Abstract
Effective chemotherapeutic strategies for uterine sarcoma are lacking; existing therapies achieve poor response rates. Previous studies have identified the prostaglandin 15-deoxy-Δ12,14-prostaglandin J2 (15d-PGJ2) as a potential anticancer treatment; however, its effectiveness in uterine sarcoma has not been examined. Furthermore, the molecular mechanisms underlying the cytotoxic mechanism of 15d-PGJ2 remain unclear. Here, we evaluated the effects of 15d-PGJ2 alone and in combination with the tyrosine kinas inhibitor (TKI) dasatinib in uterine sarcoma cell lines (MES-SA, MES-SA/DX5 and SKN). 15d-PGJ2 inhibited cell growth and increased apoptosis. Western blotting demonstrated that 15d-PGJ2 treatment increased MEK and ERK phosphorylation, and decreased levels of phosphorylated AKT. Dasatinib in combination with 15d-PGJ2 significantly reduced cell proliferation compared with 15d-PGJ2 alone, and repressed both the AKT and MAPK pathways. The cell growth inhibition rate in the PGJ2 was 21.5±12.0, 35.3±5.4 and 28.3±4.2%, respectively (MES-SA, MES-SA/DX5 and SKN cell lines) and the cell growth inhibition rate in the combination therapy was significantly higher compared with 15d-PGJ2 alone (MES-SA; 64.2±0.8, MES-SA/DX5;23.9±8.2 and SKN; 41.4±17.6%). The PGJ2 IC50 determined by MTT assay was 27.41,10.46 and 17.38 µmol/l, respectively (MES-SA, MES-SA/DX5 and SKN cell lines) and the dasatinib IC50 was 6.68,17.30 and 6.25 µmol/l, respectively. Our findings demonstrate that 15d-PGJ2 suppresses proliferation by inactivating the AKT pathway in uterine sarcoma. Furthermore, combining 15d-PGJ2 with dasatinib produced a synergistic effect on cancer cell inhibition by repressing 15d-PGJ2-mediated activation of MAPK signaling, and further repressing AKT signaling. These results suggest that 15d-PGJ2 could be used in combination with dasatinib as a potential therapeutic approach for uterine sarcoma.
Keywords: 15-deoxy-Δ12,14-prostaglandin J2; uterine sarcoma; dasatinib; MAPK; ERK; AKT
Introduction
Uterine sarcoma is associated with aggressive characteristics and poor clinical outcome. The most effective treatment for this disease is a complete resection of the primary lesion at an early stage. However, if surgical remission cannot be achieved, the clinical outcome is poor. When postoperative chemotherapy is selected for uterine carcinosarcoma, regimens include ifosfamide, platinum-based drugs, and paclitaxel (1). However, in uterine leiomyosarcoma, chemotherapy only achieves a 30% response rate (2). Standard therapies have not been established due to difficulties with early diagnosis and drug-resistant phenotypes. Thus, the development of new therapeutic approaches is necessary to treat this disease.
15-deoxy-Δ12,14-prostaglandin J2 (15d-PGJ2, Fig. 1) is a type of prostaglandin (PG). PGs are produced from cyclooxygenase-2 (Cox-2) enzyme oxidation of arachidonic acid in response to various stress stimulations. Previous studies have shown that 15d-PGJ2 significantly inhibits cell growth and induces apoptosis in cancer cells, indicating it as a potential cancer treatment (3,4). 15d-PGJ2 induces a variety of cellular responses including activation of mitogen-activated protein kinase (MAPK) (5), modulation of Cox-2 (6), inhibition of vascular smooth muscle cell proliferation and upregulation of antioxidant response genes (7). In osteosarcoma and other cancers, 15d-PGJ2 has been demonstrated to inhibit cancer cell growth (8,9). However, its role in uterine sarcoma has not been reported.
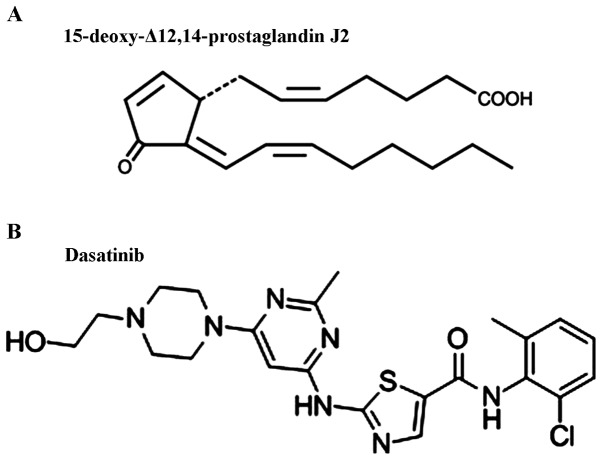
Figure 1.
(A) Chemical structure of 15-deoxy-Δ12,14-prostaglandin J2. (B) Chemical structure of dasatinib.
Peroxisome proliferator-activated receptors (PPAR) ο, β and γ are nuclear hormone receptors that regulate a multitude of downstream metabolic processes (10). In particular, PPAR-γ plays a variety of roles in antiproliferation, proapoptotic and antiangiogenic pathways (5,9–11). Numerous studies have demonstrated that PPAR-γ ligands exert antiproliferative effects in several cancers, including breast (4), prostate (12,13), and colon cancer (14,15). PPAR-γ ligands include endogenous ligands such as the eicosanoid cascade and 15d-PGJ2. PPAR-γ agonists have shown anti-cancer activity both in vitro and in vivo in combination with conventional anticancer drugs including platinum-based drugs (16), taxanes (17,18), and irinotecan (17). However, the pathways affected by treatment with PPAR-γ agonists and the most suitable combination therapy based on theoretical signaling mechanisms remain unclear.
A previous study showed inhibition of uterine sarcoma cell growth by suppression of tyrosine kinase (19). Dasatinib (V. 1) is an orally available tyrosine kinase inhibitor (TKI) that inhibits SRC protein. This drug is currently being examined in patients with acute myeloid leukemia and chronic lymphocytic leukemia (20,21) and also in several solid tumors (18,22).
In this study, we evaluated the possibility of combination therapy of PGJ2 and dasatinib in the treatment of uterine sarcoma. The results of this study may provide a novel therapeutic strategy for treatment of uterine sarcoma.
Materials and methods
Cell lines
The human uterine sarcoma cell lines MES-SA and MES-SA/DX5 and human uterine leiomyosarcoma cell line SKN were purchased from the European Collection of Cell Cultures (Salisbury, Wiltshire UK) and the Japan Health Science Foundation (Osaka, Japan), respectively. MES-SA and MES-SA/DX5 cells were cultured in McCoy medium whereas SKN cells were maintained in Ham's F12 medium supplemented with 10% fetal bovine serum, penicillin (100 U/ml), and streptomycin (100 µ/ml) at 37°C in 5% CO2.
Reagents
PJ2 was purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). Dasatinib was obtained from Focus Biomolecules (Plymouth Meeting, PA, USA) and dissolved in DMSO with a final concentration of 0.05% in the culture medium. MEK and phospho-MEK (ser217/221) antibodies were purchased from Santa Cruz Biotechnology, Inc., and SRC, phospho-SRC (tyr416), ERK, phospho-ERK (tyr204), AKT, phospho-AKT and β-actin antibodies were purchased from Cell Signaling Technology, Inc. (Beverly, MA, USA).
In vitro analyses of cell proliferation and apoptosis
Cells were seeded in 96-well culture plates at a density of 1.0×104 cells/well in a volume of 100 µl. At 24 h later, PGJ2, dasatinib or PGJ2+dasatinb were added at various concentrations and cells were incubated overnight. Next, 10 µl of 2-(4-iodophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium monosodium salt solution (WST1; Roche Diagnostics GmbH, Mannheim Germany) was added to the wells and the plates ware incubated for additional 2 h at 37°C. Absorbance at 450 nm was measured by a plate reader.
To measure cellular apoptosis, quantitative caspase-3/7 enzyme assay (Promega Corp., Madison, WI, USA) was performed. Apoptosis was also assayed by detecting DNA fragmentation using in situ terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling (TUNEL) (Promega Corp.). Cells treated with DNaseI (Promega Corp.) were used as positive controls.
Immunoblotting
Cells were collected on ice, washed with PBS, and lysed with 1x cell lysis buffer (Thermo Scientific Pierce; Life Technologies, Carlsbad, CA, USA), adding 2.5 µl protease inhibitor (WAKO, Osaka, Japan) immediately before use. Cell lysate was collected after centrifugation (12,000 rpm, 10 min) and protein concentration was determined using the Pearce BSA protein assay kit (Thermo Scientific Pierce). Equal amounts of protein samples were separated by SDS-PAGE on 7.5% acrylamide minigels and transferred to a nitrocellulose membrane. After incubation in 3% BSA blocking buffer for 2 h, the membrane was treated with one of the primary antibodies, followed by the respective secondary antibodies. The signal was detected by an ECLPlus system (GE Healthcare, Buckinghamshire, England, UK). We reprobed the membrane after incubation in 15 ml stripping buffer (Takara Bio Inc., Kusatsu, Japan) for 1 h at room temperature. The band intensities were quantified using the ImageJ software program [National Institutes of Health (NIH), Bethesda, MA, USA].
Analysis of drug synergism
The combination index (CI) was calculated for the analysis of the synergistic, antagonistic or additive effects of the two drugs. CompuSyn program (Chou and Martin) was used to compute a CI for drug combinations studied with growth assays. The Chou-Talalay combination-index method for drug combination is based on the median-effect equation which is the unified theory that provides the common link between single entity and multiple entities (23) CI>1 indicates antagonism, CI=1 indicates an additive effect and CI<1 indicates synergy.
Statistical analysis
Data shown are means ± SEM. Significant differences were determined by applying Student's t-test or ANOVA with Bonferroni's adjustment as appropriate. P<0.05 was considered to indicate a statistically significant difference.
Results
PGJ2 inhibits sarcoma cell growth and increases apoptosis
To evaluate the effect of 15d-PGJ2 on the growth of uterine sarcoma cells, we used the drug sensitive human sarcoma cell line MES-SA, its multidrug resistant counterpart MES-SA/Dx5 and the uterine leiomyosarcoma cell line SKN for experimental analyses. Cells were treated with various concentrations of 15d-PGJ2 (1, 5, 10, 20 and 50 µmol/l) for 24 h and examined by WST1 assay (Fig. 2A-C). We detected a significant reduction in cell growth starting at 10 µmol/l 15d-PGJ2 in all of the cell lines.
Figure 2.
15d-PGJ2 inhibits the growth of uterine sarcoma cells. (A-C) MES-SA, MES-SA/DX5, and SKN cells were seeded 1 day before treatment with various concentrations of 15d-PGJ2 for 24 h or DMSO as control. Cell viability was assessed using WST1 assays and expressed as a percentage of viability under controlled culture conditions. (D-F) Cells were treated with 15d-PGJ2 (10 µmol/l) and incubated for various times. WST1 assays were performed and cell viability was determined. All data represent the mean ± SD of three independent experiments. *P<0.05.
We next examined the effects of 10 µmol/l 15d-PGJ2 on cell proliferation over time. We treated cells with 10 µmol/l 15d-PGJ2 and incubated cells for various times (0, 3, 6, 12 or 24 h) (Fig. 2D-F). We detected a significant reduction in cell growth starting at 24 h treatment in all of the cell lines. Together our results showed that 15d-PGJ2 significantly inhibited the growth of all three uterine sarcoma cell lines in a dose- and time-dependent manner.
We then investigated whether 15d-PGJ2 induced apoptosis of uterine sarcoma cell lines. We treated all three uterine sarcoma cell lines with 15d-PGJ2 at different dose levels and examined the effects on cellular apoptosis using the caspase-3/7 assay after 12 h of culture (Fig. 3A-C) and by TUNEL assays (Fig. 3D). The results from both assays showed that 15d-PGJ2 treatment resulted in increased apoptosis. These studies indicated that 15d-PGJ2 exerts a cytotoxic effect, inhibiting uterine sarcoma cell growth.
Figure 3.
15d-PGJ2 induces apoptosis of uterine sarcoma cells and downregulates the AKT pathway. (A-C) Cellular apoptosis levels in MES-SA, MES-SA/DX5, and SKN cells treated with 15d-PGJ2 (10 µmol/l) for 24 h were quantified using the caspase-3/7 assay. All data represent the mean ± SD of three independent experiments. *P<0.05 vs. control. (D) DNA fragmentation was detected by in situ TUNEL staining in MES-SA cells after treatment with various concentrations of 15d-PGJ2. Nucleic acids are stained with DAPI (blue). Representative images are shown. Cells were treated with 100 µl DNaseI were included as positive controls. (E) Western blot analysis of MES-SA and MES-SA/Dx5 cells treated with DMSO (C), 15d-PGJ2 (P) and relabeling the fig. lanes as C, P, for 24 h using antibodies against MEK, p-MEK, ERK, p-ERK, AKT, and p-AKT. The band intensities were quantified using the ImageJ software program. The presented values were normalized against those of DMSO (C).
PGJ2 treatment activates the MAPK pathway
Previous studies showed that PGJ2 alters phosphorylation of PPAR-γ, suggesting that this compound might affect the activity of upstream kinases. Other reports demonstrated that 15-d-PGJ2 induced ERK activation (24,25). To test whether PGJ2 affects the MAPK pathway in uterine sarcoma cells, MES-SA and MES-SA/Dx5 cells were treated with various concentrations of PGJ2 for 24 h and total cell lysates were analyzed by western blot analysis (Fig. 3E). The western blot results showed that both MEK and ERK were phosphorylated in treated cells compared with controls (22). The AKT pathway is another important survival pathway. A previous study reported downregulation of the AKT pathway by antitumor inhibitors (26). The western blot results showed that phosphorylation of AKT was decreased by 15d-PGJ2 in uterine sarcoma cells, indicating that 15d-PGJ2 represses the AKT pathway.
In vitro effects of combination therapy with PGJ2 and dasatinib
Our results suggest that the antiproliferative effects of 15d-PGJ2 were associated with inactivation of AKT. However the MAPK has been activated. We then speculated that combining drugs that suppressed AKT and MAPK would result in enhanced anti-tumor effects. We therefore used the TKI dasatinib to suppress MAPK signaling in combination with 15d-PGJ2.
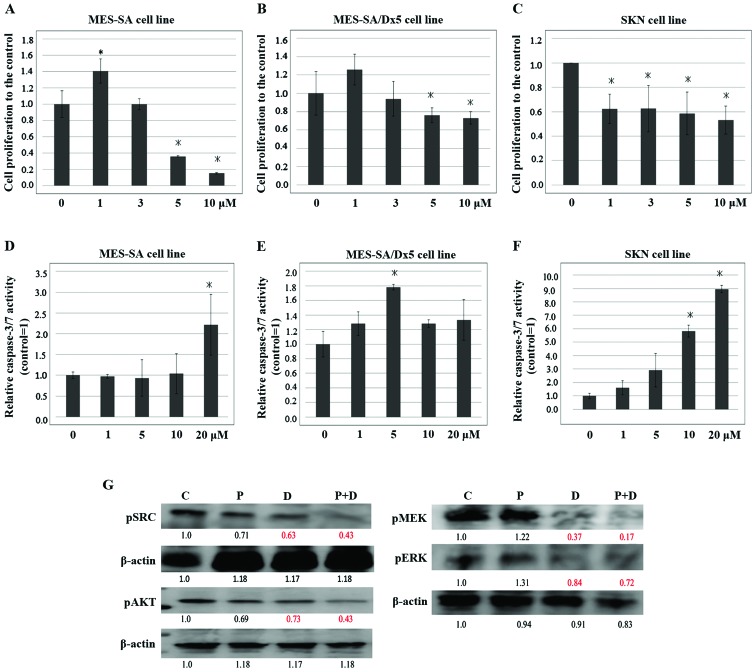
MES-SA cells were treated with 15d-PGJ2 (10 µmol/l) and various concentrations of dasatinb (1, 3, 5 and 10 µmol/l) for 24 h and examined by WST-1 assay (Fig. 4A-C). We also examined the effects of 10 µmol/l 15d-PGJ2 and 5 µmol/l dasatinb on cellular apoptosis using the caspase-3/7 assay after 12 h of culture (Fig. 4D-F). The results showed that dasatinib in combination with 15d-PGJ2 significantly reduced cell proliferation and increased apoptosis compared with 15d-PGJ2 alone in concentration dependent manners.
Figure 4.
Dasatinib enhances the inhibitory effect of 15d-PGJ2 on cell proliferation. (A-C) MES-SA cells were seeded 1 day before treatment with 15d-PGJ2 (10 µmol/l) and various concentrations of dasatinib, and then cultured for 24 h. WST1 assays were performed to assess cell viability and results were normalized to those of cells treated with 15d-PGJ2 (10 µmol/l) alone. (D-F) Cellular apoptosis levels in MES-SA, MES-SA/DX5, and SKN cells treated with 15d-PGJ2 (10 µmol/l) and arious concentrations of dasatinib and then cultured for 24 h were quantified using the caspase-3/7 assay. All data represent the mean ± SD of three independent experiments. *P<0.05. (G) Western blot analysis of MES-SA cells treated with DMSO (C), 15d-PGJ2 (10 µmol/l) (P), dasatinib (5 µmol/l) (D), or 15d-PGJ2 (10 µmol/l) combined with dasatinib (5 µmol/l) (P+D) for 24 h using the indicated antibodies. The band intensities were quantified using the ImageJ software program. The values were normalized against those of the control.
The CI was used to analyze the synergistic effect. The CI was 0.489,0.36428 and 0.36301 in the MES-SA cell, MES-SA/DX5 and SKN cell line, indicating that combined PGJ2 and dasatinib generates synergistic effect (Table I). We also found that the combination therapy induced a significant downregulation of AKT and MAPK pathways compared with treatment of either 15d-PGJ2 or dasatinb alone (Fig. 4G). Our results showed that the combination therapy exerts antitumor effects with more pronounced compared with either treatment alone.
Table I.
Combination index.
| Cell line | ED50 | ED75 | ED90 | ED95 |
|---|---|---|---|---|
| MEA-SA | 0.48970 | 0.33910 | 0.25557 | 0.22472 |
| MES-SA/Dx5 | 0.36428 | 0.41357 | 0.49295 | 0.56954 |
| SKN | 0.36301 | 0.35493 | 0.36386 | 0.37558 |
Discussion
PPAR-γ is targeted by endogenous ligands such as Δ12.15 prostaglandin J2 and functions as a transcriptional factor in vivo (9,15). PPAR-γ plays a variety of roles in antiproliferation and proapoptotic pathways. In this study, our results showed that 15d-PGJ2 inhibited uterine sarcoma cell proliferation by inducing apoptosis. This finding is in agreement with the results of previous studies of 15d-PGJ2 in other cell lines (27). However, the molecular mechanisms underlying the cytotoxic mechanism of 15d-PGJ2-induced apoptosis remained unclear. The present study shows that 15d-PGJ2 induced downregulation of the AKT pathway with subsequent apoptosis.
In this study, we found that 15d-PGJ2 induced antiproliferative effects in uterine sarcoma cells. Two main survival signaling pathways, MAPK and AKT pathways are downregulated by antitumor inhibitors (26). Our results showed that 15d-PGJ2 inhibited phosphorylation of AKT and also promoted phosphorylation of MAPK. Because of its activation of MAPK, the anti-tumor effects of 15d-PGJ2 are limited. Recent studies reported ERK-dependent negative feedback in several cancers (28). This ERK-dependent negative feedback was lost after treatment with a MEK inhibitor, and the ability of receptor tyrosine kinase ligands to activate growth signaling was markedly enhanced (24,25).
Significant tumor regression may be typical for treatments combining PPAR-γ agonists with conventional cytotoxic anticancer agents (15,16). However, the antiproliferative effects and mechanism of action of PGJ2 in combination with molecular-targeted agents are unclear. Importantly, our results showed that MAPK signaling was activated by 15d-PGJ2. Activation of these pathways may limit the antiproliferative effects of 15d-PGJ2, and thus the addition of molecular-targeted agents with PGJ2 may help to suppress these growth pathways.
The orally available TKI dasatinib inhibits SRC, which was found to activate receptor tyrosine kinases that induce trastuzumab de novo and acquired resistance. This drug is currently being examined in cancer patients (20,21) and also in several solid tumors (18,22,29,30). Previous studies showed that in nasopharyngeal carcinoma treated with dasatinib, phospho-AKT, phospho-MEK, and phosphor-ERK levels were significantly reduced (31). This suggests that dasatinib exhibits antitumor effects in uterine sarcoma by downregulating MAPK and AKT pathway activity. SRC phosphorylation was inhibited to a higher degree in the dasatinib treatment group compared with the control group, and inactivation of both the AKT and MAPK pathways was observed in the combination treatment group (Fig. 4). This effect of dasatinib appears to be mediated by inhibition of SRC phosphorylation. These results showed that inhibition of SRC has sustained effects on the MAPK cascade and AKT in uterine sarcoma.
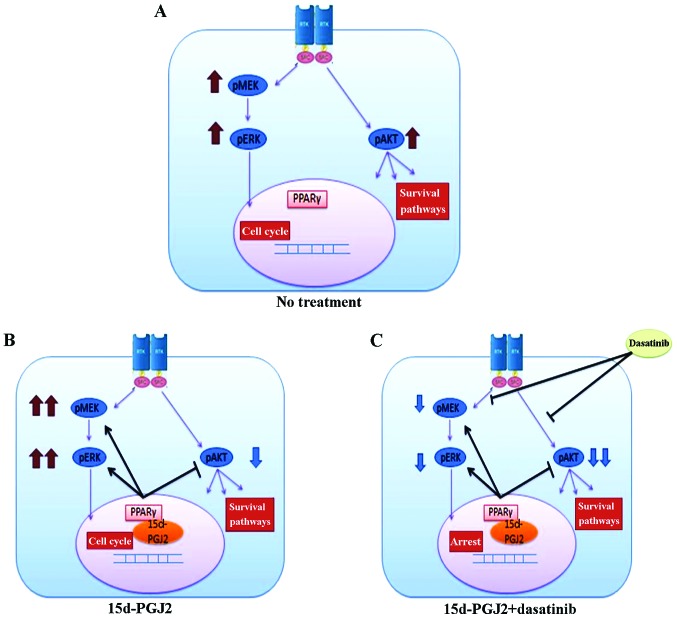
Fig. 5 presents a schematic model of the mechanisms of 15d-PGJ2 and dasatinib in uterine sarcoma cells. The MAPK pathway is activated by 15d-PGJ2 treatment, and combined treatment with 15d-PGJ2 and dasatinib suppresses both the AKT and MAPK pathways, leading to synergistic antiproliferative effects.
Figure 5.
Schematic for model of the effects of 15d-PGJ2 alone and in combination with dasatinib in uterine sarcoma cells. (A) No treatment. AKT and MAPK pathways are activated. (B) 15d-PGJ2 inactivates AKT. However, the MAPK pathways are activated. (C) The combination of 15d-PGJ2 with dasatinib suppresses both AKT and MAPK pathways and leads to synergistic antiproliferative effects.
In conclusion, this study demonstrated the tumor-suppressive effects and major underlying mechanism of 15d-PGJ2 in uterine sarcoma involving inactivation of the AKT pathway. Treatment with 15d-PGJ2 combined with dasatinib produced a synergistic effect by negatively regulating both AKT and MAPK pathways. These results suggest that 15d-PGJ2 could be used in combination with dasatinib as a potential therapeutic approach for uterine sarcoma.
Acknowledgements
The authors would like to thank Dr Iwasa for valuable research advice. This study was supported by Support Center for Advanced Medical Sciences, Institute of Biomedical Sciences, Tokushima University Graduate School.
References
- 1.Ebina Y, Katabuchi H, Mikami M, Nagase S, Yaegashi N, Udagawa Y, Kato H, Kubushiro K, Takamatsu K, Ino K, Yoshikawa H. Japan Society of Gynecologic Oncology guidelines 2013 for the treatment of uterine body neoplasms. Int J Clin Oncol. 2016;21:419–434. doi: 10.1007/s10147-016-0981-1. [DOI] [PubMed] [Google Scholar]
- 2.Akahira J, Tokunaga H, Toyoshima M, Takano T, Nagase S, Yoshinaga K, Tase T, Wada Y, Ito K, Niikura H, et al. Prognoses and prognostic factors of carcinosarcoma, endometrial stromal sarcoma and uterine leiomyosarcoma: A comparison with uterine endometrial adenocarcinoma. Oncology. 2006;71:333–340. doi: 10.1159/000107107. [DOI] [PubMed] [Google Scholar]
- 3.Chen YX, Zhong XY, Qin YF, Bing W, He LZ. 15d-PGJ2 inhibits cell growth and induces apoptosis of MCG-803 human gastric cancer cell line. World J Gastroenterol. 2003;9:2149–2153. doi: 10.3748/wjg.v9.i10.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clay CE, Atsumi GI, High KP, Chilton FH. Early de novo gene expression is required for 15-deoxy-Delta 12, 14-prostaglandin J2-induced apoptosis in breast cancer cells. J Biol Chem. 2001;276:47131–47135. doi: 10.1074/jbc.C100339200. [DOI] [PubMed] [Google Scholar]
- 5.Lim HJ, Lee KS, Lee S, Park JH, Choi HE, Go SH, Kwak HJ, Park HY. 15d-PGJ2 stimulates HO-1 expression through p38 MAP kinase and Nrf-2 pathway in rat vascular smooth muscle cells. Toxicol Appl Pharmacol. 2007;223:20–27. doi: 10.1016/j.taap.2007.04.019. [DOI] [PubMed] [Google Scholar]
- 6.Kim EH, Na HK, Kim DH, Park SA, Kim HN, Song NY, Surh YJ. 15-Deoxy-Delta12,14-prostaglandin J2 induces COX-2 expression through Akt-driven AP-1 activation in human breast cancer cells: A potential role of ROS. Carcinogenesis. 2008;29:688–695. doi: 10.1093/carcin/bgm299. [DOI] [PubMed] [Google Scholar]
- 7.Levonen AL, Dickinson DA, Moellering DR, Mulcahy RT, Forman HJ, Darley-Usmar VM. Biphasic effects of 15-deoxy-delta (12,14)-prostaglandin J(2) on glutathione induction and apoptosis in human endothelial cells. Arterioscler Thromb Vasc Biol. 2001;21:1846–1851. doi: 10.1161/hq1101.098488. [DOI] [PubMed] [Google Scholar]
- 8.Yen CC, Hsiao CD, Chen WM, Wen YS, Lin YC, Chang TW, Yao FY, Hung SC, Wang JY, Chiu JH, et al. Cytotoxic effects of 15d-PGJ2 against osteosarcoma through ROS-mediated and cell cycle inhibition. Oncotarget. 2014;5:716–725. doi: 10.18632/oncotarget.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suh N, Wang Y, Williams CR, Risingsong R, Gilmer T, Willson TM, Sporn MB. A new ligand for the peroxisome proliferator-activated receptor-gamma (PPAR-gamma), GW7845, inhibits rat mammary carcinogenesis. Cancer Res. 1999;59:5671–5673. [PubMed] [Google Scholar]
- 10.Michalik L, Desvergne B, Wahli W. Peroxisome-proliferator-activated receptors and cancers: Complex stories. Nat Rev Cancer. 2004;4:61–70. doi: 10.1038/nrc1254. [DOI] [PubMed] [Google Scholar]
- 11.Rosen ED, Spiegelman BM. PPARgamma: A nuclear regulator of metabolism, differentiation, and cell growth. J Biol Chem. 2001;276:37731–37734. doi: 10.1074/jbc.R100034200. [DOI] [PubMed] [Google Scholar]
- 12.Qin L, Ren Y, Chen AM, Guo FJ, Xu F, Gong C, Cheng P, Du Y, Liao H. Peroxisome proliferator-activated receptor γ ligands inhibit VEGF-mediated vasculogenic mimicry of prostate cancer through the AKT signaling pathway. Mol Med Rep. 2014;10:276–282. doi: 10.3892/mmr.2014.2198. [DOI] [PubMed] [Google Scholar]
- 13.Santha S, Viswakarma N, Das S, Rana A, Rana B. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-troglitazone-induced apoptosis in prostate cancer cells involve AMP-activated protein kinase. J Biol Chem. 2015;290:21865–21875. doi: 10.1074/jbc.M115.663526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dionne S, Levy E, Levesque D, Seidman EG. PPARgamma ligand 15-deoxy-delta12,14-prostaglandin J2 sensitizes human colon carcinoma cells to TWEAK-induced apoptosis. Anticancer Res. 2010;30:157–166. [PubMed] [Google Scholar]
- 15.Osawa E, Nakajima A, Wada K, Ishimine S, Fujisawa N, Kawamori T, Matsuhashi N, Kadowaki T, Ochiai M, Sekihara H, Nakagama H. Peroxisome proliferator-activated receptor gamma ligands suppress colon carcinogenesis induced by azoxymethane in mice. Gastroenterology. 2003;124:361–367. doi: 10.1053/gast.2003.50067. [DOI] [PubMed] [Google Scholar]
- 16.Girnun GD, Naseri E, Vafai SB, Qu L, Szwaya JD, Bronson R, Alberta JA, Spiegelman BM. Synergy between PPARgamma ligands and platinum-based drugs in cancer. Cancer Cell. 2007;11:395–406. doi: 10.1016/j.ccr.2007.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shimazaki N, Togashi N, Hanai M, Isoyama T, Wada K, Fujita T, Fujiwara K, Kurakata S. Anti-tumour activity of CS-7017, a selective peroxisome proliferator-activated receptor gamma agonist of thiazolidinedione class, in human tumour xenografts and a syngeneic tumour implant model. Eur J Cancer. 2008;44:1734–1743. doi: 10.1016/j.ejca.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 18.Araujo JC, Mathew P, Armstrong AJ, Braud EL, Posadas E, Lonberg M, Gallick GE, Trudel GC, Paliwal P, Agrawal S, Logothetis CJ. Dasatinib combined with docetaxel for castration-resistant prostate cancer: Results from a phase 1–2 study. Cancer. 2012;118:63–71. doi: 10.1002/cncr.26204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Makino K, Kawamura K, Sato W, Kawamura N, Fujimoto T, Terada Y. Inhibition of uterine sarcoma cell growth through suppression of endogenous tyrosine kinase B signaling. PLoS One. 2012;7:e41049. doi: 10.1371/journal.pone.0041049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amrein PC, Attar EC, Takvorian T, Hochberg EP, Ballen KK, Leahy KM, Fisher DC, Lacasce AS, Jacobsen ED, Armand P, et al. Phase II study of dasatinib in relapsed or refractory chronic lymphocytic leukemia. Clin Cancer Res. 2011;17:2977–2986. doi: 10.1158/1078-0432.CCR-10-2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Apperley JF, Cortes JE, Kim DW, Roy L, Roboz GJ, Rosti G, Bullorsky EO, Abruzzese E, Hochhaus A, Heim D, et al. Dasatinib in the treatment of chronic myeloid leukemia in accelerated phase after imatinib failure: The START a trial. J Clin Oncol. 2009;27:3472–3479. doi: 10.1200/JCO.2007.14.3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen B, Xu X, Luo J, Wang H, Zhou S. Rapamycin Enhances the Anti-Cancer Effect of Dasatinib by Suppressing Src/PI3K/mTOR Pathway in NSCLC Cells. PLoS One. 2015;10:e0129663. doi: 10.1371/journal.pone.0129663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chou TC. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010;70:440–446. doi: 10.1158/0008-5472.CAN-09-1947. [DOI] [PubMed] [Google Scholar]
- 24.Sawayama H, Ishimoto T, Watanabe M, Yoshida N, Sugihara H, Kurashige J, Hirashima K, Iwatsuki M, Baba Y, Oki E, et al. Small molecule agonists of PPAR-γ exert therapeutic effects in esophageal cancer. Cancer Res. 2014;74:575–585. doi: 10.1158/0008-5472.CAN-13-1836. [DOI] [PubMed] [Google Scholar]
- 25.Lito P, Pratilas CA, Joseph EW, Tadi M, Halilovic E, Zubrowski M, Huang A, Wong WL, Callahan MK, Merghoub T, et al. Relief of profound feedback inhibition of mitogenic signaling by RAF inhibitors attenuates their activity in BRAFV600E melanomas. Cancer Cell. 2012;22:668–682. doi: 10.1016/j.ccr.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McLean GW, Carragher NO, Avizienyte E, Evans J, Brunton VG, Frame MC. The role of focal-adhesion kinase in cancer-a new therapeutic opportunity. Nat Rev Cancer. 2005;7:505–515. doi: 10.1038/nrc1647. [DOI] [PubMed] [Google Scholar]
- 27.Montero-Conde C, Ruiz-Llorente S, Dominguez JM, Knauf JA, Viale A, Sherman EJ, Ryder M, Ghossein RA, Rosen N, Fagin JA. Relief of feedback inhibition of HER3 transcription by RAF and MEK inhibitors attenuates their antitumor effects in BRAF-mutant thyroid carcinomas. Cancer Discov. 2013;3:520–533. doi: 10.1158/2159-8290.CD-12-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prahallad A, Sun C, Huang S, Di Nicolantonio F, Salazar R, Zecchin D, Beijersbergen RL, Bardelli A, Bernards R. Unresponsiveness of colon cancer to BRAF(V600E) inhibition through feedback activation of EGFR. Nature. 2012;483:100–103. doi: 10.1038/nature10868. [DOI] [PubMed] [Google Scholar]
- 29.Brooks HD, Glisson BS, Bekele BN, Johnson FM, Ginsberg LE, El-Naggar A, Culotta KS, Takebe N, Wright J, Tran HT, Papadimitrakopoulou VA. Phase 2 study of dasatinib in the treatment of head and neck squamous cell carcinoma. Cancer. 2011;117:2112–2119. doi: 10.1002/cncr.25769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Finn RS, Dering J, Ginther C, Wilson CA, Glaspy P, Tchekmedyian N, Slamon DJ. Dasatinib, an orally active small molecule inhibitor of both the src and abl kinases, selectively inhibits growth of basal-type/‘triple-negative’ breast cancer cell lines growing in vitro. Breast Cancer Res Treat. 2007;105:319–326. doi: 10.1007/s10549-006-9463-x. [DOI] [PubMed] [Google Scholar]
- 31.Li YJ, He YF, Han XH, Hu B. Dasatinib suppresses invasion and induces apoptosis in nasopharyngeal carcinoma. Int J Clin Exp Pathol. 2015;8:7818–7824. [PMC free article] [PubMed] [Google Scholar]