Abstract
This document is an international evidence-based guideline on the diagnosis and management of idiopathic pulmonary fibrosis, and is a collaborative effort of the American Thoracic Society, the European Respiratory Society, the Japanese Respiratory Society, and the Latin American Thoracic Association. It represents the current state of knowledge regarding idiopathic pulmonary fibrosis (IPF), and contains sections on definition and epidemiology, risk factors, diagnosis, natural history, staging and prognosis, treatment, and monitoring disease course. For the diagnosis and treatment sections, pragmatic GRADE evidence-based methodology was applied in a question-based format. For each diagnosis and treatment question, the committee graded the quality of the evidence available (high, moderate, low, or very low), and made a recommendation (yes or no, strong or weak). Recommendations were based on majority vote. It is emphasized that clinicians must spend adequate time with patients to discuss patients' values and preferences and decide on the appropriate course of action.
Keywords: idiopathic pulmonary fibrosis; usual interstitial pneumonia; evidence-based medicine, diagnosis, therapeutics
CONTENTS
Introduction
Objective
Methods
Committee Composition
Disclosure of Conflicts of Interest
Committee Meetings and Evidence Review Process
Document Preparation
Document Structure
Formulation of the Topic Sections and Questions
Literature Review and Preparation of Evidence Profiles
Quality of Evidence and Strength of Recommendations
External Review Process
Significance of Evidence-based Recommendations to Clinicians for the Management of IPF
Summary Conclusions and Treatment Recommendations
Conclusions
Treatment Recommendations
Definition and Epidemiology
Definition
Clinical Presentation
Incidence and Prevalence
Potential Risk Factors
Genetic Factors
Definition Of UIP Pattern
UIP Pattern: HRCT Features
UIP Pattern: Histopathology Features
Diagnosis
Diagnostic Criteria
Exclusion of Other Known Causes
Bronchoalveolar Lavage Cellular Analysis
Transbronchial Lung Biopsy
Serological Testing for Connective Tissues Disease
Multidisciplinary Discussion
Natural History of IPF
Acute Exacerbation of IPF
Vital Statistics
Staging and Prognosis
Demographics
Dyspnea
Physiology
HRCT Features
Composite Scoring Systems
Six-Minute-Walk Testing
Histopathology
Pulmonary Hypertension
Emphysema
Serum and Bronchoalveolar Lavage Biomarkers
Treatment
Pharmacologic Therapies
Nonpharmacologic Therapies
Selected Complications and Comorbid Conditions
Palliative Care
Monitoring the Clinical Course of Disease
Monitoring for Progressive Disease
Monitoring for Worsening Symptoms
Monitoring for Worsening Oxygenation
Monitoring for Complications and Comorbidities
Summary of Clinical Management of IPF
Future Directions
Idiopathic pulmonary fibrosis (IPF) is defined as a specific form of chronic, progressive fibrosing interstitial pneumonia of unknown cause, occurring primarily in older adults, and limited to the lungs. It is characterized by progressive worsening of dyspnea and lung function and is associated with a poor prognosis. The American Thoracic Society and European Respiratory Society (ATS/ERS), in collaboration with the American College of Chest Physicians (ACCP), published an international consensus statement in 2000 on the diagnosis and management of IPF (1). Importantly, the statement recognized IPF as a distinct clinical entity associated with the histologic appearance of usual interstitial pneumonia (UIP), and provided specific recommendations for clinicians regarding its diagnosis and management. Since the publication of the 2000 ATS/ERS statement, studies have used the ATS/ERS statement recommendations to further our understanding of the clinical manifestations and course of IPF. The accumulated data and observations made in these studies allow us to provide new guidelines for the diagnosis and management of IPF based on the best available evidence using ATS/ERS methodology.
OBJECTIVE
This document is an international evidence-based guideline on the diagnosis and management of IPF. The purpose of these guidelines is to analyze the additional evidence accumulated since the publication of the 2000 ATS/ERS consensus statement and to provide evidence-based recommendations for management, with an emphasis on diagnosis and treatment. This document is intended to replace the previous ATS/ERS IPF consensus statement, and will be updated when appropriate in accordance with the policy of the sponsoring societies.
The primary objective of this document is to provide recommendations based on a thorough review of the evidence published to date using the GRADE methodology (see below) to clinicians in a transparent manner. It is intended to empower clinicians to interpret these recommendations in the context of individual patient values and preferences, and to make appropriate decisions regarding all aspects of disease management, tailored to the patient with typical IPF.
METHODS
Committee Composition
This guideline is a collaborative effort between the ATS, ERS, Japanese Respiratory Society (JRS), and Latin American Thoracic Association (ALAT). The project chair (G.R.) nominated two co-chairs (J.J.E. and F.J.M.) and a group of experts in IPF and/or evidence-based methodology from North America, Europe, Asia, and South America. This group consisted of clinicians with recognized expertise in IPF and interstitial lung diseases (24 pulmonologists, 4 radiologists, and 4 pathologists), 4 methodologist (also a general pulmonologist), and 1 chief librarian, assisted by 2 librarians experienced with literature searches for pulmonary diseases. This group was approved by and represented the membership of the four sponsoring societies.
Disclosure of Conflicts of Interest
Panel members disclosed all potential conflicts of interest. The chair discussed and resolved all potential conflicts of interest with committee members. All potential conflicts of interest (including those of the chair and co-chairs) were discussed with the chair of the Ethics and Conflict of Interest Committee of the ATS.
During all deliberations, members with perceived conflicts of interest abstained from voting on specific recommendations related to the conflict of interest. Furthermore, members were reminded to consider their own and other members' potential conflicts of interest when discussing and voting on recommendations. In addition, other potential conflict of interest, if any (e.g., academic conflicts of interest), that were not apparent in the formal disclosures were left to be resolved by individual committee members based on their own conscience, judgment, and discretion in making recommendations (i.e., voting). The reference librarians did not participate in voting for any of the recommendations.
Committee Meetings and Evidence Review Process
The committee was divided into subgroups, and each subgroup was provided with articles relevant to their respective sections and/or questions. The subgroups were tasked with reviewing the literature, developing relevant questions, and developing preliminary section drafts. Four face-to-face meetings were held in which the subgroup drafts were reviewed. For certain sections, evidence-based recommendations were discussed, voted on, and finalized by the entire committee.
Document Preparation
The chair and a member of the committee (H.R.C.) integrated the draft sections and voting results into a preliminary document that was circulated among the committee members for further input. Input from the committee members was incorporated into the document which was read and edited further by an editing committee (G.R., H.R.C., J.H.R., J.B., M.E., K.R.F., and H.J.S.) via live webinar-teleconference. A final draft document was reviewed by the full committee, finalized, approved, and submitted to the ATS and ERS for peer review. The document was revised to incorporate the pertinent comments suggested by the external reviewers and the input provided by the editor of the ATS documentation and implementation committee. The drafted revised document was read and edited via webinar-teleconference (G.R., J.J.E., F.J.M., H.R.C., and H.J.S.) and circulated to the entire committee for further input. A pre-final draft of the revised document was subsequently finalized via webinar-teleconference (G.R., J.J.E., F.J.M., H.R.C., and H.J.S.). Concerns raised by some committee members regarding the choice of most appropriate words to convey the significance of recommendations were resolved by consensus reached by all concerned, which included the chair (G.R.), co-chairs (F.J.M. and J.J.E.), and committee members (H.J.S., H.R.C., A.U.W., U.C., and J.B.), and incorporated in the document. One committee member (R.D.B.) requested not to be a co-author of the final document due to his concerns regarding the methodology used for the treatment section. Since he participated in voting and document preparation, he is listed as a committee member. The revised document was reviewed by the authors, finalized, approved, and submitted to the editor of the ATS documentation and implementation committee.
Document Structure
This document is structured to provide an evidence-based review of the current state of knowledge regarding IPF, and contains guidelines for the management of IPF that include definition and epidemiology; risk factors; natural history; staging and prognosis; monitoring disease course; future directions. For the diagnosis and treatment sections, pragmatic GRADE evidence-based methodology was applied (2, 3). These sections were organized around specific questions as described below. The committee performed a complete systematic review of the literature for the questions focused on treatment. The literature searches and assessment of the evidence followed the GRADE approach to rate the quality of evidence and strength of the recommendations for all questions in the diagnosis and treatment sections. The remaining sections were written after a thorough review of the available literature in a narrative review format.
Formulation of the Topic Sections and Questions
Relevant section topics and questions were identified by committee members. Additional input was sought from general pulmonologists in the community and at academic centers.
Literature Review and Preparation of Evidence Profiles
An evidence profile was created for each question using the GRADE methodology (2, 3). A MEDLINE search from 1996 to December 2006 was performed at the beginning of the committee's work, with periodic updates during document development and finalization through May 31, 2010. Searching the literature before 1996 was not done systematically, since it had been searched extensively for the 2000 Consensus Statement (1). The current search was augmented by searches of EMBASE and committee member files. The literature search was limited to manuscripts published in the English language and English abstracts available from articles published in other languages. For the section on IPF treatment, we utilized the methodology of systematic review, which included meta-analysis of studies where appropriate (4–7). This review examined all relevant studies including randomized controlled trials, cohort studies, case-control studies, and cross-sectional studies. A few studies were not included in this question-based document due to the preliminary nature of their observations (8–11). For details of the literature search methodology and results, please see the online supplement.
Quality of Evidence and Strength of Recommendations
The quality of evidence was determined according to the ATS GRADE criteria (3) (Tables 1 and 2). The GRADE approach identifies all outcomes that are of importance to patients and differentiates the critical outcomes from the important but not critical ones. Recommendations depend on the evidence for all patient-important outcomes and the quality of evidence for each of those outcomes. GRADE evidence profiles are tabulated in this document for randomized controlled trials (see Treatment below). For each question, the committee graded the quality of the evidence available (high, moderate, low, or very low), and made a recommendation for or against. Recommendations were decided on the basis of majority vote. There were 31 voting members of the committee (the reference librarians were not voting members). The number of votes for, against, abstaining, and absent are reported for all treatment votes. Recommendations were either “strong” or “weak.” The strength of a recommendation reflects the extent to which one can, across the range of patients for whom the recommendation is intended, be confident that desirable effects outweigh undesirable effects (3).
TABLE 1.
QUALITY OF EVIDENCE DETERMINATION
|
Quality of Evidence |
Study Design |
Lower If: |
Higher If: |
|---|---|---|---|
| High | Randomized controlled trial |
|
|
| Moderate | Downgraded randomized controlled trial or upgraded observational study | ||
| Low | Well done observational study with control groups | ||
| Very low |
Any other evidence (e.g.,
case reports, case series) |
TABLE 2.
QUALITY OF THE EVIDENCE RATING AND IMPLICATIONS
|
Quality of the Evidence (GRADE) |
The quality of the evidence is a judgment about the extent to which we can be confident that the estimates of effect are correct. These judgments are made using the GRADE system, and are provided for each outcome. The judgments are based on the type of study design (randomized trials versus observational studies), the risk of bias, the consistency of the results across studies, and the precision of the overall estimate across studies. For each outcome, the quality of the evidence is rated as high, moderate, low, or very low using the following definitions: |
| High (⊕⊕⊕⊕) | Further research is very unlikely to change our confidence in the estimate of effect. |
| Moderate (⊕⊕⊕○) | Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. |
| Low (⊕⊕○○) | Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. |
| Very low (⊕○○○) | We are very uncertain about the estimate. (For more information about the GRADE system, see: www.gradeworkinggroup.org) |
All recommendations were made after face-to-face, detailed discussions of the evidence profile and quality by committee members present at the face-to-face discussions. While the recommendation on the use of pirfenidone had been made by the committee members during the face-to-face discussions, the question was revisited because of the subsequent release of substantial additional scientific evidence. The ATS and ERS also recommended including the additional scientific data from just-completed clinical trials of pirfenidone that had been released to the scientific and public domain in the committee's recommendation. This new evidence, including a meta-analysis of the available pirfenidone data, was sent to all members of the committee electronically, and the final voting for pirfenidone was made by e-mail. Thus, the total number of votes for the pirfenidone question reflects all the voting members of the committee; that is, it included the votes of the members who were not present during the prior face-to-face discussions of pirfenidone and other topics.
Newer data published subsequent to the final formal face-to-face voting was not considered for evidence-based recommendations because there was not sufficient time for a thorough review and consideration of the data by the committee members. These newer data that were not subjected to formal face-to-face discussion are provided as a summarized narrative in the text of the document. These and all other new pertinent published data will be considered for formal evidence-based recommendations in future updates of this document.
External Review Process
This document was subjected to review by the ATS Board of Directors and ERS Science Committee as well as external peer review. The final document met the approval of the governing bodies of the ATS, ERS, JRS, and ALAT.
SIGNIFICANCE OF EVIDENCE-BASED RECOMMENDATIONS TO CLINICIANS FOR THE MANAGEMENT OF IPF
Over the last decade, there has been an increasing body of evidence pertinent to the clinical management of IPF. This committee has reviewed the extensive literature published to date, and recommendations are provided based on a robust and transparent methodology. Since the process is transparent, the recommendations provided empower the clinician confronted with the patient with typical IPF to make the most appropriate decisions tailored to the patient's values and preferences.
Clinicians need guidance to interpret evidence-based recommendations, in particular the direction and strength of a recommendation (Table 3). Recommendations against certain interventions are particularly important if an expert committee (guideline panel) is concerned that current practice needs to change and if the evidence indicates that there may be more harm than benefit from an intervention that is frequently used. It should be emphasized that evidence-based recommendations are for typical patients. For individual patients, the best decision may sometimes not be the one recommended by evidence-based guidelines. Factors that influence such decisions are primarily related to patients' values and preferences. Some patients may be willing to accept possible adverse consequences even if expected benefits are small; others may not.
TABLE 3.
IMPLICATIONS OF RECOMMENDATIONS FOR PATIENTS, CLINICIANS, AND POLICY MAKERS
|
Strong |
Weak |
|||||
|---|---|---|---|---|---|---|
| “Strong
Yes” |
“Strong
No” |
“Weak
Yes” |
“Weak
No” |
|||
| Patients | Most people in this situation would want the intervention and only a small proportion would not | Most people in this situation would not want the intervention and only a small proportion would | The majority of people in this situation would want the intervention, but many would not | The majority of people in this situation would not want the intervention, but many would | ||
| Clinicians | Most patients should receive the recommended course of action | Be more prepared to help patients to make a decision that is consistent with the patient's own values | ||||
| Policy Makers |
The recommendation can be
adopted as a policy in most situations |
There is a need for
substantial debate and involvement of stakeholders |
||||
The strength of the recommendations is either strong or weak based on the quality of evidence and the voting of the committee members. When the recommendation is for the use of a specific treatment (or a specific question), it is denoted as a “YES,” and when the recommendation is against the use of the specific treatment (or a specific question), it is denoted as a “NO.” Thus the recommendations are either (1) STRONG–YES, (2) STRONG–NO, (3) WEAK–YES, or (4) WEAK–NO.
A strong recommendation implies that most patients would want the recommended course of action. A weak recommendation implies that the majority of patients would want the intervention, but many would not. Specifically, a weak negative recommendation implies that the majority of patients would not want the intervention, but many would. In the case of a weak recommendation, clinicians are especially required to spend adequate time with patients to discuss patients' values and preferences. Such an in-depth discussion is necessary for the patient to make the best decision. This may lead a significant proportion of patients to choose an alternative approach. Fully informed patients are in the best position to make decisions that are consistent with the best evidence and patients' values and preferences.
The committee recognizes that regulatory agencies review applications seeking their approval for use of specific drugs for treatment of IPF, and decisions regarding approval are made according to set policies and procedures of the agencies.
SUMMARY CONCLUSIONS AND TREATMENT RECOMMENDATIONS
Conclusions
IPF is defined as a specific form of chronic, progressive fibrosing interstitial pneumonia of unknown cause, occurring primarily in older adults, limited to the lungs, and associated with the histopathologic and/or radiologic pattern of UIP.
- The diagnosis of IPF requires:
- Exclusion of other known causes of interstitial lung disease (ILD) (e.g., domestic and occupational environmental exposures, connective tissue disease, and drug toxicity).
- The presence of a UIP pattern on high-resolution computed tomography (HRCT) in patients not subjected to surgical lung biopsy.
-
Specific combinations of HRCT and surgical lung biopsy pattern in patients subjected to surgical lung biopsy.The major and minor criteria proposed in the 2000 ATS/ERS Consensus Statement have been eliminated.
The accuracy of the diagnosis of IPF increases with multidisciplinary discussion between pulmonologists, radiologists, and pathologists experienced in the diagnosis of ILD.
- IPF is a fatal lung disease; the natural history is variable and unpredictable:
- Most patients with IPF demonstrate a gradual worsening of lung function over years; a minority of patients remains stable or declines rapidly.
- Some patients may experience episodes of acute respiratory worsening despite previous stability.
Disease progression is manifested by increasing respiratory symptoms, worsening pulmonary function test results, progressive fibrosis on HRCT, acute respiratory decline, or death.
Patients with IPF may have sub-clinical or overt co-morbid conditions including pulmonary hypertension, gastroesophageal reflux, obstructive sleep apnea, obesity, and emphysema. The impact of these conditions on the outcome of patients with IPF is unclear.
Treatment Recommendations
The recommendations detailed below are based on the GRADE approach outlined in the introductory section (3). The committee felt the preponderance of evidence to date suggests that pharmacologic therapy for IPF is without definitive, proven benefit. For this reason, the committee has chosen to make recommendations of varying strength against most therapies.
Treatment recommendations for specific therapies are the following (the quality of evidence is in parenthesis, presented as one to four plus signs, with zeroes as place holders where there are fewer than four plus signs):
- The recommendation against the use of the following agents for the treatment of IPF is strong:
- Corticosteroid monotherapy (⊕○○○)
- Colchicine (⊕○○○)
- Cyclosporine A (⊕○○○)
- Combined corticosteroid and immune-modulator therapy (⊕⊕○○)
- Interferon γ 1b (⊕⊕⊕⊕)
- Bosentan (⊕⊕⊕○)
- Etanercept (⊕⊕⊕○)
- The recommendation against the use of the following agents for the treatment of IPF is weak; that is, these therapies should not be used in the majority of patients with IPF, but may be a reasonable choice in a minority:
- Combined acetylcysteine and azathioprine and prednisone (⊕⊕○○)
- Acetylcysteine monotherapy (⊕⊕○○)
- Anticoagulation (⊕○○○)
- Pirfenidone (⊕⊕○○)
The recommendation for long-term oxygen therapy in patients with IPF and clinically significant resting hypoxemia is strong (⊕○○○).
The recommendation for lung transplantation in appropriate patients with IPF is strong (⊕○○○).
The recommendation against mechanical ventilation in patients with respiratory failure due to IPF is weak; that is, mechanical ventilation should not be used in the majority of patients with IPF, but may be a reasonable choice in a minority (⊕⊕○○).
The recommendation for pulmonary rehabilitation in patients with IPF is weak; that is, pulmonary rehabilitation should be used in the majority of patients with IPF, but not using pulmonary rehabilitation may be a reasonable choice in a minority (⊕⊕○○).
The recommendation for corticosteroids in patients with acute exacerbation of IPF is weak; that is, corticosteroids should be used in the majority of patients with acute exacerbation of IPF, but not using corticosteroids may be a reasonable choice in a minority (⊕○○○).
The recommendation against the treatment of pulmonary hypertension associated with IPF is weak; that is, pulmonary hypertension should not be treated in the majority of patients with IPF, but treatment may be a reasonable choice in a minority (⊕○○○).
The recommendation for the treatment of asymptomatic gastroesophageal reflux in patients with IPF is weak; that is, asymptomatic gastroesophageal reflux should be treated in the majority of patients with IPF, but not treating asymptomatic gastroesophageal reflux may be a reasonable choice in a minority (⊕○○○).
Based on the evidence published to date, there is no proven pharmacological therapy for IPF. While a few studies have suggested potential benefits from some pharmacologic agents, the recommendations made by the committee for these agents were “weak no.” For the well-informed patient who strongly desires pharmacologic treatment, it is suggested that the choice of agent may be made from therapies that received a weak recommendation against their use (“weak no”).
Continued, concerted efforts should be made by physicians, patients, and sponsors to pursue well-designed clinical trials aimed at improving outcomes, including quality of life, in patients with IPF. The committee recognizes the need to update treatment recommendation when new and pertinent high-quality evidence regarding the use of other treatment becomes available for scientific review.
DEFINITION AND EPIDEMIOLOGY
Definition
IPF is defined as a specific form of chronic, progressive fibrosing interstitial pneumonia of unknown cause, occurring primarily in older adults, limited to the lungs, and associated with the histopathologic and/or radiologic pattern of UIP defined below (1, 12, 13). The definition of IPF requires the exclusion of other forms of interstitial pneumonia including other idiopathic interstitial pneumonias and ILD associated with environmental exposure, medication, or systemic disease (1, 12).
Clinical Presentation
IPF should be considered in all adult patients with unexplained chronic exertional dyspnea, and commonly presents with cough, bibasilar inspiratory crackles, and finger clubbing (14–16). The incidence of the disease increases with older age, with presentation typically occurring in the sixth and seventh decades (16–19). Patients with IPF aged less than 50 years are rare; such patients may subsequently manifest overt features of an underlying connective tissue disease that was subclinical at the time IPF was diagnosed (20, 21). More men have been reported with IPF than women, and the majority of patients have a history of cigarette smoking (14–17, 22, 23).
Incidence and Prevalence
There are no large-scale studies of the incidence or prevalence of IPF on which to base formal estimates. The incidence of IPF was estimated at 10.7 cases per 100,000 per year for men and 7.4 cases per 100,000 per year for women in a population-based study from the county of Bernalillo, New Mexico (23). A study from the United Kingdom reported an overall incidence rate of only 4.6 per 100,000 person-years, but estimated that the incidence of IPF increased by 11% annually between 1991 and 2003 (16). This increase was not felt to be attributable to the aging of the population or increased ascertainment of milder cases. A third study from the United States estimated the incidence of IPF to be between 6.8 and 16.3 per 100,000 persons using a large database of healthcare claims in a health plan (20).
Prevalence estimates for IPF have varied from 2 to 29 cases per 100,000 in the general population (17, 22–25). The wide range in these numbers is likely explained by the previous lack of uniform definition used in identifying cases of IPF, as well as by differences in study designs and populations. A recent analysis based on healthcare claims data of a large health plan in the United States yielded a prevalence estimate of between 14.0 and 42.7 per 100,000 persons depending on the case definition used (20). It is unknown if the incidence and prevalence of IPF are influenced by geographic, ethnic, cultural, or racial factors.
Potential Risk Factors
Although idiopathic pulmonary fibrosis is, by definition, a disease of unknown etiology, a number of potential risk factors have been described.
Cigarette smoking.
Smoking is strongly associated with IPF, particularly for individuals with a smoking history of more than 20 pack-years (22, 26–31). This applies to familial as well as sporadic IPF (29).
Environmental exposures.
Increased risk for IPF has been found to be associated with a variety of environmental exposures (22, 26, 27, 30, 32–34). A significantly increased risk has been observed after exposure to metal dusts (brass, lead, and steel) and wood dust (pine) (26, 30, 33) Farming, raising birds, hair dressing, stone cutting/polishing, and exposure to livestock and to vegetable dust/animal dust have also been associated with IPF (27). Supporting an environmental etiology, increased numbers of inorganic particles have been detected in lymph nodes of patients with pulmonary fibrosis in autopsy studies (35). These observations must be interpreted with great caution, since epidemiologic studies of environmental risk factors are subject to a variety of biases and limitations.
Microbial agents.
Several studies have investigated the possible role of chronic viral infection in the etiology of IPF (30, 36–52). Most research has been focused on Epstein-Barr virus (EBV) (38, 40, 41, 44–46, 48, 50, 52) and hepatitis C (30, 36, 37, 39, 42, 47, 49). Both the protein and the DNA of EBV have been identified in lung tissue of patients with IPF, usually in the alveolar epithelial cells (38, 44). EBV genome rearrangement, which is associated with productive EBV replication, was found in 11 of 18 EBV DNA-positive IPF biopsies (48). Tang and coworkers tested for the presence of eight herpesviruses, including EBV, in lung specimens from 33 patients with IPF, and found that one or more herpesviruses were detected in almost all IPF lungs compared with one-third of the control lungs (50). The positive viruses include EBV, cytomegalovirus, human herpesvirus (HHV)-7, and HHV-8. However, negative association studies have also been reported (41, 52). Variable results have emerged from studies of hepatitis C (30, 36, 37, 39, 42, 47, 49). Elevation of serum antibodies to cytomegalovirus has been reported (43), while associations with other viruses, including BK and JC polyomaviruses, have not been found (51).
The evaluation of putative associations of virus, and other microbes, with IPF is hindered by confounding factors: patients were likely receiving immunosuppressive therapy, making infection a potential complication of therapy (40); the prevalence of EBV in general population is high: in one study, EBV DNA was detected in 96% of patients with IPF, but also in 100% of fibrotic lungs secondary to systemic sclerosis and in 71% of control lungs (45). Despite the large number of studies to date, definitive conclusions about the role of infection in IPF cannot be made.
Gastroesophageal reflux.
Several studies have suggested that abnormal acid gastroesophageal reflux (GER), through its presumed association with microaspiration, is a risk factor for IPF. Abnormal GER is common in patients with IPF (19, 53, 54). In a Veterans Administration case-control study, GER-associated erosive esophagitis was linked with a number of respiratory diseases, including pulmonary fibrosis (55). GER is clinically silent in the majority of patients with IPF (19, 53), and the typical symptoms of heartburn and regurgitation do not distinguish between those with and without GER (54). GER is frequent in the normal population as well as in patients with other advanced lung diseases such as lung fibrosis associated with scleroderma (56). Since abnormal GER may have nonacid components, alkaline GER may also be important in patients with IPF. It is unknown if changes in intrathoracic pressure, as a result of poorly compliant lung, lead to abnormal GER. Nevertheless, the putative role of GER in IPF warrants further study.
Other risk factors such as diabetes mellitus have been recently described (57).
Genetic Factors
Familial pulmonary fibrosis.
Although accounting for less than 5% of total patients with IPF, familial forms of IPF (i.e., those affecting two or more members of the same primary biological family) have been reported (58–64). The criteria used to define IPF in familial and sporadic cases are the same; familial IPF and sporadic IPF are clinically and histologically indistinguishable (59, 60), although familial forms may develop at an earlier age (59, 60, 64) and seem to have different patterns of gene transcription (65). The evidence of a “founder effect” (i.e., a significant geographical clustering of cases) of familial pulmonary fibrosis in the Finnish population supports the relevance of genetic factors in the development of pulmonary fibrosis (60). The results of a recent genome-wide search by the same authors suggest that ELMOD2, a gene of unknown biological function located on chromosome 4q31, may be a susceptibility gene for familial IPF (66). Many studies of apparent “familial IPF” are actually studies of familial pulmonary fibrosis, since at least half of the pedigrees demonstrate the presence of more than one type of idiopathic interstitial pneumonia (IIP) (e.g., IPF, nonspecific interstitial pneumonia [NSIP], cryptogenic organizing pneumonia [COP], unclassified ILD) (29).
The most likely mode of genetic transmission of pulmonary fibrosis in familial cases is autosomal-dominant with variable penetrance (29, 61, 62, 67, 68). A linkage with chromosome 14 has been suggested (68). More strong associations with familial idiopathic interstitial pneumonia have been found with mutations in the surfactant protein C gene (69), but this association has not been found in patients with the sporadic form of the disease (70–72). Rare mutations in the gene encoding another surfactant protein, A2 (SFTPA2), have been associated with familial pulmonary fibrosis and lung cancer (73); the locus was discovered by genetic linkage in a large pedigree and two rare mutations were discovered by sequencing candidate genes within the linked interval.
Recent reports by several investigators have documented that genetic variants within the human telomerase reverse transcriptase (hTERT) or human telomerase RNA (hTR) components of the telomerase gene are associated with familial pulmonary fibrosis and are present in some patients with sporadic IPF. These rare mutations can be found in up to 15% of familial pulmonary fibrosis kindreds and 3% of sporadic IIP cases (74–78), and result in telomere shortening that ultimately causes apoptosis of cells, including the alveolar epithelial cell.
Genetic factors in sporadic cases of IPF.
Polymorphisms of genes encoding for cytokines (interleukin [IL]-1 α, tumor necrosis factor-α, lymphotoxin α, IL-4, IL-6, IL-8, IL-10, and IL-12 [79–88]), enzymes (α1-antitrypsin [89, 90] and angiotensin-converting enzyme [91]), profibrotic molecules (transforming growth factor-β1 [92]), coagulation pathway genes (plasminogen activator inhibitors-1 and -2), genes for surfactant protein-A and -B (70), immunomodulatory genes (complement receptor 1, NOD2/CARD15 [93]), and matrix metalloproteinase (MMP)-1 (94) have been reported to have increased frequencies in patients with sporadic IPF. Many of these have also been related to disease progression. However, none of these findings has been validated in subsequent studies. Human leukocyte antigen (HLA) class I and class II allele haplotypes have a skewed distribution among patients with IPF (95), and ethnic background might have a role in determining clinical outcome (96). Recent data from a Mexican population suggests a relationship between MHC class I chain–related gene A (MICA) and IPF (94). These association studies need to be investigated in larger cohorts; at present there are no genetic factors that are consistently associated with sporadic IPF. Microarray analyses of gene expression will contribute to our understanding of pathogenesis, refinement of classification, and the targeting of candidates for therapy, but these are currently in an early phase of development (97).
While genetic studies in familial pulmonary fibrosis have provided useful insights into the pathogenesis of IPF, more functional studies that confirm their significance and studies investigating other mutations, associations, and gene–environment relationships are needed. In our present state of understanding, the committee does not recommend genetic testing in patients with either familial or sporadic IPF as part of clinical evaluation.
DEFINITION OF UIP PATTERN
UIP Pattern: HRCT Features
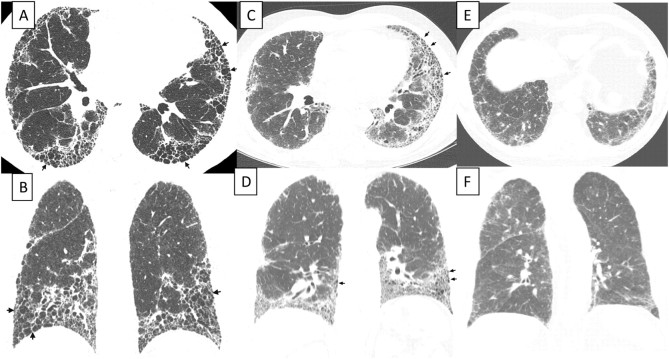
HRCT is an essential component of the diagnostic pathway in IPF (Table 4, Figure 1). The optimal HRCT technique for evaluation of ILD is provided in the online supplement (see Table E6). UIP is characterized on HRCT by the presence of reticular opacities, often associated with traction bronchiectasis (98, 99). Honeycombing is common, and is critical for making a definite diagnosis. Honeycombing is manifested on HRCT as clustered cystic airspaces, typically of comparable diameters on the order of 3–10 mm but occasionally as large as 2.5 cm. It is usually subpleural and is characterized by well-defined walls (100). Ground glass opacities are common, but usually less extensive than the reticulation. The distribution of UIP on HRCT is characteristically basal and peripheral, though often patchy. The presence of coexistent pleural abnormalities (e.g., pleural plaques, calcifications, significant pleural effusion) suggests an alternative etiology for UIP pattern. Micronodules, air trapping, nonhoneycomb cysts, extensive ground glass opacities, consolidation, or a peribronchovascular-predominant distribution should lead to consideration of an alternative diagnosis. Mild mediastinal lymph node enlargement (usually < 1.5 cm in short axis) can be seen (101, 102). The chest radiograph is less useful than HRCT in evaluating patients with suspected IPF (103).
TABLE 4.
HIGH-RESOLUTION COMPUTED TOMOGRAPHY CRITERIA FOR UIP PATTERN
|
UIP Pattern (All Four Features) |
Possible UIP Pattern (All Three Features) |
Inconsistent with UIP Pattern (Any of the Seven Features) |
|---|---|---|
| • Subpleural, basal
predominance
• Reticular abnormality
• Honeycombing
with or without traction bronchiectasis
• Absence of features
listed as inconsistent with UIP pattern (see third
column) |
• Subpleural, basal
predominance
• Reticular abnormality
• Absence of
features listed as inconsistent with UIP pattern (see third
column) |
• Upper or mid-lung
predominance
• Peribronchovascular predominance
•
Extensive ground glass abnormality (extent > reticular abnormality)
• Profuse micronodules (bilateral, predominantly upper lobes)
• Discrete cysts (multiple, bilateral, away from areas of
honeycombing)
• Diffuse mosaic attenuation/air-trapping (bilateral,
in three or more lobes)
• Consolidation in bronchopulmonary
segment(s)/lobe(s) |
Definition of abbreviation: UIP = usual interstitial pneumonia.
Figure 1.
High-resolution computed tomography (HRCT) images demonstrating usual interstitial pneumonia (UIP) pattern and possible UIP pattern. (A and B) UIP pattern, with extensive honeycombing: axial and coronal HRCT images show basal predominant, peripheral predominant reticular abnormality with multiple layers of honeycombing (arrows). (C and D) UIP pattern, with less severe honeycombing: axial and coronal CT images show basal predominant, peripheral predominant reticular abnormality with subpleural honeycombing (arrows). (E and F) Possible UP pattern: axial and coronal images show peripheral predominant, basal predominant reticular abnormality with a moderate amount of ground glass abnormality, but without honeycombing.
Several studies have documented that the positive predictive value of a HRCT diagnosis of UIP is 90 to 100% (103–108). These studies are affected by selection bias because they only included patients with biopsy-proven diagnoses. Nonetheless, a UIP pattern on HRCT is highly accurate for the presence of UIP pattern on surgical lung biopsy. If honeycombing is absent, but the imaging features otherwise meet criteria for UIP, the imaging features are regarded as representing possible UIP, and surgical lung biopsy is necessary to make a definitive diagnosis. In patients whose HRCT does not demonstrate a UIP pattern, the surgical lung biopsy may still demonstrate UIP pattern on histopathology.
UIP Pattern: Histopathology Features
The histopathologic hallmark and chief diagnostic criterion is a heterogeneous appearance at low magnification in which areas of fibrosis with scarring and honeycomb change alternate with areas of less affected or normal parenchyma (1, 12) (Table 5, Figure 2). These histopathologic changes often affect the subpleural and paraseptal parenchyma most severely. Inflammation is usually mild and consists of a patchy interstitial infiltrate of lymphocytes and plasma cells associated with hyperplasia of type 2 pneumocytes and bronchiolar epithelium. The fibrotic zones are composed mainly of dense collagen, although scattered convex subepithelial foci of proliferating fibroblasts and myofibroblasts (so-called fibroblast foci) are a consistent finding. Areas of honeycomb change are composed of cystic fibrotic airspaces that are frequently lined by bronchiolar epithelium and filled with mucus and inflammatory cells. Smooth muscle metaplasia in the interstitium is commonly seen in areas of fibrosis and honeycomb change.
TABLE 5.
HISTOPATHOLOGICAL CRITERIA FOR UIP PATTERN
|
UIP Pattern (All Four Criteria) |
Probable UIP Pattern |
Possible UIP Pattern (All Three Criteria) |
Not UIP Pattern (Any of the Six Criteria) |
|---|---|---|---|
|
|
|
|
Definition of abbreviations: HRCT = high-resolution computed tomography; UIP = usual interstitial pneumonia.
Can be associated with acute exacerbation of idiopathic pumonary fibrosis.
An isolated or occasional granuloma and/or a mild component of organizing pneumonia pattern may rarely be coexisting in lung biopsies with an otherwise UIP pattern.
This scenario usually represents end-stage fibrotic lung disease where honeycombed segments have been sampled but where a UIP pattern might be present in other areas. Such areas are usually represented by overt honeycombing on HRCT and can be avoided by pre-operative targeting of biopsy sites away from these areas using HRCT.
Figure 2.
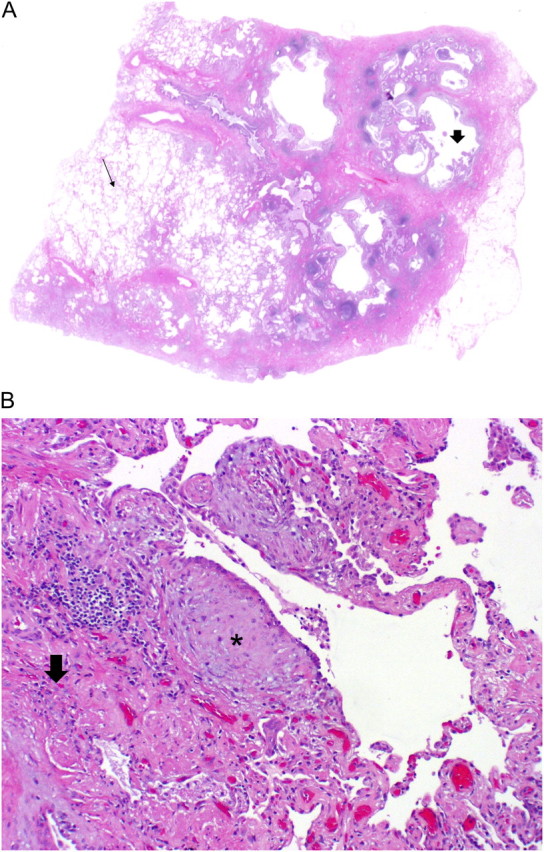
Surgical lung biopsy specimens demonstrating UIP pattern. (A) Scanning power microscopy showing a patchy process with honeycomb spaces (thick arrow), some preserved lung tissue regions (thin arrow), and fibrosis extending into the lung from the subpleural regions. (B) Adjacent to the regions of more chronic fibrosis (thick arrow) is a fibroblast focus (asterisk), recognized by its convex shape and composition of edematous fibroblastic tissue, suggestive of recent lung injury.
The differential diagnosis for UIP pattern on pathology is relatively short, especially when strict criteria for UIP are maintained. The major differential diagnostic considerations include UIP in other clinical settings such as connective tissue diseases, chronic hypersensitivity pneumonitis (extrinsic allergic alveolitis), and pneumoconioses (especially asbestosis).
Some biopsies may reveal a pattern of fibrosis that does not meet the above criteria for UIP pattern (1). These biopsies may be termed “nonclassifiable fibrosis.” In the absence of histologic features diagnostic of an alternative condition (e.g., hypersensitivity pneumonitis, sarcoidosis, etc.), such biopsies may be consistent with the diagnosis of IPF (Tables 5 and 6) in the appropriate clinical and radiologic setting and after careful multidisciplinary discussion.
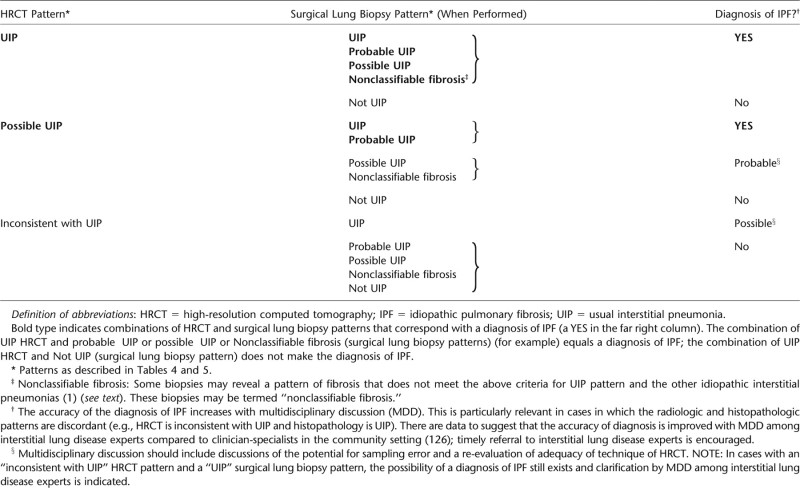
TABLE 6.
COMBINATION OF HIGH-RESOLUTION COMPUTED TOMOGRAPHY AND SURGICAL LUNG BIOPSY FOR THE DIAGNOSIS OF IPF (REQUIRES MULTIDISCIPLINARY DISCUSSION)
 |
DIAGNOSIS
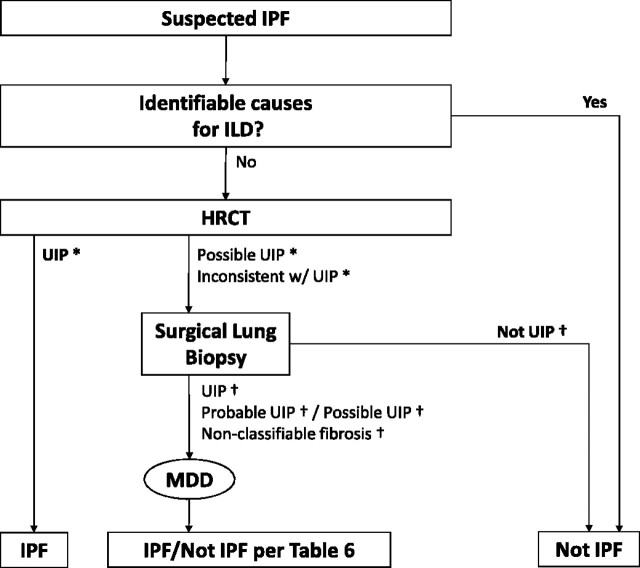
Diagnostic criteria and schema for adult patients with ILD and suspected IPF are presented in Figure 3 and Table 6. Careful exclusion of alternative etiologies through multidisciplinary discussion between pulmonologists, radiologists, and pathologists experienced in the diagnosis of ILD is of the utmost importance to an accurate diagnosis. In situations in which multidisciplinary discussion is not feasible, it is recommended that patients be referred to experienced clinical experts in ILD for consultation.
Figure 3.
Diagnostic algorithm for idiopathic pulmonary fibrosis (IPF). Patients with suspected IPF (i.e., patients with unexplained dyspnea on exertion and/or cough with evidence of interstitial lung disease [ILD]) should be carefully evaluated for identifiable causes of ILD. In the absence of an identifiable cause for ILD, an HRCT demonstrating UIP pattern is diagnostic of IPF. In the absence of UIP pattern on HRCT, IPF can be diagnosed by the combination of specific HRCT and histopathological patterns. The accuracy of the diagnosis of IPF increases with multidisciplinary discussion (MDD) among ILD experts. *Refer to Table 4 for definitions. †Refer to Table 5 for definitions.
The diagnostic criteria for IPF presented in this document have been significantly modified from those stated in the previous ATS/ERS Statement (1). Given the high-quality evidence regarding HRCT specificity for the recognition of histopathologic UIP pattern, surgical lung biopsy is not essential (104, 105, 109, 110). In the appropriate clinical setting (as described in the clinical presentation section above; this includes a thorough medical, occupational/environmental and family history, physical examination, physiological testing, and laboratory evaluation), the presence of a UIP pattern on HRCT is sufficient for the diagnosis of IPF. Thus, the major and minor criteria for the clinical (i.e., nonpathologic) diagnosis of IPF have been eliminated.
Diagnostic Criteria
The diagnosis of IPF requires the following:
Exclusion of other known causes of ILD (e.g., domestic and occupational environmental exposures, connective tissue disease, and drug toxicity).
The presence of a UIP pattern on HRCT in patients not subjected to surgical lung biopsy (see Table 4).
Specific combinations of HRCT and surgical lung biopsy pattern in patients subjected to surgical lung biopsy (see Tables 5 and 6).
Thus, the accuracy of diagnosis of IPF increases with clinical, radiologic, and histopathologic correlation and can be accomplished with a multidisciplinary discussion among experienced clinical experts in the field of ILDs (111). This is particularly relevant in cases in which the radiologic and histopathologic patterns are discordant (e.g., HRCT is inconsistent with UIP and histopathology is UIP). An HRCT or pathologic UIP pattern is not 100% specific to IPF (1, 12, 112–114). Discordant histologic patterns on surgical lung biopsy specimens obtained from different segments have been described. Cases with coexisting UIP pattern and fibrotic NSIP pattern (discordant UIP) appear to behave similarly to those with UIP pattern in all lobes (concordant UIP) (115, 116). This supports the obtainment of surgical lung biopsies from multiple lobes in patients with suspected IPF.
Several studies have compared VATS to open thoracotomy (117–120). The diagnostic yield from surgical lung biopsies obtained from VATS and open thoracotomy are similar. While VATS may be associated with lower morbidity and length of stay than open thoracotomy, the decision on which procedure to perform should be based on individual patient characteristics and surgical expertise. In patients with severe physiologic impairment or substantial comorbidity, the risks of surgical lung biopsy may outweigh the benefits of establishing a secure diagnosis of IPF. The final decision regarding whether or not to pursue a surgical lung biopsy must be tailored to the clinical situation of the individual patient.
Exclusion of Other Known Causes
The exclusion of other known causes of ILD is a broad and inherently subjective criterion, but several specific points should be made. A careful history and physical examination focusing on comorbidities, medication use, environmental exposures, and family history is essential, and physicians should utilize a standardized approach. While there are no validated tools for this, a template, such as the one available through the American College of Chest Physicians (http://www.chestnet.org/memberResources/downloads/networks/IDLDquestionnaire.pdf), may be of use. It is of particular importance to evaluate patients thoroughly for possible chronic hypersensitivity pneumonitis, since such patients may mimic IPF. The inciting antigen may not be identifiable in some patients despite a thorough search (121); bronchoalveolar lavage (BAL) showing a lymphocytosis of 40% or greater may suggest occult hypersensitivity pneumonitis in this setting, prompting further investigations for environmental exposures, and possibly a surgical lung biopsy. Patients who meet established criteria for connective tissue disease do not have IPF. Younger patients, especially women, without clinical or serologic features at presentation may subsequently manifest clinical features of connective tissue disease. Therefore, the index of suspicion for connective tissue disease in younger patients (under the age of 50 yr) should be high.
- Question: Should BAL cellular analysis be performed in the diagnostic evaluation of suspected IPF?
- Cellular analyses of BAL can be useful in the diagnosis of certain forms of ILD. In the evaluation of patients with suspected IPF, the most important application of BAL is in the exclusion of chronic hypersensitivity pneumonitis; prominent lymphocytosis (> 40%) should suggest the diagnosis. Recent retrospective data suggest that 8% of patients with an HRCT UIP pattern may have BAL findings suggestive of an alternative diagnosis (122). It is unclear whether BAL adds significant diagnostic specificity to a careful exposure history and clinical evaluation.
- Recommendation: BAL cellular analysis should not be performed in the diagnostic evaluation of IPF in the majority of patients, but may be appropriate in a minority (weak recommendation, low-quality evidence).
- Values: This recommendation places a high value on the additional risk and cost of BAL in patients with IPF and a low value on possible improved specificity of diagnosis.
- Remarks: This recommendation is only for BAL differential cell count (“cellular analysis”). It does not refer to the use of BAL in the evaluation of infection, malignancy, etc. At present, BAL cellular analysis should be considered in the evaluation of patients with IPF at the discretion of the treating physician based on availability and experience at their institution/regional laboratory. (Vote: 4 for the use of BAL, 18 against the use of BAL, 1 abstention, 8 absent.)
- Question: Should transbronchial lung biopsy be used in the evaluation of suspected IPF?
- Transbronchial lung biopsy is useful in the evaluation of selected conditions (e.g., granulomatous disorders such as sarcoidosis). A UIP pattern on HRCT makes these conditions unlikely (104, 105, 109). In cases requiring histopathology, the specificity and positive predictive value of UIP pattern identified by transbronchial biopsy has not been rigorously studied. While transbronchial biopsy specimens may show all the histologic features of UIP (123), the sensitivity and specificity of this approach for the diagnosis for UIP pattern is unknown. It is also unknown how many and from where transbronchial biopsies should be obtained.
- Recommendation: Transbronchial biopsy should not be used in the evaluation of IPF in the majority of patients, but may be appropriate in a minority (weak recommendation, low-quality evidence).
- Values: This recommendation places a high value on the additional morbidity of transbronchial lung biopsy in patients with IPF who will subsequently undergo surgical lung biopsy and low value on possible diagnostic specificity.
- Remarks: (Vote: none for the use of transbronchial biopsy, 23 against the use of transbronchial biopsy, no abstentions, 8 absent.)
- Question: Should serologic testing for connective tissues disease be used in the evaluation of suspected IPF?
- There are no reliable data on the role of screening serologies in patients with suspected IPF. Connective tissue disease can present with a UIP pattern (124), and ILD has been described as the sole clinical manifestation of these conditions and can precede the overt manifestation of a specific connective tissue disease (125).
- Recommendation: Serologic testing for connective tissue disease should be performed in the evaluation of IPF in the majority of patients, but may not be appropriate in a minority (weak recommendation, very low-quality evidence).
- Values: This recommendation places a high value on distinguishing connective tissue disease from IPF and low value on cost.
- Remarks: Serologic evaluation should be performed even in the absence of signs or symptoms of connective tissue disease, and should include rheumatoid factor, anti-cyclic citrullinated peptide, and anti-nuclear antibody titer and pattern. The routine use of other serological tests such as antisynthetase antibodies (e.g., Jo-1), creatine kinase and aldolase, Sjogren's antibodies (SS-A, SS-B), and scleroderma antibodies (scl-70, PM-1) is of unclear benefit, but may be helpful in selected cases. Patients with IPF may have a mildly positive antinuclear antibody titer and/or rheumatoid factor level without any other clinical features of connective tissue. Such patients should be carefully screened for signs and symptoms of connective tissues disease (e.g., arthritis, Raynaud's phenomenon, skin changes, abnormal esophageal motility). In the absence of additional serologic or clinical evidence to support a connective tissues diagnosis, the diagnosis of IPF is appropriate. Repeat serologic and clinical evaluation during follow up may subsequently confirm the development of a connective tissue disease; in such cases, the diagnosis should be revised. (Vote: 23 for the use of serologic testing, none against the use of serologic testing, no abstentions, 8 absent.)
- Question: Should a multi-disciplinary discussion be used in the evaluation of suspected IPF?
- The diagnosis of IPF is, by definition, multidisciplinary, drawing on the expertise of experienced clinicians, radiologists, and pathologists. Proper communication between the various disciplines involved in the diagnosis of IPF (pulmonary, radiology, pathology) has been shown to improve inter-observer agreement among experienced clinical experts as to the ultimate diagnosis (111, 126).
- Recommendation: We recommend that a multi-disciplinary discussion should be used in the evaluation of IPF (strong recommendation, low-quality evidence).
- Values: This recommendation places a high value on the accurate diagnosis of IPF and a low value on the access to and availability of experts for multidisciplinary discussion.
- Remarks: It is recognized that a formal multidisciplinary discussion (MDD) between the treating pulmonologist, radiologist, and pathologist is not possible for many practitioners. Effort should be made, however, to promote verbal communication between specialties during the evaluation of the case. There are data to suggest that the accuracy of diagnosis is improved through MDD among ILD experts compared with MDD among specialists in the community setting (126); timely referral to ILD experts is encouraged. (Vote: 23 for the use of multidisciplinary discussion, none against the use of multidisciplinary discussion, no abstentions, 8 absent.)
NATURAL HISTORY OF IPF
The natural history of IPF has been described as a progressive decline in subjective and objective pulmonary function until eventual death from respiratory failure or complicating comorbidity (127–129). Available longitudinal studies do not allow a clear assessment of median survival in IPF. Several retrospective longitudinal studies suggest a median survival time from 2 to 3 years from the time of diagnosis (130–134). However, recent data from clinical trials of patient with preserved pulmonary function suggest this may be an underestimate (135–137).
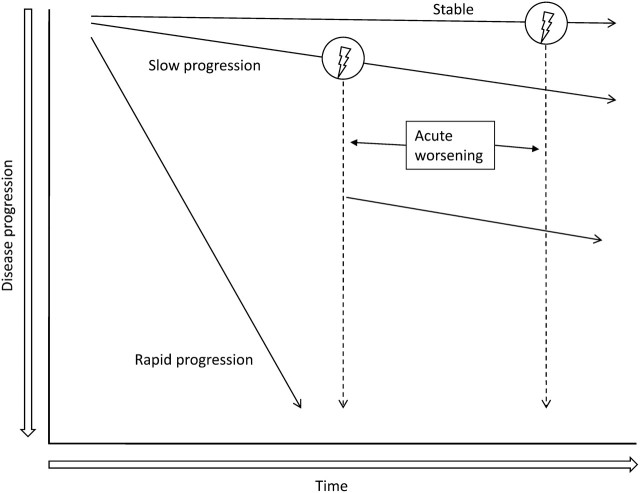
There appear to be several possible natural histories for patients with IPF (Figure 4) (138). For a given patient, the natural history is unpredictable at the time of the diagnosis. The majority of patients demonstrate a slow, gradual progression over many years. Some patients remain stable while others have an accelerated decline (139). Some patients may experience episodes of acute respiratory worsening. It is unknown if these different natural histories represent distinct phenotypes of IPF or if the natural history is influenced by geographic, ethnic, cultural, racial, or other factors. Other comorbid conditions such as emphysema and pulmonary hypertension may impact the disease course (140–142).
Figure 4.
Natural history of IPF. There appear to be several possible natural histories for patients with IPF. The majority of patients experience a slow but steady worsening of their disease (“Slow progression”). Some patients remain stable (“Stable”), while others have an accelerated decline (“Rapid progression”). A minority of patients may experience unpredictable acute worsening of their disease (lightning bolt), either from a secondary complication such as pneumonia, or for unrecognized reasons. This event may be fatal or may leave patients with substantially worsened disease. The relative frequency of each of these natural histories is unknown.
Acute Exacerbation of IPF
Recent observations have suggested that acute respiratory worsening occurs in a small minority of patients with IPF annually (approximately 5–10%) (137, 143, 144). These episodes may occur secondary to common conditions such as pneumonia, pulmonary embolism, pneumothorax, or cardiac failure (145, 146). When a cause cannot be identified for the acute respiratory decline, the term acute exacerbation of IPF has been used (144, 145, 147–157). It is presently unclear if acute exacerbation of IPF is simply a manifestation of an unidentified respiratory complication (such as pulmonary emboli, infection) contributing to an acute worsening in a patient with IPF or represents an inherent acceleration in the pathobiological processes involved in IPF. Recent data from gene expression profiling of patients with acute exacerbation of IPF do not suggest an infectious etiology (158).
Historically, criteria for acute exacerbation of IPF have included an unexplained worsening of dyspnea within 1 month, evidence of hypoxemia as defined by worsened or severely impaired gas exchange, new radiographic alveolar infiltrates, and an absence of an alternative explanation such as infection, pulmonary embolism, pneumothorax, or heart failure (143). Acute exacerbation can occur at any point in the course of IPF and occasionally can be its presenting manifestation (149, 153, 159, 160). Worsened cough, fever, and/or increased sputum have been observed (148, 149, 153). While there are no known risk factors for acute exacerbation of IPF, there have been reports of acute respiratory decompensation after thoracic surgery (161–165) and bronchoalveolar lavage (149, 166). It is unclear whether or not these events represent true acute exacerbations or complications of the respective procedures.
Acute exacerbation of IPF histologically manifests as acute or organizing diffuse alveolar damage (DAD), or, less commonly, organizing pneumonia in zones of relatively preserved lung tissue away from the most fibrotic regions (143). Anecdotal experience indicates that sampling issues in some patients may result in specimens demonstrating only uncomplicated UIP or the organizing phase of DAD without histologic evidence of underlying UIP in the sample evaluated (153).
Vital Statistics
Deaths from pulmonary fibrosis increase with increasing age (18, 167). In addition, there is evidence to suggest increasing mortality from pulmonary fibrosis over the past two decades (18, 167). A recent analysis of the death certificate data in the United States noted a significant increase in mortality from pulmonary fibrosis from 1992 to 2003 (167). When the most rigorous definition of IPF was applied, the mortality rate in the United States in 2003 was 61.2 deaths per 1,000,000 in men and 54.5 per 1,000,000 in women (167). In Japan, the mortality rate for IPF was estimated to be 33 per 1,000,000 in men and 24 per 1,000,000 in women (22). The mortality burden attributable to IPF is higher than that of some cancers (168). Recent evidence suggests that mortality from IPF in the United States is greater in the winter months (169). The most common cause of death is progressive lung disease (60% of deaths) (146, 167). Additional causes of morbidity and mortality in patients with IPF include coronary artery disease (170), pulmonary embolism, and lung cancer.
STAGING AND PROGNOSIS
The extent of disease and the severity of functional impairment of patients with IPF at the time of diagnosis are variable. The reasons for this are thought to be variation in subjective perception of symptoms and differences in providers' awareness. Recent studies have clarified predictors of survival in IPF. However, the accuracy of these predictors is limited by the retrospective nature of some of these studies and variations in study design.
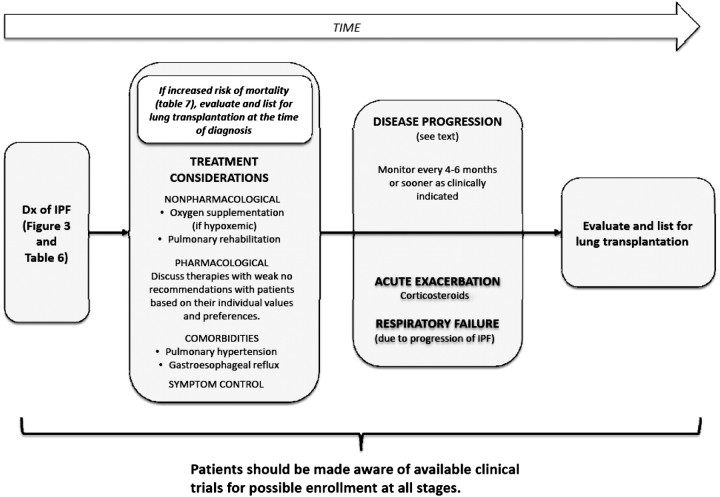
Terms such as “mild,” “moderate,” “severe,” “early,” and “advanced” have been suggested for staging disease. Proposed stages are commonly based on resting pulmonary function test measurements and/or extent of radiologic abnormalities. However, it is unknown if these staging approaches are relevant to clinical decision making. The committee recognizes the importance of identifying patients with increased risk for mortality within 2 years to prompt consideration for lung transplantation. Limited data suggest selected features commonly observed in clinical practice are associated with increased mortality (see below and Table 7). Because of variability in the natural history of IPF, it is unknown if the presence of one of more of these features identifies a subpopulation of patients with “advanced” or “end-stage” IPF.
TABLE 7.
SELECTED FEATURES ASSOCIATED WITH INCREASED RISK OF MORTALITY IN IDIOPATHIC PULMONARY FIBROSIS
|
Baseline factors* |
|---|
| Level of dyspnea† |
| DlCO < 40% predicted |
| Desaturation ≤ 88% during 6MWT |
| Extent of honeycombing on HRCT† |
| Pulmonary hypertension |
| Longitudinal factors |
| Increase in level of dyspnea† |
| Decrease in Forced Vital Capacity by ≥ 10% absolute value |
| Decrease in DlCO by ≥ 15% absolute value |
| Worsening of fibrosis
on HRCT† |
Definition of abbreviations: 6MWT = 6-minute-walk test; DlCO = diffusion capacity for carbon monoxide; HRCT = high-resolution computed tomography.
Baseline forced vital capacity is of unclear predictive value.
Currently, there is no uniformity in approach to quantification.
Demographics
Patients that are older and male have been reported as having worse prognosis in some but not all studies (15, 131, 171–177). The effect of smoking has been shown to be associated with both increased (134, 178) and decreased (131) risk of subsequent mortality. The prognostic value of geographic, ethnic, cultural, and racial factors is unknown.
Dyspnea
Baseline dyspnea has been shown to correlate with quality of life and survival in several studies (15, 179–182). A variety of different metrics for dyspnea have been used, including the medical research council scale, baseline dyspnea index, quality of life (QoL) measurement tools with respiratory questionnaires, Borg scale, University of California San Diego shortness of breath questionnaire, and the clinical-radiological-physiological dyspnea score (183–185). It remains unclear which dyspnea metric is most predictive of outcome in patients with IPF. Change in dyspnea over time has also been shown to predict survival (186).
Physiology
Baseline pulmonary function test values have shown mixed associations with survival in IPF. This may be due, in part, to comorbid conditions such as emphysema, pulmonary vascular disease, and obesity, or technical differences in testing. Baseline FVC is of unclear predictive value (15, 173, 175, 177, 180, 186–189). Diffusing capacity for carbon monoxide (DlCO, single breath, hemoglobin corrected) is more reliably predictive of survival at baseline, and a threshold of approximately 40 percent predicted has been associated with an increased risk of mortality (186, 187, 190, 191). Limited data suggest that baseline total lung capacity (TLC) and alveolar-arterial oxygen difference in partial pressures (P(A-a)O2) may be predictive of survival, but no clear threshold exists (186). Baseline cardiopulmonary exercise testing (maximal oxygen uptake) has been suggested to predict survival (192).
Longitudinal change in physiology is clearly an important predictor of mortality in IPF. A decline in FVC over 6 or 12 months has been reliably associated with decreased survival (177, 186, 187, 191, 193). Recent data indicate that in IPF, declines in FVC of 5–10% may be predictive of mortality. A decline in DlCO has also been associated with decreased survival, although less consistently (186, 187, 191, 193). Greater than 15 mm Hg change in P(A-a)O2 after 12 months has been shown to be predictive of survival (187). Six-month change in TLC and P(A-a)O2 may also be predictive of survival (186).
HRCT Features
HRCT features of fibrosis and honeycombing are strongly correlated with FVC and DlCO measurements (194). Several groups have demonstrated that the extent of fibrosis and honeycombing on HRCT are predictive of survival in IPF (109, 195–198).
Composite Scoring Systems
Composite scoring systems have been developed utilizing physiological and radiographic variables in an attempt to provide more accurate prognostic information. A composite physiologic index (CPI) has been developed that uses values from FEV1, FVC, and DlCO to predict the extent of disease on HRCT (141, 191). This CPI was a stronger predictor of mortality than individual measures of lung function such as FEV1, FVC, DlCO, TLC, PaO2, the clinical-radiographic-physiological scoring system (CRP) (183) or new CRP scoring systems (15). However, this composite approach has not been tested in any prospective clinical trials to date and its clinical utility is unknown.
Six-Minute-Walk Testing
Although the 6-minute-walk test (6MWT) is widely used in clinical practice, its prognostic value is limited due to lack of standardization of the procedure in patients with IPF. Some studies have suggested that desaturation (i.e., a decline in oxygen saturation to below 88%) during 6MWT is a marker for increased risk of mortality (188, 199, 200). Shorter walk distance and delayed heart-rate recovery after walk testing have been associated with an increased risk of subsequent mortality (188, 201–203). However, it is unclear if desaturation, distance walked, and other variables measured during 6MWT in this population are reproducible (204). A steady-state 6-minute exercise test using a walking treadmill has been used in patients with IPF in a recent clinical trial in Japan, but the clinical utility of this unvalidated test is unclear (144).
Histopathology
Varied histopathologic patterns can be found within individual patients when multiple biopsies are obtained. A pattern of UIP and NSIP has been identified in 12 to 26% of patients with multiple lobe biopsies (115, 116), highlighting the importance of obtaining biopsies from multiple lobes. The prognosis for patients with discordant UIP (pattern of UIP and NSIP within the same patient) appears to be similar to that of patients with concordant UIP (UIP in all lobes biopsied) (115, 116).
An increased number of fibroblast foci has been associated with an increased risk of mortality in some studies (134, 175, 205–207). A higher profusion of fibroblast foci has also been associated with a decline in FVC and DlCO over 6 and 12 months of follow-up (205). The utility of detailed histopathologic scoring systems in the day-to-day clinical management of patients with IPF has not been evaluated.
Pulmonary Hypertension
The majority of data regarding the presence and significance of pulmonary hypertension come from patients with IPF undergoing evaluation for lung transplantation. The presence of pulmonary hypertension (defined as a mean pulmonary artery pressure of > 25 mm Hg at rest) has been associated with increased risk of mortality for patients with IPF (140, 142, 176). In a separate series of 70 patients with IPF, receiver operator characteristic (ROC) analysis suggested a mean pulmonary artery pressure of 17 mm Hg as the best discriminator of mortality (189). These data need to be validated. Echocardiographic estimation of pulmonary artery systolic pressures does not correlate well with right heart catheterization (208–210). Increased pulmonary vascular resistance has also been linked to worse survival (211). It is not clear if IPF with pulmonary hypertension represents a distinct clinical phenotype (IPF–PH).
Emphysema
Recent retrospective data suggest that patients with IPF and coexisting emphysema have a poorer outcome than those without emphysema (140, 212). Patients with coexisting IPF and emphysema may require treatment for both conditions. Limited data suggest that patients with IPF and emphysema are likely to require long-term oxygen therapy and may have significant pulmonary hypertension. When controlling for these differences, the presence of emphysema was not significantly predictive of survival (140). Thus, it is not clear if IPF with coexisting emphysema represents a distinct clinical phenotype (combined pulmonary fibrosis and emphysema) with a distinct prognosis or whether emphysema in these cases is simply a comorbidity.
Serum and BAL Biomarkers
There are limited retrospective data on the predictive value of serum and BAL biomarkers in IPF. However, these are largely unavailable for routine clinical use. Krebs von den Lungen-6 (KL-6) is a high-molecular-weight glycoprotein, classified as human MUC1 mucin, that is produced by regenerating type II pneumocytes (213). Serum levels of KL-6 have been shown to be elevated in patients with IPF, and these levels may correlate with increased risk of subsequent mortality (214, 215). Serum levels of surfactant protein A and D are also elevated in patients with IPF and are predictive of survival (216–218). Recent data demonstrate a relationship between serum CCL18, other chemokines, and serum brain natiuretic peptide levels and mortality (219–223). Studies of plasma and BAL matrix metalloproteinase (MMP) levels suggest that MMP1 and MMP7 are increased in patients with IPF, and MMP7 levels may correlate with disease severity (224). BAL levels of SP-A appear predictive of survival (225, 226). Cellular analysis of BAL is of unclear predictive value in IPF (226, 227). Preliminary evidence suggests that the presence of circulating fibrocytes (mesenchymal progenitor cells) is associated with worse short-term survival (228).
TREATMENT
Pharmacological Therapies
The committee did not find sufficient evidence to support the use of any specific pharmacologic therapy for patients with IPF. However, clinical trials of some agents have suggested a possible benefit. The recommendations detailed below are based on the evidence-based approach outlined in the introductory section; these recommendations may change if additional and/or new data become available in publications subjected to peer review. The number of votes for, against, abstaining, and absent are reported for all treatment votes. Most abstentions were a result of panel members withholding from voting on questions with which they felt they had a potential conflict of interest.
The strength of a recommendation reflects the extent to which the committee was confident that desirable effects of a therapy outweighed its undesirable effects (3). The recommendations against most treatment therapies are strong; there is insufficient evidence to support the routine use of these therapies. Other treatment recommendations were weak, reflecting the need for better quality data and uncertainty regarding the benefits and risks of therapy. The strength of a recommendation has important implications for patients, clinicians, and policy makers (Table 3).
Therapies with a weak recommendation against their use may still be appropriate in selected patients.
Clinicians should be prepared to help patients make an appropriate decision regarding whether or not to use a specific treatment regimen with weak recommendation that is consistent with their own goals and values. For the well-informed patient who strongly desires pharmacologic treatment, it is suggested that the choice of agent be made from therapies receiving a weak recommendation against their use.
- Question: Should patients with IPF be treated with corticosteroid monotherapy?
- No randomized controlled trials have been conducted with corticosteroid monotherapy (229, 230). Retrospective uncontrolled studies have reported no survival benefits, but have suggested that a minority of patients treated with corticosteroid monotherapy improve their pulmonary function (179, 231, 232); controlled data have found no survival benefit (14, 233). There is substantial morbidity from long-term corticosteroid therapy (231).
- Recommendation: We recommend that patients with IPF should not be treated with corticosteroid monotherapy (strong recommendation, very low-quality evidence).
- Values: This recommendation places a high value on preventing treatment-related morbidity and a low value on potential improvement in pulmonary function as based on very low-quality evidence.
- Remarks: (Vote: none for use, 21 against use, 2 abstentions, 8 absent.)
- Question: Should patients with IPF be treated with colchicine?
- Colchicine has been shown to inhibit fibroblast proliferation and collagen synthesis in vitro (234), and early studies in patients with IPF suggested a potential benefit (235). Several prospective clinical trials have compared colchicine to various treatment regimens showing no difference in clinical outcomes (8, 236–238). None of these studies contained a “no therapy” arm. A retrospective study of 487 patients with IPF compared survival as a function of treatment program (14). Compared with no therapy, colchicine had no impact on survival (see Table 8).
- Recommendation: We recommend that patients with IPF should not be treated with colchicine (strong recommendation, very low-quality evidence).
- Values: This recommendation places a high value on the very low-quality evidence, suggesting no benefit.
- Remarks: (Vote: none for use, 21 against use, 2 abstentions, 8 absent.)
- Question: Should patients with IPF be treated with cyclosporin A?
- There are limited data on the use of cyclosporin A in the English language literature. Early reports in small, uncontrolled groups of patients with IPF suggested a possible benefit (239, 240). More recently, a retrospective study of 10 patients with IPF showed no apparent benefit to cyclosporine treatment (241). Two studies of small groups of post–lung transplant patients with IPF treated with cyclosporine-containing immunosuppressive regimens have shown progression of disease in the native lung (242, 243).
- Recommendation: We recommend that patients with IPF should not be treated with cyclosporine A (strong recommendation, very low quality evidence).
- Values: This recommendation places a high value on preventing side effects and cost and a low value on very low-quality evidence showing discordant results.
- Remarks: (Vote: none for use, 18 against use, 4 abstentions, 9 absent.)
- Question: Should patients with IPF be treated with combination corticosteroid and immunomodulator therapy (e.g., azathioprine or cyclophosphamide)?
- A retrospective study suggested a potential benefit of treatment with azathioprine plus prednisone in a small case series (244). A small randomized trial of corticosteroid versus corticosteroid and azathioprine showed a trend toward a survival benefit with combination therapy with corticosteroid and azathioprine (245) (Table 9). Corticosteroid and cyclophosphamide was compared with corticosteroid alone, and a survival benefit with cyclophosphamide was demonstrated (246). The results of this trial are confounded by the inclusion of patients that do not meet recent diagnostic criteria for IPF (1). Two retrospective, controlled studies of cyclophosphamide have been published. The first compared corticosteroid and cyclophosphamide therapy to no therapy in 164 patients, and found no survival difference (172). The second compared corticosteroid and cyclophosphamide therapy to corticosteroid alone in 82 patients, and found a survival benefit with combination therapy (247).
- Recommendation: We recommend that patients with IPF should not be treated with combination corticosteroid and immunomodulator therapy (strong recommendation, low-quality evidence).
- Values: This recommendation places a high value on preventing treatment-related morbidity and on recent data suggesting that the addition of acetylcysteine to this regimen slowed the decline in pulmonary function (see below). It places a lower value on possible improvement in pulmonary function.
- Remarks: The evidence was low quality and there was a variable degree of uncertainty about the balance of benefits and harms. (Vote: none for use, 21 against use, 2 abstentions, 8 absent.) The committee was not unanimous regarding the strength of this recommendation; the majority voted for a strong recommendation.
- Question: Should patients with IPF be treated with combination corticosteroid, azathioprine, and acetylcysteine therapy?
- Acetylcysteine is a precursor to the antioxidant glutathione, which may be reduced in the lungs of patients with IPF (248, 249). A randomized controlled trial comparing the effect of high-dose acetylcysteine versus placebo in patients receiving prednisone plus azathioprine has been completed (250) (Table 10). In this study, the 12-month declines in vital capacity and diffusing capacity were significantly less in the acetylcysteine-containing arm (vital capacity: 0.18 liter difference; 95% confidence interval [CI], 0.03–0.32; P = 0.02; diffusion capacity: 0.75 mmol/min/kilopascal difference; 95% CI, 0.27–1.23; P = 0.003). There was no observed difference in mortality or other secondary endpoints including dyspnea, quality of life, exercise physiology, or radiographic appearance. Limitations of this study include substantial drop-out (approximately 30%), the unclear clinical significance of the observed treatment effect, and the lack of a true “no treatment” arm (251, 252).
- Recommendation: The majority of patients with IPF should not be treated with combination corticosteroid, azathioprine, and acetylcysteine therapy, but this therapy may be a reasonable choice in a minority (weak recommendation, low-quality evidence).
- Values: This recommendation places a high value on preventing treatment-related morbidity and a low value on low-quality data, including the absence of a true “no-therapy” arm.
- Remarks: There was considerable debate about this recommendation. All committee members agreed more data are needed to definitively address this question (vote: 3 for use, 17 against use, 3 abstentions, 8 absent). This treatment may be appropriate in patients who are willing to accept possible adverse consequences even if expected benefits are small. Fully informed patients are in the best position to make decisions that are consistent with the best evidence and that patient's values and preferences.
- Question: Should patients with IPF be treated with acetylcysteine monotherapy?
- The most recent data for acetylcysteine monotherapy comes from a randomized controlled trial discussed in the corticosteroid, azathioprine, and oral acetylcysteine section above (250). In this study, the addition of oral acetylcysteine to corticosteroid and azathioprine was associated with a significantly smaller decline in pulmonary function. A previous uncontrolled study of 18 patients treated with oral acetylcysteine for 12 weeks also demonstrated improvements in lung function indices including vital capacity, diffusion capacity, and capillary PaO2 (249). Another previous study randomized 30 patients to aerosolized acetylcysteine or placebo for 12 months and documented significant improvement in the extent of ground glass on computed tomography and reduction in KL-6 levels (253) (Table 11). No differences in physiologic measurements or walk distance were found.
- Recommendation: The majority of patients with IPF should not be treated with acetylcysteine monotherapy, but this therapy may be a reasonable choice in a minority (weak recommendation, low-quality evidence).
- Values: This recommendation places a high value on the potential cost of therapy and a low value on low-quality data, including the absence of a true “no therapy” arm, and indirect evidence of a potential benefit.
- Remarks: There was considerable debate about this recommendation. All committee members agreed more data are needed to definitively address this question. The committee recognizes that there is a lack of standardization in the preparation of acetylcysteine available to the public in some countries, and the route of delivery is different between studies (oral versus aerosolized), which may affect the mechanism of action. (Vote: 5 for use, 15 against use, 3 abstentions, 8 absent.) This treatment may be appropriate in patients who are willing to accept possible adverse consequences even if expected benefits are small. Fully informed patients and clinicians are in the best position to make decisions that are consistent with the best evidence and that patient's values and preferences.
- Question: Should patients with IPF be treated with interferon -γ 1b?
- Interferon-γ 1b (IFN-γ) is an agent with antifibrotic and immunomodulatory properties that has been evaluated in two large clinical trials after a pilot study suggested benefit (254) (Table 12). The first clinical trial evaluated the time to clinical worsening or death in 330 patients with IPF, randomized 1:1 to receive IFN-γ 200 μg three times a week subcutaneously or placebo, with low-dose prednisone being allowed as concomitant medication in both groups (135). The primary endpoint was not different between groups; post hoc analysis suggested a trend toward improved survival with IFN-γ in a subgroup of patients with less severe physiological disease at baseline. A subsequent open-label study of IFN-γ compared with colchicine in patients with less severe physiology also suggested a possible benefit (237). A recent, definitive trial tested this hypothesis in more than 800 patients with physiologically mild disease and demonstrated there was no difference in overall mortality (14.5% in the IFN-γ group compared with 12.7% in the placebo arm) (136).
- Recommendation: We recommend that patients with IPF should not be treated with IFN-γ (strong recommendation, high-quality evidence).
- Values: This recommendation places a high value on the potential risks and cost of therapy.
- Remarks: (Vote: none for use, 17 against use, 6 abstentions, 8 absent.)
- Question: Should patients with IPF be treated with bosentan?
- Endothelin-1 (ET-1) is a powerful vasoconstrictor and growth factor that is involved in the pathogenesis of pulmonary hypertension and potentially of IPF. Elevated endothelin levels in serum and BAL, and exaggerated expression of endothelin receptors and ET-1 in lung tissue have been observed in patients with IPF (255). Bosentan, a dual endothelin receptor A and B antagonist, was tested in a phase II randomized controlled trial, using the change of the modified 6MWD as the primary endpoint (137) (Table 13). The primary endpoint was not reached. There were trends favoring bosentan in a predefined endpoint of time to disease progression or death, dyspnea, and quality of life. A post hoc analysis suggested that in patients who underwent surgical lung biopsy for diagnosis of IPF, bosentan had a beneficial effect on the predefined endpoint of time to disease progression or death and quality of life (256). The nature of these analyses limits the ability to interpret the results. Importantly, a successor study is ongoing to investigate whether bosentan benefits patients with IPF who have undergone surgical lung biopsy.
- Recommendation: We recommend that patients with IPF should not be treated with bosentan (strong recommendation, moderate-quality evidence).
- Values: This recommendation places a high value on the potential risks and cost of therapy and a low value on trends in secondary outcome measures.
- Remarks: The evidence was moderate quality and there was a variable degree of uncertainty about the balance of benefits and harms. (Vote: none for use, 10 against use, 13 abstentions, 8 absent.) The committee was not unanimous regarding the strength of this recommendation; the majority voted for a strong recommendation.
- Question: Should patients with IPF be treated with etanercept?
- Etanercept is a recombinant soluble human tumor necrosis factor (TNF) receptor that binds to TNF and neutralizes its activity in vitro (257). TNF has been implicated in the pathogenesis of pulmonary fibrosis (258, 259). A recent randomized controlled study of etanercept for patients with IPF failed to show a difference in the primary endpoint of change in FVC over 48 weeks, although the study was underpowered. Nonsignificant trends were observed in DlCO, 6MWT parameters, or patient-centered outcomes (260) (Table 14).
- Recommendation: We recommend that patients with IPF should not be treated with etanercept (strong recommendation, moderate-quality evidence).
- Values: This recommendation places a high value on the potential risks and cost of therapy and a low value on possible improvement in secondary outcome measures.
- Remarks: The committee recognizes that due to the underpowered nature of this trial, no definitive conclusion regarding efficacy can be drawn. (Vote: none for use, 18 against use, 4 abstentions, 9 absent.)
- Question: Should patients with IPF be treated with anticoagulants?
- Anticoagulation therapy has been evaluated for the treatment of IPF in a Japanese unblinded, randomized controlled trial that compared corticosteroids plus anticoagulation (unfractionated or low-molecular-weight heparin during follow-up when re-hospitalized and warfarin during outpatient treatment) to corticosteroids alone (152) (Table 15). A survival benefit in the anticoagulation arm was demonstrated and felt to be due to reduced mortality during hospitalization for acute exacerbations or disease progression. Significant limitations of the study included the absence of blinding, differential drop-out rates, failure to exclude pulmonary embolism as a potential cause of deterioration, and suboptimal documentation of the quality of anticoagulation during outpatient phases.
- Recommendation: The majority of patients with IPF should not be treated with anticoagulants, but this therapy may be a reasonable choice in a minority (weak recommendation, very low-quality evidence).
- Values: This recommendation places a high value on the potential risks and cost of therapy and a low value on very low quality data showing a benefit.
- Remarks: There was considerable debate about this recommendation. The evidence was very low quality and there was a variable degree of uncertainty about the balance of benefits and harms. (Vote: 1 for use, 20 against use, 2 abstentions, 8 absent.) This treatment may be appropriate in patients who are willing to accept possible adverse consequences even if expected benefits are small. Fully informed patients are in the best position to make decisions that are consistent with the best evidence and that patient's values and preferences.
- Question: Should patients with IPF be treated with pirfenidone?
-
Pirfenidone is a pyridone compound with pleiotropic, anti-inflammatory, antifibrotic, and antioxidant properties, with antagonism of TGF-β1 effects. Pilot studies suggested benefit to this drug (261, 262). A subsequent randomized controlled trial (RCT) from Japan comparing pirfenidone with placebo was stopped prematurely after a secondary endpoint—acute exacerbation—was found significantly more frequently in the placebo group as compared with the active treatment arm (144) (see Table 16). Although the data set was incomplete due to premature interruption of the trial, there was a suggestion of beneficial treatment effect on oxygen saturation during 6-minute steady-state exercise test (which was the primary endpoint) and a significantly diminished decline of vital capacity in the active treatment compared with the placebo arm. A second RCT from Japan comparing pirfenidone to placebo found a reduction in the rate of decline in vital capacity over 52 weeks in the pirfenidone arm (−90 ml vs. −160 ml, P value 0.04) (263). There was also a difference in progression-free survival (defined by death or > 10% decline in vital capacity) favoring the pirfenidone group (P = 0.03). However, there were significant limitations to this trial. These included highly selective enrolment of patients who demonstrated desaturation on an unvalidated exercise study. In addition, the primary endpoint of the study (that was the rationale for the selected patient population) was changed before unblinding.The results of two additional international RCTs of pirfenidone have been recently reviewed by the United States Food and Drug Administration (USFDA), and a detailed report is available (264–267). One trial (PIPF-004) met the primary endpoint of absolute change from baseline in percent-predicted FVC with an effect size of 4.4% favoring pirfenidone over placebo. The other trial (PIPF-006) did not meet this same primary endpoint. Some secondary efficacy variables were numerically supportive, but inconsistent between trials. A survival benefit was not established for all-cause on-treatment mortality. Pirfenidone was associated with significant gastrointestinal adverse events, liver laboratory abnormalities, photosensitivity, and rash.
- Recommendation: The majority of patients with IPF should not be treated with pirfenidone, but this therapy may be a reasonable choice in a minority (weak recommendation, low- to moderate-quality evidence)
- Values: This recommendation places a high value on side effects and cost and a lower value on the possible small reduction in pulmonary function decline.
- Remarks: The number of votes for this recommendation reflects the voting of the entire committee membership (other than the librarians) because the pirfenidone voting was updated subsequent to the face-to-face meeting electronically as detailed in Methods. Thus the total number of votes for this recommendation is more than the votes made for other recommendations by participants present at the face-to-face meetings (electronic vote: 4 for use, 10 against use, 17 abstentions, none absent). This treatment may be appropriate in patients who are willing to accept possible adverse consequences even if expected benefits are small. Fully informed patients are in the best position to make decisions that are consistent with the best evidence and that patient's values and preferences. The committee acknowledges that the methodology used in making recommendations for or against the use of therapies in this evidence-based guideline differs from that used by regulatory agencies.
-
TABLE 8.
COLCHICINE GRADE EVIDENCE PROFILE*
|
Quality Assessment |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of
Studies |
Design |
Limitations |
Inconsistency |
Indirectness |
Imprecision |
Other
Considerations |
|||||||
| Mortality (follow-up median 1.5 yr) | 1 | Randomized trials | No serious limitations‡ | No serious inconsistency | Serious§ | Very serious¶ | None | ||||||
| Pulmonary
Function (Better indicated by higher values) |
1 |
Randomized
trials |
Serious1 |
No serious
inconsistency |
Serious†† |
No serious
imprecision |
None |
||||||
| Summary of
Findings |
||||||||||
| No. of
Patients |
Effect |
|||||||||
| Colchicine |
No
colchicine |
Relative
(95% CI) |
Absolute |
Quality |
Importance† |
|||||
| Mortality (follow-up median 1.5 yr) | 10/14 (71.4%) | 10/12 (83.3%)‖ | RR, 0.86 (0.56–1.30) | 117 fewer per 1,000 (from 367 fewer to 250 more)** | ⊕○○○ Very low | Critical | ||||
| Pulmonary
Function (Better indicated by higher values) |
14‡‡ |
12 |
— |
§§ |
⊕⊕○○ Low |
Important |
||||
Data are from Reference 238.
The overall quality of evidence rating is listed in the first row and is the one used in the text of the document. The quality rating for outcomes listed in other rows may differ. How these additional outcomes are rated in terms of quality does not influence the final quality rating as they are to inform, but not to make, decisions.
Importance rating: the relative importance of the outcome for decision making. The rating “critical” indicates making recommendations on choice of testing and treatment strategies. The rating “important” indicates that the outcome is important but not critical for making recommendations.
The use of colchicine in the prednisone arm for less than 2 weeks, and of prednisone at less than 20 mg/day for less than 2 weeks in the colchicine arm was permitted for reasons other than as treatment of idiopathic pulmonary fibrosis. This would blur treatment effects.
The comparison in this trial is corticosteroids, leaving the single effect of colchicine versus no treatment somewhat uncertain.
Only 26 patients were enrolled in this trial.
This data was abstracted from the figure provided in the publication.
Please note that the baseline mortality risk is high in this trial which would produce large absolute effects based on apparent small relative effects.
The patient importance of the pulmonary function measures is questionable.
Summary estimates are not provided here, but all differences were not significant.
FVC (percent predicted) was not significantly different for prednisone- versus colchicine-treated subjects (−6.9 and −5.1, respectively, P = 0.385), although both treatment groups experienced a significant decline from baseline (P = 0.012 and P = 0.027, respectively). The change from baseline DlCO (ml/min/mm Hg) was not significantly different for prednisone- versus colchicine-treated subjects (−2.0 and −1.1, P = 0.529); however, both treatment groups experienced a significant decrease from baseline (P = 0.031 and P = 0.017, respectively).
TABLE 9.
AZATHIOPRINE GRADE EVIDENCE PROFILE*
|
Quality Assessment |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of
Studies |
Design |
Limitations |
Inconsistency |
Indirectness |
Imprecision |
Other
Considerations |
|||||||
| Mortality (follow-up mean 1 yr; directly assessed) | 1 | Randomized trials | Serious‡ | No serious inconsistency | No serious indirectness | Serious§ | None | ||||||
| Adverse Effects (follow-up mean 1 yr; study follow up) | 1 | Randomized trials | No serious limitations | No serious inconsistency | No serious indirectness | No serious imprecision | None | ||||||
| Forced vital
capacity in percent predicted (follow-up mean 1 yr; measured with:
percent predicted FVC; Better indicated by higher
values) |
1 |
Randomized
trials |
Very
serious** |
No serious
inconsistency |
Very
serious†† |
No serious
imprecision |
None |
||||||
| Summary of
Findings |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of
Patients |
Effect |
|||||||||
| Azathioprine |
No
Azathioprine |
Relative
(95% CI) |
Absolute |
Quality |
Importance† |
|||||
| Mortality (follow-up mean 1 yr; directly assessed) | 4/14 (28.6%) | 4/13 (30.8%) | HR 1.0 (not provided)¶ | Not calculated–not statistically significant | ⊕⊕○○ Low | Critical | ||||
| Adverse Effects (follow-up mean 1 yr; study follow up) | 28/14‖ (200%) | 25/13‖ (192.3%) | 1 | — | ⊕⊕⊕⊕ High | Critical | ||||
| Forced vital
capacity in percent predicted (follow-up mean 1 yr; measured with:
percent predicted FVC; Better indicated by higher
values) |
14 |
13 |
— |
Mean 4.8
higher‡‡ |
⊕○○○ Very low |
Important |
||||
Data are from Reference 245.
The overall quality of evidence rating is listed in the first row and is the one used in the text of the document. The quality rating for outcomes listed in other rows may differ. How these additional outcomes are rated in terms of quality does not influence the final quality rating as they are to inform, but not to make, decisions.
Importance rating: the relative importance of the outcome for decision making. The rating critical indicates making recommendations on choice of testing and treatment strategies. The rating important indicates that the outcome is important but not critical for making recommendations.
No effect measure was calculated. The RR is approximately 1.
Only 27 patients were randomized. The confidence intervals were very wide.
Based on follow-up after 1 year, four patients died in each group. The HR after age-adjustment and follow up for up to 9 yr was 0.26 (0.08-0.88).
Patients had more than one event.
Patients crossed over (n = 3) from one to the other group because of “clinical deterioration.” Data were available only for patients who did not die in the first year.
It is not clear how important a change in FVC% is for patients.
P value 0.87.
TABLE 10.
COMBINED CORTICOSTEROIDS, AZATHIOPRINE, AND ACETYLCYSTEINE GRADE EVIDENCE PROFILE*
|
Quality Assessment |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of
Studies |
Design |
Limitations |
Inconsistency |
Indirectness |
Imprecision |
Other
Considerations |
|||||||
| Mortality (follow-up 12 mo; study follow-up) | 1 | Randomized trials | Serious‡ | No serious inconsistency | No serious indirectness | Serious§ | None | ||||||
| Adverse Outcomes | 1 | Randomized trials** | Serious†† | No serious inconsistency | Serious‡‡ | No serious imprecision | None | ||||||
| FVC (follow-up 12 mo¶¶; measured with: liters; Better indicated by higher values) | 1 | Randomized trials | Serious¶¶ | No serious inconsistency | Serious‡‡,‖‖ | No serious imprecision*** | None††† | ||||||
| DlCO (follow-up 12 mo¶¶; measured with: mmol/min/kPa;
Better indicated by higher values) |
1 |
Randomized
trials |
Serious‡‡‡ |
No serious
inconsistency |
Serious‡‡, ‖‖ |
No serious
imprecision*** |
None††† |
||||||
| Summary of
Findings |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of
Patients |
Effect |
|||||||||
| Corticosteroids and Azathioprine and Acetylcysteine |
Azathioprine
and Corticosteroids |
Relative
(95% CI) |
Absolute |
Quality |
Importance† |
|||||
| Mortality (follow-up 12 mo; study follow-up) | 7/80 (8.8%) | 8/75 (10.7%) | RR, 0.82 (0.31–2.15)¶ | 19 fewer per 1,000 (from 74 fewer to 123 more)‖ | ⊕⊕○○ Low | Critical | ||||
| Adverse Outcomes | 322/80 (402.5%)§§ | 303/75 (404%)§§ | Approximately RR 1.0 | — | ⊕⊕○○ Low | Critical | ||||
| FVC (follow-up 12 mo¶¶; measured with: liters; Better indicated by higher values) | 71 | 68 | — | Mean 0.18 higher (0.03 to 0.32 higher) | ⊕⊕○○ Low | Critical | ||||
| DlCO (follow-up 12 mo¶¶; measured with: mmol/min/kPa;
Better indicated by higher values) |
71 |
68 |
— |
Mean 0.75
higher (0.27 to 1.23 higher) |
⊕⊕○○ Low |
Important |
||||
Data are from Reference 250.
The overall quality of evidence rating is listed in the first row and is the one used in the text of the document. The quality rating for outcomes listed in other rows may differ. How these additional outcomes are rated in terms of quality does not influence the final quality rating as they are to inform, but not to make, decisions.
Importance rating: the relative importance of the outcome for decision making. The rating “critical” indicates making recommendations on choice of testing and treatment strategies. The rating “important” indicates that the outcome is important but not critical for making recommendations.
Drop out from this study was high. Furthermore, reevaluation of inclusion of patients on the basis of lack of confirmation of usual interstitial pneumonia pattern and histological findings led to exclusion to patients after randomization. Of 180 randomized, only 155 were included in the mortality analysis.
No explanation was provided.
The P value was 0.69 for this comparison.
No absolute effect was calculated because the relative estimate has very wide confidence intervals and is not statistically significant.
We have not evaluated observational studies that compare this triple therapy with no therapy or azathioprine and corticosteroids with no therapy in this comparison.
Similar concerns regarding drop out or lack of inclusion apply to the accounting for adverse outcomes.
This study compared a combination of acetylcysteine plus corticosteroids plus azathioprine to corticosteroids plus azathioprine alone. It is possible that acetylcysteine did not act on the actual disease progression of IPF but rather prevented toxicity from what was considered standard treatment (which was not evaluated in well-done clinical studies).
Note that many patients had more than one adverse event.
There was important drop out of patients in this study. At 1-year follow-up approximately 30% of initially randomized patients were available for follow-up. A total of 71 and 68 patients in the acetylcysteine and placebo group, respectively, provided data for the 1-year follow-up results, with the last observation carried forward in the FVC analyses. A total of 53 and 51 patients in the two groups actually provided FVC data after 1-year follow-up. In part, deaths in the two groups are responsible for the use of the last observation carried forward.
There also is concern about how direct the outcomes FVC and DlCO related to patient important outcomes, such as quality of life, function, and mortality.
It is not clear how important the observed difference in pulmonary function in the analysis based on the last observation carried forward is. However, we did not downgrade further.
It is unlikely that additional trials have been performed but were not published.
There was important dropout of patients in this study. At 1-year follow-up, approximately 30% of initially randomized patients were available for follow-up. A total of 68 and 63 patients in the acetylcysteine and placebo group, respectively, provided data for the 1-year follow-up results, with the last observation carried forward in the DlCO analyses. A total of 48 and 47 patients in the two groups actually provided DlCO data after 1-year follow-up. In part, deaths in the two groups are responsible for the use of the last observation carried forward.
TABLE 11.
AEROSOLIZED ACETYLCYSTEINE MONOTHERAPY GRADE EVIDENCE PROFILE*
|
Quality Assessment |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of
Studies |
Design |
Limitations |
Inconsistency |
Indirectness |
Imprecision |
Other
Considerations |
|||||||
| Quality of Life (follow-up 12 mo; measured with SF36; better indicated by higher values) | 1 | Randomized trials | No serious limitations | No serious inconsistency | No serious indirectness | Serious‡ | None | ||||||
| Exercise Capacity (follow-up 12 mo; measured with 6-minute-walk test; better indicated by higher values) | 1 | Randomized trials | No serious limitations | No serious inconsistency | Serious¶ | Serious‡ | None | ||||||
| Adverse Effects (follow-up 12 mo; better indicated by lower values) | 1 | Randomized trials | Serious‖ | No serious inconsistency | No serious indirectness | Serious‡ | None | ||||||
| Vital Capacity (% of predicted) (follow-up 12 mo; better indicated by lower values) | 1 | Randomized trials | No serious limitations | No serious inconsistency | Serious** | Serious‡ | None | ||||||
| Oxygen
Saturation (follow-up 12 mo; measured with: SaO2; better indicated
by higher values) |
1 |
Randomized
trials |
No serious
limitations |
No serious
inconsistency |
Serious¶ |
Serious‡ |
None |
||||||
| Summary of
Findings |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of
Patients |
Effect |
|||||||||
| N-acetylcysteine |
Bromhexine
Hydrocholoride |
Relative
(95% CI) |
Absolute |
Quality |
Importance† |
|||||
| Quality of Life (follow-up 12 mo; measured with SF36; better indicated by higher values) | 10 | 12 | — | —§ | ⊕⊕⊕○ Moderate | Critical | ||||
| Exercise Capacity (follow-up 12 mo; measured with 6-minute-walk test; better indicated by higher values) | 10 | 12 | — | MD 66.4 higher (37.9 lower to 170.7 higher) | ⊕⊕○○ Low | Critical | ||||
| Adverse Effects (follow-up 12 mo; better indicated by lower values) | 10 | 12 | — | — | ⊕⊕○○ Low | Critical | ||||
| Vital Capacity (% of predicted) (follow-up 12 mo; better indicated by lower values) | 10 | 12 | — | MD 2.4 higher (6.9 lower to 11.7 higher) | ⊕⊕○○ Low | Important | ||||
| Oxygen
Saturation (follow-up 12 mo; measured with: SaO2; better indicated
by higher values) |
10 |
12 |
— |
MD 7.1
higher (5.45 to 8.75 higher) |
⊕⊕○○ Low |
Important |
||||
Data are from Reference 253.
The overall quality of evidence rating is listed in the first row and is the one used in the text of the document. The quality rating for outcomes listed in other rows may differ. How these additional outcomes are rated in terms of quality does not influence the final quality rating as they are to inform, but not to make, decisions.
Importance rating: the relative importance of the outcome for decision making. The rating “critical” indicates making recommendations on choice of testing and treatment strategies. The rating “important” indicates that the outcome is important but not critical for making recommendations.
Small sample size with wide confidence intervals leading to uncertainty about the actual magnitude of any effect.
No signficant differences on the SF36 and direction of change inconsistent among instruments indic.
Oxygen saturation is a surrogate for patient important outcomes and the direct relation unclear.
Unclear if adverse events were specifically measured, however, authors report that none were observed.
Pulmonary function is a surrogate for patient important outcomes and the direct relation unclear.
TABLE 12.
INTERFERON-GAMMA 1B GRADE EVIDENCE PROFILE*
|
Quality Assessment |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of
Studies |
Design |
Limitations |
Inconsistency |
Indirectness |
Imprecision |
Other
Considerations |
|||||||
| Mortality (follow-up 48-96 wk; Study follow up) | 3‡ | Randomized trials | No serious limitations§ | No serious inconsistency | No serious indirectness | No serious imprecision¶ | None | ||||||
| Influenza-like Illness (follow-up 48–96 wk) | 3 | Randomized trials | No serious limitations | No serious inconsistency | No serious indirectness | No serious imprecision | None | ||||||
| Fever (follow-up 48–96 wk) | 3 | Randomized trials | No serious limitations | No serious inconsistency | No serious indirectness | No serious imprecision | None | ||||||
| Fatigue (follow-up 48–96 wk) | 3 | Randomized trials | No serious limitations | No serious inconsistency | No serious indirectness | No serious imprecision | None | ||||||
| Any Adverse
Events (follow-up 48–96 wk) |
3 |
Randomized
trials |
No serious
limitations |
No serious
inconsistency |
No serious
indirectness†† |
No serious
imprecision |
None |
||||||
|
Summary of Findings |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of
Patients |
Effect |
|||||||||
| IFN-γ |
No
IFN-γ |
Relative
(95% CI) |
Absolute |
Quality |
Importance† |
|||||
| Mortality (follow-up 48-96 wk; Study follow up) | 95/722 (13.2%) | 63/452 (13.9%) | RR, 0.94 (0.69–1.28)‖ | 8 fewer per 1,000 (from 43 fewer to 39 more) | ⊕⊕⊕⊕ High | Critical | ||||
| Influenza-like Illness (follow-up 48–96 wk) | 232/713 (32.5%) | 57/443 (12.9%) | RR, 2.31 (1.78–3.01)** | 169 more per 1,000 (from 100 more to 259 more) | ⊕⊕⊕⊕ High | Critical | ||||
| Fever (follow-up 48–96 wk) | 209/713 (29.3%) | 41/443 (9.3%) | RR, 3.22 (2.35–4.41)** | 205 more per 1,000 (from 125 more to 316 more) | ⊕⊕⊕⊕ High | Critical | ||||
| Fatigue (follow-up 48–96 wk) | 221/713 (31%) | 98/443 (22.1%) | RR, 1.35 (1.10–1.67)** | 77 more per 1,000 (from 22 more to 148 more) | ⊕⊕⊕⊕ High | Critical | ||||
| Any Adverse
Events (follow-up 48–96 wk) |
710/722
(98.3%) |
439/452
(97.1%) |
RR, 1.01
(0.99–1.03) |
10 more per
1,000 (from 10 fewer to 29 more) |
⊕⊕⊕⊕ High |
Important |
||||
overall quality of evidence rating is listed in the first row and is the one used in the text of the document. The quality rating for outcomes listed in other rows may differ. How these additional outcomes are rated in terms of quality does not influence the final quality rating as they are to inform, but not to make, decisions.
Importance rating: The relative importance of the outcome for decision making. The rating “critical” indicates making recommendations on choice of testing and treatment strategies. The rating “important” indicates that the outcome is important but not critical for making recommendations.
Studies had different length of follow-up.
In the study by Ziesche and coworkers the methods of randomization, concealment, and other study characteristics were not well described. However, we did not downgrade the quality of evidence, because the results had little impact on the overall results.
The panel did not downgrade for imprecision, although the confidence intervals remain wide despite the two larger studies that have been conducted. One of the underlying reasons for not downgrading is that in the context of the downsides of therapy the still-conceivable benefit (based on the confidence intervals) of therapy likely does not outweigh the harms.
No patient (a total of 18 patients were reported) in the study by Ziesche and colleagues had died after 1 year of follow-up. These data were pooled using a fixed effect model with data from the studies by King and coworkers and by Raghu and colleagues. Ziesche and coworkers reported no death in either group.
The study by Ziesche and colleagues was not included as this outcome was not reported separately.
It is questionable whether counting of any adverse event is direct enough for decision making. It can lead to blurring the effect of important adverse effects.
TABLE 13.
BOSENTAN GRADE EVIDENCE PROFILE*
|
Quality Assessment |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of
Studies |
Design |
Limitations |
Inconsistency |
Indirectness |
Imprecision |
Other
Considerations |
|||||||
| Mortality or Disease Progression | 1 | Randomized trials | No serious limitations | No serious inconsistency | No serious indirectness | Serious‡ | None | ||||||
| Exercise
Capacity (follow-up median 12 mo; in meters) |
1 |
Randomized
trials |
No serious
limitations |
No serious
inconsistency |
Serious§ |
No serious
imprecision |
None |
||||||
|
Summary of Findings |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of
Patients |
Effect |
|||||||||
| Bosentan |
No
Bosentan |
Relative
(95% CI) |
Absolute |
Quality |
Importance† |
|||||
| Mortality or Disease Progression | 38/71 (53.5%) | 43/83 (51.8%) | RR, 1.03 (0.77–1.39) | 26 more per 1,000 (from 518 fewer to 518 fewer) | ⊕⊕⊕○ Moderate | Critical | ||||
| Exercise
Capacity (follow-up median 12 mo; in meters) |
71 |
83 |
— |
Mean 18
meters higher¶ |
⊕⊕⊕○ Moderate |
Critical |
||||
Data are from Reference 137.
The overall quality of evidence rating is listed in the first row and is the one used in the text of the document. The quality rating for outcomes listed in other rows may differ. How these additional outcomes are rated in terms of quality does not influence the final quality rating as they are to inform, but not to make, decisions.
Importance rating: the relative importance of the outcome for decision making. The rating “critical” indicates making recommendations on choice of testing and treatment strategies. The rating “important” indicates that the outcome is important but not critical for making recommendations.
We downgraded for imprecision because of few events and wide confidence intervals overall for this outcome.
Six-minute-walk distance is of questionable importance to patients.
Confidence interval for difference not provided in the study.
TABLE 14.
ETANERCEPT GRADE EVIDENCE PROFILE*
|
Quality Assessment |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of
Studies |
Design |
Limitations |
Inconsistency |
Indirectness |
Imprecision |
Other
Considerations |
|||||||
| Death or Disease Progression (follow-up 48 wk; study follow up) | 1 | Randomized trials | No serious limitations‡,§ | No serious inconsistency | Serious¶ | No serious imprecision‖ | None | ||||||
| Pulmonary function (follow-up 48 wk; better indicated by higher values) | 1 | Randomized trials | No serious limitations† | No serious inconsistency | Very serious** | No serious imprecision | None | ||||||
| Adverse
outcomes (follow-up 48 wk) |
1 |
Randomized
trials |
No serious
limitations‡‡ |
No serious
inconsistency |
Very
serious§§ |
No serious
imprecision |
None |
||||||
|
Summary of Findings |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of
Patients |
Effect |
|||||||||
| Ertanercept |
No
Ertanercpt |
Relative
(95% CI) |
Absolute |
Quality |
Importance† |
|||||
| Death or Disease Progression (follow-up 48 wk; study follow up) | 23/45 (51.1%) | 22/40 (55%) | RR, 0.91 (0.61–1.36) | 49 fewer per 1,000 (from 214 fewer to 198 more) | ⊕⊕⊕○ Moderate | Critical | ||||
| Pulmonary function (follow-up 48 wk; better indicated by higher values) | 45 | 40 | — | MD 0.1 lower†† | ⊕⊕○○ Low | Important | ||||
| Adverse
outcomes (follow-up 48 wk) |
42/46
(91.3%) |
37/41
(90.2%) |
RR, 1.01
(0.88–1.16) |
9 more per
1,000 (from 108 fewer to 144 more) |
⊕⊕○○ Low |
Critical |
||||
Data are from Reference 260.
The overall quality of evidence rating is listed in the first row and is the one used in the text of the document. The quality rating for outcomes listed in other rows may differ. How these additional outcomes are rated in terms of quality does not influence the final quality rating as they are to inform, but not to make, decisions.
Importance rating: the relative importance of the outcome for decision making. The rating “critical” indicates making recommendations on choice of testing and treatment strategies. The rating “important” indicates that the outcome is important but not critical for making recommendations.
Of 88 patients randomized, three were excluded from the anlaysis before unblinding. One of those three patients did not receive any study medication, and two were excluded because of issues related to study conduct at one site.
The overall quality of evidence is based on the quality of the outcome death or disease progression. The other outcomes are equally showing no important benefit and thus are going in the same direction.
We did not separate death from disease progression, although death is clearly a more important outcome than disease progression, and there is indirectness related to the outcome disease progression (defined as decline in FVC% of more than 10%).
We did not downgrade for imprecision given that the lack of benefit on all outcomes suggests no overall benefit against either the potential harms or benefits for the outcome death or disease progression.
FVC% is very indirect with respect to patient importance.
No confidence interval around the mean difference was reported. At 48 weeks of follow-up, patients in the etanercept group experienced a mean decline of 0.1 (SD, 0.3) L from baseline actual FVC, compared with a mean decline of 0.2 (SD, 0.3) L experienced by patients in the placebo group (P = 0.1076). For the outcome DlCOHb, atients in the etanercept group experienced a mean decline of 0.9 (SD 2.6) ml/min/mm Hg from baseline in absolute measures of DlCOHb, compared with a mean decline of 1.7 (SD 2.9) ml/min/mm Hg experienced by patients in the placebo group (P = 0.158).
This outcome is reported for 87 patients, including the 2 patients who were withdrawn from the assessment of the other outcomes.
The outcomes are mixed, including very mild to very serious events.
TABLE 15.
ANTICOAGULATION GRADE EVIDENCE PROFILE*
| Quality
Assessment |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of
Studies |
Design |
Limitations |
Inconsistency |
Indirectness |
Imprecision |
Other
Considerations |
|||||||
| Mortality (follow-up median 12 mo) | 1 |
Randomized
trials |
Very
serious‡ |
No serious
inconsistency |
No serious
indirectness |
Serious§ |
None |
||||||
| Summary of
Findings |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of
Patients |
Effect |
|||||||||
| Anticoagulation |
No
Anticoagulation |
Relative
(95% CI) |
Absolute |
Quality |
Importance† |
|||||
| Mortality (follow-up median 12 mo) | 5/23
(21.7%) |
20/33
(60.6%) |
RR, 0.36
(0.16–0.82) |
388 fewer
per 1,000 (from 109 fewer to 509 fewer) |
⊕○○○ Very low |
Critical |
||||
Data are from Reference 152.
The overall quality of evidence rating is listed in the first row and is the one used in the text of the document. The quality rating for outcomes listed in other rows may differ. How these additional outcomes are rated in terms of quality does not influence the final quality rating as they are to inform, but not to make, decisions.
Importance rating: the relative importance of the outcome for decision making. The rating “critical” indicates making recommendations on choice of testing and treatment strategies. The rating “important” indicates that the outcome is important but not critical for making recommendations.
No explanation was provided.
The confidence intervals are wide. In the context of bleeding risk and other burden from anticoagulant therapy, the panel downgraded for imprecision.
TABLE 16.
PIRFENIDONE GRADE EVIDENCE PROFILE*
|
Quality Assessment |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of
Studies |
Design |
Limitations |
Inconsistency |
Indirectness |
Imprecision |
Other
Considerations |
|||||||
| Mortality (follow-up 72 wk) | 4 | Randomized trials | No serious limitations | No serious inconsistency | No serious indirectness‡ | Serious§ | None¶ | ||||||
| Acute Exacerbation (follow-up 72 wk‖) | 4 | Randomized trials | Serious** | No serious inconsistency | No serious indirectness | Serious§ | None¶ | ||||||
| Vital Capacity (follow-up 72 wk; measured with: SMD based on FVC% predicted, VC and FVC; better indicated by higher values) | 4 | Randomized trials | No serious limitations†† | No serious inconsistency | Very serious‡‡ | No serious imprecision | None¶ | ||||||
| Photosensitivity (follow-up 72 wk) | 4 | Randomized trials | No serious limitations | No serious inconsistency | No serious indirectness | No serious imprecision | None¶ | ||||||
| Anorexia | 4 | Randomized trials | No serious limitations | No serious inconsistency | No serious indirectness | No serious imprecision | None¶ | ||||||
| Fatigue | 3 | Randomized trials | No serious limitations | No serious inconsistency | No serious indirectness‖‖ | No serious imprecision | None¶ | ||||||
| Stomach Discomfort | 4 | Randomized trials | No serious limitations | No serious inconsistency | Serious*** | No serious imprecision | None¶ | ||||||
| DlCO (better indicated by lower values) | 4 | Randomized trials | No serious limitations | No serious inconsistency | Serious††† | No serious imprecision*** | None | ||||||
| Oxygen
Saturation (follow-up 9 mo; better indicated by higher
values) |
2 |
Randomized
trials |
No serious
limitations |
No serious
inconsistency |
Serious§§§ |
No serious
imprecision |
None |
||||||
| Summary of
Findings |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of
Patients |
Effect |
|||||||||
| Pirfenidone |
No
Pirfenidone |
Relative(95% CI) |
Absolute |
Quality |
Importance |
|||||
| Mortality (follow-up 72 wk) | 30/526 (5.7%) | 39/486 (8%) | RR, 0.77 (0.49–1.21) | 18 fewer per 1,000 (from 41 fewer to 17 more) | ⊕⊕⊕○ Moderate | Critical | ||||
| Acute Exacerbation (follow-up 72 wk‖) | 10/526 (1.9%) | 14/486 (2.9%) | RR, 0.69 (0.2–2.42) | 9 fewer per 1,000 (from 23 fewer to 41 more) | ⊕⊕○○ Low | Critical | ||||
| Vital Capacity (follow-up 72 wk; measured with: SMD based on FVC% predicted, VC and FVC; better indicated by higher values) | 521 | 485 | — | SMD 0.23 higher (0.06 to 0.41 higher) | ⊕⊕○○ Low | Important§§ | ||||
| Photosensitivity (follow-up 72 wk) | 130/526 (24.7%) | 30/489 (6.1%) | RR, 5.3 (1.46–19.24) ¶¶ | 264 more per 1,000 (from 28 more to 1,119 more) | ⊕⊕⊕⊕ High | Important | ||||
| Anorexia | 78/526 (14.8%) | 18/489 (3.7%) | RR, 3.57 (2.15–5.93) ¶¶ | 95 more per 1,000 (from 42 more to 181 more) | ⊕⊕⊕⊕ High | Important | ||||
| Fatigue | 120/417 (28.8%)¶¶ | 72/382 (18.8%) | RR, 2.54 (0.53–12.18) | 290 more per 1,000 (from 89 fewer to 2,107 more) | ⊕⊕⊕⊕ High | Important | ||||
| Stomach Discomfort | 54/526 (10.3%) | 10/489 (2%) | RR, 4.2 (2.17–8.11) | 65 more per 1,000 (from 24 more to 145 more) | ⊕⊕⊕○ Moderate | Important | ||||
| DlCO (better indicated by lower values) | 526 | 486‡‡‡ | — | Not pooled | ⊕⊕⊕○ Moderate | Important | ||||
| Oxygen
Saturation (follow-up 9 mo; better indicated by higher
values) |
171 |
135 |
— |
MD 0.53 higher
(1.01 lower to 2.06 higher) |
⊕⊕⊕○ Moderate |
Important |
||||
The overall quality of evidence rating is listed in the first row and is the one used in the text of the document. The quality rating for outcomes listed in other rows may differ. How these additional outcomes are rated in terms of quality does not influence the final quality rating as they are to inform, but not to make, decisions.
Importance rating: the relative importance of the outcome for decision making. The rating “critical” indicates making recommendations on choice of testing and treatment strategies. The rating “important” indicates that the outcome is important but not critical for making recommendations.
The studies used slightly different doses of pirfenidone.
There are sparse data, leading to imprecison. The number of events and patients in the studies is too small to show an effect or exclude with confidence that no important effect on mortality is achieved. The confidence intervals are wide.
The number of studies is small and publication bias is difficult to detect given the small number of studies.
Two trials (004 and 006) with follow-up of 72 weeks.
One trial (Azuma and colleagues) stopped early because of perceived benefit in relation to exacerbations.
Data were imputed in studies 004 and 006.
It is not clear how important a change in FVC% is for patients. In one trial vital capacity and not FVC was measured.
The study period data for PFT data were used. The SMD was used because there were no standard deviations given for the absoulte difference in FVC (only for FVC in percent predicted). The FVC data used here are derived from the prespecified imputed data in the FDA document for studies 004 and 006 (Table 8).
Data for 004 and 006 were not provided separately and for the meta-analysis it was assumed that this is one study for this outcome.
It is not clear whether this outcome was continuous and how severe it was (whether it was sporadic or transient).
No explanation was provided.
DlCO is not a patient-important outcome.
It is not clear which patients had DlCO measured and the data provided in the publications do not allow for pooling of the results.
The importance of this outcome measure for patients and the relation to patient-important outcomes is uncertain.
Therapies without Recommendations: Newer Data Published Subsequent to Final Formal Face-to-Face Discussions (see Methods)
Sildenafil.
Sildenafil (an oral phosphodiesterase 5 inhibitor that has been shown to safely reduce pulmonary vascular pressures in patients with IPF [268]) has been studied in small cohorts of patients with IPF and pulmonary hypertension, and has demonstrated improved walk distance and pulmonary hemodynamics over 8 to 12 weeks (269, 270). Patients with IPF and a severely reduced DlCO are at increased risk for pulmonary vascular disease (271). Based on these observations, a phase III randomized controlled trial of sildenafil in patients with IPF and a severely reduced DlCO (< 35% predicted) has recently be completed and published online (272) (Table 17). One hundred eighty subjects were randomized to sildenafil (20 mg three times daily) or placebo for 12 weeks, with a subsequent 12-week open label phase in which all patients received active drug. The primary endpoint was categorical change of 20% in 6-minute-walk distance at 12 weeks. Key secondary endpoints were dyspnea, quality of life, gas exchange (e.g., PaO2) and pulmonary function (e.g., DlCO). There was no difference in the primary endpoint between active therapy and placebo (10.1% versus 6.6%, P = 0.39). There were statistically significant differences in the change in dyspnea, PaO2, DlCO, and quality of life favoring sildenafil. There were no differences in serious adverse events or mortality over 24 weeks. A second study randomized 29 subjects with IPF to sildenafil or placebo (273). Unlike the first study, there was no requirement for advanced disease; the average DlCO in this study group was 42% predicted. The primary endpoint was the change in 6-minute-walk distance at 6 months. There was no significant difference in the primary endpoint. There was also no difference in change in exertional dyspnea.
TABLE 17.
SILDENAFIL GRADE EVIDENCE PROFILE*
|
Quality Assessment |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Studies |
Design |
Limitations |
Inconsistency |
Indirectness |
Imprecision |
Other
Considerations |
|||||||
| Mortality (Copy) (follow-up 12–24 wk) | 2 | Randomized trials | No serious limitations | No serious inconsistency‡ | No serious indirectness | Very serious§ | None | ||||||
| Exacerbations (follow-up mean 12 wk) | 1¶ | Randomized trials | No serious limitations | No serious inconsistency | No serious indirectness | Very serious‖ | None | ||||||
| Quality of Life (SGRQ) (follow-up mean 12 wk; better indicated by lower values) | 1 | Randomized trials | No serious limitations | No serious inconsistency | No serious indirectness | Serious** | None | ||||||
| FVC (follow-up 12–24 wk; better indicated by lower values) | 2 | Randomized trials | No serious limitations | No serious inconsistency | Serious†† | Serious** | None | ||||||
| Dyspnea Change Scores Borg (follow-up 12–24 wk; better indicated by lower values) | 2 | Randomized trials | No serious limitations | No serious inconsistency | No serious indirectness‡‡ | Serious** | None | ||||||
| DlCO (better indicated by lower values) | 2 | Randomized trials | No serious limitations | No serious inconsistency | Serious§§ | Serious** | None | ||||||
| Six-Minute-Walk Distance (better indicated by lower values) | 2 | Randomized trials | No serious limitations | No serious inconsistency | Serious¶¶ | Serious** | None | ||||||
| Oxygen Saturation (better
indicated by lower values) |
2 |
Randomized
trials |
No serious
limitations |
No serious
inconsistency |
Serious‖‖ |
Serious** |
None |
||||||
| Summary of
Findings |
||||||||||
| No. of
Patients |
Effect |
|||||||||
| Sildenafil |
No Sildenafil |
Relative (95%
CI) |
Absolute |
Quality |
Importance†† |
|||||
| Mortality (Copy) (follow-up 12–24 wk) | 2/103 (1.9%) | 4/106 (3.8%) | RR 0.51 (0.1–2.72) | 18 fewer per 1,000 (from 34 fewer to 65 more) | ⊕⊕○○ Low | Critical | ||||
| Exacerbations (follow-up mean 12 wk) | 1/89 (1.1%) | 3/91 (3.3%) | RR 0.34 (0.04–3.22) | 22 fewer per 1,000 (from 32 fewer to 73 more) | ⊕⊕○○ Low | Critical | ||||
| 3.3% | 22 fewer per 1,000 (from 32 fewer to 73 more) | |||||||||
| Quality of Life (SGRQ) (follow-up mean 12 wk; better indicated by lower values) | 89 | 91 | — | MD 4.09 lower (7.31 to 0.87 lower) | ⊕⊕⊕○ Moderate | Critical | ||||
| FVC (follow-up 12–24 wk; better indicated by lower values) | 103 | 106 | — | SMD 0.07 higher (0.2 lower to 0.34 higher) | ⊕⊕○○ Low | Critical | ||||
| Dyspnea Change Scores Borg (follow-up 12–24 wk; better indicated by lower values) | 103 | 106 | — | MD 0.18 lower (0.61 lower to 0.25 higher) | ⊕⊕⊕○ Moderate | Important | ||||
| DlCO (better indicated by lower values) | 103 | 106 | — | SMD 0.01 lower (0.33 lower to 0.31 higher) | ⊕⊕○○ Low | Important | ||||
| Six-Minute-Walk Distance (better indicated by lower values) | 103 | 106 | — | MD 2.75 lower (50.99 lower to 45.5 higher) | ⊕⊕○○ Low | Important | ||||
| Oxygen Saturation (better
indicated by lower values) |
103 |
106 |
— |
SMD 0.04 lower (0.82 lower
to 0.74 higher) |
⊕⊕○○ Low |
Important |
||||
The overall quality of evidence rating is listed in the first row and is the one used in the text of the document. The quality rating for outcomes listed in other rows may differ. How these additional outcomes are rated in terms of quality does not influence the final quality rating as they are to inform, but not to make, decisions.
Importance rating: the relative importance of the outcome for decision making. The rating “critical” indicates making recommendations on choice of testing and treatment strategies. The rating “important” indicates that the outcome is important but not critical for making recommendations.
No events in the trial by Jackson.
Only six events in total.
Reported in only one of the two trials. The follow-up period was very short.
Only four events observed in an overall relatively small sample size.
There are very few patients in these trials—the continuous outcome measure may mask that there are few patients.
It is not clear how important a change in FVC% or FVC is for patients.
Dyspnea is a fairly direct outcome, and this outcome measure has been validated.
DlCO is not a patient important outcome.
There is some question whether 6-minute-walk distance is a patient-important outcome or not.
The importance of this outcome measure for patients and the relation to patient important outcomes is uncertain.
Imatinib.
Imatinib mesylate is a tyrosine kinase inhibitor with activity against platelet-derived growth factor receptors. This has led to the investigation of imatinib as an antiprolferative agent in several diseases, including IPF. In a phase II randomized controlled trial of imatinib, 121 patients with recently diagnosed (< 36 mo), progressive disease with preserved pulmonary function (FVC > 55%, DlCO > 35%) were randomized to oral imatinib (600 mg once daily) or placebo for up to 96 weeks (274). The primary endpoint was a composite measure of disease progression defined by a greater than 10% decline in FVC or death. No difference in this endpoint was observed (hazard ratio, 1.05; 95% CI, 0.56–1.96; P = 0.89). There were no meaningful differences in secondary endpoints. Imatinib was associated with a higher incidence of adverse event–related dropouts (22% versus 10%).
Nonpharmacologic Therapies
The committee recommends the use of several nonpharmacologic therapies in appropriate patients with IPF.
- Question: Should patients with IPF and resting hypoxemia receive long-term oxygen therapy?
- There are no data that directly inform the use of long-term oxygen therapy in patients with IPF. One study has retrospectively compared survival in a cohort of patients with IPF, many of whom (27%) received oxygen therapy (14). In multivariate analysis, no survival benefit was demonstrated with oxygen use. This study was limited by its retrospective design. There is limited evidence demonstrating improvement in exercise capacity in patients with resting hypoxemia using oxygen (275). Indirect evidence from two large randomized trials in obstructive lung disease has demonstrated a clear survival benefit with long-term oxygen therapy (276, 277). Variable definitions of hypoxemia were used in these studies (PaO2 of 55–65 mm Hg).
- Recommendation: We recommend that patients with IPF and clinically significant resting hypoxemia should be treated with long-term oxygen therapy (strong recommendation, very low-quality evidence).
- Values: This recommendation places a high value on evidence from other chronic lung diseases and a low value on inconvenience to patients and cost.
- Remarks: The committee was divided over the strength of this recommendation. There was a degree of uncertainty about the balance of benefits and inconvenience to patients/cost. The strong recommendation was driven by physiological rationale, ethical concern over withholding supplemental oxygen in a patient demonstrating clinically significant resting hypoxemia (commonly defined by a resting SpO2 of < 88%), and extrapolation from data in COPD. The committee is not able to specify a PaO2 cutoff for use of supplemental oxygen; for now this must be determined at the discretion of the treating physician. It is unknown if supplemental long-term oxygen therapy in patients who demonstrate only exertional hypoxemia improves survival. However, limited data suggest improved walk distance with supplemental oxygen in these patients (188). (Vote: 18 for use, none against use, 4 abstentions, 9 absent.)
- Question: Should appropriate patients with IPF undergo lung transplantation?
- Five-year survival rates after lung transplantation in IPF are estimated at 50 to 56% (278, 279). A single-center study of 46 patients referred for lung transplantation with IPF demonstrated a reduced risk of death at 5 years in patients receiving lung transplantation (280). Additional evidence suggests that patients with pulmonary fibrosis undergoing lung transplantation have favorable long-term survival compared with other disease indications (279). There are no clear data to guide precise timing of transplantation, although criteria have been proposed based on diffusion capacity and or the presence of progressive disease (190). It is unclear if the survival benefit is different in single- versus double-lung transplant recipients (281).
- Recommendation: We recommend that appropriate patients with IPF should undergo lung transplantation (strong recommendation, low-quality evidence).
- Values: This recommendation places a high value on low-quality evidence showing a survival benefit and lower value on cost and procedural risk.
- Remarks. The committee recognizes that there is variability among lung transplantation programs regarding eligibility and timing of listing for transplantation. There are major limitations to the published retrospective studies of lung transplantation for IPF. Most importantly, the patient populations in these studies include patients with other forms of fibrotic lung disease. Discussion of lung transplantation is encouraged in appropriate patients at the time of diagnosis, and detailed evaluation for lung transplantation should occur in a timely manner at the first sign of objective deterioration. (Vote: 21 for use, none against use, 1 abstention, 9 absent.)
- Question: Should patients with respiratory failure due to IPF receive mechanical ventilation?
- There are several small studies of mechanical ventilation in patients with IPF and respiratory failure, all of which show a high hospital mortality rate (149, 282–291). The inclusion criteria varied among studies, with some only including patients with respiratory failure of unknown etiology (149, 287). A representative study of 23 patients with IPF and respiratory failure who required mechanical ventilation reported a hospital mortality rate of 96% (286). The only survivor underwent lung transplantation 6 hours after intubation. A systematic review of mechanical ventilation in patients with IPF and respiratory failure reports a similarly poor hospital mortality of 87% among the 135 reported cases (292).
- Recommendation: The majority of patients with respiratory failure due to IPF should not receive mechanical ventilation, but mechanical ventilation may be a reasonable intervention in a minority (weak recommendation, low-quality evidence).
- Values: This recommendation places a high value on the high mortality observed in this patient population and on reducing unnecessary suffering.
- Remarks: Clinicians need to evaluate each patient carefully before making the decision to not pursue mechanical ventilation. Given the high mortality associated with mechanical ventilation in IPF, this therapy should only be used after discussion with patients and their caregivers regarding goals of care. Whether or not to receive mechanical ventilation in this situation is a value-laden decision that is best made by the patient, clinician, and family ahead of time (ideally during a previous clinic visit). Noninvasive positive pressure ventilation may be appropriate in some patients. In rare circumstances, mechanical ventilation may be appropriate as a bridge to lung transplantation. (Vote: 2 for use, 19 against use, 2 abstentions, 8 absent.)
- Question: Should patients with IPF receive pulmonary rehabilitation?
- Pulmonary rehabilitation programs involve aerobic conditioning, strength and flexibility training, educational lectures, nutritional interventions, and psychosocial support. Pulmonary rehabilitation has recently been studied in patients with ILD. Two controlled trials of pulmonary rehabilitation in IPF have demonstrated an improvement in walk distance and symptoms or quality of life (293, 294). Other uncontrolled studies have found similar findings (295–298). The beneficial effects of pulmonary rehabilitation may be more pronounced in patients with worse baseline functional status (295).
- Recommendation: The majority of patients with IPF should be treated with pulmonary rehabilitation, but pulmonary rehabilitation may not be reasonable in a minority (weak recommendation, low-quality evidence).
- Values: This recommendation places a high value on moderate-quality data demonstrating improvement in functional status and patient-centered outcomes and a low value on cost and uncertainty regarding duration of benefit.
- Remarks: The long-term benefit of pulmonary rehabilitation remains unclear. The committee recognizes that the components of pulmonary rehabilitation may need to be tailored to this patient population. (Vote: 19 for use, none against use, 3 abstentions, 9 absent.)
TREATMENT OF SELECTED COMPLICATIONS AND COMORBID CONDITIONS
There is an increasing awareness of complications and comorbid conditions frequently associated with IPF. These include acute exacerbation of IPF, pulmonary hypertension, gastroesophageal reflux disease, obesity, emphysema, and obstructive sleep apnea (299). It is unknown if treating these comorbidities influences clinical outcomes. There are no data on which to make recommendations for treatment of obesity, emphysema, and obstructive sleep apnea in the setting of IPF.
- Question: Should patients with acute exacerbation of IPF be treated with corticosteroids?
- Although high-dose corticosteroids are commonly prescribed for the treatment of acute exacerbation of IPF (143, 144, 147–149, 152, 153, 155, 157, 300), there are no controlled trials on which to judge efficacy. Cyclosporin A and anticoagulation have also been used without conclusive results (152, 241, 301).
- Recommendation: The majority of patients with acute exacerbation of IPF should be treated with corticosteroids, but corticosteroids may not be reasonable in a minority (weak recommendation, very low-quality evidence).
- Values: This recommendation places a high value on anecdotal reports of benefit and the high mortality of acute exacerbation of IPF.
- Remarks: Specific recommendations regarding the dose, route, and duration of corticosteroid therapy cannot be made. Intravenous corticosteroids up to a gram per day have been reported in a few case series. There was consensus that supportive care is the mainstay of therapy for acute exacerbation of IPF. (Vote: 14 for use, 5 against use, 1 abstention, 11 absent.)
- Question: Should pulmonary hypertension be treated in patients with IPF?
- There are limited data on the treatment of pulmonary hypertension (generally defined by the presence of a mean pulmonary artery pressure of > 25 mm Hg on right heart catheterization) in patients with IPF. A single dose trial of intravenous and aerosolized epoprostenol in eight patients with ILD and pulmonary hypertension (one had IPF) demonstrated improved pulmonary hemodynamics but worsened shunt flow and oxygenation (302). A retrospective study of long-term therapy with intravenous epoprostenol or oral bosentan in 19 patients with ILD and pulmonary hypertension (eight with IPF) suggested improvement in 6-minute-walk distance and quality of life over 6 months (303). A single dose of sildenafil has been shown to improve pulmonary hemodynamics without increasing shunt flow or worsening oxygenation (268). Two small, uncontrolled prospective studies of sildenafil in patients with IPF and pulmonary hypertension demonstrated improved walk distance and pulmonary hemodynamics over 8 to 12 weeks (269, 270).
- Recommendation: Pulmonary hypertension should not be treated in the majority of patients with IPF, but treatment may be a reasonable choice in a minority (weak recommendation, very low-quality evidence).
- Values: This recommendation places a high value on cost and the potential for drug-related morbidity, and a low value on very low-quality data suggesting a possible benefit in selected patients.
- Remarks: In patients with moderate to severe pulmonary hypertension documented by right heart catheterization (i.e., mean pulmonary artery pressure > 35 mm Hg), in line with the interpretation of a weak recommendation, a trial of vasomodulatory therapy may be indicated. The committee recognizes the need for clinical trials of vasomodulatory therapies in this patient population. (Vote: 8 for use, 14 against use, 1 abstention, 8 absent.)
- Question: Should asymptomatic gastroesophageal reflux disease be medically treated in patients with IPF?
- Abnormal acid gastroesophageal reflux (GER) is highly prevalent in patients with IPF, and up to one half of patients are asymptomatic (19, 53, 304). Abnormal GER is a risk factor for aspiration, which is a known cause of pneumonitis, and may contribute to chronic airways inflammation and fibrosis (305–307). Two retrospective case series describe stabilization of pulmonary function and oxygen requirements with medical and surgical management of gastroesophageal reflux (19, 308).
- Recommendation: Asymptomatic gastroesophageal reflux disease should be medically treated in the majority of patients with IPF, but treatment may not be reasonable in a minority (weak recommendation, very low-quality evidence).
- Values: This recommendation places a high value on very low-quality evidence suggesting a possible benefit, and a low value on cost and potential increased risk of pneumonia and osteoporosis with acid suppression therapy.
- Remarks: This recommendation does not extend to the potential treatment of non–acid reflux and surgical treatment with fundoplication. Treatment of abnormal GER in patients with IPF warrants further studies and clinical trials. (Vote: 15 for use, 8 against use, no abstentions, 8 absent.)
PALLIATIVE CARE
Palliative care focuses on reducing symptoms and providing comfort to patients, rather than treating patients' disease. Specific goals for palliative care include relief from physical and emotional suffering and consideration for psychological and spiritual support for patients and caregivers. Such care will need to be individualized. Palliative care should be considered an adjunct to disease-focused care.
Worsening of symptoms such as cough and dyspnea are common and difficult to treat. Limited data suggest that corticosteroids and thalidomide may be beneficial for chronic cough in IPF (309, 310). Chronic opioids may be used for severe dyspnea and cough; careful monitoring for side effects should be performed (10). Advanced directives and end-of-life care issues should be addressed in the ambulatory setting in all patients with IPF, particularly those with severe physiologic impairment and comorbid conditions. In patients who are bed-bound due to IPF, hospice care should be considered.
MONITORING THE CLINICAL COURSE OF DISEASE
Monitoring of patients with IPF is necessary to proactively identify patients with progressive disease, to appreciate worsening of symptoms and oxygenation, and to detect the development of disease or treatment complications. In addition, careful assessment of the clinical course is useful in helping patients understand their disease course and in initiating timely, appropriate therapeutic interventions, including consideration of lung transplantation.
Monitoring for Progressive Disease
Disease progression may be manifested by increasing respiratory symptoms, worsening pulmonary function test results, progressive fibrosis on HRCT, or acute respiratory decline.
In the absence of another identifiable cause, the presence of any of the following changes is consistent with progressive disease:
Progressive dyspnea (objectively assessed)
Progressive, sustained decrease from baseline in absolute FVC
Progressive, sustained decrease from baseline in absolute DlCO (corrected for hemoglobin)
Progression of fibrosis from baseline on HRCT
Acute exacerbation
Death from respiratory failure
These parameters were developed based on data from clinical trials (see Staging and Prognosis). While progressive dyspnea is an important subjective variable, objective assessment of dyspnea is encouraged (e.g., with dysnpea scores, assessment of dyspnea by validated tools such as University of California San Diego shortness of breath questionnaire). Further studies are needed to validate the inclusion of dyspnea assessment and other variables. Evidence from several clinical cohorts to date confirms that a change in absolute FVC of 10% (with or without a concomitant change in DlCO) or a change in absolute DlCO of 15% (with or without a concomitant change in FVC) is a surrogate marker of mortality and is evidence of, in the absence of an alternative explanation, disease progression (177, 186, 187, 191, 193). Smaller (5–10%) but progressive, sustained changes in FVC may also represent progression of disease (311). The committee was unable to specify the absolute minimum magnitude of FVC and DlCO change required for determination of disease progression, but isolated changes of less than 5% in FVC and less than 10% in DlCO should be interpreted with caution. Changes in this range are more likely to overlap with the intrinsic variability of the test (312–316). On average, progression of disease is monitored over periods of 3 to 6 months, but sustained changes in symptoms, physiology, and radiology over shorter periods of time may also identify disease progression.
Of the above parameters, pulmonary function testing provides the most standardized approach to objective monitoring and quantification of disease progression. Evidence suggests that progressive fibrosis leads to gradual decline in pulmonary function and worsening symptoms (317). The placebo arms of several large, randomized controlled treatment trials in IPF have suggested an average annual decline in FVC of approximately 0.2 liters in the overall population of patients with IPF with mild to moderate pulmonary function abnormalities at the time of enrollment (135, 137, 144, 260). The rate of decline in individual patients is widely variable.
A decline in absolute DlCO in the absence of an alternative explanation is consistent with progressive disease, although such a decline may also reflect changes in the pulmonary vasculature and coexistent pulmonary hypertension. Using our current techniques, longitudinal measurement of other clinical and physiological variables (e.g., TLC, P(A-a)O2) and 6-MWT variables have significant limitations and are not recommended for routine use in monitoring for disease progression at this time. Monitoring for desaturation during 6MWT is useful, however, in patients with significant exercise intolerance to assess the need for supplemental oxygen.
The physiological effect of comorbidities such as coexisting emphysema on the predictive values of serial changes in pulmonary function is unclear, but is likely to be a confounding factor (318). The committee recognizes that the presence of significant emphysema impacts FVC measurement, and thus changes in FVC alone may not be as reliable an indicator of disease progression in these circumstances (319–321). Under these circumstances, a combination of FVC and DlCO may be useful in assessing progression of disease.
The committee recommends that FVC and DlCO measurements be performed during routine monitoring in accordance with ATS/ERS standards to follow trends (312–316). While it is appropriate to routinely monitor disease course with FVC and DlCO measurements at 3- to 6-month intervals, a subset of patients with rapid progression or acute worsening may not have demonstrated progression during the preceding interval (145). The optimal time interval for repetition of FVC and DlCO has not been formally investigated. A flexible approach to monitoring for disease progression is required with a lower threshold for earlier repetition of FVC and DlCO in the presence of progressive dyspnea or other features of a more rapidly progressive course.
Monitoring for Worsening Symptoms
Identifying patients with worsening respiratory symptoms (e.g., dyspnea) has important management implications. Patients experiencing worsening respiratory symptoms require evaluation for progressive disease, assessment of oxygenation at rest and with exertion, and prompt detection of secondary complications (e.g., development of deep venous thrombosis and pulmonary embolus). In addition, some patients may benefit from symptom-based therapies. There are several research tools available for the quantification of dyspnea. It is unclear if any of these tools have clinical utility.
Monitoring for Worsening Oxygenation
Oxygen saturation by pulse oximetry should be measured at rest and with exertion in all patients regardless of symptoms to assure adequacy of oxygenation and identify the need for supplemental oxygen at baseline and during follow-up evaluation. Careful attention to the pulse oximetry tracing and signal is required to overcome potential problems related to poor circulation and inadequate signal quality. Generally, desaturation below 88% during a formal 6MWT or equivalent has been used to prescribe supplemental oxygen (322). Such measurements should be performed at baseline and during follow up at 3- to 6-month intervals. Formal cardiopulmonary exercise testing does not have a defined role and is not recommended for routine monitoring.
Monitoring for Complications and Comorbidities
Comorbidities including pulmonary hypertension, pulmonary embolism, lung cancer, and coronary artery disease are known to occur in IPF. While the development of these comorbidities may influence survival, the role of routine screening to identify such complications in patients with IPF (e.g., annual HRCT for lung cancer surveillance) is unknown. Thus, a recommendation for routine screening cannot be made. In patients demonstrating progressive disease, the identification of pulmonary hypertension may impact consideration for lung transplantation in eligible patients, and evaluation is indicated. Echocardiography is inaccurate in estimating pulmonary hemodynamics in patients with fibrotic lung disease and should not be relied upon to assess the presence and severity of pulmonary hypertension (208, 210, 271). Brain natiuretic peptide levels have been shown to correlate with the presence of moderate to severe pulmonary hypertension, but have not been thoroughly validated as a screening tool (220, 221). A clinical prediction model has also been proposed but requires independent validation (323). At the present time, right heart catheterization is required to confirm the presence of pulmonary hypertension.
Since some patients with connective tissue disease (e.g., younger women) may present with isolated pulmonary abnormalities characteristic of IPF prior to overt manifestations of systemic disease, appropriate serological monitoring for connective tissue disease should be considered in such patients when symptoms arise.
For patients manifesting acute respiratory worsening, the possibility of acute exacerbation of IPF should be entertained, and prompt evaluation for alternative etiologies of acute worsening such as pulmonary embolus, pneumothorax, respiratory infection, or aspiration should be undertaken.
Monitoring for complications associated with pharmacologic therapy will need to be tailored to the known side effect profiles of the specific treatment regimen.
Summary of Clinical Management of IPF
The committee has integrated its recommendations into a schematic pathway for clinical management based on these considerations (Figure 5).
Figure 5.
Schematic pathway for clinical management of patients with IPF. Clinicians are required to spend adequate time with patients to discuss patients' values, preferences, and prognosis. All patients should be made aware of available clinical trials for possible enrollment. Patient at increased risk of mortality should be considered for lung transplantation. Pharmacologic treatment should be limited to a carefully selected minority of patients who are willing to accept possible adverse consequences even if expected benefits are small. See text for specific recommendations of pharmacological therapies. Oxygen supplementation (if hypoxemic) and pulmonary rehabilitation are recommended treatments (strong yes and weak yes, respectively). All patients should be monitored for disease progression and identification of complications at 4 to 6 months or sooner as clinically indicated. Corticosteroids are an appropriate treatment option for acute exacerbation (weak yes). Mechanical ventilation is not recommended for the majority of patients with respiratory failure due to progression of their disease (weak no). Symptom control (palliative care) focuses on reducing symptoms (e.g., cough and dyspnea) and providing comfort to patients, rather than treating patients' disease. Advanced directives must be discussed in the ambulatory setting. See text for additional details.
FUTURE DIRECTIONS
This document represents the current state of the art in the evidence-based management of IPF. It will require amendment as new evidence emerges. Since evidence-based recommendations were only feasible for the diagnosis and treatment sections, future research needs to focus on the other areas in particular (e.g., natural history, biomarkers, monitoring).
The definition and diagnosis of IPF may require modification as more is learned about the pathogenesis and biology of the disease. For example, the measurement of differential gene expression using microarrays might provide insights on novel disease-specific patterns that will help to improve diagnostic specificity or identify distinct phenotypes within IPF that have clinically relevant differences.
Prognosis in IPF remains difficult and is limited by the lack of comprehensive approaches to identifying multivariable predictive models. Such an effort is critically important and would inform issues such as staging of disease and identification of additional surrogate endpoints (i.e., biomarkers) for clinical trials. The committee encourages the use of large, well-described cohorts for this purpose. For example, the combination of small changes in multiple physiological endpoints (e.g., FVC and DlCO) may prove useful measures of disease progression. The clinical significance of changes in small magnitudes of pulmonary function tests needs to be determined in future studies. Variables assessed during the 6MWT (e.g., walk distance, walk speed, time to desaturation) warrant additional study.
Additional high-quality, prospective, controlled, clinical trials of new therapies for IPF are required. The committee believes that successful treatment of IPF will require a combination of therapies targeting multiple pathways involved in fibroproliferation. Future clinical trials should incorporate endpoints of proven clinical value, utilize sophisticated study design and statistical methodology, investigate the impact of potential preventive measures (e.g., treatment of gastroesophageal reflux), and consider combinations of promising therapies that work through distinct mechanisms. Although improved survival is an important endpoint in clinical trials, mortality is not the only appropriate outcome measure in the committee's opinion. Endpoints for future clinical trials should be carefully chosen based on the clinical characteristics of the study population (e.g., extent and severity of disease, presence of emphysema, pulmonary hypertension, etc.) and the target of therapy. A discussion among clinical investigators and regulatory agencies is needed to reach a consensus on clinically significant and meaningful endpoints in clinical trials of IPF.
Finally, it is hoped that with continued collaboration between basic and clinical scientists, the goals of finding the cause(s) of IPF, detecting disease in preclinical and early stages, improving outcomes and quality of life, prolonging survival, and, ultimately, curing IPF will be realized. Genetic studies and preventive and regenerative strategies, including stem cell transplant research and gene therapy, hold promise and should be aggressively pursued.
This statement was prepared by the ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis.
Members of the ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis:
Ganesh Raghu, M.D. (Chair)
Jim J. Egan, M.D. (Co-Chair)
Fernando J. Martinez, M.D., M.S. (Co-Chair)
Pulmonary
Julio Ancochea, M.D.
Juergen Behr, M.D.
Demosthenes Bouros, M.D., Ph.D.
Kevin K. Brown, M.D.
Carlos Carvalho, M.D.
Harold R. Collard, M.D.
Jean-François Cordier, M.D.
Ulrich Costabel, M.D.
Roland du Bois, M.D.*
Kevin R. Flaherty, M.D.
Dong Soon Kim, M.D.
Talmadge E. King, Jr., M.D.
Yasuhiro Kondoh, M.D.
Jospeh A. Lasky, M.D.
Luca Richeldi, M.D., Ph.D.
Jay H. Ryu, M.D.
Moisés Selman, M.D.
Jeffrey J. Swigris, D.O., M.S.
Athol U. Wells, M.D.
Pulmonary and Evidence-based Approaches, Epidemiology and Biostatistics
Holger J. Schünemann, M.D., Ph.D., M.Sc.
Radiology
David M. Hansell, M.D.
Takeshi Johkoh, M.D., Ph.D.
David A. Lynch, M.D.
Nestor L. Müller, M.D. Ph.D.
Pathology
Thomas V. Colby, M.D.
Masahito Ebina, M.D.
Jeffrey Myers, M.D.
Andrew G. Nicholson, F.R.C.Path., D.M.
Reference Librarians
Rosalind F. Dudden, M.L.S.
Barbara S. Griss, M.L.S.
Shandra L. Protzko, M.L.S.
* Dr. du Bois was a member of the Committee, but requested that he not be listed as an author of this Document.
Acknowledgments
The committee acknowledges the American Thoracic Society, European Respiratory Society, Japanese Respiratory Society, and Latin American Thoracic Association for supporting this project; the staff of University College, Dublin, Ireland, and University of Modena and Reggio Emilia, Italy, for assistances with face-to-face meetings; Ms. Judy Corn, Mr. Lance Lucas and the ATS staff for administrative assistance; members of the ATS Documentation and Implementation Committee, and the many peer reviewers and community providers for providing input and recommendations during the development of this document.
Footnotes
This Official Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS), the Japanese Respiratory Society (JRS), and the Latin American Thoracic Association (ALAT) Was Approved by the ATS Board of Directors, November 2010, the ERS Executive Committee, September 2010, the JRS Board of Directors, December 2010, and the ALAT Executive Committee, November 2010
This Statement has been formally endorsed by the Society of Thoracic Radiology and by the Pulmonary Pathology Society
This document has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Author Disclosure: G.R. reported consultancies with Actelion ($10,001–$50,000), Amgen ($1,001–$5,000), Amira ($1,001–$5,000), Bayer ($1,001–$5,000), Boehringer Ingelheim ($5,001–$10,000), Celgene (anticipated: $5,001–$10,000), Centocor/Johnson & Johnson ($1,001–$5,000), Genzyme ($10,001–$50,000), Gilead Sciences ($1,001–$5,000), Oncothyreon ($1,001–$5,000), and Stromedix ($1001–$5,000); lecture fees from Actelion ($1,001–$5,000); nongovernmental research support from Actelion ($10,001–$50,000) and the Coalition for Pulmonary Fibrosis ($10,001–$50,000). H.R.C. reported consultancies with Actelion ($5,001–$10,000), Amira ($1,001–$5,000), Bayer (up to $1,000), and with CV Therapeutics, Fibrogen, Genzyme, Gilead, and Nektar ($1,001–$5,000 each). He received a development award from the ASP/CHEST Foundation ($50,001–$100,000). J.J.E. served on a Wyeth advisory committee ($1,001–$5,000). F.J.M. consulted with Adelphi, Commonhealth, Decision Resources, Eidetics, MedMark, Mpex, and Travelex (up to $1,000 each), and with Genzyme ($1,001–$5,000) and Novartis ($10,001–$50,000). He served on advisory committees of Altana/Nycomed ($10,001–$50,000), Astra Zeneca ($1,001–$5,000), Comgenix ($5,001–$10,000), Dey ($1,001–$5,000), Forest ($5,001–$10,000), Fusion MD ($1,001–$5,000), GlaxoSmithKline ($10,001–$50,000), Johnson & Johnson ($5,001–$10,000), Novartis ($1,001–$5,000), Sanofi ($1,001–$5,000), Sepracor ($1,001–$5,000), Schering ($10,001–$50,000) and Talecris ($1,001–$5,000). He received lecture fees from American Health Education ($10,001–$50,000), Astra Zeneca ($10,001–$50,000), Boehringer Ingelheim ($10,001–$50,000), GlaxoSmithKline ($100,001 or more), Pfizer ($10,001–$50,000), Schering ($5,001–$10,000), and WebMD ($5,001–$10,000). He received research support from Actelion ($50,001–$10,000) and Boehringer Ingelheim ($10,001–$50,000), and royalties from Associates in Medical Marketing ($10,001–$50,000), Castle Connolly (up to $1,000), FB Associates ($1,001–$5,000) and HHC (up to $1,000). J.B. consulted with Actelion ($5,001–$10,000), Bayer Schering Pharma ($5,001–$10,000), and Genzyme, Intermune, and Pari Pharma ($1,001–$5,000 each); served on advisory committees of Bayer Schering Pharma ($5,001–$10,000), Gilead ($1,001–$5,000), GlaxoSmithKline ($1,001–$5,000), and Lilly (up to $1,000); received lecture fees from Actelion ($10,001–$50,000), Bayer Schering Pharma ($5,001–$10,000), Boehringer Ingelheim ($5,001–$10,000), GlaxoSmithKline ($1,001–$5,000), and Pfizer ($1,001–$5,000); received nongovernmental research support from Bayer Schering Pharma ($10,001–$50,000) and Pari Pharma ($10,001–50,000). K.K.B. consulted with Actelion ($10,001–$50,000), Amgen ($1,001–$5,000), Celgene ($1001–5000), Elan ($1,001–$5,000), Fibrogen ($1,001–$5,000), Genzyme ($10,001–$50,000), MondoBiotech ($1,001–$5,000), Pacific Therapeutics ($1,001–$5,000), Phillips (up to $1,000) and Stromedix ($1,001–$5,000). He served on advisory committees of Boehringer Ingelheim, Centocor, Gilead, and Novartis ($5001–$10,000 each); received lecture fees from Biogen ($1001–$5,000); and nongovernmental research support from Actelion ($100,001 or more), Amgen ($50,001–$100,000), Genzyme ($50,001–$100,000), Gilead ($50,001–$100,000), and Novartis ($50,001–$100,000). T.V.C. reported employment by Gilead for microscopic slide review ($10,001–$50,000), and book royalties from various publishers (up to $1,000). J-F.C. consulted with Actelion, GlaxoSmithKline and Pfizer (up to $1,000 each); served on advisory committees of Actelion, GlaxoSmithKline, and Pfizer (up to $1,000 each); and received lecture fees from Actelion, GlaxoSmithKline, and Pfizer (up to $1,000 each). K.R.F. consulted with Boehringer Ingelheim, Fibrogen, GlaxoSmithKline, Gilead, and Neopharm ($1,001–$5,000 each), and received nongovernmental research support from Intermune and Johnson & Johnson ($50,001–$100,000 each). J.A.L. served on advisory committees of Actelion, Boehringer Ingelheim, and Centocor ($1,001–$5,000 each), and received nongovernmental research support from Intermune ($50,001–$100,000) and Novartis ($100,000 or more). D.A.L. consulted with Centocor, Gilead, Intermune, Novartis, and Perceptive Imaging ($1,001–$5,000 each); served on an Actelion advisory committee ($10,001–$50,000), and received nongovernmental research support from Siemens ($10,001–$50,000; institutional grant, $50,001–$100,000). J.J.S. consulted with Actelion ($1,001–$5,000) and was a site principal investigator for drug trials of Actelion, Intermune, and Novartis (payment amounts not noted). A.U.W. consulted with Actelion ($5,001–$10,000); served on advisory committees of Actelion, Centocor, Encysive, and Genzyme ($1,001–$5,000 each); and received lecture fees from Actelion ($1,001–$5,000). J.A. served on a Boehringer Ingelheim advisory committee ($5,001–$10,000); received lecture fees from Boehringer Ingelheim, GlaxoSmithKline, and Pfizer ($1,001–$5,000 each); and nongovernmental research support from GlaxoSmithKline ($10,001–$50,000). U.C. consulted with Actelion ($1,001–$5,000), Bayer (up to $1,000), Boehringer Ingelheim ($10,001–$50,000), Centocor ($5,001–$10,000) and Intermune ($10,001–$50,000). He served on a Serono advisory committee ($10,001–$50,000); received lecture fees from Astra Zeneca ($1,001–$5,000); and nongovernmental research support from Actelion and Astra Zeneca ($10,001–$50,000 each), Boehringer Ingelheim ($100,001 or more), Gilead ($10,001–$50,000), and Intermune ($100,001 or more). D.M.H. consulted with Astra Zeneca ($1,001–$5,000). D.S.K. consulted with Boehringer Ingelheim ($5,001–$10,000) and received nongovernmental research support from Actelion ($10,001–$50,000). T.E.K., Jr. consulted with Boehringer Ingelheim ($10,001–$50,000); served on advisory committees of Actelion ($10,001–$50,000), CV Therapeutics (up to $1,000), ImmuneWorks ($5,001–$10,000) and Intermune ($10,001–$50,000); and received lecture fees from Actelion (up to $1,000). Y.K. received lecture fees from Astra Zeneca, Eizai, and Shionogi (up to $1,000 each). J.M. reported a patent with the University of Michigan for a modular system of trays for managing microscopic slides and paraffin blocks, and book royalties from Blackwell and Thieme publishers (up to $1,000 each). N.L.M. consulted with Actelion and Bayer Japan ($5,001–$10,000 each), and received nongovernmental research support from GlaxoSmithKline ($100,000 or more). A.G.N. consulted with Actelion ($10,001–50,000), Astra Zeneca ($1,001–$5,000) and Boehringer Ingelheim ($10,001–$50,000). L.R. consulted with Cellestis and Oxford Immunotec ($1,001–$5,000 each); served on advisory committees of Boehringer Ingelheim ($5,001–$10,000), Celgene ($1,001–$5,000), Gilead ($1,001–$5,000), and Novartis ($5,001–$10,000); received lecture fees from Cellestis ($1,001–$5,000); and nongovernmental research support from Boehringer Ingelheim ($10,001–$50,000), Gilead ($5,001–$10,000), and Intermune ($10,001–$50,000). M.S. consulted with Boehringer Ingelheim ($5,001–$10,000). R.F.D. reported book royalties from Neal-Schuman Publishers ($1,001–$5,000). H.J.S. consulted with Pfizer ($1,001–$5,000); received royalties from GlaxoSmithKline and United Biosource ($1,001–$5,000 each) for development of a quality of life instrument; and nongovernmental research support from Barilla ($100,001 or more) and the World Allergy Organization ($100,001 or more). J.H.R., D.B., C.C., M.E., T.J., B.S.G., and S.L.P each reported no commercial interests or nongovernmental, noncommercial interests relevant to subject matter.
References
- 1.American Thoracic Society; European Respiratory Society. Idiopathic pulmonary fibrosis: diagnosis and treatment: international consensus statement. Am J Respir Crit Care Med 2000;161:646–664. [DOI] [PubMed] [Google Scholar]
- 2.Guyatt G, Gutterman D, Baumann MH, Addrizzo-Harris D, Hylek EM, Phillips B, Raskob G, Lewis SZ, Schunemann H. Grading strength of recommendations and quality of evidence in clinical guidelines: report from an American College of Chest Physicians Task Force. Chest 2006;129:174–181. [DOI] [PubMed] [Google Scholar]
- 3.Schunemann HJ, Jaeschke R, Cook DJ, Bria WF, El-Solh AA, Ernst A, Fahy BF, Gould MK, Horan KL, Krishnan JA, et al. An official ATS statement: grading the quality of evidence and strength of recommendations in ATS guidelines and recommendations. Am J Respir Crit Care Med 2006;174:605–614. [DOI] [PubMed] [Google Scholar]
- 4.Cochrane Handbook for Systematic Reviews of Interventions 5.0.2. Oxford: The Cochrane Collaboration, 2009. [online] 2009. [accessed 20 June 2010]; Available from: http://www.cochrane.org/resources/handbook/.
- 5.McGowan J, Sampson M. Systematic reviews need systematic searchers. J Med Libr Assoc 2005;93:74–80. [PMC free article] [PubMed] [Google Scholar]
- 6.Harris MR. The librarian's roles in the systematic review process: a case study. J Med Libr Assoc 2005;93:81–87. [PMC free article] [PubMed] [Google Scholar]
- 7.McKibbon KA. Systematic reviews and librarians. Libr Trends 2006;55:202–215. [Google Scholar]
- 8.Selman M, Carrillo G, Salas J, Padilla RP, Perez-Chavira R, Sansores R, Chapela R. Colchicine, D-penicillamine, and prednisone in the treatment of idiopathic pulmonary fibrosis: a controlled clinical trial. Chest 1998;114:507–512. [DOI] [PubMed] [Google Scholar]
- 9.Yoshida M, Taguchi O, Gabazza EC, Yasui H, Kobayashi T, Kobayashi H, Maruyama K, Adachi Y. The effect of low-dose inhalation of nitric oxide in patients with pulmonary fibrosis. Eur Respir J 1997;10:2051–2054. [DOI] [PubMed] [Google Scholar]
- 10.Allen S, Raut S, Woollard J, Vassallo M. Low dose diamorphine reduces breathlessness without causing a fall in oxygen saturation in elderly patients with end-stage idiopathic pulmonary fibrosis. Palliat Med 2005;19:128–130. [DOI] [PubMed] [Google Scholar]
- 11.Nadrous HF, Ryu JH, Douglas WW, Decker PA, Olson EJ. Impact of angiotensin-converting enzyme inhibitors and statins on survival in idiopathic pulmonary fibrosis. Chest 2004;126:438–446. [DOI] [PubMed] [Google Scholar]
- 12.American Thoracic Society, European Respiratory Society. American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. Am J Respir Crit Care Med 2002;165:277–304. [DOI] [PubMed] [Google Scholar]
- 13.Visscher DW, Myers JL. Histologic spectrum of idiopathic interstitial pneumonias. Proc Am Thorac Soc 2006;3:322–329. [DOI] [PubMed] [Google Scholar]
- 14.Douglas WW, Ryu JH, Schroeder DR. Idiopathic pulmonary fibrosis: Impact of oxygen and colchicine, prednisone, or no therapy on survival. Am J Respir Crit Care Med 2000;161:1172–1178. [DOI] [PubMed] [Google Scholar]
- 15.King TE Jr, Tooze JA, Schwarz MI, Brown KR, Cherniack RM. Predicting survival in idiopathic pulmonary fibrosis: scoring system and survival model. Am J Respir Crit Care Med 2001;164:1171–1181. [DOI] [PubMed] [Google Scholar]
- 16.Gribbin J, Hubbard RB, Le Jeune I, Smith CJ, West J, Tata LJ. Incidence and mortality of idiopathic pulmonary fibrosis and sarcoidosis in the UK. Thorax 2006;61:980–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scott J, Johnston I, Britton J. What causes cryptogenic fibrosing alveolitis? A case-control study of environmental exposure to dust. BMJ 1990;301:1015–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mannino DM, Etzel RA, Parrish RG. Pulmonary fibrosis deaths in the United States, 1979–1991: an analysis of multiple-cause mortality data. Am J Respir Crit Care Med 1996;153:1548–1552. [DOI] [PubMed] [Google Scholar]
- 19.Raghu G, Freudenberger TD, Yang S, Curtis JR, Spada C, Hayes J, Sillery JK, Pope CE II, Pellegrini CA. High prevalence of abnormal acid gastro-oesophageal reflux in idiopathic pulmonary fibrosis. Eur Respir J 2006;27:136–142. [DOI] [PubMed] [Google Scholar]
- 20.Raghu G, Weycker D, Edelsberg J, Bradford WZ, Oster G. Incidence and prevalence of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2006;174:810–816. [DOI] [PubMed] [Google Scholar]
- 21.Nadrous HF, Myers JL, Decker PA, Ryu JH. Idiopathic pulmonary fibrosis in patients younger than 50 years. Mayo Clin Proc 2005;80:37–40. [DOI] [PubMed] [Google Scholar]
- 22.Iwai K, Mori T, Yamada N, Yamaguchi M, Hosoda Y. Idiopathic pulmonary fibrosis: epidemiologic approaches to occupational exposure. Am J Respir Crit Care Med 1994;150:670–675. [DOI] [PubMed] [Google Scholar]
- 23.Coultas DB, Zumwalt RE, Black WC, Sobonya RE. The epidemiology of interstitial lung diseases. Am J Respir Crit Care Med 1994;150:967–972. [DOI] [PubMed] [Google Scholar]
- 24.von Plessen C, Grinde O, Gulsvik A. Incidence and prevalence of cryptogenic fibrosing alveolitis in a Norwegian community. Respir Med 2003;97:428–435. [DOI] [PubMed] [Google Scholar]
- 25.Karakatsani A, Papakosta D, Rapti A, Antoniou KM, Dimadi M, Markopoulou A, Latsi P, Polychronopoulos V, Birba G, Ch L, et al.; Hellenic Interstitial Lung Diseases Group. Epidemiology of interstitial lung diseases in Greece. Respir Med 2009;103:1122–1129. [DOI] [PubMed] [Google Scholar]
- 26.Hubbard R, Lewis S, Richards K, Johnston I, Britton J. Occupational exposure to metal or wood dust and aetiology of cryptogenic fibrosing alveolitis. Lancet 1996;347:284–289. [DOI] [PubMed] [Google Scholar]
- 27.Baumgartner KB, Samet JM, Stidley CA, Colby TV, Waldron JA. Cigarette smoking: a risk factor for idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 1997;155:242–248. [DOI] [PubMed] [Google Scholar]
- 28.Enomoto T, Usuki J, Azuma A, Nakagawa T, Kudoh S. Diabetes mellitus may increase risk for idiopathic pulmonary fibrosis. Chest 2003;123:2007–2011. [DOI] [PubMed] [Google Scholar]
- 29.Steele MP, Speer MC, Loyd JE, Brown KK, Herron A, Slifer SH, Burch LH, Wahidi MM, Phillips JA III, Sporn TA, et al. Clinical and pathologic features of familial interstitial pneumonia. Am J Respir Crit Care Med 2005;172:1146–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miyake Y, Sasaki S, Yokoyama T, Chida K, Azuma A, Suda T, Kudoh S, Sakamoto N, Okamoto K, Kobashi G, et al. Occupational and environmental factors and idiopathic pulmonary fibrosis in Japan. Ann Occup Hyg 2005;49:259–265. [DOI] [PubMed] [Google Scholar]
- 31.Taskar VS, Coultas DB. Is idiopathic pulmonary fibrosis an environmental disease? Proc Am Thorac Soc 2006;3:293–298. [DOI] [PubMed] [Google Scholar]
- 32.Johnston ID, Prescott RJ, Chalmers JC, Rudd RM. British Thoracic Society study of cryptogenic fibrosing alveolitis: current presentation and initial management. Fibrosing Alveolitis Subcommittee of the Research Committee of the British Thoracic Society. Thorax 1997;52:38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hubbard R, Cooper M, Antoniak M, Venn A, Khan S, Johnston I, Lewis S, Britton J. Risk of cryptogenic fibrosing alveolitis in metal workers. Lancet 2000;355:466–467. [DOI] [PubMed] [Google Scholar]
- 34.Gustafson T, Dahlman-Hoglund A, Nilsson K, Strom K, Tornling G, Toren K. Occupational exposure and severe pulmonary fibrosis. Respir Med 2007;101:2207–2212. [DOI] [PubMed] [Google Scholar]
- 35.Kitamura H, Ichinose S, Hosoya T, Ando T, Ikushima S, Oritsu M, Takemura T. Inhalation of inorganic particles as a risk factor for idiopathic pulmonary fibrosis: elemental microanalysis of pulmonary lymph nodes obtained at autopsy cases. Pathol Res Pract 2007;203:575–585. [DOI] [PubMed] [Google Scholar]
- 36.Ueda T, Ohta K, Suzuki N, Yamaguchi M, Hirai K, Horiuchi T, Watanabe J, Miyamoto T, Ito K. Idiopathic pulmonary fibrosis and high prevalence of serum antibodies to hepatitis C virus. Am Rev Respir Dis 1992;146:266–268. [DOI] [PubMed] [Google Scholar]
- 37.Irving WL, Day S, Johnston ID. Idiopathic pulmonary fibrosis and hepatitis C virus infection. Am Rev Respir Dis 1993;148:1683–1684. [DOI] [PubMed] [Google Scholar]
- 38.Egan JJ, Stewart JP, Hasleton PS, Arrand JR, Carroll KB, Woodcock AA. Epstein-Barr virus replication within pulmonary epithelial cells in cryptogenic fibrosing alveolitis. Thorax 1995;50:1234–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meliconi R, Andreone P, Fasano L, Galli S, Pacilli A, Miniero R, Fabbri M, Solforosi L, Bernardi M. Incidence of hepatitis C virus infection in Italian patients with idiopathic pulmonary fibrosis. Thorax 1996;51:315–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuwano K, Nomoto Y, Kunitake R, Hagimoto N, Matsuba T, Nakanishi Y, Hara N. Detection of adenovirus E1A DNA in pulmonary fibrosis using nested polymerase chain reaction. Eur Respir J 1997;10:1445–1449. [DOI] [PubMed] [Google Scholar]
- 41.Wangoo A, Shaw RJ, Diss TC, Farrell PJ, du Bois RM, Nicholson AG. Cryptogenic fibrosing alveolitis: lack of association with Epstein-Barr virus infection. Thorax 1997;52:888–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamaguchi S, Kubo K, Fujimoto K, Honda T, Sekiguchi M, Sodeyama T. Analysis of bronchoalveolar lavage fluid in patients with chronic hepatitis C before and after treatment with interferon alpha. Thorax 1997;52:33–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yonemaru M, Kasuga I, Kusumoto H, Kunisawa A, Kiyokawa H, Kuwabara S, Ichinose Y, Toyama K. Elevation of antibodies to cytomegalovirus and other herpes viruses in pulmonary fibrosis. Eur Respir J 1997;10:2040–2045. [DOI] [PubMed] [Google Scholar]
- 44.Stewart JP, Egan JJ, Ross AJ, Kelly BG, Lok SS, Hasleton PS, Woodcock AA. The detection of Epstein-Barr virus DNA in lung tissue from patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 1999;159:1336–1341. [DOI] [PubMed] [Google Scholar]
- 45.Tsukamoto K, Hayakawa H, Sato A, Chida K, Nakamura H, Miura K. Involvement of Epstein-Barr virus latent membrane protein 1 in disease progression in patients with idiopathic pulmonary fibrosis. Thorax 2000;55:958–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lok SS, Stewart JP, Kelly BG, Hasleton PS, Egan JJ. Epstein-Barr virus and wild p53 in idiopathic pulmonary fibrosis. Respir Med 2001;95:787–791. [DOI] [PubMed] [Google Scholar]
- 47.Idilman R, Cetinkaya H, Savas I, Aslan N, Sak SD, Bastemir M, Sarioglu M, Soykan I, Bozdayi M, Colantoni A, et al. Bronchoalveolar lavage fluid analysis in individuals with chronic hepatitis C. J Med Virol 2002;66:34–39. [PubMed] [Google Scholar]
- 48.Kelly BG, Lok SS, Hasleton PS, Egan JJ, Stewart JP. A rearranged form of Epstein-Barr virus DNA is associated with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2002;166:510–513. [DOI] [PubMed] [Google Scholar]
- 49.Arase Y, Ikeda K, Tsubota A, Saitoh S, Suzuki Y, Kobayashi M, Suzuki F, Someya T, Akuta N, Hosaka T, et al. Usefulness of serum KL-6 for early diagnosis of idiopathic pulmonary fibrosis in patients with hepatitis C virus. Hepatol Res 2003;27:89–94. [DOI] [PubMed] [Google Scholar]
- 50.Tang YW, Johnson JE, Browning PJ, Cruz-Gervis RA, Davis A, Graham BS, Brigham KL, Oates JA Jr, Loyd JE, Stecenko AA. Herpesvirus DNA is consistently detected in lungs of patients with idiopathic pulmonary fibrosis. J Clin Microbiol 2003;41:2633–2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Procop GW, Kohn DJ, Johnson JE, Li HJ, Loyd JE, Yen-Lieberman B, Tang YW. BK and JC polyomaviruses are not associated with idiopathic pulmonary fibrosis. J Clin Microbiol 2005;43:1385–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zamo A, Poletti V, Reghellin D, Montagna L, Pedron S, Piccoli P, Chilosi M. HHV-8 and EBV are not commonly found in idiopathic pulmonary fibrosis. Sarcoidosis Vasc Diffuse Lung Dis 2005;22:123–128. [PubMed] [Google Scholar]
- 53.Tobin RW, Pope CE II, Pellegrini CA, Emond MJ, Sillery J, Raghu G. Increased prevalence of gastroesophageal reflux in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 1998;158:1804–1808. [DOI] [PubMed] [Google Scholar]
- 54.Patti MG, Tedesco P, Golden J, Hays S, Hoopes C, Meneghetti A, Damani T, Way LW. Idiopathic pulmonary fibrosis: how often is it really idiopathic? J Gastrointest Surg 2005;9:1053–1056. [DOI] [PubMed] [Google Scholar]
- 55.el-Serag HB, Sonnenberg A. Comorbid occurrence of laryngeal or pulmonary disease with esophagitis in United States military veterans. Gastroenterology 1997;113:755–760. [DOI] [PubMed] [Google Scholar]
- 56.D'Ovidio F, Singer LG, Hadjiliadis D, Pierre A, Waddell TK, de Perrot M, Hutcheon M, Miller L, Darling G, Keshavjee S. Prevalence of gastroesophageal reflux in end-stage lung disease candidates for lung transplant. Ann Thorac Surg 2005;80:1254–1260. [DOI] [PubMed] [Google Scholar]
- 57.Gribbin J, Hubbard R, Smith C. Role of diabetes mellitus and gastro-oesophageal reflux in the aetiology of idiopathic pulmonary fibrosis. Respir Med 2009;103:927–931. [DOI] [PubMed] [Google Scholar]
- 58.Bitterman PB, Rennard SI, Keogh BA, Wewers MD, Adelberg S, Crystal RG. Familial idiopathic pulmonary fibrosis: evidence of lung inflammation in unaffected family members. N Engl J Med 1986;314:1343–1347. [DOI] [PubMed] [Google Scholar]
- 59.Marshall RP, Puddicombe A, Cookson WO, Laurent GJ. Adult familial cryptogenic fibrosing alveolitis in the UK. Thorax 2000;55:143–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hodgson U, Laitinen T, Tukiainen P. Nationwide prevalence of sporadic and familial idiopathic pulmonary fibrosis: evidence of founder effect among multiplex families in Finland. Thorax 2002;57:338–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Allam JS, Limper AH. Idiopathic pulmonary fibrosis: is it a familial disease? Curr Opin Pulm Med 2006;12:312–317. [DOI] [PubMed] [Google Scholar]
- 62.Lee H, Ryu JH, Wittmer MH, Hartman TE, Lymp JF, Tazelaar HD, Limper AH. Familial idiopathic pulmonary fibrosis: clinical features and outcome. Chest 2005;127:2034–2041. [DOI] [PubMed] [Google Scholar]
- 63.Raghu G, Hert R. Interstitial lung diseases: genetic predisposition and inherited interstitial lung diseases. Sem Respir Med 1993;14:323–332. [Google Scholar]
- 64.Mageto YN, Raghu G. Genetic predisposition of idiopathic pulmonary fibrosis. Curr Opin Pulm Med 1997;3:336–340. [DOI] [PubMed] [Google Scholar]
- 65.Yang IV, Burch LH, Steele MP, Savov JD, Hollingsworth JW, McElvania-Tekippe E, Berman KG, Speer MC, Sporn TA, Brown KK, et al. Gene expression profiling of familial and sporadic interstitial pneumonia. Am J Respir Crit Care Med 2007;175:45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hodgson U, Pulkkinen V, Dixon M, Peyrard-Janvid M, Rehn M, Lahermo P, Ollikainen V, Salmenkivi K, Kinnula V, Kere J, et al. ELMOD2 is a candidate gene for familial idiopathic pulmonary fibrosis. Am J Hum Genet 2006;79:149–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rosas IO, Ren P, Avila NA, Chow CK, Franks TJ, Travis WD, Philip MJ Jr, May RM, Wu H, Nguyen DM, et al. Early interstitial lung disease in familial pulmonary fibrosis. Am J Respir Crit Care Med 2007;176:698–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Musk AW, Zilko PJ, Manners P, Kay PH, Kamboh MI. Genetic studies in familial fibrosing alveolitis. Possible linkage with immunoglobulin allotypes (Gm). Chest 1986;89:206–210. [DOI] [PubMed] [Google Scholar]
- 69.Thomas AQ, Lane K, Phillips J III, Prince M, Markin C, Speer M, Schwartz DA, Gaddipati R, Marney A, Johnson J, et al. Heterozygosity for a surfactant protein C gene mutation associated with usual interstitial pneumonitis and cellular nonspecific interstitial pneumonitis in one kindred. Am J Respir Crit Care Med 2002;165:1322–1328. [DOI] [PubMed] [Google Scholar]
- 70.Selman M, Lin H, Montano M, Jenkins AL, Estrada A, Lin Z, Wang G, DiAngelo SL, Guo X, Umstead TM, et al. Surfactant protein A and B genetic variants predispose to idiopathic pulmonary fibrosis. Hum Genet 2003;113:542–550. [DOI] [PubMed] [Google Scholar]
- 71.Lawson WE, Grant SW, Ambrosini V, Womble KE, Dawson EP, Lane KB, Markin C, Renzoni E, Lympany P, Thomas AQ, et al. Genetic mutations in surfactant protein C are a rare cause of sporadic cases of IPF. Thorax 2004;59:977–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Markart P, Ruppert C, Wygrecka M, Schmidt R, Korfei M, Harbach H, Theruvath I, Pison U, Seeger W, Guenther A, et al. Surfactant protein C mutations in sporadic forms of idiopathic interstitial pneumonias. Eur Respir J 2007;29:134–137. [DOI] [PubMed] [Google Scholar]
- 73.Wang Y, Kuan PJ, Xing C, Cronkhite JT, Torres F, Rosenblatt RL, DiMaio JM, Kinch LN, Grishin NV, Garcia CK. Genetic defects in surfactant protein A2 are associated with pulmonary fibrosis and lung cancer. Am J Hum Genet 2009;84:52–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Armanios MY, Chen JJ, Cogan JD, Alder JK, Ingersoll RG, Markin C, Lawson WE, Xie M, Vulto I, Phillips JA III, et al. Telomerase mutations in families with idiopathic pulmonary fibrosis. N Engl J Med 2007;356:1317–1326. [DOI] [PubMed] [Google Scholar]
- 75.Tsakiri KD, Cronkhite JT, Kuan PJ, Xing C, Raghu G, Weissler JC, Rosenblatt RL, Shay JW, Garcia CK. Adult-onset pulmonary fibrosis caused by mutations in telomerase. Proc Natl Acad Sci USA 2007;104:7552–7557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cronkhite JT, Xing C, Raghu G, Chin KM, Torres F, Rosenblatt RL, Garcia CK. Telomere shortening in familial and sporadic pulmonary fibrosis. Am J Respir Crit Care Med 2008;178:729–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Alder JK, Chen JJ, Lancaster L, Danoff S, Su SC, Cogan JD, Vulto I, Xie M, Qi X, Tuder RM, et al. Short telomeres are a risk factor for idiopathic pulmonary fibrosis. Proc Natl Acad Sci USA 2008;105:13051–13056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mushiroda T, Wattanapokayakit S, Takahashi A, Nukiwa T, Kudoh S, Ogura T, Taniguchi H, Kubo M, Kamatani N, Nakamura Y; Pirfenidone Clinical Study Group. A genome-wide association study identifies an association of a common variant in TERT with susceptibility to idiopathic pulmonary fibrosis. J Med Genet 2008;45:654–656. [DOI] [PubMed] [Google Scholar]
- 79.Renzoni E, Lympany P, Sestini P, Pantelidis P, Wells A, Black C, Welsh K, Bunn C, Knight C, Foley P, et al. Distribution of novel polymorphisms of the interleukin-8 and CXC receptor 1 and 2 genes in systemic sclerosis and cryptogenic fibrosing alveolitis. Arthritis Rheum 2000;43:1633–1640. [DOI] [PubMed] [Google Scholar]
- 80.Whyte M, Hubbard R, Meliconi R, Whidborne M, Eaton V, Bingle C, Timms J, Duff G, Facchini A, Pacilli A, et al. Increased risk of fibrosing alveolitis associated with interleukin-1 receptor antagonist and tumor necrosis factor-alpha gene polymorphisms. Am J Respir Crit Care Med 2000;162:755–758. [DOI] [PubMed] [Google Scholar]
- 81.Freeburn RW, Kendall H, Dobson L, Egan J, Simler NJ, Millar AB. The 3′ untranslated region of tumor necrosis factor-alpha is highly conserved in idiopathic pulmonary fibrosis (IPF). Eur Cytokine Netw 2001;12:33–38. [PubMed] [Google Scholar]
- 82.Pantelidis P, Fanning GC, Wells AU, Welsh KI, Du Bois RM. Analysis of tumor necrosis factor-alpha, lymphotoxin-alpha, tumor necrosis factor receptor II, and interleukin-6 polymorphisms in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2001;163:1432–1436. [DOI] [PubMed] [Google Scholar]
- 83.Hutyrova B, Pantelidis P, Drabek J, Zrkova M, Kolek V, Lenhart K, Welsh KI, Du Bois RM, Petrek M. Interleukin-1 gene cluster polymorphisms in sarcoidosis and idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2002;165:148–151. [DOI] [PubMed] [Google Scholar]
- 84.Latsi P, Pantelidis P, Vassilakis D, Sato H, Welsh KI, du Bois RM. Analysis of IL-12 p40 subunit gene and IFN-gamma G5644A polymorphisms in Idiopathic Pulmonary Fibrosis. Respir Res 2003;4:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Whittington HA, Freeburn RW, Godinho SIH, Egan J, Haider Y, Millar AB. Analysis of an IL-10 polymorphism in idiopathic pulmonary fibrosis. Genes Immun 2003;4:258–264. [DOI] [PubMed] [Google Scholar]
- 86.Riha RL, Yang IA, Rabnott GC, Tunnicliffe AM, Fong KM, Zimmerman PV. Cytokine gene polymorphisms in idiopathic pulmonary fibrosis. Intern Med J 2004;34:126–129. [DOI] [PubMed] [Google Scholar]
- 87.Vasakova M, Striz I, Slavcev A, Jandova S, Dutka J, Terl M, Kolesar L, Sulc J. Correlation of IL-1alpha and IL-4 gene polymorphisms and clinical parameters in idiopathic pulmonary fibrosis. Scand J Immunol 2007;65:265–270. [DOI] [PubMed] [Google Scholar]
- 88.Vasakova M, Striz I, Slavcev A, Jandova S, Kolesar L, Sulc J. Th1/Th2 cytokine gene polymorphisms in patients with idiopathic pulmonary fibrosis. Tissue Antigens 2006;67:229–232. [DOI] [PubMed] [Google Scholar]
- 89.Geddes DM, Webley M, Brewerton DA, Turton CW, Turner-Warwick M, Murphy AH, Ward AM. alpha 1-antitrypsin phenotypes in fibrosing alveolitis and rheumatoid arthritis. Lancet 1977;2:1049–1051. [DOI] [PubMed] [Google Scholar]
- 90.Hubbard R, Baoku Y, Kalsheker N, Britton J, Johnston I. Alpha1-antitrypsin phenotypes in patients with cryptogenic fibrosing alveolitis: a case-control study. Eur Respir J 1997;10:2881–2883. [DOI] [PubMed] [Google Scholar]
- 91.Morrison CD, Papp AC, Hejmanowski AQ, Addis VM, Prior TW. Increased D allele frequency of the angiotensin-converting enzyme gene in pulmonary fibrosis. Hum Pathol 2001;32:521–528. [DOI] [PubMed] [Google Scholar]
- 92.Xaubet A, Marin-Arguedas A, Lario S, Ancochea J, Morell F, Ruiz-Manzano J, Rodriguez-Becerra E, Rodriguez-Arias JM, Inigo P, Sanz S, et al. Transforming growth factor-beta1 gene polymorphisms are associated with disease progression in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2003;168:431–435. [DOI] [PubMed] [Google Scholar]
- 93.Zorzetto M, Ferrarotti I, Campo I, Trisolini R, Poletti V, Scabini R, Ceruti M, Mazzola P, Crippa E, Ottaviani S, et al. NOD2/CARD15 gene polymorphisms in idiopathic pulmonary fibrosis. Sarcoidosis Vasc Diffuse Lung Dis 2005;22:180–185. [PubMed] [Google Scholar]
- 94.Checa M, Ruiz V, Montano M, Velazquez-Cruz R, Selman M, Pardo A. MMP-1 polymorphisms and the risk of idiopathic pulmonary fibrosis. Hum Genet 2008;124:465–472. [DOI] [PubMed] [Google Scholar]
- 95.Falfan-Valencia R, Camarena A, Juarez A, Becerril C, Montano M, Cisneros J, Mendoza F, Granados J, Pardo A, Selman M. Major histocompatibility complex and alveolar epithelial apoptosis in idiopathic pulmonary fibrosis. Hum Genet 2005;118:235–244. [DOI] [PubMed] [Google Scholar]
- 96.Lederer DJ, Arcasoy SM, Barr RG, Wilt JS, Bagiella E, D'Ovidio F, Sonett JR, Kawut SM. Racial and ethnic disparities in idiopathic pulmonary fibrosis: A UNOS/OPTN database analysis. Am J Transplant 2006;6:2436–2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zuo F, Kaminski N, Eugui E, Allard J, Yakhini Z, Ben-Dor A, Lollini L, Morris D, Kim Y, DeLustro B, et al. Gene expression analysis reveals matrilysin as a key regulator of pulmonary fibrosis in mice and humans. Proc Natl Acad Sci USA 2002;99:6292–6297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nishimura K, Kitaichi M, Izumi T, Nagai S, Kanaoka M, Itoh H. Usual interstitial pneumonia: histologic correlation with high-resolution CT. Radiology 1992;182:337–342. [DOI] [PubMed] [Google Scholar]
- 99.Johkoh T, Muller NL, Cartier Y, Kavanagh PV, Hartman TE, Akira M, Ichikado K, Ando M, Nakamura H. Idiopathic interstitial pneumonias: diagnostic accuracy of thin-section CT in 129 patients. Radiology 1999;211:555–560. [DOI] [PubMed] [Google Scholar]
- 100.Hansell DM, Bankier AA, Macmahon H, McLoud TC, Muller NL, Remy J. Fleischner Society: Glossary of terms for thoracic imaging. Radiology 2008;246:697–722. [DOI] [PubMed] [Google Scholar]
- 101.Hwang JH, Misumi S, Sahin H, Brown KK, Newell JD, Lynch DA. Computed tomographic features of idiopathic fibrosing interstitial pneumonia: comparison with pulmonary fibrosis related to collagen vascular disease. J Comput Assist Tomogr 2009;33:410–415. [DOI] [PubMed] [Google Scholar]
- 102.Souza CA, Muller NL, Lee KS, Johkoh T, Mitsuhiro H, Chong S. Idiopathic interstitial pneumonias: prevalence of mediastinal lymph node enlargement in 206 patients. AJR Am J Roentgenol 2006;186:995–999. [DOI] [PubMed] [Google Scholar]
- 103.Mathieson JR, Mayo JR, Staples CA, Muller NL. Chronic diffuse infiltrative lung disease: comparison of diagnostic accuracy of CT and chest radiography. Radiology 1989;171:111–116. [DOI] [PubMed] [Google Scholar]
- 104.Hunninghake GW, Zimmerman MB, Schwartz DA, King TE Jr, Lynch JP III, Hegele R, Waldron J, Colby T, Muller N, Lynch D, et al. Utility of a lung biopsy for the diagnosis of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2001;164:193–196. [DOI] [PubMed] [Google Scholar]
- 105.Raghu G, Mageto YN, Lockhart D, Schmidt RA, Wood DE, Godwin JD. The accuracy of the clinical diagnosis of new-onset idiopathic pulmonary fibrosis and other interstitial lung disease: A prospective study. Chest 1999;116:1168–1174. [DOI] [PubMed] [Google Scholar]
- 106.Grenier P, Valeyre D, Cluzel P, Brauner MW, Lenoir S, Chastang C. Chronic diffuse interstitial lung disease: diagnostic value of chest radiography and high-resolution CT. Radiology 1991;179:123–132. [DOI] [PubMed] [Google Scholar]
- 107.Lee KS, Primack SL, Staples CA, Mayo JR, Aldrich JE, Muller NL. Chronic infiltrative lung disease: comparison of diagnostic accuracies of radiography and low- and conventional-dose thin-section CT. Radiology 1994;191:669–673. [DOI] [PubMed] [Google Scholar]
- 108.Swensen SJ, Aughenbaugh GL, Myers JL. Diffuse lung disease: diagnostic accuracy of CT in patients undergoing surgical biopsy of the lung. Radiology 1997;205:229–234. [DOI] [PubMed] [Google Scholar]
- 109.Flaherty KR, Thwaite EL, Kazerooni EA, Gross BH, Toews GB, Colby TV, Travis WD, Mumford JA, Murray S, Flint A, et al. Radiological versus histological diagnosis in UIP and NSIP: survival implications. Thorax 2003;58:143–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Quadrelli S, Molinari L, Ciallella L, Spina JC, Sobrino E, Chertcoff J. Radiological versus histopathological diagnosis of usual interstitial pneumonia in the clinical practice: does it have any survival difference? Respiration 2010;79:32–37. [DOI] [PubMed] [Google Scholar]
- 111.Flaherty KR, King TE Jr, Raghu G, Lynch JP III, Colby TV, Travis WD, Gross BH, Kazerooni EA, Toews GB, Long Q, et al. Idiopathic interstitial pneumonia: what is the effect of a multidisciplinary approach to diagnosis? Am J Respir Crit Care Med 2004;170:904–910. [DOI] [PubMed] [Google Scholar]
- 112.Lynch JP III, Saggar R, Weigt SS, Zisman DA, White ES. Usual interstitial pneumonia. Semin Respir Crit Care Med 2006;27:634–651. [DOI] [PubMed] [Google Scholar]
- 113.Trahan S, Hanak V, Ryu JH, Myers JL. Role of surgical lung biopsy in separating chronic hypersensitivity pneumonia from usual interstitial pneumonia/idiopathic pulmonary fibrosis: analysis of 31 biopsies from 15 patients. Chest 2008;134:126–132. [DOI] [PubMed] [Google Scholar]
- 114.Silva CI, Muller NL, Lynch DA, Curran-Everett D, Brown KK, Lee KS, Chung MP, Churg A. Chronic hypersensitivity pneumonitis: differentiation from idiopathic pulmonary fibrosis and nonspecific interstitial pneumonia by using thin-section CT. Radiology 2008;246:288–297. [DOI] [PubMed] [Google Scholar]
- 115.Monaghan H, Wells AU, Colby TV, du Bois RM, Hansell DM, Nicholson AG. Prognostic implications of histologic patterns in multiple surgical lung biopsies from patients with idiopathic interstitial pneumonias. Chest 2004;125:522–526. [DOI] [PubMed] [Google Scholar]
- 116.Flaherty KR, Travis WD, Colby TV, Toews GB, Kazerooni EA, Gross BH, Jain A, Strawderman RL, Flint A, Lynch JP, et al. Histopathologic variability in usual and nonspecific interstitial pneumonias. Am J Respir Crit Care Med 2001;164:1722–1727. [DOI] [PubMed] [Google Scholar]
- 117.Bensard DD, McIntyre RC Jr, Waring BJ, Simon JS. Comparison of video thoracoscopic lung biopsy to open lung biopsy in the diagnosis of interstitial lung disease. Chest 1993;103:765–770. [DOI] [PubMed] [Google Scholar]
- 118.Miller JD, Urschel JD, Cox G, Olak J, Young JE, Kay JM, McDonald E. A randomized, controlled trial comparing thoracoscopy and limited thoracotomy for lung biopsy in interstitial lung disease. Ann Thorac Surg 2000;70:1647–1650. [DOI] [PubMed] [Google Scholar]
- 119.Carnochan FM, Walker WS, Cameron EW. Efficacy of video assisted thoracoscopic lung biopsy: an historical comparison with open lung biopsy. Thorax 1994;49:361–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ferson PF, Landreneau RJ, Dowling RD, Hazelrigg SR, Ritter P, Nunchuck S, Perrino MK, Bowers CM, Mack MJ, Magee MJ. Comparison of open versus thoracoscopic lung biopsy for diffuse infiltrative pulmonary disease. J Thorac Cardiovasc Surg 1993;106:194–199. [PubMed] [Google Scholar]
- 121.Vourlekis JS, Schwarz MI, Cherniack RM, Curran-Everett D, Cool CD, Tuder RM, King TE Jr, Brown KK. The effect of pulmonary fibrosis on survival in patients with hypersensitivity pneumonitis. Am J Med 2004;116:662–668. [DOI] [PubMed] [Google Scholar]
- 122.Ohshimo S, Bonella F, Cui A, Beume M, Kohno N, Guzman J, Costabel U. Significance of bronchoalveolar lavage for the diagnosis of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2009;179:1043–1047. [DOI] [PubMed] [Google Scholar]
- 123.Berbescu EA, Katzenstein AA, Snow JL, Zisman DA. Transbronchial biopsy in usual interstitial pneumonia. Chest 2006;129:1126–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Park JH, Kim DS, Park IN, Jang SJ, Kitaichi M, Nicholson AG, Colby TV. Prognosis of fibrotic interstitial pneumonia: idiopathic versus collagen vascular disease-related subtypes. Am J Respir Crit Care Med 2007;175:705–711. [DOI] [PubMed] [Google Scholar]
- 125.Wasicek CA, Reichlin M, Montes M, Raghu G. Polymyositis and interstitial lung disease in a patient with anti-Jo1 prototype. Am J Med 1984;76:538–544. [DOI] [PubMed] [Google Scholar]
- 126.Flaherty KR, Andrei A, King TE Jr, Raghu G, Colby TV, Wells A, Bassily N, Brown K, du Bois R, Flint A, et al. Idiopathic interstitial pneumonia: do community and academic physicians agree on diagnosis? Am J Respir Crit Care Med 2007;175:1054–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Carrington CB, Gaensler EA, Coutu RE, FitzGerald MX, Gupta RG. Natural history and treated course of usual and desquamative interstitial pneumonia. N Engl J Med 1978;298:801–809. [DOI] [PubMed] [Google Scholar]
- 128.Tukiainen P, Taskinen E, Holsti P, Korhola O, Valle M. Prognosis of cryptogenic fibrosing alveolitis. Thorax 1983;38:349–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gross TJ, Hunninghake GW. Idiopathic pulmonary fibrosis. N Engl J Med 2001;345:517–525. [DOI] [PubMed] [Google Scholar]
- 130.Bjoraker JA, Ryu JH, Edwin MK, Myers JL, Tazelaar HD, Schroeder DR, Offord KP. Prognostic significance of histopathologic subsets in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 1998;157:199–203. [DOI] [PubMed] [Google Scholar]
- 131.Flaherty KR, Toews GB, Travis WD, Colby TV, Kazerooni EA, Gross BH, Jain A, Strawderman RL III, Paine R, Flint A, et al. Clinical significance of histological classification of idiopathic interstitial pneumonia. Eur Respir J 2002;19:275–283. [DOI] [PubMed] [Google Scholar]
- 132.Nicholson AG, Colby TV, du Bois RM, Hansell DM, Wells AU. The prognostic significance of the histologic pattern of interstitial pneumonia in patients presenting with the clinical entity of cryptogenic fibrosing alveolitis. Am J Respir Crit Care Med 2000;162:2213–2217. [DOI] [PubMed] [Google Scholar]
- 133.Rudd RM, Prescott RJ, Chalmers JC, Johnston IDA, Fibrosing Alveolitis Subcommittee of the Research Committee of the British Thoracic Society. British Thoracic Society Study on cryptogenic fibrosing alveolitis: Response to treatment and survival. Thorax 2007;62:62–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.King TE Jr, Schwarz MI, Brown K, Tooze JA, Colby TV, Waldron JA Jr, Flint A, Thurlbeck W, Cherniack RM. Idiopathic pulmonary fibrosis: relationship between histopathologic features and mortality. Am J Respir Crit Care Med 2001;164:1025–1032. [DOI] [PubMed] [Google Scholar]
- 135.Raghu G, Brown KK, Bradford WZ, Starko K, Noble PW, Schwartz DA, King TE Jr, Idiopathic Pulmonary Fibrosis Study Group. A placebo-controlled trial of interferon gamma-1b in patients with idiopathic pulmonary fibrosis. N Engl J Med 2004;350:125–133. [DOI] [PubMed] [Google Scholar]
- 136.King TE Jr, Albera C, Bradford WZ, Costabel U, Hormel P, Lancaster L, Noble PW, Sahn SA, Szwarcberg J, Thomeer M, et al.; INSPIRE Study Group. Effect of interferon gamma-1b on survival in patients with idiopathic pulmonary fibrosis (INSPIRE): a multicentre, randomised, placebo-controlled trial. Lancet 2009;374:222–228. [DOI] [PubMed] [Google Scholar]
- 137.King TE Jr, Behr J, Brown KK, du Bois RM, Lancaster L, de Andrade JA, Stahler G, Leconte I, Roux S, Raghu G. BUILD-1: a randomized placebo-controlled trial of bosentan in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2008;177:75–81. [DOI] [PubMed] [Google Scholar]
- 138.Raghu G. Idiopathic pulmonary fibrosis: a rational clinical approach. Chest 1987;92:148–154. [DOI] [PubMed] [Google Scholar]
- 139.Selman M, Carrillo G, Estrada A, Mejia M, Becerril C, Cisneros J, Gaxiola M, Perez-Padilla R, Navarro C, Richards T, et al. Accelerated variant of idiopathic pulmonary fibrosis: clinical behavior and gene expression pattern. PLoS ONE 2007;2:e482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Mejia M, Carrillo G, Rojas-Serrano J, Estrada A, Suarez T, Alonso D, Barrientos E, Gaxiola M, Navarro C, Selman M. Idiopathic pulmonary fibrosis and emphysema: decreased survival associated with severe pulmonary arterial hypertension. Chest 2009;136:10–15. [DOI] [PubMed] [Google Scholar]
- 141.Wells AU, Desai SR, Rubens MB, Goh NSL, Cramer D, Nicholson AG, Colby TV, du Bois RM, Hansell DM. Idiopathic pulmonary fibrosis: a composite physiologic index derived from disease extent observed by computed tomography. Am J Respir Crit Care Med 2003;167:962–969. [DOI] [PubMed] [Google Scholar]
- 142.Lettieri CJ, Nathan SD, Barnett SD, Ahmad S, Shorr AF. Prevalence and outcomes of pulmonary arterial hypertension in advanced idiopathic pulmonary fibrosis. Chest 2006;129:746–752. [DOI] [PubMed] [Google Scholar]
- 143.Collard HR, Moore BB, Flaherty KR, Brown KK, Kaner RJ, King TE Jr, Lasky JA, Loyd JE, Noth I, Olman MA, et al.; Idiopathic Pulmonary Fibrosis Clinical Research Network Investigators. Acute exacerbations of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2007;176:636–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Azuma A, Nukiwa T, Tsuboi E, Suga M, Abe S, Nakata K, Taguchi Y, Nagai S, Itoh H, Ohi M, et al. Double-blind, placebo-controlled trial of pirfenidone in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2005;171:1040–1047. [DOI] [PubMed] [Google Scholar]
- 145.Martinez FJ, Safrin S, Weycker D, Starko KM, Bradford WZ, King TE Jr, Flaherty KR, Schwartz DA, Noble PW, Raghu G, et al.; IPF Study Group. The clinical course of patients with idiopathic pulmonary fibrosis. Ann Intern Med 2005;142:963–967. [DOI] [PubMed] [Google Scholar]
- 146.Panos RJ, Mortenson RL, Niccoli SA, King TE Jr. Clinical deterioration in patients with idiopathic pulmonary fibrosis: causes and assessment. Am J Med 1990;88:396–404. [DOI] [PubMed] [Google Scholar]
- 147.Akira M, Hamada H, Sakatani M, Kobayashi C, Nishioka M, Yamamoto S. CT findings during phase of accelerated deterioration in patients with idiopathic pulmonary fibrosis. AJR Am J Roentgenol 1997;168:79–83. [DOI] [PubMed] [Google Scholar]
- 148.Ambrosini V, Cancellieri A, Chilosi M, Zompatori M, Trisolini R, Saragoni L, Poletti V. Acute exacerbation of idiopathic pulmonary fibrosis: report of a series. Eur Respir J 2003;22:821–826. [DOI] [PubMed] [Google Scholar]
- 149.Kim DS, Park JH, Park BK, Lee JS, Nicholson AG, Colby T. Acute exacerbation of idiopathic pulmonary fibrosis: frequency and clinical features. Eur Respir J 2006;27:143–150. [DOI] [PubMed] [Google Scholar]
- 150.Kondoh Y, Taniguchi H, Kawabata Y, Yokoi T, Suzuki K, Takagi K. Acute exacerbation in idiopathic pulmonary fibrosis: analysis of clinical and pathologic findings in three cases. Chest 1993;103:1808–1812. [DOI] [PubMed] [Google Scholar]
- 151.Kondoh Y, Taniguchi H, Yokoi T, Nishiyama O, Ohishi T, Kato T, Suzuki K, Suzuki R. Cyclophosphamide and low-dose prednisolone in idiopathic pulmonary fibrosis and fibrosing nonspecific interstitial pneumonia. Eur Respir J 2005;25:528–533. [DOI] [PubMed] [Google Scholar]
- 152.Kubo H, Nakayama K, Yanai M, Suzuki T, Yamaya M, Watanabe M, Sasaki H. Anticoagulant therapy for idiopathic pulmonary fibrosis. Chest 2005;128:1475–1482. [DOI] [PubMed] [Google Scholar]
- 153.Parambil JG, Myers JL, Ryu JH. Histopathologic features and outcome of patients with acute exacerbation of idiopathic pulmonary fibrosis undergoing surgical lung biopsy. Chest 2005;128:3310–3315. [DOI] [PubMed] [Google Scholar]
- 154.Tiitto L, Bloigu R, Heiskanen U, Paakko P, Kinnula VL, Kaarteenaho-Wiik R. Relationship between histopathological features and the course of idiopathic pulmonary fibrosis/usual interstitial pneumonia. Thorax 2006;61:1091–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Rice AJ, Wells AU, Bouros D, du Bois RM, Hansell DM, Polychronopoulos V, Vassilakis D, Kerr JR, Evans TW, Nicholson AG. Terminal diffuse alveolar damage in relation to interstitial pneumonias: an autopsy study. Am J Clin Pathol 2003;119:709–714. [DOI] [PubMed] [Google Scholar]
- 156.Churg A, Muller NL, Silva CIS, Wright JL. Acute exacerbation (acute lung injury of unknown cause) in UIP and other forms of fibrotic interstitial pneumonias. Am J Surg Pathol 2007;31:277–284. [DOI] [PubMed] [Google Scholar]
- 157.Kondo A, Saiki S. Acute exacerbation in idiopathic interstitial pneumonia (IIP). In: Harasawa M, Fukuchi Y, Morinari H, editors. Interstitial pneumonia of unknown etiology. Tokyo: University of Tokyo Press; 1989. pp. 33–42.
- 158.Konishi K, Gibson KF, Lindell KO, Richards TJ, Zhang Y, Dhir R, Bisceglia M, Gilbert S, Yousem SA, Song JW, et al. Gene expression profiles of acute exacerbations of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2009;180:167–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Sakamoto K, Taniguchi H, Kondoh Y, Ono K, Hasegawa Y, Kitaichi M. Acute exacerbation of idiopathic pulmonary fibrosis as the initial presentation of the disease. Eur Respir Rev 2009;18:129–132. [DOI] [PubMed] [Google Scholar]
- 160.Kondoh Y, Taniguchi H, Kataoka K, Kato K, Suzuki R, Ogura T, Johkoh T, Yokoi T, Wells AU, Kitaichi M; Tokai Diffuse Lung Disease Study Group. Prognostic factors in rapidly progressive interstitial pneumonia. Respirology 2010;15:257–264. [DOI] [PubMed] [Google Scholar]
- 161.Kumar P, Goldstraw P, Yamada K, Nicholson AG, Wells AU, Hansell DM, Dubois RM, Ladas G. Pulmonary fibrosis and lung cancer: risk and benefit analysis of pulmonary resection. J Thorac Cardiovasc Surg 2003;125:1321–1327. [DOI] [PubMed] [Google Scholar]
- 162.Yuksel M, Ozyurtkan MO, Bostanci K, Ahiskali R, Kodalli N. Acute exacerbation of interstitial fibrosis after pulmonary resection. Ann Thorac Surg 2006;82:336–338. [DOI] [PubMed] [Google Scholar]
- 163.Utz JP, Ryu JH, Douglas WW, Hartman TE, Tazelaar HD, Myers JL, Allen MS, Schroeder DR. High short-term mortality following lung biopsy for usual interstitial pneumonia. Eur Respir J 2001;17:175–179. [DOI] [PubMed] [Google Scholar]
- 164.Zegdi R, Azorin J, Tremblay B, Destable MD, Lajos PS, Valeyre D. Videothoracoscopic lung biopsy in diffuse infiltrative lung diseases: a 5-year surgical experience. Ann Thorac Surg 1998;66:1170–1173. [DOI] [PubMed] [Google Scholar]
- 165.Kondoh Y, Taniguchi H, Kitaichi M, Yokoi T, Johkoh T, Oishi T, Kimura T, Nishiyama O, Kato K, du Bois RM. Acute exacerbation of interstitial pneumonia following surgical lung biopsy. Respir Med 2006;100:1753–1759. [DOI] [PubMed] [Google Scholar]
- 166.Hiwatari N, Shimura S, Takishima T, Shirato K. Bronchoalveolar lavage as a possible cause of acute exacerbation in idiopathic pulmonary fibrosis patients. Tohoku J Exp Med 1994;174:379–386. [DOI] [PubMed] [Google Scholar]
- 167.Olson AL, Swigris JJ, Lezotte DC, Norris JM, Wilson CG, Brown KK. Mortality from pulmonary fibrosis increased in the United States from 1992 to 2003. Am J Respir Crit Care Med 2007;176:277–284. [DOI] [PubMed] [Google Scholar]
- 168.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin 2007;57:43–66. [DOI] [PubMed] [Google Scholar]
- 169.Olson AL, Swigris JJ, Raghu G, Brown KK. Seasonal variation: mortality from pulmonary fibrosis is greatest in the winter. Chest 2009;136:16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hubbard RB, Smith C, Le Jeune I, Gribbin J, Fogarty AW. The association between idiopathic pulmonary fibrosis and vascular disease: a population-based study. Am J Respir Crit Care Med 2008;178:1257–1261. [DOI] [PubMed] [Google Scholar]
- 171.Nadrous HF, Pellikka PA, Krowka MJ, Swanson KL, Chaowalit N, Decker PA, Ryu JH. Pulmonary hypertension in patients with idiopathic pulmonary fibrosis. Chest 2005;128:2393–2399. [DOI] [PubMed] [Google Scholar]
- 172.Collard HR, Ryu JH, Douglas WW, Schwarz MI, Curran-Everett D, King TE Jr, Brown KK. Combined corticosteroid and cyclophosphamide therapy does not alter survival in idiopathic pulmonary fibrosis. Chest 2004;125:2169–2174. [DOI] [PubMed] [Google Scholar]
- 173.Hubbard R, Venn A, Smith C, Cooper M, Johnston I, Britton J. Exposure to commonly prescribed drugs and the etiology of cryptogenic fibrosing alveolitis: a case-control study. Am J Respir Crit Care Med 1998;157:743–747. [DOI] [PubMed] [Google Scholar]
- 174.Schwartz DA, Van Fossen DS, Davis CS, Helmers RA, Dayton CS, Burmeister LF, Hunninghake GW. Determinants of progression in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 1994;149:444–449. [DOI] [PubMed] [Google Scholar]
- 175.Enomoto N, Suda T, Kato M, Kaida Y, Nakamura Y, Imokawa S, Ida M, Chida K. Quantitative analysis of fibroblastic foci in usual interstitial pneumonia. Chest 2006;130:22–29. [DOI] [PubMed] [Google Scholar]
- 176.Nadrous HF, Pellikka PA, Krowka MJ, Swanson KL, Chaowalit N, Decker PA, Ryu JH. The impact of pulmonary hypertension on survival in patients with idiopathic pulmonary fibrosis. Chest 2005;128:616S–617S. [DOI] [PubMed] [Google Scholar]
- 177.Jegal Y, Kim DS, Shim TS, Lim C, Do Lee S, Koh Y, Kim WS, Kim WD, Lee JS, Travis WD, et al. Physiology is a stronger predictor of survival than pathology in fibrotic interstitial pneumonia. Am J Respir Crit Care Med 2005;171:639–644. [DOI] [PubMed] [Google Scholar]
- 178.Wells AU, Hogaboam CM. Update in diffuse parenchymal lung disease 2007. Am J Respir Crit Care Med 2008;177:580–584. [DOI] [PubMed] [Google Scholar]
- 179.Turner-Warwick M, Burrows B, Johnson A. Cryptogenic fibrosing alveolitis: response to corticosteroid treatment and its effect on survival. Thorax 1980;35:593–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Schwartz DA, Helmers RA, Galvin JR, Van Fossen DS, Frees KL, Dayton CS, Burmeister LF, Hunninghake GW. Determinants of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 1994;149:450–454. [DOI] [PubMed] [Google Scholar]
- 181.Nishiyama O, Taniguchi H, Kondoh Y, Kimura T, Ogawa T, Watanabe F, Nishimura K. Health-related quality of life in patients with idiopathic pulmonary fibrosis: what is the main contributing factor? Respir Med 2005;99:408–414. [DOI] [PubMed] [Google Scholar]
- 182.Nishiyama O, Taniguchi H, Kondoh Y, Kimura T, Kato K, Kataoka K, Ogawa T, Watanabe F, Arizono S. A simple assessment of dyspnea as a prognostic indicator in idiopathic pulmonary fibrosis. Eur Respir J 2010;36:1067–1072. [DOI] [PubMed] [Google Scholar]
- 183.Watters LC, King TE Jr, Schwarz MI, Waldron JA, Stanford RE, Cherniack RM. A clinical, radiographic, and physiologic scoring system for the longitudinal assessment of patients with idiopathic pulmonary fibrosis. Am Rev Respir Dis 1986;133:97–103. [DOI] [PubMed] [Google Scholar]
- 184.Witek TJ Jr, Mahler DA. Minimal important difference of the transition dyspnoea index in a multinational clinical trial. Eur Respir J 2003;21:267–272. [DOI] [PubMed] [Google Scholar]
- 185.Kupferberg DH, Kaplan RM, Slymen DJ, Ries AL. Minimal clinically important difference for the UCSD Shortness of Breath Questionnaire. J Cardiopulm Rehabil 2005;25:370–377. [DOI] [PubMed] [Google Scholar]
- 186.Collard HR, King TE Jr, Bartelson BB, Vourlekis JS, Schwarz MI, Brown KK. Changes in clinical and physiologic variables predict survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2003;168:538–542. [DOI] [PubMed] [Google Scholar]
- 187.King TE Jr, Safrin S, Starko KM, Brown KK, Noble PW, Raghu G, Schwartz DA. Analyses of efficacy end points in a controlled trial of interferon-gamma1b for idiopathic pulmonary fibrosis. Chest 2005;127:171–177. [DOI] [PubMed] [Google Scholar]
- 188.Hallstrand TS, Boitano LJ, Johnson WC, Spada CA, Hayes JG, Raghu G. The timed walk test as a measure of severity and survival in idiopathic pulmonary fibrosis. Eur Respir J 2005;25:96–103. [DOI] [PubMed] [Google Scholar]
- 189.Hamada K, Nagai S, Tanaka S, Handa T, Shigematsu M, Nagao T, Mishima M, Kitaichi M, Izumi T. Significance of pulmonary arterial pressure and diffusion capacity of the lung as prognosticator in patients with idiopathic pulmonary fibrosis. Chest 2007;131:650–656. [DOI] [PubMed] [Google Scholar]
- 190.Egan JJ, Martinez FJ, Wells AU, Williams T. Lung function estimates in idiopathic pulmonary fibrosis: the potential for a simple classification. Thorax 2005;60:270–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Latsi PI, du Bois RM, Nicholson AG, Colby TV, Bisirtzoglou D, Nikolakopoulou A, Veeraraghavan S, Hansell DM, Wells AU. Fibrotic idiopathic interstitial pneumonia: the prognostic value of longitudinal functional trends. Am J Respir Crit Care Med 2003;168:531–537. [DOI] [PubMed] [Google Scholar]
- 192.Fell CD, Liu LX, Motika C, Kazerooni EA, Gross BH, Travis WD, Colby TV, Murray S, Toews GB, Martinez FJ, et al. The prognostic value of cardiopulmonary exercise testing in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2009;179:402–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Flaherty KR, Mumford JA, Murray S, Kazerooni EA, Gross BH, Colby TV, Travis WD, Flint A, Toews GB, Lynch JP III, et al. Prognostic implications of physiologic and radiographic changes in idiopathic interstitial pneumonia. Am J Respir Crit Care Med 2003;168:543–548. [DOI] [PubMed] [Google Scholar]
- 194.Lynch DA, Godwin JD, Safrin S, Starko KM, Hormel P, Brown KK, Raghu G, King TE Jr, Bradford WZ, Schwartz DA, et al., Idiopathic Pulmonary Fibrosis Study Group. High-resolution computed tomography in idiopathic pulmonary fibrosis: diagnosis and prognosis. Am J Respir Crit Care Med 2005;172:488–493. [DOI] [PubMed] [Google Scholar]
- 195.Best AC, Meng J, Lynch AM, Bozic CM, Miller D, Grunwald GK, Lynch DA. Idiopathic pulmonary fibrosis: physiologic tests, quantitative CT indexes, and CT visual scores as predictors of mortality. Radiology 2008;246:935–940. [DOI] [PubMed] [Google Scholar]
- 196.Jeong YJ, Lee KS, Muller NL, Chung MP, Chung MJ, Han J, Colby TV, Kim S. Usual interstitial pneumonia and non-specific interstitial pneumonia: serial thin-section CT findings correlated with pulmonary function. Korean J Radiol 2005;6:143–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Shin KM, Lee KS, Chung MP, Han J, Bae YA, Kim TS, Chung MJ. Prognostic determinants among clinical, thin-section CT, and histopathologic findings for fibrotic idiopathic interstitial pneumonias: tertiary hospital study. Radiology 2008;249:328–337. [DOI] [PubMed] [Google Scholar]
- 198.Sumikawa H, Johkoh T, Colby TV, Ichikado K, Suga M, Taniguchi H, Kondoh Y, Ogura T, Arakawa H, Fujimoto K, et al. Computed tomography findings in pathological usual interstitial pneumonia: relationship to survival. Am J Respir Crit Care Med 2008;177:433–439. [DOI] [PubMed] [Google Scholar]
- 199.Lama VN, Flaherty KR, Toews GB, Colby TV, Travis WD, Long Q, Murray S, Kazerooni EA, Gross BH, Lynch JP III, et al. Prognostic value of desaturation during a 6-minute walk test in idiopathic interstitial pneumonia. Am J Respir Crit Care Med 2003;168:1084–1090. [DOI] [PubMed] [Google Scholar]
- 200.Enright PL. The six-minute walk test. Respir Care 2003;48:783–785. [PubMed] [Google Scholar]
- 201.Lederer DJ, Arcasoy SM, Wilt JS, D'Ovidio F, Sonett JR, Kawut SM. Six-minute-walk distance predicts waiting list survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2006;174:659–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Swigris JJ, Swick J, Wamboldt FS, Sprunger D, du Bois R, Fischer A, Cosgrove GP, Frankel SK, Fernandez-Perez ER, Kervitsky D, et al. Heart rate recovery after 6-min walk test predicts survival in patients with idiopathic pulmonary fibrosis. Chest 2009;136:841–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Caminati A, Bianchi A, Cassandro R, Mirenda MR, Harari S. Walking distance on 6-MWT is a prognostic factor in idiopathic pulmonary fibrosis. Respir Med 2009;103:117–123. [DOI] [PubMed] [Google Scholar]
- 204.Eaton T, Young P, Milne D, Wells AU. Six-minute walk, maximal exercise tests: reproducibility in fibrotic interstitial pneumonia. Am J Respir Crit Care Med 2005;171:1150–1157. [DOI] [PubMed] [Google Scholar]
- 205.Nicholson AG, Fulford LG, Colby TV, du Bois RM, Hansell DM, Wells AU. The relationship between individual histologic features and disease progression in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2002;166:173–177. [DOI] [PubMed] [Google Scholar]
- 206.Flaherty KR, Colby TV, Toews GB, Travis WD, Flint A, Gay SE, Strawderman RL III, Jain A, Lynch JP III, Martinez FJ. Differential presence of fibroblastic foci in UIP patients with or without conntective tissue disease. Am J Respir Crit Care Med 2001;163:A983. [Google Scholar]
- 207.Hanak V, Ryu JH, de Carvalho E, Limper AH, Hartman TE, Decker PA, Myers JL. Profusion of fibroblast foci in patients with idiopathic pulmonary fibrosis does not predict outcome. Respir Med 2008;102:852–856. [DOI] [PubMed] [Google Scholar]
- 208.Arcasoy SM, Christie JD, Ferrari VA, Sutton MS, Zisman DA, Blumenthal NP, Pochettino A, Kotloff RM. Echocardiographic assessment of pulmonary hypertension in patients with advanced lung disease. Am J Respir Crit Care Med 2003;167:735–740. [DOI] [PubMed] [Google Scholar]
- 209.Nathan SD, Shlobin OA, Barnett SD, Saggar R, Belperio JA, Ross DJ, Ahmad S, Saggar R, Libre E, Lynch JP III, et al. Right ventricular systolic pressure by echocardiography as a predictor of pulmonary hypertension in idiopathic pulmonary fibrosis. Respir Med 2008;102:1305–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Fisher MR, Forfia PR, Chamera E, Housten-Harris T, Champion HC, Girgis RE, Corretti MC, Hassoun PM. Accuracy of Doppler echocardiography in the hemodynamic assessment of pulmonary hypertension. Am J Respir Crit Care Med 2009;179:615–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Corte TJ, Wort SJ, Gatzoulis MA, Macdonald P, Hansell DM, Wells AU. Pulmonary vascular resistance predicts early mortality in patients with diffuse fibrotic lung disease and suspected pulmonary hypertension. Thorax 2009;64:883–888. [DOI] [PubMed] [Google Scholar]
- 212.Cottin V, Nunes H, Brillet PY, Delaval P, Devouassoux G, Tillie-Leblond I, Israel-Biet D, Court-Fortune I, Valeyre D, Cordier JF; Groupe d'Etude et de Recherche sur les Maladies Orphelines Pulmonaires (GERM O P). Combined pulmonary fibrosis and emphysema: a distinct underrecognised entity. Eur Respir J 2005;26:586–593. [DOI] [PubMed] [Google Scholar]
- 213.Stahel RA, Gilks WR, Lehmann HP, Schenker T. Third International Workshop on Lung Tumor and Differentiation Antigens: overview of the results of the central data analysis. Int J Cancer Suppl 1994;8:6–26. [DOI] [PubMed] [Google Scholar]
- 214.Kohno N, Kyoizumi S, Awaya Y, Fukuhara H, Yamakido M, Akiyama M. New serum indicator of interstitial pneumonitis activity: sialylated carbohydrate antigen KL-6. Chest 1989;96:68–73. [DOI] [PubMed] [Google Scholar]
- 215.Yokoyama A, Kondo K, Nakajima M, Matsushima T, Takahashi T, Nishimura M, Bando M, Sugiyama Y, Totani Y, Ishizaki T, et al. Prognostic value of circulating KL-6 in idiopathic pulmonary fibrosis. Respirology 2006;11:164–168. [DOI] [PubMed] [Google Scholar]
- 216.Greene KE, King TE Jr, Kuroki Y, Bucher-Bartelson B, Hunninghake GW, Newman LS, Nagae H, Mason RJ. Serum surfactant proteins-A and -D as biomarkers in idiopathic pulmonary fibrosis. Eur Respir J 2002;19:439–446. [DOI] [PubMed] [Google Scholar]
- 217.Kinder BW, Brown KK, McCormack FX, Ix JH, Kervitsky A, Schwarz MI, King TE Jr. Serum surfactant protein-A is a strong predictor of early mortality in idiopathic pulmonary fibrosis. Chest 2009;135:1557–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Takahashi H, Fujishima T, Koba H, Murakami S, Kurokawa K, Shibuya Y, Shiratori M, Kuroki Y, Abe S. Serum surfactant proteins A and D as prognostic factors in idiopathic pulmonary fibrosis and their relationship to disease extent. Am J Respir Crit Care Med 2000;162:1109–1114. [DOI] [PubMed] [Google Scholar]
- 219.Prasse A, Probst C, Bargagli E, Zissel G, Toews GB, Flaherty KR, Olschewski M, Rottoli P, Muller-Quernheim J. Serum CC-chemokine ligand 18 concentration predicts outcome in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2009;179:717–723. [DOI] [PubMed] [Google Scholar]
- 220.Leuchte HH, Baumgartner RA, Nounou ME, Vogeser M, Neurohr C, Trautnitz M, Behr J. Brain natriuretic peptide is a prognostic parameter in chronic lung disease. Am J Respir Crit Care Med 2006;173:744–750. [DOI] [PubMed] [Google Scholar]
- 221.Leuchte HH, Neurohr C, Baumgartner R, Holzapfel M, Giehrl W, Vogeser M, Behr J. Brain natriuretic peptide and exercise capacity in lung fibrosis and pulmonary hypertension. Am J Respir Crit Care Med 2004;170:360–365. [DOI] [PubMed] [Google Scholar]
- 222.Song JW, Song JK, Kim DS. Echocardiography and brain natriuretic peptide as prognostic indicators in idiopathic pulmonary fibrosis. Respir Med 2009;103:180–186. [DOI] [PubMed] [Google Scholar]
- 223.Shinoda H, Tasaka S, Fujishima S, Yamasawa W, Miyamoto K, Nakano Y, Kamata H, Hasegawa N, Ishizaka A. Elevated CC chemokine level in bronchoalveolar lavage fluid is predictive of a poor outcome of idiopathic pulmonary fibrosis. Respiration 2009;78:285–292. [DOI] [PubMed] [Google Scholar]
- 224.Rosas IO, Richards TJ, Konishi K, Zhang Y, Gibson K, Lokshin AE, Lindell KO, Cisneros J, Macdonald SD, Pardo A, et al. MMP1 and MMP7 as potential peripheral blood biomarkers in idiopathic pulmonary fibrosis. PLoS Med 2008;5:e93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Phelps DS, Umstead TM, Mejia M, Carrillo G, Pardo A, Selman M. Increased surfactant protein-A levels in patients with newly diagnosed idiopathic pulmonary fibrosis. Chest 2004;125:617–625. [DOI] [PubMed] [Google Scholar]
- 226.McCormack FX, King TE Jr, Bucher BL, Nielsen L, Mason RJ. Surfactant protein A predicts survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 1995;152:751–759. [DOI] [PubMed] [Google Scholar]
- 227.Kinder BW, Brown KK, Schwarz MI, Ix JH, Kervitsky A, King TE Jr. Baseline BAL neutrophilia predicts early mortality in idiopathic pulmonary fibrosis. Chest 2008;133:226–232. [DOI] [PubMed] [Google Scholar]
- 228.Moeller A, Gilpin SE, Ask K, Cox G, Cook D, Gauldie J, Margetts PJ, Farkas L, Dobranowski J, Boylan C, et al. Circulating fibrocytes are an indicator of poor prognosis in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2009;179:588–594. [DOI] [PubMed] [Google Scholar]
- 229.Davies HR, Richeldi L, Walters EH. Immunomodulatory agents for idiopathic pulmonary fibrosis. Cochrane Database Syst Rev 2003; (3):CD003134. [DOI] [PubMed]
- 230.Richeldi L, Davies HR, Ferrara G, Franco F. Corticosteroids for idiopathic pulmonary fibrosis. Cochrane Database Syst Rev 2003; (3):CD002880. [DOI] [PMC free article] [PubMed]
- 231.Gay SE, Kazerooni EA, Toews GB, Lynch JP III, Gross BH, Cascade PN, Spizarny DL, Flint A, Schork MA, Whyte RI, et al. Idiopathic pulmonary fibrosis: predicting response to therapy and survival. Am J Respir Crit Care Med 1998;157:1063–1072. [DOI] [PubMed] [Google Scholar]
- 232.Flaherty KR, Toews GB, Lynch JP III, Kazerooni EA, Gross BH, Strawderman RL, Hariharan K, Flint A, Martinez FJ. Steroids in idiopathic pulmonary fibrosis: a prospective assessment of adverse reactions, response to therapy, and survival. Am J Med 2001;110:278–282. [DOI] [PubMed] [Google Scholar]
- 233.Nagai S, Kitaichi M, Hamada K, Nagao T, Hoshino Y, Miki H, Izumi T. Hospital-based historical cohort study of 234 histologically proven Japanese patients with IPF. Sarcoidosis Vasc Diffuse Lung Dis 1999;16:209–214. [PubMed] [Google Scholar]
- 234.Entzian P, Schlaak M, Seitzer U, Bufe A, Acil Y, Zabel P. Antiinflammatory and antifibrotic properties of colchicine: implications for idiopathic pulmonary fibrosis. Lung 1997;175:41–51. [DOI] [PubMed] [Google Scholar]
- 235.Peters SG, McDougall JC, Douglas WW, Coles DT, DeRemee RA. Colchicine in the treatment of pulmonary fibrosis. Chest 1993;103:101–104. [DOI] [PubMed] [Google Scholar]
- 236.Fiorucci E, Lucantoni G, Paone G, Zotti M, Li BE, Serpilli M, Regimenti P, Cammarella I, Puglisi G, Schmid G. Colchicine, cyclophosphamide and prednisone in the treatment of mild-moderate idiopathic pulmonary fibrosis: comparison of three currently available therapeutic regimens. Eur Rev Med Pharmacol Sci 2008;12:105–111. [PubMed] [Google Scholar]
- 237.Antoniou KM, Nicholson AG, Dimadi M, Malagari K, Latsi P, Rapti A, Tzanakis N, Trigidou R, Polychronopoulos V, Bouros D. Long-term clinical effects of interferon gamma-1b and colchicine in idiopathic pulmonary fibrosis. Eur Respir J 2006;28:496–504. [DOI] [PubMed] [Google Scholar]
- 238.Douglas WW, Ryu JH, Swensen SJ, Offord KP, Schroeder DR, Caron GM, DeRemee RA; Lung Study Group. Colchicine versus prednisone in the treatment of idiopathic pulmonary fibrosis: a randomized prospective study. Am J Respir Crit Care Med 1998;158:220–225. [DOI] [PubMed] [Google Scholar]
- 239.Alton EW, Johnson M, Turner-Warwick M. Advanced cryptogenic fibrosing alveolitis: preliminary report on treatment with cyclosporin A. Respir Med 1989;83:277–279. [DOI] [PubMed] [Google Scholar]
- 240.Moolman JA, Bardin PG, Rossouw DJ, Joubert JR. Cyclosporin as a treatment for interstitial lung disease of unknown aetiology. Thorax 1991;46:592–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Homma S, Sakamoto S, Kawabata M, Kishi K, Tsuboi E, Motoi N, Yoshimura K. Cyclosporin treatment in steroid-resistant and acutely exacerbated interstitial pneumonia. Intern Med 2005;44:1144–1150. [DOI] [PubMed] [Google Scholar]
- 242.Grgic A, Lausberg H, Heinrich M, Koenig J, Uder M, Sybrecht GW, Wilkens H. Progression of fibrosis in usual interstitial pneumonia: serial evaluation of the native lung after single lung transplantation. Respiration 2008;76:139–145. [DOI] [PubMed] [Google Scholar]
- 243.Wahidi MM, Ravenel J, Palmer SM, McAdams HP. Progression of idiopathic pulmonary fibrosis in native lungs after single lung transplantation. Chest 2002;121:2072–2076. [DOI] [PubMed] [Google Scholar]
- 244.Winterbauer RH, Hammar SP, Hallman KO, Hays JE, Pardee NE, Morgan EH, Allen JD, Moores KD, Bush W, Walker JH. Diffuse interstitial pneumonitis: clinicopathologic correlations in 20 patients treated with prednisone/azathioprine. Am J Med 1978;65:661–672. [DOI] [PubMed] [Google Scholar]
- 245.Raghu G, Depaso WJ, Cain K, Hammar SP, Wetzel CE, Dreis DF, Hutchinson J, Pardee NE, Winterbauer RH. Azathioprine combined with prednisone in the treatment of idiopathic pulmonary fibrosis: a prospective double-blind, randomized, placebo-controlled clinical trial. Am Rev Respir Dis 1991;144:291–296. [DOI] [PubMed] [Google Scholar]
- 246.Johnson MA, Kwan S, Snell NJ, Nunn AJ, Darbyshire JH, Turner-Warwick M. Randomised controlled trial comparing prednisolone alone with cyclophosphamide and low dose prednisolone in combination in cryptogenic fibrosing alveolitis. Thorax 1989;44:280–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Pereira CAC, Malheiros T, Coletta EM, Ferreira RG, Rubin AS, Otta JS, Rocha NS. Survival in idiopathic pulmonary fibrosis-cytotoxic agents compared to corticosteroids. Respir Med 2006;100:340–347. [DOI] [PubMed] [Google Scholar]
- 248.Cantin AM, Hubbard RC, Crystal RG. Glutathione deficiency in the epithelial lining fluid of the lower respiratory tract in idiopathic pulmonary fibrosis. Am Rev Respir Dis 1989;139:370–372. [DOI] [PubMed] [Google Scholar]
- 249.Behr J, Maier K, Degenkolb B, Krombach F, Vogelmeier C. Antioxidative and clinical effects of high-dose N-acetylcysteine in fibrosing alveolitis: adjunctive therapy to maintenance immunosuppression. Am J Respir Crit Care Med 1997;156:1897–1901. [DOI] [PubMed] [Google Scholar]
- 250.Demedts M, Behr J, Buhl R, Costabel U, Dekhuijzen R, Jansen HM, MacNee W, Thomeer M, Wallaert B, Laurent F, et al.; IFIGENIA Study Group. High-dose acetylcysteine in idiopathic pulmonary fibrosis. N Engl J Med 2005;353:2229–2242. [DOI] [PubMed] [Google Scholar]
- 251.Hunninghake GW. Antioxidant therapy for idiopathic pulmonary fibrosis. N Engl J Med 2005;353:2285–2287. [DOI] [PubMed] [Google Scholar]
- 252.Wells AU. Antioxidant therapy in idiopathic pulmonary fibrosis: hope is kindled. Eur Respir J 2006;27:664–666. [DOI] [PubMed] [Google Scholar]
- 253.Tomioka H, Kuwata Y, Imanaka K, Hashimoto K, Ohnishi H, Tada K, Sakamoto H, Iwasaki H. A pilot study of aerosolized N-acetylcysteine for idiopathic pulmonary fibrosis. Respirology 2005;10:449–455. [DOI] [PubMed] [Google Scholar]
- 254.Ziesche R, Hofbauer E, Wittmann K, Petkov V, Block LH. A preliminary study of long-term treatment with interferon gamma-1b and low-dose prednisolone in patients with idiopathic pulmonary fibrosis. N Engl J Med 1999;341:1264–1269. [DOI] [PubMed] [Google Scholar]
- 255.Thannickal VJ, Flaherty KR, Martinez FJ, Lynch JP III. Idiopathic pulmonary fibrosis: emerging concepts on pharmacotherapy. Expert Opin Pharmacother 2004;5:1671–1686. [DOI] [PubMed] [Google Scholar]
- 256.Raghu G, King TE Jr, Behr J, Brown KK, du Bois RM, Leconte I, Roux S, Swigris J. Quality of life and dyspnoea in patients treated with bosentan for idiopathic pulmonary fibrosis (BUILD-1). Eur Respir J 2010;35:118–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Mohler KM, Torrance DS, Smith CA, Goodwin RG, Stremler KE, Fung VP, Madani H, Widmer MB. Soluble tumor necrosis factor (TNF) receptors are effective therapeutic agents in lethal endotoxemia and function simultaneously as both TNF carriers and TNF antagonists. J Immunol 1993;151:1548–1561. [PubMed] [Google Scholar]
- 258.Sime PJ, Marr RA, Gauldie D, Xing Z, Hewlett BR, Graham FL, Gauldie J. Transfer of tumor necrosis factor-alpha to rat lung induces severe pulmonary inflammation and patchy interstitial fibrogenesis with induction of transforming growth factor-beta1 and myofibroblasts. Am J Pathol 1998;153:825–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Piguet PF, Ribaux C, Karpuz V, Grau GE, Kapanci Y. Expression and localization of tumor necrosis factor-alpha and its mRNA in idiopathic pulmonary fibrosis. Am J Pathol 1993;143:651–655. [PMC free article] [PubMed] [Google Scholar]
- 260.Raghu G, Brown KK, Costabel U, Cottin V, du Bois RM, Lasky JA, Thomeer M, Utz JP, Khandker RK, McDermott L, et al. Treatment of idiopathic pulmonary fibrosis with etanercept: an exploratory, placebo-controlled trial. Am J Respir Crit Care Med 2008;178:948–955. [DOI] [PubMed] [Google Scholar]
- 261.Raghu G, Johnson WC, Lockhart D, Mageto Y. Treatment of idiopathic pulmonary fibrosis with a new antifibrotic agent, pirfenidone: results of a prospective, open-label Phase II study. Am J Respir Crit Care Med 1999;159:1061–1069. [DOI] [PubMed] [Google Scholar]
- 262.Nagai S, Hamada K, Shigematsu M, Taniyama M, Yamauchi S, Izumi T. Open-label compassionate use one year-treatment with pirfenidone to patients with chronic pulmonary fibrosis. Intern Med 2002;41:1118–1123. [DOI] [PubMed] [Google Scholar]
- 263.Taniguchi H, Ebina M, Kondoh Y, Ogura T, Azuma A, Suga M, Taguchi Y, Takahashi H, Nakata K, Sato A, et al., Pirfenidone Clinical Study Group in Japan. Pirfenidone in idiopathic pulmonary fibrosis. Eur Respir J 2010;35:821–829. [DOI] [PubMed] [Google Scholar]
- 264.Seymour S. Briefing Information for the March 9, 2010. Meeting of the Pulmonary-Allergy Drugs Advisory Committee. NDA 22–535. Washington, D.C.: US Food and Drug Administration, 2010. [accessed 2010 May 10.] Available from: http://www.fda.gov/ downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/Pulmonary-AllergyDrugsAdvisoryCommittee/UCM203081.pdf.
- 265.Seymour S. Division Summary: Overview of the FDA background materials for New Drug Application (NDA) 22-535, Esbriet (pirfenidone) for the treatment of patients with idiopathic pulmonary fibrosis (IPF) to reduce the decline in lung function. In: Seymour S, editor. FDA Briefing Information for the March 9, 2010. Meeting of the Pulmonary-Allergy Drugs Advisory Committee (PADAC). NDA 22–535. Washington, D.C.: US Food and Drug Administration; 2010. pp. 2–20. [accessed 2010 May 10.] Available from: http://www. fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/Pulmonary-AllergyDrugsAdvisoryCommittee/UCM203081.pdf.
- 266.Karimi-Shah B. Esbriet (pirfenidone) 2403 mg/day to reduce the decline in lung function in patients with idiopathic pulmonary fibrosis (Clinical Briefing Document). In: Seymour S, editor. FDA Briefing Information for the March 9, 2010. Meeting of the Pulmonary-Allergy Drugs Advisory Committee (PADAC).NDA 22–535. Washington, D.C.: US Food and Drug Administration; 2010. pp. 21–109. [accessed 2010 May 10.] Available from: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/Pulmonary-AllergyDrugsAdvisoryCommittee/UCM203081.pdf.
- 267.Zhou F. Pirfenidone capsules [three 267-mg capsules TID] for treatment of patients with idiopathic pulmonary fibrosis to reduce decline in lung function (Statistical Briefing Document). In: Seymour S, editor. FDA Briefing Information for the March 9, 2010. Meeting of the Pulmonary-Allergy Drugs Advisory Committee (PADAC). NDA 22–535. Washington, D.C.: US Food and Drug Administration; 2010. p. 110–158. [accessed 2010 May 10.] Available from: http://www. fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/Pulmonary-AllergyDrugsAdvisoryCommittee/UCM203081.pdf.
- 268.Ghofrani HA, Wiedemann R, Rose F, Schermuly RT, Olschewski H, Weissmann N, Gunther A, Walmrath D, Seeger W, Grimminger F. Sildenafil for treatment of lung fibrosis and pulmonary hypertension: a randomised controlled trial. Lancet 2002;360:895–900. [DOI] [PubMed] [Google Scholar]
- 269.Madden BP, Allenby M, Loke T, Sheth A. A potential role for sildenafil in the management of pulmonary hypertension in patients with parenchymal lung disease. Vascul Pharmacol 2006;44:372–376. [DOI] [PubMed] [Google Scholar]
- 270.Collard HR, Anstrom KJ, Schwarz MI, Zisman DA. Sildenafil improves walk distance in idiopathic pulmonary fibrosis. Chest 2007;131:897–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Nathan SD, Noble PW, Tuder RM. Idiopathic pulmonary fibrosis and pulmonary hypertension: connecting the dots. Am J Respir Crit Care Med 2007;175:875–880. [DOI] [PubMed] [Google Scholar]
- 272.The Idiopathic Pulmonary Fibrosis Clinical Research Network. A controlled trial of sildenafil in advanced idiopathic pulmonary fibrosis. N Engl J Med 2010;363:620–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Jackson RM, Glassberg MK, Ramos CF, Bejarano PA, Butrous G, Gomez-Marin O. Sildenafil therapy and exercise tolerance in idiopathic pulmonary fibrosis. Lung 2010;188:115–123. [DOI] [PubMed] [Google Scholar]
- 274.Daniels CE, Lasky JA, Limper AH, Mieras K, Gabor E, Schroeder DR; Imatinib-IPF Study Investigators. Imatinib treatment for idiopathic pulmonary fibrosis: randomized placebo-controlled trial results. Am J Respir Crit Care Med 2010;181:604–610. [DOI] [PubMed] [Google Scholar]
- 275.Morrison DA, Stovall JR. Increased exercise capacity in hypoxemic patients after long-term oxygen therapy. Chest 1992;102:542–550. [DOI] [PubMed] [Google Scholar]
- 276.Nocturnal Oxygen Therapy Trial Group. Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease: a clinical trial. Ann Intern Med 1980;93:391–398. [DOI] [PubMed] [Google Scholar]
- 277.Longterm domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema: report of the Medical Research Council Working Party. Lancet 1981;1:681–686. [PubMed] [Google Scholar]
- 278.Mason DP, Brizzio ME, Alster JM, McNeill AM, Murthy SC, Budev MM, Mehta AC, Minai OA, Pettersson GB, Blackstone EH. Lung transplantation for idiopathic pulmonary fibrosis. Ann Thorac Surg 2007;84:1121–1128. [DOI] [PubMed] [Google Scholar]
- 279.Keating D, Levvey B, Kotsimbos T, Whitford H, Westall G, Williams T, Snell G. Lung transplantation in pulmonary fibrosis: challenging early outcomes counterbalanced by surprisingly good outcomes beyond 15 years. Transplant Proc 2009;41:289–291. [DOI] [PubMed] [Google Scholar]
- 280.Thabut G, Mal H, Castier Y, Groussard O, Brugiere O, Marrash-Chahla R, Leseche G, Fournier M. Survival benefit of lung transplantation for patients with idiopathic pulmonary fibrosis. J Thorac Cardiovasc Surg 2003;126:469–475. [DOI] [PubMed] [Google Scholar]
- 281.Thabut G, Christie JD, Ravaud P, Castier Y, Dauriat G, Jebrak G, Fournier M, Leseche G, Porcher R, Mal H. Survival after bilateral versus single-lung transplantation for idiopathic pulmonary fibrosis. Ann Intern Med 2009;151:767–774. [DOI] [PubMed] [Google Scholar]
- 282.Blivet S, Philit F, Sab JM, Langevin B, Paret M, Guerin C, Robert D. Outcome of patients with idiopathic pulmonary fibrosis admitted to the ICU for respiratory failure. Chest 2001;120:209–212. [DOI] [PubMed] [Google Scholar]
- 283.Molina-Molina M, Badia JR, Marin-Arguedas A, Xaubet A, Santos MJ, Nicolas JM, Ferrer M, Torres A. Outcomes and clinical characteristics of patients with pulmonary fibrosis and respiratory failure admitted to an intensive care unit: a study of 20 cases. Med Clin (Barc) 2003;121:63–67. [DOI] [PubMed] [Google Scholar]
- 284.Saydain G, Islam A, Afessa B, Ryu JH, Scott JP, Peters SG. Outcome of patients with idiopathic pulmonary fibrosis admitted to the intensive care unit. Am J Respir Crit Care Med 2002;166:839–842. [DOI] [PubMed] [Google Scholar]
- 285.Nava S, Rubini F. Lung and chest wall mechanics in ventilated patients with end stage idiopathic pulmonary fibrosis. Thorax 1999;54:390–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Stern JB, Mal H, Groussard O, Brugiere O, Marceau A, Jebrak G, Fournier M. Prognosis of patients with advanced idiopathic pulmonary fibrosis requiring mechanical ventilation for acute respiratory failure. Chest 2001;120:213–219. [DOI] [PubMed] [Google Scholar]
- 287.Al-Hameed FM, Sharma S. Outcome of patients admitted to the intensive care unit for acute exacerbation of idiopathic pulmonary fibrosis. Can Respir J 2004;11:117–122. [DOI] [PubMed] [Google Scholar]
- 288.Fumeaux T, Rothmeier C, Jolliet P. Outcome of mechanical ventilation for acute respiratory failure in patients with pulmonary fibrosis. Intensive Care Med 2001;27:1868–1874. [DOI] [PubMed] [Google Scholar]
- 289.Pitsiou G, Ioannis Trigonis I, Tsiata E, Kontou P, Manolakoglou N, Stanopoulos I, Mavrofridis E, Argyropoulou P. Outcome of patients with pulmonary fibrosis admitted to the ICU for acute respiratory failure. Eur Respir J Suppl 2006;28:E650. [Google Scholar]
- 290.Mollica C, Paone G, Conti V, Ceccarelli D, Schmid G, Mattia P, Perrone N, Petroianni A, Sebastiani A, Cecchini L, et al. Mechanical ventilation in patients with end-stage idiopathic pulmonary fibrosis. Respiration 2010;79:209–215. [DOI] [PubMed] [Google Scholar]
- 291.Rangappa P, Moran JL. Outcomes of patients admitted to the intensive care unit with idiopathic pulmonary fibrosis. Crit Care Resusc 2009;11:102–109. [PubMed] [Google Scholar]
- 292.Mallick S. Outcome of patients with idiopathic pulmonary fibrosis (IPF) ventilated in intensive care unit. Respir Med 2008;102:1355–1359. [DOI] [PubMed] [Google Scholar]
- 293.Holland AE, Hill CJ, Conron M, Munro P, McDonald CF. Short term improvement in exercise capacity and symptoms following exercise training in interstitial lung disease. Thorax 2008;63:549–554. [DOI] [PubMed] [Google Scholar]
- 294.Nishiyama O, Kondoh Y, Kimura T, Kato K, Kataoka K, Ogawa T, Watanabe F, Arizono S, Nishimura K, Taniguchi H. Effects of pulmonary rehabilitation in patients with idiopathic pulmonary fibrosis. Respirology 2008;13:394–399. [DOI] [PubMed] [Google Scholar]
- 295.Ferreira A, Garvey C, Connors GL, Hilling L, Rigler J, Farrell S, Cayou C, Shariat C, Collard HR. Pulmonary rehabilitation in interstitial lung disease: benefits and predictors of response. Chest 2009;135:442–447. [DOI] [PubMed] [Google Scholar]
- 296.Ferreira G, Feuerman M, Spiegler P. Results of an 8-week, outpatient pulmonary rehabilitation program on patients with and without chronic obstructive pulmonary disease. J Cardiopulm Rehabil 2006;26:54–60. [DOI] [PubMed] [Google Scholar]
- 297.Jastrzebski D, Gumola A, Gawlik R, Kozielski J. Dyspnea and quality of life in patients with pulmonary fibrosis after six weeks of respiratory rehabilitation. J Physiol Pharmacol 2006;57:139–148. [PubMed] [Google Scholar]
- 298.Naji NA, Connor MC, Donnelly SC, McDonnell TJ. Effectiveness of pulmonary rehabilitation in restrictive lung disease. J Cardiopulm Rehabil 2006;26:237–243. [DOI] [PubMed] [Google Scholar]
- 299.Lancaster LH, Mason WR, Parnell JA, Rice TW, Loyd JE, Milstone AP, Collard HR, Malow BA. Obstructive sleep apnea is common in idiopathic pulmonary fibrosis. Chest 2009;136:772–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Kim DS, Collard HR, King TE Jr. Classification and natural history of the idiopathic interstitial pneumonias. Proc Am Thorac Soc 2006;3:285–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Sakamoto S, Homma S, Miyamoto A, Kurosaki A, Fujii T, Yoshimura K. Cyclosporin A in the treatment of acute exacerbation of idiopathic pulmonary fibrosis. Intern Med 2010;49:109–115.20075573 [Google Scholar]
- 302.Olschewski H, Ghofrani HA, Walmrath D, Schermuly R, Temmesfeld-Wollbruck B, Grimminger F, Seeger W. Inhaled prostacyclin and iloprost in severe pulmonary hypertension secondary to lung fibrosis. Am J Respir Crit Care Med 1999;160:600–607. [DOI] [PubMed] [Google Scholar]
- 303.Minai OA, Sahoo D, Chapman JT, Mehta AC. Vaso-active therapy can improve 6-min walk distance in patients with pulmonary hypertension and fibrotic interstitial lung disease. Respir Med 2008;102:1015–1020. [DOI] [PubMed] [Google Scholar]
- 304.Sweet MP, Patti MG, Leard LE, Golden JA, Hays SR, Hoopes C, Theodore PR. Gastroesophageal reflux in patients with idiopathic pulmonary fibrosis referred for lung transplantation. J Thorac Cardiovasc Surg 2007;133:1078–1084. [DOI] [PubMed] [Google Scholar]
- 305.Marik PE. Aspiration pneumonitis and aspiration pneumonia. N Engl J Med 2001;344:665–671. [DOI] [PubMed] [Google Scholar]
- 306.Barnes TW, Vassallo R, Tazelaar HD, Hartman TE, Ryu JH. Diffuse bronchiolar disease due to chronic occult aspiration. Mayo Clin Proc 2006;81:172–176. [DOI] [PubMed] [Google Scholar]
- 307.Davis RD Jr, Lau CL, Eubanks S, Messier RH, Hadjiliadis D, Steele MP, Palmer SM. Improved lung allograft function after fundoplication in patients with gastroesophageal reflux disease undergoing lung transplantation. J Thorac Cardiovasc Surg 2003;125:533–542. [DOI] [PubMed] [Google Scholar]
- 308.Linden PA, Gilbert RJ, Yeap BY, Boyle K, Deykin A, Jaklitsch MT, Sugarbaker DJ, Bueno R. Laparoscopic fundoplication in patients with end-stage lung disease awaiting transplantation. J Thorac Cardiovasc Surg 2006;131:438–446. [DOI] [PubMed] [Google Scholar]
- 309.Hope-Gill BDM, Hilldrup S, Davies C, Newton RP, Harrison NK. A study of the cough reflex in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2003;168:995–1002. [DOI] [PubMed] [Google Scholar]
- 310.Horton MR, Danoff SK, Lechtzin N. Thalidomide inhibits the intractable cough of idiopathic pulmonary fibrosis. Thorax 2008;63:749. [DOI] [PubMed] [Google Scholar]
- 311.Zappala CJ, Latsi PI, Nicholson AG, Colby TV, Cramer D, Renzoni EA, Hansell DM, du Bois RM, Wells AU. Marginal decline in forced vital capacity is associated with a poor outcome in idiopathic pulmonary fibrosis. Eur Respir J 2010;35:830–836. [DOI] [PubMed] [Google Scholar]
- 312.Miller MR, Crapo R, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Enright P, van der Grinten CP, Gustafsson P, et al.; ATS/ERS Task Force. General considerations for lung function testing. Eur Respir J 2005;26:153–161. [DOI] [PubMed] [Google Scholar]
- 313.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, et al.; ATS/ERS Task Force. Standardisation of spirometry. Eur Respir J 2005;26:319–338. [DOI] [PubMed] [Google Scholar]
- 314.Wanger J, Clausen JL, Coates A, Pedersen OF, Brusasco V, Burgos F, Casaburi R, Crapo R, Enright P, van der Grinten CP, et al. Standardisation of the measurement of lung volumes. Eur Respir J 2005;26:511–522. [DOI] [PubMed] [Google Scholar]
- 315.Macintyre N, Crapo RO, Viegi G, Johnson DC, van der Grinten CP, Brusasco V, Burgos F, Casaburi R, Coates A, Enright P, et al. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J 2005;26:720–735. [DOI] [PubMed] [Google Scholar]
- 316.Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, Coates A, van der Grinten CP, Gustafsson P, Hankinson J, et al. Interpretative strategies for lung function tests. Eur Respir J 2005;26:948–968. [DOI] [PubMed] [Google Scholar]
- 317.Selman M, King TE Jr, Pardo A; American Thoracic Society, European Respiratory Society, American College of Chest Physicians. Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann Intern Med 2001;134:136–151. [DOI] [PubMed] [Google Scholar]
- 318.Akagi T, Matsumoto T, Harada T, Tanaka M, Kuraki T, Fujita M, Watanabe K. Coexistent emphysema delays the decrease of vital capacity in idiopathic pulmonary fibrosis. Respir Med 2009;103:1209–1215. [DOI] [PubMed] [Google Scholar]
- 319.Mura M, Zompatori M, Pacilli AMG, Fasano L, Schiavina M, Fabbri M. The presence of emphysema further impairs physiologic function in patients with idiopathic pulmonary fibrosis. Respir Care 2006;51:257–265. [PubMed] [Google Scholar]
- 320.Cottin V, Cordier JF. Combined pulmonary fibrosis and emphysema: an experimental and clinically relevant phenotype. Am J Respir Crit Care Med 2005;172:1605–1606. [DOI] [PubMed] [Google Scholar]
- 321.Wiggins J, Strickland B, Turner-Warwick M. Combined cryptogenic fibrosing alveolitis and emphysema: the value of high resolution computed tomography in assessment. Respir Med 1990;84:365–369. [DOI] [PubMed] [Google Scholar]
- 322.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002;166:111–117. [DOI] [PubMed] [Google Scholar]
- 323.Zisman DA, Karlamangla AS, Kawut SM, Shlobin OA, Saggar R, Ross DJ, Schwarz MI, Belperio JA, Ardehali A, Lynch JP III, et al. Validation of a method to screen for pulmonary hypertension in advanced idiopathic pulmonary fibrosis. Chest 2008;133:640–645. [DOI] [PMC free article] [PubMed] [Google Scholar]