Abstract
Background
Vitamin D is a micronutrient important for bone growth and immune function. Deficiency can lead to rickets and has been linked to various infections, including respiratory infections. The evidence on the effects of supplementation on infections in children has not been assessed systematically.
Objectives
To evaluate the role of vitamin D supplementation in preventing pneumonia, tuberculosis (TB), diarrhoea, and malaria in children under five years of age. This includes high‐, middle‐, and low‐income countries.
Search methods
We searched the Cochrane Infectious Diseases Group Specialized Register, the Cochrane Central Register of Controlled Trials (CENTRAL), the Cochrane Library, MEDLINE, EMBASE, LILACS, the WHO International Clinical Trials Registry Platform (ICTRP; http://www.who.int/ictrp/en/) , ClinicalTrials.gov and the ISRCTN registry (http://www.isrctn.com/) up to 16 June 2016.
Selection criteria
We included randomized controlled trials (RCTs) that evaluated preventive supplementation of vitamin D (versus placebo or no intervention) in children under five years of age.
Data collection and analysis
Two review authors independently screened the titles and abstracts, extracted the data, and assessed the risk of bias of included trials.
Main results
Four trials met the inclusion criteria, with a total of 3198 children under five years of age, and were conducted in Afghanistan, Spain, and the USA. Prevalence of vitamin D deficiency varied widely in these populations (range: 73.1% in Afghanistan, 10 to 12% in USA, and 6.2% in Spain). The included trials evaluated mortality (two trials), pneumonia incidence (two trials), diarrhoea incidence (two trials), hospitalization (two trials), and mean serum vitamin D concentrations (four trials).
We do not know whether vitamin D supplementation impacts on all‐cause mortality because this outcome was underpowered due to few events (risk ratio (RR) 1.43, 95% confidence interval (CI) 0.54 to 3.74; one trial, 3046 participants, low quality evidence).
For pneumonia, episodes of 'radiologically confirmed' first or only episode of pneumonia were little different in the supplemented and unsupplemented group (Rate Ratio: 1.06, 95% confidence interval (CI) 0.89 to 1.26; two trials, 3134 participants, moderate quality evidence), and similarly for children with confirmed or unconfirmed pneumonia (RR 0.95, 95% CI 0.87 to 1.04; one trial, 3046 participants). In these two trials there were no obvious differences between supplemented and unsupplemented children regarding episodes of diarrhoea.
In the single large trial from Afghanistan, the trial authors reported that vitamin D supplementation was associated with an increase in repeat episodes of pneumonia confirmed by chest radiograph (RR 1.69, 95% CI 1.28 to 2.21; one trial, 3046 participants), but not reflected in the outcome of confirmed or unconfirmed pneumonia (RR 1.06, 95% CI 1.00 to 1.13; one trial, 3046 participants).
For hospital admission measured in one small trial, there was no difference detected (RR 0.86, 95% CI 0.20 to 3.62; one trial, 88 participants; very low quality evidence).
The mean serum vitamin D concentrations were higher in supplemented compared to unsupplemented children at the end of supplementation (MD 7.72 ng/mL, 95% CI 0.50 to 14.93; four trials, 266 participants, low quality evidence). These results were driven primarily by two smaller trials with large magnitudes of effect. In the other two bigger trials, serum vitamin D concentrations were elevated in the intervention group for most of the trial duration but not at the end of supplementation. This may be due to time elapsed at measurement from the last dose, incomplete compliance, or increased need of vitamin D with infant age.
We did not find any trial that reported on the incidence of TB, malaria or febrile illness, duration of pneumonia, duration of diarrhoea, severity of infection, and cause‐specific mortality (due to TB, diarrhoea, or malaria).
Authors' conclusions
Evidence from one large trial did not demonstrate benefit of vitamin D supplementation on the incidence of pneumonia or diarrhoea in children under five years. To our knowledge, trials that evaluated supplementation for preventing other infections, including TB and malaria, have not been performed.
23 April 2019
Update pending
Studies awaiting assessment
The CIDG is currently examining a search conducted up to April 2019 for potentially relevant studies. These studies have not yet been incorporated into this Cochrane Review. All eligible published studies found in the last search (16 Jun, 2016) were included and four ongoing studies were identified (see 'Characteristics of ongoing studies' section).
Plain language summary
Vitamin D supplementation for preventing infections in children under five
Background
Vitamin D is a micronutrient important for bone growth and immune function. Deficiency can lead to rickets and has been linked to various infections, including respiratory infections. Several studies have reported an association between vitamin D deficiency and infections among children, and is thought to be related to the role of vitamin D in the immune system. In this systematic review, Cochrane researchers examined the role of vitamin D supplementation in prevention of infections in children under five years of age. The researchers studied the infections of pneumonia, tuberculosis (TB), diarrhoea, and malaria in this review.
Study characteristics
The review authors examined the available evidence up to 17 June 2016, and included four trials with a total of 3198 children under five years of age. The included trials were conducted in Afghanistan, Spain and the USA.
Key findings
The review did not detect an effect of vitamin D supplementation on death (low quality evidence); the occurrence of the first or only episode of pneumonia; or on children with pneumonia, irrespective of whether this had been confirmed by hospital tests (moderate quality evidence). Limited evidence showed that there was no obvious difference in the first or repeat episodes of diarrhoea between supplemented and unsupplemented children. We do not know about whether Vitamin D influences hospital admissions as there was only one small study measuring this (very low quality evidence). The mean serum vitamin D concentrations were higher in the supplemented versus unsupplemented children at the end of supplementation period (low quality evidence). One large trial from Afghanistan showed an increase in repeat episodes of confirmed pneumonia but not on confirmed and unconfirmed pneumonia. None of the included trials reported on TB or malaria as outcomes.
Conclusions
One large trial has not demonstrated an effect of vitamin D on death or respiratory infections in children under five years of age. We did not find trials evaluating Vitamin D supplementation to prevent other infections such as TB and malaria.
Summary of findings
Summary of findings for the main comparison. Vitamin D versus control for preventing infections in children under five years of age.
| Vitamin D versus control for preventing infections in children under five years of age | |||||
|
Patient or population: children under five years of age
Settings: hospitals, clinics, and community
Intervention: vitamin D supplementation (daily dose of 402 IU or quarterly supplementation of 100,000 IU) Control: placebo or no supplementation | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative/absolute effect (95% CI) | Number of participants (trials) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Control | Vitamin D | ||||
| All‐cause mortality | 5 per 1000 | 7 per 1000 (3 to 19) | Risk ratio 1.43 (0.54 to 3.74) | 3046 (1) |
⊕⊕⊝⊝ low1,2 |
| Cause‐specific mortality | 3 per 1000 |
5 per 1000 (1 to 16) |
Risk ratio 1.50 (0.42 to 5.30) | 3046 (1) |
⊕⊕⊝⊝ low1,2 |
| Incidence rate radiologically confirmed first or only episode of pneumonia | 157 episodes per 1000 person‐years | 166 episodes per 1000 person years (140 to 198) | Rate ratio 1.06 (0.89 to 1.26) | 3134 (2) | ⊕⊕⊕⊝ moderate2,3 |
| Any hospital admission | 9 per 100 |
8 per 100 (2 to 33`) |
Risk ratio 0.86 (0.20 to 3.62) |
88 (1) |
⊕⊝⊝⊝ very low4,5,6 |
| TB cases | ‐ | ‐ | ‐ | 0 studies | ‐ |
| Diarrhoea cases | ‐ | ‐ | ‐ | 2 studies7 | ‐ |
| Malaria cases | ‐ | ‐ | ‐ | 0 studies | ‐ |
| Febrile illness | ‐ | ‐ | ‐ | 0 studies | ‐ |
| Mean serum vitamin D concentrations | 141 | 125 | Mean difference 7.72ng/mL higher (0.50 higher to 14.93 higher) | 266 (4) |
⊕⊕⊝⊝ low2,8 |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Abbreviations: CI: confidence interval; RR: risk ratio; GRADE: Grading of Recommendations Assessment, Development and Evaluation; TB: tuberculosis; HR: hazard ratio. | |||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | |||||
1Downgraded by 1 for imprecision: the estimate varies from 46% decrease to over 3‐fold increase for all‐cause mortality; and from 58% decrease to over 5‐fold increase for cause‐specific mortality. 2Downgraded by 1 for indirectness: data comes mainly from a single large trial conducted in Afghanistan with a high baseline prevalence of vitamin D deficiency limiting the generalizability of the estimate to developed countries. However, findings from this setting would be generalizable to majority of other developing countries. 3Imprecision: no serious imprecision as the Afghanistan trial was adequately powered to detect clinically important benefits with vitamin D. The 95% CI of the result is narrow and probably excludes clinically important benefits. 4Downgraded by 1 for high risk of bias in Alonso 2011 due to unblinding, high/differential loss to follow‐up and selective outcome reporting. 5Downgraded by 1 for indirectness: data comes mainly from one trial in developed country limiting the generalizability of the estimate to developing countries. 6The effect estimates are also imprecise with wide CIs. 7No effect however meta‐analysis could not be performed since Alonso 2011 reported the RR while Manaseki‐Holland 2012 reported the HR. 8Downgraded by 1 for imprecision: the estimate varies from 0.50 to over 14.00 ng/mL increase.
Background
Description of the condition
Vitamin D is a family of fat‐soluble molecules that are important micronutrients for humans, and two forms (D2 and D3) play a central role in bone growth by increasing the uptake of calcium from the gut (Bentley 2013). Vitamin D is therefore especially important in growing children, and a deficiency in vitamin D can lead to rickets, which is characterized by weak and deformed bones.
Humans can obtain both vitamin D2 and D3 from their diet, with fish liver oils, eggs, and milk being particularly rich in both. However, we obtain most of our vitamin D in the form of vitamin D3 by synthesizing it directly when their skin is exposed to sunlight. This powers a photochemical reaction in which a derivative of cholesterol is converted into pre‐vitamin D3, which is then transformed into vitamin D3 by the heat of the skin (Wagner 2008a).
Vitamin D2 and D3 are water‐insoluble and are transported in the blood to the liver bound to carrier proteins, mainly vitamin D‐binding protein. Here, they are converted to 25‐hydroxyvitamin D (25(OH)D), which is the major circulating form of vitamin D. In the kidneys, 25(OH)D is converted to 1,25‐dihydroxyvitamin D (1,25(OH)D) via the action of the enzyme 1α hydroxylase (Holick 2008; White 2008). This is the active form of vitamin D in the body, and is thus the true hormonal form of vitamin D. It binds to the vitamin D receptor (VDR), which is found on the nuclear membrane of many cells (Walker 2009).
Vitamin D levels in the body are best measured using the concentration of 25(OH)D in blood serum. According to the US Institute of Medicine (IOM 2010), concentrations of 25(OH)D above 50 nmol/L (20 ng/mL) can provide benefit to most in the population and are considered in the normal range. Vitamin D deficiency refers to concentrations below this cut‐off value. It is estimated that around one billion people in the world may have suboptimal vitamin D concentrations (Walker 2009). The major risk factors are lack of sunlight (especially during winter months), vegetarian diets, a dark pigmented skin (as melanin acts as a natural sunscreen), increased pollution, and wearing long‐sleeved garments or clothes completely covering the body (Williams 2008; Nimitphong 2013). Modern recommendations to avoid the sun to prevent skin cancers may also be contributing to a deficiency in vitamin D (Misra 2008).
Fortified foods such as infant formulas, breakfast cereal, cheese, and cows' milk are the major dietary sources of vitamin D in children, but they may not be consumed in sufficient quantities. Furthermore, dairy products may not be fortified in all countries. Diet contributes less than 10% to 20% of an adult’s vitamin D stores, and this proportion may be even smaller in children (Sichert‐Hellert 2006). Since vitamin D is a fat‐soluble vitamin, a diet that is extremely low in fat can also impair its absorption. Breast milk can be a poor source of vitamin D, particularly if the mother has clinical or subclinical vitamin D deficiency.
Over the past 20 years, much attention has been paid to recognition of vitamin D deficiency in children worldwide. Childhood vitamin D deficiency is highly prevalent in many developing countries, even those with abundant sunlight such as Turkey (Ozgür 1996), Iran (Salimpour 1975; Bassil 2013), Saudi Arabia (Elidrissy 1984), Jordan (Bassil 2013), the United Arab Emirates (Bassil 2013), Algeria (Garabedian 1991), India (Ghai 1991; Wayse 2004), China (Zhao 1991; Zhao 1992; Du 2001; Zhang 2013), and Nigeria (Akpede 1999; Akpede 2001). A study on the health status of children in low‐ and middle‐income countries reported that 73.1% of underprivileged children were 25(OH)D‐deficient (Manaseki‐Holland 2008). A high occurrence of vitamin D deficiency in infants and children has also been reported in many other countries, including industrialized ones (Prentice 2008) such as the USA (Mansbach 2009), the UK (Lawson 1999), Greece (Nicolaidou 2006), Finland Lehtonen‐Veromaa 1999), Canada (Ward 2007), and New Zealand (Grant 2009).
Description of the intervention
In the past, clinicians have primarily focused on the role of vitamin D in preventing and treating rickets. However, a few early researchers realized that children with rickets were more likely to have respiratory infections. Initially, clinicians presumed these infections were caused by poor lung function as a result of bone deformities and the overall compromised nutritional status associated with rickets. Since then, several studies, including case‐control and case series studies, have linked rickets with pneumonia and respiratory tract infections (Salimpour 1975; Muhe 1997; Najada 2004), and implicated vitamin D deficiency as a potential risk factor for these infections. Many epidemiological studies have also been conducted in children to assess the link between inadequate vitamin D concentrations and respiratory infections, including tuberculosis (TB) (Wayse 2004; Nnoaham 2008; Williams 2008; Karatekin 2009; McNally 2009; Roth 2009). The findings from these studies have been mixed, with some reporting strong positive associations and others no associations. A retrospective case‐series of 64 paediatric TB patients during a two‐year period in UK showed that 86% of children were either vitamin D‐deficient or vitamin D‐insufficient (Williams 2008). Other case‐control studies in Europe and Australia showed an increased risk of vitamin D deficiency in children with TB compared to healthy controls (Gray 2012; Venturini 2014), while another study from India did not find vitamin D deficiency a risk factor for TB in children (Jubulis 2014). Evidence is also emerging on the role of vitamin D deficiency as a risk factor for gastroenteritis. A prospective cohort study of school‐age children in Colombia reported a significant two‐fold increased risk of diarrhoea with vomiting over a one‐year period in vitamin D‐deficient compared to vitamin D‐sufficient children. This indicated increased susceptibility to norovirus and Salmonella or Shigella bacterial infections (Thornton 2013). Vitamin D insufficiency has also been linked to more severe malarial infections in Ugandan children 18 months to 12 years old (Cusick 2014). In animal models, vitamin D is known to inhibit the development of cerebral malaria during Plasmodium berghei infection (He 2014); and in another study, the death rate in mice from P. berghei infection reduced after addition of cod liver oil or vitamin D and dicalcium phosphate to antimalarial drugs (Sautet 1957; Luong 2015).
The precise molecular mechanisms by which vitamin D helps defend people against infectious disease are now being elucidated. It has become clear that 1,25(OH)D plays a role not only in calcium homeostasis and bone metabolism, but also in the integrity of the innate immune system (Bhutta 2008; Wagner 2008b; Dimitrov 2015). Acting via the VDR, 1,25(OH)D alters the activity of many immune system cells, including macrophages, regulatory T cells, and natural killer cells.
Based on bone health benefits of vitamin D, the US Institute of Medicine (IOM) published new dietary guidelines in 2010, with the adequate intake for infants of 400 International Units (IU) daily of vitamin D, and raised the Recommended Dietary Allowance (RDA) for children older than one year from 400 IU/day in 2008 (Wagner 2008a) to 600 IU/day (IOM 2010). Health Canada also has similar recommendations of 400 IU/day for all exclusively breastfed, healthy infants; this should be continued until the infant's diet provides at least 400 IU/day from other sources (Canadian Paediatric Society 2007). However, the IOM committee did not find sufficient conclusive evidence for effects on non‐skeletal outcomes (Shapses 2011). It is therefore not yet known if these doses are sufficient to deliver all potential non‐skeletal health benefits related to vitamin D, and some experts recommend that at least 1000 IU/day may be required to consistently raise serum 25(OH)D concentrations above 30 ng/mL (Holick 2011). Vitamin D is generally safe and well tolerated when given at appropriate doses; cases of hypercalcaemia have been documented with vitamin D toxicity (only at doses of 50,000 IU/day or more for several weeks), which may eventually lead to vascular and tissue calcification with subsequent renal and cardiovascular damage (IOM 2010).
How the intervention might work
Vitamin D influences the action of more than 200 human genes in a wide range of tissues and displays as many molecular mechanisms (Cannell 2008). In particular, it interacts with the human immune system in a wide variety of ways, and helps to protect against infectious diseases (Gunville 2013). For example, it has been known for 20 years that exposure to 1,25(OH)D stimulates anti‐mycobacterial activity in human monocytes and macrophages. Recent research suggests that this is due to vitamin D helping to generate antimicrobial peptides (AMPs) like cathelicidin and some β defensins (White 2008; Gunville 2013). These AMPs then lead to enhanced killing of intracellular Mycobacterium tuberculosis by direct membrane damage and also by acting as chemoattractants for monocytes.
Recent research also indicates that a sufficient intake of vitamin D is essential for killer T cells to fend off serious infections, by controlling T cell antigen receptor (TCR) signalling and the activation of human T cells. Besides this, 1,25(OH)D also suppresses an overzealous adaptive immune response to pathogens that may be difficult for macrophages to handle efficiently (Walker 2009). The levels of plasma interleukin‐1ß have also been shown to be low in children with vitamin D deficiency which can predispose them to infections (Liang 2010; Bentley 2013).
The relationship between vitamin D and infectious diseases is also supported by genetic studies of polymorphisms in the gene for the VDR. Researchers have found a significant link between single nucleotide polymorphisms of genes related to the innate immune function, including the VDR, and genetic susceptibility to respiratory syncytial virus (RSV) bronchiolitis (Janssen 2007).
While there is much research on the beneficial effects of vitamin D for TB infections, data are emerging from various sources about its role in fighting other bacterial and viral pathogens. Apart from the synthesis of AMPs (cathelicidin and defensins), the binding of activated vitamin D to the VDR can modulate viral lower respiratory tract disease. One of the defensins, retrocyclin‐2, inhibits infection with the influenza virus by blocking its fusion with cell membranes (Leikina 2005). Respiratory tract infections peak during the winter season when there is less sunlight and so vitamin D deficiency during this season may enhance the infectivity of influenza viruses. A randomized controlled trial (RCT) on 334 schoolchildren showed that vitamin D supplements had a beneficial effect on influenza A incidence during the four‐month study period (Urashima 2010).
Vitamin D and its role in malarial infection has also been explored. It inhibits development of cerebral malaria in animal models due to suppression of inducible systemic inflammatory responses with reduced production of cytokines (interferon‐Ƴ and tumour necrosis factor) (He 2014). Vitamin D also inhibits penetration of P. berghei into erythrocyte membranes (Sergacheva 1986) and inhibits growth of Plasmodium falciparum in red blood cells in vitro (Vial 1982). VDR expression was also higher in patients with Plasmodium vivax infection, which indicates a link between VDR polymorphism and severity of malarial infection (Ray 2012).
Cystic fibrosis (CF) is an inherited disorder seen in children, and is characterized by pancreatic insufficiency and recurrent infections. Children with CF have inadequate fat‐soluble vitamins, including vitamin D. Yim 2007 showed that there was enhanced antibacterial activity against airway pathogens, such as Pseudomonas aeruginosa and Bordetella bronchiseptica, in both normal and CF bronchial epithelial cells in participants supplemented with 1,25(OH)D. They also witnessed 1,25(OH)D‐induced production of cathelicidin in this cell type.
Why it is important to do this review
Globally, pneumonia and diarrhoea constitute the leading infectious causes of childhood deaths in children under five years of age, and the burden is concentrated in Southeast Asia and Africa. This also explains the focus of this Cochrane Review on this age range. According to 2010 estimates, there were 120 million episodes of pneumonia and 1.731 billion episodes of diarrhoea worldwide (Walker 2013). Twelve per cent of pneumonia episodes and 2% of diarrhoea episodes progressed to severe disease; the case‐fatality ratio for severe pneumonia was 8.9% (uncertainty range: 3.1 to 12.5%) and for severe diarrhoea 2.0% (1.4 to 4.4%) (Walker 2013).
Rickets is the best‐known medical condition associated with vitamin D deficiency. It has also been linked to various infectious diseases, especially respiratory infections such as pneumonia, TB, and bronchiolitis, which suggests that suboptimal concentrations of vitamin D may be responsible in the aetiology of these infections (see the 'Description of the intervention' section). Due to lack of evidence on the role of vitamin D in preventing infections, the mortality and morbidity burden of infections in children due to vitamin D deficiency has not yet been quantified.
Vitamin D supplementation is a relatively simple intervention that might decrease the incidence of many infections. A study in the UK on a small cohort reported that the cost of preventing vitamin D deficiency in a high‐risk population of Asian children was much lower than the cost of treating the general health issues linked with chronic vitamin D deficiency (Zipitis 2006). Because of its cost effectiveness and ease of administration, it can easily be applied on a large scale to children in communities or in health facilities. Vitamin D could therefore help prevent the enormous burden of morbidity and mortality in children.
Objectives
To evaluate the role of vitamin D supplementation for preventing pneumonia, tuberculosis (TB), diarrhoea, and malaria in children under five years of age. This includes high‐, middle‐, and low‐income countries.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials (RCTs), whether individually‐ or cluster‐RCTs.
Types of participants
Children under five years of age. We excluded studies on children with pre‐existing illnesses, such as rickets, human immunodeficiency virus (HIV)/ acquired immune deficiency syndrome (AIDS), meningitis, severe malnutrition, and sickle cell disease. We also excluded studies of supplementation in preterm and low birthweight infants.
Types of interventions
Interventions
We included trials of synthetic oral vitamin D supplementation of various doses and frequencies compared to a control group (placebo or no intervention). The co‐interventions (for example, multiple vitamins or adjunct mineral and nutrient supplementation) should have been identical in both groups, that is vitamin D supplementation was the only difference between the intervention and control groups. We excluded studies that evaluated the effects of food fortification or the consumption of vitamin D‐rich foods. If a trial included more than one eligible intervention group (for example, differing in dose), we combined the groups for the main analysis, although they could be treated separately for subgroup analyses.
Comparisons
Vitamin D versus placebo or no treatment.
Vitamin D plus 'other micronutrient(s)' versus 'other micronutrient(s)'.
We excluded the following comparisons.
Vitamin D plus 'other micronutrient' versus placebo or no treatment.
Vitamin D plus 'other micronutrient' versus 'different other micronutrient'.
Types of outcome measures
Primary outcomes
-
Incidence rate (number of cases or episodes per total child‐years) of the following.
Pneumonia.
Tuberculosis (TB).
Secondary outcomes
-
Incidence rate (number of cases or episodes per total child‐years) of the following.
Diarrhoea.
Malaria.
Incidence rate (cases or episodes per total child‐years) of febrile illness.
-
Duration (mean number of days of all episodes) of the following.
Pneumonia (positive clinical examination or chest radiograph findings).
Diarrhoea.
-
Severity of infection.
Moderate or severe pneumonia (as defined by the authors).
Moderate or severe diarrhoea, that is, history of loose stools more than three times per day and history of oral dehydration or intravenous fluid therapy.
All‐cause mortality (included post hoc in this review).
-
Cause‐specific mortality (included post hoc in this review).
Pneumonia.
TB.
Diarrhoea.
Malaria.
Hospital admission rate due to infections.
Change in mean serum vitamin D concentrations
Adverse outcomes
-
New cases per total children of the following.
Nausea within 72 hours.
Vomiting within 72 hours.
Headache within 72 hours.
Constipation.
Kidney stones.
We included trials that reported on at least one of the review‐defined outcomes. If a trial had information on infection‐related outcomes in the full text but data were unavailable, we contacted the trial authors to get the complete results.
Time of outcome assessment
We grouped outcomes by time: zero to 12 months, 13 to 60 months, and over 60 months. When trials reported multiple time points, we extracted the longest outcome interval in a given time period.
Search methods for identification of studies
We attempted to identify all relevant studies regardless of language, setting/region (low‐, middle‐, and high‐income countries), or publication status (published, unpublished, in press, and ongoing).
Electronic searches
We searched the following databases for relevant studies using the search terms and strategy detailed in Appendix 1: the Cochrane Infectious Diseases Group (CIDG) Specialized Register; the Cochrane Central Register of Controlled Trials (CENTRAL), published in the Cochrane Library issue 6 2016; MEDLINE (Pubmed, 1966 to 16 June 2016); EMBASE (OVID, 1980 to 16 June 2016); and LILACS (1982 to 16 June 2016). We also searched the WHO International Clinical Trials Registry Platform (ICTRP; http://www.who.int/ictrp/en/) , ClinicalTrials.gov and the ISRCTN registry (http://www.isrctn.com/) for ongoing trials, using "vitamin D" and ‘child* OR infant*’ as search term (all accessed on 16 June 2016).
Searching other resources
We contacted researchers in the field to identify additional studies that may be eligible for inclusion. We also checked the reference lists of all studies identified by the above methods.
Data collection and analysis
Selection of studies
Two review authors (MYY and FR) independently assessed studies for inclusion. They selected potentially relevant studies by screening the titles and abstracts of studies, if available. We retrieved and reviewed full‐text articles when we could not determine their relevance from titles or abstracts. Three review authors (MYY, RAS, and FR) independently screened these full‐text articles to assess the eligibility of all potentially relevant studies and filled out eligibility forms, which we designed in accordance with the specified inclusion criteria. We resolved differences of opinion about studies' suitability for inclusion by discussion between all review authors. We presented the excluded studies that appeared to meet the inclusion criteria but on further investigation of the full‐text article did not in the 'Characteristics of excluded studies' table, along with the reason(s) for their exclusion. In the case of conference abstracts, if additional data were not forthcoming, we used the information provided in the abstract for review purposes. We also attempted to contact the trial authors regarding eligibility for studies where eligibility was unclear.Figure 1 shows the study flow diagram.
1.
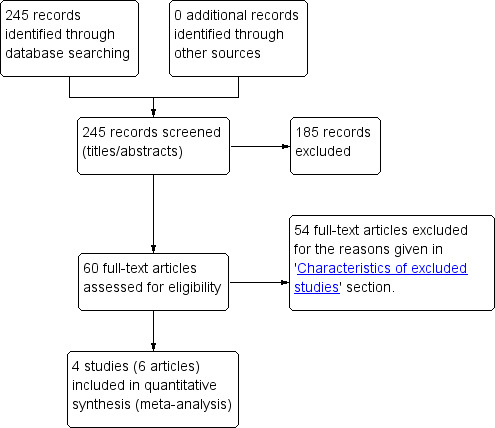
Study flow diagram.
Data extraction and management
We used a data extraction sheet to extract the following information from each included trial.
Dates.
Location (country, urban/rural).
Method of recruitment.
Inclusion criteria.
Unit of analysis.
Allocation ratio.
Risk of bias (see below).
-
Participants.
Sample size and person‐time of follow‐up.
Socio‐demographics (age, gender).
Co‐morbidities.
-
Intervention and comparison.
Number of eligible intervention groups.
-
For each intervention and comparison group of interest.
Dosage.
Duration.
Frequency.
Co‐intervention (if any).
Details of the comparison.
-
Outcomes.
Outcomes and time points (a) collected and (b) reported.
-
For each outcome of interest.
Outcome definition and unit of measurement (if relevant).
Loss to follow‐up.
-
Miscellaneous.
Key conclusions of trial authors.
References to other relevant trials.
Correspondence required.
For incidence rates (count data), we extracted the number of events in the treatment and control groups, and the total person‐time at risk in each group, or the rate ratio and a measure of variance, for example, standard error (SE) directly from the trial report. We used cumulative incidences or risks for dichotomous data such as mortality risks and adverse events for which we extracted the number of participants experiencing the condition and the total number of participants in each treatment group.
Assessment of risk of bias in included studies
Two review authors (MYY and RAS) independently assessed methodological quality using the Cochrane 'Risk of bias' assessment tool (Higgins 2011). We assessed the included trials based on the following six components.
Sequence generation (checking for selection bias and baseline exchangeability)
For each included trial we described the method used to generate allocation sequence in sufficient detail. We assessed the method as at one of the following levels of bias.
Low risk (any truly random process, for example, random number table, computer random number generation).
High risk (any quasi‐ or non‐random process, for example, odd or even date of birth; hospital or clinic record number).
Unclear risk.
Allocation concealment (checking for selection bias and baseline exchangeability)
We described for each included trial the method used to conceal the allocation sequence and determine whether intervention allocation could have been foreseen in advance of or during recruitment, or changed after assignment. We assessed the method as the following.
Low risk (for example, telephone or central randomization; consecutively numbered sealed opaque envelopes).
High risk (for example, open random allocation; unsealed or non‐opaque envelopes).
Unclear risk.
Blinding (checking for possible performance bias)
We described for each included trial the methods used, if any, to blind trial participants and personnel from knowledge of which intervention a participant received. We judged trials to be at low risk of bias if they were blinded, or if we judge that the lack of blinding could not have affected the results. We assessed the methods as follows.
Low, high, or unclear risk for participants.
Low, high, or unclear risk for personnel.
Low, high, or unclear risk for outcome assessors.
Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations)
For each included trial we described the data missing, including attrition and exclusions from the analysis. We stated whether the trial authors reported attrition and exclusions, the numbers included in the analysis at each stage (compared with the total randomized participants), and if the trial authors reported the reasons for attrition. We assessed the methods as follows.
Low risk.
High risk.
Unclear risk.
Selective reporting bias (deviation from a priori specification in the protocol)
We assessed selective reporting bias in the included trials as follows.
Low risk (where it was clear that the trial authors reported all of the trial's prespecified outcomes and all expected outcomes of interest to the review).
High risk (where the trial authors did not report all the trial's prespecified outcomes; did not prespecify one or more primary outcomes; reported the outcomes of interest incompletely and so could not be used, the trial failed to include the results of a key outcome that we would have expected the trial authors to have reported).
Unclear risk.
Other potential sources of bias
For each included trial, we described susceptibility to other possible sources of bias, for example, information bias (misclassification). We assessed whether each included trial was free of other issues that could put it at risk of bias.
Low risk.
High risk.
Unclear risk.
Overall risk of bias
We made explicit judgements about whether the included trials were at high or low risk of bias, according to the criteria given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). With reference to the risk of bias criteria we have listed above, we assessed the likely magnitude and direction of bias. We had planned to explore the impact of the level of bias through undertaking sensitivity analyses for the primary outcomes.
We presented findings in a 'Risk of bias' tables where, for each question‐based entry, our 'Risk of bias' judgement is followed by a text box that provides details on the available information that led us to each judgement. Also we presented the results in 'Risk of bias' figures. For information that was unclear based on the full‐text article(s), we attempted to contact the trial authors for clarification. We resolved any disagreements between the two review authors who performed the 'Risk of bias' assessments by discussion among all the review authors. Further details about the 'Risk of bias' assessment tool are included in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Quality of the evidence
We assessed the quality of the evidence using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach (Schünemann 2009) for the following outcomes for the main comparison of vitamin D versus placebo/control.
All‐cause mortality.
Incidence rates of pneumonia.
Incidence rates of TB.
Incidence rates of diarrhoea.
Incidence rates of febrile illness.
Incidence rates of malaria.
Mean serum vitamin D concentrations.
We used the GRADEpro Guideline Development Tool (GDT) (GRADEpro GDT 2015) to import data from Review Manager (RevMan) (RevMan 2014) to create a 'Summary of findings' table. We produced a summary of the intervention effect and a measure of quality for each of the above outcomes using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the quality of evidence for each outcome. The evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations based on assessments in these five areas.
Measures of treatment effect
Count data
We used rate ratios with 95% confidence intervals (CIs) to combine count data.
Dichotomous data
For dichotomous data, we presented results as risk ratios with 95% CIs using intention‐to‐treat (ITT) analysis results from the included trials.
Continuous data
We combined the continuous data using mean differences (MDs) if continuous data were summarized by arithmetic means and standard deviations. We combined them on the log scale using the MD where the data were summarized using geometric means, We reported medians and ranges.
Unit of analysis issues
No cluster‐randomized controlled trials (cluster‐RCTs) met the inclusion criteria of this review, and all included RCTs performed randomization and analysed at the individual level.
Dealing with missing data
We described missing data, including dropouts. Differential dropout rates can lead to biased estimates of the effect size, and bias may arise if the reasons for dropping out differ across groups. We reported the reasons for dropout. If data were missing for some cases, or if the trial authors did not report the reasons for dropping out, we contacted the trial authors. If there were missing data for dichotomous outcomes, we used ITT analyses as opposed to the complete‐case analyses because in the latter the intervention and control groups are no longer exchangeable at baseline with respect to different characteristics that may introduce confounding bias in the analysis.
Assessment of heterogeneity
We assessed the included RCTs for clinical, methodological, and statistical heterogeneity. We assessed clinical heterogeneity by comparing the distribution of important factors, such as the study participants, study setting, dose and duration of the intervention and co‐interventions. We evaluated methodological heterogeneity on the basis of factors such as the method of sequence generation, allocation concealment, blinding of outcome assessment, and loss to follow‐up. We assessed statistical heterogeneity among the included trials by visual inspection of forest plot and the I² statistic (calculated as I² statistic = 100% x (Q‐df )/Q; where Q is Cochrane’s heterogeneity statistic and df is the degree of freedom) (Higgins 2003). The I² statistic describes the percentage of variability attributable to between‐study heterogeneity rather than due to sampling error or chance. If the I² statistic value exceeded 50%, we considered heterogeneity to be substantial. We had also planned to undertake subgroup analyses to explore reasons for high levels of heterogeneity, where applicable.
Assessment of reporting biases
We planned to use funnel plots to assess reporting biases but only four trials met our inclusion criteria. See the 'Differences between protocol and review' section.
Data synthesis
We performed meta‐analyses using RevMan (RevMan 2014). We chose a fixed‐effect model when the I² statistic value less than or equal to 50%, and a random‐effects model otherwise. We used generic inverse variance (GIV) analysis for count data such as the rate ratio, where the estimates of log rate ratio and its SE were directly entered. This was to facilitate the use of rate ratios for studies where the actual denominator of person‐time was unavailable or was given in standardized units only, such as per 1000 child‐days. For dichotomous data, such as the risk ratio, we entered detailed information on the number of events and total participants rather than relying on the GIV method.
Subgroup analysis and investigation of heterogeneity
See the 'Differences between protocol and review' section.
Sensitivity analysis
See the 'Differences between protocol and review' section.
Results
Description of studies
See the 'Characteristics of included studies', 'Characteristics of excluded studies', and 'Characteristics of ongoing studies' sections.
Results of the search
We identified 245 titles and abstracts from our literature search (after we removed duplicates), which we further screened. We selected 60 abstracts for full‐text review, and four trials (six articles) met the inclusion criteria (Figure 1). Two trials reported the outcomes of pneumonia, diarrhoea, and any hospital admissions (Alonso 2011; Manaseki‐Holland 2012); for Alonso 2011, we sought detailed data on infections from the trial authors. All four included trials reported mean serum vitamin D concentrations (Greer 1981; Greer 1989; Alonso 2011; Manaseki‐Holland 2012). We did not find any studies that evaluated either tuberculosis (TB) or malaria as outcomes.
Included studies
We have presented essential information about the included trials in the 'Characteristics of included studies' table. We obtained the data on infections from the Alonso 2011 trial from the trial authors.
Greer 1981 was conducted in a single, private paediatric practice in Cincinnati, Ohio (USA) where the standard procedure was not to give breast‐fed babies vitamin D. It was a randomized double‐blind prospective trial. The trial included 18 healthy, term, exclusively breast‐fed infants and randomly divided participants into two groups. One group (N = 9) received a daily placebo of propylene glycol and the other group (N = 9) received vitamin D 400 IU/d till 12 weeks of age. The dose was in accordance with the IOM recommendations (400 IU/d). The proportion of season‐adjusted vitamin D deficiency/inadequacy in this population is reported to be 10% among male and 12% among female children during 2001 to 2006 (Looker 2011).
Greer 1989 was conducted in a private paediatric practice in Madison, Wisconsin (USA) where all mothers were planning to exclusively breast‐feed their babies for the first six months of life. It was a randomized double‐blind prospective trial. The trial included 46 healthy, term, breast‐fed white infants and randomly divided participants into two groups. One group (N = 24) received a daily placebo of propylene glycol and the other group (N = 22) received vitamin D 400 IU/d until six months of age. The dose was in accordance with the IOM recommendations (400 IU/d). The proportion of season‐adjusted vitamin D deficiency/inadequacy in this population is reported to be 10% among male and 12% among female children during 2001 to 2006 (Looker 2011).
Alonso 2011 was conducted in 11 primary healthcare centres of a community in northern Spain. The participants included healthy term infants presenting for a routine health visit in the first 15 days of life. The intervention group included 48 infants who were administered vitamin D 402 IU/day, while the control group comprised of 54 infants who did not receive either vitamin D or placebo. The trial excluded seven infants from each group before the start of the trial, with a final sample of 88 infants (intervention = 41, control = 47). There were 52.3% male participants; the follow‐up period was 12 months. The dose given was in accordance with the IOM recommendations (400 IU/d). The trial authors did not report the outcomes of incidence of pneumonia and diarrhoea in the published paper and we obtained unpublished data from the contact author Dr Maruchi Alonso. We have presented this data in Appendix 2. The proportion of vitamin D deficiency in the paediatric population in Italy with the same latitude as Spain is reported to be 6.2% (Lippi 2007).
Manaseki‐Holland 2012 was conducted in Kabul, Afghanistan, within the catchment area of a teaching hospital serving an inner‐city population. The participants included infants aged one to 11 months, with 1524 randomized to the intervention group and 1522 to placebo controls. The intervention group received quarterly oral 100,000 IU vitamin D3 supplementation versus placebo in the comparison group; the follow‐up period was 18 months. There were 52.2% male participants, and 42.4% were under six months of age. The frequency of supplementation ranged from a single dose to six doses during the follow‐up period. The dose given represented upper level of intake (1000 to 2500 IU/d) because most children were vitamin D‐deficient at baseline. The proportion of vitamin D deficiency in this paediatric population is reported to be 73.1% (Manaseki‐Holland 2008).
Excluded studies
We excluded 54 studies that did not meet our inclusion criteria. We provided further details in the 'Characteristics of excluded studies' table.
Risk of bias in included studies
All the included trials were individually randomized controlled trials (RCTs). Figure 2 and Figure 3 provide a graphical summary of the 'Risk of bias' assessments for the included trials.
2.
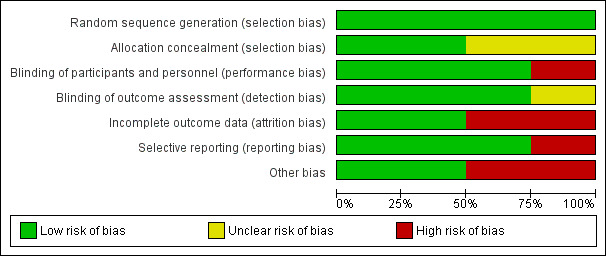
'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included trials.
3.
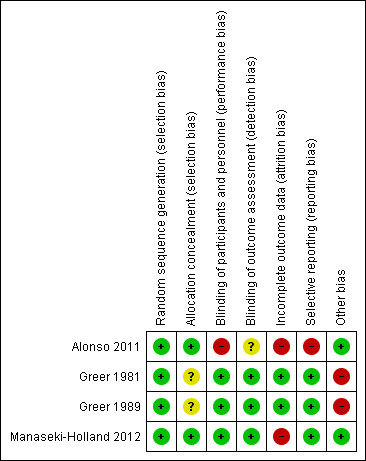
'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included trial.
Allocation
Alonso 2011 and Manaseki‐Holland 2012 were at low risk of bias for randomization as the trial used methods for random sequence generation and allocation concealment that were adequate. The randomization process in Greer 1981 and Greer 1989 was through random number tables by the pharmacist after the trial authors called in (obtained from communication with the trial authors) and so were at low risk for this bias; however, the trial authors did not describe the procedures for allocation concealment, which resulted in the judgements of unclear risk.
Blinding
Alonso 2011 was unblinded to participants and personnel and hence we rated it at high risk of bias; while the risk was unclear for the blinding of outcome assessment since it did not provide any information. Greer 1981, Greer 1989, and Manaseki‐Holland 2012 were at low risk of bias since the treatment was masked from participants and personnel and the outcome assessors were also blinded.
Incomplete outcome data
Alonso 2011 was at high risk of attrition bias since it had a high loss to follow‐up rate in the intervention group (26.8%) compared to the control group (8.5%) and the trial authors did not give the distribution of reasons for drop out in each group. Manaseki‐Holland 2012 was also at high risk of attrition bias since the attrition rate was approximately 30% in each group and the trial authors did not report the reasons for loss to follow‐up in the published paper. Greer 1981 and Greer 1989 were at low risk of attrition bias. In Greer 1981 there was no loss to follow‐up, while although in Greer 1989 the attrition rates were approximately 10% to 20%, the reasons for loss to follow‐up were similarly distributed between the two groups.
Selective reporting
The protocols were unavailable for any of the included trials, however Alonso 2011 did not report any of the infection outcomes in the published paper. Furthermore, the methods section mentioned outcomes such as child's body weight, length, and head circumference that the trial authors did not further discuss in the results section. The protocols of the Greer 1981, Greer 1989, and Manaseki‐Holland 2012 were unavailable but based on the 'methods' section of these articles, it seems the trial authors reported all expected pre‐specified outcomes.
Other potential sources of bias
Greer 1981 and Greer 1989 did not adequately describe the allocation concealment process, with a slight possibility of confounding and selection bias at baseline.
Effects of interventions
See: Table 1
Primary outcomes
Primary outcomes for our review included incidence rates of pneumonia and TB. Two trials reported incidence rates of pneumonia (Alonso 2011; Manaseki‐Holland 2012); while none reported incidence rate of TB. Since Manaseki‐Holland 2012 reported first and repeat episodes of pneumonia separately and we could not access data on overall person‐time of the children followed in the two groups, we therefore report this outcome as first and repeat episodes of pneumonia.
Incidence rate of first or only episode of pneumonia
Two trials reported incidence rate of first or only episode of pneumonia confirmed by chest radiograph (Alonso 2011; Manaseki‐Holland 2012). One trial reported confirmed or unconfirmed pneumonia (Manaseki‐Holland 2012). Moderate quality evidence showed that there was no effect of vitamin D supplementation on the incidence of first or only episode of pneumonia confirmed by chest radiograph (rate ratio (RR) 1.06, 95% confidence interval (CI) 0.89 to 1.26; 3134 participants, two trials, moderate quality evidence; Analysis 1.1; Figure 4). The result was similar for confirmed or unconfirmed pneumonia (RR 0.95, 95% CI: 0.87 to 1.04; 3046 participants, one trial) (Manaseki‐Holland 2012).
1.1. Analysis.
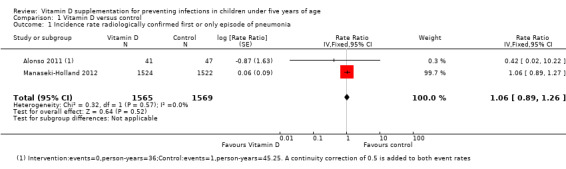
Comparison 1 Vitamin D versus control, Outcome 1 Incidence rate radiologically confirmed first or only episode of pneumonia.
4.
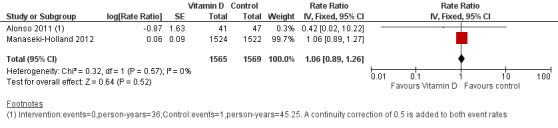
Forest plot of comparison: 1 Vitamin D versus control, outcome: 1.1 Incidence rate radiologically confirmed first or only episode of pneumonia.
Incidence rate of repeat episodes of pneumonia
One large trial from Afghanistan reported incidence rate of repeat episodes of pneumonia (Manaseki‐Holland 2012). There was a significant increase in repeat episodes pneumonia confirmed by chest radiograph (RR 1.69, 95% CI 1.28 to 2.21; 3046 participants, one trial), but not reflected in the outcome of confirmed or unconfirmed pneumonia (RR 1.06, 95% CI 1.00 to 1.13; 3046 participants; one trial).
Secondary outcomes
We reported the incidence rate of diarrhoea separately as first and repeat episodes of diarrhoea, since Manaseki‐Holland 2012 reported it separately and we could not access data on overall person‐time of the children followed in the two groups.
Incidence rate of first or only episode of diarrhoea
Two trials reported incidence rate of first or only episode of diarrhoea (Alonso 2011; Manaseki‐Holland 2012); however, we could not perform a meta‐analysis since Alonso 2011 reported rate ratios while Manaseki‐Holland 2012 reported hazard ratios (HR). The incidence of first or only episode of diarrhoea was similar in the supplemented and unsupplemented children in both trials (RR 0.14, 95% CI 0.01 to 2.59; 88 participants, Alonso 2011; and HR 1.02, 95% CI 0.95 to 1.11; 3046 participants, Manaseki‐Holland 2012).
Incidence rate of repeat episodes of diarrhoea
One trial reported the incidence of repeat episodes of diarrhoea (Manaseki‐Holland 2012). There was no effect of vitamin D supplementation on the repeat episodes of diarrhoea (HR 1.05, 95% CI 0.98 to 1.17; 3046 participants, one trial).
All‐cause mortality
One large trial from Afghanistan reported all‐cause mortality (Manaseki‐Holland 2012). Due to the low quality evidence from this trial and few events resulting in an underpowered outcome, we do not know whether vitamin D supplementation impacts on all‐cause mortality (RR 1.43, 95% CI 0.54 to 3.74; 3046 participants, one trial; Analysis 1.2; Figure 5). The risk difference showed no excess deaths in the supplementation group compared to the control group (risk difference 0.00, 95% CI −0.00 to 0.01; 3046 participants, one trial).
1.2. Analysis.
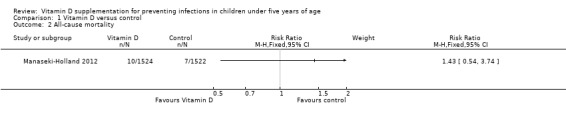
Comparison 1 Vitamin D versus control, Outcome 2 All‐cause mortality.
5.

Forest plot of comparison: 1 Vitamin D versus control, outcome: 1.2 All‐cause mortality.
Cause‐specific mortality
The same large trial in Afghanistan, Manaseki‐Holland 2012, reported no significant difference in pneumonia and septicaemia cause‐specific mortality between the supplemented and the control groups (RR 1.50, 95% CI 0.42 to 5.30; 3046 participants, one trial. There was no reported cause‐specific mortality in either group in the Alonso 2011 trial.
Hospital admissions
Two trials reported that vitamin D supplementation did not affect any hospital admissions (Alonso 2011; Manaseki‐Holland 2012). Numeric data was unavailable from Manaseki‐Holland 2012 (despite contact with the trial authors). The estimates from Alonso 2011 trial did not detect a difference, but numbers were small (RR 0.86; 95% CI 0.20 to 3.62, 88 participants, one trial; very low quality evidence).
Mean serum vitamin D concentrations
Four trials reported mean serum vitamin D concentrations (Greer 1981; Greer 1989; Alonso 2011; Manaseki‐Holland 2012). At the end of supplementation period, mean concentrations of vitamin D were higher in the supplemented relative to unsupplemented children (mean difference (MD) 7.72 ng/mL, 95% CI 0.50 to 14.93; 266 participants, four trials; low quality evidence; Analysis 1.4; Figure 6). This was driven primarily by two smaller trials (Greer 1981; Greer 1989). In the larger two trials that also contributed to outcomes of pneumonia and mortality (Alonso 2011; Manaseki‐Holland 2012), vitamin D concentrations were higher in the intervention group at other time points but could not be sustained until the end of supplementation.This may be related to time elapsed at measurement (four months) from the last dose (Manaseki‐Holland 2012), or other reasons such as incomplete compliance or increased need of vitamin D with infant age (Alonso 2011). Baseline vitamin D concentrations were available for one out of four trials (MD 0.34 ng/mL, 95% CI −3.30 to 3.98; 46 participants, one trial; Analysis 1.5). We contacted the authors of the remaining three trials but either the baseline measurement was not done (Alonso 2011), was done one week after the first dose (Manaseki‐Holland 2012), or the trial was too old for trial authors to have the data (Greer 1981).
1.4. Analysis.

Comparison 1 Vitamin D versus control, Outcome 4 End of supplementation mean serum vitamin D concentrations in ng/mL.
6.
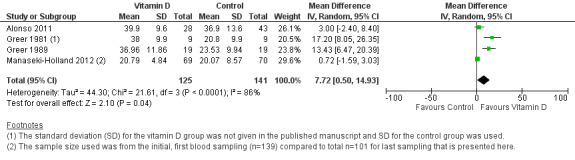
Forest plot of comparison: 1 Vitamin D versus control, outcome: 1.4 Mean serum vitamin D concentrations in ng/mL.
1.5. Analysis.
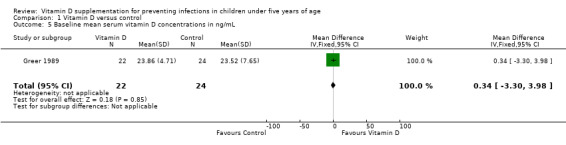
Comparison 1 Vitamin D versus control, Outcome 5 Baseline mean serum vitamin D concentrations in ng/mL.
Adverse events
None of the included trials reported any adverse events of vitamin D supplementation. In Alonso 2011, one infant had vitamin D concentrations that were too high (almost 100 ng/mL) and supplementation was suspended. An abdominal ultrasound and a subsequent analytical control were performed, and were normal (personal communication with Alonso M). Two children in Manaseki‐Holland 2012 trial had toxic concentrations of vitamin D (150 ng/mL).
None of the included trials reported other prespecified secondary outcomes including incidence rates of TB, malaria, and febrile illness; duration of pneumonia; duration of diarrhoea; severity of infection; cause‐specific mortality (due to TB, diarrhoea, or malaria); and adverse events, such as hypercalcaemia or seizures.
Subgroup analyses
We did not find sufficient trials to conduct prespecified subgroup analyses or to explore heterogeneity.
Sensitivity analyses
We did not find sufficient trials to conduct prespecified sensitivity analyses.
Discussion
This systematic review evaluated the effects of vitamin D supplementation on preventing pneumonia, tuberculosis (TB), diarrhoea, and malaria in children under five years of age.
Summary of main results
In the large trial from Afghanistan, the incidence of first or only episode of pneumonia (both radiologically confirmed, and confirmed or unconfirmed) was similar in supplemented and unsupplemented children (Manaseki‐Holland 2012). None of the included trials reported the impact of vitamin D supplementation on incidence rate of TB. Among secondary outcomes, there was no effect of vitamin D supplementation on first and repeat episodes of diarrhoea, all‐cause mortality, cause specific mortality, and any hospital admissions. The mean vitamin D concentrations at the last follow‐up were higher in intervention compared to control groups in the meta‐analysis of four, driven by two smaller trials (Greer 1981; Greer 1989). In individual results of larger two trials (Alonso 2011; Manaseki‐Holland 2012), the concentrations in the intervention and control groups were similar at the last follow‐up, although levels were higher in intervention group at other time points during the trial. There were no adverse events reported in any of the included trials. None of the included trials reported other prespecified secondary outcomes, including incidence rates of malaria, incidence rates of febrile illness, duration of pneumonia, duration of diarrhoea, severity of infection, and cause‐specific mortality (due to TB, diarrhoea, and malaria).
Overall completeness and applicability of evidence
We found that there was no benefit of vitamin D supplementation in preventing either pneumonia or diarrhoea in children under five years. However, the included trials had limitations. Alonso 2011 had a very small sample size and may not have been adequately powered to detect small differences in outcome. This trial was conducted in a developed country among infants at lower risk of vitamin D deficiency. Manaseki‐Holland 2012 was a secondary analysis of a large trial conducted in Afghanistan; a population that has both a high prevalence of vitamin D deficiency and incidence of pneumonia. This trial was adequately powered with a larger sample size; however, it is only generalizable to similar settings. Greer 1981 and Greer 1989 were small trials and the allocation process in these trials was also not adequately described. Thus, the existing evidence of vitamin D supplementation among children on infectious diseases has limited applicability since we included only two trials in separate settings in the final analysis. The higher number of repeat episodes of pneumonia in the vitamin D supplementation group is counterintuitive and based on a single trial that is likely to be a chance finding (Manaseki‐Holland 2012). However, the need for further trials in areas with low vitamin D deficiency should be assessed based on other competing resources for research.
Quality of the evidence
We judged Greer 1981 and Greer 1989 as at unclear risk of bias for allocation concealment. However, these trials were of low risk of bias for random sequence generation, blinding, incomplete outcome, and selective reporting.
We considered the Alonso 2011 trial to be at 'high risk of bias' for blinding, incomplete outcome, and selective reporting. This trial did not use a placebo, and the participants, trial personnel, and outcome assessors were not blinded to the treatment assignment, thereby introducing possible differential misclassification of outcomes that could shift the rate ratios in either direction. Alonso 2011 did not report any of the infection‐related outcomes in the published paper.
Regarding the large trial from Afghanistan, Manaseki‐Holland 2012, we judged it to be at 'low risk of bias' for randomization, allocation concealment, blinding, and selective reporting. However, the trial had a high loss to follow‐up across all intervention groups. There was potential random misclassification of episodes of pneumonia, both confirmed or unconfirmed by chest radiographs, that could have attenuated the results towards the null (Manaseki‐Holland 2012).
For important outcomes we used the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach to assess the quality of the evidence. We rated the quality of the evidence for the 'incidence rate of radiologically confirmed first or only episode of pneumonia' as moderate quality ; while 'all‐cause mortality', 'cause‐specific mortality' and 'mean serum vitamin D concentrations' were low quality ; and 'any hospital admissions' were very low quality.
Potential biases in the review process
We believe that there were minimal potential biases in this review process. There was a systematic evaluation at all stages, including literature search screening, full‐text eligibility, and data extraction. Two review authors did this independently and resolved discrepancies by discussion among all the review authors. We modified a few points in the protocol post hoc (after the data extraction) in this review to incorporate all the outcomes mentioned in the included trials, for example, all‐cause mortality and cause‐specific mortality, but most outcomes remained those prespecified in the protocol.
Agreements and disagreements with other studies or reviews
A previous meta‐analysis that covered two different trials studied the preventive effect of vitamin D supplementation on respiratory tract infections in paediatric populations (Charan 2012). The review did not evaluate pneumonia as an outcome separately from other upper and lower respiratory tract infections, such as influenza. It included the Manaseki‐Holland 2010 trial that we excluded because participant children had pneumonia at baseline at the start of supplementation. We included the additional trials of Alonso 2011 and Manaseki‐Holland 2012 This meta‐analysis did not study other infections, such as TB, diarrhoea, or malaria. Mao 2013 also did a meta‐analysis on the role of vitamin D supplementation in preventing respiratory tract infections but included both paediatric and adult studies. They included only one trial, Manaseki‐Holland 2012, from among two trials on infections selected for our review. Multiple other reviews on vitamin D supplementation studied either high‐risk children with asthma or cystic fibrosis or reported outcomes other than infections such as bone mineral density or miscellaneous other outcomes (Ferguson 2009; Winzenberg 2010; Winzenberg 2011; Theodoratou 2014; Pojsupap 2015).
Authors' conclusions
Implications for practice.
This Cochrane review has limited implications for current practice or changing guidelines regarding vitamin D supplementation to healthy children, given the evidence from only very few studies available. The large trial from Afghanistan suggested no benefit of vitamin D supplementation on pneumonia or diarrhoea incidence (Manaseki‐Holland 2012).
Implications for research.
Our findings suggest that this area of research should be prioritized as only four trials (two with infections as outcome) met our inclusion criteria. Whether further trials are worthwhile in areas with low baseline vitamin D deficiency is a judgement that needs to be made carefully against other competing resources for research. Effect modification in outcomes according to baseline vitamin D concentrations may be studied. However, it is notable that most children in the Manaseki‐Holland 2012 trial were likely to be vitamin D‐deficient (Manaseki‐Holland 2008) and yet no benefits were observed. In this trial, the vitamin D concentrations were higher in the intervention group for most of the study duration but were not different at the last measurement that was four months after the last dose. Also, whether the intermittent bolus therapy of vitamin D as used in this trial may be more efficient than a regimen of daily therapy in increasing serum vitamin D concentrations is still uncertain as the evidence is mixed (Emel 2012; Tan 2015). In conclusion, there appears to be no benefit of vitamin D with regard to preventing infections, that is pneumonia and diarrhoea. The need for further RCTs should also be carefully considered in context of baseline vitamin D deficiency prevalence and other research priorities.
Acknowledgements
We thank the Alonso 2011 trial authors for providing data on infectious outcomes from their trial. We also acknowledge the contributions of Dr Shamlan M Sheikh in conducting the initial literature search for articles pertinent to the 'Background' section for the protocol (Yakoob 2010). The editorial base for the Cochrane Infectious Diseases Group is funded by the UK Department for International Development (DFID) for the benefit of low‐ and middle‐income countries (Grant: 5242). The views expressed in this review do not necessarily reflect UK government policy.
Appendices
Appendix 1. Detailed search strategies
| Search set | CIDG SR1 | CENTRAL | MEDLINE2 | EMBASE2 | LILACS2 |
| 1 | Vitamin D | VITAMIN D | VITAMIN D | VITAMIN D | Vitamin D |
| 2 | Child* | CHOLECALCIFEROL | CHOLECALCIFEROL | COLECALCIFEROL | Child$ |
| 3 | Infant* | ERGOCALCIFEROLS | ERGOCALCIFEROLS | ERGOCALCIFEROL | Infant$ |
| 4 | Newborn* | CALCITRIOL | VITAMIN D DEFICIENCY | Vitamin D [ti, ab] | Newborn$ |
| 5 | 2 3 or 4 | Vitamin D [ti, ab] | Vitamin D [ti, ab] | 1 or 2 or 3 or 4 | 2 or 3 or 4 |
| 6 | 1 and 5 | 1 or 2 or 3 or 4 or 5 | 1 or 2 or 3 or 4 or 5 | Child* [ti, ab] | 1 and 5 |
| 7 | ‐ | Child* [ti, ab] | Child* [ti, ab] | Infant* [ti, ab] | ‐ |
| 8 | ‐ | Infant* [ti, ab] | Infant* [ti, ab] | Newborn* [ti, ab] | ‐ |
| 9 | ‐ | Newborn* [ti, ab] | Newborn* [ti, ab] | Neonatal* [ti, ab] | ‐ |
| 10 | ‐ | Neonatal* [ti, ab] | Neonatal* [ti, ab] | 6 or 7 or 8 or 9 | ‐ |
| 11 | ‐ | 7 or 8 or 9 or 10 | 7 or 8 or 9 or 10 | Infect* [ti, ab] | ‐ |
| 12 | ‐ | Infect* [ti, ab] | Infect* [ti, ab] | Malaria [ti, ab] | ‐ |
| 13 | ‐ | Malaria [ti, ab] | Malaria [ti, ab] | Diarrh* [ti, ab] | ‐ |
| 14 | ‐ | Diarrh* [ti, ab] | Diarrh* [ti, ab] | Pneumonia [ti, ab] | ‐ |
| 15 | ‐ | Pneumonia [ti, ab] | Pneumonia [ti, ab] | Tuberculosis [ti, ab] | ‐ |
| 16 | ‐ | Tuberculosis [ti, ab] | Tuberculosis [ti, ab] | 11 or 12 or 13 or 14 or 15 | ‐ |
| 17 | ‐ | 12 or 13 or 14 or 15 or 16 | 12 or 13 or 14 or 15 or 16 | 5 and 10 and 16 | ‐ |
| 18 | ‐ | 6 and 11 and 17 | 6 and 11 and 17 | ‐ | ‐ |
| 1Cochrane Infectious Diseases Group Specialized Register. 2Search terms used in combination with the search strategy for retrieving trials developed by Cochrane (Higgins 2011); Upper case: MeSH or EMTREE heading; Lower case: free‐text term. | |||||
Appendix 2. Data provided by Alonso 2011 trial authors
| Age of infants (months) |
Vitamin D prophylaxis (N = 41) |
No vitamin D prophylaxis (N = 47) |
| 3 | 1 suspected sepsis 1 upper respiratory tract infection |
2 upper respiratory tract infection 2 Atopic dermatitis 3 Febrile syndrome 1 bronchiolitis 1 Dacryocystitis |
| 6 | 4 upper respiratory tract infection 1 pyelonephritis 1 asthma |
5 upper respiratory tract infection 2 gastroenteritis 1 pneumonia 1 bronchiolitis 1 febrile syndrome |
| 12 | 6 upper respiratory tract infection 3 asthma 1 bronchiolitis 1 urinary tract infection |
8 upper respiratory tract infection 2 gastroenteritis 1 bronchiolitis |
Abbreviations: N=Number of participants
Person‐time: in the prophylaxis group, 30 infants were followed for 12 months, 6 were excluded from 3 to 6 months and 5 infants were excluded from 6 to 12 months. In the group without prophylaxis: 43 infants were followed by 12 months; 2 were excluded from 3 to 6 months and 2 were excluded from 6 to 12 months.
These data can be found in Figure 1 (Alonso 2011).
The denominator (person‐time) in the prophylaxis group is: (6 × 4.5) + (5 × 9) + (30 × 12)= 27 + 45 + 360 = 432 persons‐months.
The denominator (person‐time) in the group without prophylaxis is: (2 × 4.5) + (2 × 9) + (43 × 12) = 9 + 18 + 516 = 543 persons‐months.
Data and analyses
Comparison 1. Vitamin D versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence rate radiologically confirmed first or only episode of pneumonia | 2 | 3134 | Rate Ratio (Fixed, 95% CI) | 1.06 [0.89, 1.26] |
| 2 All‐cause mortality | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3 Any hospital admission | 1 | 88 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.20, 3.62] |
| 4 End of supplementation mean serum vitamin D concentrations in ng/mL | 4 | 266 | Mean Difference (IV, Random, 95% CI) | 7.72 [0.50, 14.93] |
| 5 Baseline mean serum vitamin D concentrations in ng/mL | 1 | 46 | Mean Difference (IV, Fixed, 95% CI) | 0.34 [‐3.30, 3.98] |
1.3. Analysis.
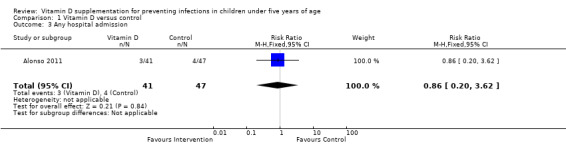
Comparison 1 Vitamin D versus control, Outcome 3 Any hospital admission.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Alonso 2011.
| Methods | Individual randomized trial. Location: Northern Spain. Setting: primary health care centres in a community. Duration: 12 months (enrolment from February 2007 through February 2008). |
|
| Participants | Number: 102 enrolled (14 excluded before start of prophylaxis, 7 in each group). Inclusion criteria: healthy term infants presenting for a routine health visit within the first 15 days of life. Exclusion criteria: infants with chronic disease, use of medications affecting vitamin D metabolism, refusal of parents, prematurity, dark skin, sunlight exclusion for cultural, religious or other reasons, and breastfeeding by vegetarian mothers. In summary, the trial excluded infants at risk of vitamin D deficiency. |
|
| Interventions | Intervention: vitamin D supplementation 402 IU/d containing 67 IU of cholecalciferol per drop (N = 41). Control: no vitamin D supplementation. No placebo was used (N = 47). |
|
| Outcomes |
|
|
| Sources of funding | The trial was funded partly by grant FIS ECO8/00238 from the Instituto de Salud Carlos III and by the Fundacion Nutricion y Crecimiento. | |
| Conflicts of interest | The trial authors did not report this information. | |
| Notes | We acquired data on infections (outcome 3), which were not reported in the published article, from the trial author. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The principal investigator made the assignment by phone using a computer software". Comment: adequately done. |
| Allocation concealment (selection bias) | Low risk | Quote: "The principal investigator made the assignment by phone using a computer software". Comment: adequately done. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "The study was not blinded to parents and investigators". Comment: unblinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: unclear. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Intervention group: 11/41 = 26.8% lost to follow‐up. Control group: 4/47 = 8.5% lost to follow‐up. The attrition rate was higher in intervention group. The trial authors mentioned the reasons for loss to follow‐up but did not give details of the distribution between groups. |
| Selective reporting (reporting bias) | High risk | The trial protocol was unavailable. However, the methods section mentions outcomes such as child's body weight, length and head circumference that the trial authors did not discuss in the results. The trial authors did not report infection outcomes and we obtained them from the unpublished data. |
| Other bias | Low risk | There was no other evidence of confounding or selection bias. |
Greer 1981.
| Methods | Double‐blind randomized prospective trial. Location: Cincinnati, Ohio. Setting: single private paediatric practice. Duration: 12 weeks. |
|
| Participants | Number: 18 enrolled. Inclusion criteria: healthy, term, exclusively breast‐fed infants between the second and third weeks of life. Exclusion criteria: Infants with major congenital anomalies, bone disorders, and gastrointestinal disease were excluded. |
|
| Interventions | Intervention: 400 IU of vitamin D2 per day diluted with propylene glycol to a concentration of 400 IU/ mL (N = 9). Control: a daily placebo of propylene glycol (N = 9). |
|
| Outcomes |
|
|
| Sources of funding | Supported in part by a grant from Ross Laboratories and the National Institute of Child Health and Human Development, HD 11725‐02. | |
| Conflicts of interest | The trial authors did not report this information. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Eighteen healthy, term, exclusively breast‐fed infants were divided randomly into two groups. The randomization was done with a random numbers table by the pharmacist after we called in" (obtained from communication with the authors). Comment: adequately done. |
| Allocation concealment (selection bias) | Unclear risk | Comment: unclear. The trial authors did not provide enough information. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Eighteen healthy, term, exclusively breast‐fed infants were divided randomly into two groups and studied prospectively in a double‐blind fashion". Comment: adequately done. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Eighteen healthy, term, exclusively breast‐fed infants were divided randomly into two groups and studied prospectively in a double‐blind fashion". Comment: adequately done. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there was no attrition or lost to follow‐up in this trial. |
| Selective reporting (reporting bias) | Low risk | The trial protocol was unavailable; but based on 'methods' section, it seems the trial authors reported all expected prespecified trial outcomes. |
| Other bias | High risk | There is a possibility of confounding and selection bias because the trial authors did not adequately describe the randomisation methods. |
Greer 1989.
| Methods | Double‐blind randomized prospective trial. Location: Madison, Wisconsin. Setting: private paediatric practice. Duration: 6 months (24 weeks). |
|
| Participants | Number: 46 enrolled from October 1985 to January 1987. Inclusion criteria: healthy, term, breast‐fed white infants during the first week of life. Exclusion criteria:Infants with major congenital anomalies, bone disorders, and gastrointestinal disease were excluded. |
|
| Interventions | Intervention: 400 IU of vitamin D2 per day diluted with propylene glycol to a concentration of 400 IU/ mL (N = 22). Control: a daily placebo of propylene glycol (N = 24). All participating families were given a small supply of vitamin D‐free formula to be used only for emergency situations. |
|
| Outcomes |
|
|
| Sources of funding | Supported by U.S. Department of Agriculture grant No. 85‐CRCR‐1‐1712. | |
| Conflicts of interest | This trial authors did not report this information. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Forty‐six term, breast‐fed infants were divided randomly into two groups. The randomization was done with a random numbers table by the pharmacist after we called in" (obtained from communication with the authors). Comment: adequately done. |
| Allocation concealment (selection bias) | Unclear risk | Comment: unclear risk. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Forty‐six term, breast‐fed infants were divided randomly into two groups and studied in a double‐blind fashion". Comment: adequately done. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Forty‐six term, breast‐fed infants were divided randomly into two groups and studied in a double‐blind fashion". Comment: adequately done. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intervention group: 3/22 = 13.6% lost to follow‐up at 6 months. Control group: 5/24 = 20.8% lost to follow‐up at 6 months. The attrition rate was high but almost similar across the two groups. The trial authors mentioned the reasons for loss to follow‐up and these were distributed equally between the two groups. |
| Selective reporting (reporting bias) | Low risk | The trial protocol was unavailable; but based on 'methods' section, it seems that the trial authors reported all expected prespecified outcomes from the trial. |
| Other bias | High risk | There is a possibility of confounding and selection bias because the trial authors did not adequately describe the randomization methods. |
Manaseki‐Holland 2012.
| Methods | Individual randomized placebo‐controlled superiority trial. Location: catchment area of the Maiwand Teaching Hospital, serving an inner‐city population in Kabul, Afghanistan. Setting: community‐based trial. Duration: 18 months, enrolment between 4 November and 4 December 2008 with follow‐up until May 2009. |
|
| Participants | Number: 3046 enrolled. Inclusion criteria: infants aged 1 to 11 months and living in the trial region. Exclusion criteria: children expected to migrate within 18 months, with diagnosis of rickets or past history of vitamin D treatment, or having kwashiorkor or marasmus. |
|
| Interventions | Intervention: quarterly supplementation of 100,000 IU (2.5 mg) of vitamin D (cholecalciferol) in olive oil (2 mL) (N = 1524). Control: 2 mL placebo (olive oil) (N = 1522). |
|
| Outcomes |
|
|
| Sources of funding | The Wellcome Trust and British Council Delphi programme funded this trial. USAID, Afghanistan and Washington State University also supported the trial author(s). | |
| Conflicts of interest | Trial authors have no known conflicts of interest. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "An independent statistician (Shabbar Jaffar) randomised unique identification numbers individually in fixed blocks of 20 to the vitamin D. or placebo group by use of a random number generator with the SAS routine". Comment: adequately done. |
| Allocation concealment (selection bias) | Low risk | Quote: "An independent statistician (Shabbar Jaffar) randomised unique identification numbers individually in fixed blocks of 20 to the vitamin D. or placebo group by use of a random number generator with the SAS routine." Comment: adequately done. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The vitamin D3 and the placebo were the same colour (pale yellow), taste, and quantity (0·5 mL) and therefore the study staff and the families did not know to which group the children were assigned". Comment: adequately done. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The masked radiographs were read by two independent paediatric radiologists". Comment: adequately done. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Intervention group: 436/1524 = 28.6% lost to follow‐up and 10/1524 died. Control group: 445/1522 = 29.2% lost to follow‐up and 7/1522 died. The attrition rate was high but similar across the two groups. The trial authors did not mention the reasons for loss to follow‐up. |
| Selective reporting (reporting bias) | Low risk | The trial protocol was unavailable; but based on 'methods' section, it seems that the trial authors reported all expected prespecified trial outcomes. |
| Other bias | Low risk | There was no other evidence of confounding or information bias (misclassification). |
Abbreviations: N: number of participants.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Al‐Shaar 2014 | This trial compared 2 different doses of vitamin D in adolescents. |
| Ala‐Houhala 1988 | This trial included children above 5 years of age. |
| Alam 2011 | This trial included children with acute diarrhoea. |
| Arpadi 2009 | This trial included HIV‐infected children aged 6 to 16 years. |
| Basile 2006 | This trial provided v itamin D supplementation to mothers and not to children. |
| Camargo 2012 | This trial targeted children above 5 years of age. |
| Camargo 2014 | This trial included children with a topic dermatitis. |
| Carpenter 1996 | This trial included children with hypophosphataemic rickets. |
| Choudhary 2012 | This trial included children with severe pneumonia. |
| Economos 2014 | This trial evaluated the impact of fortified juices. |
| Gallo 2013 | This trial did not have a placebo/control group and it compared different dosages of vitamin D. |
| Ganmaa 2012 | This trial included children above 5 years of age. |
| Gordon 2008 | This trial did not have a placebo/control group. |
| Grant 2015 | The trial supplemented b oth mothers from 27 weeks' gestation and their infants with vitamin D. |
| Hanson 2011 | This trial included preterm infants (< 32 weeks' gestational age) during initial hospitaliz ation. |
| Havens 2012 | This trial included HIV infected youth aged 18 to 24 years. |
| Hettiarachchi 2010 | This trial evaluated the impact of fortified cereal‐based food, not supplementation. |
| Hillman 2008 | This trial included children aged 3 to 15 years with juvenile arthritis. |
| Ho 1985 | This trial evaluated the impact of vitamin D obtained through sunshine and not through supplements. |
| Holmlund‐Suila 2012 | This trial did not have a placebo/control group and compared different dosages of vitamin D. |
| Kakalia 2011 | This trial included HIV‐infected children. |
| Khandelwal 2014 | This study evaluated the levels of Vitamin D in children diagnosed with tuberculosis . |
| Kilpinen‐Loisa 2007 | This trial included children aged 9 to 18 years with developmental disabilities. |
| Kumar 2011 | This trial included low birthweight term infants. |
| Kutluk 2002 | This trial included children with nutritional rickets. |
| Liakakos 1975 | This trial included epileptic children aged 5 to 14 years of age. |
| Lodha 2014 | This trial included children diagnosed with tuberculosis . |
| Lucas 1996 | This trial did not have a placebo/control group and targeted preterm infants. |
| Maalouf 2008 | This trial targeted children aged 10 to 17 years. |
| Madar 2009 | Vitamin D supplementation not the only difference between intervention and control groups (cluster‐ randomised controlled trial (cluster‐RCT)). The intervention group was infants of immigrant origin 6 weeks old who received free drops of vitamin D2 plus customiz ed information handouts compared to control group who received usual care. |
| Majak 2009 | This trial targeted asthmatic children aged 6 to 12 years. |
| Manaseki‐Holland 2010 | This trial included children with clinical episode of pneumonia at baseline. |
| Marchisio 2013 | This trial included otitis‐prone children and evaluated otitis media as outcome. |
| Morcos 1998 | This trial included children with tuberculosis. |
| Moya 1977 | This trial did not have a placebo/control group and included children with rickets. |
| Natarajan 2014 | This trial compared different doses of vitamin D and did not have a suitable placebo/control group. |
| Ndeezi 2010 | This trial did not have a suitable placebo/control group. |
| Pettifor 1986 | This trial included very‐low birthweight infants and did not have a suitable placebo/control group. |
| Rajakumar 2015 | This RCT included children 8 to 14 years old. |
| Rothberg 1982 | This trial included mother‐infant pairs, however all the infants received v itamin D and there was no control group. |
| Saadi 2009 | This trial did not have a suitable placebo/control group. |
| Sacheck 2015 | Th is RCT included schoolchildren aged 8 to 15 years. |
| Schou 2003 | This trial included asthmatic children above 5 years of age. |
| Schümann 2009 | This trial evaluated the impact of foodLETS (fortification in infant food), not supplementation. |
| Sidbury 2008 | This trial included children with atopic dermatitis. |
| Specker 1992 | This trial did not have suitable placebo/control group. |
| Stallings 2014 | This trial included human immunodeficiency virus (HIV)‐ infected children above 5 years of age. |
| Sudfeld 2015 | This trial included HIV‐ infected and HIV‐ exposed infants and did not have a suitable placebo/control group. |
| Tan 2015 | The study i ncluded Aboriginal children under 16 years of age. This was a RCT of vitamin D given as oral daily or single‐dose stoss therapy but had no placebo or control group. |
| Thacher 1999 | This trial included children with rickets. |
| Thacher 2009 | This trial compared 2 doses of vitamin D among children with rickets. |
| Urashima 2010 | This trial included children above 5 years of age. |
| Wagner 2006 | This trial supplemented mothers with different doses of v itamin D and did not have a suitable placebo/control group. |
| Zeghoud 1994 | This trial compared different doses of vitamin D and did not have a suitable placebo/control group. |
Abbreviations: HIV : human immun odeficiency virus; RCT: randomized controlled trial.
Characteristics of ongoing studies [ordered by study ID]
ACTRN12616000659404.
| Trial name or title | PREVARID ‐ PREVention of Acute Respiratory Infections with Vitamin D. Does vitamin D supplementation prevent acute respiratory infection health care visits among children under 2 years old? A randomized controlled trial |
| Methods | Parallel randomized controlled trial. |
| Participants | Children who are residents of New Zealand, are < 2 years old a the time of their acute lower respiratory tract infection (ALRI) hospital admission and reside in the Auckland District Health Board catchment area. |
| Interventions | Weekly vitamin D supplementation (5000 IU) for 12 months after ALRI hospital admission. |
| Outcomes | Number of ARI hospital admissions Number of ARI presentations to health care Number of ARI presentations to hospital emergency departments Number of antibiotic prescriptions dispensed during 12 month follow‐up Serum 25(OH)D concentration at baseline and 6 months, plus at 12 months in a 10% subsample |
| Starting date | 1 July 2016 |
| Contact information | cc.grant@auckland.ac.nz |
| Notes | The results are expected to be available by next year. No mention about stratification of ARI into pneumonia, bronchiolitis, upper respiratory tract infection, etc. |
NCT01229189.
| Trial name or title | Evaluation of the Effectiveness of Vitamin D Supplementation to Pregnant Women and Their Infants in Pakistan |
| Methods | Double‐blind randomized controlled trial. |
| Participants | Pregnant women from 20 to 22 weeks of gestation and their infants |
| Interventions | Vitamin D supplement versus placebo |
| Outcomes | Vitamin D deficiency Pre‐eclampsia Stillbirths Low birth weight Prematurity |
| Starting date | February 2010 |
| Contact information | zulfiqar.bhutta@aku.edu |
| Notes | No other details yet available |
NCT01419821.
| Trial name or title | Vitamin D and Its Affect on Growth Rates and Bone Mineral Density Until Age 5 |
| Methods | Double‐blind randomized controlled trial |
| Participants | Children between 9 to 12 months of age with normal 25(OH)D levels and those with 25(OH)D deficiency. Children with vitamin D deficiency were randomized. |
| Interventions | Vitamin D supplementation of 800 IU for one year versus placebo |
| Outcomes | Height at the age of 3 years. Bone densitometry by ultrasound. |
| Starting date | September 2011 |
| Contact information | avigdor@hadassah.org.il |
| Notes | The outcomes may be irrelevant to this Cochrane review |
NCT02046577.
| Trial name or title | A Randomized, Double‐blind, Controlled Trial of Vitamin D for the Prevention of Acute Respiratory Infections in Children Aged 18 to 36 Months in Santiago, Coyhaique and Punta Arenas, Chile. |
| Methods | Double‐blind randomized controlled trial, efficacy study, parallel assignment. |
| Participants | 276 preschool children aged 18 to 36 months attending daycare in Santiago, Coyhaique, or Punta Arenas. |
| Interventions | Oral 5600 IU vitamin D3 versus oral 11200 IU vitamin D3 versus oral placebo in liquid weekly during 6 months. |
| Outcomes | Incidence of acute respiratory tract infections at 6 months. Adverse events during 6 months. Hospitalizations due to acute respiratory tract infections during 6 months. Serum cathelicidin levels at baseline and 6 months. Serum 25(OH)D levels at baseline and 6 months. Viral etiology of acute respiratory tract infections during 6 months. Bone metabolism parameters, that is serum measurement of parathyroid hormone, alkaline phosphatases, calcium, phosphorus, and urinary calcium/creatinine ratio at baseline and 6 months. |
| Starting date | February 2014 |
| Contact information | mlreyes@med.puc.cl |
| Notes | Results of the trial not yet available. Estimated study completion date: May 2016. |
Differences between protocol and review
Two new authors joined the review author team (RAS and FR).
Under the 'Types of participants' section, we modified the exclusion criteria. We excluded studies among children with severe illnesses like rickets, human immunodeficiency virus (HIV)/acquired immune deficiency syndrome (AIDS), meningitis, severe malnutrition, sickle cell disease, etc. Similarly, we also decided to exclude studies of supplementation in preterm and low birthweight infants. These groups represent high‐risk children and might have different biological response to supplementation and extremely high baseline risk of infections, and results would not be generalizable to healthy children.
In the 'Types of interventions' section, we removed the condition of at least two weeks of supplementation. The pattern/dosage of supplementation of vitamin D is different from other micronutrients such as zinc. It can be given in large doses as a single administration and need not be given on a continuous daily basis for supplementation.
We amended the wording of 'vitamin D levels' to 'vitamin D concentrations' throughout the review.
We added the outcomes of all‐cause mortality and cause‐specific mortality post hoc because the included trials presented data on these outcomes.
We modified the 'Assessment of risk of bias in included studies' section to make it more explicit and in accordance with the latest recommendations of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
We removed sections on dealing with cluster‐randomized controlled trials (cluster‐RCTs) since we did not find any cluster‐RCTs in this review.
We did not implement the following aspects of analyses in the protocol.
Assessment of reporting biases
If we had found a sufficient number of trials that met the inclusion criteria of this Cochrane review, we would have generated funnel plots to help assess the possibility of publication bias. We would have inspected the funnel plot visually for asymmetry and also have used statistical analytical indicators such as the Begg's or Egger's P‐values. If we suspected publication bias, then we would have used the trim‐and‐fill method to infer the existence of unpublished studies, as determined from the funnel plot, and would subsequently have corrected the meta‐analysis by imputing the presence of missing studies to yield an unbiased pooled estimate.
Subgroup analyses and investigation of heterogeneity
We had planned to carry out the following three prespecified subgroup analyses for incidence of infections.
Subgroup analysis according to the age of participants: less than one year of age and one to less than five years of age.
Subgroup analysis to examine the possibility that there would be a variable response according to the dosages of vitamin D supplementation, for example, standard dosages versus high (greater than standard).
Subgroup analysis according to the duration of vitamin D supplementation, for example, low (six months or less) versus high (greater than six months).
We had also planned to explore the contribution of these variables to heterogeneity by meta‐regression.
Sensitivity analyses
We had planned to perform sensitivity analyses according to the following factors.
Method and adequacy of allocation concealment.
Blinding status of the participants.
Percentage lost to follow‐up by excluding studies with an attrition of greater than or equal to 20%.
Random‐effects model for the primary analysis.
Contributions of authors
Drs Mohammad Y Yakoob, Farhan Raza, and Rehana A Salam participated in all steps of manuscript preparation, including literature search, data extraction, analysis, and manuscript writing. Dr Zulfiqar A Bhutta conceptualized the idea of the review, edited the manuscript, and is the overall guarantor of the integrity of this work.
Sources of support
Internal sources
-
Aga Khan University, Pakistan.
Financial support
Liverpool School of Tropical Medicine, UK.
External sources
-
Department for International Development (DFID), UK.
Grant: 5242
Declarations of interest
Dr. Zulfiqar Bhutta was a co‐investigator in the Manaseki‐Holland 2012 trial in Afghanistan, which we included in this Cochrane review.
Unchanged
References
References to studies included in this review
Alonso 2011 {published and unpublished data}
- Alonso A, Rodríguez J, Carvajal I, Prieto MA, Rodríguez RM, Pérez AM, et al. Prophylactic vitamin D in healthy infants: assessing the need. Metabolism 2011;60(12):1719‐25. [DOI] [PubMed] [Google Scholar]
Greer 1981 {published data only}
- Greer FR, Searcy JE, Levin RS, Steichen JJ, Asch PS, Tsang RC. Bone mineral content and serum 25‐hydroxyvitamin D concentration in breast‐fed infants with and without supplemental vitamin D. The Journal of Pediatrics 1981;98(5):696‐701. [DOI] [PubMed] [Google Scholar]
- Greer FR, Searcy JE, Levin RS, Steichen JJ, Steichen‐Asche PS, Tsang RC. Bone mineral content and serum 25‐hydroxyvitamin D concentrations in breast‐fed infants with and without supplemental vitamin D: one‐year follow‐up. The Journal of Pediatrics 1982;100(6):919‐22. [DOI] [PubMed] [Google Scholar]
Greer 1989 {published data only}
- Greer FR, Marshall S. Bone mineral content, serum vitamin D metabolite concentrations, and ultraviolet B light exposure in infants fed human milk with and without vitamin D2 supplements. The Journal of Pediatrics 1989;114(2):204‐12. [DOI] [PubMed] [Google Scholar]
Manaseki‐Holland 2012 {published data only}
- Aluisio AR, Maroof Z, Chandramohan D, Bruce J, Mughal MZ, Bhutta Z, et al. Vitamin D₃ supplementation and childhood diarrhea: a randomized controlled trial. Pediatrics 2013;132(4):e832‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manaseki‐Holland S, Maroof Z, Bruce J, Mughal MZ, Masher MI, Bhutta ZA, et al. Effect on the incidence of pneumonia of vitamin D supplementation by quarterly bolus dose to infants in Kabul: a randomised controlled superiority trial. The Lancet 2012;379(9824):1419‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Ala‐Houhala 1988 {published data only}
- Ala‐Houhala M, Koskinen T, Koskinen M, Visakorpi JK. Double blind study on the need for vitamin D supplementation in prepubertal children. Acta Paediatrica Scandinavica 1988;77(1):89‐93. [DOI] [PubMed] [Google Scholar]
Alam 2011 {published data only}
- Alam NH, Ashraf H, Gyr NE, Meier RF. Efficacy of L‐isoleucine supplemented food and vitamin D in the treatment of acute diarrhea in children. Gastroenterology 2011;140(5 Suppl 1):S571. [Google Scholar]
Al‐Shaar 2014 {published data only}
- Al‐Shaar L, Mneimneh R, Nabulsi, Maalouf J, Fuleihan Gel‐H. Vitamin D3 dose requirement to raise 25‐hydroxyvitamin D to desirable levels in adolescents: results from a randomized controlled trial. Journal of Bone and Mineral Research 2014;29(4):944‐51. [DOI] [PubMed] [Google Scholar]
Arpadi 2009 {published data only}
- Arpadi SM, McMahon D, Abrams EJ, Bamji M, Purswani M, Engelson ES, et al. Effect of bimonthly supplementation with oral cholecalciferol on serum 25‐hydroxyvitamin D concentrations in HIV‐infected children and adolescents. Pediatrics 2009;123(1):e121‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Basile 2006 {published data only}
- Basile LA, Taylor SN, Wagner CL, Horst RL, Hollis BW. The effect of high‐dose vitamin D supplementation on serum vitamin D levels and milk calcium concentration in lactating women and their infants. Breastfeeding Medicine 2006;1(1):27‐35. [DOI] [PubMed] [Google Scholar]
Camargo 2012 {published data only}
- Camargo CA Jr, Ganmaa D, Frazier AL, Kirchberg FF, Stuart JJ, Kleinman K, et al. Randomized trial of vitamin d supplementation and risk of acute respiratory infection in Mongolia. Pediatrics 2012;130(3):e561‐7. [DOI] [PubMed] [Google Scholar]
Camargo 2014 {published data only}
- Camargo CA Jr, Ganmaa D, Sidbury R, Erdenedelger K, Radnaakhand N, Khandsuren B. Randomized trial of vitamin D supplementation for winter‐related atopic dermatitis in children. The Journal of Allergy and Clinical Immunology 2014;134(4):831‐5.e.1. [DOI] [PubMed] [Google Scholar]
Carpenter 1996 {published data only}
- Carpenter TO, Keller M, Schwartz D, Mitnick M, Smith C, Ellison A, et al. 24,25 Dihydroxyvitamin D supplementation corrects hyperparathyroidism and improves skeletal abnormalities in X‐linked hypophosphatemic rickets‐‐a clinical research center study. Journal of Clinical Endocrinology and Metabolism 1996;81(6):2381‐8. [DOI] [PubMed] [Google Scholar]
Choudhary 2012 {published data only}
- Choudhary N, Gupta P. Vitamin D supplementation for severe pneumonia‐‐a randomized controlled trial. Indian Pediatrics 2012;49(6):449‐54. [DOI] [PubMed] [Google Scholar]
Economos 2014 {published data only}
- Economos CD, Moore CE, Hyatt RR, Kuder J, Chen T, Meydani SN, et al. Multinutrient‐fortified juices improve vitamin D and vitamin E status in children: a randomized controlled trial. Journal of the Academy of Nutrition and Dietetics 2014;114(5):709‐17. [DOI] [PubMed] [Google Scholar]
Gallo 2013 {published data only}
- Gallo S, Comeau K, Vanstone C, Agellon S, Sharma A, Jones G, et al. Effect of different dosages of oral vitamin D supplementation on vitamin D status in healthy, breastfed infants: a randomized trial. JAMA 2013;309(17):1785‐92. [DOI] [PubMed] [Google Scholar]
Ganmaa 2012 {published data only}
- Ganmaa D, Giovannucci E, Bloom BR, Fawzi W, Burr W, Batbaatar D, et al. Vitamin D, tuberculin skin test conversion, and latent tuberculosis in Mongolian school‐age children: a randomized, double‐blind, placebo‐controlled feasibility trial. American Journal of Clinical Nutrition 2012;96(2):391‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gordon 2008 {published data only}
- Gordon CM, Williams AL, Feldman HA, May J, Sinclair L, Vasquez A, et al. Treatment of hypovitaminosis D in infants and toddlers. Journal of Clinical Endocrinology and Metabolism 2008;93(7):2716‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Grant 2015 {published data only}
- Grant CC, Kaur S, Waymouth E, Mitchell EA, Scragg R, Ekeroma A, et al. Reduced primary care respiratory infection visits following pregnancy and infancy vitamin D supplementation: a randomised controlled trial. Acta Paediatrica 2015;104(4):396‐404. [DOI] [PubMed] [Google Scholar]
Hanson 2011 {published data only}
- Hanson C, Armas L, Lyden E, Anderson‐Berry A. Vitamin D status and associations in newborn formula‐fed infants during initial hospitalization. Journal of the American Dietetic Association 2011;111(12):1836‐43. [DOI] [PubMed] [Google Scholar]
Havens 2012 {published data only}
- Havens PL, Mulligan K, Hazra R, Flynn P, Rutledge B, Loan MD, et al. Serum 25‐hydroxyvitamin D response to vitamin D3 supplementation 50,000 IU monthly in youth with HIV‐1 infection. Journal of Clinical Endocrinology and Metabolism 2012;97(11):4004‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hettiarachchi 2010 {published data only}
- Hettiarachchi M, Lekamwasam S, Liyanage C. Long‐term cereal‐based nutritional supplementation improved the total spine bone mineral density amongst Sri Lankan preschool children: a randomized controlled study. Journal of Pediatric Endocrinology & Metabolism 2010;23(6):555‐63. [DOI] [PubMed] [Google Scholar]
Hillman 2008 {published data only}
- Hillman LS, Cassidy JT, Chanetsa F, Hewett JE, Higgins BJ, Robertson JD. Percent true calcium absorption, mineral metabolism, and bone mass in children with arthritis: effect of supplementation with vitamin D3 and calcium. Arthritis and Rheumatism 2008;58(10):3255‐63. [DOI] [PubMed] [Google Scholar]
Ho 1985 {published data only}
- Ho ML, Yen HC, Tsang RC, Specker BL, Chen XC, Nichols BL. Randomized study of sunshine exposure and serum 25‐OHD in breast‐fed infants in Beijing, China. The Journal of Pediatrics 1985;107(6):928‐31. [DOI] [PubMed] [Google Scholar]
Holmlund‐Suila 2012 {published data only}
- Holmlund‐Suila E, Viljakainen H, Hytinantti T, Lamberg‐Allardt C, Andersson S, Mäkitie O. High‐dose vitamin D intervention in infants—effects on vitamin D status, calcium homeostasis, and bone strength.. Journal of Clinical Endocrinology and Metabolism 2012;97(11):4139‐47. [DOI] [PubMed] [Google Scholar]
Kakalia 2011 {published data only}
- Kakalia S, Sochett EB, Stephens D, Assor E, Read SE, Bitnun A. Vitamin D supplementation and CD4 count in children infected with human immunodeficiency virus. The Journal of Pediatrics 2011;159(6):951‐7. [DOI] [PubMed] [Google Scholar]
Khandelwal 2014 {published data only}
- Khandelwal D, Gupta N, Mukherjee A, Lodha R, Singh V, Grewal HM, et al. Vitamin D levels in Indian children with intrathoracic tuberculosis. The Indian Journal of Medical Research 2014;140(4):531‐7. [PMC free article] [PubMed] [Google Scholar]
Kilpinen‐Loisa 2007 {published data only}
- Kilpinen‐Loisa P, Nenonen H, Pihko H, Mäkitie O. High‐dose vitamin D supplementation in children with cerebral palsy or neuromuscular disorder. Neuropediatrics 2007;38(4):167‐72. [DOI] [PubMed] [Google Scholar]
Kumar 2011 {published data only}
- Kumar GT, Sachdev HS, Chellani H, Rehman AM, Singh V, Arora H, et al. Effect of weekly vitamin D supplements on mortality, morbidity, and growth of low birthweight term infants in India up to age 6 months: randomised controlled trial. BMJ 2011;342:d2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kutluk 2002 {published data only}
- Kutluk G, Cetinkaya F, Başak M. Comparisons of oral calcium, high dose vitamin D and a combination of these in the treatment of nutritional rickets in children. Journal of Tropical Pediatrics 2002;48(6):351‐3. [DOI] [PubMed] [Google Scholar]
Liakakos 1975 {published data only}
- Liakakos D, Papadopoulos Z, Vlachos P, Boviatsi E, Varonos DD. Serum alkaline phosphatase and urinary hydroxyproline values in children receiving phenobarbital with and without vitamin D. The Journal of Pediatrics 1975;87(2):291‐6. [DOI] [PubMed] [Google Scholar]
Lodha 2014 {published data only}
- Lodha R, Mukherjee A, Singh V, Singh S, Friis H, Faurholt‐Jepsen D, et al. Effect of micronutrient supplementation on treatment outcomes in children with intrathoracic tuberculosis: a randomized controlled trial. The American Journal of Clinical Nutrition 2014;100(5):1287‐97. [DOI] [PubMed] [Google Scholar]
Lucas 1996 {published data only}
- Lucas A, Fewtrell MS, Morley R, Lucas PJ, Baker BA, Lister G, et al. Randomized outcome trial of human milk fortification and developmental outcome in preterm infants. The American Journal of Clinical Nutrition 1996;64(2):142‐51. [DOI] [PubMed] [Google Scholar]
Maalouf 2008 {published data only}
- Maalouf J, Nabulsi M, Vieth R, Kimball S, El‐Rassi R, Mahfoud Z, et al. Short‐ and long‐term safety of weekly high‐dose vitamin D3 supplementation in school children. The Journal of Clinical Endocrinology and Metabolism 2008;93(7):2693‐701. [DOI] [PMC free article] [PubMed] [Google Scholar]
Madar 2009 {published data only}
- Madar AA, Klepp KI, Meyer HE. Effect of free vitamin D(2) drops on serum 25‐hydroxyvitamin D in infants with immigrant origin: a cluster randomized controlled trial. European Journal of Clinical Nutrition 2009;63(4):478‐84. [DOI] [PubMed] [Google Scholar]
Majak 2009 {published data only}
- Majak P, Rychlik B, Stelmach I. The effect of oral steroids with and without vitamin D3 on early efficacy of immunotherapy in asthmatic children. Clinical and Experimental Allergy 2009;39(12):1830‐41. [DOI] [PubMed] [Google Scholar]
Manaseki‐Holland 2010 {published data only}
- Manaseki‐Holland S, Qader G, Isaq Masher M, Bruce J, Zulf Mughal M, Chandramohan D, et al. Effects of vitamin D supplementation to children diagnosed with pneumonia in Kabul: a randomised controlled trial. Tropical Medicine & International Health 2010;15(10):1148–55. [DOI] [PubMed] [Google Scholar]
Marchisio 2013 {published data only}
- Marchisio P, Consonni D, Baggi E, Zampiero A, Bianchini S, Terranova L, et al. Vitamin D supplementation reduces the risk of acute otitis media in otitis‐prone children. The Pediatric Infectious Disease Journal 2013;32(10):1055‐60. [DOI] [PubMed] [Google Scholar]
Morcos 1998 {published data only}
- Morcos MM, Gabr AA, Samuel S, Kamel M, Baz M, Beshry M, et al. Vitamin D administration to tuberculous children and its value. Bollettino Chimico Farmaceutico 1998;137(5):157‐64. [PubMed] [Google Scholar]
Moya 1977 {published data only}
- Moya M, Beltran J, Colomer J. Therapeutic and collateral effects of 25‐hydroxycholecalciferol in vitamin D deficiency. European Journal of Pediatrics 1977;127(1):49‐55. [DOI] [PubMed] [Google Scholar]
Natarajan 2014 {published data only}
- Natarajan CK, Sankar MJ, Agarwal R, Pratap OT, Jain V, Gupta N, et al. Trial of daily vitamin D supplementation in preterm infants. Pediatrics 2014;133(3):e628‐34. [DOI] [PubMed] [Google Scholar]
Ndeezi 2010 {published data only}
- Ndeezi G, Tylleskär T, Ndugwa CM, Tumwine JK. Effect of multiple micronutrient supplementation on survival of HIV‐infected children in Uganda: a randomized, controlled trial. Journal of the International AIDS Society 2010;13:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pettifor 1986 {published data only}
- Pettifor JM, Stein H, Herman A, Ross FP, Blumenfeld T, Moodley GP. Mineral homeostasis in very low birth weight infants fed either own mother's milk or pooled pasteurized preterm milk. Journal of Pediatric Gastroenterology and Nutrition 1986;5(2):248‐53. [PubMed] [Google Scholar]
Rajakumar 2015 {published data only}
- Rajakumar K, Moore CG, Yabes J, Olabopo F, Haralam MA, Comer D, et al. Effect of vitamin D3 supplementation in black and in white children: a randomized, placebo‐controlled trial. The Journal of Clinical Endocrinology and Metabolism 2015;100(8):3183‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rothberg 1982 {published data only}
- Rothberg AD, Pettifor JM, Cohen DF, Sonnendecker EW, Ross FP. Maternal‐infant vitamin D relationships during breast‐feeding. The Journal of Pediatrics 1982;101(4):500‐3. [DOI] [PubMed] [Google Scholar]
Saadi 2009 {published data only}
- Saadi HF, Dawodu A, Afandi B, Zayed R, Benedict S, Nagelkerke N, et al. Effect of combined maternal and infant vitamin D supplementation on vitamin D status of exclusively breastfed infants. Maternal & Child Nutrition 2009;5(1):25‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sacheck 2015 {published data only}
- Sacheck JM, Rompay MI, Olson EM, Chomitz VR, Goodman E, Gordon CM, et al. Recruitment and retention of urban schoolchildren into a randomized double‐blind vitamin D supplementation trial. Clinical Trials 2015;12(1):45‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schou 2003 {published data only}
- Schou AJ, Heuck C, Wolthers OD. Does vitamin D administered to children with asthma treated with inhaled glucocorticoids affect short‐term growth or bone turnover?. Pediatric Pulmonology 2003;36(5):399‐404. [DOI] [PubMed] [Google Scholar]
Schümann 2009 {published data only}
- Schümann K, Longfils P, Monchy D, Xylander S, Weinheimer H, Solomons NW. Efficacy and safety of twice‐weekly administration of three RDAs of iron and folic acid with and without complement of 14 essential micronutrients at one or two RDAs: a placebo‐controlled intervention trial in anemic Cambodian infants 6 to 24 months of age. European Journal of Clinical Nutrition 2009;63(3):355‐68. [DOI] [PubMed] [Google Scholar]
Sidbury 2008 {published data only}
- Sidbury R, Sullivan AF, Thadhani RI, Camargo CA Jr. Randomized controlled trial of vitamin D supplementation for winter‐related atopic dermatitis in Boston: a pilot study. The British Journal of Dermatology 2008;159(1):245‐7. [DOI] [PubMed] [Google Scholar]
Specker 1992 {published data only}
- Specker BL, Ho ML, Oestreich A, Yin TA, Shui QM, Chen XC, et al. Prospective study of vitamin D supplementation and rickets in China. The Journal of Pediatrics 1992;120(5):733‐9. [DOI] [PubMed] [Google Scholar]
Stallings 2014 {published data only}
- Stallings VA, Schall JI, Hediger ML, Zemel BS, Tuluc F, Dougherty KA, et al. High‐dose vitamin D3 supplementation in children and young adults with HIV: a randomized, placebo‐controlled trial. The Pediatric Infectious Disease Journal 2014;34(2):32‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sudfeld 2015 {published data only}
- Sudfeld CR, Duggan C, Aboud S, Kupka R, Manji KP, Kisenge R, et al. Vitamin D status is associated with mortality, morbidity, and growth failure among a prospective cohort of HIV‐infected and HIV‐exposed Tanzanian infants. The Journal of Nutrition 2015;145(1):121‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tan 2015 {published data only}
- Tan JK, Kearns P, Martin AC, Siafarikas A. Randomised controlled trial of daily versus stoss vitamin D therapy in Aboriginal children. Journal of Paediatrics Child Health 2015;51(6):626‐31. [DOI] [PubMed] [Google Scholar]
Thacher 1999 {published data only}
- Thacher TD, Fischer PR, Pettifor JM, Lawson JO, Isichei CO, Reading JC, et al. A comparison of calcium, vitamin D, or both for nutritional rickets in Nigerian children. The New England Journal of Medicine 1999;341(8):563‐8. [DOI] [PubMed] [Google Scholar]
Thacher 2009 {published data only}
- Thacher TD, Obadofin MO, O'Brien KO, Abrams SA. The effect of vitamin D2 and vitamin D3 on intestinal calcium absorption in Nigerian children with rickets. The Journal of Clinical Endocrinology and Metabolism 2009;94(9):3314‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Urashima 2010 {published data only}
- Urashima M, Segawa T, Okazaki M, Kurihara M, Wada Y, Ida H. Randomized trial of vitamin D supplementation to prevent seasonal influenza A in schoolchildren. The American Journal of Clinical Nutrition 2010;91(5):1255‐60. [DOI] [PubMed] [Google Scholar]
Wagner 2006 {published data only}
- Wagner CL, Hulsey TC, Fanning D, Ebeling M, Hollis BW. High‐dose vitamin D3 supplementation in a cohort of breastfeeding mothers and their infants: a 6‐month follow‐up pilot study. Breastfeeding Medicine 2006;1(2):59‐70. [DOI] [PubMed] [Google Scholar]
Zeghoud 1994 {published data only}
- Zeghoud F, Ben‐Mekhbi H, Djeghri N, Garabédian M. Vitamin D prophylaxis during infancy: comparison of the long‐term effects of three intermittent doses (15, 5, or 2.5 mg) on 25‐hydroxyvitamin D concentrations. The American Journal of Clinical Nutrition 1994;60(3):393‐6. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
ACTRN12616000659404 {unpublished data only}
- PREVARID ‐ PREVention of Acute Respiratory Infections with Vitamin D. Does vitamin D supplementation prevent acute respiratory infection health care visits among children under 2 years old? A randomized controlled trial. Ongoing study 1 July 2016.
NCT01229189 {unpublished data only}
- NCT01229189. Evaluation of the Effectiveness of Vitamin D Supplementation to Pregnant Women and Their Infants in Pakistan. clinicaltrials.gov/show/NCT01229189 (accessed 18 January 2014).
NCT01419821 {unpublished data only}
- NCT01419821. Vitamin D and Its Affect on Growth Rates and Bone Mineral Density Until Age 5 (VitD). clinicaltrials.gov/show/NCT01419821 (accessed 18 January 2014).
NCT02046577 {published data only}
- Study of Vitamin D for the Prevention of Acute Respiratory Infections in Children. clinicaltrials.gov/ct2/show/NCT02046577 Accessed: 15 June 2016.
Additional references
Akpede 1999
- Akpede GO, Omotara BA, Ambe JP. Rickets and deprivation: a Nigerian study. The Journal of the Royal Society for the Promotion of Health 1999;119(4):216‐22. [DOI] [PubMed] [Google Scholar]
Akpede 2001
- Akpede GO, Solomon EA, Jalo I, Addy EO, Banwo AI, Omotara BA. Nutritional rickets in young Nigerian children in the Sahel savanna. East African Medical Journal 2001;78(11):568‐75. [DOI] [PubMed] [Google Scholar]
Bassil 2013
- Bassil D, Rahme M, Hoteit M, Fuleihan Gel‐H. Hypovitaminosis D in the Middle East and North Africa: Prevalence, risk factors and impact on outcomes. Dermato‐Endocrinology 2013;5(2):274‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bentley 2013
- Bentley J. Vitamin D deficiency: identifying gaps in the evidence base. Nursing Standard 2013;27(46):35‐41. [DOI] [PubMed] [Google Scholar]
Bhutta 2008
- Bhutta ZA. Vitamin D and child health: some emerging issues. Maternal & Child Nutrition 2008;4(2):83‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Canadian Paediatric Society 2007
- Canadian Paediatric Society. Vitamin D supplementation: Recommendations for Canadian mothers and infants. Paediatrics & Child Health 2007;12(7):583‐9. [PMC free article] [PubMed] [Google Scholar]
Cannell 2008
- Cannell JJ, Hollis BW. Use of vitamin D in clinical practice. Alternative Medicine Review 2008;13(1):6‐20. [PubMed] [Google Scholar]
Charan 2012
- Charan J, Goyal JP, Saxena D, Yadav P. Vitamin D for prevention of respiratory tract infections: A systematic review and meta‐analysis. Journal of Pharmacology & Pharmacotherapeutics 2012;3(4):300‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cusick 2014
- Cusick SE, Opaka RO, Lund TC, John CC, Polgreen LE. Vitamin D insufficiency is common in Ugandan children and is associated with severe malaria. PLoS One 2014;9(12):e113185. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dimitrov 2015
Du 2001
- Du X, Greenfield H, Fraser DR, Ge K, Trube A, Wang Y. Vitamin D deficiency and associated factors in adolescent girls in Beijing. The American Journal of Clinical Nutrition 2001;74(4):494‐500. [DOI] [PubMed] [Google Scholar]
Elidrissy 1984
- Elidrissy AT, Sedrani SH, Lawson DE. Vitamin D deficiency in mothers of rachitic infants. Calcified Tissues International 1984;36(3):266‐8. [DOI] [PubMed] [Google Scholar]
Emel 2012
- Emel T, Doğan DA, Erdem G, Faruk O. Therapy strategies in vitamin D deficiency with or without rickets: efficiency of low‐dose stoss therapy. Journal of Pediatric Endocrinology and Metabolism 2012;25(1‐2):107‐10. [DOI] [PubMed] [Google Scholar]
Ferguson 2009
- Ferguson JH, Chang AB. Vitamin D supplementation for cystic fibrosis. Cochrane Database of Systematic Reviews 2014, Issue 5. [DOI: 10.1002/14651858.CD007298.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Garabedian 1991
- Garabedian M, Ben‐Mekhbi H. Is vitamin D deficiency rickets a public health problem in France and Algeria?. In: Glorieux FH editor(s). Rickets. New York: Raven, 1991:215‐21. [Google Scholar]
Ghai 1991
- Ghai OP, Koul PB. Rickets in India. In: Glorieux FH editor(s). Rickets. New York: Raven, 1991:247‐52. [Google Scholar]
GRADEpro GDT 2015
- GRADEpro GDT: GRADEpro Guideline Development Tool [Software]. McMaster University, 2015 (developed by Evidence Prime, Inc.). Available from www.gradepro.org.
Grant 2009
- Grant CC, Wall CR, Crengle S, Scragg R. Vitamin D deficiency in early childhood: prevalent in the sunny South Pacific. Public Health Nutrition 2009;12(10):1893‐901. [DOI] [PubMed] [Google Scholar]
Gray 2012
- Gray K, Wood N, Gunasekera H, Sheikh M, Hazelton B, Barzi F, et al. Vitamin d and tuberculosis status in refugee children. The Pediatric Infectious Disease Journal 2012;31(5):521‐3. [DOI] [PubMed] [Google Scholar]
Gunville 2013
- Gunville CF, Mourani PM, Ginde AA. The role of vitamin D in prevention and treatment of infection. Inflammation & Allergy‐Drug Targets 2013;12(4):239‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
He 2014
- He X, Yan J, Zhu X, Wang Q, Pang W, Qi Z, et al. Vitamin D inhibits the occurrence of experimental cerebral malaria in mice by suppressing the host inflammatory response. Journal of Immunology 2014;193(3):1314‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Holick 2008
- Holick MF. Vitamin D: a D‐Lightful health perspective. Nutrition Reviews 2008;66(10 Suppl 2):S182‐94. [DOI] [PubMed] [Google Scholar]
Holick 2011
- Holick MF, Binkley NC, Bischoff‐Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. Journal of Clinical Endocrinology and Metabolism 2011;96(7):1911‐30. [DOI] [PubMed] [Google Scholar]
IOM 2010
- Institute of Medicine. Dietary Reference Intakes for Calcium and Vitamin D. www.iom.edu/Reports/2010/Dietary‐Reference‐Intakes‐for‐Calcium‐and‐Vitamin‐D.aspx (accessed November 23, 2012).
Janssen 2007
- Janssen R, Bont L, Siezen CL, Hodemaekers HM, Ermers MJ, Doornbos G, et al. Genetic susceptibility to respiratory syncytial virus bronchiolitis is predominantly associated with innate immune genes. The Journal of Infectious Diseases 2007;196(6):826‐34. [DOI] [PubMed] [Google Scholar]
Jubulis 2014
- Jubulis J, Kinikar A, Ithape M, Khandave M, Dixit S, Hotalkar S, et al. Modifiable risk factors associated with tuberculosis disease in children in Pune, India. The International Journal of Tuberculosis and Lung Disease 2014;18(2):198‐204. [DOI] [PMC free article] [PubMed] [Google Scholar]
Karatekin 2009
- Karatekin G, Kaya A, Salihoğlu O, Balci H, Nuhoğlu A. Association of subclinical vitamin D deficiency in newborns with acute lower respiratory infection and their mothers. European Journal of Clinical Nutrition 2009;63(4):473‐7. [DOI] [PubMed] [Google Scholar]
Lawson 1999
- Lawson M, Thomas M. Vitamin D concentrations in Asian children aged 2 years living in England: population survey. BMJ 1999;318(7175):28. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lehtonen‐Veromaa 1999
- Lehtonen‐Veromaa M, Möttönen T, Irjala K, Kärkkäinen M, Lamberg‐Allardt C, Hakola P, et al. Vitamin D intake is low and hypovitaminosis D common in healthy 9‐ to 15‐year‐old Finnish girls. European Journal of Clinical Nutrition 1999;53(9):746‐51. [DOI] [PubMed] [Google Scholar]
Leikina 2005
- Leikina E, Delanoe‐Ayari H, Melikov K, Cho MS, Chen A, Waring AJ, et al. Carbohydrate‐binding molecules inhibit viral fusion and entry by crosslinking membrane glycoproteins. Nature Immunololgy 2005;6(10):995‐1001. [DOI] [PubMed] [Google Scholar]
Liang 2010
- Liang L, Chantry C, Styne DM, Stephensen CB. Prevalence and risk factors for vitamin D deficiency among healthy infants and young children in Sacramento, California. European Journal of Pediatrics 2010;169(11):1337‐44. [DOI] [PubMed] [Google Scholar]
Lippi 2007
- Lippi G, Montagnana M, Targher G. Vitamin D deficiency among Italian children. Canadian Medical Association Journal 2007;177(12):1529‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Looker 2011
- Looker AC, Johnson CL, Lacher DA, Pfeiffer CM, Schleicher RL, Sempos CT. Vitamin D Status: United States, 2001‐2006. National Center for Health Statistics Data Brief 2011;59:1‐8. [PubMed] [Google Scholar]
Luong 2015
- Luong KV, Nguyen LT. The role of vitamin D in malaria. Journal of Infection in Developing Countries 2015;9(1):8‐19. [DOI] [PubMed] [Google Scholar]
Manaseki‐Holland 2008
- Manaseki‐Holland S, Zulf Mughal M, Bhutta Z, Qasem Shams M. Vitamin D status of socio‐economically deprived children in Kabul, Afghanistan. International Journal for Vitamin and Nutrition Research 2008;78(1):16‐20. [DOI] [PubMed] [Google Scholar]
Mansbach 2009
- Mansbach JM, Ginde AA, Camargo CA Jr. Serum 25‐hydroxyvitamin D levels among US children aged 1 to 11 years: do children need more vitamin D?. Pediatrics 2009;124(5):1404‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mao 2013
- Mao S, Huang S. Vitamin D supplementation and risk of respiratory tract infections: a meta‐analysis of randomized controlled trials. Scandinavian Journal of Infectious Diseases 2013;45(9):696‐702. [DOI] [PubMed] [Google Scholar]
McNally 2009
- McNally JD, Leis K, Matheson LA, Karuananyake C, Sankaran K, Rosenberg AM. Vitamin D deficiency in young children with severe acute lower respiratory infection. Pediatric Pulmonology 2009;44(10):981‐8. [DOI] [PubMed] [Google Scholar]
Misra 2008
- Misra M, Pacaud D, Petryk A, Collett‐Solberg PF, Kappy M, Drug and Therapeutics Committee of the Lawson Wilkins Pediatric Endocrine Society. Vitamin D deficiency in children and its management: review of current knowledge and recommendations. Pediatrics 2008;122(2):398‐417. [DOI] [PubMed] [Google Scholar]
Muhe 1997
- Muhe L, Lulseged S, Mason KE, Simoes EA. Case‐control study of the role of nutritional rickets in the risk of developing pneumonia in Ethiopian children. The Lancet 1997;349(9068):1801‐4. [DOI] [PubMed] [Google Scholar]
Najada 2004
- Najada AS, Habashneh MS, Khader M. The frequency of nutritional rickets among hospitalized infants and its relation to respiratory diseases. Journal of Tropical Pediatrics 2004;50(6):364‐8. [DOI] [PubMed] [Google Scholar]
Nicolaidou 2006
- Nicolaidou P, Hatzistamatiou Z, Papadopoulou A, Kaleyias J, Floropoulou E, Lagona E, et al. Low vitamin D status in mother‐newborn pairs in Greece. Calcified Tissues International 2006;78(6):337‐42. [DOI] [PubMed] [Google Scholar]
Nimitphong 2013
- Nimitphong H, Holick MF. Vitamin D status and sun exposure in southeast Asia. Dermato‐Endocrinology 2013;5(1):34‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nnoaham 2008
- Nnoaham KE, Clarke A. Low serum vitamin D levels and tuberculosis: a systematic review and meta‐analysis. International Journal of Epidemiology 2008;37(1):113‐9. [DOI] [PubMed] [Google Scholar]
Ozgür 1996
- Ozgür S, Sümer H, Koçoğlu G. Rickets and soil strontium. Archives of Disease in Childhood 1996;75(6):524‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pojsupap 2015
- Pojsupap S, Iliriani K, Sampaio TZ, O'Hearn K, Kovesi T, Menon K, et al. Efficacy of high‐dose vitamin D in pediatric asthma: a systematic review and meta‐analysis. The Journal of Asthma 2015;52(4):382‐90. [DOI] [PubMed] [Google Scholar]
Prentice 2008
- Prentice A. Vitamin D deficiency: a global perspective. Nutrition Reviews 2008;66(10 Suppl 2):S153‐64. [DOI] [PubMed] [Google Scholar]
Ray 2012
- Ray S, Kamath KS, Srivastava R, Raghu D, Gollapalli K, Jain R, et al. Serum proteome analysis of vivax malaria: An insight into the disease pathogenesis and host immune response. Journal of Proteomics 2012;75(10):3063‐80. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roth 2009
- Roth DE, Shah R, Black RE, Baqui AH. Vitamin D status and acute lower respiratory infection in early childhood in Sylhet, Bangladesh. Acta Paediatrica 2009;99(3):389‐93. [DOI] [PubMed] [Google Scholar]
Salimpour 1975
- Salimpour R. Rickets in Tehran. Study of 200 cases. Archives of Disease in Childhood 1975;50(1):63‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sautet 1957
- Sautet J, Vuillet J, Arnaud G. Effects of the immediate adjunction of cod liver oil or vitamin D and calcium biphosphate to antimalarial drugs used in the treatment of Plasmodium berghei infections. II. Bulletin de la Société de la Pathologie Exotique et de ses Filiales 1957;50(1):44‐9. [PubMed] [Google Scholar]
Schünemann 2009
- Schünemann HJ. GRADE: from grading the evidence to developing recommendations. A description of the system and a proposal regarding the transferability of the results of clinical research to clinical practice [GRADE: Von der Evidenz zurEmpfehlung. Beschreibung des Systems und Losungsbeitrag zur Ubertragbarkeit von Studienergebnissen]. Zeitschrift für Evidenz, Fortbildung und Qualität im Gesundheitswesen 2009;103(6):391‐400. [DOI] [PubMed] [Google Scholar]
Sergacheva 1986
- Sergacheva IuIu, Sokanenkova TL, Soprunov FF, Lur'e AA. Effect of vitamins D and E on the development of Plasmodium berghei infection in mice. Meditsinskaia Parazitologiia i Parazitarnye Bolezni (Mosk) 1986;4:15‐8. [PubMed] [Google Scholar]
Shapses 2011
- Shapses SA, Manson JE. Vitamin D and prevention of cardiovascular disease and diabetes: why the evidence falls short. JAMA 2011;305(24):2565‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sichert‐Hellert 2006
- Sichert‐Hellert W, Wenz G, Kersting M. Vitamin intakes from supplements and fortified food in German children and adolescents: results from the DONALD study. The Journal of Nutrition 2006;136(5):1329‐33. [DOI] [PubMed] [Google Scholar]
Tan 2015
- Tan JK, Kearns P, Martin AC, Siafarikas A. Randomised controlled trial of daily versus stoss vitamin D therapy in Aboriginal children. Journal of Paediatric Child Health 2015;51(6):626‐31. [DOI] [PubMed] [Google Scholar]
Theodoratou 2014
- Theodoratou E, Tzoulaki I, Zgaga L, Ioannidis JP. Vitamin D and multiple health outcomes: umbrella review of systematic reviews and meta‐analyses of observational studies and randomised trials. BMJ 2014;348:g2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thornton 2013
- Thornton KA, Marín C, Mora‐Plazas M, Villamor E. Vitamin D deficiency associated with increased incidence of gastrointestinal and ear infections in school‐age children. The Pediatric Infectious Disease Journal 2013;32(6):585‐93. [DOI] [PubMed] [Google Scholar]
Venturini 2014
- Venturini E, Facchini L, Martinez‐Alier N, Novelli V, Galli L, Martino M, et al. Vitamin D and tuberculosis: a multicenter study in children. BMC Infectious Diseases 2014;14:652. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vial 1982
- Vial HJ, Thuet MJ, Philippot JR. Inhibition of the in vitro growth of Plasmodium falciparum by D vitamins and vitamin D‐3 derivatives. Molecular Biochemistry and Parasitology 1982;5(3):189‐98. [DOI] [PubMed] [Google Scholar]
Wagner 2008a
- Wagner CL, Greer FR, American Academy of Pediatrics Section on Breastfeeding, American Academy of Pediatrics Committee on Nutrition. Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics 2008;122(5):1142‐52. [DOI] [PubMed] [Google Scholar]
Wagner 2008b
- Wagner CL, Taylor SN, Hollis BW. Does vitamin D make the world go 'round'?. Breastfeeding Medicine 2008;3(4):239‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Walker 2009
- Walker VP, Modlin RL. The vitamin D connection to pediatric infections and immune function. Pediatric Research 2009;65(5 Pt 2):106R‐13R. [DOI] [PMC free article] [PubMed] [Google Scholar]
Walker 2013
- Walker CL, Rudan I, Liu L, Nair H, Theodoratou E, Bhutta ZA, et al. Global burden of childhood pneumonia and diarrhoea. The Lancet 2013;381(9875):1405‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ward 2007
- Ward LM, Gaboury I, Ladhani M, Zlotkin S. Vitamin D‐deficiency rickets among children in Canada. Canadian Medical Association Journal 2007;177(2):161‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wayse 2004
- Wayse V, Yousafzai A, Mogale K, Filteau S. Association of subclinical vitamin D deficiency with severe acute lower respiratory infection in Indian children under 5 y. European Journal of Clinical Nutrition 2004;58(4):563‐7. [DOI] [PubMed] [Google Scholar]
White 2008
- White JH. Vitamin D signaling, infectious diseases, and regulation of innate immunity. Infection and Immunity 2008;76(9):3837‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Williams 2008
- Williams B, Williams AJ, Anderson ST. Vitamin D deficiency and insufficiency in children with tuberculosis. The Pediatric Infectious Disease Journal 2008;27(10):941‐2. [DOI] [PubMed] [Google Scholar]
Winzenberg 2010
- Winzenberg TM, Powell S, Shaw KA, Jones G. Vitamin D supplementation for improving bone mineral density in children. Cochrane Database of Systematic Reviews 2010, Issue 10. [DOI: 10.1002/14651858.CD006944.pub2] [DOI] [PubMed] [Google Scholar]
Winzenberg 2011
- Winzenberg T, Powell S, Shaw KA, Jones G. Effects of vitamin D supplementation on bone density in healthy children: systematic review and meta‐analysis. BMJ 2011;342:c7254. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yim 2007
- Yim S, Dhawan P, Ragunath C, Christakos S, Diamond G. Induction of cathelicidin in normal and CF bronchial epithelial cells by 1,25‐dihydroxyvitamin D(3). Journal of Cystic Fibrosis 2007;6(6):403‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2013
- Zhang W, Stoecklin E, Eggersdorfer M. A glimpse of vitamin D status in Mainland China. Nutrition 2013;29(7‐8):953‐7. [DOI] [PubMed] [Google Scholar]
Zhao 1991
- Zhao XH. Rickets in China. In: Glorieux FH editor(s). Rickets. New York: Raven, 1991:253‐61. [Google Scholar]
Zhao 1992
- Zhao XH. Nutritional situation of Beijing residents. Southeast Asian Journal of Tropical Medicine and Public Health 1992;23 Suppl 3:65‐8. [PubMed] [Google Scholar]
Zipitis 2006
- Zipitis CS, Markides GA, Swann IL. Vitamin D deficiency: prevention or treatment?. Archives of Disease in Childhood 2006;91(12):1011‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Yakoob 2010
- Yakoob MY, Bhutta ZA. Vitamin D supplementation for preventing infections in children less than five years of age. Cochrane Database of Systematic Reviews 2010, Issue 11. [DOI: 10.1002/14651858.CD008824] [DOI] [PMC free article] [PubMed] [Google Scholar]


