Abstract
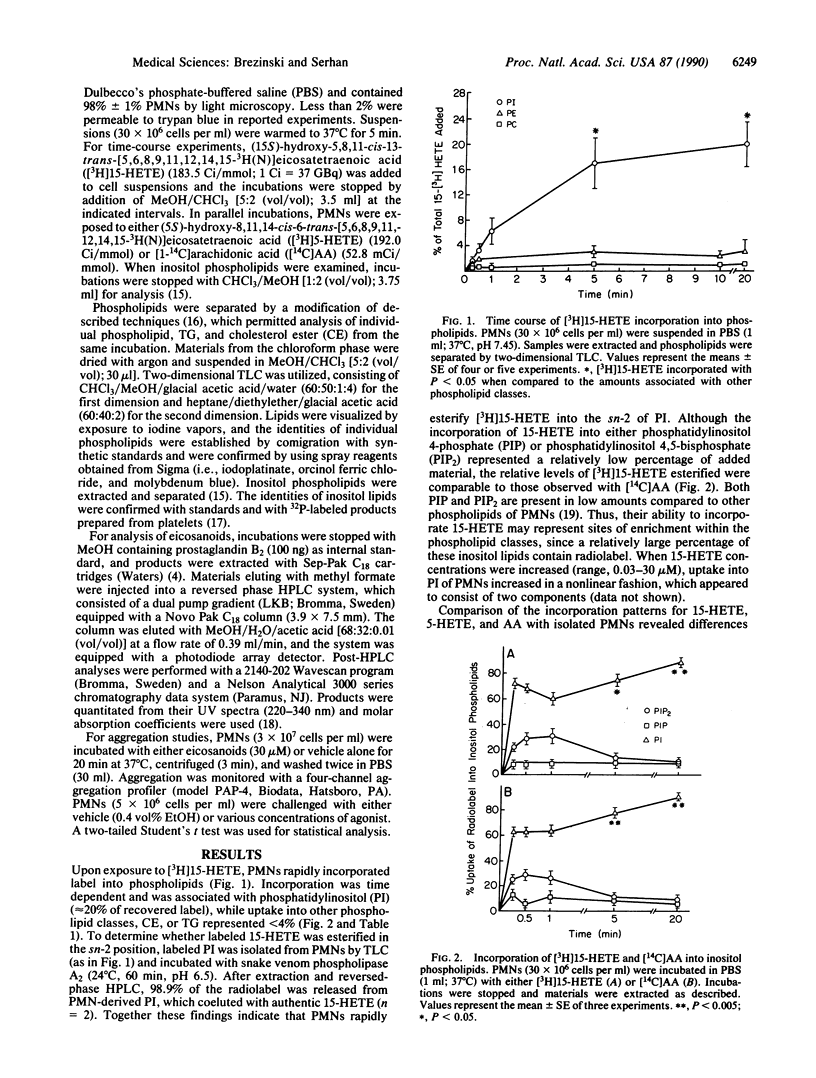
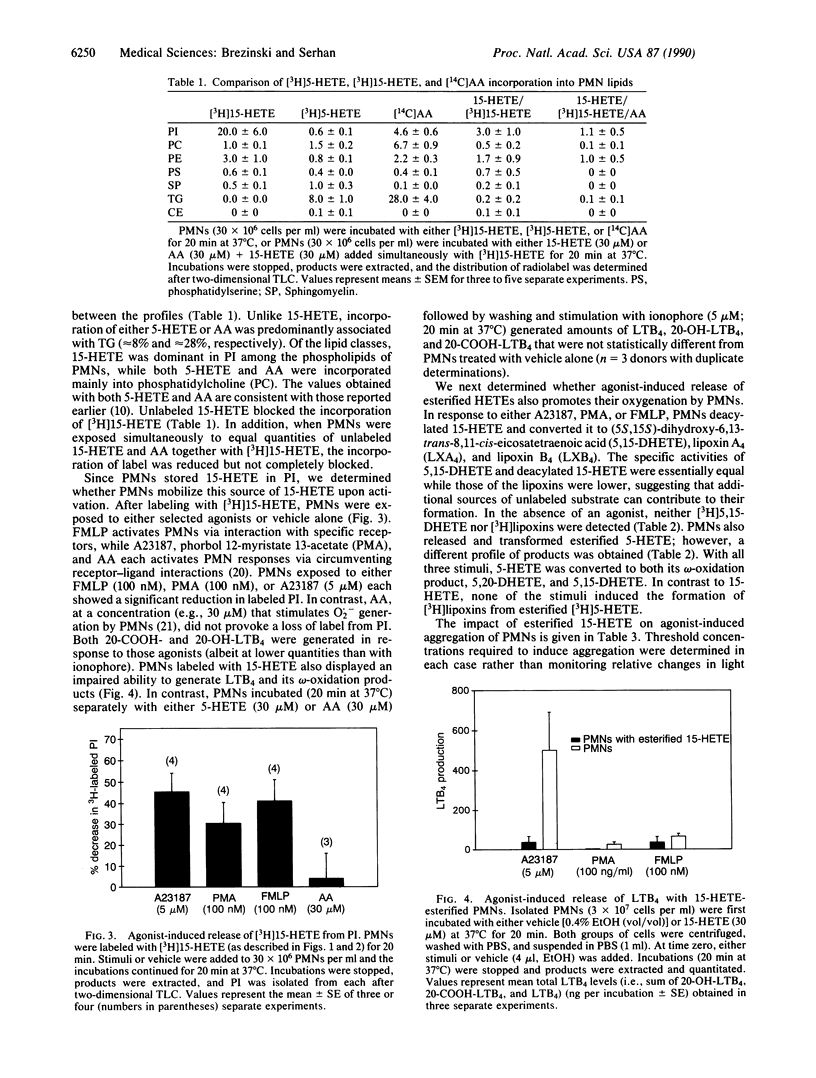
The uptake and mobilization of (15S)-hydroxy-5,8,11-cis-13-trans-eicosatetraenoic acid (15-HETE), a major product of arachidonic acid metabolism, was examined with human neutrophils (polymorphonuclear leukocytes; PMNs). Upon exposure to labeled 15-HETE, PMNs rapidly (15 sec to 20 min) incorporated approximately 20% of the label into phosphatidylinositol, while less than 4% was associated with other phospholipid classes and neutral lipids. This pattern was distinct from that of either labeled arachidonate or labeled(5S)-hydroxy-8,11,14-cis-6-trans-eicosatetraenoic acid (5-HETE), which within 20 min were predominantly associated with triglycerides and phosphatidylcholine. After reversed-phase HPLC, greater than 98% of the label in phosphatidylinositol, isolated from PMNs, was released with phospholipase A2. Upon exposure to either chemotactic peptide (FMLP), phorbol 12-myristate 13-acetate, or an ionophore (A23187), 15-HETE-labeled PMNs released 15-HETE from phosphatidylinositol and displayed an impaired ability to generate leukotriene B4 (LTB4), 20-OH-LTB4, and 20-COOH-LTB4. Deacylated [3H]15-HETE was converted to (5S,15S)-dihydroxy-6,13-trans-8,11-cis-eicosatetraenoic acid (5,15-DHETE), lipoxin A4, and lipoxin B4, each carrying 3H label. PMNs labeled with 5-HETE also released and transformed this HETE when stimulated. However, the profile of labeled products differed between PMNs with either esterified 15-HETE or 5-HETE. When activated, 5-HETE-labeled PMNs generated both 5,20-DHETE and 5,15-DHETE but not labeled lipoxins. Threshold aggregation induced by FMLP with 15-HETE-labeled PMNs was inhibited (approximately 2 orders of magnitude), while the threshold response was relatively unimpaired with either A23187 or phorbol 12-myristate 13-acetate-induced aggregation. Results indicate that 15-HETE is rapidly esterified into phosphatidylinositol of PMNs, which can be mobilized and transformed upon exposure of the cells to a second signal. Moreover, they suggest that eicosanoid intermediates other than arachidonic acid can be stored by cells, released via signal transduction, and oxygenated to generate alternative profiles of eicosanoids.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Badr K. F., DeBoer D. K., Schwartzberg M., Serhan C. N. Lipoxin A4 antagonizes cellular and in vivo actions of leukotriene D4 in rat glomerular mesangial cells: evidence for competition at a common receptor. Proc Natl Acad Sci U S A. 1989 May;86(9):3438–3442. doi: 10.1073/pnas.86.9.3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badwey J. A., Curnutte J. T., Karnovsky M. L. cis-Polyunsaturated fatty acids induce high levels of superoxide production by human neutrophils. J Biol Chem. 1981 Dec 25;256(24):12640–12643. [PubMed] [Google Scholar]
- Becker E. L., Kermode J. C., Naccache P. H., Yassin R., Munoz J. J., Marsh M. L., Huang C. K., Sha'afi R. I. Pertussis toxin as a probe of neutrophil activation. Fed Proc. 1986 Jun;45(7):2151–2155. [PubMed] [Google Scholar]
- Berridge M. J. The Albert Lasker Medical Awards. Inositol trisphosphate, calcium, lithium, and cell signaling. JAMA. 1989 Oct 6;262(13):1834–1841. [PubMed] [Google Scholar]
- Bonser R. W., Siegel M. I., Chung S. M., McConnell R. T., Cuatrecasas P. Esterification of an endogenously synthesized lipoxygenase product into granulocyte cellular lipids. Biochemistry. 1981 Sep 1;20(18):5297–5301. doi: 10.1021/bi00521a032. [DOI] [PubMed] [Google Scholar]
- Brash A. R., Ingram C. D. Lipoxygenase metabolism of endogenous and exogenous arachidonate in leukocytes: GC-MS analyses of incubations in H2(18)O buffers. Prostaglandins Leukot Med. 1986 Aug;23(2-3):149–154. doi: 10.1016/0262-1746(86)90178-2. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Chilton F. H., Murphy R. C. Remodeling of arachidonate-containing phosphoglycerides within the human neutrophil. J Biol Chem. 1986 Jun 15;261(17):7771–7777. [PubMed] [Google Scholar]
- Duell E. A., Ellis C. N., Voorhees J. J. Determination of 5,12, and 15-lipoxygenase products in keratomed biopsies of normal and psoriatic skin. J Invest Dermatol. 1988 Nov;91(5):446–450. doi: 10.1111/1523-1747.ep12476562. [DOI] [PubMed] [Google Scholar]
- Fogh K., Hansen E. S., Herlin T., Knudsen V., Henriksen T. B., Ewald H., Bünger C., Kragballe K. 15-Hydroxy-eicosatetraenoic acid (15-HETE) inhibits carrageenan-induced experimental arthritis and reduces synovial fluid leukotriene B4 (LTB4). Prostaglandins. 1989 Feb;37(2):213–228. doi: 10.1016/0090-6980(89)90058-0. [DOI] [PubMed] [Google Scholar]
- Fogh K., Søgaard H., Herlin T., Kragballe K. Improvement of psoriasis vulgaris after intralesional injections of 15-hydroxyeicosatetraenoic acid (15-HETE). J Am Acad Dermatol. 1988 Feb;18(2 Pt 1):279–285. doi: 10.1016/s0190-9622(88)70040-7. [DOI] [PubMed] [Google Scholar]
- Gans K. R., Lundy S. R., Arner E. C., Munzer D. A., Dowling R. L., Galbraith W. Eicosanoid production and cell accumulation induced by intrapleural injection of sodium arachidonate in the rat. Characterization of the model. Biochem Pharmacol. 1989 Nov 15;38(22):4151–4154. doi: 10.1016/0006-2952(89)90698-9. [DOI] [PubMed] [Google Scholar]
- Haurand M., Flohé L. Leukotriene formation by human polymorphonuclear leukocytes from endogenous arachidonate. Physiological triggers and modulation by prostanoids. Biochem Pharmacol. 1989 Jul 1;38(13):2129–2137. doi: 10.1016/0006-2952(89)90067-1. [DOI] [PubMed] [Google Scholar]
- Hawkins D. J., Kühn H., Petty E. H., Brash A. R. Resolution of enantiomers of hydroxyeicosatetraenoate derivatives by chiral phase high-pressure liquid chromatography. Anal Biochem. 1988 Sep;173(2):456–462. doi: 10.1016/0003-2697(88)90214-x. [DOI] [PubMed] [Google Scholar]
- Hedqvist P., Raud J., Palmertz U., Haeggström J., Nicolaou K. C., Dahlén S. E. Lipoxin A4 inhibits leukotriene B4-induced inflammation in the hamster cheek pouch. Acta Physiol Scand. 1989 Dec;137(4):571–572. doi: 10.1111/j.1748-1716.1989.tb08805.x. [DOI] [PubMed] [Google Scholar]
- Herlin T., Fogh K., Ewald H., Hansen E. S., Knudsen V. E., Holm I., Kragballe K., Bunger C. Changes in lipoxygenase products from synovial fluid in carrageenan induced arthritis in dogs. APMIS. 1988 Jul;96(7):601–604. doi: 10.1111/j.1699-0463.1988.tb00917.x. [DOI] [PubMed] [Google Scholar]
- Holtzman M. J., Pentland A., Baenziger N. L., Hansbrough J. R. Heterogeneity of cellular expression of arachidonate 15-lipoxygenase: implications for biological activity. Biochim Biophys Acta. 1989 Jun 8;1003(2):204–208. doi: 10.1016/0005-2760(89)90257-9. [DOI] [PubMed] [Google Scholar]
- Lee T. H., Crea A. E., Gant V., Spur B. W., Marron B. E., Nicolaou K. C., Reardon E., Brezinski M., Serhan C. N. Identification of lipoxin A4 and its relationship to the sulfidopeptide leukotrienes C4, D4, and E4 in the bronchoalveolar lavage fluids obtained from patients with selected pulmonary diseases. Am Rev Respir Dis. 1990 Jun;141(6):1453–1458. doi: 10.1164/ajrccm/141.6.1453. [DOI] [PubMed] [Google Scholar]
- Lee T. H., Horton C. E., Kyan-Aung U., Haskard D., Crea A. E., Spur B. W. Lipoxin A4 and lipoxin B4 inhibit chemotactic responses of human neutrophils stimulated by leukotriene B4 and N-formyl-L-methionyl-L-leucyl-L-phenylalanine. Clin Sci (Lond) 1989 Aug;77(2):195–203. doi: 10.1042/cs0770195. [DOI] [PubMed] [Google Scholar]
- McKinney M. Blockade of receptor-mediated cyclic GMP formation by hydroxyeicosatetraenoic acid. J Neurochem. 1987 Aug;49(2):331–341. doi: 10.1111/j.1471-4159.1987.tb02870.x. [DOI] [PubMed] [Google Scholar]
- Meade C. J., Turner G. A., Bateman P. E. The role of polyphosphoinositides and their breakdown products in A23187-induced release of arachidonic acid from rabbit polymorphonuclear leucocytes. Biochem J. 1986 Sep 1;238(2):425–436. doi: 10.1042/bj2380425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray J. J., Tonnel A. B., Brash A. R., Roberts L. J., 2nd, Gosset P., Workman R., Capron A., Oates J. A. Release of prostaglandin D2 into human airways during acute antigen challenge. N Engl J Med. 1986 Sep 25;315(13):800–804. doi: 10.1056/NEJM198609253151304. [DOI] [PubMed] [Google Scholar]
- O'Flaherty J. T., Wykle R. L., Redman J., Samuel M., Thomas M. Metabolism of 5-hydroxyicosatetraenoate by human neutrophils: production of a novel omega-oxidized derivative. J Immunol. 1986 Nov 15;137(10):3277–3283. [PubMed] [Google Scholar]
- Richards C. F., Campbell W. B. Incorporation of hydroxyeicosatetraenoic acids into cellular lipids of adrenal glomerulosa cells: inhibition of aldosterone release by 5-HETE. Prostaglandins. 1989 Nov;38(5):565–580. doi: 10.1016/0090-6980(89)90150-0. [DOI] [PubMed] [Google Scholar]
- Samuelsson B., Dahlén S. E., Lindgren J. A., Rouzer C. A., Serhan C. N. Leukotrienes and lipoxins: structures, biosynthesis, and biological effects. Science. 1987 Sep 4;237(4819):1171–1176. doi: 10.1126/science.2820055. [DOI] [PubMed] [Google Scholar]
- Samuelsson B. Leukotrienes: mediators of immediate hypersensitivity reactions and inflammation. Science. 1983 May 6;220(4597):568–575. doi: 10.1126/science.6301011. [DOI] [PubMed] [Google Scholar]
- Schafer A. I., Maas A. K., Ware J. A., Johnson P. C., Rittenhouse S. E., Salzman E. W. Platelet protein phosphorylation, elevation of cytosolic calcium, and inositol phospholipid breakdown in platelet activation induced by plasmin. J Clin Invest. 1986 Jul;78(1):73–79. doi: 10.1172/JCI112576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan C. N. On the relationship between leukotriene and lipoxin production by human neutrophils: evidence for differential metabolism of 15-HETE and 5-HETE. Biochim Biophys Acta. 1989 Aug 8;1004(2):158–168. doi: 10.1016/0005-2760(89)90264-6. [DOI] [PubMed] [Google Scholar]
- Serhan C. N., Sheppard K. A. Lipoxin formation during human neutrophil-platelet interactions. Evidence for the transformation of leukotriene A4 by platelet 12-lipoxygenase in vitro. J Clin Invest. 1990 Mar;85(3):772–780. doi: 10.1172/JCI114503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setty B. N., Stuart M. J. 15-Hydroxy-5,8,11,13-eicosatetraenoic acid inhibits human vascular cyclooxygenase. Potential role in diabetic vascular disease. J Clin Invest. 1986 Jan;77(1):202–211. doi: 10.1172/JCI112277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen X. Y., Figard P. H., Kaduce T. L., Spector A. A. Conversion of 15-hydroxyeicosatetraenoic acid to 11-hydroxyhexadecatrienoic acid by endothelial cells. Biochemistry. 1988 Feb 9;27(3):996–1004. doi: 10.1021/bi00403a024. [DOI] [PubMed] [Google Scholar]
- Spector A. A., Gordon J. A., Moore S. A. Hydroxyeicosatetraenoic acids (HETEs). Prog Lipid Res. 1988;27(4):271–323. doi: 10.1016/0163-7827(88)90009-4. [DOI] [PubMed] [Google Scholar]
- Stenson W. F., Nickells M. W., Atkinson J. P. Esterification of monohydroxyfatty acids into the lipids of a macrophage cell line. Prostaglandins. 1983 Aug;26(2):253–264. doi: 10.1016/0090-6980(83)90093-x. [DOI] [PubMed] [Google Scholar]
- Stenson W. F., Parker C. W. 12-L-hydroxy-5,8,10,14-eicosatetraenoic acid, a chemotactic fatty acid, is incorporated into neutrophil phospholipids and triglyceride. Prostaglandins. 1979 Aug;18(2):285–292. doi: 10.1016/0090-6980(79)90115-1. [DOI] [PubMed] [Google Scholar]
- Stenson W. F., Parker C. W. Metabolism of arachidonic acid in ionophore-stimulated neutrophils. Esterification of a hydroxylated metabolite into phospholipids. J Clin Invest. 1979 Nov;64(5):1457–1465. doi: 10.1172/JCI109604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderhoek J. Y., Bryant R. W., Bailey J. M. Inhibition of leukotriene biosynthesis by the leukocyte product 15-hydroxy-5,8,11,13-eicosatetraenoic acid. J Biol Chem. 1980 Nov 10;255(21):10064–10066. [PubMed] [Google Scholar]
- Waldman J. S., Marcus A. J., Soter N. A., Lim H. W. Cutaneous inflammation: effects of hydroxy acids and eicosanoid pathway inhibitors on vascular permeability. J Invest Dermatol. 1989 Jan;92(1):112–116. doi: 10.1111/1523-1747.ep13071322. [DOI] [PubMed] [Google Scholar]
- Yuli I., Snyderman R. Light scattering by polymorphonuclear leukocytes stimulated to aggregate under various pharmacologic conditions. Blood. 1984 Sep;64(3):649–655. [PubMed] [Google Scholar]
- van Dongen C. J., Zwiers H., Gispen W. H. Microdetermination of phosphoinositides in a single extract. Anal Biochem. 1985 Jan;144(1):104–109. doi: 10.1016/0003-2697(85)90090-9. [DOI] [PubMed] [Google Scholar]



