Abstract
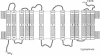
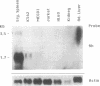
cDNA clones encoding a human blood group Rh polypeptide were isolated from a human bone marrow cDNA library by using a polymerase chain reaction-amplified DNA fragment encoding the known common N-terminal region of the Rh proteins. The entire primary structure of the Rh polypeptide has been deduced from the nucleotide sequence of a 1384-base-pair-long cDNA clone. Translation of the open reading frame indicates that the Rh protein is composed of 417 amino acids, including the initiator methionine, which is removed in the mature protein, lacks a cleavable N-terminal sequence, and has no consensus site for potential N-glycosylation. The predicted molecular mass of the protein is 45,500, while that estimated for the Rh protein analyzed in NaDodSO4/polyacrylamide gels is in the range of 30,000-32,000. These findings suggest either that the hydrophobic Rh protein behaves abnormally on NaDodSO4 gels or that the Rh mRNA may encode a precursor protein, which is further matured by a proteolytic cleavage of the C-terminal region of the polypeptide. Hydropathy analysis and secondary structure predictions suggest the presence of 13 membrane-spanning domains, indicating that the Rh polypeptide is highly hydrophobic and deeply buried within the phospholipid bilayer. In RNA blot-hybridization (Northern) analysis, the Rh cDNA probe detects a major 1.7-kilobase and a minor 3.5-kilobase mRNA species in adult erythroblasts, fetal liver, and erythroid (K562, HEL) and megakaryocytic (MEG01) leukemic cell lines, but not in adult liver and kidney tissues or lymphoid (Jurkat) and promyelocytic (HL60) cell lines. These results suggest that the expression of the Rh gene(s) might be restricted to tissues or cell lines expressing erythroid characters.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agre P., Saboori A. M., Asimos A., Smith B. L. Purification and partial characterization of the Mr 30,000 integral membrane protein associated with the erythrocyte Rh(D) antigen. J Biol Chem. 1987 Dec 25;262(36):17497–17503. [PubMed] [Google Scholar]
- Arfin S. M., Bradshaw R. A. Cotranslational processing and protein turnover in eukaryotic cells. Biochemistry. 1988 Oct 18;27(21):7979–7984. doi: 10.1021/bi00421a001. [DOI] [PubMed] [Google Scholar]
- Avent N. D., Ridgwell K., Mawby W. J., Tanner M. J., Anstee D. J., Kumpel B. Protein-sequence studies on Rh-related polypeptides suggest the presence of at least two groups of proteins which associate in the human red-cell membrane. Biochem J. 1988 Dec 15;256(3):1043–1046. doi: 10.1042/bj2561043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballas S. K., Clark M. R., Mohandas N., Colfer H. F., Caswell M. S., Bergren M. O., Perkins H. A., Shohet S. B. Red cell membrane and cation deficiency in Rh null syndrome. Blood. 1984 May;63(5):1046–1055. [PubMed] [Google Scholar]
- Blanchard D., Bloy C., Hermand P., Cartron J. P., Saboori A. M., Smith B. L., Agre P. Two-dimensional iodopeptide mapping demonstrates that erythrocyte Rh D, c, and E polypeptides are structurally homologous but nonidentical. Blood. 1988 Oct;72(4):1424–1427. [PubMed] [Google Scholar]
- Bloy C., Blanchard D., Dahr W., Beyreuther K., Salmon C., Cartron J. P. Determination of the N-terminal sequence of human red cell Rh(D) polypeptide and demonstration that the Rh(D), (c), and (E) antigens are carried by distinct polypeptide chains. Blood. 1988 Aug;72(2):661–666. [PubMed] [Google Scholar]
- Catterall W. A. Structure and function of voltage-sensitive ion channels. Science. 1988 Oct 7;242(4875):50–61. doi: 10.1126/science.2459775. [DOI] [PubMed] [Google Scholar]
- Colin Y., Le Van Kim C., Tsapis A., Clerget M., d'Auriol L., London J., Galibert F., Cartron J. P. Human erythrocyte glycophorin C. Gene structure and rearrangement in genetic variants. J Biol Chem. 1989 Mar 5;264(7):3773–3780. [PubMed] [Google Scholar]
- Dunstan R. A., Simpson M. B., Rosse W. F. Erythrocyte antigens on human platelets. Absence of Rh, Duffy, Kell, Kidd, and Lutheran antigens. Transfusion. 1984 May-Jun;24(3):243–246. doi: 10.1046/j.1537-2995.1984.24384225031.x. [DOI] [PubMed] [Google Scholar]
- Engelman D. M., Steitz T. A., Goldman A. Identifying nonpolar transbilayer helices in amino acid sequences of membrane proteins. Annu Rev Biophys Biophys Chem. 1986;15:321–353. doi: 10.1146/annurev.bb.15.060186.001541. [DOI] [PubMed] [Google Scholar]
- Falkenburg J. H., Fibbe W. E., van der Vaart-Duinkerken N., Nichols M. E., Rubinstein P., Jansen J. Human erythroid progenitor cells express Rhesus antigens. Blood. 1985 Sep;66(3):660–663. [PubMed] [Google Scholar]
- Favaloro J., Treisman R., Kamen R. Transcription maps of polyoma virus-specific RNA: analysis by two-dimensional nuclease S1 gel mapping. Methods Enzymol. 1980;65(1):718–749. doi: 10.1016/s0076-6879(80)65070-8. [DOI] [PubMed] [Google Scholar]
- Gahmberg C. G., Karhi K. K. Association of Rho(D) polypeptides with the membrane skeleton in Rho(D)-positive human red cells. J Immunol. 1984 Jul;133(1):334–337. [PubMed] [Google Scholar]
- Gahmberg C. G. Molecular characterization of the human red cell Rho(D) antigen. EMBO J. 1983;2(2):223–227. doi: 10.1002/j.1460-2075.1983.tb01409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahmberg C. G. Molecular identification of the human Rho (D) antigen. FEBS Lett. 1982 Apr 5;140(1):93–97. doi: 10.1016/0014-5793(82)80528-0. [DOI] [PubMed] [Google Scholar]
- Green F. A. Erythrocyte membrane sulfhydryl groups and Rh antigen activity. Immunochemistry. 1967 Jul;4(4):247–257. doi: 10.1016/0019-2791(67)90186-3. [DOI] [PubMed] [Google Scholar]
- Green F. A. The mode of attenuation of erythrocyte membrane Rh0(D) antigen activity by 5,5'-dithiobis-(2-nitrobenzoic acid) and protection against loss of activity by bound anti-Rh0(D) antibody. Mol Immunol. 1983 Jul;20(7):769–775. doi: 10.1016/0161-5890(83)90055-x. [DOI] [PubMed] [Google Scholar]
- Hartmann E., Rapoport T. A., Lodish H. F. Predicting the orientation of eukaryotic membrane-spanning proteins. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5786–5790. doi: 10.1073/pnas.86.15.5786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hediger M. A., Coady M. J., Ikeda T. S., Wright E. M. Expression cloning and cDNA sequencing of the Na+/glucose co-transporter. 1987 Nov 26-Dec 2Nature. 330(6146):379–381. doi: 10.1038/330379a0. [DOI] [PubMed] [Google Scholar]
- Hughes-Jones N. C., Bloy C., Gorick B., Blanchard D., Doinel C., Rouger P., Cartron J. P. Evidence that the c, D and E epitopes of the human Rh blood group system are on separate polypeptide molecules. Mol Immunol. 1988 Sep;25(9):931–936. doi: 10.1016/0161-5890(88)90132-0. [DOI] [PubMed] [Google Scholar]
- Knoth K., Roberds S., Poteet C., Tamkun M. Highly degenerate, inosine-containing primers specifically amplify rare cDNA using the polymerase chain reaction. Nucleic Acids Res. 1988 Nov 25;16(22):10932–10932. doi: 10.1093/nar/16.22.10932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopito R. R., Lodish H. F. Primary structure and transmembrane orientation of the murine anion exchange protein. Nature. 1985 Jul 18;316(6025):234–238. doi: 10.1038/316234a0. [DOI] [PubMed] [Google Scholar]
- Kozak M. An analysis of 5'-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987 Oct 26;15(20):8125–8148. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuypers F., van Linde-Sibenius-Trip M., Roelofsen B., Tanner M. J., Anstee D. J., Op den Kamp J. A. Rhnull human erythrocytes have an abnormal membrane phospholipid organization. Biochem J. 1984 Aug 1;221(3):931–934. doi: 10.1042/bj2210931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauf P. K., Joiner C. H. Increased potassium transport and ouabain binding in human Rhnull red blood cells. Blood. 1976 Sep;48(3):457–468. [PubMed] [Google Scholar]
- Marsh W. L., Chaganti R. S., Gardner F. H., Mayer K., Nowell P. C., German J. Mapping human autosomes: evidence supporting assignment of rhesus to the short arm of chromosome No. 1. Science. 1974 Mar 8;183(4128):966–968. doi: 10.1126/science.183.4128.966. [DOI] [PubMed] [Google Scholar]
- Marshall R. D. The nature and metabolism of the carbohydrate-peptide linkages of glycoproteins. Biochem Soc Symp. 1974;(40):17–26. [PubMed] [Google Scholar]
- Martin D. I., Zon L. I., Mutter G., Orkin S. H. Expression of an erythroid transcription factor in megakaryocytic and mast cell lineages. Nature. 1990 Mar 29;344(6265):444–447. doi: 10.1038/344444a0. [DOI] [PubMed] [Google Scholar]
- Mattei M. G., Sardet C., Franchi A., Pouysségur J. The human amiloride-sensitive Na+/H+ antiporter: localization to chromosome 1 by in situ hybridization. Cytogenet Cell Genet. 1988;48(1):6–8. doi: 10.1159/000132575. [DOI] [PubMed] [Google Scholar]
- Moore S., Woodrow C. F., McClelland D. B. Isolation of membrane components associated with human red cell antigens Rh(D), (c), (E) and Fy. Nature. 1982 Feb 11;295(5849):529–531. doi: 10.1038/295529a0. [DOI] [PubMed] [Google Scholar]
- Mueckler M., Caruso C., Baldwin S. A., Panico M., Blench I., Morris H. R., Allard W. J., Lienhard G. E., Lodish H. F. Sequence and structure of a human glucose transporter. Science. 1985 Sep 6;229(4717):941–945. doi: 10.1126/science.3839598. [DOI] [PubMed] [Google Scholar]
- Nash R., Shojania A. M. Hematological aspect of Rh deficiency syndrome: a case report and a review of the literature. Am J Hematol. 1987 Mar;24(3):267–275. doi: 10.1002/ajh.2830240306. [DOI] [PubMed] [Google Scholar]
- Paradis G., Bazin R., Lemieux R. Protective effect of the membrane skeleton on the immunologic reactivity of the human red cell Rho(D) antigen. J Immunol. 1986 Jul 1;137(1):240–244. [PubMed] [Google Scholar]
- Rearden A., Masouredis S. P. Blood group D antigen content of nucleated red cell precursors. Blood. 1977 Dec;50(6):981–986. [PubMed] [Google Scholar]
- Reynolds J. A., Tanford C. The gross conformation of protein-sodium dodecyl sulfate complexes. J Biol Chem. 1970 Oct 10;245(19):5161–5165. [PubMed] [Google Scholar]
- Ridgwell K., Tanner M. J., Anstee D. J. The Rhesus (D) polypeptide is linked to the human erythrocyte cytoskeleton. FEBS Lett. 1984 Aug 20;174(1):7–10. doi: 10.1016/0014-5793(84)81066-2. [DOI] [PubMed] [Google Scholar]
- Romeo P. H., Prandini M. H., Joulin V., Mignotte V., Prenant M., Vainchenker W., Marguerie G., Uzan G. Megakaryocytic and erythrocytic lineages share specific transcription factors. Nature. 1990 Mar 29;344(6265):447–449. doi: 10.1038/344447a0. [DOI] [PubMed] [Google Scholar]
- Saboori A. M., Smith B. L., Agre P. Polymorphism in the Mr 32,000 Rh protein purified from Rh(D)-positive and -negative erythrocytes. Proc Natl Acad Sci U S A. 1988 Jun;85(11):4042–4045. doi: 10.1073/pnas.85.11.4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Bugawan T. L., Horn G. T., Mullis K. B., Erlich H. A. Analysis of enzymatically amplified beta-globin and HLA-DQ alpha DNA with allele-specific oligonucleotide probes. Nature. 1986 Nov 13;324(6093):163–166. doi: 10.1038/324163a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardet C., Franchi A., Pouysségur J. Molecular cloning, primary structure, and expression of the human growth factor-activatable Na+/H+ antiporter. Cell. 1989 Jan 27;56(2):271–280. doi: 10.1016/0092-8674(89)90901-x. [DOI] [PubMed] [Google Scholar]
- Schiffer M., Edmundson A. B. Use of helical wheels to represent the structures of proteins and to identify segments with helical potential. Biophys J. 1967 Mar;7(2):121–135. doi: 10.1016/S0006-3495(67)86579-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shows T. B., Eddy R. L., Byers M. G., Fukushima Y., Dehaven C. R., Murray J. C., Bell G. I. Polymorphic human glucose transporter gene (GLUT) is on chromosome 1p31.3----p35. Diabetes. 1987 Apr;36(4):546–549. doi: 10.2337/diab.36.4.546. [DOI] [PubMed] [Google Scholar]
- Shull G. E., Schwartz A., Lingrel J. B. Amino-acid sequence of the catalytic subunit of the (Na+ + K+)ATPase deduced from a complementary DNA. Nature. 1985 Aug 22;316(6030):691–695. doi: 10.1038/316691a0. [DOI] [PubMed] [Google Scholar]
- Strader C. D., Sigal I. S., Dixon R. A. Structural basis of beta-adrenergic receptor function. FASEB J. 1989 May;3(7):1825–1832. doi: 10.1096/fasebj.3.7.2541037. [DOI] [PubMed] [Google Scholar]
- Sturgeon P. Hematological observations on the anemia associated with blood type Rhnull. Blood. 1970 Sep;36(3):310–320. [PubMed] [Google Scholar]
- Suyama K., Goldstein J. Enzymatic evidence for differences in the placement of Rh antigens within the red cell membrane. Blood. 1990 Jan 1;75(1):255–260. [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessels H. P., Spiess M. Insertion of a multispanning membrane protein occurs sequentially and requires only one signal sequence. Cell. 1988 Oct 7;55(1):61–70. doi: 10.1016/0092-8674(88)90009-8. [DOI] [PubMed] [Google Scholar]
- de Vetten M. P., Agre P. The Rh polypeptide is a major fatty acid-acylated erythrocyte membrane protein. J Biol Chem. 1988 Dec 5;263(34):18193–18196. [PubMed] [Google Scholar]
- von Heijne G. Analysis of the distribution of charged residues in the N-terminal region of signal sequences: implications for protein export in prokaryotic and eukaryotic cells. EMBO J. 1984 Oct;3(10):2315–2318. doi: 10.1002/j.1460-2075.1984.tb02132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]