Abstract
From the point of view of implant dentistry, this review discusses the development and clinical use of demineralized dentin matrix (DDM) scaffolds, produced from the patient's own extracted teeth, to repair alveolar bone defects. The structure and the organic and inorganic components of DDM are presented to emphasize the similarities with autogenous bone. Studies of DDM properties, such as osteoinductive and osteoconductive functions as well as efficacy and safety, which are mandatory for its use as a bone graft substitute, are also presented. The clinical applications of powder, block, and moldable DDM are discussed, along with future developments that can support growth factor and stem cell delivery.
Keywords: Bone graft substitute, demineralized dentin matrix, tooth
INTRODUCTION
Autogenous bone graft is considered the gold standard for the repair of alveolar bone defects, but it is associated with donor complications and morbidity and also suffers from a limited supply. To avoid and overcome these disadvantages, bone substitutes are under development as an alternative to autogenous bone.
Bone and dentin are mineralized tissues of almost similar chemical composition. They consist of 18% collagen, 2% noncollagenous proteins (NCPs), 70% hydroxyapatite (HA), and 10% body fluid (percentages indicating weight/volume). Their matrix is a repository for growth factors, such as bone morphogenetic proteins (BMPs), transforming growth factor-β, insulin-like growth factor, and basic fibroblast growth factor. Several NCPs, such as osteocalcin (OCN) and osteopontin (OPN), are common in bone and dentin, whereas dentin phosphoprotein is an NCP found specifically in dentin [Figure 1].[1]
Figure 1.
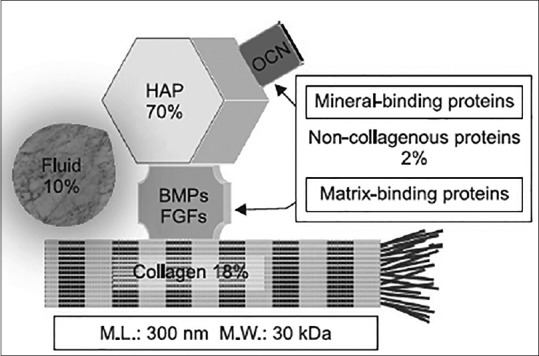
The common components in dentin and bone. HAP: Hydroxyapatite, OCN: osteocalcin, BMP: bone morphogenetic protein, FGFs: Fibroblast growth factors
Research on the bone-inducing properties of dentin began with a report in 1967. After Urist proposed that BMPs in dentin and bone are major stimulants with osteoinductive properties, Yeomans and Urist[2] were the first to show the regenerative properties of autogenous demineralized dentin matrix (DDM). Bang and Urist[3] also found that the collagenous dentin matrix, similar to bone matrix, can induce bone formation.
In vivo studies indicated that DDM was a more effective bone-inducing matrix than calcified dentin matrix (CDM), as CDM granules induced the formation of only a scanty amount of bone after a latent period of 8–12 weeks. The delayed inductive properties of calcified dentin may be related to the inhibition of BMP-release by apatite crystals [Figure 2].[4]
Figure 2.
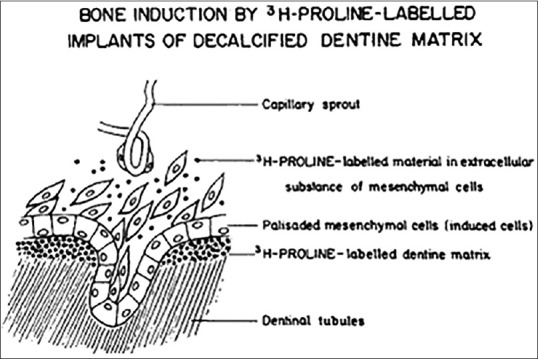
Diagrammatic illustration of the transfer of degradation products of radioisotope-labeled dentin matrix
Bessho et al.[5] successfully isolated BMP from human dentin matrix. Although human dentin-derived BMP was different from human bone-derived BMP, the two types of BMP had similar functions in the body. Reddi et al.[6,7] found that fibroblast transformation to form cartilage and bone by allogenic demineralized whole-tooth transplants of different shapes was profoundly influenced by the transplant geometry, which determines oxygen tension from surrounding tissues.
Human DDM is one of the most acid-soluble scaffolds, containing a collagenous matrix and osteoinductive growth factors, in addition to a mineral phase, and is an ideal bone substitute. Of clinical importance, DDM-based scaffolds are reprocessed, acellular, and nanoporous. This paper reviews in vitro and in vivo studies of DDM and relates them to the previous studies, as well as clinical applications and outcomes achieved so far.
DISCUSSION
Dentin matrix demineralization
Dentin demineralization with 0.6 N HCl results in the elimination of the major part of the mineral phase and immunogenic components, while retaining a very low fraction of minerals, and the majority of type I collagen and NCPs, providing an osteoconductive and osteoinductive scaffold-containing several growth factors.[8]
Two percent HNO3 has also been successfully used for dentin decalcification for both research purposes and clinical applications.[9,10] Inoue et al.[11] compared the capacity of dentin to induce cartilage and bone formation after demineralization with 0.6 N HCl or 3 M (9 N) citric acid. They found cartilage formation on the internal surface of the citric acid-demineralized dentin roll but significantly less than that on dentin demineralized with HCl.
Inorganic and organic components of demineralized dentin matrix
In general, DDM in powder or block form contains 5%–10% or 10%–30% mineral, respectively. X-ray diffraction analysis revealed four types of calcium phosphate, including HA, beta-tricalcium phosphate (TCP), amorphous calcium phosphate, and octacalcium phosphate, consisting of low-crystalline HA with relatively low Ca/P ratio. The amount and incorporation pattern of calcium and phosphorus in DDM were very similar to those of autogenous cortical bone.[12,13,14]
Based on sodium dodecyl sulfate polyacrylamide gel electrophoresis and western blotting analyses, BMP was not detected in DDM powder from dried tooth and fresh wisdom tooth. However, minor bands at 76–102 kDa were detected by electrophoresis, corresponding to the rest of NCPs, such as phosphophoryn, sialoprotein, glycoprotein, proteoglycan, OPN, OCN, and dentin matrix protein-1.[15] Type I collagens were identified at approximately 110–120 kDa.[16] In vivo, osteonectin was found in the dentinal tubules of DDM.[17]
Structure of demineralized dentin matrix
At present, DDM is available in two forms, powder and block. The importance of DDM geometry has already been emphasized by many researchers.[6,7] DDM powder is prepared by crushing dentin into particles 300–800 μm in size, and it possesses dentinal tubule-derived micropores (diameter: 1.0–3.0 μm, approximately 50,000 tubules/mm2) [Figure 3].[12] These pores are too small for cell infiltration and ingrowth. Instead, enlarged dentinal tubules and loosened collagen matrix after demineralization may serve as channels for releasing proteins that are essential for osteoblast growth and differentiation and also enhance surface microroughness or microtexture to increase dentin absorbability for blood or other body fluids.[8,9] Although Reddi and Huggins[6] documented osteoinduction by dentin particles 74–420 μm in size, Togari et al.[18] demonstrated that dentin particles 250–500 μm in size were highly efficient in osteoinduction. Recently, Koga et al.[19] recommended the use of 1000-μm particles, following partial demineralization with 2% HNO3, compared to nondemineralized or completely demineralized dentin.
Figure 3.
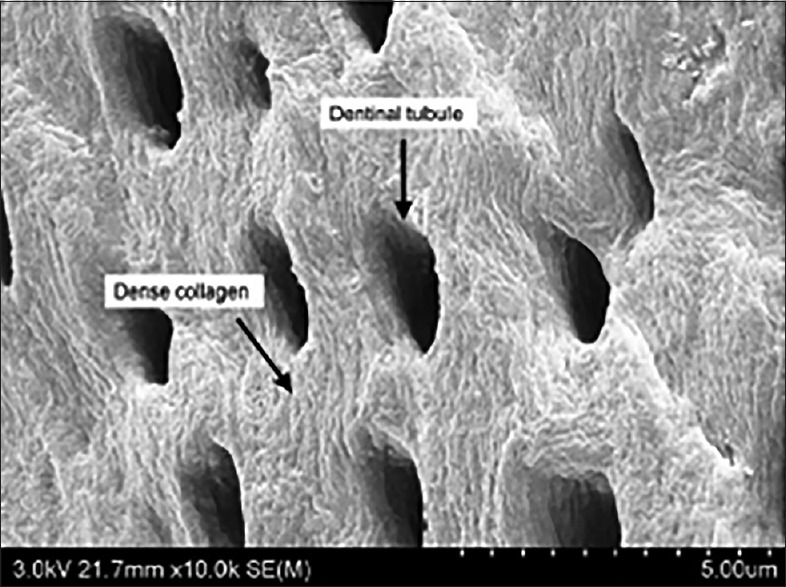
Demineralized dentin matrix powder (300–800 μm) with enlarged dentinal tubules
DDM powder is usually mixed with other suitable materials to form a paste that can be easily molded at the bone defect site. A rat tooth milled to a size of 10–50 μm mixed with hydroxypropyl cellulose (HPC) as base material promoted early healing and bone formation, while HPC, which is chemically stable in vivo, had an osteoconductive effect in the socket.[20]
A DDM block is fabricated from the root cut at the cementoenamel junction. The artificial macropores throughout are approximately 300–400 μm in diameter. Such porosity can influence the osteoconductive characteristics of the scaffold by creating spaces for osteoblast attachment, differentiation, and growth and vascular invasion from surrounding tissues [Figure 4].[21] Recently, Kabir et al.[22] reported that perforated root dentin matrix blocks with artificial macropores (1 mm in diameter) contributed to active bone ingrowth in critical-size bone defects of the iliac crest in adult sheep. Artificial macropores and supersonic demineralization increased the effective surface area of the dentin scaffold and created multiple centers for blood vessel invagination.
Figure 4.

Demineralized dentin matrix block with macropores (300–400 μm)
Several studies using transplantation of human dentin blocks (5–6 mm in diameter; 3 mm in thickness) for rabbit bone defects showed progressive dentin-bone ankylosis after 3 months, with osseous replacement resorption and no sign of inflammation.[23,24,25] Furthermore, the use of porous dentin scaffolds supports rapid microvascular ingrowth, as well as osseointegration, in mice, highlighting their potential as bone engineering materials.[26]
Osteoinductivity of demineralized dentin matrix
Gomes et al.[27] reported that autogenous DDM slices in the parietal bone of rabbits stimulated new bone formation and were completely incorporated into the newly formed bone tissue, having been resorbed during bone remodeling. Togari et al.[18] illustrated that in rat skull defects new bone formation occurred from individual bovine DDM granules within the defect, and not just from its margin. They concluded that DDM serves as a scaffold for bone regeneration by inducing a high degree of new bone formation soon after surgery.
Murata et al.[9,28] confirmed that human wisdom tooth-derived DDM granules induced independently bone and cartilage formation in subcutaneous tissues of nude mice, and the sequence for bone induction was similar to demineralized bone matrix.
Kim et al.[29] performed an in vivo study to evaluate tissue responses to human DDM inserted into the dorsum of athymic mice. In 2 weeks, new lining cells had attached to the DDM powder without any inflammation, suggesting its excellent biocompatibility. Kim et al.[15,29,30] also showed that grafted human DDM in muscle tissue of nude mice induced independently cartilage and bone formation. The newly deposited osteoid on DDM powder illustrates its osteoinductivity.
Osteoconductivity of demineralized dentin matrix
Gomes et al.[31] and Carvalho et al.[32] evaluated the osteoconductive properties of autogenous DDM on parietal bone defects and 5-mm defects of the mandibular buccal bone in rabbits. The DDM was completely incorporated in the newly formed bone tissue and was resorbed during bone remodeling. de Oliveira et al.[33] performed BMP-2 and BMP-4 immunostaining in osteoblasts during the healing process of rat upper second molar sockets treated with human DDM. The results suggest that human DDM acts as a scaffold for osteoblast differentiation, actively producing new bone through, at least in part, matrix degradation and consequent controlled delivery of BMP-2 and BMP-4.
Kim et al.[34,35] confirmed the excellent osteoconductive healing capacity of human DDM in critical size defects of minipig crania and in the porcine sinus [Figure 5]. The excellent osteoconductivity and bone remodeling abilities of human DDM might be attributed to its minerals, such as low-crystallinity HA and TCP.
Figure 5.
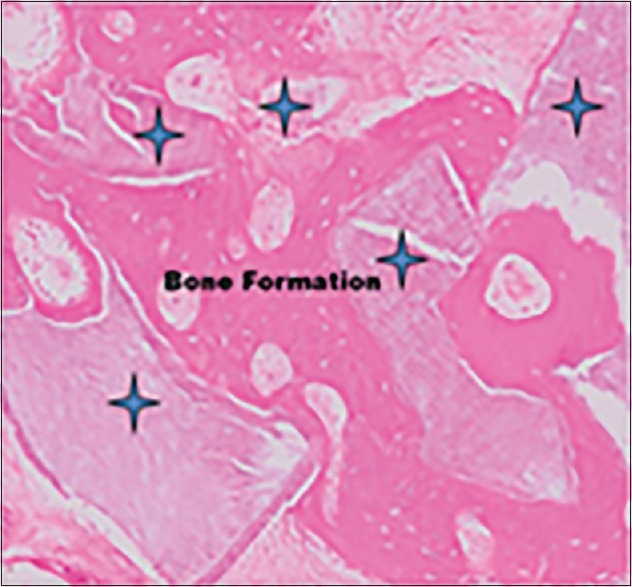
Extensive new bone formation around demineralized dentin matrix granules. Asterisks indicate demineralized dentin matrix. Staining by hematoxylin and eosin (×100, magnification)
In addition to DDM, studies have been performed with mineralized dentin matrix as well, yielding different results. Atiya et al.[36] and Al-Namnam et al.[37] demonstrated the osteoconductive and osteoinductive properties of autogenous and allogenic mineralized dentin treated only with liquid nitrogen in a rabbit femur model. Moharamzadeh et al.[38] reported that mineralized bovine dentine has the potential to be an osteoconductive bone substitute, using a rat femur model (five walls).
However, these processed bovine mineralized dentin products were not ideal bone materials for large rabbit calvarial defects (4-wall defects), as there was evidence of some soft-tissue invasion into defects.[39] In a dog model, autogenous mineralized dentin grafts offered did not improve bone regeneration in alveolar extraction sockets.[40]
Human mineralized dentin blocks (6 mm in diameter; 3 mm in thickness) were inserted in cavities penetrating into the marrow space in New Zealand rabbit tibias and were incorporated in the bone without inflammation and gradually resorbed and replaced by new bone.[24]
Demineralized dentin matrix scaffolds as recombinant human bone morphogenetic protein-2 carriers
Since surface demineralized root dentin induces new bone formation, slow mineral resorption, and recombinant human bone morphogenetic protein-2 (rhBMP-2) release, Ike and Urist[41] suggested already in 1998 that it may be recycled for use as a rhBMP-2 carrier.
In 2005, Murata et al.[42,43] showed that rhBMP-2 increased the bone-inductive potential of DDM in rat subcutaneous tissues and suggested that human recycled DDM is a unique, absorbable, and osteoconductive matrix that should be an effective scaffold for BMP-2 delivery.
Kim et al.[17] compared in vitro the kinetics of rhBMP-2 release from various scaffolds and found that rhBMP-2 release was most prominent from DDM powders, tentatively concluded that DDM could be better than synthetic TCP and inorganic bovine bone as a rhBMP-2 carrier. An in vivo study of protein marker expression showed that osteonectin was expressed strongly in dentinal tubules at an early stage, and its expression in muscle tissue of nude mice was better sustained on DDM combined with rhBMP-2. Both in vitro and in vivo findings suggest that the microporous dentinal tubules (1.0–3.0 μm in diameter) contribute to both rhBMP-2 loading and longer release. In rabbit calvarial defects, the remarkable increase in bone volume after treatment with DDM combined with rhBMP-2 was illustrated, and the gradual resorption of DDM, as described also by Ike and Urist[41] in 1998, seemed to play a secondary role in the increased osteoinductive potential of DDM combined with rhBMP-2.[44]
Clinical applications of autogenous demineralized dentin matrix
Demineralized dentin matrix powder
Gomes et al.[45] conducted a study in humans where 27 lower third molar sockets were selected and filled with autogenous DDM, resulting in superior bony architecture.
In 2010, Kim et al.[12] reported the histological evaluation of six patients at the time of their second surgery who received simultaneously DDM powder and an implant. The results show that DDM underwent gradual resorption, and 46%–74% of it was replaced by new bone through osteoinduction and osteoconduction. Six years later, they examined by cone beam computed tomography five of these patients and found that the corticocancellous bone that had formed was successfully maintained around the implant after an average time of 5 years.[46]
Many clinical studies on guided bone regeneration (GBR), socket preservation, and ridge augmentation showed that new bone was formed by osteoinduction and/or osteoconduction, and the crestal bone resorption during the follow-up period (3, 5, or 6 months) was 0.29 mm on average (0–3.0 mm).[47,48]
In the GBR group (14 implants), the average bone loss 8 months after prosthetic loading was 0.29 mm, whereas in the sinus graft group (14 implants), it was 0.66 mm 7.6 months after prosthetic loading.[49] A GBR case series study with 15 patients and a 31-month follow-up period showed 0.47 mm crestal bone loss.[50] A retrospective cohort study with a 33-month follow-up period showed 0.33 ± 0.63 mm bone loss after implant placement (54 implants), demonstrating remarkable healing property [Figure 6].[51]
Figure 6.
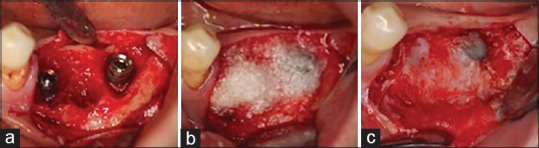
(a) The defect around the implant, (b) defects are covered by the demineralized dentin matrix particles, (c) texture of newly formed bone after 3 months. Most of the demineralized dentin matrix powder underwent resorption and bone remodeling
Another case series study of extraction socket preservation showed that the average amount of crestal bone loss around the implant was 0.05 mm during an average of 22.5 months (12–34 months) after functional loading. Newly formed tissues were evident in the 3-month specimen owing to its osteoconductivity and bone remodeling properties.[52] A prospective, randomized clinical trial that compared the clinical efficacy and histological outcome of autogenous DDM in postextraction alveolar bone augmentation with those of inorganic bovine bone (Bio-Oss, Geistlich, Switzerland) showed that autogenous DDM was as effective as inorganic bovine bone.[53]
Demineralized dentin matrix powder mixed with hydroxypropylmethyl cellulose
Ku et al.[54] reported a clinical study using DDM mixed with hydroxypropylmethyl cellulose (HPMC) in 58 patients and 93 implants for GBR, ridge augmentation, and socket preservation, aimed at investigating implant stability at the time of installation and of the second surgery. They concluded that DDM mixed with HPMC showed outstanding performance with respect to implant stability in the graft site. When DDM mixed with HPMC was applied to sinus-related alveolar bone defects, the bone-forming capacity was not less than that of conventional DDM, regardless of the characteristics of the defect and different pathways of blood supply. Therefore, HPMC powders might be an effective base material for conventional DDM powders.[55]
Demineralized dentin matrix blocks
The first clinical report using autogenous DDM blocks for socket preservation indicated excellent bone formation and strong integration of the DDM block into the recipient bone, in 12 patients.[21] The alveolar bone volume was well maintained both vertically and horizontally, and the formed bone was not resorbed during the early stages. Histological examination showed aponeurotic fusions between the gingiva and the DDM block, osteocytic embedding, osteoclastic resorption, and vascular invasion into the DDM block. One of these cases was followed-up for 5 years, and excellent results were reported in 2014 [Figure 7].[56] The same researchers examined DDM block remodeling based on radiological evaluations and reported that the grafted block was replaced completely by newly formed bone from the host after 14 or 15 months of prosthetic loading.[57]
Figure 7.
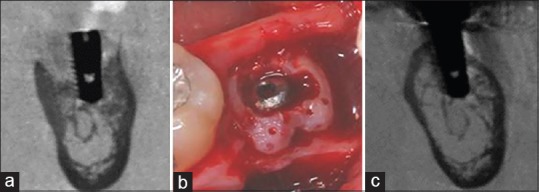
(a) The vertical and horizontal alveolar defect around the implant, (b) the defect repaired by the demineralized dentin matrix block and blood clot aggregation, (c) complete formation of corticocancellous bone. Over time, the demineralized dentin matrix block underwent gradual resorption and became less visible
A case series study to evaluate the fate of DDM blocks during long-term follow-up observations based on 22 patients, who received a single implant with a DDM block in the posterior area of the maxilla (12 patients) or the mandible (10 patients), was performed with an average follow-up period of 44 months. The findings were consistent with those of previous short-term studies and indicate that DDM blocks are capable of continuous remodeling under a functional load with appropriate volume maintenance.[58]
Sinus bone graft of demineralized dentin matrix powder
The first clinical case of a sinus lifting procedure using autogenous DDM was reported in the 2003 IADR Congress by Dr. Murata.[59] Lee et al.[60] performed a histomorphometric study to compare the efficacy of DDM with that of various other scaffolds in sinus. After 4 months, all groups showed new bone formation around the graft material and implant in the sinus. Jeong et al.[61] performed a retrospective clinical study on 100 implants in 51 patients, from July 2009 to November 2010, to evaluate the effectiveness of DDM scaffold in the sinus. The overall implant survival rate was 96.15%, supported by gradual resorption and new bone formation on DDM through osteoconduction and osteoinduction, as assessed by histologic examination.
On microcomputed tomographic and histological examinations of tissue specimens 9 months after the sinus graft, the total bone volume (DDM + new bone) was 76.45%, and the proportion of new bone was 45.4%.[62] In a prospective clinical study with 14 patients receiving inorganic bovine bone and 18 patients receiving DDM, biopsy specimens were analyzed by microcomputed tomography and histomorphometry to assess the sinus bone graft procedure. The results showed that DDM performance in the sinus was not inferior to that of inorganic bovine bone.[63]
The amount of bone resorption in the sinus, performed by crestal approach, was measured in patients treated with DDM or synthetic materials (11 patients/group). The bone resorption height, measured 1 year after the graft, was 0.76 mm on average in DDM and 0.53 mm in synthetic materials, indicating that DDM is a good alternative material to synthetic bone graft for bone-added sinus lift.[64]
CONCLUSION
Third molar and premolars extracted for orthodontic purposes have been already used in elderly patients belonging to the host's family for the purpose of alveolar bone engineering.[65] Furthermore, autogenous DDM combined with rhBMP-2 is under clinical trial for receiving approval as a medical device.[66] In addition, a retrospective clinical study of allogenic DDM for alveolar bone repair has been published early this year.[67] Kim et al.[8] summarized their 5-year experience in tooth banking by analyzing the written documents as well as histopathologic and microbiologic examinations that support the safety of the tooth banking system. Even though uniform guidelines and standards for tooth bank management are still lacking, we believe that the banking procedures for DDM will be valuable for developing and improving DDM scaffolds in the future.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
This study was supported by the Korea Health Industry Development Institute (KHIDI) grant that is funded by the ministry of Health & Welfare, Republic of Korea under Grant HI15C3136.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Murata M. Collagen biology for bone regenerative surgery. J Korean Assoc Oral Maxillofac Surg. 2012;38:321–5. [Google Scholar]
- 2.Yeomans JD, Urist MR. Bone induction by decalcified dentine implanted into oral, osseous and muscle tissues. Arch Oral Biol. 1967;12:999–1008. doi: 10.1016/0003-9969(67)90095-7. [DOI] [PubMed] [Google Scholar]
- 3.Bang G, Urist MR. Bone induction in excavation chambers in matrix of decalcified dentin. Arch Surg. 1967;94:781–9. doi: 10.1001/archsurg.1967.01330120035008. [DOI] [PubMed] [Google Scholar]
- 4.Urist MR, Dowell TA, Hay PH, Strates BS. Inductive substrates for bone formation. Clin Orthop Relat Res. 1968;59:59–96. [PubMed] [Google Scholar]
- 5.Bessho K, Tanaka N, Matsumoto J, Tagawa T, Murata M. Human dentin-matrix-derived bone morphogenetic protein. J Dent Res. 1991;70:171–5. doi: 10.1177/00220345910700030301. [DOI] [PubMed] [Google Scholar]
- 6.Reddi AH, Huggins CB. Influence of geometry of transplanted tooth and bone on transformation of fibroblasts. Proc Soc Exp Biol Med. 1973;143:634–7. doi: 10.3181/00379727-143-37381. [DOI] [PubMed] [Google Scholar]
- 7.Reddi AH. Bone matrix in the solid state: Geometric influence on differentiation of fibroblasts. Adv Biol Med Phys. 1974;15:1–18. doi: 10.1016/b978-0-12-005215-8.50007-3. [DOI] [PubMed] [Google Scholar]
- 8.Kim YK, Um IW, Murata M. Tooth bank system for bone regeneration-safety report. J Hard Tissue Biol. 2014;23:371–6. [Google Scholar]
- 9.Murata M, Akazawa T, Takahata M, Ito M, Tazaki J, Hino J, et al. Bone induction of human tooth and bone crushed by newly developed automatic mill. J Ceram Soc Jpn. 2010;118:434–7. [Google Scholar]
- 10.Kabir MA, Murata M, Kusano K, Zakaria SM, Noor AM, Khuda F, et al. Radiological evaluation of human dentin autografts in Bangladesh. J Hard Tissue Biol. 2014;23:363–70. [Google Scholar]
- 11.Inoue T, Deporter DA, Melcher AH. Induction of cartilage and bone by dentin demineralized in citric acid. J Periodontal Res. 1986;21:243–55. doi: 10.1111/j.1600-0765.1986.tb01456.x. [DOI] [PubMed] [Google Scholar]
- 12.Kim YK, Kim SG, Byeon JH, Lee HJ, Um IU, Lim SC, et al. Development of a novel bone grafting material using autogenous teeth. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109:496–503. doi: 10.1016/j.tripleo.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 13.Kim YK, Kim SG, Oh JS, Jin SC, Son JS, Kim SY, et al. Analysis of the inorganic component of autogenous tooth bone graft material. J Nanosci Nanotechnol. 2011;11:7442–5. doi: 10.1166/jnn.2011.4857. [DOI] [PubMed] [Google Scholar]
- 14.Kim YK, Kim SG, Yun PY, Yeo IS, Jin SC, Oh JS, et al. Autogenous teeth used for bone grafting: A comparison with traditional grafting materials. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;117:e39–45. doi: 10.1016/j.oooo.2012.04.018. [DOI] [PubMed] [Google Scholar]
- 15.Kim YK, Lee JH, Kim KW, Um IW, Murata M, Ito K. Analysis of organic components and osteoinductivity in autogenous tooth bone graft material. J Korean Assoc Maxillofac Plast Reconstr Surg. 2013;35:353–9. [Google Scholar]
- 16.Ahn GJ, Kim YK, Um IW, Kim JY. Evaluation of prognosis of autogenous tooth bone graft material according to the condition of donor tooth. J Dent Implant Res. 2015;341:7–11. [Google Scholar]
- 17.Kim YK, Um IW, An HJ, Kim KW, Hong KS, Murata M. Effects of demineralized dentin matrix used as an rhBMP-2 carrier for bone regeneration. J Hard Tissue Biol. 2014;23:415–22. [Google Scholar]
- 18.Togari K, Miyazawa K, Yagihashi K, Tabuchi M, Maeda H, Kawai T, et al. Bone regeneration by demineralized dentin matrix in skull defects of rats. J Hard Tissue Biol. 2011;21:25–34. [Google Scholar]
- 19.Koga T, Minamizato T, Kawai Y, Miura K, IT, Nakatani Y, et al. Bone regeneration using dentin matrix depends on the degree of demineralization and particle size. PLoS One. 2016;11:e0147235. doi: 10.1371/journal.pone.0147235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miyata Y, Ozawa S, Kojima N, Kondo Y, Matuskawa R, Tanaka Y. An experimental study of bone grafting using rat milled tooth. Int J Oral Maxillofac Implants. 2011;26:1210–6. [PubMed] [Google Scholar]
- 21.Kim YK, Kim SG, Um IW, Kim KW. Bone grafts using autogenous tooth blocks: A case series. Implant Dent. 2013;22:584–9. doi: 10.1097/ID.0000000000000011. [DOI] [PubMed] [Google Scholar]
- 22.Kabir MA, Murata M, Akazawa T, Kusano K, Yamada K, Ito M. Evaluation of perforated demineralized dentin scaffold on bone regeneration in critical-size sheep iliac defects? Clin Oral Implants Res. 2017 doi: 10.1111/clr.13000. doi: 1.01111/clr13000. [DOI] [PubMed] [Google Scholar]
- 23.Andersson L, Ramzi A, Joseph B. Studies on dentin grafts to bone defects in rabbit tibia and mandible; development of an experimental model. Dent Traumatol. 2009;25:78–83. doi: 10.1111/j.1600-9657.2008.00703.x. [DOI] [PubMed] [Google Scholar]
- 24.Andersson L. Dentin xenografts to experimental bone defects in rabbit tibia are ankylosed and undergo osseous replacement. Dent Traumatol. 2010;26:398–402. doi: 10.1111/j.1600-9657.2010.00912.x. [DOI] [PubMed] [Google Scholar]
- 25.Qin X, Raj RM, Liao XF, Shi W, Ma B, Gong SQ, et al. Using rigidly fixed autogenous tooth graft to repair bone defect: An animal model. Dent Traumatol. 2014;30:380–4. doi: 10.1111/edt.12101. [DOI] [PubMed] [Google Scholar]
- 26.Bormann KH, Suarez-Cunqueiro MM, Sinikovic B, Kampmann A, von See C, Tavassol F, et al. Dentin as a suitable bone substitute comparable to ß-TCP – An experimental study in mice. Microvasc Res. 2012;84:116–22. doi: 10.1016/j.mvr.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 27.Gomes MF, dos Anjos MJ, Nogueira TO, Guimarães SA. Histologic evaluation of the osteoinductive property of autogenous demineralized dentin matrix on surgical bone defects in rabbit skulls using human amniotic membrane for guided bone regeneration. Int J Oral Maxillofac Implants. 2001;16:563–71. [PubMed] [Google Scholar]
- 28.Murata M, Sato D, Akazawa T, Taira T, Sasaki T, Arisue M. Bone and cartilage induction in nude mice by human demineralized dentin matrix. J Hard Tissue Biol. 2003;11:110–4. [Google Scholar]
- 29.Kim YK, Lee JK, Kim KW, Um IW, Murata M. Healing mechanism and clinical application of autogenous tooth bone graft material. In: Pignatello R, editor. Advances in Biomaterials Science and Biomedical Applications. Croatia: Intech Publisher; 2013. pp. 405–35. [Google Scholar]
- 30.Kim KW. Bone induction by demineralized dentin matrix in nude mouse muscles. Maxillofac Plast Reconstr Surg. 2014;36:50–6. doi: 10.14402/jkamprs.2014.36.2.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gomes MF, dos Anjos MJ, Nogueira Tde O, Catanzaro Guimarães SA. Autogenous demineralized dentin matrix for tissue engineering applications: Radiographic and histomorphometric studies. Int J Oral Maxillofac Implants. 2002;17:488–97. [PubMed] [Google Scholar]
- 32.Carvalho VA, Tosello Dde O, Salgado MA, Gomes MF. Histomorphometric analysis of homogenous demineralized dentin matrix as osteopromotive material in rabbit mandibles. Int J Oral Maxillofac Implants. 2004;19:679–86. [PubMed] [Google Scholar]
- 33.de Oliveira GS, Miziara MN, Silva ER, Ferreira EL, Biulchi AP, Alves JB. Enhanced bone formation during healing process of tooth sockets filled with demineralized human dentine matrix. Aust Dent J. 2013;58:326–32. doi: 10.1111/adj.12088. [DOI] [PubMed] [Google Scholar]
- 34.Kim JY, Kim KW, Um IW, Kim YK, Lee JK. Bone healing capacity of demineralized dentin matrix materials in a mini-pig cranium defect. J Korean Dent Sci. 2012;5:21–8. [Google Scholar]
- 35.Lee du H, Yang KY, Lee JK. Porcine study on the efficacy of autogenous tooth bone in the maxillary sinus. J Korean Assoc Oral Maxillofac Surg. 2013;39:120–6. doi: 10.5125/jkaoms.2013.39.3.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Atiya BK, Shanmuhasuntharam P, Huat S, Abdulrazzak S, Oon H. Liquid nitrogen-treated autogenous dentin as bone substitute: An experimental study in a rabbit model. Int J Oral Maxillofac Implants. 2014;29:e165–70. doi: 10.11607/jomi.te54. [DOI] [PubMed] [Google Scholar]
- 37.Al-Namnam NM, Shanmuhasuntharam R, Ha KO, Siar C. Processed allogenic dentine as a scaffold for bone healing: An in vivo study. Aust J Basic Appl Sci. 2010;4:5932–40. [Google Scholar]
- 38.Moharamzadeh K, Freeman C, Blackwood K. Processed bovine dentine as a bone substitute. Br J Oral Maxillofac Surg. 2008;46:110–3. doi: 10.1016/j.bjoms.2007.07.209. [DOI] [PubMed] [Google Scholar]
- 39.Hussain I, Moharamzadeh K, Brook IM, José de Oliveira Neto P, Salata LA. Evaluation of osteoconductive and osteogenic potential of a dentin-based bone substitute using a calvarial defect model. Int J Dent. 2012;2012:396316. doi: 10.1155/2012/396316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kadkhodazadeh M, Ghasemianpour M, Soltanian N, Sultanian GR, Ahmadpour S, Amid R. Effects of fresh mineralized dentin and cementum on socket healing: A preliminary study in dogs. J Korean Assoc Oral Maxillofac Surg. 2015;41:119–23. doi: 10.5125/jkaoms.2015.41.3.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ike M, Urist MR. Recycled dentin root matrix for a carrier of recombinant human bone morphogenetic protein. J Oral Implantol. 1998;24:124–32. doi: 10.1563/1548-1336(1998)024<0124:RDRMFA>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- 42.Murata M. Bone engineering using human demineralized dentin matrix and recombinant human BMP-2. J Hard Tissue Biol. 2005;14:80–1. [Google Scholar]
- 43.Murata M, Kawai T, Kawakami T, Akazawa T, Tazaki J, Ito K, et al. Human acid-insoluble dentin with BMP-2 accelerates bone induction in subcutaneous and intramuscular tissues. J Ceram Soc Jpn. 2010;118:438–41. [Google Scholar]
- 44.Um IW, Hwang SH, Kim YK, Kim MY, Jun SH, Ryu JJ, et al. Demineralized dentin matrix combined with recombinant human bone morphogenetic protein-2 in rabbit calvarial defects. J Korean Assoc Oral Maxillofac Surg. 2016;42:90–8. doi: 10.5125/jkaoms.2016.42.2.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gomes MF, Abreu PP, Morosolli AR, Araújo MM, Goulart MD. Densitometric analysis of the autogenous demineralized dentin matrix on the dental socket wound healing process in humans. Braz Oral Res. 2006;20:324–30. doi: 10.1590/s1806-83242006000400008. [DOI] [PubMed] [Google Scholar]
- 46.Kim YK, Lee JH, Um IW, Cho WJ. Guided bone regeneration using demineralized dentin matrix: Long-term follow-up. J Oral Maxillofac Surg. 2016;74:515.e1–9. doi: 10.1016/j.joms.2015.10.030. [DOI] [PubMed] [Google Scholar]
- 47.Kim YK, Lee HJ, Kim KW, Kim SG, Um IW. Guided bone regeneration using autogenous teeth: Case reports. J Korean Assoc Maxillofac Surg. 2011;37:142–7. [Google Scholar]
- 48.Park SM, Um IW, Kim YK, Kim KW. Clinical application of auto-tooth bone graft material. J Korean Assoc Oral Maxillofac Surg. 2012;38:2–8. [Google Scholar]
- 49.Han MW, Lee JK. Clinical study on the efficacy of the autogenous tooth bone graft material (AutoBT) J Korean Assoc Maxillofac Plast Reconstr Surg. 2013;35:221–6. [Google Scholar]
- 50.Kim YK, Kim SG, Bae JH, Um IW, Oh JS, Jeong KI. Guided bone regeneration using autogenous tooth bone graft in implant therapy: Case series. Implant Dent. 2014;23:138–43. doi: 10.1097/ID.0000000000000046. [DOI] [PubMed] [Google Scholar]
- 51.Lee JY, Kim YK. Retrospective cohort study of autogenous tooth bone graft. Oral Biol Res. 2012;36:39–43. [Google Scholar]
- 52.Kim YK, Yun PY, Um IW, Lee HJ, Yi YJ, Bae JH, et al. Alveolar ridge preservation of an extraction socket using autogenous tooth bone graft material for implant site development: Prospective case series. J Adv Prosthodont. 2014;6:521–7. doi: 10.4047/jap.2014.6.6.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pang KM, Um IW, Kim YK, Woo JM, Kim SM, Lee JH, et al. Autogenous demineralized dentin matrix from extracted tooth for the augmentation of alveolar bone defect: A prospective randomized clinical trial in comparison with anorganic bovine bone? Clin Oral Implants Res. 2016 doi: 10.1111/clr.12885. doi: 10.1111/clr.12885. [DOI] [PubMed] [Google Scholar]
- 54.Ku JK, Kim YK, Yun PY. Bone graft using the moldable autogenous tooth bone graft (M-AutoBT) J Korean Assoc Oral Maxillofac Surg. 2015;41(Suppl 1):357. [Google Scholar]
- 55.Kim YK, Um IW, Cho WJ, Murata M, Jun SH, Lee JK. Applications of moldable autogenous tooth bone graft (M-AutoBT) mixed with hydroxypropylmethyl cellulose for sinus lifting. J Hard Tissue Biol. 2015;24:391–6. [Google Scholar]
- 56.Um IW, Acosta J. Tooth-derived bone graft materials: Demineralised dentin matrix (DDM) Dent Asia. 2014;1:45–50. [Google Scholar]
- 57.Lee EG, Lee JY, Kim YK, Um IW, Choi JH. Delayed implant placement after extraction socket reconstruction and ridge augmentation using autogenous tooth bone graft material. Dentistry. 2013;3:174. [Google Scholar]
- 58.Kim YK, Pang KM, Yun PY, Leem DH, Um IW. Long-term follow-up of autogenous tooth bone graft blocks with dental implants. Clin Case Rep. 2017;5:108–18. doi: 10.1002/ccr3.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Murata M. Autogenous demineralized dentin matrix for maxillary sinus augmentation in human: The first clinical report. J Dent Res. 2003;82:B243. [Google Scholar]
- 60.Lee JY, Kim YK, Kim SG, Lim SC. Histomorphometric study of sinus bone graft using various graft material. J Dent Rehabil Appl Sci. 2011;27:141–7. [Google Scholar]
- 61.Jeong KI, Kim SG, Kim YK, Oh JS, Jeong MA, Park JJ. Clinical study of graft materials using autogenous teeth in maxillary sinus augmentation. Implant Dent. 2011;20:471–5. doi: 10.1097/ID.0b013e3182386d74. [DOI] [PubMed] [Google Scholar]
- 62.Kim YK, Jun SH, Um IW, Kim SY. Evaluation of the healing process of autogenous tooth bone graft material nine months after sinus bone graft: Micromorphometric and histological evaluation. J Korean Assoc Maxillofac Plast Reconstr Surg. 2013;35:310–5. [Google Scholar]
- 63.Jun SH, Ahn JS, Lee JI, Ahn KJ, Yun PY, Kim YK. A prospective study on the effectiveness of newly developed autogenous tooth bone graft material for sinus bone graft procedure. J Adv Prosthodont. 2014;6:528–38. doi: 10.4047/jap.2014.6.6.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim YK, Lee J, Yun JY, Yun PY, Um IW. Comparison of autogenous tooth bone graft and synthetic bone graft materials used for bone resorption around implants after crestal approach sinus lifting: A retrospective study. J Periodontal Implant Sci. 2014;44:216–21. doi: 10.5051/jpis.2014.44.5.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Um IW, Lee BK. Familial tooth bone graft. In: Murata M, Um IW, editors. Advances in Oral Tissue Engineering. Chicago, USA: Quintessence Publishing Co; 2014. pp. 67–72. [Google Scholar]
- 66.Kim YK, Jang HJ, Um IW. A case report of allogenic demineralized dentin matrix loaded with recombinant human bone morphogenetic proteins for alveolar bone repair. J Dent Oral Health. 2016;2:45. [Google Scholar]
- 67.Kim YK, Bang KM, Murata M, Mitsugi M, Um IW. Retrospective clinical study of allogenic demineralized dentin matrix for alveolar bone repair. J Hard Tissue Biol. 2017;26:95–102. [Google Scholar]


