Abstract
Background
Rural residents diagnosed with cardiovascular disease (CVD) or with CVD-related risks are underrepresented in behavioral intervention trials based on an extensive review of published studies. The low participation rate of rural residents weakens both the internal and external validity of published studies. Moreover, compared to urban residents, limited research exists to describe the unique barriers that limit the participation of rural residents in behavioral intervention trials.
Objective
The purpose of this review is to identify a conceptual framework (CF) underpinning common barriers faced by rural CVD patients to enroll in behavioral intervention trials.
Methods
We conducted a literature review using several electronic databases to obtain a representative sample of research articles, synthesized the evidence, and developed a CF to explain the barriers that may affect the research participation rate of rural residents with CVD or related risks.
Results
We found our evidence-based CF well explained the barriers for rural CVD patients to take part in behavioral intervention trials. Besides contextual factors (i.e. patient, community and research levels), other common factors impacting rural patients’ intent to enroll are lack of awareness and understanding about behavioral trials, limited support from their healthcare providers and social circles, unfavorable attitudes, and the lack of opportunity to participating research.
Conclusion and Implication of result
The findings demonstrate the evidence-based model consisting of interlinked multi-level factors may help our understanding of the barriers encountered by rural CVD patients participating interventions to promote behavioral change. The implication for researchers is that identifying and developing strategies to overcome the barriers precedes conducting studies in rural communities.
Keywords: rural CVD patients, recruitment and retention barriers, behavior interventions
I. INTRODUCTION
A. Background and Conceptual Framework
Despite the increased prevalence of cardiovascular disease (CVD) and related risks in rural communities [1, 2], studies report that rural residents with CVD and related risks are underrepresented in clinical trials [3, 4]. Also, as members of the rural population age, the percentage of rural residents with CVD and related risks is expected to increase [5–7]. Behavioral modifications, including engagement in healthy lifestyles, can help slow the progression of CVD and reduce risks [8]. However, the impact of behavioral interventions on rural participants’ CVD progression and risk reduction is uncertain due to the low participation rate of rural residents in clinical trials designed to promote behavioral modification [9]. Thus, effective approaches to recruit and retain participants from rural communities are needed to generate conclusive evidence to support the use of behavioral interventions to reduce CVD among rural residents. Likewise, the low rate of research participation for rural individuals threatens the internal and external validity of study results [10]. Furthermore, the low participation rate makes it challenging to identify feasible and sustained strategies to implement an efficacious behavioral intervention program in rural populations [3, 4, 11, 12]. Consequently, it is critical to examine the unique barriers encountered by rural participants taking part in behavioral intervention trials. To date, few studies report on these barriers. Therefore, the purpose of this review is to identify common barriers to participation in behavioral intervention trials for rural residents with CVD or related risks.
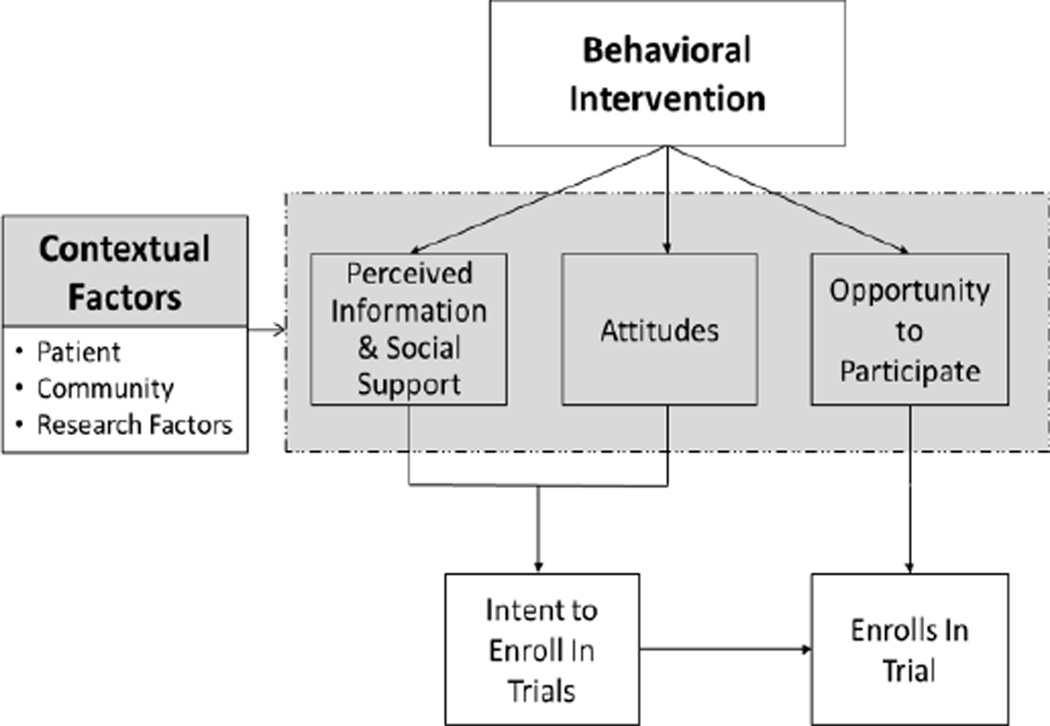
When studying the factors influencing the main outcome of interest, the researchers often propose a conceptual framework that provides a visual representation of variables involved and their relationships {{947 Jabareen, Yosef Rafeq 2009; 948 Kerlinger, Fred Nichols 1979}}. Things need to be taken into account in developing a conceptual framework include 1) identifying the research question needed to be addressed; 2) searching variables related to the main outcome of interest with a thorough literature review; 3) specifying relations among variables of interest; and 4) defining the scope of population {{948 Kerlinger, Fred Nichols 1979}}. For instance, to help understand the barriers of underrepresented populations to participate in cancer clinical trials, Ford et al. [11] developed a conceptual framework using Icek Ajzen’s Theory of Planned Behavior (TPB), which is widely used as a theoretical model to predict and explain human behavior in specified health-related contexts [13–16]. According to TPB, a person’s actual action with respect to a given behavior is determined by his/her intention to act and his/her perceived control over the behavior (e.g. opportunity to participate). The intention to act is further guided by the person’s attitude towards the behavior (e.g. belief) and the subjective norm (i.e. perceived social pressure and significant others’ appraisal). Overall, a person is more likely to act on a behavior if he or she believes in the desirable outcome of the behavior, receives support, and feels control over the behavior [15]. A conceptual framework based on TPB theory is used to help organize the barriers to participate in behavioral trial in rural populations (Figure 1). Based on our conceptual framework, we believe that a rural participant living with CVD or related risks is less likely to enroll in a behavioral intervention trial if he/she receives poor support (e.g. being provided no or inadequate information and social support), has unfavorable attitudes (e.g. disbelieves the intervention), and perceives no control over the situation (i.e. no opportunity to access the intervention). In addition, we believe the contextual factors such as participants’ characteristics, community and research related factors also contribute to the barriers to participation (Figure 1).
Figure 1.
Conceptual Framework of Barriers to Participate Behavioral Intervention Trials
II. METHODS
To obtain a representative sample of the research articles, we conducted the keyword search using several electronic databases, including Cumulative Index of Nursing and Allied Health Literature (CINAHL), Embase, Medline, PubMed, and the Cochrane Library. The keywords used alone and in combination included “rural”, “cardiovascular disease risk”, “heart disease risk”, “circulatory disease risk”, “behavior” or “behavioral”, “randomized control trials”, “research subject”, “enrolment”, “recruitment”, “retention”, “barrier”, “obstacle”, and “impediment”. First, two researchers and a reference librarian independently conducted the initial search using aforementioned keywords and retrieved 5,027 article titles. Secondly, a total of 1,026 article titles were selected based on the inclusion and exclusion criteria. Following the title screening, 105 articles were included for the abstracts review based on the inclusion/exclusion criteria. The abstract review resulted in a total of 35 eligible articles. The reference lists from the eligible articles were also examined for relevance. Last, the full-text articles were retrieved and screened based on the inclusion/exclusion criteria. Studies were included if they (1) were published in English, (2) were published between January 1, 1978 to September 30, 2014 (i.e., search end date), (3) included study participants who lived in rural areas and had CVD and/or risks, (4) examined or reviewed the effects of behavioral intervention on CVD progression or risk reduction, and (5) reported recruitment and retention barriers to participate behavioral intervention trials. Studies were excluded if (1) the participants were under 21 years of age, (2) the study examined the effects of a behavioral change intervention on only mental health-related symptom outcomes (e.g., depression, anxiety), (3) the target population was minority-specific or cultural specific, but not for rural populations, (4) the target population had pregnancy and/or birth-related cardiac conditions, (5) the abstract or complete text was not available, and (6) the barriers and challenges to recruitment and retention were not discussed. In addition, to establish the methodological quality of the articles used for the review, we used a rating system recommended by the agency for Healthcare Research and Quality (AHRQ){{946 West, S 2002}}. Two reviewers independently assessed and graded the quality of each article and discrepancies in the quality grades were resolved by further discussion. Studies with higher scores were included to the review.
The most frequent reasons for exclusion during title screening and abstract review were that (1) the target populations were not relevant to rural populations, (2) no abstract/article was available, (3) the article did not address barriers, and (4) the article did not discuss behavioral intervention trials. The selected articles were published between 2003 and 2014, and consisted of trials, review articles, and descriptive studies reporting barriers related to conducting clinical trials in rural communities as well as means to promote behavioral change and reduce CVD risk factors. The studies were mainly conducted in the United States [4, 11, 17–27], Canada [28], and Australia [29].
III. RESULTS
Overall, the proposed model (Figure 1) explicitly demonstrate that multi-level factors contribute to the lower participation rate of rural individuals in behavioral intervention trials compared to urban residents, which indicates the presence of unique recruitment/retention barriers in rural areas[3, 4, 9, 18, 20].
A. Contextual Factors
1) Patient Factors
It is difficult to recruit subjects who (1) are from minority ethnic groups and/or males [18, 22, 30], (2) have low health literacy [19, 20, 29, 31], (3) have low socioeconomic status [4, 18, 20, 20, 29], (4) have high disease burden [19, 20, 23], and (5) have other priorities in one’s personal life, such as personal issues or caregiver burden [19, 23]. Bergeron et al reported the percentage of rural residents with educational levels of high school and above is lower than individuals in urban areas [4]. Miyamoto et al. theorized that this reduced level of education could cause issues related to comprehension of research materials, often written with complicated medical jargon, and in turn, decrease the willingness of rural individuals to participate in clinical research studies [19]. In addition to disparities in education, several reported that in rural areas, more individuals live below the poverty line compared with urban areas, which could limit the ability of rural individuals to meet research-related demands, such as cost for transportation, diagnostic testing and medication, as well as the time required to participate in clinical trials [18, 19, 22, 23]. Further, two reports concluded that compared with urban residents, rural individuals are more likely to report reduced health statuses and suffer from chronic conditions that compromise their ability to participate clinical research studies [24, 32]. In some cases, competing priorities in caring for their own chronic conditions and those of family members, as well as busy farming and ranching schedules, precluded individuals from participation [19, 22, 29]. As a result, Pribulick concluded that “being too busy” was the most frequently reported dropout reason in her study [23].
2) Community Factors
In additional to patient level factors, living in remote area affects the participation of research study. Several articles reported difficulty in fulfilling recruitment requirement because rural residents often live in dispersed and sparsely populated areas [19, 23, 28, 29, 33]. Insufficient infrastructure and research resources are major barriers to conducting clinical trials in rural communities [18, 19, 29]. The cultural and social characteristics of diverse rural communities create further challenges in recruiting and retaining study participants [19]. Furthermore, when the intervention program is perceived as a duplicate service competing with a local existing service, the community is reluctant to accept the research program [19, 29]. On the other hand, rural participants are less likely to complete the intervention when their communities have limited resources to support behavioral change, such as limited access to unprocessed foods, lack of indoor exercise facilities, and increased cost of fresh fruits and vegetables [29].
3) Research Factors
Commonly reported research factors that influence recruitment and retention include (1) the study’s design, (2) complex research documents (e.g., informed consent, regulatory approvals, documentation), (3) strict ethical regulations (e.g., Health Insurance Portability and Accountability Act [HIPAA]), and (4) misperceptions of researchers on rural cultures and values [18, 19, 23, 28, 29, 29]. HIPAA requires the study recruiters must have the legal access to the potential participants [34]. It takes time and effort to identify, hire, and train the local recruiters, and the training process itself is lengthy [18, 19]. However, there is a short window of opportunity to recruit participants for studies. As a result, the multiple steps and the length of time required to meet HIPAA regulations contribute to missed opportunities for recruitment and delays in implementing the intervention [18, 19]. This lengthy process also discourages potential candidates from participating in clinical trials [18]. Furthermore, several studies implied that urban researchers often have misperceptions about rural cultures and values, which contributes to barriers in recruitment and retention of participants [4, 20]. Moreover, the recruitment process requires long hours of travels, vehicle expenses, overnight stays, and rigid schedules for the research team, resulting in challenges in recruitment and follow-up data collection [18, 19, 23]. Due to the extra cost of transportation, training local research staff, additional technology support, more intensive recruitment efforts, and extended study period, additional funds and human resources are needed to conduct research in rural areas compared with urban areas [4, 18–20, 23].
B. Perceived Information and Social Support
1) Potential Lack of Knowledge, Understanding, or Awareness
It was reported that members of rural communities have little to no prior exposure to research [19].Consequently, rural residents were more likely to lack knowledge, understanding, and awareness of clinical trials than the general public [4, 18, 19, 22, 23, 35]. Comis [36] reported that this lack of awareness of clinical trials is the one of the biggest obstacles to recruiting and retaining rural participants because it can result in uncertainty regarding the risks and benefits of taking part in the trials, leading to unwillingness to participate [23].
2) Lack of Provider Referrals
Physician referrals are one of the most effective ways to recruit participants to clinical trials [18, 31, 35]. However, the findings by Tanner [18] suggest the rural healthcare providers lack awareness of ongoing clinical trials. Lack of communication and miscommunication between investigators and rural providers can further hinder rural providers’ understanding of study trials [18, 28]. Without knowledge of how a study would benefit their patients and practice, rural health providers are reluctant to assist in recruitment [18, 19, 28]. As a result, Tanner [18] reported that the top perceived barrier to recruitment in rural areas was “doctors unaware of ongoing trials.”
3) Lack of Social Support
Several reports discuss resistance from family members as one of the reasons that rural residents decline to participate in a study [19, 28]. Tanner [18] reported that potential participants often seek support and reassurance from family members and friends during the decision-making process. Often family members and friends are acting out of concern for the wellbeing of their loved ones, as well as their own personal responsibilities (e.g., availability, being needed for transportation) [20]. Thus, family members and friends may discourage potential candidates from participating.
C. Participant Attitudes
Rural residents’ perceptions of health and health related research can affect their decision to participate in clinical trials [18, 20]. According to Long and Weinert [37], the cultural and life perspective that rural residents hold are unique compared with urban counterparts. With respect to rural healthcare practices, Long and Weinert [1989] identified several unique concepts, including work and health beliefs, self-reliance, outsider/insider, and old-timer/newcomer perceptions. In their study, they found rural residents generally believe health is attained through work, being productive, and maintaining their current functioning, therefore, work needs are often put above health needs [20, 37]. Thus, rural individuals may be reluctant to enroll and participate in research studies that interrupt work schedules [20, 22]. Further, rural individuals often desire independence and self-sufficiency [20, 37], as well as have a tendency to not trust “outsiders,” healthcare systems, and government agencies [20, 23]. As a result, more often, rural individuals are reluctant to accept help and services from “outsiders” and instead rely on their family, neighbors, and friends for healthcare needs and information, which affects their willingness to participate clinical trials conducted by “outsiders” or government agencies [20, 37]. In addition, other commonly reported attitudes toward clinical research are fears, concerns related to cost, potential harm, breach of confidentiality [18, 23], disbelief of intervention efficacy [19, 29], which contributes to the refusal to participate in clinical research.
D. Opportunity to Participate
1) Lack of transportation
The often distant, isolated areas where many rural residents reside affect their accessibility to healthcare and clinical trial sites [20, 37]. Lack of transportation is one of major barriers to recruiting rural residents to participate in clinical research studies [19, 20, 23]. Compared with urban residents, rural residents are less likely to have private or public transportation available [4]. Even with reliable transportation, it is still more costly for rural residents to travel longer distances as compared to urban areas [23, 29].
2) Lack of Technology Support
Without adequate technological support, the use of telehealth to conduct clinical trials in rural communities is not possible [28]. Several studies identify challenges in conducting telehealth-delivered interventional research, including the continual loss of internet connection, broken communication between the researcher and participant due to weak videoconferencing connection, lack of on-site staff to trouble-shoot technological issues, and lags in the internet connection [23, 28]. Thus, while telehealth can provide access to remote areas, connectivity issues can sharply hinder the use of this tool to conduct the intervention.
IV. DISCUSSION
This is the first report to apply a conceptual framework to guide a comprehensive review of the barriers to recruiting and retaining rural patients with CVD risks to participate in behavioral intervention trials. This review demonstrates distinct barriers encountered by research investigators when conducting clinical trials in rural areas. Like the conceptual model Ford et al. [11] used to explain barriers to recruiting underrepresented populations to cancer research, our proposed conceptual framework based on the Theory of Planned Behavior accounts for the barriers addressed in the relevant literature.
For the contextual factors, the patient level barriers include reduced (1) health literacy, (2) socioeconomic status, and (3) health status, as well as (4) competing priorities in personal lives (e.g., personal issues and caregiver burden). The four primary community level barriers include lack of (1) awareness of research studies, (2) research infrastructure, (3) local resources, and (4) environmental support for healthy living. Furthermore, because rural participants reside in sparsely populated remote areas, the potential participant pool in rural areas is very limited compared to urban areas. From a research perspective, a lack of resources is one of the common factors that hampers the participation rate of rural residents. As suggested by this review, additional time, effort, and extensive resources are required to conduct research in rural areas due to the extra cost of transportation, staff training, and technological support [4, 18–20, 23]. In turn, inadequate funding for research personnel can create great barriers to conducting research that focuses on rural individuals as subjects. The complex study regulations and documentation requirements are other common factors that impede the research team. Furthermore, urban researchers may lack understanding of rural culture and beliefs, which may contribute barriers in recruitment and retention [4, 20]
According to our conceptual framework (Figure 1), in addition to the aforementioned contextual factors, other predictors of intention to participate in research include perceived information, social support, attitudes, and opportunity to participate. Rural residents received limited information about the research, resulting in a lack awareness and understanding of behavioral intervention trials [4, 18, 19, 22, 23, 29]. The limited support from both healthcare providers and their social circles (e.g., family members, friends, and neighbors) further impact their intent to participate in research studies [18, 19, 29, 31]. Common attitudes affecting the participation rate are distrust and disbelief [19, 20, 23, 29, 37]. Furthermore, due to the lack of local resources, rural residents with CVD or related risks may become frustrated if the interventions are not helpful or feasible to follow without adequate support [19]. Therefore, it is important that researchers recognize these types of challenges in changing risk behaviors in rural, remote areas where resources are scarce. It has been reported that rural residents are given little opportunity to participate in behavioral trials due to the lack of accessibility and transportation to research sites [19, 20, 23]. The added burdens of financial concerns and time constraints are other barriers to participation in rural studies [23, 29]. The rural residents’ lives are often scheduled around farm work and they often need to prioritize work before they can attend to healthcare needs. For example, it is difficult to recruit, conduct interventions, or collect follow-up data during harvest time in the late summer and early fall [37]. Thus, to help overcome barriers, researchers need to be educated on the busiest times of the year, particularly for rural farmers and ranchers. For example, it would not be as beneficial to schedule a behavioral intervention during calving season if the rural community of interest is involved in raising cattle. Thus, the schedules of the individuals in the rural community need to be taken into account.
A. Limitations
We applied strict criteria to guide our literature search and focused our review on the distinct population of rural individuals; therefore, additional articles related to barriers to participation in clinical were not discussed if they did not meet the search criteria, particularly with foci on both rural participants and clinical behavioral interventions. Further, only a small number of studies report on barriers to recruitment and retention that are specific to behavioral reduction of CVD risks in rural areas. However, we used a systematic approach to locate appropriate articles, with the assistance of reference librarians and two research staff. The literature search process was intensive. Furthermore, each stage of the search was performed by at least two research personnel to cross-validate the quality of studies. Therefore, the authors are confident that this review includes a comprehensive list of studies conducted in rural communities that are specific to CVD risk reduction and behavioral intervention clinical trials. The generalizability and comprehensiveness of the review is also influenced by the selected studies that have their own limitations in terms of the heterogeneity of study design, quality of data collection and reporting, and rural population representativeness. Further, the rural communities are diverse in nature, and generalizations may not apply to each. For example, while identifying common barriers is expected help researchers moving forward, it is still important to consider that belief systems, even within the same geographical region, can vary from town to town, and that each rural community is unique. The most unifying factor would be that they are located in remote settings.
Still, despite the limited number of existing studies, this review is the first to provide a comprehensive overview of the general barriers against recruiting and retaining rural participants in clinical trials that promote CVD risk behavior reduction. Further, this review is the first to propose a conceptual framework to organize barriers that rural individuals encounter when participating in behavioral intervention trials.
B. Implications in Future Research
We developed the conceptual framework to help researchers identify potential barriers to the recruitment and retention within rural populations. Future studies can potentially utilize this framework to predict the participation rate and identify the barriers in conducting behavioral intervention among rural residents.
V. CONCLUSION
The evidence on effective interventions to reduce risk behaviors among rural CVD patients is very limited. Without strong and sufficient evidence, the development of effective programs and healthcare policies cannot be fully achieved [19]. Therefore, additional clinical trials with adequate sample sizes are needed to generate evidence to promote behavioral change. However, without overcoming the barriers for recruitment and retention, there is little opportunity to conduct fully powered research. Therefore, future studies are needed to improve recruitment and retention of rural participants with CVD risks.
Acknowledgments
This research was funded by National institute of Health and National Institute of Nursing Research (NIH/NINR) through grant number 1R15NR13769-01A1. The authors kindly thank Laura A Hull for the professional editing of this manuscript.
Biographies

Dr. Lufei Young, RN, APRN-NP, is an assistant professor in the College of Nursing at the University of Nebraska Medical Center. She received her PhD in nursing at University of Nebraska Medical Center, College of Nursing. Dr. Young has extensive training and expertise in various research methodologies, such as cost-effective analysis, meta-analysis, systematic review and structure equation modeling. Her research focus is to address health disparity in rural population living with chronic consuming conditions by developing patient centered care continuum, care-coordination process, and assisting self-management behavior change.
Melody Montgomery is the researcher and writer at the University of Nebraska Medical Center. She has been involved in grant proposal development.
Dr. Susan Barnason, FAHA, FAAN, is a professor in the College of Nursing at the University of Nebraska Medical Center.
Cynthia Schmidt, MD, is the reference librarian at the University of Nebraska Medical Center.
Van Do, MD, is a doctoral student of Health Services Administration and Policy Department in the College of Public, Health at the University of Nebraska Medical Center.
REFERENCES
- 1.National Center for Health Statistics. Health, United States, 2011: With Special Feature on Socioeconomic Status and Health. Hyattsville (MD): 2012. [PubMed] [Google Scholar]
- 2.Health Data Interactive [Google Scholar]
- 3.Baquet CR, Commiskey P, Daniel Mullins C, Mishra SI. Recruitment and participation in clinical trials: socio-demographic, rural/urban, and health care access predictors. Cancer Detect. Prev. 2006;30:24–33. doi: 10.1016/j.cdp.2005.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergeron C, Foster C, Friedman D, Tanner A, Kim S, Friedman, Bergeron Caroline, Foster Caroline, Daniela, Kim ATS. Clinical trial recruitment in rural South Carolina: a comparison of investigators’ perceptions and potential participant eligibility. Rural and Remote Health. 2013;13 [PubMed] [Google Scholar]
- 5.Folta SC, Lichtenstein AH, Seguin RA, Goldberg JP, Kuder JF, Nelson ME. The StrongWomen–Healthy Hearts program: reducing cardiovascular disease risk factors in rural sedentary, overweight, and obese midlife and older women. Am. J. Public Health. 2009;99:1271. doi: 10.2105/AJPH.2008.145581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tai-Seale T, Chandler C. Nutrition and overweight concerns in rural areas: a literature review. Rural Healthy People. 2010;2 [Google Scholar]
- 7.Cromartie J, Nelson PB. Baby boom migration and its impact on rural America. 2009 [Google Scholar]
- 8.Artinian NT, Fletcher GF, Mozaffarian D, Kris-Etherton P, Van Horn L, Lichtenstein AH, Kumanyika S, Kraus WE, Fleg JL, Redeker NS. Interventions to promote physical activity and dietary lifestyle changes for cardiovascular risk factor reduction in adults a scientific statement from the American Heart Association. Circulation. 2010;122:406–441. doi: 10.1161/CIR.0b013e3181e8edf1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baquet CR, Henderson K, Commiskey P, Morrow JN. Clinical trials: The art of enrollment. Seminars in Oncology Nursing. 2008:262–269. doi: 10.1016/j.soncn.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friedman LM, Furberg C, DeMets DL. Fundamentals of clinical trials. 2010 [Google Scholar]
- 11.Ford JG, Howerton MW, Lai GY, Gary TL, Bolen S, Gibbons MC, Tilburt J, Baffi C, Tanpitukpongse TP, Wilson RF, Powe NR, Bass EB. Barriers to recruiting underrepresented populations to cancer clinical trials: a systematic review. Cancer. 2008 Jan 15;112:228–242. doi: 10.1002/cncr.23157. [DOI] [PubMed] [Google Scholar]
- 12.Sateren WB, Trimble EL, Abrams J, Brawley O, Breen N, Ford L, McCabe M, Kaplan R, Smith M, Ungerleider R, Christian MC. How sociodemographics, presence of oncology specialists, and hospital cancer programs affect accrual to cancer treatment trials. J. Clin. Oncol. 2002 Apr 15;20:2109–2117. doi: 10.1200/JCO.2002.08.056. [DOI] [PubMed] [Google Scholar]
- 13.Armitage CJ, Conner M. Efficacy of the Theory of Planned Behaviour: a meta-analytic review. Br. J. Soc. Psychol. 2001 Dec;40:471–499. doi: 10.1348/014466601164939. [DOI] [PubMed] [Google Scholar]
- 14.Godin G, Kok G. The theory of planned behavior: a review of its applications to health-related behaviors. Am. J. Health Promot. 1996 Nov-Dec;11:87–98. doi: 10.4278/0890-1171-11.2.87. [DOI] [PubMed] [Google Scholar]
- 15.Ajzen I. The theory of planned behavior. Organ. Behav. Hum. Decis. Process. 1991;50:179–211. [Google Scholar]
- 16.Godin G. The theories of reasoned action and planned behavior: Overview of findings, emerging research problems and usefulness for exercise promotion. Journal of Applied Sport Psychology. 1993;5:141–157. [Google Scholar]
- 17.Befort CA, Klemp JR, Fabian C, Perri MG, Sullivan DK, Schmitz KH, Diaz FJ, Shireman T. Protocol and recruitment results from a randomized controlled trial comparing group phone-based versus newsletter interventions for weight loss maintenance among rural breast cancer survivors. Contemporary Clinical Trials. 2014;37:261–271. doi: 10.1016/j.cct.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tanner A, Kim S, Friedman DB, Foster C, Bergeron CD. Barriers to medical research participation as perceived by clinical trial investigators: communicating with rural and African American communities. J. Health Commun. 2014:1–9. doi: 10.1080/10810730.2014.908985. [DOI] [PubMed] [Google Scholar]
- 19.Miyamoto S, Henderson S, Young H, Ward D, Santillan V. Recruiting Rural Participants for a Telehealth Intervention on Diabetes Self-Management. The Journal of Rural Health. 2013;29:69–77. doi: 10.1111/j.1748-0361.2012.00443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dibartolo M, McCrone S. Recruitment of rural community-dwelling older adults: barriers, challenges, and strategies. Aging & Mental Health. 2003;7:75–82. doi: 10.1080/1360786031000072295. [DOI] [PubMed] [Google Scholar]
- 21.Gillies CL, Abrams KR, Lambert PC, Cooper NJ, Sutton AJ, Hsu RT, Khunti K. Pharmacological and lifestyle interventions to prevent or delay type 2 diabetes in people with impaired glucose tolerance: systematic review and meta-analysis. BMJ. 2007 Feb 10;334:299. doi: 10.1136/bmj.39063.689375.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parra-Medina D, D'antonio A, Smith SM, Levin S, Kirkner G, Mayer-Davis E POWER study. Successful recruitment and retention strategies for a randomized weight management trial for people with diabetes living in rural, medically underserved counties of South Carolina: the POWER study. J. Am. Diet. Assoc. 2004 Jan;104:70–75. doi: 10.1016/j.jada.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 23.Pribulick M, Willams IC, Fahs PS. Strategies to Reduce Barriers to Recruitment and Participation. Online J. Rural Nurs. Health. Care. 2010;10:22–33. [PMC free article] [PubMed] [Google Scholar]
- 24.Bull CN, Krout JA, Rathbone-McCuan E, Shreffler MJ. Access and issues of equity in remote/rural areas. The Journal of Rural Health. 2001;17:356–359. doi: 10.1111/j.1748-0361.2001.tb00288.x. [DOI] [PubMed] [Google Scholar]
- 25.Cudney S, Craig C, Nichols E, Weinert C. Barriers to recruiting an adequate sample in rural nursing research. Online Journal of Rural Nursing and Health Care. 2012;4:78–88. [Google Scholar]
- 26.Paskett ED, Cooper MR, Stark N, Ricketts TC, Tropman S, Hatzell T, Aldrich T, Atkins J. Clinical trial enrollment of rural patients with cancer. Cancer Pract. 2002;10:28–35. doi: 10.1046/j.1523-5394.2002.101006.x. [DOI] [PubMed] [Google Scholar]
- 27.King G, Farmer J. What older people want: evidence from a study of remote Scottish communities. Rural and Remote Health. 2009;9:1166. [PubMed] [Google Scholar]
- 28.Taylor D, Stone S, Huijbregts M. Remote participants' experiences with a group-based stroke self-management program using videoconference technology. Rural Remote Health. 2012;12:1–15. [PubMed] [Google Scholar]
- 29.Reddy P, Hernan AL, Vanderwood KK, Arave D, Niebylski ML, Harwell TS, Dunbar JA. Implementation of diabetes prevention programs in rural areas: Montana and south-eastern Australia compared. Aust. J. Rural Health. 2011;19:125–134. doi: 10.1111/j.1440-1584.2011.01197.x. [DOI] [PubMed] [Google Scholar]
- 30.Trauth JM, Jernigan JC, Siminoff LA, Musa D, Neal-Ferguson D, Weissfeld J. Factors affecting older african american women's decisions to join the PLCO Cancer Screening Trial. J. Clin. Oncol. 2005 Dec 1;23:8730–8738. doi: 10.1200/JCO.2004.00.9571. [DOI] [PubMed] [Google Scholar]
- 31.Befort CA, Bennett L, Christifano D, Klemp JR, Krebill H. Effective recruitment of rural breast cancer survivors into a lifestyle intervention. Psycho -Oncology. 2014 doi: 10.1002/pon.3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gamm L, Hutchison L, Dabney B, Dorsey A. Rural Healthy People 2010. 2003 See Http://srph.Tamhsc.edu/centers/rhp2010/Volume_3/Vol3rhp2010.Pdf. [Google Scholar]
- 33.Melvin CL, Corbie-Smith G, Kumanyika SK, Pratt CA, Nelson C, Walker ER, Ammerman A, Ayala GX, Best LG, Cherrington AL, Economos CD, Green LW, Harman J, Hooker SP, Murray DM, Perri MG, Ricketts TC Workshop Working Group on CVD Prevention in High-Risk Rural Communities. Developing a research agenda for cardiovascular disease prevention in high-risk rural communities. Am. J. Public Health. 2013 Jun;103:1011–1021. doi: 10.2105/AJPH.2012.300984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.The AHRQ Informed Consent and Authorization Toolkit for Minimal Risk Research [Google Scholar]
- 35.Reddy P, Dunn AB, White CM, Tsikouris JP, Giri S, Kluger J. An economic analysis of amiodarone versus placebo for the prevention of atrial fibrillation after open heart surgery. Pharmacotherapy. 2002 Jan;22:75–80. doi: 10.1592/phco.22.1.75.33498. [DOI] [PubMed] [Google Scholar]
- 36.Comis RL, Miller JD, Aldige CR, Krebs L, Stoval E. Public attitudes toward participation in cancer clinical trials. J. Clin. Oncol. 2003 Mar 1;21:830–835. doi: 10.1200/JCO.2003.02.105. [DOI] [PubMed] [Google Scholar]
- 37.Long KA, Weinert C. Rural nursing: developing the theory base. Sch. Inq. Nurs. Pract. 1989 Summer;3:113–127. [PubMed] [Google Scholar]