Abstract
In recent years the emergence of multiple drug resistance microbes has become a global public health problem. The aim of the present investigation was to evaluate possible antibiofilm efficacy of a gallotannin 1,2,6-tri-O-galloyl-β-D-glucopyranose from Terminalia chebula fruits alone and in combination with gentamicin and trimethoprim against preformed biofilms of multidrug-resistant (MDR) uropathogenic E. coli isolates using microbroth dilution, checkerboard titration and kill kinetics methods. Test gallotannin showed > 50% antibiofilm efficacy after 24 h when administered alone whereas gentamicin and trimethoprim failed to do so. But in combination, test gallotannin/gentamicin and test gallotannin/trimethoprim showed 71.24±6.75% and 93.4±8.46% antibiofilm activity respectively. On the basis of FICI values, test gallotannin/gentamicin showed synergistic interactions against 71.42% and test gallotannin/trimethoprim against 85.71% biofilm forming test bacterial isolates. Kill-kinetics study confirmed their synergistic interactions. Thus, gentamicin and trimethoprim in combination with test gallotannin may have potential for treatment of urinary tract infections caused by biofilm forming MDR uropathogenic E. coli.
Introduction
In recent years the emergence of multidrug resistance microbes has become a global public health problem. Despite the recent introduction of highly potent newer antibiotics such as the aminoglycosides, fluoroquinolones and the third generation of cephalosporins into clinical practice, multidrug-resistant pathogens pose a major therapeutic problem for clinicians worldwide [1]. This drug resistance problem is considered to be associated mostly by their biofilm forming ability [2].
Bacterial biofilms are surface associated communities of cells embedded in a self-produced matrix. This matrix consists of extracellular polymeric substances (EPS) and it is one of the most important factors contributing to increase tolerance to antibiotics associated with bacterial biofilms [3]. Bacteria embedded in biofilms are less susceptible to host defenses and more resistant (10–1000 times) to antimicrobial products than their planktonic counterparts [4]. According to the National Institutes of Health (NIH), biofilm forming bacteria involved up to 80% of all infections [5]. In drug resistant bacteria, the antibiotic concentration necessary to eliminate biofilms often exceeds the highest deliverable concentration of antibiotics compared to planktonic bacteria which in subsequent rendered the conventional antibiotics ineffective against the biofilm-forming drug-resistant microbes [6].
Among the biofilm forming bacterial pathogens, Escherichia coli is the most common biofilm-forming bacterial species in urinary tract infections. It is also frequently responsible for other types of infections in our body [7]. In recent years, the emergence of multidrug resistant (MDR) uropathogenic E. coli has become a global public health problem. Most of the conventional antibiotics rendered ineffective against this bacterial pathogen. This problem demands that an alternative approach should be made to make the conventional antibiotics effective against the MDR E. coli by augmenting their antimicrobial efficacy.
Our previous study on combination effects of test gallotannin 1,2,6-tri-O-galloyl-β-D-glucopyranose with selected antibiotics (amoxicillin, ceftazidime, ciprofloxacin, gentamicin and trimethoprim) against planktonic cells of MDR uropathogenic E. coli isolates revealed that test gallotannin exhibited synergistic antibacterial activity against the studied bacteria in combination with gentamicin and trimethoprim and additive effects with amoxicillin, ciprofloxacin and ceftazidime [8]. In the present investigation, an attempt has therefore been made to evaluate whether this synergistic antibacterial activity of test gallotannin in combination with antibiotics gentamicin and trimethoprim against MDR uropathogenic E. coli is associated with their antibiofilm activity or not.
Materials and methods
Test compound
A gallotannin, 1,2,6–tri-O-galloyl-β-D-glucopyranose (Fig 1) was isolated from hydro ethanol extract of T. chebula fruits by bioassay-guided fractionation and characterized by extensive spectroscopic analyses (FT-IR, 1H-NMR, 13C-NMR and ESI-MS) [8]. This isolated gallotannin (Fig 1) was used in the present investigation.
Fig 1. Chemical structure of test gallotannin (1,2,6-tri-O-galloyl-β-D-glucopyranose).
Microorganisms
Seven multidrug-resistant clinical isolates of Escherichia coli from urine samples collected from patients having indwelling catheter for more than two weeks, and urine microscopy shows pyuria were used in the present investigation. Isolates were kindly provided by the Department of Microbiology, NRS Medical College and Hospital, Kolkata, India. Their antibiotics resistance profiles are summarized in Table 1. Internal quality assurance was ensured using reference E. coli (ATCC 8739) strain procured from National Chemical Laboratory, Pune, India. The isolates were maintained on nutrient agar slants at 4°C.
Table 1. Bacterial strains used and their resistance profile.
| Organism | Type | Resistance profile |
|---|---|---|
| E. coli (EC1601) | MDR | GMR, CIPR, TMPR |
| E. coli (EC1602) | MDR | CEFR, GMR, TMPR, NORR |
| E. coli (EC1603) | MDR | IMPR, GMR, TMPR, CIPR |
| E. coli (EC1604) | MDR | CEFR, NORR, IMPR, GMR |
| E. coli (EC1605) | MDR | GMR, CEFR, TMPR |
| E. coli (EC1606) | MDR | GMR, TMPR, NORR, IMPR |
| E. coli (EC1607) | MDR | CEFR, IMPR, TMPR, GMR |
| E. coli (ATCC 8739) | Type strain | ---------------------------- |
MDR: Multidrug-resistant; CEF: Cefotaxime; CIP: Ciplofloxacin; GM: Gentamicin; IMP: Imipenem; NOR: Norfloxacin; TMP: Trimethoprim; R: Resistant
Inoculum preparation
The inoculum size of the test bacterial isolates was standardized following the National Committee for Clinical Laboratory Standards guidelines [9]. The bacterial isolates were inoculated in Mueller Hinton Broth (MHB) (Hi-media, Mumbai, India) and incubated at 37°C in a shaker water bath for 3–6 h until the culture attained a turbidity of 0.5 McFarland unit. For experimental purposes, inoculum size of test isolates was adjusted to 5 × 105 CFU/ml.
Determination of minimum inhibitory concentration (MIC)
Microbroth dilution susceptibility test was performed for minimum inhibitory concentration (MIC) determination in flat-bottom 96-well microtiter plates containing MHB medium (90 μl) in each well. The test compound reconstituted in 0.5% dimethyl sulphoxide (DMSO) was diluted twofold serially with MHB ranging from 2 μg/ml to 512 μg/ml. 100 μl of diluted solution was given in each well containing broth. 10 μl of working inoculum suspension (5 × 105 CFU/ml) was added to the wells. A number of wells were reserved in each plate for control of sterility (no inoculum added), inoculums viability (no sample solution added) and DMSO inhibitory effect. The plates were then incubated for 18 h at 37°C. After incubation, 10 μl of Alamar Blue was added in each well and further incubated for 4 h for a colour change from blue to pink. A blue colour in the well was interpreted as no growth, and a pink indicated growth. MICs were determined as the lowest concentration of the drug that prevented the colour change from blue to pink [10]. Each experiment was repeated thrice.
Determination of antibiofilm activity of test gallotannin and antibiotics alone and in combination
Biofilm formation was achieved by aliquoting 100 μl of test E. coli culture (5 × 105 CFU/ml) into wells of 96-well microtiter plate. The plate was incubated for 72 h at 37°C to allow biofilm formation. After incubation, supernatant was removed and each well was washed thoroughly with sterile distilled water to remove free-floating cells; thereafter plates were air-dried for 30 min and the biofilm formed was stained with 100 μl of 0.1% aqueous solution of crystal violet (CV) and kept for 15 min at room temperature. After staining, the excess of stain was removed washing the plate three times with sterile distilled water. Finally, the dye bound to the cells was solubilized by adding 200 μl of 95% ethanol to each well and after 15 min of incubation, absorbance was measured at 595 nm. CV absorbance of all test isolates were found to be ≥1.5 which indicates that the test bacterial isolates have high biofilm forming ability [11]. After biofilm formation, 100 μl of test gallotannin and antibiotics alone and in combination (1:1) at their MIC doses were given in wells of microtiter plate. Well containing equal volume of DMSO (0.5%) in place of test antimicrobials served as control. Plates were further incubated at 37°C for 24 h. Absorbance of the control and treated wells (OD595nm) were determined and percent inhibition of biofilm biomass of test gallotannin and antibiotics alone and in combination were calculated using the following formula [12]. Each experiment was repeated thrice.
Determination of fractional inhibitory concentration index (FICI)
For determining FICI values, effects of test gallotannin and antibiotics alone and in combination on MDR uropathogenic E. coli biofilms was evaluated using the method of Ramage et al. (2001) [13]. Biofilm formation of test bacterial strains was achieved following the same protocol as described above. After biofilm formation, the medium was aspirated gently and non-adherent cells were removed by washing the biofilms three times with sterile PBS. Then 100 μl of 2-fold serial dilutions (1/32 × MIC to 4 × MIC) of test gallotannin and antibiotics were added to each biofilm containing wells and microtiter plates were incubated for 24 h at 37°C. Minimum biofilm eradication concentrations (MBECs) were determined by the XTT reduction assay following the method of Ramage et al. (2001) [13]. FICindices were calculated using the formula: FICindex = (MBEC of antibiotic in presence of test gallotannin/MBEC of antibiotic alone) + (MBEC of test gallotannin in presence of antibiotic/MBEC of test gallotannin alone). The results were interpreted according to FICindices as follows: ‘synergy’ (FICI ≤ 0.5), ‘additive’ (FICI > 0.5–4) and ‘antagonism’ (FICI > 4) [14]. All the experiments were repeated thrice.
Kill kinetics study
To evaluate the type of antibiofilm interactions (synergistic, additive or antagonistic) of test gallotannin in combination with antibiotics, time-kill assay was performed. Here, the combination of ¼ × MBEC was applied [14, 15]. For this, biofilms of most susceptible test isolate [E. coli (EC1606)] were allowed to form in 96-well microtiter plates following the same procedure as described above and challenged with test gallotannin and antibiotics alone and in combination (1:1) at their ¼ MBEC doses. Microtiter plates were incubated at 37°C for 24 h. Viable cells were measured over a series of time intervals (0, 2, 4, 6, 8 and 24 h) and plotted. Each experiment was repeated thrice. Synergy was defined as > 100-fold or >2log10 decrease in colony count at 24 h by the combination when compared with their single agent [14].
The types of interactions were considered as follows.
‘synergistic’: > 2 log10 CFU/ml decrease; ‘additive’: < 2 log10 CFU/ml decrease; and ‘antagonistic’: > 2 log10 CFU/ml increase in bacterial colony count at 24 h by combination treatment in comparison with the killing by the most active single agent [14, 15].
MTT assay using human normal colon cell line
Cell culture
Human normal colon cell line was obtained from American Type Culture Collection (ATCC, USA) and maintained in EMEM medium which was supplemented with 10% fetal bovine serum (FBS), penicillin (100 units/ml), streptomycin (100 μg/ml) and 1% sodium pyruvate. The cells were incubated at 37°C in a humidified 5% CO2 incubator.
MTT assay
Cytotoxic potential of test galotannin that showed synergistic interactions was tested in triplicate by MTT [3-(4,3-dimethylthiazole-2-yl)-2,5-diphenyltetrazolium bromide] assay [16] using human normal colon cell line (CCD-18Co) with slight modification. Briefly, after being harvested from culture flasks the cells (100μl) were seeded at a density of 1 × 105 cells/ml in each well of 96 well plate containing 100 μl of fresh growth medium per well and cells were permitted to adhere for 24h at 37°C. The medium was removed after 24 h of incubation and 100 μl of fresh medium containing different concentrations (2 μg/ml—512 μg/ml) of test gallotannin were added. To control wells only culture medium (100μl) was used. Following 72 h of incubation, 20 μl of MTT (5 mg/ml) was added into each well and further incubated for another 4h. The formation of insoluble purple formazan from yellowish MTT by enzymatic reduction was dissolved in DMSO (100 μl) after removal of medium. The plates were shaken for 5 min and the absorbance was measured in a microplate reader at 570 nm with 630 nm as reference wavelength. The percent cell inhibition was determined using the following formula:
A dose-response curve was plotted from which IC50 was calculated.
Statistical analysis
Data were statistically analysed using SPSS software: Version 18.0. Two-way analysis of variance (ANOVA) and Student’s t-test were applied for analysis of data with a level of significance set at p < 0.05.
Results
The bacterial strains used in the study and their resistance profile are shown in Table 1.
MIC values of test gallotannin and antibiotics gentamicin and trimethoprim against the studied bacteria are cited in Table 2. The MIC values of test gallotannin, gentamicin and trimethoprim against the test clinical isolates were found to be 14.09 ± 3.49 μg/ml, 60.95 ± 9.62 μg/ml and 30.47 ± 4.81 μg/ml respectively whereas these values against type strains were 10.66 ± 4.61 μg/ml, 26.66 ± 9.23 μg/ml and 13.33 ± 4.61 μg/ml respectively (Table 2).
Table 2. Minimum inhibitory concentration (MIC) values of test gallotannin and antibiotics gentamicin and trimethoprim against planktonic cells of multidrug- resistant uropathogenic E. coli.
| Microorganisms | TG | GM | TMP |
|---|---|---|---|
| E. coli (ATCC 8739) | 10.66 ± 4.61 | 26.66 ± 9.23 | 13.33 ± 4.61 |
| E. coli (EC1601) | 13.33 ± 4.61 | 53.33 ± 18.47 | 26.66 ± 9.23 |
| E. coli (EC1602) | 16.00 ± 0.00 | 64.00 ± 0.00* | 26.66 ± 9.23 |
| E. coli (EC1603) | 13.33 ± 4.61 | 53.33 ± 18.47 | 32.00 ± 0.00* |
| E. coli (EC1604) | 13.33 ± 4.61 | 64.00 ± 0.00* | 32.00 ± 0.00* |
| E. coli (EC1605) | 13.33 ± 4.61 | 64.00 ± 0.00* | 32.00 ± 0.00* |
| E. coli (EC1606) | 16.00 ± 0.00 | 64.00 ± 0.00* | 32.00 ± 0.00* |
| E. coli (EC1607) | 13.33 ± 4.61 | 64.00 ± 0.00* | 32.00 ± 0.00* |
Values are mean ± SD of triplicate experiment
*p < 0.05 in comparison with type strain
TG: Test gallotannin; GM: Gentamicin; TMP: Trimethoprim;
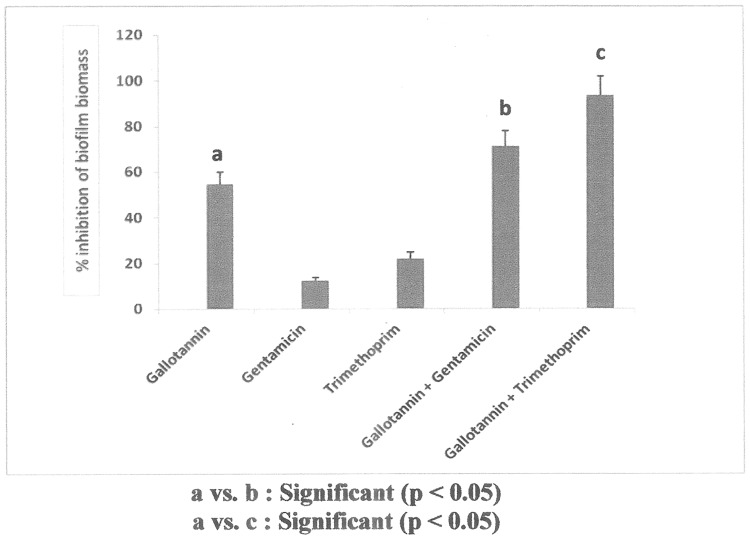
After determining the MIC values of test samples against the studied bacteria, their alone as well as combination effects on biofilms produced by the test bacterial isolates were observed. The results of antibiofilm efficacy of test samples alone and in combination are shown in Fig 2. From Fig 2, it was observed that the test gallotannin showed 54.60 ± 5.27% inhibition on biofilm biomass of test bacterial isolates whereas these values for gentamicin and trimethoprim were 12.50 ± 1.21% and 21.87 ± 2.84% respectively after 24 h of administration. But test gallotannin/ gentamicin and test gallotannin/ trimethoprim combinations showed 71.24 ± 6.75% and 93.40 ± 8.46% inhibition on biofilm biomass respectively.
Fig 2. Antibiofilm efficacy of test gallotannin and antibiotics gentamicin and trimethoprim alone and in combination against MDR uropathogenic E. coli biofilms.
Table 3 shows the FICI values of test gallotannin/ gentamicin and test gallotannin/ trimethoprim combinations on biofilms formed by the studied bacteria. It was observed that both test gallotannin/ gentamicin (FICI: 0.156–0.375) and test gallotannin/ trimethoprim (FICI: 0.093–0.50) combinations exhibited synergistic antibiofilm activity against most of the studied bacteria.
Table 3. Fractional inhibitory concentration values (FICI) of test gallotannin in combination with antibiotics gentamicin and trimethoprim against MDR uropathogenic E. coli biofilms.
| Microorganism | Combinations | |||||
|---|---|---|---|---|---|---|
| TG + GM | TG + TMP | |||||
| FIC | FICI | Remarks | FIC | FICI | Remarks | |
| E. coli (EC1601) | 0.250 (TG) | 0.375 | S | 0.031 (TG) | 0.093 | S |
| 0.125 (GM) | 0.062 (TMP) | |||||
| E. coli (EC1602) | 0.125 (TG) | 0.250 | S | 0.062 (TG) | 0.312 | S |
| 0.125 (GM) | 0.250 (TMP) | |||||
| E. coli (EC1603) | 0.250 (TG) | 1.25 | ADD | 0.062 (TG) | 0.124 | S |
| 1.00 (GM) | 0.062 (TMP) | |||||
| E. coli (EC1604) | 0.031 (TG) | 0.156 | S | 0.500 (TG) | 1.00 | ADD |
| 0.125 (GM) | 0.500 (TMP) | |||||
| E. coli (EC1605) | 0.125 (TG) | 0.625 | ADD | 0.25 (TG) | 0.50 | S |
| 0.500 (GM) | 0.25 (TMP) | |||||
| E. coli (EC1606) | 0.031 (TG) | 0.281 | S | 0.062 (TG) | 0.187 | S |
| 0.250 (GM) | 0.125 (TMP) | |||||
| E. coli (EC1607) | 0.250 (TG) | 0.375 | S | 0.125 (TG) | 0.250 | S |
| 0.125 (GM) | 0.125 (TMP) | |||||
TG: Test gallotannin; GM: Gentamicin; TMP: Trimethoprim
S: Synergistic; ADD: Additive
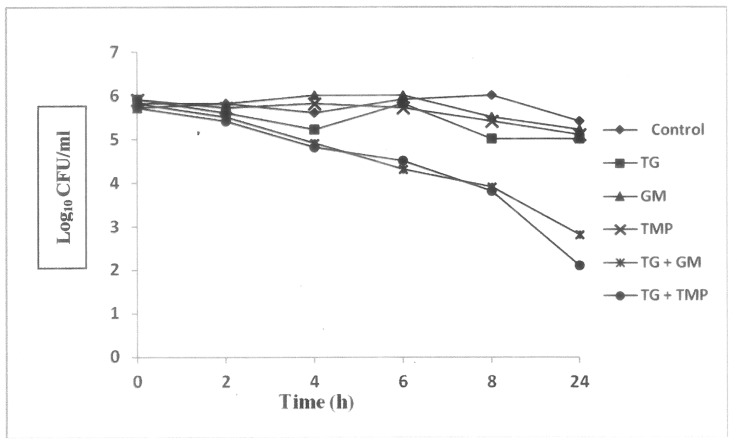
Fig 3 shows the results of kill-kinetics study of test gallotannin and antibiotics alone and in combination against the studied bacterial biofilms. It was observed that a reduction > 2 Log10 CFU/ml decrease in colony count was observed in both test gallotannin/ gentamicin and test gallotannin/ trimethoprim combinations in comparison with the killing by the most active single agent.
Fig 3. Kill kinetics curves on antibiofilm efficacy of test gallotannin and antibiotics gentamicin and trimethoprim alone and in combination against uropathogenic MDR E. coli biofilms.
[TG: Test gallotannin; GM: Gentamicin; TMP: Trimethoprim].
The results of MTT assay revealed that test gallotannin failed to show any cytotoxic effect on human normal colon cell line at recommended dosage level and the IC50 value was found to be > 512 μg/ml (Table 4).
Table 4. Cytotoxic potential of test galloatannin 1,2,6–tri-O-galloyl-β-D-glucopyranose using MTT assay.
| Concentration (μg/ml) | % cell death (72 h) | IC50 (μg/ml) |
|---|---|---|
| 0 | 0 | > 512 |
| 2 | 0 | |
| 4 | 0 | |
| 8 | 0 | |
| 16 | 0 | |
| 32 | 0 | |
| 64 | 7.86 ± 0.85 | |
| 128 | 11.70 ± 1.05 | |
| 256 | 17.4 ± 1.21 | |
| 512 | 21.7 ± 1.16 |
Values are mean ± SD of triplicate experiments
Discussion
Synergistic combinations of antimicrobials have been introduced in recent years as more successful strategies to combat infections involving MDR bacterial pathogens. Generally, synergistic combinations have proven to be an essential feature of antimicrobial treatment due to a number of important considerations viz. (i) they increase activity through the use of compounds with synergistic activity; (ii) they thwart drug resistance; (iii) they decrease required doses, reducing both cost and adverse/toxic side effects and (iv) they increase the spectrum of antimicrobial activity. Several studies recommend synergistic combination therapy as the treatment of choice in biofilm-associated infections [17, 18].
In the present investigation, to evaluate possible synergistic antibiofilm efficacy of test gallotannin against MDR uropathogenic E. coli biofilms with conventional antibiotics gentamicin and trimethoprim that were rendered ineffective against these studied clinical bacterial isolates, at first MIC values of these test antimicrobials against planktonic cells of MDR uropathogenic E. coli isolates were determined. It was observed that at their alone effects the MIC values of test gallotannin against studied clinical bacterial isolates were much lower than the MIC values of gentamicin and trimethoprim. Besides, there was no significant difference in MIC values of test gallotannin against test clinical isolates compared to type strains. But the MIC values of both gentamicin and trimethoprim were significantly (p < 0.05) different between clinical isolates and type strains (Table 2).
Using the MIC values of test antimicrobials, their antibiofilm efficacy alone and in combination against the studied bacterial biofilms were evaluated. A reduction in > 50% in biofilm biomass by test antimicrobials was considered as active. When administered individually, the test gallotannin showed > 50% inhibition in biofilm biomass whereas gentamicin and trimethoprim failed to show > 50% antibiofilm efficacy after 24 h of administration. But in combination with test gallotannin both gentamicin and trimethoprim showed synergistic antibiofilm efficacy. These findings suggest that gallotannin has the ability to inhibit biofilm biomass formed by studied bacteria and also to augment the antibiofilm efficacy of conventional antibiotics gentamicin and trimethoprim (Fig 2).
To evaluate the type of antibiofilm interactions (synergistic, additive or antagonistic), FICI values of test antimicrobials using checkerboard titration method were determined. From FICI values (Table 3), it was observed that test gallotannin/ gentamicin combination showed synergistic antibiofilm efficacy against 71.42% test bacterial isolates whereas this value for test gallotannin/ trimethoprim combination was 85.71%.
In order to confirm the synergistic antibiofilm efficacy of test gallotannin in combination with gentamicin and trimethoprim, kill-kinetics study was performed. A reduction in > 2 Log10 CFU/ml decrease in bacterial colony count at 24 h in comparison with the killing by the most active single agent was considered as synergistic [14]. From kill-kinetics curve, it was observed that test gallotannin/ gentamicin and test gallotannin/ trimethoprim combinations decreased the bacterial colony count by 2.2 Log10 CFU/ml and 2.9 Log10 CFU/ml respectively at 24 h compared to most active single agent test gallotannin (Fig 3) confirming the synergistic antibiofilm efficacy of test gallotanin with antibiotics gentamicin and trimethoprim against the studied bacterial biofilms. Our results corroborate with the findings of other workers [19, 20] where they found synergistic antibiofilm activity of plant extracts and/ or phytocompounds with conventional antibiotics against disease-producing bacterial and fungal biofilms. Possible mechanisms behind the synergistic interactions of test gallotannin in combination with gentamicin and trimethoprim is not clear right now. Studies have shown that two different anti-biofilm mechanisms are able to modulate biofilm formation: inhibition of bacterial surface attachment and interruption of quorum sensing (QS) [21]. Whether synergistic anti-biofilm activity of test gallotannin in combination with antibiotics gentamicin and trimethoprim is due to inhibition of bacterial surface attachment or interruption of quorum sensing that needs to be investigated.
Furthermore, MTT assay is a rapid and high accuracy colorimetric approach that widely used to determine cell growth and cytotoxicity, particularly in the development of new drug. It measures cell membrane integrity by determining mitochondrial activity through enzymatic reaction on the reduction of yellow tetrazolium MTT to a purple formazan. So the amount of formazan produced reflected the number of metabolically active viable cells [22]. The cytotoxic effect of test gallotannin was investigated in vitro on human normal colon cell line using MTT assay and found that the test gallotannin failed to show any toxic potential against human normal colon cell line with IC50 > 512 μg/ml which suggests that the test gallotannin can be considered as a safe substance ordinarily.
Thus from the foregoing findings, it was observed that the test gallotannin 1,2,6-tri-O-galloyl-β-D-glucopyranose isolated from T. chebula fruit has the ability to eradicate biofilms produced by MDR uropathogenic E. coli alone and in combination with conventional antibiotics gentamicin and trimethoprim in vitro. Antibiofilm efficacy of test gentamicin and trimethoprim was also significantly enhanced in presence of test gallotannin. The results provide evidence that the test gallotannin alone and in combination with gentamicin and trimethoprim may serve as a potential source for treatment of biofilm-associated urinary tract infections caused by MDR E. coli. Further studies in in vivo systems are required for their practical applications. This report may serve as a footstep on these important aspects.
Acknowledgments
Authors are thankful to UGC, New Delhi; the Director, Indian Statistical Institute, Kolkata; and Prof. N.K. Pal, Department of Microbiology, NRS Medical College, Kolkata, for their kind cooperation and help to carry out the work.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by the University Grants Commission, New Delhi, India provided Post Doctoral Fellowship [F.15-1/2012-13/PDFWM-2012-13-GE-WES-18557(SA-II)] to AB. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Karlowsky JA, Kelly LJ, Thornsberry C, Jones ME, Sahm DF. Trend in antimicrobial resistance among urinary tract infection isolates Escherichia coli from female out- patients in the united states. Antimicrobial Agents and Chemotherapy. 2002; 46: 2540–2545. 10.1128/AAC.46.8.2540-2545.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Subramanian P, Shanmugam N, Sivaraman U, Kumar S, Selvaraj S. Antibiotic resistance pattern of biofilm-forming uropathogens isolated from catheterized patients in Pondicherry, India. Australas Medical Journal. 2012; 5(7): 344–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mah TC, O’Toole GA. Mechanisms of biofilm resistance to antimicrobial agents. Trends in Microbiology. 2001; 9: 34–39. [DOI] [PubMed] [Google Scholar]
- 4.Donlan RM, Costerton JW. Biofilms: survival mechanisms of clinically relevant microorganisms. Clinical Microbiology Reviews. 2002; 15: 167–193. 10.1128/CMR.15.2.167-193.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Costerton JW, Stewart PS, Greenbarg EP. Bacterial biofilms: a common cause of persistence infections. Science. 1999; 284: 1318–1322. [DOI] [PubMed] [Google Scholar]
- 6.Musk DJ, Hergenrother PJ. Chemical counter measures for the control of bacterial biofilms: effective compounds and promising targets. Current Medicinal Chemistry 2006; 13: 2163–2177. [DOI] [PubMed] [Google Scholar]
- 7.Foxman B. Epidemiology of urinary tract infections: incidence, morbidity and economic costs. The American Journal of Medicine. 2002; 113 (Suppl 1A): 5S–13S. [DOI] [PubMed] [Google Scholar]
- 8.Bag A, Bhattacharyya SK, Chattopadhyay RR. Isolation and identification of a gallotannin 1,2,6-tri-O-galloyl-β-D-glucopyranose from hydroalcoholic extract of Terminalia chebula fruits effective against multidrug-resistant uropathogens. Journal of Applied Microbiology. 2013; 115: 390–397. 10.1111/jam.12256 [DOI] [PubMed] [Google Scholar]
- 9.Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Disk Susceptibility Tests, Approved Standard, 7th ed., CLSI document M02-A11. Clinical and Laboratory Standards Institute, 950 West Valley Road, Suite 2500, Wayne, Pennsylvania 19087, USA; 2012.
- 10.Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing: Fifteenth Informational Supplement M100–S15. Weyne, PA: Clinical and Laboratory Standards Institute; 2005.
- 11.Sherry L, Rajendran R, Lappin DF, Borghi E, Perdoni F, Falleni M, et al. Biofilms formed by Candida albicans bloodstream isolates display phenotypic and transcriptional heterogeneity that are associated with resistance and pathogenicity. BMC Microbiology. 2014; 14(1): 182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rogers SA, Melander C. Construction and screening of a 2-aminoimidazole library identifies a small molecule capable of inhibiting and dispersing biofilms across bacterial order, class, and phylum. Angewandte Chemie International Edition. 2008; 47: 5229–5231. 10.1002/anie.200800862 [DOI] [PubMed] [Google Scholar]
- 13.Ramage G, Vande-Walle K, Wickes B, López-Ribot JL. Standardized method for in vitro antifungal susceptibility testing of Candida albicans biofilms. Antimicrobial Agents and Chemotherapy. 2001; 45: 2475–2479. 10.1128/AAC.45.9.2475-2479.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leclercq R, Bingen E, Su QH, Lambert-Zechovski N, Courvalin P, Duval J. Effects of combinations of β-lactams, daptomycin, gentamicin and glycopeptides against glycopeptides-resistant enterococci. Antimicrobial Agents and Chemotherapy. 1991; 35: 92–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Messick CR, Rodvold KA, Pendland SL. Modified time—kill assay againstmultidrug-resistant Enterococcus faecium with novel antimicrobial combinations. Journal of Antimicrobial Chemotherapy. 1999; 44(6): 831–834. [DOI] [PubMed] [Google Scholar]
- 16.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. Journal of Immunological Methods. 1983; 65: 55–63. [DOI] [PubMed] [Google Scholar]
- 17.Barapatre A, Aadil KR, Jha H. Synergistic antibacterial and antibiofilm activity of silver nanoparticles biosynthesized by lignin-degrading fungus. Bioresources and Bioprocessing. 2016; 3(8): 1–13. [Google Scholar]
- 18.Dosler S, Karaaslan E, Alev Gerceker A. Antibacterial and anti-biofilm activities of melittin and colistin, alone and in combination with antibiotics against Gram-negative bacteria. Journal of Chemotherapy. 2016; 28(2): 95–103. 10.1179/1973947815Y.0000000004 [DOI] [PubMed] [Google Scholar]
- 19.Khan MS, Ahmad I. Antibiofilm activity of certain phytocompounds and their synergy with fluconazole against Candida albicans biofilms. Journal of Antimicrobial Chemotherapy. 2012; 67(3): 618–621. 10.1093/jac/dkr512 [DOI] [PubMed] [Google Scholar]
- 20.Dineshbabu J, Darsini DTP, Srinivasan P, Everlyne IM, Manimekalai K. Synergistic anti-biofilm activity of medicinal plants against biofilm forming Streptococcus pyogenes from pharyngitis patients. Indo American Journal of Pharmaceutical Research. 2015; 5(8): 2598–2606. [Google Scholar]
- 21.Miquel S, Lagrafeuille R, Souweine B, Forestier C. Anti-biofilm activity as a Health Issue. Frontier Microbiology. 2016; 7: 592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sieuwerts AM, Klijn JGM, Peters HA, Foekens JA. The MTT tetrazolium salt assay scrutinized: How to use this assay reliably to measure metabolic activity of cell cultures in vitro for the measure metabolic activity of cell cultures, IC50-values and cell survival. European Journal of Clinical Chemistry and Clinical Biochemistry. 1995; 33: 813–823. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.