Abstract
Background
We aimed to increase human immunodeficiency virus (HIV) counseling, testing, referral (CTR), and knowledge of HIV serostatus of close contacts of tuberculosis patients and improve tuberculosis screening and treatment of HIV-infected contacts.
Methods
Of close contacts to infectious tuberculosis patients reported from December 2002 to November 2003, investigators (1) offered HIV CTR, (2) identified factors associated with HIV testing, and (3) assessed study costs.
Results
Of 614 contacts, 569 (93%) were provided HIV information and offered HIV CTR. Of the 569, 58 (10%) were previously HIV tested; 165 (29%) were newly HIV tested; and 346 (61%) were not tested. None of the 165 newly HIV tested contacts were HIV infected. Contacts more likely to be newly HIV tested (vs not tested) included those aged 18–24, Hispanic, or non-Hispanic Black. Of 24 HIV-infected contacts, 71 percent received chest-radiograph screening for tuberculosis disease; 56 percent of 18 eligible for latent-tuberculosis-infection treatment started and half completed. It cost $1 per patient to provide HIV information and $5–$8 to offer HIV CTR.
Conclusion
The project increased HIV CTR of close contacts of infectious tuberculosis patients. The important factor for success in knowing contacts’ HIV serostatus was simply for TB program staff to ask about it and offer the test to those who did not know their status.
Keywords: contacts, costs, HIV, tuberculosis
Persons with human immunodeficiency virus (HIV) and Mycobacterium tuberculosis coinfection have approximately a 10-fold greater risk of progression to tuberculosis (TB) disease than HIV-uninfected persons.1–5 TB is an AIDS-defining disease.6 In 2002, a minimum of nine percent of all TB patients in the United States and 16 percent of those aged 25–44 were known to be HIV infected.7 Of patients aged 25–44 in New York City (NYC), 29 percent were known to be HIV infected.
In 2002, Manhattan had the highest percentage of persons living with HIV or AIDS (PLWHA) (1.8% or 1,800 per 100,000) among all boroughs in NYC, and accounted for 30 percent of NYC AIDS diagnoses in the first quarter of 2003.8 Nearly a quarter of those diagnosed with HIV in Manhattan in 2001 first learned that they were HIV infected upon diagnosis with AIDS.9 In 2000 and 2001, Manhattan also had the greatest number of pulmonary TB patients and accounted for 37 percent of HIV-infected TB patients in NYC (New York City Department of Health and Mental Hygiene [NYCDOHMH] unpublished data).
The US Centers for Disease Control and Prevention (CDC) has issued recommendations for voluntary HIV counseling and testing, beginning in 198610 and 198711 for high-risk TB patients and then in 1989 for all TB patients and suspects.12 These guidelines stress the importance of offering HIV counseling and voluntary testing with the provision of on-site services in high HIV prevalence areas by trained and qualified TB program staff or through coordination with HIV programs.13 In 1992, at the height of the US TB/HIV outbreaks, the CDC and the American Thoracic Society (ATS) emphasized the importance of knowing the HIV serostatus of contacts to TB patients as well.6,14 In 2001 and again in 2003, CDC guidelines added that HIV information and prevention counseling, voluntary HIV testing, and referral to medical, prevention, and support services (HIV counseling, testing, referral [CTR]) should be routinely recommended to TB suspects, TB patients, and their contacts.15,16
Contact investigations of TB patients currently rely on the tuberculin skin test (TST) as the critical tool for detecting latent TB infection (LTBI) and as an indicator for evaluating further for TB disease. Immune suppression in HIV-infected contacts reduces TST reactivity.17 A contact who has cutaneous anergy because of HIV infection may have a negative reaction to the TST despite true infection, and may miss being diagnosed with LTBI or disease and receiving treatment. Knowing the HIV serostatus of contacts to TB patients provides an opportunity for identifying or preventing TB because of undetected infection.
For most TB programs, however, provision of HIV CTR for contacts has not been a routine part of contact investigation procedures. In a study of 1996–1997 data from 11 urban TB programs, only 13 percent of 6,225 close contacts had HIV serostatus recorded in their medical charts.18 Of those contacts with known HIV serostatus, 13 percent of the HIV infected were identified with TB disease compared with two percent of the uninfected contacts. The study also found that more than one fourth of those known to be HIV infected were not screened completely for M tuberculosis infection and disease, and a third of eligible HIV-infected contacts started and a sixth completed treatment for LTBI to prevent progression to disease.19 Another study of 1,169 close contacts found similar results, with HIV serostatus known for 19 percent.20 In NYC in 2000, the policy was not explicit regarding routine offering of HIV CTR to all contacts. HIV serostatus was known for only two percent of close contacts, of whom approximately one percent were HIV infected.
The NYCDOHMH TB Bureau implemented a study in conjunction with CDC to offer HIV CTR to close contacts to persons with infectious TB reported in Manhattan, NYC, from December 1, 2002, through November 30, 2003. The goals were to increase HIV CTR and knowledge of HIV serostatus of close contacts, to screen all HIV-infected contacts for TB disease, to prevent progression to TB disease in HIV-infected contacts through LTBI treatment, and to prevent additional AIDS opportunistic infections through referral to and accessing of care for HIV. In this article, we report on the process and results of study implementation, factors associated with increased HIV testing, and the cost of implementing HIV CTR in the program.
Methods
Study population
This study sample included close contacts of all pulmonary TB patients verified in Manhattan. The NYCDOHMH TB Bureau defines a close contact as a person who has had prolonged, intense, or frequent contact with the TB patient, or spent on average eight or more hours per week with the patient during the infectious period. Contacts were eligible for the study if (1) they were close contacts of a patient who had a sputum smear result positive for acid-fast bacilli, culture positive for M tuberculosis from a pulmonary or laryngeal source, or had cavitary lesions on chest radiograph; (2) the index TB patient was verified between December 1, 2002, and November 30, 2003; and (3) they were 13 years of age or older. A close contact younger than 13 was eligible only when the biological mother was the index TB patient and was HIV infected.
Study activities
In 2002, the NYCDOHMH TB Bureau began emphasizing the importance of HIV testing among close contacts. As part of this effort, the NYCDOHMH TB Bureau received CDC funding for this study in June 2002. After finalization of the study protocol in September 2002, the study was approved by the institutional review board (IRB) at the NYCDOHMH and received a determination of not human subjects research by the CDC, since its primary purpose for participating CDC staff was to improve patient management.
Project start-up involved several steps. Study personnel obtained support from all levels of the NYC-DOHMH TB Bureau. HIV CTR policies were revised from providing HIV CTR to contacts aged 18 years or more receiving medical evaluation in a NYCDOHMH chest clinic to offering HIV CTR to all close contacts aged 13 years or more regardless of their TST result. The contact investigation evaluation form was modified to reflect the new HIV CTR policy by adding the following questions:
What is your HIV status?
Do you consider yourself at risk for HIV?
Have you been tested for HIV?
All close contacts were provided HIV information and offered HIV CTR at the time of TST placement by outreach workers in the field. For those accepting HIV CTR, HIV counseling and testing was provided in the clinics following standard NYC HIV/AIDS Service Administration (HASA) procedures, which included risk assessment and completion of standard HIV risk forms sent to HASA. The NYCDOHMH TB Bureau did not collect or use the HIV risk assessment data. TB/HIV educational pamphlets for contacts were developed in four different languages, including English, Spanish, Chinese, and Creole. A “Dear Colleague” letter on the importance of HIV CTR of TB contacts was sent to private physicians who provided treatment of TB or LTBI in NYC. The project was announced in routine meetings attended by administrators and medical physicians in the NYCDOHMH TB Bureau.
To assist staff including field workers with discussing HIV, we developed a script to guide staff in offering HIV testing (Figure 1) and a card that described HIV-risk behaviors. We also provided project staff with a 1-week training in HIV CTR, certified by the NYC-DOHMH. In the clinics and the field office, logs were developed to identify persons not being offered HIV information. Project staff accompanied field workers at the beginning of the project to reensure training and procedures. Procedures of offering HIV CTR were reviewed and reinforced in periodic staff meetings.
FIGURE 1.
HIV counseling, testing, referral script. (TB indicates tuberculosis; TST, tuberculin skin test; and HIV, human immunodeficiency virus.)
The TB case registry provided routinely collected demographic, laboratory, and treatment information on all TB patients and their contacts. A study database was used to track the date, location, and results of HIV CTR, as well as homeless status, history of drug use, and incarceration. Information on the time spent and the number of efforts made to offer HIV CTR was also collected. HIV CTR costs were gathered, including personnel costs for providing HIV information and for offering and providing CTR, and follow-up; laboratory costs for HIV testing; HIV CTR supplies; and transportation costs related to HIV CTR. Data collection was conducted prospectively in person or by telephone.
At the time of TST placement, outreach workers used the predetermined script to educate contacts about the importance of HIV testing, and ask whether they would like to have HIV CTR in a chest clinic. HIV rapid testing was not available at the time this project was conducted. Therefore, contacts agreeing to HIV CTR were given an appointment at an NYCDOHMH TB Bureau chest clinic. Contacts not making an HIV CTR appointment were given a card to use at an NYCDOHMH TB Bureau chest clinic in the future, giving them priority in scheduling for HIV CTR when they returned. If the contact declined the initial HIV CTR offer, study staff reoffered HIV CTR on the date of TST reading, TST placement after the window period, their appointment with the physician, or by telephone, as appropriate. In some situations, home visits were made by study staff to reoffer HIV CTR. For contacts who preferred to receive HIV CTR from their private medical provider, we encouraged the medical provider to offer HIV CTR to the contact. All self-reported HIV results were verified by medical chart review. If a self-reported HIV result was performed within the preceding 12 months, the result was recorded in the study database. Otherwise, we classified the HIV serostatus of non–HIV-infected contacts as unknown if they were not retested. HIV retesting was encouraged. For contacts who were HIV infected, medical charts were reviewed to obtain information on years since HIV diagnosis, results of the most recent CD4+ T-lymphocyte count, and HIV treatment.
Contacts had the option of either confidential or anonymous HIV testing. A contact choosing anonymous HIV counseling and testing was referred to one of the NYCDOHMH clinics, which provide the services for free. Only those who consented to a confidential HIV test were tested by study staff. Blood samples collected in the NYCDOHMH TB Bureau chest clinics were sent to the DOHMH public health laboratories for analysis of HIV antibodies according to existing procedure, which included screening by enzyme immunoassay (EIA) for all specimens and confirmation by Western blot assay for all EIA reactive specimens.
Data management and analysis
Measured outputs included (1) the number and percentage of close contacts provided HIV information and offered HIV CTR, and the number and percentage newly counseled and HIV tested; (2) the number and percentage of HIV-infected contacts screened for TB disease by chest radiograph, and the number and percentage who started and who completed LTBI treatment; and (3) the costs of the project. Linkage to care for HIV-infected persons was also included as one of the outputs.
The major outcome measure of interest was HIV serostatus. Among contacts with known HIV serostatus, newly tested contacts were defined as those in whom HIV testing was performed by project efforts; previously tested were contacts who were HIV tested within the past year by other providers. Those with unknown HIV serostatus were defined as not tested. We conducted qualitative interviews of contacts who were not tested to assess reasons. The characteristics of interest for associations with outcomes were age, race and ethnicity, sex, TST result, result of chest radiograph, relationship of the contact to the index case, and HIV serostatus of the index patient.
Data analysis included descriptive summaries of project outputs and comparisons of newly HIV-tested and previously HIV-tested to not-tested contacts. The associations between characteristics of interest and the outcome of HIV testing were examined using risk ratios (RR). In multivariate analyses, RRs and 95 percent confidence intervals were estimated using log-binomial models.21,22 Variables with P < .25 in the bivariate analyses were examined in the multivariate modeling. Homelessness, drug use, and incarceration were not included in the models because the numbers of observations in those categories were too small. For the model of previous HIV testing, the following variables were collapsed to obtain efficient estimates: race/ethnicity (Asian vs non-Asian), type of contacts (family and leisure vs work site), and contact of HIV-index case (yes vs no).
Data were entered and managed with Microsoft Access 2000 (9.0.3821 SR-1). All data analyses were performed using SAS software (Release 8.2, SAS Institute Inc, Cary, North Carolina). Cost data were analyzed using models set up in Microsoft Excel spreadsheets.
Results
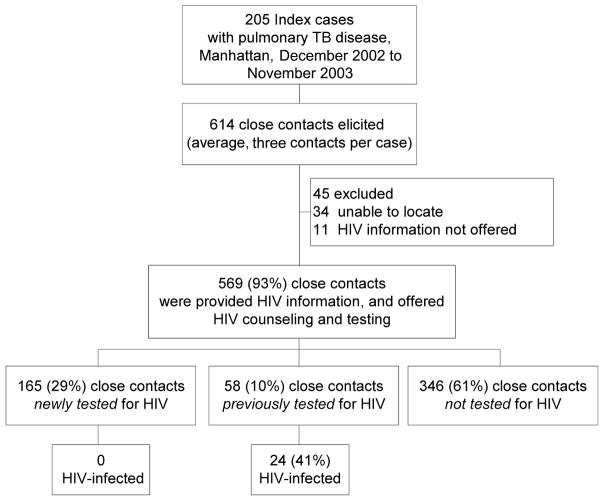
During the project period in Manhattan, 205 patients with infectious pulmonary TB disease were eligible, from whom 614 close contacts were identified and eligible. Figure 2 displays the classification of close contacts and the project outputs.
FIGURE 2.
Classification of study subjects and measured outputs. (TB indicates tuberculosis; HIV, human immunodeficiency virus.)
Knowledge of contacts’ HIV serostatus
Of the 614 contacts, 34 could not be reached; 11 more were reached for TST, but HIV CTR was unintentionally not offered. For the remaining 569 (93%) close contacts, HIV information was provided by project staff and HIV CTR offered to all; 346 (61%) were not tested for HIV, 58 (10%) were previously tested for HIV (24 were HIV infected and 34 were HIV uninfected); and 165 (29%) were newly HIV tested during the project period. HIV testing of contacts by the HIV serostatus of the index case is shown in Table 1. Twenty-four contacts (4%) were HIV infected; all were contacts of HIV-infected index patients and had previously known their HIV serostatus. By asking contacts about their HIV serosta-tus, project staff identified 11 of the 24 HIV-infected contacts who would not have been identified without project procedures. The remaining 13 lived in single-room occupancy hotels funded by HASA and therefore were already known to be HIV infected when the contact investigation was conducted. None of the contacts newly tested for HIV during the project period were found to be HIV infected.
TABLE 1.
Human immunodeficiency virus (HIV) testing and results of close contacts (N = 569) by HIV serostatus of index cases
| Close contact | Index Case
|
Total
|
||||||
|---|---|---|---|---|---|---|---|---|
| HIV-infected
|
HIV-uninfected
|
HIV-unknown
|
||||||
| No. | % | No. | % | No. | % | No. | % | |
| Total close contacts | 112 | 327 | 130 | 569 | ||||
| Previously tested for HIV | 37 | 33.0* | 17 | 5.2 | 4 | 3.1 | 58 | 10.2 |
| Eligible for HIV testing | 75 | 67.0 | 310 | 94.8 | 126 | 96.9 | 511 | 89.8 |
| Accepted and newly tested for HIV† | 25 | 33.3‡ | 115 | 37.1 | 25 | 19.8 | 165 | 32.3 |
| HIV positive from previous test | 24 | 21.4 | 0 | 0.0 | 0 | 0.0 | 24 | 4.2 |
| HIV positive from new test | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
Significantly greater than that of contacts of HIV-uninfected or HIV-unknown index cases (P < .001).
Denominator for percentage was the number of those eligible for HIV testing; otherwise, the number of close contacts, if not indicated.
Significantly greater than that of contacts of HIV-unknown index cases (P < .01). But not different from those of HIV-uninfected index cases (P = .543).
Of close contacts, 346 (61%) were not tested for HIV (Table 2). Half were not tested because they felt they were not at risk for HIV. The reasons for not being tested for HIV in contacts of HIV-infected index cases were not statistically different from those in contacts of HIV-uninfected index cases. Eight (2%) refused HIV testing after completing the counseling session, six stating that they were not at risk for HIV, one from fear of having blood drawn, and one because of not wanting to know the HIV test result.
TABLE 2.
Reasons for not being tested for human immunodeficiency virus (HIV) in close contacts (N = 346) by HIV serostatus of index cases
| Reason | Total
|
Index Case
|
||||||
|---|---|---|---|---|---|---|---|---|
| HIV-infected
|
HIV-uninfected
|
HIV-unknown
|
||||||
| No. | % | No. | % | No. | % | No. | % | |
| Claimed not at risk | 176 | 50.9 | 22 | 44.0* | 86 | 44.1* | 68 | 67.3 |
| Refused, and gave no reasons | 59 | 17.1 | 11 | 22.0* | 38 | 19.5 | 10 | 9.9 |
| No show or lost to follow-up | 19 | 5.5 | 1 | 2.0 | 16 | 8.2* | 2 | 2.0 |
| Recent previous HIV-negative, unverified | 16 | 4.6 | 4 | 8.0 | 5 | 2.6 | 7 | 6.9 |
| Nonrecent previous HIV-negative, unverified | 17 | 4.9 | 4 | 8.0 | 8 | 4.1 | 5 | 5.0 |
| Moved | 9 | 2.6 | 0 | 0.0 | 8 | 4.1 | 1 | 1.0 |
| Phone disconnected after initial contact | 6 | 1.7 | 1 | 2.0 | 5 | 2.6 | 0 | 0.0 |
| Language barrier | 3 | 0.9 | 1 | 2.0 | 2 | 1.0 | 0 | 0.0 |
| Other† | 19 | 5.5 | 4 | 8.0 | 10 | 5.1 | 5 | 5.0 |
| Unknown | 22 | 6.4 | 2 | 4.0 | 17 | 8.7 | 3 | 3.0 |
P< .05 (Pearson’s chi-square test) for comparison to close contacts of cases with unknown HIV serostatus; otherwise, there were no statistically significant differences between groups.
Included the reasons, such as being afraid of needles or blood, waiting for response or partner to be tested, didn’t want to know, and wanted to be tested by private physicians.
In bivariate comparisons to those not tested for HIV, contacts who were newly tested for HIV were more likely to be aged 18–24, Asian, Hispanic, non-Hispanic Black, non–US-born, and, if non–US-born, to have resided in the United States for 5–10 years, to be newly TST-positive, to have received a chest radiograph, or to have started LTBI treatment (Table 3). Contacts of HIV-infected TB patients were not more likely to be newly tested for HIV than contacts of HIV-uninfected index cases. In the multivariate analysis, being newly tested for HIV was significantly associated with age 18–24 years (RRadj = 1.6, 95% CI = 1.2, 2.2), Hispanic ethnicity (RRadj = 3.3, 95% CI = 1.3, 8.8), or being newly TST-positive (RRadj = 2.0, 95% CI = 1.4, 2.7).
TABLE 3.
Unadjusted risk ratio of being tested for HIV associated with demographic characteristics, TST, chest radiograph, and treatment of latent TB infection (TLTBI) in close contacts, Manhattan, New York City (N = 569)*
| Variable | (1) Newly tested (n = 165) | (2) Previously tested (n = 58) | (3) Not tested (n = 346) | (1) vs (3)
|
(2) vs (3)
|
||
|---|---|---|---|---|---|---|---|
| No. | No. | No. | RR | 95% CI | RR | 95% CI | |
| Age, y | |||||||
| <13 | 0 | 4 | 0 | NA | NA | NA | NA |
| 13–17 | 4 | 4 | 14 | 0.8 | 0.3–1.9 | 1.8 | 0.7–4.6 |
| 18–24 | 37 | 8 | 44 | 1.6 | 1.2–2.3 | 1.2 | 0.6–2.6 |
| 25–44 | 71 | 22 | 147 | 1.2 | 0.9–1.6 | 1.0 | 0.6–1.8 |
| >44 | 53 | 20 | 138 | Referent | Referent | ||
| Unknown | 0 | 0 | 3 | NA | NA | NA | NA |
| Sex | |||||||
| Male | 86 | 24 | 174 | 1.1 | 0.8–1.4 | 0.7 | 0.5–1.2 |
| Female | 79 | 34 | 172 | Referent | Referent | ||
| Race/ethnicity | |||||||
| Asian | 46 | 2 | 100 | 3.5 | 1.3–9.1 | 0.2 | 0.04–0.9 |
| Hispanic | 73 | 22 | 93 | 4.8 | 1.9–12.5 | 1.7 | 0.7–4.3 |
| Black, non-Hispanic | 42 | 29 | 112 | 3.0 | 1.1–7.9 | 1.9 | 0.8–4.5 |
| White, non-Hispanic | 4 | 5 | 40 | Referent | Referent | ||
| Other | 0 | 0 | 1 | NA | NA | NA | NA |
| Country of birth | |||||||
| Non–US-born | 133 | 27 | 225 | 1.7 | 1.2–2.4 | 0.5 | 0.3–0.8 |
| US-born | 32 | 31 | 115 | Referent | Referent | ||
| Unknown | 0 | 0 | 6 | NA | NA | NA | NA |
| Time in United States among the non–US-born†, y | |||||||
| <5 | 51 | 8 | 73 | 1.3 | 0.9–1.8 | 1.2 | 0.5–3.1 |
| 5–10 | 41 | 10 | 40 | 1.6 | 1.2–2.3 | 2.5 | 1.0–5.8 |
| >10 | 41 | 8 | 90 | Referent | Referent | ||
| Close contact | |||||||
| Household or family | 36 | 15 | 80 | 1.0 | 0.7–1.4 | 2.4 | 1.1–5.4 |
| Leisure | 77 | 35 | 152 | 1.1 | 0.8–1.4 | 2.9 | 1.4–5.9 |
| Work site | 52 | 8 | 114 | Referent | Referent | ||
| Homelessness when identified | |||||||
| Yes | 0 | 6 | 3 | NA | NA | 5.1 | 3.0–8.6 |
| No | 165 | 52 | 342 | Referent | Referent | ||
| Unknown | 0 | 0 | 1 | NA | NA | NA | NA |
| Ever incarcerated | |||||||
| Yes | 1 | 3 | 1 | 1.3 | 0.3–5.3 | 5.7 | 3.0–10.8 |
| No | 155 | 38 | 252 | Referent | Referent | ||
| Unknown | 9 | 17 | 93 | 0.2 | 0.1–0.4 | 1.2 | 0.7–2.0 |
| Ever used substances | |||||||
| Yes | 0 | 8 | 2 | NA | NA | 5.9 | 3.9–9.1 |
| No | 156 | 39 | 250 | Referent | Referent | ||
| Unknown | 9 | 11 | 94 | 0.2 | 0.1–0.4 | 0.8 | 0.4–1.5 |
| Index case HIV serostatus | |||||||
| Positive | 25 | 37 | 50 | 0.9 | 0.6–1.3 | 5.3 | 3.2–8.9 |
| Negative | 115 | 17 | 195 | Referent | Referent | ||
| Refused | 19 | 3 | 59 | 0.7 | 0.4–1.0 | 0.6 | 0.2–2.0 |
| Unknown | 6 | 1 | 42 | 0.3 | 0.2–0.7 | 0.3 | 0.04–2.1 |
| TST result | |||||||
| Newly positive | 98 | 14 | 117 | 2.1 | 1.6–2.8 | 0.7 | 0.4–1.2 |
| Prior positive | 16 | 9 | 34 | 1.5 | 0.9–2.4 | 1.3 | 0.7–2.6 |
| Negative | 49 | 33 | 177 | Referent | Referent | ||
| Not done/refused | 2 | 2 | 18 | 0.5 | 0.1–1.8 | 0.6 | 0.2–2.5 |
| Chest radiograph among eligible‡ | |||||||
| Done | 113 | 33 | 130 | 10.7 | 1.5–69.8 | 0.8 | 0.4–1.6 |
| Not done | 1 | 7 | 21 | 0.1 | 0.01–0.7 | 1.5 | 0.7–3.1 |
| Started TLTBI among eligible§ | |||||||
| Yes | 87 | 17 | 91 | 3.3 | 1.5–6.9 | 0.6 | 0.3–1.3 |
| No | 6 | 11 | 34 | Referent | Referent | ||
NA = not available.
Of 385 non–US-born contacts, 362 (94%) had information on date of arrival in the United States.
Limited to those eligible for CXR: n = 114 for newly tested, n = 40 for previously tested, and n = 151 for not tested.
Limited to those eligible for TLTBI: n = 93 for newly tested, n = 28 for previously tested, and n = 125 for not tested.
In bivariate analyses of contacts who were previously tested for HIV in comparison with those not tested for HIV, previously tested contacts were more likely to be household members or leisure contacts of the index cases, homeless, ever incarcerated, substance abusers, or contacts to an HIV-infected index case, and were less likely to be Asian or non–US-born. In multivariate analysis, contacts previously tested for HIV were more likely to be household members or leisure contacts of the index cases (RRadj = 2.9, 95% CI = 1.4, 5.7) or contacts to an HIV-infected index case (RRadj = 4.4, 95% CI = 2.7, 7.1), or less likely to be Asian (RRadj = 0.2, 95% CI = 0.04, 0.7).
TB case management of HIV-infected contacts
Nine (1.6%) patients with TB were identified from the 569 close contacts: 8 (4%) of 199 HIV-uninfected and 1 (4%) of 24 HIV-infected contacts. In addition, Table 4 shows TST, chest radiograph results, and starting and completion of treatment for LTBI for HIV-infected close contacts. All 24 HIV-infected contacts were already receiving HIV care prior to the contact investigation. CD4+ T-lymphocyte count was available for 21 HIV-infected contacts. Eighteen (85.7% of 21) had CD4+ T-lymphocyte counts 200/mm3 or more (range: 200–1,221), 3 of whom were newly TST positive, 3 were prior positive, and 12 were TST negative, with 1 newly TST-positive confirmed to have active TB and 3 others having abnormal chest radiographs. Three had CD4+ T-lymphocyte counts fewer than 200/mm3 (ranges: 48–103/mm3), none of whom were newly TST positive, but one had a history of positivity, one was TST negative, and one refused the TST.
TABLE 4.
TB evaluation and treatment of latent TB infection (TLTBI) among HIV-infected contacts (N = 24)*
| Variable | CD4 < 200 (n = 3) | CD4 ≥ 200 (n = 18) | CD4 Unknown (n = 3) |
|---|---|---|---|
| TST result | |||
| Newly positive | 0 | 3 | 0 |
| Newly negative | 1 | 12 | 3 |
| Prior positive | 1 | 3 | 0 |
| Refused | 1 | 0 | 0 |
| Chest radiograph among eligible | |||
| Abnormal | 0 | 4† | 0 |
| Normal | 2 | 10 | 1 |
| Not done | 1 | 4 | 2 |
| Eligible for TLTBI | 2 | 13 | 3 |
| Started TLTBI among eligible | 1 | 8 | 1 |
| Completed TLTBI | 0 | 4 | 1 |
TST indicates tuberculin skin test; TLTBI, treatment of latent tuberculosis infection.
One had active tuberculosis, three had abnormal chest radiographs inconsistent with tuberculosis disease.
Seventy-one percent of the 24 HIV-infected contacts received chest radiographic examination. The remaining 29 percent of HIV-infected contacts who did not receive chest radiographic examination could not be reached or did not keep their appointments. The study did not identify any active TB disease that had gone undetected from the use of the TST as the sole diagnostic tool among known HIV-infected contacts, since the one HIV-infected contact who was diagnosed as a TB patient was TST positive. Thus, the prevention of TB transmission and deaths from undetected TB identified early due to project procedures were not estimated.
Eighteen of the 24 HIV-infected close contacts were eligible for LTBI treatment regardless of TST result according to CDC guidelines, compared with only three who would have been considered for LTBI treatment before the study procedures because they had positive TST results. Ten of 18 eligible HIV-infected contacts started LTBI treatment; 5 completed. The risk of progression to TB disease is estimated at 18 percent over 10 years for untreated HIV-infected contacts.23,* LTBI treatment reduces this risk to approximately five percent over 10 years.24,† Thus, completion of LTBI treatment in the five contacts results in approximately one TB case prevented [18 × 0.18 = 3.24 cases without LTBI treatment minus (5 × 0.05) + (13 × 0.18) = 2.34 cases with LTBI treatment], plus prevention of possible death from TB and secondary cases among contacts to pulmonary TB patients.
Some of the barriers to receiving medical evaluation and LTBI treatment among HIV-infected contacts included (1) contacts’ homelessness that precluded locating them for follow-up medical evaluations and treatment; (2) higher priorities in their lives than TB evaluation; and (3) noncompliance with HIV treatment, since lack of contact with the HIV provider limited access to the patient for TB evaluation and treatment.
Project costs
Variable direct medical costs in 2003 US dollars to implement HIV CTR in Manhattan were calculated on the basis of values of supplies (eg, syringes, gloves, and incentives), laboratory costs of EIA and Western blot HIV tests, and personnel time (salaries plus benefits times the number of minutes per service) to provide HIV information, offer HIV CTR, conduct counseling, perform testing, conduct posttest counseling, and follow up to obtain or provide results.
The total annual variable cost (in 2003 dollars) to implement HIV CTR in Manhattan was $10,361. The cost per contact for each service is as follows. Personnel costs for the provision of HIV information (averaging 2 minutes per patient) were $1 per patient. The offering of HIV CTR averaged $5 for those tested and $8 for those not tested, since more attempts were needed to ascertain a refusal. The provision of HIV pretest counseling cost $14 per patient (for an average of 36 minutes per session). The cost of HIV testing was approximately $24 per patient, including personnel time, laboratory test, and supplies. Posttest counseling cost $7 per patient for an average of 18 minutes. An average of 60 minutes at a cost of $23 per patient was needed for follow-up to provide HIV test results. Averaging all variable costs ($10,361) for the 569 contacts results in a cost of approximately $18 per contact.
Fixed costs included training, transportation, and administrative costs. An automobile was rented for 1 year for the project; its cost was divided by the number of those tested for HIV ($42 each), as transportation for clients to and from the clinic was provided. HIV CTR training was paid for by the Division of HIV Services and provided to 6 project staff members at a value of $1,735. Administrative costs were approximately $286,000 during the project period. If we assume that these administrative costs assisted only in evaluating the HIV CTR effort or provided support to HIV CTR start-up efforts city-wide, then they do not have to be considered part of project implementation costs that would be needed to sustain a continued HIV CTR effort. Likewise, the NYCDOHMH provided training in HIV CTR to staff throughout the city in 2002.
Discussion
This project evaluates the implementation of HIV CTR as a routine component of contact investigation in a TB program setting in the United States. TB programs need to know the HIV serostatus of close contacts to enable optimal TB screening, TB prevention through LTBI treatment, and for additional AIDS prevention through referrals to HIV care. HIV CTR also promotes HIV prevention through provision of HIV information and HIV prevention counseling. Preventing HIV also contributes to TB prevention, as HIV-infected persons are among the highest risk groups for progression to TB if exposed and infected.
The results of this study demonstrate that HIV CTR of close contacts can be done. But, additional efforts are needed to improve the acceptance of HIV CTR. Knowledge of close contacts’ HIV serostatus increased from 2 percent of contacts in 2000 to 39 percent in the 2003 project period. The important factor for success in finding out contacts’ HIV serostatus was simply for TB program staff to ask about it and offer the test to those who did not know their status. By asking for HIV serostatus, staff identified 11 additional contacts who were HIV infected, which guided their timely medical evaluation (including chest radiograph) and placement on LTBI treatment. By offering HIV CTR to those not already known to be HIV infected, project staff newly counseled and HIV tested 32 percent of contacts and potentially prevented new HIV infections. Our experience with this project taught us that successful project implementation required thorough planning to increase the staff confidence level in discussing HIV with contacts, strong support from all levels of program staff, and an effective project team effort.
Factors that affected the acceptance of new HIV CTR in this study varied. The most common reason given for not being tested was not feeling at risk for HIV, which is consistent with other studies.25,26 Contacts who had a positive TST or had a chest radiograph done were more likely to be newly tested for HIV, probably because of the additional staff opportunities to offer HIV testing when clients came to the clinic for further medical evaluation. Reoffering HIV testing, especially at the time of clinic visits, may be an effective way to increase the acceptance of HIV counseling and testing. The project also showed an increase in new HIV counseling and testing among 18- to 24-year-olds and Hispanics or non-Hispanic Blacks, groups that are at high risk for HIV or, in the case of youth, who will soon enter the high-risk group for HIV. Estimated AIDS case rates in 2004 were highest for non-Hispanic Blacks (56 per 100,000), followed by Hispanics (19/100,000 population).27 In 2001, Hispanics and Blacks accounted for almost 70 percent of AIDS cases, but only 25 percent of the US population. It is hoped that, while no new HIV cases were detected, study provision of HIV information and HIV prevention counseling will prevent future HIV infections in these groups.
In this Manhattan setting, the study documented greater rates of previous HIV testing among contacts to HIV-infected index cases, homeless persons, substance abusers, and those who had been incarcerated. Persons who lived in a single-room occupancy hotel for persons with HIV/AIDS, or whose sexual partner or biological parent were HIV infected may have been previously tested for HIV because they were at high risk for HIV infection. Our project found it reassuring that all known HIV-infected contacts were already receiving HIV care, which may have accounted for relatively low TB incidence in this group. Provision of access to HIV care could be a big benefit of similar project activities in other areas, as it was found in a recent study of HIV-infected persons not receiving antiretroviral treatment that 42 percent were unaware they were HIV infected.28
Routine offering of HIV CTR to contacts to TB patients is now recommended by the CDC15 and has been shown to be effective and cost-effective in populations other than contacts of TB patients.29–32 With decreased funding of TB programs, it will be a challenge to sustain the additional efforts required to make HIV CTR a routine part of contact investigation and to evaluate its effects. To sustain this effort, it may be useful to provide staff training needed to reduce staff reluctance to talk about HIV risks and status, provide sample scripts for offering HIV information, streamline procedures for walk-in HIV CTR in the clinics, use “Dear Colleague” letters to enhance participation of private non-DOHMH providers, and adjust data-collection forms to prompt for HIV serostatus. We found that the simple practice of providing HIV information and asking for HIV serostatus uses few resources—$1 per patient for 2 minutes of providing information and asking for status. Offering HIV CTR, at $5–$8 per patient, may possibly have high yields in patients agreeing to new testing. Referrals to HIV programs for counseling and testing might save TB program resources and help HIV programs achieve their goals of testing high-risk populations. Another possible strategy to conserve resources is to prioritize HIV CTR for close contacts of HIV-infected TB patients, since we found that 21 percent of these contacts were HIV infected, compared with no contacts of HIV-uninfected index patients. The use of a social networking approach, in which patients identify social contacts who in turn identify other social contacts, may be an additional effective way to identify HIV-infected contacts, which may or may not be cost-effective compared with other approaches.33,34
There are some limitations of this study. In New York City in 2001, new HIV diagnoses occurred in 0.04 percent of those aged 13–24, 0.16 percent of those aged 25–44, 0.1 percent of those aged 45–64, and 0.01 percent of those aged 65 or more.9 The zero yield of HIV positives among those newly tested is likely due to the relatively small number (n = 165) of persons tested; we did not have the power to detect any new cases. Another study limitation is that we were unable to use a rapid HIV diagnostic test, and thus lost an opportunity to compare acceptance by different HIV testing methods. The use of rapid HIV tests may increase acceptance of HIV testing by as much as 25 percent, as shown in New York state35 and in studies of testing women in labor.36
Despite the limitations, the results of this study on implementation of HIV CTR among close contacts to infectious TB patients will be useful to other TB programs. Further improvements are possible as a result of rapid HIV tests that have become available. Since September 2004, rapid HIV diagnostic tests (with results available within 20 minutes) have been used in health department chest clinics in NYC, resulting in a 30 percent to 40 percent increase in HIV testing (NYC-DOHMH, unpublished data). Rapid HIV tests that use oral fluid specimens will further increase acceptance among those who fear blood draws, eliminate the need for phlebotomists to conduct the testing, and facilitate HIV testing in field settings.37
Among the cohort of contacts, we identified 24 HIV-infected contacts, for prevalence of 4 percent among all offered HIV CTR or of 11 percent among the 223 having known HIV serostatus. It is possible that some HIV-infected contacts were among the 61 percent having unknown HIV status.
The main impact of the project was in increasing HIV CTR by routinely offering it to a population with high HIV prevalence (≥1%), which resulted in provider knowledge of patient HIV infection so that recommended testing for TB disease and treatment for LTBI could take place, regardless of TST result. While we did not identify any HIV-infected persons who did not already know their status, all contacts received HIV information and newly tested HIV-uninfected contacts received HIV prevention counseling. We showed that this program effort was feasible and that providing basic HIV information and asking contacts about their HIV status cost little.
Acknowledgments
This project was funded by the Centers for Disease Control and Prevention and the Tuberculosis Epidemiologic Studies Consortium.
The authors thank Bill Bower and Ken Holley from Charles P. Felton National TB Center at Harlem Hospital for providing HIV training to NYCDOHMH TB Bureau field staff. We also thank NYCDOHMH TB Bureau staff Guy Dorsinville, Elizabeth Drackett, Darrin Taylor, and Ericka Moore for their assistance in project plan development; and Carla Clark for creating an MS Access data entry and data management system. The authors also express great appreciation to all TB Bureau staff who contributed to the success of the project in many ways. Without their support and participation, this project would have not been possible. The authors also thank Drs Andrew Vernon, Phillip LoBue, and Michael F. Iademarco from the US CDC for their critical reviews of the manuscript.
Footnotes
The 18% risk over 10 years for HIV-infected persons developing TB without LTBI treatment was calculated on the basis of unpublished data from the Markowitz study23 (N. Hansen, personal communication, 5 years of annual data, August 2002) projected over 10 years. Note: Since this study was conducted prior to HAART, it does not include reductions in TB incidence due to HAART.
The 5% risk over 10 years for HIV-infected persons developing TB after 9 months of isoniazid was calculated on the basis of unpublished data from the Gordin international trial of 12 months of isoniazid24 (J. Matts, personal communication, 5 years of annual data, May 2002) projected over 10 years. Note: It is assumed that the risk of TB with 9 months of isoniazid is equivalent to the risk with 12 months, based on studies done by Dr George Comstock.
Contributor Information
Jiehui Li, Deputy Director of Epidemiology, Bureau of Tuberculosis Control, New York City Department of Health and Mental Hygiene, New York.
Suzanne M. Marks, Epidemiologist of the Division of TB Elimination, Centers for Disease Control and Prevention, Atlanta, Georgia.
Cynthia R. Driver, Director of Epidemiology, Bureau of Tuberculosis Control, New York City Department of Health and Mental Hygiene, New York. She is currently Director of Surveillance and Epidemiology, Diabetes Prevention and Control Program, New York City Department of Health and Mental Hygiene, New York.
Francisco A. Diaz, Epidemiologist at the Bureau of Tuberculosis Control, New York City Department of Health and Mental Hygiene, New York. He is currently with New York State Department of Health AIDS Institute.
Alejandro F. Castro, III, Epidemiologist at the Bureau of Tuberculosis Control, New York City Department of Health and Mental Hygiene, New York. He is currently with Johns Hopkins University, Bloomberg School of Public Health.
Annette Figueroa de Regner, Research Assistant at the Bureau of Tuberculosis Control, New York City Department of Health and Mental Hygiene, New York.
Aurelia E. Gibson, Senior Public Health Advisor at the Bureau of Tuberculosis Control, New York City Department of Health and Mental Hygiene, New York.
Kinta Dokubo-Okereke, Senior Public Health Advisor at the Bureau of Tuberculosis Control, New York City Department of Health and Mental Hygiene, New York.
Sonal S. Munsiff, Director of the Bureau of Tuberculosis Control, and Assistant Commissioner of the New York City Department of Health and Mental Hygiene, New York.
References
- 1.Selwyn PA, Hartel D, Lewis VA, et al. A prospective study of the risk of tuberculosis among intravenous drug users with human immunodeficiency virus infection. N Engl J Med. 1989;320:545–550. doi: 10.1056/NEJM198903023200901. [DOI] [PubMed] [Google Scholar]
- 2.Guelar A, Gatell JM, Verdejo J, et al. A prospective study of the risk of tuberculosis among HIV-infected patients. AIDS. 1993;7:1345–1349. doi: 10.1097/00002030-199310000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Markowitz N, Hansen NI, Hopewell PC, et al. Incidence of tuberculosis in the United States among HIV-infected persons. The pulmonary complications of HIV Infection Study Group. Ann Intern Med. 1997;126:123–132. doi: 10.7326/0003-4819-126-2-199701150-00005. [DOI] [PubMed] [Google Scholar]
- 4.Daley CL, Hahn JA, Moss AR, et al. Incidence of tuberculosis in injection drug users in San Francisco: impact of anergy. Am J Respir Crit Care Med. 1998;157:19–22. doi: 10.1164/ajrccm.157.1.9701111. [DOI] [PubMed] [Google Scholar]
- 5.Cohn DL, El-Sadr WM. Treatment of latent tuberculosis infection. In: Reichman LB, Hershfield E, editors. Tuberculosis: A Comprehensive International Approach. 2. New York: Marcel Dekker Inc; 2000. pp. 471–502. [Google Scholar]
- 6.Centers for Disease Control and Prevention. 1993 Revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep. 1992;41(RR-17):1–19. [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. Reported Tuberculosis in the United States, 2003. Atlanta Ga: US Department of Health and Human Services, CDC; 2004. [Google Scholar]
- 8.New York City Department of Health and Mental Hygiene. HIV Epidemiology Program: 1st Quarter Report. 2004. [Google Scholar]
- 9.Centers for Disease Control and Prevention. Implementation of named HIV reporting—New York City, 2001. MMWR Morb Mortal Wkly Rep. 2004;52(51):1248–1252. [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. Diagnosis and management of mycobacterial infection and disease in persons with human T-lymphotropic virus type III/lymphadenopathy-associated virus infection. MMWR Morb Mortal Wkly Rep. 1986;35:448–452. [PubMed] [Google Scholar]
- 11.American Thoracic Society and Centers for Disease Control and Prevention. Mycobacterioses and the acquired immunodeficiency syndrome. Joint position paper of the American Thoracic Society and the Centers for Disease Control. Am Rev Respir Dis. 1987;136:492–496. doi: 10.1164/ajrccm/136.2.492. [DOI] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention. Tuberculosis and human immunodeficiency virus infection: recommendations of the Advisory Committee for the Elimination of Tuberculosis (ACET) MMWR Morb Mortal Wkly Rep. 1989;38:236–250. [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention. Essential components of a tuberculosis prevention and control program. Recommendations of the Advisory Council for the Elimination of Tuberculosis. MMWR Recomm Rep. 1995;44:1–16. [PubMed] [Google Scholar]
- 14.American Thoracic Society. Control of tuberculosis in the United States. Am Rev Respir Dis. 1992;146:1623–1633. doi: 10.1164/ajrccm/146.6.1623. [DOI] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. Revised guidelines for HIV counseling, testing, and referral. MMWR Recomm Rep. 2001;50:1–57. [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention. Advancing HIV prevention: new strategies for a changing epidemic—United States, 2003. MMWR Morb Mortal Wkly Rep. 2003;52:329–332. [PubMed] [Google Scholar]
- 17.Markowitz N, Hansen NI, Wilcosky TC, et al. Tuberculin and anergy testing in HIV-seropositive and HIV-seronegative persons. Pulmonary complications of HIV Infection Study Group. Ann Intern Med. 1993;119:185–193. doi: 10.7326/0003-4819-119-3-199308010-00002. [DOI] [PubMed] [Google Scholar]
- 18.Marks SM, Taylor Z, Qualls NL, et al. Outcomes of contact investigations of infectious tuberculosis patients. Am J Respir Crit Care Med. 2000;162:2033–2038. doi: 10.1164/ajrccm.162.6.2004022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention. Missed opportunities for prevention of tuberculosis among persons with HIV infection–selected locations, United States, 1996–1997. MMWR Morb Mortal Wkly Rep. 2000;49:685–687. [PubMed] [Google Scholar]
- 20.Reichler MR, Bur S, Reves R, et al. Results of testing for human immunodeficiency virus infection among recent contacts of infectious tuberculosis cases in the United States. Int J Tuberc Lung Dis. 2003;7:S471–S478. [PubMed] [Google Scholar]
- 21.Greenland S. Model-based estimation of relative risks and other epidemiologic measures in studies of common outcomes and in case-control studies. Am J Epidemiol. 2004;160:301–305. doi: 10.1093/aje/kwh221. [DOI] [PubMed] [Google Scholar]
- 22.Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol. 2005;162:199–205. doi: 10.1093/aje/kwi188. [DOI] [PubMed] [Google Scholar]
- 23.Markowitz N, Hansen NI, Hopewell PC, et al. Incidence of tuberculosis in the United States among HIV-infected persons. Ann Intern Med. 1997;126:123–132. doi: 10.7326/0003-4819-126-2-199701150-00005. [DOI] [PubMed] [Google Scholar]
- 24.Gordin F, Chaisson RE, Matts JP, et al. Rifampin and pyrazinamide vs isoniazid for prevention of tuberculosis in HIV-infected persons: an international randomized trial. JAMA. 2000;283(11):1445–1450. doi: 10.1001/jama.283.11.1445. Terry Beirn Community Programs for Clinical Research on AIDS, the Adult AIDS Clinical Trials Group, the Pan American Health Organization, and the Centers for Disease Control and Prevention Study Group. [DOI] [PubMed] [Google Scholar]
- 25.Kellerman SE, Lehman JS, Lansky A, et al. HIV testing within at-risk populations in the United States and the reasons for seeking or avoiding HIV testing. J Acquir Immune Defic Syndr. 2002;31:202–210. doi: 10.1097/00126334-200210010-00011. [DOI] [PubMed] [Google Scholar]
- 26.Aragon R, Kates J, Hoff T. The AIDS Epidemic at 20 Years: The View From America. Menlo Park, Calif: Kaiser Family Foundation; 2001. [Google Scholar]
- 27.Centers for Disease Control and Prevention. HIV/AIDS Surveillance Report, 2004. Vol. 16. Atlanta, Ga: US Department of Health and Human Services, Centers for Disease Control and Prevention; 2005. p. 14. [Google Scholar]
- 28.Teshale E, Kamimoto L, Harris N, et al. Estimated number of HIV-infected persons eligible for and receiving HIV antiretroviral therapy, 2003–United States [Section 42, #167]. Paper presented at: The 12th Conference on Retroviruses and Opportunistic Diseases; February 25, 2005; Boston, Mass. [Google Scholar]
- 29.Phillips KA, Fernyak S. The cost-effectiveness of expanded HIV counseling and testing in primary care settings: a first look. AIDS. 2000;14:2159–2169. doi: 10.1097/00002030-200009290-00013. [DOI] [PubMed] [Google Scholar]
- 30.Walensky RP, Weinstein MC, Kimmel AD, et al. Routine in-patient HIV testing: a clinical and economic evaluation of national guidelines. Abstract book of the 2003 National HIV Prevention Conference; July 27–30, 2003; Atlanta, Ga. Abstract T3-E1102. [Google Scholar]
- 31.Paltiel AD, Weinstein MC, Kimmel AD, et al. Expanded screening for HIV in the United States—an analysis of cost-effectiveness. N Engl J Med. 2005;352:586–595. doi: 10.1056/NEJMsa042088. [DOI] [PubMed] [Google Scholar]
- 32.Walensky RP, Losina E, Malatesta L, et al. Effective HIV case identification through routine HIV screening at urgent care centers in Massachusetts. Am J Public Health. 2005;95:71–73. doi: 10.2105/AJPH.2003.031310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Latkin CA, Knowlton AR. Micro-social structural approaches to HIV prevention: a social ecological perspective. AIDS Care. 2005;(suppl 1):S102–S113. doi: 10.1080/09540120500121185. [DOI] [PubMed] [Google Scholar]
- 34.Amirkhanian YA, Kelly JA, McAuliffe TL. Identifying, recruiting, and assessing social networks at high risk for HIV/AIDS: methodology, practice, and a case study in St Petersburg, Russia. AIDS Care. 2005;17(1):58–75. doi: 10.1080/09540120412331305133. [DOI] [PubMed] [Google Scholar]
- 35.Branson BM, Burstein G. Retarding HIV prevention: initial results from OraQuick postmarketing surveillance. Paper presented at: Centers for Disease Control and Prevention, Division of HIV/AIDS Prevention; August 2003; Atlanta, Ga. [Google Scholar]
- 36.Bulterys M, Jamieson DJ, O’Sullivan MJ, et al. Rapid HIV-1 testing during labor: a multicenter study. JAMA. 2004;292:219–223. doi: 10.1001/jama.292.2.219. [DOI] [PubMed] [Google Scholar]
- 37.U.S. Food and Drug Administration. Tests waived by FDA from January 2000 to present. 2005 Available at: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfClia/testswaived.cfm.