and **P=0.005 vs. children. VEE, volume expansion efficiency.
The VEE ratio of adults to that of children obtained as median data is demonstrated in Fig. 4B. The VEE ratio was 1.05 at 5 min after initiation of infusion; thereafter, the ratio increased and peaked at 2.26 at 60 min after initiation of infusion. VEE ratio subsequently decreased to 0.06 at the end of the study. In the present study, the urine output was recorded at the end of the study when the extravascular volume expansion was 51 (28–66)% in adults and 53 (−14–71)% in children (P=0.486) of the infused fluid. When comparing the intravascular and extravascular volume expansion, it was revealed that more fluid was retained in the extravascular space than in the vascular system in both groups. This difference was not statistically significant for children (21 vs. 53% of infused fluid; P=0.759); however, in adults there was a significant difference (P<0.001), with 53% infused fluid in the extravascular space and 18% in the intravascular system.
Hemodynamic and myocardial oxygen consumption
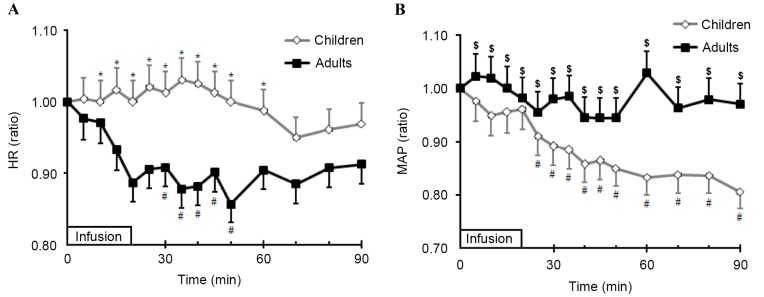
The baseline HR in children was 118 (112–125) bpm and 84 (71–92) bpm in adults (P<0.01). The HR in children rose slightly during the tests; however, the adult HR remained relatively unchanged. When comparing the relative changes in HR in the two groups (HR at each time point/baseline HR), it was observed that the relative changes were significantly greater in children than in adults 30–50 min after infusion initiation (P<0.01). Following this, the relative changes in HR were comparable between the groups (Fig. 5A). The baseline MAP in children and adults was 89 (84–93) and 94 (80–109) mmHg, respectively, and there was no significant difference between the two groups (P=0.05). MAP declined significantly from 25–90 min after infusion initiation in children (P<0.05) compared to adults, where MAP remained relatively constant (Fig. 5B). Simple regression analysis revealed a positive relationship between the relative changes of MAP and the plasma dilution. The reduction of MAP in children may, therefore, explain 47% of the variation in plasma dilution (R2=0.47; P=0.007); however, the relative changes of MAP in adults had no effect on plasma dilution (r=−0.22; P=0.697). Multiple regression analysis revealed that no other factors, including the changes in HR in children and adults (P>0.05), age and weight, correlated with plasma dilution.
Figure 5.
Comparison of relative changes in (A) HR and (B) MAP during and after infusion of Ringer's solution between children and adults. Student's t-tests for comparisons between children and adults were performed during and after infusion of fluid. One-way analysis of variance was performed for comparison between MAP or HR baseline values and values following infusion of fluids. Data are expressed as the mean ± standard deviation. *P<0.01 vs. adults; $P<0.05 vs. children; #P<0.05 vs. baseline values for each group. HR, heart rate; MAP, mean arterial blood pressure.
Myocardial oxygen consumption calculated as a ratio of RPP at different time points post-infusion/baseline RPP was roughly equivalent in both groups from infusion initiation up to 60 min after infusion initiation. Thereafter, the relative changes in RPP were significantly higher in adults than in children (P<0.01). Following infusion, all RPP values decreased compared to the baseline data; however, these differences were not statistically significant before 20 min in children and 10 min in adults after infusion initiation (P>0.05). Thereafter, RPP values became significantly lower than the baseline data (P<0.01; Fig. 6).
Figure 6.
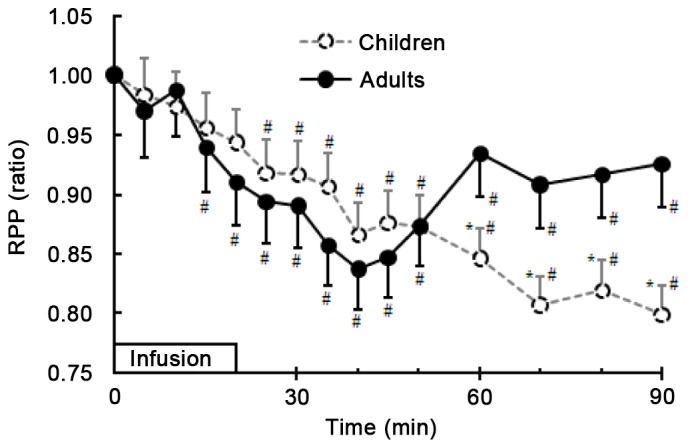
Relative changes in RPP during and after infusion of Ringer's solution between children and adults. Student's t-tests were performed for comparison between children and adults during and after infusion of fluid. One-way analysis of variance was performed for comparison between RPP baseline values and values following infusion of fluids. Data are expressed as the mean ± standard deviation. *P<0.01 vs. adults; #P<0.01 vs. baseline values for each group. RPP, rate-pressure-product.
Discussion
The present study was the first to examine the difference in hemodynamics and dynamics induced by LR infusion between adults and children prior to surgery. Results demonstrated that i.v. infusion of 10 ml/kg LR over 20 min produced different dynamics of intravascular and extravascular volume expansion in different age groups. Infused fluid produced the most pronounced plasma VE in adults compared with children, which peaked at the end of LR infusion. In the subsequent 60 min, a gradual decrease in VE was observed and the VE values for adults and children converged at the end of study. When VEE was calculated by the volume expansion divided by the infusion volume, it was observed that the majority of infused fluid cleared quickly through distribution to the extravascular space or by passing urine in both groups. The infused fluid mostly left the intravascular space by the way of urine output in children, whereas it was distributed to the extravascular space in adults. VEE was significantly higher in adults than in children 20, 30 and 60 min after infusion initiation. VEE was <1 at all times in both groups after LR infusion. Furthermore, the fluid in children attained to the balance of distribution according to a ‘4:1 rule’ 30 min after infusion initiation; however, in adults at the time of 90 min post-infusion initiation, it was indicated that 20% of infused LR solution was retained in the intravascular space. Previous research has demonstrated that children had a more rapid LR elimination than adults, which was indicated by volume kinetic parameters of the LR solution and may be explained by the different durations of preoperative fasting times (7). Less prominent plasma dilution following infusion of fluid has been considered to represent reduced dehydration (2,9). Other research has demonstrated that larger and more sustained volume expansion in animals or humans is obtained under conditions of hypovolemia (10,11), which is consistent with the results of the present study. Low blood volume due to various reasons, such as anesthesia (12), increases anti-diuretic hormone (ADH) and aldosterone, resulting in reduction of renal blood flow. This may explain why reduced urine output is observed in adults compared with children. It is not possible to rule out that the biological differences between children and adults may lead to marked differences in the fluid shift between the intravascular and extravascular space and the kidneys.
A significant limitation of the present study is that urine output was only measured at the end of the study, thus only one extravascular space volume was calculated. As a result, the change in trend of fluid distribution or elimination was not displayed. Another limitation is that the blood level of water-electrolyte metabolism hormones, such as ADH and aldosterone, was not measured; however, there is no evidence to suggest that the different urine volumes between the two groups in the present study was related to the above hormones. Further study is required to determine which factors may influence the different fluid disposition.
In the present study, the baseline MAP in both groups was comparable. MAP declined significantly from 25–90 min after the initiation of infusion in children. Simple regression analysis demonstrated a positive relationship between the relative changes in MAP and the plasma dilution, and the reduction of MAP may explain 47% of the variation of plasma dilution. Thus, the relative change in MAP in children following infusion of LR solution was an independent factor associated with plasma dilution in children. The study also demonstrated that the blood pressure in adults after infusion of fluid remained stable and that the plasma dilution in adults was greater than in children; however, the relative change of MAP in adults is unable to explain the variation in plasma dilution. Our previous study has reported conflicting findings concerning the relationship between the changes of MAP and plasma dilution (2). It was demonstrated that induction of general or epidural anesthesia resulted in excessive plasma dilution, which was most likely dependent upon the greater extent of reduction of MAP. This was supported by the negative relationship between the value of MAP and plasma dilution (r=−0.50; P<0.001) (2). Relative hypovolemia, caused by general or local anesthetics during the induction of anesthesia, triggered an increase in plasma dilution. It was indicated that increasing plasma dilution was a mechanism for maintaining normal blood volume after fluid infusion (2). Whereas, in the present study, adults may have exhibited dehydration or hypovolemia; however they were able to maintain blood pressure within the normal range. After fluid therapy to correct dehydration, heart rate decreased, which conformed to normal physiological regulation. Contrastingly, the hemodynamic changes in children appeared inconsistent with those in adults. The mechanism responsible for the difference in hemodynamics between adults and children requires further investigation.
It is well known that the purposes of fluid therapy are to raise the cardiac output, improve microcirculation in tissues and to improve tissue oxygen delivery (DO2). Our previous study demonstrated that fluid administration in patients who had already been in stroke volume index (SVI) optimization exhibited reduced DO2. This was explained by the fact that DO2 is heavily influenced by the infusion fluid-induced reciprocal correlation between the concentration of Hb and cardiac output (CO) (11). Furthermore, the study revealed that only patients with dehydration or hypovolemia were able to experience benefits in DO2 in response to fluid therapy (11). In the present study, CO of the patients was not measured, which was an additional limitation. Little is known about the myocardial oxygen consumption after fluid infusion before surgery. In the present study, fluid infusion induced hemodynamic variation, resulting in myocardial oxygen consumption (calculated from the rate-pressure-product) decrease in both patient groups scheduled for minor surgery. After 60 min of initiation of fluid infusion, myocardial oxygen consumption in adults increased, whereas it decreased further in children. When MAP or HR change, an increase in myocardial oxygen consumption may occur. This response may be regarded as a risk factor for patients with coronary artery disease when loading fluid, and it is necessary for changes in their hemodynamics, dehydration and hypovolemia to be monitored.
In conclusion, the results of the present study suggest that children and adults scheduled for minor surgery handle the infusion of LR solution using different processing modes prior to surgery. Infused fluid is often detained in the intravascular space, evoking more pronounced plasma dilution, and subsequently reaches the theoretical ‘4:1’ equilibrium of distribution between intra- and extravascular space more slowly in adults compared with children. In addition, the infused fluid was eliminated by urine output in children. The hemodynamic changes after infusion presented different trends in both age groups and changes in myocardial oxygen consumption also demonstrated two different trends in the two groups. These results indicate that infusion is much safer for children under the premise of strict dosage-control and infusion speed.
Acknowledgements
The authors thank Dr Hu Yaoqin, an anesthesiologist, for her assistance during the experiment. The study was supported by Zhejiang Province Natural Fund (grant no. LY15H030013), Zhejiang Medical Science and Technology Project (grant no. 2014KYB277) and Shaoxing City Science and Technology Project (grant no. 2015B70058).
References
- 1.Kaye AD, Grogono AW. Fluid and Electrolyte Physiology. In: Miller RD, editor. Anesthesia. 5th. New York: Churchill Livingstone; 2000. pp. 1586–1603. [Google Scholar]
- 2.Li YH, Lou XF, Bao FP. Dynamics of vascular volume and hemodilution of lactated ringer's solution in patients during induction of general and epidural anesthesia. J Zhejiang Univ Sci B. 2006;7:738–744. doi: 10.1631/jzus.2006.B0738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vane LA, Prough DS, Kinsky MA, Williams CA, Grady JJ, Kramer GC. Effects of different catecholamines on the dynamics of volume expansion of crystalloid infusion. Anesthesiology. 2004;101:1136–1144. doi: 10.1097/00000542-200411000-00013. [DOI] [PubMed] [Google Scholar]
- 4.Li YH, Zhu HB, Zheng X, Chen HJ, Shao L, Hahn RG. Low doses of esmolol and phenylephrine act as diuretics during intravenous anesthesia. Crit Care. 2012;16:R18. doi: 10.1186/cc11175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drobin D, Hahn RG. Distribution and elimination of crystalloid fluid in pre-eclampsia. Clin Sci (Lond) 2004;106:307–313. doi: 10.1042/CS20030349. [DOI] [PubMed] [Google Scholar]
- 6.Zdolsek JH, Bergek C, Lindahl TL, Hahn RG. Colloid osmotic pressure and extravasation of plasma proteins following infusion of ringer's acetate and hydroxyethyl starch 130/0.4. Acta Anaesthesiol Scand. 2015;59:1303–1310. doi: 10.1111/aas.12558. [DOI] [PubMed] [Google Scholar]
- 7.Li Y, Hahn RG, Hu Y, Xiang Y, Zhu S. Plasma and renal clearances of lactated ringer's solution in pediatric and adult patients just before anesthesia is induced. Paediatr Anaesth. 2009;19:682–687. doi: 10.1111/j.1460-9592.2009.03047.x. [DOI] [PubMed] [Google Scholar]
- 8.Retzlaff JA, Tauxe WN, Kiely JM, Stroebel CF. Erythrocyte volume, plasma volume and lean body mass in adult men and women. Blood. 1969;33:649–661. [PubMed] [Google Scholar]
- 9.Li Y, Zhu S, Hahn RG. The kinetics of ringer's solution in young and elderly patients during induction of general anesthesia with propofol and epidural anesthesia with ropivacaine. Acta Anaesthesiol Scand. 2007;51:880–887. doi: 10.1111/j.1399-6576.2007.01351.x. [DOI] [PubMed] [Google Scholar]
- 10.Zdolsek J, Li Y, Hahn RG. Detection of dehydration by using volume kinetics. Anesth Analg. 2012;115:814–822. doi: 10.1213/ANE.0b013e318261f6ba. [DOI] [PubMed] [Google Scholar]
- 11.Li Y, He R, Ying X, Hahn RG. Dehydration, haemodynamics and fluid volume optimization after induction of general anaesthesia. Clinics (Sao Paulo) 2014;69:809–816. doi: 10.6061/clinics/2014(12)04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hahn RG. Volume kinetics for infusion fluids. Anesthesiology. 2010;113:470–481. doi: 10.1097/ALN.0b013e3181dcd88f. [DOI] [PubMed] [Google Scholar]