Abstract
We have previously shown that the transcription factor HNF4A is required for the formation of hepatic progenitor cells from endoderm that has been derived from human induced pluripotent stem cells (iPSCs). We reasoned that we could uncover regulatory pathways with new roles in hepatocyte differentiation by identifying cellular processes that regulate HNF4A. We therefore performed a screen of 1120 small molecules with well-characterized mechanisms of action to detect those that affect the abundance of HNF4A in iPSC-derived hepatic progenitor cells. This approach uncovered several small molecules that depleted HNF4A. Of those, we chose to focus on an inhibitor of heat shock protein 90 beta (HSP90β). We show that mutation of the gene encoding HSP90β represses hepatocyte differentiation during the formation of hepatocytes from iPSCs. We reveal that HSP90β, although dispensable for expression of HNF4A mRNA, directly interacts with HNF4A protein to regulate its half-life. Our results demonstrate that HSP90β has an unappreciated role in controlling hepatic progenitor cell formation and highlight the efficiency of using small-molecule screens during the differentiation of iPSCs to reveal new molecular mechanisms that control hepatocyte formation.
KEY WORDS: Liver development, Small-molecule screening, iPSC-derived hepatocytes
Highlighted Article: The heat shock protein HSP90β regulates the half-life of HNF4A and is necessary for efficient differentiation of hepatocytes from human iPSCs.
INTRODUCTION
Much effort has been made to elucidate the molecular mechanisms that underlie liver development. Success in identifying proteins that control hepatic specification and hepatocyte differentiation has facilitated the generation of protocols that can be used to produce hepatocyte-like cells from human pluripotent stem cells (Cai et al., 2007; Hay et al., 2008; Basma et al., 2009; Si-Tayeb et al., 2010b; Sullivan et al., 2010; Touboul et al., 2010). Several reviews have discussed the molecular basis of liver development in depth (Lemaigre, 2009; Si-Tayeb et al., 2010a; Iwafuchi-Doi and Zaret, 2016). Briefly, the parenchymal cells of the liver originate from the ventral foregut endoderm. Competence of the foregut endoderm to adopt a hepatic fate is influenced by pioneer transcription factors, such as forkhead box A (FOXA) and GATA binding proteins (GATAs) (Gualdi et al., 1996; Bossard and Zaret, 1998). Fibroblast growth factors (FGFs) from the developing heart, bone morphogenetic proteins (BMPs) from the septum transversum mesenchyme, and dynamic regulation by wingless-type MMTV integration site (WNT) proteins, induce endoderm cells to differentiate into hepatic progenitors expressing transcription factors that drive hepatocyte differentiation (Jung et al., 1999; Rossi et al., 2001; Ober et al., 2006; McLin et al., 2007).
Hepatocyte nuclear factor 4 alpha (HNF4A) is a transcription factor in the nuclear hormone family that is essential for hepatocyte formation. Previous work has shown that HNF4A is expressed at the beginning of hepatic progenitor cell formation and regulates the onset of hepatic gene expression (Duncan et al., 1994). Therefore, depletion of HNF4A in human pluripotent stem cells prevents the endoderm from adopting a hepatic fate (Delaforest et al., 2011). Although several studies emphasize the importance of HNF4A as a central regulator of hepatocyte differentiation, little is known about the control of HNF4A expression during early development of the liver.
We rationalized that identifying cellular processes that control HNF4A protein levels could provide new insight into cellular mechanisms that govern hepatocyte formation during hepatogenesis. To find pathways that are required to maintain HNF4A protein during the transition of human induced pluripotent stem cell (iPSC)-derived endoderm to a hepatic fate, we performed a screen of 1120 small molecules that have known mechanisms of action. We report that disruption of the molecular chaperone heat shock protein 90 alpha family class B member 1 (HSP90AB1, referred to here as HSP90β) causes a dramatic reduction in the levels of HNF4A protein and, as a consequence, negatively affects the conversion of the endoderm to a hepatic fate. We also reveal that HSP90β directly interacts with HNF4A to control its half-life. These findings indicate that heat shock proteins can facilitate differentiation by fine-tuning the levels of transcriptional regulators that determine cell fate.
RESULTS
The differentiation of iPSCs into hepatocyte-like cells in 96-well plates is compatible with screening for inhibitors of HNF4A
We have previously described a protocol to generate human hepatocyte-like cells from iPSCs (Si-Tayeb et al., 2010b). We initially optimized this protocol for the differentiation of cells in six-well plates. Unfortunately, such a culture format was incompatible with our proposal to screen large numbers of compounds. To establish a platform that could facilitate medium-throughput analyses of small molecules, we therefore sought to scale down the differentiation to 96-well plates. After initial trials, we noted that after the formation of endoderm, the cells had a tendency to peel off from the surface of tissue culture plates coated with Matrigel. We speculated that cell peeling was a consequence of transport of fluids to the basal surface resulting in an increase in basal pressure that could drive epithelial detachment. However, we found that transiently exposing iPSC-derived endoderm cells to 0.02% EDTA (pH 7.2) at day 5 of differentiation substantially reduced the peeling. This treatment had no effect on cell viability or differentiation because the differentiated cells robustly expressed HNF4A and albumin (ALB; Fig. S1).
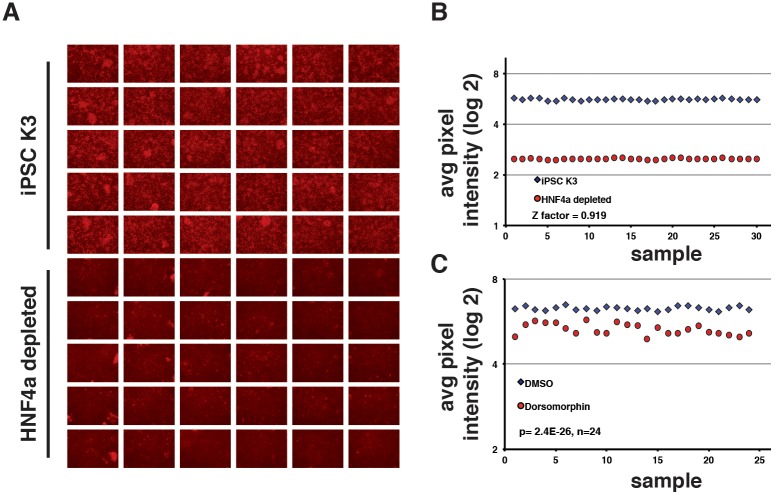
To measure the impact of small-molecule treatment on the formation of hepatic progenitors, we next developed an assay suitable for quantification of HNF4A protein in a medium-throughput screen. We chose to focus on measuring HNF4A protein levels rather than generating cells with a reporter gene regulated by the HNF4A promoter because we believed that such an approach could identify cell processes that reached beyond transcriptional regulation. We performed immunostaining using an antibody to detect endogenous HNF4A protein. To confirm that this method was compatible with screening, we used an HNF4A-depleted iPSC line that we had generated previously (Delaforest et al., 2011). Immunostaining detected HNF4A in hepatic progenitors generated from wild-type iPSCs but was undetectable in the HNF4A-depleted cells (Fig. 1A). A statistical measurement called a z-factor is commonly used to evaluate the suitability of using an assay for high-throughput screening (Zhang et al., 1999). A z-factor of 1 indicates that the test is perfect, between 0.5 to 1.0 is excellent, whereas a score below 0.5 suggests that the assay is marginal and incompatible with screening. We therefore calculated the z-factor of HNF4A immunostaining to determine with a small-molecule screen. Wild-type cells (n=30 wells) and HNF4A-depleted cells (n=30 wells) were differentiated to hepatic progenitor cells and then HNF4A immunostaining was performed. Images were processed using ImageJ software to calculate the average pixel intensity in each well. The level of HNF4A in each well was highly reproducible; however, we identified a clear distinction between the positive and negative (HNF4A-depleted) groups, which computed to a z-factor of 0.9 (Fig. 1B).
Fig. 1.
Differential expression of HNF4A protein can be quantified by immunocytochemistry. (A) Micrographs showing the results of immunostaining to detect HNF4A in hepatic progenitor cells after 8 days of differentiation from control K3 iPSCs (Si-Tayeb et al., 2010c) and HNF4A-depleted iPSCs (Delaforest et al., 2011). (B) Graphs showing the result of quantification of HNF4A immunostaining by measuring average pixel intensity using ImageJ software in each of 30 independent wells of differentiated control (blue diamonds) or HNF4A-depleted cells (red circles). Results were plotted and used to calculate the z-factor (z′robust=0.919). (C) Graphs showing the ability to detect differences in the level of HNF4A protein by immunostaining after inhibition of BMP signaling by dorsomorphin. K3 iPSCs were differentiated and treated with DMSO (blue diamonds) or dorsomorphin (5 μM; red circles) from day 6 to day 8. Immunostaining was performed to detect HNF4A on day 8 of differentiation (n=24). The average pixel intensity in each well was quantified using ImageJ and significance calculated by Student's unpaired t-test (P=2.4×10−26).
To confirm that the assay was capable of identifying a small-molecule that inhibited hepatic specification, we treated differentiating iPSCs with 5 µM dorsomorphin. Dorsomorphin inhibits BMP signaling, which is required for the onset of hepatic development (Rossi et al., 2001). HNF4A immunostaining was performed on both untreated (n=24 wells) and treated cells (n=24 wells). As before, the images were processed by ImageJ software to calculate pixel intensity. The immunostaining revealed that the cells treated with dorsomorphin had a reduced level of HNF4A compared with control cells. A comparison of the pixel intensity between control and treated cells revealed that HNF4A levels were significantly lower compared with untreated cells (P=2.4×10−26, n=24; Fig. 1C). Based on these results, we concluded that the immunostaining assay was compatible with a medium-throughput small-molecule screen and could quantitatively and reproducibly detect differences in HNF4A protein levels.
A screen of small molecules reveals several pathways that regulate HNF4A protein levels
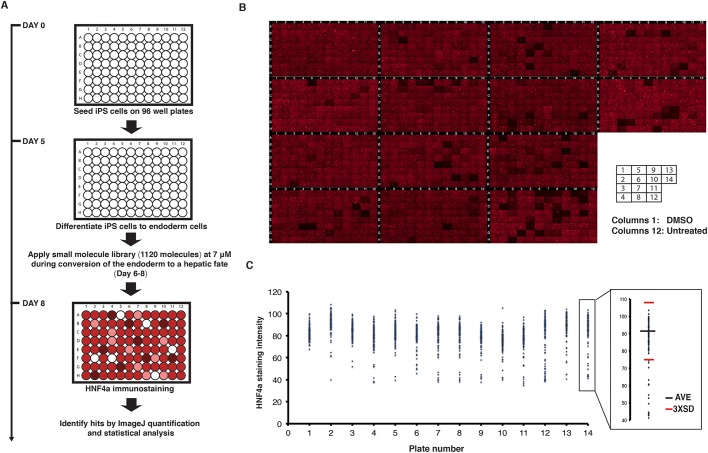
To identify pathways that affect the formation of hepatic progenitors, we examined the effect of 1120 small molecules on HNF4A protein during the differentiation of iPSCs toward a hepatic fate (Fig. 2A). Human K3 iPSCs were plated on 96-well plates (day 0) and induced to differentiate into hepatic progenitor cells. After the formation of the definitive endoderm at day 6, individual small molecules were added to each well at a concentration of 7 µM. For each 96-well plate, one column of wells was treated with DMSO (vehicle) and one was untreated. Thus, for the full screening, fourteen 96-well plates were used comprising 1120 experimental wells, 112 untreated wells and 112 wells treated with DMSO (vehicle). By the end of day 8, all cells were fixed and stained for HNF4A protein by immunocytochemistry and counterstained with 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI) to reveal the number of cells. We captured a representative fluorescent microscopic image for each well (Fig. 2B). HNF4A protein levels appeared similar in DMSO and untreated wells. In contrast to the control wells, the micrographs revealed that several wells treated with small molecules showed a substantial change in the level of HNF4A staining.
Fig. 2.
Identification of small molecules that reduce HNF4A in iPSC-derived hepatic progenitor cells. (A) A schematic representation of the small-molecule screening approach. (B) Micrographs showing the result of immunostaining for HNF4A in hepatic progenitor cells following treatment with 1120 small molecules. The first column in each plate was treated with vehicle (DMSO) and the last column (column 12) was untreated. (C) Graphs showing quantification of HNF4A immunostaining following the screen. The average pixel intensity in each well was quantified using ImageJ. On each plate, drugs that affected HNF4A levels by ±3 s.d. (inset: red bars) compared with the average reading per plate (inset: blue bar) were considered hits. AVE, average (mean).
Next, we processed all 1344 images with ImageJ software to calculate the pixel intensity. The quantitative values were plotted and used for statistical analysis. We set as a threshold ±3 s.d. from the average level of HNF4A expression. The treatments that met these criteria were considered to be primary ‘hits’ (Fig. 2C). In the screening, ∼85% of the small molecules had no effect on the expression of HNF4A, whereas ∼15% (189 of 1120) were found either to cause a change in HNF4A level or to affect cell viability. To verify these data, we repeated the treatment of all 189 hits in 24-well plates of differentiations and found that 132/189 (∼70%) were consistent with the initial screen (Fig. S2). A complete list of small molecules tested, in addition to those affecting HNF4A, is presented in Table S1.
We next attempted to identify biological processes that could impact the formation of hepatic progenitors by performing bioinformatic analyses. A software tool called STITCH (‘Search Tool for Interacting Chemicals’) has recently been developed that allows investigators to explore interactions between chemicals and proteins (Szklarczyk et al., 2016). The STITCH 4.0 database describes interactions between 300,000 small molecules and 2.6 million proteins from diverse organisms. Using STITCH software, we identified 396 proteins that interacted with the small molecules identified as hits in our screen (Table S1). Gene ontology analyses were then performed on the target proteins to identify cellular processes that were affected by the small molecules using PANTHER (Protein ANalysis THrough Evolutionary Relationships; Mi et al., 2013; Fig. S3). As expected, PANTHER revealed that several of the small molecules affected pathways targeting biological processes that are indispensable for cell survival, such as protein synthesis (anisomycin), cell cycle progression (aminopurvalanol A) and cell survival (AT101). In our screen, treatment with such small molecules usually resulted in a detrimental effect on cell viability as evidenced by loss of DAPI staining. We also identified small molecules targeting signaling pathways that are necessary for hepatic progenitor cell formation from pluripotent stem cells, including the FGF (PD161570) and WNT (XAV939) pathways (Twaroski et al., 2015). In addition to the known pathways, several of the small molecules that affected HNF4A protein levels were agonists or antagonists of kinases or signaling receptors. These included the SRC family of kinases (1-naphthyl PP1), spleen tyrosine kinase (SYK; ER27319 maleate) and transient receptor potential vanilloid 1 (TRPV1; AMG9810). Network analyses using STITCH revealed that these pathways also form a complex interacting network of cellular functions that influence HNF4A levels (Fig. S4). Such pathways might, therefore, make unappreciated contributions to regulation of the early stages of hepatocyte differentiation.
HSP90β plays a role in hepatocyte differentiation by regulating HNF4A levels
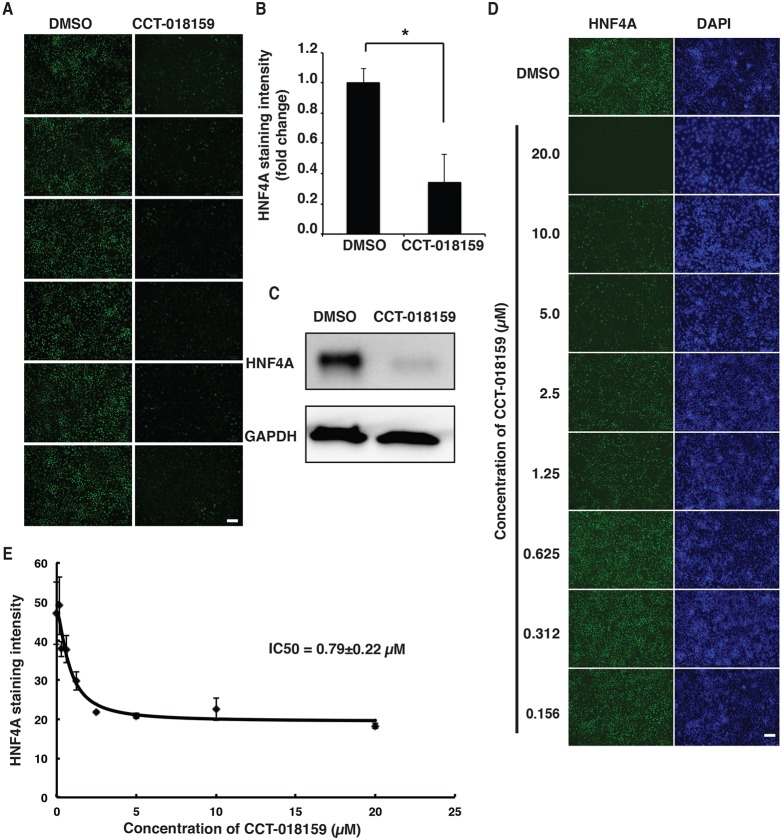
Although gene ontology analyses had identified several provocative pathways, we chose to focus on the effect of CCT-018159. This small-molecule targets HSP90β (Sharp et al., 2007), which is an ATP-dependent chaperone. HSP90β stabilizes and promotes the folding of a broad repertoire of client proteins (Karagöz and Rüdiger, 2015). Moreover, our STITCH analyses of the small-molecule targets had identified HSP90β as being part of a network of cell functions that impact HNF4A levels (Fig. S4). Several transcription factors rely on HSP90β, including nuclear hormone receptors (Sanchez, 2012); however, regulation of HNF4A has not been previously described. We therefore sought to determine whether HSP90β contributes to hepatic progenitor cell formation by regulating HNF4A protein levels. Initially, we repeated the CCT-018159 treatment in multiple wells of iPSCs from day 6 to day 8 of differentiation, which allowed us to exclude experimental artifacts and assess reproducibility (Fig. 3A,B). The result confirmed that CCT-018159 treatment significantly reduces HNF4A protein levels by at least threefold (P≤0.0001). We verified the reduction of HNF4A protein in response to CCT-018159 treatment by immunoblot analysis (Fig. 3C).
Fig. 3.
An inhibitor of HSP90β reduces the level of HNF4A protein in hepatic progenitor cells. (A) Micrographs showing the result of immunostaining for HNF4A in iPSC-derived hepatic progenitor cells treated between days 6 and 8 of differentiation with either DMSO (n=6 wells) or the HSP90β inhibitor CCT-018159 (n=6 wells). Scale bar: 100 µm. (B) Bar graph showing the quantification of HNF4A levels by measuring the average pixel intensity using ImageJ. Significance was determined by Student's unpaired t-test (mean±s.e.m., P≤0.001, n=6). (C) Representative western blot used to measure the steady-state levels of intracellular HNF4A in iPSC-derived hepatic progenitor cells treated with either DMSO (control) or CCT-018159 between days 6 and 8 of differentiation. GAPDH was used as a loading control. (D) Micrographs showing immunostaining for HNF4A in hepatic progenitor cells treated with DMSO or 20, 10, 5, 2.5, 1.25, 0.625, 0.312 and 0.156 mM CCT-018159. DAPI staining was used as a indication of cell viability. Scale bar: 100 µm. (E) Graph showing the CCT-018159 dose-response curve, determined by quantification of HNF4A immunostaining by ImageJ, that was used to calculate the IC50 (http://ic50.tk) (n=3).
The half-maximal inhibitory concentration (IC50) value for inhibition of HSP90β by CCT-018159 is 3.2 µM; however, at 50 µM CCT-018159 has been found to inhibit the activity of some kinases, including GSK3β, LCK and PDGFRa (Sharp et al., 2007). We therefore examined the effect of different doses of CCT-018159 on HNF4A protein levels by immunostaining (Fig. 3D). Doses as low as 1.25 µM visibly reduced HNF4A protein in the iPSC-derived hepatic progenitor cells, and the calculated IC50 was 0.79 µM (Fig. 3E).
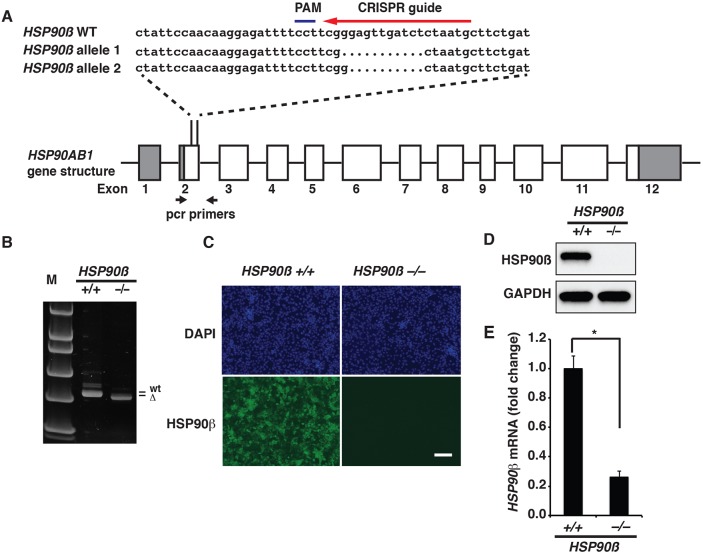
Although the dose-response data suggested that the reduction in HNF4A levels after CCT-018159 treatment was probably attributable to inhibition of HSP90β, we recognized that off-target effects could potentially confound interpretation. We believed that if CCT-018159 acted by specifically inhibiting HSP90β then mutating the HSP90AB1 gene (encoding HSP90β) using clustered regularly interspaced short palindromic repeats/CRISPR-associated protein 9 (CRISPR/Cas9; Ran et al., 2013) should recapitulate the effect of CCT-018159. A CRISPR guide RNA was therefore designed to target exon 2 of the HSP90AB1 gene (Fig. 4A). We detected insertions and deletions (indels) by PCR amplification of genomic DNA and confirmed the nature of the mutations by nucleotide sequencing (Fig. 4A,B). Through this approach, we identified a cell line that contained deletions of 10 bp in one allele and 11 bp in the other allele of HSP90AB1. These mutations introduce frameshifts (p.[Glu42LeufsTer56];[Glu42Ter]) that are predicted to disrupt HSP90β function. For simplicity, we referred to this iPSC cell line as HSP90β−/−.
Fig. 4.
Generation of HSP90β−/− iPSCs. (A) Schematic illustration of the HSP90AB1 gene structure showing the position of the CRISPR/Cas9 guide nucleotide sequence (red arrow) and PAM sequence (blue line) used to target exon 2. The nucleotide sequences of the HSP90AB1 wild-type (WT) allele (HSP90β−/−) and both alleles (HSP90β allele 1 and 2) that were mutated in HSP90β−/− iPSCs are shown. Black arrows indicate the relative position of PCR primers used to identify indels. (B) Image showing the result of PAGE to identify PCR amplicons of the PAM region in control and HSP90β−/− cells. Indels result in an electrobility shift in the amplicon derived from HSP90β−/− cells compared with HSP90β+/+ cells and the DNA standards (M). (C) Immunostaining reveals depletion of HSP90β protein (green) in endoderm derived from HSP90β−/− iPSCs. The total number of cells was revealed by DAPI staining (blue). Scale bar: 100 µm. (D) Western blot of HSP90β protein in endoderm derived from HSP90β+/+ and HSP90β−/− iPSCs. GAPDH was used as the loading control. (E) Bar graph showing the relative level of HSP90AB1 mRNA detected by qRT-PCR in HSP90β+/+- and HSP90β−/−-derived endoderm. Significance was determined by Student's unpaired t-test (mean±s.e.m., *P≤0.0001).
It has been reported that HSP90β is expressed ubiquitously during embryogenesis in multiple species (Krone and Sass, 1994; Voss et al., 2000; Dugyala et al., 2002; Vanmuylder et al., 2002). We used RT-PCR to confirm the presence of HSP90β mRNA in the developing liver bud throughout hepatic development in mouse embryos ranging from embryonic day (E)10.5 to E18.5 (Fig. S5A). We also confirmed that HSP90β protein was present in undifferentiated human iPSCs and in iPSC-derived endoderm (day 5), hepatic progenitors (day 8) and hepatocytes (day 20) (Fig. S5B). To determine whether the introduction of deletions within the HSP90AB1 gene caused a loss of function, we compared the expression of HSP90β protein by immunostaining and immunoblot analyses between control and HSP90β−/− iPSC-derived endoderm (Fig. 4C,D). Although we observed HSP90β in the control endoderm, it was undetectable in endoderm derived from HSP90β−/− iPSCs. Likewise, RT-qPCR revealed that HSP90AB1 mRNA was reduced by approximately fivefold in HSP90β−/− endoderm compared with control cells (Fig. 4E). The reduction in HSP90AB1 mRNA in HSP90β−/− endoderm is likely to reflect nonsense-mediated decay of the transcript. Based on these data, we conclude that the frameshift deletions in exon 2 of HSP90AB1 result in loss of HSP90β protein.
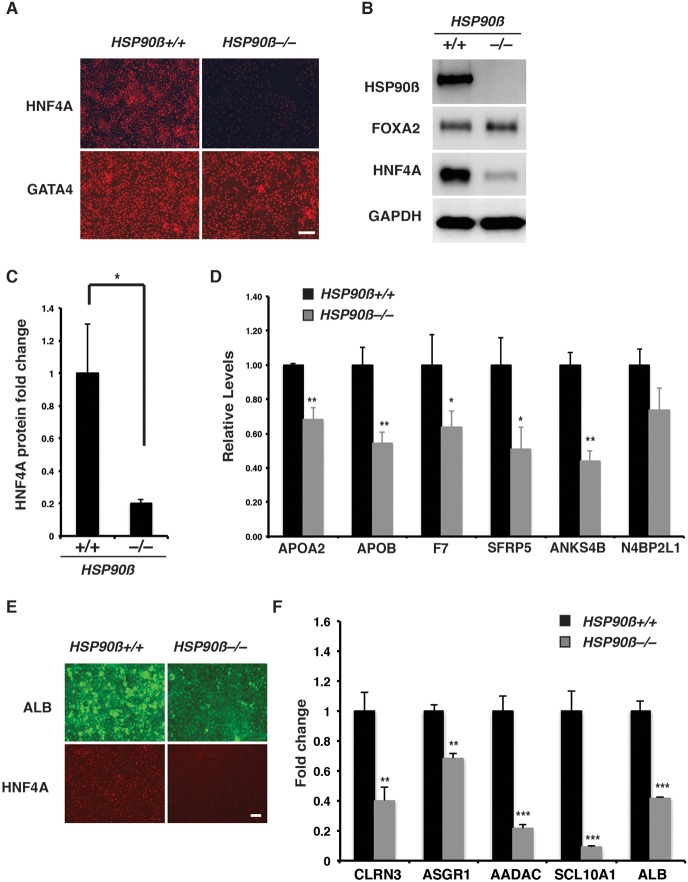
We next examined the impact of the loss of HSP90β on hepatic progenitor cell formation. We measured the levels of characteristic endoderm and hepatic progenitor cell markers by RT-qPCR, immunoblot analyses and immunostaining (Fig. 5). We observed uniform expression of HNF4A protein in hepatic progenitors derived from HSP90β+/+ iPSCs, whereas the protein was barely detectable in HSP90β−/− cells at an equivalent stage of differentiation (Fig. 5A). Quantification by immunoblot analyses revealed that the amount of HNF4A was approximately fivefold lower in HSP90β−/− cells compared with control cells (Fig. 5B,C). No morphological change was observed in the HSP90β−/− endoderm cells (Fig. S6A), and the endoderm markers GATA4, FOXA2, CXCR4 and SOX17 were expressed at similar levels in both HSP90β+/+ and HSP90β−/− differentiated cells, demonstrating that loss of HSP90β did not significantly impact endoderm formation (Fig. 5A,B; Fig. S6B).
Fig. 5.
Loss of HSP90β inhibits the differentiation of hepatocyte-like cells from iPSCs. (A) Micrographs showing immunostaining for HNF4A and GATA4 in HSP90β+/+ and HSP90β−/− hepatic progenitor cells on day 8 of differentiation. Scale bar: 100 µm. (B) Western blot of HSP90β, FOXA2 and HNF4A in HSP90β+/+ and HSP90β−/− cells on day 8 of differentiation. GAPDH was used as a loading control. (C) Quantification of western blot analyses of HNF4A by densitometry. HNF4A protein level was normalized to total protein and significance determined by Student's unpaired t-test (mean±s.e.m., *P≤0.05, n=3). (D) Bar graph showing fold change in the steady-state level of mRNA encoding HNF4A target genes in HSP90β+/+ and HSP90β−/− cells at day 8 of differentiation. Significance was determined by Student's unpaired t-test (mean±s.e.m., *P≤0.05, **P≤0.01, n=3). (E) Micrographs showing immunostaining of ALB and HNF4A in hepatocyte-like cells derived from HSP90β+/+ and HSP90β−/− iPSCs at day 20 of differentiation. Scale bar: 100 µm. (F) Bar graph shows relative steady-state level of characteristic hepatocyte mRNAs in hepatocyte-like cells derived from HSP90β+/+ and HSP90β−/− iPSCs at day 20 of differentiation. Significance was determined by Student's unpaired t-test (mean±s.e.m., **P≤0.01, ***P≤0.001, n=3).
Given the essential role of HNF4A in regulating hepatic progenitor cell formation (Delaforest et al., 2011), we predicted that an HSP90β-mediated reduction of HNF4A protein would impact hepatocyte differentiation from iPCSs by affecting the expression of hepatic progenitor cell mRNAs. We have previously defined a series of genes that are direct targets of HNF4A (Odom et al., 2004; Bolotin et al., 2010; Delaforest et al., 2011). We therefore performed RT-qPCR to determine the mRNA level of these genes in hepatic progenitor cells generated from wild-type and HSP90β−/− iPSCs. We observed a substantial reduction in the steady-state mRNA levels encoded by these genes in the HSP90β−/− hepatic progenitor cells compared with control cells (Fig. 5D). When we extended the differentiations to day 20, the majority of HSP90β+/+ cells expressed ALB, HNF4A and other markers that are characteristic of relatively mature hepatocytes (Fig. 5E,F). In contrast to the HSP90β+/+ cells, day 20 hepatocyte-like cells derived from HSP90β−/− iPSCs exhibited a marked reduction in ALB and HNF4A protein levels. The levels of mRNA encoding CLRN3, ASGR1, AADAC and SLC10A1, all of which are characteristic of late stages of hepatocyte differentiation, were also severely reduced in the absence of HSP90β. Although the impact on expression of markers such as ALB was reduced to 30% of control values, we noted that the impact of losing HSP90β was less dramatic than in HNF4A−/− iPSC-derived hepatocytes, where ALB was undetectable (Fig. S7). The retention of some marker expression presumably reflects the impact of residual levels of HNF4A that are retained in the HSP90β−/− cells. Cumulatively, these data confirm that, although HSP90β is dispensable for differentiation of iPSCs into endoderm, it is essential for maintenance of normal HNF4A protein levels and is required for the efficient differentiation of hepatocytes from iPSCs.
HSP90β maintains HNF4A levels by regulating HNF4A protein turnover
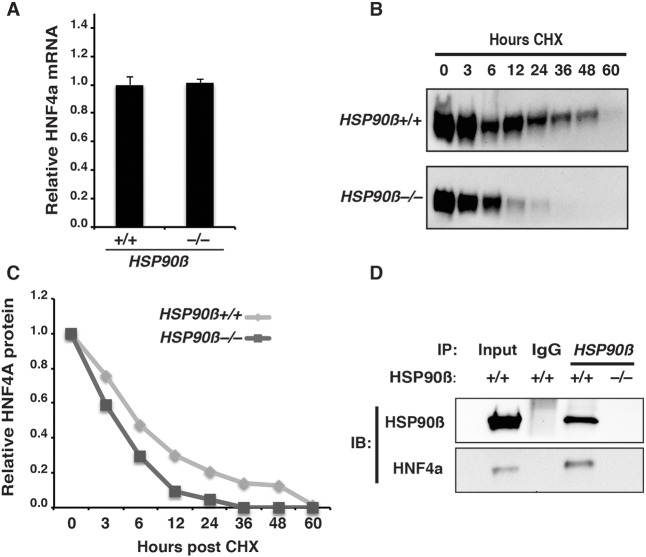
Finally, we sought to determine the mechanism through which HSP90β controls the level of HNF4A. We first considered the possibility that HSP90β interfered with HNF4A mRNA expression by repressing the FGF signaling pathway. FGF is crucial for hepatic specification and tissue growth through the activation of the mitogen-activated protein kinase (MAPK) and phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K) pathways (Jung et al., 1999; Calmont et al., 2006; Twaroski et al., 2015). It has been reported that HSP90β inhibition blocks MAPK1/3 (ERK1/2) and v-akt murine thymoma viral oncogene homolog 1 (AKT) activation, which are downstream effectors of the FGFR (Hackl et al., 2010). However, immunoblot analyses revealed that the levels of phosphorylated ERK and phosphorylated AKT were indistinguishable in hepatic progenitor cells derived from either HSP90β+/+ or HSP90β−/− iPSCs (Fig. S8). Moreover, despite our finding that the absence of HSP90β significantly reduced the levels of HNF4A protein, RT-qPCR revealed that the steady-state level of HNF4A mRNA was comparable between control and HSP90β−/− cells (Fig. 6A). These data imply that the regulation of HNF4A by HSP90β is post-transcriptional and independent of FGFR activity.
Fig. 6.
HSP90β interacts with HNF4A to regulate protein half-life. (A) Bar graph showing relative levels of HNF4A mRNA in HSP90β+/+ and HSP90β−/− cells at day 8 of differentiation (mean±s.e.m.). (B) Western blots of cycloheximide (CHX) chase analyses of HNF4A protein in day 8 hepatic progenitor cells derived from HSP90β+/+ and HSP90β−/− iPSCs. Samples were collected at 0, 3, 6, 12, 24, 36, 48 and 60 h after incubation of cells with 100 µM CHX to inhibit protein synthesis. (C) Quantification of HNF4A protein levels by densitometry of western blots performed on CHX chase samples. The level of HNF4A was calculated at each time point and expressed relative to the level of HNF4A in untreated cells (0 h). (D) Western blots to detect HNF4A and HSP90β after immunoprecipitation of HSP90β+/+ and HSP90β−/− day 8 hepatic progenitor cell lysates with an HSP90β antibody. Wild-type iPSC-derived hepatic progenitor cell lysate was used to measure total HNF4A and HSP90β, and immunoprecipitation of the same lysate using IgG was used as a negative control.
As an ATP-dependent chaperone, HSP90β protein aids in the folding and stability of its clients (Karagöz and Rüdiger, 2015). We therefore considered the possibility that HSP90β directly regulates HNF4A protein turnover. To test this hypothesis, we determined the half-life of HNF4A protein in hepatic progenitor cells in the presence and absence of HSP90β. Control cells and HSP90β−/− cells were differentiated until they formed hepatic progenitor cells at day 8. The cells were then cultured with cycloheximide (CHX, 100 µM) to prevent the synthesis of nascent protein, and we measured HNF4A protein levels over time by immunoblot (Fig. 6B). Given that the level of HNF4A was lower in HSP90β−/− cells (Fig. 5B), we normalized the loading of lysates to ensure equivalent levels of HNF4A at time 0 h (Fig. 6B). In contrast to HSP90β+/+ cells, where HNF4A was easily detected after 24 h of cycloheximide treatment, HNF4A was barely identifiable in HSP90β−/− cells. Quantification of the immunoblots after normalization to total protein revealed that the half-life of HNF4A protein in HSP90β+/+ hepatic progenitor cells was ∼6 h, whereas in HSP90β−/− cells the HNF4A half-life was closer to 3 h (Fig. 6C). HNF4A is a member of the nuclear hormone receptor class of transcription factors. Several other transcription factors within this family have been described as interacting with HSP90β, raising the possibility that HNF4A is an HSP90β client (Sanchez, 2012). We therefore performed co-immunoprecipitation experiments to determine whether endogenous HNF4A and HSP90β physically interact in iPSC-derived hepatic progenitor cells. Immunoprecipitations were performed on cells differentiated from either HSP90β+/+ or HSP90β−/− hepatic progenitor cells using anti-HSP90β or IgG antibodies. The presence of HNF4A and HSP90β in the immune-precipitate was detected by immunoblot. HNF4A was co-precipitated from HSP90β+/+ cell extracts using an anti-HSP90β antibody but was not precipitated from HSP90β−/− cells or when we performed precipitations with IgG (Fig. 6D). Based on these data, we conclude that HSP90β interacts with HNF4A and controls its steady-state levels and, as a consequence, is required for the efficient differentiation of hepatocyte-like cells from human iPCSs.
DISCUSSION
Developmental biologists have focused on identifying growth factors, signaling pathways and transcription factors with the belief that gene expression controls cell fate. Such studies have been very successful, with significant practical implications. For example, by recapitulating the molecular events that drive cell differentiation during embryonic development, investigators have been able to design approaches that allow the generation of cell lineages from pluripotent stem cells. Despite such success, the focus on regulation of gene expression, through either growth or transcription factors, often overlooks the complexities of cell biology that govern cell behavior. Such biological processes might indirectly affect cell fate by regulating, for example, cell metabolism, protein function, intracellular protein transport or subcellular structure. Given that intracellular functions are intricately linked, it seems logical to assume that cellular processes exist that have an unappreciated impact on cell fate decisions. Support for such a view comes from genetic studies demonstrating that cilia and intraflagellar transport control signal transduction through the Hedgehog pathway (Huangfu et al., 2003). Likewise, cell junctions recruit and process specific miRNAs that impact multiple cell properties (Kourtidis et al., 2015).
One challenge that limits our ability to identify cellular mechanisms that control cell differentiation is the availability of suitable models that can mimic the developmental process. Genetic approaches are powerful, but in screening for cellular processes that affect development they are in general limited to low-throughput studies. Pluripotent stem cells offer a cell culture model that can dynamically recapitulate cell differentiation. Such a platform opens the possibility of using chemical screens to reveal new mechanisms that affect cell fate. Here, we successfully used the differentiation of iPSCs to hepatocytes to identify cellular pathways that regulate the conversion of the endoderm to a hepatic fate.
The mechanisms underlying the development of the liver have been studied extensively (Lemaigre, 2009; Si-Tayeb et al., 2010a; Iwafuchi-Doi and Zaret, 2016). Such studies have identified several growth factors and transcription factors that contribute to hepatocyte formation. HNF4A is a particularly appealing marker of hepatic progenitor cells because it is expressed at the onset of hepatic progenitor cell formation. Moreover, previous work from our group demonstrated that HNF4A is essential for the generation of the hepatic lineage from iPSCs (Delaforest et al., 2011). We therefore reasoned that cellular pathways that impact HNF4A protein levels would have important roles in controlling hepatocyte differentiation and formation. Although we could have used a reporter gene targeted to the HNF4A locus as a read-out of expression, we felt that this approach would restrict us to identifying processes that affected transcriptional regulation of the HNF4A gene. Instead, we elected to measure the impact of small molecules with known mechanisms of action on the endogenous HNF4A protein levels, which we believed would capture a broader class of regulatory mechanisms.
Our screen of a library of 1120 small molecules identified 132 that could reproducibly impact HNF4A protein levels. The hits included chemicals that affect signaling pathways involved in the development of hepatic cells, including FGF (PD161570) and WNT (XAV939, endo-IWR 1, BIO) signaling. The successful identification of proteins known to control hepatic fate provided confidence in the fidelity of the screen. Although our studies focused on small molecules that reduced the level of HNF4A without affecting cell viability, several of the hits resulted in a loss of cells. Many of the small molecules that repressed processes vital for cell survival, such as protein and mRNA synthesis, were not considered further. However, it is important to note that cell death per se should not be considered a criterion for exclusion because disruption of many developmentally important pathways can manifest in a cell death phenotype. For example, acute inhibition of FGF signaling blocks specification, but when the FGFR is chronically repressed it diminishes cell viability (Twaroski et al., 2015). With this in mind, if we avoid exclusion based solely on viability, several additional small molecules could be considered provocative. This group would include those that target pathways that control an array of liver functions but have not so far been implicated in the conversion of the endoderm to a hepatic fate. For example, PHA 665752 is an inhibitor of MET proto-oncogene, receptor tyrosine kinase (MET), which is a receptor for hepatocyte growth factor (HGF). Both Hgf−/− and Met−/− mice die during embryogenesis between embryonic days 13.5 and 16.5 because of defects in the development of multiple tissues, including liver (Schmidt et al., 1995). For this reason, HGF is included in many protocols that generate hepatocyte-like cells from iPSCs (Cai et al., 2007; Hay et al., 2008; Basma et al., 2009; Si-Tayeb et al., 2010b; Sullivan et al., 2010; Touboul et al., 2010). Whether signaling through MET or related receptors affected by PHA 665752 is required for hepatic specification has not been determined, and we believe that the use of an iPSC-based differentiation model will clarify its role.
In addition to processes known to regulate hepatocyte function, our screen surprisingly identified many agonists and antagonists of receptors that have predominantly been studied in the nervous system. Dopamine, 5-hydroxytryptamine (serotonin), adrenergic and ryanodine receptors accounted for ∼30% of all hits. Although we recognize that the effect exhibited on hepatic progenitor cell formation by these small molecules might, in some cases, reflect off-target effects, in many instances multiple drugs purported to target a given receptor all repressed HNF4A levels. For example, the Sigma-1 receptor that modulates calcium signaling is targeted by five individual small molecules with distinct chemical structures (Bd 1047, astemizole, Gbr 12935, sertraline and vanoxerine). Likewise, six molecules in the library target the dopamine receptors and eight are 5-HT receptor antagonists. All of these receptor classes have subtypes that are expressed in the liver (Nassar et al., 1986; Klouz et al., 2002). Whether they affect liver development has not been described, but our data suggest that such a role should be investigated.
At the completion of the screen, we chose to focus on HSP90β. HSP90β is a protein chaperone abundantly expressed in all eukaryotic cell types (Johnson, 2012). It binds to client proteins and regulates their maturation (Wayne et al., 2011), localization (Kazlauskas et al., 2001) and activation (Vaughan et al., 2008). The clients of HSP90β include transcription factors (Sato et al., 2003), kinases (Xu et al., 1999) and receptors (Morishima et al., 2000) that affect diverse functions of cells (Taipale et al., 2010; Karagöz and Rüdiger, 2015). HSP90β has been primarily studied as a target for cancer therapy because many HSP90β clients are oncoproteins, and the expression of HSP90β is upregulated in several types of malignancies (Trepel et al., 2010). Although the perturbation of expression leads to developmental abnormalities (Voss et al., 2000), the role of HSP90β in the context of organogenesis and development of the liver is not understood.
We show that pharmacological inhibition or mutation of the gene encoding HSP90β substantially reduced HNF4A protein levels during the formation of hepatic progenitor cells from iPSCs without causing cell death. In addition to its role in hepatocyte differentiation in the fetus, HNF4A also has significant roles in adults. For example, a haploinsufficiency of HNF4A causes maturity-onset diabetes of the young (Yamagata et al., 1996). Moreover, HNF4A regulates the expression of a wide variety of genes in both fetal and adult hepatocytes and is intimately associated with control of many liver functions, including cholesterol homeostasis, carbohydrate metabolism, secretion of serum factors and xenobiotic responses (Odom et al., 2004; Battle et al., 2006; Bolotin et al., 2010). It seems likely, therefore, that HSP90β, through its regulation of HNF4A protein levels, will also contribute to the control of the adult liver function.
Consistent with the fact that signaling pathways that control HNF4A expression were not affected, we found that HNF4A mRNA levels were normal in HSP90β−/− cells. Given that loss of HSP90β reduces the half-life of HNF4A protein and that HSP90β directly interacts with HNF4A, we believe it most likely that HNF4A is a client of the HSP90β chaperone. HSP90β regulates ligand binding, inactivation, protein transport and degradation of various nuclear receptors (Pratt and Toft, 1997). However, no one has reported a role for HSP90β in controlling HNF4A. Based on our understanding of the mechanism of action of HSP90β in the context of other nuclear receptors, we favor a model whereby disruption of HSP90β results in a failure to control maturation of HNF4A protein structure. As a consequence of improper folding, HNF4A is degraded by the proteasome in HSP90β−/− cells. Other studies have documented that turnover of HNF4A can occur through ubiquitin-mediated proteasomal degradation (Zhou et al., 2012). Unfortunately, treatment of iPSC-derived hepatic progenitors with the proteasome inhibitor MG 132 resulted in extensive and rapid cell death (not shown). This toxicity, therefore, prevented us from directly testing whether loss of HNF4A in HSP90β−/− hepatic progenitors was mediated by the proteasome. Although we have demonstrated that HSP90β regulates HNF4A protein levels, it is also important to acknowledge that the impact of HSP90β on hepatocyte differentiation could be multifaceted. As we have discussed, the list of HSP90β clients is broad; therefore, it seems likely that other client proteins could contribute, and experiments to address this possibility are currently ongoing.
In summary, we have combined the use of a small-molecule screen with the differentiation of human iPSCs to identify new cellular pathways that impact the conversion of the endoderm to a hepatic fate. One caveat of using chemicals for screening is that small molecules can have off-target effects; therefore, they require significant follow-up analyses. Nevertheless, small molecules have several advantages compared with genetic approaches. For example, chemicals can be applied at a particular stage of the differentiation procedure, thereby circumventing any early requirement for a target pathway that would limit traditional mutagenesis screens. In addition, the availability of small molecules that affect diverse targets provides an unbiased opportunity to identify contributions made by cellular processes that could not have been predicted. Although our study directly establishes the role of HSP90β in controlling the fate of the endoderm, we are also confident that further work on other targets that influence HNF4A protein levels will advance our general understanding of hepatic development.
MATERIALS AND METHODS
Culture and differentiation of human iPSCs
Human K3 iPSCs (Si-Tayeb et al., 2010c) were regularly tested for contamination and cultured in mTeSR is the brand name of the medium, not an abbreviation medium (Ludwig et al., 2006) with 4 ng/ml zebrafish basic fibroblast growth factor on an E-cadherin-IgG Fc fusion protein matrix (Nagaoka and Duncan, 2010) in 4% O2-5% CO2 in air. K3 cells were seeded on Matrigel (2 mg/ml)-coated tissue culture plates 24 h before differentiation. Cells were induced to form hepatocyte-like cells as described in a stepwise protocol published previously (Mallanna and Duncan, 2013).
Small-molecule screening
Human K3 iPSCs were seeded on fourteen 96-well plates and induced to form endoderm. Small molecules from the Tocriscreen Mini library (Tocris, #2890) were individually applied between day 6 and day 8 of differentiation. In each 96-well plate, eight wells were untreated, eight wells were treated with DMSO, and the remaining wells were treated with small molecules at a concentration of 7 μM. At the end of day 8, cells were fixed for immunostaining to detect HNF4A and the number of cells was determined by DAPI staining.
Immunostaining
Cultured cells were fixed with 4% paraformaldehyde for 30 min and made permeable using 0.5% Triton X-100 in PBS for 15 min. Cells were treated with 3% bovine serum albumin in PBS for 30 min followed by overnight incubation with primary antibody at 4°C. Antibodies used were HNF4A (Santa Cruz, #sc-1556, 1:250), GATA4 (Santa Cruz, #sc-1237, 1:250) and HSP90β (Abcam, #ab32568, 1:500). Cells were rinsed with PBS three times, each for 5 min, and incubated with DAPI (1 µg/ml) and secondary antibody for 1 h at room temperature. Alexa fluor antibodies (594 nm anti-goat, Invitrogen, #A-11058; 488 nm anti-rabbit, Invitrogen, #A11008; 488 nm anti-goat, Invitrogen, #A-11055) were used at 1:1000 dilution. Images for quantitative analysis were captured using identical microscopy and image settings for each sample. ImageJ software was used to measure the pixel intensity of the whole image over a linear range (Schneider et al., 2012). After analyses, the images were processed using Adobe Photoshop to optimize brightness and contrast. Control and experimental wells were processed identically.
Immunoblot and immunoprecipitation
Whole cell lysates were collected using NP-40 buffer with protease inhibitor cocktail (ThermoFisher Scientific, #78443). Total protein (30 µg) was separated by SDS-PAGE using Any kD Mini-protean TGX stain-free precast gels (BioRad, #4568123), and transferred to PVDF membranes using the Trans-Blot Turbo Transfer System (BioRad, #1704155). Membranes were incubated overnight with antibodies against HNF4A (Santa Cruz, #sc-1556, 1:1000), HSP90β (Abcam, #ab32568, 1:100,000), phospho-ERK (Cell Signaling Technology, #9101, 1:2000), Phospho-AKT (Cell Signaling Technologies, #4060, 1:2000), pan-AKT (Cell Signaling Technology, #4691, 1:2000), pan-ERK (Cell Signaling Technology, #4695, 1:2000) or GAPDH (Novus Biologicals, #NB600-502, 1:6000) at 4°C. Horseradish peroxidase-conjugated secondary antibodies were used at a dilution of 1:2000. Protein levels were calculated using BioRad stain-free Imaging System and normalized to total protein using Image Lab software from BioRad. To determine HNF4A half-life, cells were cultured in the presence of 100 μg/ml CHX and lysed at different time points. Collected samples were used for western blot. To detect interaction between HNF4A and HSP90, co-immunoprecipitation was performed using Catch and Release V2.0 kit (EMD Millipore, #17-500), following the manufacturer's directions.
Quantitative real-time PCR analysis
RNA was isolated from K3 cells or iPSC-derived hepatocyte-like cells using the RNeasy Mini Kit (Qiagen, #74106). Genomic DNA was removed using the TURBO DNA-free Kit (ThermoFisher/Ambion, #AM1907). First-strand cDNA was synthesized using M-MLV Reverse Transcriptase (ThermoFisher/Invitrogen, #28025-013). Quantitative real-time PCR was performed on a BioRad CFX384 real-time PCR machine using TaqMan Gene Expression assay (ThermoFisher/Applied Biosystems, #4369016) or Power SYBR Green PCR assays (ThermoFisher/Applied Biosystems, #4367659), following the manufacturer's directions. SYBR green primers and Taqman assays are listed in Table S2.
CRISPR/Cas9 genome editing
CRISPR guide RNAs targeting exon 2 of the HSP90AB1 gene were designed following the protocol established by Zhang and colleagues (Ran et al., 2013). A guide sequence (GTAATCTCTAGTTGAGGGCT) was cloned into PX459 pSPCas9(BB)-2A-Puro vector (Ran et al., 2013). The plasmid was introduced into K3 cells by electroporation using a BTX electroporator. Electroporated iPSCs were cultured on Matrigel for 24 h in the presence of ROCK inhibitor Y27632 (StemRD, #146986-50-7) and then treated with 1 μg/ml puromycin for 2 days. Cells that survived the selection were expanded until clones could be collected. Genomic DNA was extracted from the clones using QuickExtract DNA extraction solution (Epicentre, #QE09050). The targeted region of the HSP90AB1 gene was amplified using Herculase Fusion Polymerase (Agilent, #600675) and run on Novex 4-20% TBE gels (ThermoFisher/Invitrogen, #EC6225BOX) to detect indels (forward AGGAGGAGGTGGAGACTTT, reverse AGGCCAAACCACTCCTTTC). Amplicons were cloned into plasmid and subjected to nucleotide sequencing to confirm the identity of the indels.
Statistical analysis
The z-factor was defined based on the means and standard deviations of both the positive and negative control values and calculated using the online z-factor calculator (http://www.screeningunit-fmp.net/tools/z-prime.php). Student's unpaired t-test was used to determine the significance of the difference between control and experimental results generated by immunostaining pixel intensity measurement, immunoblot densitometry and RT-qPCR assays.
Acknowledgements
We thank Lauren Tolliver for technical support and Lindy Keane Carter for editing the manuscript prior to submission.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: R.J., S.A.D.; Methodology: R.J., S.A.D.; Validation: R.J.; Formal analysis: R.J., C.B.D., S.A.D.; Investigation: R.J., C.B.D., S.A.D.; Resources: S.A.D.; Data curation: R.J., S.A.D.; Writing - original draft: R.J.; Writing - review & editing: S.A.D.; Visualization: S.A.D.; Supervision: S.A.D.; Project administration: S.A.D.; Funding acquisition: S.A.D.
Funding
This work was supported by gifts from the Marcus Family, the Phoebe R. and John D. Lewis Foundation, the South Carolina Smart State Endowed Chair in Regenerative Medicine, and by National Institutes of Health (DK102716, HG006398 and HD08257). Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at http://dev.biologists.org/lookup/doi/10.1242/dev.146845.supplemental
References
- Basma H., Soto-Gutiérrez A., Yannam G. R., Liu L., Ito R., Yamamoto T., Ellis E., Carson S. D., Sato S., Chen Y. et al. (2009). Differentiation and transplantation of human embryonic stem cell-derived hepatocytes. Gastroenterology 136, 990-999.e4. 10.1053/j.gastro.2008.10.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battle M. A., Konopka G., Parviz F., Gaggl A. L., Yang C., Sladek F. M. and Duncan S. A. (2006). Hepatocyte nuclear factor 4alpha orchestrates expression of cell adhesion proteins during the epithelial transformation of the developing liver. Proc. Natl. Acad. Sci. USA 103, 8419-8424. 10.1073/pnas.0600246103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolotin E., Liao H., Ta T. C., Yang C., Hwang-Verslues W., Evans J. R., Jiang T. and Sladek F. M. (2010). Integrated approach for the identification of human hepatocyte nuclear factor 4alpha target genes using protein binding microarrays. Hepatology 51, 642-653. 10.1002/hep.23357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossard P. and Zaret K. S. (1998). GATA transcription factors as potentiators of gut endoderm differentiation. Development 125, 4909-4917. [DOI] [PubMed] [Google Scholar]
- Cai J., Zhao Y., Liu Y., Ye F., Song Z., Qin H., Meng S., Chen Y., Zhou R., Song X. et al. (2007). Directed differentiation of human embryonic stem cells into functional hepatic cells. Hepatology 45, 1229-1239. 10.1002/hep.21582 [DOI] [PubMed] [Google Scholar]
- Calmont A., Wandzioch E., Tremblay K. D., Minowada G., Kaestner K. H., Martin G. R. and Zaret K. S. (2006). An FGF response pathway that mediates hepatic gene induction in embryonic endoderm cells. Dev. Cell 11, 339-348. 10.1016/j.devcel.2006.06.015 [DOI] [PubMed] [Google Scholar]
- Delaforest A., Nagaoka M., Si-Tayeb K., Noto F. K., Konopka G., Battle M. A. and Duncan S. A. (2011). HNF4A is essential for specification of hepatic progenitors from human pluripotent stem cells. Development 138, 4143-4153. 10.1242/dev.062547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugyala R. R., Claggett T. W., Kimmel G. L. and Kimmel C. A. (2002). HSP90alpha, HSP90beta, and p53 expression following in vitro hyperthermia exposure in gestation day 10 rat embryos. Toxicol. Sci. 69, 183-190. 10.1093/toxsci/69.1.183 [DOI] [PubMed] [Google Scholar]
- Duncan S. A., Manova K., Chen W. S., Hoodless P., Weinstein D. C., Bachvarova R. F. and Darnell J. E. Jr (1994). Expression of transcription factor HNF-4 in the extraembryonic endoderm, gut, and nephrogenic tissue of the developing mouse embryo: HNF-4 is a marker for primary endoderm in the implanting blastocyst. Proc. Natl. Acad. Sci. USA 91, 7598-7602. 10.1073/pnas.91.16.7598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gualdi R., Bossard P., Zheng M., Hamada Y., Coleman J. R. and Zaret K. S. (1996). Hepatic specification of the gut endoderm in vitro: cell signaling and transcriptional control. Genes Dev. 10, 1670-1682. 10.1101/gad.10.13.1670 [DOI] [PubMed] [Google Scholar]
- Hackl C., Mori A., Moser C., Lang S. A., Dayoub R., Weiss T. S., Schlitt H. J., Geissler E. K., Hellerbrand C. and Stoeltzing O. (2010). Effect of heat-shock protein-90 (HSP90) inhibition on human hepatocytes and on liver regeneration in experimental models. Surgery 147, 704-712. 10.1016/j.surg.2009.10.061 [DOI] [PubMed] [Google Scholar]
- Hay D. C., Zhao D., Fletcher J., Hewitt Z. A., McLean D., Urruticoechea-Uriguen A., Black J. R., Elcombe C., Ross J. A., Wolf R. et al. (2008). Efficient differentiation of hepatocytes from human embryonic stem cells exhibiting markers recapitulating liver development in vivo. Stem Cells 26, 894-902. 10.1634/stemcells.2007-0718 [DOI] [PubMed] [Google Scholar]
- Huangfu D., Liu A., Rakeman A. S., Murcia N. S., Niswander L. and Anderson K. V. (2003). Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature 426, 83-87. 10.1038/nature02061 [DOI] [PubMed] [Google Scholar]
- Iwafuchi-Doi M. and Zaret K. S. (2016). Cell fate control by pioneer transcription factors. Development 143, 1833-1837. 10.1242/dev.133900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. L. (2012). Evolution and function of diverse Hsp90 homologs and cochaperone proteins. Biochim. Biophys. Acta 1823, 607-613. 10.1016/j.bbamcr.2011.09.020 [DOI] [PubMed] [Google Scholar]
- Jung J., Zheng M., Goldfarb M. and Zaret K. S. (1999). Initiation of mammalian liver development from endoderm by fibroblast growth factors. Science 284, 1998-2003. 10.1126/science.284.5422.1998 [DOI] [PubMed] [Google Scholar]
- Karagöz G. E. and Rüdiger S. G. D. (2015). Hsp90 interaction with clients. Trends Biochem. Sci. 40, 117-125. 10.1016/j.tibs.2014.12.002 [DOI] [PubMed] [Google Scholar]
- Kazlauskas A., Sundström S., Poellinger L. and Pongratz I. (2001). The hsp90 chaperone complex regulates intracellular localization of the dioxin receptor. Mol. Cell. Biol. 21, 2594-2607. 10.1128/MCB.21.7.2594-2607.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klouz A., Sapena R., Liu J., Maurice T., Tillement J.-P., Papadopoulos V. and Morin D. (2002). Evidence for sigma-1-like receptors in isolated rat liver mitochondrial membranes. Br. J. Pharmacol. 135, 1607-1615. 10.1038/sj.bjp.0704626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourtidis A., Ngok S. P., Pulimeno P., Feathers R. W., Carpio L. R., Baker T. R., Carr J. M., Yan I. K., Borges S., Perez E. A. et al. (2015). Distinct E-cadherin-based complexes regulate cell behaviour through miRNA processing or Src and p120 catenin activity. Nat. Cell Biol. 17, 1145-1157. 10.1038/ncb3227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krone P. H. and Sass J. B. (1994). HSP 90 alpha and HSP 90 beta genes are present in the zebrafish and are differentially regulated in developing embryos. Biochem. Biophys. Res. Commun. 204, 746-752. 10.1006/bbrc.1994.2522 [DOI] [PubMed] [Google Scholar]
- Lemaigre F. P. (2009). Mechanisms of liver development: concepts for understanding liver disorders and design of novel therapies. Gastroenterology 137, 62-79. 10.1053/j.gastro.2009.03.035 [DOI] [PubMed] [Google Scholar]
- Ludwig T. E., Bergendahl V., Levenstein M. E., Yu J., Probasco M. D. and Thomson J. A. (2006). Feeder-independent culture of human embryonic stem cells. Nat. Methods 3, 637-646. 10.1038/nmeth902 [DOI] [PubMed] [Google Scholar]
- Mallanna S. K. and Duncan S. A. (2013). Differentiation of hepatocytes from pluripotent stem cells. Curr. Protoc. Stem Cell Biol. 26, Unit 1G.4.1-1G.4.13. 10.1002/9780470151808.sc01g04s26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLin V. A., Rankin S. A. and Zorn A. M. (2007). Repression of Wnt/beta-catenin signaling in the anterior endoderm is essential for liver and pancreas development. Development 134, 2207-2217. 10.1242/dev.001230 [DOI] [PubMed] [Google Scholar]
- Mi H., Muruganujan A., Casagrande J. T. and Thomas P. D. (2013). Large-scale gene function analysis with the PANTHER classification system. Nat. Protoc. 8, 1551-1566. 10.1038/nprot.2013.092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishima Y., Kanelakis K. C., Silverstein A. M., Dittmar K. D., Estrada L. and Pratt W. B. (2000). The Hsp organizer protein hop enhances the rate of but is not essential for glucocorticoid receptor folding by the multiprotein Hsp90-based chaperone system. J. Biol. Chem. 275, 6894-6900. 10.1074/jbc.275.10.6894 [DOI] [PubMed] [Google Scholar]
- Nagaoka M. and Duncan S. A. (2010). Transcriptional control of hepatocyte differentiation. Prog. Mol. Biol. Transl. Sci. 97, 79-101. 10.1016/B978-0-12-385233-5.00003-9 [DOI] [PubMed] [Google Scholar]
- Nassar C. F., Karkaji E. G., Habbal Z. M. and Nasser M. G. (1986). Dopamine receptors in normal and diabetic liver plasma membrane. Gen. Pharmacol. 17, 367-370. 10.1016/0306-3623(86)90057-1 [DOI] [PubMed] [Google Scholar]
- Ober E. A., Verkade H., Field H. A. and Stainier D. Y. R. (2006). Mesodermal Wnt2b signalling positively regulates liver specification. Nature 442, 688-691. 10.1038/nature04888 [DOI] [PubMed] [Google Scholar]
- Odom D. T., Zizlsperger N., Gordon D. B., Bell G. W., Rinaldi N. J., Murray H. L., Volkert T. L., Schreiber J., Rolfe P. A., Gifford D. K. et al. (2004). Control of pancreas and liver gene expression by HNF transcription factors. Science 303, 1378-1381. 10.1126/science.1089769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt W. B. and Toft D. O. (1997). Steroid receptor interactions with heat shock protein and immunophilin chaperones. Endocr. Rev. 18, 306-360. 10.1210/er.18.3.306 [DOI] [PubMed] [Google Scholar]
- Ran F. A., Hsu P. D., Wright J., Agarwala V., Scott D. A. and Zhang F. (2013). Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 8, 2281-2308. 10.1038/nprot.2013.143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi J. M., Dunn N. R., Hogan B. L. M. and Zaret K. S. (2001). Distinct mesodermal signals, including BMPs from the septum transversum mesenchyme, are required in combination for hepatogenesis from the endoderm. Genes Dev. 15, 1998-2009. 10.1101/gad.904601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez E. R. (2012). Chaperoning steroidal physiology: lessons from mouse genetic models of Hsp90 and its cochaperones. Biochim. Biophys. Acta 1823, 722-729. 10.1016/j.bbamcr.2011.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato N., Yamamoto T., Sekine Y., Yumioka T., Junicho A., Fuse H. and Matsuda T. (2003). Involvement of heat-shock protein 90 in the interleukin-6-mediated signaling pathway through STAT3. Biochem. Biophys. Res. Commun. 300, 847-852. 10.1016/S0006-291X(02)02941-8 [DOI] [PubMed] [Google Scholar]
- Schmidt C., Bladt F., Goedecke S., Brinkmann V., Zschiesche W., Sharpe M., Gherardi E. and Birchmeler C. (1995). Scatter factor/hepatocyte growth factor is essential for liver development. Nature 373, 699-702. 10.1038/373699a0 [DOI] [PubMed] [Google Scholar]
- Schneider C. A., Rasband W. S. and Eliceiri K. W. (2012). NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671-675. 10.1038/nmeth.2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp S. Y., Boxall K., Rowlands M., Prodromou C., Roe S. M., Maloney A., Powers M., Clarke P. A., Box G., Sanderson S. et al. (2007). In vitro biological characterization of a novel, synthetic diaryl pyrazole resorcinol class of heat shock protein 90 inhibitors. Cancer Res. 67, 2206-2216. 10.1158/0008-5472.CAN-06-3473 [DOI] [PubMed] [Google Scholar]
- Si-Tayeb K., Lemaigre F. P. and Duncan S. A. (2010a). Organogenesis and Development of the Liver. Dev. Cell 18, 175-189. 10.1016/j.devcel.2010.01.011 [DOI] [PubMed] [Google Scholar]
- Si-Tayeb K., Noto F. K., Nagaoka M., Li J., Battle M. A., Duris C., North P. E., Dalton S. and Duncan S. A. (2010b). Highly efficient generation of human hepatocyte-like cells from induced pluripotent stem cells. Hepatology 51, 297-305. 10.1002/hep.23354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si-Tayeb K., Noto F. K., Sepac A., Sedlic F., Bosnjak Z. J., Lough J. W. and Duncan S. A. (2010c). Generation of human induced pluripotent stem cells by simple transient transfection of plasmid DNA encoding reprogramming factors. BMC Dev. Biol. 10, 81 10.1186/1471-213X-10-81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan G. J., Hay D. C., Park I.-H., Fletcher J., Hannoun Z., Payne C. M., Dalgetty D., Black J. R., Ross J. A., Samuel K. et al. (2010). Generation of functional human hepatic endoderm from human induced pluripotent stem cells. Hepatology 51, 329-335. 10.1002/hep.23335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szklarczyk D., Santos A., von Mering C., Jensen L. J., Bork P. and Kuhn M. (2016). STITCH 5: augmenting protein-chemical interaction networks with tissue and affinity data. Nucleic Acids Res. 44, D380-D384. 10.1093/nar/gkv1277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taipale M., Jarosz D. F. and Lindquist S. (2010). HSP90 at the hub of protein homeostasis: emerging mechanistic insights. Nat. Rev. Mol. Cell Biol. 11, 515-528. 10.1038/nrm2918 [DOI] [PubMed] [Google Scholar]
- Touboul T., Hannan N. R. F., Corbineau S., Martinez A., Martinet C., Branchereau S., Mainot S., Strick-Marchand H., Pedersen R., Di Santo J. et al. (2010). Generation of functional hepatocytes from human embryonic stem cells under chemically defined conditions that recapitulate liver development. Hepatology 51, 1754-1765. 10.1002/hep.23506 [DOI] [PubMed] [Google Scholar]
- Trepel J., Mollapour M., Giaccone G. and Neckers L. (2010). Targeting the dynamic HSP90 complex in cancer. Nat. Rev. Cancer 10, 537-549. 10.1038/nrc2887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twaroski K., Mallanna S. K., Jing R., DiFurio F., Urick A. and Duncan S. A. (2015). FGF2 mediates hepatic progenitor cell formation during human pluripotent stem cell differentiation by inducing the WNT antagonist NKD1. Genes Dev. 29, 2463-2474. 10.1101/gad.268961.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanmuylder N., Werry-Huet A. E., Rooze M. and Louryan S. E. (2002). Heat shock protein HSP86 expression during mouse embryo development, especially in the germ-line. Anat. Embryol. 205, 301-306. 10.1007/s00429-002-0258-5 [DOI] [PubMed] [Google Scholar]
- Vaughan C. K., Mollapour M., Smith J. R., Truman A., Hu B., Good V. M., Panaretou B., Neckers L., Clarke P. A., Workman P. et al. (2008). Hsp90-dependent activation of protein kinases is regulated by chaperone-targeted dephosphorylation of Cdc37. Mol. Cell 31, 886-895. 10.1016/j.molcel.2008.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss A. K., Thomas T. and Gruss P. (2000). Mice lacking HSP90beta fail to develop a placental labyrinth. Development 127, 1-11. [DOI] [PubMed] [Google Scholar]
- Wayne N., Mishra P. and Bolon D. N. (2011). Hsp90 and client protein maturation. Methods Mol. Biol. 787, 33-44. 10.1007/978-1-61779-295-3_3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Singer M. A. and Lindquist S. (1999). Maturation of the tyrosine kinase c-src as a kinase and as a substrate depends on the molecular chaperone Hsp90. Proc. Natl. Acad. Sci. USA 96, 109-114. 10.1073/pnas.96.1.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata K., Furuta H., Oda N., Kaisaki P. J., Menzel S., Cox N. J., Fajans S. S., Signorini S., Stoffel M. and Bell G. I. (1996). Mutations in the hepatocyte nuclear factor-4alpha gene in maturity-onset diabetes of the young (MODY1). Nature 384, 458-460. 10.1038/384458a0 [DOI] [PubMed] [Google Scholar]
- Zhang J.-H., Chung T. D. Y. and Oldenburg K. R. (1999). A Simple Statistical Parameter for Use in Evaluation and Validation of High Throughput Screening Assays. J. Biomol. Screen. 4, 67-73. 10.1177/108705719900400206 [DOI] [PubMed] [Google Scholar]
- Zhou W., Hannoun Z., Jaffray E., Medine C. N., Black J. R., Greenhough S., Zhu L., Ross J. A., Forbes S., Wilmut I. et al. (2012). SUMOylation of HNF4α regulates protein stability and hepatocyte function. J. Cell Sci. 125, 3630-3635. 10.1242/jcs.102889 [DOI] [PMC free article] [PubMed] [Google Scholar]