Abstract
Research about antibody specificity spectra was conducted to develop single-specific antibodies or broad-specific antibodies. Aflatoxins, as one class of high-toxicity mycotoxins, were selected as the research targets to investigate the effect of the immunogen dose on antibody specificity spectra. For this aim, 16 monoclonal antibodies were induced by low or high doses of aflatoxin B1-BSA, and 34 monoclonal antibodies were induced by low or high doses of aflatoxin M1-BSA. The specificities of the antibodies induced, whether by aflatoxin B1 conjugate or aflatoxin M1 conjugate, indicated that the low dose of the immunogen induced a narrow spectrum of antibody specificity, while the high dose of the immunogen showed an advantage to form a broad spectrum of antibody specificity. Therefore, this report provides important information for the development of new antibodies against small molecules like aflatoxins.
Keywords: specificity spectrums, dose of immuogen, antibodies, aflatoxin
1. Introduction
Due to immunochemical specificity, using the same immunogen could induce different spectra of antibody specificity. Narrow spectra of antibody specificity are assemblages of specific antibodies that recognize a single target with high specificity. However, broad spectra of antibody specificity are collectives of specific antibodies that can detect various related compounds in one simple test [1,2]. With respect to the research, the specificity spectra of antibodies contributes to the development of narrow-spectrum antibody specificity or broad-spectrum antibody specificity more easily.
Until 2000, researchers put their main efforts into the development of narrow-spectrum antibody specificity [3,4,5]. However, with the advent of congener toxins in food, such as sulfonamides, triazine herbicides, organophosphorus (OP), aflatoxins, etc., multi-analyte determination has attracted considerable interest when screening large numbers of food samples [6,7,8]. Instrumental methods have the potential for simultaneous determination of multiple analogues and may be more specific and sensitive than immunoassays. However, they are expensive and need a larger amount of time for sample preparation before analysis, which has inhibited extending the scope of monitoring, particularly in field-screening scenarios [9]. As an alternative, broad-specificity immunoassays are extraordinarily effective for monitoring and detecting samples of multi-analyte residues in food and environmental samples [10,11], and the development of broad-specificity immunoassays demands the preparation of a broad spectrum of antibody specificity to all target analytes. For example, several attempts have been made to develop broad-specificity immunoassays for OP pesticide residues by the production of a broad-spectrum-specific antibody against OP pesticide [12].
The most commonly used method to produce a broad-specificity immunoassay is to produce an antibody having broad-specificity by using a “generic hapten”, which should demonstrate the common characteristics of all target analytes [12]. Due to the lack of understanding of the specific interactions between antibodies and target analytes or haptens, the antibody specificity resulting from the newly-designed hapten is often unpredictable, and this result comes only after laborious and time-consuming animal experiments. Sometimes an apparent rationally-designed “generic hapten” is unable to generate antibodies with the desirable sensitivity and specificity [13,14,15]. The diverse exposure of an antigenic determinant could form a broad spectrum of the antibody specificity. The most common method of raising the diverse exposure of the antigenic determinant is improve the coupling ratio of the coupling reaction between the hapten and the carrier protein. Another approach to raise the diverse exposure of the antigenic determinant is to use the flexible connection arm with the proper length, and increase the chance of diversity exposure of the hapten. However, the specificity of the antibody was not only impressed by the different structures of the antigen, but also by the immunogenicity or efficiency of the antigen [16], such as the dose of the immunogen. The aim of the present study was to research whether the dose of the immunogen was a key influencing factor to obtain a broad spectrum of antibody specificity or not.
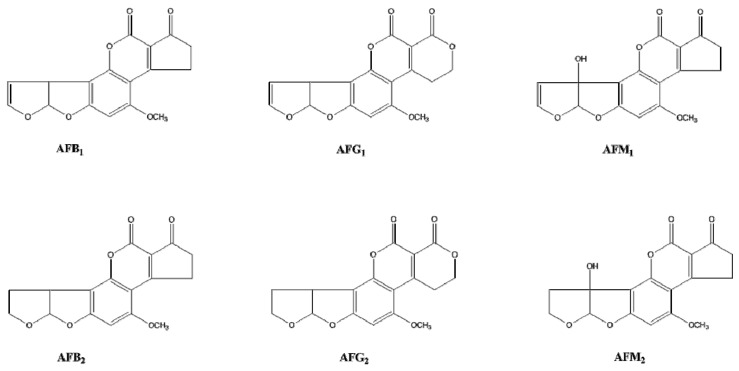
Aflatoxins (AF) are members of the coumarin family and have become a main threat worldwide because they are teratogenic, extremely toxic, mutagenic, and carcinogenic. Due to the varying structure of different aflatoxins causing an issue in the development of diagnostic techniques, aflatoxins were chosen as research subjects in this paper [17]. Since all aflatoxins have a similar core structure (Figure 1), it should be possible to develop a single antibody that is able to screening a single target with high specificity, or obtain a generic immunoassay for simultaneous recognition of multiple aflatoxins. Additionally, the strong, rigid structure of aflatoxin molecules are an advantage to study the antigen-antibody interaction [18]. On the other hand, based on 10 years of research on aflatoxins, our team had accumulated a significant amount of hybridoma and monoclonal antibodies, which function against aflatoxins. As it turns out, we found the dose of the immunogen greatly contributes to the specificity spectra of antibodies against aflatoxins. Low doses of the immunogen helped to obtain a narrow spectrum of antibody specificity, while a high dose of the immunogen would help to form a broad spectrum of the antibody specificity.
Figure 1.
Structures of the main aflatoxins.
2. Results and Discussion
2.1. The Influence on the Dose of the Immunogen against AFB1
In each experiment, three female Balb/c mice were subcutaneously immunized with the different doses of the immunogen (AFB1-BSA) in multiple sites, and three subsequent subcutaneous injections followed. After the procedure of cell fusion and cloning six times, the stable hybridoma lines were injected into Balb/c hybrid mice and 10–15 mL ascitic fluid was collected from mAbs (monoclonal antibody) from each mouse. Thus, nine mAbs with high sensitivity were obtained in mice immunized with 33 µg every time, and seven were screened successfully in the same way in mice immunized with 150 µg every time.
The mAb specificity was estimated by cross-reactivity (CR) with AFB2, AFG1, AFG2, and AFM1 via indirect competitive enzyme-linked immunosorbent assay (ic-ELISA). As the structure of AFB2, AFG1, AFG2, and AFM1 are very similar to AFB1, they were selected. The value of CR was used to estimate the specificity of ic-ELISA. The cross-reactivity values for different aflatoxins was determined by comparing the IC50 values of analytes and calculated according to the following equation: CR (%) = [IC50 (AFB1)/IC50 (analyte)] × 100 [8]. The detailed CR data are summarized in Table 1.
Table 1.
Comparison of the cross-reactivity of anti-AFB1 antibodies.
| Dose of Immunogen | mAb | IC50 for AFB1 (ng mL−1) | Cross-Reactivity (%) | ||||
|---|---|---|---|---|---|---|---|
| (µg per Balb/c Mice) | AFB1 | AFB2 | AFG1 | AFG2 | AFM1 | ||
| 33 | 1B5 | 0.012 | 100 | 4 | 3 | <0.1 | <0.1 |
| 33 | 2F12 | 0.010 | 100 | 5 | 2 | 0.2 | <0.1 |
| 33 | 2C7 | 0.020 | 100 | 6 | 8 | 3 | <0.1 |
| 33 | 1F11 | 0.052 | 100 | 7 | 2 | <0.1 | <0.1 |
| 33 | 3H3 | 0.023 | 100 | 12 | 4 | <1 | <0.1 |
| 33 | 7H7 | 0.052 | 100 | 26 | 5 | 5 | <0.1 |
| 33 | 5E2 | 0.013 | 100 | 33 | 3 | 2 | <0.1 |
| 33 | 7B12 | 0.012 | 100 | 61 | 6 | 6 | <0.1 |
| 33 | 6B7 | 0.027 | 100 | 72 | 5 | 10 | <0.1 |
| 150 | 1D3 | 0.44 | 100 | 54 | 115 | 16 | 33 |
| 150 | 4F12 | 0.086 | 100 | 90 | 85 | 43 | 21 |
| 150 | 1C11 | 0.001 | 100 | 92 | 53 | 7 | 9 |
| 150 | 10C9 | 2.09 | 100 | 94 | 95 | 65 | 71 |
| 150 | 8F6 | 1.70 | 100 | 104 | 100 | 47 | 65 |
| 150 | 10G4 | 0.73 | 100 | 136 | 155 | 50 | 51 |
| 150 | 4F3 | 0.29 | 100 | 171 | 200 | 57 | 108 |
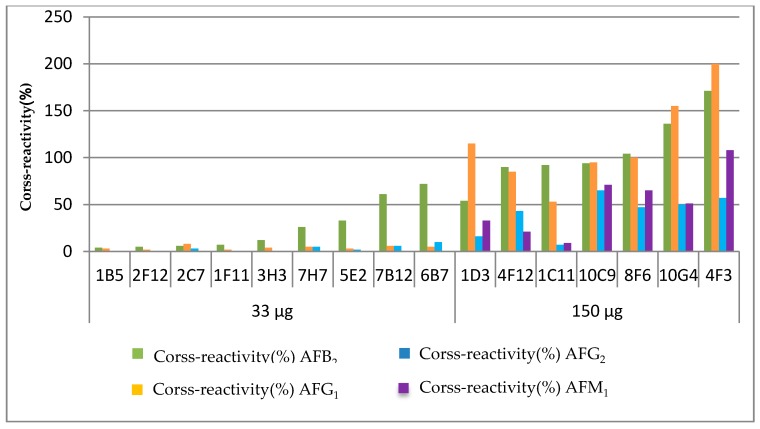
The results demonstrate that the CR values of antibodies screened from Mouse Group 1 (the mice immunized with 33 μg) were under 10% when against AFG1 and AFG2, and showed no cross-reaction with AFM1. The cross-reactivity was partly evident against AFB2, but the CR values of seven mAbs obtained from Mouse Group 1 were under 50%, only two mAbs were higher than 50%. Compared with Mouse Group 2 (the mice immunized with 150 μg), the dose of the immunogen was nearly five times that of Mouse Group 1, and the specificity spectra of the antibodies were completely different. The seven mAbs that were obtained from Mouse Group 2 showed broad-specificity towards all of the analogues. Figure 2 shows the distribution of the specificity spectra of the 16 antibodies. For example, the CR values of AFB2 ranged from 54% up to 171%, and all exceeded 50%. With the low dose of the immunogen, the CR values of AFB2 ranged from 4 to 72%. It seems that the mAbs obtained from Mouse Group 1 showed narrow spectra of antibody specificity, and were broader with the higher dose of the immunogen.
Figure 2.
The distribution of specificity spectrums of antibodies against AFB1.
2.2. The Influence on the Dose of the Immunogen against AFM1
To verify whether the dose of the immunogen contributes to the specificity spectra of the antibodies against aflatoxin or not, a similar test was conducted which used AFM1 as the analyte. Based on works we had conducted before, the dose of the immunogen, comparing between 16 µg per Balb/c mice and 65 µg per Balb/c mice, resulted in us obtaining 34 kinds of monoclonal antibodies against AFM1. The aflatoxins AFB1, AFB2, AFG1, and AFG2 as analogues of AFM1 were tested for cross-reactivities (CR). The sensitivity and cross-reactivity are displayed in Table 2.
Table 2.
Comparison of the cross-reactivity of anti-AFM1.
| Dose of Immunogen (µg per Balb/c Mice) |
mAb | IC50 for AFM1 (ng mL−1) | Cross-Reactivity (%) | ||||
|---|---|---|---|---|---|---|---|
| AFM1 | AFB1 | AFB2 | AFG1 | AFG2 | |||
| 16 | 2C9 | 0.067 | 100 | <1 | <1 | <1 | <1 |
| 16 | 3C4 | 0.043 | 100 | <1 | <1 | <1 | <1 |
| 16 | 1D7 | 0.058 | 100 | <1 | <1 | <1 | <1 |
| 65 | LM43 | 0.014 | 100 | <1 | <1 | <1 | <1 |
| 65 | LM3 | 0.029 | 100 | 1 | <1 | <1 | <1 |
| 65 | LM10 | 0.034 | 100 | 1 | <1 | <1 | <1 |
| 65 | LM14 | 0.017 | 100 | 1 | <1 | <1 | <1 |
| 65 | LM47 | 0.020 | 100 | 1 | <1 | <1 | <1 |
| 65 | LM15 | 0.006 | 100 | 1 | <1 | <1 | <1 |
| 65 | LM20 | 0.011 | 100 | 2 | <1 | <1 | <1 |
| 65 | LM39 | 0.069 | 100 | 2 | <1 | <1 | <1 |
| 65 | LM37 | 0.017 | 100 | 3 | <1 | <1 | <1 |
| 65 | LM54 | 0.029 | 100 | 3 | <1 | <1 | <1 |
| 65 | LM16 | 0.030 | 100 | 3 | <1 | 1 | <1 |
| 65 | LM40 | 0.029 | 100 | 5 | <1 | 2 | <1 |
| 65 | LM7 | 0.052 | 100 | 5 | <1 | 2 | <1 |
| 65 | LM4 | 0.014 | 100 | 6 | <1 | 3 | <1 |
| 65 | LM17 | 0.069 | 100 | 14 | 6 | <1 | 2 |
| 65 | LM13 | 0.011 | 100 | 20 | 3 | <1 | 2 |
| 65 | LM41 | 0.015 | 100 | 11 | 10 | 1 | 11 |
| 65 | LM9 | 0.014 | 100 | 11 | 8 | 1 | 3 |
| 65 | LM21 | 0.011 | 100 | 19 | 18 | 2 | 22 |
| 65 | LM30 | 0.023 | 100 | 33 | 20 | 9 | 10 |
| 65 | LM38 | 0.014 | 100 | 40 | 22 | 4 | 16 |
| 65 | LM46 | 0.011 | 100 | 40 | 22 | 25 | 18 |
| 65 | LM48 | 0.012 | 100 | 74 | 57 | 21 | 65 |
| 65 | LM28 | 0.012 | 100 | 75 | 47 | 18 | 102 |
| 65 | LM44 | 0.019 | 100 | 78 | 15 | 43 | 9 |
| 65 | LM11 | 0.019 | 100 | 86 | 18 | 14 | 15 |
| 65 | LM49 | 0.019 | 100 | 92 | 30 | 66 | 74 |
| 65 | LM33 | 0.013 | 100 | 101 | 97 | 13 | 11 |
| 65 | LM50 | 0.035 | 100 | 112 | 39 | 31 | 32 |
| 65 | LM32 | 0.046 | 100 | 114 | 67 | 60 | 110 |
| 65 | LM51 | 0.023 | 100 | 175 | 29 | 59 | 39 |
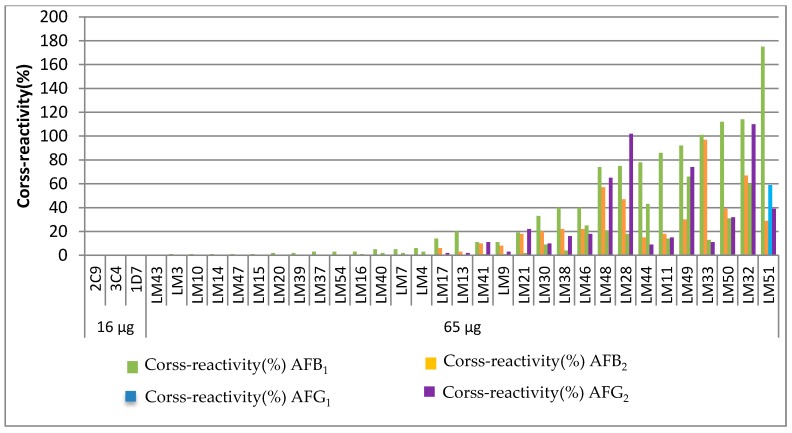
The sensitivities of antibodies were basically similar, but the cross-reactivities were different. No significant cross-reactivity was observed against aflatoxin B1, B2, G1, and G2 for the mAb of 2C9, 3C4, or 1D7. However, when the dose of the immunogen was increased from 16 µg to 65 µg, the specificity spectra of antibodies changed. The antibodies showed good cross-reactivity and could be classified broadly into five distinct groups. LM43 was assigned to Group 1, and the CR value was equal to that of 2C9 mAb, which could not cross-react with aflatoxin B1, B2, G1, and G2. Nine monoclonal antibodies were assigned to Group 2: LM3, LM10, LM14, LM47, LM15, LM20, LM39, LM37, and LM54, which had weak reaction efficiency with B1 but showed no cross-reaction with B2, G1, G2. Group 3 contained four monoclonal antibodies, LM16, LM40, LM7, and LM4, which had low CR values of B1 and G1 and showed no cross-reaction with B2 and G2. LM17 and LM13, with low CR values of B1, B2, and G1, were assigned to Group 4. The remaining were assigned to Group 5, which had similar reaction intensities with the other four aflatoxins. In particular, the antibody LM32 showed a high reactive specificity and sensitivity for the five toxins, as well as cross-reactivity with closely-related toxins. Longitudinally, when the dose of the immunogen increased to 65 µg, the CR values of AFB1 ranged from 1 to 175%, the CR values of AFB2 ranged from 1 to 97%, the CR values of AFG1 ranged from 1 to 66%, and the CR values of AFG1 ranged from 1 to 110%. We can draw a conclusion from the data of the five distinct groups that the higher the dose of the immunogen resulted in a wider range of CR values. Figure 3 shows the distribution of the specificity spectra of five types of antibodies. All of the results are in agreement with what might have been predicted by using different doses of the immunogen that exhibited different specificity spectra of antibodies. The greater the dose of the immunogen, the wider the specificity spectra of the antibodies may be.
Figure 3.
The distribution of specificity spectrums of antibodies against AFM1.
2.3. Discussion
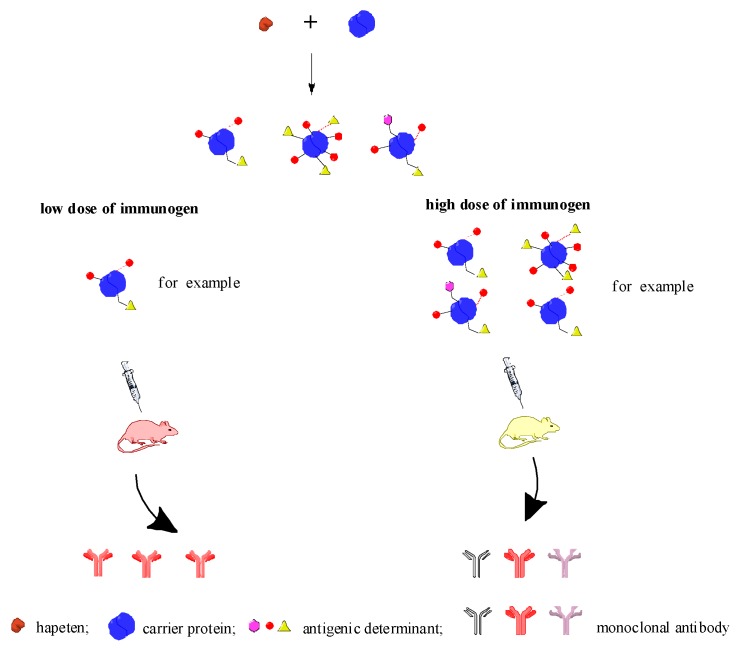
The study provides a potential method for the development of narrow/broad spectra of antibody specificity by controlling the dose of the immunogen. Antibody–antigen interactions are indispensable to immunoassay, although the interactions at the molecular level are, in general, undetermined. It is suggested that the antigen–antibody recognition is based on steric criteria and on interactions resulting from the electronic properties of the molecules [19], so the position space or quantity of antigen epitopes may play a significant role in antigen–antibody interaction. For example, in the sketch in Figure 4, the same hapten conjugates to the carrier protein may turn up different antigen epitopes, which means the antigenic determinants are different, with the difference containing the length of the spacer arm, position space of the antigen epitopes, the coupling ratio of the hapten and the carrier protein, etc. When increasing the dose of the immunogen, the antigenic determinant became more diversely exposed. Thus, the opportunity to select various monoclonal antibodies arose, which means a low dose of the immunogen was helpful to obtain a narrow spectrum of antibody specificity, while a high dose of the immunogen helped to form a broad spectrum of antibody specificity.
Figure 4.
Model for different dose of immunogen acting on Balb/c mice.
3. Conclusions
In this study, the contribution to the specificity spectra of antibodies against aflatoxins was discussed. It turned out that a low dose of the immunogen helped obtain a narrow spectrum of antibody specificity while a high-dose of the immunogen helped to form a broad spectrum of antibody specificity. To verify the point, we used different doses of the immunogen to develop zearalenone mAb and capsaicine mAb, and the result was found to be consistent.
In conclusion, the findings provide a foundation for the development of specificity spectra of antibodies and for the establishment of broad-spectrum rapid screening methods for toxins.
4. Experimental Section
4.1. Chemicals and Instruments
Aflatoxin M1-BSA conjugate (Lot # 083m4109v, 4.24 mole AFM1 per mole BSA, coupling through active ester with engineered Aflatoxin M1), aflatoxin B1-BSA conjugate (Lo t# 093m405080, 8.7 mole AFB1 per mole BSA, coupling through active ester with engineered Aflatoxin B1), Aflatoxin M1, B1, B2, G1, and G2 standard solution, incomplete and complete Freund’s adjuvants, 3,3′,5,5′-tetramethylbenzidine (TMB), goat anti-mouse IgG-horseradish peroxidase (IgG-HRP), polyethylene glycol 1450, hypoxanthine-thymidine, and hypoxanthine–aminopterin–thymidine, were all obtained from Sigma-Aldrich(St. Louis, MO, USA). Fetal bovine serum, streptomycin (10,000 Lg/mL), and penicillin (10,000 U/mL) were from Gibco. RPMI-1640 medium, l-glutamine, and HEPES (2-[4-(2-Hydroxyethyl)-1-piperazinyl]ethanesulfonic acid) were from HyClone. All other reagents were of analytical reagent grade or better, unless otherwise stated. Female Balb/c mice were obtained from the Centers for Disease Control and Prevention of Hubei Province. Water was obtained from a MilliQ purification system.
Cell culture plates were purchased from Iwaki Co. (Iwaki, Japan). Absorbance was measured at a wavelength of 450 nm using a SpectraMax M2e microplate reader from PerkinElmer (Waltham, MA, USA). Polystyrene 96-well microtiter plates were obtained from Costar (Cambridge, MA, USA). The mice were approved by The Laboratory Animal Monitoring Committee of Hubei Province (identification code: 42000600015661; date of approval: 2016.07.05).
4.2. Immunization
In the first immunization, different doses of the immunogen were dissolved in sterilized 0.85% NaCl solution and then emulsified with an equal volume of Freund’s complete adjuvant, and the final water-in-oil emulsion was injected into multiple sites subcutaneously into three eight-week-old female Balb/c mice.
Different doses of the immunogen were conducted with the same doses using Freund’s incomplete adjuvant and injected on the fourth week, seventh week, and ninth week after the initial immunization. At the seventh day after the fourth injection, antisera were gathered from the caudal vein of each mouse and assayed for anti-aflatoxin B1 or M1 antibodies by indirect competitive ELISAs (ic-ELISAs) with aflatoxin M1, B1, B2, G1, and G2 as the competitors. The mice whose antiserum exhibited higher sensitivity were given an intraperitoneal booster three days before the spleen was removed. The booster injection used a two-fold dose of antigen without emulsification with adjuvant.
4.3. Production of mAbs
The hybridoma cells were obtained by fusion of SP2/0 murine myeloma cells with the spleen cells isolated from the selected mice using PEG 2000 [20]. After cell fusion, when the hybridoma cells were grown to approximately 40% confluence in wells at 7–10 days, culture supernatants were collected and screened using indirect ELISA for the presence of antihapten antibodies. Selected hybridomas were cloned by limiting dilution, and stable antibody-producing clones were expanded. An indirect competitive ELISA (ic-ELISA) was employed to screen if the antibodies could recognize the analytes. Antibodies were generated by ascites growth using the selected clones. Ascites fluids were collected and purified using the method of caprylic acid-ammonium sulfate precipitation and were used in the following ELISA [21].
4.4. Evaluation of Antibody Sensitivity and Cross-Reactivity
The indirect competitive ELISA format as described was used to evaluate the sensitivity of each monoclonal antibody. The procedure of the ic-ELISA was as follows: flat-bottom polystyrene ELISA plates were coated with AFM1-BSA/AFBI-BSA (100 μL/well) in carbonate buffer (pH 9.6) at 37 °C overnight, and the each well was blocked with 200 μL 4% skim milk in PBST solution at 37 °C for 1 h. Each well was incubated with 50 μL of the analyte in methanol-PBS and 50 μL of optimized dilutions of antibodies were added. After incubation at 37 °C for 40 min, goat anti-rabbit IgG-HRP diluted to 1:5000 was added (100 μL/well) to each well, and the plates were incubated for 30 min at 37 °C. Then, 100 μL per well of TMB solution was added and incubated for 10 min at 37 °C. After each step, a PBST washing step was carried out. The reaction was stopped by addition of 50 μL of 2 M H2SO4 and the OD values were recorded at 450 nm. The optimum dilution of antibody required as a working concentration was defined as the dilution which gave an absorbance closest to 1.0 [22]. Each competition reaction was carried out in duplicate with at least seven concentrations of aflatoxin, and the last well was blank to contrast. %B/B0 could express these data while the absorbance in the absence of analyte was B0, and the value of B represents the absorbance at each concentration of analyte. The cross-reactivity values, CR, for different aflatoxins was determined by comparing the IC50 values of the analytes and calculated according to the following equation: % CR = (IC50 AFB1 or AFM1/IC50 analyte) × 100 [23].
Acknowledgments
This work was supported by the National Natural Science Foundation of China (31471650), International Science & Technology Cooperation Program of China (2016YFE0112900), and the key project in the Hubei Science & Technology Pillar Plan (2014BBB017).
Author Contributions
Peiwu Li and Qi Zhang conceived and designed the experiments; Jing Wu, Xiaoqian Tang, Zhaowei Zhang and Wen Zhang performed the experiments; Qi Zhang and Li Zhang analyzed the data; Zhiyong Fan, Tingting Yu and Feng Jiang contributed reagents/materials/analysis tools; Peiwu Li and Jing Wu wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Wang S.T., Gui W.J., Guo Y.R., Zhu G.N. Preparation of a multihapten antigen and broad specificity polyclonal antibodies for a multiple pesticide immunoassay. Anal. Chim. Acta. 2007;587:287–292. doi: 10.1016/j.aca.2007.01.052. [DOI] [PubMed] [Google Scholar]
- 2.Wang Z., Zhu Y., Ding S., He F., Beier R.C., Li J., Jiang H., Feng C., Wan Y., Zhang S., et al. Development of a monoclonal antibody-based broad-specificity ELISA for fluoroquinolone antibiotics in foods and molecular modeling studies of cross-reactive compounds. Anal. Chem. 2007;79:4471–4483. doi: 10.1021/ac070064t. [DOI] [PubMed] [Google Scholar]
- 3.Chu F.S., Steinert B.W., Gaur P.K. Production and characterization of antibody against aflatoxin G1. J. Food Saf. 1985;7:161–170. doi: 10.1111/j.1745-4565.1985.tb00539.x. [DOI] [Google Scholar]
- 4.Albrecht R. Development of antibacterial agents of the nalidixic acid type. Drug Res. 1977;21:91–104. doi: 10.1007/978-3-0348-7098-6_1. [DOI] [PubMed] [Google Scholar]
- 5.Brimfield A.A., Lenz D.E., Graham C., Hunter K.W. Mouse monoclonal antibodies against paraoxon: Potential reagents for immunoassay with constant immunochemical characteristics. J. Agric. Food Chem. 1985;33:1237–1242. doi: 10.1021/jf00066a055. [DOI] [Google Scholar]
- 6.Bucknall S., Silverlight N.J., Coldham L., Thorne R. Antibodies to the quinolones and fluoroquinolones for the development of generic and specific immunoassays for detection of these residues in animal products. Food Addit. Contam. 2003;20:221–228. doi: 10.1080/0265203021000055388. [DOI] [PubMed] [Google Scholar]
- 7.Xu Z.L., Shen Y.D., Zheng W.X., Beier R.C., Xie G.M., Dong J.X., Yang J.Y., Wang H., Lei H.T., She Z.G., et al. Broad-specificity immunoassay for O,O-diethyl organophosphorus pesticides: Application of molecular modeling to improve assay sensitivity and study antibody recognition. Anal. Chem. 2010;82:9314–9321. doi: 10.1021/ac1018414. [DOI] [PubMed] [Google Scholar]
- 8.Kato M., Ihara Y., Nakata E., Miyazawa M., Sasaki M., Kodaira T., Nakazawa H. Development of enrofloxacin ELISA using a monoclonal antibody tolerating an organic solvent with broad cross-reactivity to other new quinolones. Food Agric. Immunol. 2007;18:179–187. doi: 10.1080/09540100701763365. [DOI] [Google Scholar]
- 9.Anklam E., Stroka J., Boenke A. Acceptance of analytical methods for implementation of EU legislation with a focus on mycotoxins. Food Control. 2002;13:173–183. doi: 10.1016/S0956-7135(01)00098-6. [DOI] [Google Scholar]
- 10.Ma F., Wu R., Li P.W., Yu L. Analytical approaches for measuring pesticides, mycotoxins and heavy metals in vegetable oils: A review. Eur. J. Lipid Sci. Technol. 2016;118:339–352. doi: 10.1002/ejlt.201400535. [DOI] [Google Scholar]
- 11.Zhang D.H., Li P.W., Zhang Q., Yang Y., Zhang W., Guan D., Ding X.X. Extract-free immunochromatographic assay for on-site tests of aflatoxin M1 in milk. Anal. Methods. 2012;4:3307–3313. doi: 10.1039/c2ay25205h. [DOI] [Google Scholar]
- 12.Xu Z.L., Xie G.M., Li Y.H., Wang B.F., Lei H.L., Wang H., Shen Y.D., Sun Y.M. Production and characterization of a broad-specificity polyclonal antibody for O,O-diethyl organophosphorus pesticides and a quantitative structure-activity relationship study of antibody recognition. Anal. Chim. Acta. 2009;647:90–96. doi: 10.1016/j.aca.2009.05.025. [DOI] [PubMed] [Google Scholar]
- 13.Anfossi L., Calderara M., Baggiani C., Giovannoli C., Arletti E., Giraudi G. Development and application of solvent-free extraction for the detection of aflatoxin M1 in dairy products by enzyme immunoassay. J. Agric. Food Chem. 2008;56:1852–1857. doi: 10.1021/jf073133d. [DOI] [PubMed] [Google Scholar]
- 14.Cao L., Kong D., Sui J., Jiang T., Li J., Ma L., Lin H. Broad-specific antibodies for a generic immunoassay of quinolone: Development of a molecular model for selection of haptens based on molecular field-overlapping. Anal. Chem. 2009;81:3246–3251. doi: 10.1021/ac802403a. [DOI] [PubMed] [Google Scholar]
- 15.Zeng H.P., Chen J.H., Zhang C.J., Huang X.A., Sun Y.M., Xu Z.L., Lei H.T. Broad-Specificity chemiluminescence enzyme immunoassay for (fluoro)quinolones: Hapten design and molecular modeling study of antibody recognition. Anal. Chem. 2016;88:3909–3916. doi: 10.1021/acs.analchem.6b00082. [DOI] [PubMed] [Google Scholar]
- 16.Yuan M., Liu B., Liu E.M., Sheng W., Zhang Y., Crossan A., Kennedy I., Wang S. Immunoassay for phenylurea herbicides: Application of molecular modeling and quantitative structure-activity relationship analysis on an antigen-antibody interaction study. Anal. Chem. 2011;83:4767–4774. doi: 10.1021/ac200227v. [DOI] [PubMed] [Google Scholar]
- 17.Aly S.E. Distrubution of aflatoxins in product and by-products during glucose production from contaminated corn. Nahrung. 2002;46:341–344. doi: 10.1002/1521-3803(20020901)46:5<341::AID-FOOD341>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 18.Gacem M.A., El-Hadj-Khelil A.O. Toxicology, biosynthesis, bio-control of aflatoxin and new methods of detection. Asian Pac. J. Trop. Biomed. 2016;9:808–814. doi: 10.1016/j.apjtb.2016.07.012. [DOI] [Google Scholar]
- 19.Horn P.R., Mao Y., Head-Gordon M. Defining the contributions of permanent electrostatics, Pauli repulsion, and dispersion in density functional theory calculations of intermolecular interaction energies. J. Chem. Phys. 2016;144:114107. doi: 10.1063/1.4942921. [DOI] [PubMed] [Google Scholar]
- 20.Davis J.M., Pennington J.E., Kubler A.M., Conscience J.F. A simple, single-step technique for selecting and cloning hybridomas for the production of monoclonal antibodies. J. Immunol. Methods. 1982;50:161–171. doi: 10.1016/0022-1759(82)90222-8. [DOI] [PubMed] [Google Scholar]
- 21.Zhang J.Y., Li P.W., Zhang W., Zhang Q., Ding X.X., Chen X.M. Production and Characterization of Monoclonal Antibodies against Aflatoxin G1. Hybridoma. 2009;28:67–70. doi: 10.1089/hyb.2008.0064. [DOI] [PubMed] [Google Scholar]
- 22.Holtzapple C.K., Buckley S., Stanker L.H. Production and characterization of monoclonal antibodies against sarafloxacin and cross-reactivity studies of related fluoroquinolones. J. Agric. Food Chem. 1997;45:1984–1990. doi: 10.1021/jf960754q. [DOI] [Google Scholar]
- 23.Wang J., Wang Y.L., Pan Y.H. Preparation of a generic monoclonal antibody and development of a highly sensitive indirect competitive ELISA for the detection of phenothiazines in animal feed. Food Chem. 2017;221:1004–1013. doi: 10.1016/j.foodchem.2016.11.062. [DOI] [PubMed] [Google Scholar]