Abstract
Background
Tuberculosis (TB) of the gastrointestinal tract and any other organ within the abdominal cavity is abdominal TB, and most guidelines recommend the same six‐month regimen used for pulmonary TB for people with this diagnosis. However, some physicians are concerned whether a six‐month treatment regimen is long enough to prevent relapse of the disease, particularly in people with gastrointestinal TB, which may sometimes cause antituberculous drugs to be poorly absorbed. On the other hand, longer regimens are associated with poor adherence, which could increase relapse, contribute to drug resistance developing, and increase costs to patients and health providers.
Objectives
To compare six‐month versus longer drug regimens to treat people that have abdominal TB.
Search methods
We searched the following electronic databases up to 2 September 2016: the Cochrane Infectious Diseases Group Specialized Register, the Cochrane Central Register of Controlled Trials (CENTRAL), PubMed, Embase (accessed via OvidSP), LILACS, INDMED, and the South Asian Database of Controlled Clinical Trials. We searched the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) and ClinicalTrials.gov for ongoing trials. We also checked article reference lists.
Selection criteria
We included randomized controlled trials (RCTs) that compared six‐month regimens versus longer regimens that consisted of isoniazid, rifampicin, pyrazinamide, and ethambutol to treat adults and children that had abdominal TB. The primary outcomes were relapse, with a minimum of six‐month follow‐up after completion of antituberculous treatment (ATT), and clinical cure at the end of ATT.
Data collection and analysis
Two review authors independently selected trials, extracted data, and assessed the risk of bias in the included trials. For analysis of dichotomous outcomes, we used risk ratios (RR) with 95% confidence intervals (CIs). Where appropriate, we pooled data from the included trials in meta‐analyses. We assessed the quality of the evidence using the GRADE approach.
Main results
We included three RCTs, with 328 participants, that compared six‐month regimens with nine‐month regimens to treat adults with intestinal and peritoneal TB. All trials were conducted in Asia, and excluded people with HIV, those with co‐morbidities and those who had received ATT in the previous five years. Antituberculous regimens were based on isoniazid, rifampicin, pyrazinamide, and ethambutol, and these drugs were administered daily or thrice weekly under a directly observed therapy programme. The median duration of follow‐up after completion of treatment was between 12 and 39 months.
Relapse was uncommon, with two cases among 140 participants treated for six months, and no events among 129 participants treated for nine months. The small number of participants means we do not know whether or not there is a difference in risk of relapse between the two regimens (very low quality evidence). At the end of therapy, there was probably no difference in the proportion of participants that achieved clinical cure between six‐month and nine‐month regimens (RR 1.02, 95% CI 0.97 to 1.08; 294 participants, 3 trials, moderate quality evidence). For death, there were 2/150 (1.3%) in the six‐month group and 4/144 (2.8%) in the nine‐month group. All deaths occurred in the first four months of treatment, so was not linked to the duration of treatment in the included trials. Similarly, the number of participants that defaulted from treatment was small in both groups, and there may be no difference between them (RR 0.50, 95% CI 0.10 to 2.59; 294 participants, 3 trials, low quality evidence). Only one trial reported on adherence to treatment, with only one participant allocated to the nine‐month regimen presenting poor adherence to treatment. We do not know whether six‐month regimens are associated with fewer people experiencing adverse events that lead to treatment interruption (RR 0.53, 95% CI 0.18 to 1.55; 318 participants, 3 trials, very low quality evidence).
Authors' conclusions
We found no evidence to suggest that six‐month treatment regimens are inadequate for treating people that have intestinal and peritoneal TB, but numbers are small. We did not find any incremental benefits of nine‐month regimens regarding relapse at the end of follow‐up, or clinical cure at the end of therapy, but our confidence in the relapse estimate is very low because of size of the trials. Further research is required to make confident conclusions regarding the safety of six‐month treatment for people with abdominal TB. Larger studies that include HIV‐positive people, with long follow‐up for detecting relapse with reliability, would help improve our knowledge around this therapeutic question.
2 April 2019
Up to date
All studies incorporated from most recent search
All eligible published studies found in the last search (2 Sep, 2016) were included
Plain language summary
Six‐month therapy for people with abdominal tuberculosis
What is abdominal tuberculosis and why is duration of treatment important?
Abdominal tuberculosis (TB) is a type of TB that affects the gut, the peritoneum (the lining of the abdominal cavity), abdominal lymph nodes, and, more rarely, the solid organs in the abdomen (liver, pancreas, and spleen). Abdominal TB leads to severe illness in adults and children, and can cause complications, such as bowel rupture, which can lead to death.
Most current guidelines recommend treating people that have abdominal TB with antituberculous treatment (ATT) for six months, but some clinicians treat for longer periods due to concerns that six months is not adequate to achieve cure and prevent relapse of the disease after the end of treatment. Longer ATT regimens have disadvantages: patients may find it more difficult to adhere to the tablets; patients are exposed to the risk of side effects of ATT for longer periods; and the cost to health systems and to patients is greater.
What the evidence shows
Cochrane researchers examined the available evidence up to the 2 September 2016. We included three trials with 328 participants that compared six‐month ATT with nine‐month ATT; two were from India and one was from South Korea. The trials were mostly of high quality, although two had concerns of risk of bias for detecting relapse of the disease. All the trials included HIV‐negative adults with TB of the gut (gastrointestinal TB), and one also included TB of the peritoneum (peritoneal TB).
The results show that relapse was an uncommon event, but we are uncertain whether or not there is a difference between the six‐month and nine‐month groups as numbers of participants are small (very low quality evidence). Six‐month and nine‐month regimens are probably similarly effective in terms of the chances of achieving cure (moderate quality evidence). Death was uncommon in both groups, and all deaths occurred during the first four months of ATT, which suggests that duration of treatment did not have an effect on risk of death. Few people had poor treatment compliance, and few participants experienced side effects that led to their treatment being stopped or changed, and it was not possible to detect a difference between the groups.
Six‐month regimens are probably as good as nine‐month regimens in terms of numbers of people cured. We found no evidence to suggest that six‐month regimens are less safe for gastrointestinal and peritoneal TB than nine‐month regimens, but we still do not know whether there is a difference in risk of relapse between the two regimens. Further studies are needed to increase our confidence as to whether six‐month regimens are as good as nine‐month regimens for preventing relapse; and to provide information about treating abdominal TB in children and in people with HIV.
Background
Description of the condition
Tuberculosis (TB) is an infectious disease that is caused by infection with bacterial species of the Mycobacterium tuberculosis complex. The World Health Organization (WHO) estimated that 9.6 million people developed the disease in 2014 (WHO 2015a). Alongside HIV, TB remains a leading cause of death worldwide and caused 1.5 million deaths in 2014, mostly in low‐ and middle‐income countries (WHO 2015a). There is also increasing incidence of TB in developed countries due to HIV co‐infection, the increased use of immunosuppressive therapy, and migration from high TB burden countries (Debi 2014; Kim 2003).
TB mainly affects the lungs (pulmonary TB), but can spread to other organs (extrapulmonary TB, EPTB). The term abdominal TB refers to TB infection in any of the structures within the abdominal cavity, which includes the gastrointestinal tract, the peritoneum (the lining of the abdominal cavity), the lymph nodes within the abdomen, and any of the solid organs in the abdomen (liver, pancreas, or spleen). Appendix 1 outlines the various forms of abdominal TB. Abdominal TB can present as isolated involvement of the gastrointestinal tract, the peritoneum, lymph nodes, or solid organs, or with the involvement of multiple sites (Debi 2014). The most common forms of abdominal TB affect the gastrointestinal tract, with the junction between the small bowel and large bowel (the ileocaecal area) being the most common site involved, and the peritoneum (Bolukbas 2005). In children, adhesive peritonitis and lymphadenopathy are the most common forms of abdominal TB (Tinsa 2010). Routine data collection by most national TB programmes worldwide does not currently report EPTB cases by organ system affected and estimates of prevalence vary considerably for abdominal TB, ranging from 3% to 17% of EPTB cases (Khan 2006; Sharma 2004; Sheer 2003). Sheer 2003 reported abdominal TB as the sixth most frequent site of EPTB.
Although abdominal TB can be detected in individuals of any age, young adults between 25 and 45 years are most commonly affected (Lazarus 2007). Abdominal TB can result from swallowing infected sputum, ingestion of contaminated milk products or meat, haematogenous spread from a tubercular focus in any other organ, spread via lymphatics from infected nodes, and contiguous spread from adjacent organs (Debi 2014; Lazarus 2007). The clinical presentation depends on the site infected. Abdominal pain, abdominal distension, diarrhoea, and constitutional symptoms of TB, such as weight loss and fever, are frequent manifestations of intestinal TB (Bolukbas 2005; Mamo 2013). The onset is insidious in most cases, but intestinal TB may present acutely with complications, such as intestinal obstruction and perforation. In addition to the common manifestations of abdominal TB, other symptoms may be present depending on the infected site. Colonic TB may present with chronic diarrhoea or recurrent partial intestinal obstruction and, uncommonly, with bleeding from the gastrointestinal tract, and rectal lesions such as anal fissures, fistulae, or perirectal abscesses (Golden 2005). Tuberculous peritonitis commonly presents with ascites (Debi 2014).
Microbiological diagnosis of abdominal TB by in vitro culture of M. tuberculosis is difficult, and the diagnosis is usually based on histopathological and radiological findings (Debi 2014; Mamo 2013). Barium contrast and abdominal computerized tomography with enterography (CT‐E) are helpful in establishing the diagnosis of gastrointestinal TB. Biopsy of the area affected in the gastrointestinal tract can be obtained by endoscopy or even laparotomy, in order to increase chances of definite diagnosis by identification of M. tuberculosis. Regarding TB peritonitis, examination of ascitic fluid usually shows characteristics of exudate, with high protein content, lymphocytic predominance, and high adenosine deaminase levels. In vitro culture of peritoneal (ascitic) fluid has very low sensitivity for isolation of M. tuberculosis, although concentration methods, such as centrifugation, may improve the yield. In vitro culture of peritoneal biopsy specimens has a higher sensitivity. Peritoneal specimens can be obtained with ultrasound guidance or via laparoscopy/laparotomy (Golden 2005).
Abdominal TB is frequently mistaken for other diseases that involve the abdomen, as the clinical presentation can mimic several other conditions. Regarding intestinal TB, the differential diagnosis includes Crohn’s disease, cancers, and other infectious diseases such as amoebiasis, gastrointestinal histoplasmosis, and Yersinia enterocolitis (Bolukbas 2005). Therefore, a high index of suspicion is required to make a prompt diagnosis and to start antituberculous treatment (ATT), which is essential to limit complications and prevent death (Balasubramanian 1997; Lazarus 2007).
Description of the intervention
Standardized international recommendations for treating people that have pulmonary TB consist of six‐month antituberculous regimens, which include isoniazid (H), rifampicin (R), and pyrazinamide (Z), usually with ethambutol (E) as a fourth drug during the first two months of treatment (intensive phase), followed by isoniazid and rifampicin for four additional months (continuation phase) (WHO 2010). More recently, some national TB programmes have amended the continuation phase to include ethambutol because of emerging isoniazid monoresistance (WHO 2014). In a person suffering from TB, M. tuberculosis is present in replicating and slow‐ or non‐replicating states. Bacilli in slow‐ or non‐replicating states are tolerant to some antituberculous drugs, and it is believed that these are responsible for the need for long antituberculous regimens with a combination of drugs (Raffetseder 2014; Zumla 2014). The discovery of new antituberculous drugs over the last 50 years, along with trials that have assessed different combinations and doses of antituberculous drugs, has allowed the shortening of treatment duration for pulmonary TB to six months, also known as short‐course ATT (Menzies 2009; Zumla 2014). The basic principles of ATT for pulmonary TB have been extrapolated to EPTB, with exceptions such as TB meningitis. Most current guidelines recommend the same six‐month regimen to treat people with drug‐sensitive abdominal TB as to treat people with pulmonary TB (American Thoracic Society 2003; WHO 2010). However, these recommendations have not been supported by high quality evidence. Trials that evaluated the effectiveness of six‐month ATT excluded EPTB cases because of difficulties in establishing a microbiological diagnosis and due to the lack of clear and reliable parameters for assessing treatment outcome (Kim 2003). There is reluctance among physicians, especially in low‐ and middle‐income countries, to treat abdominal TB with a six‐month regimen. This is based on concerns that short‐course ATT may not be long enough to eliminate slow‐ or non‐replicating bacilli in the infected site in order to prevent relapse of the disease, and because of the difficulties in assessing treatment response in abdominal TB (Park 2009). Thus, despite the current recommendations, many clinicians still treat patients with abdominal TB for more than six months (Debi 2014; Makharia 2015a).
How the intervention might work
Some trials have reported that six‐month antituberculous regimens are as effective as longer regimens in the treatment of people with abdominal TB (Balasubramanian 1997; Makharia 2015a). Long regimens are associated with poor adherence and loss of participants to follow‐up, which can lead to increased relapse rates and mortality. Poor adherence to ATT also facilitates the development of drug‐resistant TB strains (Zumla 2014). Finally, the other disadvantages of longer regimens are increased cost, and increased exposure to antituberculous drugs which may lead to increased drug toxicity (Park 2009).
On the other hand, relapse of the disease remains a concern when treating people with abdominal TB for six months. Short‐course regimens may not be long enough to eliminate slow‐ or non‐replicating mycobacteria in the infected sites, which may lead to higher relapse rates. Some manifestations of abdominal TB present with malabsorption, which raises the possibility that absorption of antituberculous drugs could be affected (Lazarus 2007).
According to the literature on pulmonary TB, most relapses occur within the first six to 12 months after completion of ATT (American Thoracic Society 2003; Park 2009). By extrapolating basic principles of pulmonary TB treatment to EPTB treatment due to a lack of data for abdominal TB treatment, a minimum of six‐month follow‐up after treatment completion is required to assess the relapse outcome. TB infection can relapse many years after initial treatment, so ideally long follow‐up periods are required to assess relapse rates. However, most deaths associated with abdominal TB seem to occur within the first weeks after diagnosis (Mamo 2013). Deaths are reduced by prompt diagnosis and early initiation of ATT (Debi 2014), and the role of duration of ATT in reducing deaths is uncertain.
Why it is important to do this review
The key concern for acceptance of a six‐month regimen for abdominal TB is whether six‐month regimens achieve successful treatment rates that are as good as longer regimens without significantly increasing the number of relapses. Few trials have assessed the effectiveness of six‐month regimens versus longer regimens for the treatment of this form of TB (Makharia 2015a; Park 2009; Tony 2008). As relapse is a relatively uncommon event, large numbers of participants are required to assess this outcome, and existing trials may be underpowered to detect a difference in relapse rates. Therefore, a meta‐analysis may be helpful to estimate the effect of six‐month ATT on relapse rates in people with abdominal TB.
Two review authors (SJu and HR) conducted an evidence review to compare the effects of treatment with the six‐month first‐line regimen 2RHZE/4RH versus the nine‐month regimen 2RHZE/7RH for abdominal TB for the Indian Extra‐Pulmonary TB (INDEX‐TB) guidelines, which forms the preliminary work for this Cochrane Review (INDEX‐TB 2016).
Objectives
To compare six‐month versus longer drug regimens to treat people that have abdominal tuberculosis (TB).
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials (RCTs) or quasi‐RCTs.
Types of participants
Adults and children with a diagnosis of presumed drug‐sensitive abdominal TB as defined by the trial authors, from all settings and countries.
Types of interventions
Short‐course regimens
Six‐month antituberculous regimens that contained a two‐month intensive phase with isoniazid, rifampicin, pyrazinamide, and ethambutol, followed by a continuation phase of four months that included at least isoniazid and rifampicin.
Prolonged‐course regimens
Antituberculous regimens of more than six months that contained a two‐month intensive phase with isoniazid, rifampicin, pyrazinamide, and ethambutol, followed by a continuation phase that included at least isoniazid and rifampicin.
Types of outcome measures
Primary outcomes
Relapse: participants who had new symptoms and signs of TB after resolution of disease and completion of antituberculous treatment (ATT).
Clinical cure: participants who completed treatment according to the original treatment plan without evidence of treatment failure at the end of treatment (WHO 2013).a
aThe World Health Organization (WHO)'s definitions for TB outcomes are primarily based on the assessment of pulmonary TB patients, so sputum smear and culture status are important factors in defining outcomes. In general, repeating biopsy of the infected tissue for histopathology and culture at the end of ATT is not done routinely in patients with abdominal TB. Therefore, in practice, bacteriological status is not part of the definition of cure or successful treatment. Consequently, we reported here the participants who the trial investigators considered cured based on signs and symptoms. We reported cure confirmed with complete healing of active lesions, documented by endoscopy for intestinal TB for example, as a secondary outcome.
Secondary outcomes
Death from any cause.
Treatment failure: failure to improve with ATT, or deterioration following initial improvement while on ATT.
Default: participants who discontinued ATT before the end of treatment, or participants whose treatment was interrupted for eight weeks or more consecutively (WHO 2013).
Poor adherence: lack of compliance with the treatment regimen, as reported by the trial authors, but did not meet the definition of ‘default’ outlined above.
Complete healing of active lesions, documented by endoscopy or histopathology.
Adverse events
Serious adverse events that were life‐threatening or led to hospitalization.
Adverse events that led to the discontinuation or modification of ATT.
Other adverse events related to ATT.
Timing of outcome assessment
For RCTs that reported on relapse, we included those with a minimum median of follow‐up of six months after ATT completion.
Search methods for identification of studies
We attempted to identify all relevant trials regardless of language or publication status (published, unpublished, in press, and ongoing).
Electronic searches
We searched the following databases, using the search terms and strategy we have described in Appendix 2: the Cochrane Infectious Disease Group Specialized Register; the Cochrane Central Register of Controlled Trials (CENTRAL, published in the Cochrane Library, Issue 1 2016); PubMed (1966 to 2 September 2016); Embase (OVID, 1980 to 2 September 2016); LILACS (1982 to 2 September 2016); INDMED (indmed.nic.in/, 2 September 2016); and the South Asian Database of Controlled Clinical trials (www.cochrane‐sadcct.org/, 2 September 2016). We also searched ClinicalTrials.gov and the search portal of the WHO International Clinical Trials Registry Platform (ICTRP) (www.who.int/trialsearch) (both accessed on 2 September 2016) to identify ongoing trials, and used “tuberculosis” and “abdominal or intestinal or hepatic or liver" as search terms.
Searching other resources
We checked the reference lists of existing reviews and of all trials identified by the above methods that meet our eligibility criteria, for other potentially relevant trials.
Data collection and analysis
Selection of studies
Two review authors (SJu and SJa) independently screened the titles and abstracts of the studies identified by the literature search for studies that may have met the eligibility criteria. We retrieved the full‐text articles of potentially eligible studies. SJu and SJa then independently assessed the full‐text studies for study eligibility using an eligibility form based on the predefined inclusion and exclusion criteria. We resolved any disagreements by discussion. We listed the excluded studies and their reasons for exclusion in a 'Characteristics of excluded studies' table. Also, we constructed a PRISMA diagram to illustrate the study selection process.
Data extraction and management
One review author (SJu) piloted the data extraction form on two included trials. Based on the pilot results, we modified and finalized the data extraction form. Two review authors (SJu and SJa) independently extracted data from the included trials using the agreed data extraction tool. We compared the data extracted by the two review authors to identify possible errors, and resolved any discrepancies through discussion and by referring to the original articles. We extracted the following data, when available.
Country, setting, when the trial was conducted, study design, inclusion and exclusion criteria applied, number of participants recruited to each trial arm.
Participant characteristics: age, gender, epidemiological data such as known contact with a TB patient, duration of the disease at presentation, severity of disease at presentation (as reported by the trial authors), features of malabsorption, site of the disease, comorbidity (HIV, malnutrition, other immunosuppressive conditions, and other diseases), co‐existing pulmonary TB or concurrent TB infection in any other organ, diagnostic methods and results (PPD skin test in mm, microscopy, culture, histology and cytology of ascitic fluid, lymph node aspirate or biopsy, other tissue biopsy, chest X‐ray, abdominal X‐ray, barium enema, computed tomography (CT) of the abdomen, endoscopy, laparoscopy, surgery), number of bacteriologically‐confirmed and clinically‐diagnosed cases of abdominal TB, and history of previous ATT received.
Intervention data: antituberculous drugs, dose, route of administration in both the intensive and continuation phases, and duration of each phase. Administration of other drugs or therapeutic procedures. Administration of treatment under directly observed therapy or unsupervised/home treatment.
Outcome data: for relapse, we extracted data on the participants who relapsed, clinical severity of relapse, method of diagnosis, and the time between end of treatment and relapse. For clinical cure, we extracted the exact definition used by the trial authors. For death, we extracted data on the time the death occurred related to the start of ATT, and the cause of death. For assessment of defaulters and adherence, we examined the methods for assuring adherence, including clinical history, direct observation, and tablet counting. We extracted the number of defaulters, and the number of participants with poor compliance, based on the definitions in the 'Secondary outcomes' section. For all the outcomes, we extracted, if available, data on site of disease and on HIV status.
Follow‐up: length of follow‐up, the way participants were followed up, the number and characteristics of losses to follow‐up.
For each established outcome, we extracted the number of participants randomized and the number of participants analysed in each treatment group. For dichotomous outcomes, we extracted the number of participants that experienced the event. For count data outcomes, such as adverse events, we extracted the number of events in the intervention and control groups.
Assessment of risk of bias in included studies
Two review authors (SJu and SJa) independently assessed the methodological quality of each included trial using the Cochrane 'Risk of bias' assessment tool, which addresses sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting, and other biases (Higgins 2011). As blinding of outcome assessment would introduce different risk of detection bias for objective and subjective outcomes, we have assessed these two groups of outcomes separately. Indeed we considered both objective outcomes, death, default, and poor adherence, and subjective outcomes (based on clinician judgment), relapse, clinical cure, complete healing of active lesions, and treatment failure. We collected the outcomes of this review at two endpoints: at the end of ATT (clinical cure, complete healing of active lesions, death, treatment failure, default, and poor adherence) and at the end of the follow‐up period (relapse), thus we have assessed the risk of attrition bias accordingly.
For each component, we classified our judgments as either at 'low', 'high', or 'unclear' risk of bias, according to Jüni 2001. For attrition and reporting biases, we based our judgments on the following criteria.
Incomplete outcome data (attrition bias) for outcomes assessed at the end of ATT (clinical cure, complete healing of active lesions, death, treatment failure, default, and poor adherence)
Low risk: less than 5% of participants lost to follow‐up during ATT.
Unclear risk: between 5% and 10% of participants lost to follow‐up during ATT.
High risk: more than 10% of participants lost to follow‐up during ATT.
Incomplete outcome data (attrition bias) for outcomes assessed at the end of the follow‐up period (relapse)
Low risk: less than 5% of participants lost to follow‐up at the end of the follow‐up period.
Unclear risk: between 5% and 10% of participants lost to follow‐up at the end of the follow‐up period.
High risk: more than 10% of participants lost to follow‐up at the end of the follow‐up period.
Selective reporting (reporting bias)
Low risk: trial investigators stated in the introduction or method sections the outcomes they would look at, and they reported all of them in the results section.
Unclear risk: trial investigators did not state in the introduction or method sections the outcomes they would look at.
High risk: trial investigators stated in the introduction or method sections the outcomes they would look at but they did not report all of them in the results section.
We resolved any discrepancies through discussion between the two review authors and we contacted a third review author when required. We summarized the results of the assessment in 'Risk of bias' graphs and 'Risk of bias' tables, with supporting evidence from the trial reports.
Measures of treatment effect
We calculated the risk ratio (RR) for dichotomous outcomes and presented the effect estimates with 95% confidence intervals (CIs). We analysed the count data with the same methods as for dichotomous outcomes.
Unit of analysis issues
There were no cluster RCTs.
Dealing with missing data
To assess the impact of missing data on the estimate of effect, it is possible to impute data using best‐ and worst‐case scenario analyses (that is, the ‘best‐case’ scenario is that all participants with missing outcomes in the experimental intervention group had good outcomes, and all those with missing outcomes in the control intervention group had poor outcomes; the ‘worst‐case’ scenario is the converse). However this is an extreme adjustment, especially where outcomes are rare, as it would be very unlikely that all participants with missing data experienced an event for either treatment arm. Instead, we performed imputations using the event proportion observed in the available data. As an available‐case analysis implicitly assumes that the proportion of events observed also apply to the missing data, we varied the observed event proportions within reasonable limits, and applied these varied event proportions to the missing data, so that the resulting sensitivity analyses represented plausible scenarios that may have occurred within the missing data. This allowed us to investigate how plausible missing data scenarios impacted the overall effect estimate.
Assessment of heterogeneity
We assessed clinical and methodological diversity by looking at the variability in participants, interventions, outcomes, study design, and risk of bias in the included trials. We assessed statistical heterogeneity by inspecting the forest plots for overlapping CIs, by applying the Chi² test with a P value of 0.10 used to indicate statistical significance, and by using the I² statistic with a value of 50% used to denote a moderate level of heterogeneity.
Assessment of reporting biases
We intended to construct a funnel plot to assess publication bias, but this was not possible as we included fewer than 10 trials.
Data synthesis
We summarized all included trials in the 'Characteristics of included studies' tables. We analysed the data with Review Manager (RevMan) (RevMan 2014). To describe the effect of estimates, we used RR values as a summary statistic for dichotomous data, with 95% CIs. We conducted meta‐analyses using a fixed‐effect model, as we found low heterogeneity. We assessed the quality of the evidence using the GRADE principles (Guyatt 2011), and constructed a 'Summary of findings' table using the GRADEpro Guideline Development Tool (GRADEpro GDT 2014).
Subgroup analysis and investigation of heterogeneity
We could not conduct formal subgroup analysis due to the limited number of included trials.
Sensitivity analysis
We explored the effect of missing data on the primary outcomes by performing imputations using the event proportions we observed in the available data. We would have explored the impact of risk of bias on the results if more trials met the inclusion criteria of the review.
Results
Description of studies
Results of the search
We identified 250 records from the literature search. We did not identify any additional records through other sources. By screening titles and abstracts, we selected seven records and retrieved the full‐text articles of these references (Figure 1).
1.
Study flow diagram.
Included studies
We included three RCTs which included 328 participants (Makharia 2015a; Park 2009; Tony 2008). For a summary of the included trial characteristics, see Table 1, and for a detailed description of each trial see the 'Characteristics of included studies' tables.
1. Description of the included trials.
| Trial ID | Makharia 2015a | Park 2009 | Tony 2008 | |||
| Setting | ||||||
| Country | India | South Korea | India | |||
| Centre | 3 tertiary centres | Single tertiary centre | Single tertiary centre | |||
| Participants | ||||||
| Number randomized | 191a | 90 | 47 | |||
| Group age | 15 to 65 years | 18 to 75 years | Adultsb | |||
| HIV‐positive people | Excluded | Excluded | Excluded | |||
| Nutritional status | Underweight (BMI < 18.5 kg/m²): 40/80 (50%) versus 41/71 (57.8%) | Not reported | Not reported | |||
| Site of abdominal TB | Gastrointestinal tract (77% versus 73%), peritoneum (23% versus 19.8%), or both (2% versus 1%) | Intestinal TB | Intestinal TB: ileocaecal region, colon, or both. | |||
| Cases confirmed on bacteriological testing, histological testing (caseating granuloma), or both | 54% versus 57% | 77% | "Epithelioid granuloma and Langhan's giant cells" in all participants | |||
| Intervention and comparator | ||||||
| Duration of ATT | 6 months | 9 months | 6 months | 9 months | 6 months | 9 months |
| Number of participants allocated | 100 | 91 | 45 | 45 | 23 | 24 |
| Regimen | 2(HRZE)3/4(HR)3 | 2(HRZE)3/7(HR)3 | 2HRZE/4HRE | 2HRZE/7HRE | 2(HRZE)3/4(HR)3 | 2HRZE/7HR |
| Directly observed therapy | Yes | Yes | No | No | Yes | No |
| Median duration of FU after completing ATT (range) | 12 months | 12 months | 39 months (6 to 131) |
32 months (10 to 127) |
27 months (3 to 55) |
26 months (3 to 52) |
| Lost to FU during ATT | 11/100 (11%)c | 8/91 (8.8%)c | 0/45 (0%) | 0/45 (0%) | 0/23 (0%) | 1/24 (4.2%) |
| Lost to FU after completing ATT | 4/80 (5%) | 4/72 (5.6%) | 2/45 (4.4%) | 3/45 (6.7%) | 0/23 (0%) | 0/23 (0%) |
| Outcomes reported | ||||||
| Relapse | Yes | Yes | Yes | |||
| Clinical cure | Yes | Yes | Yes | |||
| Complete healing of active lesions | Yes | Yes | Yes | |||
| Death from any cause | Yes | Deducible | Deducible | |||
| Treatment failure | Yes (“no response”) | Deducible | Deducible | |||
| Default | Deducible | Deducible | Unclear | |||
| Poor adherence | Yes | No | No | |||
Abbreviations: ATT: antituberculous treatment, BMI: body mass index; E: ethambutol; FU: follow‐up; H: isoniazid; HIV: human immunodeficiency virus; R: rifampicin; TB: tuberculosis; vs: versus; Z: pyrazinamide.
aMakharia 2015a randomized 197 participants and excluded six participants after randomization owing to misdiagnosis. bMean age (standard deviation): 39.9 (13.5) years in the 6‐month ATT group versus 37.8 (11.6) years in the 9‐month ATT group. cSeven additional participants in each group did not follow the directly observed therapy protocol.
Setting
The three included trials were conducted in tertiary centres in Asia (Makharia 2015a and Tony 2008 in India; Park 2009 in South Korea). Makharia 2015a recruited participants from three centres, and the other two trials were conducted in a single centre.
Participants
The three trials compared 168 adults who received six months of antituberculous treatment (ATT) with 160 adults who received nine months of ATT. All trials excluded HIV‐positive people, those with co‐morbidities, and those who had received ATT in the previous five years. Makharia 2015a reported the nutritional status at baseline for 79% of the participants recruited; 50% of those that received six months of ATT and 58% of those that received nine months of ATT were underweight, with a body mass index (BMI) under 18.5 kg/m². Two trials reported features of malabsorption: between 33% and 56% of the participants in both arms had hypoalbuminaemia at baseline, which the trial authors defined as albumin levels under 3.3 g/dL (Park 2009), and 3.5 g/dL (Makharia 2015a).
Makharia 2015a included participants with gastrointestinal TB, peritoneal TB, or both, and the two other trials included participants with intestinal TB (TB of the ileocaecal region, colon, or both) (Park 2009; Tony 2008). For the diagnosis, all trials conducted endoscopic examination with biopsies for histology. Makharia 2015a and Park 2009 also performed acid‐fast bacilli (AFB) stain and culture of the specimens. We did not find any study that looked at participants with abdominal lymph nodes TB or visceral TB.
ATT regimens
In the three included trials, the two‐month intensive phase was comprised of isoniazid, rifampicin, pyrazinamide, and ethambutol (as stated in our inclusion criteria), followed by a continuation phase with isoniazid and rifampicin for four or seven additional months. Park 2009 retained ethambutol in the continuation phase, based on high rates of primary drug resistance in the setting of the trial (South Korea). Park 2009 administered the drugs daily, Makharia 2015a treated participants thrice weekly under a directly observed therapy programme, while Tony 2008 compared six‐month ATT given thrice weekly under directly observed therapy with nine‐month ATT given daily without directly observed therapy programme. The dosage of the antituberculous drugs were very similar in all three trials, with some adjustments between daily or thrice weekly administration.
Co‐intervention
Two participants with intestinal obstruction in Makharia 2015a received surgical intervention. Park 2009 established indications for surgery, although no participants finally required it. Park 2009 also specified that no participants received corticosteroids. Tony 2008 did not report any co‐interventions.
Length of follow‐up
Makharia 2015a followed up all participants for 12 months after completing ATT; Park 2009 followed‐up the participants treated for six months for a median of 39 months (ranging from 6 to 131 months) and those treated for nine months for a median of 32 months (ranging from 10 to 127 months); and Tony 2008 followed up participants that received six‐ and nine‐month treatment for a median of 27 (range 3 to 55) and 26 (range 3 to 52) months respectively. Makharia 2015a and Park 2009 had planned visits for following‐up the participants once ATT was completed, but Tony 2008 did not describe the method of follow‐up after completion of ATT.
Outcomes
The three included trials reported relapse. Park 2009 gave a detailed definition of this outcome: "endoscopic documentation of recurrent lesions after achieving complete response", and performed endoscopic examinations for evaluating the disease status of the participants one year after the end of the treatment. The other two trials assessed participants clinically during the follow‐up period.
The three trials reported on clinical cure, and complete healing of active lesions documented by endoscopy or histopathology at six months after starting ATT or at the end of ATT. Makharia 2015a reported death from any cause and treatment failure, and we were able to deduce these outcomes in Park 2009 and Tony 2008 based on the findings of the other outcomes. Park 2009 clearly reported default, while reporting of this outcome was unclear in Tony 2008 and was deducible from the other outcomes in Makharia 2015a. Only Makharia 2015a assessed poor adherence to treatment. All included trials reported on adverse events that led to the discontinuation or modification of ATT, but did not report uniformly on other adverse events related to ATT.
Excluded studies
We excluded four reports and listed the reasons for exclusion in the 'Characteristics of excluded studies' table.
Risk of bias in included studies
See Figure 2 for a summary of the 'Risk of bias' assessment. The 'Risk of bias' tables provide further details for the supporting evidence in the 'Characteristics of included studies' tables.
2.

'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included trial.
Allocation
The generation of the allocation sequence was adequate in all three trials, and the allocation concealment was adequate in Makharia 2015a but unclear in Park 2009 and Tony 2008.
Blinding
In the three trials, participants were not blinded to the intervention, but we considered that these trials were at low risk of performance bias for all outcomes. In Park 2009 and Makharia 2015a, the personnel that assessed the outcomes were not blinded. Tony 2008 did not provide any details on whether the personnel that assessed objective outcomes, such as death, default, and poor adherence to treatment, were blinded or not. However, although unblinded, we considered this would be at low risk of detection bias for objective outcomes. For detecting subjective outcomes, the personnel were not blinded in Park 2009 and Makharia 2015a, and thus we judged these studies as at high risk of detection bias, while in Tony 2008 the personnel performing endoscopy examination were blinded to the treatment allocation and we judged this study as at low risk of detection bias.
Incomplete outcome data
During ATT, more than 5% of the participants were lost to follow‐up in both arms of the Makharia 2015a trial, and we considered this as high risk of attrition bias. In Tony 2008, only one participant was lost to follow‐up, and there were no participants lost to follow‐up during ATT in Park 2009, thus we considered both studies at low risk of attrition bias regarding the outcomes assessed at the end of ATT.
After completing ATT, between 5% and 10% of the participants were lost to follow‐up in Makharia 2015a and Park 2009, with unknown reasons, and so we assessed both studies as at unclear risk of bias. Tony 2008 did not describe any participants as lost to follow‐up during the follow‐up period (range three to 55 months), but the large range of follow‐up duration means it is unclear whether there was significant attrition or not, and so we assessed this study as at unclear risk of bias.
Selective reporting
We did not detect evidence of selective outcome reporting in the included trials. Thus all included studies were at low risk of selective reporting bias.
Other potential sources of bias
Tony 2008 conducted an interim evaluation of the findings: the trial investigators found that the results were similar at six months from the start of ATT and considered it was unethical to proceed with the trial. Consequently, they stopped trial earlier than originally planned because the investigators felt the effects were similar in both groups, rather than because they observed harm in one group or the other. As effects are known to fluctuate during trials, we cannot know whether a difference between the groups would have been detected had this trial continued. We therefore assessed Tony 2008 as unclear risk of bias. We did not identify any other sources of bias in Makharia 2015a and Park 2009, and so we assessed them as low risk of bias.
Effects of interventions
See 'Summary of findings' table (Figure 3) and Table 2 for a descriptive summary of trial findings of all outcomes.
3.
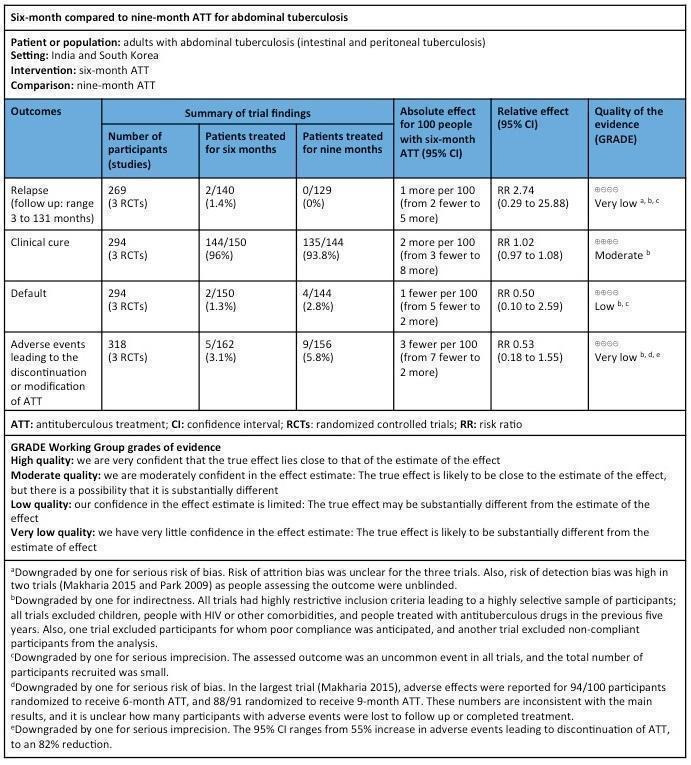
'Summary of findings' table.
2. Summary of findings by complete‐case analysis.
| Duration of ATT | Makharia 2015a | Park 2009 | Tony 2008 | |||
| 6 months | 9 months | 6 months | 9 months | 6 months | 9 months | |
| Primary outcomes | ||||||
| Relapse | 1/76a (1.3%) |
0/68 (0%) |
1/41b (2.4%) |
0/38 (0%) |
0/23 (0%) |
0/23 (0%) |
| Clinical cure | 78/82c (95.1%) |
71/76d (93.4%) |
43/45 (95.6%) |
41/45 (91.1%) |
23/23 (100%) |
23/23 (100%) |
| Secondary outcomes | ||||||
| Complete healing of active lesions | NDDe | NDDf | 42/45 (93.3%) |
41/45 (91.1%) |
23/23 (100%) |
23/23 (100%) |
| Death from any cause | 2/82g (2.4%) |
4/76g (5.2%) |
0/45 (0%) |
0/45 (0%) |
0/23 (0%) |
0/23 (0%) |
| Treatment failure | 2/82 (2.4%) |
1/76 (1.3%) |
0/45h (0%) |
0/45 (0%) |
0/23 (0%) |
0/23 (0%) |
| Default | 0/82i (0%) |
0/76i (0%) |
2/45j (4.4%) |
4/45j (8.9%) |
0/23 (0%) |
0/23 (0%) |
| Poor adherence | 0/82 (0%) |
1/76 (1.3%) |
NR | NR | NR | NR |
Abbreviations: ATT: antituberculous treatment; NDD: no disaggregated data; NR: not reported. aOne participant presented relapse of TB at another site (lymph node); intestinal lesions were endoscopically healed. bNo participant suffered a bacteriologically or histologically confirmed relapse. However, the trial authors reported 1 participant with recurrence of the endoscopic lesion, with 1 tiny ulcer on colonoscopy: “Although this finding did not fulfil our diagnostic criteria for intestinal TB, the patient was retreated for 12 months with anti‐TB medications identical to those previously received and later achieved complete response without any relapse.” cAccording to the trial authors' definitions, 75 participants presented "complete clinical response" and 3 participants presented "partial clinical response" at the end of treatment. All the 78 participants presented resolution of clinical manifestations, which fulfilled the definition of "clinical cure" in this review. dAccording to the trial authors' definitions, 69 participants presented "complete clinical response" and 2 participants presented "partial clinical response" at the end of treatment. All the 71 participants presented resolution of clinical manifestations, which fulfilled the definition of "clinical cure" in this review. eAmong 62 participants with gastrointestinal TB, 31 participants agreed to undergo colonoscopy control (31/31 colonoscopy showed mucosal healing) and 38/62 participants with gastrointestinal TB showed healing of lesions on either colonoscopy or imaging. Among 18 eligible participants with peritoneal TB, 11 undertook imaging at the end of treatment and 11/11 showed resolution of the lesions on imaging. fAmong 57 participants with gastrointestinal TB, 24 participants agreed to undergo colonoscopy control (24/24 colonoscopy showed mucosal healing) and 31/57 participants with gastrointestinal TB showed healing of lesions on either colonoscopy or imaging. Among 15 eligible participants with peritoneal TB, 3 undertook imaging at the end of treatment and 3/3 showed resolution of the lesions on imaging. gThe trial authors also reported 9/302 deaths in participants who were screened but died before randomization, and thus excluded them from the analysis. hIn one participant, symptoms had disappeared at the time of completing ATT, but a “tiny residual ulcer, associated with extensive granulation tissue was seen on colonoscopy”. According to the trial authors, “although the endoscopic lesion seemed to improve without further therapy”, this participant was maintained on one additional month on ATT and thus received 7 months of therapy, as the trial authors “felt it was ethical to do so”. However, this participant did not meet our definition of treatment failure, and we classified it as clinical cure, with incomplete healing of active lesions. iBy deduction from data given for compliance. jTwo participants in the 6‐month ATT group and 4 participants in the 9‐month ATT group withdrew before completing treatment because of drug toxicity or intolerance. They fulfilled our definition of default and we therefore classified them as such in this review.
Relapse
Across all three included studies, two out of the 140 adults treated for six months relapsed, and none of the 129 adults treated for nine months relapsed. In Makharia 2015a, one participant presented relapse of TB at 11 months follow‐up. This participant no longer had intestinal TB but presented with a new cervical lymph node, and fine‐needle aspiration showed caseation and AFB, with negative culture. He responded to retreatment with nine‐month duration (directly observed therapy, category II). The second participant reported to relapse had recurrence of a colonic lesion seen on endoscopy (Park 2009). Park 2009 described finding one tiny ulcer on colonoscopy, without bacteriological or histological confirmation of TB. Although this finding did not fulfil their diagnostic criteria for intestinal TB, they decided to retreat this participant with 12 months of ATT, and achieved a complete response. The trial authors did not specify the time between the end of ATT and detection of relapse.
Methods of evaluation and duration of follow‐up are the two main factors that would influence detection of relapse. Park 2009 followed participants treated for six months for a median of 39 months (range six to 131 months) and those treated for nine months for a median of 32 months (range 10 to 127), and performed endoscopic examinations on all 79 participants who completed ATT 12 months after the end of the treatment. The other two included trials assessed participants clinically during the follow‐up period, and it is unclear whether they had prespecified criteria to prompt repeat endoscopic examination. In Makharia 2015a, the participant who presented with cervical lymph node TB had his intestinal disease evaluated again at this stage, but it is unclear whether other participants underwent endoscopic examination during the follow‐up period. In addition, duration of follow‐up in Tony 2008 ranged from three to 55 months, which meant that some participants were followed‐up for less than six months. Consequently, investigators in Park 2009 may have been more likely to detect relapse than in the other two trials.
The meta‐analysis shows a very imprecise estimate of no difference between six‐month and nine‐month regimens (RR 2.74, 95% CI 0.29 to 25.88; 269 participants, 3 trials; Analysis 1.1).
1.1. Analysis.
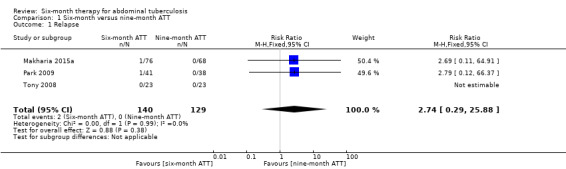
Comparison 1 Six‐month versus nine‐month ATT, Outcome 1 Relapse.
Overall, relapse was an uncommon event regardless of duration of treatment, and the findings did not change substantially when we explored the effect of missing data by performing imputation for participants lost to follow‐up, using the event proportions observed in the complete‐case analysis (see Table 3 and Analysis 2.1).
3. Summary of main findings, after imputation of missing data.
| Duration of ATT | Makharia 2015a | Park 2009 | Tony 2008 | |||
| 6 months | 9 months | 6 months | 9 months | 6 months | 9 months | |
| Primary outcomes | ||||||
| Relapse | 1/80 (1.3%) |
0/72 (0%) |
1/43 (2.3%) |
0/41 (0%) |
0/23 (0%) |
0/23 (0%) |
| Clinical cure | 95/100 (95%) |
86/91 (94.5%) |
43/45 (95.6%) |
41/45 (91.1%) |
23/23 (100%) |
24/24 (100%) |
Abbreviations: ATT: antituberculous treatment.
2.1. Analysis.
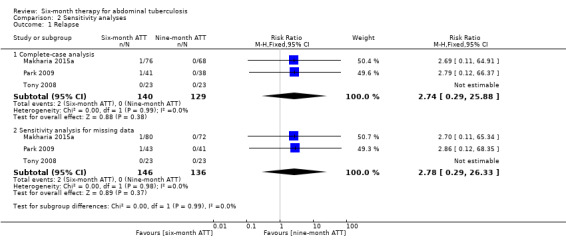
Comparison 2 Sensitivity analyses, Outcome 1 Relapse.
Clinical cure
At the time of ATT completion, there was no difference in the proportion of participants that achieved clinical cure between six‐month and nine‐month ATT regimens (RR 1.02, 95% CI 0.97 to 1.08; 294 participants, 3 trials; Analysis 1.2; Figure 4).
1.2. Analysis.
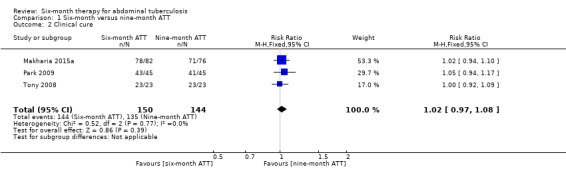
Comparison 1 Six‐month versus nine‐month ATT, Outcome 2 Clinical cure.
4.
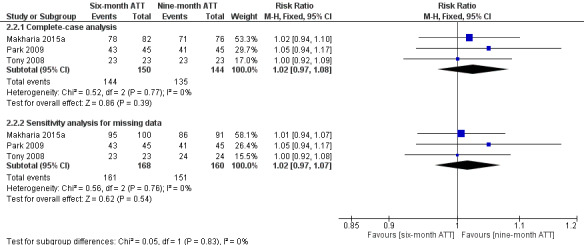
Forest plot of comparison: 2 Six‐month ATT versus nine‐month ATT, outcome: 2.2 Clinical cure.
The effect of missing data, which we explored by applying the event proportions observed in the complete‐case analysis to the participants lost to follow‐up, did not substantially change these findings (Analysis 2.2, Figure 4).
2.2. Analysis.
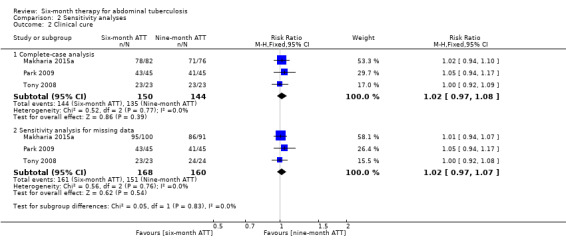
Comparison 2 Sensitivity analyses, Outcome 2 Clinical cure.
Complete healing of active lesions, documented by endoscopy or histopathology
Tony 2008 performed endoscopic assessment of healing of lesions at six months of ATT and Park 2009 performed it at the end of ATT. There was no difference in the proportion of participants that achieved complete healing of active lesions between six‐ and nine‐month ATT (RR 1.02, 95% CI 0.94 to 1.10; 136 participants, 2 trials; Analysis 1.3).
1.3. Analysis.
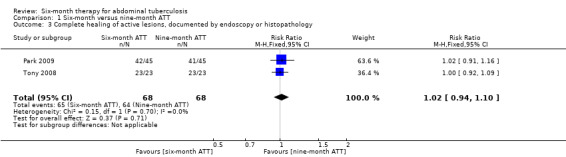
Comparison 1 Six‐month versus nine‐month ATT, Outcome 3 Complete healing of active lesions, documented by endoscopy or histopathology.
Death from any cause
The included trials reported few deaths, which led to an imprecise estimate of no difference in risk of death between participants treated for either six months or nine months (RR 0.46, 95% CI 0.09 to 2.46; 294 participants, 3 trials; Analysis 1.4).
1.4. Analysis.
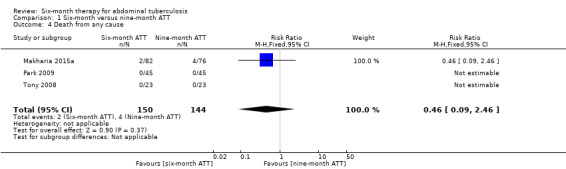
Comparison 1 Six‐month versus nine‐month ATT, Outcome 4 Death from any cause.
Only Makharia 2015a reported any deaths: 2/82 (2.4%) participants died in the six‐month ATT group, and 4/76 (5.3%) participants died in the nine‐month ATT group. Two males (aged 33 and 22 years) allocated in the six‐month ATT group died at one and three months after starting treatment. Both participants had ileocolonic TB and were malnourished. The four participants who died in the nine‐month ATT group consisted of one male (26 years) and three females (16, 25, and 43 years) who died between two and four months after starting ATT. All three had ileocolonic TB: two developed intestinal obstruction and one developed ileal perforation and died of related complications. The fourth participant was a 16‐year‐old female with peritoneal TB and the cause of death was unclear.
Treatment failure
At the time of ATT completion, there was no significant difference in treatment failure between participants treated for six and nine months (RR 1.85, 95% CI 0.17 to 20.03; 294 participants, 3 trials; Analysis 1.5).
1.5. Analysis.
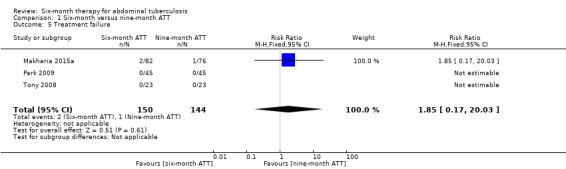
Comparison 1 Six‐month versus nine‐month ATT, Outcome 5 Treatment failure.
Makharia 2015a reported three participants with "no response" to ATT, which the trial authors defined as persistent clinical symptoms and inflammatory lesions at the end of treatment: 2/82 participants in the six‐month ATT group and 1/76 participants in the nine‐month ATT group. Both participants from the six‐month ATT group required surgical intervention due to intestinal obstruction (at five and six months after starting ATT), and completed ATT (one of them preferred daily instead of thrice weekly drug regimen). The participant with peritoneal TB with no response to treatment from the nine‐month ATT group had persistent ascites, and responded to three additional months of treatment. All three participants remained well after one year follow‐up after ATT completion.
Default
There were few participants reported to have defaulted from treatment, which led to an imprecise estimate of no difference in risk of default between participants that received either six‐month or nine‐month ATT (RR 0.50, 95% CI 0.10 to 2.59; 294 participants, 3 trials; Analysis 1.6).
1.6. Analysis.
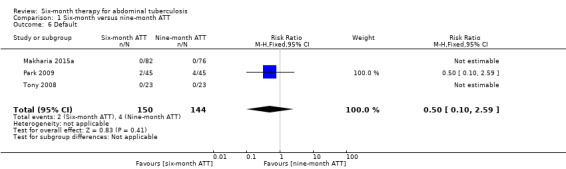
Comparison 1 Six‐month versus nine‐month ATT, Outcome 6 Default.
Overall, six participants in Park 2009 withdrew before treatment completion because of drug toxicity or intolerance: 2/45 (4.4%) in the six‐month ATT group, and 4/45 (8.9%) in the nine‐month ATT group.
Poor adherence
Only Makharia 2015a reported adherence to treatment. The trial investigators defined poor adherence to treatment as a participant who took drugs for less than 80% of the intended days. One out of the 76 participants allocated to the nine‐month ATT group fulfilled this definition, while all the 82 participants allocated to the six‐month ATT group with available data at the time of completing ATT had good adherence to treatment.
Adverse events
See Table 4 for a summary of the findings regarding all adverse events.
4. Adverse events.
| Trial ID | Participants with serious AE that are life‐threatening or lead to hospitalization | Participants with AE that lead to the discontinuation or modification of ATT | Participants with other AE relating to ATTa | |||
| 6‐month ATT | 9‐month ATT | 6‐month ATT | 9‐month ATT | 6‐month ATT | 9‐month ATT | |
| Makharia 2015a | NR | NR | 3/94b | 5/88b | 20/94 | 16/88 |
| Park 2009 | NR | NR | 2/45 | 4/45 | NDDc | NDDc |
| Tony 2008 | 0/23 | 0/23 | 0/23 | 0/23 | 7/23 | 10/23 |
Abbreviations: AE: adverse events; ATT: antituberculous treatment; NDD: no disaggregated data; NR: not reported. aWe have provided descriptions of these adverse events in Table 5. bOverall, 8 participants had drug‐induced hepatitis: 3 in the 6‐month ATT group, and 5 in the 9‐month ATT group. Drug‐induced hepatitis were managed by replacing isoniazid, rifampicin, and pyrazinamide with quinolones and streptomycin. After resolution of hepatitis, isoniazid, rifampicin and pyrazinamide were reintroduced. Total duration of interruption was compensated by prolongation of the treatment duration. All recovered. cThere were AEs observed in "some" participants.
Serious adverse events that were life‐threatening or led to hospitalization
None of the included trials clearly reported this outcome. We do not know whether participants with drug‐induced hepatitis in Makharia 2015a and Park 2009 were hospitalized or not. Based on the available data in Tony 2008, we can reasonably deduce that no participants had serious adverse events that were life‐threatening or led to hospitalization.
Adverse events that lead to the discontinuation or modification of ATT
There were fewer participants who had their ATT interrupted due to adverse events related to antituberculous drugs in the six‐month ATT group than in the nine‐month ATT group, although the difference was not statistically significant (RR 0.53, 95% CI 0.18 to 1.55; 318 participants, 3 trials; Analysis 1.7). The study authors did not report the timing of these adverse events. Therefore, it is not possible to estimate whether the duration of treatment was plausibly associated with the difference between the groups.
1.7. Analysis.
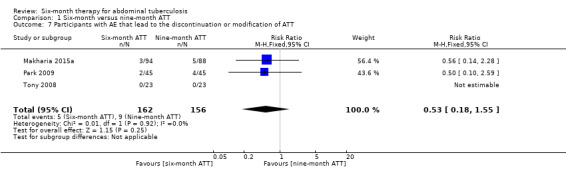
Comparison 1 Six‐month versus nine‐month ATT, Outcome 7 Participants with AE that lead to the discontinuation or modification of ATT.
Other adverse events relating to ATT
See Table 5 for a description of the adverse events as reported in the included trials.
5. Description of the 'other adverse events relating to ATT'.
| Trial ID | 6‐month ATT | 9‐month ATT |
| Makharia 2015a | Overall, 20 participants developed 1 or more AE, described for 94 participants.
|
Overall, 16 participants developed one or more AE, described for 88 participants.
|
| Park 2009 | The trial authors stated that "drug‐related AE were observed in some patients". “Gastrointestinal reactions such as nausea and poor appetite were most common but were effectively managed with symptomatic therapy." | |
| Tony 2008 |
|
|
Abbreviations: AE: adverse event; ATT: antituberculous treatment; ULN: upper limit of normal.
There were no disaggregated data between participants allocated to the six‐ and nine‐month ATT groups in Park 2009. Both Makharia 2015a and Tony 2008 reported symptomatic adverse events related to antituberculous drugs (vomiting, epigastric pain, anorexia) and participants with elevation of liver enzymes. Overall, there was no difference in the proportion of participants with adverse events between participants treated for six months and nine months (RR 0.99, 95% CI 0.62 to 1.59; 228 participants, 2 trials; Analysis 1.8).
1.8. Analysis.
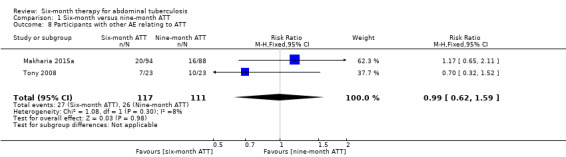
Comparison 1 Six‐month versus nine‐month ATT, Outcome 8 Participants with other AE relating to ATT.
Discussion
Summary of main results
We included three randomized controlled trials (RCTs) (328 participants) that compared six‐month regimens with nine‐month regimens for treating adults that had intestinal and peritoneal tuberculosis (TB). No trial examined participants with abdominal lymph nodes TB or visceral TB.
Relapse was an uncommon event with 2/140 events among participants treated for six months and 0/129 events among those treated for nine months. Proportions of clinical cure at the end of treatment, and complete healing of active lesions documented by endoscopy or histology were high in both participants treated with six‐month and nine‐month antituberculous treatment (ATT), with no significant difference between both groups. There were few deaths, and all deaths occurred in the first four months of treatment, which indicated that the risk of death was not linked with the duration of treatment in the included trials. There were few episodes of treatment failure and episodes of default in both groups of participants treated for six months and nine months. There was no statistically significant difference between the groups regarding the number of participants who had adverse events that led to interruption of ATT.
Overall completeness and applicability of evidence
The three trials were conducted in Asia: two in India, which is one of the 22 TB 'high burden countries' according to the World Health Organization (WHO) (WHO 2015b), and one in South Korea. Therefore, the results of this review are likely to be applicable to regions where TB burden is high.
In this review, we restricted trial inclusion to those that used ATT regimens that consisted of isoniazid, rifampicin, pyrazinamide, and ethambutol, as those are currently the most commonly used regimens. The included trials comprised both daily drug administration and intermittent dosages given under directly observed therapy. The three trials recruited participants with intestinal TB, and one of the trials also recruited participants with peritoneal TB, which means that the results of this review may not be applicable to TB that affects the solid organs within the abdominal cavity.
We also restricted trial inclusion to those that evaluated sensitive strains of M. tuberculosis. However, there is uncertainty whether some cases of relapse of the disease could actually be a primary infection caused by drug‐resistant strains.
Only one trial, Makharia 2015a, included participants who required surgical intervention. This was also the only trial that reported any deaths. This raises questions about whether participants included in the other two trials had less severe disease, and consequently, whether the findings of this review can be applied to people with severe forms of abdominal TB and to those who require surgical interventions.
All trials had highly restrictive inclusion criteria, which led to a highly‐selected sample of participants: all trials excluded children, people with HIV or other comorbidities, pregnant women, and people treated with antituberculous drugs in the previous five years. Also, one trial excluded participants for whom poor compliance was anticipated, and another trial excluded non‐compliant participants from the analysis. This means that the findings of this review apply to a population with similar characteristics to those in the included trials, and must be interpreted with caution for other groups, such as people with HIV. Indeed, although risk factors for relapse in people with abdominal TB are unclear, poor compliance and default may be important factors. There are different reasons why we may expect that adherence to treatment and interruption of ATT would differ in groups excluded from the trials. For example, HIV‐positive people that take antiretroviral therapy are more likely to experience drug interactions with antituberculous drugs, and people with HIV or other comorbidities may struggle to adhere to ATT because of high pill burden. Finally, adherence to treatment is likely to be affected by several factors, including duration of treatment but also treatment delivery, for example, directly observed therapy programmes.
Quality of the evidence
For all outcomes, we downgraded the quality of the evidence for 'indirectness'. Indeed, we considered that the trials had restrictive inclusion criteria, leading to a highly selected sample of participants, which limits the applicability of the evidence.
The second main reason for downgrading the quality of the evidence was 'imprecision', as the three included trials were small, and there were few or no events for most of the outcomes. Relapse was an uncommon outcome, and therefore large trials with long follow‐up periods are required to detect this outcome with sufficient reliability.
Finally, we considered that there was high 'risk of bias' in the included trials which could potentially affect the effect estimated for relapse. People that assessed this subjective outcome were unblinded in two trials, which introduces a high risk of detection bias, and there was unclear risk of attrition bias during the follow‐up period in the three trials.
Potential biases in the review process
We attempted to limit bias by following the rigorous methods provided by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). The Cochrane Infectious Diseases Group Information Specialist performed the literature search without language restrictions, and as such it is unlikely that we missed any large studies. However, it is possible that we may have missed small, unpublished studies. We attempted to limit bias in the selection of studies for inclusion, data extraction, and assessment of risk of bias of the included studies by conducting these processes independently and by comparing results between at least two review authors. One of the review authors (VA) is an author of one of the included trials, Makharia 2015a, and so SJu and SJa completed the data extraction and the 'Risk of bias' assessment for this study without VA's involvement. We were unable to formally assess publication bias because we included fewer than 10 trials.
Agreements and disagreements with other studies or reviews
One trial that compared a six‐month regimen of isoniazid, rifampicin, and pyrazinamide with a 12‐month regimen of isoniazid, ethambutol, and streptomycin found similar results to the findings of this review, with no cases of relapse among the 147 participants who completed five‐year follow‐up (Balasubramanian 1997).
Evidence from the existing literature and this systematic review support the current recommendations of most international guidelines on the duration of treatment for people with drug‐sensitive abdominal TB, which consist of the same six‐month regimen as used for the treatment of pulmonary TB (American Thoracic Society 2003; WHO 2010).
Authors' conclusions
Implications for practice.
We found no evidence to suggest that six‐month regimens are inadequate for treating people that have intestinal and peritoneal TB. We did not find any incremental benefits of nine‐month regimens regarding number of relapse at the end of follow‐up, or clinical cure at the end of therapy. However, our confidence in the effect estimate for relapse is very low.
Implications for research.
Further research is required to make confident conclusions regarding the safety of six‐month ATT for people with abdominal TB. Randomized controlled trials (RCTs) with long follow‐up of participants are required to detect relapse with reliability, and should be more inclusive to reflect the range of abdominal TB patients seen in clinical practice. Large multicentre trials are needed to provide sufficient statistical power to yield conclusive results. The World Health Organization (WHO) estimated that in 2014, 12% of the 9.6 million new TB cases were HIV‐positive (WHO 2015a). Therefore, such trials should involve HIV‐positive participants in order to obtain data that could apply to this group of participants. Given the potential impact of adherence to treatment on TB outcomes, treatment adherence should be carefully monitored and reported in future studies. Efforts should also be made to limit the risk of detection bias by ensuring outcome assessors are blind to the duration of treatment, where possible.
The three trials included participants with intestinal or peritoneal TB. We did not find any data that evaluated the duration of treatment for other forms of abdominal TB, such as TB of the solid organs and abdominal lymph node TB. Despite their relatively low prevalence, further research is needed to establish the optimal duration of treatment for these other forms of abdominal TB.
In this review, we noted that there was a lack of consistency in the included studies regarding the methods for detection of relapse and case definitions of relapse. Firstly, participants were followed‐up for different durations of time, and longer follow‐up of all participants is likely to lead to a higher detection of cases of relapse. Secondly, only one trial seemed to have systematically assessed participants with endoscopy examinations in order to detect relapse, while the other two trials evaluated participants for relapse based on clinical assessment. While the diagnosis of new cases is already difficult, diagnosis of relapse of the disease may be even more difficult. On the one hand, research is ongoing to improve diagnostic accuracy for abdominal TB, and on the other hand, investigators should design trials with comprehensive methods for evaluating relapse of abdominal TB and with at least 12 months of follow‐up after ATT completion for all the participants recruited.
Finally, we also noted a lack of consistency in the included studies when reporting default, adherence to treatment and adverse events. Efforts towards using standardized definitions and better reporting of these outcomes should be encouraged for future studies.
Acknowledgements
We are grateful to Vittoria Lutje, the Information Specialist of the Cochrane Infectious Diseases Group (CIDG) for help with the literature search strategy. Many thanks to Paul Garner and David Sinclair, CIDG Co‐ordinating Editors, for their feedback and support. The CIDG editorial base is funded by UK aid from the UK Government for the benefit of developing countries (Grant: 5242). The views expressed in this Cochrane Review do not necessarily reflect UK government policy.
Appendices
Appendix 1. Forms of abdominal TB
| Form | Anatomical site |
| Abdominal lymph node TB (abdominal TB lymphadenitis) | Lymph nodes (mesenteric, omental, at porta hepatis, at coeliac axis) |
| Peritoneal TB (TB peritonitis) | Peritoneum |
| Gastrointestinal TB | Ileocaecal area (ileocolonic TB) involving the ileum and caecum |
| Jejunum | |
| Colon | |
| Oesophagus, stomach, duodenum | |
| Visceral TB | Liver, spleen, pancreas |
Appendix 2. Detailed search strategies
PubMed
1 tuberculosis [MeSH]
2 tuberculosis [ti, ab]
3 Abdominal OR gastroenteric OR gastrointestinal OR intestinal OR enterocolitis OR peritonitis OR hepatic OR liver OR splenic [ti, ab]
4 1 or 2
5 3 and 4
6 "Peritonitis, tuberculous" [Mesh]
7 "Tuberculosis, Gastrointestinal"[Mesh]
8 "Tuberculosis, Hepatic"[Mesh]
9 "Tuberculosis, Splenic"[Mesh]
10 6 or 7 or 8 or 9
11 5 or 10
12 randomised controlled trial.pt
13 controlled clinical trial.pt
14 randomised or randomized ti, ab
15 placebo ti, ab
16 randombly ti, ab
17 trial ti, ab
18 12 or 13 or 14 or 15 or 16 or 17
19 11 and 18
Embase
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 tuberculosis.mp. or tuberculosis/ 2 limit 1 to human 3 (Abdominal or gastroenteric or gastrointestinal or intestinal or enterocolitis or peritonitis or hepatic or liver or splenic).ab. or (Abdominal or gastroenteric or gastrointestinal or intestinal or enterocolitis or peritonitis or hepatic or liver or splenic).ti. 4 2 and 3 5 abdominal tuberculosis/ 6 limit 5 to human 7 tuberculous peritonitis/ 8 liver tuberculosis.mp. 9 4 or 5 or 7 or 8 10 randomized controlled trial/ 11 controlled clinical trial/ 12 10 or 11 13 double blind procedure/ 14 12 or 13 15 9 and 14
The Cochrane Library
#1"abdominal tuberculosis":ti,ab,kw or "intestinal tuberculosis":ti,ab,kw or "enteric tuberculosis":ti,ab,kw or "enterocolitis tubercul*":ti,ab,kw or "tuberculo* peritonitis":ti,ab,kw (Word variations have been searched) #2MeSH descriptor: [Peritonitis, Tuberculous] explode all trees #3MeSH descriptor: [Tuberculosis, Splenic] explode all trees #4MeSH descriptor: [Tuberculosis, Hepatic] explode all trees #5MeSH descriptor: [Tuberculosis, Gastrointestinal] explode all trees #6#1 or #2 or #3 or #4 or #5
LILACS, INDMED, South Asian Database of Controlled Clinical Trials
tuberculosis AND (Abdominal or gastroenteric or gastrointestinal or intestinal or enterocolitis or peritonitis or hepatic or liver or splenic)
Data and analyses
Comparison 1. Six‐month versus nine‐month ATT.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Relapse | 3 | 269 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.74 [0.29, 25.88] |
| 2 Clinical cure | 3 | 294 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.97, 1.08] |
| 3 Complete healing of active lesions, documented by endoscopy or histopathology | 2 | 136 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.94, 1.10] |
| 4 Death from any cause | 3 | 294 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.09, 2.46] |
| 5 Treatment failure | 3 | 294 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.85 [0.17, 20.03] |
| 6 Default | 3 | 294 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.10, 2.59] |
| 7 Participants with AE that lead to the discontinuation or modification of ATT | 3 | 318 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.18, 1.55] |
| 8 Participants with other AE relating to ATT | 2 | 228 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.62, 1.59] |
Comparison 2. Sensitivity analyses.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Relapse | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Complete‐case analysis | 3 | 269 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.74 [0.29, 25.88] |
| 1.2 Sensitivity analysis for missing data | 3 | 282 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.78 [0.29, 26.33] |
| 2 Clinical cure | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Complete‐case analysis | 3 | 294 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.97, 1.08] |
| 2.2 Sensitivity analysis for missing data | 3 | 328 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.97, 1.07] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Makharia 2015a.
| Methods | Study design: multicentre randomized controlled trial (RCT). Follow‐up method
Length of follow‐up after completing treatment: 1 year. Losses to follow‐up: during ATT, 11/100 (11%) participants were lost to follow‐up and 7/100 (7%) did not follow the DOTs protocol in the 6‐month regimen group, and 8/91 (8.8%) participants were lost to follow‐up and 7/91 (7.7%) did not follow the DOTs protocol in the 9‐month regimen group. After completing ATT, 4/80 (5%) participants treated for 6 months and 4/72 (5.6%) participants treated for 9 months were lost to follow‐up. |
|
| Participants |
Inclusion criteria Consecutive patients aged 15 to 65 years with new diagnosis of either gastrointestinal or peritoneal TB or both.
Exclusion criteria
Participants recruited Number: 191 enrolled (100 in the 6‐month ATT group versus 91 in the 9‐month ATT group). Mean age (SD): 34 (14.2) years versus 34.9 (13.9) years; 53% versus 57% males. HIV status: HIV negative participants; HIV‐seropositivity was used as an exclusion criterion. Nutritional status: underweight as defined by the trial authors (BMI < 18.5 kg/m²): 40/80 versus 41/71. Features of malabsorption: low albumin (defined as < 3.5 g/dL) 30/90 versus 37/83. Radiological evidence of active pulmonary TB: not reported. Diagnosis procedures
Site of TB within the abdomen
|
|
| Interventions | 6‐month regimen: 2(HRZE)3 /4(HR)3 under DOTS. 9‐month regimen: 2(HRZE)3 /7(HR)3 under DOTS. 3 times weekly oral dosages:
Co‐intervention: surgical intervention for intestinal obstruction in 2 participants. |
|
| Outcomes |
|
|
| Notes | Location: 3 tertiary centres in India.
Study dates: From September 2008 to April 2014. Funding: The Central Tuberculosis Division, Ministry of Health and Family Welfare, government of India. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “The randomization was done for each center separately using computer‐generated table by a person not involved in the study.” |
| Allocation concealment (selection bias) | Low risk | “The randomized treatment allocation (ie. 6 or 9 months) was printed and concealed in sealed envelopes bearing the serial number of the patient (separately for each site).” |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and personnel were not blinded (personal communication with the trial authors). However, we considered this to be at low risk of performance bias for all outcomes. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | People assessing outcomes were not blinded (personal communication with trial authors). However, we considered this would be at low risk of detection bias for objective outcomes. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | People that assessed the outcomes and endoscopy were not blinded (personal communication with authors of the trial), which introduce high risk of bias for detecting subjective outcomes. Endoscopic healing of lesions was an important criterion in assessing treatment response in the trial. |
| Incomplete outcome data (attrition bias) Outcomes assessed at the end of ATT | High risk | 11/100 (11%) of participants treated for 6 months and 8/91 (8.8%) of participants treated for 9 months were lost to follow‐up during ATT, with unknown reasons. |
| Incomplete outcome data (attrition bias) Outcomes assessed at the end of follow‐up | Unclear risk | After completing ATT, 4/80 (5%) participants treated for 6 months and 4/72 (5.6%) participants treated for 9 months were lost to follow‐up, with unknown reasons. |
| Selective reporting (reporting bias) | Low risk | We did not observe any evidence of selective reporting. The trial authors reported all the outcomes they had specified in the methods in the results section. |
| Other bias | Low risk | We did not identify any other sources of bias. |
Park 2009.
| Methods | Study design: single‐centre randomized open trial. Follow‐up method
Length of follow‐up after completing treatment: 79 participants were successfully followed up 12 months after completing ATT, with a median duration of 39 months (range 6 to 131) in the 6 months group, and a median duration of 32 months (range 10 to 127) in the 9 months group. Losses to follow‐up: no participants was lost during ATT; 5 were lost after ATT (2 from the 6‐month ATT group, 3 from the 9‐month ATT group). |
|
| Participants |
Inclusion criteria Participants with a diagnosis of intestinal TB. A definite diagnosis of intestinal TB was made if the participant met at least 1 of the following criteria.
Exclusion criteria
Participants recruited Number: 90 enrolled (45 in each arm). Median age (range): 36 (18 to 71) years versus 42 (20 to 71) years; 40% versus 49% males. HIV status: HIV negative participants, HIV‐seropositivity was used as an exclusion criterion. Nutritional status: not reported. Features of malabsorption: hypoalbuminaemia, defined as albumin of < 3.3 g/dL: 33% versus 56%. Radiological evidence of active pulmonary TB: 20/45 (44%) participants in the 6‐month ATT group and 24/45 (53%) in the 9‐month ATT group. Diagnosis procedures
Site of TB within the abdomen All patients with intestinal TB (38 ileocaecal area, 25 ascending colon, 10 transverse colon, 4 descending colon, 2 sigmoid colon, 3 rectum versus 41 ileocaecal area, 28 ascending colon, 15 transverse colon, 5 descending colon, 6 sigmoid colon, 4 rectum). |
|
| Interventions | 6‐month regimen: 2HRZE/4HRE. 9‐month regimen: 2HRZE/7HRE. Daily dosages
The decision to retain ethambutol in the continuation phase was based on high rates of primary drug resistance in South Korea. Drug susceptibility tests were performed in 33/49 culture‐positive: 2 in the 6‐month group and 3 in the 9‐month group were isoniazid‐resistant strains. However, these patients did not receive second‐line drugs and achieved complete responses after their scheduled therapies. Co‐interventions: no surgery in any patient (also indicated if intestinal obstruction, perforation or fistula), and no corticosteroids given. |
|
| Outcomes |
|
|
| Notes | Location: the Asan Medical Centre, a University Hospital in Seoul, South Korea.
Study dates: from October 1995 to October 2005. Funding: none stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was performed using a computer‐generated list." |
| Allocation concealment (selection bias) | Unclear risk | The trial authors did not provide any details. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | “The trial was not double blind” but we considered this to be at low risk of performance bias for all outcomes. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | "The trial was not double blind”, which introduces low risk of detection bias for objective outcomes. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | "The trial was not double blind”, which introduces high risk of detection bias for subjective outcomes, as criteria for cure and relapse were centred on endoscopic findings. |
| Incomplete outcome data (attrition bias) Outcomes assessed at the end of ATT | Low risk | No participants were lost during ATT. |
| Incomplete outcome data (attrition bias) Outcomes assessed at the end of follow‐up | Unclear risk | In this trial, 5/84 (6.0%) participants were lost to follow‐up after completing ATT, with unknown reasons. |
| Selective reporting (reporting bias) | Low risk | We did not observe any evidence of selective reporting. The trial authors reported all the outcomes they had specified in the methods in the results section. |
| Other bias | Low risk | We did not identify any other sources of bias. |
Tony 2008.
| Methods | Study design: single‐centre RCT. Follow‐up method
Length of follow‐up after completing treatment: median follow‐up of 27 months (range 3 to 55) in participants treated for 6 months and median follow‐up of 26 months (range 3 to 52) in participants treated for 9 months. Losses to follow‐up: 1 participant in the 9‐month regimen arm was lost after 2 months from starting ATT. After completing ATT, the trial authors did not mention any other additional participant as lost to follow‐up. |
|
| Participants |
Inclusion criteria Participants diagnosed with TB of the ileocaecal region, colon or both sites, on the basis of clinical, radiological, and endoscopic features and with histological evidence of epithelioid granuloma with or without caseation and AFB positivity. The trial also included patients without microbiological confirmation but with resolution of symptoms, increase in weight, and healing of lesions after treatment. Exclusion criteria
Participants recruited Number: 47 enrolled (23 in the 6‐month ATT group versus 24 in the 9‐month ATT group). Mean age (SD): 39.9 (13.5) years versus 37.8 (11.6) years; 57% versus 58% males. HIV status: HIV‐negative participants. The trial used HIV‐seropositivity as an exclusion criterion. Nutritional status: not reported. Features of malabsorption: not reported. Radiological evidence of active pulmonary TB: 4/23 participants in the 6‐month ATT group, and 3/24 participants in the 9‐month ATT group. Diagnosis procedures
Site of TB within the abdomen: all participants with TB in ileocaecal region, colon, or both sites. |
|
| Interventions | 6‐month regimen: 2(HRZE)3/4(HR)3 under DOTS. Thrice weekly dosages
9‐month regimen: 2HRZE/7HR no DOTS. Daily dosages
Co‐interventions: none reported. |
|
| Outcomes |
|
|
| Notes | Location: Calicut medical college, Kerala, India.
Study dates: from January 2002 to December 2006. Funding: none stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Random numbers were generated by a computer program." |
| Allocation concealment (selection bias) | Unclear risk | The trial authors did not provide any details. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | “Absence of blinding of participants” and personnel as well (as 1 arm received the treatment through DOTS while the other did not). However, we considered this to be at low risk of performance bias for all outcomes. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | The trial authors did not provide any details. However, although unblinded, it would be at low risk of detection bias for objective outcomes. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | “Colonoscopy was done at 2 and 6 months by two seniors consultants in the department who were not aware of the treatment allocation.” |
| Incomplete outcome data (attrition bias) Outcomes assessed at the end of ATT | Low risk | One participant (4.2%) allocated to the 9‐month regimen group was lost to follow‐up during treatment. |
| Incomplete outcome data (attrition bias) Outcomes assessed at the end of follow‐up | Unclear risk | The trial authors did not provide any details on the number of participants who completed the follow‐up period. Large follow‐up range are provided for both arms (3 to 52 and 3 to 55 months respectively) and it is unclear whether these ranges comprise all participants. |
| Selective reporting (reporting bias) | Low risk | We did not observe any evidence of selective reporting. The trial authors reported all the outcomes they had specified in the methods in the results section. |
| Other bias | Unclear risk | The trial authors calculated a sample size of 45 in each arm for detecting a difference of 30% between conventional and DOTS regimens, with a 90% power. The investigators evaluated interim findings after enrolling 47 participants and considered that the results were similar at 6 months. Consequently, they decided to stop recruiting as they considered it was unethical to proceed. The trial authors did not state in the methods that investigators would conduct an interim evaluation of the findings. It would not have been unethical to continue the trial as outcomes were similar in each arm with no excess adverse events observed in either arm. It is unclear how stopping the trial early might have affected the overall findings. The authors considered the results similar between the 2 groups, but the findings could have changed if the trial had continued. |
AFB: acid‐fast bacilli; ATT: antituberculous treatment; CT: computerized tomography; DOTS: directly observed short‐course therapy; EOT: end of therapy; ESR: erythrocyte sedimentation rate; GI: gastrointestinal; PCR: polymerase chain reaction; RCT: randomized controlled trial; SD: standard deviation; TB: tuberculosis.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Balasubramanian 1997 | This trial compared a 6‐month regimen based on rifampicin, isoniazid, and pyrazinamide, with a 12‐month regimen based on isoniazid, ethambutol, and streptomycin. |
| Makharia 2014a | Duplicated data with Makharia 2015a. |
| Makharia 2014b | Duplicated data with Makharia 2015a. |
| Makharia 2015b | Duplicated data with Makharia 2015a. |
Differences between protocol and review
We amended the objective of the review from "To compare the effects of six months versus longer regimens for abdominal TB, consisting of a two months intensive phase with rifampicin, isoniazid, pyrazinamide, and ethambutol followed by a four months or longer continuation phase including at least isoniazid and rifampicin" to the current title to simplify the wording.
We changed the definition of relapse from "participants who have new symptoms and signs of abdominal TB after resolution of disease and completion of antituberculous treatment (ATT)" to "participants who have new symptoms and signs of TB after resolution of disease and completion of ATT". We classified all forms of TB that occurred in participants who had completed ATT and were considered cured as 'relapse' for the purposes of this review.
We stated in our protocol that when RR value was inappropriate to evaluate the effect estimate of uncommon events, we would use risk difference. In the review, we used RR for all outcomes even when there were few events. However, we presented them together with the absolute effect to avoid any misleading interpretation of the findings.
We modified the outcome "participants with adverse events that lead to the discontinuation of ATT" to "participants with adverse events that lead to the discontinuation or modification of ATT", as this better reflects clinical practice.
Contributions of authors
SJu and Sja assessed the eligibility of the studies, extracted the data, and assessed the methodological quality of the included studies. SJu and HR assessed the quality of the evidence using the GRADE approach. SJu drafted the text with input from all the other review authors.
Sources of support
Internal sources
Cochrane Infectious Diseases Group, Liverpool School of Tropical Medicine, UK.
All India Institute of Medical Sciences, New Delhi, India.
Liverpool School of Tropical Medicine, UK.
External sources
-
Department for International Development (DFID), UK.
Grant: 5242
Declarations of interest
SJu and HR are employed by the CIDG, which is funded by a grant from the Department for International Development (DFID), UK. SJa has no known conflicts of interest. VA is an author of one of the included trials, Makharia 2015a; he was not involved in the data extraction and the 'Risk of bias' assessment of this study.
Unchanged
References
References to studies included in this review
Makharia 2015a {published data only}
- Makharia GK, Ghoshal UC, Ramakrishna BS, Agnihotri A, Ahuja V, Chowdhury SD, et al. Intermittent directly observed therapy for abdominal tuberculosis: a multicenter randomized controlled trial comparing 6 months versus 9 months of therapy. Clinical Infectious Diseases 2015;61(5):750‐7. [DOI: 10.1093/cid/civ376] [DOI] [PubMed] [Google Scholar]
Park 2009 {published data only}
- Park SH, Yang SK, Yang DH, Kim KJ, Yoon SM, Choe JW, et al. Prospective randomized trial of six‐month versus nine‐month therapy for intestinal tuberculosis. Antimicrobial Agents and Chemotherapy 2009;53(10):4167‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tony 2008 {published data only}
- Tony J, Sunilkumar K, Thomas V. Randomized controlled trial of DOTS versus conventional regime for treatment of ileocecal and colonic tuberculosis. Indian Journal of Gastroenterology 2008;27(1):19‐21. [PubMed] [Google Scholar]
References to studies excluded from this review
Balasubramanian 1997 {published data only}
- Balasubramanian R, Nagarajan M, Balambal R, Tripathy SP, Sundararaman R, Venkatesan P, et al. Randomised controlled clinical trial of short course chemotherapy in abdominal tuberculosis: a five‐year report. International Journal of Tuberculosis and Lung Disease 1997;1(1):44‐51. [PubMed] [Google Scholar]
Makharia 2014a {published data only}
- Makharia GK, Agnihotri A, Chaudhary S, Ghoshal U, Pathak MK, Mishra A, et al. A comparison of efficacy of 6 months and 9 months of intermittent anti‐tuberculous therapy for abdominal tuberculosis (intestinal and peritoneal): a randomized controlled trial. Asian Pacific Digestive Week Conference, 2014 Nov 22‐25; Bali, Indonesia. Journal of Gastroenterology and Hepatology (Australia) 2014;29:238. [Google Scholar]
Makharia 2014b {published data only}
- Makharia GK, Agnihotri A, Ghoshal UC, Ramakrishna BS, Chaudhary S, Pathak MK, et al. A comparison between efficacy of 6 months and 9 months of intermittent anti‐tuberculous therapy for abdominal tuberculosis (intestinal and peritoneal): A randomized controlled trial. 55th Annual Conference of the Indian Society of Gastroenterology, ISGCON ‐ 2014, 2014 Dec 19‐23; Mumbai. Indian Journal of Gastroenterology 2014;33(1 Suppl 1):A18. [Google Scholar]
Makharia 2015b {published data only}
- Makharia GK, Ghoshal UC, Ramakrishna BS, Agnihotri A, Ahuja V, Chowdhury SD, et al. Intermittent supervised therapy for abdominal tuberculosis: a multicenter randomized controlled trial comparing 6 months versus 9 months duration of therapy. Version civ376v1. http://cid.oxfordjournals.org/content/early/2015/05/11/cid.civ376 (accessed 7 September 2016). [DOI: 10.1093/cid/civ376] [DOI] [PubMed]
Additional references
American Thoracic Society 2003
- American Thoracic Society, Centers for Disease Control and Prevention, Infectious Diseases Society of America. Treatment of tuberculosis. Morbidity and Mortality Weekly Report 2003;52(RR‐11):1‐77. [PubMed] [Google Scholar]
Bolukbas 2005
- Bolukbas C, Bolukbas FF, Kendir T, Dalay RA, Akbayir N, Sokmen MH, et al. Clinical presentation of abdominal tuberculosis in HIV seronegative adults. BMC Gastroenterology 2005;5:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Debi 2014
- Debi U, Ravisankar V, Prasad KK, Sinha SK, Sharma AK. Abdominal tuberculosis of the gastrointestinal tract: revisited. World Journal of Gastroenterology 2014;20(40):14831‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Golden 2005
- Golden MP, Vikram HR. Extrapulmonary tuberculosis: an overview. American Family Physician 2005;72(9):1761‐8. [PubMed] [Google Scholar]
GRADEpro GDT 2014 [Computer program]
- GRADE Working Group, McMaster University. GRADEpro GDT. Version accessed 06 September 2016. Hamilton (ON): GRADE Working Group, McMaster University, 2014.
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction‐GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S, editor(s) Cochrane Handbook for Systematic Reviews of Interventions (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
INDEX‐TB 2016
- INDEX‐TB Guidelines Group. INDEX‐TB Guidelines ‐ Guidelines on Extra‐Pulmonary Tuberculosis for India. http://www.icmr.nic.in/guidelines/TB/Index‐TB%20Guidelines%20‐%20green%20colour%202594164.pdf. Date accessed 13th September 2016.. First. New Delhi: Central TB Division, Ministry of Health and Family Welfare, Government of India, 2016.
Jüni 2001
- Jüni P, Altman DG, Egger M. Systematic reviews in health care: assessing the quality of controlled clinical trials. BMJ 2001;323(7303):42‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Khan 2006
- Khan R, Abid S, Jafri W, Abbas Z, Hameed K, Ahmad Z. Diagnostic dilemma of abdominal tuberculosis in non‐HIV patients: an ongoing challenge for physicians. World Journal of Gastroenterology 2006;12(39):6371‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2003
- Kim SG, Kim JS, Jung HC, Song IS. Is a 9‐month treatment sufficient in tuberculous enterocolitis? A prospective, randomized, single‐centre study. Alimentary Pharmacology & Therapeutics 2003;18(1):85‐91. [DOI] [PubMed] [Google Scholar]
Lazarus 2007
- Lazarus AA, Thilagar B. Abdominal tuberculosis. Disease‐a‐Month 2007;53(1):32‐8. [DOI] [PubMed] [Google Scholar]
Mamo 2013
- Mamo JP, Brij SO, Enoch DA. Abdominal tuberculosis: a retrospective review of cases presenting to a UK district hospital. Quarterly Journal of Medicine 2013;106(4):347‐54. [DOI] [PubMed] [Google Scholar]
Menzies 2009
- Menzies D, Benedetti A, Paydar A, Martin I, Royce S, Pai M, et al. Effect of duration and intermittency of rifampin on tuberculosis treatment outcomes: a systematic review and meta‐analysis. PLoS Medicine 2009;6(9):e1000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
Raffetseder 2014
- Raffetseder J, Pienaar E, Blomgran R, Eklund D, Patcha Brodin V, Andersson H, et al. Replication rates of Mycobacterium tuberculosis in human macrophages do not correlate with mycobacterial antibiotic susceptibility. PLoS One 2014;9(11):e112426. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sharma 2004
- Sharma SK, Mohan A. Extrapulmonary tuberculosis. Indian Journal of Medical Research 2004;120(4):316‐53. [PubMed] [Google Scholar]
Sheer 2003
- Sheer TA, Coyle WJ. Gastrointestinal tuberculosis. Current Gastroenterology Reports 2003;5(4):273‐8. [DOI] [PubMed] [Google Scholar]
Tinsa 2010
- Tinsa F, Essaddam L, Fitouri Z, Brini I, Douira W, Ben Becher S, et al. Abdominal tuberculosis in children. Journal of Pediatric Gastroenterology and Nutrition 2010;50(6):634‐8. [DOI] [PubMed] [Google Scholar]
WHO 2010
- World Health Organization. Guidelines for treatment of tuberculosis, 4th edition. 2010. apps.who.int/iris/bitstream/10665/44165/1/9789241547833_eng.pdf?ua=1&ua=1 (accessed 18 September 2015).
WHO 2013
- World Health Organization. Definitions and reporting framework for tuberculosis ‐ 2013 revision (updated December 2014). apps.who.int/iris/bitstream/10665/79199/1/9789241505345_eng.pdf (accessed 18 March 2016).
WHO 2014
- World Health Organization. Standards for TB care in India. 2014. www.searo.who.int/india/mediacentre/events/2014/stci_book.pdf (accessed 28 April 2016).
WHO 2015a
- World Health Organization. Global tuberculosis report 2015, 20th edition. apps.who.int/iris/bitstream/10665/191102/1/9789241565059_eng.pdf?ua=1 (accessed 16 December 2015).
WHO 2015b
- World Health Organization. Use of high burden country lists for TB by WHO in the post‐2015 era. www.who.int/tb/publications/global_report/high_tb_burdencountrylists2016‐2020.pdf?ua=1 (accessed 19 April 2016).
Zumla 2014
- Zumla AI, Gillespie SH, Hoelscher M, Philips PPJ, Cole ST, Abubakar I, et al. New antituberculosis drugs, regimens, and adjunct therapies: needs, advances, and future prospects. Lancet. Infectious Diseases 2014;14(4):327‐40. [DOI] [PubMed] [Google Scholar]


