Abstract
Bilingualism represents an interesting model of possible experience-dependent alterations in brain structure. The current study examines whether interhemispheric adaptations in brain structure are associated with bilingualism. Corpus callosum volume and cortical thickness asymmetry across 13 regions of interest (selected to include critical language and bilingual cognitive control areas) were measured in a sample of Spanish-English bilinguals and age- and gender-matched monolingual individuals (N = 39 per group). Cortical thickness asymmetry of the anterior cingulate region differed across groups, with thicker right than left cortex for bilinguals and the reverse for monolinguals. In addition, two adjacent regions of the corpus callosum (mid-anterior and central) had greater volume in bilinguals. The findings suggest that structural indices of interhemispheric organization in a critical cognitive control region are sensitive to variations in language experience.
Keywords: bilingualism, cortical thickness, corpus callosum, anterior cingulate, lateralization
Speakers of more than one language must meet demands during language use that monolingual speakers do not face. Determining which language to speak in a given situation, suppressing features of the unintended language, and smoothly switching between languages when necessary requires additional resources beyond those recruited by single language users (Green & Abutalebi, 2013). Behavioral studies have demonstrated that bilingualism is associated with some enhanced cognitive functions (Bialystok, Craik & Luk, 2012, but see also Paap & Greenberg, 2013) and functional neuroimaging research reveals recruitment of additional neural systems during bilingual language use. For example, during picture naming, French-German bilinguals had increased activation in the left anterior cingulate and left caudate in a language switching context relative to a single language context (Abutalebi et al., 2008). Effects of bilingualism can also be seen in a single language context when bilinguals encounter similar words that have different meanings in their two languages (interlingual homographs, van Heuven, Schriefers, Dijkstra, & Hagoort, 2008). Dutch-English bilinguals evidenced greater activation for such homographs, relative to control English words, in the left inferior prefrontal cortex; response conflict engendered by the homographs in an English-only lexical decision task additionally produced increased activation in medial prefrontal regions (pre-supplementary motor and anterior cingulate regions). Neither effect was seen in English monolingual participants (van Heuven et al., 2008). A meta-analysis revealed that bilingual language switching involved activation in left inferior frontal, middle frontal, and middle temporal gyri, right precentral and superior temporal gyri, supplementary motor regions and bilateral caudate nuclei (Luk, Green, Abutalebi, & Grady, 2012). Hence, in addition to left hemisphere language areas, bilingual language use involves a network of cognitive control1 regions. Li (2015) and Abutalebi & Green (2007) identify the dorsolateral prefrontal cortex, anterior cingulate, basal ganglia and inferior parietal cortex as comprising a cognitive control network for bilingual language use.
Such functional data raise the question about whether adaptations in brain structure accompany bilingualism. In other domains, experience-dependent alterations in regional cortical volume/density (Draganski & May, 2008; Schmidt-Wilck et al., 2010) and thickness (Engvig et al., 2010; Metzler-Baddeley et al., 2016; Taubert et al., 2016; Wenger et al., 2012) have been found. Despite a burgeoning functional neuroimaging literature, the issue of potential neuroanatomical correlates of bilingualism has received much less scrutiny. A recent thorough review of such neuroanatomical correlates revealed several brain regions in which cortical volume and/or density was greater for bilinguals than for monolinguals (Li, Legault & Litcofsky, 2014). These regions include the anterior cingulate cortex (Abutalebi et al., 2015), inferior parietal lobule (Della Rossa et al., 2013; Mechelli et al., 2004), anterior temporal pole (Abutalebi et al., 2014), orbitofrontal cortex (Abutalebi et al., 2014), Heschl’s gyrus (Ressel et al., 2012), and caudate nucleus (Zou et al., 2012). These volumetric increases were sometimes observed bilaterally (Abutalebi et al., 2014, 2015; Michelli et al., 2004; Ressel et al., 2012), and in other cases only within the left hemisphere (Della Rosa et al., 2012; Zou et al., 2012). Neurobiological interpretations of such volumetric measures are difficult because volume estimates do not separate the contributions of cortical surface area and thickness, and these two measures can vary independently, having different genetic underpinnings (Chen et al., 2013; Panizzon et al., 2009), developmental trajectories (Hogstrom et al., 2013; Raznahan et al., 2011), structural network features (Sanabria-Diaz et al., 2010) and asymmetries (Meyer et al., 2014). Thus it is unclear whether the above-mentioned findings indicate expansion of surface area and/or cortical thickening in bilingual individuals.
One prior study compared cortical thickness in bilingual and monolingual persons (Klein, Mok, Chen, & Watkins, 2014)2. Increased cortical thickness in the left anterior inferior frontal gyrus (IFG) was observed for early and late bilinguals, relative to monolinguals. In the homologous region of the right hemisphere reduced thickness was observed in bilinguals who acquired the second language (L2) after age 3, as compared to monolinguals or early bilinguals. These data indicate that cortical thickness can vary with bilingual language experience. Further, although asymmetry indices were not calculated, the findings imply that the need to coordinate multiple languages may influence structural lateralization in at least one language-relevant brain region. Such data speak to a long-standing hypothesis that functional language lateralization may be more bilateral for bilinguals which could suggest that language experience contributes to individual differences in lateralization. Meta-analyses of behavioral lateralization studies (divided visual field, dichotic listening), for example, indicate greater right hemisphere involvement in language for early bilinguals, relative to monolinguals or late bilinguals (Hull & Vaid, 2006, 2007). However, to our knowledge no prior study has directly compared structural lateralization between monolingual and bilingual participants, and this issue was not considered in a recent review of structural indices of bilingualism (Li et al., 2014).
If interhemispheric organization varies with bilingual language experience one might also expect to observe differences in corpus callosum anatomy between monolingual and bilingual persons as the callosum is the major structural link that enables interhemispheric interaction (Shulte & Müller-Oehring, 2010). Increases in callosum size have been correlated with alterations in structural and functional asymmetry, although the direction of the relationship varies with callosal region, subject characteristics, and task (e.g., Gootjes et al., 2006; Josse et al., 2008; Moffat et al., 1998). An early study reported increased cross-sectional area of the anterior midbody portion of the corpus callosum in middle-aged bilinguals, relative to monolinguals (Coggins, Kennedy, & Armstrong, 2004). Area/volume studies of the corpus callosum have a long history (e.g, Ardekani, Figarsky, & Sidtis, 2012; Bishop & Wahlsten, 1997; Byne et al., 1988; Welcome et al., 2009) but to date only Coggins et al. (2004) have considered potential impacts of bilingualism. It is important to note, however, that greater callosal area or volume need not imply greater numbers of axons (Banich, 1995; Bloom & Hynd, 2005). We will return to this point in the Discussion section.
More recent diffusion tensor imaging (DTI) findings of the corpus callosum have explored whether fractional anisotropy (FA) differs with language experience. FA indexes the extent to which water diffuses in a particular direction in axon bundles and is an indication of the number, alignment, density and myelination of fiber tracts (Beaulieu, 2009). One study examined FA in two regions of the corpus callosum in 8–11 year old children, a frontal portion connecting orbitofrontal cortex and the anterior callosum midbody (Mohades et al., 2012). Relative to monolinguals, bilingual children who learned both languages simultaneously had lower FA in the orbitofrontal callosal portion, but no differences were seen in the anterior midbody. In another study, adult sequential bilinguals (mean age = 31.9 yrs) had higher FA values in the genu, body, and anterior splenium regions of the callosum than did bilinguals (Pliatsikas et al., 2015). Two other investigations measured callosal FA in older adults (Gold, Johnson, & Powell, 2013; Luk, Bialystok, Craik, & Grady, 2011). In one case higher FA values were observed for lifelong bilinguals for most of the corpus callosum, excepting the most anterior and posterior portions (Luk et al., 2011, Figure 1a, mean age = 70.5 years), similar to the Pliatsikas et al. (2015) findings for younger adults. However, older bilinguals matched for cognitive status to similar aged monolinguals (mean age = 64 years) had lower FA values in the genu, anterior to midbody, and splenium of the corpus callosum (Gold et al., 2013, Figure 2). Although the latter finding may be an indication of enhanced bilingual cognitive reserve, the direction and location of FA differences in the corpus callosum are somewhat inconsistent across studies and may be age-dependent. However, both DTI and callosal size findings suggest that variations in corpus callosum structure may be associated with differences in language experience.
In the current investigation we examined both cortical structural asymmetry and callosal morphology in monolingual and bilingual young adults. Group differences would be expected if the demand to coordinate multiple languages has structural correlates in language and/or cognitive control regions across hemispheres. To examine these issues we measured asymmetry in cortical thickness and surface area in regions of interest selected to include both perisylvian language cortex and areas implicated in bilingual language control, and corpus callosum volume. The study had several objectives. First, surface area and cortical thickness were assessed separately to more precisely identify any language experience differences in cortical structure. Second, regional asymmetries in each metric were compared across groups to test whether the degree and direction of asymmetry would differ between monolingual and bilingual persons. If bilingualism is associated with either reduced (Hull & Vaid, 2006) or enhanced (Klein et al., 2014) asymmetry, then group differences in cortical structure might be expected. Third, regional corpus callosum volume was also measured for each group to determine whether this structural correlate of interhemispheric communication would vary with bilingual language experience. This could occur if bilingualism or increased cognitive control demands required alterations in interhemispheric coordination. The findings will contribute to our understanding of how language experience may sculpt brain structure.
Materials and Methods
Participants
Participants included 39 monolingual English-speaking individuals (26 females; mean age 21.2 yrs, age range: 18–34) from the University of California, Riverside (UCR) and 39 Spanish-English bilinguals (26 females; mean age 22.2 yrs, age range: 18–34) from the University of Houston (UH). Consent was obtained from all participants in accord with procedures approved by the Institutional Review Boards of the University of California, Riverside and the University of Houston. Groups did not differ in age, t(76) < 1. All were right handed and had normal or corrected-to-normal vision. The bilingual participants learned Spanish first (L1) and mean age of acquisition for English (L2) was 6.6 years (range: birth – 17 years). Bilinguals reported currently using English 70.5% of the time and Spanish 29.5%, t(38) = 10.50, p < .001. Bilinguals rated their English speech (6.31) higher, on average, than their Spanish (5.97), t(38) = 1.52, p > .05. L1 and L2 proficiency for bilingual participants was assessed using a composite score from the reading sentence comprehension and picture vocabulary subsections of the Woodcock-Munoz Language Survey—Revised (Woodcock, Muñoz-Johnson, Ruef, & Alvarado, 2005). Although there was some variability in English and Spanish proficiencies, on average L1 and L2 proficiencies did not differ, t < 1.
MRI Acquisition
Each UCR participant was scanned twice, to increase the signal to noise ratio of the averaged image, on a 1.5-T GE Signa scanner (3-D SPGR, 1.2 mm thick sagittal images). Imaging parameters: TR 11 ms, TE 2.2 ms, flip angle 25°, field of view 24 cm, acquisition time 4.4 min. Each UH participant was scanned twice on a 3-T Siemens Magnetom Trio scanner (MPRAGE, 1mm thick transversal images) at the Center for Advanced MR Imaging (CAMRI), Baylor School of Medicine, Houston, Texas. Imaging parameters included the following: TR 1200 ms, TE 2.66 ms, flip angle 12°, field of view 24.5 cm, acquisition time 4.5 min.
Anatomical Measurements
Cortical reconstruction and volumetric segmentation for both monolingual and bilingual samples was performed using the Freesurfer v 5.3 analysis suite (Dale, Fischl, & Sereno, 1999; Fischl, Sereno, & Dale, 1999; Fischl, Sereno, Tootell, & Dale, 1999), which is documented and freely available for download online (htttp://surfer.nmr.mgh.harvard.edu/). Briefly, processing includes motion correction and coregistration of T1 weighted images, removal of non-brain tissue, automated Talairach transformation, segmentation of deep gray and subcortical white matter volumetric structures, intensity normalization, tessellation of gray and white matter boundaries, automated topology correction, and surface deformation after intensity gradients optimally identify boundaries based on greatest intensity shifts. Manual inspection of the gray/white segmentation for all 156 hemispheres was performed.
Once the cortical models were complete, parcellation of the cerebral cortex into units based on gyral and sulcal structure, and a variety of surface based data including maps of cortical thickness representations were created using both intensity and continuity information from the entire three-dimensional MR volume. Procedures for the measurement of cortical thickness have been validated against histological analysis (Rosas et al., 2002) and manual measurements (Kuperberg et al., 2003; Salat et al., 2004). FreeSurfer morphometric procedures have been demonstrated to show good test–retest reliability across scanner manufacturers and across field strengths (Han et al., 2006; Reuter, Schmansky, Rosas, & Fischl, 2012). A potential problem with the use of different scanners is that the differing magnetic fields and acquisition parameters could have affected the measurements. By examining asymmetry (discussed below), which is a relative measure (ratio of left-right difference relative to total), baseline scanner-related differences should be minimized. This issue will be addressed further in light of the results.
Cortical thickness and surface area values were automatically extracted for each hemisphere by the FreeSurfer software. During processing, surface images were produced and mapped onto an averaged surface for each hemisphere where the parcellations were performed. The individual surfaces were then nonlinearly warped back into individual subject space. We examined 13 parcellations produced by FreeSurfer’s automated procedure. These regions were selected to include perisylvian language/speech areas with asymmetries in cortical surface structure and/or thickness (Chiarello et al., 2013; Daily et al., 2013), and/or were regions identified as important for bilingual language control in fMRI studies (Garbin et al., 2010; Hernandez, 2009): pars triangularis, pars opercularis, anterior insula, planum temporale, Heschl’s gyrus, lateral superior temporal gyrus (STG), posterior ramus of STG, superior temporal sulcus (STS), middle temporal gyrus (MTG), angular gyrus, supramarginal gyrus, anterior cingulate, and inferior frontal sulcus (DLPFC). Thickness and surface area asymmetries for each parcellation were calculated in the typical manner (see Chiarello et al., in press; Zhou et al., 2013) by subtracting the right value from the left value and dividing by the average, so that the degree of hemisphere difference is evaluated relative to each individual’s mean surface area or thickness. Leftward asymmetries yield positive coefficients.
Corpus callosum volume and segmentation was obtained from the FreeSurfer volume stream (aseg) (https://surfer.nmr.mgh.harvard.edu/pub/docs/wiki/mri_cc.help.xml.html). Measurements were made for a 5 mm lateral extent centered on the mid-sagittal plane. Callosum volume was regressed against total intracranial volume and the residuals were used to obtain callosum volume estimates unbiased by overall brain size. FreeSurfer divides the callosum into five segments of equal length along the primary eigendirection (long axis) of this structure: anterior, mid-anterior, central, mid-posterior, posterior (Rosas et al., 2010). Much prior work on callosum size uses estimates based on cross-sectional area at the most medial slice (e.g., Ardekani, Figarsky, & Sidtis, 2013; Bishop & Wahlsten, 1997; Welcome et al., 2009). This provides a reasonable metric, but does not take into account callosal measurements at somewhat more lateral positions. A recent paper (Wade et al., 2013) indicated that measurements that also extend past the most medial position provide a more stable metric than a single midsaggital slice. As the FreeSurfer volume measure includes a 5 mm lateral extent it seems preferable to the earlier “single slice” cross-sectional measure. A volumetric measure of the callosum is a natural extension of the standard cross-sectional method that has been in use for decades.
Results
Asymmetry Findings
The analyses reported below were conducted on both surface area and thickness asymmetries. As no group differences were observed for any analysis of surface area those data can be found in Supplementary Materials. Mean thickness asymmetries for each ROI are given in Table 1 for monolingual and bilingual participants.
Table 1.
Cortical thickness asymmetries (sd) for each region of interest (FreeSurfer parcellation number) and t-test of group difference in asymmetry.
| Monolingual | Bilingual | t(76) | |
|---|---|---|---|
| Anterior Cingulate Gyrus and Sulcus (6) |
.026 (.054) |
−.041 (.057) |
−5.31, p < .0001 |
| Pars Triangularis (14) | −.026 (.057) |
.009 (.054) |
2.79, p <.01 |
| Pars Opercularis (12) | −.020 (.064) |
.006 (.054) |
1.89 |
| Inferior Frontal Sulcus (52) | −.016 (.050) |
.014 (.054) |
2.48, p < .02 |
| Anterior Insula (18) | .035 (.071) |
.012 (.056) |
−1.53 |
| Heschl’s Gyrus (33) | −.030 (.072) |
−.044 (.105) |
−0.71 |
| Posterior Ramus of Lateral Sulcus (41) | −.039 (.074) |
−.012 (.048) |
1.91 |
| Lateral Superior Temporal Gyrus (34) | −.038 (.052) |
−.010 (.062) |
2.20, p < .05 |
| Planum Temporale (36) | −.033 (.080) |
−.002 (.077) |
1.71 |
| Superior Temporal Sulcus (73) | −.035 (.035) |
−.020 (.041) |
1.73 |
| Middle Temporal Gyrus (38) | −.032 (.054) |
−.022 (.047) |
0.87 |
| Supramarginal Gyrus (26) | −.008 (.045) |
−.008 (.038) |
0.07 |
| Angular Gyrus (25) | −.024 (.065) |
−.011 (.046) |
1.02 |
Note. Boldfaced values indicate significance at Bonferroni correction of .003.
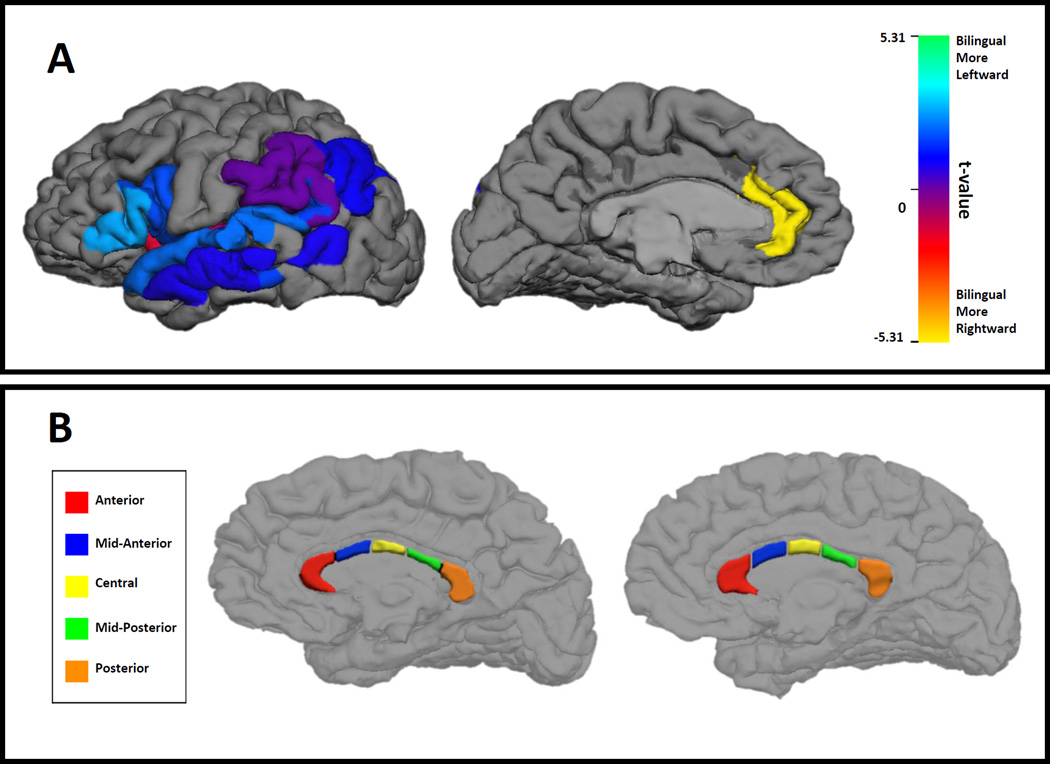
Group comparisons of thickness asymmetry within each ROI were computed and t-values are given in Table 1, and graphically displayed in Figure 1A. We adopted a conservative significance cut-off of .003, the Bonferroni correction, to account for the multiple t-tests. These analyses revealed that the anterior cingulate was the only region which differed reliably across groups, t(76) = −5.31, p < .0001. In this area, bilinguals had significant rightward asymmetry, t(38) = −4.88, p < .0001 (i.e., right anterior cingulate thicker than left), whereas monolinguals had significant leftward asymmetry, t(38) = 2.98, p < .005. The differing asymmetries appear to be mainly due to variations in right hemisphere thickness. The right anterior cingulate was significantly thicker for bilinguals (2.94) than for monolinguals (2.83), t(76) = 3.12, p < .01; the left anterior cingulate was thinner in bilinguals (2.83) than monolinguals (2.91), but this difference was not significant, t(76) = 1.9, p =.06.
Figure 1.
A. T-test of the bilingual/monolingual differences in cortical thickness asymmetry in the 13 regions of interest. Warmer colors indicate more rightward asymmetry for bilingual participants.
B. Illustration of corpus callosum segmentation for two female participants (left monolingual, right bilingual).
Because the thickness asymmetry of the anterior cingulate varied with language experience, we performed a post-hoc exploratory analysis to examine the role of L1/L2 proficiency and age of acquisition (AOA) on this asymmetry among the bilingual participants. L1 proficiency, L2 proficiency, Age, and AOA were entered in a simultaneous multiple regression to predict anterior cingulate thickness asymmetry. Only L1 proficiency accounted for a significant amount of variance, standardized estimate = .51, p < .02 (overall model fit R2 = .18). Bilinguals with greater L1 (Spanish) proficiency had less rightward asymmetry of the anterior cingulate, r = .23 p = .05.
Table 1 suggests that the asymmetries for two frontal regions, the pars triangularis and the adjacent inferior frontal sulcus, may differ between monolinguals and bilinguals, although these areas did not meet our stringent significance cut-off. In these areas, rightward asymmetries were observed for monolinguals, whereas the asymmetries were more leftward for bilinguals.
Corpus Callosum Findings
Table 2 contains mean callosal volumes for monolingual and bilingual participants, and Figure 1B displays the FreeSurfer corpus callosum segmentations for two participants. All statistical analyses were conducted on the residual callosal volumes after adjustment for total intracranial volume. Group differences in callosal volumes were examined for each callosal segment and total callosum, using a Bonferroni adjusted p-value cut-off of .008. As indicated in Table 2, the groups did not differ in total callosal volume, but the mid-anterior segment of the corpus callosum was significantly larger for bilinguals relative to monolinguals. The adjacent central callosal segment was marginally larger for bilinguals than monolinguals. Because the callosal regions demonstrating group differences were adjacent, we also summed the volumes for the mid-anterior and central segments, and then re-computed the residual volume after adjusting for total intracranial volume. The group difference for this combined callosal region was highly reliable, t(76) = 3.24, p < .002.
Table 2.
Mean corpus callosum volume (mm3) and t-test of group difference.
| Monolingual | Bilingual | t(76) | |
|---|---|---|---|
| Anterior | 866.9 (131.1) |
859.2 (128.3) |
0.26 |
| Mid-anterior |
451.7 (71.5) |
522.8 (123.9) |
3.32, p < .005 |
| Central | 455.3 (82.5) |
509.6 (98.7) |
2.65, p = .01 |
| Mid-posterior | 427.4 (75.1) |
441.2 (81.2) |
0.93 |
| Posterior | 925.5 (116.2) |
897.3 (143.2) |
−0.69 |
| Total corpus callosum | 3126.8 (385.2) |
3230.1 (395.2) |
1.59 |
Note. Boldfaced values indicate significance at Bonferroni correction of .008.
We also performed a post-hoc exploratory analysis to examine the role of L1/L2 proficiency and age of acquisition (AOA) on mid-anterior and central callosal volume (and the combined segments) among the bilingual participants. None of the predictors were significant.
A final analysis examined whether there was any relation between thickness asymmetry of the anterior cingulate and the two callosal segments that demonstrated group differences. There was a weak relationship between the mid-anterior callosal volume and anterior cingulate asymmetry, r = −.24, p < .05: as callosal volume increased, the cingulate asymmetry became increasingly rightward. A negative relationship was also observed between central callosal volume and anterior cingulate asymmetry, but this was not significant, r = −.19, p < .10. However, a significant correlation of the anterior cingulate asymmetry with the combined mid-anterior/central callosal volume was observed, r = −.23, p < .05.
Discussion
The current study revealed two neurostructural effects associated with differences in language experience. Relative to monolinguals, our bilingual participants had reversed cortical thickness asymmetry in the anterior cingulate, and larger corpus callosa in a central callosal region just posterior to the genu. These findings imply that some alteration in structural interhemispheric organization is associated with bilingual language experience. Our results partially support and extend prior findings on cortical structural correlates of bilingualism (Li et al., 2014).
As we noted earlier, most prior bilingualism research on cortical structure has relied on volumetric measures (Li et al., 2014). Current tools now allow us to separately examine two independent contributors to volume: surface area and thickness. In our investigation, there was no evidence for differences in surface area asymmetry in any of our regions of interest (see Supplementary Materials). Only cortical thickness asymmetry varied between monolinguals and bilinguals. Although many language-relevant regions are strongly asymmetrical for surface area (e.g., Chiarello et al., 2013, in press), this neurobiological feature may not be sensitive to differences in language experience. Post-natal differences in cortical surface area can be attributed to alterations in the width and spacing of cortical columns due to increased intracortical myelination which “stretches” the cortical surface, increased synaptic density, and larger dendritic fields (Buxhoeveden et al., 2001; Hill et al., 2010; Hogstrom et al., 2012), whereas cortical thickness can vary due to proliferation or pruning of neuropil across cortical layers and/or changes in myelination at the gray/white matter interface (Sowell et al., 2004; Vandekar et al., 2015;Wu et al., 2014). Across species, variation in cortical thickness has been attributed to differences in the number of glial cells (Carlo & Stevens, 2013). Developmentally, cortical thinning during adolescence correlates both with sulci widening (perhaps attributable to a gray matter reduction) and with thickening of the underlying white matter (implying shifts in the gray/white boundary) (Aleman-Gomez et al., 2013; but see also Wu et al., 2014). The current data suggest that language experience can affect the complex sculpting of laminar neural features that occur postnatally, at least within a few critical regions.
We observed robust group differences in the thickness asymmetry of the anterior cingulate, and a trend for differences in two anterior inferior frontal regions: the pars triangularis and inferior frontal sulcus. The latter findings, although they did not meet our strict significance criterion, are reminiscent of the prior findings of Klein et al. (2014). In that study, young adult sequential bilinguals had greater left hemisphere thickness in the pars triangularis and orbitalis, and reduced right hemisphere thickness in the pars orbitalis, relative to monolinguals. Although asymmetry indices were not calculated, the findings imply increased leftward (or decreased rightward) asymmetry for bilinguals in anterior IFG. This parallels the asymmetry differences we observed for anterior inferior frontal regions (rightward asymmetries for monolinguals, but leftward asymmetries for bilinguals, see Table 1). Although we will refrain from further discussion of these findings, it is worth noting that group differences in thickness asymmetries were observed only for regions within frontal cortex.
The most pronounced mono/bilingual difference that we observed was for the anterior cingulate region. Here thickness asymmetries were found for both groups, but in opposing directions. For bilinguals the right ACC was thicker than the left, while for monolinguals the left ACC was thicker than right. Several prior studies have reported cortical thickness asymmetry for the anterior cingulate. Luders et al. (2006) and Plessen et al. (2014) found leftward asymmetry of this region while Zhou et al. (2013) observed a rightward asymmetry. No information about the language background of the participants was provided in these studies. Although methodological differences cannot be discounted, our findings suggest that differential language experience may have contributed to the differing results. Since we observed ACC asymmetry in opposite directions for monolinguals and bilinguals, discrepancies across samples with varying numbers of mono/bilinguals might be expected. In a study comparing older bilinguals and monolinguals, Abutalebi et al. (2015) found that the only structural difference between the groups was greater volume in bilinguals for the left and right anterior cingulate, although no asymmetry measure was calculated. As this study reported volume, it is unclear whether differences in thickness or surface area may have been present.
Our findings raise an important question about the genesis of the differing ACC thickness asymmetries. On the one hand, the rightward asymmetry for bilinguals could reflect enhanced growth or reduced pruning of right cortex, relative to left (Rosen, Sherman, & Galaburda, 1992; Rosen, 1996). On the other hand, the asymmetry could reflect greater white matter expansion for the left cingulate, relative to right (Sowell et al., 2004; Vandekar et al., 2015). It was recently reported that the anterior cingulate is one of the cortical regions with a negative relation between cortical thickness and FA of the underlying superficial white matter (Wu et al., 2014). This finding was independent of age (10–18 years) and indicates that thinner cortex in this area is associated with increased white matter integrity. Because thickness variations can be attributed to changes in either gray or white matter (as discussed above), and we do not have measures of underlying white matter, the current data are somewhat ambiguous. However, consideration of developmental data may be helpful. Cortical thickness increases during early childhood, followed by a protracted period of thinning that accelerates during adolescence (Nie et al., 2013; Shaw et al., 2008; Zhou et al., 2013, 2015). Our measurements were taken in early adulthood after considerable cortical thinning would have occurred. In the absence of longitudinal data to document when structural development may have diverged for our participants, suggestions about neurobiological mechanisms are speculative. Nevertheless, on the basis of the developmental (Nie et al., 2013; Shaw et al., 2008; Zhou et al., 2013, 2015) and training/plasticity (Metzler-Baddeley et al., 2016; Taubert, et al., 2016; Wenger et al., 2012) literatures, one can suggest that thinner cortex is more mature and perhaps more specialized, while thicker cortex is less mature and may be somewhat more plastic. Following this logic, our findings imply that bilingual language experience may be associated with a more mature cortical organization in the left ACC, relative to right ACC, whereas single language experience is associated with more mature right ACC cortex. As the group differences we observed were more reliable for the right than the left hemisphere, one possible interpretation is that bilingualism may prolong a more “juvenile” plastic cortical organization in the right ACC. Longitudinal structural studies comparing monolingual and bilingual individuals will be needed to support this conjecture.
Although no existing study has investigated changes in structure across development for bilinguals, fMRI findings are consistent with developmental changes in ACC recruitment. Archila-Suerte, Zevin, Ramos, and Hernandez (2013) tested a group of young children (ages 6–7), older children (ages 8–10) and young adults (ages 18–24) on a passive auditory task with nonsense syllables. For bilinguals, increased activity was observed for the older children, relative to both young children and adults, in cognitive control areas including the left and right ACC. This pattern of activity stood in stark contrast to that exhibited by monolinguals where there was a trend toward greater activation in adults relative to children in areas involved in auditory processing. Taken together these results reveal how development in sequential bilinguals may involve reliance on cognitive control during periods of language improvement in L2. One possibility is that the use of this area may also be associated with changes in the structure of the ACC during development.
Functional imaging research with bilingual adults presents a mixed picture of lateralization of processing in the ACC. While some researchers have found bilateral ACC activity during language switching tasks (Abutalebi et al., 2012; Guo et al., 2011), others report ACC activity in the left hemisphere only (Abutalebi et al., 2008; Wang et al., 2007). Abutalebi et al. (2012) compared brain activity in highly proficient bilingual and monolingual college students during verbal and nonverbal conflict monitoring tasks and found reduced activity in bilateral dorsal ACC in bilinguals relative to monolinguals for conflict monitoring. In addition, regression analyses demonstrated a stronger positive relationship between gray matter volume and activity during this task in bilinguals, but not in monolinguals. Given these findings, the authors suggested that in order to resolve verbal and nonverbal conflicts, bilinguals may employ the ACC more efficiently than monolinguals. Along the same lines, Guo et al. (2011) examined the neural correlates of conflict resolution during a picture-naming task. They found greater activity during trial-by-trial language switching in bilateral ACC and supplementary motor areas, and suggested that these regions had an important role in local inhibition processes. Wang et al. (2007) examined a group of late bilinguals to investigate neurofunctional correlates of switching from a highly proficient to a less proficient language. During object naming, switching from the more proficient to the less proficient language engendered increased activity in the left ACC, among other areas, relative to the opposite comparison. This may reflect an increased demand for inhibitory processes in order to suppress use of the more proficient language. Thus, when bilinguals perform effortful cognitive tasks, both bilateral ACC and left predominant ACC activity is found. One possibility is that the distribution of function across right and left ACC in bilinguals varies dynamically as task demands change, implying an increased need for interhemispheric coordination. As discussed further below, our findings of greater bilingual corpus callosum volume may be relevant to this point.
It is currently debatable whether young adult bilinguals have enhanced cognitive control abilities relative to monolinguals (Bialystok, Craik, & Luk, 2012; García-Pentón et al., 2016; Paap & Greenberg, 2013). Nevertheless, it is notable that we observed significant cortical thickness differences between our young adult groups in the anterior cingulate. In the absence of functional or behavioral data we cannot determine whether the differing lateral organizations would have any impact on cognitive control abilities. However, even if mono- and bilingual individuals achieve similar performance in cognitive control tasks, this could be mediated by a different distribution of function across left and right regions.
Our exploratory analysis indicated that variation in ACC asymmetry among bilinguals was weakly predicted by L1 proficiency, but not L2 proficiency or age of acquisition. The absence of AOA effects is not surprising given that approximately 80% of our participants acquired L2 within a narrow age range of 3–8 years. It is interesting that individuals with higher L1 proficiency had less rightward/more leftward asymmetry of the ACC, a pattern more similar to our monolingual participants. Our sample size does not allow us to disentangle the ways in which learning of a second language alters the structure of the ACC. However, Green and Abutalebi (2013) have discussed the ways in which different types of language experience might play a role in the development of cognitive control. To the extent that our ACC asymmetry findings reflect alterations in cognitive control, varying L1 experience may moderate the distribution of control functions across hemispheres, perhaps due to differential demand for local inhibitory processes (Guo et al., 2011).
Variations in corpus callosum volume provided the second indication of altered interhemispheric organization associated with language experience. Bilinguals had greater volume in two adjacent regions of the corpus callosum. These regions were just posterior to the genu and extended to include the middle portion of the corpus callosum body (see Figure 1B), and included the area Coggins et al. (2004) previously identified as having greater area for bilinguals in a small sample of adults (mean age = 38 years). We note that the regions of increased bilingual callosal volume overlap substantially with areas having higher bilingual FA values in two prior DTI studies (Luk et al., 2011; Pliatsikas et al., 2015). DTI tractography studies suggest that the callosal regions we identified interconnect the superior frontal gyri/sulci including supplementary motor areas (Hofer & Frahm, 2006; Park et al., 2008; Vergani et al., 2014) and the cingulate gyrus (Park et al., 2008). Although some caution is needed to interpret tractography results due to the problem of crossing fibers, post-mortem data confirm the supplementary motor area findings (Vergani et al., 2014). Non-human primate studies also identify interhemispheric fibers from the anterior cingulate and supplementary motor regions crossing in post-genual to midbody sections of the corpus callosum (Pandya & Seltzer, 1986). There are strong within-hemisphere connections between the anterior cingulate and the anteromedial supplementary motor area (Li et al., 2013; Vergani et al., 2014), which Li et al. (2013) suggested comprise part of a cognitive control network. We conjecture that the callosal regions having increased volume for bilinguals may be implicated in altered interhemispheric coordination of cognitive control functions. The association we observed between anterior cingulate thickness asymmetry and the volume of the two critical callosal regions additionally suggests that our two major findings are not coincidental.
It is important to note several limitations of the current study. First, the data from monolingual and bilingual participants was obtained using different scanners and acquisition parameters. This is not ideal and may have influenced the measures we obtained. However, we relied on a relative asymmetry measure that should adjust for overall scanner differences in the measurement of cortical surface area and thickness. In addition, all surface area and most cortical thickness measures did not evidence group differences, and the group differences that we did obtain occurred in regions that have been found to differ based on language experience in other studies (Abutalebi et al., 2015; Luk et al., 2012). Hence, it is unlikely, but not impossible, that differing acquisition parameters could have led to spurious group differences in a single brain region.
Second, we examined only sequential bilinguals, most of whom acquired the second language near school age. There is no way to know whether simultaneous bilinguals, or those who acquire L2 as adolescents or adults, would have neurostructural features similar to those we observed for the current sample. Third, our data were collected at only one time point in early adulthood. Both cortical thickness and callosal structure change throughout the lifespan (Hasan et al., 2009; Hogstrom et al., 2013; Nie et al., 2013), and longitudinal studies are critically needed to examine how and when bilingualism might affect the trajectory of brain structure development. Also, as described above, the neurobiological basis of our cortical thickness data must remain ambiguous in the absence of concurrent measures of the organization of underlying white matter. Fourth, we do not have functional data or cognitive control measures for our mono/bilingual comparisons. Hence, it is unclear whether the functional lateralization of ACC activity would parallel our structural findings, nor whether the group differences in brain structure might be associated with any processing advantages.
Finally, our corpus callosum findings are limited to volumetric measures and should be further studied using diffusion techniques. Variations in human corpus callosum size can reflect differences in numbers of fibers, axonal size and degree of myelination, and/or packing density (Aboitiz et al., 1992; Highley et al., 1999). Hence it is premature to conclude that increased callosal volume in bilinguals necessarily implies an increase in interhemispheric connectivity, as opposed to experience-dependent variation in callosal organization. Nevertheless, the current data imply that structural features of interhemispheric organization for cognitive control differ between monolinguals and some bilingual individuals.
In summary, we report several findings that suggest some alteration in interhemispheric brain structure between monolinguals and bilinguals, both in frontal brain regions and in the corpus callosum. However, we found no structural evidence to support a more bilateral brain organization for bilinguals (Hull & Vaid, 2006, 2007). Rather, our findings suggest that bilingualism may alter the direction of structural asymmetry, as well as the structural conduit for interhemispheric communication, for critical cognitive control regions. These findings contribute to the emerging view that variations in language experience can have profound effects on brain structure (Li et al., 2014).
Supplementary Material
Thickness asymmetry of anterior cingulate is reversed for bilingual vs monolinguals
Rightward asymmetry for bilinguals, leftward for monolinguals
Mid-anterior/central corpus callosum volume greater for bilinguals
Brain structure in a cognitive control region varies with language experience
Differing interhemispheric organization in brain structure for bilinguals
Acknowledgments
This research was supported by NIH grant DC006957 to CC as well as HD059103 and HD079873 to AH. We thank Ronald Otto, M.D. for facilitating this research, and Laura K. Halderman, Janelle Julagay, Suzanne Welcome, and Adam Daily for assistance with data collection and/or analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Botvinick & Braver (2015, pg. 84) define cognitive control as “functions that regulate more basic attention-, memory-, language-, and action-related faculties and coordinate their activity in the service of specific tasks.”
Martensson et al. (2012) also measured cortical thickness in a 3-month language training study. The training was associated with increased cortical thickness in portions of the left dorsal MFG, left IFG, and left STG.
References
- Abutalebi J, Annoni JM, Zimine I, Pegna AJ, Seghier ML, Lee-Jahnke H, Khateb A. Language control and lexical competition in bilinguals: An event-related fMRI study. Cerebral Cortex. 2008;18(7):1496–1505. doi: 10.1093/cercor/bhm182. [DOI] [PubMed] [Google Scholar]
- Abutalebi J, Canini M, Della Rosa PA, Sheung LP, Green DW, Weekes BS. Bilingualism protects anterior temporal lobe integrity in aging. Neurobiology of Aging. 2014;35(9):2126–2133. doi: 10.1016/j.neurobiolaging.2014.03.010. [DOI] [PubMed] [Google Scholar]
- Abutalebi J, Della Rossa PA, Green DW, Hernandez M, Scifo P, Keim R, Cappa SF, Costa A. Bilingualism tunes the anterior cingulate cortex for conflict monitoring. Cerebral Cortex. 2012;22:2076–2086. doi: 10.1093/cercor/bhr287. [DOI] [PubMed] [Google Scholar]
- Abutalebi J, Green D. Bilingual language production: The neurocognition of language representation and control. Journal of Neurolinguistics. 2007;20(3):242–275. [Google Scholar]
- Abutalebi J, Guidi L, Borsa V, Canini M, Della Rossa PA, Parris BA, Weeks BS. Bilingualism provides a neural reserve for aging populations. Neuropsychologia. 2015;69:201–210. doi: 10.1016/j.neuropsychologia.2015.01.040. [DOI] [PubMed] [Google Scholar]
- Alemán-Gómez Y, Janssen J, Schnack H, Balaban E, Pina-Camacho L, Alfaro-Almagro F, Desco M. The human cerebral cortex flattens during adolescence. The Journal of Neuroscience. 2013;33(38):15004–15010. doi: 10.1523/JNEUROSCI.1459-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archila-Suerte P, Zevin J, Ramos AI, Hernandez AE. The neural basis of non-native speech perception in bilingual children. Neuroimage. 2013;67:51–63. doi: 10.1016/j.neuroimage.2012.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardekani BA, Figarsky K, Sidtis JJ. Sexual dimorphism in the human corpus callosum: An MRI study using the OASIS Brain Database. Cerebral Cortex. 2013;23:2514–2520. doi: 10.1093/cercor/bhs253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banich MT. Interhemispheric processing: Theoretical considerations and empirical approaches. In: Davidson RJ, Hugdahl K, editors. Brain Asymmetry. Cambridge: MIT Press; 1995. pp. 427–490. [Google Scholar]
- Beaulieu C. The biological basis of diffusion anisotropy. In: Johansen-Berg H, Behrens TEJ, editors. Diffusion MRI: From quantitative measurement to in vivo neuroanatomy. San Diego: Elsevier; 2009. pp. 105–126. [Google Scholar]
- Bialystok E, Craik FIM, Luk G. Bilingualism: Consequences for mind and brain. Trends in Cognitive Sciences. 2012;16(4):240–250. doi: 10.1016/j.tics.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop KM, Wahlsten D. Sex differences in the human corpus callosum: Myth or reality? Neuroscience and Biobehavioral Reviews. 1997;21:581–601. doi: 10.1016/s0149-7634(96)00049-8. [DOI] [PubMed] [Google Scholar]
- Bloom JS, Hynd GW. The role of the corpus callosum in interhemispheric transfer of information: Excitation or inhibition? Neuropsychology Review. 2005;15:59–71. doi: 10.1007/s11065-005-6252-y. [DOI] [PubMed] [Google Scholar]
- Booth JR. Brain bases of learning and development of language and reading. In: Coch D, editor. Human Behavior, Learning, and the Developing Brain. New York: Guilford Press; 2007. pp. 279–300. [Google Scholar]
- Botvinick M, Braver T. Motivation and cognitive control: from behavior to neural mechanism. Annual Review of Psychology. 2015;66(1):83–113. doi: 10.1146/annurev-psych-010814-015044. [DOI] [PubMed] [Google Scholar]
- Buxhoeveden DP, Switala AE, Roy E, Litaker M, Casanova MF. Morphological differences between minicolumns in human and nonhuman primate cortex. American Journal of Physical Anthropology. 2001;115(4):361–371. doi: 10.1002/ajpa.1092. [DOI] [PubMed] [Google Scholar]
- Byne W, Bleier R, Houston L. Variations in human corpus callosum do not predict gender: A study using magnetic resonance imaging. Behavioral Neuroscience. 1988;102:222–227. doi: 10.1037//0735-7044.102.2.222. [DOI] [PubMed] [Google Scholar]
- Carlo CN, Stevens CF. Structural uniformity of neocortex, revisited. Proceedings of the National Academy of Sciences. 2013;110(4):1488–1493. doi: 10.1073/pnas.1221398110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CH, Fiecas M, Gutierrez ED, Panizzon MS, Eyler LT, Vuoksimaa E, Kremen WS. Genetic topography of brain morphology. Proceedings of the National Academy of Sciences. 2013;110(42):17089–17094. doi: 10.1073/pnas.1308091110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiarello C, Vazquez D, Daily A, Felton A, Leonard C. A whole-brain analysis of cortical asymmetries. Poster presented at the Cognitive Neuroscience Society annual meeting; San Francisco, CA. 2013. [Google Scholar]
- Chiarello C, Vazquez D, Felton A, Leonard CM. Structural asymmetry of anterior insula: Behavioral correlates and individual differences. Brain and Language. 2013;126(2):109–122. doi: 10.1016/j.bandl.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiarello C, Vazquez D, Felton A, McDowell A. Structural asymmetry of the human cerebral cortex: Regional and between-subject variability of surface area, cortical thickness, and local gyrification. Neuropsychologia. doi: 10.1016/j.neuropsychologia.2016.01.012. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coggins PE, Kennedy TJ, Armstrong TA. Bilingual corpus callosum variability. Brain and Language. 2004;89(1):69–75. doi: 10.1016/S0093-934X(03)00299-2. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis: I. Segmentation and surface reconstruction. Neuroimage. 1999;9(2):179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Daily A, Vazquez D, Felton A, Chiarello C. Individual differences in cortical structure III: Variability predicts degree of structural hemispheric asymmetry. Poster presented at the Cognitive Neuroscience Society annual meeting; Boston, MA. 2014. [Google Scholar]
- Della Rosa PA, Videsott G, Borsa VM, Canini M, Weekes BS, Franceschini R, Abutalebi J. A neural interactive location for multilingual talent. Cortex. 2012;49:605–608. doi: 10.1016/j.cortex.2012.12.001. http://dx.doi.org/10.1016/j.cortex.2012.12.001. [DOI] [PubMed] [Google Scholar]
- Draganski B, May A. Training-induced structural changes in the adult human brain. Behavioural Brain Research. 2008;192(1):137–142. doi: 10.1016/j.bbr.2008.02.015. [DOI] [PubMed] [Google Scholar]
- Engvig A, Fjell AM, Westlye LT, Moberget T, Sundseth Ø, Larsen VA, Walhovd KB. Effects of memory training on cortical thickness in the elderly. Neuroimage. 2010;52(4):1667–1676. doi: 10.1016/j.neuroimage.2010.05.041. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis: II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9(2):195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Tootell RB, Dale AM. High-resolution intersubject averaging and a coordinate system for the cortical surface. Human Brain Mapping. 1999;8(4):272–284. doi: 10.1002/(SICI)1097-0193(1999)8:4<272::AID-HBM10>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbin G, Sanjuan A, Forn C, Bustamante JC, Rodríguez-Pujadas A, Belloch V, Ávila C. Bridging language and attention: Brain basis of the impact of bilingualism on cognitive control. Neuroimage. 2010;53(4):1272–1278. doi: 10.1016/j.neuroimage.2010.05.078. [DOI] [PubMed] [Google Scholar]
- García-Pentón L, Fernández García Y, Costello B, Duñabeitia JA, Carreiras M. The neuroanatomy of bilingualism: How to turn a hazy view into the full picture. Language, Cognition and Neuroscience. 2015;31(3):1–25. http://dx.doi.org/10.1080/23273798.2015.1068944. [Google Scholar]
- Gold BT, Johnson NF, Powell DK. Lifelong bilingualism contributes to cognitive reserve against white matter integrity declines in aging. Neuropsychologia. 2013;51(13):2841–2846. doi: 10.1016/j.neuropsychologia.2013.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DW, Abutalebi J. Language control in bilinguals: The adaptive control hypothesis. Journal of Cognitive Psychology. 2013;25(5):515–530. doi: 10.1080/20445911.2013.796377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gootjes L, Bouma A, Van Strien JW, Van Schinjndel R, Barkhof F, Scheltens Ph. Corpus callosum size correlates with asymmetric performance on a dichotic listening task in healthy aging but not in Alzheimer’s Disease. Neuropsychologia. 2006;44:208–217. doi: 10.1016/j.neuropsychologia.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Guo T, Liu H, Misra M, Kroll JF. Local and global inhibition in bilingual word production: fMRI evidence from Chinese–English bilinguals. NeuroImage. 2011;56(4):2300–2309. doi: 10.1016/j.neuroimage.2011.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Jovicich J, Salat D, van der Kouwe A, Quinn B, Czanner S, Fischl B. Reliability of MRI-derived measurements of human cerebral cortical thickness: The effects of field strength, scanner upgrade and manufacturer. Neuroimage. 2006;32(1):180–194. doi: 10.1016/j.neuroimage.2006.02.051. [DOI] [PubMed] [Google Scholar]
- Hasan KM, Kamali A, Ifikhar A, Kramer LA, Papanicolaou AC, Fletcher JM, Ewing-Coggs L. Diffusion tensor tractography quantification of the human corpus callosum fiber pathways across the lifespan. Brain Research. 2009;1249:91–100. doi: 10.1016/j.brainres.2008.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez AE. Language switching in the bilingual brain: What’s next? Brain and Language. 2009;109(2):133–140. doi: 10.1016/j.bandl.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Hill J, Inder T, Neil J, Dierker D, Harwell J, Van Essen D. Similar patterns of cortical expansion during human development and evolution. Proceedings of the National Academy of Sciences. 2010;107(29):13135–13140. doi: 10.1073/pnas.1001229107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer S, Frahm J. Topography of the human corpus callosum revisited – Comprehensive fiber tractography using diffusion tensor magnetic resonance imaging. NeuroImage. 2006;32:989–994. doi: 10.1016/j.neuroimage.2006.05.044. [DOI] [PubMed] [Google Scholar]
- Hogstrom LJ, Westlye LT, Walhovd KB, Fjell AM. The structure of the cerebral cortex across adult life: age-related patterns of surface area, thickness, and gyrification. Cerebral Cortex. 2013;23:2521–2530. doi: 10.1093/cercor/bhs231. [DOI] [PubMed] [Google Scholar]
- Hull R, Vaid J. Laterality and language experience. Laterality. 2006;11(5):436–464. doi: 10.1080/13576500600691162. [DOI] [PubMed] [Google Scholar]
- Hull R, Vaid J. Bilingual language lateralization: A meta-analytic tale of two hemispheres. Neuropsychologia. 2007;45(9):1987–2008. doi: 10.1016/j.neuropsychologia.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Josse G, Seghier ML, Kherif F, Price CJ. Explaining function with anatomy: Language lateralization and corpus callosum size. Journal of Neuroscience. 2008;28:14132–14139. doi: 10.1523/JNEUROSCI.4383-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein D, Mok K, Chen JK, Watkins KE. Age of language learning shapes brain structure: A cortical thickness study of bilingual and monolingual individuals. Brain and Language. 2014;131:20–24. doi: 10.1016/j.bandl.2013.05.014. [DOI] [PubMed] [Google Scholar]
- Kuperberg GR, Broome MR, McGuire PK, David AS, Eddy M, Ozawa F, Fischl B. Regionally localized thinning of the cerebral cortex in schizophrenia. Archives of general psychiatry. 2003;60(9):878–888. doi: 10.1001/archpsyc.60.9.878. [DOI] [PubMed] [Google Scholar]
- Li P. Bilingualism as a dynamic process. In: MacWhinney Be., O’Grady W., editors. The handbook of language emergence. Wiley-Blackwell; 2015. pp. 511–536. [Google Scholar]
- Li P, Legault J, Litcofsky KA. Neuroplasticity as a function of second language learning: anatomical changes in the human brain. Cortex. 2014;58:301–324. doi: 10.1016/j.cortex.2014.05.001. [DOI] [PubMed] [Google Scholar]
- Li W, Qin W, Liu H, Fan L, Wang J, Jiang T, Yu C. Subregions of the human superior frontal gyrus and their connections. NeuroImage. 2013;78:46–58. doi: 10.1016/j.neuroimage.2013.04.011. [DOI] [PubMed] [Google Scholar]
- Luders E, Narr KL, Thompson PM, Rex DE, Jancke L, Toga AW. Hemispheric asymmetries in cortical thickness. Cerebral Cortex. 2006;16(8):1232–1238. doi: 10.1093/cercor/bhj064. [DOI] [PubMed] [Google Scholar]
- Luk G, Bialystok E, Craik FI, Grady CL. Lifelong bilingualism maintains white matter integrity in older adults. The Journal of Neuroscience. 2011;31(46):16808–16813. doi: 10.1523/JNEUROSCI.4563-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk G, Green DW, Abutalebi J, Grady C. Cognitive control for language switching in bilinguals: A quantitative meta-analysis of functional neuroimaging studies. Language and Cognitive Processes. 2012;27(10):1479–1488. doi: 10.1080/01690965.2011.613209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mårtenssen J, Eriksson J, Bodammer NC, Lindgren M, Johansson M, Nyberg L, Lövdén M. Growth of language-related brain areas after foreign language learning. NeuroImage. 2012;63:240–244. doi: 10.1016/j.neuroimage.2012.06.043. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Crinion JT, Noppeney U, O'Doherty J, Ashburner J, Frackowiak RS, Price CJ. Structural plasticity in the bilingual brain. Nature. 2004;431(7010):757–757. doi: 10.1038/431757a. [DOI] [PubMed] [Google Scholar]
- Metzler-Baddeley C, Caeyenberghs K, Foley S, Jones DK. Task complexity and location specific changes of cortical thickness in executive and salience networks after working memory training. NeuroImage. 2016;130:48–62. doi: 10.1016/j.neuroimage.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer M, Liem F, Hirsiger S, Jancke L, Hanggi J. Cortical surface area and cortical thickness demonstrate differential structural asymmetry in auditory-related areas of the human cortex. Cerebral Cortex. 2014;24(10):2541–2552. doi: 10.1093/cercor/bht094. [DOI] [PubMed] [Google Scholar]
- Moffat SD, Hampson E, Lee DL. Morphology of the planum temporale and corpus callosum in left handers with evidence of left and right speech representation. Brain. 1998;121:2369–2379. doi: 10.1093/brain/121.12.2369. [DOI] [PubMed] [Google Scholar]
- Mohades SG, Struys E, Van Schuerbeek P, Mondt K, Van De Craen P, Luypaert R. DTI reveals structural differences in white matter tracts between bilingual and monolingual children. Brain Research. 2012;1435:72–80. doi: 10.1016/j.brainres.2011.12.005. [DOI] [PubMed] [Google Scholar]
- Nie J, Li G, Shen D. Development of cortical anatomical properties from early childhood to early adulthood. NeuroImage. 2013;76:216–224. doi: 10.1016/j.neuroimage.2013.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paap KR, Greenberg ZI. There is no coherent evidence for a bilingual advantage in executive processing. Cognitive Psychology. 2013;66(2):232–258. doi: 10.1016/j.cogpsych.2012.12.002. [DOI] [PubMed] [Google Scholar]
- Pandya DN, Seltzer B. The topography of commissural fibers. In: Lepore F, Ptito M, Jasper HH, editors. Two hemispheres – one brain: Functions of the corpus callosum. New York: Alan R. Liss; 1986. pp. 47–71. [Google Scholar]
- Panizzon MS, Fennema-Notestine C, Eyler LT, Jernigan TL, Prom-Wormley E, Neale M, Kremen WS. Distinct genetic influences on cortical surface area and cortical thickness. Cerebral Cortex. 2009;19:2728–2735. doi: 10.1093/cercor/bhp026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H-J, Kim JJ, Lee S-K, Seok JH, Chun J, Kim DI, Lee JD. Corpus callosal connection mapping using cortical gray matter parcellation and DT-MRI. Human Brain Mapping. 2008;29:503–516. doi: 10.1002/hbm.20314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plessen KJ, Hugdahl K, Bansal R, Hao X, Peterson BS. Sex, age, and cognitive correlates of asymmetries in thickness of the cortical mantle across the life span. The Journal of Neuroscience. 2014;34(18):6294–6302. doi: 10.1523/JNEUROSCI.3692-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pliatsikas C, Moschopoulou E, Saddy JD. The effects of bilingualism on the white matter structure of the brain. Proceedings of the National Academy of Sciences. 2015;112:1334–1337. doi: 10.1073/pnas.1414183112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raznahan A, Shaw P, Lalonde F, Stockman M, Wallace GL, Greenstein D, Giedd JN. How does your cortex grow? The Journal of Neuroscience. 2011;31(19):7174–7177. doi: 10.1523/JNEUROSCI.0054-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressel V, Pallier C, Ventura-Campos N, Díaz B, Roessler A, Ávila C, Sebastián-Gallés N. An effect of bilingualism on the auditory cortex. The Journal of Neuroscience. 2012;32(47):16597–16601. doi: 10.1523/JNEUROSCI.1996-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter M, Schmansky NJ, Rosas HD, Fischl B. Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage. 2012;61(4):1402–1418. doi: 10.1016/j.neuroimage.2012.02.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas HD, Lee SY, Bender AC, Zaleta AK, Vangel M, Yu P, Hersch SM. Altered white matter microstructure in the corpus callosum in Huntington's disease: Implications for cortical “disconnection”. Neuroimage. 2010;49(4):2995–3004. doi: 10.1016/j.neuroimage.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas HD, Liu AK, Hersch S, Glessner M, Ferrante RJ, Salat DH, Fischl B. Regional and progressive thinning of the cortical ribbon in Huntington’s disease. Neurology. 2002;58:695–701. doi: 10.1212/wnl.58.5.695. [DOI] [PubMed] [Google Scholar]
- Rosen GD. Cellular, morphometric, ontogenetic and connectional substrates of anatomical asymmetry. Neuroscience and Biobehavioral Reviews. 1996;20:607–615. doi: 10.1016/0149-7634(95)00073-9. [DOI] [PubMed] [Google Scholar]
- Rosen GD, Sherman GF, Galaburda AM. Biological substrates of anatomic asymmetry. Progress in Neurobiology. 1992;39:507–515. doi: 10.1016/0301-0082(92)90004-x. [DOI] [PubMed] [Google Scholar]
- Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RS, Busa E, Fischl B. Thinning of the cerebral cortex in aging. Cerebral Cortex. 2004;14(7):721–730. doi: 10.1093/cercor/bhh032. [DOI] [PubMed] [Google Scholar]
- Sanabria-Diaz G, Melie-García L, Iturria-Medina Y, Alemán-Gómez Y, Hernández-González G, Valdés-Urrutia L, Valdés-Sosa P. Surface area and cortical thickness descriptors reveal different attributes of the structural human brain networks. Neuroimage. 2010;50(4):1497–1510. doi: 10.1016/j.neuroimage.2010.01.028. [DOI] [PubMed] [Google Scholar]
- Schmidt-Wilcke T, Rosengarth K, Luerding R, Bogdahn U, Greenlee MW. Distinct patterns of functional and structural neuroplasticity associated with learning Morse code. Neuroimage. 2010;51(3):1234–1241. doi: 10.1016/j.neuroimage.2010.03.042. [DOI] [PubMed] [Google Scholar]
- Shaw P, Kabani NJ, Lerch JP, Eckstrand K, Lenroot R, Gogtay N, Wise SP. Neurodevelopmental trajectories of the human cerebral cortex. The Journal of Neuroscience. 2008;28(14):3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulte T, Müller-Oehring EM. Contribution of callosal connections to the interhemispheric integration of visuomotor and cognitive processes. Neuropsychology Review. 2010;20:174–190. doi: 10.1007/s11065-010-9130-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, Toga AW. Longitudinal mapping of cortical thickness and brain growth in normal children. The Journal of Neuroscience. 2004;24(38):8223–8231. doi: 10.1523/JNEUROSCI.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taubert M, Mehnert J, Pleger B, Villringer A. Rapid and specific gray matter changes in M1 induced by balance training. NeuroImage. 2016;133:399–407. doi: 10.1016/j.neuroimage.2016.03.017. [DOI] [PubMed] [Google Scholar]
- Turkeltaub P, Eden G, Jones K, Zeffiro T. Meta-analysis of the functional neuroanatomy of single-word reading: Method and validation. Neuroimage. 2002;16:765–780. doi: 10.1006/nimg.2002.1131. [DOI] [PubMed] [Google Scholar]
- Vandekar SN, Shinohara RT, Raznahan A, Roalf DR, Ross M, DeLeo N, Satterthwaite TD. Topologically dissociable patterns of development of the human cerebral cortex. The Journal of Neuroscience. 2015;35(2):599–609. doi: 10.1523/JNEUROSCI.3628-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergani F, Lacerda L, Martino J, Attems J, Morris C, Mitchell P, de Schotten MT, Dell’Acqua F. White matter connections of the supplementary motor area in humans. Journal of Neurology, Neurosurgery, & Psychiatry. 2014;85:1377–1385. doi: 10.1136/jnnp-2013-307492. [DOI] [PubMed] [Google Scholar]
- van Heuven WJB, Schriefers H, Dijkstra T, Hagoort P. Language conflict in the bilingual brain. Cerebral Cortex. 2008;18(11):2706–2716. doi: 10.1093/cercor/bhn030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade BSC, Stockman M, McLaughlin MJ, Raznahan A, Lalonde F, Giedd JN. Improved corpus callosum area measurements by analysis of adjoining parasagittal slices. Psychiatry Research: Neuroimaging. 2013;211:221–225. doi: 10.1016/j.pscychresns.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Xue G, Chen C, Xue F, Dong Q. Neural bases of asymmetric language switching in second-language learners: An ER-fMRI study. NeuroImage. 2007;35(2):862–870. doi: 10.1016/j.neuroimage.2006.09.054. [DOI] [PubMed] [Google Scholar]
- Welcome SE, Chiarello C, Towler S, Halderman LK, Otto R, Leonard CM. Behavioral correlates of corpus callosum size: Anatomical/behavioral relationships vary across sex/handedness groups. Neuropsychologia. 2009;47:2427–2435. doi: 10.1016/j.neuropsychologia.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenger E, Schaefer S, Noack H, Kühn S, Mårtensson J, Heinze HJ, Lövdén M. Cortical thickness changes following spatial navigation training in adulthood and aging. Neuroimage. 2012;59(4):3389–3397. doi: 10.1016/j.neuroimage.2011.11.015. [DOI] [PubMed] [Google Scholar]
- Woodcock RW, Muñoz-Sandoval AF, Ruef ML, Alvarado CG. Woodcock-Munoz Language Survey: Normative Update. Itasca, IL: Riverside Publishing; 2005. [Google Scholar]
- Wu M, Lu LH, Lowes A, Yang S, Passarotti AM, Zhou XJ, Pavuluri MN. Development of superficial white matter and its structural interplay with cortical gray matter in children and adolescents. Human Brain Mapping. 2014;35:2806–2816. doi: 10.1002/hbm.22368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D, Lebel C, Evans A, Beaulieu C. Cortical thickness asymmetry from childhood to older adulthood. NeuroImage. 2013;83:66–74. doi: 10.1016/j.neuroimage.2013.06.073. [DOI] [PubMed] [Google Scholar]
- Zhou D, Lebel C, Treit S, Evans A, Beaulieu C. Accelerated longitudinal cortical thinning in adolescence. NeuroImage. 2015;104:138–145. doi: 10.1016/j.neuroimage.2014.10.005. [DOI] [PubMed] [Google Scholar]
- Zou L, Ding G, Abutalebi J, Shu H, Peng D. Structural plasticity of the left caudate in bimodal bilinguals. Cortex. 2012;48(9):1197–1206. doi: 10.1016/j.cortex.2011.05.022. http://dx.doi.org/10.1016/j.cortex.2011.05.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.