Abstract
Rapid growth of residual tumors can occur as a result of their recurrence and progression. The present study aimed to investigate the expression of hypoxia inducible factor-2 subunit α (HIF-2α), vascular endothelial growth factor A (VEGFA), erythropoietin-producing hepatocellular A2 (EphA2) and angiogenesis in residual hepatocellular carcinoma (HCC), following treatment with high-intensity focused ultrasound (HIFU) ablation, in order to investigate the association between protein expression and tumor recurrence and growth. Athymic BALB/c (nu/nu) mice were subcutaneously inoculated with the HCC cell line HepG2, in order to create xenograft tumors. Approximately 30 days post-inoculation, eight mice were treated with HIFU, whereas eight mice received no treatment and acted as the control group. Residual tumor tissues were obtained from the experimental groups after one month. Levels of HIF-2α, VEGFA, EphA2 and cluster of differentiation 31 (CD31) expression was measured by immunohistochemical staining. CD31-positive vascular endothelial cells were counted to calculate microvascular density (MVD), and western blot analysis was performed to determine levels of HIF-2α, VEGFA, and EphA2 protein. It was found that the expression levels of HIF-2α, VEGFA, EphA2, and MVD proteins in residual HCC tissues were significantly higher than in the control group tissues (P<0.05). Tumor MVD was strongly correlated with VEGFA (R=0.957, P<0.01) and EphA2 (R=0.993, P<0.01) protein expression levels. Furthermore, there was a significant positive correlation between HIF-2α and EphA2 expression (R=0.991, P<0.01). The correlation between VEGFA and EphA2 expression was also positive (R=0.985, P<0.01). These data suggest that overexpression of HIF-2α, VEGFA and EphA2 is related to angiogenesis in residual HCC following HIFU ablation, potentially via their association with key mediators of recurrence.
Keywords: hypoxia inducible factor-2 subunit α, residual hepatocellular carcinoma, angiogenesis, vascular endothelial growth factor A, erythropoietin-producing hepatocellular A2
Introduction
Hepatocellular carcinoma (HCC) is the most prevalent type of primary liver cancer, with a high occurrence rate in Asia and an increasing incidence in Western countries (1). For patients with HCC who have undergone surgical resection, the 5-year survival rate is 40–70%, though for patients with early-stage HCC who have undergone liver transplantation, this rate increases marginally to 60–70% (2). However, the majority of patients are diagnosed at an intermediate or advanced stage of the disease, when few effective therapies are available (3). Due to recent advances in technology, high-intensity focused ultrasound (HIFU) ablation is now considered a key adjuvant treatment for unresectable HCC, as it is an effective and safe method of treatment (4). However, despite the use of thorough HIFU ablation, residual tumors may still develop due to tumor recurrence (5). The molecular mechanisms leading to the recurrence and development of residual tumors are not well understood. However, studies have indicated that tumor angiogenesis serves a potential role in the recurrence and growth of HCC; a process tightly regulated by numerous angiogenic factors (6,7).
Hypoxia inducible factor-2 subunit α (HIF-2α) is a key factor in angiogenesis, tumor growth and endothelial growth during the remodeling process of tumorigenesis (8). In addition, vascular endothelial growth factor (VEGF) is a major factor that contributes to angiogenesis and metastasis in numerous types of tumor, such as HCC, gastric cancer and breast cancer, and its overexpression is associated with tumor progression and poor clinical outcomes (9). VEGFA is part of the VEGF structural family of proteins, and is among the most potent angiogenic factors expressed in different types of human cancer, including HCC, breast cancer, clear cell renal carcinoma and gastric cancer (10,11). Erythropoietin-producing hepatocellular A2 (EphA2) is also implicated in HCC. It has been found that EphA2 expression is prominent in invasive hepatoma cells and is significantly correlated with poor survival of HCC patients, suggesting that EphA2 is a potential serum marker for the detection of HCC development and progression (12). Furthermore, there is increasing evidence that high-levels of EphA2 may promote angiogenesis, tumor growth and invasion (13,14).
The association between the expression of the factors HIF-2α, VEGFA and EphA2, and angiogenesis in residual HCC following HIFU ablation and in normal tumor tissue, remains unknown. Therefore, the present study examined the levels of HIF-2α, VEGFA and EphA2 expression in HCC with and without HIFU to determine the potential correlation between residual tumor angiogenesis and recurrence.
Materials and methods
HCC cell line and antibodies
Human HCC HepG2 cells were purchased from the Cell Resource Center at the Chinese Academy of Medical Sciences, Peking Union Medical College (Beijing, China). Cells were cultured in 100-cm2 plastic tissue flasks at 37°C in a humidified 5% CO2 atmosphere in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (Hyclone; GE Healthcare, Logan, UT, USA). Rabbit polyclonal anti-HIF-2α (cat. no. ab20654) and mouse monoclonal anti-VEGFA (cat. no. ab105219) antibodies were obtained from Abcam (Cambridge, UK), anti-EphA2 (cat. no. BS8759) and anti-cluster of differentiation 31 (CD31; cat. no. BS1574) antibodies were obtained from BIOSS (Beijing, China) and Bioworld Technology, Inc. (St. Louis Park, MN, USA), respectively.
Animals and treatment protocol
A total of 16 athymic BALB/c (nu/nu) 4–6 week old male nude mice (weight, 20±2 g) were purchased from the Animal Center of Chongqing Medical University (Chongqing, China). Mice were maintained under specific-pathogen-free conditions and exposed to a 12-h light/dark cycle at a temperature of 25°C and 50% humidity. Water and food were autoclaved and provided for mice ad libitum. HepG2 cells (5×106 cells/mouse) in 0.2 ml DMEM were subcutaneously injected into the flanks of the mice. When the xenografted tumors reached ~1,000 mm3 in volume, tuweekmor-bearing mice were randomly divided into a treatment and negative control group (n=8 each). Each mouse in the treatment groups received Seapostar HIFU therapy using a CZF Ultrasonic Therapeutic Apparatus (Haifu Medical Technology Co., Ltd., Chongqing, China), at 8.6 MHz, 5 W for 30 sec, until ~10% residual tumor tissue remained (2–5 min), and were monitored with computed tomography to identify clinical tumor recurrence following HIFU treatment. Experiments with mice were conducted according to institutional guidelines (National Institutes of Health Guidelines and legal requirements in China) and approved by the Animal Care and Use Committee of Chongqing Medical University.
Immunohistochemical analysis
Following one month of growth, mice were anesthetized with sodium pentobarbital (30 mg/kg; cat. no. P3761; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and sacrificed via cervical dislocation, and tumor tissues were harvested and fixed in 4% neutral-buffered formalin. After 24 h, the samples were paraffin-embedded, sliced into 4 µm sections, and stained with hematoxylin and eosin. The sections were blocked with 2.5% hydrogen peroxide in methanol at 37°C for 30 min and microwaved at 100°C for 3 min for heat-induced antigen retrieval and incubated in 2% bovine serum albumin (cat. no. SH30022.01B; Hyclone; GE Healthcare) with the primary antibodies (anti-HIF-2α, 1:2,000; anti-VEGFA, 1:50; anti-EphA2, 1:100; and anti-CD31, 1:100) overnight at 4°C. Sections were then washed with PBS and reacted with horseradish peroxidase (HRP)-conjugated anti-immunoglobulin (Ig) G secondary antibody (1:50; cat. no. A0208; Beyotime Institute of Biotechnology, Haimen, China) at 37°C for 20 min. Following nucleic acid detection with 3,3′-diaminobenzidine stain, sections were counterstained with hematoxylin. A light microscope was used to take representative images of the tumor tissues.
Analysis of the immunostained tumors was performed as described previously (15,16). Briefly, two independent investigators scored the sections; the total immunostaining score of each section was calculated by the percentage of positive-stained tumor cells (0–5% staining, 0 points; 6–50% staining, 2 points; >50% staining, 3 points) and the staining intensity (weak intensity, 1 point; moderate intensity, 2 points; strong intensity, 3 points). The specimens detected as grade 3 (moderate staining: 3–4 points) and 4 (strong staining: 5–6 points) were recorded as positive results in the statistical analysis.
Microvascular density (MVD) was determined according to the criteria introduced by Tian et al (17). Briefly, the area with the highest CD31-positive vessel density was screened at ×40 magnification, then counted at ×200 in 10 random fields. The mean number of micro-vessels in each field was regarded as the level of MVD.
Western blot analysis
Tumor tissues were lysed using cell lysis buffer containing protease inhibitors (Roche Applied Science, Penzberg, Germany) and a protein extraction kit (cat. no. P0027; Beyotime Institute of Biotechnology). Protein concentration was determined using a Bradford assay kit (cat. no. P0006; Beyotime Institute of Biotechnology) and equivalent quantities of protein (35 µg) were loaded onto an SDS-PAGE gel (6–8%), subjected to electrophoresis, and subsequently transferred to a polyvinylidene fluoride membrane. The blots were blocked with 5% non-fat dry milk at 37°C for 1 h prior to incubation overnight at 4°C with primary antibodies (anti-HIF-2α, 1:1,000; anti-VEGFA, 1:200; anti-EphA2, 1:100), and anti-β-actin antibody (cat. no. sc-47778; 1:800; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) as a positive control. Blots were washed and treated at room temperature for 2 h with horseradish peroxidase-conjugated (HRP) anti-IgG secondary antibody (1:5,000; Santa Cruz Biotechnology, Inc.) and signals were detected using an enhanced chemiluminescence detection system (Quantity One software; version 4.62 Bio-Rad Laboratories GmbH, Munich, Germany). The reaction was performed in triplicate.
Statistical analysis
Data were expressed as the mean ± standard deviation and analyzed using Student's two-tailed unpaired t-test. The correlations among HIF-2α, VEGFA, EphA2 and MVD were determined using the Pearson correlation coefficient. Statistical analyses were performed using SPSS 17.0 software (SPSS Inc., Chicago, IL, USA). P<0.05 was considered to represent a statistically significant difference.
Results
Residual tumor tissue and pathological analysis
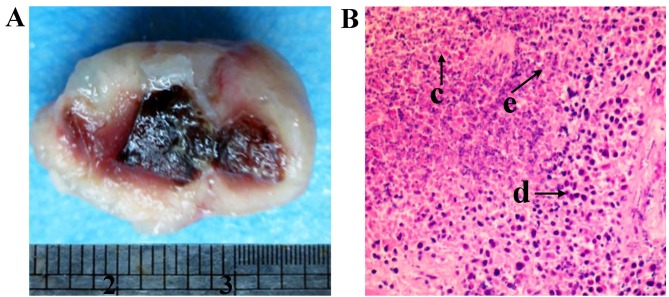
Following HIFU ablation, the residual hepatic tumor tissue was obtained. The tissue displayed a ‘fish-flesh’ cut surface and partial central liquefactive necrosis (Fig 1A). Necrosis was consistent with an infiltration of inflammatory cells in the surrounding areas (Fig. 1B).
Figure 1.
Residual hepatic tumor tissue following high intensity focused ultrasound ablation. (A) The cut surface of the residual tumor tissue. (B) Residual tumor tissue with hematoxylin and eosin staining, revealing (c) necrotic cells, (d) normal hepatoma cells, and (e) inflammatory cells, Magnification, ×200.
Expression of HIF-2α, VEGF, and EphA2 protein
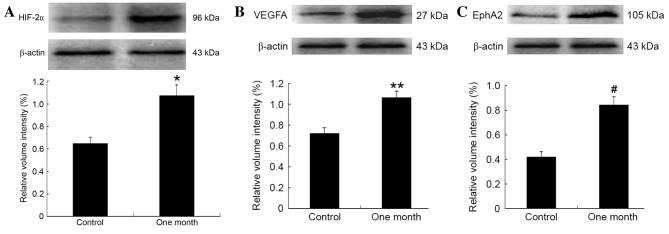
One month after tissue samples were harvested, the expression of HIF-2α (4–5 points), VEGFA (4–6 points) and EphA2 (3–6 points) protein was more intense in the experimental than in the control group (all 1–4 points; Fig. 2A-C). Positive expression of CD31 was mainly observed as brown/brownish-yellow granules in the cytoplasm of vascular endothelial cells. The mean MVD (35.8±5) in the experimental groups was significantly higher than that in the control group 26.2±2; t=10.42, P<0.01; data not shown. Western blot analysis confirmed that the protein expression levels of HIF-2α, VEGFA and EphA2 were significantly increased in the residual hepatic tumor tissues (t=7.32, P<0.01; t=10.41, P<0.01; t=16.67, P<0.01, respectively; Fig. 3).
Figure 2.
Immunohistochemical analysis of HIF-2α, VEGFA, EphA2 and CD31 expression. The staining indicates protein levels in tumor tissue of (A-D) the control group vs. residual hepatic tumor tissue of (E-H) the experimental group one month after HIFU treatment. Magnification, ×200. HIF-2α, hypoxia-inducible factor-2α; VEGFA, vascular endothelial growth factor A; EphA2, ephrin A2 receptor; CD31, cluster of differentiation 31; HIFU, high intensity focused ultrasound.
Figure 3.
Western blot analysis of HIF-2α, VEGFA, and EphA2 expression, β-actin was used as internal control. Relative protein expression of (A) HIF-2α, (B) VEGFA, and (C) EphA2 were quantified and normalized against β-actin. *t=7.32, P<0.01; **t=10.41, P<0.01; #t=16.67, P<0.01. HIF-2α, hypoxia-inducible factor-2α; VEGFA, vascular endothelial growth factor A; EphA2, ephrin A2 receptor.
Relationship between CD31, HIF-2α, VEGFA and EphA2 expression
There was a significant positive correlation between MVD and VEGFA expression (r=0.957, P<0.01), and between MVD and EphA2 expression (r=0.993, P<0.01), in the residual hepatic tumor tissues. There was also a significant positive correlation between HIF-2α and EphA2 expression (r=0.991, P<0.01), and between VEGFA and EphA2 expression (r=0.985, P<0.01; Fig. 4).
Figure 4.
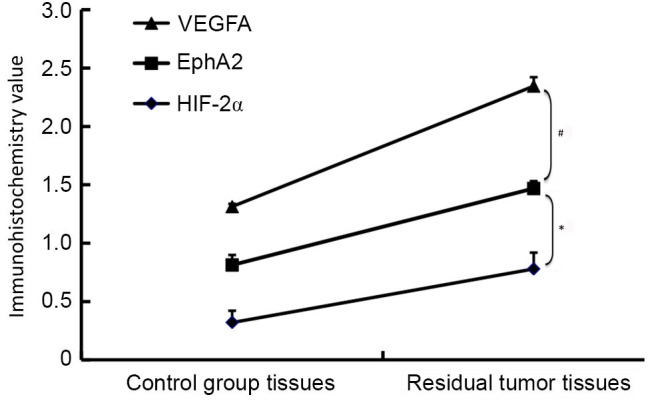
Increased HIF-2α, EphA2 and VEGFA protein expression in residual tumor tissue. Based on immunohistochemical detection, EphA2 expression was found to be significantly correlated with HIF-2α expression and with VEGFA expression in residual hepatic tumor tissue. *r=0.991, P<0.01; #r=0.985, P<0.01.
Discussion
HIFU ablation is becoming established as a non-invasive, safe and effective technique of treating unresectable HCC (18). However, in numerous clinical centers it has been observed that radical HIFU ablation can lead to the development of residual tumors, due to tumor recurrence and rapid tumor progression. Previous research has demonstrated that insufficient HIFU ablation promotes angiogenesis in residual carcinoma tissue over time (16). Furthermore, numerous studies have shown that active tumor angiogenesis is associated with the recurrence, invasion and metastasis of HCC (19–21). However, the molecular mechanisms leading to angiogenesis and residual tumor recurrence remain unclear.
HIF is a heterodimeric protein composed of α- and β-subunits, which together bind to hypoxia-response elements in the promoters of target genes. HIF is considered to be a key driver of cellular adaption to hypoxia, as well as a fundamental molecular link between hypoxia and angiogenesis in tumorigenic tissues (22,23). Ubiquitous HIF-1α and hepatocyte-specific HIF-2α are part of the HIF family, which collectively regulates a range of genes encoding proteins involved in angiogenesis, glycolysis, cell growth and metastasis (24). HIF-2α is known to promote angiogenesis and vascular function, and in the liver it is the primary regulatory factor of erythropoietin production and angiogenic gene expression (25,26). In residual hepatic carcinoma, it has been indicated that HIF-2α protein expression is markedly increased, with this increase being significantly correlated with VEGFA expression (27).
Increased levels of VEGF are associated with the recurrence and progression of HCC. VEGFA is among the most potent angiogenic factors expressed in various human cancers, including HCC (28). VEGFA is a hexose-modified multifunctional protein that acts specifically on vascular endothelial cells, whereby it induces micro-angiogenesis. In tumorigenic tissues, this activity results in tumor invasion, metastasis and recurrence (6). It has been indicated that HIF-2α may regulate VEGFA expression at numerous levels, namely by enhancing the transcriptional activity and mRNA stability of the growth factor (29). In the present study, VEGFA expression was significantly higher relative to the control group, indicating that HIF-2α may be an upstream regulator of VEGF expression. As it is established that HIF-2a is upregulated under hypoxic conditions, including within tumor tissue (30), this indicates that HIF-2a may be an upstream regulator. These results are consistent with past reports that have detected significant regulation of VEGFA expression by HIF-2α (29,31). In the present study, the correlation between HIF-2α and VEGFA expression was found to be negative, possibly due to an insufficient quantity and/or quality of samples.
The ephrin (Eph) receptors are the largest known family of tyrosine kinases and are divided into two subclasses: Eph-A and Eph-B (32). EphA2 belongs to the EphA subclass, and is expressed at a minimal level in epithelial cells (33). There is evidence that high levels of EphA2 promote various aspects of the malignant phenotype, including cell growth, angiogenesis, migration, invasion and survival of cancer cells (34,35). A previous study reported that there is a correlation between EphA2 and high levels of angiogenesis markers, in particular, VEGF expression and MVD counts, in HCC tumorigenesis (36,37). Furthermore, EphA2 overexpression is involved in vascular mimicry (VM), as it promotes the formation of vasculogenic-like networks in vitro, which in turn affects tumor cell plasticity (38,39). It has been indicated that part of the red blood cell compartment is able to trans-differentiate into endothelial cells, thereby acquiring a number of endothelial cell functions, along with VM capability, possibly through the EphA2/phosphoinositide 3-kinase pathway (40). Thus, EphA2 is considered to play a major role in the recurrence and development of cancer, via neovascularization prior to tumorigenesis. The present study revealed that expression of EphA2 was significantly higher in residual tumor tissue. An increase in VEGFA expression appeared to be significantly correlated with increased levels of EphA2 and MVD, indicating that VEGFA may be an upstream regulator of EphA2 expression. In addition, it was found that matrix metalloproteinase-2 (MMP2) and MMP9, which act as effector molecules and are induced by EphA2, are also controlled by VEGF (41). Furthermore, a significant correlation between HIF-2α and EphA2 expression was observed in the present study. However, the mechanism by which EphA2 is induced by VEGFA or HIF-2α remains unclear and warrants further study.
MVD is a representative index of tumor angiogenesis (42) and is related to the oxygen availability within tumors, along with the invasion, metastasis and proliferation of tumor cells. In the present study, the level of MVD in residual tumors was significantly higher than that seen in the control group. There was also a significant positive correlation between the MVD level and the expression levels of VEGFA and EphA2, which is in accordance with previous reports (43,44). These results indicate that overexpression of VEGFA and EphA2 may induce residual tumor angiogenesis, although the corresponding mechanism warrants further study.
In conclusion, the levels of HIF-2α, VEGFA and EphA2 protein expression were significantly increased to similar quantities in residual hepatic tumor tissue following HIFU ablation, relative to control group tissues that received no HIFU treatment. In tissues harvested after one month, the expression patterns of VEGF-A, EphA2 and MVD were also similar, and there was a significant positive correlation between MVD and the expression of HIF-2α, VEGFA and EphA2. These results indicate that overexpression of HIF-2α, VEGFA and EphA2 are potential risk factors that may induce tumor angiogenesis and recurrence. Furthermore, the present study indicates potential in the use of HIF-2α-suppressive agents alone or in conjunction with other anti-angiogenic cancer therapies, may be developed to prevent residual tumor angiogenesis and recurrence following HIFU ablation in patients with HCC.
Acknowledgements
The present study was supported by the Natural Science Foundation of Hubei Provincial Department of Education (grant no. Q20162112), the Natural Science Foundation of the Bureau of Science and Technology of Shiyan City (grant no. 16Y61), and the School Foundation for Hubei University of Medicine (grant no. FDFR201614).
References
- 1.Lafaro KJ, Demirjian AN, Pawlik TM. Epidemiology of hepatocellular carcinoma. Surg Oncol Clin N Am. 2015;24:1–17. doi: 10.1016/j.soc.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 2.Xu G, Luo G, He L, Li J, Shan H, Zhang R, Li Y, Gao X, Lin S, Wang G. Follow-Up of high-intensity focused ultrasound treatment for patients with hepatocellular carcinoma. Ultrasound Med Biol. 2011;37:1993–1999. doi: 10.1016/j.ultrasmedbio.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 3.Meng M, Wang H, Zeng X, Zhao L, Yuan Z, Wang P, Hao X. Stereotactic body radiation therapy: A novel treatment modality for inoperable hepatocellular carcinoma. Drug Discov Ther. 2015;9:372–379. doi: 10.5582/ddt.2015.01056. [DOI] [PubMed] [Google Scholar]
- 4.Chan AC, Cheung TT, Fan ST, Chok KS, Chan SC, Poon RT, Lo CM. Survival analysis of high-intensity focused ultrasound therapy versus radiofrequency ablation in the treatment of recurrent hepatocellular carcinoma. Ann Surg. 2013;257:686–692. doi: 10.1097/SLA.0b013e3182822c02. [DOI] [PubMed] [Google Scholar]
- 5.Fukuda H, Numata K, Nozaki A, Morimoto M, Kondo M, Tanaka K, Maeda S, Ohto M, Ito R, Zhu H, Wang ZB. Findings of multidetector row computed tomography of HCCs treated by HIFU ablation. Eur J Radiol. 2012;81:e239–e243. doi: 10.1016/j.ejrad.2011.01.101. [DOI] [PubMed] [Google Scholar]
- 6.Yang X, Zhang XF, Lu X, Jia HL, Liang L, Dong QZ, Ye QH, Qin LX. MicroRNA-26a suppresses angiogenesis in human hepatocellular carcinoma by targeting hepatocyte growth factor-cMet pathway. Hepatology. 2014;59:1874–1885. doi: 10.1002/hep.26941. [DOI] [PubMed] [Google Scholar]
- 7.Wang Z, Dabrosin C, Yin X, Fuster MM, Arreola A, Rathmell WK, Generali D, Nagaraju GP, El-Rayes B, Ribatti D, et al. Broad targeting of angiogenesis for cancer prevention and therapy. Semin Cancer Biol. 2015;35(Suppl):S224–S243. doi: 10.1016/j.semcancer.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahmad A, Ahmad S, Malcolm KC, Miller SM, Hendry-Hofer T, Schaack JB, White CW. Differential regulation of pulmonary vascular cell growth by hypoxia-inducible transcription factor-1α and hypoxia-inducible transcription factor-2α. Am J Respir Cell Mol Biol. 2013;49:78–85. doi: 10.1165/rcmb.2012-0107OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kitajima Y, Miyazaki K. The critical impact of HIF-1a on gastric cancer biology. Cancers (Basel) 2013;5:15–26. doi: 10.3390/cancers5010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu A, Huang C, Cai X, Xu J, Yang D. Twist promotes angiogenesis in pancreatic cancer by targeting miR-497/VEGFA axis. Oncotarget. 2016;7:25801–25814. doi: 10.18632/oncotarget.8269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dong S, Xia J, Wang H, Sun L, Wu Z, Bin J, Liao Y, Li N, Liao W. Overexpression of TRIB3 promotes angiogenesis in human gastric cancer. Oncol Rep. 2016;36:2339–2348. doi: 10.3892/or.2016.5017. [DOI] [PubMed] [Google Scholar]
- 12.Cui XD, Lee MJ, Yu GR, Kim IH, Yu HC, Song EY, Kim DG. EFNA1 ligand and its receptor EphA2: Potential biomarkers for hepatocellular carcinoma. Int J Cancer. 2010;126:940–949. doi: 10.1002/ijc.24798. [DOI] [PubMed] [Google Scholar]
- 13.Wada H, Yamamoto H, Kim C, Uemura M, Akita H, Tomimaru Y, Hama N, Kawamoto K, Kobayashi S, Eguchi H, et al. Association between ephrin-A1 mRNA expression and poor prognosis after hepatectomy to treat hepatocellular carcinoma. Int J Oncol. 2014;45:1051–1058. doi: 10.3892/ijo.2014.2519. [DOI] [PubMed] [Google Scholar]
- 14.Fang WB, Brantley-Sieders DM, Parker MA, Reith AD, Chen J. A kinase-dependent role for EphA2 receptor in promoting tumor growth and metastasis. Oncogene. 2005;24:7859–7868. doi: 10.1038/sj.onc.1208937. [DOI] [PubMed] [Google Scholar]
- 15.Merritt WM, Kamat AA, Hwang JY, Bottsford-Miller J, Lu C, Lin YG, Coffey D, Spannuth WA, Nugent E, Han LY, et al. Clinical and biological impact of EphA2 over-expression and angiogenesis in endometrial cancer. Cancer Biol Ther. 2010;10:1306–1314. doi: 10.4161/cbt.10.12.13582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu L, Fu Z, Zhou S, Gong J, Liu CA, Qiao Z, Li S. HIF-1α and HIF-2α: Siblings in promoting angiogenesis of residual hepatocellular carcinoma after high-intensity focused ultrasound ablation. PLoS One. 2014;13:e88913. doi: 10.1371/journal.pone.0088913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tian T, Nan KJ, Wang SH, Liang X, Lu CX, Guo H, Wang WJ, Ruan ZP. PTEN regulates angiogenesis and VEGF expression through phosphatase-dependent and -independent mechanisms in HepG2 cells. Carcinogenesis. 2010;31:1211–1219. doi: 10.1093/carcin/bgq085. [DOI] [PubMed] [Google Scholar]
- 18.Cheung TT, Poon RT, Jenkins CR, Chu FS, Chok KS, Chan AC, Tsang SH, Dai WC, Yau TC, Chan SC, et al. Survival analysis of high-intensity focused ultrasound therapy vs. transarterial chemoembolization for unresectable hepatocellular carcinomas. Liver Int. 2014;34:e136–e143. doi: 10.1111/liv.12474. [DOI] [PubMed] [Google Scholar]
- 19.Yuan SX, Yang F, Yang Y, Tao QF, Zhang J, Huang G, Yang Y, Wang RY, Yang S, Huo XS, et al. Long noncoding RNA associated with microvascular invasion in hepatocellular carcinoma promotes angiogenesis and serves as a predictor for hepatocellular carcinoma patients' poor recurrence-free survival after hepatectomy. Hepatology. 2012;56:2231–2241. doi: 10.1002/hep.25895. [DOI] [PubMed] [Google Scholar]
- 20.Song R, Song H, Liang Y, Yin D, Zhang H, Zheng T, Wang J, Lu Z, Song X, Pei T, et al. Reciprocal activation between ATPase inhibitory factor 1 and NF-κB drives hepatocellular carcinoma angiogenesis and metastasis. Hepatology. 2014;60:1659–1673. doi: 10.1002/hep.27312. [DOI] [PubMed] [Google Scholar]
- 21.Yang X, Zhang XF, Lu X, Jia HL, Liang L, Dong QZ, Ye QH, Qin LX. MicroRNA-26a suppresses angiogenesis in human hepatocellular carcinoma by targeting hepatocyte growth factor-cMet pathway. Hepatology. 2014;59:1874–1885. doi: 10.1002/hep.26941. [DOI] [PubMed] [Google Scholar]
- 22.He C, Sun XP, Qiao H, Jiang X, Wang D, Jin X, Dong X, Wang J, Jiang H, Sun X. Downregulating hypoxia-inducible factor-2α improves the efficacy of doxorubicin in the treatment of hepatocellular carcinoma. Cancer Sci. 2012;103:528–534. doi: 10.1111/j.1349-7006.2011.02177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilson WR, Hay MP. Targeting hypoxia in cancer therapy. Nat Rev Cancer. 2011;11:393–410. doi: 10.1038/nrc3064. [DOI] [PubMed] [Google Scholar]
- 24.Keith B, Johnson RS, Simon MC. HIF1α and HIF2α: Sibling rivalry in hypoxic tumor growth and progression. Nat Rev Cancer. 2011;12:9–22. doi: 10.1038/nrc3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao D, Zhai B, He C, Tan G, Jiang X, Pan S, Dong X, Wei Z1, Ma L, Qiao H, et al. Upregulation of HIF-2α induced by sorafenib contributes to the resistance by activating the TGF-α/EGFR pathwayin hepatocellular carcinoma cells. Cell Signal. 2014;26:1030–1039. doi: 10.1016/j.cellsig.2014.01.026. [DOI] [PubMed] [Google Scholar]
- 26.Skuli N, Liu L, Runge A, Wang T, Yuan L, Patel S, Iruela-Arispe L, Simon MC, Keith B. Endothelial deletion of hypoxia-inducible factor-2alpha (HIF-2alpha) alters vascular function and tumor angiogenesis. Blood. 2009;114:469–477. doi: 10.1182/blood-2008-12-193581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ju C, Colgan SP, Eltzschig HK. Hypoxia-inducible factors as molecular targets for liver diseases. J Mol Med (Berl) 2016;94:613–627. doi: 10.1007/s00109-016-1408-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kong J, Kong J, Pan B, Ke S, Dong S, Li X, Zhou A, Zheng L, Sun WB. Insufficient radiofrequency ablation promotes angiogenesis of residual hepatocellular carcinoma via HIF-1α/VEGFA. PLos one. 2012;7:e37266. doi: 10.1371/journal.pone.0037266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park SJ, Lee KS, Kim SR, Chae HJ, Yoo WH, Kim DI, Jeon MS, Lee YC. AMPK activation reduces vascular permeability and airway inflammation by regulating HIF/VEGFA pathway in a murine model of toluene diisocyanate-induced asthma. Inflamm Res. 2012;61:1069–1083. doi: 10.1007/s00011-012-0499-6. [DOI] [PubMed] [Google Scholar]
- 30.Lanikova L, Reading NS, Hu H, Tashi T, Burjanivova T, Shestakova A, Siwakoti B, Thakur BK, Pun CB, Sapkota A, et al. Evolutionary selected Tibetan variants of HIF pathway and risk of lung cancer. Oncotarget. 2016 Dec 28; doi: 10.18632/oncotarget.14340. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakajima T, Nakajima E, Shearer TR, Azuma M. Concerted inhibition of HIF-1α and −2α expression markedly suppresses angiogenesis in cultured RPE cells. Mol Cell Biochem. 2013;383:113–122. doi: 10.1007/s11010-013-1760-1. [DOI] [PubMed] [Google Scholar]
- 32.Xi HQ, Wu XS, Wei B, Chen L. Eph receptors and ephrins as targets for cancer therapy. J Cell Mol Med. 2012;16:2894–2909. doi: 10.1111/j.1582-4934.2012.01612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barquilla A, Pasquale EB. Eph receptors and ephrins: Therapeutic opportunities. Annu Rev Pharmacol Toxicol. 2015;55:465–487. doi: 10.1146/annurev-pharmtox-011112-140226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Youngblood V, Wang S, Song W, Walter D, Hwang Y, Chen J, Brantley-Sieders DM. Elevated Slit2 activity impairs VEGF-induced angiogenesis and tumor neovascularization in ephA2-deficient endothelium. Mol Cancer Res. 2015;13:524–537. doi: 10.1158/1541-7786.MCR-14-0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lennon FE, Mirzapoiazova T, Mambetsariev N, Mambetsariev B, Salgia R, Singleton PA. Transactivation of the receptor-tyrosine kinase ephrin receptor A2 is required for the low molecular weight hyaluronan-mediated angiogenesis that is implicated in tumor progression. J Biol Chem. 2014;289:24043–24058. doi: 10.1074/jbc.M114.554766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Merritt WM, Kamat AA, Hwang JY, Bottsford-Miller J, Lu C, Lin YG, Coffey D, Spannuth WA, Nugent E, Han LY, et al. Clinical and biological impact of EphA2 overexpression and angiogenesis in endometrial cancer. Cancer Biol Ther. 2010;10:1306–1314. doi: 10.4161/cbt.10.12.13582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tandon M, Vemula SV, Mittal SK. Emerging strategies for EphA2 receptor targeting for cancer therapeutics. Expert Opin Ther Targets. 2011;15:31–51. doi: 10.1517/14728222.2011.538682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hendrix MJ, Seftor EA, Hess AR, Seftor RE. Vasculogenic mimicry and tumour-cell plasticity: Lessons from melanoma. Nat Rev Cancer. 2003;3:411–421. doi: 10.1038/nrc1092. [DOI] [PubMed] [Google Scholar]
- 39.Guo JQ, Zheng QH, Chen H, Chen L, Xu JB, Chen MY, Lu D, Wang ZH, Tong HF, Lin S. Ginsenoside Rg3 inhibition of vasculogenic mimicry in pancreatic cancer through downregulation of VE-cadherin/EphA2/MMP9/MMP2 expression. Int J Oncol. 2014;45:1065–1072. doi: 10.3892/ijo.2014.2500. [DOI] [PubMed] [Google Scholar]
- 40.Zhang LN, Zhao GQ, Wang Q, Niu YJ, Xu Q, Yang WY. In vitro study on expression of tumor stem cell biomarker and transdifferentiation towards endothelial cells of retinoblastoma cells under hypoxia condition. Zhonghua Yan Ke Za Zhi. 2013;49:736–743. (In Chinese) [PubMed] [Google Scholar]
- 41.Miao Z, Dong Y, Fang W, Shang D, Liu D, Zhang K, Li B, Chen YH. VEGF increases paracellular permeability in brain endothelial cells via upregulation of EphA2. Anat Rec (Hoboken) 2014;297:964–972. doi: 10.1002/ar.22878. [DOI] [PubMed] [Google Scholar]
- 42.Moghaddam N Afshar, Mahsuni P, Taheri D. Evaluation of endoglin as an angiogenesis marker in glioblastoma. Iran J Pathol. 2015;10:89–96. [PMC free article] [PubMed] [Google Scholar]
- 43.Shao Z, Zhang WF, Chen XM, Shang ZJ. Expression of EphA2 and VEGF in squamous cell carcinoma of the tongue: Correlation with the angiogenesisand clinical outcome. Oral Oncol. 2008;44:1110–1117. doi: 10.1016/j.oraloncology.2008.01.018. [DOI] [PubMed] [Google Scholar]
- 44.Sáinz-Jaspeado M, Huertas-Martinez J, Lagares-Tena L, Liberal J Martin, Mateo-Lozano S, de Alava E, de Torres C, Mora J, Del Muro XG, Tirado OM. EphA2-induced angiogenesis in ewing sarcoma cells works through bFGF production and is dependent on caveolin-1. PLoS One. 2013;8:e71449. doi: 10.1371/journal.pone.0071449. [DOI] [PMC free article] [PubMed] [Google Scholar]