Abstract
Rationale: Sarcoidosis is a systemic inflammatory disorder characterized by distinct up-regulation of Th1 cytokines, such as tumor necrosis factor (TNF)-α and IL-12. The mechanism underlying this up-regulation remains unclear. Recognition of microbial moieties through Toll-like or Nod-like receptors evokes sequential activation of mitogen-activated protein kinases (MAPKs), which plays a role in Th1-immune response.
Objectives: To test the hypothesis that dysregulation in MAPK signaling in response to microbial stimulation is important in mediating Th1 response in sarcoidosis.
Methods: Ex vivo cultured bronchoalveolar lavage (BAL) cells isolated from patients with sarcoidosis and control subjects were stimulated with low-dose Toll-like receptor 4 (TLR4) and nucleotide-binding oligomerization domain 1 (NOD1) ligands as a model of microbial stimulation, and MAPK signaling and inflammatory response were analyzed.
Measurements and Main Results: BAL cells from patients with sarcoidosis exhibited higher basal p38 activity, greater p38 phosphorylation, and more robust production of TNF-α and IL-12/IL-23p40 on stimulation with NOD1 and TLR4 agonists than cells isolated from control subjects. In contrast, control BAL cells had greater basal extracellular signal–regulated kinase (ERK) activity and NOD1 and TLR4 agonists preferentially activated the ERK pathway. Inhibition of p38, but not ERK, attenuated production of both IL12/IL23p40 and TNF-α. Interestingly, stimulation of cells from patients with sarcoidosis with either NOD1 or TLR4 ligand failed to induce MAPK phosphatase 1 (MKP-1). Adenovirus-mediated overexpression of MKP-1 attenuated p38 activation and decreased the production of IL12/IL23p40 and TNF-α in sarcoid BAL cells.
Conclusions: Our results suggest that enhanced p38 signaling in response to microbial products is caused by abnormal regulation of MKP-1 and contributes to heightened inflammation in sarcoidosis.
Keywords: sarcoidosis, MAP kinase, p38 MAP kinase, dual specificity phosphatase, cytokines
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
Current knowledge suggests that the immunopathogenic mechanisms underlying sarcoidosis are likely to be shaped by both environmental and genetic factors, but no unifying pathogen has yet been identified. More importantly, the signaling pathway(s) underlying chronic Th1-mediated pathology in sarcoidosis are unknown. The pattern-recognition receptors, nucleotide-binding oligomerization domain (NOD)-like receptors and Toll-like receptors, have been shown to play a role in shaping innate and adaptive immunity.
What This Study Adds to the Field
We show that sustained activation of p38 MAPK in response to specific microbial moieties, such as NOD1 or TLR4 agonists, and a lack of negative feedback loop through MKP1 lead to persistent inflammation. Overexpression of mitogen-activated protein kinase MKP1 or pharmacological inhibition of p38 activation abrogated NOD1/Toll-like receptor 4 mediated Th1 cytokine induction.
Sarcoidosis is a complex systemic disorder characterized by granuloma formation affecting barrier organs, such as lungs, skin, and eyes (1). Several studies have demonstrated elevated levels of predominantly Th1 cell cytokines, including IL-6, tumor necrosis factor (TNF)-α, and IL-12, in serum of patients with sarcoidosis. Furthermore, it has been shown that isolated peripheral blood monocytes (PBMCs) and alveolar macrophages (AMs) of patients with sarcoidosis produce greater amounts of Th1 cytokines ex vivo (2, 3). Several investigators have isolated bacteria such as Propionibacterium acnes and Mycobacterium tuberculosis from the tissues of these patients. Although the role of these microbes in disease pathogenesis remains unclear (4–6), its similarities to infectious granulomatous diseases and its association with major histocompatibility (MHC) loci suggest a major role for inciting microbial triggers. Nonetheless, how specific antigen signaling might lead to such an immune response and sustained inflammation is still largely unknown.
Recognition of microbial constituents occurs through leucine-rich repeated domains of pattern-recognition receptors, such as Toll-like receptors (TLRs) and Nod-like receptors (NLRs) (7). Recent findings suggest that both TLRs and NLRs contribute to the onset of antigen-specific immune responses (8). One important NLR, nucleotide-binding oligomerization domain 1 (NOD1), is activated by peptidoglycan fragments containing γ-D-glutamyl-meso-diaminopimelic acid (iE-DAP), a product of all gram-negative bacteria, including Mycobacteria, and some gram-positive bacteria (9). On microbial sensing, NOD1 homo-oligomerizes and recruits the serine-threonine kinase RICK (Rip-like interacting caspase-like apoptosis-regulatory protein kinase), leading to activation of nuclear factor-κB (NF-κB) and MAPKs with subsequent up-regulation of cytokine transcripts (10, 11). Aberrant sensing through the NOD1 receptor has been implicated in sarcoidosis: a Japanese study found a frequent 796 A-type allele in the NOD1 gene and impaired response to P. acnes in sarcoidosis (12). Activation of MAPKs is crucial for the syntheses of numerous cytokines and chemokines necessary for eradication of pathogens (13). Recognition of microbial components by TLRs and NLRs sequentially activate the MAPK cascades leading to dual phosphorylation at both threonine and tyrosine at the TXY motif of three principal kinases: p38, c-Jun N-terminal kinases (JNKs), and extracellular signal–regulated kinases (ERKs) (14, 15). Phosphorylation of the MAPKs by cognate MAPK kinases (MKKs) leads to conformational change of the MAPKs and stimulates their catalytic activities. For example, p38 is activated by dual phosphorylation at residues Thr180 and Tyr182, mediated by MKK3 and MKK6. Activated p38 not only plays a critical role in the transcription of IL-12, TNF-α, and IL-1 (IL-1α and IL-1β) genes but also is crucial for the production of many cytokines through altering the stability, transport, and translation of mRNA transcripts of those cytokines in response to different stimuli (13, 16, 17). There is compelling evidence that activated p38 plays an essential role in Th1 commitment. MAPKs are primarily deactivated through dephosphorylation by MAPK phosphatases (MKPs), a group of more than 10 dual-specificity phosphatases that dephosphorylate the regulatory residues of their targeted MAPKs (17, 18). It has been demonstrated that rapid induction of MKP-1 in immune cells deactivates p38 to restrain the excessive production of inflammatory cytokines (19, 20). Failure of adequate induction of MKPs may result in sustained phosphorylation of MAPKs and persistent inflammation and autoimmunity.
Although increased Th1-mediated cytokines in sarcoidosis have been well documented, the signaling pathway underlying this abnormality remains largely unknown. Based on these considerations we set out to investigate the effect of NOD1 and TLR4 ligation on cellular signaling in sarcoidosis. Using bronchoalveolar cells and PBMCs from control subjects and subjects with sarcoidosis, we show that stimulation of BAL cells of subjects with sarcoidosis with low-level NOD1 and/or TLR4 agonists evokes a sustained p38 phosphorylation without a significant MKP-1 induction. Pharmacological inhibition of p38 abrogates NOD1/TLR4-mediated Th1 cytokine induction. Moreover adenovirus-mediated overexpression of MKP-1 in BAL cells of patients attenuates p38 phosphorylation and TNF-α and IL-12 production. Thus, dysregulation of p38 activation and MKP-1 induction leads to sustained production of Th1 cytokines in sarcoidosis. Some of the results of these studies have been previously reported in abstract form (21).
METHODS
Chemicals
Chemicals were purchased from Sigma Chemical (St. Louis, MO) unless specified otherwise. Inhibitors PD98059 and SB203580 were purchased from Calbiochem (San Diego, CA). All ligands, LPS, and iE-DAP (D-g-Glu-mDAP), were purchased from InvivoGen (San Diego, CA). Anti–phospho ERK1/2, anti–phospho JNK1/2, total ERK, total JNK, α-tubulin, and β-actin antibodies were purchased from Cell Signaling Technology (Beverly, MA). Antibodies against total p38, MKP-1, MKP-3, and phospho p38 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Horseradish peroxidase–conjugated anti-mouse IgG, anti-goat IgG, and anti-Rabbit IgG antibodies were purchased from Cell Signaling Technology.
Study Design
The Committee for Investigations Involving Human Subjects at Wayne State University approved the protocol of obtaining alveolar macrophages by bronchoalveolar lavage (BAL) and blood monocytes by phlebotomy from control subjects and patients with sarcoidosis. Sarcoidosis diagnosis was made based on the guidelines of the American Thoracic Society (1). The criteria for enrollment in the diseased group were: presence of noncaseating granulomas in tissue biopsy of the lungs or mediastinal lymph nodes and compatible clinical phenotype. Subjects were excluded who (1) were smokers, (2) were receiving immune suppressive medication (defined as corticosteroid alone and/or in combination with immune modulatory medications), (3) had identification of positive microbial culture in routine laboratory examinations or viral infection; or (4) had known hepatitis or HIV infections. Criteria for enrollment in the control group were: (1) absence of any chronic respiratory diseases (defined as normal pulmonary function tests), (2) absence of noncaseating granuloma in tissue biopsy, (3) negative microbial culture, (4) lifetime nonsmoker, and (5) absence of HIV or hepatitis infection.
A total of 61 subjects underwent bronchoscopy with tissue biopsies and BAL. Among those, 34 cases were diagnosed to have noncaseating granuloma on their lung tissue biopsies with negative culture results for fungal and mycobacterial pathogens. Three patients whose bronchoscopically obtained tissues were nondiagnostic underwent mediastinoscopy with lymph node excisions confirming the presence of noncaseating granulomas and were also included in the study group, making a total of 37 patients with sarcoidosis in the disease group. Sixteen out of 61 cases who underwent bronchoscopy with BAL and had negative tissue biopsies and absence of sarcoid phenotype served as the control group. The rest of the assessed patients either had positive microbial cultures or had alternative diagnoses and were excluded from the study. The medical records of all patients were reviewed, and data regarding demographics, radiography stages, pulmonary function tests, and organ involvements were recorded. Of note, due to restricted use of human materials each subject underwent only one-time bronchoscopy with BAL collection.
BAL and the Preparation of Cell Suspensions
Alveolar macrophages were obtained during BAL during bronchoscopy after administration of local anesthesia and before tissue biopsies. Briefly, a total of 150 to 200 ml of sterile saline solution was injected via fiberoptic bronchoscopy; the BAL fluid was retrieved, measured, and centrifuged. Cells recovered from the BAL fluid were washed three times with phosphate-buffered saline (PBS), resuspended in endotoxin-free RPMI 1640 medium (Sigma) supplemented with l-glutamine, 80 μg/ml of gentamicin, and 1% fetal calf serum (Sigma), and then counted. Macrophages, lymphocytes, neutrophils, and eosinophils on cytocentrifuged smears stained with Wright-Giemsa were differentially counted, for a total count of 300 cells, according to morphological criteria. Cells were cultured in special nonadherent plates from Costar (Lowell, MA) to minimize the effect of adherence on signaling.
Separation of Adherent from Nonadherent Cells
BAL cells were suspended in RPMI 1640 medium and were cultured on adherent plates for 1 hour at 37°C in air containing 5% CO2. Nonadherent cells were removed by aspiration. Adherent plates were washed several times and the supernatant collected. Supernatants containing nonadherent cells were centrifuged, counted, and transferred into a new plate. Viability of these populations was routinely about 97% and by morphologic criteria the adherent cells were in excess of 98% AMs.
Isolation of PBMC
PBMCs were isolated from heparinized blood using Ficoll-Hypaque (Mediatech Inc., Manassas, VA) density gradient separation followed by washing twice with PBS. Cells were counted and resuspended in complete media and cultured in 12-well plates for different time periods.
ELISA
The levels of human TNF-α, IL-1β, IL-6, and IL12/IL23p40 in culture medium were measured by ELISA according to the manufacturer's instructions (ELISA DuoKits; R&D Systems, Minneapolis, MN).
Protein Extraction and Immunoblotting
Cells were harvested after the appropriate treatment and washed with PBS. Total cellular proteins were extracted by addition of RIPA buffer including a protease inhibitor cocktail and antiphosphatase I and II (Sigma Chemicals). Protein concentration was measured with the BCA assay (Bio-Rad, Hercules, CA). Equal amounts of proteins (10–50 μg) were mixed 1/1 (v/v) with sample buffer (20% glycerol, 4% sodium dodecyl sulfate, 10% 2-ME, 0.05% bromophenol blue, and 1.25 M Tris-HCl, pH 6.8), and fractionated on a 10% sodium dodecyl sulfate–polyacrylamide gel. Proteins were transferred onto a polyvinylidene difluoride membrane (Bio-Rad) for 30 minutes at 20 V using a SemiDry Transfer Cell (Bio-Rad). The polyvinylidene difluoride membrane was blocked with 5% nonfat dry milk in TBST (Tris-buffered saline with 0.1% Tween 20) for 1 hour, washed, and incubated with primary Abs (1/1000) overnight at 4°C. The blots were washed with TBST and then incubated for 1 hour with the horseradish peroxidase–conjugated secondary anti-IgG Ab using a dilution of 1/10,000 in 5% nonfat dry milk in TBST. Membranes were washed four times in TBST. Immunoreactive bands were visualized using a chemiluminescent kit (ECL-Plus; GE Healthcare, Piscataway, NJ). Images were captured on Hyblot CL film (Denville; Scientific, Inc; Metuchen, NJ). Optical density analysis of signals was performed using ImageQuant software from Molecular Dynamics (version 5).
Cell Viability
Cell viability was measured using the MTT (3[4,5] dimethylthiazol-2, 5-diphenyltetrazolium bromide) assay as described previously (22).
Adenovirus Infection
The recombinant adenoviruses expressing MKP-1 (AdMKP-1) or enhanced green fluorescent protein (AdEGFP) have been described previously (23, 24). These adenoviruses were amplified by the Virus Core Facility of the Research Institute at Nationwide Children's Hospital (23). AMs were infected with AdEGFP or AdMKP-1 for 2 hours at 37°C in RPMI 1640 medium without fetal bovine serum. After incubation, an equal volume of RPMI 1640 medium containing 10% fetal bovine serum was added to reach a serum concentration of 5%, and the infections were continued for 24 hours. We studied various multiplicities of infection (MOI) to determine the most effective concentration of virus particles for infection of AMs. Adenovirus infection at an MOI of 400 particles per cell of AMs resulted in greater than 75% infection (determined by fluorescence of cells infected with AdEGFP vector) without any effect on cell viability at 24 hours as measured by trypan blue staining.
RNA Extraction and Quantitative Reverse Transcriptase/Real Time-Polymerase Chain Reaction
Total RNA was extracted using Stat 60 (Iso-Tex Diagnostics, Friendswood, TX) and reverse transcribed using the Reverse Transcription System (Promega, Madison, WI). Primers for IL-12 p40, TNF-α, IL-1β, IL-10, NF-κB, and β-actin were used to amplify the corresponding cDNAs. Quantitative analysis of mRNA expression was performed using iQ SYBR Green Supermix (Bio-Rad) with an MX3000p instrument (Stratagene, La Jolla, CA). Polymerase chain reaction (PCR) amplification was performed in a total volume of 20 μl containing 2 μl of cDNA and 20 pg primers (Invitrogen, Carlsbad, CA). The PCR amplification protocol consisted of one initial denaturation step for 10 minutes at 95°C followed by 45 cycles: denaturation for 10 seconds at 95°C, annealing for 20 seconds at 60°C, and extension at 72°C for 20 seconds. Relative mRNA levels were calculated after normalization to β-actin. The specificity of amplification was assessed for each sample by melting curve analysis (for all above 82°C) and the size of some amplicons was checked by electrophoresis.
Primer sequences (5′ to 3′) were: IL12p40: forward AGATGGATCACCTG GACCTG, and reverse TCCTTTGTGACAGGTGTACTGG; TNF-α: forward CCATCCTATGAAGAGACAGTGG, and reverse GGCTGGGTATAATACGAAGGAG; IL-1β: forward CTTCGACACATGGGATAACG, and reverse ATGGACCAGACATCACCAAG; TLR4: forward AAGCCGAAAGGTGATTGTTG, and reverse CTGAGCAGGGTCTTCTCCAC; NOD1: forward TCCGAGTTCTTCCTCTACTTGC, and reverse AGTGTTGACCACGACTTTGCTC; and β-actin: forward CTTCTACAATGAGCTGCGTGTG, and reverse CATGATCTGGGTCATCTTCTCG.
Statistical Analyses
Statistical analyses were performed using SPSS software, version 17.0 (SPSS Inc., Chicago, IL). One-way analysis of variance test and post hoc repeated measure comparisons (least significant difference) were performed to identify differences between groups. ELISA results were expressed as mean ± SEM. For all analyses, two-tailed P values of less than 0.05 were considered significant.
RESULTS
Baseline Cytokine Levels in Cultured BAL Cells from Patients with Sarcoidosis and Healthy Subjects
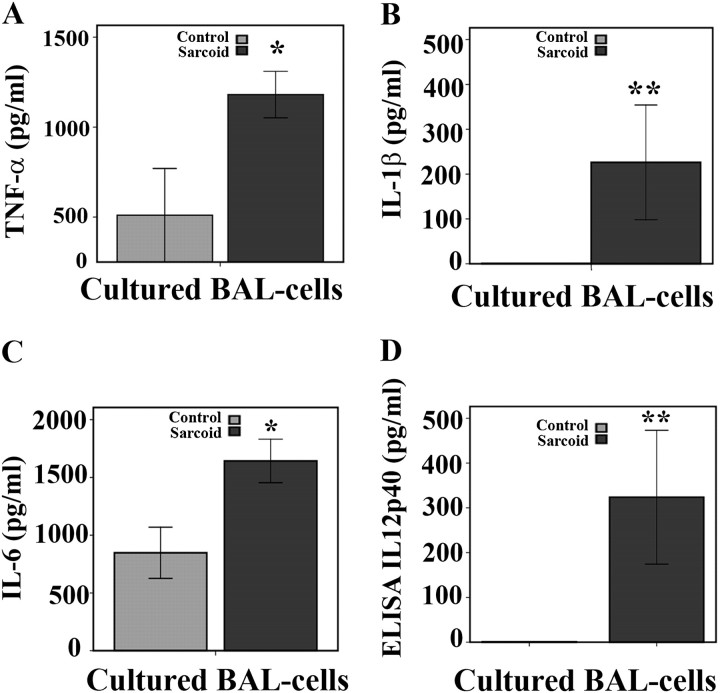
All enrolled patients with sarcoidosis were ambulatory outpatient subjects with predominant lung involvement and mostly radiologic stage 2 (see Table 1). Previous studies showed that AMs and BAL cells obtained from the sarcoid lung and cultured ex vivo spontaneously produce several proinflammatory cytokines (2, 3). To mimic the cellular environment of the lung we chosen to use total BAL cells obtained from patients with sarcoidosis and healthy control subjects (Table 2 shows the characteristics of BAL samples among patients with sarcoidosis and control subjects). We first compared spontaneous release of several proinflammatory cytokines by cultured BAL cells in both groups. The cells obtained from patients and control subjects were cultured for 16 hours as described in Methods, and the cytokines released into medium were assessed by using ELISA. Figure 1 shows levels of TNF-α (A), IL-1β (B), IL-6 (C), and IL12/IL23p40 (D) in the medium of cultured BAL cells in both groups. In culture, BAL cells of patients with sarcoidosis exhibited significantly elevated spontaneous production of all four cytokines compared with cells of control subjects (Figure 1).
TABLE 1.
SUBJECT DEMOGRAPHICS
|
Characteristic |
Patients |
Control Subjects |
||
|---|---|---|---|---|
| Age, yr | 35.7 ± 7.4 | 37 ± 7.4 | ||
| BMI | 29 ± 4.4 | 28 ± 3.6 | ||
| Sex, no. (%) | ||||
| Female | 27 (75) | 11 (68) | ||
| Male | 10 (25) | 5 (32) | ||
| Race, no. (%) | ||||
| African American | 33 (89) | 9 (56) | ||
| White | 6 (11) | 7 (44) | ||
| CXR stage, no. (%) | ||||
| 0 | 0 (0) | NA | ||
| 1 | 9 (27) | NA | ||
| 2 | 24 (72) | NA | ||
| Organ Involvements, no. (%)* | ||||
| Lung | 37 (100) | NA | ||
| Skin | 10 (27) | NA | ||
| Multiorgan |
20 (52) |
NA |
||
Definition of abbreviations: BMI = body mass index; CXR = chest X-ray; NA = not applicable.
Some patients had multiple organ involvements.
TABLE 2.
CHARACTERISTICS OF BRONCHOALVEOLAR LAVAGE
|
Group |
No. |
Age, yr |
BAL cells (x 104 /ml) |
AMs |
%Total BAL Cells (N/L/E) |
|---|---|---|---|---|---|
| Control | 16 | 37 ± 7.4 | 17.4 ± 11.5 | 86.6 ± 10.4 | 2.8 ± 1.8/22 ± 14.1/0.5 ± 0.8 |
| Sarcoidosis |
37 |
35.7 ± 7.4 |
20.1 ± 6.2 |
75 ± 9. 1 |
1.9 ± 0.8/29.1 ±
11.7/0.2 ± 0.1 |
Definition of abbreviations: AM = alveolar macrophages; BAL = bronchoalveolar lavage; E = eosinophils; L = lymphocytes; N = neutrophils.
Data are expressed as mean ± SEM.
Figure 1.
Baseline cytokine profiles of cultured bronchoalveolar lavage (BAL) cells from patients and control subjects. Cells obtained during BAL from patients with and without sarcoidosis were washed twice with phosphate-buffered saline, counted, and cultured in a density of 106 cells per well in complete media containing 1% serum to decrease effects of growth factors on cytokine response. After 16 hours, spontaneous release of cytokines from BAL cells was measured in supernatants via ELISA for (A) tumor necrosis factor (TNF)-α, (B) IL-1β, (C) IL-6, and (D) IL-12/IL23p40. Data are presented as mean ± SEM of 24 patients and 10 control subjects, each measured in triplicate. Using analysis of variance Mann-Whitney U test *P < 0.05 and **P < 0.001. Statistically significant differences were found for TNF-α (P < 0.05), and for IL-1β, IL-6, and IL12/IL23p40 (P < 0.001).
NOD1 and TLR4 Stimulation Enhances TNF-α and IL12/IL23p40 Production in Cultured BAL Cells of Patients with Sarcoidosis
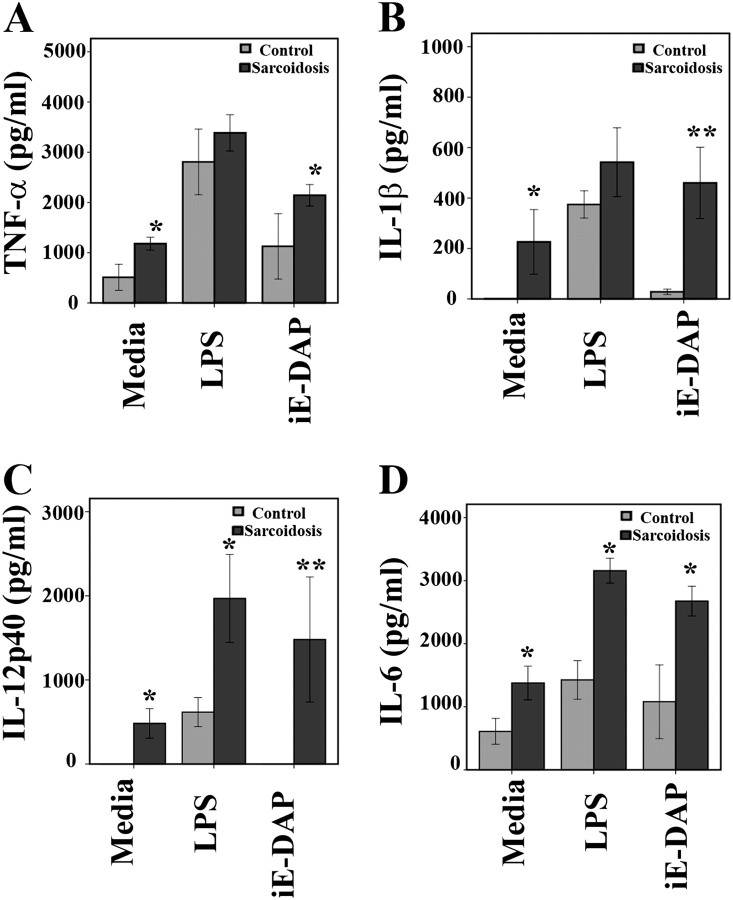
Recent studies have suggested that NOD1 and NOD2 signaling can induce antigen-specific immunity (8). Therefore, we examined the role of NOD1 in the initiation of immune responses in sarcoidosis. Several microorganisms have been implicated in sarcoidosis. However, most of these studies have detected lower levels of microbial products or less virulent pathogens in tissues and BAL. Therefore, low amounts of microbial products are likely to be more relevant in chronic inflammatory disorders, such as sarcoidosis, than higher ligand doses used in acute inflammatory models, such as sepsis. We first performed a dose–response curve and determined the lowest effective doses of LPS (1 ng/ml) and NOD1-agonist (iE-DAP: 1 μg/ml). We then applied these low doses throughout our subsequent experiments. BAL cells were cultured in media containing 1% serum in the presence or absence of LPS or iE-DAP for 16 hours, and levels of cytokine secreted into the media by BAL cells were assessed. As shown in Figure 2, BAL cells of subjects in both groups responded to LPS with an increase of TNF-α, IL-1β, IL12/IL23p40, and IL-6 (Figure 2). Interestingly, stimulation of BAL cells from healthy subjects with iE-DAP evoked only a minimal cytokine production. Remarkably, BAL cells from patients with sarcoidosis responded to iE-DAP more robustly than cells from healthy subjects, as indicated by substantially elevated inflammatory cytokine production.
Figure 2.
Effect of nucleotide-binding oligomerization domain 1 (NOD)1 and Toll-like receptor 4 (TLR 4) activation on cytokine profiles in cultured bronchoalveolar lavage (BAL) cells. BAL cells from patients with and without sarcoidosis were washed twice with phosphate-buffered saline, counted, and 106 cells were cultured in media containing 1% serum. Cells were incubated with γ-D-glutamyl-meso-diaminopimelic acid (iE-DAP) (1 μg/ml) or LPS (1 ng/ml) for 16 hours or left without treatment. After 16 hours, cytokines were measured in culture supernatants via ELISA for (A) tumor necrosis factor (TNF)-α, (B) IL-1β, (C) IL12/IL23p40, and (D) IL-6. Data are presented as mean ± SEM of 24 patients and 10 control subjects measured in triplicate. It is important to note that there were differences within each group in terms of response to ligands and cytokine production. Using analysis of variance Mann-Whitney U test P < 0.05 was considered significant. *P < 0.05; **P < 0.001. Significant differences were found for IL-1β and IL12/IL23p40 with iE-DAP stimulation (P < 0.001) between the groups.
Furthermore, we confirmed these data at the mRNA level using quantitative reverse transcriptase–PCR (qRT-PCR). After 2 hours of treatment with NOD1 or TLR4 agonist, mRNA extracted from cultured BAL cells of patients with sarcoidosis (n = 10) and control subjects (n = 10) were assessed for TNF-α, IL-1β, and IL12/IL23p40 expression. As shown in Figure E1, cells from patients with sarcoidosis responded to stimulation of either NOD1 or TLR4 with more robust increases in expression of TNF-α, IL-1β, and IL12/IL23p40 than cells from healthy control subjects. These results were not due to a significant difference in the relative gene expression of NOD1 and TLR4 receptors between the two groups (10 each group) as determined by qRT-PCR from RNA extract of BAL cells (Figure E2).
NOD1/TLR4 Signaling Induces ERK and p38 Phosphorylation in BAL Cells
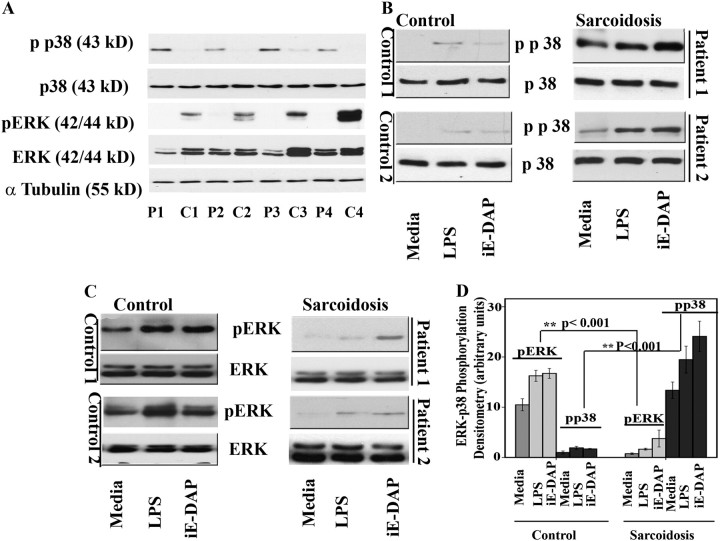
The MAPK activation in response to TLR and NLR is a fundamental regulatory mechanism in shaping both innate and adaptive immunity (13). Several studies have demonstrated the crucial role of p38 activation mediated in the production of Th1 cytokines, especially TNF-α (20) and IL-12 (25, 26). Additionally, several reports highlighted the importance of constitutively active (phosphorylated) ERK in the survival of AMs in human lungs (27). First, we investigated the levels of total ERK, total p38, and their active forms of BAL cells obtained from patients with sarcoidosis or control subjects under basal condition. Western blot analysis was performed using protein extracted from BAL cells of both groups side by side and under identical conditions. Surprisingly, we observed consistently the presence of phosphorylated p38 (i.e., the activated form) in BAL cells of all patients but not in the control subjects (four subjects each group) (Figure 3A). Control BAL cells exhibited higher levels of active (i.e., phosphorylated) ERK than did cells from the patients with sarcoidosis. There are clear differences in the total ERK levels among the human subjects (Figure 3A). The differences in total ERK levels were not experimental artifact, because comparable levels of α-tubulin were detected in these samples. Our findings are significant and may indicate diversity in protein expression for different kinases in human subjects.
Figure 3.
Nucleotide-binding oligomerization domain 1 (NOD1) and Toll-like receptor 4 (TLR 4) stimulation leads to p38 and extracellular signal–regulated kinase (ERK) mitogen-activated protein (MAP) kinase phosphorylation in cultured human bronchoalveolar lavage (BAL) cells. Whole cell extracts were prepared from freshly obtained cells collected during BAL of control subjects and patients, and 30 μg of proteins were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blot analysis. (A) Whole cell lysates of four control subjects (C1–C4) and four patients (P1–P4). Blots were probed with antibodies against the phosphorylated forms of ERK and p38 as well as total ERK and total p38. Equal loading was confirmed using α-tubulin antibody. (B) BAL cells obtained from control subjects and patients were placed in culture immediately after isolation with and without γ-D-glutamyl-meso-diaminopimelic acid (iE-DAP) (1 μg/ml) or LPS (1 ng/ml) for 45 minutes. Phosphorylation of p38 in BAL cells of patients and control subjects was analyzed using whole cell lysates and specific antibodies against the phosphorylated form (Thr180/Tyr182) and total p38. Representative results are shown for two patients and two control subjects out of a total of n = 12 in each group. (C) Phosphorylation of ERK in BAL cells of patients and control subjects in response to LPS, iE-DAP, and a combination of both. Western blot analysis was performed using antibodies against the phosphorylated form of ERK (Thr202/Tyr204). Equal loading was determined using total ERK. (D) Densitometric values (mean ± SEM) of phosphorylated form of ERK and p38 of eight different experiments. Using analysis of variance Mann-Whitney U test P < 0.05 was considered significant. *P < 0.05; **P < 0.001. Significant differences were found in phosphorylated form of both p38 and ERK kinase in disease and control subjects.
Next, we assessed the effects of low dose of NOD1 or TLR4 agonists on activation of the MAPK members, including p38, ERK, and JNK in BAL cells. Cultured BAL cells were stimulated with iE-DAP or LPS for 45 minutes or left unstimulated. Total cell lysates were then subjected to immunoblotting using a specific anti–phospho p38 (Thr180/Tyr182) antibody. As shown above, cells isolated from patients with sarcoidosis contained higher levels of active p38 even under the unstimulated condition (Figure 3B). In vitro stimulation of BAL cells from patients with either iE-DAP or low-dose LPS resulted in a further increase in p38 phosphorylation. In contrast, healthy control subjects lacked constitutive active p38 and demonstrated only a minimal increase in p38 phosphorylation in response to NOD1/TLR4 stimulation.
ERK kinase activity was also determined via Western blot analysis using an antibody specific for phosphorylated ERK (Thr202/Tyr204) or for total ERK. In contrast to the lower p38 activity, cells isolated from control subjects exhibited substantially higher basal ERK activity. ERK activity was further enhanced in response to iE-DAP and LPS (Figure 3C). Our finding that BAL cells of healthy subjects have constitutively active ERK agrees with a previous study done in healthy AMs (27). In contrast, the BAL cells of diseased subjects not only contained very low basal ERK activity but also failed to respond to ligand stimulation with a robust ERK phosphorylation. This was not due to lack of total ERK in BAL cells of patients. In fact, Western blot analysis performed using total ERK antibody indicated that total ERK in some cases was actually higher in diseased cells. Figure 3D depicts graphically the sum of densitometry of Western blots from BAL cell lysates for pERK and pp38 of patients with sarcoidosis and control subjects with and without exposure to LPS and iE-DAP (n = 4). There were significant differences in phosphorylation of both ERK and p38 kinases in unstimulated and stimulated conditions between the two groups (P < 0.001). We also determined the kinetics of ERK and p38 activation in BAL cells of subjects with sarcoidosis in response to iE-DAP and LPS. BAL cells of patients with sarcoidosis responded to iE-DAP with a late and nonsustained ERK activation, whereas a sustained activation was observed for p38 (Figure E3). The peak ERK phosphorylation was at 45 minutes post stimulation with iE-DAP and LPS (data not shown). In contrast, enhanced p38 phosphorylation in response to ligands began as early as 15 minutes post stimulation and was sustained up to 24 hours (data not shown). These findings indicate that BAL of patients with sarcoidosis exhibits constitutive activation of p38 and reduced activation of ERK in response to NOD1 and TLR4.
We also evaluated the JNK activation by performing Western blot analysis. Total cell lysates of BAL cells of sarcoid and control subjects that were left untreated or treated with NOD1 or TLR4 ligands were subjected to immunoblotting using an antibody specific for phosphorylated JNK (Thr183/Tyr185). Figure E4 shows phospho JNK in BAL cells of one control subject and two subjects with sarcoidosis. In contrast to significant differences seen in p38 and ERK phosphorylation between patients and control subjects, there were no significant differences in JNK phosphorylation both at the baseline and in response to stimulation with TLR4 and NOD1 ligands between the two groups. We concluded that the major difference between BAL cells of control subjects and subjects with sarcoidosis is the differential activation of p38 and ERK but not JNK.
As shown in Table 2, BAL cells of patients with sarcoidosis had a higher percentage of lymphocytes than control subjects. Because we assessed the response of total BAL cells to TLR4 and NOD1 ligands, the differences in response to ligands can be due to presence of different cell populations. To address this issue we separated nonadherent cells (lymphocytes) from adherent cells (AMs) using adherent plates (see Methods). As comparison, we also cultured total BAL cells in nonadherent plates. All three cell preparations were stimulated side by side with LPS (1 ng/ml) or with iE-DAP (1 μg/ml) or kept in media. TNF-α and IL-12p40 were measured 16 hours post stimulation (Figures E5A and E5B). Additionally, the effect of iE-DAP and LPS on p38 phosphorylation was investigated in these three cell populations. Figure E5C shows that nonadherent cells (lymphocytic cell population) failed to respond to such stimulation as assessed by analysis of p38 phosphorylation. In contrast, both adherent cells (AMs) and BAL cells responded to both ligands with significant p38 phosphorylation.
Production of IL-12p40 and TNF-α in Response to NOD1 and TLR4 Agonists is Mediated by p38 Activity
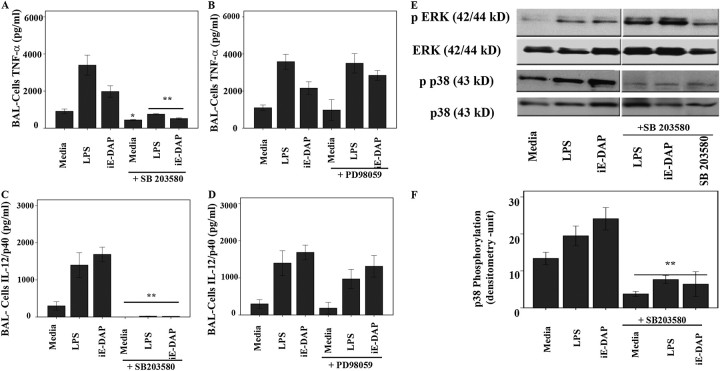
It has been shown in different cell culture models that NOD1 stimulation regulates chemokine and cytokine production via activation of RICK kinase. This leads to NF-κB and MAPK activation and results in induction of several cytokine genes (28, 29). Having shown that both ERK and p38 respond to ligands differentially in sarcoid and healthy BAL cells, we next determined which MAPK plays a prominent role in mediating Th1-type cytokine production in this setting. We assessed the effects of SB203580, the inhibitor of p38 and RICK kinase, and PD98059, a specific inhibitor of MEK/ERK pathway (30), on iE-DAP– and LPS-mediated cytokine responses. BAL cells obtained from the lungs of patients with sarcoidosis were cultured and pretreated with PD98059, SB203580, or vehicle (dimethyl sulfoxide) 30 minutes before stimulation with NOD1- and TLR4-agonists. Figure 4 shows cytokine levels in the medium 16 hours after stimulation in the presence or absence of inhibitors. We focused on two major cytokines, TNF-α and IL12/IL23p40. Patients with sarcoidosis responded to both iE-DAP and LPS with an increased production of both TNF-α (Figures 4A and 4B) and IL-12/IL23p40 (Figures 4C and 4D). The specific ERK inhibitor PD98059 had little effect on the production of either TNF-α (Figure 4B) or IL-12p40 (Figure 4D). In contrast, inhibition of p38/RICK kinase using SB203580 completely abolished the stimulatory effect of iE-DAP and LPS on the production of both TNF-α (Figure 4A) and IL-12p40 (Figure 4C). To investigate whether the decrease of cytokines in the presence of SB203580 was not due to increased cell death, we assessed the viability of the whole BAL cells in the presence and absence of SB203580 with and without iE-DAP or LPS up to 36 hours using the MTT assay. Neither iE-DAP nor LPS treatment led to adverse effect on cell survival, and the pretreatment with SB203580 did not change the cellular viability (data not shown). These results suggest that p38 and RICK kinase play a critical role in NOD1/TLR4-mediated TNF-α and IL-12/IL23p40 induction in BAL cells.
Figure 4.
Effect of inhibition of extracellular signal–regulated kinase (ERK) and p38 phosphorylation on tumor necrosis factor (TNF)-α and IL-12p40 production in bronchoalveolar lavage (BAL) cells of patients with sarcoidosis. Cells obtained from patients during BAL were counted and placed in culture immediately after isolation. Cells were pretreated either with (A and C) SB203580 or (B and D) PD98059 30 minutes before treatment with γ-D-glutamyl-meso-diaminopimelic acid (iE-DAP) (1 μg/ml) or LPS (1 ng/ml) for 16 hours. (A and B) TNF-α and (C and D) IL-12/IL-23p40 immunoreactivity in supernatants was analyzed by ELISA. Data presented are mean ± SEM of eight patients analyzed in triplicate. Using analysis of variance Mann-Whitney U test P < 0.05 was considered significant. *P < 0.05; **P < 0.001. (E) SB203580 abolishes p38 phosphorylation but increases ERK phosphorylation in BAL cells. Cells (5 × 107) obtained from patients with sarcoidosis (n = 4) were placed in culture immediately after isolation in the presence or absence of SB203580 30 minutes before treatment with iE-DAP (1 μg/ml) or LPS (1 ng/ml) for 45 minutes or left in media without any treatment (control). Whole cell lysates were subjected to Western blot analysis and immunoblotting using antibodies recognizing the active (phosphorylated) form of ERK and p38. Several treatments with other ligands as well as PD98059 were cut from the original blot and excluded in the final image for clarity of image. Equal loading of proteins was confirmed using total ERK and p38 antibodies. As shown, SB203580 inhibited phosphorylation of p38 while increasing ERK phosphorylation. The results were replicated in six different patients. (F) Densitometric values (mean ± SEM) of phosphorylated form of p38 of four different experiments. Using analysis of variance Mann-Whitney U test P < 0.05 was considered significant. **P < 0.001. Significant differences were found between the densitometric units of pp38 in presence and absence of SB203580.
To confirm the inhibitory effects of SB203580 on p38 activity, BAL cells of patients were incubated with SB203580 30 minutes before adding iE-DAP or LPS. Whole cell lysates were subjected to Western blot analysis using phospho-specific antibodies against ERK and p38. As shown in Figure 4E, SB203580 pretreatment abrogated p38 phosphorylation in response to both ligands and even decreased the baseline p38 phosphorylation in BAL cells of patients with sarcoidosis. Interestingly, the presence of SB203580 not only decreased p38 phosphorylation but also led to an increased ERK phosphorylation. These data are in agreement with another study showing that inhibition of p38 pathway via SB203580 in human AMs leads to enhanced ERK phosphorylation in response to asbestos exposure (20). These data suggest that p38 plays an important role in the biological response to NOD1 and TLR4 stimulation in sarcoidosis and that p38 negatively regulates ERK phosphorylation.
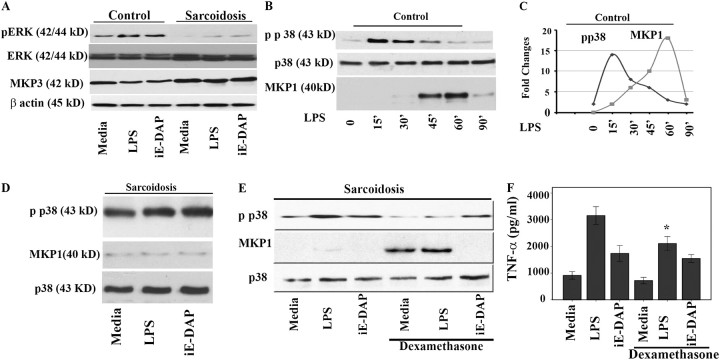
MKP-1 Expression Is Significantly Attenuated in Sarcoid BAL Cells
Phosphorylation of MAPKs in response to NLR and TLR ligation are crucial in the acute inflammatory response. Conversely, their deactivation through dephosphorylation by a recently recognized class of dual-specificity phosphatases (DUSPs or MKPs) is important to control excessive inflammatory responses and autoimmunity (17, 19). It has been shown that MKP-1 negatively regulates p38 activation, whereas MKP-3 preferentially targets ERK (17, 19, 20). Furthermore, it has been shown that MKP-3 is highly expressed in lungs and human AMs (20). First, we determined the MKP-3 expression at the steady state and in response to ligands in BAL cells of patients and control subjects. Cells were cultured with iE-DAP (1 μg/ml) and LPS for 45 minutes; after completing the experiment total cell lysates were subjected to immunoblotting using specific MKP-3, anti–phospho ERK and total ERK antibodies. As shown in Figure 5A, the baseline of MKP-3 expression in BAL cells of patients with sarcoidosis was higher than that in healthy control subjects. The healthy control subjects responded to iE-DAP and LPS with a down-regulation of MKP-3 with a robust ERK phosphorylation, whereas BAL cells of patients had a blunt response to this stimulation and a lesser ERK activation (Figure 5A).
Figure 5.
Mitogen-activated protein kinase phosphate (MKP)-1 and MKP-3 expression in bronchoalveolar lavage (BAL) cells and healthy peripheral blood monocytes (PBMCs). (A) MKP-3 is down-regulated in response to nucleotide-binding oligomerization domain–like receptor (NLR) and Toll-like receptor 4 (TLR 4) ligands. BAL cells (5 × 107 cells) obtained from patients with sarcoidosis and control subjects were placed in culture immediately after isolation and treated with γ-D-glutamyl-meso-diaminopimelic acid (iE-DAP) (1 μg/ml) or LPS (1 ng/ml) for 45 minutes or left in media without any treatment (control). Whole cell lysates were subjected to Western blot analysis using antibodies recognizing the active phosphorylated form of extracellular signal–regulated kinase (ERK) and a polyclonal antibody against human MKP-3. Equal loading of protein was confirmed using total ERK or β-actin antibodies. Patients demonstrated higher basal levels of MKP3 compared with control subjects. Both groups responded with a down-regulation of MKP3 after nucleotide-binding oligomerization domain 1 (NOD1) and LPS treatment, with patients responding to a lesser degree. Three other treatments with different ligands were cut from the original blot and excluded from the present image. Representative Western blot analyses from six separate experiments (patients) are shown. (B) PBMCs of healthy volunteers were isolated and cells were cultured to a density of 5 × 107 per well and treated with 100 ng/ml LPS for different periods of time. Total cell lysates from human monocytes (n = 3) were subjected to Western blot analysis using antibodies specific for the phosphorylated form of p38 (Thr180/Tyr182), total p38, and MKP-1. Two lanes (treated with different ligands) from the image for the total p38 and phospho p38 were excluded. (C) Dynamic relation of p38 and MKP-1 in isolated PBMC of healthy subjects. Densitometric quantifications from Western blots were performed from three separate experiments for phospho p38 and MKP-1 in response to LPS. The intensity of bands for phospho p38 and MKP1 are plotted against time. (D) Lack of MKP-1 induction in BAL cells of patients with sarcoidosis. BAL cells (5 × 107 cells) obtained from patients with sarcoidosis (n = 12) were placed in culture and treated with iE-DAP (1 μg/ml) or LPS (1 ng/ml) for 60 minutes or left in media without any treatment (media). Whole cell lysates were subjected to Western blot analyses using antibodies specific to phospho p38 (upper panel), MKP-1, and total p38 (as control). (E) Dexamethasone induces MKP-1 in BAL cells. Cells of patients (n = 3) were pretreated with dexamethasone (100 ng/ml) for 45 minutes or kept in media. The cells were treated with NOD1 or TLR4 ligands as indicated. Immunoblotting was performed using phospho p38 (Thr180/Tyr182), and MKP1 antibodies. Equal loading was determined using total p38 antibody. This is a representative Western blot from three different experiments. (F) BAL cells obtained from patients were counted and placed in culture immediately after isolation. Cells were pretreated either with 100 ng/ml dexamethasone for 45 minutes before stimulation with iE-DAP (1 μg/mL) or LPS (1 ng/mL) for 16 hours. Tumor necrosis factor (TNF)-α immunoreactivity in supernatants was analyzed by ELISA. Using analysis of variance Mann-Whitney U test P < 0.05 was considered significant. *P < 0.05.
Because BAL cells of our patients with sarcoidosis demonstrated higher levels of p38 activity both at baseline and in response to treatment than cells of the healthy subjects, we asked the question whether the negative regulation of p38 by MKP-1 is defective in this disease. MKP-1 is highly expressed by innate immune cells and belongs to the immediate-early gene family (18). It has been shown in murine macrophages that TLR-mediated signaling events, active ERK, and glucocorticosteroids are positive regulators of MKP-1 expression (31–33). We determined the kinetics of MKP-1 induction and p38 activation in isolated PBMCs of healthy control subjects after treatment with LPS (100 ng/ml). In response to LPS, MKP-1 protein expression was first detected after 30 minutes, whereas its maximal expression was reached at 60 minutes (18-fold increased, P < 0.001) (Figures 5B and 5C). Maximal activity of p38 was found between 15 and 45 minutes. Initial robust p38 activation in response to LPS was followed by MKP-1 induction and p38 dephosphorylation. Figure 5C depicts the kinetic association between the LPS-mediated MKP-1 induction and p38 deactivation.
We then explored whether stimulation with low levels of NOD1 and TLR4 agonists leads to up-regulation of MKP-1 in BAL cells of patients. We observed only a minimal expression of MKP-1 at baseline in BAL cells and after stimulation with low-dose iE-DAP or LPS for 60 minutes (Figure 5D). Treatment of BAL cells of patients with iE-DAP or LPS did not significantly alter MKP-1 protein levels, although the phosphorylation of p38 was significantly enhanced. To assess the possibility of a delayed MKP-1 response in BAL cells of patients, we also repeated the same experiments (in two different patients) with higher doses of LPS (100 ng/ml) and longer incubation time (up to 90 min). We again observed sustained p38 activation and a lack of MKP-1 expression in BAL cells of patients (data not shown).
Corticosteroids are a mainstay of therapy of sarcoidosis and it has been suggested that their effect is partly due to induction of MKP-1 and thus negative regulation of p38 (34). Therefore, we investigated whether BAL cells of patients with sarcoidosis respond to dexamethasone with MKP-1 induction. BAL cells of patients with active sarcoidosis were cultured with LPS or iE-DAP for 1 hour (the highest amount of MKP-1 induction occurs at 1 h) with and without pretreatment with dexamethasone (100 ng/ml). Figure 5E shows the effect of dexamethasone on MKP-1 expression in BAL cells of subjects with sarcoidosis. Interestingly, we observed a robust MKP-1 induction by dexamethasone either alone or in combination with LPS, whereas iE-DAP–treated cells showed a lack of MKP1 induction. Dexamethasone did not completely block p38 phosphorylation (Figure 5D). Additionally, we measured TNF-α and IL12/IL23p40 production in response to LPS and iE-DAP in the presence and absence of dexamethasone. Figure 5E shows TNF-α level 16 hours after stimulation in the presence or absence of dexamethasone in three patients with sarcoidosis. Presence of dexamethasone significantly decreased both TNF-α (Figure 5F) and IL12/IL23/p40 (Figure E6). The inhibitory effect of dexamethasone on both cytokines was less pronounced as compared with the effect of the direct p38 inhibitor (Figure 4).
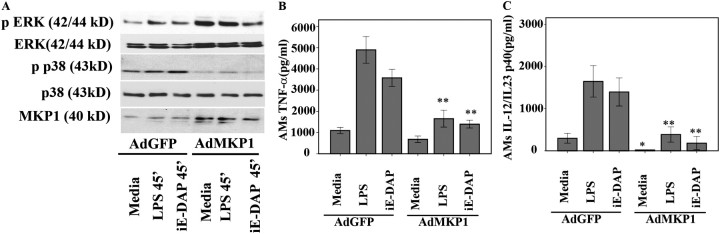
MKP-1 Overexpression Down-Regulates p38 Activation and Abrogates TNF-α and IL-12 Production in Response to TLR4 and NOD1 Agonists in BAL Cells of Sarcoidosis
To further investigate the role of MKP-1 in persistent p38 activation and exaggerated proinflammatory cytokine production in sarcoidosis, we examined the effect of MKP-1 overexpression on p38 and proinflammatory cytokine production in BAL cells. BAL cells of patients were infected either with adenovirus-expressing GFP (AdGFP) or adenovirus-expressing MKP-1 (AdMKP-1) at an MOI of 400. The same MOI (400) of AdGFP infection resulted in 75 to 80% GFP-positive cells at 24 hours (Figure E7). Using adenovirus particles containing either EGFP or MKP-1, we infected BAL cells collected from four different patients. After 24 hours, infected cells were stimulated with LPS or iE-DAP for an additional 45 minutes to evaluate the effects on MAPK activation or for additional 16 hours to evaluate the effects on cytokine production. Western blot analysis of protein extracts obtained 24 hours after adenovirus-mediated gene transfer demonstrated a significant increase of cellular MKP-1 (Figure 6A). MKP-1–overexpressed BAL cells clearly show decreased phosphorylation of p38 as compared with adenovirus constructs containing EGFP, both at baseline and in response to both treatments (Figure 6A). The decrease of p38 phosphorylation was associated with a decreased TNF-α and IL-12 production in response to ligands (Figures 6B and 6C).
Figure 6.
Adenovirus-mediated mitogen-activated protein kinase phosphate (MKP-1) overexpression in bronchoalveolar lavage (BAL) cells of patients with sarcoidosis abrogates p38 phosphorylation and tumor necrosis factor (TNF)-α and IL-12p40 production in response to Toll-like receptor 4 (TLR 4) and nucleotide-binding oligomerization domain 1 (NOD1) agonists. BAL cells of patients (n = 4) were infected with adenovirus-expressing GFP (AdGFP) or adenoviruses expressing MKP-1 (AdMKP-1) at a multiplicity of infection (MOI) of 400. Twenty-four hours post infection cells were stimulated with γ-D-glutamyl-meso-diaminopimelic acid (iE-DAP) (1 μg/ml) or LPS (1 ng/ml) for 45 minutes. Immunoblotting was performed using phospho-p38 (Thr180/Tyr182), phospho-extracellular signal–regulated kinase (ERK) (Thr202/Tyr204), and MKP-1 antibodies. (A) Equal loading was evaluated using total p38 or total ERK antibodies. Different MOI of AdMKP1 were used for the experiments; in the present figure some lanes representing the MOI of 200 were excluded (spliced out). Representative Western blot is shown from four separate experiments. Infected BAL cells were treated with iE-DAP (1 μg/ml) or LPS (1 ng/ml) or kept in media for 16 hours. After 16 hours cytokines were measured in culture supernatants via ELISA for (B) TNF-α and (C) IL12/IL23p40. Data are presented as mean ± SEM of four patients. Using analysis of variance Mann-Whitney U test P < 0.05 was considered significant. *P < 0.05; **P < 0.001.
DISCUSSION
The signaling pathways underlying the systemic inflammation in sarcoidosis remain poorly understood. Immunologic hallmarks of sarcoidosis are increased Th1 cytokine production at sites of inflammation but also increased circulatory cytokines (35). No study, until now, investigated the signaling pathway leading to Th1-mediated immunology in this disorder. In this study, we analyzed the signaling pathway in BAL cells of patients with sarcoidosis in response to low doses of NOD1 and TLR4 ligands (Figures 2 and 3). BAL cells of patients with sarcoidosis respond to low-dose iE-DAP and LPS with strikingly increased and sustained p38 phosphorylation (Figure 3). Such enhanced p38 activation provides a mechanistic explanation for the excessive production of proinflammatory, predominantly Th1 cytokines in sarcoidosis (Figure 2). The p38 inhibitor SB203580 abolishes the stimulatory effect of both NOD1 and TLR4 agonists and decreases TNF-α and IL-12p40 (Figure 4). Furthermore, the persistent p38 phosphorylation is associated with a lack of MKP-1 up-regulation in BAL cells of patients with sarcoidosis (Figure 5). Overexpression of MKP-1 via AdMKP-1 decreases p38 phosphorylation and ligand-induced TNF-α and IL-12p40 production (Figure 6).
Sarcoidosis is a complex inflammatory disorder with significant heterogeneity in disease presentation but with a high incidence of lung involvement (>90% of cases). In the lungs, AMs play a critical role in initiating an inflammatory response to microbial triggers and inhaled toxins both locally and in distantly located organs. As part of innate immunity, AMs recognize conserved pathogen-associated molecular patterns (PAMPs) through their elaborate pattern-recognition receptors. Indeed, the stimulation of adherent cells (AMs) or total BAL cells, but not nonadherent cells (lymphocytes), by TLR4 or NOD1 agonists leads to p38 phosphorylation and cytokine production (Figure E5), suggesting a pivotal role for AMs in recognition of microbial constituents.
Both TLRs and NLRs have been associated with autoimmunity. A Japanese study implicated a frequent mutation in the NOD1 gene among patients with sarcoidosis as compared with control subjects (12). The NOD1 receptor recognizes peptidoglycan-associated molecules containing the dipeptide iE-DAP present in all gram-negative bacteria (10, 36). Detection of peptidoglycan by the NOD1 receptor leads to rapid receptor oligomerization and a transient recruitment of serine threonine kinase RICK (RIP2) (8). The NOD1-RIP2 complex then recruits TAK1, whose activation is required for the activation of MAP kinase and NF-κB (37, 38). Although genetic variations in NOD1 and TLRs (e.g., TLR2) have been implicated in sarcoidosis (12, 39), abnormalities in MAP kinase signaling have not been reported in this disease. We have observed the active form of p38 (phosphorylated) in BAL cells of most patients with sarcoidosis even under unstimulated conditions and increased and sustained p38 activation after NOD1 or TLR4 stimulation (Figure 3). The sustained activation of p38 MAPK plays a critical role in inflammatory diseases, such as Crohn disease and rheumatoid arthritis, and directly controls several transcription factors and cytokine genes related to sarcoidosis. Our novel finding that BAL cells and especially AMs of subjects with sarcoidosis show differential MAPK activation in response to microbial constituents, with a sustained p38 activation (Figures 2 and 3), might be an explanation for the persistent production of several inflammatory cytokines, such as TNF-α and IL-12, in sarcoidosis. This notion is consistent with the observation that pretreatment of BAL cells in vitro with SB 203580 inhibited production of TNF-α and IL12/IL23p40 (Figure 4), two major Th1 cytokines associated with granulomatous disorders (40, 41). It is worth noting that differential activation of p38/ERK pathway has been shown to play a role in autoimmunity and T-cell anergy (a clinical feature of sarcoidosis) (2, 42). Previously, it has been shown that SB203580 pretreatment decreases phosphorylation of p38 in a murine model of Crohn disease. Such inhibition was associated with a decreased production of Th1 cytokines and a milder disease pattern (43). Thus, therapeutic targeting of the p38 pathway in sarcoidosis may be more promising than inhibiting or neutralizing one cytokine at a time. Interestingly, we found that inhibition of p38 via SB203580 results in enhanced phosphorylation of ERK kinase (Figure 5E). It has been demonstrated that the ability of AMs to survive in alveoli despite exposure to chemical pollutants and pathogens is partly due to constitutively active ERK (27, 44). In our study, the control subjects demonstrated constitutively active ERK even in the absence of stimulation (Figure 3A), unlike BAL cells of subjects with sarcoidosis. Chen and colleagues have shown that inhibiting the ERK pathways using U0126, a MEK1/2 inhibitor, enhances p38 activity in LPS-stimulated murine macrophages, at least in part by preventing the induction of MKP-1 (45). These findings suggest an additional network of crosstalk between the two MAP kinase pathways.
Sequential phosphorylations and activations of MAPKs are essential for initiation of the acute inflammatory response necessary for the effective clearance of pathogens. The persistent activation of MAPKs may lead to uncontrolled inflammation and chronic inflammatory diseases (17). A major negative feedback mechanism is the rapid induction of dual specific phosphatases, among them MKP-1 and MKP-3 (17, 19), which are important for inactivation of p38 and ERK, respectively. Although the exact mechanisms responsible for the induction of these phosphatases are still not well understood, their expression depends on both enhanced gene transcription and post-translational modifications (46–48). It appears that the activation of p38 and ERK is regulated through differential expression of MKP-1 and MKP-3 (20). Indeed, we observed higher expression of MKP-3 and higher p38 activation in BAL cells of patients with sarcoidosis (Figure 5A). Another novel observation of this study is the fact that BAL cells of patients with sarcoidosis lack induction of MKP-1 in response to low-dose LPS or iE-DAP stimulation despite presence of active p38 (Figure 5D). As MKP-1 is a critical negative regulator of p38, lower MKP-1 expression explains the elevated p38 activity and the proinflammatory phenotype of patients with sarcoidosis. In a murine model of sepsis, it has been shown that MKP-1 deficiency leads to sustained activation of p38 MAPK with increased TNF-α and IL-6 production. Interestingly, recently it has been shown that MKP-1–deficient animals show decreased antigen clearance despite increased inflammation in response to pathogens (49, 50). Post-translationally, MKP-1 can be phosphorylated and acetylated, with increased stability in the former and increased affinity to phospho p38 and enzymatic activity in the latter (51). Serine phosphorylation of MKP-1 by ERK kinase decreases the proteasomal degradation of MKP-1 and increases its stability (52). As shown in Figure 3, BAL cells of patients show a decreased ERK activation, likely due to elevated MKP-3 expression in these cells. The decline in basal ERK activity and the defect in triggering a robust ERK activation in these cells provide a plausible explanation for the defective MKP-1 induction and the persistent p38 activation and chronic Th1 cytokine production.
MKP-1 is induced by multiple immunosuppressive agents, including glucocorticoids, the mainstay treatment for sarcoidosis. Dexamethasone treatment of BAL cells of patients with sarcoidosis partially induced MKP-1 and decreased the LPS-mediated p38 activation as well as TNF-α and IL-12 induction (Figures 5D and 5E). This effect was less pronounced in cells treated with iE-DAP (Figure 5D), suggesting the possible importance of signal intensity and type (TLR4 vs. NOD1) in MKP-1 up-regulation. Corticosteroid pretreatment of BAL cells of patients with sarcoidosis (in three patients) induced MKP-1, suggesting the presence of an intact MKP-1 gene at least in some patients. Previously it has been shown that glucocorticoids increase the expression of the MKP-1 gene through enhancing the promoter activity and attenuating its proteasomal degradation (32). Clinically, it is known that some patients with sarcoidosis do not respond favorably to steroid treatment, raising the possibility of aberrant MKP-1 promoter response to steroids in these cases. Further studies are needed to investigate whether increased proteasomal degradation and/or lack of adequate expression of MKP-1 play a major role in this disease. Our findings confirm that sarcoidosis, similar to other autoinflammatory diseases, such as Crohn disease and rheumatoid arthritis, is associated with a persistent state of increased proinflammatory cytokine levels. We have found that in cultured BAL cells ex vivo several proinflammatory cytokines, such as IL-6, TNF-α, IL-1β, and IL-12, are increased. This study also strongly suggests a pivotal role for sustained p38 activation as a consequence of attenuated MKP-1 expression in chronic inflammation of sarcoidosis. It is tempting to speculate that exposure to shared specific bacterial moieties, which triggers activation of p38 pathway, results in this phenotype, and the reexposure to these moieties leads to relapsing disease due to lack of MKP-1 induction. The functional role of dual-specificity phosphatases in inflammation still is emerging and their roles in diverse human diseases remain to be discovered.
In this study we provided a mechanistic explanation for increased Th1-mediated cytokines in sarcoidosis. Additionally, we identified a novel therapeutic target for rational drug development with the goal of either inhibiting p38 activation or enhancing MKP-1 induction.
Supported by a joint grant from American Thoracic Society and Foundation for Sarcoidosis Research (L.S.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201005-0792OC on September 17, 2010
Author Disclosure: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Statement on sarcoidosis. Joint statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med 1999;160:736–755. [DOI] [PubMed] [Google Scholar]
- 2.Mathew S, Bauer KL, Fischoeder A, Bhardwaj N, Oliver SJ. The anergic state in sarcoidosis is associated with diminished dendritic cell function. J Immunol 2008;181:746–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prior C, Knight RA, Herold M, Ott G, Spiteri MA. Pulmonary sarcoidosis: patterns of cytokine release in vitro. Eur Respir J 1996;9:47–53. [DOI] [PubMed] [Google Scholar]
- 4.Chen ES, Wahlstrom J, Song Z, Willett MH, Wiken M, Yung RC, West EE, McDyer JF, Zhang Y, Eklund A, et al. T cell responses to mycobacterial catalase-peroxidase profile a pathogenic antigen in systemic sarcoidosis. J Immunol 2008;181:8784–8796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Furukawa A, Uchida K, Ishige Y, Ishige I, Kobayashi I, Takemura T, Yokoyama T, Iwai K, Watanabe K, Shimizu S, et al. Characterization of propionibacterium acnes isolates from sarcoid and non-sarcoid tissues with special reference to cell invasiveness, serotype, and trigger factor gene polymorphism. Microb Pathog 2009;46:80–87. [DOI] [PubMed] [Google Scholar]
- 6.Ishige I, Usui Y, Takemura T, Eishi Y. Quantitative PCR of mycobacterial and propionibacterial DNA in lymph nodes of Japanese patients with sarcoidosis. Lancet 1999;354:120–123. [DOI] [PubMed] [Google Scholar]
- 7.Uematsu S, Akira S. Toll-like receptors and innate immunity. J Mol Med 2006;84:712–725. [DOI] [PubMed] [Google Scholar]
- 8.Fritz JH, Le Bourhis L, Sellge G, Magalhaes JG, Fsihi H, Kufer TA, Collins C, Viala J, Ferrero RL, Girardin SE, et al. Nod1-mediated innate immune recognition of peptidoglycan contributes to the onset of adaptive immunity. Immunity 2007;26:445–459. [DOI] [PubMed] [Google Scholar]
- 9.Inohara C, McDonald C, Nunez G. NOD-LRR proteins: role in host-microbial interactions and inflammatory disease. Annu Rev Biochem 2005;74:355–383. [DOI] [PubMed] [Google Scholar]
- 10.Inohara N, Ogura Y, Chen FF, Muto A, Nunez G. Human Nod1 confers responsiveness to bacterial lipopolysaccharides. J Biol Chem 2001;276:2551–2554. [DOI] [PubMed] [Google Scholar]
- 11.Inohara N, Ogura Y, Fontalba A, Gutierrez O, Pons F, Crespo J, Fukase K, Inamura S, Kusumoto S, Hashimoto M, et al. Host recognition of bacterial muramyl dipeptide mediated through Nod2. Implications for Crohn's disease. J Biol Chem 2003;278:5509–5512. [DOI] [PubMed] [Google Scholar]
- 12.Tanabe T, Ishige I, Suzuki Y, Aita Y, Furukawa A, Ishige Y, Uchida K, Suzuki T, Takemura T, Ikushima S, et al. Sarcoidosis and NOD1 variation with impaired recognition of intracellular Propionibacterium acnes. Biochim Biophys Acta 2006;1762:794–801. [DOI] [PubMed] [Google Scholar]
- 13.Dong C, Davis RJ, Flavell RA. Map kinases in the immune response. Annu Rev Immunol 2002;20:55–72. [DOI] [PubMed] [Google Scholar]
- 14.Garrington TP, Johnson GL. Organization and regulation of mitogen-activated protein kinase signaling pathways. Curr Opin Cell Biol 1999;11:211–218. [DOI] [PubMed] [Google Scholar]
- 15.Lee CL, Sit WH, Jiang PP, So IW, Wan JM. Polysaccharopeptide mimics ciclosporin-mediated Th1/Th2 cytokine balance for suppression of activated human T cell proliferation by MAPKp38 and STAT5 pathways. J Pharm Pharmacol 2008;60:1491–1499. [DOI] [PubMed] [Google Scholar]
- 16.Inoue T, Boyle DL, Corr M, Hammaker D, Davis RJ, Flavell RA, Firestein GS. Mitogen-activated protein kinase kinase 3 is a pivotal pathway regulating p38 activation in inflammatory arthritis. Proc Natl Acad Sci USA 2006;103:5484–5489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jeffrey KL, Camps M, Rommel C, Mackay CR. Targeting dual-specificity phosphatases: manipulating map kinase signalling and immune responses. Nat Rev Drug Discov 2007;6:391–403. [DOI] [PubMed] [Google Scholar]
- 18.Li L, Chen SF, Liu Y. MAP kinase phosphatase-1, a critical negative regulator of the innate immune response. Int J Clin Exp Med 2009;2:48–67. [PMC free article] [PubMed] [Google Scholar]
- 19.Chi H, Flavell RA. Acetylation of MKP-1 and the control of inflammation. Sci Signal 2008;1:pe44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tephly LA, Carter AB. Asbestos-induced MKP-3 expression augments TNF-alpha gene expression in human monocytes. Am J Respir Cell Mol Biol 2008;39:113–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Samavati LDW, Rastogi R, Nuñez G. Nod1 engagement leads to increased il12/il23 p40 and decreased il-10 expressions in sarcoidosis as compared to healthy subjects [abstract]. J Immunol 2009;98.17:182. [Google Scholar]
- 22.Samavati L, Rastogi R, Du W, Huttemann M, Fite A, Franchi L. STAT3 tyrosine phosphorylation is critical for interleukin 1 beta and interleukin-6 production in response to lipopolysaccharide and live bacteria. Mol Immunol 2009;46:1867–1877. [DOI] [PubMed] [Google Scholar]
- 23.Zhao Q, Shepherd EG, Manson ME, Nelin LD, Sorokin A, Liu Y. The role of mitogen-activated protein kinase phosphatase-1 in the response of alveolar macrophages to lipopolysaccharide: attenuation of proinflammatory cytokine biosynthesis via feedback control of p38. J Biol Chem 2005;280:8101–8108. [DOI] [PubMed] [Google Scholar]
- 24.Pratt PF, Bokemeyer D, Foschi M, Sorokin A, Dunn MJ. Alterations in subcellular localization of p38 MAPK potentiates endothelin-stimulated cox-2 expression in glomerular mesangial cells. J Biol Chem 2003;278:51928–51936. [DOI] [PubMed] [Google Scholar]
- 25.Hickey FB, Brereton CF, Mills KH. Adenylate cyclase toxin of Bordetella pertussis inhibits TLR-induced IRF-1 and IRF-8 activation and IL-12 production and enhances IL-10 through MAPK activation in dendritic cells. J Leukoc Biol 2008;84:234–243. [DOI] [PubMed] [Google Scholar]
- 26.Pan K, Wang H, Liu WL, Zhang HK, Zhou J, Li JJ, Weng DS, Huang W, Sun JC, Liang XT, et al. The pivotal role of p38 and NF-kappaB signal pathways in the maturation of human monocyte-derived dendritic cells stimulated by streptococcal agent OK-432. Immunobiology 2009;214:350–358. [DOI] [PubMed] [Google Scholar]
- 27.Monick MM, Powers LS, Barrett CW, Hinde S, Ashare A, Groskreutz DJ, Nyunoya T, Coleman M, Spitz DR, Hunninghake GW. Constitutive ERK MAPK activity regulates macrophage ATP production and mitochondrial integrity. J Immunol 2008;180:7485–7496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hasegawa M, Fujimoto Y, Lucas PC, Nakano H, Fukase K, Nunez G, Inohara N. A critical role of RICK/RIP2 polyubiquitination in Nod-induced NF-kappaB activation. EMBO J 2008;27:373–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park JH, Kim YG, Shaw M, Kanneganti TD, Fujimoto Y, Fukase K, Inohara N, Nunez G. Nod1/RICK and TLR signaling regulate chemokine and antimicrobial innate immune responses in mesothelial cells. J Immunol 2007;179:514–521. [DOI] [PubMed] [Google Scholar]
- 30.Samavati L, Monick MM, Sanlioglu S, Buettner GR, Oberley LW, Hunninghake GW. Mitochondrial K(ATP) channel openers activate the ERK kinase by an oxidant-dependent mechanism. Am J Physiol Cell Physiol 2002;283:C273–C281. [DOI] [PubMed] [Google Scholar]
- 31.Chi H, Barry SP, Roth RJ, Wu JJ, Jones EA, Bennett AM, Flavell RA. Dynamic regulation of pro- and anti-inflammatory cytokines by MAPK phosphatase 1 (MKP-1) in innate immune responses. Proc Natl Acad Sci USA 2006;103:2274–2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kassel O, Sancono A, Kratzschmar J, Kreft B, Stassen M, Cato AC. Glucocorticoids inhibit map kinase via increased expression and decreased degradation of MKP-1. EMBO J 2001;20:7108–7116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hammer M, Mages J, Dietrich H, Schmitz F, Striebel F, Murray PJ, Wagner H, Lang R. Control of dual-specificity phosphatase-1 expression in activated macrophages by IL-10. Eur J Immunol 2005;35:2991–3001. [DOI] [PubMed] [Google Scholar]
- 34.King EM, Holden NS, Gong W, Rider CF, Newton R. Inhibition of NF-kappaB-dependent transcription by MKP-1: transcriptional repression by glucocorticoids occurring via p38 MAPK. J Biol Chem 2009;284:26803–26815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hata M, Sugisaki K, Miyazaki E, Kumamoto T, Tsuda T. Circulating IL-12 p40 is increased in the patients with sarcoidosis, correlation with clinical markers. Intern Med 2007;46:1387–1393. [DOI] [PubMed] [Google Scholar]
- 36.Inohara N, Koseki T, Lin J, del Peso L, Lucas PC, Chen FF, Ogura Y, Nunez G. An induced proximity model for NF-kappa B activation in the Nod1/RICK and RIP signaling pathways. J Biol Chem 2000;275:27823–27831. [DOI] [PubMed] [Google Scholar]
- 37.Kanneganti TD, Lamkanfi M, Nunez G. Intracellular NOD-like receptors in host defense and disease. Immunity 2007;27:549–559. [DOI] [PubMed] [Google Scholar]
- 38.Shaw MH, Reimer T, Kim YG, Nunez G. NOD-like receptors (NLRs): bona fide intracellular microbial sensors. Curr Opin Immunol 2008;20:377–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Veltkamp M, Wijnen PA, van Moorsel CH, Rijkers GT, Ruven HJ, Heron M, Bekers O, Claessen AM, Drent M, van den Bosch JM, et al. Linkage between Toll-like receptor (TLR) 2 promotor and intron polymorphisms: functional effects and relevance to sarcoidosis. Clin Exp Immunol 2007;149:453–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shigehara K, Shijubo N, Ohmichi M, Takahashi R, Kon S, Okamura H, Kurimoto M, Hiraga Y, Tatsuno T, Abe S, et al. IL-12 and IL-18 are increased and stimulate IFN-gamma production in sarcoid lungs. J Immunol 2001;166:642–649. [DOI] [PubMed] [Google Scholar]
- 41.Taha RA, Minshall EM, Olivenstein R, Ihaku D, Wallaert B, Tsicopoulos A, Tonnel AB, Damia R, Menzies D, Hamid QA. Increased expression of IL-12 receptor mRNA in active pulmonary tuberculosis and sarcoidosis. Am J Respir Crit Care Med 1999;160:1119–1123. [DOI] [PubMed] [Google Scholar]
- 42.Adler HS, Kubsch S, Graulich E, Ludwig S, Knop J, Steinbrink K. Activation of MAP kinase p38 is critical for the cell-cycle-controlled suppressor function of regulatory T cells. Blood 2007;109:4351–4359. [DOI] [PubMed] [Google Scholar]
- 43.Hollenbach E, Vieth M, Roessner A, Neumann M, Malfertheiner P, Naumann M. Inhibition of RICK/nuclear factor-kappaB and p38 signaling attenuates the inflammatory response in a murine model of Crohn disease. J Biol Chem 2005;280:14981–14988. [DOI] [PubMed] [Google Scholar]
- 44.Monick MM, Powers LS, Gross TJ, Flaherty DM, Barrett CW, Hunninghake GW. Active ERK contributes to protein translation by preventing JNK-dependent inhibition of protein phosphatase 1. J Immunol 2006;177:1636–1645. [DOI] [PubMed] [Google Scholar]
- 45.Chen P, Li J, Barnes J, Kokkonen GC, Lee JC, Liu Y. Restraint of proinflammatory cytokine biosynthesis by mitogen-activated protein kinase phosphatase-1 in lipopolysaccharide-stimulated macrophages. J Immunol 2002;169:6408–6416. [DOI] [PubMed] [Google Scholar]
- 46.Liu Y, Shepherd EG, Nelin LD. MAPK phosphatases—regulating the immune response. Nat Rev Immunol 2007;7:202–212. [DOI] [PubMed] [Google Scholar]
- 47.Bermudez O, Marchetti S, Pages G, Gimond C. Post-translational regulation of the ERK phosphatase DUSP6/MKP3 by the mTOR pathway. Oncogene 2008;27:3685–3691. [DOI] [PubMed] [Google Scholar]
- 48.Furukawa T, Tanji E, Xu S, Horii A. Feedback regulation of DUSP6 transcription responding to MAPK1 via ETS2 in human cells. Biochem Biophys Res Commun 2008;377:317–320. [DOI] [PubMed] [Google Scholar]
- 49.Wang X, Meng X, Kuhlman JR, Nelin LD, Nicol KK, English BK, Liu Y. Knockout of MKP-1 enhances the host inflammatory responses to gram-positive bacteria. J Immunol 2007;178:5312–5320. [DOI] [PubMed] [Google Scholar]
- 50.Frazier WJ, Wang X, Wancket LM, Li XA, Meng X, Nelin LD, Cato AC, Liu Y. Increased inflammation, impaired bacterial clearance, and metabolic disruption after gram-negative sepsis in Mkp-1-deficient mice. J Immunol 2009;183:7411–7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cao W, Bao C, Padalko E, Lowenstein CJ. Acetylation of mitogen-activated protein kinase phosphatase-1 inhibits toll-like receptor signaling. J Exp Med 2008;205:1491–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brondello JM, Pouyssegur J, McKenzie FR. Reduced map kinase phosphatase-1 degradation after p42/p44MAPK-dependent phosphorylation. Science 1999;286:2514–2517. [DOI] [PubMed] [Google Scholar]