Abstract
Aims and Objective:
The purpose of the present study was to evaluate and compare flexural strength and Staphylococcus aureus adhesion of heat-activated poly (methyl methacrylate [MMA]) resin modified with a comonomer of methacrylic acid (MAA) and MMA monomer.
Materials and Methods:
Comonomer preparation was done with the addition of varying concentration of MAA (0, 15, 20, and 25 wt %) to the MMA of conventional heat-activated denture base resin to prepare the specimens. Prepared specimens were stored in distilled water at 37°C for 1 day and 1 week before the evaluation of flexural strength and microbial adhesion. Flexural strength was measured using a universal testing machine at a crosshead speed for 2 mm/min (n = 10). Microbial adhesion (colony-forming unit [CFU]) was evaluated against S. aureus using a quadrant streaking method (n = 5). Data were subjected to one-way ANOVA, and the significant differences among the results were subjected to Tukey's HSD test. P < 0.05 was considered statistically significant.
Results:
Addition of MAA to the MMA monomer was found to significantly reduce the adhesion of S. aureus for all the groups. Reduction of CFU of S. aureus was found be more significant for Group 3 as compared to control, both at 1-day (P < 0.001) and 1-week (P < 0.002) storage in distilled water. However, no statistically significant changes in the flexural strength were observed with the addition of MAA at 1-day (P = 0.52) and 1-week (P = 0.88) time interval.
Conclusion:
Addition of MAA to conventional denture base resin reduced the microbial adhesion without significantly affecting the flexural strength.
Keywords: Flexural strength, heat polymerized acrylic resin, methacrylic acid, microbial adhesion, Staphylococcus aureus
INTRODUCTION
The use of denture prosthesis is indispensable for the functional and esthetic rehabilitation of edentulous patients. Therefore, denture prosthesis should simulate, as closely as possible, the natural structures or tissues lost. The most commonly used denture base material is based on poly (methyl methacrylate) (PMMA). It exhibits excellent esthetics, good compressive and tensile strength, low water sorption, and is cost effective to enumerate few desirable properties. Nevertheless, they are prone to microbial adhesion, commonly Candida albicans[1,2,3,4,5] leading to chronic atrophic candidiasis, also known as denture stomatitis.[6,7,8,9] High degree of adhesion of both C. albicans and Staphylococcus aureus on the dentures is reported to be associated with angular cheilitis lesions also.[10]
Although S. aureus plays a considerable role in the bionetwork of the normal oral flora, it is a known pathogen in many clinical conditions such as angular cheilitis, parotitis, and mucositis in several debilitating elderly patients.[11] Sumi et al.[12] observed the high prevalence of adherence of respiratory pathogens, including S. aureus, on the denture surfaces and stated that these microorganisms may cause life-threatening situations such as aspirational pneumonia in elderly patients.[11,13,14,15] In a study conducted by Powell et al.,[16] it was found that 67% of all materials transported from dental laboratories were cross contaminated with various potentially pathogenic bacteria including S. aureus.[17,18]
Several attempts have been made previously to reduce the microbial adhesion of the denture by the incorporation of materials such as self-bonding polymer, phosphate group of ethylene glycol methacrylate phosphate, silver–zinc zeolite, and aluminum oxide powder. The attempted modifications of the acrylic resins either change the surface charge or the hydrophilic properties of the acrylic surface and thereby reduce microbial adhesion.[19,20,21] In the present study, methacrylic acid (MAA) has been added as a comonomer to impede microbial adhesion to the conventional denture base material. MAA is colorless, viscous carboxylic acid liquid soluble in most organic solvents. It is manufactured industrially on a large scale as a precursor to its esters, especially methyl methacrylate (MMA). Since the addition of MAA alters the microbial adhesion[19] and can affect the mechanical properties[22,23] of denture base resins, these modified acrylic resins with increasing concentrations of MAA must be investigated for regular use by elderly and medically compromised or physically disabled patients who cannot maintain their oral hygiene.
MATERIALS AND METHODS
Commercially available heat cure denture base resin was procured from dental products of India, Mumbai, India. MAA of 97% purity was purchased from Burgoyne Burbidges and Co., Mumbai, India. Standard strain of S. aureus (ATCC25923) was procured from Himedia lab, Mumbai, India. The entire study was divided into four groups based on the percentage of MAA: 0%, 15%, 20%, and 25%, namely control, Group I, II, and III, respectively, for both time interval, namely, at 1 day and 1 week.
Sample preparation for flexural strength
Comonomer preparation was done by mixing MMA and MAA in the appropriate ratio as per the groups in a closed container. Samples were prepared by mixing polymer and comonomer at 2.5:1 ratio by volume. The proportioned material was then enclosed in a ceramic cup until the mixture reached dough stage at which time it is packed into aluminum mold of 65 mm × 10 mm × 3 mm under pressure of 2000 psi[24] as per ISO 1567. The mold along with the packed dough was then transferred to a water bath at room temperature after bench curing for 30 min. Polymerization cycle was initiated by raising the temperature of water bath to 74°C in 30 min, and the same temperature was maintained for 8 h. At the end of 8 h, the temperature of the water bath was increased to 100°C and maintained for 30 min.[25] The mold was left in the water bath until it reached room temperature after which the specimens were retrieved, finished, and polished (n = 10). The polished samples were then rinsed and stored in distilled water for 1 day at 37°C. Another set of samples of the same number was then made in accordance with the protocol mentioned above and stored in distilled water for 1 week at 37°C to simulate oral condition [Figure 1].
Figure 1.
Finished and polished samples for flexural strength
Measurement of flexural strength
The flexural strength at 1 day and 1 week of the aforementioned samples was evaluated by three-point bending test using universal testing machine (Instron 3366, United Kingdom). The equipment consisted of a loading wedge and a pair of adjustable supporting wedges placed 50 mm apart. The specimens were centered on the device in such a way that the loading wedge, set to travel at a crosshead speed of 2 mm/min, engaged the center of the upper surface of the specimens. Specimens were loaded until fracture occurred, and the maximum load applied during the load was automatically recorded and used for the measurement of flexural strength [Figures 2 and 3].
Figure 2.
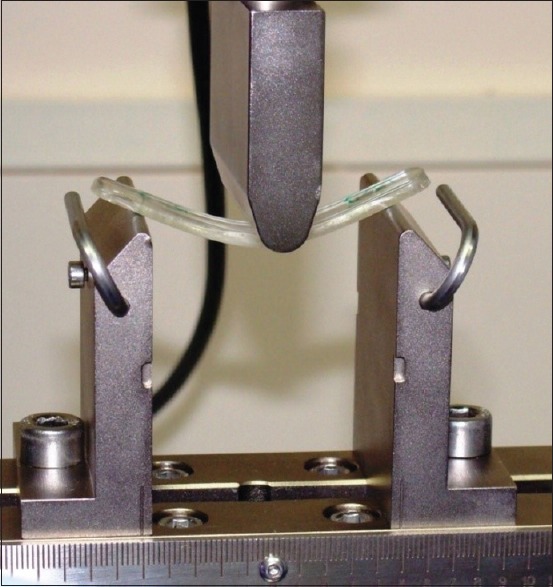
Samples under three bending test (INSTRON 3366) to evaluate flexural strength
Figure 3.

Fractured samples after testing
Preparation of culture media
Hot sterilized brain–heart infusion (BHI) agar media (Himedia, Mumbai, India) was allowed to cool to 55°C and poured into the sterilized petri dishes under aseptic condition. The media were allowed to solidify, and the plates were left overnight at room temperature.
Sample preparation for Staphylococcus aureus adhesion
Wax patterns were fabricated with dimensions of 5 mm × 5 mm × 3 mm in a silicon mold. Prepared wax patterns were invested and dewaxed to make a plaster mold in a dental flask for sample preparation. Manipulation of the modified acrylic resin and processing was done in the same manner as for flexural strength. Prepared samples were finished and polished [Figure 4] in a conventional manner (n = 5). All the samples were sterilized with chlorhexidine 2%[15,26] and washed with sterilized broth to remove chlorhexidine residues.
Figure 4.
Prepared samples for Staphylococcus aureus adhesion studies
Measurement of adhesion of Staphylococcus aureus
The BHI broth volume was maintained with a suspension and turbidity equivalent to a McFarland standard of 0.5. 5 ml of BHI broth for each sample was transferred to individual test tubes and inoculated with S. aureus (ATCC25923). Prepared acrylic blocks were introduced individually (using a sterile pointed forceps) into test tubes containing 5 ml of BHI broth inoculated with S. aureus. Subsequently, the test tubes were incubated at 37°C aerobically for 18 h. Later, the acrylic blocks were removed from the test tubes and washed using sterile BHI broth to remove nonadherent cells of S. aureus. Transferring of primary inoculum was done by 2 mm diameter sterilized nichrome wire loop onto the agar plate [Figure 5]. Primary inoculum was spread over the agar plate with continuous quadrant streaking method.[26,27] and incubated at 37° C for 24 h.[28] After incubation, the colony counts were noted either at 1-day or 1-week stored samples for all the groups [Figure 6].
Figure 5.

Preparation for primary inoculum on agar plate for colony-forming unit
Figure 6.
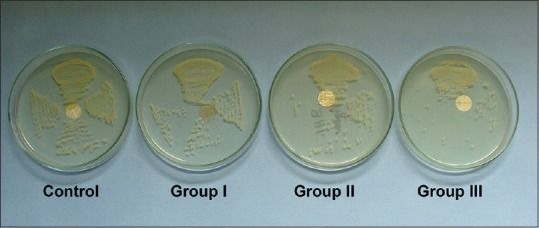
Reduction of colony-forming unit of Staphylococcus aureus on agar plate with increasing concentration of methacrylic acid
RESULTS
Statistical analysis
The descriptive analysis was done to evaluate the mean value and standard deviation of the flexural strength and S. aureus colony-forming unit (CFU) using IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp. Multiple comparisons among the groups were done using post hoc Tukey HSD test.
Evaluation of flexural strength
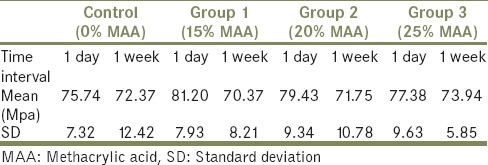
The mean and the standard deviation values of flexural strength of the control and test groups are presented in Graph 1 and Table 1. From the graph, it can be observed that the flexural strength did not change significantly with the addition of MAA. These results indicate that the addition of MAA to heat cure acrylic-based denture materials will not significantly affect their flexural strength.
Graph 1.
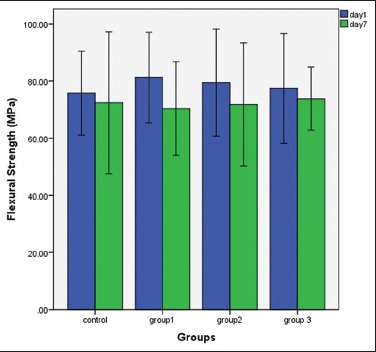
Comparisons of flexural strength among the groups at 1-day and 1-week time interval
Table 1.
Comparisons of flexural strength among the groups at 1-day and 1-week time interval
Evaluation of adhesion of Staphylococcus aureus
Mean and standard deviation values of the adherence of S. aureus to the PMMA surface with and without MAA are presented in Graph 2 and Table 2. The control group showed highest CFU of S. aureus at 1 day and 1 week, whereas Group 3 showed an extreme reduction of S. aureus CFU as compared to control group at 1-day (<0.001) and 1-week (<0.002) interval. Reduction of S. aureus CFU was observed for Group 1; however, the reduction was not statistically significant at both the time intervals. Reduction of S. aureus CFU was observed for Group 2 at 1 day and 1 week; however, the reduction was statistically significant at 1-week (<0.038) time interval as compared to 1 day. The longevity of the effect of MAA was observed for Group 3 for 1 week as compared to other groups.
Graph 2.
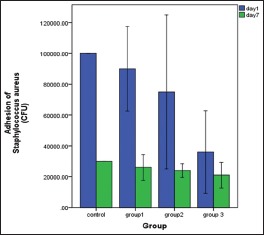
Comparisons of adhesion of Staphylococcus aureus (colony-forming units) among the groups at 1-day and 1-week time interval
Table 2.
Comparisons of adhesion of Staphylococcus aureus (colony forming units) among the groups at 1-day and 1-week time interval

DISCUSSION
The microbial adhesion on the denture surface depends on a variety of factors ranging from the type of microorganism, structure of microbe, and the surface properties of denture base itself. The microbial constitution of oral cavity is very diverse, and they do not cause disease in healthy patients. However, in elderly patients and in immunocompromised patients, even bacteria constituting the normal flora can cause diseases. To avoid accumulation of such microorganisms, denture surfaces have been modified to reduce their potential adhesion and colonization. Such attempts are realized by adding antimicrobial additives to denture base formulations in the form of comonomers. In the present study, MAA has been added to denture base resin formulation to reduce the CFU of S. aureus on the prosthesis. This addition of MAA would be helpful in preventing overgrowth of opportunistic pathogens in medically compromised and elderly patients and will also be helpful in preventing cross-contamination from laboratory to the patient.
Continuous modifications are required for a material to attain better mechanical, physical, and biological properties, which will ultimately enhance the worth of a material. This concept of modification has been applied to this study, whereby the MMA has been modified with 97% of pure MAA in an attempt to attain better mechanical as well as effective antimicrobial properties.
Cross-contamination from dental laboratory to clinics has been studied by various authors,[16,29,30,31,32] and they have concluded that various microorganisms including fungi and bacteria were transferred from dental laboratory to dental clinics and hence to the patient's mouth, which is unsafe and should be prevented.
Contamination of denture base resins has been reduced by means of various modalities including meticulous oral hygiene, disinfection with various disinfecting solutions,[17,33,34,35] microwave energy,[36] and altering the surface energy of denture base resins.[37] PMMA is highly attributed to microbial adhesion on its surface owing to its inherent hydrophobic nature. The advantage of using the MAA for modification of PMMA is that it aids in disinfecting external as well as internal part of the acrylic resin by its property to create a net effective negative charge.[38,39] The examination of the physiochemical interactions of the PMAA–g-EG nanosphere system (copolymer of MAA and g-ethylene glycol) with Caco-2 cell monolayer revealed that these systems possessed low cytotoxicity.[40,41]
The ability of microorganisms to adhere to polymeric surfaces has been correlated with attractive hydrophobic and repulsive electrostatic forces.[42,43,44,45] For hydrophobic surfaces such as PMMA, monomer units on the surface, relate with the hydrophobic provinces on a protein in the cell membrane of the microorganism by strong hydrophobic bonds. Such interactions would result in a tendency for microorganisms to adhere more readily to hydrophobic surfaces than to hydrophilic surfaces.
The contribution of electrostatic interaction is secondary to the hydrophobic interaction since the adherence process takes place in the presence of repulsive forces.[46]
The cell surface of S. aureus, as in most bacteria, has a moderately negative net charge at neutral pH, which is probably due to the fact that the teichoic acids contain fewer positively charged D-alanine residues than negatively charged phosphate groups. Nevertheless, S. aureus can adhere to hydrophobic or slightly negatively charged surfaces such as polystyrene or glass, respectively.[47] Thus, it can be postulated that the addition of MAA to MMA increases net negative charge, which probably leads to a pronounced increase in the repulsive forces, thereby disabling any adherence of the S. aureus to PMMA resin.
Flexural strength, also known as modulus of rupture, bend strength, transverse strength, or fracture strength, is essentially a strength test of a bar supported at each end, or a thin disk supported along a lower support circle, under a static load, or it may also be referred to as a mechanical parameter for brittle materials, which can be defined as a material's ability to resist deformation under load. The flexural strength represents the highest stress experienced within the material at its moment of rupture. It is measured in terms of stress.
Flexural strength was used as one of the parameters for the evaluation of mechanical properties of denture base resins in this study because this test will closely simulate the type of loading conditions in vivo.[48] The measurement of flexural strength is more important compared to tensile and compressive strength as flexural failure of denture base resins is considered the primary mode of clinical failure.[49]
Before flexural strength testing, the width and thickness of each specimen were measured with a digital caliper, with measuring accuracy of ± 0.1 mm. This procedure was necessary because after the trimming and polishing procedures, the original size of each specimen was altered.
Flexural testing was conducted using an INSTRON 3366, employing a three-point or four-point loading system. It was suggested that the four point loading test is attractive for routine testing since a considerable length of the beam is subjected to a stress system which is uniform along it and the peak stress is not concentrated near the loading noses. However, they also stated that it is seldom used for routine testing purposes since it is experimentally more difficult. It is for the same reason that a three point testing was carried out in the present study avoiding the technical sensitivity of the four point testing.[50,51]
CONCLUSION
Within the limitations of the present study, it can be concluded that MAA up to 25% can be added to conventional denture base formulations to impart antimicrobial property without adversely affecting its mechanical properties. Such an addition may be useful in case of medically compromised and elderly patients where occurrence of S. aureus infections is very common. However, further investigations are needed before its clinical application.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Park SE, Periathamby AR, Loza JC. Effect of surface-charged poly (methyl methacrylate) on the adhesion of Candida albicans. J Prosthodont. 2003;12:249–54. doi: 10.1016/s1059-941x(03)00107-4. [DOI] [PubMed] [Google Scholar]
- 2.Dhir G, Berzins DW, Dhuru VB, Periathamby AR, Dentino A. Physical properties of denture base resins potentially resistant to Candida adhesion. J Prosthodont. 2007;16:465–72. doi: 10.1111/j.1532-849X.2007.00219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Samaranayake LP, McCourtie J, MacFarlane TW. Factors affecting the in-vitro adherence of Candida albicans to acrylic surfaces. Arch Oral Biol. 1980;25:611–5. doi: 10.1016/0003-9969(80)90076-x. [DOI] [PubMed] [Google Scholar]
- 4.Segal E, Lehrman O, Dayan D. Adherence in vitro of various Candida species to acrylic surfaces. Oral Surg Oral Med Oral Pathol. 1988;66:670–3. doi: 10.1016/0030-4220(88)90315-5. [DOI] [PubMed] [Google Scholar]
- 5.Cannon RD, Chaffin WL. Oral colonization by Candida albicans. Crit Rev Oral Biol Med. 1999;10:359–83. doi: 10.1177/10454411990100030701. [DOI] [PubMed] [Google Scholar]
- 6.Ramage G, Tomsett K, Wickes BL, López-Ribot JL, Redding SW. Denture stomatitis: A role for Candida biofilms. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;98:53–9. doi: 10.1016/j.tripleo.2003.04.002. [DOI] [PubMed] [Google Scholar]
- 7.Davenport JC. The oral distribution of Candida albicans in denture stomatitis. Br Dent J. 1970;129:151–6. doi: 10.1038/sj.bdj.4802540. [DOI] [PubMed] [Google Scholar]
- 8.Budtz-Jörgensen E. Clinical aspects of Candida infection in denture wearers. J Am Dent Assoc. 1978;96:474–9. doi: 10.14219/jada.archive.1978.0088. [DOI] [PubMed] [Google Scholar]
- 9.Webb BC, Thomas CJ, Willcox MD, Harty DW, Knox KW. Candida-associated denture stomatitis. Aetiology and management: A review. Part 3 Treatment of oral candidosis. Aust Dent J. 1998;43:244–9. doi: 10.1111/j.1834-7819.1998.tb00172.x. [DOI] [PubMed] [Google Scholar]
- 10.Aguirre JM, Verdugo F, Zamacona JM, Quindos G, Ponton J. Cytological changes in oral mucosa in denture stomatitis. Gerodontology. 1996;13:63–7. doi: 10.1111/j.1741-2358.1996.tb00152.x. [DOI] [PubMed] [Google Scholar]
- 11.Smith AJ, Jackson MS, Bagg J. The ecology of Staphylococcus species in the oral cavity. J Med Microbiol. 2001;50:940–6. doi: 10.1099/0022-1317-50-11-940. [DOI] [PubMed] [Google Scholar]
- 12.Sumi Y, Miura H, Sunakawa M, Michiwaki Y, Sakagami N. Colonization of denture plaque by respiratory pathogens in dependent elderly. Gerodontology. 2002;19:25–9. doi: 10.1111/j.1741-2358.2002.00025.x. [DOI] [PubMed] [Google Scholar]
- 13.Mylotte JM. Nursing home-acquired pneumonia. Clin Infect Dis. 2002;35:1205–11. doi: 10.1086/344281. [DOI] [PubMed] [Google Scholar]
- 14.Terpenning MS, Taylor GW, Lopatin DE, Kerr CK, Dominguez BL, Loesche WJ. Aspiration pneumonia: Dental and oral risk factors in an older veteran population. J Am Geriatr Soc. 2001;49:557–63. doi: 10.1046/j.1532-5415.2001.49113.x. [DOI] [PubMed] [Google Scholar]
- 15.Altieri KT, Sanitá PV, Machado AL, Giampaolo ET, Pavarina AC, Vergani CE. Effectiveness of two disinfectant solutions and microwave irradiation in disinfecting complete dentures contaminated with methicillin-resistant Staphylococcus aureus. J Am Dent Assoc. 2012;143:270–7. doi: 10.14219/jada.archive.2012.0152. [DOI] [PubMed] [Google Scholar]
- 16.Powell GL, Runnells RD, Saxon BA, Whisenant BK. The presence and identification of organisms transmitted to dental laboratories. J Prosthet Dent. 1990;64:235–7. doi: 10.1016/0022-3913(90)90185-f. [DOI] [PubMed] [Google Scholar]
- 17.Yilmaz H, Aydin C, Bal BT, Ozçelik B. Effects of disinfectants on resilient denture-lining materials contaminated with Staphylococcus aureus, Streptococcus sobrinus, and Candida albicans. Quintessence Int. 2005;36:373–81. [PubMed] [Google Scholar]
- 18.Schwartz R, Kinyon T, Mayhew R. Infection control in the dental laboratory: A review of the literature. Mil Med. 1991;156:1–4. [PubMed] [Google Scholar]
- 19.Park SE, Blissett R, Susarla SM, Weber HP. Candida albicans adherence to surface-modified denture resin surfaces. J Prosthodont. 2008;17:365–9. doi: 10.1111/j.1532-849X.2007.00292.x. [DOI] [PubMed] [Google Scholar]
- 20.Casemiro LA, Gomes Martins CH, Pires-de-Souza Fde C, Panzeri H. Antimicrobial and mechanical properties of acrylic resins with incorporated silver-zinc zeolite – Part I. Gerodontology. 2008;25:187–94. doi: 10.1111/j.1741-2358.2007.00198.x. [DOI] [PubMed] [Google Scholar]
- 21.Puri G, Berzins DW, Dhuru VB, Raj PA, Rambhia SK, Dhir G, et al. Effect of phosphate group addition on the properties of denture base resins. J Prosthet Dent. 2008;100:302–8. doi: 10.1016/S0022-3913(08)60210-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aldana L, Marker VA, Kolstad R, Iacopino AM. Effects of Candida treatment regimens on the physical properties of denture resins. Int J Prosthodont. 1994;7:473–8. [PubMed] [Google Scholar]
- 23.Ellakwa AE, Morsy MA, El-Sheikh AM. Effect of aluminum oxide addition on the flexural strength and thermal diffusivity of heat-polymerized acrylic resin. J Prosthodont. 2008;17:439–44. doi: 10.1111/j.1532-849X.2008.00318.x. [DOI] [PubMed] [Google Scholar]
- 24.Yau WF, Cheng YY, Clark RK, Chow TW. Pressure and temperature changes in heat-cured acrylic resin during processing. Dent Mater. 2002;18:622–9. doi: 10.1016/s0109-5641(01)00092-6. [DOI] [PubMed] [Google Scholar]
- 25.Anusavice KJ. Phillips Science of Dental Materials. 10th ed. Philadelphia: Saunders; 1996. pp. 249–50. [Google Scholar]
- 26.Bajaj N, Tandon S. The effect of Triphala and Chlorhexidine mouthwash on dental plaque, gingival inflammation, and microbial growth. Int J Ayurveda Res. 2011;2:29–36. doi: 10.4103/0974-7788.83188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sitges-Serra A, Liñares J. Limitations of semiquantitative method for catheter culture. J Clin Microbiol. 1988;26:1074–6. doi: 10.1128/jcm.26.5.1074-1076.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dutta D, Willcox MD. A laboratory assessment of factors that affect bacterial adhesion to contact lenses. Biology (Basel) 2013;2:1268–81. doi: 10.3390/biology2041268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Katberg JW., Jr Cross-contamination via the prosthodontic laboratory. J Prosthet Dent. 1974;32:412–9. doi: 10.1016/0022-3913(74)90350-3. [DOI] [PubMed] [Google Scholar]
- 30.Williams HN, Falkler WA, Jr, Smith AG, Hasler JF. The isolation of fungi from laboratory dental pumice. J Prosthet Dent. 1986;56:737–40. doi: 10.1016/0022-3913(86)90155-1. [DOI] [PubMed] [Google Scholar]
- 31.Wakefield CW. Laboratory contamination of dental prostheses. J Prosthet Dent. 1980;44:143–6. doi: 10.1016/0022-3913(80)90125-0. [DOI] [PubMed] [Google Scholar]
- 32.Kahn RC, Lancaster MV, Kate W., Jr The microbiologic cross-contamination of dental prostheses. J Prosthet Dent. 1982;47:556–9. doi: 10.1016/0022-3913(82)90309-2. [DOI] [PubMed] [Google Scholar]
- 33.Polyzois GL, Zissis AJ, Yannikakis SA. The effect of glutaraldehyde and microwave disinfection on some properties of acrylic denture resin. Int J Prosthodont. 1995;8:150–4. [PubMed] [Google Scholar]
- 34.Bell JA, Brockmann SL, Feil P, Sackuvich DA. The effectiveness of two disinfectants on denture base acrylic resin with an organic load. J Prosthet Dent. 1989;61:580–3. doi: 10.1016/0022-3913(89)90280-1. [DOI] [PubMed] [Google Scholar]
- 35.da Silva FC, Kimpara ET, Mancini MN, Balducci I, Jorge AO, Koga-Ito CY. Effectiveness of six different disinfectants on removing five microbial species and effects on the topographic characteristics of acrylic resin. J Prosthodont. 2008;17:627–33. doi: 10.1111/j.1532-849X.2008.00358.x. [DOI] [PubMed] [Google Scholar]
- 36.Dixon DL, Breeding LC, Faler TA. Microwave disinfection of denture base materials colonized with Candida albicans. J Prosthet Dent. 1999;81:207–14. doi: 10.1016/s0022-3913(99)70250-7. [DOI] [PubMed] [Google Scholar]
- 37.Moura JS, da Silva WJ, Pereira T, Del Bel Cury AA, Rodrigues Garcia RC. Influence of acrylic resin polymerization methods and saliva on the adherence of four Candida species. J Prosthet Dent. 2006;96:205–11. doi: 10.1016/j.prosdent.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 38.Hogt AH, Gregonis DE, Andrade JD, Kim SW, Dankert J, Feijen J. Wettability and potentials of a series of methacrylate polymers and copolymers. J Colloid Interface Sci. 1985;106:289–98. [Google Scholar]
- 39.Lin JJ, Cameron SM, Runyan DA, Craft DW. Disinfection of denture base acrylic resin. J Prosthet Dent. 1999;81:202–6. doi: 10.1016/s0022-3913(99)70249-0. [DOI] [PubMed] [Google Scholar]
- 40.Cosmetic Ingredient Review Expert Panel. Final report of the safety assessment of methacrylic acid. Int J Toxicol. 2005;24(Suppl 5):33–51. doi: 10.1080/10915810500434191. [DOI] [PubMed] [Google Scholar]
- 41.Torres-Lugo M, García M, Record R, Peppas NA. Physicochemical behavior and cytotoxic effects of P (methacrylic acid-g-ethylene glycol) nanospheres for oral delivery of proteins. J Control Release. 2002;80:197–205. doi: 10.1016/s0168-3659(02)00027-5. [DOI] [PubMed] [Google Scholar]
- 42.Minagi S, Miyake Y, Inagaki K, Tsuru H, Suginaka H. Hydrophobic interaction in Candida albicans and Candida tropicalis adherence to various denture base resin materials. Infect Immun. 1985;47:11–4. doi: 10.1128/iai.47.1.11-14.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Klotz SA, Drutz DJ, Zajic JE. Factors governing adherence of Candida species to plastic surfaces. Infect Immun. 1985;50:97–101. doi: 10.1128/iai.50.1.97-101.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Horbett TA, Waldburger JJ, Ratner BD, Hoffman AS. Cell adhesion to a series of hydrophilic-hydrophobic copolymers studied with a spinning disc apparatus. J Biomed Mater Res. 1988;22:383–404. doi: 10.1002/jbm.820220503. [DOI] [PubMed] [Google Scholar]
- 45.Nikawa H, Iwanaga H, Kameda M, Hamada T. In vitro evaluation of Candida albicans adherence to soft denture-lining materials. J Prosthet Dent. 1992;68:804–8. doi: 10.1016/0022-3913(92)90206-p. [DOI] [PubMed] [Google Scholar]
- 46.Koda T, Tsuchiya H, Yamauchi M, Ohtani S, Takagi N, Kawano J. Leachability of denture-base acrylic resins in artificial saliva. Dent Mater. 1990;6:13–6. doi: 10.1016/0109-5641(90)90037-f. [DOI] [PubMed] [Google Scholar]
- 47.Gross M, Cramton SE, Götz F, Peschel A. Key role of teichoic acid net charge in Staphylococcus aureus colonization of artificial surfaces. Infect Immun. 2001;69:3423–6. doi: 10.1128/IAI.69.5.3423-3426.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Power JM, Sakaguchi RL. Craig's Restorative Dental Materials. 12th ed. Philadelphia: Mosby Elsevier; 2006. pp. 517–20. [Google Scholar]
- 49.Barbosa DB, de Souza RF, Pero AC, Marra J, Compagnoni MA. Flexural strength of acrylic resins polymerized by different cycles. J Appl Oral Sci. 2007;15:424–8. doi: 10.1590/S1678-77572007000500010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chitchumnong P, Brooks SC, Stafford GD. Comparison of three- and four-point flexural strength testing of denture-base polymers. Dent Mater. 1989;5:2–5. doi: 10.1016/0109-5641(89)90082-1. [DOI] [PubMed] [Google Scholar]
- 51.Thean HP, Chew CL, Goh KI, Norman RD. An evaluation of bond strengths of denture repair resins by a torsional method. Aust Dent J. 1998;43:5–8. doi: 10.1111/j.1834-7819.1998.tb00143.x. [DOI] [PubMed] [Google Scholar]


