Abstract
Context
Tuberculosis (TB) rates among US homeless persons cannot be calculated because they are not included in the US Census. However, homelessness is often associated with TB.
Objectives
To describe homeless persons with TB and to compare risk factors and disease characteristics between homeless and nonhomeless persons with TB.
Design and Setting
Cross-sectional analysis of all verified TB cases reported into the National TB Surveillance System from the 50 states and the District of Columbia from 1994 through 2003.
Main Outcome Measures
Number and proportion of TB cases associated with homelessness, demographic characteristics, risk factors, disease characteristics, treatment, and outcomes.
Results
Of 185 870 cases of TB disease reported between 1994 and 2003, 11 369 were among persons classified as homeless during the 12 months before diagnosis. The annual proportion of cases associated with homelessness was stable (6.1%–6.7%). Regional differences occurred with a higher proportion of TB cases associated with homelessness in western and some southern states. Most homeless persons with TB were male (87%) and aged 30 to 59 years. Black individuals represented the highest proportion of TB cases among the homeless and nonhomeless. The proportion of homeless persons with TB who were born outside the United States (18%) was lower than that for non-homeless persons with TB (44%). At the time of TB diagnosis, 9% of homeless persons were incarcerated, usually in a local jail; 3% of nonhomeless persons with TB were incarcerated. Compared with nonhomeless persons, homeless persons with TB had a higher prevalence of substance use (54% alcohol abuse, 29.5% noninjected drug use, and 14% injected drug use), and 34% of those tested had coinfection with human immunodeficiency virus. Compared with nonhomeless persons, TB disease in homeless persons was more likely to be infectious but not more likely to be drug resistant. Health departments managed 81% of TB cases in homeless persons. Directly observed therapy, used for 86% of homeless patients, was associated with timely completion of therapy. A similar proportion in both groups (9%) died from any cause during therapy.
Conclusions
Individual TB risk factors often overlap with risk factors for homelessness, and the social contexts in which TB occurs are often complex and important to consider in planning TB treatment. Nevertheless, given good case management, homeless persons with TB can achieve excellent treatment outcomes.
Numerous tuberculosis (TB) outbreaks have originated among homeless persons in the United States.1–4 Homelessness is associated with an increased risk of exposure to Mycobacterium tuberculosis,5 undetected and untreated infection, and subsequent progression to TB disease.6 Homeless persons with TB are more likely to be hospitalized, and to be hospitalized longer, than nonhomeless persons with TB; the public sector pays most of those costs.7
Better understanding of the characteristics of homeless persons with TB disease is important for creating strategies to reduce TB incidence in this high-risk population in the United States. In 1993, the Centers for Disease Control and Prevention (CDC) standardized national monitoring of TB disease among homeless persons by asking health departments to indicate whether annually reported TB cases occurred in homeless persons. Homeless status was reported incompletely (ie, 70%) that first year but increased to at least 90% each subsequent year. Thus, 1994 through 2003 represents the first full decade of national TB surveillance that includes an assessment of homelessness. This article describes homeless TB patients and compares risk factors and disease characteristics between homeless and nonhomeless TB patients from 1994–2003.
METHODS
The US Census Bureau does not enumerate the national homeless population, so we were unable to use TB surveillance data to determine disease rates among homeless persons. Instead, we calculated the proportion of all reported TB cases that occurred in homeless persons.
Health departments have been reporting all TB cases into the US National TB Surveillance System at the CDC since 1953. The system expanded in 1993 to permit collection of additional information (eg, homeless status, human immunodeficiency virus [HIV] status, anti-TB drug resistance) and has not changed since then. Cases must meet the surveillance case definition (ie, laboratory or clinical evidence of active disease due to M tuberculosis complex) to be included in the national count.8 Standard case reports to the surveillance system also indicate whether patients have a previous diagnosis of TB disease (ie, >12 months before current diagnosis) but not history of vaccinations, latent TB infection, or any TB treatment. Molecular epidemiology results are not currently incorporated.
Our analysis included all cases reported from the 50 states and the District of Columbia from January 1, 1994, through December 31, 2003, based on reports submitted to the CDC as of March 30, 2004. In case reports, homelessness is defined by any of the criteria specified by the McKinney Act (ie, the lack of regular access to a conventional, nontemporary nighttime residence) during the 12 months before diagnosis.8,9 Case reports also note whether persons were residents of a correctional facility on the day of their TB diagnosis. Therefore, no one incarcerated continuously for more than 12 months could also be reported as homeless, but more recently incarcerated persons might be reported as both homeless and incarcerated.
Homeless and nonhomeless persons with TB were compared on the basis of demographic characteristics, including sex, age, country of birth, and self-reported ethnicity/race. Stratifying TB incidence by ethnicity/race is valuable because disparities in TB case rates have persisted.10
We examined self-reported excessive alcohol use and any unprescribed drug use, either injected (ie, intravenous, subcutaneous, or intramuscular) or noninjected (ie, ingested, inhaled, or smoked), during the 12 months before TB diagnosis. We calculated prevalence ratios (PRs) with 95% confidence intervals (CIs) to compare substance use between homeless and nonhomeless persons with TB.11 Because the age distribution of homeless patients was centered, we adjusted for the effect of age by recalculating each PR with the subset of persons aged 30 to 59 years.
All states reported positive HIV test results, and every state except California also reported negative results to the CDC. After excluding California and including only reports with HIV test results (ie, positive, negative, or indeterminate) in the denominator, we calculated the prevalence of documented TB-HIV coinfection.
We examined the infectiousness of TB disease by ascertaining the frequency of pulmonary disease that was also culture-confirmed or had a positive sputum smear for acid-fast bacilli.12 In addition, the frequency of cavitary pulmonary disease, a sign of advanced TB, was reviewed.
We noted differences in the susceptibility of initial cultures to first-line anti-TB medications (ie, isoniazid, rifamycins, pyrazinamide, and ethambutol). Primary isoniazid resistance was defined as resistance to isoniazid, and primary multidrug-resistant TB was defined as resistance to at least isoniazid and rifampin in patients without a previous history of TB.8 We also examined drug resistance patterns among patients with a previous TB diagnosis. Because drug-resistant TB strains can be associated with overseas TB treatment, we stratified patients with previous TB into those born inside or outside the United States and recalculated for each stratum the prevalence of drug resistance by homeless status.
Treatment guidelines for TB did not substantially change during the 10-year period of this study. For evaluating treatment and outcomes, analysis was restricted to 1994–2001 because health departments continue to collect and submit those data for up to 2 years after reporting a case. We noted who provided care (ie, health department, private clinician, or both) and type of treatment (ie, whether patient received a recommended initial 4-drug anti-TB regimen and/or directly observed therapy [DOT]).13 For all patients who began an anti-TB drug regimen, we examined whether treatment was eventually completed (regardless of length of time). The Healthy People 2010 initiative of the US Department of Health and Human Services sets the goal that 90% of TB patients will complete therapy within 1 year.14 Using previously described criteria for evaluating the timeliness of treatment completion,8 we excluded all patients who died or whose therapy might be reasonably expected to take longer (eg, rifampin resistance).
Analysis of the deidentified data from the National TB Surveillance System was performed with SAS 8.02 (SAS Institute, Cary, NC) and Microsoft Excel 2002 (Microsoft, Redmond, Wash). There was greater than 85% completeness of reporting on each analyzed variable except for HIV status. The χ2 test for comparisons between the homeless and nonhomeless groups consistently yielded P values that were ≤.01 (as would be expected given the data set size), so those are not individually listed.
RESULTS
Reported TB Cases
Patients were categorized as either homeless or nonhomeless in 178 517 (96%) of the 185 870 total TB case reports sent to the CDC from the 50 states and the District of Columbia for 1994 through 2003. The 7353 reports missing information on homeless status were excluded from all subsequent analyses.
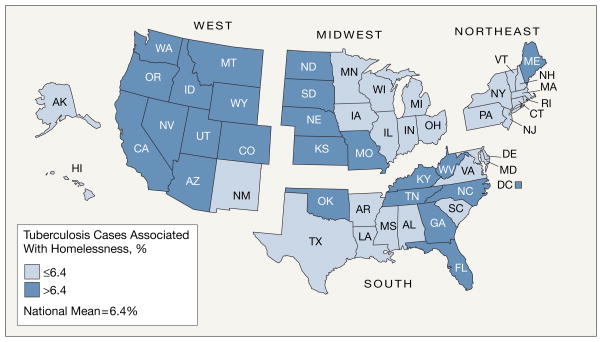
The annual number of cases of TB reported to occur in homeless persons decreased from 1392 in 1994 to 913 in 2003, while the total number of TB cases decreased from 24 205 to 14 874. The proportion of TB cases associated with homelessness was stable, ranging annually from 6.1% to 6.7% (overall mean: 6.4%). Regional differences were apparent, with western and some southern states reporting a higher proportion of cases associated with homelessness (Figure).
Figure.
Proportion of Tuberculosis Cases Associated With Homelessness, United States, 1994–2003
Demographic Characteristics
Characteristics of patients with TB according to homeless status during the 12 months before diagnosis are summarized in the Table. Males accounted for 87% of TB cases in homeless persons during the 1994–2003 period. This proportion was stable over time. Among nonhomeless TB patients, male predominance was less marked (61%). The age distribution of homeless persons at the time of TB diagnosis was centered (ie, 82% of persons were aged 30–59 years), with a slight aging trend from a median age of 41 years in 1994 to 46 years in 2003. Unsurprisingly, the age distribution of nonhomeless TB patients was wider (ie, 47% were aged 30–59 years), with the median essentially unchanged (44–45 years) during the 10-year period.
Table.
Characteristics of Tuberculosis (TB) Patients and Disease, by Homeless Status, 1994–2003
| Characteristic | No. (%) | |
|---|---|---|
| Homeless Patients (n = 11 369) | Nonhomeless Patients (n = 167 148) | |
| Male sex | 9834 (86.5) | 101 752 (60.9) |
| Age, y* <30 |
1080 (9.5) | 39 586 (23.7) |
| 30–39 | 2824 (24.8) | 28 836 (17.3) |
| 40–49 | 4067 (35.8) | 28 206 (16.9) |
| 50–59 | 2402 (21.1) | 21 168 (12.7) |
| ≥60 | 995 (8.8) | 49 326 (29.5) |
| Birth outside the United States | 2009 (17.7) | 72 866 (43.6) |
| Ethnicity/race Hispanic |
2223 (19.6) | 38 464 (23.0) |
| White | 3400 (29.9) | 39 577 (23.7) |
| Black | 5091 (44.8) | 51 366 (30.7) |
| American Indian | 358 (3.1) | 2088 (1.2) |
| Asian | 250 (2.2) | 33 690 (20.2) |
| All other, including multiple and unknown | 47 (0.4) | 1963 (1.2) |
| Incarcerated at time of TB diagnosis | 1032 (9.1) | 5228 (3.1) |
| Excessive alcohol use | 6121 (53.8) | 19 545 (11.7) |
| Injected drug use | 1549 (13.6) | 3385 (2.0) |
| Noninjected drug use | 3352 (29.5) | 8830 (5.3) |
| HIV test result reported† | 6608 (76.0) | 69 895 (52.8) |
| Positive result‡ | 2223 (33.6) | 14 221 (20.3) |
| Pulmonary disease | 10 576 (93.0) | 134 706 (80.6) |
| Culture-positive case§ | 9484 (89.7) | 109 727 (81.5) |
| Sputum smear obtained§ | 9987 (94.4) | 113 310 (84.1) |
| Smear-positive TB|| | 6018 (60.3) | 58 109 (51.3) |
| Cavitary disease§ | 3049 (28.8) | 33 096 (24.6) |
| No previous diagnosis of TB¶ | 10 420 (91.7) | 156 984 (93.9) |
| Culture confirmation# | 9221 (88.5) | 124 967 (79.6) |
| Anti-TB drug susceptibility results** | 8833 (95.8) | 118 021 (94.4) |
| Primary isoniazid resistance†† | 540 (6.1) | 8918 (7.6) |
| Primary multidrug resistance†† | 79 (0.9) | 1467 (1.2) |
| Previous diagnosis of TB¶ | 814 (7.2) | 8553 (5.1) |
| Culture confirmation (current episode)# | 700 (86.0) | 6798 (79.5) |
| Anti-TB drug susceptibility results** | 661 (94.4) | 6399 (94.1) |
| Isoniazid resistance, current episode†† | 48 (7.3) | 987 (15.4) |
| Multidrug resistance, current episode†† | 18 (2.7) | 341 (5.3) |
Abbreviation: HIV, human immunodeficiency virus.
Age distribution excludes 1 homeless patient and 26 nonhomeless patients whose ages were unknown.
Number and percentage exclude 2674 homeless patients and 34 880 nonhomeless patients who were residents of California.
Percentage is based on number of non-California cases with an HIV test result reported (provided in above row).
Percentage is based on total pulmonary disease cases (provided in above row).
Percentage is based on number of pulmonary cases with a sputum smear obtained (provided in above row).
Numbers exclude 135 (1.2%) homeless and 1611 (1.0%) nonhomeless persons for whom it was unknown whether there was a prior diagnosis of TB.
Percentage is based on total number of cases in that category (provided in above row).
Number is based on susceptibility results for at least isoniazid. Percentage is based on number of culture-confirmed TB cases in that category (provided in above row).
Percentage is based on number of culture-confirmed TB cases with drug susceptibility results in that category (provided in above row). Trends were similar after stratifying for country of birth, except for primary isoniazid resistance among US-born persons, which was 5.3% (383 of 7258) among homeless vs 4.8% (3127 of 64 974) among nonhomeless.
Among all reported TB cases, a higher proportion of nonhomeless were born outside the United States (44% vs 18%). However, persons born outside the United States accounted for a growing proportion of TB among homeless persons (from 14% in 1994 to 23% in 2003), with a mean of 11 years since immigration. Mexico was the country of birth for 47% of the homeless persons with TB who were born outside the United States.
Among all reported cases of TB disease during the study period, black individuals represented the highest proportions among the homeless and nonhomeless. Among homeless TB patients from 1994–2003, the annual number who were white declined from 415 to 254. Outside the South, the annual number who were black also decreased from 450 to 155, but numbers in the southern states remained constant (ie, 207 blacks in 1994 and 2003). During this period, the annual number of Hispanic homeless patients with TB decreased from 256 to 215, although their number as a proportion of total homeless TB patients increased from 18% to 24%.
TB Risk Factors
Nine percent of the TB cases (n=1032) associated with homelessness were diagnosed in persons who were residents of correctional facilities on the day of their TB diagnosis. The type of facility was usually a local jail (83%), with a smaller proportion in a state or federal prison (13%). Three percent (n=5228) of nonhomeless persons with TB were incarcerated on the day of their diagnosis.
The prevalence of excessive alcohol use was 4.6 times greater among homeless (54%) than among nonhomeless (12%) persons with TB (95% CI, 4.5–4.7). Homeless TB patients also had a higher prevalence of noninjected (29.5% vs 5%; PR, 5.6; 95% CI, 5.4–5.8) and injected (14% vs 2%; PR, 6.7; 95% CI, 6.4–7.1) drug use. All PR point estimates remained greater than 3 when this comparison was restricted to patients aged 30 to 59 years.
Homeless TB patients during 1994–2003 were more likely than nonhomeless TB patients to obtain an HIV test (76% vs 53%). Among TB patients with HIV test results reported into the National TB Surveillance System (California excluded), TB-HIV coinfection was higher among homeless (34%) than nonhomeless (20%) patients. A difference persisted (35% vs 29%) when analysis was limited to the 30- to 59-year-old age group.
Disease Characteristics
Compared with nonhomeless patients, homeless patients were more likely to have pulmonary disease (93% vs 81%), and those with pulmonary disease were more likely to have culture-confirmed or smear-positive disease. The prevalence of cavitary pulmonary disease was also slightly higher among homeless persons (29% vs 25%). However, neither homeless persons with a primary episode of TB nor homeless persons with a previous diagnosis of TB were more likely than nonhomeless persons to have a drug-resistant strain of M tuberculosis. Although homeless persons were more likely to have a previous history of TB, nonhomeless persons with a previous history of TB were about twice as likely to be infected with a drug-resistant strain 5.3% vs 2.7%), even after controlling for country of birth.
TB Treatment and Outcomes
Health departments managed 81% of the TB cases associated with homelessness during 1994–2001. Most homeless TB patients received an initial regimen of 4 anti-TB drugs (81%) and were managed with DOT (86%).
Fewer homeless (77%) than non-homeless (84%) TB patients eventually completed TB treatment. A similar proportion in both groups died during therapy (9%), but more homeless prematurely stopped treatment for reasons such as moving away or being lost to follow-up (12%, in contrast to 7% of nonhomeless). However, among homeless patients, those who received DOT were 1.4 times (95% CI, 1.3–1.5) more likely to complete therapy in 1 year or less, compared with those who did not receive DOT.
COMMENT
Homelessness has persistently cultivated a “pocket of endemicity” for TB,15 which continues to be associated with poverty.16,17 Although the number of TB cases associated with homelessness during the 1994–2003 decade decreased, certain states had higher proportions of TB cases associated with homelessness, and the annual number of black homeless patients with TB in southern states was unchanged.
Of note, risk factors for TB overlap with many of the risk factors associated with persistent homelessness (eg, being male, or having a history of incarceration or substance abuse).18 Excessive alcohol and illicit drug use may have importance at several points across the infection-to-disease continuum. For example, social settings in which alcohol or drugs are shared may facilitate transmission of M tuberculosis.19,20 Alcohol17,21 and inhaled22,23 or injected23 drugs enhance susceptibility to respiratory tract infections. Even in the pre-HIV era, drug dependency was associated with more rapid progression to TB disease.24 Untreated HIV infection remains an important risk factor for TB.6,12 Testing for HIV is recommended for all homeless shelter clients25; new rapid tests, with results available within 20 minutes, can be done in nonmedical settings.26
Our surveillance system could not capture all the variables of interest for understanding the complex social contexts in which TB often occurs. For example, we were unable to examine the relationship of socioeconomic indicators such as education and income variables with homeless status or ethnicity/ race. In addition, psychiatric disorders are frequent among homeless persons,16,18,27 but we had no data to assess the prevalence of mental health problems among homeless TB patients. These are important considerations, not only in understanding the epidemiology of TB, but also in planning its treatment, among homeless persons.
The most urgent priority for controlling TB in the United States is interrupting new transmission of M tuberculosis.28 Opportunities for transmission arise when homeless persons with infectious TB frequent homeless shelters,1–4 emergency departments,29 and jails.2,30 Locations in a particular community where homeless persons typically congregate are appropriate places to focus resources to prevent, identify, and treat TB. Nine percent of homeless TB patients were residents of a correctional facility on the day of their TB diagnosis. The burden of TB that is associated with the incarceration of homeless persons is likely much higher, but no further incarceration history is recorded for TB surveillance purposes. The 1996 National Survey of Homeless Assistance Providers and Clients found that 49% of homeless persons reported at least 1 incarceration of at least 5 days in a jail, and 18% reported incarceration in a prison, at some point during their lifetime.27 Although a history of incarceration is a well-documented risk factor for TB,6,31 it might also be regarded as a unique opportunity for more outreach in this hard-to-reach population. Local public health staff could collaborate with correctional facility staff (ie, for staff training, information sharing, and discharge planning) to initiate supervised treatment of latent TB infection and TB disease.32
We had no means to determine the extent to which homeless persons experienced delays in obtaining an evaluation for TB, but the slightly higher prevalence of cavitary pulmonary TB suggests more advanced disease at the time of diagnosis. Once diagnosed, however, homeless TB patients received good case management, including laboratory diagnostic evaluation, appropriate use of a 4-drug regimen, and excellent treatment outcomes for persons given DOT (recommended for all TB patients). Controlling this public health problem demands considerable resources but is integral to responding to the Institute of Medicine’s call to eliminate TB in the United States.28
Acknowledgments
Funding/Support: All financial and material support for the design and conduct of this study; collection, management, analysis, and interpretation of the data; and preparation, review, and approval of the manuscript were provided by the Division of Tuberculosis Elimination at the Centers for Disease Control and Prevention. All the authors are full-time employees of the US Department of Health and Human Services. Analysis of data in the National TB Surveillance System is part of the authors’ usual work duties.
We gratefully acknowledge the staff from local and state health departments throughout the United States who have collected and reported data about TB cases for more than 50 years. We also thank Mar-tha Burt, PhD, for assistance with understanding the National Survey of Homeless Assistance Providers and Clients; Robert H. Pratt, BSIE, for support with SAS coding; the National TB Surveillance System Analytic Steering Committee for guidance with study objectives and design; and Jose Becerra, MD, MPH, Lauren Lambert, MPH, and Ann Lanner, BA, for review of the manuscript.
Footnotes
Financial Disclosures: None reported.
Previous Presentation: Presented in part at the 132nd Annual Meeting of the American Public Health Association, Washington, DC, November 6–10, 2004.
Author Contributions: Ms Haddad had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Haddad, Wilson, Ijaz, Marks.
Acquisition of data: Wilson, Moore.
Analysis and interpretation of data: Haddad, Wilson, Marks.
Drafting of the manuscript: Haddad, Wilson.
Critical revision of the manuscript for important intellectual content: Haddad, Wilson, Ijaz, Marks, Moore.
Statistical analysis: Haddad, Wilson, Marks.
Administrative, technical, or material support: Wilson, Moore.
Study supervision: Ijaz.
References
- 1.Centers for Disease Control and Prevention. Tuberculosis outbreak among homeless persons—King County, Washington, 2002–2003. MMWR Morb Mortal Wkly Rep. 2003;52:1209–1210. [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Tuberculosis outbreak in a homeless population—Portland, Maine, 2002–2003. MMWR Morb Mortal Wkly Rep. 2003;52:1184–1185. [PubMed] [Google Scholar]
- 3.Curtis AB, Ridzon R, Novick LF, et al. Analysis of Mycobacterium tuberculosis transmission patterns in a homeless shelter outbreak. Int J Tuberc Lung Dis. 2000;4:308–313. [PubMed] [Google Scholar]
- 4.Lathan M, Mukasa LN, Hooper N, et al. Cross-jurisdictional transmission of Mycobacterium tuberculosis in Maryland and Washington, D.C., 1996–2000, linked to the homeless. Emerg Infect Dis. 2002;8:1249–1251. doi: 10.3201/eid0811.020245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnes PF, el Hajj H, Preston-Martin S, et al. Transmission of tuberculosis among the urban homeless. JAMA. 1996;275:305–307. [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. Targeted tuberculin testing and treatment of latent tuberculosis infection. MMWR Morb Mortal Wkly Rep. 2000;49(RR-6):1–54. [PubMed] [Google Scholar]
- 7.Marks SM, Taylor Z, Burrows NR, Qayad MG, Miller B. Hospitalization of homeless persons with tuberculosis in the United States. Am J Public Health. 2000;90:435–438. doi: 10.2105/ajph.90.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. Reported Tuberculosis in the United States, 2003. Atlanta, Ga: US Dept of Health and Human Services; 2004. [Google Scholar]
- 9.McKinney Act. 42 USC, §119 (July 22, 1987).
- 10.Centers for Disease Control and Prevention. Racial disparities in tuberculosis—selected southeastern states, 1991–2002. MMWR Morb Mortal Wkly Rep. 2004;53:556–559. [PubMed] [Google Scholar]
- 11.Thompson ML, Myers JE, Kriebel D. Prevalence odds ratio or prevalence ratio in the analysis of cross sectional data: what is to be done? Occup Environ Med. 1998;55:272–277. doi: 10.1136/oem.55.4.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iseman MD. A Clinician’s Guide to Tuberculosis. Philadelphia, Pa: Lippincott Williams & Wilkins; 2000. [Google Scholar]
- 13.American Thoracic Society; Centers for Disease Control and Prevention; Infectious Diseases Society of America. Treatment of tuberculosis. MMWR Recomm Rep. 2003;52(RR-11):1–77. [PubMed] [Google Scholar]
- 14.US Department of Health and Human Services. Healthy People 2010. Washington, DC: US Government Printing Office; 2000. [Google Scholar]
- 15.Nolan CM, Elarth AM, Barr H, Saeed AM, Risser DR. An outbreak of tuberculosis in a shelter for homeless men. Am Rev Respir Dis. 1991;143:257–261. doi: 10.1164/ajrccm/143.2.257. [DOI] [PubMed] [Google Scholar]
- 16.Rossi PH, Wright JD, Fisher GA, Willis G. The urban homeless: estimating composition and size. Science. 1987;235:1336–1341. doi: 10.1126/science.2950592. [DOI] [PubMed] [Google Scholar]
- 17.Knopf SA. Tuberculosis as a cause and result of poverty. JAMA. 1914;63:1720–1725. [Google Scholar]
- 18.Phelan JC, Link BG. Who are “the homeless”? reconsidering the stability and composition of the homeless population. Am J Public Health. 1999;89:1334–1338. doi: 10.2105/ajph.89.9.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kline SE, Hedemark LL, Davies SF. Outbreak of tuberculosis among regular patrons of a neighborhood bar. N Engl J Med. 1995;333:222–227. doi: 10.1056/NEJM199507273330404. [DOI] [PubMed] [Google Scholar]
- 20.Perlman DC, Perkins MP, Paone D, et al. “Shotgunning” as an illicit drug smoking practice. J Subst Abuse Treat. 1997;14:3–9. doi: 10.1016/s0740-5472(96)00182-1. [DOI] [PubMed] [Google Scholar]
- 21.Omidvari K, Casey R, Nelson S, Olariu R, Shellito JE. Alveolar macrophage release of tumor necrosis factor-alpha in chronic alcoholics without liver disease. Alcohol Clin Exp Res. 1998;22:567–572. doi: 10.1111/j.1530-0277.1998.tb04294.x. [DOI] [PubMed] [Google Scholar]
- 22.Baldwin GC, Tashkin DP, Buckley DM, Park AN, Dubinett SM, Roth MD. Marijuana and cocaine impair alveolar macrophage function and cytokine production. Am J Respir Crit Care Med. 1997;156:1606–1613. doi: 10.1164/ajrccm.156.5.9704146. [DOI] [PubMed] [Google Scholar]
- 23.Mayaud C, Boussaud V, Saidi F, Parrot A. Bronchopulmonary disease in drug abusers. Rev Pneumol Clin. 2001;57:259–269. [PubMed] [Google Scholar]
- 24.Reichman LB, Felton CP, Edsall JR. Drug dependence, a possible new risk factor for tuberculosis disease. Arch Intern Med. 1979;139:337–339. [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention. Revised guidelines for HIV counseling, testing, and referral. MMWR Recomm Rep. 2001;50(RR-19):1–57. [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention. Advancing HIV prevention: new strategies for a changing epidemic—United States, 2003. MMWR Morb Mortal Wkly Rep. 2003;52:329–332. [PubMed] [Google Scholar]
- 27.The Urban Institute. Homelessness Programs and the People They Serve: Findings of the National Survey of Homeless Assistance Providers and Clients. Washington, DC: US Dept of Housing and Urban Development; 1999. [Google Scholar]
- 28.Institute of Medicine. Ending Neglect: The Elimination of Tuberculosis in the United States. Washington, DC: National Academies Press; 2000. [PubMed] [Google Scholar]
- 29.Asch S, Leake B, Knowles L, Gelberg L. Tuberculosis in homeless patients: potential for case finding in public emergency departments. Ann Emerg Med. 1998;32:144–147. doi: 10.1016/s0196-0644(98)70128-3. [DOI] [PubMed] [Google Scholar]
- 30.Centers for Disease Control and Prevention. Tuberculosis transmission in multiple correctional facilities—Kansas, 2002–2003. MMWR Morb Mortal Wkly Rep. 2004;53:734–738. [PubMed] [Google Scholar]
- 31.Centers for Disease Control and Prevention. Controlling TB in Correctional Facilities. Atlanta, Ga: US Dept of Health and Human Services; 1995. [Google Scholar]
- 32.Reichard AA, Lobato MN, Roberts CA, Bazerman LA, Hammett TM. Assessment of tuberculosis screening and management practices of large jail systems. Public Health Rep. 2003;118:500–507. doi: 10.1016/S0033-3549(04)50286-8. [DOI] [PMC free article] [PubMed] [Google Scholar]