Abstract
Rationale: Mortality rates from rheumatoid arthritis–associated interstitial lung disease (RA-ILD) are largely unknown.
Objectives: We sought to determine mortality rates from rheumatoid arthritis–associated interstitial lung disease in the United States from 1988 through 2004.
Methods: Using data from the National Center for Health Statistics, we calculated age-adjusted mortality rates from the deaths of persons with rheumatoid arthritis–associated interstitial lung disease, determined the prevalence of interstitial lung disease in all decedents with rheumatoid arthritis, and compared the age and underlying cause of death in these two cohorts of decedents.
Measurements and Main Results: From 1988 to 2004, there were 39,138,394 deaths in U.S. residents and 162,032 rheumatoid arthritis–associated deaths. Of these deaths, 10,725 (6.6%) met criteria for rheumatoid arthritis–associated interstitial lung. Mortality rates from rheumatoid arthritis fell over the course of this study in both women and men. However, mortality rates from rheumatoid arthritis–associated interstitial lung disease increased 28.3% in women (to 3.1 per million persons in 2004) and declined 12.5% in men (to 1.5 per million persons in 2004). Because the rate of decline in rheumatoid arthritis outpaced rheumatoid arthritis–associated interstitial lung disease in men, the prevalence of rheumatoid arthritis–associated interstitial lung disease increased in both sexes over time.
Conclusions: Clinically significant RA-ILD occurs in nearly 10% of the RA population, and is associated with shortened survival and more severe underlying disease. Whereas overall mortality rates for RA have fallen, those associated with RA-ILD have increased significantly in older age groups.
Keywords: interstitial lung disease, rheumatoid arthritis, mortality rates, epidemiology, prevalence
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
Rheumatoid arthritis–associated interstitial lung disease mortality rates and prevalence are largely unknown.
What This Study Adds to the Field
We found that the burden of rheumatoid arthritis–associated interstitial lung disease is increasing and occurs in approximately 10% of the rheumatoid arthritis population.
Rheumatoid arthritis (RA) is a systemic inflammatory disorder that classically involves the diarthrodial joints (1). This autoimmune disease is common, afflicting approximately 1% of the population, or 3 million people, in the United States (2). Extraarticular manifestations of disease are frequent, with an estimated prevalence of 40% (3), and account for a significant proportion of the excess mortality reported for RA (4). In small cohort studies, pulmonary complications of RA (including infections, RA treatment–induced lung diseases, and RA-associated interstitial lung disease [RA-ILD]) account for the death of as many as 20% of subjects. Few studies, however, have focused on the epidemiology of RA-ILD, and none have investigated RA-ILD on a large, population-based scale (3, 5).
Although available data suggest that RA-ILD results in significant morbidity when present (6), there has been widespread belief that the extraarticular manifestations of RA (including RA-ILD) are becoming less common in the era of biological agents (i.e., tumor necrosis factor [TNF]-α antagonist therapy), although no available data support this assertion (3). At the same time, concerns have been raised that the use of anti–TNF-α agents in those with concurrent fibrotic ILD may hasten the pulmonary disease process (7, 8).
In this study, we used death certificate data to examine RA-ILD on a large, population-based scale in an attempt to advance understanding of its epidemiology and to help clarify certain controversial issues related to RA-ILD. The four specific aims of this study were as follows: (1) to assess changes in mortality rates of RA-ILD over time; (2) to determine the prevalence of ILD in decedents with RA; (3) to compare age and underlying cause of death (UCD) in RA decedents with and without ILD; and (4) to examine the influence of biological agents on the prevalence of ILD among patients with RA.
METHODS
Database
We analyzed data from 1988 to 2004 stored in the U.S. Multiple Cause-of-Death (MCOD) mortality database (9). The National Center for Health Statistics (NCHS) compiles data from all death certificates in the United States and releases the figures in yearly public use files. An annual file contains more than 2 million decedent records, and each record contains decedent demographics, MCOD codes (which identify up to 20 conditions related to death within a section of the record the NCHS calls the “record axis”), and a UCD—defined by the World Health Organization as “the disease or injury which initiated the train of events leading to death” (10, 11).
In this database, from 1988 to 1998, the NCHS coded conditions related to death with the Ninth Revision of the International Classification of Diseases (ICD-9) (12). After 1998, the NCHS coded conditions related to death with the Tenth Revision of the ICD (ICD-10) (10). Additional specifics of this database related to coding, data maintenance, data quality, and accessibility have previously been described (13). All data contained in these database files have been deidentified and are on public record; therefore, Institutional Review Board (IRB) approval for this study was not required.
Case Definitions
We included files from any decedent with “rheumatoid arthritis” (RA) in the record axis or from the UCD. From 1988 to 1998, we captured all decedents with ICD-9 codes 714.0–714.9 (excluding codes 714.3 for juvenile RA and 714.4 for chronic postrheumatic arthropathy [Jaccoud's syndrome]). After 1998, we captured all decedents with RA defined by ICD-10 codes M05.0–M06.9. Under the ICD-10 coding system, juvenile arthritis and postrheumatic arthropathy were not included within this range of codes.
To capture those with RA-ILD, we identified decedents with axis codes for both “rheumatoid arthritis” and “interstitial lung disease” (ILD). From 1988 to 1998, ILD was defined by ICD-9 codes 714.8 (rheumatoid lung disease), 515 (postinflammatory pulmonary fibrosis), and 516.3 (idiopathic pulmonary fibrosis). From 1999 to 2004, interstitial lung disease was defined by similar, corresponding ICD-10 codes M05.1 and/or J99.0 (rheumatoid lung disease), and J84.1 (a code that combined both postinflammatory pulmonary fibrosis and idiopathic pulmonary fibrosis). ICD codes for the UCD are reported in the Appendix.
Methods for Mortality Rate Calculations
We used July 1 intercensal population estimates (from 1988 to 1999) and July 1 population projections (from 2000 to 2004), obtained from the U.S. Census Bureau to determine denominators for corresponding yearly mortality rates. We used the 2000 U.S. Census population to standardize mortality rates (14). Mortality rates were calculated with Microsoft Office Excel 2003 SP2 (Microsoft Corporation, Redmond, WA). For age-adjusted mortality rate 95% confidence intervals, the method by Keyfitz was used (15).
Statistical Analysis
Poisson multivariable regression analysis was used to evaluate differences in mortality rates by age group and year of death for each sex. When comparing the ultimate UCD between those decedents with RA and those with RA-ILD, the Fisher exact test was used. A two-sample t test was used to compare the mean age of death (a continuous variable) between those with RA and RA-ILD. A P value less than 0.05 was considered to represent statistical significance. All data were analyzed with SAS version 9.1 (SAS Institute, Cary, NC). The SAS “GENMOD procedure” was used to perform the Poisson regression analysis.
RESULTS
Mortality Rates from RA-associated Deaths
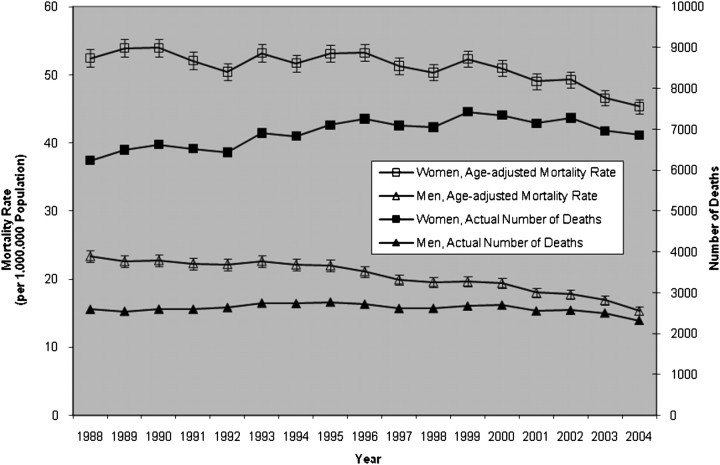
From 1988 to 2004, there were 39,138,394 deaths in U.S. residents. Of these deaths, a total of 162,032 records (or 0.41%) contained a diagnostic code for RA. For this subset, the average age-adjusted mortality rate from RA over this time period was 51.1 per 1,000,000 population in women and 20.4 per 1,000,000 population in men. From 1988 to 2004, age-adjusted mortality from RA fell 13.6% (from 52.4 per 1,000,000 in 1988 to 45.3 per 1,000,000 in 2004) in women and declined 34.2% (from 23.3 per 1,000,000 in 1988 to 15.3 per 1,000,000 in 2004) in men (Figure 1).
Figure 1.
Age-adjusted mortality rates (per 1,000,000 population) and actual number of deaths per year among decedents with rheumatoid arthritis (RA), 1988 through 2004 (error bars, 95% confidence intervals).
When we stratified the data set by sex and age grouping, mortality rates on average were higher in women than in men for any given age group (Table 1) and RA-associated mortality increased with increasing age group in both men and women (see Figure E1 in the online supplement). However, over the course of this study, for both men and women, mortality rates decreased in each age grouping, with the largest decline (as measured by percent change from 1988) occurring in those 55 to 64 years of age (Table 1). For any given age group, mortality rates declined to a greater degree in men than women with each passing year of the study (Table 1); thus, the mortality rate sex ratio of women to men increased from 2.2 in 1988 to 3.0 in 2004.
TABLE 1.
AGE-STRATIFIED MORTALITY RATES (IN 2004) AND PERCENT DECREASE IN MORTALITY RATES (FROM 1988) AMONG DECEDENTS WITH RHEUMATOID ARTHRITIS–ASSOCIATED DEATH
|
Women |
Men |
|||||
|---|---|---|---|---|---|---|
| Age Stratum (yr) |
Mortality Rate, Deaths per 1,000,000 Persons (2004) |
Percent Change (from 1988) |
Mortality Rate, Deaths per 1,000,000 Persons (2004) |
Percent Change (from 1988) |
||
| 45–54 | 9.0 ± 1.3 | −19.5 | 4.8 ± 1.0 | −35.4 | ||
| 55–64 | 38.6 ± 3.1 | −34.4 | 20.5 ± 2.4 | −51.9 | ||
| 65–74 | 136.9 ± 7.2 | −26.7 | 71.6 ± 5.7 | −41.9 | ||
| 75–84 | 365.2 ± 13.5 | −7.0 | 176.3 ± 11.4 | −27.9 | ||
| >85 |
549.8 ± 25.3 |
−1.9 |
248.2 ± 25.4 |
−6.5 |
||
Mortality rates are reported with the 95% confidence interval.
For men or women, by using Poisson regression models, we found that mortality rates from RA significantly increased with increasing age strata. Although the year was not significant in these models, all second-order interactions between age group and year (as a continuous variable) in either women or men were significant, indicating that the declining mortality rates over time resulted from significantly different rates of decline within any given age group over time (Figures E2 and E3).
Mortality Rates from RA-ILD
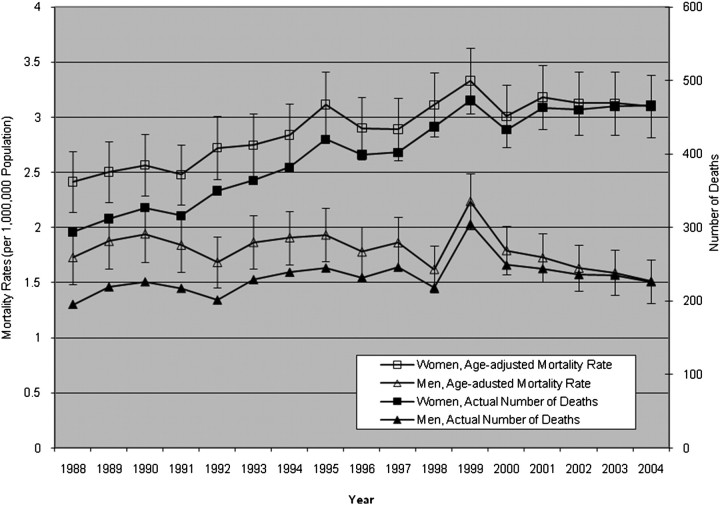
Of the total 162,032 records that contained a diagnostic code for RA, 10,725 (6.6%) met our definition of RA-ILD. Over the course of the study, the average age-adjusted mortality from RA-ILD was 2.9 per 1,000,000 population in women and 1.8 per 1,000,000 population in men. From 1988 to 2004, age-adjusted RA-ILD–associated mortality rates increased 28.3% (from 2.4 per 1,000,000 in 1988 to 3.1 per 1,000,000 in 2004) in women and fell 12.5% (from 1.7 per 1,000,000 in 1988 to 1.5 per 1,000,000 in 2004) in men. Overall, age-adjusted mortality rates in decedents with RA-ILD were higher in women than in men (Figure 2).
Figure 2.
Age-adjusted mortality rates (per 1,000,000 population) and actual number of deaths per year among decedents with rheumatoid arthritis–interstitial lung disease (RA-ILD), 1988 through 2004 (error bars, 95% confidence intervals).
When we stratified decedents with RA-ILD by sex and age, mortality rates on average were higher in women than in men for any given age group. For men or women, by using Poisson regression, we found that RA-ILD–associated mortality significantly increased with increasing age strata until the 74- to 85-year age group and then declined in those greater than 85 years of age, regardless of sex (Table 2) (Figures E4–E7). Whereas the year as a continuous variable was not significant in women (and thus taken out of the model), second-order interactions between the year and age grouping were significant for those in the 65–74, 75–84, and greater than 85-year age groups, indicating that for those age groups, RA-ILD–associated mortality rates increased significantly over time (Figures E4–E6). In men, mortality rates from RA-ILD fell significantly over time, whereas significant second-order interactions between year and age grouping indicate that, similar to women, mortality rates from RA-ILD did increase in two groups: those 75–84 years old and those greater than 85 years of age (Table 2) (Figures E4 and E7).
TABLE 2.
AGE-STRATIFIED MORTALITY RATES (IN 2004) AND PERCENT DECREASE IN MORTALITY RATES (FROM 1988) AMONG DECEDENTS WITH RHEUMATOID ARTHRITIS–INTERSTITIAL LUNG DISEASE
|
Women |
Men |
|||||
|---|---|---|---|---|---|---|
| Age Stratum (yr) |
Mortality Rate, Deaths per 1,000,000 Persons (2004) |
Percent Change (from 1988) |
Mortality Rate, Deaths per 1,000,000 Persons (2004) |
Percent Change (from 1988) |
||
| 45–54 | 0.9 ± 0.4 | −8.1 | 0.6 ± 0.3 | −42.6 | ||
| 55–64 | 3.3 ± 0.9 | −35.8 | 2.1 ± 0.8 | −50.8 | ||
| 65–74 | 12.5 ± 2.2 | 26.8 | 8.8 ± 2.0 | −6.7 | ||
| 75–84 | 26.6 ± 3.6 | 54.4 | 16.9 ± 3.5 | 6.4 | ||
| >85 |
18.4 ± 4.6 |
32.1 |
12.2 ± 5.6 |
64.4 |
||
Mortality rates are reported with the 95% confidence interval.
Comparison of Decedents with RA Alone with Those with RA-ILD
Prevalence of RA-ILD in the RA cohort.
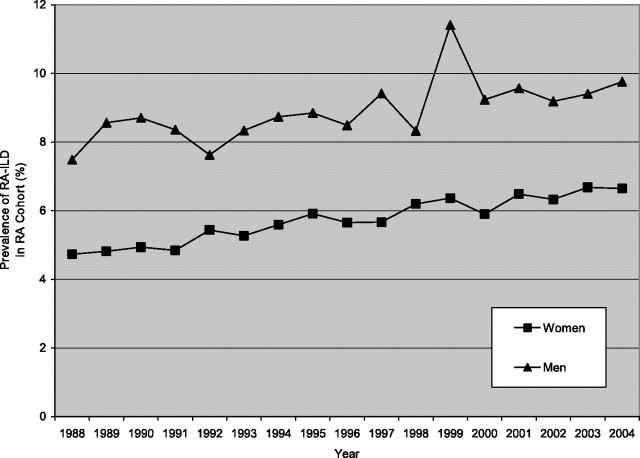
As mortality rates from RA-associated death fell for both sexes in all groups and mortality rates from RA-ILD increased in women greater than 65 years old and men greater than 75 years old over time, the prevalence of RA-ILD increased over time (the ratio of RA cases with ILD to cases without ILD) (Figure 3).
Figure 3.
Prevalence of rheumatoid arthritis–interstitial lung disease (RA-ILD) in women and men in the RA cohort, from 1988 to 2004.
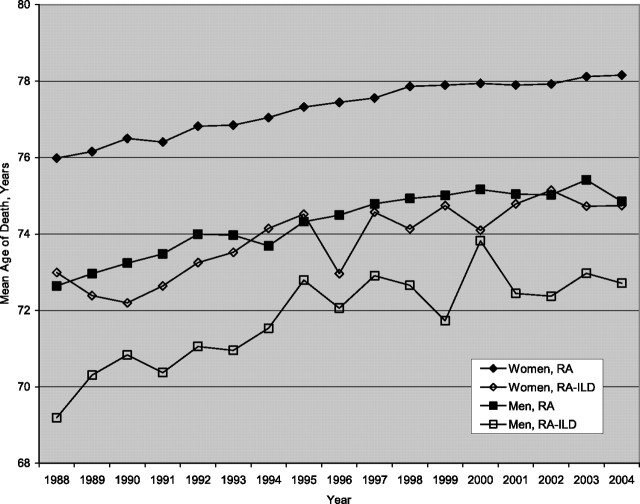
Average age at death.
Over the duration of the study, the mean age at death in the RA subgroup with ILD (for any decedent older than 45 yr) was significantly less than that for the RA subgroup without ILD (P < 0.05) (Figure 4); on average, the mean age at death of women with ILD was 3.52 years (standard deviation, 0.51 yr) less than that of women without ILD, and the mean age at death of men with ILD was 2.62 years (standard deviation, 0.51 yr) less than that of men without ILD.
Figure 4.
Mean age at death of those with rheumatoid arthritis (RA) compared with rheumatoid arthritis–interstitial lung disease (RA-ILD), by year. (For each year, a comparison between RA and RA-ILD by sex was performed using the Student t test; all P values < 0.05.)
Underlying cause of death.
Significant differences were noted in the UCD between decedents with ILD and those without ILD (Table 3). Overall, either RA-ILD or RA itself was coded as the UCD in more than 70% of decedents in the RA-ILD subgroup, whereas RA itself was coded as the UCD in approximately 20% of decedents without ILD. A smaller percentage of decedents with ILD had as the UCD other pulmonary conditions, cardiac diseases, cerebrovascular accident (CVA)/stroke, infectious diseases, and other miscellaneous causes of death (P < 0.0001 for the comparison of each UCD between groups). The risk of dying from tuberculosis was similar in both groups (P = 0.7).
TABLE 3.
AVERAGE ANNUAL UNDERLYING CAUSE OF DEATH FOR DECEDENTS WITH RHEUMATOID ARTHRITIS–INTERSTITIAL LUNG DISEASE AND RHEUMATOID ARTHRITIS ALONE OVER THE ENTIRE STUDY PERIOD
|
Underlying Cause of Death |
RA-ILD: % (SD, %) (n = 10,725) |
RA (no ILD): % (SD, %) (n = 151,322) |
OR (95% CI) |
P Value |
||||
|---|---|---|---|---|---|---|---|---|
| Rheumatoid arthritis complications | 35.0 (4.1) | 20.4 (3.9) | 2.1 (2.0–2.2) | <0.0001 | ||||
| Pulmonary diseases | ||||||||
| Interstitial lung disease | 35.3 (6.4) | — | — | — | ||||
| Pulmonary embolism | 0.3 (0.4) | 0.5 (0.1) | 0.66 (0.47–0.93) | 0.0168 | ||||
| Chronic obstructive lung disease | 1.6 (1.3) | 2.5 (2.3) | 0.68 (0.59–0.79) | <0.0001 | ||||
| Lung cancer | 2.1 (0.5) | 3.5 (0.3) | 0.58 (0.50–0.66) | <0.0001 | ||||
| Cardiac diseases | ||||||||
| Acute MI | 2.6 (0.9) | 7.8 (1.3) | 0.31 (0.27–0.34) | <0.0001 | ||||
| Chronic ischemic heart disease | 4.0 (0.9) | 11.7 (0.7) | 0.32 (0.29–0.35) | <0.0001 | ||||
| Congestive heart failure/cardiomyopathy | 1.3 (0.5) | 3.1 (0.2) | 0.43 (0.37–0.51) | <0.0001 | ||||
| Total cardiac diseases | 7.9 (1.6) | 22.6 (1.7) | 0.29 (0.27–0.31) | <0.0001 | ||||
| CVA/stroke | 0.9 (0.4) | 5.4 (0.4) | 0.16 (0.13–0.20) | <0.0001 | ||||
| Infectious diseases | ||||||||
| Pneumonia | 2.1 (1.2) | 4.4 (2.8) | 0.44 (0.39–0.51) | <0.0001 | ||||
| Sepsis | 0.6 (0.3) | 1.8 (0.2) | 0.33 (0.26–0.42) | <0.0001 | ||||
| TB | 0.08 (0.10) | 0.09 (0.04) | 0.89 (0.45–1.74) | 0.7293 | ||||
| Total infectious diseases | 2.7 (1.0) | 6.3 (2.7) | 0.41 (0.37–0.46) | <0.0001 | ||||
| Other |
14.0 (2.4) |
38.8 (2.2) |
0.25 (0.24–0.28) |
<0.0001 |
||||
Definition of abbreviations: CI = confidence interval; CVA = cerebrovascular accident; ICD = International Classification of Diseases; ILD = interstitial lung disease; MI = myocardial infarction; OR = odds ratio; RA = rheumatoid arthritis; TB = tuberculosis.
ICD-9 and ICD-10 codes used for each of the categories are presented in the Appendix.
DISCUSSION
Our results provide comprehensive data on mortality in decedents with RA and RA-ILD in the United States between 1988 and 2004. We analyzed nearly 40,000,000 death files, identified more than 160,000 decedents with RA, and found 10,725 that met our definition of RA-ILD. Over the course of the study, RA-associated mortality rates declined 20.8% in men and women combined, and the average age-adjusted mortality rate from RA was 36.2 per 1,000,000 people in the general population.
However, we found that RA-ILD–associated mortality rates increased over time in older women and older men, and the overall, average, age-adjusted mortality rate for women was 3.2 per 1,000,000 population and for men was 2.0 per 1,000,000 population. For men, declines in non–ILD-RA–associated mortality outpaced the decline in RA-ILD–associated mortality; thus, the prevalence of RA-ILD in RA-associated deaths rose over the course of the study (from 7.5 to 9.8%). In women, overall mortality rates from RA-ILD increased over time, and the prevalence of RA-ILD in RA-associated deaths also rose over time (from 4.7 to 6.8%).
Previously reported data regarding the prevalence of RA-ILD in patients with RA vary depending on the method used to diagnose disease and the population under study. Studies in which investigators used high-resolution computed tomography (HRCT) to detect lung disease in subjects with RA have revealed that the majority have interstitial abnormalities (16, 17); however, available limited data suggest that far fewer have clinically significant disease. Using a community-based cohort of 609 patients from Olmsted County, Minnesota, diagnosed with RA between 1955 and 1994 and monitored for a median of 11.8 years, Turesson and colleagues reported clinically significant lung fibrosis in 6.8% of patients (standard error, 1.9%) (3). In 1997, Gabbay and colleagues observed that among 36 patients with RA and joint manifestations of less than 2 years' duration, despite HRCT findings consistent with ILD in 33% of patients, only 14% of patients had clinically significant disease (5). In an examination of 582 patients with RA monitored for a mean of 16.4 years, Bongartz and colleagues reported a lifetime risk of 7.7% for developing ILD (18). Similar to these results, our data suggest that the prevalence of clinically significant RA-ILD, defined as such because it was recorded on a death certificate as a contributor to the death process, is at least 6.8% in women and 9.8% in men (as of 2004) at the time of death. The development and universal use of HRCT scans (19), along with the conduct of several, large multicenter trials of agents for idiopathic pulmonary fibrosis (20), have both likely contributed to the increased clinical awareness of underlying lung disease in RA.
Not only did we find the prevalence of clinically significant RA-ILD on the rise over the course of the study, we found that this was partly the result of increasing RA-ILD mortality rates in older age groups and of declining mortality rates in RA that outpaced any decreases noted for RA-ILD in men. Several factors may account for these findings. As more effective therapies have become available for the treatment of RA joint disease (e.g., disease-modifying agents and TNF-α antagonists) and its associated comorbid conditions (i.e., heart disease), the relative burden of RA-ILD—a condition that, at least when fibrosis is present, has no reliably effective therapy—has increased (21). The time frame of our study extends beyond the period that saw widespread implementation of biological agents for RA; the absence of a decline in the prevalence of RA-ILD over these years suggests that biologic agents do not prevent the development of (or death from) ILD among patients with RA (22).
Regardless of the contributing factors, our findings do not suggest that RA-ILD is becoming less common. In fact, mortality rates from RA-ILD appear to be increasing in those who are older. Further, we found that the mean age of death in RA decedents with ILD was less than in those without ILD, suggesting that clinically significant RA-ILD is associated with early mortality. We also found that decedents with RA-ILD were more likely to die of RA itself or ILD progression than of non-ILD pulmonary conditions, cardiac disease, stroke, or infectious disease. Thus, although the data would suggest that improvements in therapies for RA (e.g., biological agents) have contributed to a decline in death from RA, for certain subgroups with ILD this has not held true. It remains unclear whether such drugs (i.e., TNF-α antagonists) are detrimental in patients with RA-ILD and this issue requires additional investigation.
For context, the average age- and sex-adjusted mortality rate from RA over the course of this study was 36.2 per 1,000,000 and this figure is on the same order as mortality rates from pulmonary fibrosis (50.8 per 1,000,000) over a similar time course (13), lung cancer (526 per 1,000,000), and chronic obstructive pulmonary disease (424 per 1,000,000) (23). Further, the age- and sex-adjusted mortality rates from RA-ILD over the course of this study are similar to mortality rates from influenza (1 per 1,000,000) (23).
This study has several limitations. The accuracy of the data depends entirely on death certifiers to identify RA and RA-ILD, and on the accurate coding of these conditions on the death certificate. We were unable to identify any peer-reviewed studies regarding the accuracy of rheumatological disease coding; however, previous investigations have found that “pulmonary fibrosis” (defined by ICD-9 codes 516.3 [IFP] and 515 [postinflammatory pulmonary fibrosis, PIPF] and ICD-10 code J84.1 [a combination of IPF and PIPF]) is underreported on death certificates (24, 25). We do not know whether underreporting occurs in other diagnostic codes for ILD, but it probably does. However, if underreporting occurred for RA-ILD, then the prevalence of RA-ILD and mortality rates from RA-ILD would be higher than we report and our results describe the minimal value for the scope of this problem. The database used ICD-9 codes to identify patients with RA and RA-ILD from 1988 to 1998 and ICD-10 codes after 1999. The ICD-10 codes used to define this cohort may or may not reflect a cohort of patients similar to the one defined by ICD-9 codes. One French study found good comparability between ICD-9 and ICD10 codes for all musculoskeletal diseases (26). We did not observe any significant discontinuity in the RA- or RA-ILD–associated mortality around the time of the coding change.
Although other, population-based, epidemiological studies investigating RA-ILD are not available for comparison, large-scale studies from France and Sweden have reported RA-associated mortality trends similar to those we found (27, 28). Ziadé and colleagues examined all French death certificates (n = 17,806,923) from 1970 to 2002 and found an 18% decline (27), and data from Sweden also indicate a decrease in RA-associated mortality over the past 20 years (28). These results, taken together, further support our overall findings and suggest that therapies for RA have impacted survival in some patients with RA, and that RA-ILD may be a growing complication of disease.
CONCLUSIONS
In the United States from 1988 to 2004, although mortality rates from RA declined, there was an increasing prevalence of RA. Patients with RA-ILD die at a younger age than those with RA and they more often die of either lung disease or RA itself than from other causes, suggesting that this extraarticular manifestation of disease is more severe than RA alone. Although therapy for RA may be resulting in declining mortality from certain RA-related manifestations, the same trend does not appear to be holding true for RA-ILD. Further investigation is required to examine these findings.
APPENDIX
From 1988 through 1998, “acute myocardial infarction” (AMI) was defined by ICD-9 codes 410.0 through 411.8, “chronic ischemic heart disease” (CIHD) was defined by ICD-9 codes 414.0 through 414.9, “congestive heart failure” (CHF) was defined by ICD-9 codes 428.0 through 428.9, cardiomyopathy (CM) was defined by ICD-9 codes 425.0 through 425.9, “chronic obstructive pulmonary disease” (COPD) was defined by ICD-9 codes 490 through 492.8, including 493.2 (asthma associated with COPD), “pulmonary embolism” (PE) was defined by ICD-9 code 415.1, “lung cancer” was defined by ICD-9 codes 162.2 through 162.9, “pneumonia” was defined by ICD-9 codes 480.0 through 487.8, “sepsis” was defined by ICD-9 codes 038.0 through 038.9, “tuberculosis” (TB) was defined by ICD-9 codes 010.0 through 018.9, and “cerebrovascular disease” (CVD) was defined by ICD-9 codes 430.0 through 438.0.
After 1998, AMI was defined by ICD-10 codes I21.0 through I24.9, CIHD was defined by ICD-10 codes I25.0 through I25.9, CHF was defined by ICD-10 codes I50.0 thought I50.9, CM was defined by ICD-10 codes I42.0 through I42.9, COPD was defined by ICD-10 codes J40 through J44.9, PE was defined by ICD-10 codes I26.0 through I26.9, lung cancer was defined by ICD-10 codes C34.0 through C34.9, pneumonia was defined by ICD-10 codes J09 through J18.9, sepsis was defined by ICD-10 codes A40.0 through A41.9, TB was defined by ICD-10 codes A15.0 through A19.9, and CVD was defined by ICD-10 codes I60.0 through I69.8.
This article has an online supplement, which is available from the issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201004-0622OC on September 17, 2010
Author Disclosure: A.L.O. received a training grant from the NIH ($50,001–$100,000). J.J.S. was a consultant for Actelion Pharmaceuticals, Inc. ($1,001–$5,000) and received grant support from the NIH (more than $100,001). He has served as a site principal investigator for drug trials sponsored by Actelion Pharmaceuticals, Intermune, and Novartis. D.B.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. A.F. was on the Board or Advisory Board for Actelion ($5,001–$10,000). She received lecture fees from Actelion ($5,001–$10,000) and Gilead ($1,001–$5,000). E.R.F.-P. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.M. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.C. was a consultant for UCB and Genentech ($1,001–$5,000), and was on the Board or Advisory Board for UCB ($1,001–$5,000). G.R. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. K.K.B. was a consultant for Genzyme, Actelion ($10,001–$50,000), MondoBiotech ($1,001–$5,000), Phillips (up to $1,000), Amgen, Pacific Therapeutics, Celgene, Elan, Stromedix, and Fibrogen ($1,001–$5,000). He was on the Board or Advisory Board for Novartis, Boehringer-Ingelheim, Centocor, and Gilead ($5,001–$10,000) and received lecture fees from Biogen ($1,001–$5,000). He received grant support from Genzyme ($50,001–$100,000), Actelion (more than $100,001), Novartis, Gilead, and Amgen ($50,001 - $100,000), and the NIH-NHLBI (more than $100,001).
References
- 1.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HS, et al. The American Rheumatism Association 1987. revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31:315–324. [DOI] [PubMed] [Google Scholar]
- 2.Gabriel SE, Crowson CS, O'Fallon WM. The epidemiology of rheumatoid arthritis in Rochester, Minnesota, 1955–1985. Arthritis Rheum 1999;42:415–420. [DOI] [PubMed] [Google Scholar]
- 3.Turesson C, O'Fallon WM, Crowson CS, Gabriel SE, Matteson EL. Extra-articular disease manifestations in rheumatoid arthritis: incidence trends and risk factors over 46 years. Ann Rheum Dis 2003;62:722–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turesson C, Matteson EL. Management of extra-articular disease manifestations in rheumatoid arthritis. Curr Opin Rheumatol 2004;16:206–211. [DOI] [PubMed] [Google Scholar]
- 5.Gabbay E, Tarala R, Will R, Carroll G, Adler B, Cameron D, Lake FR. Interstitial lung disease in recent onset rheumatoid arthritis. Am J Respir Crit Care Med 1997;156:528–535. [DOI] [PubMed] [Google Scholar]
- 6.Park JH, Kim DS, Park IN, Jang SJ, Kitaichi M, Nicholson AG, Colby TV. Prognosis of fibrotic interstitial pneumonia: idiopathic versus collagen vascular disease–related subtypes. Am J Respir Crit Care Med 2007;175:705–711. [DOI] [PubMed] [Google Scholar]
- 7.Ramos-Casals M, Brito-Zerón P, Muñoz S, Soria N, Galiana D, Bertolaccini L, Cuadrado MJ, Khamashta MA. Autoimmune disease induced by TNF-targeted therapies: analysis of 233 cases. Medicine (Baltimore) 2007;86:242–251. [DOI] [PubMed] [Google Scholar]
- 8.Ledingham J, Deighton C. Update on the British Society for Rheumatology guidelines for prescribing TNFα blockers in adults with rheumatoid arthritis (update of previous guidelines of April 2001). Rheumatology (Oxford) 2005;44:157–163. [DOI] [PubMed] [Google Scholar]
- 9.National Center for Health Statistics, Centers for Disease Control and Prevention. Public use data tape documentation: multiple cause of death for ICD-9 1992 data. Hyattsville, MD: U.S. Department of Health and Human Services; 1994.
- 10.World Health Organization. ICD-10: international statistical classification of diseases and related health problems, 10th revision. Geneva, Switzerland: World Health Organization; 2003.
- 11.Redelings MD, Sorvillo F, Simon P. A comparison of underlying cause and multiple causes of death. Epidemiology 2006;17:100–103. [DOI] [PubMed] [Google Scholar]
- 12.U.S. Public Health Service. International classification of diseases, 9th revision. DHHS Publication No. (PHS) 80-1260. Washington, DC: U.S. Government Printing Office; 1980.
- 13.Olson AL, Swigris JJ, Lezotte DC, Norris JM, Wilson CG, Brown KK. Mortality from pulmonary fibrosis increased in the United States from 1992 to 2003. Am J Respir Crit Care Med 2007;176:277–284. [DOI] [PubMed] [Google Scholar]
- 14.U.S. Census Bureau. Intercensal population estimates [Internet]. Available from: http://www.census.gov [accessed October 2010].
- 15.Keyfitz N. Sampling variance of standardized mortality rates. Hum Biol 1966;38:309–317. [PubMed] [Google Scholar]
- 16.Zrour SH, Touzi M, Bejia I, Golli M, Rouatbi N, Sakly N, Younes M, Tabka Z, Bergaoui N. Correlations between high-resolution computed tomography of the chest and clinical function in patients with rheumatoid arthritis. Joint Bone Spine 2005;72:41–47. [DOI] [PubMed] [Google Scholar]
- 17.Biglici A, Ulusoy H, Kuru O, Celenk C, Unsal M, Danaci M. Pulmonary involvement in rheumatoid arthritis. Rheumatol Int 2005;25:429–435. [DOI] [PubMed] [Google Scholar]
- 18.Bongartz T, Nannini C, Medina-Velasquez YF, Achenbach SJ, Crowson CS, Ryu JH, Vassallo R, Babriel SE, Matteson EL. Incidence and mortality of interstitial lung disease in rheumatoid arthritis: a population based study. Arthritis Rheum 2010;62:1583–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lynch JP III, Saaggar R, Weight SS, Zisman DA, White ES. Usual interstitial pneumonia. Semin Respir Crit Care Med 2006;27:634–651. [DOI] [PubMed] [Google Scholar]
- 20.Rhagu G, Brown KK, Bradford WZ, Starko K, Nobel PW, Schwartz DA, King TE Jr. A placebo-controlled trial of interferon γ-1b in patients with idiopathic pulmonary fibrosis. N Engl J Med 2004;350:125–133. [DOI] [PubMed] [Google Scholar]
- 21.Brown KK. Rheumatoid lung disease. Proc Am Thorac Soc 2007;4:443–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kelly C, Hamilton J. What kills patients with rheumatoid arthritis? [editorial]. Rheumatology 2007;46:183–184. [DOI] [PubMed] [Google Scholar]
- 23.National Center for Health Statistics, Centers for Disease Control and Prevention. Deaths and mortality [Internet]. Available from: http://www.cdc.gov/nchs/fastats/deaths.htm [accessed October 2010].
- 24.Coultas DB, Zumwalt RE, Black WC, Sobonya RE. The epidemiology of interstitial lung diseases. Am J Respir Crit Care Med 1994;150:967–972. [DOI] [PubMed] [Google Scholar]
- 25.Johnson I, Britton J, Kinnear W, Logan R. Rising mortality from cryptogenic fibrosing alveolitis. BMJ 1990;301:1017–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pavillon G, Boileau J, Renaud G. Conséquences des changements de codage des causes médicales de décès sur les données nationals de mortalité en France, à partir de l'année 2000 [in French]. Bull Epidemiol Hebdomadaire 2005;4:13–16. [Google Scholar]
- 27.Ziadé N, Jougla E, Coste J. Population-level influence of rheumatoid arthritis on mortality and recent trends: a multiple cause-of-death analysis in France, 1970–2002. J Rheumatol 2008;35:1950–1957. [PubMed] [Google Scholar]
- 28.Björnådal L, Baecklund E, Yin L, Granath F, Klareskog L, Ekbom A. Decreasing mortality in patients with rheumatoid arthritis: results from a large population based cohort in Sweden, 1964–95. J Rheumatol 2002;29:906–912. [PubMed] [Google Scholar]