SUMMARY
SETTING
Tuberculosis (TB) patients and their contacts enrolled in nine states and the District of Columbia from 16 December 2009 to 31 March 2011.
OBJECTIVE
To evaluate characteristics of TB patients that are predictive of tuberculous infection in their close contacts.
DESIGN
The study population was enrolled from a list of eligible African-American and White TB patients from the TB registry at each site. Information about close contacts was abstracted from the standard reports of each site.
RESULTS
Close contacts of African-American TB patients had twice the risk of infection of contacts of White patients (adjusted risk ratio [aRR] 2.1, 95%CI 1.3–3.4). Close contacts of patients whose sputum was positive for acid-fast bacilli on sputum smear microscopy had 1.6 times the risk of tuberculous infection compared to contacts of smear-negative patients (95%CI 1.1–2.3). TB patients with longer (>3 months) estimated times to diagnosis did not have higher proportions of infected contacts (aRR 1.2, 95%CI 0.9–1.6).
CONCLUSION
African-American race and sputum smear positivity were predictive of tuberculous infection in close contacts. This study did not support previous findings that longer estimated time to diagnosis predicted tuberculous infection in contacts.
Keywords: epidemiologic factors, contact tracing, infection
TUBERCULOSIS (TB) control programs need to know which TB patient characteristics best predict transmission in order to allocate limited resources to screening of contacts most at risk for latent tuberculous infection (LTBI) and disease progression.1,2 Previously identified predictors of transmission include longer times from symptom onset to TB diagnosis, presence of acid-fast bacilli (AFB) in sputum smears, and pulmonary cavities on chest radiography.3–6
We hypothesized that TB cases with delayed diagnosis, defined as a longer time from symptom onset to TB diagnosis, would be more likely to transmit TB to their contacts. We evaluated delayed time to TB diagnosis as a risk factor for transmission of Mycobacterium tuberculosis from US-born African-American and White TB cases to their contacts.
STUDY POPULATION AND METHODS
The Tuberculosis Epidemiologic Studies Consortium (TBESC) was funded by the Centers for Disease Control and Prevention (CDC; Atlanta, GA, USA) to conduct research on TB prevention and control at 21 sites across the United States and Canada.7 One study focused on the differences in time to diagnosis between African Americans and Whites. This study was conducted at seven TBESC sites and three additional collaborating TB programs that accounted for 36.8% of TB cases among US-born Blacks and 15.8% of cases among US-born Whites reported to the CDC’s National Tuberculosis Surveillance System during the enrollment period from 16 December 2009 to 31 March 2011 (Table 1). The following analysis was conducted on contact investigation data collected for the study.
Table 1.
Catchment areas for participant recruitment
| TB patients (n = 277) | Contacts (n = 3301) | |||
|---|---|---|---|---|
| TBESC site | Recruitment area | Dates of enrollment | n | n |
| Maryland | Statewide, District of Columbia*, and Virginia (statewide)* | Maryland: December 2009–March 2011; District of Columbia: May 2010–March 2011; Virginia: January 2010–September 2010 | 42 | 417 |
| North Carolina | Statewide | December 2009–March 2011 | 56 | 334 |
| New Jersey | Statewide and Philadelphia, Pennsylvania* | New Jersey: December 2009–March 2011; Philadelphia: September 2010–March 2011 | 4 | 12 |
| Georgia | Clayton Health District, Cobb/Douglas Health District, DeKalb Health District, Fulton Health District, Gwinnett/Rockdale Health Districts, East Central Health District, West Central Health District, and North Central Health District | January 2010–March 2011 | 39 | 1751 |
| Tennessee | Shelby County, Davidson County, Mid- Cumberland Region: Stewart County, Houston County, Humphreys County, Montgomery County, Dickson County, Robertson County, Cheathum County, Williamson County, Sumner County, Truesdale County, Wilson County, and Rutherford County | Shelby County, Davidson County: December 2009–March 2011; Mid-Cumberland Region: December 2010–March 2011 | 37 | 257 |
| New York Texas | New York City | December 2009–March 2011 | 30 | 147 |
| City of Houston, and Dallas County | December 2009–March 2011 | 69 | 383 |
District of Columbia and Virginia were sub-contract sites of Maryland; Philadelphia, Pennsylvania was a sub-contract site of New Jersey.
TBESC = Tuberculosis Epidemiologic Studies Consortium; TB = tuberculosis.
To be eligible, patients listed on the site’s TB registry had to 1) have a TB diagnosis that met CDC case verification criteria,8 2) be US-born; 3) be non-Hispanic; 4) be African American, White, or a combination that included either race; 5) be at least 15 years old at the time of diagnosis; and 6) be first reported to a local health department as a presumptive TB case from 16 August 2009 to 31 December 2010.
The institutional review boards at the CDC and the local institutions approved the study. All living participants provided written informed consent.
Data collection on tuberculosis patients
TB patients were interviewed in person about their demographics, behavioral risk factors, medical history, TB symptoms and symptom onset dates, and care seeking for TB and other conditions. Clinical information was abstracted from health department records and CDC Reported Verified Case of Tuberculosis (RVCT) surveillance reports. For eligible persons who died before they could be interviewed, study researchers abstracted relevant information from death certificates, health department records, and RVCT reports.
Data collection on tuberculosis contacts
TB contacts’ demographics, places and duration of exposure to the TB patient, LTBI and TB disease history, and local determination of whether the contact was close, high risk, or high priority, were abstracted from health department contact investigation reports; no personal identifiers were collected.
Study variables
Tuberculous infection in a contact was determined from the results of the tuberculin skin test (TST) or either of the commercially available interferon-gamma release assays (IGRAs)—QuantiFERON® (Cellestis, Carnegie, VIC, Australia) and T-SPOT®.TB (Oxford Immunotec, Abingdon, UK)—as recorded in the health department contact investigation records. Contacts were defined as having tuberculous infection if they had 1) a positive interpretation noted on the first or second IGRA or TST; 2) ≥5 mm induration recorded on the first or second TST; or 3) a diagnosis of TB disease. Contacts were defined as not having tuberculous infection if they had negative interpretations of their most recent IGRAs or TSTs, or <5 mm induration recorded as the most recent TST result. Contacts with positive test results were regarded as recently infected with Mycobacterium tuberculosis if they did not have a previous positive TB test, LTBI diagnosis, or TB diagnosis.9
TB patients were asked to provide the month and year of symptom onset, and the 15th of the month was selected as the day. For those who did not remember or had died before the interview, the earliest symptom date in the medical record was used. The date of TB diagnosis was defined as the recorded date the patient first received TB medication. In preliminary analyses, time from symptom onset to diagnosis was treated as a continuous and dichotomous variable with values based on a review of the literature4,10 and the distribution of the study data. Two dichotomous variables were used for the final analyses: <2 months vs. ≥2 months, and <3 months vs. ≥3 months.
Statistical analysis
As outcomes of tuberculous infection in contacts of the same TB patient were unlikely to be independent of each other,4,11,12 generalized estimating equations (GEE) were used to control for intra-cluster dependence.13 Relative risks (RRs) and 95% confidence intervals (CIs) were calculated using adjusted and unadjusted GEE models. All predictor variables with a P value of <0.25 in unadjusted analyses were included in the adjusted model. Although time to diagnosis did not meet the threshold for inclusion, it was included in the model as it was the main exposure variable of interest. Final models were formed using a backward elimination strategy and the Z-statistic. Data were analyzed using Statistical Analysis Software (SAS, version 9.3; SAS Inc, Cary, NC, USA).
RESULTS
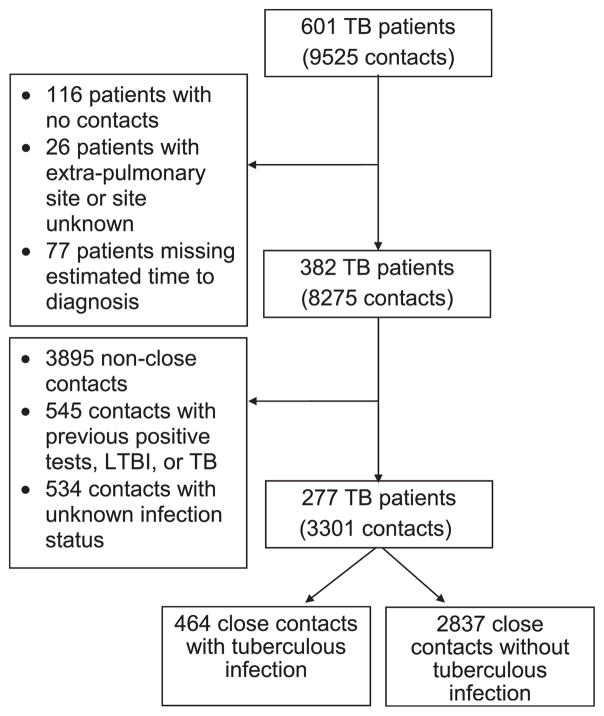
Of 1014 eligible patients, 128 (13%) died before they could be interviewed and 886 (87%) were approached for recruitment. A final 601 patients were enrolled, 126 deceased and 475 living, with a total of 9525 contacts.
Patients were excluded from analysis if 1) no contacts were listed (n = 116); 2) they had an unknown site of disease or had no pulmonary, laryngeal, or pleural involvement, as transmission from such patients was unlikely (n = 26);14,15 or 3) time to diagnosis could not be determined (n = 77) (Figure 1). After exclusion of these patients, 382 patients and 8275 contacts remained.
Figure 1.
Selection of TB patients and contacts: included TB patients and contacts are on the right, exclusions on the left. TB = tuberculosis; LTBI = latent tuberculous infection.
Contacts other than those designated by health departments as close, high priority, or high risk (hereafter, referred to as close contacts) were excluded, to focus on those most likely to acquire tuberculous infection (n =3895) (Figure 1).15–18 Also excluded were contacts likely to have old infections2 (previous positive TB test, LTBI diagnosis, or TB diagnosis, n=545) and those with unknown infection status (n = 534).
After these exclusions, 277 patients (46% of total) remained, 33 deceased and 244 living, and their 3301 (35% of total) close contacts. Compared to the patients excluded from the study, the included patients were younger at TB diagnosis, less likely to have had a previous TB diagnosis, less educated, more likely to report drug and alcohol use, more likely to report a cough lasting at least 2 weeks in the year before TB diagnosis, to have positive sputum smears, and to have pulmonary cavities (Table 2).
Table 2.
Characteristics of TB patients included and excluded in final analysis
| TB patients included (n = 277) | TB patients excluded (n = 324) | ||
|---|---|---|---|
| Characteristics | n (column %)* | n (column %)* | P value† |
| Race | |||
| African American | 214 (77) | 243 (75) | 0.52 |
| White | 63 (23) | 81 (25) | |
| Sex | |||
| Male | 184 (66) | 224 (69) | 0.48 |
| Female | 93 (34) | 100 (31) | |
| Age at TB diagnosis, years | |||
| <49 | 138 (50) | 111 (34) | 0.0003 |
| ≥49 | 138 (50) | 213 (66) | |
| Data missing | 1 (0) | 0 | |
| Highest level of schooling completed | |||
| <High school | 107 (39) | 112 (35) | 0.01 |
| ≥High school | 164 (59) | 187 (58) | |
| Data missing | 6 (2) | 25 (8) | |
| Homeless | |||
| Yes | 42 (15) | 52 (16) | 0.62 |
| No | 235 (85) | 271 (84) | |
| Data missing | 0 | 1 (0) | |
| Excess alcohol use | |||
| Yes | 93 (34) | 64 (20) | <0.0001 |
| No | 183 (66) | 249 (77) | |
| Data missing | 1 (<1) | 11 (3) | |
| Drug use | |||
| Yes | 99 (36) | 69 (21) | 0.0003 |
| No | 172 (62) | 243 (75) | |
| Data missing | 6 (2) | 12 (4) | |
| Intravenous drug use | |||
| Yes | 6 (2) | 12 (4) | 0.43 |
| No | 267 (96) | 305 (94) | |
| Data missing | 4 (1) | 7 (2) | |
| HIV status | |||
| Positive | 50 (18) | 67 (21) | 0.002 |
| Negative | 214 (77) | 217 (67) | |
| Data missing | 13 (5) | 40 (12) | |
| Sputum smear status | |||
| Positive | 161 (58) | 115 (35) | <0.0001 |
| Negative | 112 (40) | 185 (57) | |
| Data missing | 4 (1) | 24 (7) | |
| Pulmonary cavities | |||
| Yes | 127 (46) | 87 (27) | <0.0001 |
| No | 141 (51) | 186 (57) | |
| Data missing | 9 (3) | 51 (16) | |
| Previous diagnosis of TB | |||
| Yes | 12 (4) | 19 (6) | 0.04 |
| No | 261 (94) | 289 (89) | |
| Data missing | 4 (1) | 16 (5) | |
| Cough in year before TB diagnosis | |||
| Yes | 219 (79) | 106 (33) | <0.0001 |
| No | 49 (18) | 155 (48) | |
| Data missing | 9 (3) | 63 (19) | |
Column percentages may not add up to 100% due to rounding.
χ2 test.
TB = tuberculosis; HIV = human immunodeficiency virus.
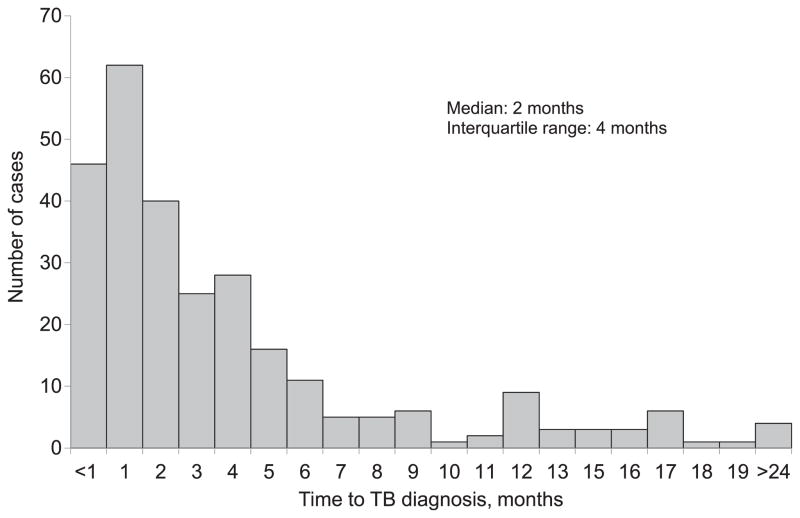
The 214 African-American and 63 White TB patients had a median of three close contacts (range 1–438). Compared to Whites, African Americans with TB disease were more likely to have human immunodeficiency virus (HIV) infection, be less educated, and younger (data not shown). The median estimated time to diagnosis was 2 months (range <1 month–237 months; interquartile range 4 months) (Figure 2). Of the 3301 close contacts included in the analysis, 464 (14%) had tuberculous infection; 22 (4.7%) of those infected had TB disease. Of the close contacts with TB disease, 19 (86.4%) were African-American, one contact (4.6%) was White, and two contacts (9.1%) were of unknown race. Of the 2870 close contacts who were negative on their initial TST/IGRA, 1104 (38.5%) did not have a second TST/IGRA result available. Sixty-three contacts (2%) who were negative on their initial TST/IGRA were positive on their second TST/IGRA. The majority of contacts (89%) were tested using TST; 5% of Whites and 3% of African-Americans were tested using IGRAs (P < 0.05). Although a large amount of information was missing for contacts for most variables (>65%), available data for sex (85% complete) and race (73% complete) showed that the majority of close contacts were male (63%) and African American (53%). Infection was more common among females than males (16% vs. 12%, P < 0.01), and among African Americans than Whites (15% vs. 9%, P < 0.0001). African-American TB patients (81%) were more likely than Whites (12%) to have African-American close contacts (P < 0.0001). In unadjusted GEE analysis, close contacts were at higher risk of tuberculous infection if their index TB patient was African-American, had pulmonary cavities, was sputum smear-positive, or reported drug use. Close contacts were at lower risk of tuberculous infection if their index patient was homeless or had a previous diagnosis of TB (Table 3). Time to diagnosis at the 3-month cut-off had a smaller P value (P = 0.44) than the 2-month cut-off (P = 0.70), and was therefore included in the adjusted model (Table 4).
Figure 2.
Distribution of estimated time to TB diagnosis. TB = tuberculosis.
Table 3.
Unadjusted association of TB patient characteristics with tuberculous infection in close contacts
| TB patients (n = 277) | Close contacts with tuberculous infection (n = 464) | Close contacts without tuberculous infection (n = 2837) | GEE unadjusted | |
|---|---|---|---|---|
| Characteristics | n (column %) | n (row %) | n (row %) | P value |
| Time from symptom onset to diagnosis, months | ||||
| ≥2 | 169 (61) | 320 (17) | 1546 (83) | 0.70 |
| <2 | 108 (39) | 144 (10) | 1291 (90) | |
| Time from symptom onset to diagnosis, months | ||||
| ≥3 | 129 (47) | 278 (19) | 1176 (81) | 0.44 |
| <3 | 148 (53) | 186 (10) | 1661 (90) | |
| Race | ||||
| African American | 214 (77) | 431 (15) | 2491 (85) | 0.01 |
| White | 63 (23) | 33 (9) | 346 (91) | |
| Sex | ||||
| Male | 184 (66) | 357 (14) | 2271 (86) | 0.62 |
| Female | 93 (34) | 107 (16) | 566 (84) | |
| Age at TB diagnosis, years | ||||
| <49 | 138 (50) | 352 (14) | 2098 (86) | 0.39 |
| ≥49 | 138 (50) | 112 (13) | 736 (87) | |
| Highest level of schooling completed | ||||
| <High school | 107 (39) | 221 (12) | 1674 (88) | 0.77 |
| ≥High school | 164 (59) | 242 (17) | 1143 (83) | |
| Homeless | ||||
| Yes | 42 (15) | 114 (18) | 517 (82) | 0.20 |
| No | 235 (85) | 350 (13) | 2320 (87) | |
| Excess alcohol use | ||||
| Yes | 93 (33) | 205 (15) | 1128 (85) | 0.99 |
| No | 183 (66) | 258 (13) | 1702 (87) | |
| Drug use | ||||
| Yes | 99 (36) | 231 (12) | 1670 (88) | 0.22 |
| No | 172 (62) | 227 (17) | 1138 (83) | |
| Intravenous drug use | ||||
| Yes | 6 (2) | 1 (5) | 19 (95) | 0.26 |
| No | 267 (96) | 461 (14) | 2808 (86) | |
| HIV status | ||||
| Positive | 50 (18) | 75 (11) | 632 (89) | 0.95 |
| Negative | 214 (77) | 385 (15) | 2161 (85) | |
| Sputum smear status | ||||
| Positive | 161 (58) | 353 (16) | 1849 (84) | 0.03 |
| Negative | 112 (40) | 111 (10) | 982 (90) | |
| Pulmonary cavities | ||||
| Yes | 127 (46) | 309 (16) | 1610 (84) | 0.03 |
| No | 141 (51) | 145 (11) | 1155 (89) | |
| Previous diagnosis of TB | ||||
| Yes | 12 (4) | 3 (6) | 51 (94) | 0.14 |
| No | 261 (94) | 460 (14) | 2761 (86) | |
| Cough in year before TB diagnosis | ||||
| Yes | 219 (79) | 395 (15) | 2209 (85) | 0.29 |
| No | 49 (18) | 65 (10) | 609 (90) | |
TB = tuberculosis; GEE = generalized estimating equations; HIV = human immunodeficiency virus.
Table 4.
TB patient characteristics in unadjusted and adjusted GEE model that describe tuberculous infection among close contacts
| Characteristics | GEE unadjusted RR (95%CI) | P value | GEE adjusted RR (95%CI) | P value |
|---|---|---|---|---|
| Time from symptom onset to diagnosis, months | ||||
| ≥3 | 1.14 (0.82–1.58) | 0.44 | 1.18 (0.86–1.63) | 0.31 |
| <3 | Referent | Referent | ||
| Race | ||||
| African American | 1.98 (1.20–3.27) | 0.01 | 2.09 (1.27–3.43) | 0.004 |
| White | Referent | Referent | ||
| Homeless | ||||
| Yes | 0.73 (0.46–1.18) | 0.20 | ||
| No | Referent | |||
| Drug use | ||||
| Yes | 1.24 (0.88–1.73) | 0.22 | ||
| No | Referent | |||
| Sputum smear status | ||||
| Positive | 1.50 (1.05–2.18) | 0.03 | 1.58 (1.10–2.27) | 0.01 |
| Negative | Referent | Referent | ||
| Pulmonary cavities | ||||
| Yes | 1.44 (1.03–2.02) | 0.03 | ||
| No | Referent | |||
| Previous diagnosis of TB | ||||
| Yes | 0.41 (0.13–1.34) | 0.14 | ||
| No | Referent |
TB = tuberculosis; GEE = generalized estimating equations; RR = risk ratio; CI = confidence interval.
In the adjusted model, close contacts of African-American TB patients had twice the risk of tuberculous infection as close contacts of White patients. Further analyses were conducted to determine the specific differences in factors associated with infectiousness between African-American and White TB patients. However, models stratified on race did not produce results that changed conclusions of the study. Close contacts of sputum smear-positive patients had 1.6 times the risk of tuberculous infection compared to close contacts of sputum smear-negative patients (Table 4). The TB patients’ time to diagnosis was not associated with tuberculous infection in their contacts (aRR 1.18, 95%CI 0.86–1.63). Controlling for study site in the adjusted model did not change these conclusions (data not shown).
DISCUSSION
The presence of AFB on sputum smear microscopy is a marker of both the severity of TB disease and its infectiousness.19 Sputum smear positivity has long been associated with increased risk for tuberculous infection in contacts, and our results support other studies that found an increased risk of tuberculous infection in contacts exposed to patients with advanced TB disease.2,5–7,16,18,20–23
While some studies have found an association between African-American race and TB transmission,4,5,21,24–27 others did not.19,28–32 Our study found that close contacts of US-born non-Hispanic African Americans were twice as likely to have tuberculous infection as close contacts of US-born non-Hispanic Whites; African-American patients were also more likely than White patients to have African-American close contacts. However, the association between race and tuberculous infection may be a reflection of the generally higher prevalence of LTBI in the African-American community rather than an indication of recent TB transmission. The 2008 National Health and Nutrition Examination Survey (NHANES) found that US-born non-Hispanic African Americans had a significantly higher prevalence of LTBI than non-Hispanic Whites (5.7% vs. 1.1%).33
Previous studies found that longer time to diagnosis results in TB transmission in the community. A Maryland study that included 54 US-born patients and their 310 close contacts reported that 40% of close contacts of patients who had a time to diagnosis of ≥90 days had positive TSTs compared to 24% of contacts with <90 days’ delay (P < 0.01).4 While our definition of a close, high risk, or high priority contact varied across sites, the local health departments in Maryland shared a similar definition of close contact,4 reducing the variance of exposure and increasing the power to find an association between time to diagnosis and TB transmission. The Maryland study reported more than twice as many contacts with tuberculous infection (35%)4 as found in our study (14%), while earlier studies reported even higher proportions of close contacts with tuberculous infection (18–43%).1,2,16,18 The lower proportion of infection in our study may reflect the continuing reduction in overall TB rates in the US-born population.34 In addition, our study used IGRAs as well as the TST to diagnose infection in contacts, while previous studies relied primarily on TST.1,2,4,16,18 Higher specificity has been reported for IGRAs than for the TST,35 and may have influenced our study’s tuberculous infection results. Lastly, our study had a smaller proportion of patients with delayed diagnosis (47%) than the Maryland study (56%).4 The lower proportion of delayed diagnosis in patients, coupled with the lower proportion of infection in contacts, may have contributed to our inability to determine time to diagnosis as a risk factor for TB transmission.
One possible limitation of our study is that over one third of the contacts had a negative result on their initial TSTor IGRA, and a second TSTor IGRA result was unavailable. Some of these contacts could thus have been misclassified as negative, as they might have been tested before their immune systems mounted a reaction to tuberculous infection. However, our data show that only a small percentage of contacts (2%) who had negative results on the initial TSTor IGRA had positive results on their second test. The effect of misclassification on our study results was therefore probably minimal.
Differences in testing methods by race could also have caused systematic bias. However, the great majority of contacts (89%) were tested using TST, and the difference in proportions of White contacts tested with IGRAs (5%) compared to African Americans (3%), while statistically significant, was small. An analysis that excluded contacts who received IGRAs did not change the study conclusions.
The study excluded a large number of patients (54%) and their contacts (65%) from the analysis. However, 36% of those who were excluded had no contacts listed, and 69% of the eliminated contacts were not close, high priority, or high risk. We therefore do not believe inclusion of these patients and contacts would have changed our study results.
Despite these limitations, a major strength of our study is that the study population included a large number of patients and their contacts from diverse jurisdictions, which supports both its reliability and generalizability.
CONCLUSIONS
Our large, multisite study found African American race and sputum smear positivity to be independent risk factors for M. tuberculosis infection in close contacts. Sputum smear positivity has long been incorporated into contact investigation screening algorithms, and our results support the findings of higher LTBI prevalence in US-born African Americans observed in previous publications. The lower proportion of infection in our study may correspond to the lower overall rates of TB in the US-born population, possibly a reflection of the success of the TB program. Similarly, the lower proportion of patients with delayed diagnosis may demonstrate the efforts made by clinicians and health departments to reduce the time from symptom onset to TB diagnosis and treatment in order to reduce TB disease severity and sequelae. The CDC has designated the treatment of LTBI as a major tool in achieving the national goal of TB elimination.9 Our study emphasizes the importance of contact investigations as a method to find persons with LTBI who could benefit from preventive treatment.
Acknowledgments
The authors thank the investigators and staff at all the TB Epidemiologic Studies Consortium (TBESC) participating sites from Atlanta, GA (G Albritton, H M Blumberg, M Leonard, J Mack, J Tapia); New York, NY (H Anger, P Colson, H Ortega); Research Triangle Park, NC (M Clayton, S Harris, B Levine, Q Xia); Centers for Disease Control and Prevention (S Ghosh, G B Grant, E Magee, J Wing); Houston, TX (E A Graviss, P Williams); Richmond, VA (S Keller, B Mayes); Dallas, TX (K Kemp, C Lovely); Baltimore, MD (W Cronin, F Maurer, B Munk, H Rutz); Nashville, TN (T Chavez-Lindell, J Pinilla, T Stein-Hart, J Warkentin); Newark, NJ (A Sevilla, J Vergeon); and Austin, TX (C Wallace).
This research was funded by the Centers for Disease Control and Prevention (CDC), TBESC.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC.
Conflicts of interest: none declared.
References
- 1.Bailey WC, Gerald LB, Kimerling ME, et al. Predictive model to identify positive tuberculosis skin test results during contact investigations. JAMA. 2002;287:996–1002. doi: 10.1001/jama.287.8.996. [DOI] [PubMed] [Google Scholar]
- 2.Gerald LB, Tang S, Bruce F, et al. A decision tree for tuberculosis contact investigation. Am JRespir Crit Care Med. 2002;166:1122–1127. doi: 10.1164/rccm.200202-124OC. [DOI] [PubMed] [Google Scholar]
- 3.Chin DP, Crane CM, Diul MY, et al. Spread of Mycobacterium tuberculosis in a community implementing recommended elements of tuberculosis control. JAMA. 2000;283:2968–2974. doi: 10.1001/jama.283.22.2968. [DOI] [PubMed] [Google Scholar]
- 4.Golub JE, Bur S, Cronin WA, et al. Delayed tuberculosis diagnosis and tuberculosis transmission. Int J Tuberc Lung Dis. 2006;10:24–30. [PubMed] [Google Scholar]
- 5.Cronin WA, Golub JE, Lathan MJ, et al. Molecular epidemiology of tuberculosis in a low- to moderate-incidence state: are contact investigations enough? Emerg Infect Dis. 2002;8:1271–1279. doi: 10.3201/eid0811.020261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rodrigo T, Cayla JA, Garcia de Olalla P, et al. Characteristics of tuberculosis patients who generate secondary cases. Int J Tuberc Lung Dis. 1997;1:352–357. [PubMed] [Google Scholar]
- 7.Katz D, Albalak R, Wing JS, Combs V. Setting the agenda: a new model for collaborative tuberculosis epidemiologic research. Tuberculosis (Edinb) 2007;87:1–6. doi: 10.1016/j.tube.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. Reported tuberculosis in the United States, 2011. Atlanta, GA, USA: US Department of Health and Human Services, CDC; 2012. [Google Scholar]
- 9.Centers for Disease Control and Prevention. Guidelines for the investigation of contacts of persons with infectious tuberculosis. Recommendations from the National Tuberculosis Controllers Association and CDC. MMWR Recomm Rep. 2005;54(RR-15):1–47. [PubMed] [Google Scholar]
- 10.Riley RL, Moodie AS. Infectivity of patients with pulmonary tuberculosis in inner city homes. Am Rev Respir Dis. 1974;110:810–812. doi: 10.1164/arrd.1974.110.6P1.810. [DOI] [PubMed] [Google Scholar]
- 11.Bosley AR, George G, George M. Outbreak of pulmonary tuberculosis in children. Lancet. 1986;1:1141–1143. doi: 10.1016/s0140-6736(86)91848-9. [DOI] [PubMed] [Google Scholar]
- 12.Packe GE, Patchett PA, Innes JA. Tuberculosis outbreak among Rastafarians in Birmingham. Lancet. 1985;1:627–628. doi: 10.1016/s0140-6736(85)92157-9. [DOI] [PubMed] [Google Scholar]
- 13.Liang K-Y, Scott LZ. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 14.Erkens CG, Kamphorst M, Abubakar I, et al. Tuberculosis contact investigation in low prevalence countries: a European consensus. Eur Respir J. 2010;36:925–949. doi: 10.1183/09031936.00201609. [DOI] [PubMed] [Google Scholar]
- 15.Braden CR. Infectiousness of a university student with laryngeal and cavitary tuberculosis. Investigative team. Clin Infect Dis. 1995;21:565–570. doi: 10.1093/clinids/21.3.565. [DOI] [PubMed] [Google Scholar]
- 16.Marks SM, Taylor Z, Qualls NL, Shrestha-Kuwahara RJ, Wilce MA, Nguyen CH. Outcomes of contact investigations of infectious tuberculosis patients. Am J Respir Crit Care Med. 2000;162:2033–2038. doi: 10.1164/ajrccm.162.6.2004022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Behr MA, Hopewell PC, Paz EA, Kawamura LM, Schecter GF, Small PM. Predictive value of contact investigation for identifying recent transmission of Mycobacterium tuberculosis. Am J Respir Crit Care Med. 1998;158:465–469. doi: 10.1164/ajrccm.158.2.9801062. [DOI] [PubMed] [Google Scholar]
- 18.Reichler MR, Reves R, Bur S, et al. Evaluation of investigations conducted to detect and prevent transmission of tuberculosis. JAMA. 2002;287:991–995. doi: 10.1001/jama.287.8.991. [DOI] [PubMed] [Google Scholar]
- 19.Carvalho AC, DeRiemer K, Nunes ZB, et al. Transmission of Mycobacterium tuberculosis to contacts of HIV-infected tuberculosis patients. Am J Respir Crit Care Med. 2001;164:2166–2171. doi: 10.1164/ajrccm.164.12.2103078. [DOI] [PubMed] [Google Scholar]
- 20.Aissa K, Madhi F, Ronsin N, et al. Evaluation of a model for efficient screening of tuberculosis contact subjects. Am J Respir Crit Care Med. 2008;177:1041–1047. doi: 10.1164/rccm.200711-1756OC. [DOI] [PubMed] [Google Scholar]
- 21.Ellis BA, Crawford JT, Braden CR, McNabb SJ, Moore M, Kammerer S. Molecular epidemiology of tuberculosis in a sentinel surveillance population. Emerg Infect Dis. 2002;8:1197–1209. doi: 10.3201/eid0811.020403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Driver CR, Balcewicz-Sablinska MK, Kim Z, Scholten J, Munsiff SS. Contact investigations in congregate settings, New York City. Int J Tuberc Lung Dis. 2003;7(Suppl 3):S432–S438. [PubMed] [Google Scholar]
- 23.Gessner BD, Weiss NS, Nolan CM. Risk factors for pediatric tuberculosis infection and disease after household exposure to adult index cases in Alaska. J Pediatr. 1998;132(3 Pt 1):509–513. doi: 10.1016/s0022-3476(98)70029-0. [DOI] [PubMed] [Google Scholar]
- 24.Small PM, Hopewell PC, Singh SP, et al. The epidemiology of tuberculosis in San Francisco. A population-based study using conventional and molecular methods. N Engl J Med. 1994;330:1703–1709. doi: 10.1056/NEJM199406163302402. [DOI] [PubMed] [Google Scholar]
- 25.Talarico S, Ijaz K, Zhang X, et al. Identification of factors for tuberculosis transmission via an integrated multidisciplinary approach. Tuberculosis (Edinb) 2011;91:244–249. doi: 10.1016/j.tube.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.El Sahly HM, Adams GJ, Soini H, Teeter L, Musser JM, Graviss EA. Epidemiologic differences between United States-and foreign-born tuberculosis patients in Houston, Texas. J Infect Dis. 2001;183:461–468. doi: 10.1086/318079. [DOI] [PubMed] [Google Scholar]
- 27.Borgdorff MW, Behr MA, Nagelkerke NJ, Hopewell PC, Small PM. Transmission of tuberculosis in San Francisco and its association with immigration and ethnicity. Int J Tuberc Lung Dis. 2000;4:287–294. [PubMed] [Google Scholar]
- 28.Geng E, Kreiswirth B, Driver C, et al. Changes in the transmission of tuberculosis in New York City from 1990 to 1999. N Engl J Med. 2002;346:1453–1458. doi: 10.1056/NEJMoa012972. [DOI] [PubMed] [Google Scholar]
- 29.Bishai WR, Graham NM, Harrington S, et al. Molecular and geographic patterns of tuberculosis transmission after 15 years of directly observed therapy. JAMA. 1998;280:1679–1684. doi: 10.1001/jama.280.19.1679. [DOI] [PubMed] [Google Scholar]
- 30.Weis SE, Pogoda JM, Yang Z, et al. Transmission dynamics of tuberculosis in Tarrant county, Texas. Am J Respir Crit Care Med. 2002;166:36–42. doi: 10.1164/rccm.2109089. [DOI] [PubMed] [Google Scholar]
- 31.Alland D, Kalkut GE, Moss AR, et al. Transmission of tuberculosis in New York City. An analysis by DNA fingerprinting and conventional epidemiologic methods. N Engl J Med. 1994;330:1710–1716. doi: 10.1056/NEJM199406163302403. [DOI] [PubMed] [Google Scholar]
- 32.Barnes PF, Yang Z, Preston-Martin S, et al. Patterns of tuberculosis transmission in Central Los Angeles. JAMA. 1997;278:1159–1163. [PubMed] [Google Scholar]
- 33.Bennett DE, Courval JM, Onorato I, et al. Prevalence of tuberculosis infection in the United States population: the national health and nutrition examination survey, 1999–2000. Am J Respir Crit Care Med. 2008;177:348–355. doi: 10.1164/rccm.200701-057OC. [DOI] [PubMed] [Google Scholar]
- 34.Alami NN, Yuen CM, Miramontes R, Pratt R, Price SF, Navin TR. Trends in tuberculosis—United States, 2013. MMWR Morb Mortal Wkly Rep. 2014;63:229–233. [PMC free article] [PubMed] [Google Scholar]
- 35.Mazurek GH, Jereb J, Vernon A, LoBue P, Goldberg S, Castro K. Updated guidelines for using interferon gamma release assays to detect Mycobacterium tuberculosis infection—United States, 2010. MMWR Recomm Rep. 2010;59(RR-5):1–25. [PubMed] [Google Scholar]