Summary
Background
In 2011, the Centers for Disease Control and Prevention modified its 2008 protocol for flight-related tuberculosis contact investigation initiation. The 2011 Modified protocol was implemented and replaced the 2008 CDC protocol based on comparative epidemiologic and economic analyses; this publication reports the economic analysis results.
Methods
A return on investment model compared relative changes in tuberculosis disease treatment costs resulting from expenditures on tuberculosis contact investigations and latent tuberculosis infection treatment for the 2008 CDC and Modified protocols.
Results
At moderate/high rates of latent tuberculosis infection and tuberculosis disease, positive returns on investment indicated each $1.00 spent on tuberculosis contact investigations and latent tuberculosis treatment resulted in more than $1.00 of savings from reduced tuberculosis disease treatment costs. Low rates of latent tuberculosis infection and tuberculosis disease resulted in negative returns on investment, indicating economic losses from tuberculosis disease treatment costs. There were smaller economic losses at low latent tuberculosis infection and tuberculosis disease rates with the Modified protocol in comparison to the 2008 CDC protocol, while both identified comparable numbers of persons at risk for tuberculosis.
Conclusion
The Modified protocol for conducting flight-related tuberculosis contact investigations represents a better use of resources and protects public health.
Keywords: Tuberculosis, Return on investment, Contact tracing
Introduction
The Centers for Disease Control and Prevention (CDC), Division of Global Migration and Quarantine (DGMQ), has regulatory authority to prevent introduction of infectious diseases, including tuberculosis (TB), into the United States [1]. A case of TB disease in a passenger traveling into the United States is usually reported to DGMQ days to weeks after arrival, depending on when the passenger (case-traveler) was evaluated and diagnosed [2]. The CDC protocol for flight-related TB contact investigations (TBCI) includes criteria for determining whether a TBCI is warranted based on the case-traveler’s clinical data, date of the flight relative to diagnosis, along with subsequent notification by a public health official to CDC, and flight duration [3]. The protocol also defines which passengers are considered contacts of the case-traveler. If case-traveler and flight criteria are met, DGMQ obtains passenger information and provides it to Public Health Departments (HDs). HDs follow national guidelines for contact tracing [3] and voluntarily report health outcomes to DGMQ. Information on foreign nationals without US contact information is provided to public health authorities in the country of residence [4–8].
TBCIs in the community are tools traditionally used to prevent the spread of TB disease [2,3]. During flight-related TBCIs, HDs notify persons identified as close contacts of a case-traveler and recommend testing for latent TB infection (LTBI) with a tuberculin skin test (TST) or interferon gamma release assay (IGRA) test. If the test is positive, HDs then recommend a chest radiograph (CXR) to test for, and identify possible TB disease. If the CXR does not indicate TB disease, case-passenger contacts then receive education on LTBI and may be offered treatment to prevent progression from LTBI to TB disease [3].
The economic study reported in this article was initiated to determine whether or not flight-related TBCIs and LTBI treatment maximize resources and efficiency in protecting public health. During informal meetings and phone conversations with CDC, some health departments had expressed concerns about the use of scarce resources for, and the utility of, flight-related TBCIs. Articles published from 2006 to 2010 suggested that the public health impact of flight-related TBCIs was not well established [3–8] and supported the HDs’ viewpoint that spending money on flight-related TBCIs could be an inefficient allocation of resources [3]. In response, CDC developed three alternative protocols with more restrictive criteria than the original 2008 protocol for comparative purposes in determining when to conduct flight-related TBCIs. The goal of all three alternative protocols was to reduce the number of TBCIs and associated resource use, while still protecting public health. Complete descriptions of the original CDC 2008 protocol and the three alternative protocols and risk analysis are provided separately in Marienau et al., where protocols are labeled 2008 CDC Protocol, 2008 WHO Guidelines, Modified CDC Protocol, and Multidrug resistant isolates [9].
In 2010 and 2011, we conducted epidemiologic risk analyses on the three alternative protocols and compared them with the 2008 CDC protocol. The primary result of the epidemiologic risk analyses was that the Modified CDC protocol (Modified) was the best public health alternative because even with more restrictive criteria (Table 1), it provided public health protection against TB disease comparable to the 2008 CDC [9]. After completion of the risk analysis, we then did the economic analysis comparing the 2008 CDC and Modified protocols. This economics-focused article describes in detail the return-on-investment analysis conducted to determine the costs and benefits of the 2008 CDC as compared with the Modified protocols, and whether flight-related TBCIs and treatment of LTBI were an efficient use of resources for the control and prevention of TB.
Table 1.
Comparison of index case flight and clinical criteria for conducting flight-related TB contact investigations for the 2008 CDC Protocol and Modified CDC Protocol.
| Criteria | 2008 CDC protocol | Modified CDC protocol |
|---|---|---|
| Flight duration | at least 8 h (gate-to-gate) | at least 8 h (gate-to-gate) |
| TB diagnosis relative to flight date | Within 3 months of flight | Within 3 months of flight |
| Time from flight to notification of agency | Within 6 months of flight | Within 3 months of flight |
| Isolate:susceptible to isoniazid or rifampin | Sputum smear positive for M. tuberculosis by culture or NAATa
AND Sputum smear positive for AFBb (with or without cavitation detected on CXR) OR Sputum smear negative (x3) and cavitation detected on CXRc |
Sputum positive for M. tuberculosis by culture or NAATa AND Sputum smear positive for AFBb and cavitation detected on CXRc |
| Isolate:multidrug-resistant (resistant to at least isoniazid and rifampin) | Alld | Alld |
NAAT: nucleic acid amplification test.
AFB: acid-fast bacilli.
CXR: chest radiograph.
Regardless of sputum smear or CXR results.
Methods
A return on investment model (ROI) was used to estimate whether the investment of resources by HDs and DGMQ to conduct flight-related TBCIs, along with screening for and treating LTBI, cost more or less than the costs associated with TB disease cases that may have occurred among the contacts of a case-traveler. ROIs were calculated with the following equation:
Positive ROI indicated that Investment Gain > Investment Cost
Negative ROI indicated that Investment Gain < Investment Cost
Cost of investment was defined as DGMQ and HD resources spent on flight-related TBCIs plus the costs of testing and treatment for LTBI. Gain from investment was defined as the reduction in TB treatment costs resulting from testing and treatment for LTBI. Costs were all adjusted to and calculated in 2009 U.S. dollars.
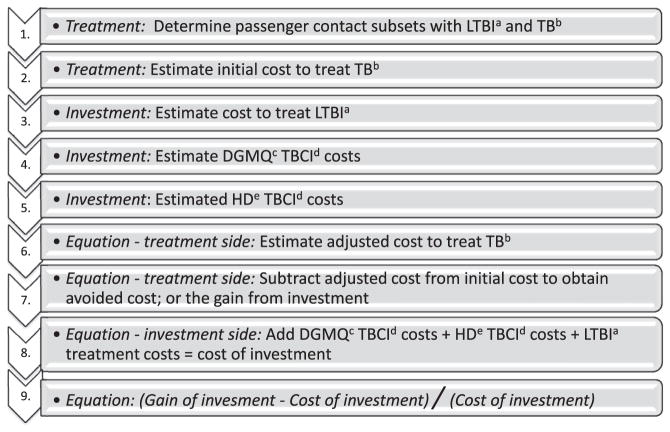
Each section below corresponds to a step listed in the Model Flowchart (Fig. 1).
Figure 1.
Flowchart and sequencing of treatment, investment, and equation components. a. LTBI = latent tuberculosis infection b. TB = tuberculosis c. DGMQ = Division of Global Migration and Quarantine d. TBCI = tuberculosis contact investigation e. HD = health department.
Step 1. Determine passenger contact subsets with LTBI and TB disease
The population that would be reached by contact tracing and offered medical treatment where necessary was comprised of contacts of a case-traveler who had TB. DGMQ identified 9248 passenger-contacts of TB case-travelers during flight-related TBCIs conducted during 2007–2009. Of those 9,248, telephone or postal address contact information in the United States was available for 7924 passenger contacts and was provided to HDs for notification, evaluation, and treatment (if indicated). The contact tracing model treated the 7924 passenger contacts as a cross-sectional cohort for analytic purposes to estimate what numbers of persons might be diagnosed with LTBI and potential TB disease.
Estimated LTBI rates calculated by CDC risk analyses [9] for 2008 CDC were 1.1%–24% for the cohort of passenger-contacts. For Modified the passenger-contact LTBI rates were 1.4%–19% [9]. The population in the cohort of passenger-travelers was then multiplied by these LTBI rates, creating four groups in two LTBI prevalence categories associated with each protocol: low prevalence (1.1%, 1.4%) and high prevalence (19%, 24%). The lifetime risk range of contracting TB once exposed to Mycobacterium tuberculosis is about 5–10% [10]. For the model, we assume risks of conversion from LTBI to TB disease as a range of three rates (5.25%, 7.35% and 10.25%) [10]. The three TB disease conversion rates were multiplied by the four LTBI rates to estimate the number of TB disease cases that might occur under both protocols. The result was a grid of the estimated number of persons in the cohort that might have LTBI and the proportion of those who would be expected to progress to TB disease without LTBI treatment (Table 2).
Table 2.
Estimated number of passenger contacts with latent tuberculosis infection (LTBI) and cases of active tuberculosis (TB) for a cohort of 7924 passenger contacts, based on risk of acquiring LTBI and risk of progression to TB disease.
| Latent tuberculosis infection (LTBI) risk | 2008 CDC low 1.1% | Modified low 1.4% | Modified high 19% | 2008 CDC high 24% |
|---|---|---|---|---|
| Estimated number of passenger contacts with LTBI, based on risk of acquiring LTBI | 87 | 111 | 1506 | 1902 |
| Estimated number of active TB cases, based on risk of progressing to TB disease | ||||
| TB disease risk = 5.25% | 4.6 | 5.8 | 79.2 | 99.6 |
| TB disease risk = 7.35% | 6.4 | 8.4 | 110.7 | 139.8 |
| TB disease risk = 10.25% | 8.9 | 12.2 | 154.4 | 195.0 |
Components common to TB and LTBI evaluation and management (see Fig. 1 steps 2 and 3)
Several screening, testing, and treatment components were the same for TB disease and LTBI, such as initial TST or IGRA tests, CXR, some drugs, private and public clinic medical visit costs, amount of patient time to engage in medical care (opportunity costs), and the price of patient transportation. We used allowable billing charges [11] as cost data, on the assumption that billing charges were the costs of the test or service from the perspective of the patient or the insurer. Where a low/high range of allowable billing charges was noted, we calculated cost point estimates as a weighted average of 75% low and 25% high allowable private insurance billing charge for each Current Procedural Terminology (CPT) code in the Physicians’ Fee and Coding Guide [11]. Where available, we used Medicare allowable billing charges as the low cost estimate. Cost estimates were weighted to the low end on the assumption that many patients are probably Medicaid eligible or would have no insurance and be in low income brackets eligible for sliding fee scales.
Testing [11]
Different HDs and medical practices engaged in different billing practices. To be inclusive, we calculated billing charges for all CPT codes that appeared in a variety of online billing guidance documents from clinics and HDs found with multiple Google searches. In some cases, to obtain a standard cost estimate, multiple types of the same test, e.g., CXRs, were weighted based on their estimated frequency of use: CXR 71,035 (45%), CXR 71,020 (45%), and CXR 71,030 (10%); TST 86,580 (70%) or IGRA 86,480 (30%). Other tests included were sputum smears and cultures; homogenization and isolation of culture; 10% of the weighted sputum induction price (reflecting need for induction in 10% of patients); and first-line drug susceptibility tests.
Drugs [12]
All drugs had multiple price, packaging, and dose formulations. Formulations not matching CDC dosing recommendations were omitted. Remaining product package prices were divided by the number of pills in a pack to obtain a price-per-dose and then averaged for rifampin, isoniazid, ethambutol, pyrazinamide, and an isoniazid/rifampin combination.
Private medical visits [11]
We calculated the costs of private medical visits by using the average of high and low allowable billing charges, where the initial diagnostic visit was set at complexity level 2 (out of a range of 1–5), weighted 10% new 90% established patient. We calculated the costs of subsequent visits by using allowable charges for follow-up visits. Nurse or medical paraprofessional visits were assigned 35% of the cost of an established patient visit by assumption, because there were no specific allowable billing charges for these types of visits.
Public health department medical visits [13–15]
Estimations of HD medical visit costs could not be calculated in the same manner as private medical visits because no comparable HD billing data exist. Therefore, HD costs were calculated as labor costs from national wage databases [13,14], including a 33% benefit rate [15].
Patient opportunity costs and transportation to/from clinic/hospital [16]
Patients engaged in medical visits for treatment are not engaged in other routine economic activities they would normally participate in, as reflected in the Gross Domestic Product (GDP). We estimated this lost economic activity by dividing the 2009 per-capita U.S. GDP [16] of $46,400 by annual minutes (525,600) or $0.09 per minute. The $0.09 was multiplied by the minutes a patient spent in treatment plus 30 min round-trip travel time per treatment visit (medical or hospital). Also added was the average of mass transit system fares for 2009 in New York City, Washington DC, Chicago, Boston, and San Francisco.
Step 2. Estimate the initial cost to treat TB disease
In addition to tests, drugs, private medical visits, public clinic medical visits, patient opportunity costs, and transportation, as already described, estimated costs for treating TB disease included hospitalization. This section first describes the hospitalization component and then describes the process of estimating total costs of TB disease treatment.
Hospitalization
Costs for hospitalizations for TB disease where patients survive the hospitalization and where drug resistance does not develop were based on a published report of TB hospital stays [17] and adjusted to 2009 dollars. Costs for single-drug resistance or deceased hospital patients were adjusted upwards based on published studies that indicated higher hospital costs when patients were high risk or died [17,18]. It is difficult to ascertain what percentage of TB patients were hospitalized, and the data included a wide range of observations about TB hospitalizations [17,18]. The most recent reference for TB hospitalizations by the Agency for Healthcare Research and Quality mentions the annual number of hospital stays related to TB but not hospital stays as a proportion of TB cases: “In 2006, there were 58,500 hospital stays related to TB.” [17] Further, the range of likelihood of being hospitalized changed significantly with geographic area, race, age, comorbid conditions, and insurance status [17,18], and also it was likely that some persons with comorbid conditions would have multiple hospitalizations. Lacking clear-cut data, we simply assumed that 55% of TB cases would be hospitalized where TB was either a primary or secondary condition (e.g., HIV might be the primary, and TB the secondary condition).
Estimating the total cost of TB disease treatment
We used component prices to estimate costs of testing and drug regimens, medical visits (e.g., physician or nurse visits), and patient time and transportation.
Testing costs were calculated at four points
At both initial diagnosis and end of treatment: CXR, TST/IGRA (done only once during initial screening), 3 sputum smears, culture, 10% of the cost of sputum induction (reflecting need for induction in 10% of patients), and 4 first-line drug susceptibility tests.
During treatment at the 2-week and 2-month points: 3 sputum smears, culture, and 10% of the cost of sputum induction.
Drug dosing was based on CDC recommendations [19] and body weight. From 2001 to 2008, 62% of TB disease cases were in males, and 38% were in females [20]. Drug dose costs were calculated by gender, based on average body weights; then drug costs were averaged, although there was very little gender difference between the two dosing regimens. About 5% of patients were assumed to have strains resistant to isoniazid.
The standard of medical care for the initial phase of TB disease treatment is directly observed therapy (DOT). We assumed private care would be too costly for most patients and that most private practices were not set up for DOT. Therefore, all TB disease treatment took place in HDs. However, 10% of the cost of a private medical visit was included in the cost of the first HD visit, on the assumption that some patients were referred by private medical practices. In the second phase of treatment, we assumed that patients received 50% DOT and 50% self-administered therapy (SAT).
Patient opportunity costs vary depending on whether the patients receive completely DOT or combined DOT/SAT and whether they are hospitalized. We assumed that all patients used the following time: 60 min initial TB diagnosis, 6 min for DOT drug administration, and 30 min for transportation. Patient time for a hospital stay was 15 days, based on the literature [18].
The final step was to sum testing, drugs, medical visits, hospitalization (assuming a 55% hospitalization rate), and patient opportunity costs. The average per-case treatment cost for TB disease was $26,605. This amount is increased to $67,058 for a small number of patients who died or whose TB strain was single-drug resistant, or who had other high-risk factors (e.g., HIV, age, drug addiction). The model also incorporated a separate treatment cost of $131,000 for MDR patients [21].
Step 3. Estimating the cost to treat LTBI
We assumed 15% of patients were treated in private medical practices and 85% in HDs; all treatment of LTBI was SAT; patients starting, but not finishing, received 30 days of treatment; patients who finished LTBI drug therapy were accorded 270 days of treatment. LTBI treatment cost categories were tests, medical visits, drugs, and patient opportunity costs.
Step 4. Estimating DGMQ TB contact investigation costs
For each flight for which a TBCI was conducted, DGMQ staff obtained passenger flight manifests from airlines and additional data from the Department of Homeland Security. In our study, DGMQ obtained and processed contact information for 9248 passengers who were identified as contacts of case-travelers. Of these passenger-contacts, 7924 had US addresses and/or telephone numbers that were provided to HDs for contact tracing.
The estimates of the federal government costs of TBCIs were based on the labor time spent by DGMQ personnel on each reported TB disease case for which a TBCI was conducted and on obtaining, processing, and distributing passenger-contact information associated with each incident. DGMQ staff routinely involved with TBCIs provided a list of the required routine tasks, along with the numbers of personnel normally involved and personnel job titles. Employee wages were calculated [13,14] by using the job titles, and a component was added for benefits to estimate total compensation in 2009 dollars [15].
An examination of the personnel task categories showed that some tasks were associated with an entire incident, for example, collecting case information and report writing, while some tasks were related to the number of passenger-contacts associated with each incident. In economic terms, the incident-related time became ‘fixed’ labor time and the per-contact time became ‘variable’ labor time. Fixed labor time was constant per incident, but decreased per contact with more contacts. For example, a 1-h incident involving a single contact was 60 min per contact, while 60 contacts were 1 min per contact. Variable time increased with each additional contact, and the average variable time per contact was 11.65 min. Labor time was averaged for multiple events to obtain a range of average minutes per contact. These averages were multiplied by wages per minute to calculate the average cost per contact across all events. The average cost-per-contact used in the model was $16.76.
Step 5. Estimate HD TB contact investigation costs
TBCI processes vary by state. A range of HD costs was developed to account for a wide variety in HD TBCI practices [22–24]. The range of dollar values chosen represents the wide range of HD expenditures per contact during TBCIs: $28, $47, $134, and $164.
Step 6. The equation treatment side: estimate adjusted cost to treat TB disease
Data from the literature show that approximately 28% of people who are diagnosed with LTBI do not start treatment [25]. Thirty six percent of the 72% who do start treatment do not complete treatment. Those who do completed treatment substantially reduced their risk of progressing to active TB disease [25–29]. One study found that persons completing LTBI treatment reduced their risk of progression to TB disease by 80%, while another study found a range of reduced efficacy for population subgroups that included 80% [27,29]. For the 46% of the study cohort estimated to have started and completed LTBI treatment, the risk of TB disease incidence was estimated to be reduced by 80% compared with those who did not start and complete LTBI treatment. In our analysis, the costs of treating TB disease were applied to those in the cohort who were estimated to develop TB disease; therefore, TB disease treatment costs were reduced by 80% for 46% of the cohort population.
Step 7. The equation treatment side: subtract adjusted cost of TB disease treatment from initial cost to obtain avoided cost, or the gain from investment
The ROI model calculated total treatment costs for the cohort subset estimated to progress to TB disease (Table 2). We assumed that 90% of TB cases were infected with isolates susceptible to all first-line drugs, 5% with single-drug resistant isolates, and 5% with MDR isolates. Using these assumptions, we calculated TB disease treatment costs.
A factor for the value of a statistical life (VSL) was added to account for hospital-related deaths. VSL is used by multiple government agencies to assign a monetary value reflecting the loss of economic activity and emotional relations from premature loss of life. [30,31] To estimate hospital TB disease-related deaths, we used two sources. One placed the 2006 TB disease death rate at 4.7% [32], and another placed the 2000 hospital-specific TB disease-related death rate at a 4.9% average [18]. The model estimated that 4.7% of hospitalized cases died from TB.
There are multiple VSL values [30,31] because different government agencies use different VSL estimation methods. One study developed an age-related VSL to value death from influenza [33], and we used this value in the ROI model because deaths from both influenza and TB disease increase with age.
The total initial cost to treat TB disease, which included treatment plus VSL, was calculated for each of 12 groups of TB cases (Table 2). The total initial cost was then reduced by 46% for each group to calculate the ROI gain of reduced costs of TB disease treatment.
Step 8. The equation investment side: add DGMQ and HD contact investigation costs and the costs of LTBI treatment for the cost of investment
To calculate DGMQ’s investment in TBCIs, the average labor cost per contact of $16.76 was multiplied by 9248 passenger-contacts. The HD costs per contact of $28, $47, $134, and $164 were multiplied by 7924, the number of passenger-contacts whose name and contact information DGMQ provided to HDs. The number of passenger-contacts provided to HDs, rather than the number of persons finally reached by HDs, was used to calculate costs because HDs are under no obligation to report back to CDC the actual number of persons reached. HD costs were added to DGMQ costs, resulting in 4 levels of TBCI costs associated with each level of HD spending. Cost per person of treating LTBI, weighted by 36% for those estimated to start LTBI treatment and 64% for those estimated to complete treatment, was multiplied by the number in each group of passenger contacts with LTBI (Table 2) and added to TBCI costs.
Step 9. Equation: (gain of investment – cost of investment)/(cost of investment)
The ROI was calculated as the net gain or loss: the change in TB disease treatment costs that resulted from TBCI and LTBI treatment expenditures.
Results
LTBI per-person treatment costs were $949.51 for full (270 doses) treatment courses.
Estimated numbers of passenger contacts with LTBI using 2008 CDC ranged from 87 to 1902 and for Modified from 111 to 1506 (Table 2). Estimated numbers of passenger contacts progressing to TB disease ranged from 5 to 195 cases for 2008 CDC and from 6 to 155 cases for Modified.
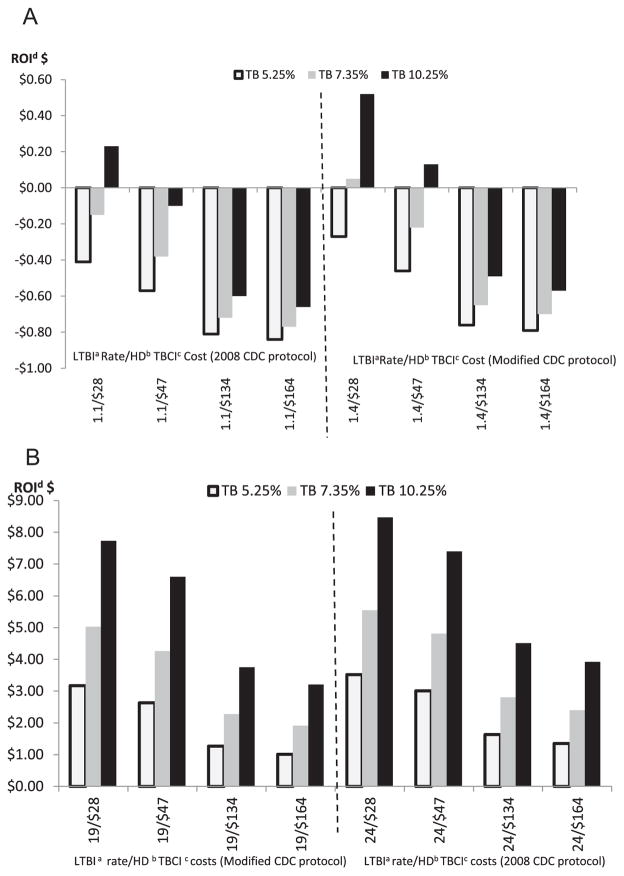
The results of the ROI analysis were driven by incidence rates for LTBI and active TB disease, as well as the costs for HD contact investigations and testing/treating TB disease and LTBI (Fig. 2(A) and (B)).
Figure 2.
A. Estimated US health department return on investment for tuberculosis (TB) contact investigations for latent TB infection rates of 1.1% and 1.4%, at risks of progression to TB disease of 5.25%, 7.35%, and 10.25%. B. Estimated US health department return on investment for tuberculosis (TB) contact investigations for latent TB infection rates of 19% and 24%, at risks of progression to TB disease of 5.25%, 7.35%, and 10.25% aLTBI = latent tuberculosis Infection bHD = health department cTBCI = tuberculosis contact investigation dROI = Return on Investment.
Positive ROIs indicated that every $1.00 spent on TBCIs and LTBI treatment resulted in more than $1.00 reduction in TB disease treatment costs. Negative ROIs indicated TB disease treatment costs were reduced by less than $1.00. ROI gains and losses for Modified were somewhat less widespread than the gains and losses associated with 2008 CDC. For 2008 CDC at a 24% LTBI rate, every $1.00 spent on TBCIs and treatment of LTBI resulted in a range of reduced TB disease treatment costs of approximately $1.50 to $8.50. For Modified at a 19% rate of LTBI, every $1.00 spent resulted in reduced TB disease treatment costs of approximately $1 to $7.80. Both protocols resulted in negative ROIs (net losses) of 1.1% and 1.4% rates of LTBI respectively, except where HD TBCI per-contact costs were the lowest ($28).
Discussion
At lower rates of LTBI and TB disease, adopting the Modified protocol ameliorated the resource losses associated with treatment (Fig. 2(A)). Even though Modified contained a shorter post-flight time frame to notify government agencies of TB disease and more restrictive diagnostic criteria than 2008 CDC, the results of the risk analyses [9] indicated that both protocols were comparable in protecting the public against TB. At the same time, the costs of Modified were lower than 2008 CDC. At higher rates of LTBI and TB disease, conducting flight-related TBCIs was economically beneficial for both 2008 CDC and Modified protocols (Fig. 2(B)).
Where ROIs were positive, most savings accrued to health insurers in the form of avoided hospitalizations because 70% of the costs of TB disease were due to the costs of hospitalizing patients. For CDC and HDs, some savings resulted from limiting the numbers of contact tracing events.
The ROI model had some weaknesses. The wide risk range, along with the uncertainty that any individual would progress from LTBI to TB, was a primary motivator not to discount the benefits of LTBI treatment. We felt that any assumptions made to model discounting a future stream of benefits would be unreliable without a more certain time frame and likelihood of contracting TB. However, the wide ranges also function as natural sensitivity boundaries and can be viewed as providing all of the options that would arise out of a sensitivity analysis.
The list of CPT codes used by medical practices to bill for screening and treating TB and LTBI varied from practice to practice, insurer to insurer, and state to state. Further, specific codes that were ‘allowable’ changed annually in the complex world of medical billing. It is likely that not all the CPT codes we included in our estimates of screening and treating were always used, and also likely that some practices used CPT codes not included in our analysis. For example, we did include the costs of first-line drug susceptibility testing, but not the costs of second-line drug susceptibility testing. However, we feel confident that our estimates of screening and treating costs were a reliable benchmark of what many insurers (public and private) paid for screening and treatment of TB and LTBI.
The model and results did not fully reflect two wide ranges of costs. One was high-end per-contact tracing costs in the $200–400 range, reported by a few HDs. State and HD policies governing the numbers and types of personnel involved in, and the amount of effort devoted to, TBCIs varied tremendously. Further, the component parts or definitions of TBCI processes were very diverse. Because HD TBCI practices varied, different HD per-contact tracing costs were not clearly comparable. We believed that the range of HD costs in this study of $28, $47, $134, and $164 covered the practices of most states.
The second wide range of costs not reflected in the model was hospitalizations for MDR TB patients. Personal communication with DGMQ personnel indicated that at least one person with MDR TB who was put under a federal isolation order after entry to the United States incurred hospital costs of more than $600,000. While $131,000 [21] probably reflected a relatively accurate average cost for MDR TB hospitalization, in verifiable cases extended hospitalization costs were substantially higher.
Conclusions
Global air travel from countries with high rates of LTBI and TB disease will continue to be a risk factor for the spread of TB. Conducting flight-related TBCIs under the Modified protocol (flights at 8 h duration, TB diagnosis and flight notification within 3 months of flight, sputum culture and smear positive AND cavitation on CXR, or MDR TB) rather than under the 2008 CDC protocol (same as Modified except flight notification within 6 months of flight, sputum culture and smear positive OR cavitation on CXR) for flights into the United States is worthwhile from both an epidemiologic and economic perspective because doing so moderates public health resource use without jeopardizing TB prevention and control. As with any change in policy that has the potential for unanticipated effects on public health, it will be important to conduct periodic evaluations to determine whether the revised policy is adequate and to monitor TB cases that might be the consequence of airline exposure.
Acknowledgments
Dr. Elaine H. Cramer, Contractor, Global Health Sector, SRA International.
Footnotes
Disclaimer
The opinions expressed by authors contributing to this journal do not necessarily reflect the opinions of the Centers for Disease Control and Prevention or the institutions with which the authors are affiliated.
Conflict of interest
None.
References
- 1.The Centers for Law and the Public Health, CDC Collaborating Center for Public Health Legal Preparedness, WHO/PAHO Collaborating Center on Public Health Law and Human Rights. [accessed May 2012];Tuberculosis control laws and public policies: a handbook for public health and legal practitioners. 2009 Oct 1; http://www.cdc.gov/tb/programs/TBlawPolicyHandbook.pdf.
- 2.Centers for Disease Control and Prevention. Controlling tuberculosis in the United States. Recommendations from the American Thoracic Society, CDC, and the Infectious Diseases Society of America. MMWR Morb Mortal Wkly Rep. 2005;54(RR-12):1–81. [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Guidelines for the investigation of contacts of persons with infectious tuberculosis. Recommendations from the National Tuberculosis Controllers Association and CDC. MMWR Morb Mortal Wkly Rep. 2005;54(RR-15):1–47. [PubMed] [Google Scholar]
- 4.Marienau KJ, Burgess GW, Cramer EH, Averhoff FM, Buff AM, Russell M, et al. Tuberculosis investigations associated with air travel: U. S. Centers for disease control and prevention, Jan 2007–June 2008. Travel Med Infect Dis. 2010;8:104–12. doi: 10.1016/j.tmaid.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 5.Abubakar I, Welfare R, Moore J, Watson J. Surveillance of air-travel-related tuberculosis incidents, England and Wales: 2007–2008. Euro Surveill. 2008;13(23):1–3. [PubMed] [Google Scholar]
- 6.Abubakar I. Tuberculosis and air travel: a systematic review and analysis of policy. Lancet Infect Dis. 2010;10:176–83. doi: 10.1016/S1473-3099(10)70028-1. [DOI] [PubMed] [Google Scholar]
- 7.Chemardin J, Paty MC, Renard-Dubois S, Veziris N, Antoine D. Contact tracing of passengers exposed to an extensively drug-resistant tuberculosis case during an air flight from Beirut to Paris, October 2006. Euro Surveill. 2007;12(49):3325. doi: 10.2807/esw.12.49.03325-en. [DOI] [PubMed] [Google Scholar]
- 8.Kornylo-Duong K, Kim C, Cramer EH, Rodriguez D, Buff AM, Doyle J, et al. Three air travel-related contact investigations associated with infectious tuberculosis, 2007–2008. Travel Med Infect Dis. 2010;8:120–8. doi: 10.1016/j.tmaid.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 9.Marienau KJ, Cramer EH, Coleman MS, Marano N, Cetron MS. Flight related tuberculosis contact investigations in the United States: comparative risk and economic analysis of alternate protocols. Travel Med Infect Dis. 2013 doi: 10.1016/j.tmaid.2013.09.007. in press. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. CDC grand rounds: the TB/HIV syndemic. MMWR Morb Mortal Wkly Rep. 2012 Jul 26;61(26):484–9. [PubMed] [Google Scholar]
- 11.MAG Mutual Health Care Solutions, Inc. Physicians’ fee and coding guide [Source for CPT code pricing] 2009 www.magmutual.com/hsi.
- 12.Reuters Thomson. Red book: pharmacy’s fundamental reference. 2009 Edition, www.PDRbookstore.com. Source for drug prices.
- 13.Bureau of Labor Statistics, United States Department of Labor. [accessed March 2011];Occupational employment statistics. http://www.bls.gov/oes/
- 14.U. S. Office of Personnel Management. [accessed March 2011];General schedule (GS) locality pay tables. 2009 http://www.opm.gov/oca/09tables/indexgs.asp.
- 15.Congressional Budget Office. Characteristics and pay of federal civilian employees. Washington, DC: Congressional Budget Office; 2007. [Google Scholar]
- 16.Central Intelligence Agency. [accessed March 2011];The world Factbook. https://www.cia.gov/library/publications/the-world-factbook/geos/us.html.
- 17.Healthcare Cost and Utilization Project, Agency for Healthcare Research and Quality. Holmquist L, Russo CA, Elixhauser A. [accessed March 2011];Statistical brief #60: tuberculosis stays in hospitals 2006. 2008 Oct; http://www.hcup-us.ahrq.gov/reports/statbriefs/sb60.pdf. [PubMed]
- 18.Hansel NN, Merriman B, Haponik EF, Diette GB. Hospitalizations for tuberculosis in the United States in 2000: predictors of in-hospital mortality. Chest. 2004;126:1079–86. doi: 10.1378/chest.126.4.1079. [DOI] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention, American Thoracic Society, and Infectious Diseases Society of America. Treatment of tuberculosis. [accessed May 2012];MMWR Recomm Rep. 2003 Jun 20;52(RR11):1–77. http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5211a1.htm#tab3. [Google Scholar]
- 20.CDC WONDER. [accessed May 2012];Online tuberculosis information center. http://wonder.cdc.gov/tb.html.
- 21.Marks SM, Flood J, Seaworth B, Hirsch-Moverman Y, Armstrong L, Mase S, et al. Treatment practices, outcomes, and cost of multidrug-resistant and extensively drug resistant tuberculosis in the United States. Poster presentation. In: American Thoracic Society 2012 conference; San Francisco, California. May 21 2012. [Google Scholar]
- 22.Brown RE, Miller B, Taylor WR, Palmer C, Bosco L, Nicola RM, et al. Health-care expenditures for tuberculosis in the United States. Arch Intern Med. 1995;155:1595–600. [PubMed] [Google Scholar]
- 23.Coleman MS, Garbat-Welch L, Burke H, Weinberg MS, Humbaugh K, Tindall A, et al. Direct cost of one case of refugee-imported measles in Kentucky. Vaccine. 2012 Jan 5;30(2):317–21. doi: 10.1016/j.vaccine.2011.10.091. Epub 2011 Nov 12. [DOI] [PubMed] [Google Scholar]
- 24.Jereb J, Etkind SC, Joglar OT, Moore M, Taylor Z. Tuberculosis contact investigations: outcomes in selected areas of the United States, 1999. Int J Tuberc Lung Dis. 2003;7(12):S384–90. [PubMed] [Google Scholar]
- 25.Hirsch-Moverman Y, Daftary A, Franks J, Colson PW. Adherence to treatment for latent tuberculosis infection: systematic review of studies in the US and Canada. Int J Tuberc Lung Dis. 2008;12(11):1235–54. [PubMed] [Google Scholar]
- 26.Lobue PA, Menzies D. Treatment of latent tuberculosis infection: an update. Respirology. 2010;15:603–22. doi: 10.1111/j.1440-1843.2010.01751.x. [DOI] [PubMed] [Google Scholar]
- 27.Lobue PA, Moser KS. Use of isoniazid for latent tuberculosis infection in a public health clinic. Am J Respir Crit Care Med. 2003;168:443–7. doi: 10.1164/rccm.200303-390OC. [DOI] [PubMed] [Google Scholar]
- 28.Hess K, Goad J, Wu J, Johnson K. Isoniazid completion rates for latent tuberculosis infection among college students managed by a community pharmacist. J Am Coll Health. 2009;57(5):553–5. doi: 10.3200/JACH.57.5.553-556. [DOI] [PubMed] [Google Scholar]
- 29.Anger H, Proops D, Harris T, Ahuja S. Preventable tuberculosis cases arising from contacts to pulmonary cases in New York city. Am J Respir Crit Care Med. 2010;181:A5382. [Google Scholar]
- 30.National Center for Health Economics. [accessed March 2011];Value of statistical life analysis and environmental policy: a white paper for presentation to science advisory board – environmental economics advisory committee. 2009 May 28; http://yosemite.epa.gov/ee/epa/eerm.nsf/vwFUS/41DD6DBAE46D241B85256E89005C2989.
- 31.Department of Transportation, Federal Aviation Administration. [accessed March 2011];Revised departmental guidance: treatment of the value of preventing fatalities and injuries in preparing economic analyses. http://www.faa.gov/regulations_policies/policy_guidance/benefit_cost/media/Revised%20Value%20Of%20Life%20Guidance%20Feburary%202008.pdf.
- 32.American Lung Association. [accessed May 2012];Trends in tuberculosis morbidity and mortality. 2010 Feb; http://www.lungusa.org/finding-cures/our-research/trend-reports/TB-Trend-Report.pdf.
- 33.Molinari NA, Ortega-Sanchez IR, Messonnier ML, Thompson WW, Wortley PM, Weintraub E, et al. The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine. 2007;25(27):5086–96. doi: 10.1016/j.vaccine.2007.03.046. Epub 2007 Apr 20. [DOI] [PubMed] [Google Scholar]