Abstract
Objectives
Several features necessary for polymer composite materials in practical applications such as dental restorative materials were investigated in photo-curable CuAAC (copper(I)-catalyzed azide-alkyne cycloaddition) thermosetting resin-based composites with varying filler loadings and compared to a conventional BisGMA/TEGDMA based composite.
Methods
Tri-functional alkyne and di-functional azide monomers were synthesized for CuAAC resins and incorporated with alkyne-functionalized silica microfillers for CuAAC composites. Polymerization kinetics, in situ temperature change, and shrinkage stress were monitored simultaneously with a tensometer coupled with FTIR spectroscopy and a data-logging thermocouple. The glass transition temperature was analyzed by dynamic mechanical analysis. Flexural modulus/strength and flexural toughness were characterized in three-point bending on a universal testing machine.
Results
The photo-CuAAC polymerization of composites containing between 0 and 60 wt% microfiller achieved ~99% conversion with a dramatic reduction in the maximum heat of reaction (~20 °C decrease) for the 60 wt% filled CuAAC composites as compared with the unfilled CuAAC resin. CuAAC composites with 60 wt% microfiller generated more than twice lower shrinkage stress of 0.43±0.01 MPa, equivalent flexural modulus of 6.1±0.7 GPa, equivalent flexural strength of 107±9 MPa, and more than 10 times higher energy absorption of 10±1 MJ m−3 when strained to 11% relative to BisGMA-based composites at equivalent filler loadings.
Significance
Mechanically robust and highly tough, photo-polymerized CuAAC composites with reduced shrinkage stress and a modest reaction exotherm were generated and resulted in essentially complete conversion.
Keywords: Photopolymerization, CuAAC, composites, dental materials, stress, modulus, flexural strength, toughness, click chemistry, step-growth polymerization
INTRODUCTION
Conventional resin-based dental restorative composites are formulated predominantly from inorganic fillers dispersed in matrices comprised of BisGMA or other related dimethacrylate-based monomer mixtures. These composites are esthetically appealing, mechanically stiff, and biocompatible thermosetting materials with rapid photo-curing kinetics upon incident visible light activation [1–3]. Typically, a high volume fraction of inorganic silica/glass fillers is used in combination with the resin in dental composites, for instance, 30–65 v/v% or 40–80 wt/wt% of 0.4–1.0 μm fillers/resins [4]. The roles of the filler involve enhanced mechanical performance including properties such as hardness, strength, and wear resistance, along with improved biocompatibility and moisture resistance [3,5] as well as reduced thermal expansion coefficient and polymerization-induced volumetric shrinkage that leads to significant stress [6]. However, extensive filler loading in resin-based composites can hinder photopolymerization kinetics due to light scattering and also influence handling properties due to the substantial increase in initial viscosity of the uncured composite paste as compared to the unfilled resin [7]. Therefore, the influence of filler types, shapes, sizes, concentrations, and functionalization on kinetics, mechanics, rheology, biocompatibility, and handling properties of resin-based composites has been investigated widely in the search for a more durable and practical dental restorative composite material [1,8–12].
Different classifications of inorganic fillers include metal oxides (eg: silica, aluminum oxide, titanium dioxide), alkaline silicate glass, bioactive or biomimetic fillers (eg: hydroxyapatite), and organic-inorganic hybrids [7]. Silicon dioxides are often utilized in resin-based dental composites [11] due to their mechanical performance along with reduced water-sorption and solubility [7]. Chemical functionalization of the filler that leads to covalent interactions between the filler particles and the polymer matrix after polymerization improves mechanical performance and hydrophobicity of composites as well as handling properties of the uncured pastes [1,13,14]. Silane derivatives with reactive functional groups, e.g., methacrylates, are used as coupling agents to chemically modify the surface of inorganic particles, enhance particle dispersion, and promote interfacial bonding between the resin and filler via copolymerization [1,14]. In addition to bulk silica particles, mesoporous silica nanofillers have also been utilized where their porous structure facilitates mechanical interlocking with the polymerizable resin phase [15,16]. In contrast to micro-sized fillers (0.1–5 μm) with maximum particle loadings of 70 vol% or 85 wt%, nano-sized fillers (5–100 nm) are more restricted with respect to the maximum loading levels in photo-curable dental composites due to particle aggregation, viscosity increase, and light scattering associated with the significant increase in specific surface area of the particles [3,11,16,17]. As a consequence, nano-filled composites typically have reduced mechanical performance while enabling other positive attributes related to reduced opacity and improved polishability, hardness and wear resistance [11]. Alternatively, hybrid fillers consisting of both micro- and nano-sized particles are often employed in resin-based composites with enhanced packing fraction and surface smoothness [4].
Despite significant advances in filler development, failure of practical resin-based dental composites have been associated with high polymerization shrinkage stress [6], brittleness [18], monomer toxicity, and extractables [19] as well as the formation of biofilms [20]. These issues, which shorten the service lifetime of composite restoratives, arise in part due to the nature of chain-growth polymerization associated with the conventional curing of methacrylate-based composites [2]. The free-radical photopolymerization of BisGMA-based dimethacrylates generates highly crosslinked, glassy networks that are structurally heterogeneous and highly brittle [18], while also being prone to hydrolytic and enzymatic degradation in moist environments due to the presence of ester functionalities in the monomer structures [10]. In addition, the low gel point conversion that occurs in a chain-growth polymerization of dimethacrylate resins necessitates that significant polymerization-induced shrinkage stress will arise while the relatively low conversion at which vitrification occurs limits the maximum conversion and may lead to significant amount of residual monomer [6,21]. The shrinkage and stress development can lead to micro-cracks, adhesive failures, and secondary caries [22]. Over the past decade, alternative chemistries/mechanisms to minimize or eliminate the detrimental characteristics associated with traditional methacrylate-based dental resins have been explored. Ring-opening polymerizations utilizing epoxy, silorane, or cyclopropyl functional groups in resins as well as on nanofillers generated low volumetric shrinkage [23–26], and step-growth polymerization of thiol-ene or CuAAC (copper(I)-catalyzed azide-alkyne cycloaddition) resins achieved reduced polymerization shrinkage stress via delayed gelation [27–29]. In addition, hybrid polymerizations of ternary systems including chain-growth polymerization of methacrylates with step-growth polymerization of thiol-ene/thiol-yne, produced interpenetrating polymer networks (IPNs) with a dramatic reduction in shrinkage stress [30–32]. Further, incorporation of allyl sulfide or trithiocarbonate functionalities in dimethacrylate-based or other polymerized networks effectively lowered polymerization shrinkage stress via addition-fragmentation chain transfer [33–36].
Previously, we introduced the photo-initiated copper(I)-catalyzed azide-alkyne cycloaddition (photo-CuAAC) polymerization [37,38] (Scheme 1) as a means to prepare a glassy thermoset with significantly reduced polymerization shrinkage stress at complete conversion while simultaneously maintaining improved mechanical performance [29]. The preeminent benefits of the photo-CuAAC polymers over conventional BisGMA-based dimethacrylate polymers lie not only in the nature of the step-growth polymerization and the associated delayed gel point conversion, which contributes to reduced shrinkage stress [29], but also on the formation of rigid triazole linkages as a product that significantly enhance the overall mechanical behavior [38–40]. Furthermore, owing to the “click” chemistry of a highly orthogonal CuAAC reaction without forming a byproduct [41,42], the kinetics and mechanics of the CuAAC resin-based crosslinked photopolymers have been explored with varying monomer structures, photoinitiators, copper salts, and/or light exposure conditions [38,39,43–45]. Herein, we investigate polymerization kinetics coupled with in situ measurement of reaction temperature and (thermo-) mechanical properties of photo-polymerized CuAAC composites. These experimental formulations with various silica microfiller compositions use visible light photo-activation to achieve rapid photo-curable materials with excellent mechanical performance. For dental restorative applications, polymerization shrinkage stress, flexural modulus (or Young’s modulus), flexural strength, and flexural toughness are considered as essential characteristics in dental composites, and these are compared between CuAAC composites and a BisGMA-based dimethacrylate composite used as a control.
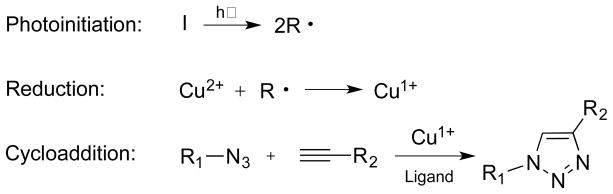
Scheme 1.
A general scheme for the photoinitiated CuAAC reaction using a radical generating photoinitiator comprised of three reaction steps. 1. photoinitiation of radicals 2. reduction of Cu(II) to Cu(I) 3. copper catalyzed cycloaddition of azides and alkynes to generate 1,2,3-triazoles.
EXPERIMENTAL SECTION
1. Materials
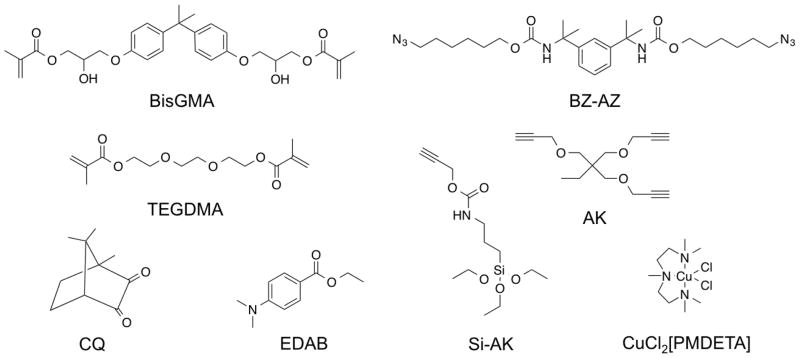
1,3-Bis(2-isocyanatopropan-2-yl)benzene, dibutyltin dilaurate, tetrahydrofuran, 6-chloro-1-hexanol, sodium azide, 1,1,1-tris(hydroxymethyl)propane, propargyl bromide, propargyl alcohol, 3-(triethoxysilyl)propyl isocyanate, copper(II) chloride, N,N,N′,N′,N″-pentamethyldiethylenetriamine (PMDETA), camphorquinone (CQ), ethyl 4-(dimethylamino)benzoate (EDAB), toluene, and acetonitrile were used as received from Sigma Aldrich. Propylamine, sodium hydroxide, dimethyl sulfoxide, dimethylformamide, methanol, and sodium sulfate were used as received from Fisher Scientific. The BisGMA/TEGDMA (70/30) comonomer mixture was used as donated from ESSTECH. Fusion silane coupling agent was used as received from George Taub Products & Fusion Co., Inc. Schott glass (mean particle size of 0.4 μm) with both untreated surface and surface treated with γ–methacryloxypropyltrimethoxysilane were used as received from ESSTECH. Bis(6-azidohexyl) (1,3-phenylenebis(propane-2,2-diyl))dicarbamate (BZ-AZ), 1-(prop-2-yn-1-yloxy)-2,2-bis((prop-2-yn-1-yloxy)methyl)butane (AK), and prop-2-yn-1-yl (3-(triethoxysilyl)propyl)carbamate (Si-AK) were synthesized according to previously reported procedures [29,39,43]. All synthetic procedures and NMR predictions are provided in the Supporting Information. Azides were synthesized according to the azide safety rules and handled with appropriate precaution when working with monomers, resins, and polymers in small quantities [46].
2. Methods
BisGMA/TEGDMA composite preparation
A BisGMA:TEGDMA (70:30 weight ratio) mixture with 0.6 wt% CQ and 1.6 wt% EDAB was prepared by physical mixing. Methacrylated microfillers (Schott, 0.4 μm) were added to the resin mixture and blended in a speedmixer (DAC 150 FVZ, Flakteck) to ensure uniform dispersion of the fillers.
CuAAC composites preparation
Stoichiometric mixtures of di-azide and tri-alkyne (a mole ratio of 1:1 to azide:alkyne) with 2 mole percentage of CuCl2[PMDETA] per functionality, 0.6 wt% CQ, 1.6 wt% EDAB were prepared. Methanol was added to homogenize the mixture and later removed in vacuo. The residual solvent content of each resin was verified by 1H-NMR using a Bruker Avance-III 400 MHz spectrometer with 16 scans and 1 s of relaxation time. For all experiments, the residual methanol concentration was <0.5 wt%. Alkyne-functionalized microfillers (Schott, 0.4 μm) were added to the resin mixture and blended in a speedmixer (DAC 150 FVZ, Flakteck) to ensure uniform dispersion of fillers.
Filler functionalization [47]
Silica particles (20 g; Schott, 0.4 μm) were placed in a glass tube and dried for 3.5 h at 180 °C in vacuo using a Buchi drying glass oven. To a round bottom flask containing 400 ml of anhydrous toluene, 6 ml of prop-2-yn-1-yl (3-(triethoxysilyl)propyl)carbamate (Si-AK), 0.4 g of propylamine, and 20 g of the dried microparticles were added while purging under nitrogen. The reaction mixture was stirred at 75 °C overnight to accommodate the silanization process. The liquid suspension was centrifuged 4 times with excess toluene (3000 rpm for 5 min) followed by centrifuging 3 times with methylene chloride in dilution (3000 rpm for 15 min). The solid pellets were collected after each centrifugation cycle and redispersed in excess solvent for the additional cycles. The final washed filler particles were dried in vacuo at 70 °C overnight and 100 °C for 2 h. Thermogravimetry (TG) and diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) were used to analyze the functionalized particles (see Fig. S1, Fig. S2).
Fourier Transform Infrared Spectroscopy
An FTIR spectrometer (Nicolet 6700) connected to a tensometer via fiber optic cables was used to monitor the real-time polymerization kinetics in concert with stress measurements. Samples were placed between two cylindrical quartz rods, and 600 mW cm−2 of light was irradiated from the bottom rod using a light guide connected to a mercury lamp (Acticure 4000, EXFO) with 400–500 nm bandgap filter. A radiometer (Model 100, Demetron Research) was used to measure the light intensity transmitted from the end of the quartz rod. The overtone signal of the alkyne was monitored between 6538–6455 cm−1, and the overtone signal of the methacrylate was measured between 6250–6096 cm−1.
Polymerization shrinkage stress measurement
A tensometer (American Dental Association Health Foundation, ADAHF-PRC) was utilized to monitor polymerization post-gel shrinkage stress using cantilever beam deflection theory [48] with in situ polymerization kinetic measurements [49]. The sample (6mm in diameter, 1mm in thickness) was placed in a cavity between two cylindrical quartz rods, which were previously treated with silane (methacrylate silane coupling agent or Si-AK) to improve the interfacial adhesion, and sealed with a polytetrafluoroethylene (PTFE) sleeve to prevent oxygen inhibition. 600 mW cm−2 of light was transmitted through the bottom rod using a light guide connected to a mercury lamp (Acticure 4000, EXFO) with a 400–500 nm bandgap filter. The deflection of the aluminum beam, caused by a tensile force exerted with bonded sample shrinkage, was measured with a linear variable differential transformer (LVDT) and converted to stress based upon beam calibration constant and cross-sectional area of the sample. A simultaneous measurement of functional group conversion with polymerization shrinkage stress by fiber optic cables was recorded for 15 min.
Dynamic mechanical analysis
A DMA Q800 (TA instruments) in multi-frequency-strain mode with a frequency of 1Hz and a heating rate of 3 °C min−1 was used to measure the storage modulus and the glass transition temperature (Tg), which was taken as the peak of the tan δ (a ratio of E″/E′: the storage and loss moduli) curve. An irradiance of ~300 mW cm−2 at 455 nm from a light emitting diode (LED Thorlabs) was directed to one side of the sample for 60 s at room temperature followed immediately by an analogous cure on the opposite side of the sample. The rectangular dimensions of each sample specimen were 0.25 × 5 × 25 mm (t × w × l). The samples were placed in the oven at 70 °C overnight and 150 °C for 1 h.
Three-point flexural test
Three-point bending (MTS 858 Mini Bionix II) with a strain rate of 1 mm min−1 and a span of 20 mm was used to obtain flexural modulus, flexural strength, and flexural toughness. Flexural modulus was calculated from the initial slope between 0.5 and 1% strain, and flexural strength was obtained using the following equation: , where F is the maximum load, L is the length of span, B is the width of the sample specimen, H is the height of the sample specimen [50]. Photo-activation was conducted at ~300 mW cm−2 of 455 nm light from an LED (Thorlabs). The irradiation was conducted for 60 s at room temperature on one side of the sample followed by immediate inversion to cure for 60 s on the opposite side of the sample. The samples were placed in the oven at 70 °C overnight and 150 °C for 30 min and used for the experiment within 1 day after the thermal cure. The rectangular dimensions of each sample specimen were 2 × 2 × 25 mm (t × w × l).
Viscosity measurement
A rheometer (ARES, TA Instruments) was used to measure the viscosity of the monomers in the torque ranges from 2·10−6 to 2·10−2 N m, using 200 μm thick samples placed between 8 mm diameter quartz plates.
Thermal gravimetric analysis
Thermogravimetric analysis (TGA Pyris 1, Perkin Elmer) was used to analyze the functionalized fillers. Each sample was run in a nitrogen atmosphere (20 ml min−1) from 50 °C to 850 °C at a heating rate of 10 °C min−1.
Statistical analysis
Statistical analysis of the experiments was performed via one-way analysis of variance (ANOVA), and multiple pair-wise comparisons were conducted via a Tukey’s post-hoc test with a significance level of 0.05. The number of repetitions for each experiment were as follows: dynamic mechanical analysis (n=3), polymerization shrinkage stress (n=3), three-point flexural test (n=5), and viscosity measurement (n=3).
RESULTS AND DISCUSSION
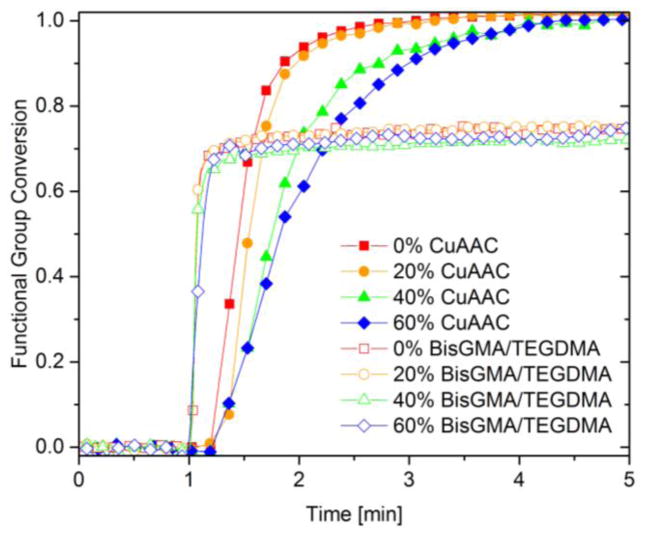
In situ polymerization kinetics for the CuAAC composites and BisGMA-based composites are presented with varying microfiller loadings using 600 mW cm−2 of 400–500 nm light for 4 min irradiation at ambient temperature (Fig. 2). For all filler loadings (0–60%), the CuAAC composites achieved quantitative conversion within 3 min although the presence of 40–60% filler in the composites did lead to a slower polymerization rate. This outcome may be due to the CuAAC resin being more sensitive than the BisGMA/TEGDMA resin to filler-induced effects such as light attenuation, increased viscosity, and a reduced reaction exotherm due to the thermal mass of the filler. The effects of microfiller loading on the initial composite viscosity and the reaction exotherm generated during polymerization are depicted in Table 1 and Fig. 3, respectively. For both the CuAAC and BisGMA/TEGDMA composites, the viscosity of the unpolymerized composite paste as measured via rheometry dramatically increased as the filler concentration increased from 40 to 60% (Table 1). Although the CuAAC composites at high loadings were less fluid than the analogous BisGMA/TEGDMA composite at a shear rate of 50 s−1, the CuAAC composites displayed more dramatic non-Newtonian shear thinning behavior over a range of shear rates (10–100 s−1) as compared with BisGMA/TEGDMA, leading to less viscous and more flowable composites at a high shear rates (Fig. S3).
Figure 2.
Polymerization kinetics of CuAAC composites (closed symbol) and BisGMA/TEGDMA composites (open symbol) with variable microfiller loadings including 0% (red square), 20% (orange circle), 40% (green triangle), and 60% (blue rhombus), as measured by FTIR. Each mixture was irradiated for 4 min at ambient temperature with 600 mW cm−2 of 400–500 nm light following 1 min in the dark to establish a baseline.
Table 1.
The viscosity of unpolymerized CuAAC and BisGMA/TEGDMA composite pastes with variable microfiller loading including 0%, 20%, 40%, and 60%, as measured at a shear rate of 50 s−1. The letters indicate statistically significant differences (p<0.05) via a one-way ANOVA and a Tukey’s post-hoc test.
| BisGMA/TEGDMA | CuAAC | |||||||
|---|---|---|---|---|---|---|---|---|
| Filler loadings [wt%] | 0 | 20 | 40 | 60 | 0 | 20 | 40 | 60 |
| Viscosity [Pa·s] | 1.9±0.03C | 3.2±0.03C | 6.6±0.03C | 40.4±2.3B | 0.8±0.02C | 1.7±0.03C | 6.1±0.06C | 96±10A |
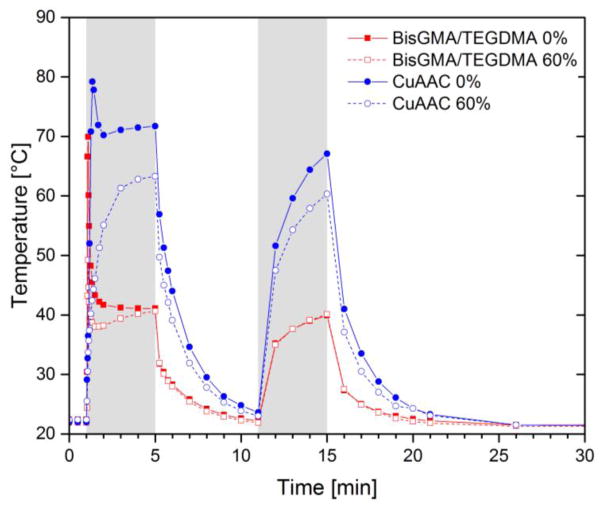
Figure 3.
Dynamic temperature rise for the CuAAC resin and composite (blue closed/open circle) and for the BisGMA/TEGDMA resin and composite (red closed/open square) as measured during polymerization by a digital thermometer. The same reaction conditions were used as described in Fig. 2: each mixture was irradiated for 4 min at ambient temperature with 600 mW cm−2 of 400–500 nm light following 1 min in the dark to establish a baseline. Each sample was exposed to the same light again for 4 min after 6 min of dark polymerization.
To monitor the temperature change in the sample during polymerization due to the exothermic reaction and relatively high light intensity utilized, a micro-tip thermocouple connected to a digital thermometer (Omega, HH11) was inserted in the middle of each sample (6mm in diameter, 1mm in thickness), and the temperature was recorded as a function of time (Fig. 3). Simultaneously, in situ real-time FTIR measurement of the functional group conversion was performed on each sample to monitor the polymerization (Fig. S4). To standardize the sample size and allow simultaneous in situ kinetic measurements, each sample was placed in the tensometer between two quartz rods having 6 mm in diameter and covered with a PTFE sleeve to prevent oxygen inhibition. Although this approach enabled precise control of the sample size, the PTFE sleeve acts as an insulator that limits heat dissipation. For both CuAAC and BisGMA/TEGDMA resins/composites, only 0 and 60% filler concentrations were chosen for the study. Each sample was exposed to 600 mW cm−2 of 400–500 nm light for 4 min following 1 min in the dark. A second identical irradiation was performed again on the photo-cured sample after the temperature of each sample had reequilibrated to ambient following the polymerization. Specifically, the specimens were irradiated for 4 min following a 1 min delay interval, and then at 11 min, the specimen was exposed for an additional 4 min as presented in Fig. 3. During the second light exposure, no substantial polymerization occurred for either the CuAAC or BisGMA/TEGDMA systems as indicated by the FTIR measurement; therefore, the temperature rise associated with the secondary exposure occurs primarily from absorption of light from the curing light.
During the first light exposure, both the CuAAC resin and the 60% loaded composite yielded quantitative conversion within 3 min (Fig. S4). The unfilled CuAAC resin showed a sharp thermal increase with a maximum temperature rise of ~55 °C during the first 30 s of light exposure. Alternatively, no or significantly less temperature change occurred for the CuAAC composite due to the presence of inorganic filler that reduced the reactive functional group density and increased the heat capacity. Additionally, the slower polymerization rate in CuAAC composites mitigated the accumulated heat rise. This filler-based reduction in the temperature increase during polymerization was more apparent in the Bis-GMA/TEGDMA system. As shown in Fig. S4, both the BisGMA/TEGDMA resin and the 60% composite materials yielded analogous polymerization rates and final maximum conversion within 20 s; however, BisGMA/TEGDMA resins developed approximately 20 °C higher maximum temperature increase during the polymerization compared to the composite, which is indicative of the exotherm modulation connected with filler incorporation. During the second light exposure as shown between 11 and 15 min in Fig. 3, the CuAAC resin and composite exhibited more than twice the thermal increase due to light in contrast to the BisGMA/TEGDMA resin and composite. This exotherm enabled maintaining the reaction temperature close to Tg for the CuAAC system during the light irradiation and enhancing mobility of these materials to achieve quantitative conversion, where a Tg at ~99% conversion for CuAAC resin and composite was found to be approximately 64 °C (Table 2).
Table 2.
Storage modulus at 40 °C and glass transition temperature (Tg) for CuAAC composites and BisGMA/TEGDMA composites with varying microfiller loadings as measured by DMA. For a representative DMA plot of 60% composites, see Fig. S5. Within each row, the letters indicate statistically significant differences (p<0.05) via a one-way ANOVA and a Tukey’s post-hoc test.
| BisGMA/TEGDMA | CuAAC | |||||
|---|---|---|---|---|---|---|
| Filler loading [%] | 20 | 40 | 60 | 20 | 40 | 60 |
| E′ @ 40 °C [GPa] | 2.5±0.3C | 3.7±0.8C | 5.7±0.7B | 3.0±0.2C | 5.3±0.3B | 7.0±0.3A |
| Tg [°C] | 151±2A | 154±3A | 157±5A | 63±2B | 64±1B | 64±1B |
Thermo-mechanical properties of both CuAAC and BisGMA/TEGDMA composites, such as Tg and storage modulus at 40 °C, are addressed in Table 2 with varying microfiller loadings. Each sample was photo-cured using ~300 mW cm−2 of 455 nm LED light for 1 min on each side at ambient temperature and then post-cured at 70 °C overnight followed by 150 °C for 1 h, to ensure the maximum degree of polymerization in both the experimental and control systems. The CuAAC and BisGMA/TEGDMA composites with different filler loadings had statistically similar Tg values, suggesting that the functional group conversion within each composite system is nearly equivalent despite the different filler loadings. This behavior indicates that the progressive enhancement in storage modulus with increasing filler content is almost exclusively based on the increased filler incorporation. Despite the modest glass transition temperature of the CuAAC composites as compared with BisGMA/TEGDMA composites, the CuAAC materials generated slightly higher or analogous glassy modulus as measured at 40 °C at equivalent filler loadings. This behavior arises at least in part because the CuAAC systems had much narrower glass transition widths than their methacrylate counterparts (Fig. S5) [29,39].
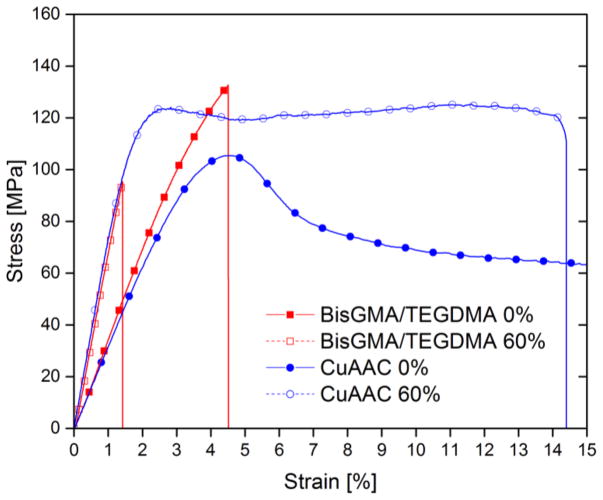
Mechanical properties including flexural modulus, flexural strength, and flexural toughness of CuAAC and BisGMA/TEGDMA resins/composites were further analyzed via three-point bend tests performed on a universal testing machine (MTS) and presented in Fig. 4 and Table 3. Fig. 4 displays a representative stress-strain curve of the CuAAC resin and 60% composite in comparison with the BisGMA/TEGDMA resin and 60% composite. The functional group conversion was monitored via FTIR prior to photopolymerization and after thermal cure as described in the Experimental Section and presented in Fig. S6. As the filler concentration increased from 0 to 60% in both the CuAAC and BisGMA/TEGDMA materials, the flexural modulus (or Young’s modulus) calculated from the initial slope of the stress-strain curve dramatically increased from approximately 3 GPa to 6 GPa (Table 3). However, the flexural strength of the CuAAC and BisGMA/TEGDMA composites at varying filler loadings of 20–60% was maintained around 100 MPa, indicative of a well-bonded interface between functional groups on the fillers and resin matrices. In spite of and due to the improved elastic modulus of the composites, the flexural toughness of the BisGMA/TEGDMA composites with 60% fillers yielded only 1.1±0.3 MJ m−3, which was 3–4 times lower than the BisGMA/TEGDMA resin system. Contrary to the low strain failure of BisGMA/TEGDMA composites, about half of the CuAAC composite samples at 60% filler fractured above 13% strain. In addition, only a few of the experimental specimens with 60 wt% filler displayed even a small crack on the bottom of the sample where tensile forces develop during the bending test, and no fracture occurred in any of the CuAAC samples with less than 60% filler loading. Therefore, we calculated energy absorbed when strained to 11% where samples still maintain horizontal contact with the fixture as a relative flexural toughness for the CuAAC resin and composites. Despite their higher modulus, the CuAAC composite consisting of 60% filler exhibited even higher absorbed energy (10.0±1 MJ m−3) when strained to 11% as compared with the unfilled CuAAC resin (8.0±0.1 MJ m−3) at equivalent strain. This combination of excellent ductility of the CuAAC thermosetting composites along with improved stiffness and strength suggests an interesting potential for high durability restorative materials.
Figure 4.
A representative stress-strain curve for the CuAAC resin denoted as CuAAC 0% (blue closed circle), CuAAC composite denoted as CuAAC 60% (blue open circle), dimethacrylate-based resin denoted as BisGMA/TEGDMA 0% (red closed square), and dimethacrylate-based composite denoted as BisGMA/TEGDMA 60% (red open square) via three-point bending on a universal testing machine. Due to geometric limits on the instrument, reliable flexural data was collected up to 11% strain if the samples had not yet broken which was the case for most of the CuAAC based resins and composites.
Table 3.
Comparison of resin and composite flexural modulus, flexural strength, flexural toughness (or work of fracture), and energy required to strain the experimental materials to 11% without fracture from the three-point bend testing. Within each category of columns, the letters indicate statistically significant differences (p<0.05) via a one-way ANOVA and a Tukey’s post-hoc test.
| BisGMA/TEGDMA | CuAAC | |||||
|---|---|---|---|---|---|---|
| Filler loading [%] | Flexural modulus [GPa] | Flexural strength [MPa] | Toughness [MJ m−3] | Flexural modulus [GPa] | Flexural strength [MPa] | Energy to strain 11% [MJ m−3] |
| 0 | 3.5±0.5D | 131±19A | 3.7±0.8C | 3.1±0.04D | 105±1A,B | 8.0±0.1B |
| 20 | 3.9±0.3C,D | 98±9B | 1.8±0.4D | 3.7±0.2D | 96±5B | 8.0±0.4B |
| 40 | 4.9±0.3B | 89±23B | 1.1±0.6D | 4.5±0.3B,C | 99±6B | 8.8±0.5B |
| 60 | 6.6±0.3A | 101±18B | 1.1±0.3D | 6.1±0.7A | 107±9A,B | 10±1A |
Incorporation of fillers not only enhances the mechanical performance but also reduces shrinkage stress developed during polymerization. Table 4 summarizes the final polymerization shrinkage stress obtained after 15 min of reaction time and corresponding to functional group conversions at varying filler loadings for both the CuAAC and BisGMA/TEGDMA systems. To release any residual heat accumulated during the polymerization from both the reaction exotherm and light absorption as described in Fig. 3, the final polymerization shrinkage stress of each sample was taken after 15 min although no substantial conversion change occurred after 4 min of light exposure. When the filler loading was increased from 0 to 60% in both the CuAAC and BisGMA/TEGDMA samples, approximately 20% reduction in final shrinkage stress was observed in 60% filled CuAAC and BisGMA/TEGDMA composites in contrast to resins at relatively equivalent conversion (Table 4). It appears that the reduced polymerization shrinkage associated with the decrease in reactive functional group density in composites as compared with the unfilled resins leads to lower polymerization shrinkage stress despite the higher elastic moduli (Table 3).
Table 4.
Polymerization shrinkage stress for CuAAC composites and BisGMA/TEGDMA composites with varying microfiller loadings taken after 15 min of reaction time as a function of the functional group conversion using a tensometer coupled with the FTIR. Each mixture was irradiated for 4 min at ambient temperature with 600 mW cm−2 of 400–500nm light. Within each category of columns, the letters indicate statistically significant differences (p<0.05) via a one-way ANOVA and a Tukey’s post-hoc test.
| BisGMA/TEGDMA | CuAAC | |||
|---|---|---|---|---|
| Filler loading [%] | Conversion [%] | Shrinkage stress [MPa] | Conversion [%] | Shrinkage stress [MPa] |
| 0 | 76±4B | 1.39±0.05A | ~99A | 0.60±0.02D |
| 20 | 73±4B | 1.35±0.08A,B | ~99A | 0.60±0.09D |
| 40 | 72±2B | 1.22±0.05B,C | ~99A | 0.64±0.06D |
| 60 | 77±4B | 1.10±0.04C | ~99A | 0.43±0.01E |
CONCLUSIONS
Triazole-containing thermosetting composites formed via photo-CuAAC polymerization are high energy-absorbing glassy materials with sufficient mechanical stiffness and strength, while generating low polymerization shrinkage stress. Although high loadings of inorganic microparticles in the CuAAC composites leads to a slower rate of photo-CuAAC polymerization, quantitative conversion within 3 min of visible light irradiation with further reduced polymerization shrinkage stress was achieved for highly filled CuAAC composites. The addition of filler also provides a lower heat of reaction, which otherwise is significantly greater than methacrylate-based resin system. Moreover, CuAAC composites exhibit remarkable ductility upon deformation without fracture while achieving analogous elastic moduli as compared with BisGMA-based composites, which provides merits associated with high toughness dental restorative materials.
Supplementary Material
Figure 1.
Monomer libraries of difunctional azides BZ-AZ, trifunctional alkynes AK, difunctional methacrylates BisGMA, TEGDMA, photoinitiator CQ, co-initiator EDAB, copper catalyst CuCl2[PMDETA], alkyne silane Si-AK as previously reported [29].
Acknowledgments
The authors acknowledge financial support from the National Institutes of Health (NIH:5U01DE023774) and the National Science Foundation (NSF:CHE1214109). The authors thank Dr. Parag K. Shah for the help with TGA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cramer NB, Stansbury JW, Bowman CN. Recent advances and developments in composite dental restorative materials. J Dent Res. 2011;90:402–16. doi: 10.1177/0022034510381263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anseth KS, Newman SM, Bowman CN. Biopolym. II. Springer; Berlin Heidelberg: 1995. Polymeric Dental Composites : Properties and Reaction Behavior of Multimethacrylate Dental Restorations; pp. 177–217. [DOI] [Google Scholar]
- 3.Ferracane JL. Current trends in dental composites. Crit Rev Oral Biol Med. 1995;6:302–18. doi: 10.1177/10454411950060040301. [DOI] [PubMed] [Google Scholar]
- 4.Anusavice KJ. Phillips’ Science of dental materials. 11. Elsevier Health Sciences; 2003. [Google Scholar]
- 5.Moszner N, Salz U. New developments of polymeric dental composites. Prog Polym Sci. 2001;26:535–76. doi: 10.1016/S0079-6700(01)00005-3. [DOI] [Google Scholar]
- 6.Braga RR, Ballester RY, Ferracane JL. Factors involved in the development of polymerization shrinkage stress in resin-composites: A systematic review. Dent Mater. 2005;21:962–70. doi: 10.1016/j.dental.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 7.Habib E, Wang R, Wang Y, Zhu M, Zhu XX. Inorganic Fillers for Dental Resin Composites: Present and Future. ACS Biomater Sci Eng. 2016;2:1–11. doi: 10.1021/acsbiomaterials.5b00401. [DOI] [PubMed] [Google Scholar]
- 8.Shah PK, Stansbury JW. Role of filler and functional group conversion in the evolution of properties in polymeric dental restoratives. Dent Mater. 2014;30:586–93. doi: 10.1016/j.dental.2014.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drummond JL. Degradation, Fatigue, and Failure of Resin Dental Composite Materials. J Dent Res. 2008;87:710–9. doi: 10.1177/154405910808700802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Santerre JP, Shajii L, Leung BW. Relation of dental composite formulations to their degradation and the release of hydrolyzed polymeric-resin-derived products. Crit Rev Oral Biol Med. 2001;12:136–51. doi: 10.1177/10454411010120020401. [DOI] [PubMed] [Google Scholar]
- 11.Klapdohr S, Moszner N. New Inorganic Components for Dental Filling Composites. Monatshefte Für Chemie. 2005;136:21–45. doi: 10.1007/s00706-004-0254-y. [DOI] [Google Scholar]
- 12.Lee J, Um C, Lee I. Rheological properties of resin composites according to variations in monomer and filler composition. Dent Mater. 2006;22:515–26. doi: 10.1016/j.dental.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 13.Karabela MM, Sideridou ID. Effect of the structure of silane coupling agent on sorption characteristics of solvents by dental resin-nanocomposites. Dent Mater. 2008;24:1631–9. doi: 10.1016/j.dental.2008.02.021. [DOI] [PubMed] [Google Scholar]
- 14.Matinlinna JP, Lassila LV, Özcan M, Yli-Urpo A, Vallittu PK. An introduction to silanes and their clinical applications in dentistry. Int J Prosthodont. 2004;17:155–64. [PubMed] [Google Scholar]
- 15.Ji X, Hampsey JE, Hu Q, He J, Yang Z, Lu Y. Mesoporous silica-reinforced polymer nanocomposites. Chem Mater. 2003:3656–62. doi: 10.1021/cm0300866. [DOI] [Google Scholar]
- 16.Samuel SP, Li S, Mukherjee I, Guo Y, Patel AC, Baran G, et al. Mechanical properties of experimental dental composites containing a combination of mesoporous and nonporous spherical silica as fillers. Dent Mater. 2009;25:296–301. doi: 10.1016/j.dental.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 17.García AH, Lozano MAM, Vila JC, Escribano AB, Galve PF. Composite resins. A review of the materials and clinical indications. Med Oral Patol Oral Cir Bucal. 2006;11:215–20. [PubMed] [Google Scholar]
- 18.Matsukawa S, Hayakawa T, Nemoto K. Development of high-toughness resin for dental applications. Dent Mater. 1994;10:343–6. doi: 10.1016/0109-5641(94)90057-4. [DOI] [PubMed] [Google Scholar]
- 19.Goldberg M. In vitro and in vivo studies on the toxicity of dental resin components : a review. Clin Oral Investig. 2008;12:1–8. doi: 10.1007/s00784-007-0162-8. [DOI] [PubMed] [Google Scholar]
- 20.Busscher HJ, Rinastiti M, Siswomihardjo W, van der Mei HC. Biofilm Formation on Dental Restorative and Implant Materials. J Dent Res. 2010;89:657–65. doi: 10.1177/0022034510368644. [DOI] [PubMed] [Google Scholar]
- 21.Abu-Elenain DA, Lewis SH, Stansbury JW. Property evolution during vitrification of dimethacrylate photopolymer networks. Dent Mater. 2013;29:1173–81. doi: 10.1016/j.dental.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mantri SP, Mantri SS. Management of Shrinkage Stresses in Direct Restorative Light-Cured Composites: A Review. J Esthet Restor Dent. 2013;25:305–13. doi: 10.1111/jerd.12047. [DOI] [PubMed] [Google Scholar]
- 23.Weinmann W, Thalacker C, Guggenberger R. Siloranes in dental composites. Dent Mater. 2005;21:68–74. doi: 10.1016/j.dental.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 24.Soh MS, Yap AUJ, Sellinger A. Physicomechanical evaluation of low-shrinkage dental nanocomposites based on silsesquioxane cores. Eur J Oral Sci. 2007;115:230–8. doi: 10.1111/j.1600-0722.2007.00449.x. [DOI] [PubMed] [Google Scholar]
- 25.Eick JD, Kotha SP, Chappelow CC, Kilway KV, Giese GJ, Glaros AG, et al. Properties of silorane-based dental resins and composites containing a stress-reducing monomer. Dent Mater. 2007;23:1011–7. doi: 10.1016/j.dental.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 26.Contreras PP, Tyagi P, Agarwal S. Low volume shrinkage of polymers by photopolymerization of 1,1-bis(ethoxycarbonyl)-2-vinylcyclopropanes. Polym Chem. 2015;6:2297–304. doi: 10.1039/C4PY01705F. [DOI] [Google Scholar]
- 27.Lu H, Carioscia Ja, Stansbury JW, Bowman CN. Investigations of step-growth thiol-ene polymerizations for novel dental restoratives. Dent Mater. 2005;21:1129–36. doi: 10.1016/j.dental.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 28.Podgórski M, Becka E, Claudino M, Flores A, Shah PK, Stansbury JW, et al. Ester-free thiol-ene dental restoratives-Part A : resin development. Dent Mater. 2015;31:1255–62. doi: 10.1016/j.dental.2015.08.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song HB, Sowan N, Shah PK, Baranek A, Flores A, Stansbury JW, et al. Reduced shrinkage stress via photo-initiated copper(I)-catalyzed cycloaddition polymerizations of azide-alkyne resins. Dent Mater. 2016;32:1332–42. doi: 10.1016/j.dental.2016.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee TY, Carioscia J, Smith Z, Bowman CN. Thiol-Allyl Ether-Methacrylate Ternary Systems. Evolution Mechanism of Polymerization-Induced Shrinkage Stress and Mechanical Properties Macromolecules. 2007;40:1473–9. doi: 10.1021/ma0624954. [DOI] [Google Scholar]
- 31.Cramer NB, Couch CL, Schreck KM, Carioscia JA, Boulden JE, Stansbury JW, et al. Investigation of thiol-ene and thiol-ene-methacrylate based resins as dental restorative materials. Dent Mater. 2010;26:21–8. doi: 10.1016/j.dental.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ye S, Cramer NB, Smith IR, Voigt KR, Bowman CN. Reaction Kinetics and Reduced Shrinkage Stress of Thiol-Yne-Methacrylate and Thiol-Yne-Acrylate Ternary Systems. Macromolecules. 2011;44:9084–90. doi: 10.1021/ma2018809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leung D, Bowman CN. Reducing Shrinkage Stress of Dimethacrylate Networks by Reversible Addition–Fragmentation Chain Transfer. Macromol Chem Phys. 2012;213:198–204. doi: 10.1002/macp.201100402. [DOI] [Google Scholar]
- 34.Park H, Kloxin CJ, Fordney MF, Bowman CN. Stress relaxation of trithiocarbonate-dimethacrylate-based dental composites. Dent Mater. 2012;28:888–93. doi: 10.1016/j.dental.2012.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park HY, Kloxin CJ, Scott TF, Bowman CN. Covalent adaptable networks as dental restorative resins: Stress relaxation by addition-fragmentation chain transfer in allyl sulfide-containing resins. Dent Mater. 2010;26:1010–6. doi: 10.1016/j.dental.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kloxin CJ, Scott TF, Bowman CN. Stress Relaxation via Addition-Fragmentation Chain Transfer in a Thiol-ene Photopolymerization. Macromolecules. 2009;42:2551–6. doi: 10.1021/ma802771b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Adzima BJ, Tao Y, Kloxin CJ, DeForest Ca, Anseth KS, Bowman CN. Spatial and temporal control of the alkyne-azide cycloaddition by photoinitiated Cu(II) reduction. Nat Chem. 2011;3:256–9. doi: 10.1038/nchem.980. [DOI] [PubMed] [Google Scholar]
- 38.Gong T, Adzima BJ, Baker NH, Bowman CN. Photopolymerization reactions using the photoinitiated copper (I)-catalyzed azide-alkyne cycloaddition (CuAAC) reaction. Adv Mater. 2013;25:2024–8. doi: 10.1002/adma.201203815. [DOI] [PubMed] [Google Scholar]
- 39.Baranek A, Song HB, McBride M, Finnegan P, Bowman CN. Thermomechanical Formation–Structure–Property Relationships in Photopolymerized Copper-Catalyzed Azide–Alkyne (CuAAC) Networks. Macromolecules. 2016;49:1191–200. doi: 10.1021/acs.macromol.6b00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sandmann B, Happ B, Hager MD, Vitz J, Rettler E, Burtscher P, et al. Efficient Cu(I) acetate-catalyzed cycloaddition of multifunctional alkynes and azides: From solution to bulk polymerization. J Polym Sci Part A Polym Chem. 2014;52:239–47. doi: 10.1002/pola.26996. [DOI] [Google Scholar]
- 41.Kolb HC, Finn MG, Sharpless KB. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew Chemie - Int Ed. 2001;40:2004–21. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 42.Barner-Kowollik C, Du Prez FE, Espeel P, Hawker CJ, Junkers T, Schlaad H, et al. “Clicking” polymers or just efficient linking: What is the difference? Angew Chemie - Int Ed. 2011;50:60–2. doi: 10.1002/anie.201003707. [DOI] [PubMed] [Google Scholar]
- 43.Song HB, Baranek A, Bowman CN. Kinetics of bulk photo-initiated copper(I)-catalyzed azide-alkyne cycloaddition (CuAAC) polymerizations. Polym Chem. 2016;7:603–12. doi: 10.1039/C5PY01655J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shete AU, El-Zaatari BM, French JM, Kloxin CJ. Blue-light activated rapid polymerization for defect-free bulk Cu(I)-catalyzed azide-alkyne cycloaddition (CuAAC) crosslinked networks. Chem Commun. 2016;52:10574–7. doi: 10.1039/c6cc05095f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sandmann B, Happ B, Vitz J, Hager MD, Burtscher P, Moszner N, et al. Photoinduced polyaddition of multifunctional azides and alkynes. Polym Chem. 2013;4:3938–42. doi: 10.1039/c3py00356f. [DOI] [Google Scholar]
- 46.Bräse S, Gil C, Knepper K, Zimmermann V. Organic azides: An exploding diversity of a unique class of compounds. Angew Chemie - Int Ed. 2005;44:5188–240. doi: 10.1002/anie.200400657. [DOI] [PubMed] [Google Scholar]
- 47.Podgórski M, Becka E, Claudino M, Flores A, Shah PK, Stansbury JW, et al. Ester-free thiol–ene dental restoratives—Part B: composite development. Dent Mater. 2015;31:1263–70. doi: 10.1016/j.dental.2015.08.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lu H, Stansbury JW, Dickens SH, Eichmiller FC, Bowman CN. Probing the origins and control of shrinkage stress in dental resin-composites: I. Shrinkage stress characterization technique. J Mater Sci Mater Med. 2004;15:1097–103. doi: 10.1023/B:JMSM.0000046391.07274.e6. [DOI] [PubMed] [Google Scholar]
- 49.Lu H, Stansbury JW, Dickens SH, Eichmiller FC, Bowman CN. Probing the origins and control of shrinkage stress in dental resin composites. II. Novel method of simultaneous measurement of polymerization shrinkage stress and conversion. J Biomed Mater Res Part B Appl Biomater. 2004;71:206–13. doi: 10.1002/jbm.b.30088. [DOI] [PubMed] [Google Scholar]
- 50.Rodrigues SA, Junior, Zanchi CH, de Carvalho RV, Demarco FF. Flexural strength and modulus of elasticity of different types of resin-based composites. Braz Oral Res. 2007;21:16–21. doi: 10.1590/S1806-83242007000100003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.