Abstract
Opioids classically regulate the excitability of neurons by suppressing synaptic GABA release from inhibitory neurons. Here, we report a role for opioids in modulating excitatory synaptic transmission. By activating ubiquitously clustered μ-opioid receptor (MOR) in excitatory synapses, morphine caused collapse of preexisting dendritic spines and decreased synaptic α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors. Meanwhile, the opioid antagonist naloxone increased the density of spines. Chronic treatment with morphine decreased the density of dendritic spines even in the presence of Tetrodotoxin, a sodium channel blocker, indicating that the morphine's effect was not caused by altered activity in neural network through suppression of GABA release. The effect of morphine on dendritic spines was absent in transgenic mice lacking MORs and was blocked by CTOP (D-Phe-Cys-Tyr-D-Trp-Orn-Thr-Pen-ThrNH2), a μ-receptor antagonist. These data together with others suggest that endogenous opioids and/or constitutive activity of MORs participate in maintaining normal morphology and function of spines, challenging the classical model of opioids. Abnormal alteration of spines may occur in drug addiction when opioid receptors are overactivated by exogenous opiates.
Keywords: synaptic plasticity
The children of mothers who abuse opiate narcotics during pregnancy are known to exhibit various clinical deficits in brain growth and psychological behaviors (1, 2). Chronic exposure to opiates can impair learning and memory in humans and other mammals (3, 4). In the classical model, opioids acutely affect the excitability of neurons by suppressing the presynaptic release of GABAergic inhibitory neurons (5, 6). However, there is an unsolved puzzle for this classical model. In addition to GABAergic neurons, μ-opioid receptors (MORs) extensively exist in glutamatergic neurons such as pyramidal neurons in the cortex and the hippocampus (7). It has been previously reported that the density of dendritic spines in animals that have been chronically treated with morphine is lower than that in untreated control animals (8, 9). This difference could be caused by altered formation, maturation, plasticity, and/or stabilization. To distinguish these possibilities, time-lapse, live-imaging experiments are required to examine the temporal dynamics in the morphine-induced changes in dendritic spines. Hence, in this study, we have used a longitudinal live-imaging system to examine opioid-induced changes in dendritic spines in the same neurons for several days. Our live-imaging data together with electrophysiological data revealed additional roles for opioids: destabilizing dendritic spines and decreasing synaptic α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors. Plasticity of dendritic spines and trafficking of AMPA receptors play important roles in synaptic plasticity, a cellular model for learning and memory (10, 11). Opiate tolerance and dependence have been proposed to present a pathological form of learning and memory (12). Recent studies indicate that AMPA receptors, particularly GluR1 subunits, are important for morphine tolerance and dependence (13, 14). Therefore, the observed destabilization of dendritic spines and decrease of synaptic AMPA receptors could contribute to opiate addiction.
Methods
Neuronal Cultures and Transfection. A 25-mm glass coverslip (thickness, 0.08 mm) was glued to the bottom of a 35-mm Petri dish with a 22-mm hole by using silicone sealant as described (15). Dissociated neuronal cultures from rats (the hippocampus) and mice (the whole cortex) at postnatal days 1 and 2 were prepared as described (16–18). The genetic background of WT mice is similar to that of MOR knockout mice. Neurons were plated onto such 35-mm Petri dishes at a density of 1 × 106 cells per dish. The age of cultured neurons was counted from the day of plating (day 1 in vitro). To label dendrites, neurons (5–9 days in vitro) were transfected with plasmids encoding EGFP (Clontech). The standard calcium phosphate coprecipitation method was used for such transfections (17).
Immunocytochemistry and Apoptosis Test. As described (16), cultured neurons were fixed and permeabilized successively with 4% paraformaldehyde, 100% methanol, and 0.2% Triton X-100. Antibodies against AMPA receptors (fluorescent dye-conjugated rabbit antibodies), NMDA receptors (mouse), and synaptophysin proteins (mouse) have been described (16, 19). Antisera against MOR were produced against a synthesized 15-residue peptide (NHQLENLEAETAPLP) corresponding to amino acids 384–398 (7). Fixed and permeabilized neurons were incubated with the antisera (1:200 in 10% donkey sera) for 1–2 h at room temperature and washed five to six times before adding a secondary anti-rabbit antibody. In blocking experiments (Fig. 1A Right), the above peptide (2 μg/ml) was added to 10% donkey sera in PBS ≈1 h before the incubation of antisera. In cases where several rabbit antibodies were used, neurons were stained by a previously well characterized method (20). To detect apoptosis, fixed neurons were stained with TUNEL by using an ApopTag In Situ Apoptosis Detection kit (S7160, Chemicon) and counterstained with DAPI (Molecular Probes).
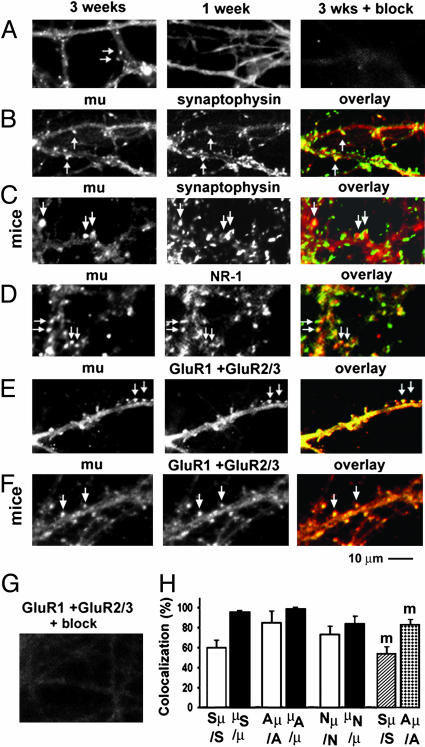
Fig. 1.
MORs were clustered in mature synapses in both cultured cortical and hippocampal neurons. (A) A 3-week-old neuron (Left) and a 1-week-old neuron (Center) were stained with a polyclonal rabbit antibody against MORs. (Right) The appropriate peptide blocked the staining of MORs. (B–F) MORs (Left, red) were double-stained with synaptophysin proteins (B and C), NMDA receptors (D), or AMPA receptors (E and F Center, green). (Right) Overlays of the Left and Center images. (C and F) Mouse cortical neurons. (B, D, and E) Rat hippocampal neurons. (G) An experiment that was similar to E Center but with peptide block. (H) Quantification of colocalization. Sμ/S, synaptophysin clusters that were colocalized with MORs (Sμ) versus total number of synaptophysin clusters (S). μS/μ, MOR clusters that were colocalized with synaptophysin (μS) versus total number of MORs (μ). Aμ/A and μA/μ, similar quantifications for MORs and AMPA receptors; Nμ/N andμN/μ, quantifications for MORs and NMDA receptors. The last two bars (m) show data from mouse cortical neurons. Arrows denote clusters of MORs and their colocalization with synaptophysin proteins, NMDA receptors, and AMPA receptors. Note that synaptophysin clusters sometimes surrounded MOR clusters (C Left, indicated by two arrows).
Image Analysis. All digital images were analyzed with a MetaMorph Imaging System (Universal Imaging, Downingtown, PA). Unless otherwise stated, all images on live neurons were taken as stacks and averaged into one image before further analysis. In addition to simple averaging, stacks of images were processed by deconvolution analyses with metamorph software with the nearest planes. A stack of deconvoluted images was further averaged into one single image. A dendritic protrusion with an expanded head that was 50% wider than its neck was defined as a spine. The number of spines or nonspine protrusions from one neuron was manually counted and normalized per 100 μm of dendritic length. Two-way ANOVA (it is also called repeated-measures one-way ANOVA) was used for comparison (n, sample size, the number of neurons; P < 0.05, significant). If the ANOVA test indicated that there were significant differences between parameters that were collected at different time points, paired t tests were used to further test the significance of such changes (21). These tests were used to detect longitudinal changes, which would not be affected by variation among individual neurons (21). To analyze the colocalization of dendritic spines and N-GluR1 staining, the digital images of EGFP were colored green, and the images of N-GluR1 staining were colored red in the metamorph program. We visually determined which spine had (+) or did not have (–) surface GluR1 subunits by comparing three images (GFP, GluR1, and overlay). Similar methods were used to analyze the colocalization in other double-staining images (MORs vs. synaptophysin proteins, NMDA receptors, and AMPA receptors). To highlight dendritic protrusions and spines in the measurement of protrusion size (see Fig. 5E), a detection threshold was first set at 50–75% of the fluorescent intensity in the middle of the dendritic shaft in an image that was acquired before the application of drugs. The same percentage was used to set the threshold in the same dendrite in images that were acquired later on. All data are reported as mean ± standard error: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
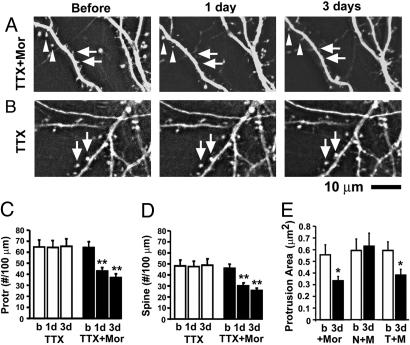
Fig. 5.
Chronic treatment with morphine changed the morphology of dendritic spines even in the presence of TTX. (A) Changes in dendrites before and after treatment with morphine in a neuron that has been chronically treated with TTX for 1 week. TTX was continuously present during the treatment of morphine. Triangles indicate spines that gradually shrank to become tiny philopodia. Arrows indicate where protrusions disappeared. (B) A control neuron that has been treated with TTX alone for 1 week is shown. Arrows indicate no changes in spines. (C and D) Density of protrusions and spines before (b) and after the application of morphine (1–3 days). (E) Chronic treatment with morphine significantly decreased the size of dendritic protrusions (+Mor). Morphine's effect was blocked by naloxone (N+M) but not by TTX (T+M).
Electrophysiology. Miniature excitatory postsynaptic currents (mEPSCs) were recorded in 3-week-old cultured neurons that had been chronically treated with opioid agonists and antagonists for 3–6 days as described (ref. 19; holding potential, approximately –55 to –60 mV; filtered in 1 KHz). Input and series resistances were checked before and after the recording of mEPSCs, which lasted ≈10–30 min. There was no significant difference in the series resistances and input resistance among various groups of experiments. One recording sweep lasting 200 ms was sampled for every 1 s. All mEPSCs were analyzed with a program designed by Synaptosoft (Decatur, GA). Detection criteria for mEPSCs included peak amplitudes >3 pA, a fast rise time, and a slow decay time. The time course and amplitude of every detected mEPSC was calculated, and such parameters of all mEPSCs in each neuron were furthered averaged and treated as single samples. mEPSCs were recorded in cultured dissociated neurons in standard Earle's balanced salt solution at room temperature with 200 μM 2-amino-5-phosphonovaleric acid (NMDA receptor blocker; D,L-form), 1 μM Tetrodotoxin (TTX) (block sodium current), and 100 μM picrotoxin (GABA receptor blocker), gassed with 95% air and 5% CO2. To increase the number of mEPSCs, extra 2 mM Ca2+ and 1 mM Mg2+ were added to the bath solution. The internal solution in the patch pipette contained 100 mM cesium gluconate, 0.2 mM EGTA, 0.5 mM MgCl2, 2 mM ATP, 0.3 mM GTP, and 40 mM Hepes (pH 7.2 with CsOH). To examine the acute effect of morphine, mEPSCs were recorded for 5 min and then were recorded for another 5–20 min after application of 10 μM morphine. In our pilot experiments, no significant change was found during acute morphine treatment. Our acute experiments would not be able to address the issue of “disinhibition” as all mEPSCs are recorded in the presence of picrotoxin (a GABA receptor antagonist) and the internal solution contained cesium, which blocked potassium currents.
Results
MORs have been believed to play important roles in opiate addiction and analgesia (12). Previous immunohistochemical studies suggest that MORs exist abundantly in glutamatergic neurons such as pyramidal neurons in the hippocampus and neocortex (7, 22). A previous ultrastructural study shows that MORs are abundant in the postsynaptic density of dendritic spines in the caudate-putamen in adult rats but are absent in such spines in 1-week-old rats (23). However, it is still unknown whether MOR exists in glutamatergic synapses because dendritic spines in caudate-putamen could be dopaminergic synapses (24). Furthermore, it is not known whether MORs are concentrated in synapses as they also have been detected in dendritic shafts and soma in ultrastructural studies (23). We have used a polyclonal rabbit antibody to detect MOR in cultured dissociated rat hippocampal neurons and mouse cortical neurons (7). Interestingly, MORs formed numerous distinct clusters along dendrites in neurons at 3 weeks in culture, whereas such receptors were diffusely distributed in dendritic shafts in neurons at 1 week in culture (Fig. 1A). MOR clusters were colocalized with synaptophysin proteins (Fig. 1 B and C), NMDA receptors (Fig. 1D), and AMPA receptors (Fig. 1 E and F) after 3 weeks in culture. A total of 60 ± 7% of total synapses (Sμ/S, n = 5 dishes), 85 ± 11% of AMPA receptor-containing synapses (Aμ/A, n = 5), and 73 ± 8% NMDA receptor-containing synapses (Nμ/N, n = 5) contain clustered MORs in cultured rat hippocampal neurons (Fig. 1H). Similarly in cultured mouse cortical neurons, 53 ± 6% of total synapses (Fig. 1H, slashed bar, n = 5) and 82 ± 5% of AMPA receptor-containing synapses (Fig. 1H, dotted bar, n = 5) contained clustered MORs. The shape of MOR clusters was similar to that of AMPA receptor clusters but not to that of synaptophysin clusters, suggesting that MORs were likely to be postsynaptic. This evidence indicates that MORs are clustered in glutamatergic synapses. In addition, it showed that most synapses, particularly AMPA receptor-containing synapses, contained clustered opioid receptors. The ubiquitous presence of MOR in glutamatergic synapses suggests that opioids may play a broad role in modulating the morphology and/or function of such synapses.
To examine how chronic treatment with morphine affects the function of excitatory synapses, a whole-cell voltage clamp was used to record mEPSCs in 3-week-old cultured neurons that had been incubated with 10 μM morphine for 3–6 days (Fig. 2 A and B). Chronic morphine treatment significantly decreased the frequency (by 46.6%), amplitude (31.5%), rise time (27.8%), and decay time (43.8%) of mEPSCs in 3-week-old cultured neurons (Fig. 2 C–F; nine neurons in each group of experiments). The effects of morphine were blocked by either CTOP (D-Phe-Cys-Tyr-D-Trp-Orn-Thr-Pen-ThrNH2, a selective MOR antagonist) or naloxone (a nonselective opiate receptor antagonist). Interestingly, chronic treatment with naloxone alone significantly increased the frequency of mEPSCs by 47% (Fig. 2C), suggesting that endogenous opioids and/or the constitutive activity of opioid receptors can regulate synaptic transmission. Chronic morphine treatment had little effect on mEPSCs in 1-week-old neurons (Fig. 2 G–I; n = 9 in each group). Dendritic spines have not yet been formed at 1 week in vitro, and most excitatory synapses exist on dendritic shafts during this time (16, 25, 26). Thus, this result suggests that opioids may affect mainly the function of excitatory synapses by regulating dendritic spines.
Fig. 2.
Morphine and endogenous opioids modulate excitatory synaptic transmission. (A)(Left) Five consecutive traces of mEPSCs that were recorded from a 3-week-old control neuron. (Right) Similar traces from a morphine-treated neuron. (B)(Left) An averaged trace recorded from a morphine-treated neuron for 15 min (black trace) was compared with a similar averaged trace from a control neuron (gray trace). (Right) The black trace was rescaled to have the same amplitude as the gray trace. Note that morphine changed the time course of mEPSC responses by shortening both the rising and decaying phases. (C–F) Various parameters of mEPSCs were compared among five groups of experiments (all were 3-week-old neurons). Ctl, untreated; Mor, morphine-treated; M+Ctp, morphine plus CTOP; M+Nal, morphine plus naloxone; Nal, naloxone alone. (G–I) Various parameters of mEPSCs were compared between a 1-week-old untreated neuron (open bars) and a morphine-treated neuron (filled bars).
According to the classical model proposed by del Castillo and Katz (27), a decrease in mEPSC frequency can be caused by a decrease in the density of dendritic spines (fewer synapses) during chronic morphine treatment. To test this possibility, we developed a time-lapse, live-imaging system to take images on the same EGFP-labeled neuron for several days before and after the application of morphine (Fig. 3). To repeatedly find and take images in the same neuron, we used the coordinates of an X-Y translation stage to determine the location of this neuron (accuracy, 4 μm). The culture dish would immediately be put back to a tissue culture incubator after each observation. Morphine (10 μM) was added in cultured rat neurons expressing EGFP at 16–20 days in vitro. EGFP-labeled dendritic spines were photographed before and at various times (3 h, 1 day, 3 days) after the application of morphine (Fig. 3). A serial stack of 15 images at various focal planes was gathered over 0.5-μm intervals (total 7.5 μm) for each fluorescent channel. Of these 15 images, a serial stack of seven to eight images that were in focus was selected and averaged into one single image. We visually identified all protrusions from three to four dendrites in a neuron, and a dendritic protrusion with an expanded head that was 50% wider than its neck was defined as a spine. The density of dendritic protrusions (number per 100 μm of dendrite) was significantly decreased by 18% after 1 day of morphine treatment (n = 8, P < 0.05, Fig. 3E) and was decreased by 29% after 3 days of such treatment (n = 8, P < 0.01, Fig. 3E; P < 0.001, ANOVA test for all time points). The density of spines was also significantly decreased by 26% after 1 day (P < 0.05) and 35% after 3 days (P < 0.01) of morphine treatment (P < 0.001, n = 8, ANOVA test for all time points; Fig. 3F, black bars). In contrast, the density of protrusions and spines was not significantly changed in untreated control neurons in 3 days (n = 8, P > 0.05, Fig. 3 E and F, open bars). Consistent with previous studies (22–24), dendritic spines are stable in mature, untreated control neurons. To test the specificity of morphine's effect, naloxone (10 μM, a nonselective morphine antagonist) was added with morphine (10 μM) to cultured neurons. Surprisingly, such treatment significantly increased the density of protrusions by 19% after 1 day (n = 8, P < 0.05) and 40% after 3 days (n = 8, P < 0.01; Fig. 3E, gray bars). It also significantly increased the density of spines by 47% after 3 days of treatment (P < 0.01, Fig. 3F, gray bars).
Fig. 3.
Morphine facilitated the retraction of dendritic spines, whereas naloxone had opposite effects. (A) Images of an EGFP-labeled cultured rat neuron were taken before and at various times after morphine application. Arrows denote loss of preexisting protrusions. (B) A rat neuron that was treated with both morphine and naloxone. Arrows denote the emergence of new spines after 3 days. (C) An untreated rat neuron. Arrows denote unchanged spines. (D) A neuron that was treated with naloxone alone. Arrows indicate new spines that emerged after only 3 h of naloxone application. The triangle indicates that the general shape of dendrites also was changed after 3 days. (E and F) The density of dendritic protrusions and spines in four groups of neurons. (G)(Upper) Morphine-treated neurons were double-stained with DAPI (Left) and TUNEL (Right). (Lower) Staurosporin-treated neurons were positive control. (H) The proportion of apoptotic cells versus total number of cells in four groups of neuronal cultures: Ctl, untreated control; Act, Actinomycin D; Sta, staurosporin; Mor, morphine. (I) The density of neurons (number of neurons per mm2 area) that are estimated from differential interference contrast images in morphine-treated (+M) and untreated (–M) cultures.
Treatment with naloxone alone (10 μM) promoted the growth of spines (Fig. 3D; slashed bars in Fig. 3 E and F). Interestingly, new spines emerged within only 3 h after naloxone treatment in three of eight examined neurons (Fig. 3D, arrows). Naloxone treatment significantly increased the density of protrusions and spines after 1–3 days (Fig. 3 E and F, slashed bars; by 56.7% in spine density after 3 days; n = 8, P < 0.01). As dendritic spines were stable in untreated control neurons (Fig. 3C and refs. 28–30), such results would support an important role of endogenous opioids and/or constitutive activity of opioid receptors in maintaining the normal density of dendritic spines. Although it has been previously shown that naloxone increases the density of spines in rats in comparison with untreated control rats (31), our report shows the temporal dynamics of naloxone-induced changes in dendritic spines. Previous in vivo studies only examined the density of spines at one time point (8, 9, 31), therefore, previously reported morphine- and naloxone-induced changes could be caused by many possibilities, including alterations in neuronal differentiation, neuronal death, synaptic formation, synaptic maturation, and stabilization of spines. Our time-lapse imaging data indicate that opioids mainly affect the stabilization of dendritic spines because chronic morphine treatment caused loss of preexisting spines (Figs. 3A and 4A), whereas new spines emerged within hours after naloxone treatment (Fig. 3D).
Fig. 4.
MORs are important for the morphine modulation of dendritic spines. (A–C) Images were processed with deconvolution. (A) A cortical neuron that was cultured from control mice before and after application of morphine is shown. Top two arrows indicate two dendritic spines that gradually retracted and became thin tiny protrusions. Bottom two arrows indicate two dendritic spines that disappeared in 3 days. (B) A neuron that was cultured from MOR knockout mice before and after morphine treatment is shown. (C) A rat neuron before and after the double treatment of CTOP and morphine is shown. Arrows in B and C denote an increase in the density of dendritic spines. (D) The number of dendritic spines per 100 μm of dendrites in morphine-treated control mouse neurons (open bars) and μ receptor knockout mouse neurons (filled bars). (E) The density of spines in three groups of drug-treated rat neurons. (F) An untreated control and a morphine-treated EGFP-labeled neuron were stained alive with Cy3 (red)-conjugated antibody against the N terminus of GluR1 subunits. Arrows denote protrusions that contain N-GluR1; triangles denote protrusions that contain no detectable GluR1. (G) The proportion of dendritic protrusions (Left) and spines (Right) that contain AMPA receptors in morphine-treated neurons (+M) and untreated neurons (–M).
Some studies suggest that morphine induces apoptosis of glia and neurons (32), whereas others suggest that morphine protects neurons from cell death (33). Morphine-induced changes in spines could be caused by loss of neurons due to apoptosis. To test this possibility, we used double staining of DAPI and TUNEL (Chemicon) to detect apoptosis in four groups of 3-week-old cultured hippocampal neurons (Fig. 3H). To induce apoptosis as positive controls, we treated such cultured neurons with actinomycine D (1 μM) for 12 h (n = 5) and staurosporin (1 μM) for 6 h (n = 5). About 49% of actinomycine D-treated cells (P < 0.001) and ≈85% of staurosporin-treated cells were apoptotic (Fig. 3 G and H; P < 0.001, compared with untreated). In contrast, as in untreated cells, the nuclei of morphine-treated cells were smooth, and such cells had little TUNEL staining (Fig. 3G). Only 2.8% of untreated control neurons (n = 5) and 2.9% of morphine-treated neurons (n = 5) were apoptotic, and there was no significant difference between these two groups of neurons, documenting the lack of any morphine-induced apoptosis (n = 5; Fig. 3H). To further examine possible loss of neurons, cultured neurons were treated with morphine (10 μM) for 6 days, fixed, and mounted on a cell counter. The cell bodies of neurons were visually identified, and the number of neurons per 1-mm2 area was calculated. The density of morphine-treated neurons is not significantly different from untreated control neurons (n1 = 6 dishes, n2 = 6 dishes, P > 0.05, group t test; Fig. 3I). These results indicate that the morphine-induced changes in spines were unlikely to be largely caused by cell death.
As shown in Fig. 2, chronic treatment with morphine decreased the rise time and decay time of mEPSCs. This finding might result from a decrease in the size of spines because the time to reach maximal response (rise time) and the time to remove released neurotransmitters (decay time) would be faster in a smaller synapse than a larger synapse. We expect that there are more receptors in a larger synapse. If the probability for a glutamate molecule to bind to a receptor is the same at any given time, it would take more time for glutamate molecules to bind to most of the AMPA receptors in a larger synapse. To clearly visualize the sequential morphological changes of individual spines during chronic opioid treatment, we processed stacks of live images with a deconvolution program (Figs. 4 A–E and 5; calculated with nearest planes; MetaMorph). Deconvolution analysis is a mathematical tool that would reduce the blurring from nearby bright objects and thus improve the identification of fine structures. In our experiments, both image averaging (Fig. 3) and deconvolution (Fig. 4) detect the same number of dendritic spines, and thus both should be appropriate for quantification of spine density. The deconvolution analyses in our studies demonstrated that dendritic spines gradually retracted and became tiny protrusions during chronic morphine treatment (Figs. 4A and 5A). This result revealed that chronic treatment with morphine could indeed decrease the size of dendritic spines, and thus alter the time course of mEPSCs.
Based on pharmacological studies, three major subtypes of opioid receptors have been identified: μ, δ, and κ (12). To examine the role of MORs in opioid modulation of dendritic spines, we made cultured dissociated neurons from the cortex of MOR knockout mice (34) and control mice (Fig. 4). Morphine (10 μM) significantly decreased the density of spines in control mice (Fig. 4D, open bars, n = 8, P < 0.05). The density of spines was increased in MOR knockout mice after 3 days even in the presence of morphine (Fig. 4 B and D, gray bars, n = 8, P < 0.05). In cultured rat hippocampal neurons, the morphine's effect was blocked by CTOP, and CTOP alone significantly increased the density of spines after 3 days (Fig. 4 C and E). It suggests that endogenous μ-opioid agonists, such as enkephalin and endomorphin, and/or constitutive activity of MORs, might play a role in the stabilization of dendritic spines. This evidence shows that MORs mediate opioid modulation of dendritic spines during chronic morphine treatment. However, the participation of other subtypes of opioid receptors cannot be excluded.
Synaptic responses in excitatory synapses under normal resting potentials are believed to be mediated through AMPA receptors (35, 36). To test whether morphine can alter synaptic functions by changing the distribution of AMPA receptors in individual dendritic protrusions and spines, cultured rat neurons at 16–20 days in vitro were chronically treated with morphine (10 μM) for 6 days. To detect surface AMPA receptors, EGFP-labeled neurons were incubated with a Cy3-conjugated (red) rabbit polyclonal antibody against the N terminus of GluR1 subunits (Fig. 4F). Chronic morphine treatment significantly decreased the proportion of dendritic protrusions that contained AMPA receptors (from 77.2 ± 4.1% to 45.7 ± 3.2%, n1 = 10 cells, n2 = 10 cells, P < 0.001, Fig. 4G) and the proportion of spines that contained AMPA receptors (from 88.4 ± 3.7% to 56.5 ± 3.5%, P < 0.001, Fig. 4G). These results revealed that chronic treatment with morphine could indeed reduce the amount of synaptic AMPA receptors.
It is well known that activation of opioid receptors suppresses the presynaptic release of GABA, which may alter the neural network activity, and thus indirectly change the function and morphology of dendritic spines (5, 6). To test this possibility, TTX (1 μM, a sodium channel blocker, blocking neural network) was applied to 2-week-old cultured neurons, which were allowed to grow in the continuous presence of TTX for another week. At 3 weeks in culture, GFP-labeled dendrites were photographed before and at 1 and 3 days after addition of 10 μM morphine (Fig. 5A). TTX was continuously present during the period of treatment with morphine. Treatment with morphine significantly decreased the density of dendritic protrusions and spines even in the presence of TTX (Fig. 5 C and D, black bars, n = 8, P < 0.001). Chronic treatment with morphine also changed the size of dendritic protrusions and spines even in the presence of TTX (Fig. 5A for an example; Fig. 5E, T+M, n = 9, P < 0.05). Chronic treatment with morphine alone significantly decreased the size of dendritic protrusions (Fig. 5E, +Mor, n = 8, P < 0.05) and morphine's effect was blocked by naloxone (Fig. 5E, N+M). These results indicate that morphine's effect on spines is unlikely to be caused by an indirect alteration in neural network activity.
Discussion
Glutamate receptors, protein kinases, and PDZ domain-containing proteins are well known molecules that play critical roles in modulating the morphology and function of glutamatergic synapses (37–39). One distinct feature of these molecules is that they often cluster in dendritic spines (37–39). In this study, we found that MORs, which are G protein-coupled receptors, clustered in glutamatergic synapses (Fig. 1). In a previous immunohistochemical study, anti-MOR antibodies detected numerous puncta along the dendrites of pyramidal neurons in cortical and hippocampal slices (7). Such puncta could be the synaptic clusters of MORs in this study. The ubiquitous presence of MORs in glutamatergic synapses suggests that opioids may play a broad role in modulating the morphology and/or function of such synapses. Indeed, chronic treatment with morphine or naloxone profoundly altered the function of excitatory synapses (Fig. 2). Morphine decreased the frequency, amplitude, rise time, and decay time of mEPSCs. Naloxone treatment alone increased the frequency but not the amplitude and time course of mEPSCs. The simple explanation is that these two changes are mediated through different mechanisms. An alternative explanation is that newly formed synapses after naloxone treatment are small synapses, which would be expected to have a small amount of AMPA receptors and have short rise times and decay times. In addition, chronic treatment morphine or naloxone profoundly altered the morphology of dendritic spines. Previous in vivo studies have shown that chronic morphine administration decreased the density of spines in rats in comparison with untreated control rats, whereas naltrexone increased the density of spines (8, 9, 31). However, the morphine-induced decrease in the density of spines can be caused by either a loss of preexisting spines or a suppression of the growth of new spines. Our time-lapse imaging data demonstrated that preexisting spines gradually shrank to small tiny protrusions, eventually disappearing after 3 days of treatment with morphine (Fig. 3A), whereas new spines emerged within hours after naloxone treatment (Fig. 3D). Our results indicate that opioids mainly affect the stabilization of dendritic spines instead of suppressing the formation and maturation of such spines. Furthermore, these morphological changes are accompanied by functional changes consistent with such morphological changes (Fig. 2).
The effect of morphine on dendrite spines was blocked in MOR knockout mice and by CTOP (Fig. 4), suggesting that the morphine's effect is at least partially mediated through MORs. However, the participation of other subtypes of opioid receptors (δ and κ) cannot be excluded because it is well documented that δ-opioid receptors and MORs can functionally and physically interact with each other (40, 41). The concentrations of opioid agonists and antagonists used in this study are similar to those used in previous work using cultured cells (42, 43).
Previous studies showed that acute application of MOR agonists can activate G protein-coupled inwardly rectifying potassium channels in GABAergic neurons in the hippocampus, indicating that the activation of MORs may suppress the presynaptic release of GABA, thus resulting in disinhibition of neural circuits (5, 6). One possible scenario is that the acute disinhibition by morphine may lead to overactivation of excitatory synapses, which eventually decrease the amplitude of mEPSCs by “scaling” (44). It seems unlikely that all chronic morphine-induced changes are caused by such disinhibition because MORs are predominantly postsynaptic (7, 22, 23) and clustered in ≈80% of AMPA receptor-containing synapses (Fig. 1). Contrary to the scaling hypothesis, chronic treatment with picrotoxin, a GABA receptor antagonist, actually increased the density of dendritic spines in 18-day-old cultured hippocampal neurons, suggesting mechanisms other than homeostasis are involved (45). In our study, morphine decreased the density of spines, which is opposite to the effect of picrotoxin, indicating the effect of morphine is not mediated through inhibition of presynaptic release of GABA. Furthermore, in current studies, we observed morphine-induced loss of preexisting spines even in the presence of TTX, a sodium channel blocker (Fig. 5), suggesting that morphine is likely to directly act on the clustered opioid receptors in glutamatergic synapses and not indirectly via an alteration in neural network activity.
As shown in this article, opioid receptors were ubiquitously present in excitatory synapses. Both endogenous and exogenous opioids can modulate the morphology and function of dendritic spines. Endogenous opioids may maintain the normal density of dendritic spines by preventing abnormal overgrowth of dendritic spines, whereas exogenous opiates cause such spines to collapse. The latter might have a role in the pathological basis for drug addiction. In addition to acting on MORs in GABAergic synapses (12), opioids may act directly on MORs in glutamatergic synapses as shown in this study. The interaction between these two distinct mechanisms is not yet known. One possibility is that the acute effect of morphine is mediated mainly through changes in GABAergic synapses, whereas the chronic effect is mediated through changes in glutamatergic synapses.
Acknowledgments
We thank Drs. Richard Huganir, George Wilcox, and Eric Newman for helpful comments and Dr. Martin Wessendorf (University of Minnesota, Minneapolis) for providing an anti-MOR antibody. This work was supported by grants from the Whitehall Foundation, the McManus Trust for Drug Abuse, and the Minnesota Medical Foundation (to D.L.) and National Institute on Drug Abuse Grants DA01806, DA007339, DA000564, K05-DA70554, and K05-DA000513 (to H.H.L. and P.Y.L.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; MOR, μ-opioid receptor; TTX, Tetrodotoxin; EPSC, excitatory postsynaptic current; mEPSC, miniature EPSC; CTOP, D-Phe-Cys-Tyr-D-Trp-Orn-Thr-Pen-ThrNH2.
References
- 1.Greene, C. M. & Goodman, M. H. (2003) Neonatal Network 22, 15–25. [DOI] [PubMed] [Google Scholar]
- 2.Kaltenbach, K. A. & Finnegan, L. P. (1989) Neurotoxicology 10, 597–604. [PubMed] [Google Scholar]
- 3.Davis, P. E., Liddiard, H. & McMillan, T. M. (2002) Drug Alcohol Depend. 67, 105–108. [DOI] [PubMed] [Google Scholar]
- 4.Pu, L., Bao, G. B., Xu, N. J., Ma, L. & Pei, G. (2002) J. Neurosci. 22, 1914–1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wimpey, T. L. & Chavkin, C. (1991) Neuron 6, 281–289. [DOI] [PubMed] [Google Scholar]
- 6.Madison, D. V. & Nicoll, R. A. (1988) J. Physiol. (London) 398, 123–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arvidsson, U., Riedl, M., Chakrabarti, S., Lee, J. H., Nakano, A. H., Dado, R. J., Loh, H. H., Law, P. Y., Wessendorf, M. W. & Elde, R. (1995) J. Neurosci. 15, 3328–3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ricalde, A. A. & Hammer, R. P. (1990) Neurosci. Lett. 115, 137–143. [DOI] [PubMed] [Google Scholar]
- 9.Robinson, T. E., Gorny, G., Savage, V. R. & Kolb, B. (2002) Synapse 46, 271–279. [DOI] [PubMed] [Google Scholar]
- 10.Matsuzaki, M., Honkura, N., Ellis-Davies, G. C. & Kasai, H. (2004) Nature 429, 761–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malinow, R., Mainen, Z. F. & Hayashi, Y. (2000) Curr. Opin. Neurobiol. 10, 352–357. [DOI] [PubMed] [Google Scholar]
- 12.Williams, J. T., Christie, M. J. & Manzoni, O. (2001) Physiol. Rev. 81, 299–343. [DOI] [PubMed] [Google Scholar]
- 13.Vekovischeva, O. Y., Zamanillo, D., Echenko, O., Seppala, T., Uusi-Oukari, M., Honkanen, A., Seeburg, P. H., Sprengel, R. & Korpi, E. R. (2001) J Neurosci. 21, 4451–4459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stephens, D. N. & Mead, A. N. (2003) Trends Neurosci. 26, 181–182. [DOI] [PubMed] [Google Scholar]
- 15.Lin, H., Huganir, R. & Liao, D. (2004) Biochem. Biophys. Res. Commun. 316, 501–511. [DOI] [PubMed] [Google Scholar]
- 16.Liao, D., Zhang, X., O'Brien, R., Ehlers, M. D. & Huganir, R. L. (1999) Nat. Neurosci. 2, 37–43. [DOI] [PubMed] [Google Scholar]
- 17.Ghosh, A. & Greenberg, M. E. (1995) Neuron 15, 89–103. [DOI] [PubMed] [Google Scholar]
- 18.Banker, G. A. & Cowan, W. M. (1977) Brain Res. 126, 397–425. [DOI] [PubMed] [Google Scholar]
- 19.Liao, D., Scannevin, R. H. & Huganir, R. (2001) J. Neurosci. 21, 6008–6017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O'Brien, R. J., Mammen, A. L., Blackshaw, S., Ehlers, M. D., Rothstein, J. D. & Huganir, R. L. (1997) Neuroscience 17, 7339–7350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosner, B. (1999) Fundamentals of Biostatistics (Duxbury Press, Belmont, CA), 3rd Ed.
- 22.Ding, Y. Q., Kaneko, T., Nomura, S. & Mizuno, N. (1996) J. Comp. Neurol. 367, 375–402. [DOI] [PubMed] [Google Scholar]
- 23.Wang, H., Cuzon, V. C. & Pickel, V. M. (2003) Neuroscience 118, 695–708. [DOI] [PubMed] [Google Scholar]
- 24.Ambrose, L. M., Unterwald, E. M. & Van Bockstaele, E. J. (2004) Anat. Rec. 279, 583–591. [DOI] [PubMed] [Google Scholar]
- 25.Rao, A. & Craig, M. (1997) Neuron 19, 801–812. [DOI] [PubMed] [Google Scholar]
- 26.Rao, A., Kim, E., Sheng, M. & Craig, A. M. (1998) J. Neurosci. 18, 1217–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.del Castillo, J. & Katz, B. (1954) J. Physiol. (London) 124, 560–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dailey, M. E. & Smith, S. J. (1996) J. Neurosci. 16, 2983–2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ziv, N. E. & Smith, S. J. (1996) Neuron 17, 91–102. [DOI] [PubMed] [Google Scholar]
- 30.Grutzendler, J., Kasthuri, N. & Gan, W. B. (2002) Nature 420, 812–816. [DOI] [PubMed] [Google Scholar]
- 31.Hauser, K. F., McLaughlin, P. J. & Zagon, I. S. (1989) J. Comp. Neurol. 281, 13–22. [DOI] [PubMed] [Google Scholar]
- 32.Hu, S., Sheng, W. S., Lokensgard, J. R. & Peterson, P. K. (2002) Neuropharmacology 42, 829–836. [DOI] [PubMed] [Google Scholar]
- 33.Iglesias, M., Segura, M. F., Comella, J. X. & Olmos, G. (2003) Neuropharmacology 44, 482–492. [DOI] [PubMed] [Google Scholar]
- 34.Guo, X. H., Fairbanks, C. A., Stone, L. S. & Loh, H. H. (2003) Pain 104, 209–217. [DOI] [PubMed] [Google Scholar]
- 35.Liao, D., Hessler, N. A. & Malinow, R. (1995) Nature 375, 400–404. [DOI] [PubMed] [Google Scholar]
- 36.Isaac, J. T., Nicoll, R. A. & Malenka, R. C. (1995) Neuron 15, 427–434. [DOI] [PubMed] [Google Scholar]
- 37.Kim, E. & Sheng, M. (2004) Nat. Rev. Neurosci. 5, 771–781. [DOI] [PubMed] [Google Scholar]
- 38.Bredt, D. S. & Nicoll, R. A. (2003) Neuron 40, 361–379. [DOI] [PubMed] [Google Scholar]
- 39.Sheng, M. (2001) Proc. Natl. Acad. Sci. USA 98, 7058–7061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith, A. P. & Lee, N. M. (2003) Life Sci. 73, 1873–1893. [DOI] [PubMed] [Google Scholar]
- 41.Gomes, I., Gupta, A., Filipovska, J., Szeto, H. H., Pintar, J. E. & Devi, L. A. (2004) Proc. Natl. Acad. Sci. USA 101, 5135–5139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eisinger, D. A., Ammer, H. & Schulz, R. (2002) J. Neurosci. 22, 10192–10200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Celver, J., Xu, M., Jin, W., Lowe, J. & Chavkin, C. (2004) Mol. Pharmacol. 65, 528–537. [DOI] [PubMed] [Google Scholar]
- 44.Turrigiano, G. G. & Nelson, S. B. (2004) Nat. Rev. Neurosci. 5, 97–107. [DOI] [PubMed] [Google Scholar]
- 45.Papa, M. & Segal, M. (1996) Neuroscience 71, 1005–1011. [DOI] [PubMed] [Google Scholar]