Abstract
Staphylococcus aureus is a major cause of clinical infections in humans and its enterotoxins cause foodborne disease. In the present study, we tested a total of 51 isolates of S. aureus from small-ruminant dairy farms with artisan dairy facilities, all located in Latium, Italy. The farms have a known history of a high prevalence of methicillin-resistant S. aureus (MRSA). Most of the MRSA isolates (27 of 51) belonged to spa-type t127 (43.1%), followed by t2678 (3.9%), t044 (2%), t1166 (2%), and t1773 (2%). PFGE performed on mecA positive strains identified one cluster (≥ 80% of similarity), comprising 22 MRSA. Nine of twenty-two MRSA isolates were assigned human host origin, and 13 isolates did not belong to a specific host. During the characterization study, one strain isolated from bulk tank milk samples harbored the pvl gene; the strain was not enterotoxigenic with a non-specific host according to the biotyping scheme, highlighting the possible emerging risk of transmission of bacterial virulence factors by foods, the environment, and foodhandlers. These findings stress the importance of hygienic measures at all processing steps of the food production chain and underline that monitoring for the presence of MRSA throughout the food chain is essential for public health.
Keywords: Staphylococcus aureus, PFGE, Panton-Valentine leukocidin, MRSA, typing
1. Introduction
S. aureus is one of the most important etiological agents of intramammary infections in small ruminants [1]. In sheep it is responsible for clinical mastitis in 5–11% of cases and for sub-clinical mastitis in 0.22–2.06% of cases [2,3]. Its presence is a major economic and public health concern in the milk and dairy product sector [4]. S. aureus contamination of dairy products can occur anywhere in the food chain, while intra-farm spread mainly occurs through milking procedures [5], where infected mammary glands are the main reservoir [6].
Previous studies have examined a limited number of strains isolated from sheep and goat livestock that showed a host specificity and close relatedness [3,7]. Despite host specificity, S. aureus can switch and adapt to different host species, leading to host restriction [8].
S. aureus virulence isolates may harbor several staphylococcal enterotoxin genes [9], including genetic variants related to specific hosts [10]. In addition, S. aureus strains may also harbor pore-forming toxins involved in the pathophysiology of skin infections, such as Panton-Valentine Leukocidin (PVL) [11], and antibiotic-resistance genes, including methicillin resistance mediated by the mecA gene chromosomally located in the staphylococcal cassette chromosome mec (SCCmec) [12].
Recent reports of an increasing number of MRSA isolates in pets and livestock have demonstrated that the livestock-associated MRSA (LA-MRSA), belonging to the clonal complex CC398, is the most frequently isolated [13,14]. In Italy, the community-acquired MRSA (CA-MRSA) ST(CC) 1 spa-type t127 is reported among the main lineages of MRSA isolated in livestock productions [15,16,17,18,19,20,21]. CA-MRSA clones often harbor the PVL virulence genes, more than CA-MSSA, suggesting that methicillin resistance has contributed to the success of pvl-positive S. aureus strains [22]. Furthermore, exposure to MRSA from livestock is a route for horizontal transmission between animals and people working in contact with livestock [21,23,24,25,26], and for its possible introduction in the community through the food chain [27].
Although S. aureus is frequently isolated in ovine milk and dairy products [16,28,29], the occurrence of MRSA in the ovine dairy chain appears to be low [17,18,29,30,31]. The aim of this study was to investigate the genetic diversity, as determined by pulsed-field gel electrophoresis (PFGE) and spa-types, of MRSA and MSSA isolated from samples of udder milk, raw milk, cheeses, nasal swabs of workers, and skin swabs of ewes taken from dairy sheep farms with a history of MRSA in bulk tank milk, as documented in a previous survey. Additionally, genes encoding for PVL and staphylococcal enterotoxins (SEs) were detected.
2. Results
Twenty-seven of the 51 isolates of S. aureus (52.9%) showed resistance to methicillin (MRSA), while 24 (47.1%) were susceptible (MSSA). Twenty-seven strains harbored the mecA gene, confirming methicillin resistance, while no isolates harbored the mecC gene (mecALGA251). The 11 genes coding for SEs were investigated for all 51 strains, and 41 isolates (80.4%) tested positive for at least one SE gene. The combination of the results generated six different SE profiles, including the isolates that tested negative for all SE genes (Table 1). The seh gene was the most frequent profile (33.3%), followed by the negative profile (19.7%), sec (17.6%), and seg (15.7%) genes. Seven strains (13.7%) harbored both seg-sei genes carried together on the enterotoxin gene cluster (egc) [32], including two profiles: seg-sei detected in five isolates (9.8%) and sec-seg-sei in two isolates (3.9%). The genes sea, seb, sed, see, selj, selp, ser were not detected, and two of the negative isolates for SE genes tested positive for the mecA gene.
Table 1.
Results of the enterotoxin genotyping scheme.
| Staphylococcoal Enterotoxin (SE) Profile | Staphylococcal Enterotoxin Gene(s) | No. of Isolates (%) |
|---|---|---|
| 1 | seh | 17 (33.3) |
| 2 | Negative | 10 (19.7) |
| 3 | sec | 9 (17.6) |
| 4 | seg | 8 (15.7) |
| 5 | seg-sei | 5 (9.8) |
| 6 | sec-seg-sei | 2 (3.9) |
seh: staphylococcal enterotoxin type H gene; sec: staphylococcal enterotoxin type C gene; seg: staphylococcal enterotoxin type G gene; sei: staphylococcal enterotoxin type I gene.
Six farms participated in this study, and 831 individual milk samples were obtained from sheep. Among 14 samples positive for S. aureus, 8 indicated MRSA (Supplemental Table S1). The number of workers is reported in Table 2; for each worker (14), nasal, oral and skin swabs were sampled. Among the 12 S. aureus isolated, 5 indicated MRSA.
Table 2.
Farm owners and farm workers tested.
| Farm Number | Farm Workers/Owners Tested | S. aureus | MRSA | |
|---|---|---|---|---|
| 1 | 5 | 2 | 1 | |
| 2 | 2 | 4 | 2 | |
| 3 | 4 | 3 | 1 | |
| 4 | 1 | 3 | 1 | |
| 5 | 1 | 0 | 0 | |
| 6 | 1 | 0 | 0 | |
| Total | 6 | 14 | 12 | 5 |
MRSA: methicillin-resistant S. aureus.
A total of 51 isolates were typed by spa-typing, generating 12 profiles. Among the mecA positive isolates (27 of 51), 22 had spa-type t127 (43.1%), two had t2678 (3.9%), and one (2.0%) had the spa-types t044, t1166, and t1773. Among the 24 isolates that tested negative for mecA and mecC genes, four were spa-type t528 (7.8%), three were spa-types st127, t1166, and t1773 (5.9% each), two were spa-types t050, t709, t902, and t3583 (3.9% each), and one was spa-types t084, t998, and t2678 (2.0% each).
The exotoxin PVL gene was detected in one MRSA strain isolated from bulk tank milk that tested negative for SE genes and not-specific host type 2 (NSH2) in biotyping (Table 3).
Table 3.
Results of the typing scheme.
| ID | pvl | mecA | SEsP | spa-type | SmaI | Biotype | ID | pvl | mecA | SEsP | spa-type | SmaI | Biotype |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | − | + | 4 | t1166 | 6 | NSH4 | 27 | + | + | 2 | t044 | 5 | NSH2 |
| 2 | − | + | 4 | t1773 | 7 | Human | 28 | − | + | 1 | t127 | 6 | NSH3 |
| 3 | − | + | 4 | t127 | 6 | NSH3 | 29 | − | + | 1 | t127 | 6 | NSH6 |
| 4 | − | + | 4 | t127 | 6 | NSH3 | 30 | − | + | 1 | t127 | 7 | Human |
| 5 | − | + | 4 | t127 | 7 | Human | 31 | − | − | 5 | t709 | 2 | Human |
| 6 | − | + | 4 | t127 | 7 | Human | 32 | − | − | 5 | t709 | 2 | Human |
| 7 | − | + | 4 | t2678 | 7 | Human | 33 | − | − | 6 | t050 | 8 | Human |
| 8 | − | − | 2 | t1773 | 12 | NB | 34 | − | + | 1 | t127 | 4 | Human |
| 9 | − | − | 3 | t3583 | 13 | Ovine | 35 | − | − | 6 | t050 | 8 | Human |
| 10 | − | − | 3 | t3583 | 13 | Ovine | 36 | − | + | 1 | t127 | 6 | NSH3 |
| 11 | − | − | 3 | t1773 | 13 | Ovine | 37 | − | + | 1 | t127 | 6 | NSH3 |
| 12 | − | − | 3 | t1166 | 13 | Ovine | 38 | − | + | 1 | t127 | 6 | NSH3 |
| 13 | − | + | 2 | t2678 | 7 | Human | 39 | − | + | 1 | t127 | 6 | NSH3 |
| 14 | − | − | 2 | t127 | 12 | Ovine | 40 | − | + | 1 | t127 | 6 | NSH3 |
| 15 | − | − | 3 | t1773 | 13 | Ovine | 41 | − | + | 1 | t127 | 6 | NSH3 |
| 16 | − | − | 3 | t2678 | 14 | Ovine | 42 | − | + | 1 | t127 | 9 | Human |
| 17 | − | + | 4 | t127 | 6 | NSH3 | 43 | − | − | 2 | t084 | 10 | NSH6 |
| 18 | − | − | 2 | t528 | 1 | Ovine | 44 | − | + | 1 | t127 | 6 | Human |
| 19 | − | − | 2 | t528 | 1 | Ovine | 45 | − | − | 3 | t998 | 11 | Ovine |
| 20 | − | − | 5 | t902 | 2 | Human | 46 | − | − | 3 | t1166 | 11 | Ovine |
| 21 | − | − | 2 | t127 | 3 | NSH2 | 47 | − | − | 3 | t1166 | 14 | Ovine |
| 22 | − | + | 1 | t127 | 4 | NSH3 | 48 | − | + | 1 | t127 | 6 | Human |
| 23 | − | + | 1 | t127 | 4 | NSH3 | 49 | − | − | 5 | t902 | 2 | Human |
| 24 | − | − | 2 | t528 | 3 | NSH3 | 50 | − | + | 1 | t127 | 6 | Human |
| 25 | − | − | 2 | t528 | 3 | NSH2 | 51 | − | − | 5 | t127 | 2 | Human |
| 26 | − | + | 1 | t127 | 4 | NSH3 |
ID: Identification number of the isolate; pvl: Panton-Valentine Leukocidin; mecA: methicillin resistance gene; SEsP: staphylococcal enterotoxin profile resulting from the combination of the 11 SE genes detected; spa-type: staphylococcal protein A genotype; smaI: PFGE enzyme smaI result; NB: combination of the results did not belong to any biotype described in the literature.
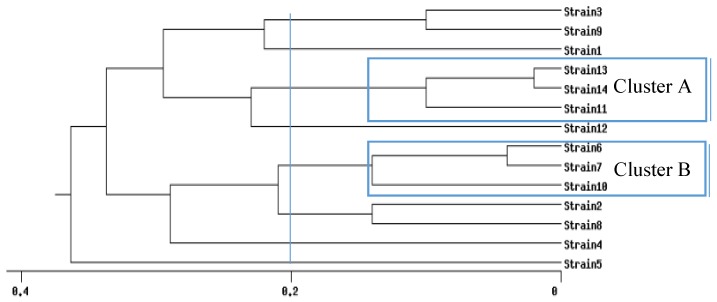
Pulsed-field gel electrophoresis (PFGE) (http://insilico.ehu.es/dice_upgma/index.php) identified 13 S. aureus pulse types. According to Mello and colleagues [33], analysis of the PFGE profiles highlighted two S. aureus clusters that simultaneously included ≥ 3 isolates with similarity ≥ 80% (Figure 1).
Figure 1.
Determination of the clonal profile of Staphylococcus aureus strain profiles highlighted by PFGE. Dendrogram generated using the Dice/Unweighted pair group method using arithmetic averages (UPGMA) analysis (BioNumerics, Applied Maths, Version 7.1, Sint-Martens-Latem, Belgium, 2016).
Cluster A consisted of three strains all harboring the gene sec coding for SE type C. In terms of host specificity, all strains were related to an ovine origin. This cluster comprised nine isolates from farm 3 (Table 4), and all strains were MSSA.
Table 4.
Strains belonging to cluster A.
| ID | Farm | Type of Sample | pvl | SEs Gene | Biotyping | spa-type | smaI | |
|---|---|---|---|---|---|---|---|---|
| 45 | 3 | Mastitis udder swab | − | sec | Ovine | t998 | 11 | |
| 46 | 3 | Udder | − | sec | Ovine | t1166 | 11 | |
| 9 | 3 | Individual milk | − | sec | Ovine | t3583 | 13 | |
| 10 | 3 | Individual milk | − | sec | Ovine | t3583 | 13 | |
| 11 | 3 | Mammary pustules | − | sec | Ovine | t1773 | 13 | |
| 12 | 3 | Mammary pustules | − | sec | Ovine | t1166 | 13 | |
| 15 | 3 | Swab skin (sheep) | − | sec | Ovine | t1773 | 13 | |
| 47 | 3 | Swab skin (sheep) | − | sec | Ovine | t1166 | 14 | |
| 16 | 3 | Swab skin (sheep) | − | sec | Ovine | t2678 | 14 | |
| Total | 9 | 1 | sec (100.0%) |
ID: Identification number of the isolate; Farm: number of the farm where the strain was isolated; pvl: Panton-Valentine Leukocidin gene; SEs: staphylococcal enterotoxin gene genotype; spa: staphylococcal protein A typing result; smaI: PFGE enzyme smaI result.
Cluster B comprised 22 MRSA isolated from different farms (1, 2, 3, and 4). In terms of host specificity, nine isolates were assigned to human host origin and 13 isolates did not belong to a specific host (NSH3, NSH6, and NSH4) (Table 5).
Table 5.
Strains belonging to cluster B.
| ID | Farm | Type of Sample | pvl | SEs Gene | Biotyping | spa-type | smaI | |
|---|---|---|---|---|---|---|---|---|
| 1 | 4 | Bulk milk | − | seg | NSH4 | t1166 | 6 | |
| 2 | 3 | Bulk milk | − | seg | Human | t1773 | 7 | |
| 3 | 1 | Bulk milk | − | seg | NSH3 | t127 | 6 | |
| 4 | 1 | Cheese | − | seg | NSH3 | t127 | 6 | |
| 5 | 3 | Individual milk | − | seg | Human | t127 | 7 | |
| 6 | 3 | Individual milk | − | seg | Human | t127 | 7 | |
| 7 | 3 | Individual milk | − | seg | Human | t2678 | 7 | |
| 13 | 3 | Swab skin (ewe) | - | neg | Human | t2678 | 7 | |
| 17 | 4 | Cheese | − | seg | NSH3 | t127 | 6 | |
| 28 | 4 | Individual milk | − | seh | NSH3 | t127 | 6 | |
| 29 | 4 | Individual milk | − | seh | NSH6 | t127 | 6 | |
| 30 | 4 | Swab skin (human) | − | seh | Human | t127 | 7 | |
| 36 | 2 | Bulk milk | − | seh | NSH3 | t127 | 6 | |
| 37 | 1 | Bulk milk | − | seh | NSH3 | t127 | 6 | |
| 38 | 1 | Bulk milk | − | seh | NSH3 | t127 | 6 | |
| 39 | 1 | Bulk milk | − | seh | NSH3 | t127 | 6 | |
| 40 | 1 | Udder half | − | seh | NSH3 | t127 | 6 | |
| 41 | 1 | Environmental swab | − | seh | NSH3 | t127 | 6 | |
| 43 | 1 | Nasal swab | − | neg | NSH6 | t084 | 10 | |
| 44 | 1 | Bulk milk | − | seh | Human | t127 | 6 | |
| 48 | 3 | Nasal swab | − | seh | Human | t127 | 6 | |
| 50 | 3 | Nasal swab | − | seh | Human | t127 | 6 | |
| Total | 22 | 4 |
ID: Identification number of the isolate; Farm: number of the farm where the strain was isolated; pvl: Panton-Valentine Leukocidin; SEs: staphylococcal enterotoxin gene genotype; spa-type: staphylococcal protein A typing result; smaI: PFGE enzyme smaI result.
3. Discussion
In this study, a PVL positive strain (lukS-lukF+) was highlighted during a study for the screening of MRSA in six ovine farms that tested positive in a previous study. PVL is one of the most important virulence factors produced by S. aureus, contributing to the pathogenicity of this microorganism. This toxin is associated with different diseases in humans, such as pneumonia and necrotizing dermatitis [34]. The Panton-Valentin leukocydin gene (pvl) gene has also been identified in S. aureus strains isolated from cases of mastitis in animals [35,36,37]. In Europe, the most common clone is the European ST80 [38]; however, this is the first report of S. aureus isolated from ovine raw milk. The strain tested positive for the mecA gene and negative for the enterotoxin genes tested (SEs profile number 2); it belonged to spa-type t044 and showed a non-host specificity (NSH2) in biotyping. The PFGE profile of the strain and the phylogenetic study showed a high genomic distance (strain 5, Figure 1) from the population studied, and it belonged to ST80, which, according to the spa-ridom server, is widely disseminated in Europe.
This study revealed the presence of multiple MRSA strains among animals at the farms with possible human contamination. In fact, 11 of the mecA positive strains confirmed by PCR (27 strains in total) indicated a human host origin in biotyping. The five strains isolated from the operators (nasal swabs, oral swab, and skin swab) from two farms (3 and 4) were considered the common infectious agent for the individual milk samples (3 isolates), bulk tank milk (2 samples), and ovine swab skin (1 isolate). Although the isolates showed the same spa-type (t127), they highlighted a different PFGE profile using SmaI between the strains isolated from two operators (spa-type t127 and PFGE profile 6) and the strains isolated from the individual milk samples of two animals (t127 and PFGE profile 7), even though the strains are genetically correlated and belong to the same cluster (Figure 1). Surprisingly, the strain isolated from the operator from farm 4 (not correlated with farm 3) had the same genetic profiles (t127 and PFGE profile 7) as the two strains isolated from the individual milk samples of animals from farm 3. Furthermore, when all the isolates with different biotyping results but belonging to cluster B were compared, the MRSA strains were present at all four farms (1, 2, 3, and 4) and were isolated from different samples, including the cheeses ready for commercialization (farms 1 and 4). Interestingly, four isolates (ID 2, ID8, ID11, and ID15) had spa-type t1773 and one carried the mecA gene (negative for the mecALGA251), contrary to previous studies [39,40,41,42] that reported spa-type t1773 isolates positive for mecALGA251. In those studies, the strains were isolated from cats (skin) [41,42] and humans [39,40]. In the present study, the ID2 MRSA isolate (mecA positive) was isolated from bulk tank milk (Table S1) and had a human origin according to the biotyping scheme. The other three strains were isolated from an individual milk sample (ID8), mammary pustules (ID11), and skin swab (ID15) from the same animal and had ovine origin according to the biotyping scheme.
S. aureus usually shows limited host specificity, though transfer between different host species may occur [43]. The transmission of milk-associated S. aureus strains between cows and humans was suggested by Lee [44] who found MRSA in milk samples with comparable antibiotypes as those in humans, but the transfer to humans was not demonstrated as, for example, the transmission from ovines to humans and vice versa. While molecular epidemiological typing did not reveal the spread of MRSA from animals to humans, it did reveal the spread of MRSA strains among ovines on two farms.
It should be emphasized that the study of S. aureus populations isolated from small ruminants holds importance since information is scarce. Recent detection of new spa types [45] and the pvl strain in this study suggests that the characterization of S. aureus isolates from ovine and caprine livestock should be encouraged.
Detection of MRSA in sheep herds is unexpected, given that the farm management practices differ from those for cattle. In fact, in small ruminant herds, breeders often prefer culling to antimicrobial treatments, resulting in a less selective pressure on S. aureus populations.
The presence of MRSA in farm animals is a serious health hazard for the community and could pose a difficult challenge for treating infections in humans. In Italy, Alba and colleagues observed that the MRSA strains isolated from cattle have a very high genetic relatedness with human isolates [20]. Therefore, it might be important to genetically correlate strains isolated from sheep farms to human isolates in the same area, investigating genomic features, including the arginine catabolic mobile element (ACME), which was identified in a specific clone of MRSA, called USA300 [46]. Lastly, the investigation of these genes can be useful for control the spread of possible new MRSA clones, studying their genetic evolution and the horizontal acquisition of novel genetic elements of strains isolated in different host species.
4. Conclusions
Characterization of SE genes in strains isolated in dairy farms is helpful for the indirect monitoring of eventual enterotoxigenic strains present in the food chain. The enterotoxin G and H genes exhibited the highest prevalence in the MRSA isolates in this study. Since there are no methods available for the identification of these enterotoxins in foodstuffs, an underestimation of the risks for humans can be assumed [47,48,49]. In addition, the potential risk for antimicrobial resistance in the food chain has to be taken into account [47,48,49]. On the other hand, the reported enterotoxin type C gene found in the MSSA, but not in the MRSA isolates, confirmed its diffusion in ovine isolates, as previously reported [28,50,51]. The description of an isolate harboring the gene for pvl poses a risk for the dissemination of this toxin in sheep herds. There are no directives for mandatory screening of this virulence factor in food sources. The results of this study highlight the need for more detailed investigations to improve consumer protection and public health safety.
5. Materials and Methods
5.1. Sample Collection
A total of 51 S. aureus strains were collected from six dairy sheep farms, including two farms with artisan dairy facilities, all located in Latium, Italy, between 2013 and 2015. The farms had a history of MRSA in bulk tank milk, as documented in a previous survey carried out between 2012 and 2015. Five farms operated a semi-extensive breeding system, two of which also had an artisan cheese factory, while the sixth farm operated an intensive system. The circulation of MRSA and of MSSA was investigated in four of the six positive farms. The flock size ranged from 83 to 556 lactating ewes. The ewes were milked twice daily using a milking machine. The workers wore dedicated coveralls and boots, but not gloves, when handling the animals. Teat-washing before milking and treatment with antibiotics at dry-off were not performed. The dairies made raw milk cheeses, and the farm owners were usually involved in milking and cheese making.
Individual milk samples were collected from all lactating ewes in all four herds. At the end of milking, bulk tank milk was collected. Nasal and skin samples were taken using cotton-tipped swabs (Amies Agar Gel with Charcoal, Laboindustria s.p.a., Italy) and Sodibox® wipes (Sodibox, Névez, France), respectively, from the farm owners and workers. None of the subjects reported recent hospitalization or the presence of a healthcare worker in the household. Two raw cheese samples were taken from each dairy plant. All samples were immediately transported to the laboratory in ice-cooled containers and analyzed within 24 h.
Overall, the isolates were obtained from udder milk (n = 17), mammary pustules (n = 2), bulk tank milk (n = 11), nasal swabs of workers (n = 8), skin swabs of workers (n = 4), skin swabs of ewes (n = 4), environmental swabs (n = 3), and raw cheese (n = 2). Isolates were confirmed as S. aureus by modified species-specific PCR, using primers targeting the femA gene (132 bp) [52] and screened for methicillin resistance using the disk diffusion method according to Clinical Laboratory Standards Institute (CLSI) criteria. All human samples were obtained voluntarily, and the farm owner consented to animal sampling (in Italy, MRSA infection in animals is not a notifiable disease). All procedures followed were in accordance with ethical standards of the relevant national and institutional committees on experimentation and with the Helsinki Declaration of 1975, as revised in 2008. Farm workers gave oral informed consent to participate in the study.
5.2. Typing of the Bacterial Isolates
All isolates were analyzed by phenotypic tests for growth on Baird Parker rabbit plasma fibrinogen agar (Merck, Darmstadt, Germany). DNA was extracted using the InstaGene™ Matrix (Bio-Rad, Milan, Italy). Staphylococcal enterotoxin genes (SEs) were detected using two multiplex PCR (mPCR) assays according to the protocols of the European Union Reference Laboratory for Coagulase-Positive Staphylococci, including Staphylococcus aureus (EU-RL CPS) (ANSES—Mesons Alfort, France) [53]. The first mPCR assay detects genes encoding SEs A to E and R; the second detects genes encoding SEs G to J and P. Reference strains of S. aureus provided by the EU-RL CPS, FRI S6 (sea, seb), FRI 137 (seg, seh, sei), FRI 326 (see), FRI 361 (sec, sed, ser), and HMPL 280 (seg, sei, selj, selp) were used as positive controls. The biotyping scheme described by Devriese [54] was utilized to assign specific ecological variants (ecovars) associated with different host species; the method includes the production of β-hemolysin, bovine plasma coagulation, staphylokinase, and crystal violet growth reaction. The possible results are: bovine, human, ovine, and six different non-specific hosts (NSH1 to NSH6) [8].
All S. aureus isolates were analyzed for the presence of mecA, the new mecA homologue gene (mecC, formerly named mecALGA251), and Panton-Valentine Leukocidin (PVL) (lukS-PV/lukF-PV) genes by PCR, as described previously [39]. PCR assays were performed on a GeneAmp System 9700 thermal cycler (Applied Biosystems, Foster City, CA, USA). All isolates were typed by DNA macrorestriction digestion analysis using a SmaI restriction enzyme and pulsed-field gel electrophoresis (PFGE) according to the EU-RL CPS protocol [55] modified as follows. Briefly, electrophoretic separation of DNA fragments was performed on 1% agarose gel; electrophoresis was performed using the CHEF MAPPER System (Bio-Rad Laboratories, Hercules, CA, USA); the gel was stained with GelRed (Biotium, Hayward, CA, USA) 1X solution in ultrapure water for 30 min. The restriction profiles were analyzed with BioNumerics software ver. 7.1 (Applied Maths, Sint-Martens-Latem, Belgium) using the Dice correlation coefficient and then visualized as a dendrogram by the unweighted pair-group method using arithmetic averages (UPGMA), with 1% tolerance and 1% optimization settings. Similarity cutoff values of 90%, 75%, and 70% were used to define a cluster. The polymorphic X region of the protein A gene (spa) was sequenced to assign the spa-type [56] using BioNumerics software ver. 7.1.
Supplementary Materials
The following are available online at www.mdpi.com/2072-6651/9/5/161/s1, Table S1: Type of sample and number of the farm where the strain was isolated.
Author Contributions
G.M. conceived the study, designed the experiments, performed the analysis, analyzed the data and wrote the paper. G. Giacinti analyzed the data, coordinated the sampling and wrote the paper. A.B. drafted the experimental section and analyzed the data. S.G. and D.M.B. assisted with data analysis. D.S., N.M. and G. Giangolini assisted with sampling and performed the microbiology analysis. L.D. and S.A. coordinated the investigation and critically reviewed the manuscript. All the authors have read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Bergonier D., de Crémoux R., Rupp R., Lagriffoul G., Berthelot X. Mastitis of dairy small ruminants. Vet. Res. 2003;34:28, 689–716. doi: 10.1051/vetres:2003030. [DOI] [PubMed] [Google Scholar]
- 2.De Almeida L.M., Zilta M., De Almeida P., De Mendonça C.L., Mamizuka E.M. Novel sequence types (STs) of Staphylococcus aureus isolates causing clinical and subclinical mastitis in flocks of sheep in the northeast of Brazil. J. Dairy Res. 2011;78:373–378. doi: 10.1017/S0022029911000379. [DOI] [PubMed] [Google Scholar]
- 3.Mørk T., Waage S., Tollersrud T., Kvitle B., Sviland S. Clinical mastitis in ewes; bacteriology, epidemiology and clinical features. Scand. Acta Vet. 2007;49:1–8. doi: 10.1186/1751-0147-49-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Buyser M.-L., Dufour B., Maire M., Lafargé V., De M.-L. Implication of milk and milk products in food-borne diseases in France and in different industrialised countries. Int. J. Food Microbiol. 2001;67:1–17. doi: 10.1016/S0168-1605(01)00443-3. [DOI] [PubMed] [Google Scholar]
- 5.Keefe G. Update on control of Staphylococcus aureus and Streptococcus agalactiae for management of mastitis. Vet. Clin. North Am. Food Anim. Pract. 2012;28:203–216. doi: 10.1016/j.cvfa.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 6.Vautor E., Abadie G., Guibert J.M., Huard C., Pépin M. Genotyping of Staphylococcus aureus isolated from various sites on farms with dairy sheep using pulsed-field gel electrophoresis. Vet. Microbiol. 2003;96:69–79. doi: 10.1016/S0378-1135(03)00207-4. [DOI] [PubMed] [Google Scholar]
- 7.Concepcion Porrero M., Hasman H., Vela A.I., Fernandez-Garayzabal J.F., Dominguez L., Aarestrup F.M. Clonal diversity of Staphylococcus aureus originating from the small ruminants goats and sheep. Vet. Microbiol. 2012;156:157–161. doi: 10.1016/j.vetmic.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 8.Guinane C.M., Ben Zakour N.L., Tormo-Mas M.A., Weinert L.A., Lowder B.V., Cartwright R.A., Smyth D.S., Smyth C.J., Lindsay J.A., Gould K.A., et al. Evolutionary genomics of Staphylococcus aureus reveals insights into the origin and molecular basis of ruminant host adaptation. Genome Biol. Evol. 2010;2:454–466. doi: 10.1093/gbe/evq031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Argudín M.Á., Mendoza M.C., Rodicio M.R. Food poisoning and Staphylococcus aureus enterotoxins. Toxins (Basel) 2010;2:1751–1773. doi: 10.3390/toxins2071751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johler S., Sihto H.-M., Macori G., Stephan R. Sequence variability in staphylococcal enterotoxin genes seb, sec, and sed. Toxins (Basel) 2016;8:169. doi: 10.3390/toxins8060169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hodille E., Cuerq C., Badiou C., Bienvenu F., Steghens J.-P., Cartier R., Bes M., Tristan A., Plesa A., Le V.T.M., et al. Delta Hemolysin and Phenol-Soluble Modulins, but Not Alpha Hemolysin or Panton-Valentine Leukocidin, Induce Mast Cell Activation. Front. Cell. Infect. Microbiol. 2016;6:1–11. doi: 10.3389/fcimb.2016.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Traversa A., Gariano G.R., Gallina S., Bianchi D.M., Orusa R., Domenis L., Cavallerio P., Fossati L., Serra R., Decastelli L. Methicillin resistance in Staphylococcus aureus strains isolated from food and wild animal carcasses in Italy. Food Microbiol. 2015;52:154–158. doi: 10.1016/j.fm.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 13.De Neeling A.J., van den Broek M.J.M., Spalburg E.C., van Santen-Verheuvel M.G., Dam-Deisz W.D. C., Boshuizen H.C., van de Giessen A.W., van Duijkeren E., Huijsdens X.W. High prevalence of methicillin resistant Staphylococcus aureus in pigs. Vet. Microbiol. 2007;3:366–372. doi: 10.1016/j.vetmic.2007.01.027. [DOI] [PubMed] [Google Scholar]
- 14.Argudín M.A., Tenhagen B.A., Fetsch A., Sachsenröder J., Käsbohrer A., Schroeter A., Hammer J.A., Hertwig S., Helmuth R., Bräunig J., et al. Virulence and resistance determinants of German Staphylococcus aureus ST398 isolates from nonhuman sources. Appl. Environ. Microbiol. 2011;77:3052–3060. doi: 10.1128/AEM.02260-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pilla R., Piccinini R., Gelain M.E., Lorenzi V., Castiglioni V., Scanziani E., Anjum M. Long-term study of MRSA ST1, t127 mastitis in a dairy cow. Vet. Rec. 2012;170:1–3. doi: 10.1136/vr.100510. [DOI] [PubMed] [Google Scholar]
- 16.Carfora V., Caprioli A., Marri N., Sagrafoli D., Boselli C., Giacinti G., Giangolini G., Sorbara L., Dottarelli S., Battisti A., et al. Enterotoxin genes, enterotoxin production, and methicillin resistance in Staphylococcus aureus isolated from milk and dairy products in Central Italy. Int. Dairy J. 2015;42:12–15. doi: 10.1016/j.idairyj.2014.10.009. [DOI] [Google Scholar]
- 17.Carfora V., Giacinti G., Sagrafoli D., Marri N., Giangolini G., Alba P., Feltrin F., Sorbara L., Amoruso R., Caprioli A., et al. Methicillin-resistant and methicillin-susceptible Staphylococcus aureus in dairy sheep and in-contact humans: An intra-farm study. J. Dairy Sci. 2016;99:4251–4258. doi: 10.3168/jds.2016-10912. [DOI] [PubMed] [Google Scholar]
- 18.Caruso M., Latorre L., Santagada G., Fraccalvieri R., Miccolupo A., Sottili R., Palazzo L., Parisi A. Methicillin-resistant Staphylococcus aureus (MRSA) in sheep and goat bulk tank milk from Southern Italy. Small Rumin. Res. 2016;135:26–31. doi: 10.1016/j.smallrumres.2015.12.023. [DOI] [PubMed] [Google Scholar]
- 19.Cortimiglia C., Bianchini V., Franco A., Caprioli A., Battisti A., Colombo L., Stradiotto K., Vezzoli F., Luini M. Short communication: Prevalence of Staphylococcus aureus and methicillin-resistant S. aureus in bulk tank milk from dairy goat farms in Northern Italy. J. Dairy Sci. 2015;98:2307–2311. doi: 10.3168/jds.2014-8923. [DOI] [PubMed] [Google Scholar]
- 20.Alba P., Feltrin F., Cordaro G., Porrero M.C., Kraushaar B., Argudín M.A., Nykäsenoja S., Monaco M., Stegger M., Aarestrup F.M., et al. Livestock-associated methicillin resistant and methicillin susceptible Staphylococcus aureus sequence type (CC)1 in European farmed animals: High genetic relatedness of isolates from Italian cattle herds and humans. PLoS One. 2015;10:1–10. doi: 10.1371/journal.pone.0137143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Franco A., Hasman H., Iurescia M., Lorenzetti R., Stegger M., Pantosti A., Feltrin F., Ianzano A., Porrero M.C., Liapi M., et al. Molecular characterization of spa-type t127, sequence type 1 methicillin-resistant Staphylococcus aureus from pigs. J. Antimicrob. Chemother. 2011;66:1231–1235. doi: 10.1093/jac/dkr115. [DOI] [PubMed] [Google Scholar]
- 22.Boyle-Vavra S., Daum R.S. Community-acquired methicillin-resistant Staphylococcus aureus: The role of Panton–Valentine leukocidin. Lab. Investig. 2007;87:3–9. doi: 10.1038/labinvest.3700501. [DOI] [PubMed] [Google Scholar]
- 23.Brennan G.I., Abbott Y., Burns A., Leonard F., McManus B.A., O’Connell B., Coleman D.C., Shore A.C. The emergence and spread of multiple livestock-associated clonal complex 398 methicillin-resistant and methicillin-susceptible Staphylococcus aureus strains among animals and humans in the Republic of Ireland, 2010–2014. PLoS One. 2016;11:1–11. doi: 10.1371/journal.pone.0149396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wulf M.W.H., Markestein A., van der Linden F.T., Voss A., Klaassen C., Verduin C.M. First outbreak of methicillin-resistant Staphylococcus aureus ST398 in a Dutch hospital, June 2007. Euro Surveill. 2008;13:1–2. [PubMed] [Google Scholar]
- 25.Juhász-Kaszanyitzky E., Jánosi S., Somogyi P., Dán A., Van Der Graaf-van Bloois L., Van Duijkeren E., Wagenaar J.A. MRSA transmission between cows and humans. Emerg. Infect. Dis. 2007;13:630–632. doi: 10.3201/eid1304.060833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hanselman B.A., Kruth S.A., Rousseau J., Low D.E., Willey B.M., McGeer A., Weese J.S. Methicillin resistant Staphylococcus aureuscolonization in veterinary personnel. Emerg. Infect. Dis. 2006;12:1933–1938. doi: 10.3201/eid1212.060231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kluytmans J.A.J.W. Methicillin-resistant Staphylococcus aureus in food products: Cause for concern or case for complacency? Clin. Microbiol. Infect. 2010;16:11–15. doi: 10.1111/j.1469-0691.2009.03110.x. [DOI] [PubMed] [Google Scholar]
- 28.Basanisi M.G., Nobili G., La Bella G., Russo R., Spano G., Normanno G., La Salandra G. Molecular characterization of Staphylococcus aureus isolated from sheep and goat cheeses in southern Italy. Small Rumin. Res. 2016;135:17–19. doi: 10.1016/j.smallrumres.2015.12.024. [DOI] [Google Scholar]
- 29.Spanu V., Scarano C., Cossu F., Pala C., Spanu C., De Santis E.P.L. Antibiotic resistance traits and molecular subtyping of Staphylococcus aureus isolated from raw sheep milk cheese. J. Food Sci. 2014;16:11–15. doi: 10.1111/j.1469-0691.2009.03110.x. [DOI] [PubMed] [Google Scholar]
- 30.Hernández M., Fernández-Natal I., Rodríguez-Lázaro D. Methicillin-resistant Staphylococcus aureus harboring mecC in livestock in Spain. J. Clin. Microbiol. 2014;52:4067–4069. doi: 10.1128/JCM.01815-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pexara A., Solomakos N., Sergelidis D., Angelidis A.S., Govaris A., Gr A.U. Occurrence and antibiotic resistance of enterotoxigenic Staphylococcus aureus in raw ovine and caprine milk in Greece. Dairy Sci. Technol. 2016;96:345–357. doi: 10.1007/s13594-015-0272-z. [DOI] [Google Scholar]
- 32.Blaiotta G., Fusco V., von Eiff C., Villani F., Becker K. Biotyping of enterotoxigenic Staphylococcus aureus by enterotoxin gene cluster (egc) polymorphism and spa typing analyses. Appl. Environ. Microbiol. 2006;72:6117–6123. doi: 10.1128/AEM.00773-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mello P.L., Riboli D.F.M., Pinheiro L., Martins L. de A., Brito M.A.V.P., da Cunha M. de L.R. de S. Detection of enterotoxigenic potential and determination of clonal profile in Staphylococcus aureus and coagulase-negative staphylococci isolated from bovine subclinical mastitis in different Brazilian states. Toxins (Basel). 2016;8:1–10. doi: 10.3390/toxins8040104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Del Giudice P., Blanc V., De Rougemont A., Bes M., Lina G., Hubiche T., Roudière L., Vandenesch F., Etienne J. Primary skin abscesses are mainly caused by panton-valentine leukocidin-positive Staphylococcus aureus strains. Dermatology. 2009;219:299–302. doi: 10.1159/000232391. [DOI] [PubMed] [Google Scholar]
- 35.Zecconi A., Cesaris L., Liandris E., Daprà V., Piccinini R. Role of several Staphylococcus aureus virulence factors on the inflammatory response in bovine mammary gland. Microb. Pathog. 2006;40:177–183. doi: 10.1016/j.micpath.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 36.Aires-de-Sousa M. Methicillin-resistant Staphylococcus aureus among animals: Current overview. Clin. Microbiol. Infect. 2016:S1198–743X(16)30557–2. doi: 10.1016/j.cmi.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 37.Esposito S., Purrello S.M., Bonnet E., Novelli A., Tripodi F., Pascale R., Unal S., Milkovich G. Central venous catheter-related biofilm infections: An up-to-date focus on meticillin-resistant Staphylococcus aureus. J. Glob. Antimicrob. Resist. 2013;1:71–78. doi: 10.1016/j.jgar.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 38.Macedo-Viñas M., Conly J., Francois P., Aschbacher R., Blanc D.S., Coombs G., Daikos G., Dhawan B., Empel J., Etienne J., et al. Antibiotic susceptibility and molecular epidemiology of Panton-Valentine leukocidin-positive meticillin-resistant Staphylococcus aureus: An international survey. J. Glob. Antimicrob. Resist. 2014;2:43–47. doi: 10.1016/j.jgar.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 39.Stegger M., Andersen P.S., Kearns A., Pichon B., Holmes M.A., Edwards G., Laurent F., Teale C., Skov R., Larsen A.R. Rapid detection, differentiation and typing of methicillin-resistant Staphylococcus aureus harbouring either mecA or the new mecA homologue mecALGA251. Clin. Microbiol. Infect. 2012;18:395–400. doi: 10.1111/j.1469-0691.2011.03715.x. [DOI] [PubMed] [Google Scholar]
- 40.Cuny C., Layer F., Werner G., Harmsen D., Daniels-Haardt I., Jurke A., Mellmann A., Witte W., Köck R. State-wide surveillance of antibiotic resistance patterns and spa-types of methicillin-resistant Staphylococcus aureus from blood cultures in North Rhine-Westphalia, 2011–2013. Clin. Microbiol. Infect. 2015;21:750–757. doi: 10.1016/j.cmi.2015.02.013. [DOI] [PubMed] [Google Scholar]
- 41.Walther B., Wieler L.H., Vincze S., Antão E.M., Brandenburg A., Stamm I., Kopp P.A., Kohn B., Semmler T., Lübke-Becker A. MRSA variant in companion animals. Emerg. Infect. Dis. 2012;18:2017–2020. doi: 10.3201/eid1812.120238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vincze S., Brandenburg A.G., Espelage W., Stamm I., Wieler L.H., Kopp P.A., Lübke-Becker A., Walther B. Risk factors for MRSA infection in companion animals: Results from a case-control study within Germany. Int. J. Med. Microbiol. 2014;304:787–793. doi: 10.1016/j.ijmm.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 43.Van Leeuwen W.B., Melles D.C., Alaidan A., Al-Ahdal M., Boelens H.A.M., Snijders S.V., Wertheim H., Van Duijkeren E., Peeters J.K., Van Der Spek P.J., et al. Host- and tissue-specific pathogenic traits of Staphylococcus aureus. J. Bacteriol. 2005;187:4584–4591. doi: 10.1128/JB.187.13.4584-4591.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee J.H. Methicillin (Oxacillin)-Resistant Staphylococcus aureus Strains Isolated from Major Food Animals and Their Potential Transmission to Humans. Appl. Environ. Microbiol. 2003;69:6489–6494. doi: 10.1128/AEM.69.11.6489-6494.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Merz A., Stephan R., Johler S. Staphylococcus aureus isolates from goat and sheep milk seem to be closely related and differ from isolates detected from bovine milk. Front. Microbiol. 2016;7:1–7. doi: 10.3389/fmicb.2016.00319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Diep B.A., Gill S.R., Chang R.F., Phan T.H., Chen J.H., Davidson M.G., Lin F., Lin J., Carleton H.A. Complete genome sequence of USA300, an epidemic clone of community-acquired meticillin-resistant Staphylococcus aureus. Lancet. 2006;367:731–739. doi: 10.1016/S0140-6736(06)68231-7. [DOI] [PubMed] [Google Scholar]
- 47.Puah S.M., Chua K.H., Mary Anne Tan J.A. Virulence factors and antibiotic susceptibility of Staphylococcus aureus isolates in ready-to-eat foods: Detection of S. aureus contamination and a high prevalence of virulence genes. Int. J. Environ. Res. Public Health. 2016;13:1–9. doi: 10.3390/ijerph13020199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Johler S., Giannini P., Jermini M., Hummerjohann J., Baumgartner A., Stephan R. Further evidence for staphylococcal food poisoning outbreaks caused by egc-Encoded enterotoxins. Toxins (Basel). 2015;7:997–1004. doi: 10.3390/toxins7030997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yu F., Chen Z., Liu C., Zhang X., Lin X., Chi S., Zhou T., Chen Z., Chen X. Prevalence of Staphylococcus aureus carrying Panton-Valentine leukocidin genes among isolates from hospitalised patients in China. Clin. Microbiol. Infect. 2008;14:381–384. doi: 10.1111/j.1469-0691.2007.01927.x. [DOI] [PubMed] [Google Scholar]
- 50.Morandi S., Brasca M., Andrighetto C., Lombardi A., Lodi R. Phenotypic and Genotypic Characterization of Staphylococcus aureus Strains from Italian Dairy Products. Int. J. Microbiol. 2010;2009 doi: 10.1155/2009/501362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scherrer D., Corti S., Muehlherr J.E., Zweifel C., Stephan R. Phenotypic and genotypic characteristics of Staphylococcus aureus isolates from raw bulk-tank milk samples of goats and sheep. Vet. Microbiol. 2004;101:101–107. doi: 10.1016/j.vetmic.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 52.Mehrotra M., Wang G., Johnson W.M. Multiplex PCR for detection of genes for Staphylococcus aureus enterotoxins, exfoliative toxins, toxic shock syndrome toxin 1, and methicillin resistance. J. Clin. Microbiol. 2000;38:1032–1035. doi: 10.1128/jcm.38.3.1032-1035.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kérouanton A., Hennekinne J.A., Letertre C., Petit L., Chesneau O., Brisabois A., De Buyser M. Characterization of Staphylococcus aureus strains associated with food poisoning outbreaks in France. Int. J. Food Microbiol. 2007;115:369–375. doi: 10.1016/j.ijfoodmicro.2006.10.050. [DOI] [PubMed] [Google Scholar]
- 54.Devriese L.A. A simplified system for biotyping Staphylococcus aureus strains isolated from animal species. J. Appl. Bacteriol. 1984;56:215–220. doi: 10.1111/j.1365-2672.1984.tb01341.x. [DOI] [PubMed] [Google Scholar]
- 55.Hennekinne J.A., Kerouanton A., Brisabois A., De Buyser M.L. Discrimination of Staphylococcus aureus biotypes by pulsed-field gel electrophoresis of DNA macro-restriction fragments. J. Appl. Microbiol. 2003;94:321–329. doi: 10.1046/j.1365-2672.2003.01837.x. [DOI] [PubMed] [Google Scholar]
- 56.Harmsen D., Claus H., Witte W., Rothgänger J., Claus H., Turnwald D., Vogel U. Typing of Methicillin-Resistant Staphylococcus aureus in a University Hospital Setting by Using Novel Software for spa Repeat Determination and Database Management. J. Clin. Microbiol. 2003;41:5442–5448. doi: 10.1128/JCM.41.12.5442-5448.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.