Abstract
We have recently described a molecular gatekeeper of the hypothalamic-pituitary-gonadal axis with the observation that G protein-coupled receptor 54 (GPR54) is required in mice and men for the pubertal onset of pulsatile luteinizing hormone (LH) and follicle-stimulating hormone (FSH) secretion to occur. In the present study, we investigate the possible central mode of action of GPR54 and kisspeptin ligand. First, we show that GPR54 transcripts are colocalized with gonadotropin-releasing hormone (GnRH) neurons in the mouse hypothalamus, suggesting that kisspeptin, the GPR54 ligand, may act directly on these neurons. Next, we show that GnRH neurons seem anatomically normal in gpr54–/– mice, and that they show projections to the median eminence, which demonstrates that the hypogonadism in gpr54–/– mice is not due to an abnormal migration of GnRH neurons (as occurs with KAL1 mutations), but that it is more likely due to a lack of GnRH release or absence of GnRH neuron stimulation. We also show that levels of kisspeptin injected i.p., which stimulate robust LH and FSH release in wild-type mice, have no effect in gpr54–/– mice, and therefore that kisspeptin acts directly and uniquely by means of GPR54 signaling for this function. Finally, we demonstrate by direct measurement, that the central administration of kisspeptin intracerebroventricularly in sheep produces a dramatic release of GnRH into the cerebrospinal fluid, with a parallel rise in serum LH, demonstrating that a key action of kisspeptin on the hypothalamo-pituitary-gonadal axis occurs directly at the level of GnRH release. The localization and GnRH release effects of kisspeptin thus define GPR54 as a major control point in the reproductive axis and suggest kisspeptin to be a neurohormonal effector.
Keywords: hypothalamus, neurons, gonadotropin axis, reproduction
The pulsatile release of gonadotropin-releasing hormone (GnRH) into the hypophyseal circulation occurs during late fetal development or early neonatal development in mammals and is then suppressed until puberty, when the reactivation of dormant hypothalamic GnRH-secreting neurons is the key event underlying the onset of pubertal development (1). The molecular control of this process has remained largely obscure, although genetic studies have revealed the requirement of molecules such as KAL1 (2, 3) and netrins (4) in the migration of GnRH neurons themselves, and mutations in the GnRH and GnRH receptor genes reveal this ligand–receptor pairing as the common pathway to gondadotropin release from the pituitary. We have recently described, however, that loss-of-function mutations in human and mouse G protein-coupled receptor 54 (GPR54) show that this receptor is absolutely required in mammals for puberty to occur (5).
GPR54 (also called AX0R12, or hot7t175) was first discovered in 1999 in the rat, as a novel orphan G protein-coupled receptor sharing 45% identity with Galanin receptors (6) and is thus clustered with the galanin-like receptors, although there is no evidence that galanin-like peptides bind to the receptor with significant affinity. GPR54 was shown to be highly expressed in the brain, and in particular in the hypothalamus, limbic system and basal ganglia, with an expression pattern that resembles that of galanin receptors (6–8). GPR54 is also expressed peripherally in the placenta, pituitary, spinal cord, pancreas, heart, muscle, kidney, liver, intestine, thymus, lung, and testis.
In 2001, four independent groups identified a high-affinity RF-amide (Arg-Phe-NH2) peptide ligand for GPR54 (7, 9–11) named kisspeptin (also known as metastin), the processed product of the kiss-1 gene. Kisspeptin had previously been identified as an orphan peptide that altered the migration properties of metastatic melanoma cells in vitro (12). The kiss-1 protein is a 145-aa peptide from the RF-amide family, with an Arg-Phe-Nh2 C-terminal sequence characteristic of this family of peptides. It is processed into various C-terminal fragments, referred to as kisspeptin-54, -14, -13, and -10, that all bind and activate GPR54 (8, 9, 13). Subsequently, cell biological experiments suggested that activation of the GPR54 receptor introduced in cell lines can alter migratory behavior based on cytoskeletal dynamics. Ohtaki et al. (10) and Hori et al. (14) demonstrated that kisspeptin inhibits chemotaxis and invasion of GPR54-expressing cells in vitro and attenuates pulmonary metastasis of GPR54-transfected melanomas in vivo (14). Recent studies have suggested that placental-derived kisspeptin may regulate syncytiotrophoblast invasion in the uterus (15, 16). Small-scale and limited clinical studies of human tumor material seem inconclusive with respect to oncogenic mechanisms, because both amplification and deletion of loci have been described. However, a recent study correlating transcript levels with clinical outcome suggested a more favorable prognosis for high peptide-expressing malignant cells (14, 17, 18).
The unprecedented central functions of GPR54 in regulating mammalian reproductive development were, however, only recently described with genetic studies. Humans and mice deficient for GPR54 protein display phenocopy syndromes characterized as isolated hypogonadotropic hypogonadism (5, 19, 20). In these studies, we have shown that, despite the absence of normal release of gonadotropins in gpr54-deficient mice and humans, pituitary gonadotropes remain functionally intact and capable of responding to exogenous GnRH with luteinizing hormone (LH) and follicle-stimulating hormone (FSH) release (5). Furthermore, we showed that the hypothalamic production of GnRH peptide in knockout mice is unaffected by the gpr54 mutation (5), ruling out a mechanism directly linked to GnRH synthesis. These observations pointed firmly to a potential role of gpr54 in modulating GnRH release and suggested that kisspeptins could be neurohormonal effectors of gonadotropin release. Mechanistically, one hypothesis suggested by this study was that GPR54 may act on GnRH neurons to modulate GnRH release. To address this question, we have in this study addressed whether kisspeptin acts uniquely by means of the GPR54 receptor, studied the colocalization of GnRH peptide and GPR54 transcripts, and demonstrated the direct effects of kisspeptin on GnRH release.
Materials and Methods
Mouse Experiments. Animals. The transgenic mice (gpr54–/–) were maintained as an inbred stock on a 129S6/SvEv genetic background. The mice were maintained on a 12-h day and 12-h night cycle and were provided with food and water ad libitum. All experiments were performed under the authority of a U.K. Home Office Project License and were approved by a local ethics panel.
Colocalization of GPR54 and GnRH neurons. For GnRH immunohistochemistry, adult male and female gpr54–/– mice and control littermates (n = 5 each) were perfused with 4% paraformaldehyde in PBS. The brains were then dissected and postfixed for 2 h at room temperature. After sucrose treatment, tissues were frozen-sectioned at a 50-μm thickness. All sections from preoptic area to median eminence were collected. The sections were incubated in 3% H2O2, then incubated overnight at 4°C with a primary rabbit polyclonal antibody against GnRH (LR1, a gift from R. Benoit, which was provided by means of F. Ebling, University of Nottingham, Nottingham, U.K.) diluted at 1:10,000, with 0.1% Triton X-100 and 1% milk powder in PBS. Detections were done by using biotinylated secondary antibodies and avidin–biotin complex (ABC; Vector Laboratories), with 3′3′-diaminobenzidine (DAB, Sigma) as chromogen.
For colocalization of β-galactosidase and GnRH, two male adult gpr54–/– mice were perfused with 4% paraformaldehyde in PBS, and the brains were dissected and postfixed for 1 h at 4°C. After sucrose treatment overnight at 4°C, tissues were frozen-sectioned at a 50-μm thickness. To detect β-galactosidase activity, sections were incubated overnight at room temperature in staining buffer containing 1 mM MgCl2, 0.5 mg/ml X-Gal (Melford Laboratories, Chelsworth, Ipswich, Suffolk, U.K.), 5 mM potassium ferrocyanide, and 5 mM potassium ferricyanide. The sections were immediately fixed by 4% paraformaldehyde in PBS for 10 min at room temperature. After washes in PBS, sections were immunostained for GnRH as described above.
Kisspeptin injections in wild-type and gpr54–/– mice. Wild-type (n = 6) and gpr54–/– (n = 6) 2- to 4-month-old male mice received four i.p. injections of 100 μlof10 μM mouse kisspeptin in 0.1 M PBS (mouse kisspeptin 105-119, Peptide Protein Research, Eastleigh, U.K.) at 30-min intervals. The mice were killed by CO2 exposure 30 min after the last injection. Blood was collected in a heparinized syringe from the vena cava and centrifuged at 1,000 × g for 5 min at room temperature. The serum supernatant samples were collected and stored at –80°C until assayed.
Mouse hormone assays. LH and FSH were measured by mouse immunoradiometric assay (IRMA) and mouse RIA, respectively, by the Ligand Assay and Analysis Core Laboratory, Center for Research in Reproduction, University of Virginia, Charlottesville. The sensitivity of the IRMA for LH was 0.07 ng/ml (intraassay variation, 6.0%; interassay variation, 12.5%), and the sensitivity of the RIA for FSH was 2 ng/ml (intraassay variation, 10%; interassay variation, 18%).
Sheep Experiments. Two experiments were conducted during the anoestrus season by using 2- to 3-yr-old Ile de France ewes. One month before the first experiment, ewes (n = 8) were ovariectomized and treated immediately with a 10-mm s.c. SILASTIC silicon tubing (Dow–Corning) implant containing estradiol (inserted under the skin in the internal face of one of the back legs) to strongly reduce GnRH/LH secretion as described (21). Simultaneously, two guide tubes were implanted aseptically under general anesthesia into the ventricular system (one in the lateral ventricle and one in the third ventricle) according to the procedure described by Skinner et al. (22). All experimental procedures were performed in accordance with local animal regulations (Authorization A37801 of the French Ministry of Agriculture).
Effect of kisspeptin on LH release. The day preceding the experiment, a catheter was inserted into the jugular vein to allow collection of blood samples, and ewes were restrained in small pens so that they could not turn around but were able to move forwards and backwards. To prevent the stress of social isolation, ewes were always in contact with other sheep and food and water were available ad libitum. The day of the experiment, blood samples were taken every 10 min for 10 h. After 4 h, a polyethylene catheter (Biotrol, Paris; o.d., 0.7 mm; i.d., 0.3 mm) was inserted through the third ventricle guide cannula so that the distal tip ended at the tip of the guide cannula. Animals were then treated intracerebroventricularly for 4 h by means of the catheter connected to a portable syringe pump (Graseby Medical, Watford, Hertfordshire, U.K.) either with human kisspeptin 112-121 (Phoenix Europe, Karlsruhe, Germany) diluted in sterile, pyrogen-free saline (flow rate 3 μl/min; concentrations of 0.2 μg/min; n = 4) or with vehicle (n = 4). The total quantity of peptide administered over the 4 h was 50 nmol. Blood collection was continued for 2 h more, and, at hour 9, animals were treated i.v. with a GnRH injection (250 ng in 2 ml of saline) to test the pituitary responsiveness. Plasma samples were stored at –18°C until assayed.
Effect of kisspeptin on GnRH release. The first experiment revealed that kisspeptin administered centrally [into the cerebrospinal fluid (CSF)] induced a strong increase of plasma LH concentration. To determine whether this increase is due to an increase of GnRH secretion, samples of CSF and jugular blood were collected simultaneously before and after infusion of the peptide.
Six of the eight animals of the previous experiment were used. A polyethylene catheter was inserted through the third ventricle guide cannula and connected to a peristaltic pump (Minipuls, Gilson, France). Samples of CSF were withdrawn at a flow rate of 300–400 μl every 15 min for 10 h. At the end of each 15-min period, a blood sample was taken from a jugular catheter inserted the day before. After 4 h, a second polyethylene catheter was inserted through the lateral ventricle guide cannula, and kisspeptin was infused with a portable syringe pump (flow rate 3 μl/min; concentrations of 90 μg/ml) for 4 h. Plasma samples, as well as CSF samples, were stored at –18°C until assayed.
Sheep hormone assays. Blood samples were assayed for LH in duplicate 100-μl aliquots of plasma by using a previously described RIA method (23). All samples from an experiment were run in a single assay. Intraassay coefficient of variation averaged 9%, and assay sensitivity was 0.16 ng/ml standard 1051-CY-LH (i.e., 0.31 ng/ml of NIH-LH-S1).
CSF samples were assayed for GnRH by using the method of Caraty et al. (24). All samples from an experiment were measured in duplicate in the same assay, and the intraassay coefficient of variation (CV) averaged 15% (six assays).
Statistical Analysis. Comparisons between treated groups and genotypes were done by using Student's t test or an unpaired Mann–Whitney test.
Results
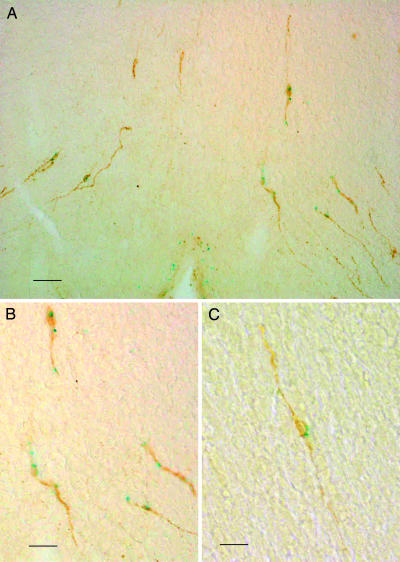
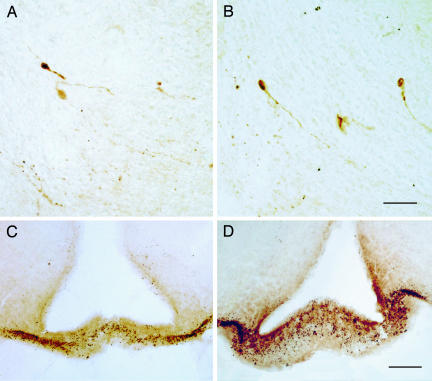
Colocalization of gpr54 and GnRH Neurons. Fig. 1 shows the presence of gpr54 expression in GnRH-immunoreactive neuronal cell bodies (A and B) and dendrites, establishing that the receptor is colocalized with GnRH neurons. Of 20 clearly identifiable GnRH-positive neuronal cell bodies in two animals (representative field shown in Fig. 1C) 11 (55%) were also β-galactosidase-positive. Fig. 2 shows the presence of GnRH-immunoreactive nerve terminals in the median eminence of homozygous mutant animals and wild-type mice (n = 5 for both mutants and wild-type controls).
Fig. 1.
Localization of GPR54 to GnRH neurons. (A and B) Coronal sections from gpr54–/– mice preoptic hypothalami, showing neurons and dendrites double stained for β-galactosidase (blue) and GnRH (brown). (C) Representative medium-power field of preoptic areas. [Scale bars: 35 μm(A), 80 μm(B), and 45 μm(C).]
Fig. 2.
GnRH neuron morphology and localization in gpr54–/– mice. Illustrated are coronal sections showing immunostained GnRH neurons in the preoptic area (A and B) (cell bodies and processes) and the median eminence (C and D) (nerve endings) of wild-type and mutant mice. [Scale bars: 45 μm(A and B) and 75 μm(C and D).]
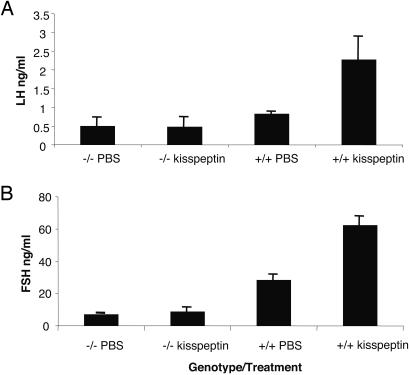
Injections of Kisspeptin in Wild-Type and gpr54–/– Mice. Fig. 3 shows that wild-type mice exhibit a robust and significant rise in plasma LH (P < 0.05) and FSH (P < 0.01) in response to kisspeptins, whereas mutant mice under the same administration regime fail to mount any detectable response.
Fig. 3.
Effect of i.p. kisspeptin injection on LH (A) and FSH (B) release in male wild-type (+/+) and mutant (–/–) mice.
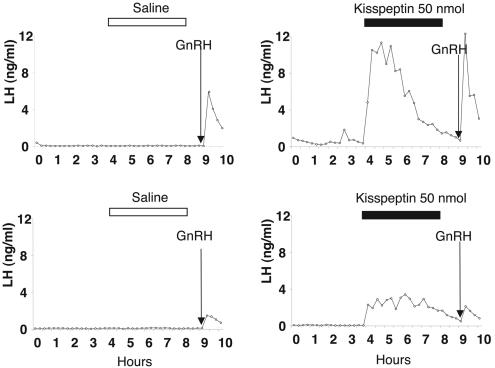
Effect of Kisspeptin on LH and GnRH Release in Sheep. In the first experiment, kisspeptin infusion in the third ventricle induced a rapid and sustained increase in LH secretion in all four animals in the experimental group and none of four animals in the control group (Fig. 4). For all four test animals, plasma LH increased in the first sample after the start of the infusion i.e., between 5 and 10 min later. Higher levels were reached during the first 2 h; then there was a progressive decline before the end of the peptide infusion. Administration of GnRH (250 ng i.v.) 1 h after the end of the infusion was able to induce an LH pulse in all animals. LH level (mean ± SEM) during the 4 h of kisspeptin infusion was significantly higher compared with the preceding 4 h (4.74 ± 0.42 ng/ml vs. 0.22 ± 0.25 ng/ml, P < 0.02). In contrast, saline infusion did not induce any change in LH secretion (mean ± SEM) for the 4 h during saline infusion vs. the 4 h before (0.62 ± 0.49 ng/ml vs. 0.47 ± 0.42 ng/ml). In all saline-treated ewes, GnRH injection also induced an LH pulse in the peripheral circulation.
Fig. 4.
Effect of kisspeptin on LH release in sheep. Representative profiles of serum LH secretion for two experimental sheep receiving 50 nmol of kisspeptin (intracerebroventricularly) over a 4-h infusion period (Right) or control animal receiving the vehicle (Left). The arrow indicates the time of the i.v. injection of 250 ng of GnRH.
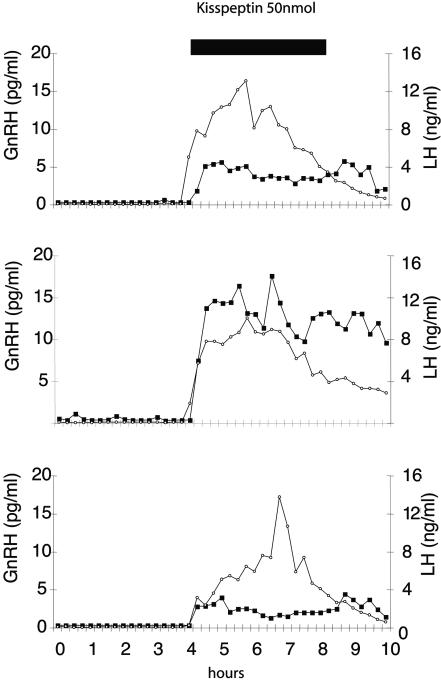
In the second experiment, plasma LH measurements made in tandem with CSF sampling for GnRH measurements showed an abrupt increase in GnRH secretion in the CSF at the start of the kisspeptin infusion (Fig. 5). As in the first group of experiments, LH secretion began to decrease before the end of the peptide infusion period; however, GnRH secretion persisted. Indeed, for two of six animals, there was no sign of a decline in GnRH levels up to 2 h after the end of the peptide infusion period.
Fig. 5.
Effect of kisspeptin on GnRH and LH secretion in sheep. Shown are CSF GnRH (filled squares) and serum LH (open circles) secretory profiles for three representative sheep before and after central administration of kisspeptin (50 nmol given over a 4-h period).
Overall, mean GnRH and LH levels were significantly increased during the 4 h of kisspeptin infusion compared with the preceding 4 h (respectively, 0.48 ± 0.16 pg/ml vs. 5.78 ± 1.56 pg/ml, P < 0.01 and 0.25 ± 0.15 ng/ml vs. 8.83 ± 0.53 ng/ml, P < 0.001).
Discussion
Using genetic approaches in mouse and man, we have previously shown that the GPR54 receptor (and by implication the ligands of GPR54), represent molecular “gatekeepers” of mammalian pubertal LH and FSH secretion (5). GnRH-containing neurons are normally found in the medial preoptic area, with axonal projections to the median eminence, where GnRH is released into the hypophyseal circulation. It is well established that proteins that affect migration of GnRH neurons will produce hypothalamic hypogonadism (5). Because there is cell biological evidence (see Introduction) that, in some contexts, GPR54 signaling may regulate cell migratory behavior, it has been hypothesized (25) that GPR54 could be acting as a major determinant of GnRH neuron migration or maturation. Our original observation that hypothalamic GnRH content is normal in gpr54 mutant mice and the observation that isolated idiopathic hypogonadotropic hypogonadism (IHH) patients with gpr54 mutations are normosmic argue against this possibility. However, to rule out a major requirement of GPR54 in this context, we searched for immunoreactive GnRH neurons in the hypothalamus of wild-type and gpr54 homozygous mutant mice. As shown above, not only are GnRH neurons found in the same location in the hypothalamus of mutant mice, but they also show similar morphology and projections to the median eminence. This finding refutes the hypothesis that a major requirement for GPR54 is in GnRH neuronal migration or maturation (25).
Because the mechanism suggested by the aggregate of the above studies is that GPR54 may regulate GnRH release from GnRH neurons, we next asked whether GPR54 is colocalized in GnRH neurons in the mammalian brain. To address this question, we took advantage of the lacZ reporter in the mutant mice, and, as shown above, β-galactosidase activity can be found coincident with GnRH immunoreactive cell bodies. Counting of clearly distinguishable and immunoreactive cell bodies showed ≈55% of GnRH neurons to also coexpress β-galactosidase.
This finding strongly suggests that kisspeptin ligands could act as GnRH-releasing peptides. This result is consistent with a recent study in cichlid fish (25) that demonstrated, using laser capture microdissection, that GnRH neurons express GPR54.
Although kisspeptins have been previously described as high-affinity ligands for the GPR54 receptor (7, 9–11), it has not been shown that they are the only ligands, or crucially, that the effects of kisspeptins on gonadotropin secretion act only by means of the GPR54 receptor. In the latter case, it is predicted that the potent gonadotropin-releasing effect of kisspeptins in vivo should be ablated in GPR54 receptor-deficient animals. To address this question, we conducted identical peripheral peptide administration regimes in wild-type and gpr54 homozygous knockout mice, measuring gonadotropin release in each case. Mutant mice fail to mount any detectable response with these kisspeptin administration regimes, showing that the effects of kisspeptin on gonadotropin release are uniquely by means of the GPR54 receptor. Moreover, we have previously shown that gpr54–/– mice can release LH in response to exogenous GnRH so their lack of response to kisspeptin is not due to pituitary failure (5).
Finally, we asked whether kisspeptin can stimulate GnRH release, by directly measuring GnRH secretion into the CSF. To address this question, we used a sheep model of intracerebroventricular peptide administration, with CSF and plasma sampling as described (22). The data presented above support the predictions and demonstrate kisspeptin to be a potent GnRH releasing factor. We have shown, by direct measurement, that kisspeptin administration provokes GnRH peptide release as measured in the CSF of sheep. This response is extremely rapid, with a sharp rise in both GnRH and LH within minutes of kisspeptin infusion. This result suggests a direct action of kisspeptin at the levels of GnRH neurons. Because we have also shown that GPR54 is colocalized in GnRH neurons, the most likely mechanistic hypothesis is that GPR54 could act to modulate GnRH neuronal activity and/or to stimulate release of GnRH peptide at the median eminence.
The GnRH-releasing effect of kisspeptins is very strong because GnRH concentrations observed for some animals are in a similar range to the CSF GnRH values observed during a preovulatory GnRH surge (22). Moreover, the effect lasted as long as the molecule was present because none of the animals showed a sign of desensitization over the time of the perfusion. Interestingly, however, LH secretion began to decrease before the end of the kisspeptin infusion period, whereas GnRH secretion persisted in some animals, indicating that gonadotrope refractory response most likely accounts for the decline in serum LH. Interestingly, kisspeptin levels in human plasma have also been shown to rise sharply by four orders of magnitude during the course of pregnancy and, together with the above observations, suggest that there could also be some role for this control mechanism in the physiology of pregnancy.
Overall, the results presented here are consistent with our previous genetic definition of the absolute requirement of GPR54 function in the gonadotropic axis; crucially, however, we have also now established that the sole gonadotropic activity of these peptides in vivo is by means of the GPR54 receptor and have observed directly the dynamics of GnRH release in a mammal. These data establish the unprecedented and fundamental role of GPR54 signaling in the physiology of the reproductive axis, with consequent diagnostic and therapeutic implications for reproductive human and animal health. Future studies are urgently needed to understand the precise neural basis of the control mechanisms for GPR54 regulation of GnRH and the physiological relationships of kisspeptin secretion to the endocrinology of the reproductive axis.
Acknowledgments
We thank Dr. Robert Benoit (Montreal General Hospital, Montreal) for the gift of LR1, which was provided by means of Dr. Fran Ebling (University of Nottingham, Nottingham, U.K.). We also thank the University of Virginia Center for Research in Reproduction, Ligand Assay and Analysis Core Laboratory, for performing the mouse assays. The Ligand Assay and Analysis Core Laboratory is supported by the National Institute of Child Health and Human Development/National Institutes of Health through a cooperative agreement (USA HD28934) as part of the Specialized Cooperative Centers Program in Reproduction Research.
Abbreviations: GnRH, gonadotropin-releasing hormone; GPR54, G protein-coupled receptor 54; LH, luteinizing hormone; FSH, follicle-stimulating hormone; CSF, cerebrospinal fluid.
Note. During the preparation of this article, we became aware of studies showing that the stimulation of LH release by kisspeptin can be blocked by GnRH receptor antagonists (26, 27) and that peripheral as well as central administration of kisspeptins can provoke robust gonadotropin release (26–29). However, none of these studies has formally demonstrated a hypothalamic site of action on GnRH release.
References
- 1.Ebling, F. J. & Cronin, A. S. (2000) NeuroReport 11, R23–R33. [DOI] [PubMed] [Google Scholar]
- 2.Franco, B., Guioli, S., Pragliola, A., Incerti, B., Bardoni, B., Tonlorenzi, R., Carrozzo, R., Maestrini, E., Pieretti, M., Taillon-Miller, P., et al. (1991) Nature 353, 529–536. [DOI] [PubMed] [Google Scholar]
- 3.Legouis, R., Hardelin, J. P., Levilliers, J., Claverie, J. M., Compain, S., Wunderle, V., Millasseau, P., Le Paslier, D., Cohen, D., Caterina, D., et al. (1991) Cell 67, 423–435. [DOI] [PubMed] [Google Scholar]
- 4.Schwarting, G. A., Raitcheva, D., Bless, E. P., Ackerman, S. L. & Tobet, S. (2004) Eur. J. Neurosci. 19, 11–20. [DOI] [PubMed] [Google Scholar]
- 5.Seminara, S. B., Messager, S., Chatzidaki, E. E., Thresher, R. R., Acierno, J. S., Jr., Shagoury, J. K., Bo-Abbas, Y., Kuohung, W., Schwinof, K. M., Hendrick, A. G., et al. (2003) N. Engl. J. Med. 349, 1614–1627. [DOI] [PubMed] [Google Scholar]
- 6.Lee, D. K., Nguyen, T., O'Neill, G. P., Cheng, R., Liu, Y., Howard, A. D., Coulombe, N., Tan, C. P., Tang-Nguyen, A. T., George, S. R. & O'Dowd, B. F. (1999) FEBS Lett. 446, 103–107. [DOI] [PubMed] [Google Scholar]
- 7.Muir, A. I., Chamberlain, L., Elshourbagy, N. A., Michalovich, D., Moore, D. J., Calamari, A., Szekeres, P. G., Sarau, H. M., Chambers, J. K., Murdock, P., et al. (2001) J. Biol. Chem. 276, 28969–28975. [DOI] [PubMed] [Google Scholar]
- 8.Stafford, L. J., Xia, C., Ma, W., Cai, Y. & Liu, M. (2002) Cancer Res. 62, 5399–5404. [PubMed] [Google Scholar]
- 9.Kotani, M., Detheux, M., Vandenbogaerde, A., Communi, D., Vanderwinden, J. M., Le Poul, E., Brezillon, S., Tyldesley, R., Suarez-Huerta, N., Vandeput, F., et al. (2001) J. Biol. Chem. 276, 34631–34636. [DOI] [PubMed] [Google Scholar]
- 10.Ohtaki, T., Shintani, Y., Honda, S., Matsumoto, H., Hori, A., Kanehashi, K., Terao, Y., Kumano, S., Takatsu, Y., Masuda, Y., et al. (2001) Nature 411, 613–617. [DOI] [PubMed] [Google Scholar]
- 11.Clements, M. K., McDonald, T. P., Wang, R., Xie, G., O'Dowd, B. F., George, S. R., Austin, C. P. & Liu, Q. (2001) Biochem. Biophys. Res. Commun. 284, 1189–1193. [DOI] [PubMed] [Google Scholar]
- 12.Lee, J. H., Miele, M. E., Hicks, D. J., Phillips, K. K., Trent, J. M., Weissman, B. E. & Welch, D. R. (1996) J. Natl. Cancer Inst. 88, 1731–1737. [DOI] [PubMed] [Google Scholar]
- 13.Terao, Y., Kumano, S., Takatsu, Y., Hattori, M., Nishimura, A., Ohtaki, T. & Shintani, Y. (2004) Biochim. Biophys. Acta 1678, 102–110. [DOI] [PubMed] [Google Scholar]
- 14.Hori, A., Honda, S., Asada, M., Ohtaki, T., Oda, K., Watanabe, T., Shintani, Y., Yamada, T., Suenaga, M., Kitada, C., et al. (2001) Biochem. Biophys. Res. Commun. 286, 958–963. [DOI] [PubMed] [Google Scholar]
- 15.Horikoshi, Y., Matsumoto, H., Takatsu, Y., Ohtaki, T., Kitada, C., Usuki, S. & Fujino, M. (2003) J. Clin. Endocrinol. Metab. 88, 914–919. [DOI] [PubMed] [Google Scholar]
- 16.Bilban, M., Ghaffari-Tabrizi, N., Hintermann, E., Bauer, S., Molzer, S., Zoratti, C., Malli, R., Sharabi, A., Hiden, U., Graier, W., et al. (2004) J. Cell Sci. 117, 1319–1328. [DOI] [PubMed] [Google Scholar]
- 17.Lee, J. H. & Welch, D. R. (1997) Cancer Res. 57, 2384–2387. [PubMed] [Google Scholar]
- 18.Lee, J. H. & Welch, D. R. (1997) Int. J. Cancer 71, 1035–1044. [DOI] [PubMed] [Google Scholar]
- 19.de Roux, N., Genin, E., Carel, J. C., Matsuda, F., Chaussain, J. L. & Milgrom, E. (2003) Proc. Natl. Acad. Sci. USA 100, 10972–10976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Funes, S., Hedrick, J. A., Vassileva, G., Markowitz, L., Abbondanzo, S., Golovko, A., Yang, S., Monsma, F. J. & Gustafson, E. L. (2003) Biochem. Biophys. Res. Commun. 312, 1357–1363. [DOI] [PubMed] [Google Scholar]
- 21.Evans, N. P., Dahl, G. E., Glover, B. H. & Karsch, F. J. (1994) Endocrinology 134, 1806–1811. [DOI] [PubMed] [Google Scholar]
- 22.Skinner, D. C., Malpaux, B., Delaleu, B. & Caraty, A. (1995) Endocrinology 136, 3230–3237. [DOI] [PubMed] [Google Scholar]
- 23.Montgomery, G. W., Martin, G. B. & Pelletier, J. (1985) J. Reprod. Fertil. 73, 173–183. [DOI] [PubMed] [Google Scholar]
- 24.Caraty, A., Antoine, C., Delaleu, B., Locatelli, A., Bouchard, P., Gautron, J. P., Evans, N. P., Karsch, F. J. & Padmanabhan, V. (1995) Endocrinology 136, 3452–3460. [DOI] [PubMed] [Google Scholar]
- 25.Parhar, I. S., Ogawa, S. & Sakuma, Y. (2004) Endocrinology 145, 3613–3618. [DOI] [PubMed] [Google Scholar]
- 26.Gottsch, M. L., Cunningham, M. J., Smith, J. T., Popa, S. M., Acohido, B. V., Crowley, W. F., Seminara, S., Clifton, D. K. & Steiner, R. A. (2004) Endocrinology 145, 4073–4077. [DOI] [PubMed] [Google Scholar]
- 27.Matsui, H., Takatsu, Y., Kumano, S., Matsumoto, H. & Ohtaki, T. (2004) Biochem. Biophys. Res. Commun. 320, 383–388. [DOI] [PubMed] [Google Scholar]
- 28.Navarro, V. M., Castellano, J. M., Fernandez-Fernandez, R., Barreiro, M. L., Roa, J., Sanchez-Criado, J. E., Aguilar, E., Dieguez, C., Pinilla, L. & Tena-Sempere, M. (2004) Endocrinology 145, 4565–4574. [DOI] [PubMed] [Google Scholar]
- 29.Navarro, V. M., Castellano, J. M., Fernandez-Fernandez, R., Tovar, S., Roa, J., Mayen, A., Nogueiras, R., Vazquez, M. J., Barreiro, M. L., Magni, P., Aguilar, E., Dieguez, C., Pinilla, L. & Tena-Sempere, M. (2004) Endocrinology 146, 156–163 [DOI] [PubMed] [Google Scholar]