Abstract
The aim of the present study was to investigate the molecular mechanism associated with the traditional Chinese medicine formula Gui Zhu Yi Kun formula (GZYKF), in the treatment of polycystic ovary syndrome (PCOS). In this study, granulosa cells (GCs) of rats with PCOS were cultured and treated with testosterone propionate (TP) alone or with serum from rats treated with different doses of GZYKF. The effect of TP on cell growth was assayed using the MTT method. Expression levels of Beclin-1, light chain (LC)3, mechanistic target of rapamycin (mTOR), tumor suppressor p53 (p53), adenosine monophosphate-activated protein kinase (AMPK), sestrin2 and tuberous sclerosis protein 1/2 were evaluated using quantitative polymerase chain reaction and western blotting. It was demonstrated that TP increased the expression of Beclin-1 and LC3, whereas GZYKF significantly decreased the TP-induced expression of Beclin-1 (P<0.01). Additionally, GCs treated with GZYKF exhibited significant increases in mTOR, phosphorylated mTOR and AMPKα expression levels, and significant reductions in p53 and sestrin2 expression levels were observed. In conclusion, the findings of the present study suggest that a reduction in ovarian GCs in rats with PCOS may be associated with GC autophagy. Furthermore, the effects of GZYKF in mediating the p53/AMPK pathway may inhibit GC autophagy, which suggests a possible novel mechanism underlying the treatment of PCOS with GZYKF.
Keywords: autophagic cell death, Chinese herbal medicine, mechanistic target of rapamycin, polycystic ovary syndrome
Introduction
Polycystic ovary syndrome (PCOS) is one of the most prevalent endocrine disorders in gynecology (1). PCOS may lead to various reproductive endocrine disorders and long-term health complications, and is therefore a potential threat to the health of women. Despite its health impacts, the pathophysiology of PCOS remains to be elucidated. In a previous study, it was reported that treatment of PCOS using Gui Zhu Yi Kun formula (GZYKF) was effective (2). However, the underlying mechanism associated with the therapeutic effects GZYKF also remains to be elucidated.
Autophagy is a cellular process which is known to participate in the pathological progression of various diseases. Autophagy is able to induce cell death that is distinct from that initiated by apoptosis (3). In the heart, excessive autophagy may be induced by severe stress, which results in cell death (4). Autophagic cell death may also occur if unfavorable metabolic conditions are established during cancer progression (5) and the inhibition of autophagy may be a potential avenue for anticancer therapy (6). Therefore, it is important to elucidate the molecular mechanisms associated with autophagy-induced cell death.
In the last decade, autophagy signaling pathways and major protein regulators have been identified. The rat microtubule-associated protein 1 light chain (LC)3, is associated with autophagosome membranes following processing (7). Beclin-1 has also been demonstrated to serve a critical role in autophagosome formation (8). Furthermore, previous studies have identified tumor suppressor p53 (p53) as a dual modulator of autophagy in regulating cell death and survival (9,10). At low energy levels, adenosine monophosphate-activated protein kinase (AMPK), which is activated by p53, is able to activate tuberous sclerosis complex (TSC)2 and thus inhibit mammalian target of rapamycin (mTOR) activity and ultimately increase autophagy (11).
The authors of the present study have previously demonstrtated that the autophagy rate of granulosa cells (GCs) in rats with PCOS is significantly increased as compared with normal control rats, and that the decrease of ovarian GCs in rats with PCOS may be associated with autophagic cell death (12).
Therefore, in the present study, GCs of rats with PCOS were used as an experimental model and testosterone propionate (TP) was used to induce autophagy. The aim of the present study was to investigate the molecular mechanism associated with the traditional Chinese medicine GZYKF in the treatment of PCOS.
Materials and methods
Antibodies and reagents
Dulbecco's modified Eagle's medium/nutrient mixture F-12 (DMEM/F12) and fetal bovine serum (FBS) were obtained from Gibco (Thermo Fisher Scientific, Inc., Waltham, MA, USA). Penicillin, streptomycin, and dimethylsulfoxide were purchased from Sigma-Aldrich (Merck Millipore, Darmstadt, Germany). Anti-p53 (cat. no. TDY075), -GAPDH (cat. no. REK0005) and -β-actin antibodies (cat. no. TDY041) were obtained from Beijing TDY Biotech Co., Ltd., (Beijing, China). Anti-follicle-stimulating hormone receptor (FSHR) antibody (cat. no. orb213952) was purchased from Biorbyt (Cambridge UK). Anti-LC3 (cat. no. 4108), -Beclin-1 (cat. no. 3495), -phosphorylated (p-)mTOR (cat. no. 5536), -mTOR (cat. no. 2983) and -AMPKα (cat. no. 2532) antibodies, and bovine serum albumin (cat. no. 9998) were purchased from Cell Signaling Technology, Inc., (Danvers, MA, USA). Anti-sestrin antibody (cat. no. 10795–1) was purchased from ProteinTech Group, Inc., (Chicago, IL, USA). GZYKF was obtained from Dongfang Hospital of Beijing University of Chinese Medicine (Beijing, China).
Cell culture
A total of 50 immature (23–25 days old) female Sprague-Dawley rats, with a weight of 60±10 g, were obtained from Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China; certificate no: SCXK 2006–0008). GCs were obtained from these Sprague-Dawley rats, to which 40 IU pregnant mare serum gonadotropin (cat. no. HOR-272; Prospec-Tany TechnoGene, Ltd., East Brunswick, NJ, USA) had been subcutaneously administered. All experimental procedures were conducted in accordance with the guidelines of National Institutes of Health for the Care and Use of Laboratory Animals. Rats were raised under a 12-h light/dark cycle, at 22±2°C and with humidity of 35–45% with ad libitum access to food and water. All rats were sacrificed via cervical dislocation and GCs were subsequently isolated using standard protocols (13), and 10 million cells were seeded in a 25-cm2 culture flask with DMEM/F12 culture medium containing 1% penicillin/streptomycin, and 10% FBS, and incubated for 5 days in an atmosphere containing 5% CO2 at 37°C. Following 24 h of incubation, non-adherent cells were removed and culture medium was changed every two days.
Immunohistochemistry
GCs were characterized by immunohistochemical staining to detect the expression of FSHR, which is a specific GC marker (14). Stably transfected GCs were plated on glass slides prior to fixation in 4% paraformaldehyde. Endogenous peroxidases were blocked by incubation in 3% H2O2 at room temperature for 10 min and 0.1% Triton X-100 was added for 10 min at room temperature. Following washing with PBS three times (3 min each), nonspecific binding was blocked by incubation in PBS containing 5% bovine serum albumin. Anti-FSHR antibody (1:100) was added at 4°C overnight and non-immune IgG (1:100; cat. no. NIR-IG; Affinity Biologicals, Inc., Ancaster, Canada) was used as a negative control. Following washing with PBS three times (3 min each), horseradish peroxidase-conjugated goat anti-rabbit IgG (1:100; cat. no. S001; Beijing TDY Biotech Co., Ltd.) was added in the dark at 37°C for 45 min. Antigenic sites were localized using a 3,3′-diaminobenzidine kit (cat. no. ZLI-9018; ZSGB-BIO, Beijing, China). Images were captured on a light microscope.
Cell cytotoxicity assay
The effect of testosterone propionate (TP) on cell growth was assayed using the MTT method (15). Cells were cultured in 96-well plates (104 cells/well) at 37°C and stimulated with different concentrations of TP (10−4, 10−5, 10−6, 10−7, 10−8 and 0 mol/l) in DMEM/F12 medium. Following 24, 48 and 72 h of stimulation with TP, MTT was added to each well (final concentration, 0.5 mg/ml) and incubated for a further 4 h. The viable cell number was directly proportional to the production of formazan following solubilization with 10% sodium dodecyl sulfate. Color intensity was measured at 490 nm. Each condition was performed in triplicate, and data were obtained from at least three separate experiments.
Preparation of GZYKF-containing rat serum
Water soluble extracts of GZYKF were prepared as follows: GZYKF included 20 g Tu Si-zi (Semen Cuscutae), 15 g Bai zhu (Rhizoma Atractylodis Macrocephalae), 15 g Dang gui (Angelica sinensis), 12 g Sha Shen (Adenophora tetraphylla), 15 g Che Qian-zi (Plantago asiatica), 12 g Qian Cao (Rubia cordifolia) and 1 0 g Si Gua-luo (Luffa cylindrica) were used. Herbs were initially subjected to soaking for 1 h in water at room temperature, followed by 1 h of decoction at 100°C. Extracts harvested from the decoction were vacuum dried, and water soluble extracts of GZYKF were dissolved in double distilled water (ddH2O) at 1.8 g/ml.
A total of 40 6-week-old female Sprague-Dawley rats (weight, 200±10 g; Vital River Laboratory Animal Technology Co., Ltd.) were randomly divided into four groups (n=10 each), and housed under the same conditions as the rats used for GC culture. Rats in low, medium and high dose GZYKF groups were administered GZYKF via gavage in doses of 5, 10 and 20 g/kg daily, respectively, whereas rats from the vehicle control group were administered volume-matched ddH2O via gavage. Daily dosages of GZYKF were divided into equal shares and administered to rats in the low, medium and high dose groups once, twice or thrice per day for 4 days, respectively. On day 5, following 12 h of fasting, rats were anesthetized by intraperitoneal injection of 0.4% pentobarbital (10 ml/kg; cat. no. 1507002; Sigma-Aldrich; Merck MIllipore) and rat aortic blood was harvested. The majority of rats succumbed to mortality due to blood loss; however, when this was not the case, rats were sacrificed via cervical dislocation. Blood serum was obtained via centrifugation (1,509 × g) of blood at 4°C for 20 min. Serum was subsequently heat-inactivated at 56°C for 30 min. Finally, the GZYKF-containing serum was sterilized via filtration through 0.22-μm cellulose ester membranes.
Treatment
GCs were divided into six groups, and each group contained ~3×105 cells. Groups 1 (control group) and 6 were treated with 15% FBS. Groups 2 to 6 were treated with 10−5 mol/l TP for 24 h. Groups 2, 3 and 4 were subsequently treated for 72 h with high-dose, medium-dose and low-dose GZYKF-containing serum (2% FBS + 13% rat serum containing GZYKF), respectively. Group 5 was treated with 2% FBS + 13% rat serum without GZYKF.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
GC total RNA was extracted using TRIzol reagent (Beijing CWBio Co., Ltd., Beijing, China). DNase I kit (cat. no. CW2090; CWBio Co., Ltd.) was used to remove genomic DNA. Total RNA was reverse-transcribed into cDNA, using a HiFi-MMLV cDNA kit (cat. no. CW0744; CWBio Co., Ltd.) according to the manufacturer's protocol. qPCR was performed using the Ultra SYBR Mixture (cat. no. CW0956; CWBio Co., Ltd.) and GoldStar Taq DNA Polymerase according to the manufacturer's protocol. Each PCR reaction was performed in a 20 µl system including the following: 10 µl 2X Ultra SYBR Mixture, 0.4 µl forward primer (10 µM), 0.4 µl reverse primer (10 µM), 2 µl cDNA, and 7.2 µl ultrapure water. Gene-specific primer pairs are presented in Table I. PCR was performed at 95°C for 2 min, followed by 35 cycles of 95°C for 15 sec, 55°C for 15 sec, and 68°C for 20 sec. GAPDH was quantified as an internal control. Results were analyzed using the 2−ΔΔCq method (16). Three independent experiments were performed.
Table I.
Gene-specific primer pairs.
| Gene | Primer sequence (5′ to 3′) | Amplicon (bp) |
|---|---|---|
| Beclin-1 | F: CAGTGTTGTTGCTCCATGCT | 202 |
| R: TGCACACAGTCCAGAAAAGC | ||
| LC3 | F: TTCTTCCTCCTGGTGAATGG | 253 |
| R: GTGGGTGCCTACGTTCTGAT | ||
| GAPDH | F: TGGAGTCTACTGGCGTCTT | 138 |
| R: TGTCATATTTCTCGTGGTTCA | ||
| p53 | F: CCTCACCATCATCACACTGGAAGAC | 91 |
| R: GTCTCTCCCAGGACAGGCACAAAC | ||
| AMPK | F: ACAGGAGAATAATGAATGAAGCCAA | 203 |
| R: ATGCCATTTTGCTTTCCTTACACCT | ||
| TSC1 | F: TTGACTGTTGTAATGACGGGTGCTC | 131 |
| R: TCCACCACCTCTGCTTCCACTACT | ||
| TSC2 | F: ACGAGTCAAACAAGCCAATCCTG | 97 |
| R: TTGTGGGTGTCGTATGATGGGAT | ||
| mTOR | F: AAAACCTCAGCATCCAGAGATACGC | 191 |
| R: CATCAGAGTCAAGTGGTCATAGTCCG | ||
| sestrin2 | F: TACAATACCATCGCCATGCACAGT | 121 |
| R: GGTTCACCTCCCCATAATCATAGTCA | ||
| β-actin | F: ACTTAGTTGCGTTACACCCTT | 156 |
| R: GTCACCTTCACCGTTCCA |
F, forward; R, reverse; LC, light chain; p53, tumor suppressor p53; AMPK, adenosine monophosphate-activated protein kinase; TSC, tuberous sclerosis protein; mtor, mechanistic target of rapamycin.
Western blot analysis
From each group, ~3×106 cells were harvested, and a protein extraction kit (cat. no. WB0001; Beijing TDY Biotech Co., Ltd.) was used on ice for 10 min, followed by centrifugation at 18,000 × g at 4°C for 20 min. The supernatants were collected, and protein concentration was determined using a BCA assay kit (cat. no. WB0028; Beijing TDY Biotech Co., Ltd.). Equal amounts of protein (10 µg) were separated by 5 (stacking gel) or 10–15% (separation gel) SDS-PAGE and transferred onto a PVDF membrane. The blot was subsequently incubated with 5% non-fat milk in PBS at room temperature for 1 h to block non-specific binding, and membranes were incubated with the following primary antibodies: p53 monoclonal antibody (1:1,000), p-mTOR monoclonal antibody (1:1,000), mTOR monoclonal antibody (1:1,000), AMPKα antibody (1:4,000), LC3 monoclonal antibody (1:2,000), Beclin-1 monoclonal antibody (1:2,000), sestrin antibody (1:2,000), β-actin monoclonal antibody (1:5,000) and GAPDH monoclonal antibody (1:20,000) overnight at 4°C, and an appropriate peroxidase-conjugated secondary antibody (1:10,000; goat anti-rabbit IgG, cat. no. 111035003; or goat anti-mouse IgG, cat. no. 115035003; both Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA) was added for 1 h. Following the final washing with TBST, signals were developed via an electrochemiluminescence detection system (cat. no. WBKLS0500; Merck Millipore), and relative photographic density was quantitated via gel documentation and analysis using the Image-Pro Plus 6.0 software (Media Cybernetics, Inc., Rockville, MD, USA). Three independent experiments were performed.
Statistical analysis
Values are presented as the mean ± standard deviation. Statistical significances of differences were analyzed via t-test for two groups, or one-way analysis of variance test for three or more groups. Analyses were performed using SPSS 22.0 software (IBM SPSS, Armonk, NY, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
GC isolation and characterization
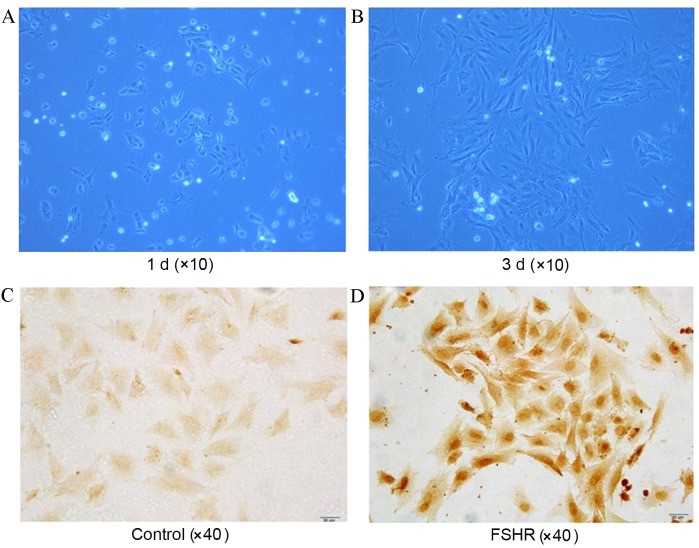
Cultured cells adhered gradually and displayed spindle-shaped morphology (Fig. 1A and B). Cells were identified as GCs by the presence of FSHR-stained antibodies (Fig. 1C and D).
Figure 1.
GC culture and characterization. Morphology (phase-contrast images; magnification, ×10) of rat ovary GCs in culture at (A) 1 d and (B) 3 d time points. Images of (C) control and (D) anti-FSHR antibody stained with cultured GCs. GCs with FSHR were stained brown. GC, granulosa cell; d, day(s); FSHR, follicle-stimulating hormone receptor.
Cell cytotoxic effects of TP on GCs
To determine the cytotoxicity of TP, GCs were treated with different concentrations of TP (10−4, 10−5, 10−6, 10−7 or 10−8 mol/l) for 24, 48 and 72 h. Results from an MTT assay of cell viability indicated that, when the concentration of TP was >10−6 mol/l, TP was able to inhibit the growth of GCs in a time- and dose-dependent manner (Fig. 2). The minimum TP stimulation time was 24 h. As the number of GCs is lower than normal in the ovaries of rats with PCOS, the optimal concentration of TP was set at 10−5 mol/l. Cell viability was significantly decreased following 24 h of treatment with 10−5 mol/l TP, compared with the control group (t=3.68; P=0.021).
Figure 2.
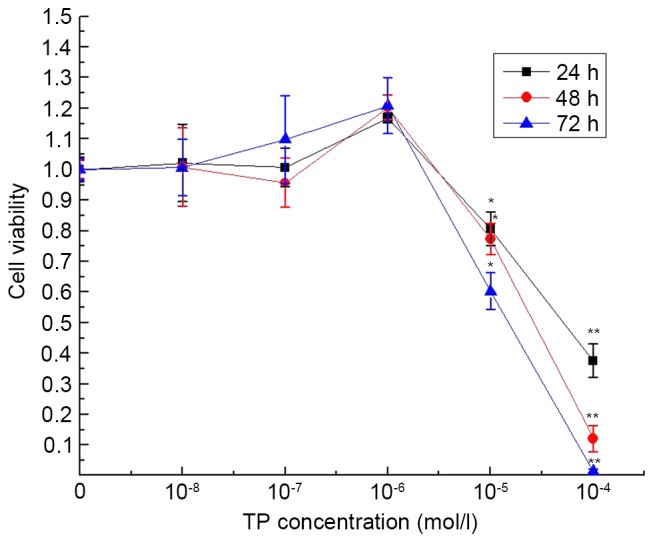
Cell cytotoxicity assay. The concentrations of TP used were 10−10, 10−8, 10−6, 10−4 and 10−2 mol/l. When concentration of TP was >10−6 mol/l, cell growth was inhibited in a time- and dose-dependent manner. The mean inhibitory rate following treatment with 10−5 mol/l TP for 24 h was 20%, and cell viability was significantly decreased compared with the control group (t=3.68; P=0.021). *P<0.05, **P<0.01 vs. control group. TP, testosterone propionate.
GZYKF decreases the autophagy rate of GCs
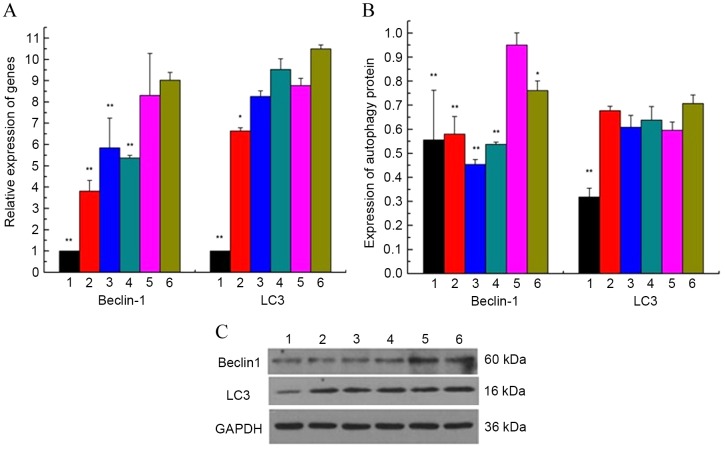
Beclin-1 and LC3 mRNA and protein levels in cultured GCs were quantified via RT-qPCR and western blotting, respectively, to evaluate the correlation between the reduction of GCs in the ovary with PCOS and autophagy, and the effect of GZYKF on the autophagy rate of GCs. LC3 is a typically-used marker for autophagy activation due to its critical role as a structural protein in autophagosome formation (17). Beclin-1 was analyzed as a marker of autophagy in the participation and regulation of autophagosomes. The mRNA expression of Beclin-1 and LC3 was demonstrated to be significantly increased in group 5 compared with the control group (both P<0.01). Furthermore, when compared with group 5, high-, medium- and low-dosages of GZYKF, were all able to significantly inhibit mRNA expression of Beclin-1 (all P<0.01), whereas only high-dose GZYKF significantly inhibited the mRNA expression of LC3 (P<0.05; Fig. 3A). Western blot analysis indicated a significant increase in Beclin-1 and LC3 protein expression levels in groups 5 compared with the control group (both P<0.01). GZYKF significantly decreased the protein expression levels of Beclin-1 (all P<0.01), whereas LC3 was not significantly affected by GZYKF (Fig. 3B and C).
Figure 3.
(A) mRNA expression of autophagy-related genes Beclin-1 and LC3, detected by reverse transcription-quantitative polymerase chain reaction, were higher in groups 5 compared with group 1. (B and C) Protein expression of Beclin-1 and LC3 were detected by western blotting. The results indicated that expression levels of autophagy-related proteins Beclin-1 and LC3 were significantly higher in groups 5 than in group 1. GZYKF decreased the protein expression levels of Beclin-1, and high-dose GZYKF decreased the protein expression levels of LC3. Data are presented as mean ± standard error of the mean of three independent experiments. *P<0.05, **P<0.01 vs. group 5. LC, light chain; TP, testosterone propionate; GZYKF, Gui Zhu Yi Kun formula; group 1, control; group 2, treated with TP and high-dose GZYKF-containing serum; group 3, treated with TP and medium-dose GZYKF-containing serum; group 4, treated with TP and low-dose GZYKF-containing serum; group 5, treated with TP and serum without GZYKF; group 6, treated with TP.
Effect of GZYKF on the p53/AMPK signaling pathway
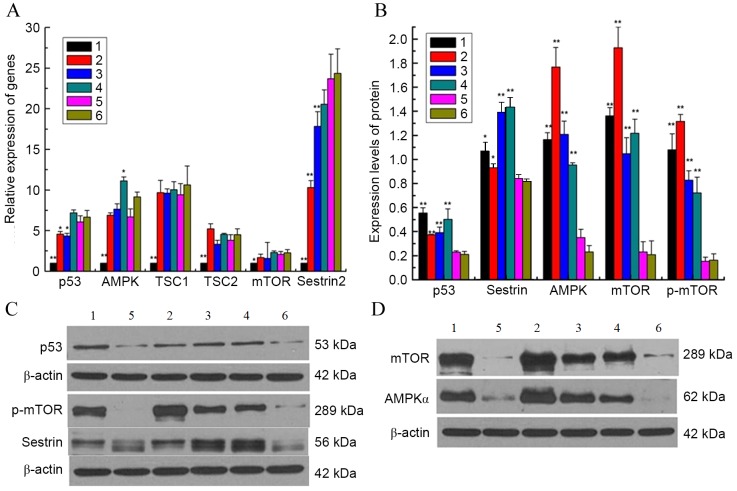
RT-qPCR and western blotting were used to detect changes in molecules from the p53/AMPK signaling pathway following culture of GCs with GZYKF-containing serum. mTOR has a role in the inhibition of autophagy, whereas p53, AMPK, sestrin2, TSC1 and TSC2 promote autophagy (18). In the present study, mRNA expression levels of mTOR, p53, AMPK, sestrin2, TSC1 and TSC2 were significantly higher in group 5 than the control group (all P<0.05). Furthermore, in groups 2 and 3, significantly lower levels of p53 (both P<0.05) and sestrin2 mRNA (both P<0.01) as compared with group 5; however no significant difference was observed between groups 4 and 5. Furthermore, no significant difference was observed among the expression levels of TSC1, TSC2 and mTOR mRNA in groups 2, 3, 4 and 5. When compared with group 5, the level of AMPK mRNA was significantly higher in group 4 (P<0.05; Fig. 4A). Results of western blot analysis demonstrated that mTOR, p-mTOR, p53, AMPKα and sestrin protein levels in group 5 were significantly decreased compared with those in the control group (all P<0.05). Significantly higher levels of these proteins were exhibited in groups 2 (all P<0.05), 3 (all P<0.01) and 4 (all P<0.01) than in group 5. The results for groups 2, 3 and 4 further revealed a dose-dependent relationship between GZYKF and these proteins, as and increasing dose of GZYKF has a positive association with mTOR, p-mTOR, AMPKα and sestrin activation, whereas increasing inhibition of p53 was observed when the GZYKF dosage was increased (Fig. 4B-D).
Figure 4.
(A) mRNA expression of p53/AMPK signaling pathway-related genes, which were detected via reverse transcription-quantitative polymerase chain reaction, indicated markedly higher expression levels in groups 2, 3, 4, 5 and 6 compared with group 1. (B-D) Western blotting was used to detect the expression levels of p53/AMPK signaling pathway-related proteins. The expression levels of mTOR, p-mTOR, p53, AMPKα and sestrin were increased in the experimental groups compared with group 1. Furthermore, when compared with group 5, p53, mTOR, p-mTOR, AMPKα and sestrin levels were significantly increased in groups 2, 3 and 4. Results for groups 2, 3 and 4 suggest that GZYKF activates mTOR, p-mTOR, AMPKα and sestrin, and inhibits p53 in a dose-dependent manner. Data are presented as mean ± standard error of the mean of three independent experiments. *P<0.05, **P<0.01, vs. group 5. p53, tumor suppressor p53; AMPK, adenosine monophosphate-activated protein kinase; TSC, tuberous sclerosis protein; mTOR, mechanistic target of rapamycin; p, phosphorylated; TP, testosterone propionate; GZYKF, Gui Zhu Yi Kun formula; group 1, control; group 2, treated with TP and high-dose GZYKF-containing serum; group 3, treated with TP and medium-dose GZYKF-containing serum; group 4, treated with TP and low-dose GZYKF-containing serum; group 5, treated with TP and serum without GZYKF; group 6, treated with TP.
Discussion
In the present study, data generated from the MTT assay for cell viability indicated that TP inhibits GC growth when applied at a concentration of >10−6 mol/l. Prevalent and characteristic traits of PCOS are hyperandrogenemia and a reduced number of ovarian GCs (19). Therefore, TP may be used to induce a PCOS cell model in vitro. Various studies have reported autophagic cell death as one of the major mechanisms of cell death (20–22). The present findings suggest that TP activates autophagy, and that reduced ovarian GCs in PCOS may be associated with autophagy. The present study has also demonstrated that GZYKF may have the ability to decrease Beclin-1 protein, Beclin-1 mRNA and LC3 mRNA expression, which suggests that GZYKF potentially increases GC numbers by reducing autophagic cell death.
Finally, the results of the present study suggest that GZYKF mediates the p53/AMPK signaling pathway, which is closely associated with cell autophagy. There is a growing body of evidence supporting the ability of p53 to both upregulate and downregulate autophagic cell death (23–25). The results of the present study suggest that GZYKF may increase the expression levels of mTOR to inhibit autophagy, which may be associated with p53, AMPK and sestrin activation. p53, which is activated by GZYKF, further activates mTOR to inhibit autophagy in the cytoplasm. When p53 is activated by GZYKF in the nucleus, it also activates AMPK and sestrin, which acts as a feedback in mTOR inhibition, thereby activating autophagy. Therefore, on a cellular level, regulation of these proteins in the cytoplasm may be the primary mechanism associated with the therapeutic effects of GZYKF. A recent study by An et al (26) also reported that reductions in p53 and p-mTOR levels may promote autophagy. However, the disaccord between gene and protein expression of p53, AMPK and mTOR may suggest that the mechanism underlying GZYKF-regulated mediation of the p53/AMPK signaling pathway is associated with the transcription of mRNA and protein expression. Alternative hypotheses are that high-dose GZYKF is able to inhibit protein degradation, or that GZYKF may have multiple targeting functions.
In conclusion, the present study suggests that TP is able to induce a PCOS cell model in vitro, and that GZYKF mediates the p53/AMPK signaling pathway, and thereby inhibits GC autophagy, which may be the underlying mechanism of the therapeutic effects of GZYKF on PCOS.
Acknowledgements
This study was supported by a grant from the National Natural Science Foundation of China (grant no. 81202720).
References
- 1.Lang J, editor. Clinical practice guideline of gynecology. 1th. Ren Min Wei Sheng Chu Ban She; Beijing: 2012. Peking Union Medical College hospital: Polycystic ovary syndrome/Endocrine disorders of gynecology; p. 258. [Google Scholar]
- 2.Hua L, Wu YN, Zhang JM. Clinical study of yishen jianpi yangxue tongli therapy in treating polycystic ovary syndrome. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2003;23:819–822. (In Chinese) [PubMed] [Google Scholar]
- 3.Canu N, Tufi R, Serafino AL, Amadoro G, Ciotti MT, Calissano P. Role of the autophagic-lysosomal system on low potassium-induced apoptosis in cultured cerebellar granule cells. J Neurochem. 2005;92:1228–1242. doi: 10.1111/j.1471-4159.2004.02956.x. [DOI] [PubMed] [Google Scholar]
- 4.Nishida K, Kyoi S, Yamaguchi O, Sadoshima J, Otsu K. The role of autophagy in the heart. Cell Death Differ. 2009;16:31–38. doi: 10.1038/cdd.2008.163. [DOI] [PubMed] [Google Scholar]
- 5.Lum JJ, Bauer DE, Kong M, Harris MH, Li C, Lindsten T, Thompson CB. Growth factor regulation of autophagy and cell survival in the absence of apoptosis. Cell. 2005;120:237–248. doi: 10.1016/j.cell.2004.11.046. [DOI] [PubMed] [Google Scholar]
- 6.Janku F, McConkey DJ, Hong DS, Kurzrock R. Autophagy as a target for anticancer therapy. Nat Rev Clin Oncol. 2011;8:528–539. doi: 10.1038/nrclinonc.2011.71. [DOI] [PubMed] [Google Scholar]
- 7.Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, Kominami E, Ohsumi Y, Yoshimori T. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000;19:5720–5728. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.von Hoven G, Kloft N, Neukirch C, Ebinger S, Bobkiewicz W, Weis S, Boller K, Janda KD, Husmann M. Modulation of translation and induction of autophagy by bacterial exoproducts. Med Microbiol Immunol: 2012;201:409–418. doi: 10.1007/s00430-012-0271-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vousden KH, Ryan KM. P53 and metabolism. Nat Rev Cancer. 2009;9:691–700. doi: 10.1038/nrc2715. [DOI] [PubMed] [Google Scholar]
- 10.Zhang XD, Qin ZH, Wang J. The role of p53 in cell metabolism. Acta Pharmacol Sin. 2010;31:1208–1212. doi: 10.1038/aps.2010.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feng Z, Hu W, de Stanchina E, Teresky AK, Jin S, Lowe S, Levine AJ. The regulation of AMPK beta1, TSC2, and PTEN expression by p53: Stress, cell and tissue specificity, and the role of these gene products in modulating the IGF-1-AKT-mTOR pathways. Cancer Res. 2007;67:3043–3053. doi: 10.1158/0008-5472.CAN-06-4149. [DOI] [PubMed] [Google Scholar]
- 12.Tong Q, Jin Z, Shen XP. Investigate the autophagy rate of granulosa cell in polycystic ovary syndrome rat. Yi Nan Bing Za Zhi. 2011;10:364–365. (In Chinese) [Google Scholar]
- 13.Campbell KL. Ovarian granulosa cells isolated with EGTA and hypertonic sucrose: Cellular integrity and function. Biol Reprod. 1979;21:773–786. doi: 10.1095/biolreprod21.4.773. [DOI] [PubMed] [Google Scholar]
- 14.Papadimitriou K, Kountourakis P, Kottorou AE, Antonacopoulou AG, Rolfo C, Peeters M, Kalofonos HP. Follicle-Stimulating Hormone Receptor (FSHR): A promising tool in oncology? Mol Diagn Ther. 2016;20:523–530. doi: 10.1007/s40291-016-0218-z. [DOI] [PubMed] [Google Scholar]
- 15.Ho ML, Hsieh YS, Chen JY, Chen KS, Chen JJ, Kuo WH, Lin SJ, Chen PN. Antimetastatic potentials of dioscorea nipponica on melanoma in vitro and in vivo. Evid Based Complement Alternat Med. 2011;2011:507920. doi: 10.1155/2011/507920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 17.He C, Klionsky DJ. Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet. 2009;43:67–93. doi: 10.1146/annurev-genet-102808-114910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Budanov AV, Karin M. p53 target genes sestrin1 and sestrin2 connect genotoxic stress and mTOR signaling. Cell. 2008;134:451–460. doi: 10.1016/j.cell.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cataldo NA, Dumesic DA, Goldsmith PC, Jaffe RB. Immu-nolocalization of Fas and Fas ligand in ovaries of women with polycystic ovary syndrome: Relationship to apoptosis. Hum Reprod. 2000;15:1889–1897. doi: 10.1093/humrep/15.9.1889. [DOI] [PubMed] [Google Scholar]
- 20.Kroemer G, Levine B. Autophagic cell death: The story of a misnomer. Nat Rev Mol Cell Biol. 2008;9:1004–1010. doi: 10.1038/nrm2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen Y, Mcmillan-Ward E, Kong J, Israels SJ, Gibson SB. Oxidative stress induces autophagic cell death independent of apoptosis in transformed and cancer cells. Cell Death Differ. 2008;15:171–182. doi: 10.1038/sj.cdd.4402233. [DOI] [PubMed] [Google Scholar]
- 22.McPhee CK, Logan MA, Freeman MR, Baehrecke EH. Activation of autophagy during cell death requires the engulfment receptor Draper. Nature. 2010;465:1093–1096. doi: 10.1038/nature09127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maiuri MC, Galluzzi L, Morselli E, Kepp O, Malik SA, Kroemer G. Autophagy regulation by p53. Curr Opin Cell Biol. 2010;22:181–185. doi: 10.1016/j.ceb.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 24.Levine B, Abrams J. p53: The Janus of autophagy? Nat Cell Biol. 2008;10:637–639. doi: 10.1038/ncb0608-637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maiuri MC, Malik SA, Morselli E, Kepp O, Criollo A, Mouchel PL, Carnuccio R, Kroemer G. Stimulation of autophagy by the p53 target gene Sestrin2. Cell Cycle. 2009;8:1571–1576. doi: 10.4161/cc.8.10.8498. [DOI] [PubMed] [Google Scholar]
- 26.An HK, Kim KS, Lee JW, Park MH, Moon HI, Park SJ, Baik JS, Kim CH, Lee YC. Mimulone-Induced autophagy through p53-mediated AMPK/mTOR pathway increases caspase-mediated apoptotic cell death in a549 human lung cancer cells. Plos One. 2014;9:e114607. doi: 10.1371/journal.pone.0114607. [DOI] [PMC free article] [PubMed] [Google Scholar]