Abstract
SMC complexes include three major classes: cohesin, condensin and SMC5/6. However, the localization pattern and genetic requirements for the SMC5/6 complex during mammalian oogenesis have not previously been examined. In mouse oocytes, the SMC5/6 complex is enriched at the pericentromeric heterochromatin, and also localizes along chromosome arms during meiosis. The infertility phenotypes of females with a Zp3-Cre-driven conditional knockout (cKO) of Smc5 demonstrated that maternally expressed SMC5 protein is essential for early embryogenesis. Interestingly, protein levels of SMC5/6 complex components in oocytes decline as wild-type females age. When SMC5/6 complexes were completely absent in oocytes during meiotic resumption, homologous chromosomes failed to segregate accurately during meiosis I. Despite what appears to be an inability to resolve concatenation between chromosomes during meiosis, localization of topoisomerase IIα to bivalents was not affected; however, localization of condensin along the chromosome axes was perturbed. Taken together, these data demonstrate that the SMC5/6 complex is essential for the formation of segregation-competent bivalents during meiosis I, and findings suggest that age-dependent depletion of the SMC5/6 complex in oocytes could contribute to increased incidence of oocyte aneuploidy and spontaneous abortion in aging females.
KEY WORDS: SMC5/6, Cohesin, Condensin, Meiosis, Chromosome segregation, Aneuploidy
Summary: The SMC5/6 complex is essential for female fertility in mice, controlling chromosome condensation and the formation of segregation-competent bivalents during meiosis I in mouse oocytes.
INTRODUCTION
Meiosis is a specialized cell division required for the formation of haploid gametes. Following pre-meiotic DNA replication, homologous chromosomes pair and recombine. DNA recombination occurs within the context of a proteinaceous scaffold known as the synaptonemal complex (SC), which ensures close juxtaposition of homologs (Handel and Schimenti, 2010). After desynapsis, homologous chromosomes remain linked via chiasmata, which are a visible manifestation of crossover recombination. Chiasmata are biologically essential as they ensure that homologous chromosomes bi-orient and thus segregate from each other during the first meiotic division (meiosis I). Subsequently, sister chromatids segregate during meiosis II, resulting in the formation of haploid gametes.
Regulation of meiosis is sexually dimorphic in mammals. Research using the mouse as a model has helped to delineate the dimorphic features that are also observed in humans. In most male mammals, meiosis is initiated postnatally, with continual production of spermatocytes undergoing meiosis throughout life. In female mice, meiosis is initiated during fetal development but arrests in a prolonged diplotene, or dictyate, stage of prophase I. Cohorts of dictyate stage oocytes begin growth shortly after birth and meiosis does not resume in vivo until after the preovulatory surge of luteinizing hormone (LH) in post-pubescent mice. However, fully grown oocytes undergo spontaneous, LH-independent, resumption of meiosis after isolation and culture under supportive conditions (Pincus and Enzmann, 1935). Meiosis, whether occurring in vivo or in vitro, becomes arrested again after progression to metaphase II and is completed only after fertilization or parthenogenic activation.
Cohorts of oocytes resume meiosis throughout the reproductive lifespan and therefore can reflect aging effects. As women age, their oocytes become more susceptible to chromosome missegregation, which can lead to infertility and developmental abnormalities (Hassold and Hunt, 2001). Therefore, it is important to determine the molecular pathways that are prone to error in oocytes, especially the proteins required for monitoring and facilitating chromosome segregation (MacLennan et al., 2015).
The structural maintenance of chromosomes (SMC) complexes are important regulators of chromosome dynamics and structure during mitosis and meiosis. Each member of the SMC family, which includes cohesin, condensin and SMC5/6, comprises a V-shaped SMC protein heterodimer. The SMC proteins each have a hinge domain that is flanked by long coiled-coil domains, which allows the proteins to fold back on themselves. The C and N globular heads interact with each other, forming an ATP-binding and ATP hydrolysis site. The ATPase domains are bridged together by non-SMC elements (Nasmyth and Haering, 2005).
Cohesin is a SMC1/3 heterodimer that is linked by an α-kleisin and a stromal antigen protein. During mitosis, cohesin is required to maintain sister chromatid cohesion before the metaphase-to-anaphase transition (Remeseiro and Losada, 2013). However, to ensure that sister chromatids segregate together during meiosis I, centromeric cohesin is maintained until meiosis II (Petronczki et al., 2003). In addition, cohesin complexes are required for accurate recombination and synapsis between homologous chromosomes (Rankin, 2015). Meiosis-specific cohesin components, including SMC1β, two α-kleisins (REC8 and RAD21L) and a stromal antigen protein (STAG3), are important for these additional requirements of cohesins during meiosis (Bannister et al., 2004; Fukuda et al., 2014; Herrán et al., 2011; Hopkins et al., 2014; Llano et al., 2014; Revenkova et al., 2004; Winters et al., 2014; Xu et al., 2005). Mutation of meiosis-specific cohesin components in female mice results in an increased frequency of oocyte aneuploidy and premature ovarian failure (Herrán et al., 2011; Hodges et al., 2005; Murdoch et al., 2013).
The two condensin complexes (I and II) are composed of the SMC2 and SMC4 heterodimers, but their kleisin subunit and pair of HEAT repeat elements are unique (Hirano, 2015). Condensins localize to the longitudinal axes of bivalents following meiotic resumption in mouse oocytes, and both complexes are required for chromosome compaction before meiosis I (Houlard et al., 2015; Lee et al., 2011). However, only condensin II is essential for disentanglement of chromosomes prior to their segregation.
SMC5/6 heterodimers are linked by NSMCE4, a kleisin subunit (Verver et al., 2015). Two additional subunits, NSMCE1 and NSMCE3, interact with one another and with NSMCE4 (Palecek et al., 2006; Pebernard et al., 2008). NSMCE1 contains a RING-finger domain, common to E3 ubiquitin ligases, and NSMCE3 contains a MAGE (melanoma-associated antigen gene) domain. NSMCE3 enhances the E3 ubiquitin ligase activity of NSMCE1 (Doyle et al., 2010). NSMCE2, which contains an SP-RING domain, binds to the coiled-coil region of SMC5 and can function as an E3 SUMO ligase (Andrews et al., 2005; Potts and Yu, 2007; Zhao and Blobel, 2005).
Studies assessing the SMC5/6 complex in mammalian germ cells have been limited to analyses of its localization pattern during mammalian spermatogenesis (Gómez et al., 2013; Verver et al., 2014, 2013). Because the regulation of meiosis is sexually dimorphic, there may be temporal and functional differences in the roles of SMC5/6 in females versus males. This study demonstrates that the SMC5/6 complex is enriched at the pericentromeric regions and is also detected along chromosome arms during female meiosis. To determine the function of the SMC5/6 complex following meiotic resumption in mouse oocytes, an oocyte-specific conditional knockout (cKO) mouse was created, deleting a floxed Smc5 allele using the Zp3-Cre transgene, which is expressed in growing oocytes before meiotic resumption (Lan et al., 2004; Lewandoski et al., 1997). Analysis of the female Smc5 cKO mutants led to two major findings: (1) maternal expression of SMC5 before meiotic resumption is essential for embryogenesis; and (2) absence of SMC5/6 during meiotic resumption results in oocyte aneuploidy due to an inability to resolve chromosomes during meiosis I. Furthermore, protein levels of SMC5/6 components in oocytes decline as wild-type females age, implicating the SMC5/6 complex as a potential contributor to oocyte aneuploidy and infertility in aging females.
RESULTS
SMC5/6 is enriched at oocyte pericentromeric heterochromatin during meiosis
Chromatin spreads were prepared to assess the localization of the SMC5/6 complex during female meiosis via immunofluorescence microscopy with antibodies raised against SMC5, SMC6 and NSMCE1 (Fig. 1; Fig. S1). Meiotic prophase sub-stages were determined by assessing chromosome axis morphology (synaptonemal complex protein, SYCP3) and centromere pairing (anti-centromere autoantibody, CEN; also known as ACA and CREST). During leptonema, SMC6 localized throughout the spread chromatin (Fig. 1A). By early zygonema, SMC6 was enriched at pericentromeric heterochromatin. At pachynema, SMC6 remained enriched at pericentromeric heterochromatin, and was also evident at lower intensity along the arms of chromosomes. These localization patterns were partially resistant to DNase treatment (Fig. S2). Additionally, SMC6 was observed as foci along chromosome axes and chromosome ends (Fig. 1B). SMC6 foci were not always evident on pachytene stage chromatin spreads, and did not overlap with MLH1 foci (Fig. S3), suggesting that they may be transient and stage specific. At early diplonema, SMC6 remained enriched at the pericentromeric heterochromatin; however, this enrichment was decreased by late diplonema. Analysis of SMC5, NSMCE1 and an additional antibody raised against SMC6 resulted in similar localization patterns (Fig. S1). Differences in localization patterns are likely to be due to epitope accessibility, as is the case with mouse prophase spermatocytes (Gómez et al., 2013), SMC6 localization to the pericentromeric heterochromatin in oocytes overlaps with that observed for TOP2A (Fig. 1C).
Fig. 1.
SMC5/6 localization during female meiosis. (A-C) Chromatin spread preparations of wild-type ovarian germ cells at different stages of meiotic prophase I. (A,B) Immunolabeled with antibodies against CEN (blue, kinetochore/centromere marker), the SC lateral element protein SYCP3 (red) and SMC6 (green, ab18039). (B) SMC6 localization on the pericentromeric heterochromatin, along chromosome arms and foci on chromosome axes during pachynema. (C) TOP2A (green) localization at pachynema. (D) Wild-type metaphase I whole oocyte preparation; DAPI (DNA, blue), SMC6 (green, ab18039), α-tubulin (red) and CEN (red). (E) Wild-type metaphase I chromatin spread; DAPI (blue), SMC6 (red, ab18039) and CEN (green). (F) Chromatin spread of an embryonic ovarian germ cell from a Rec8 mutant; SYCP3 (red) and SMC6 (green, ab18039). Boxed regions in B,C,E are magnified three times. Complementary data using additional SMC5/6 antibodies in Fig. S1. Scale bar: 10 µm.
Following meiotic resumption, SMC6 was enriched at the pericentromeric heterochromatin during meiosis I and remained present at metaphase II (MII), when oocytes arrest (Fig. 1D). Chromosome spread preparations of metaphase I (MI) oocytes demonstrated that there was also some SMC6 protein along chromosome arms (Fig. 1E).
Contrasting data have been reported on whether mutation of cohesin component, REC8, affects Smc5/6 axis loading during meiosis in budding yeast (Copsey et al., 2013; Lilienthal et al., 2013). Localization of SMC6 was assessed using a Rec8 mouse mutant (Bannister et al., 2004). The enrichment of SMC6 to the pericentromeric heterochromatin and localization to chromosome arms was not affected in Rec8 mutants (Fig. 1F), demonstrating that REC8 was not required for SMC6 localization. This finding is supported by observations made using mouse spermatocytes, where mutation of Smc1β did not affect SMC5/6 localization (Gómez et al., 2013).
Oocyte-specific conditional mutation of Smc5 results in infertility
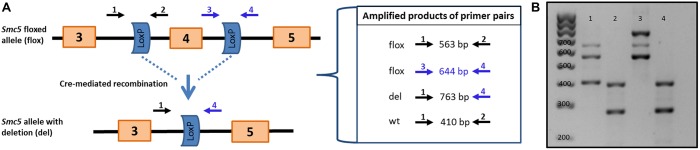
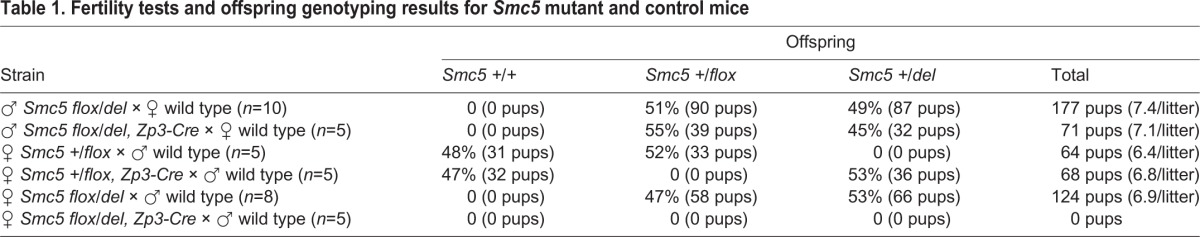
Mice that harbored a conditional knockout (cKO) allele of Smc5 were used to assess the requirement of the SMC5/6 complex for the meiotic divisions and formation of blastocysts (Fig. 2A,B, see Materials and Methods). Exon 4 of Smc5 was flanked by loxP Cre recombinase target sequences and this allele was termed Smc5 flox (Fig. 2A). Breeding heterozygous Smc5 flox mice to mice expressing the Cre recombinase transgene generated a KO allele termed Smc5 del. The heterozygous Smc5 del mice exhibited no gross morphological abnormalities during development and adult life. No offspring homozygous for the Smc5 del mice allele were produced, indicating that homozygosity for the deletion allele is lethal. Therefore, to determine whether Smc5 is essential for oogenic meiotic divisions, a hemizygous Cre recombinase transgene under the control of the promoter for the zona-pelucida protein 3 gene (Zp3-Cre tg/0) was used. This transgene is expressed exclusively in growing dictyate oocytes before resumption of the first meiotic division (Lan et al., 2004; Lewandoski et al., 1997). Breeding Smc5 +/flox, Zp3-Cre tg/0 (control) females to wild-type males showed that mutation of the Smc5 flox allele mediated by Zp3-Cre was 100% efficient (Table 1). The Smc5 flox/del, Zp3-Cre tg/0 (Smc5 cKO) females failed to produce litters (n=5), despite having normal ovarian morphology and equivalent oocyte numbers (Fig. S4A,B).
Fig. 2.
Conditional mutation of Smc5 using the Zp3-Cre recombinase results in female infertility. (A) Schematic of mouse Smc5 floxed allele containing loxP sites, flanking exon 4 and the resulting Smc5-deleted allele after excision of exon 4 by Cre recombinase. Arrows represent primers for PCR genotyping of mice. (B) DNA agarose gel image of PCR products for genotyping. Lanes 1 and 2 represent a control genotype (Smc5 +/flox, Zp3-cre tg/0). Lane 1 (Smc5 +/flox): 410 bp wild-type allele, 563 bp and 644 bp flox allele. Lane 2 (Zp3-cre tg/0): 420 bp internal control and 281 bp Zp3-Cre transgene. Lanes 3 and 4 represent Smc5 cKO (Smc5 flox/del, Zp3-cre tg/0). Lane 3 (Smc5 flox/del): 563 bp and 644 bp flox allele, and 763 bp del allele. Lane 4 (Zp3-cre tg/0): 420 bp internal control and 281 bp Zp3-Cre transgene (same as lane 2).
Table 1.
Fertility tests and offspring genotyping results for Smc5 mutant and control mice
Smc5 cKO oocytes are incapable of mature blastocyst formation following IVF
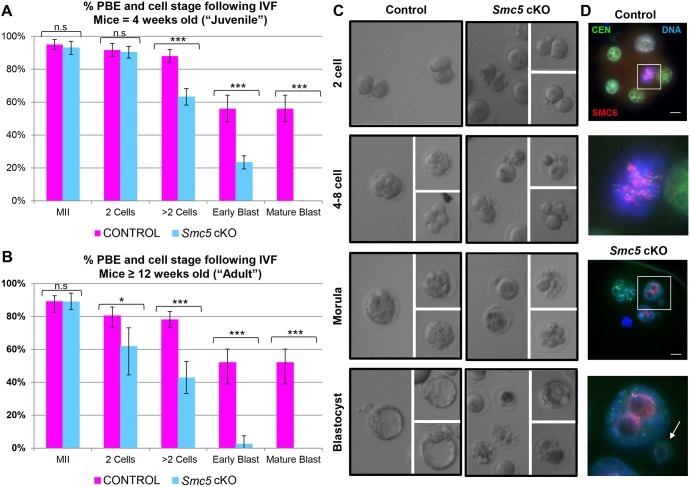
In vitro oocyte maturation (IVM) and fertilization (IVF) was used to determine whether blastocysts could be obtained from Smc5 cKO oocytes. Fully grown germinal vesicle (GV) oocytes were isolated from the large antral follicles of Smc5 cKO (Smc5 flox/del, Zp3-Cre tg/0) and control (Smc5 +/flox, Zp3-Cre tg/0) female ovaries aged between 4 and 12 weeks, and cultured in media that supported meiotic resumption in vitro (IVM). There was no observable delay in GV breakdown (GVBD), indicative of meiotic resumption (Fig. S4C), and likewise no reduction in frequency of oocytes that underwent polar body extrusion (PBE) and metaphase II (MII) arrest (Fig. 3A,B). However, following IVF using sperm from a wild-type mouse, fertilized oocytes from Smc5 cKO females failed to form mature blastocysts, with many embryos arresting at the 4- to 16-cell stages (Fig. 3A-C). Intriguingly, there was a difference in IVF results between oocytes from mice that were 4 weeks of age (considered as the ‘juvenile’ cohort), and mice that were between 12 and 16 weeks of age (considered the ‘adult’ cohort). In the ‘juvenile’ cohort, fertilized oocytes progressed to the 2-cell stage at levels comparable with their littermate controls (Fig. 3A). By contrast, the cohort of ‘adult’ fertilized oocytes displayed a significant decrease in 2-cell stage embryos following IVF (Fig. 3B). In addition, although there was a significant decrease in embryos progressing beyond the 2-cell stage compared with the littermate control, the ‘juvenile’ cohort of embryos collectively progressed further than the ‘adult’ cohort (Fig. 3A,B). Embryos from the ‘juvenile’ cohort were assessed via light and immunofluorescence microscopy. Cells and nuclei from the control embryos displayed similar shape and size, and the nuclei harbored an SMC6 signal (Fig. 3C,D; Fig. S5). By contrast, embryos from the Smc5 cKO embryos contained low or undetectable levels of SMC6 protein, and nuclei were irregular in size, which is consistent with defects during mitosis and imbalanced chromosome segregation during cell division.
Fig. 3.
Smc5 cKO oocytes fail to form mature blastocysts. (A-C) Assessment of PBE and blastocyst formation following IVF. (A) PBE and IVF data obtained for ‘juvenile’ (4 weeks of age) control (123 oocytes, Smc5 +/flox, Zp3-Cre tg/0) and Smc5 cKO (150 oocytes, Smc5 flox/del, Zp3-Cre tg/0). P values (one-tailed paired t-test) for the indicated comparisons are P=0.0771 (n.s.), MII; P=0.1085 (n.s.), 2 cells; ***P=0.0003, >2 cells; ***P<0.0002, early blastocysts; and ***P<0.0001, mature blastocysts. (B) PBE and IVF data obtained for the ‘adult’ (≥12 weeks of age) control (105 oocytes) and Smc5 cKO (134 oocytes). The error bars represent the variation between three independent experiments. The P values (one-tailed paired t-test) for the indicated comparisons are P=0.4287 (n.s.), MII; *P=0.0282, 2 cells; ***P=0.0004, >2 cells; ***P<0.0001, early blastocysts; and ***P<0.0001, mature blastocysts. (C,D) Example images of cell morphology following IVF for control and Smc5 cKO. (D) Embryos stained with DAPI (blue, DNA), SMC6 (red) and CEN (green). Boxed regions are magnified three times. Arrow indicates a nucleus with irregular size. Collectively, IVF was performed six times using a total of 10 mice for each control and Smc5 cKO cohort. Scale bars: 10 µm.
Only the ‘adult’ Smc5 cKO oocytes display aneuploidy at metaphase II
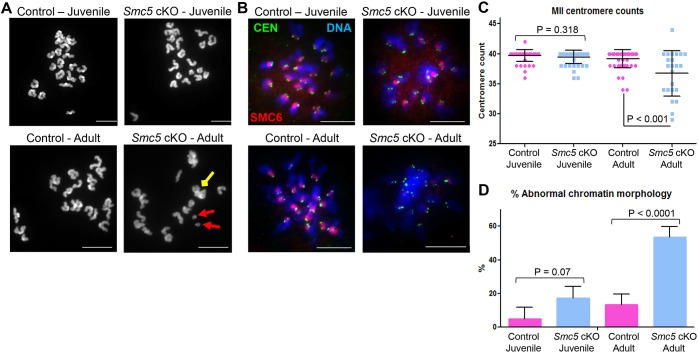
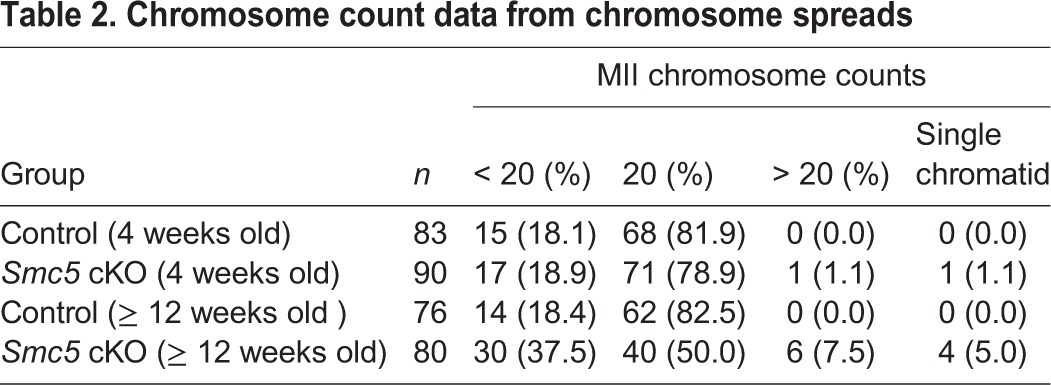
To determine whether the observed failure to form blastocysts was due to defects in chromosome segregation during meiosis, the number and morphology of chromosomes in oocytes arrested at MII were assessed. Owing to the age-related differences observed in the IVF studies, MII oocytes from ‘juvenile’ and ‘adult’ mice were assessed separately. MII chromosome spread preparations of the ‘juvenile’ Smc5 cKO oocytes did not exhibit significant increases in aneuploidy or chromosome abnormalities (Fig. 4A, Table 2). By contrast, chromosome spread preparations from the ‘adult’ Smc5 cKO females displayed abnormal chromosome number and morphology, and separated sister chromatids were observed (Fig. 4A, Table 2). Chromosome number and morphology were also assessed within the confines of the cell by treating the oocytes with monastrol. Monastrol binds to and disrupts the function of the kinesin protein KIF11, resulting in monopolar spindles making it easier to distinguish each sister chromatid pair (Stein and Schindler, 2011). Centromere number was counted using an anti-centromere autoantibody (CEN). In addition, the presence of the SMC5/6 complex was determined using an SMC6 antibody. Complementary to the chromosome spread preparations (Fig. 4A, Table 2), the monastrol-treated MII oocytes from the ‘juvenile’ Smc5 cKO cohort did not exhibit significant differences compared with the control oocytes with respect to centromere counts or chromatin morphology (Fig. 4B-D). Furthermore, most (83%) of the oocytes from the ‘juvenile’ Smc5 cKO harbored SMC6 protein signal. By contrast, the majority (61%) of monastrol treated oocytes from the ‘adult’ Smc5 cKO cohort lacked SMC6 signal, and presented significant differences with regards to centromere counts compared with littermate controls (Fig. 4B,C; Fig. S6A). Additionally, it was not possible to obtain centromere counts from more than 50% of the monastrol-treated Smc5 cKO oocytes from the ‘adult’ mice, because the chromatin was grossly abnormal, demonstrating stretched morphology, and indistinguishable sister chromatid pairs (Fig. 4B-D; Fig. S6A). Furthermore, 5% of Smc5 cKO MII oocytes displayed abnormal morphology that was indicative of oocyte degeneration (Fig. S6B,C).
Fig. 4.
Metaphase II oocytes from ‘adult’ Smc5 cKO have aneuploidy and abnormal chromosome morphology. Control (Smc5 +/flox, Zp3-Cre tg/0) and Smc5 cKO (Smc5 flox/del, Zp3-Cre tg/0) oocytes arrested at MII were assessed for chromosome number, centromere number and chromosome morphology using two separate age groups (‘juvenile’=4 weeks old, and ‘adult’ ≥12 weeks old). (A) Examples of chromosome spreads of control and Smc5 cKO MII oocytes. Red arrows indicate single chromatids and the yellow arrow shows an example of abnormal chromosome morphology. (B) MII oocytes treated with monastrol; DAPI (blue, DNA), SMC6 (red) and CEN (green). (C) Scatter dot-plot graph of centromere counts from monastrol-treated MII oocytes obtained from ‘juvenile’ cohorts of control (average=39.6, n=50) and Smc5 cKO mice (average=39.4, n=50) and ‘adult’ cohorts of control (average=39.1, n=50) and Smc5 cKO mice (average=36.7, n=25). Mean and standard deviation of the columns of each graph are represented by the black bars and P values are given for indicated comparisons (Mann–Whitney, two-tailed test). (D) Bar graph of percentage of oocytes with abnormal chromosome morphology from ‘juvenile’ cohorts of control (average=4.84%, n=62) and Smc5 cKO mice (average=17.24%, n=50), and ‘adult’ cohorts of control (average=13.25%, n=83) and Smc5 cKO mice (average=53.49%, n=86). Mean and standard error measurement of the columns of each graph are represented by the black bars and P values are given for indicated comparisons (chi-squared test). Collectively, at least 10 mice for each group were used to obtain the data. Scale bars: 10 µm.
Table 2.
Chromosome count data from chromosome spreads
Oocyte SMC5/6 protein levels decrease in aging females
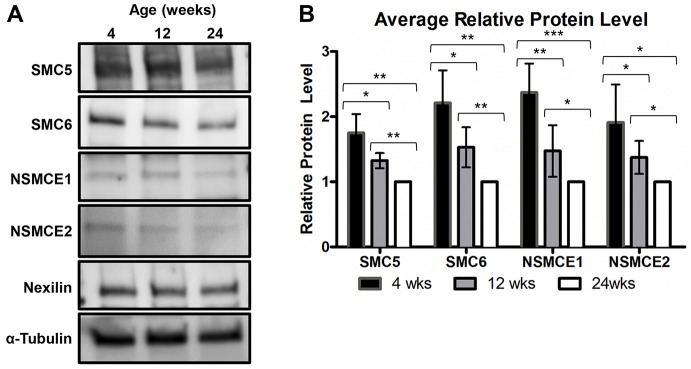
Excision of the floxed 4th exon of Smc5 driven by the Zp3-Cre transgene was shown to be 100% efficient based on mating tests, PCR analysis and the IVF data (Fig. 2B,C, Fig. 3A). However, data from monastrol-treated MII oocytes demonstrated that the SMC6 protein was still present in most oocytes of the ‘juvenile’ Smc5 cKO cohort (Fig. 4B). These data suggest that SMC5/6 protein levels present before Cre-mediated deletion of Smc5 are sufficient to support proficient meiosis, but not embryogenesis. Furthermore, the majority of oocytes from the ‘adult’ cohort do not harbor residual SMC6 protein, and fail to form chromosomally normal MII oocytes (Fig. 4, Table 2). As fertility and genome integrity are negatively correlated with age, it can be postulated that SMC5/6 levels within GV oocytes of wild-type mice may decrease with age. To test this hypothesis, oocyte protein extracts from three groups of C57BL6/J wild-type mice aged 4, 12 and 24 weeks were assessed for SMC5, SMC6, NSMCE1 and NSMCE2 protein levels (Fig. 5A,B). From this analysis, it was determined that protein levels for all four SMC5/6 components decreased significantly in oocytes isolated from older mice.
Fig. 5.
SMC5/6 protein levels decrease in oocytes as mice age and Smc5 is essential for embryogenesis. (A,B) Protein was extracted from oocytes of wild-type C57BL6/J mice that were 4, 12 and 24 weeks of age. (A) Protein extracts from 150 oocytes from wild-type C57BL/6J mice were loaded onto each lane of a 4-15% SDS PAGE gradient gel and assessed for SMC5, SMC6, NSMCE1 and NSMCE2 protein levels. Nexilin and α-tubulin were loading controls. (B) Bar graph of the average relative protein levels for each SMC5/6 complex component. Protein band signal intensities were normalized against the nexilin loading control. Error bars represent standard deviation. Two-tailed paired t-tests were performed to compare each group and P values were defined as *P<0.05, **P<0.005 and ***P<0.0005. The data are based on five sets of 150 oocytes isolated from three separate rounds of oocyte harvest.
Smc5 is a maternal-effect gene
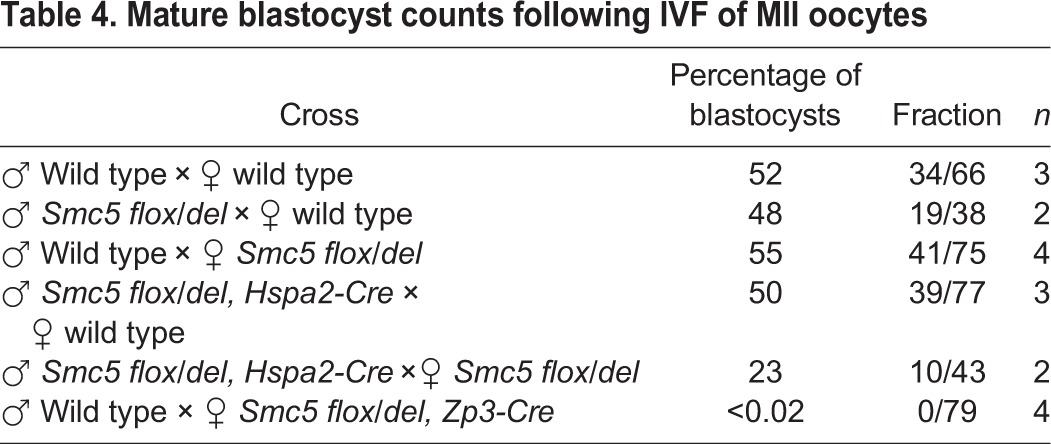
As there were residual levels of SMC6 detected in the oocytes isolated from ‘juvenile’ Smc5 cKO mice, it was hypothesized that SMC5/6 levels were adequate to facilitate chromosome segregation during meiosis, but was insufficient for sustaining proper mitotic segregation during the early embryogenesis. To further assess the relationship between Smc5 mutation and the capacity to form mature blastocysts, wild-type, heterozygous Smc5 del male and female mice were used for IVF to test effects of paternal versus maternal inheritance of the mutant allele. The oocytes used in these assays were from 4-week-old mice, and therefore equivalent to the designated ‘juvenile’ age group. In addition, Smc5 cKO male mice (Smc5 flox/del, Hspa2-Cre tg/0), which are fertile and produce sperm that almost exclusively carry the Smc5 del allele, were used for IVF. Based on mating tests with C57BL6/J wild-type females, 98% of progeny from the Smc5 flox/del, Hspa2-Cre males carry the Smc5 del allele (Table 3). When sperm from the heterozygous Smc5 del and Smc5 flox/del, Hspa2-Cre males were combined with wild-type oocytes the levels of mature blastocysts obtained were equivalent to the wild-type IVF (Table 4), showing that presence of the paternally inherited Smc5 del allele does not affect early embryogenesis. When female heterozygous Smc5 del oocytes were fertilized with wild-type sperm, levels of mature blastocysts were equivalent to the wild-type IVF results, suggesting that the expression of Smc5 during oocyte growth is essential for supporting early stages of embryogenesis. When the heterozygous Smc5 del oocytes were fertilized with sperm from the Smc5 flox/del, Hspa2-Cre males, the level of blastocysts obtained reduced by approximately half, which supports the fact that early stages of embryonic development are affected in embryos homozygous for mutation of Smc5. Homozygous mutation of other components of the SMC5/6 complex, Smc6 and Nsmce2, have also been shown to cause embryonic lethality (Jacome et al., 2015; Ju et al., 2013). Taken together with the IVF and MII data obtained for the ‘juvenile’ Smc5 cKO females (Figs 3 and 4, Table 2; Fig. S5), these results suggest that Smc5 expression during oocyte growth, before meiotic resumption, is crucial for embryogenesis and, therefore, Smc5 is a maternal-effect gene.
Table 3.
Mating test and genotyping data for Smc5 flox/del (control) and Smc5 flox/del, Hspa2-Cre cKO males mated to C57BL6/J females
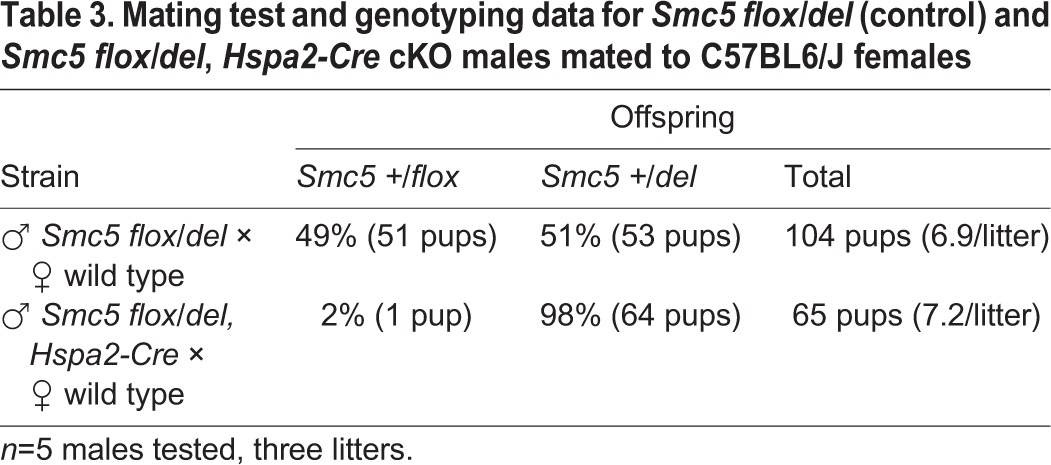
Table 4.
Mature blastocyst counts following IVF of MII oocytes
Oocyte-specific cKO of Smc5 causes chromosome stretching during meiosis I
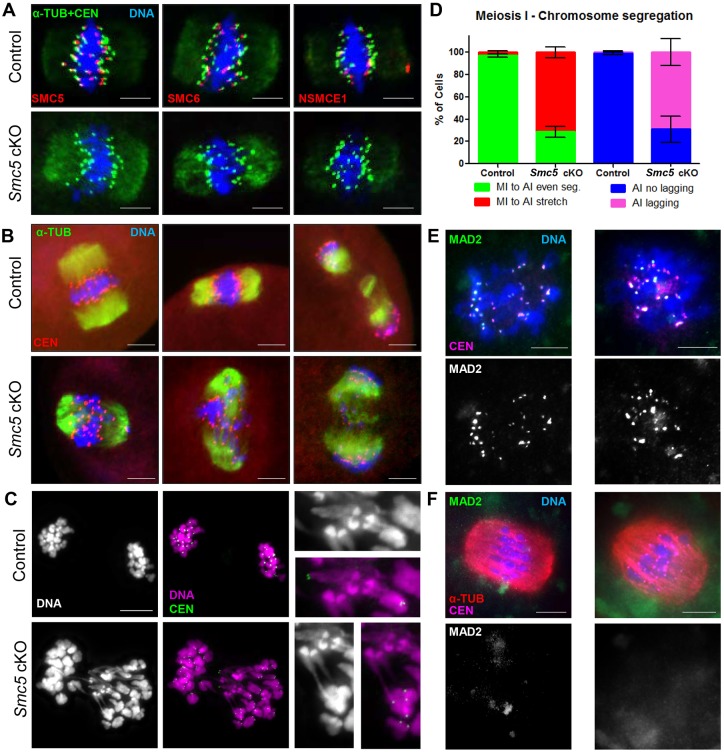
Because abnormal chromosome morphology was observed in oocytes from the ‘adult’ Smc5 cKO group at metaphase II arrest, it is possible that chromosome morphology and segregation earlier, during meiosis I, was perturbed. The localization of SMC5/6 components in the Smc5 cKO and control oocytes during meiosis I was assessed. SMC5/6 components SMC5, SMC6 and NSMCE1 were enriched at the pericentromeric heterochromatin during the metaphase-to-anaphase I transition in control oocytes, but were absent in the Smc5 cKO oocytes (Fig. 6A). Oocytes were assessed during the metaphase-to-anaphase I transition (Fig. 6B-D). In the majority (95%, n=144) of the control oocytes, proficient segregation of homologous chromosomes was observed. In sharp contrast, the majority (62%, n=220) of Smc5 cKO experimental oocytes displayed chromosome stretching and lagging chromosomes. The severe chromatin stretching observed between homologous chromosomes (Fig. 6C) suggests that deletion of Smc5 prevented decatenation of homologous chromosomes.
Fig. 6.
Smc5 cKO oocytes display lagging and stretched chromosomes during meiosis I. (A) Metaphase I oocytes from control (Smc5 +/flox, Zp3-Cre tg/0) and Smc5 cKO (Smc5 flox/del, Zp3-Cre tg/0) mice, DAPI (blue, DNA), α-tubulin (green, α-TUB), CEN (green) and a subunit of the SMC5/6 complex (SMC5, SMC6, NSMCE1, red). (B) Oocytes transitioning from metaphase I to anaphase I; DAPI (blue, DNA), α-TUB (green) and CEN (red). (C) Smc5 cKO oocyte undergoing metaphase I to anaphase I transition displaying chromatin stretching; DAPI (purple, DNA) and CEN (green). Images with a 3× magnification of chromosome stretches on the right. (D) Bar graph of percentages of oocytes (n=104 for control and n=167 for Smc5 cKO) showing even metaphase I to anaphase I chromosome segregation (MI to AI even), chromosome stretching (MI to AI stretch); and in anaphase I (n=40 for control and n=53 for Smc5 cKO) showing no lagging chromosomes (AI no lagging) and lagging chromosomes (AI lagging). The P values (one-tailed paired t-test) for the indicated comparisons are P=0.004 (MI to AI) and P=0.0078 (AI). (E) Pro-metaphase I and (F) metaphase I oocytes were stained with DAPI (blue, DNA), MAD2 (green) and CEN (purple). Collectively, at least 10 mice for each group were used to obtain the data. Scale bars: 10 µm.
Given the meiotic abnormalities described above, the spindle assembly checkpoint (SAC) was assessed in the Smc5 cKO oocytes. SAC satisfaction during the metaphase to anaphase I transition was indirectly determined by assessing the SAC protein MAD2, which normally localizes to kinetochores during prometaphase, and remains there until ubiquitous bipolar microtubule-kinetochore attachment satisfies the SAC (Lara-Gonzalez et al., 2012). MAD2 staining was present at the kinetochores at prometaphase in both control and Smc5 cKO oocytes (Fig. 6F). MAD2 signal at the kinetochore was absent in both control and Smc5 cKO oocytes undergoing the metaphase to anaphase I transition (Fig. 6G). These observations suggest that mutation of Smc5 does not affect the temporal pattern of MAD2 localization, and therefore may not affect SAC function, consistent with the lack of MI oocyte arrest. Additionally, a cell cycle kinase, PLK1, localized to kinetochores in control and Smc5 cKO oocytes (Fig. S7).
Absence of the SMC5/6 complex causes aberrant localization of condensin
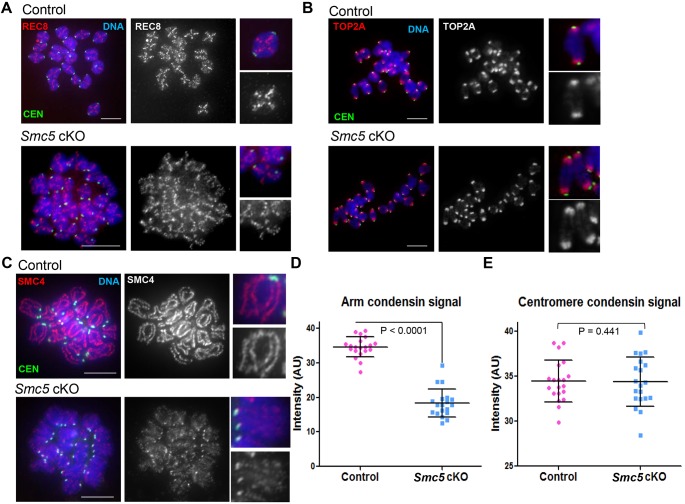
Premature depletion of REC8 before the meiosis I division in oocytes is associated with chromosome missegregation (Chiang et al., 2010; Tachibana-Konwalski et al., 2010). Therefore, localization of REC8 was assessed using metaphase I chromosome spreads. REC8 was present along the axes of the bivalents in control and Smc5 cKO oocytes from ‘adult’ mice, with no apparent difference between them (Fig. 7A). These results suggest that mutation of Smc5 before meiotic resumption does not significantly affect localization of REC8-containing cohesins.
Fig. 7.
Condensin signal is reduced along chromosome arms in Smc5 cKO oocytes during meiosis I. (A-C) Chromatin spreads of metaphase I oocytes from control (Smc5 +/flox, Zp3-Cre tg/0) and Smc5 cKO (Smc5 flox/del, Zp3-Cre tg/0) mice. Metaphase I chromosomes were stained with DAPI (blue, DNA), CEN (green) and either (A) the cohesin component REC8, (B) topoisomerase IIα (TOP2A) or (C) the condensin component SMC4 in red. Representative chromosomes with a 3× magnification are present to the right of each chromosome spread. (D,E) Quantification of signal intensity of condensin (SMC4) signal along chromosome arms (D) and associated with the centromere (E) (overlapping with CEN). P values are given for indicated comparisons (Mann–Whitney, one-tailed test) (n=20). Scale bars: 10 µm.
SMC5/6 colocalizes with TOP2A in mouse oocytes (Fig. 1C), and similar to the Smc5 cKO oocytes from the ‘adult’ cohort, inhibition of TOP2A function results in severe defects in chromosome condensation and homologous chromosome separation (Li et al., 2013). Therefore, the effect of Smc5 cKO on the localization of TOP2A during meiosis I was determined using ‘adult’ mice. TOP2A was enriched at the pericentromeric regions in control oocytes, and was also detected along chromosome arms (Fig. 7B). No detectable change in TOP2A localization was observed in the Smc5 cKO oocytes (Fig. 7B).
Condensins are required to ensure chromosome segregation during meiosis I in mouse oocytes (Houlard et al., 2015). Similar to the results presented here for Smc5 cKO oocytes, conditional mutation of a condensin II component, Ncaph2, resulted in chromosome stretching during meiosis I due to an inability to disentangle chromosomes. To determine whether condensin localization is affected in the absence of the SMC5/6 complex, localization of the condensin I and II subunit SMC4 was assessed using ‘adult’ cohorts of mice. In control metaphase I chromosome spread preparations, SMC4 was present along the longitudinal axes of each bivalent (Fig. 7C). By contrast, in chromosome spread preparations from Smc5 cKO metaphase I oocytes, there was a significant reduction in SMC4 signal along chromosome arms (Fig. 7C,D). In addition, the SMC4 signal on chromosome arms was discontinuous and the normal linear pattern along chromosomes axes was difficult to distinguish. However, there was no apparent reduction in condensin signal that colocalized with the kinetochore/centromeric regions in Smc5 cKO metaphase I chromosome spread preparations compared with control (Fig. 7E).
DISCUSSION
This study of a genetic model for oocyte depletion of SMC5 has demonstrated that the SMC5/6 complex is essential for ensuring accurate chromosome segregation following meiotic resumption and during early embryogenesis. Furthermore, the data suggest that SMC5/6 complex protein levels diminish as mice age, and Smc5 is a maternal-effect gene.
SMC5/6 localization pattern implicates multiple functions during meiosis
SMC5/6 is enriched at the pericentromeric heterochromatin regions throughout meiosis in mouse oocytes, which is consistent with what was found in mouse spermatocytes (Gomez et al., 2013; Verver et al., 2013). The pericentromeric heterochromatin region consists of densely packed repetitive sequences and is at high risk of aberrant recombination events when double-strand breaks within these regions are repaired via homologous recombination (HR) (Goodarzi and Jeggo, 2012). SMC5/6 prevents HR within repetitive sequences such as rDNA in yeast, and heterochromatin in Drosophila mitotic cells (Torres-Rosell et al., 2007; Chiolo et al., 2011). Taken together, studies using mouse spermatocytes and oocytes suggest that SMC5/6 performs a similar function at the pericentromeric heterochromatin during meiosis (Gomez et al., 2013; Verver et al., 2013).
Although lower in signal intensity, SMC5/6 also localized throughout the chromatin during meiosis. This is consistent with what has been reported for mouse spermatocytes (Gomez et al., 2013; Verver et al., 2013). SMC5/6 was also visible along chromosome axes at pachynema in oocytes, which was also detected in mouse spermatocytes (Gomez et al., 2013). In addition, transient foci of SMC6 were detected along the chromosome arms in female germ cells during pachynema, suggesting a role during meiotic recombination, which has previously been reported using budding yeast and Caenorhabditis elegans (Bickel et al., 2010; Hong et al., 2016; Checchi et al., 2014; Copsey et al., 2013; Lilienthal et al., 2013; Xaver et al., 2013). In mammals, every chromosome pair obtains many recombination sites but generally yields only one to two crossover sites (Kauppi et al., 2004). Designations of which recombination sites become crossovers involve antagonistic roles between ubiquitin E3 ligase HEI10 and SUMO E3 ligase RNF212 (Reynolds et al., 2013; Qiao et al., 2014; Rao et al., 2017; Ahuja et al., 2017). It is possible that the SMC5/6 complex is a substrate of HEI10 and RNF212. Therefore, these SMC6 foci could indicate that SMC5/6 plays a role in regulating recombination during mammalian meiosis.
Differences between the Smc5 cKO oocytes isolated from ‘juvenile’ and ‘adult’ mice
SMC6 protein was detected in the majority of oocytes in the ‘juvenile’ Smc5 cKO cohort. However, SMC6 was not detected in the majority of ‘adult’ Smc5 cKO oocytes. As a consequence of this difference, oocytes from ‘juvenile’ Smc5 cKO mice progress to MII without aberrant chromosome configurations (Fig. 4, Table 2), whereas oocytes from ‘adult’ Smc5 cKO mice fail to accurately segregate chromosomes during meiosis I (Fig. 6). Despite evidence for proficient meiosis from analysis of MII ploidy and chromosome morphology in oocytes from the ‘juvenile’ Smc5 cKO cohort, these oocytes failed to form mature blastocysts when fertilized with sperm bearing a wild-type Smc5 gene. This failure to form mature blastocysts is attributed to aberrant chromosome segregation during mitosis (Fig. 3; Fig. S5). This phenotype is reminiscent of the mitotic catastrophe observed in Smc5 cKO mouse embryonic stem cells (Pryzhkova and Jordan, 2016).
The phenotypes observed and differences between ‘juvenile’ and ‘adult’ Smc5 cKO mice implies the following hypotheses. First, SMC5/6 protein levels before oocyte growth are important for proficient chromosome segregation during meiotic resumption (Figs 4 and 6, Table 2). Second, SMC5/6 protein levels present in oocytes diminish as mice age (Fig. 5, Tables 3 and 4). Third, there is a critical level of SMC5 protein that is required for proficient chromosome segregation during oocyte meiosis (Fig. 4, Table 2). Fourth, expression of Smc5 during the oocyte growth phase is crucial during early embryogenesis (Figs 3 and 4, Table 2).
SMC5/6 protein levels are diminished in aging oocytes
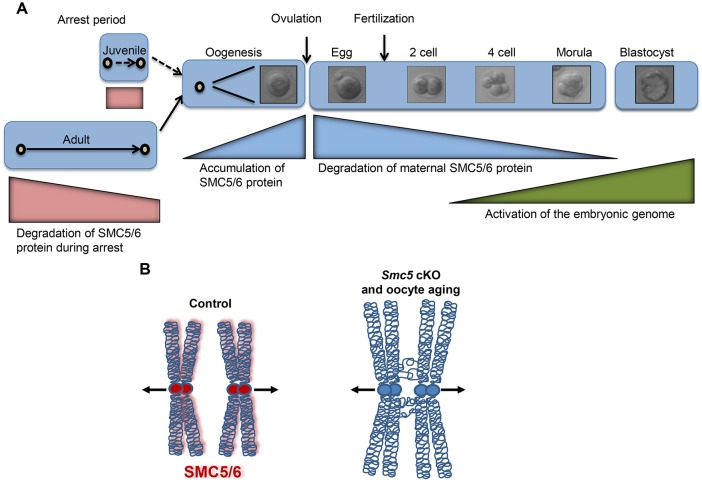
Frequency of meiotic segregation errors increases as women age, especially after the age of ∼35, resulting in dramatically increased incidence of miscarriage and birth defects (Hassold and Hunt, 2001). During the long prophase arrest that precedes meiosis I in female mammals, cohesin levels decline gradually and in aged oocytes this reduction in cohesin causes destabilization of chiasmata and separation of sister centromeres, which can result in chromosome missegregation during meiosis I (Lister et al., 2010; Tachibana-Konwalski et al., 2010; Tsutsumi et al., 2014). This current study determined that SMC5/6 protein levels decrease in oocytes isolated from older mice, and by correlation of phenotypes, this could also contribute to age-related aneuploidy and infertility (Fig. 8). Using an inducible transgene of Rec8, it was recently shown that cohesin is established in fetal oocytes during DNA replication, and there is no detectable turnover of cohesin in arrested oocytes, or during meiotic resumption (Burkhardt et al., 2016). Development of inducible, tagged version of an SMC5/6 component could be used to determine whether the SMC5/6 complex is replenished during meiotic resumption, or it remains stably associated with the chromatin for months following meiotic arrest.
Fig. 8.
Smc5 is a maternal-effect gene, and SMC5/6 is required for the formation of bivalent chromosomes capable of segregation during meiosis I in mouse oocytes. (A) SMC5/6 levels diminish in oocytes as mice age (red bars), leading to increased incidence of chromosome missegregation during meiosis. Regardless of age, maternal expression of SMC5 during oocyte maturation and early embryogenesis (blue bars), prior to activation of the embryonic genome (green bar), is essential for the formation of a functional blastocyst. (B) The SMC5/6 complex ensures that homologous chromosomes are accurately resolved and segregated during female meiosis I. Depletion of the SMC5/6 in aging oocytes may be a source of chromosome segregation error.
Heterozygous mutants of cohesin components lead to age-related increases in oocyte aneuploidy (Murdoch et al., 2013). Therefore, it is possible that a heterozygous mutation of a SMC5/6 component could lead to age-related errors during oogenesis too. Supporting this notion, it has been shown that heterozygous mutation of Nscme2 results in increased incidences of micronuclei and polynucleation in MEFs (Jacome et al., 2015).
Smc5 is a maternal-effect gene
Early stages of embryogenesis are almost entirely dependent on the oocyte for subcellular organelles and proteins before the robust activation of the embryonic genome at cleavage-stage development (Fig. 8A). These maternal proteins are encoded by maternal-effect genes (Li et al., 2010). Approximately, 45-50 maternal-effect genes have been identified in mammals, and many of these are involved in chromatin structure, modification and genome integrity (Zhang and Smith, 2015). Reduced levels of maternal-effect genes have been associated with the reduced oocyte developmental competence that is characteristic of ovarian aging (Guglielmino et al., 2011; Hamatani et al., 2004; Pan et al., 2008; Zhang and Smith, 2015). The IVF experiments presented in this study show that embryogenesis is aberrant only when Smc5 is mutated during the oocyte growth phase, and provision of a functional Smc5 gene from sperm is insufficient to facilitate embryogenesis. These data suggest that Smc5 is a maternal-effect gene in mouse. Recently, it was reported that smc5 and smc6 of Drosophila melanogaster are also maternal-effect genes (Tran et al., 2016), suggesting that this feature is conserved in many sexually reproducing organisms.
SMC5/6 may be required to assist condensin functions and TOP2A-dependent decatenation
Inhibition of TOP2A function in mouse oocytes and RNAi-mediated depletion in fly oocytes during meiosis I cause similar chromosome segregation defects observed in the Smc5 conditional knockout mouse oocytes (Hughes and Hawley, 2014; Li et al., 2013). Components of the SMC5/6 complex colocalize with TOP2A during prophase and following meiotic resumption in mouse oocytes. This is supported by previous observations made using mouse spermatocytes (Gómez et al., 2013). RNAi knockdown of SMC5 and SMC6 in human RPE-1 cells alters chromosomal localization properties of TOP2A (Gallego-Paez et al., 2013). Therefore, it was hypothesized that mutation of Smc5 would affect TOP2A localization in mouse oocytes. However, no defects in TOP2A localization were observed, which corresponds to what has been reported for Smc5 cKO in mouse embryonic stem cells (Pryzhkova and Jordan, 2016). Studies of yeast SMC5/6 have shown that the complex is linked with TopoII-dependent catenation/decatenation functions (Jeppsson et al., 2014; Kanno et al., 2015; Kegel et al., 2011). Furthermore, meiotic depletion of Top2 in budding yeast affects Smc5 localization (Copsey et al., 2013). Although TOP2A localization is unaffected by mutation of Smc5 in mouse oocytes, the functionality of TOP2A may still be affected.
Analysis of metaphase I chromosome spreads revealed that SMC5/6 is required for normal localization of condensin along chromosome arms. The phenotypes observed here for the Smc5 cKO mutant are reminiscent of the Ncaph2 condensin II cKO mutant (Houlard et al., 2015), as both display abnormal chromosome morphology, similar stretching of chromosomes and chromosome segregation defects during meiosis I. There is mounting evidence for a functional link between SMC5/6 and condensin. RNAi depletion of SMC5 and SMC6 in human RPE-1 cells resulted in defective axial localization of condensin (Gallego-Paez et al., 2013). Abnormal condensin localization was also observed using Smc5 cKO mouse embryonic stem cells (Pryzhkova and Jordan, 2016). Furthermore, mutation of smc-5 in C. elegans leads to abnormal distribution of condensin along bivalents during meiosis I (Hong et al., 2016). However, previous studies were not able to determine whether the defects in condensin localization were specific to the prophase to metaphase transition. Using the Zp3-Cre transgene to mutate Smc5 suggests that there is a functional relationship between condensin and SMC5/6 that is specific to meiotic resumption.
It has been shown that condensin and TOP2A activities are coordinated to ensure efficient chromosome condensation, sister chromatid decatenation and subsequent segregation in budding yeast (Charbin et al., 2014; Leonard et al., 2015). Based on the collective observations made using human and mouse systems, it is proposed that the aberrant localization of condensin observed in Smc5 mutant oocytes results in the loss of coordination between condensin and TOP2A, leading to an inhibition of chromosome resolution during meiosis (Fig. 8B).
Conclusions
The data demonstrate that SMC5/6 levels diminish in oocytes as mice age, leading to increased incidence of chromosome missegregation during meiosis (Fig. 8A). Furthermore, Smc5 is a maternal-effect gene and its expression during oocyte maturation is crucial for early stages of embryogenesis (Fig. 8A). The SMC5/6 complex ensures that chromosomes are accurately resolved and segregated during female meiosis (Fig. 8B), and influences the localization of condensing; based on published work, this likely affects the function of TOP2A. Like cohesin and condensin, the SMC5/6 complex is crucial to chromosome integrity in oocytes following their long arrested state. Protein levels of SMC5/6 components in oocytes are diminished in aging mice, suggesting that SMC5/6 levels are correlated with age-related oocyte and embryo chromosomal abnormalities. These data present the possibility that genetic and expression variations of SMC5/6 components are linked with fertility differences between individuals and defects may cause premature ovarian failure.
MATERIALS AND METHODS
Ethics statement
All mice were bred at The Jackson Laboratory (JAX) and Johns Hopkins University (JHU) in accordance with the National Institutes of Health and US Department of Agriculture criteria. Protocols for their care and use were approved by the Institutional Animal Care and Use Committees (IACUC) of JAX and JHU.
Mice
Mice harboring Smc5 with a floxed exon 4 (designated Smc5flox) and deleted exon 4 (designated Smc5del) have been previously described (Pryzhkova and Jordan, 2016). Heterozygous Smc5del mice were bred to mice harboring the Zp3-Cre transgene [C57BL/6-Tg(Zp3-cre)93Knw/J], which resulted in progeny heterozygous for the Smc5del allele and hemizygous for the Zp3-Cre transgene (Smc5+/del, Zp3-Cre tg/0). Male Smc5+/del, Zp3-Cre tg/0 mice were bred to homozygous Smc5flox female mice to derive Smc5 cKO (Smc5flox/del, Zp3-Cre tg/0) and control (Smc5+/flox, Zp3-Cre tg/0) genotypes. The Smc5flox/del genotype was used as an additional control. The same mating strategy was employed to create the male Smc5flox/del, Hspa2-Cre tg/0 cKO mice, using mice harboring the Hspa2-Cre transgene [C57BL/6-Tg(Hspa2-cre)1Eddy/J].
PCR genotyping
Primers used are described in Fig. 2 and Table S1. PCR conditions: 90°C for 2 min; 30 cycles of 90°C for 20 s; 58°C for annealing; and 72°C for 1 min.
Oocyte harvesting, culture and IVF
Female mice were injected intraperitoneally with 5 IU of equine chorionic (eCG; Sigma) to stimulate ovarian follicle development. GV-staged oocytes were harvested from ovaries 44 to 48 h later. Oocytes were cultured in MEMα medium supplemented with 5% fetal bovine serum (FBS; Gibco) and 3 mg/ml bovine serum albumin (BSA; Sigma-Aldrich). To harvest oocytes at metaphase II (MII) stage, mice were injected intraperitoneally with 5 IU of eCG (Sigma) and then with human chorionic gonadotropin (hCG; Sigma) 44-48 h later. After 15-16 h, MII oocytes were harvested from the ampulla of the oviduct. Ovulated oocyte-cumulus cell complexes were exposed to 300 IU/ml of hyaluronidase (Sigma) in MEMα medium supplemented with 3 mg/ml BSA to denude oocytes of surrounding cumulus cells.
For GVBD analysis, oocytes were harvested into MEMα medium supplemented with 5% FBS, 3 mg/ml BSA and 200 µM IBMX (Sigma-Aldrich). The oocytes were then washed and cultured in MEMα medium supplemented with 5% FBS, 3 mg/ml BSA and assessed for GVBD.
For IVF studies, eCG-primed oocytes were first cultured in MEMα medium supplemented with 5% FBS, 3 mg/ml BSA and 2.5 µl epidermal growth factor (EGF; 10 ng/ml) overnight. Following hyaluronidase treatment (Sigma), oocytes with a polar body indicative of progression to MII were counted. Oocytes were washed and cultured in MEMα medium supplemented with 3 mg/ml BSA and 10 µl of sperm extracted from an adult male mouse epididymis. Following IVF, oocytes were washed and cultured in KSOM media and observed each day to assess embryogenesis.
For monastrol treatment, MII oocytes were incubated in 10 mM monastrol (Sigma-Aldrich) in MEMα medium for 1.5 h at 37°C. Oocytes were washed in MEMα medium prior to fixation. All cultures were incubated at 37°C in a 5% CO2, 5% O2 and 90% N2 atmosphere.
Microscopy
Prophase-stage oocyte chromatin spreads, whole-oocyte and embryo mounts, MII chromosome spreads for chromosome number counts, and MI and MII chromosome spreads for immunofluorescence microscopy analyses were performed using techniques previously described (Susiarjo et al., 2009). Primary antibodies used and dilutions are listed in Table S2. Secondary antibodies against mouse, rabbit and human IgG and conjugated to Alexa 488, 568 or 633 (Life Technologies) were used at 1:500 dilution. Oocytes were then mounted with Vectashield+DAPI medium (Vector Laboratories) or Clearmount (Invitrogen). DNase I treatment, chromatin spreads were treated with 100 U/ml of DNase I in DNase I buffer (1% BSA, 10 mM MnCl2, 1 mM CaCl2, 50 mM Tris pH 7.5) for 1 h at 37°C prior to staining.
Images were captured using a Zeiss Cell Observer Z1 linked to an ORCA-Flash 4.0 CMOS camera (Hamamatsu) and analyzed with the Zeiss ZEN 2012 blue edition image software. Photoshop (Adobe) was used to prepare figure images.
Western blot analyses
Protein lysate from eCG-primed oocytes were isolated from C57BL6/J mice using methods previously described (Marangos, 2016). Protein extracts containing 150 oocytes were run on 4-15% gradient SDS PAGE gels (Bio-Rad) and transferred to PVDF membranes. Primary antibodies and dilutions used are presented in Table S2. At a 1:10,000 dilution, goat anti-mouse and goat anti-rabbit horseradish peroxidase-conjugated antibodies (Invitrogen) were used as secondary antibodies. Antibody signal was detected via treatment with Bio-Rad ECL western blotting substrate and captured using Syngene XR5 system. Protein levels were assessed using ImageJ (NIH).
Acknowledgements
We thank John Schimenti for providing the Rec8 mutant mouse and Karen Schindler for the REC8 antibody. We thank Marina Pryzhkova for technical assistance.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
G.H., J.J.E., M.A.H. and P.W.J. conceived the project and wrote the manuscript. Experiments performed by G.H., F.S., M.O. and P.W.J.
Funding
This work was supported by a UK-US Fulbright Commission Distinguished Scholar Award to P.W.J., by the National Institutes of Health (NIH) (K99/R00 HD069458 to P.W.J. and HD33816 to M.A.H.), by an institutional NIH Cancer Center award (CA34196 to The Jackson Laboratory, NIH R01 GM117155 to P.W.J.), and by a training grant fellowship from the National Cancer Institute (NIH) (CA009110) to G.H. Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at http://dev.biologists.org/lookup/doi/10.1242/dev.145607.supplemental
References
- Ahuja J. S., Sandhu R., Mainpal R., Lawson C., Henley H., Hunt P. A., Yanowitz J. L. and Börner G. V. (2017). Control of meiotic pairing and recombination by chromosomally tethered 26S proteasome. Science 355, 408-411. 10.1126/science.aaf4778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews E. A., Palecek J., Sergeant J., Taylor E., Lehmann A. R. and Watts F. Z. (2005). Nse2, a component of the Smc5-6 complex, is a SUMO ligase required for the response to DNA damage. Mol. Cell. Biol. 25, 185-196. 10.1128/MCB.25.1.185-196.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannister L. A., Reinholdt L. G., Munroe R. J. and Schimenti J. C. (2004). Positional cloning and characterization of mouse mei8, a disrupted allele of the meiotic cohesin Rec8. Genesis 40, 184-194. 10.1002/gene.20085 [DOI] [PubMed] [Google Scholar]
- Bickel J. S., Chen L., Hayward J., Yeap S. L., Alkers A. E. and Chan R. C. (2010). Structural maintenance of chromosomes (SMC) proteins promote homolog-independent recombination repair in meiosis crucial for germ cell genomic stability. PLoS Genet. 6, e1001028 10.1371/journal.pgen.1001028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhardt S., Borsos M., Szydlowska A., Godwin J., Williams S. A., Cohen P. E., Hirota T., Saitou M. and Tachibana-Konwalski K. (2016). Chromosome cohesion established by Rec8-cohesin in fetal oocytes is maintained without detectable turnover in oocytes arrested for months in mice. Curr. Biol. 26, 678-685. 10.1016/j.cub.2015.12.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charbin A., Bouchoux C. and Uhlmann F. (2014). Condensin aids sister chromatid decatenation by topoisomerase II. Nucleic Acids Res. 42, 340-348. 10.1093/nar/gkt882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Checchi P. M., Lawrence K. S., Van M. V., Larson B. J. and Engebrecht J. (2014). Pseudosynapsis and decreased stringency of meiotic repair pathway choice on the hemizygous sex chromosome of Caenorhabditis elegans males. Genetics 197, 543-560. 10.1534/genetics.114.164152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang T., Duncan F. E., Schindler K., Schultz R. M. and Lampson M. A. (2010). Evidence that weakened centromere cohesion is a leading cause of age-related aneuploidy in oocytes. Curr. Biol. 20, 1522-1528. 10.1016/j.cub.2010.06.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiolo I., Minoda A., Colmenares S. U., Polyzos A., Costes S. V. and Karpen G. H. (2011). Double-strand breaks in heterochromatin move outside of a dynamic HP1a domain to complete recombinational repair. Cell 144, 732-744. 10.1016/j.cell.2011.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copsey A., Tang S., Jordan P. W., Blitzblau H. G., Newcombe S., Chan A. C.-h., Newnham L., Li Z., Gray S., Herbert A. D. et al. (2013). Smc5/6 coordinates formation and resolution of joint molecules with chromosome morphology to ensure meiotic divisions. PLoS Genet. 9, e1004071 10.1371/journal.pgen.1004071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle J. M., Gao J., Wang J., Yang M. and Potts P. R. (2010). MAGE-RING protein complexes comprise a family of E3 ubiquitin ligases. Mol. Cell 39, 963-974. 10.1016/j.molcel.2010.08.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda T., Fukuda N., Agostinho A., Hernández-Hernández A., Kouznetsova A. and Höög C. (2014). STAG3-mediated stabilization of REC8 cohesin complexes promotes chromosome synapsis during meiosis. EMBO J. 33, 1243-1255. 10.1002/embj.201387329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallego-Paez L. M., Tanaka H., Bando M., Takahashi M., Nozaki N., Nakato R., Shirahige K. and Hirota T. (2013). Smc5/6-mediated regulation of replication progression contributes to chromosome assembly during mitosis in human cells. Mol. Biol. Cell 25, 302-317. 10.1091/mbc.E13-01-0020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez R., Jordan P. W., Viera A., Alsheimer M., Fukuda T., Jessberger R., Llano E., Pendás A. M., Handel M. A. and Suja J. A. (2013). Dynamic localization of SMC5/6 complex proteins during mammalian meiosis and mitosis suggests functions in distinct chromosome processes. J. Cell Sci. 126, 4239-4252. 10.1242/jcs.130195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodarzi A. A. and Jeggo P. A. (2012). The heterochromatic barrier to DNA double strand break repair: how to get the entry visa. Int. J. Mol. Sci. 13, 11844-11860. 10.3390/ijms130911844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guglielmino M. R., Santonocito M., Vento M., Ragusa M., Barbagallo D., Borzì P., Casciano I., Banelli B., Barbieri O., Astigiano S. et al. (2011). TAp73 is downregulated in oocytes from women of advanced reproductive age. Cell Cycle 10, 3253-3256. 10.4161/cc.10.19.17585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamatani T., Falco G., Carter M. G., Akutsu H., Stagg C. A., Sharov A. A., Dudekula D. B., VanBuren V. and Ko M. S. H. (2004). Age-associated alteration of gene expression patterns in mouse oocytes. Hum. Mol. Genet. 13, 2263-2278. 10.1093/hmg/ddh241 [DOI] [PubMed] [Google Scholar]
- Handel M. A. and Schimenti J. C. (2010). Genetics of mammalian meiosis: regulation, dynamics and impact on fertility. Nat. Rev. Genet. 11, 124-136. 10.1038/nrg2723 [DOI] [PubMed] [Google Scholar]
- Hassold T. and Hunt P. (2001). To err (meiotically) is human: the genesis of human aneuploidy. Nat. Rev. Genet. 2, 280-291. 10.1038/35066065 [DOI] [PubMed] [Google Scholar]
- Herrán Y., Gutiérrez-Caballero C., Sánchez-Martín M., Hernández T., Viera A., Barbero J. L., de Álava E., de Rooij D. G., Suja J. A., Llano E. et al. (2011). The cohesin subunit RAD21L functions in meiotic synapsis and exhibits sexual dimorphism in fertility. EMBO J. 30, 3091-3105. 10.1038/emboj.2011.222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano T. (2015). Chromosome dynamics during mitosis. Cold Spring Harbor Perspect. Biol. 7, a015792 10.1101/cshperspect.a015792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges C. A., Revenkova E., Jessberger R., Hassold T. J. and Hunt P. A. (2005). SMC1β-deficient female mice provide evidence that cohesins are a missing link in age-related nondisjunction. Nat. Genet. 37, 1351-1355. 10.1038/ng1672 [DOI] [PubMed] [Google Scholar]
- Hong Y., Sonneville R., Agostinho A., Meier B., Wang B., Blow J. J. and Gartner A. (2016). The SMC-5/6 complex and the HIM-6 (BLM) helicase synergistically promote meiotic recombination intermediate processing and chromosome maturation during Caenorhabditis elegans meiosis. PLoS Genet. 12, e1005872 10.1371/journal.pgen.1005872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins J., Hwang G., Jacob J., Sapp N., Bedigian R., Oka K., Overbeek P., Murray S. and Jordan P. W. (2014). Meiosis-specific cohesin component, Stag3 is essential for maintaining centromere chromatid cohesion, and required for DNA repair and synapsis between homologous chromosomes. PLoS Genet. 10, e1004413 10.1371/journal.pgen.1004413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houlard M., Godwin J., Metson J., Lee J., Hirano T. and Nasmyth K. (2015). Condensin confers the longitudinal rigidity of chromosomes. Nat. Cell Biol. 17, 771-781. 10.1038/ncb3167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes S. E. and Hawley R. S. (2014). Topoisomerase II is required for the proper separation of heterochromatic regions during Drosophila melanogaster female meiosis. PLoS Genet. 10, e1004650 10.1371/journal.pgen.1004650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacome A., Gutierrez-Martinez P., Schiavoni F., Tenaglia E., Martinez P., Rodríguez-Acebes S., Lecona E., Murga M., Méndez J., Blasco M. A. et al. (2015). NSMCE2 suppresses cancer and aging in mice independently of its SUMO ligase activity. EMBO J. 34, 2604-2619. 10.15252/embj.201591829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeppsson K., Kanno T., Shirahige K. and Sjögren C. (2014). The maintenance of chromosome structure: positioning and functioning of SMC complexes. Nat. Rev. Mol. Cell Biol. 15, 601-614. 10.1038/nrm3857 [DOI] [PubMed] [Google Scholar]
- Ju L., Wing J., Taylor E., Brandt R., Slijepcevic P., Horsch M., Rathkolb B., Rácz I., Becker L., Hans W. et al. (2013). SMC6 is an essential gene in mice, but a hypomorphic mutant in the ATPase domain has a mild phenotype with a range of subtle abnormalities. DNA Repair 12, 356-366. 10.1016/j.dnarep.2013.02.006 [DOI] [PubMed] [Google Scholar]
- Kanno T., Berta D. G. and Sjögren C. (2015). The Smc5/6 complex is an ATP-dependent intermolecular DNA linker. Cell Rep. 12, 1471-1482. 10.1016/j.celrep.2015.07.048 [DOI] [PubMed] [Google Scholar]
- Kauppi L., Jeffreys A. J. and Keeney S. (2004). Where the crossovers are: recombination distributions in mammals. Nat. Rev. Genet. 5, 413-424. 10.1038/nrg1346 [DOI] [PubMed] [Google Scholar]
- Kegel A., Betts-Lindroos H., Kanno T., Jeppsson K., Strom L., Katou Y., Itoh T., Shirahige K. and Sjogren C. (2011). Chromosome length influences replication-induced topological stress. Nature 471, 392-396. 10.1038/nature09791 [DOI] [PubMed] [Google Scholar]
- Lan Z.-J., Xu X. and Cooney A. J. (2004). Differential oocyte-specific expression of cre recombinase activity in GDF-9-iCre, Zp3cre, and Msx2Cre transgenic mice. Biol. Reprod. 71, 1469-1474. 10.1095/biolreprod.104.031757 [DOI] [PubMed] [Google Scholar]
- Lara-Gonzalez P., Westhorpe F. G. and Taylor S. S. (2012). The spindle assembly checkpoint. Curr. Biol. 22, R966-R980. 10.1016/j.cub.2012.10.006 [DOI] [PubMed] [Google Scholar]
- Lee J., Ogushi S., Saitou M. and Hirano T. (2011). Condensins I and II are essential for construction of bivalent chromosomes in mouse oocytes. Mol. Biol. Cell 22, 3465-3477. 10.1091/mbc.E11-05-0423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard J., Sen N., Torres R., Sutani T., Jarmuz A., Shirahige K. and Aragón L. (2015). Condensin relocalization from centromeres to chromosome arms promotes top2 recruitment during anaphase. Cell Rep. 13, 2336-2344. 10.1016/j.celrep.2015.11.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewandoski M., Wassarman K. M. and Martin G. R. (1997). Zp3–cre, a transgenic mouse line for the activation or inactivation of loxP-flanked target genes specifically in the female germ line. Curr. Biol. 7, 148-151. 10.1016/S0960-9822(06)00059-5 [DOI] [PubMed] [Google Scholar]
- Li L., Zheng P. and Dean J. (2010). Maternal control of early mouse development. Development 137, 859-870. 10.1242/dev.039487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X.-M., Yu C., Wang Z.-W., Zhang Y.-L., Liu X.-M., Zhou D., Sun Q.-Y. and Fan H.-Y. (2013). DNA topoisomerase II is dispensable for oocyte meiotic resumption but is essential for meiotic chromosome condensation and separation in mice. Biol. Reprod. 89, 118 10.1095/biolreprod.113.110692 [DOI] [PubMed] [Google Scholar]
- Lilienthal I., Kanno T. and Sjögren C. (2013). Inhibition of the Smc5/6 complex during meiosis perturbs joint molecule formation and resolution without significantly changing crossover or non-crossover levels. PLoS Genet. 9, e1003898 10.1371/journal.pgen.1003898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lister L. M., Kouznetsova A., Hyslop L. A., Kalleas D., Pace S. L., Barel J. C., Nathan A., Floros V., Adelfalk C., Watanabe Y. et al. (2010). Age-related meiotic segregation errors in mammalian oocytes are preceded by depletion of cohesin and Sgo2. Curr. Biol. 20, 1511-1521. 10.1016/j.cub.2010.08.023 [DOI] [PubMed] [Google Scholar]
- Llano E., Gomez-H L., García-Tuñón I., Sánchez-Martín M., Caburet S., Barbero J. L., Schimenti J. C., Veitia R. A. and Pendas A. M. (2014). STAG3 is a strong candidate gene for male infertility. Hum. Mol. Genet. 23, 3421-3431. 10.1093/hmg/ddu051 [DOI] [PubMed] [Google Scholar]
- MacLennan M., Crichton J. H., Playfoot C. J. and Adams I. R. (2015). Oocyte development, meiosis and aneuploidy. Semin. Cell Dev. Biol. 45, 68-76. 10.1016/j.semcdb.2015.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marangos P. (2016). Preparation of cell lysate from mouse oocytes for western blotting analysis. In Oogenesis: Methods and Protocols (ed. Nezis P. I.), pp. 209-215. New York, NY: Springer. [DOI] [PubMed] [Google Scholar]
- Murdoch B., Owen N., Stevense M., Smith H., Nagaoka S., Hassold T., McKay M., Xu H., Fu J., Revenkova E. et al. (2013). Altered cohesin gene dosage affects mammalian meiotic chromosome structure and behavior. PLoS Genet. 9, e1003241 10.1371/journal.pgen.1003241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasmyth K. and Haering C. H. (2005). The structure and function of smc and kleisin complexes. Annu. Rev. Biochem. 74, 595-648. 10.1146/annurev.biochem.74.082803.133219 [DOI] [PubMed] [Google Scholar]
- Palecek J., Vidot S., Feng M., Doherty A. J. and Lehmann A. R. (2006). The Smc5-Smc6 DNA repair complex. J. Biol. Chem. 281, 36952-36959. 10.1074/jbc.M608004200 [DOI] [PubMed] [Google Scholar]
- Pan H., Ma P., Zhu W. and Schultz R. M. (2008). Age-associated increase in aneuploidy and changes in gene expression in mouse eggs. Dev. Biol. 316, 397-407. 10.1016/j.ydbio.2008.01.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pebernard S., Perry J. J. P., Tainer J. A. and Boddy M. N. (2008). Nse1 RING-like domain supports functions of the Smc5-Smc6 holocomplex in genome stability. Mol. Biol. Cell 19, 4099-4109. 10.1091/mbc.E08-02-0226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petronczki M., Siomos M. F. and Nasmyth K. (2003). Un ménage à quatre: the molecular biology of chromosome segregation in meiosis. Cell 112, 423-440. 10.1016/S0092-8674(03)00083-7 [DOI] [PubMed] [Google Scholar]
- Pincus G. and Enzmann E. V. (1935). The comparative behavior of mammalian eggs in vivo and in vitro: I. The activation of ovarian eggS. J. Exp. Med. 62, 665-675. 10.1084/jem.62.5.665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potts P. R. and Yu H. (2007). The SMC5/6 complex maintains telomere length in ALT cancer cells through SUMOylation of telomere-binding proteins. Nat. Struct. Mol. Biol. 14, 581-590. 10.1038/nsmb1259 [DOI] [PubMed] [Google Scholar]
- Pryzhkova M. V. and Jordan P. W. (2016). Conditional mutation of Smc5 in mouse embryonic stem cells perturbs condensin localization and mitotic progression. J. Cell Sci. 129, 1619-1634. 10.1242/jcs.179036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao H., Prasada Rao H. B. D., Yang Y., Fong J. H., Cloutier J. M., Deacon D. C., Nagel K. E., Swartz R. K., Strong E., Holloway J. K. et al. (2014). Antagonistic roles of ubiquitin ligase HEI10 and SUMO ligase RNF212 regulate meiotic recombination. Nat. Genet. 46, 194-199. 10.1038/ng.2858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin S. (2015). Complex elaboration: making sense of meiotic cohesin dynamics. FEBS J. 282, 2426-2443. 10.1111/febs.13301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao H. B. D. P., Qiao H., Bhatt S. K., Bailey L. R. J., Tran H. D., Bourne S. L., Qiu W., Deshpande A., Sharma A. N., Beebout C. J. et al. (2017). A SUMO-ubiquitin relay recruits proteasomes to chromosome axes to regulate meiotic recombination. Science 355, 403-407. 10.1126/science.aaf6407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remeseiro S. and Losada A. (2013). Cohesin, a chromatin engagement ring. Curr. Opin. Cell Biol. 25, 63-71. 10.1016/j.ceb.2012.10.013 [DOI] [PubMed] [Google Scholar]
- Revenkova E., Eijpe M., Heyting C., Hodges C. A., Hunt P. A., Liebe B., Scherthan H. and Jessberger R. (2004). Cohesin SMC1β is required for meiotic chromosome dynamics, sister chromatid cohesion and DNA recombination. Nat. Cell Biol. 6, 555-562. 10.1038/ncb1135 [DOI] [PubMed] [Google Scholar]
- Reynolds A., Qiao H., Yang Y., Chen J. K., Jackson N., Biswas K., Holloway J. K., Baudat F., de Massy B., Wang J. et al. (2013). RNF212 is a dosage-sensitive regulator of crossing-over during mammalian meiosis. Nat. Genet. 45, 269-278. 10.1038/ng.2541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein P. and Schindler K. (2011). Mouse oocyte microinjection, maturation and ploidy assessment. J. Vis. Exp. e2851 10.3791/2851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susiarjo M., Rubio C. and Hunt P. (2009). Analyzing mammalian female meiosis. Methods Mol. Biol. 558, 339-354. 10.1007/978-1-60761-103-5_20 [DOI] [PubMed] [Google Scholar]
- Tachibana-Konwalski K., Godwin J., van der Weyden L., Champion L., Kudo N. R., Adams D. J. and Nasmyth K. (2010). Rec8-containing cohesin maintains bivalents without turnover during the growing phase of mouse oocytes. Genes Dev. 24, 2505-2516. 10.1101/gad.605910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Rosell J., Sunjevaric I., De Piccoli G., Sacher M., Eckert-Boulet N., Reid R., Jentsch S., Rothstein R., Aragon L. and Lisby M. (2007). The Smc5-Smc6 complex and SUMO modification of Rad52 regulates recombinational repair at the ribosomal gene locus. Nat. Cell Biol. 9, 923-931. 10.1038/ncb1619 [DOI] [PubMed] [Google Scholar]
- Tran M., Tsarouhas V. and Kegel A. (2016). Early development of Drosophila embryos requires Smc5/6 function during oogenesis. Biol. Open 5, 928-941. 10.1242/bio.019000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsumi M., Fujiwara R., Nishizawa H., Ito M., Kogo H., Inagaki H., Ohye T., Kato T., Fujii T. and Kurahashi H. (2014). Age-related decrease of meiotic cohesins in human oocytes. PLoS ONE 9, e96710 10.1371/journal.pone.0096710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verver D. E., van Pelt A. M., Repping S. and Hamer G. (2013). Role for rodent Smc6 in pericentromeric heterochromatin domains during spermatogonial differentiation and meiosis. Cell Death Dis. 4, e749 10.1038/cddis.2013.269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verver D. E., Langedijk N. S. M., Jordan P. W., Repping S. and Hamer G. (2014). The SMC5/6 complex is involved in crucial processes during human spermatogenesis. Biol. Reprod. 91, 22 10.1095/biolreprod.114.118596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verver D. E., Hwang G. H., Jordan P. W. and Hamer G. (2015). Resolving complex chromosome structures during meiosis: versatile deployment of Smc5/6. Chromosoma 125, 15-27. 10.1007/s00412-015-0518-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters T., McNicoll F. and Jessberger R. (2014). Meiotic cohesin STAG3 is required for chromosome axis formation and sister chromatid cohesion. EMBO J. 33, 1243-1255. 10.1002/embj.201387330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xaver M., Huang L., Chen D. and Klein F. (2013). Smc5/6-Mms21 prevents and eliminates inappropriate recombination intermediates in meiosis. PLoS Genet. 9, e1004067 10.1371/journal.pgen.1004067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H., Beasley M. D., Warren W. D., van der Horst G. T. and McKay M. J. (2005). Absence of mouse REC8 cohesin promotes synapsis of sister chromatids in meiosis. Dev. Cell 8, 949-961. 10.1016/j.devcel.2005.03.018 [DOI] [PubMed] [Google Scholar]
- Zhang K. and Smith G. W. (2015). Maternal control of early embryogenesis in mammals. Reprod. Fertil. Dev. 27, 880-896. 10.1071/RD14441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X. and Blobel G. (2005). A SUMO ligase is part of a nuclear multiprotein complex that affects DNA repair and chromosomal organization. Proc. Natl. Acad. Sci. USA. 102, 4777-4782. 10.1073/pnas.0500537102 [DOI] [PMC free article] [PubMed] [Google Scholar]