Abstract
This article presents the evaluation of water-soluble palladium nanoparticles with hydrophobic active sites that are ideal for the biphasic colloidal catalysis of water-insoluble organic substrates in aqueous solution. Palladium nanoparticles stabilized with ω-carboxylate-functionalized alkanethiolate are first synthesized using ω-carboxylate-S-alkylthiosulfate as their ligand precursor. The biphasic catalysis is carried out for the reaction of hydrophobic allylic alcohols without using any additional mixing solvent or surfactant, which results in the complete consumption of substrates under the atmospheric pressure of H2 gas and at room temperature in less than 24 h. Systematic investigations on the influence of pH and substrate size are also performed to examine the utility of these thiolate-capped palladium nanoparticles as structurally stable and water-soluble micellar catalysts for the biphasic reaction.
Keywords: Biphasic, Catalysis, Palladium, Nanoparticles, Hydrogenation, Isomerization
Graphic Abstract
Introduction
Due to the economical, non-flammable and environmentally friendly characteristics of water, there have been significant interests from both academic and industrial sectors on using water as a solvent for various applications including catalysis.1–3 The utilization of water is also one of the most popular tactics currently being sought for the green catalysis and biocatalysis of metal nanoparticles.4,5 To take advantage of high potential activity of homogeneous nanoparticle catalysis, the synthesis of water-soluble catalytic metal nanoparticles has drawn increased attention.6–10
Since the phase-separation of hydrophobic organic products from the aqueous reaction medium takes place instantly at the completion of reaction, the aqueous biphasic condition using water-soluble metal nanoparticle catalysts would make the product isolation and catalyst recovery simple and straightforward.11–14 However, biphasic catalytic reactions are generally considered to be quite challenging because the catalytic transformation happens only in the interphase region where the catalysts and the substrates encounter. Therefore, biphasic catalysis usually suffers from the slow kinetics and low activity,2,15 and requires high temperature and high pressure to compensate the said limitations.16 For example, the sulfonated tetrahydrosalen-Pd(II) complexes investigated by Joó et al. generated almost 80 % of hydrogenation product after 1 h reaction. However, the reaction required high temperature (≥80°C) and high pressure (5 bar) of H2 gas for obtaining successful results.14
In many other cases of biphasic reactions, additional additives such as organic co-solvents, surfactants or promoter ligands were added to ease the solubility and mixing problems of hydrophobic substrates in water and to increase the activity of biphasic catalytic systems.17–19 However, the additives have often caused problems during the product separation and/or added extra steps for the purification. Water-soluble ligand-stabilized metal nanoparticles with partial hydrophobic characteristics, therefore, would offer a structurally intact platform that eliminates the need for additives in aqueous environments.13,20 The synthesis of stable and isolable ligand-stabilized metal nanoparticles with good catalytic activities has been regarded as quite challenging because of the surfactant-induced poisoning of nanoparticle catalysts.21,22 To increase the catalytic activities of water-soluble metal nanoparticles, others have used approaches based on the stabilization of nanoparticles using some specialized ligands or polymers such as functionalized adamantane or semi-natural cellulose, respectively, providing access points to active surface sites of metal nanoparticles.9,13
Recently, our group was able to prepare water-soluble palladium nanoparticles (PdNP) stabilized by simple ω-functionalized alkanethiolate ligands that exhibit a good catalytic activity towards the hydrogenation of small water-soluble allyl alcohol, 2-propen-1-ol.23 The synthetic method was based on the modified Brust-Schiffrin reaction using sodium ω-carboxyl-S-alkanethiosulfates as the thiolate ligand precursor. The studies have shown that the ionic heads (COO−) of PdNP would stay out towards the aqueous environment while the long hydrocarbon chains would stay inside to form hydrophobic balls. Therefore, the configuration of produced PdNP highly resembled that of surfactant micelles, with the nanoparticle core serving as an active catalytic centre in a stable “micellar” structure.16,24 Since chemical reactions in micellar structures can take place with enhanced reaction rates or selectivity, the PdNP would be an ideal candidate as biphasic catalysts that can stabilize the transition state of the reaction by establishing a favourable interaction with the hydrophobic reactant molecules.25,26 In the previous work, we found that the water-soluble Pd nanoparticles stabilized with functionalized thiolate ligands are excellent in catalyzing the hydrogenation of small water-soluble 2-propen-1-ol. Since this reaction was performed in one phase system using water, the previous work failed to fully capitalize on the water solubility of Pd nanoparticles and their phase separation from oil-based substrates and products. To take advantage of this biphasic condition, the current work investigates the catalytic activity and selectivity of the micellar water-soluble PdNP for hydrophobic allylic alcohols with long alkyl chains. The effects of solution pH and substrate chain length on the catalytic property of PdNP are also discussed in details.
Materials and methods
The following materials were purchased from the indicated suppliers and used as received: Potassium tetrachloropalladate (K2PdCl4), tetra-n-octylammonium bromide (TOAB), 6-bromohexanoic acid and sodium borohydride (NaBH4) were purchased from ACROS. 1-Octen-3-ol was obtained from Alfa Aesar. 1-Nonen-3-ol, 1-decen-3-ol and 8-bromooctanoic acid were purchased from Sigma-Aldrich. Sodium thiosulfate (Na2S2O3.5H2O), phosphate buffered saline (PBS, pH 7.4), toluene, methanol and ethyl alcohol were obtained from Fisher Scientific. Chloroform-d and deuterium oxide were purchased from Cambridge Isotope Laboratories. Water was purified by using a Barnstead NANO pure Diamond ion exchange resins purification unit. Spectra/Por cellulose ester (CE) dialysis membrane (M.Wt. 8,000–10,000 Daltons) were purchased from Spectrum Laboratories, Inc.
Synthesis of sodium ω-carboxyl-S-alkanethiosulfate ligands
The ligand precursor, ω-carboxyl-S-hexanethiosulfate sodium salt, was prepared according to the previously published procedure.23 6-Bromohexanoic acid (25 mmol) in 50 mL of ethanol and sodium thiosulfate pentahydrate (25 mmol) in 50 mL of water were placed in a 500 mL round bottom flask equipped with a reflux condenser and refluxed for 3 h. The solvents were removed under vacuum. The crude product was dissolved in hot ethanol and the insoluble materials were removed by filtration in order to allow recrystallization of the desired product once the ethanolic solution was cooled down. The same procedure was applied for the synthesis of sodium ω-carboxyl-S-octanethiosulfate. 1H NMR (400 MHz, D2O, Fig. S1): δ 3.13 ppm (t, 2H, CH2S2O3−), δ 2.43 ppm (t, 2H, CH2COO−), δ 1.80 ppm (m, 2H, CH2), δ 1.66 ppm (m, 2H, CH2), and δ 1.47 ppm (m, 2H, CH2); sodium ω-carboxyl-S-octanethiosulfate δ 3.09 ppm (t, 2H, CH2S2O3−), δ 2.41 ppm (t, 2H, CH2COO−), δ 1.77 ppm (m, 2H, CH2), δ 1.63 ppm (m, 2H, CH2), and δ 1.30–1.42 ppm (m, 6H, CH2CH2).
General procedure for synthesis of Pd nanoparticles
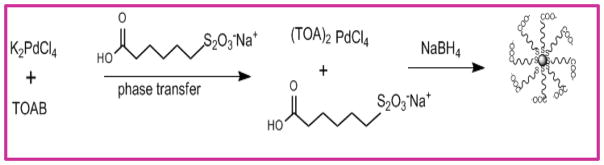
The PdNP catalysts were synthesized by the published procedure using sodium S-alkanethiosulfates (Scheme 1).23 Briefly, potassium tetrachloropalladate (0.13 g, 0.4 mmol) was dissolved in 50 mL of nanopure water in a 500 mL round bottom flask and followed by the addition of TOAB (1.09 g, 2.0 mmol) in 50 mL of toluene. The reaction mixture was continuously stirred for 15 min. Once the phase transfer was completed, the aqueous layer was discarded. Second fold of TOAB (1.09 g, 2.0 mmol) in powder form and sodium-ω-carboxyl-S-hexanthiosulfate (0.19g, 0.8 mmol) dissolved in 20 mL of 25 % methanol were sequentially added to the reaction mixture with continuous stirring for additional 15 min. A freshly prepared sodium borohydride (0.30 g, 8.0 mmol) solution in 7 mL of nanopure water was delivered to the reaction mixture over ca. 60 sec. The appearance of darkened colour indicated the formation of nanoparticles. The organic layer was separated from the aqueous layer after the completion of 3 h reaction. Water was removed under vacuum and the remaining crude products were washed with several aliquots of methanol, ethanol and chloroform. The washed nanoparticles were dialyzed for 24 h to remove excess salts. The collected nanoparticles were dried in vacuum overnight at a pressure of 25 psi.
Scheme 1.
Two-phase synthesis of water-soluble palladium nanoparticles.
Instrumentation
1H NMR was acquired on a Bruker Avance II 400 MHz or a Bruker Fourier 300 MHz at 298 K. NMR data was processed using iNMR 3.5.1 software. A residual solvent peak at δ 7.26 ppm was used as an internal reference. JEOL 1200 Ex II transmission electron microscopy (TEM) was used to take TEM images of nanoparticles at 120 keV. TEM samples were prepared by dropping 25 μL of 1 mg PdNP/1 mL THF onto a 400 mesh standard carbon-coated copper grids and allowing the grid to dry in air for 30 min. Images were analysed with Scion Image Beta Release 2 for particle size distribution. Thermogravimetric analysis (TGA) was conducted using a TA instrument SDT Q600 with a flow rate of 100 mL/min of N2 with heating from room temperature to 900 °C at a heating rate of 20 °C/min.
General procedure for catalytic hydrogenation reactions
Catalysis experiments were performed by placing 3 mL of H2O (or PBS, pH 7.4) in a 50 mL round bottom flask along with 5–10 mol% PdNP catalyst. The reaction mixture was purged with H2 gas for 10 min and 50 μL of substrate (1-octen-3-ol) was injected into the septum sealed flask. The hydrogen balloon was attached to the reaction flask for equilibrium of H2 gas. The reaction mixture was constantly stirred under atmospheric pressure and at room temperature. The product in organic phase was separated from the aqueous nanoparticle phase after the addition of CDCl3 and transferred to an NMR tube to obtain 1H NMR.
Results and discussion
Synthesis of water-soluble palladium nanoparticle catalysts
As the prior studies from our group revealed, the synthesis of water-soluble ω-carboxyl-1-hexanethiolate-capped Pd nanoparticles (PdNP-1) was successfully achieved by using sodium ω-carboxyl-S-hexanethiosulfate sodium salts as a ligand precursor in a two-phase system.23 The thiosulfate underwent S-S bond cleavage on Pd nanoparticles to form Pd-thiolate bonds.23,27
Due to the presence of ionic carboxylate groups on the surface ligand of nanoparticles, the produced PdNP-1 was eventually transferred to the aqueous layer at the completion of the reaction. After the purification of stabilized PdNP-1 in water using dialysis, the average core size of nanoparticles (1.8 ± 0.9 nm) and the organic weight fraction (26 % organic vs 74 % Pd) were obtained using TEM (Fig. S2) and TGA (Fig. S3), respectively. Ligand surface coverage calculation was based on the surface atoms of theoretical models, which showed the approximate surface ligand density of 0.38 ligands per surface atom.28 Other spectroscopic results including NMR and UV-vis spectra confirmed the ligand composition and the absence of any impurity (Fig. S4 and S5). ω-Carboxyl-1-octanethiolate-capped PdNP (PdNP-2) were also synthesized under the same synthetic conditions and the characterization results are shown in supporting information (Fig. S2–S5).
The atomistic molecular dynamics simulations of small gold nanoparticles coated with alkanethiol ligands have recently proved that the significant local bundling of longer alkyl chains creates highly asymmetric coatings and induces aggregation of nanoparticles in aqueous solution.29,30 Our studies showed that the synthesized PdNP-1 and PdNP-2, which have C6 and C8 alkyl chains, could maintain a good solubility in aqueous environments. In comparison, the PdNP capped with ω-carboxyl-1-undecanethiolate ligands underwent aggregations in aqueous environments in less than a few weeks. The results confirmed that PdNP could only maintain symmetric micellar structures and long-term water solubility when the alkyl chain lengths of ligands on PdNP are relatively short.
Biphasic reaction of 1-octen-3-ol with PdNP-1
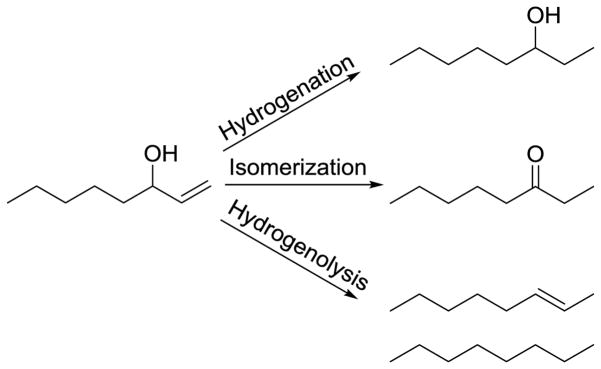
The catalytic reactions of allylic alcohols under H2 gas environment are known to produce many different products such as saturated alcohols via hydrogenation, carbonyls via isomerization, and alkenes or alkanes by hydrogenolysis as shown in Scheme 2.8,31–33 Our group has shown that the colloidal catalysis of alkanethiolate-capped Pd nanoparticles in nonpolar organic solvent such as CHCl3 resulted in a highly selective conversion of 2-propen-1-ol to propanal, the isomerization product.34 In polar protic solvent, however, the heterogeneous reaction of alkanethiolate-capped Pd nanoparticles with 2-propen-1-ol produced exclusively 1-propanol, the hydrogenation product, as a result of different solvent-induced ligand conformation.35 In comparison, the colloidal catalysis of water-soluble palladium nanoparticles (PdNP-1) exhibited a good selectivity towards the hydrogenation product, 1-propanol.23 For all these cases in both heterogeneous and colloidal homogeneous catalysis of small allylic alcohols, the formation of hydrogenolysis products has not been detected.
Scheme 2.
Possible reactions of allyl alcohols in the presence of H2 gas and metal catalysts.
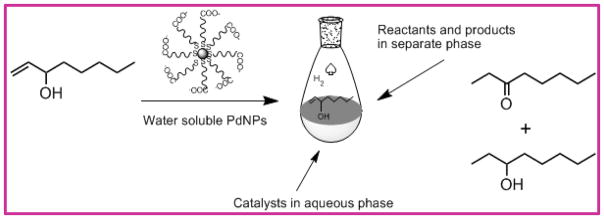
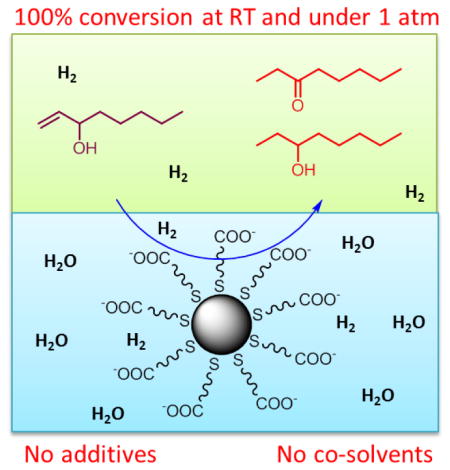
The catalytic activity of PdNP-1 for the reaction of 1-octen-3-ol (Scheme 3) was examined under various conditions with different catalyst loadings (5 mol% and 10 mol%) and hydrogen gas (from limited amount (~5 mmol) to excess using a balloon). In the absence of PdNP-1 during the control experiment, all substrates were quantitatively recovered. When 5 mol % PdNP-1 catalysts along with limited amount of H2 gas were introduced, the catalytic reactions resulted in only 25% conversion of 1-octen-3-ol to hydrogenation and isomerization products after 24 h (Table 1, entry 1). When the reaction took place in a single phase, the previous results indicated that the catalytic reactions of allyl alcohols were mostly completed in 3 to 4 hours with 5 mol% of PdNP catalysts.28,34 Since the catalytic reactions of 1-octen-3-ol are biphasic in nature, the overall reaction clearly slowed down requiring the larger amounts of PdNP-1 (10 mol%) to sufficiently enhance the kinetic efficiency of reaction. Even when 10 mol% of PdNP-1 and limited amount of H2 gas were introduced to the reaction, however, the conversion of 1-octen-3-ol was limited to less than 45 % (Table 1, entry 2). As the results in entry 3 in Table 1 indicated, the increased amount of H2 gas was required for the complete conversion of 1-octen-3-ol by PdNP-1 during the biphasic catalytic reaction in water, which was likely instigated by the strong hydrophobicity of H2 gas. The complete conversion of 1-octen-3-ol to either hydrogenation or isomerization products was only observed when at least 10 mol% of PdNP-1 and excess H2 gas were introduced to the reaction mixture. In entry 3, the results showed that the PdNP-1 could produce the hydrogenation product as the major product (73 %) with the formation of some isomerization product (27 %) after 24 h reaction. The formation of hydrogenolysis products was not observed. The kinetic profiling of 1-octen-3-ol catalysis indicated that the complete conversion of reactants required approximately 18 to 24 h. Considering that the complete reaction took place without the use of any additional surfactants or mixing solvents, the water-soluble PdNP-1 clearly offers several advantages including facile product-phase separation and catalyst recycling over many other catalytic systems based on Pd complexes or supported Pd catalysts.33,36–38
Scheme 3.
Biphasic catalytic reaction of hydrophobic allylic alcohols in water.
Table 1.
Catalysis results by PdNP-1 for the biphasic reaction of 1-octen-3-ol.a
| Entry | Mole % PdNP-1 | Time (h) | Conversionc (%) | Catalysis yield (%)c
|
||
|---|---|---|---|---|---|---|
| Isomerization | Hydrogenation | Hydrogenolysis | ||||
| 1 | 5 | 24 | 25 | 5 | 20 | 0 |
| 2 | 10 | 24 | 45 | 10 | 35 | 0 |
| 3b | 10 | 24 | 100d | 27 | 73 | 0 |
The reactions were performed under atmospheric pressure and at room temperature.
Excess H2 gas was provided using a balloon attachment.
The product ratios and conversion yield were determined by analysing the 1H NMR integration results.
Turn-over-frequency (TOF) based on the active sites on PdNP surface was estimated to be 1.43 site−1hr−1.
Previously, our group reported a high selectivity towards the hydrogenation of small and water-soluble 2-propen-1-ol for the aqueous colloidal catalysis using the same PdNP-1 (> 90% selectivity).23 For the catalytic reaction of larger 1-octen-3-ol, however, PdNP-1 produced an increased amount of isomerization product (23 %) indicating a contribution of long chain alkyl group in 1-octen-3-ol for the formation of branched mono-σ-Pd-alkyl intermediate.35,39 This intermediate was found to be a key intermediate necessary for the formation of the isomerization product. To examine the direct involvement of H2O as a hydride source, the reactions were also performed in 100 % D2O. The catalysis results confirmed no incorporation of deuterium in the hydrogenated or isomerized products, indicating the absence of Pd-D formation from D2O during the catalytic reactions.
Influence of pH for biphasic catalysis of 1-octen-3-ol by PdNP-1
Due to the micellar nature of PdNP-1 catalysts, the pH of the catalyst solution would likely impact the activity and selectivity of these nanoparticle-based biphasic catalytic reactions. To begin with, the solubility of ω-carboxylate-functionalized nanoparticles would be contingent upon the pH of aqueous solution. The decrease in the pH of nanoparticle solution would lead to the protonation of carboxylate groups and the subsequent precipitation of nanoparticles caused by multiple hydrogen bond-induced agglomerations. The catalytic condition would then be heterogeneous at the lower pH compared to the homogeneously mixed colloidal condition at the higher pH. The high pH of PdNP-1 solution would preserve carboxylate groups to remain deprotonated and the ionic characteristics of carboxylate groups would stabilize the colloidal solution through electrostatic repulsion.
Joó’s group has shown the pH effect for the aqueous-organic biphasic reaction utilizing the Pd(II)-sulfosalan complexes for the hydrogenation and isomerization of allylic alcohols.14 Although, the overall activity of their catalysts was only slightly affected by pH variation, the significant changes in the selectivity of hydrogenation/isomerization of allylic alcohols were observed in their investigation. As predicted above, the solubility and subsequent activity of PdNP-1 were greatly influenced by the changes in pH of solution. The results in Table 2 clearly indicate that the catalytic reactions of 1-octen-3-ol at the lower pH (2–6) were very slow for PdNP-1. Especially at pH 2 and 3, only small conversion of 1-octen-3-ol to either hydrogenation or isomerization products were observed. As the pH of nanoparticle solution increases to 4–6 range, the conversion rate increased about twice without any change in the selectivity between isomerization and hydrogenation products. When the pH of solution increased to above 7, PdNP-1 which have ionic carboxylate groups exhibited a good colloidal stability and a high solubility in the aqueous environment. The catalytic conversion of 1-octen-3-ol was completed in 24 h with the formation of both hydrogenation and isomerization products in 73:27 ratio indicating the increase in both activity and selectivity towards the hydrogenation pathway. As the pH of solution was further increased to pH 10.0, however, no significant changes in both activity and selectivity of the catalytic reaction were observed. This suggested that the high solubility of PdNP-1 in water is important for enhancing the activity of unsupported nanoparticle catalysts.
Table 2.
Catalysis results for the biphasic reaction of 1-octen-3-ol with PdNP-1 (10 mol% Pd with excess of H2 gas, 24 h).
| pH of solution | Conversiona (%) | Catalysis yields (%)a
|
|
|---|---|---|---|
| Isomerization | Hydrogenation | ||
| 2.2 | 15 | 7 | 8 |
| 3.2 | 15 | 6 | 7 |
| 4.0 | 28 | 12 | 16 |
| 6.0 | 30 | 13 | 17 |
| 7.8 | 100 | 27 | 73 |
| 10.0 | 98 | 32 | 66 |
Hydrogenolysis products were not produced for any condition. The product ratios and conversion yield were determined by analysing the 1H NMR integration results.
Influence of substrate size of allylic alcohols for the biphasic catalytic reactions
The catalytic properties of PdNP-1 and PdNP-2 were investigated at pH 7.8 with various hydrophobic allylic alcohols (Table 3). The catalytic conversions of allylic alcohols were all completed in 24 h and produced both isomerization and hydrogenation products. The selectivity turned out to be substrate dependent, however, resulting in more hydrogenation products for the catalysis of 1-octen-3-ol and 1-nonen-3-ol, but not for the reaction of 1-decen-3-ol. The results showed that the catalytic selectivity of longer chain allylic alcohol (1-decen-3-ol) became lower. This might be due to the increase in the substrate-ligand interaction of 1-decen-3-ol with hydrophobic alkyl chains. This interference likely caused a disruption of ligands creating the surface space required for the formation of the branched mono-σ-Pd-alkyl intermediate, which is critical for the formation of isomerization product. When PdNP-2 were used for the biphasic catalytic reactions of hydrophobic allylic alcohols, the conversion of allylic alcohols was all completed in less than 24 h indicating the similar activity compared to PdNP-1. The selectivity for hydrogenation or isomerization product was also remained mostly identical without significant changes.
Table 3.
Catalysis results by PdNP-1 and PdNP-2 for the biphasic reaction of various allylic alcohols (10 mol% Pd with excess of H2 gas, 24 h)
| Entry | Allylic alcohols | Catalysis yields (%)a
|
|
|---|---|---|---|
| Isomerization | Hydrogenation | ||
| i | 1-octen-3-ol | 27(36)b | 73(64)b |
| ii | 1-nonen-3-ol | 42(33)b | 58(67)b |
| iii | 1-decen-3-ol | 57(52)b | 43(48)b |
The product ratios were determined by analysing the 1H NMR integration results.
The yields in parentheses are obtained from the catalytic reactions of PdNP-2.
Recycling of Pd nanoparticles for biphasic catalytic reactions
The recyclability of PdNP-1 was tested in order to evaluate the stability of the catalysts in water. The results showed that the activity and selectivity of PdNP-1 for the reaction of 1-octen-3-ol mostly remained the same even after the third cycle of the catalyst recycling (100 % starting material consumption and 63–73 % hydrogenation selectivity). However, it was found that the part of PdNP-1 occasionally lose its solubility in water even after only one to two catalytic cycles with occasional fluctuations in catalytic activity. A change in pH of the solution from ~7.8 to ~5.5 was observed after the reaction and could be accounted for the nanoparticle aggregation which is induced by the partial protonation of carboxylate functional groups. Indeed, the UV-vis spectra of recycled PdNP-1 solution exhibited the presence of broad aggregation bands at >600 nm (Fig. S6). As shown in Fig. S5, the UV-vis spectra of small spherical Pd nanoparticles only show an exponential decay in absorbance with a decrease in energy lacking surface plasmon resonance bands.23
As an attempt to improve the colloidal stability of PdNP-1, the catalytic reaction of 1-octen-3-ol was performed in aqueous PBS solution instead of nanopure water (Table 4). No significant change in the activity and selectivity of the catalyst PdNP-1 was observed after the 3rd recycling. In addition, The recycling studies of PdNP-1 in the buffered solution suggested that the elimination of pH change during the reaction could enhance the colloidal stability of PdNP-1 even after multiple cycles of catalytic reactions. The enhanced colloidal stability of nanoparticles during the catalytic reaction was demonstrated by the UV-vis spectra of recycled PdNP-1 in buffered solution which were absent of any aggregation bands observed above for the reactions in nanopure water system (Fig. S6 and S7). The results also suggested that unlike the most other micelle structures in water,11 the stable micellar structure of PdNP-1 was not disrupted or affected by the presence of highly concentrated salts in aqueous solution. The catalytic reactions of 1-nonen-3-ol and 1-decen-3-ol were also studied for PdNP-1 in PBS buffer solution, which generated similar results in both the activity and selectivity for the biphasic reaction of allylic alcohols.
Table 4.
Results obtained from catalyst recycling studies using PdNP-1 for the biphasic reaction of 1-octen-3-ol in PBS solution (10 mol% Pd with excess of H2 gas, 24 h, pH 7.4).
| Cycle | Conversiona | Catalysis yields (%)a
|
|
|---|---|---|---|
| Isomerization | Hydrogenation | ||
| 1 | 100 % | 23 | 77 |
| 2 | 100 % | 23 | 77 |
| 3 | 100 % | 21 | 79 |
The product ratios and conversion yield were determined by analysing the 1H NMR integration results.
Conclusions
The biphasic catalysis of water-soluble alkanethiolate-capped Pd nanoparticles as structurally stable micelle catalysts in aqueous phase was investigated. The micellar characteristics of Pd nanoparticles allowed the hydrophobic substrate to momentarily enter the near surface hydrophobic region of the catalysts with adequate stirring force. No other surfactants and co-solvents were necessary for the complete reaction of substrates even at room temperature and under the atmospheric pressure, making the recycling of catalysts and the purification of products very simple. The catalysis results suggested that both the pH of nanoparticle solution and the size of allylic alcohol substrates are important factors in determining the activity and selectivity of water-soluble micellar Pd nanoparticles. The colloidal stability and recyclability of Pd nanoparticles could be further enhanced by using phosphate buffered solution (pH 7.4) as a reaction medium. In the future, introducing the different chemical environments in the interior of the unimolecular micelle nanoparticle catalysts will allow us to have more fundamental understanding of the hydrophobic effects of nanocatalysts used in the biphasic catalysis.
Supplementary Material
Highlights.
The biphasic catalysis of water-soluble alkanethiolate-capped Pd nanoparticles as structurally stable micelle catalysts in aqueous phase is investigated.
The biphasic catalysis is completed without using any additional mixing solvent or surfactant under the atmospheric pressure and at room temperature.
Both the pH of nanoparticle solution and the size of allylic alcohol substrates are important factors in determining the activity and selectivity of water-soluble micellar Pd nanoparticles.
The colloidal stability and recyclability of Pd nanoparticles could be further enhanced by using phosphate buffered solution (pH 7.4) as a reaction medium.
Acknowledgments
This study was funded by the National Institute of General Medical Science (#SC3GM089562) of the National Institutes of Health and the Undergraduate Education Grant Program of the W. M. Keck Foundation. NMR instrumentation was provided for by the National Science Foundation (MRI CHE-1337559).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sheldon RA. Chem Soc Rev. 2012;41:1437–1451. doi: 10.1039/c1cs15219j. [DOI] [PubMed] [Google Scholar]
- 2.Shaughnessy KH. Chem Rev. 2009;109:643–710. doi: 10.1021/cr800403r. [DOI] [PubMed] [Google Scholar]
- 3.Breslow R. In: Handbook of Green Chemistry, Volume 5: Reactions in Water. Li Chao-Jun., editor. Wiley-VCH; Weinheim: 2010. pp. 1–29. [Google Scholar]
- 4.Tao F. Metal Nanoparticles for Catalysis: Advances and Applications. The Royal Society of Chemistry; Cambridge: 2014. [Google Scholar]
- 5.Kang W, Liu J, Wang J, Nie Y, Guo Z, Xia J. Bioconjugate Chem. 2014;25:1387–1394. doi: 10.1021/bc5002399. [DOI] [PubMed] [Google Scholar]
- 6.Biondi I, Laurenczy G, Dyson PJ. Inorg Chem. 2011;50:8038–8045. doi: 10.1021/ic200334m. [DOI] [PubMed] [Google Scholar]
- 7.Yan N, Xiao C, Kou Y. Coord Chem Rev. 2010;254:1179–1218. [Google Scholar]
- 8.Pietrantonio KD, Coccia F, Tonucci L, d’Alessandro N, Bressan M. RSC Adv. 2015;5:68493–68499. [Google Scholar]
- 9.Zhang H, Yang Y, Dai W, Yang D, Lu S, Ji Y. Catal Sci Technol. 2012;2:1319–1323. [Google Scholar]
- 10.Alvarez J, Liu J, Roman E, Kaifer AE. Chem Commun. 2000;36:1151–1152. [Google Scholar]
- 11.Liu C, Li X, Jin Z. Catal Today. 2015;247:82–89. [Google Scholar]
- 12.Mao H, Yu H, Chen J, Liao X. Sci Rep. 2013;3:2226. doi: 10.1038/srep02226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Capporali M, Guerriero A, Lenco A, Caporali S, Peruzzini M, Gonsalvi L. ChemCatChem. 2013;5:2517–2526. [Google Scholar]
- 14.Voronova K, Purgel M, Udvardy A, Bényei AC, Kathó Á, Joó F. Organometallics. 2013;32:4391–4401. [Google Scholar]
- 15.Cornils B, Herrmann WA. Aqueous-Phase Organometallic Catalysis: Concepts and Applications. 2. Wiley-VCH; Weinheim: 2004. [Google Scholar]
- 16.Zhang S, Zhao Y. Chem Commun. 2012;48:9998–1000. doi: 10.1039/c2cc33012a. [DOI] [PubMed] [Google Scholar]
- 17.Hanson BE, Davis ME. J Chem Edu. 1987;64:928. [Google Scholar]
- 18.Cabou J, Bricout H, Hapiot F, Monflier E. Catal Commun. 2004;5:265. [Google Scholar]
- 19.Bergbreiter DE, Sung SD. Adv Synth Catal. 2006;348:1352. [Google Scholar]
- 20.Cargnello M, Wieder NL, Canton P, Montini T, Giambastiani G, Benedetti A, Gorte RJ, Fornasiero PA. Chem Mater. 2011;23:3961–3969. [Google Scholar]
- 21.Gan LY, Zhang YX, Zhao J. J Phys Chem C. 2010;114:996–1003. [Google Scholar]
- 22.Gavia DJ, Shon YS. ChemCatChem. 2015;7:892–900. doi: 10.1002/cctc.201402865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gavia DJ, Maung MS, Shon YS. ACS Appl Mater Interfaces. 2013;5:12432–12440. doi: 10.1021/am4035043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gao GQ, Lin L, Fan CM, Zhu Q, Wang RX, Xu AW. J Mater Chem A. 2013;1:12206–12212. [Google Scholar]
- 25.Bianchini C, Meli A, Oberhauser W. New J Chem. 2001;25:11–12. [Google Scholar]
- 26.Pieters G, Pezzato C, Prins LJ. Langmuir. 2013;29:7180–7185. doi: 10.1021/la304316z. [DOI] [PubMed] [Google Scholar]
- 27.Sadeghmoghaddam E, Lam C, Choi D, Shon YS. J Mater Chem. 2011;21:307–312. [Google Scholar]
- 28.Gavia D, Shon YS. Langmuir. 2012;28:14502–14508. doi: 10.1021/la302653u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bolintineanu DS, Lane JMD, Grest GS. Langmuir. 2014;30:11075–11085. doi: 10.1021/la502795z. [DOI] [PubMed] [Google Scholar]
- 30.Yang Z, Li Y, Zhou G, Chen X, Tao D, Hu N. J Phys Chem C. 2015;119:1768–1781. [Google Scholar]
- 31.Moreno M, Kissell LN, Jasinski JB, Zamborini FP. ACS Catal. 2012;2:2602–2613. [Google Scholar]
- 32.Lorenzo-Luis P, Romerosa A, Serrano-Ruiz M. ACS Catal. 2012;2:1079–1086. [Google Scholar]
- 33.Ganchegui B, Bouquillon S, Hénin F, Muzart J. J Mol Catal, A. 2004;214:65–69. [Google Scholar]
- 34.Sadeghmoghaddam E, Gaïeb K, Shon YS. Appl Catal A: Gen. 2011;405:137–141. [Google Scholar]
- 35.Sadeghmoghaddam E, Gu H, Shon YS. ACS Catal. 2012;2:1838–1845. doi: 10.1021/cs300270d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zharmagambetova AK, Ergozhin EE, Sheludyakov YL, Mukhamedzhanova SG, Kurmanbayeva IA, Selenova BA, Utkelov BA. J Mol Catal, A. 2001;177:165–170. [Google Scholar]
- 37.Musolino MG, De Maio P, Donato A, Pietropaolo R. J Mol Catal, A. 2004;208:219–224. [Google Scholar]
- 38.Uma R, Grévisy C, Grée R. Chem Rev. 2003;103:27–52. doi: 10.1021/cr0103165. [DOI] [PubMed] [Google Scholar]
- 39.Zhu JS, Shon YS. Nanoscale. 2015;7:17786–17790. doi: 10.1039/c5nr05090a. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.