SUMMARY
SETTING
A large randomized controlled trial recently showed that for treating latent tuberculous infection (LTBI) in persons at high risk of progression to tuberculosis (TB) disease, a 12-dose regimen of weekly rifapentine plus isoniazid (3HP) administered as directly observed treatment (DOT) can be as effective as 9 months of daily self-administered isoniazid (9H).
OBJECTIVES
To assess the cost-effectiveness of 3HP compared to 9H.
DESIGN
A computational model was designed to simulate individuals with LTBI treated with 9H or 3HP. Costs and health outcomes were estimated to determine the incremental costs per active TB case prevented and per quality-adjusted life year (QALY) gained by 3HP compared to 9H.
RESULTS
Over a 20-year period, treatment of LTBI with 3HP rather than 9H resulted in 5.2 fewer cases of TB and 25 fewer lost QALYs per 1000 individuals treated. From the health system and societal perspectives, 3HP would cost respectively US$21 525 and $4294 more per TB case prevented, and respectively $4565 and $911 more per QALY gained.
CONCLUSIONS
3HP may be a cost-effective alternative to 9H, particularly if the cost of rifapentine decreases, the effectiveness of 3HP can be maintained without DOT, and 3HP treatment is limited to those with a high risk of progression to TB disease.
Keywords: rifapentine, cost-effectiveness, LTBI, model, computer simulation
Approximately 300 000 patients are treated for latent tuberculous infection (LTBI) each year in the United States,1 with the majority of the costs of treatment borne by tuberculosis (TB) control programs in the public health sector. The American Thoracic Society, the Centers for Disease Control and Prevention (CDC) and the Infectious Diseases Society of America have endorsed the treatment of LTBI to prevent the development of TB disease as a central strategy for controlling and eliminating TB in the United States.2
A large randomized controlled trial recently showed that a 12-dose combination regimen of weekly rifapentine (RPT) plus isoniazid (INH; 900 mg each, 3HP) administered as directly observed treatment (DOT) by a health care worker can be an effective alternative to the standard 9-month regimen of daily isoniazid (300 mg) self-administered by the patient (9H) for the treatment of LTBI among persons at high risk of progression to TB disease.3 Based on the evidence of this and other clinical trials,4,5 the CDC issued guidelines in December 2011 recommending 3HP given weekly with DOT from a health care worker as an equivalent treatment alternative to daily self-administered 9H (the longstanding standard of care) for otherwise healthy adults with LTBI who are at high risk for developing TB disease.6 The shorter treatment duration of the 12-dose regimen makes it an attractive alternative to 9H for TB control programs, as patients undergoing the 12-dose treatment are expected to have increased acceptance, better adherence and higher rates of treatment completion.3 On the other hand, at current prices the cost of medication is substantially higher for 12 doses of RPT than for 270 doses of INH, and the current recommendation that the 12-dose regimen be administered via DOT adds appreciably to the treatment cost of this regimen for TB control programs. We analyzed and report on the costs and health outcomes associated with each LTBI treatment regimen to inform clinicians, policy makers and public health programs about the relative cost-effectiveness of 9H and 3HP for the treatment of LTBI among individuals at high risk for progressing to TB in the United States.
METHODS
We compared the relative cost and effectiveness of the two LTBI treatment regimens, 9H and 3HP, using an individual-based stochastic simulation model implemented using NetLogo (Center for Connected Learning and Computer-Based Modeling, Northwestern University, Evanston, IL, USA),7 a free, publicly available modeling platform. The model tracks the costs and health outcomes of two simulated populations of 100 000 individuals with LTBI, one population treated with 9H and the other with 3HP, over 20 years following the start of LTBI treatment. The primary health outcomes considered were the occurrence of TB disease and the loss of quality-adjusted life years (QALYs) due to the burden of LTBI treatment, LTBI treatment-related toxicity, TB disease and death. Costs are reported from two perspectives: the health system perspective, which includes hospital and clinic personnel time, medication, supplies, and medical and diagnostic procedures; and the societal perspective, which consists of health system costs plus patient out-of-pocket expenses and the economic value of lost patient productivity. Costs are in 2010 US dollars, and all future costs and health outcomes have been discounted at an annual rate of 3%.
The model input parameters were derived from four sources: a clinical trial comparing 9H and 3HP among persons at high risk for TB disease,3 data from a TB control program in the Nashville Public Health Department (a recruitment site for the clinical trial), data provided by United States government agencies and published literature. The base case analysis used the input parameter values shown in Table 1 and yielded the primary results in Table 2. In sensitivity analysis, each input parameter was allowed to vary independently from its base case value over a specified range (Table 3) to explore the variability of model output in response to changing input values. We also conducted multivariate sensitivity analyses in which two or more input parameters were varied simultaneously. Confidence intervals (CIs) quantifying stochastic variability in the model output were obtained by bootstrap resampling of simulation data.
Table 1.
Inputs for a cost-effectiveness model comparing 3HP with direct observation by a health care worker to 9H for treatment of LTBI; values are those used in the base case analysis
| Cost | Societal cost US$ | Health system cost US$ | Source/reference* |
|---|---|---|---|
| LTBI treatment | |||
| Cost of initial visit (excluding medicines) | 203 | 166 | Nashville Public Health Department |
| Cost per follow-up visit (excluding medicines) | 50 | 28 | Nashville Public Health Department |
| DOT, cost per dose | 20 | 18 | Nashville Public Health Department |
| Cost per dose, 9H | 0.05 | 0.05 | Department of Veterans Affairs |
| Cost per dose, 3HP | 12.31 | 12.31 | Department of Veterans Affairs |
| LTBI drug toxicity | 8, 9 | ||
| Toxicity without hospitalization | 169 | 169 | |
| Toxicity with hospitalization | 6 650 | 5 677 | |
| Active TB | |||
| Diagnostic costs | 298 | 259 | 9–11 |
| Out-patient treatment | 2 958 | 2 725 | |
| Hospitalization (if required) | 28 831 | 25 495 | |
| Quality of life adjustments | QALY lost per year | 8 | |
|
|
|||
| Health state | |||
| LTBI treatment | 0.03 | ||
| Drug toxicity | 0.25 | ||
| Hospitalization | 0.50 | ||
| Treatment of active TB | 0.10 | ||
| Previous active TB | 0.05 | ||
| Baseline TB risk, years 1–3 after treatment start | % of patients | Annual risk of progression to TB % | |
|
|
|||
| 9H doses taken | Tuberculosis Trials Consortium Data Management Center | ||
| 0–90 | 17 | 0.36 | |
| 91–240 | 15 | 0.28 | |
| 241+ | 68 | 0.08 | |
| 3HP doses taken | Tuberculosis Trials Consortium Data Management Center | ||
| 0–8 | 16 | 0.13 | |
| 9+ | 84 | 0.05 | |
| LTBI drug toxicity, probabilities per treatment start | 9H | 3HP | |
|
|
|||
| Probability of drug toxicity | 5.5 | 8.2 | 3 |
| Probability of hospitalization from toxicity | 0.1 | 0.3 | 3 |
| Probability of death from drug toxicity | 0.014 | 0.014 | 12, 13 |
| Other | Value | ||
|
|
|||
| Age at LTBI treatment start | Distribution | 3 | |
| Probability of death from TB | 5.8% | Tuberculosis Epidemiologic Studies Consortium, CDC | |
| Background mortality rate | Age-dependent | 14 | |
| Patient time valuation (2010 US$) | |||
| Hourly (clinic visits) | $12.73 | 15 | |
| Daily (overnight hospitalization) | $139.00 | 16 | |
| Probability of hospitalization for TB | 0.49 | 9 | |
| Secondary TB cases per primary case | 0.46 | 17–20 | |
Unreferenced sources refer to unpublished data; see Acknowledgements.
3HP = 3 months of weekly isoniazid plus rifapentine; 9H = 9 months of daily, self-administered isoniazid; LTBI = latent tuberculous infection; DOT = directly observed treatment; TB = tuberculosis; CDC = Centers for Disease Control and Prevention.
Table 2.
Cost per TB case prevented and cost/QALY gained comparing 3HP with direct observation by a health care worker vs. 9H*
| Mean cost/patient (2.5%, 97.5%) | Mean QALY loss/1000 patients (2.5%, 97.5%) | Mean TB cases/1000 patients (2.5%, 97.5%) | Incremental cost/TB case prevented (2.5%, 97.5%) | Incremental/life year gained (2.5%, 97.5%) | |
|---|---|---|---|---|---|
| Health system perspective | |||||
| Regimen | |||||
| 9H | 511 (497, 522) | 44 (40, 47) | 9.1 (8.5, 9.8) | Reference | Reference |
| 3HP | 623 (616, 632) | 19 (17, 22) | 3.9 (3.5, 4.4) | 21 525 (16 807, 28 520) | 4 565 (3 584, 5 965) |
| Societal perspective | |||||
| Regimen | |||||
| 9H | 705 (691, 718) | 44 (40, 47) | 9.1 (8.5, 9.8) | Reference | Reference |
| 3HP | 728 (719, 737) | 19 (17, 22) | 3.9 (3.5, 4.4) | 4 294 (1 156, 8 908) | 911 (268, 1 826) |
Base case results using input values given in Table 1. Simulation run using 100 000 simulated individuals for each regimen with an analytic horizon of 20 years. All costs and health outcomes have been discounted at an annual rate of 3%. Costs are in 2010 US dollars.
TB = tuberculosis; QALY = quality-adjusted life year; 3HP = 3 months of weekly isoniazid plus rifapentine; 9H = 9 months of daily, self-administered isoniazid.
Table 3.
Results of univariate sensitivity analysis for cost per TB case prevented and cost per QALY gained comparing 3HP to 9H*
| Parameter | Health system perspective
|
Societal perspective
|
||
|---|---|---|---|---|
| Incremental cost/TB case prevented | Incremental cost/QALY gained | Incremental cost/TB case prevented | Incremental cost/QALY gained | |
| DOT cost/dose ($17.76) | ||||
| $0 | <0 | <0 | <0 | <0 |
| $35 | 56124 | 11902 | 38893 | 8248 |
| 3HP medication cost per dose ($12.31) | ||||
| $5 | 6 854 | 1 454 | <0 | <0 |
| $25 | 46992 | 9965 | 29762 | 6311 |
| $35 | 67061 | 14221 | 39796 | 8439 |
| TB hospitalization probability (49%) | ||||
| 0% | 32855 | 6983 | 17188 | 3653 |
| 75% | 15140 | 3207 | <0 | <0 |
| Proportional change in risk of progression to TB disease (100%) | ||||
| 50% | 47861 | 7163 | 19007 | 2845 |
| 150% | 8 002 | 2 001 | <0 | <0 |
| 200% | 1 941 | 594 | <0 | <0 |
| Patient hourly time value ($12.73) | ||||
| $0 | 21525 | 4565 | 18669 | 3959 |
| $7.50 | 21525 | 4565 | 10200 | 2163 |
| $17.00 | 21525 | 4565 | <0 | <0 |
| Secondary TB cases per primary case (0.46) | ||||
| 0 | 34895 | 6436 | 12153 | 2242 |
| 0.6 | 18753 | 4104 | 2665 | 583 |
| 9H completion proportion (68%) | ||||
| 63% | 14906 | 3248 | 1145 | 250 |
| 57% | 15170 | 3464 | 2648 | 605 |
| 51% | 11378 | 3183 | 1043 | 292 |
| 3HP completion proportion (83%) | ||||
| 76% | 16969 | 3209 | <0 | <0 |
| 69% | 19259 | 3922 | <0 | <0 |
| 62% | 15220 | 2518 | <0 | <0 |
| 3HP toxicity probability (8.2%) | ||||
| 3% | 16629 | 3749 | <0 | <0 |
| 13% | 25458 | 5158 | 7686 | 1557 |
| Probability of death from TB disease (5.8%) | ||||
| 2% | 21332 | 5655 | 3828 | 1015 |
| 15% | 21424 | 3856 | 3862 | 695 |
| Cost of hospitalization for TB ($25 495) | ||||
| $10000 | 28411 | 6025 | 11181 | 2371 |
| $40000 | 15078 | 3197 | <0 | <0 |
Base case values are reported in parentheses. Entries with values <0 (where the incremental cost-effectiveness ratios are negative) represent scenarios where 3HP is cost-saving compared to 9H. Simulation run for each set of parameter values with 100 000 simulated individuals for each regimen, with an analytic horizon of 20 years. All costs and health outcomes have been discounted at an annual rate of 3%. Costs are in 2010 US dollars. An expanded version of this table appears in the Appendix.
TB = tuberculosis; QALY = quality-adjusted life year; 3HP = 3 months of weekly isoniazid plus rifapentine; 9H = 9 months of daily, self-administered isoniazid; DOT = directly observed treatment.
At the start of the simulation, each individual is treated with 9H or 3HP. The age of each individual is drawn from the distribution of ages of participants at the time of enrollment in the clinical trial, and the number of doses taken follows the distribution of doses completed by clinical trial participants. Individuals are each assumed to undergo an initial clinic visit, and thereafter one follow-up visit for each month on treatment, with 3HP patients incurring additional costs related to the administration of each dose by DOT from the health care personnel. Costs associated with LTBI treatment clinic visits are based on data from the Nashville Public Health Department (Figure 1). Medication costs for RPT and INH ($0.05 per 9H dose, $12.31 per 3HP dose) are prices available to the US Department of Veterans Affairs through the Pharmaceutical Prime Vendor Program in 2010 (unpublished data, see Acknowledgements).
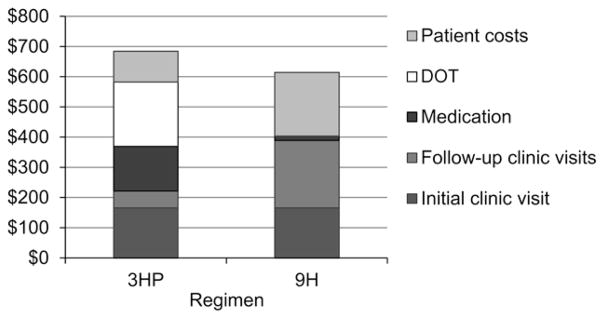
Figure 1.
Cost of a complete course of treatment for latent tuberculous infection with either 9H or 3HP administered by DOT from a health care worker. Patients receiving 9H have an initial clinic visit and eight later clinic visits; those receiving 3HP have an initial clinic visit, two later clinic visits and 12 visits by a health care worker for DOT. Patient costs include out-of-pocket expenses and lost productivity; other costs are costs to the health system. Costs are in 2010 US dollars. 3HP = 3 months of weekly isoniazid plus rifapentine; 9H = 9 months of daily, self-administered isoniazid; DOT = directly observed treatment.
While undergoing treatment for LTBI, simulated individuals have a probability of drug toxicity or hospitalization based on the frequency of treatment-related adverse events and hospitalizations among clinical trial participants.3 As no treatment-related deaths were observed in the clinical trial, simulated individuals receiving LTBI treatment with either 9H or 3HP were assumed to experience a risk of death from drug toxicity equal to that previously observed in patients taking INH for up to 12 months (Table 1).12,13
During each year of simulation, individuals have a risk of progressing to TB disease that depends on the regimen and number of doses completed; for each infected individual, the risk of progression from LTBI to TB disease is assumed to be highest soon after infection and to decline over time.21 Individuals developing TB disease are assumed to receive treatment and incur costs based on this treatment, and to experience an elevated risk of death from TB disease. All individuals experience an age-specific annual risk of death from causes unrelated to TB.14 Each case of primary TB disease is assumed to give rise to additional secondary cases due to transmission based on data from genotyping studies and contact investigations.17–20 For the societal perspective only, patient time spent in clinic visits, receiving DOT or being hospitalized due to severe LTBI drug toxicity or TB disease is valued based on economic productivity estimates for the US population (Table 1).15,16 Individuals experiencing drug toxicity or TB disease are assumed to have additional costs and reduced quality of life. Quality of life estimates used are those published in a previous cost-effectiveness analysis of LTBI treatment regimens (Table 1).8
A detailed description of the model and its input parameters, as well as the model itself with modifiable parameters and the associated source code, are available in the Appendix to this report.*
As human subjects were not involved, ethics approval was not required for this study.
RESULTS
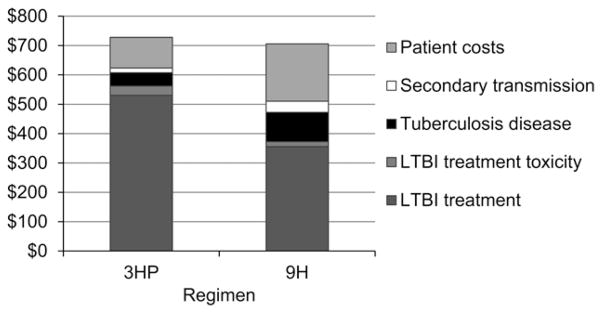
In the base case analysis, using the specified input parameter values (Table 1), treatment with 3HP prevents an average of 5.2 (95%CI 4.5–5.9) additional TB cases and 25 (95%CI 21–29) lost QALYs per 1000 LTBI treatment starts when compared to 9H. From the health system perspective, 3HP costs more than 9H by $112 (95%CI 99–129) per person treated, while from the societal perspective 3HP is more costly by $23 (95%CI 7–41) per person treated (Table 2). The upfront cost of LTBI treatment is higher for 3HP than for 9H, with 3HP recovering some of those costs over time by preventing more cases of active TB (Figure 2).
Figure 2.
Total costs per person treated for LTBI. Average costs are over a 20-year period for individuals treated for LTBI with either 9H or 3HP administered by DOT from a health care worker. Costs include those of LTBI treatment and the eventual development of tuberculosis disease in some individuals. Patient costs include out-of-pocket expenses and lost productivity; other costs are costs to the health system. Based on 100 000 simulated individuals per regimen with a 20-year analytic horizon; costs are reported in 2010 US dollars and have been discounted at an annual rate of 3%. LTBI = latent tuberculous infection; 3HP = 3 months of weekly isoniazid plus rifapentine; 9H = 9 months of daily, self-administered isoniazid; DOT = directly observed treatment.
From the health system perspective, the incremental cost per TB case prevented by 3HP vs. 9H is $21 525 (95%CI 16 807–28 520), and the cost per QALY gained is $4565 (95%CI 3584–5965). From the societal perspective, the incremental cost per TB case prevented by 3HP vs. 9H is $4294 (95%CI 1156–8908), and the cost per QALY gained is $911 (95%CI 268–1826; Table 2). Although there is no official threshold for determining the cost-effectiveness of public health interventions in the United States, interventions costing less than $50 000/QALY gained have been deemed cost-effective by some health economists.22 Until estimates of willingness to pay and opportunity costs of expenditures on LTBI treatment become available, our analysis suggests that 3HP might be a cost-effective and affordable alternative to 9H for the treatment of LTBI in individuals at high risk for developing TB in the United States.
In univariate sensitivity analyses, individual input parameters were independently varied while holding all other inputs at their base case values. The results of the univariate sensitivity analyses are shown in Table 3, with the shaded cells denoting scenarios where 3HP is cost-saving compared to 9H. The incremental cost per TB case prevented by 3HP compared to 9H ranged widely, but the incremental cost per QALY gained was consistently below $20 000.
The cost of RPT and the cost of providing DOT are both very influential in determining the cost-effectiveness of 3HP relative to 9H, as they are both upfront costs incurred with each dose taken and apply only to patients receiving 3HP. A decrease in the cost of RPT or the cost of DOT has the potential to improve the cost-effectiveness of 3HP considerably. An RPT cost of $10 per 900 mg dose is the value at which 3HP becomes cost-saving relative to 9H from the societal perspective, and setting the DOT cost to $0 (as for self-administered treatment) renders 3HP cost-saving compared to 9H from both the health system and societal perspectives. On the other hand, the current average wholesale price of RPT is $24.89 per 900 mg dose,23 about double the cost used in the base case analysis based on prices available to the United States Department of Veterans Affairs. Similarly, approximately doubling the cost of DOT from $17.76 per dose to $35 per dose more than doubles the incremental costs of 3HP per TB case prevented and per QALY gained compared to 9H (Table 3).
The baseline risk of progression from LTBI to TB disease was varied from 50% to 200% of the base case value for all individuals, regardless of regimen, to explore the possible effects of treating individuals with lower or higher risk of progression from LTBI to TB disease than those studied in the clinical trial. When the risk of progression to TB disease is lower, the cost-effectiveness of 3HP decreases substantially. As the risk of progression to TB disease increases for all individuals, 3HP is found to be increasingly cost-effective relative to 9H. Similarly, higher rates of secondary transmission and higher costs of treating TB disease lead to 3HP being more cost-effective relative to 9H (Table 3).
Decreasing levels of adherence to either 9H or 3HP while maintaining other model inputs at their base case values leads to more TB cases, but the increased cost due to TB disease is offset in part by lower LTBI treatment costs. However, as there are very few data on the TB risk of individuals completing a partial course of 3HP, it is difficult to predict the effect of decreased adherence, making sensitivity analyses around 3HP adherence particularly speculative.
In the referenced clinical trial, patients receiving 3HP developed TB at approximately half the rate of those receiving 9H, and our base case analysis is based on these data. The clinical trial investigators concluded that 3HP is at least as effective in preventing TB disease as 9H.3 In sensitivity analysis, we tested the case that 3HP and 9H have equivalent effectiveness by setting the baseline risk of progression to TB disease for both regimens to the level observed among all individuals receiving 9H in the clinical trial. Under this scenario, as expected, there was on average an equal number of TB cases among individuals receiving 3HP as 9H, and the incremental cost per QALY gained increased while remaining below $20 000. Additional results and discussion of multivariate sensitivity analyses are provided in the Appendix.
DISCUSSION
The 3HP regimen is more costly than 9H due mainly to the higher cost of medication and the current recommendation that doses be administered by DOT from health care personnel. Over the long term, 3HP recovers some of these upfront costs by preventing more cases of TB. When compared to 9H, 3HP reduces the number of clinic visits during LTBI treatment and the number of future cases of TB.
Over a wide range of sensitivity analyses, the incremental cost per QALY gained by 3HP compared to 9H was found to be less than $20 000. As 3HP, when compared to 9H for the treatment of LTBI, incurs incremental costs per QALY gained that are within the range of values for many commonly accepted public health interventions, 3HP could be judged to be a cost-effective alternative to 9H for the treatment of LTBI.22
Lowering the cost of RPT to public health programs has the potential to dramatically improve the cost-effectiveness of the 3HP regimen. Similarly, if it is possible to eliminate or relax the requirement for DOT while maintaining acceptable levels of adherence, effectiveness and safety, the cost-effectiveness of 3HP would improve substantially. For instance, in the base case the model assumes that each individual receives each dose by DOT from a health care worker who travels to the patient. If the patient is instead assumed to receive one 3HP dose by DOT during each scheduled clinic visit (so that a complete 12-dose course of 3HP involves 9 visits from a health care worker rather than 12), then 3HP becomes cost-saving relative to 9H from the societal perspective.
In populations where the risk of developing TB is lower than among the high-risk patients enrolled in the clinical trial,3 the cost-effectiveness of 3HP is projected to be less favorable because the number of cases of TB prevented by 3HP (relative to 9H) will be lower. Conversely, in populations with a higher risk of developing TB, the cost-effectiveness of 3HP has the potential to be more favorable because the number of TB cases prevented by 3HP (relative to 9H) will be higher. Care should be taken in extrapolating our findings to high-risk subpopulations (e.g., children and people infected with the human immunodeficiency virus), for whom a limited amount of data is available on the safety and effectiveness of 3HP. Our analysis projects that the costs related to LTBI drug toxicity are relatively minor for both regimens in comparison to the costs arising from LTBI treatment and future TB disease (Figure 2).
While 3HP appears to be an attractive alternative to standard LTBI treatment with 9H from the health system and societal perspectives, local and individual circumstances need to be considered when choosing a regimen. For example, drug costs, the cost of providing DOT, program feasibility, perceived risk of progression to TB disease and the length of the window of opportunity for intervention are important factors when making decisions regarding LTBI treatment. To further refine strategies and decisions regarding LTBI treatment by programs and clinicians, additional studies are needed to assess both the adherence to and the level of protection offered by a self-administered 3HP regimen, and the relative risk of progressing to TB disease among various groups of persons with LTBI.
Acknowledgments
The authors acknowledge the valuable assistance of the Nashville Public Health Department for gathering detailed data about the cost of LTBI treatment; J Jolly of the US Department of Veterans Affairs for providing information about drug costs; the TB Trials Consortium Data Management Center for detailed, unpublished information on the number of doses taken by study participants; S Beavers of the TB Epidemiologic Studies Consortium in the Division of TB Elimination (DTBE) at the Centers for Disease Control and Prevention (CDC) for recent, unpublished data on death rates due to TB disease in the United States; A Vernon, P LoBue, K Castro, K Cain, A Hill and J Becerra, DTBE, CDC, Atlanta, GA, USA; D Menzies and K Schwartzman, Montreal Chest Institute and McGill University, Montreal, QC, Canada; O Oxlade, Harvard School of Public Health, Boston, MA, USA; C Gopalappa, Futures Institute, Glastonbury, CT, USA; and F Shepardson for critical evaluation and feedback, and L Nguyen, Mount Holyoke College, South Hadley, MA, USA, for assistance with NetLogo modeling. D Shepardson thanks the Steven M Teutsch Prevention Effectiveness Fellowship Program at the CDC, and Mount Holyoke College.
Funding for this study was provided by the DTBE, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, CDC, Atlanta, GA, USA. No funding bodies had any role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The findings and conclusions in this article are those of the authors and do not necessarily represent the views of the CDC or the Agency for Toxic Substances and Disease Registry.
Disclosure: Sanofi-Aventis provided a gift to the CDC Foundation and in-kind drug donation for the Prevent TB clinical trial on which this secondary analysis is based, but provided no direct contribution to support the current study.
APPENDIX. MODEL INPUTS AND ASSUMPTIONS
Tuberculosis disease progression
Tuberculosis (TB) activation rates for the first 3 years after treatment initiation are taken to be the rates per 100 person-years observed in the clinical trial population1 based on the number of doses taken. The breakdown by doses completed was obtained from the TB Trials Consortium Data Coordinating Center and is presented in Table A.1. Activation risk is assumed to drop by 50% each year thereafter, to a minimum of 5% of the original (year 0) risk, based on the rate of decline in case rates among foreign-born persons after arrival in the United States, as presented in Figure A.1 from Cain et al.2 The assumption of an annual decrease of 50% and minimum TB rate of 5% of the original gives a steeper decline and lower floor than appears in Cain et al.,2 making it conservative with respect to the 3HP regimen (12-dose regimen of weekly rifapentine plus isoniazid), as a higher background TB activation rate improves the relative cost-effectiveness of 3HP in the base case analysis.
Individuals developing active TB were assumed to receive treatment, and costs were assessed accordingly (see below). Individuals developing active TB were assumed to have a one-time risk of death of 5.8% for a reason related to their TB disease in the same simulated year, the most up-to-date estimate for the US population based on unpublished surveillance data (personal communication, S Beavers, Surveillance, Epidemiology, and Outbreak Investigations Branch, Division of Tuberculosis Elimination, Centers for Disease Control and Prevention [CDC]) finding that 5.8% of US TB patients die of TB-related causes either before diagnosis or during anti-tuberculosis treatment. In sensitivity analysis, this risk of death varied between 2% and 15%, with relatively minor effects on the relative cost-effectiveness of 3HP as compared to 9H (see Table A.10, and Table 3 in the main article).
Lost productivity due to premature death from TB is not included in the model. These estimates are sometimes included in economic analyses conducted from the societal perspective, based on expected lifetime production of individuals who die prematurely, and can dramatically improve the apparent cost-effectiveness of health interventions. Grosse et al. estimate the present value of lifetime production for individuals in their early 40s at approximately 1 million 2007 US dollars, with a 3% annual discount rate.3 Estimates of future individual productivity, however, are somewhat speculative and do not include future societal costs that individuals dying prematurely would otherwise have incurred over a longer lifetime, and can therefore give an inflated estimate of the total societal cost of premature deaths. Inclusion of lost productivity due to premature TB-related death would tend to improve the cost-effectiveness of 3HP relative to 9H (9 months of daily, self-administered isoniazid) under the base case assumption that 3HP prevents more future cases of active TB and deaths from TB disease.
Table A.1.
Tuberculosis case rates and doses taken for patients participating in a clinical trial comparing 3HP with 9H;1 the follow-up period was 33 months
| Cases | py follow-up | Cases per 100 py follow-up | |
|---|---|---|---|
| 9H | |||
| 0–90 doses | 5 | 1377 | 0.36 |
| 91–240 doses | 4 | 1413 | 0.28 |
| 241+ doses | 6 | 7134 | 0.08 |
| 3HP | |||
| 0–8 doses | 2 | 1503 | 0.13 |
| 9+ doses | 5 | 9116 | 0.05 |
3HP = 12-dose regimen of weekly rifapentine plus isoniazid; 9H = 9 months of daily, self-administered isoniazid; py = person-years.
Figure A.1.
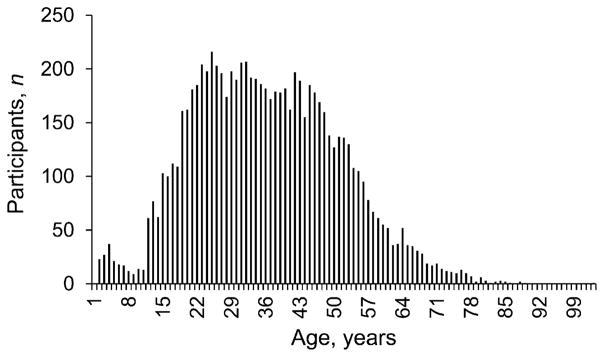
Distribution of ages of clinical trial participants at the time of enrollment in the clinical trial: mean: 36 years; median: 35 years; maximum: 103 years; minimum: 2 years. Data provided by the Tuberculosis Trials Consortium Data Coordinating Center.
Secondary cases due to transmission
The average number of secondary TB cases due to transmission from an individual with active TB, including secondary cases that develop soon after the transmission occurs and secondary cases that develop later due to latent tuberculous infection (LTBI) acquired directly from the primary source case, were estimated from the following sources.
Recent cross-sectional studies of TB genotyping data in the United States estimate that 23% of TB cases in the United States (13.2% in the US-born population and 9.8% in the foreign-born population) can be attributed to recent transmission (as opposed to reactivation of previously acquired LTBI).4,5 Assuming that US TB incidence rates are nearly constant over the short term and that cases due to recent transmission were infected by previously documented US TB cases, each primary case must generate 0.23 secondary TB cases in the short term via recent transmission. Under the common assumption that an equal number of cases will arise due to later activation of LTBI acquired via transmission (see, for example, Dye6), each primary active TB case will eventually lead to 0.46 secondary TB cases.
A recent study in the Netherlands used genotyping data for 5185 pulmonary TB patients to determine that on average 0.24 secondary TB cases occur per primary case within 2 years of diagnosis of the primary case.7 Assuming, as above, that an equal number of secondary cases will arise due to later activation of transmitted LTBI, this suggests that each primary active TB case eventually leads to 0.48 secondary TB cases. Although TB incidence in the Netherlands is 7.3 per 100 000 population, higher than the US rate of 4.1/100 000, the estimated fraction of cases due to recent transmission is very similar in the US population4,5 and the Dutch population.7
A 1999 genotyping study involving TB patients in San Francisco, CA, United States, found 183 secondary cases and 1059 primary cases, or 0.17 secondary cases per primary case over the period 1991–1996.8 Assuming that an equal number of cases will arise due to later activation of transmitted LTBI, we get 0.34 secondary TB cases per primary case.
A 2002 study of contacts of 349 US patients with culture-positive pulmonary TB yielded 3824 contacts.9 Of these, 2095 were screened, with 24 found to have active TB and 655 found to have LTBI (initial tuberculin skin test [TST] positive or skin test converter). Extrapolating these results to those who were not screened, we would expect 44 TB cases and 1196 contacts with LTBI from the 349 primary TB cases. Assuming that 10% of those with LTBI will eventually develop active TB, this gives 164 secondary TB cases from 349 primary cases, or 0.47 secondary TB cases per primary case.
In a 2000 study of close contacts of pulmonary smear-positive TB patients in the United States,10 the mean number of close contacts per patient was 6. Total TST positivity among contacts was 36%, and 2% had active TB. On this basis, and assuming, as is conventional,6 that 10% of those who are TST-positive will eventually develop active TB, the number of secondary TB cases per smear-positive primary case is estimated to be (2%*6 + 36%*10%*6) = 0.34. A 1999 study involving TB patients in San Francisco found that smear-negative primary TB cases were responsible for 17% of TB transmission,8 which suggests that the transmission rate for smear-negative individuals is 0.07. A 2004 study in Canada found 17–22%,11 while a 2008 study in the Netherlands found 13%.12 As 33% of US TB cases are thought to be smear-positive,13 the number of secondary cases per primary case is therefore estimated to be (0.33*0.34 + 0.67*0.07) = 0.16. This study reported infection and disease status only for close contacts (45% of all contacts identified) of primary TB cases; unpublished data from the same study (personal communication, S Marks, Division of TB Elimination, CDC) shows that 21% of casual contacts were TST-positive and 0.24% had active TB. There were seven casual contacts per index case. Using the same calculations described above, we get an additional (0.24%*7 + 21%* 10%*7) = 0.16 secondary TB cases per smear-positive primary case from smear-positive casual contacts, and (0.33* 0.16 + 0.67*0.03) = 0.07 secondary TB cases per primary case (any smear status) in casual contacts. Thus, when casual contacts are included, this gives 0.23 secondary TB cases per primary TB case among those identified as contacts. The estimates in both #4 and #5 are subject to the limitations that not all contacts with TB or LTBI will have acquired this via transmission from the index case, and the set of contacts investigated may not include every individual who will eventually develop TB due to transmission from an index case.
In a modeling paper from 1998,14 a differential equation model for TB in the US population was fitted to US TB surveillance data. The authors found that R = 0.55 achieved a good fit for most of the previous century’s TB incidence data, where R represents the number of secondary TB cases per primary TB case. They used R = 0.55 to project 1.2 additional TB cases per primary TB case, assuming an infinite transmission chain, whereas we consider only secondary TB cases from transmission in the present analysis.
The base case value used in the model was 0.46 secondary TB cases due to transmission per primary case based on the estimate using genotyping data for the US population (#1), which is consistent with estimates based on genotyping in the Netherlands (#2) and one study of contacts to individuals with TB in the United States (#4). In consideration of the alternative estimates and sources above, the parameter was varied over the range 0.0–0.6 in sensitivity analysis. For the purpose of discounting, one third of secondary TB cases due to transmission are assumed to occur immediately following exposure to the infected primary case, with an additional third after 5 years, and the remaining third after 25 years. The model does not consider transmission beyond a secondary case. Inclusion of additional links in the transmission chain would favor the regimen that prevents more primary cases of TB in the cost-effectiveness analysis.
Patients’ indirect costs
Hourly indirect costs for LTBI patients due to lost productivity during LTBI treatment are estimated based on average per capita income for the United States,15 based on dividing 2010 annual per capita income ($26 487) by 2080* annual working hours to get $12.73 per hour. Lost patient productivity due to overnight or multiple-day hospitalization is valued at $139 per day hospitalized based on economic productivity estimates for the US population in 2007,3 with values adjusted for inflation to 2010 US dollars based on average weekly earnings from the Current Population Survey.16
LTBI treatment
LTBI treatment costs
LTBI treatment costs for 3HP and 9H were calculated based on detailed personnel time and supply cost data collected from the Nashville Public Health Department. Fixed overhead costs were not included in the analysis. Simulated patients in the model underwent an initial clinic visit and one monthly follow-up clinic visit for each month of treatment for LTBI with either 9H or 3HP. The initial clinic visit included chest X-ray, liver function test (LFT), complete blood count (CBC), human immunodeficiency virus (HIV) testing, and examination by a provider. Follow-up clinic visits included examination by a clinic nurse, and for 5% of follow-up clinic visits additional laboratory work and examination by a provider was also required. Laboratory and supply costs for initial and monthly follow-up clinic visits are tabulated in Table A.2. Personnel and patient time for initial and follow-up clinic visits are given in Table A.3 and Table A.4, respectively.
Salaries for clinic personnel were estimated based on the Occupational Employment Statistics data reported by the Bureau of Labor Statistics,18 and are given in Table A.5. Personnel costs were calculated including wages plus estimated fringe benefits, as recommended in Haddix et al.19
Personnel costs for initial and follow-up clinic visits were calculated by multiplying personnel hours per visit (Table A.3 and A.4) by the hourly personnel cost (Table A.5). Total patient and personnel costs for initial and follow-up visits were calculated by multiplying personnel hours by hourly wage are given in Table A.6.
Table A.2.
Laboratory and supply costs (excluding medication) for initial and monthly scheduled follow-up clinic visits for LTBI treatment by either 9H or 3HP*
| Initial visit US$ | Follow-up visit US$ | |
|---|---|---|
| Reception | ||
| Paper | 0.02 | 0.01 |
| Chart × 1 | 0.16 | |
| Filing stickers | 0.18 | |
| Nurse’s office | ||
| Paper forms | 0.06 | 0.01 |
| Temperature probe covers × 1 | 0.03 | 0.03 |
| Language LINE interpreter services | 5.59† | 2.95‡ |
| Appointment cards | 0.03 | 0.03 |
| Diagnostic/screening tests | ||
| Gloves × 2 pairs | 0.18 | |
| Urine container × 1 | 0.17 | 0.01 |
| Pregnancy test kit × 1 | 2.50 | |
| Vacutainers × 1 | 0.15 | 0.01 |
| Needle vacutainers × 1 | 0.15 | 0.01 |
| Biohazard bags for transporting blood samples | 0.04 | 0.00 |
| Chest X-ray × 1 | 12.00§ | |
| Paper gowns × 1 | 0.32 | |
| LFT laboratory cost | 5.40 | 0.27 |
| HIV laboratory cost | 19.65¶ | |
| Laboratory cost of complete blood count | 5.40 | |
| Provider’s office | ||
| Language line interpreter services | 4.05† | 0.16‡ |
| Exam table paper | 0.03 | 0.03 |
| Otoscope covers | 0.04 | 0.04 |
| Total (female) | 56.15 | 3.56 |
| Total (male) | 53.65 | 3.56 |
| Total (average) | 54.90 | 3.56 |
Except for HIV laboratory cost,17 data were collected from the Nashville Public Health Department; costs for follow-up visits are based on patients seeing a provider or requiring more extensive workup during 5% of follow-up visits.
Average Language Line Services cost per month for all new treatment starts is $578.40. The clinic averages 60 new LTBI treatment starts per month, which amounts to $9.64 per patient. Patients average 58% of visit time with clinic nurses and 42% with providers during the initial clinic visit.
Average Language Line Services cost per month for all follow-up visits is $746.60. The clinic averages 240 LTBI follow-up visits per month, which amounts to $3.11 per patient.
Cost is for acquisition of X-ray only; personnel cost of reading the X-ray is included under physician time in Table A.3.
Center for Medicare and Medicaid Services 2010 Clinical Laboratory Fee Schedule, http://www.cms.gov.17
LTBI = latent tuberculous infection; 9H = 9 months of daily, self-administered isoniazid; 3HP = 12-dose regimen of weekly rifapentine plus isoniazid; LFT = lung function test; HIV = human immunodeficiency virus.
The cost of administering medication via directly observed therapy (DOT) was gathered from the Nashville Public Health Department based on the value of staff time and transportation (Table A.7). DOT doses were administered by outreach workers who traveled to the patients. Outreach worker time per dose administered was calculated by dividing the number of weekly outreach worker hours (160 total weekly hours for four full-time outreach workers) by the average number of weekly DOT doses administered in the field by the program (286 doses) to get a mean of 34 min per DOT dose administered. Outreach workers traveled an average of 4 miles per dose administered, and were reimbursed at a rate of $0.51 per mile. Patient time was 8.5 min per DOT dose received. Outreach worker average travel time per DOT dose was significantly less than patient travel time to the clinic due to 1) more than one DOT dose being administered (to more than one patient) during some DOT visits and 2) outreach workers traveled by car, while many patients traveled by public transportation. DOT costs are presented in Table A.7. Simulated patients receiving the 3HP regimen were assumed to have one clinic visit and four weekly DOT visits for each month on treatment (three clinic visits and 12 DOT visits for those who complete all 12 doses). Simulated patients receiving the 9H regimen, which is self-administered by the patient, did not require DOT and were assumed to have one monthly clinic visit for each month on treatment (nine clinic visits for those completing all 270 doses); 8.5 min of patient time was included for each dose taken by DOT based on data reported by the Nashville Department of Public Health for the societal perspective analysis. No additional per-dose patient time was added for patients taking 9H (although for 270 doses a small amount of patient time per dose does add up: 30 s per dose would add more than 2 h of patient time and $29 in patient costs to the societal perspective analysis for complete 270-dose treatment). Inclusion of this additional patient time would make 3HP more cost-effective than 9H from the societal perspective.
Table A.3.
Patient and personnel time for initial LTBI treatment clinic visits; data were collected from the Nashville Public Health Department
| Patient | Receptionist | Clinic nurse | CXR technician | Provider | Interpreter (if needed)* | |
|---|---|---|---|---|---|---|
| Travel time | ||||||
| From home/work to clinic | 27.5 | |||||
| From clinic to home/work | 27.5 | |||||
| Reception | ||||||
| Sign-in | 2.5 | 2.5 | ||||
| Reception processing | 8.0 | |||||
| Patient wait time to see nurse | 12.0 | |||||
| Nurse’s office before patient sees provider | ||||||
| Moving to nurse’s office | 1.0 | 1.0 | 1.0 | |||
| Urine collection (pregnancy test) | 4.0 | 4.0 | ||||
| LTBI education with nurse | 10.0 | 10.0 | 10.0 | |||
| LTBI education without nurse | 10.5 | 10.5 | ||||
| Initial workup (sputum smear, medical history, vital signs) | 13.5 | 13.5 | 13.5 | |||
| Blood collection | 6.0 | 6.0 | 6.0 | |||
| Diagnostic/screening tests | ||||||
| CXR | 12.0 | 12.0 | 12.0 | |||
| LFT paperwork/processing | 2.0 | |||||
| CBC paperwork/processing | 2.0 | |||||
| HIV paperwork/processing | 2.0 | |||||
| Pregnancy paperwork/processing | 2.0 | |||||
| Provider’s office | ||||||
| Escort to provider’s office | 1.5 | 1.5 | 1.5 | |||
| Patient’s wait time for provider | 8.0 | 8.0 | ||||
| Review CXR results | 2.0 | 2.0 | 2.0 | |||
| History taken | 12.5 | 12.5 | 12.5 | |||
| Physical examination | 4.5 | 4.5 | 4.5 | |||
| Documentation | 5.0 | |||||
| Nurse’s office after patient sees provider | ||||||
| Medication prepared | 2.0 | |||||
| Medication dispensed | 3.0 | 3.0 | 3.0 | |||
| Appointment scheduling | 3.5 | 3.5 | 5.0 | |||
| Documentation | 4.5 | |||||
| Return chart to reception | 1.0 | 1.0 | ||||
| Total (female) | 164.5 | 11.5 | 54.0 | 12.0 | 24.0 | 93.5 |
| Total (male) | 160.5 | 11.5 | 52.0 | 12.0 | 24.0 | 89.5 |
| Total (average) | 162.5 | 11.5 | 53.0 | 12.0 | 24.0 | 24.76 |
54% of LTBI patients (new and follow-up) were non-English speaking and needed an interpreter. Of those, half spoke languages for which there were on-site interpreters (Spanish/Arabic/Somali/Amharic); Language Line services were therefore not needed. The average interpreter time per patient is therefore 27% of the interpreter time for each individual who required an interpreter.
LTBI = latent tuberculous infection; CXR = chest X-ray; LFT = liver function test; HIV = human immunodeficiency virus.
Medication costs are estimated based on those paid by the Department of Veterans Affairs in 2010 under the Pharmaceutical Prime Vendor program (32 × 150 mg rifapentine [RPT] tablets cost $64.82, 100 × 300 mg isoniazid [INH] tablets cost $5.28). The cost per 300 mg dose of INH for the 9H regimen is $0.053. For the 3HP regimen, the cost per (900 mg RPT + 900 mg INH) dose is $12.31.
The total cost of treatment by 9H or 3HP is presented in Table A.8, including supply and personnel costs of clinic visits, medication costs, DOT cost, and (for the societal perspective only) patient direct and indirect costs.
Distribution of doses taken
Patient costs were computed in the model based on the number of doses taken by each simulated patient (e.g., patients who completed only 5 doses of the 3HP regimen would incur costs for the initial clinic visit, one monthly follow-up visit, 5 doses of medication, and 5 DOT visits). The distribution of doses taken by patients in the simulation was the distribution of doses completed by participants in the clinical trial.1 This distribution was not published, but was obtained from the Tuberculosis Trials Consortium Data Coordinating Center and is reproduced in Table A.9.
Table A.4.
Patient and personnel time for monthly follow-up LTBI treatment clinic visits*
| Patient | Receptionist | Clinic nurse | Provider | Interpreter (if needed)† | |
|---|---|---|---|---|---|
| Travel time | |||||
| From home/work to clinic | 27.5 | ||||
| From clinic to home/work | 27.5 | ||||
| Reception | |||||
| Sign-in | 2.0 | 2.0 | |||
| Reception processing | 8.0 | ||||
| Patient wait time to see nurse | 12.0 | ||||
| Nurse | |||||
| Moving to nurse’s office | 1.0 | 1.0 | 1.0 | ||
| History/vital signs/symptoms | 9.0 | 9.0 | 9.0 | ||
| Dispensing meds | 5.0 | 5.0 | 5.0 | ||
| Documentation | 4.5 | ||||
| Return chart to reception | 1.0 | ||||
| Appointment scheduling | 2.0 | 2.0 | |||
| Urine collection, if needed‡ | 4.0 | 4.0 | |||
| Blood collection, if needed‡ | 6.0 | 6.0 | 6.0 | ||
| Provider if needed (5% of patients) | |||||
| Escort to provider’s office | 1.0 | 1.0 | 1.0 | ||
| Patient’s wait time for provider | 7.5 | 7.5 | |||
| Assessing issue | 7.5 | 7.5 | 7.5 | ||
| Documentation | 2.5 | ||||
| Contact patient for missed visit (25% of patients) | |||||
| Attempt 1 | 7.5 | ||||
| Attempt 2 | 3.5 | ||||
| Attempt 3 | 3.5 | ||||
| Total | 87.3 | 13.6 | 22.9 | 0.5 | 4.47 |
Data were collected from the Nashville Public Health Department: all times are reported in minutes and have been rounded to the nearest minute.
54% of LTBI patients (new and follow-up) were non-English speaking and needed an interpreter. Of these, half spoke languages for which there were on-site interpreters (Spanish/Arabic/Somali/Amharic); Language Line services were therefore not needed. The average interpreter time per patient is therefore 27% of the interpreter time for each individual who required an interpreter.
Applies to 5% of patients.
LTBI = latent tuberculous infection.
Table A.5.
Clinic personnel wages for LTBI treatment clinic visits*
| Position | BLS occupation title | BLS code | Mean hourly wage US$ | Hourly wage + benefits† US$ | Mean annual wage US$ | Annual wage + benefits† US$ |
|---|---|---|---|---|---|---|
| Receptionist | Medical records and health information technicians | 292017 | 15.12 | 20.83 | 31 440 | 43 306 |
| Clinic nurse | Registered nurses | 291111 | 32.47 | 44.72 | 67 550 | 93 044 |
| X-ray technician | Radiologic technologists and technicians | 292037 | 25.76 | 35.48 | 53 590 | 73 815 |
| Provider | Internists, general | 291063 | 93.96 | 129.42 | 195440 | 269201 |
| Interpreter‡ | Medical records and health information technicians | 292017 | 15.12 | 20.83 | 31 440 | 43 306 |
| Outreach worker | Licensed practical and licensed vocational nurses | 292061 | 20.14 | 27.74 | 41 890 | 57 699.72 |
From the May 2010 Occupational Employment Statistics Survey by the Bureau of Labor Statistics (Industry: Outpatient Care Centers, North American Industry Classification System code 621400).18
Fringe benefits calculated based on assumption that wages account for 72.6% of employee compensation (Haddix et al., Appendix I).19
For the purpose of calculating cost, interpreter duties are assumed to be performed by personnel with the hourly wage rate of a clinic receptionist.
LTBI = latent tuberculous infection; BLS = Bureau of Labor Statistics.
Table A.6.
Patient and clinic personnel costs for initial clinic visit and monthly follow-up visit for LTBI treatment; medication and supply costs not included in totals
| Hourly compensation US$ | Initial visit
|
Follow-up visit
|
|||
|---|---|---|---|---|---|
| Time min | Cost US$ | Time min | Cost US$ | ||
| Receptionist | 20.83 | 11.5 | 3.99 | 13.625 | 4.73 |
| Clinic nurse | 44.72 | 53 | 39.51 | 22.85 | 17.03 |
| CXR technician | 35.48 | 12 | 7.10 | 0 | 0.00 |
| Provider | 129.42 | 24 | 51.77 | 0.5 | 1.08 |
| Interpreter* | 20.83 | 24.705 | 8.58 | 4.401 | 1.53 |
| Patient | 12.73 | 159.5 | 33.85 | 87.3 | 18.53 |
| Clinic personnel total | 110.94 | 24.37 | |||
| Patient round trip bus fare | 3.20 | 3.20 | |||
| Patient total | 37.05 | 21.73 | |||
The average interpreter time is 27% of the interpreter time for each individual who required an interpreter (see note to Tables A.3 and A.4).
LTBI = latent tuberculous infection; CXR = chest X-ray.
Table A.7.
DOT costs per dose administered*
| Cost | |
|---|---|
| DOT component | US$ |
| Patient time per dose, min | 8.5 |
| DOT worker time per dose, min | 34 |
| DOT worker cost per dose | 15.72 |
| Driving cost per dose (6.44 km @ $0.82/km, from Nashville site) | 2.04 |
| Patient cost per dose | 1.80 |
| Total (health system) | 17.76 |
| Total (societal) | 19.56 |
Patient and outreach worker time per dose and driving cost per dose are from the Nashville Public Health Department. Patient time is valued at $12.73 per hour here and elsewhere based on 2010 US per capita income; outreach worker time is valued at $27.74 per hour (see Table A.5). Health system costs do not include costs to the patient; societal costs include all health system costs plus patient costs.
DOT = directly observed therapy.
Table A.8.
Cost of LTBI treatment by 9H or 3HP*
| Category | Cost US$ | Source |
|---|---|---|
| Initial clinic visit | ||
| Personnel cost | 110.94 | Table A.2 |
| Patient cost | 37.05 | |
| Supply cost | 54.90 | Table A.2 |
| Total health system | 165.84 | Supply cost + personnel cost |
| Total societal | 202.89 | Supply cost + personnel cost + patient cost |
| Follow-up clinic visit | ||
| Personnel cost | 24.37 | Table A.6 |
| Patient cost | 21.73 | |
| Supply cost | 3.56 | Table A.2 |
| Total health system | 27.92 | Supply cost + personnel cost |
| Total societal | 49.65 | Supply cost + personnel cost + patient cost |
| DOT, per dose | Table A.7 | |
| Health system | 17.76 | |
| Societal | 19.56 | |
| Medication cost, per month on treatment† | Department of Veterans Affairs Pharmaceutical Prime Vendor Program | |
| 9H (30 × 300 mg INH) | 1.58 | |
| 3HP (4 × 900 mg INH + 4 × 900 mg RPT) | 49.25 | |
| 9H total treatment cost, per patient completing 270 doses‡ | Based on 1 initial clinic visit, 8 monthly follow-up clinic visits, and 9 months of medication | |
| Health system | 403.45 | |
| Societal | 614.32 | |
| 3HP total treatment cost, per patient completing 12 doses‡ | Based on 1 initial clinic visit, 2 monthly follow-up clinic visits, 3 months of medication, and 12 DOT visits | |
| Health system | 582.55 | |
| Societal | 684.71 | |
Health system costs do not include costs to the patient; societal costs include all health system costs plus patient costs. Clinic visit costs include supplies, clinic personnel time, and (for the societal perspective only) patient time and bus fare. Medication costs are not included in clinic visit costs.
Medication costs are borne by the health system.
LTBI treatment costs for simulated patients depend on the number of doses taken; on average they are less than the cost of completed treatment because of the possibility of non-adherence.
LTBI = latent tuberculous infection; 9H = 9 months of daily, self-administered INH; 3HP = 12-dose regimen of weekly RPT plus INH; INH = isoniazid; RPT = rifapentine.
Table A.9.
| 3HP
|
9H
|
||||
|---|---|---|---|---|---|
| Doses taken | Patients % | Cumulative % | Months on treatment | Patients % | Cumulative % |
| 0 | 2.53 | 2.53 | 1 | 9.42 | 9.42 |
| 1 | 2.39 | 4.92 | 2 | 3.76 | 13.18 |
| 2 | 1.83 | 6.76 | 3 | 3.66 | 16.84 |
| 3 | 2.39 | 9.14 | 4 | 2.69 | 19.52 |
| 4 | 2.29 | 11.44 | 5 | 2.53 | 22.06 |
| 5 | 1.47 | 12.91 | 6 | 2.20 | 24.26 |
| 6 | 1.13 | 14.04 | 7 | 1.87 | 26.13 |
| 7 | 1.09 | 15.13 | 8 | 5.42 | 31.55 |
| 8 | 1.18 | 16.31 | 9 | 66.89 | 98.44 |
| 9 | 0.63 | 16.94 | 10 | 1.56 | 100.00 |
| 10 | 0.29 | 17.23 | |||
| 11 | 1.91 | 19.13 | |||
| 12 | 80.63 | 99.76 | |||
| 13 | 0.22 | 99.98 | |||
| 14 | 0.00 | 99.98 | |||
| 15 | 0.00 | 99.98 | |||
| 16 | 0.00 | 99.98 | |||
| 17 | 0.02 | 100.00 | |||
Data from the Tuberculosis Trials Consortium Data Coordinating Center.
9H = 9 months of daily, self-administered isoniazid; 3HP = 12-dose regimen of weekly rifapentine plus isoniazid.
Tuberculosis disease costs
Tuberculosis disease diagnostic costs (incurred by all patients in the model who develop active tuberculosis)
Burman et al. reported $354 for diagnosis costs in 1994 dollars based on the cost of three mycobacterial cultures, chest X-ray, CBC count, serum chemistry panel, 1 h of physician time and 2 h of nurse time.20 Our estimate includes three smears* (3 × $7.70), 3 cultures† (3 × $15.48), and chest X-ray‡ ($30.97).19 As in Burman et al.,20 we add 1 h of physician time at $93.96/h and 2 h of nurse time at $32.47/h (using hourly wage estimates from the Bureau of Labor Statistics,17 as described above in the section on LTBI Treatment Costs), to obtain ($100.51 + $93.96 + $64.94) = $259 diagnosis cost to the health system. For societal cost, we include 3 h patient lost productivity for a total of $298, where patient time is valued at an hourly rate of $12.73 based on US Census Bureau data,15 as described in the section on Patient Time Valuation above.
TB disease out-patient costs (incurred by all patients in the model who develop TB disease)
Simulated individuals developing active TB were assumed to receive treatment with a standard regimen of 8 weeks of INH, rifampin (RMP), pyrazinamide and ethambutol (5 days per week, 40 doses), followed by 18 weeks of INH and RMP (5 days/week, 90 doses),21 with all doses given by DOT. Health system costs are those incurred by TB programs for medications and personnel to conduct DOT. Societal costs include health system costs as well as patient productivity losses. The cost of DOT was gathered from the Nashville Public Health Department ($17.76 health system or $19.56 societal cost per dose. The societal cost includes patient time of 8.5 min per dose; see Table A.7). This DOT cost may be an underestimate for patients with TB disease, as it was gathered for LTBI patients, many of whom receive DOT in congregate settings; patients with TB disease are much less likely to receive DOT in congregate settings. Underestimating the cost of out-patient treatment will tend to favor the cost-effectiveness of the regimen that prevents fewer cases of TB disease (9H, in the base case analysis) and is conservative with respect to the cost-effectiveness of the 3HP regimen.
As most TB programs purchase their medications through the Public Health Service (PHS), estimates of PHS drug prices are used. The estimated PHS cost of medication for the 130 dose regimen is $390 in 2008 dollars or $416 in 2010 dollars (updated using the medical care index of the Consumer Price Index [CPI]22).
Patients being treated for TB disease are assumed to undergo six monthly clinic visits while receiving anti-tuberculosis treatment. The health system and societal costs of these visits are assumed to be the same as those for follow-up visits during LTBI treatment ($27.92 health system, $49.65 societal; see Table A.8). In addition, patients being treated for TB disease are assumed to incur additional costs for two chest X-rays (2*$30.97) and two sputum cultures (2*$15.48).19 The health system cost of TB treatment is:
The societal cost of TB treatment is:
Table A.10.
Results of univariate sensitivity analysis: cost per TB case prevented and cost per QALY gained by 3HP (compared to 9H) when varying one parameter value at a time*
| Parameter | Health system perspective
|
Societal perspective
|
||
|---|---|---|---|---|
| Incremental cost per TB case prevented US$ | Incremental cost per QALY gained US$ | Incremental cost per TB case prevented US$ | Incremental cost per QALY gained US$ | |
| Base case scenario | 21525 | 4565 | 4294 | 911 |
| Directly observed therapy cost/dose ($17.76) | ||||
| $0 | <0 | <0 | <0 | <0 |
| $35 | 56124 | 11902 | 38893 | 8248 |
| 3HP medication cost per dose ($12.31) | ||||
| $5 | 6 854 | 1 454 | <0 | <0 |
| $25 | 46992 | 9965 | 29762 | 6311 |
| $35 | 67061 | 14221 | 39796 | 8439 |
| TB hospitalization probability (49%) | ||||
| 0% | 32855 | 6983 | 17188 | 3653 |
| 75% | 15140 | 3207 | <0 | <0 |
| Proportional change in risk of progression to TB disease (100%) | ||||
| 50% | 47861 | 7163 | 19007 | 2845 |
| 150% | 8 002 | 2 001 | <0 | <0 |
| 200% | 1 941 | 594 | <0 | <0 |
| Patient hourly time value ($12.73) | ||||
| $0 | 21525 | 4565 | 18669 | 3959 |
| $7.50 | 21525 | 4565 | 10200 | 2163 |
| $17.00 | 21525 | 4565 | <0 | <0 |
| Secondary TB cases per primary case (0.46) | ||||
| 0 | 34895 | 6436 | 12153 | 2242 |
| 0.6 | 18753 | 4104 | 2665 | 583 |
| 9H completion proportion (68%) | ||||
| 63% | 14906 | 3248 | 1145 | 250 |
| 57% | 15170 | 3464 | 2648 | 605 |
| 51% | 11378 | 3183 | 1043 | 292 |
| 3HP completion proportion (83%) | ||||
| 76% | 16969 | 3209 | <0 | <0 |
| 69% | 19259 | 3922 | <0 | <0 |
| 62% | 15220 | 2518 | <0 | <0 |
| 3HP toxicity probability (8.2%) | ||||
| 3% | 16629 | 3749 | <0 | <0 |
| 13% | 25458 | 5158 | 7686 | 1557 |
| Probability of death from TB disease (5.8%) | ||||
| 2% | 21332 | 5655 | 3828 | 1015 |
| 15% | 21424 | 3856 | 3862 | 695 |
| Cost of hospitalization for TB ($25 495) | ||||
| $10000 | 28411 | 6025 | 11181 | 2371 |
| $40000 | 15078 | 3197 | <0 | <0 |
| Annual QALY loss from LTBI treatment (0.03) | ||||
| 0 | 21525 | 9132 | 4294 | 1822 |
| 0.06 | 21525 | 3043 | 4294 | 607 |
| Annual QALY loss from previous TB (0.05) | ||||
| 0 | 21525 | 5961 | 4294 | 1189 |
| 0.10 | 21525 | 3698 | 4294 | 738 |
| Annual QALY loss from drug toxicity (0.25) | ||||
| 0 | 21525 | 4520 | 4294 | 902 |
| 0.50 | 21525 | 4610 | 4294 | 920 |
| Annual QALY loss from hospitalization (0.50) | ||||
| 0 | 21525 | 4571 | 4294 | 912 |
| 1.00 | 21525 | 4558 | 4294 | 909 |
| Annual QALY loss from anti-tuberculosis treatment (0.10) | ||||
| 0 | 21525 | 4613 | 4294 | 920 |
| 0.20 | 21525 | 4517 | 4294 | 901 |
| Discount rate (3%) | ||||
| 0% | 15 825 | 2 573 | 959 | 156 |
| 5% | 24766 | 5732 | 6184 | 1431 |
| Simulation duration (20 years) | ||||
| 5 years | 29468 | 5717 | 8914 | 1729 |
| 10 years | 25052 | 5078 | 6342 | 1286 |
| 50 years | 17321 | 3931 | 1633 | 371 |
| Cost of initial clinic visit ($166)† | ||||
| $125 | 21525 | 4565 | 4294 | 911 |
| $205 | 21525 | 4565 | 4294 | 911 |
| Cost of follow-up clinic visit ($28) | ||||
| $20 | 28559 | 6056 | 11328 | 2402 |
| $35 | 15237 | 3231 | <0 | <0 |
Base case values are reported in parentheses. Shaded entries (where the incremental cost-effectiveness ratios are negative) represent scenarios where 3HP is cost-saving compared to 9H. Simulation run for each set of parameter values with 100 000 simulated individuals for each regimen. Costs are in 2010 US dollars.
As the same cost of the initial clinic visit is incurred for all individuals regardless of regimen, it has no bearing on the relative cost-effectiveness of the two regimens.
TB = tuberculosis; QALY = quality-adjusted life years; 3HP = 12-dose regimen of weekly rifapentine plus isoniazid; 9H = 9 months of daily, self-administered isoniazid; LTBI = latent tuberculous infection.
Tuberculosis disease hospitalization costs (incurred by those patients in the model who are hospitalized for tuberculosis disease)
Of patients who develop TB disease, 49% are assumed to be hospitalized due to TB disease.23 As per Taylor et al.,23 the average hospitalization cost per study participant excluding physician fees is $10 881 799/733 = $14 846 (1998 dollars) or $23 820 (2010 dollars, updated using the CPI Medical Care Index). As this amount does not include physician fees, these were added separately. The mean length of hospitalization per patient was 24 days (S Marks, personal communication of unpublished data from Taylor et al.23). Brown et al. found that ‘the mean length of stay for patients with TB was 19.9 days’.24 Physician fees per day for ‘Level 2’ hospitalization are $69.78*.19 The total mean health system cost of hospitalization for TB disease, then, is estimated to be ($23 820 + 24*$69.78) = $25 495. Using $139 as the indirect cost to the patient of one day of hospitalization,3 the indirect patient cost due to hospitalization is (24* $139) = $3336 per patient hospitalized for active TB, and the total mean societal cost of hospitalization for active TB is estimated to be ($25 495 + $3336) = $28 831. In recognition of the fact that the data for the cost of hospitalization for TB are more than 10 years old and these costs could be high, we considered in sensitivity analysis the extreme case that all individuals with TB disease are treated as out-patients by setting the model parameter governing the probability of hospitalization for TB disease to zero. See Table A.10 for the result of this and other sensitivity analyses.
The costs of patients diagnosed in hospital are not subtracted from the total hospitalization costs calculated, as detailed above (i.e., the 49% of TB patients who are hospitalized incur the $259 associated with diagnostic procedures in addition to the $25 495 associated with hospitalization). The costs associated with diagnostic procedures are computed using costs from the Center for Medicare and Medicaid Services 2010 Clinical Laboratory Fee Schedule, and are therefore reported in 2010 dollars.19 (The 1994 costs computed by Burman et al.20 are provided above for the sake of comparison only.)
Quality of life adjustments
Quality of life adjustments (also known as ‘health state utilities’ to health economists) for patient health states (LTBI treatment, LTBI drug toxicity, hospitalization, TB disease and previous TB disease) are those reported in Holland et al.25 These quality of life adjustments are applied over the duration of LTBI treatment, 2 weeks (drug toxicity), 6 months (active TB), the duration of hospitalization (7 days for severe toxicity, 24 days for TB) or the duration of life following TB activation, respectively. Simulated individuals are assumed not to incur reduced quality of life following LTBI treatment as long as they do not develop active TB, reflecting the fact that there is no evidence that individuals with LTBI who do not develop TB suffer any adverse health effects. Simulated individuals accrue no additional quality adjusted life years after death.
A number of studies have attempted to quantify the quality of life associated with TB-related health states.26–32 This is necessarily a subjective process, and there is no consensus on how best to measure quality of life. We allow quality-adjusted life year (QALY) values to vary widely in sensitivity analysis in recognition of the uncertainty associated with these parameters. In particular, the extent to which LTBI treatment and previous active TB disease have an impact on quality of life is unclear (a recent cost-effectiveness analysis of screening strategies for LTBI applied quality of life adjustments of 1 for time on LTBI treatment by INH and time with previous TB disease33). To explore this scenario, we performed a multivariate sensitivity analysis in which the quality of life adjustment for time on LTBI treatment and time with previous TB disease were simultaneously set to 1 to test these assumptions from Linas et al.,33 while holding other input parameters at their base case values.
Latent tuberculosis infection drug toxicity
LTBI drug toxicity rates for the 9H and 3HP regimens are estimated based on the rates of treatment-related adverse events and hospitalizations observed in the clinical trial,1 which found 332 treatment-related adverse events and 12 treatment-related hospitalizations in 4040 treatment starts in the 3HP arm (8.2% and 0.3% of treatment starts, respectively) and 206 treatment-related adverse events and 4 treatment-related hospitalizations in 3759 treatment starts in the 9H arm (5.5% and 0.1% of treatment starts, respectively).* As there were no drug toxicity-related deaths in the clinical trial, we assume the death rate due to drug toxicity to be 0.014% per treatment start for both 9H and 3HP, based on Saukkonen et al.34 and the International Union Against Tuberculosis’ follow-up of INH preventive therapy.35
The cost of LTBI drug toxicity is estimated as in a previous cost-effectiveness analysis comparing alternative treatment regimens for LTBI,25 with the costs updated to 2010 US dollars and an assumed 1 week of patient hospitalization time added (with patient time valued at $139 per day, as above) in the case of toxicity-related hospitalization for the societal perspective analysis. From Holland et al.,25 which relies on cost data from Taylor et al.,23 the health system cost for drug toxicity without hospitalization is taken to be $158 (2008 dollars), or $169 in 2010 US dollars; the societal cost is assumed to be the same as the health system cost for toxicity without hospitalization. The health system cost for toxicity with 7 days of hospitalization is $5321 in 2008 dollars, or $5677 in 2010 US dollars; the societal cost for toxicity with 7 days of hospitalization is $5677 + 7*$139 = $6650 in 2010 US dollars.
Background mortality and age distribution
In the base case analysis, patients were simulated for 20 years after starting treatment with either 9H or 3HP and experienced an annual risk of death unrelated to TB or LTBI treatment depending on their age (other simulation durations were explored in sensitivity analysis, see Table A.10). The age-specific risk of death due to events unrelated to TB or LTBI treatment was calculated using the 2007 US Life Tables.36 The distribution of ages of simulated patients at treatment start had a mean of 36 years and was the distribution of ages at enrollment in the clinical trial comparing 3HP and 9H1 (the age distribution of participants was not published, but was obtained from the authors).
Model description
The model is an individual-based (also known as ‘agent-based’) stochastic simulation developed using NetLogo 5.0 software (NorthWestern University, Evanston, IL, USA), which is freely available online.37 The model simulates two populations of individuals with LTBI. One simulated population is treated with 3HP, and the other is treated with 9H. Simulated individuals are followed for 20 simulated years after LTBI treatment, and costs and health outcomes related to LTBI treatment and TB disease are tracked during this time.
Initialization
During model initialization, two populations of individuals with LTBI are created, one receiving 3HP and one receiving 9H. Each simulated individual has an assigned set of attributes that are tracked over the course of the simulation. These include the individual’s age, LTBI regimen taken, number of doses completed, current risk of TB, and current state of health (on LTBI treatment, healthy post-LTBI treatment, TB disease, post-TB disease and dead). Time spent, cost and QALYs lost receiving treatment for LTBI, LTBI drug toxicity or TB disease are also tracked for each individual.
During the initialization phase, the random number generator for the stochastic simulation is first initialized with the seed 73939133. Each individual’s age is assigned by drawing from the distribution of ages of clinical trial participants at the time of enrollment (Figure A.1). Each individual is also assigned to complete an amount of treatment that is drawn from the distribution of the number of doses taken by participants in the referenced clinical trial for the appropriate regimen (Table A.9). For both regimens, individuals are assumed to have one initial clinic visit, followed by one follow-up clinic visit for each month on treatment starting with the second (a full 12-week course of 3HP therefore entails 1 initial clinic visit and 2 follow-up visits; a full 9-month course of 9H entails 1 initial clinic visit and 8 follow-up visits). Each individual’s LTBI treatment costs are calculated based on the regimen and number of doses taken as follows:
For 3HP, the individual’s treatment cost is: (cost of initial clinic visit) + (cost of follow-up clinic visit)* (number of follow-up clinic visits) + (medication cost per dose + DOT cost per dose)*(number of doses taken).
For 9H the individual’s treatment cost is: (cost of initial clinic visit) + (cost of follow-up clinic visit)* (number of follow-up clinic visits) + (medication cost per month)*(number of months on treatment).
For the societal perspective only, patient costs are added based on the number of minutes spent receiving treatment. For both regimens, individuals are assumed to spend (including travel time) 159.5 min at the initial clinic visit, and 87.3 min at each follow-up clinic visit (Tables A.4, A.5). For 3HP, individuals spend an additional 8.5 min for each dose taken receiving DOT from a health care worker (Table A.7). Patient time is valued at an hourly rate of $12.73, as described above. Individual patients are assumed to incur out-of-pocket costs of $3.20 for each clinic visit for a round trip bus fare.
Individuals experience lost QALYs due to time on LTBI treatment based on the amount of time on treatment (number of doses completed) at a rate of 0.03 lost QALYs per year on treatment, as described in the Model Inputs and Assumptions section.
Simulation
The program goes through one time step for each year of simulation. At each time step, the program does the following:
Each individual’s risk of progressing to TB disease during the current simulation year is calculated. For the first 3 years of simulation, as described in the Model Inputs and Assumptions section, this is the baseline risk set during the initialization phase based on clinical trial data. Thereafter, the TB risk for each individual declines by a factor of two each year to a minimum of 1/20 of the individual’s baseline value in approximation of the declining risk of TB disease observed among recent immigrants to the United States from high TB burden countries.2
Each individual’s risk of death during the current simulation year from causes unrelated to TB is set based on the individual’s current age using data from the 2007 US Life Tables.36 Each individual undergoes a check for death from causes unrelated to TB or LTBI treatment; individuals who die enter the ‘dead’ state and accumulate no further costs or lost QALYs.
- If it is the first year of simulation (i.e., the year during which individuals receive LTBI treatment), individuals undergo checks for drug toxicity (fatal toxicity, toxicity with hospitalization or toxicity without hospitalization) with the probabilities as described in the Model Inputs and Assumptions section above. Those experiencing toxicity have their individual toxicity-related costs and toxicity-related lost QALYs adjusted as described in the Model Inputs and Assumptions section. Individuals who die from toxicity lose one QALY for each lost year of life, where their expected future years of life are calculated based on the 2007 US Life Tables.36 As with all costs and health outcomes in the model (including QALYs, number of TB cases and number of deaths), the lost QALYs due toxicity-related death are discounted at annual rate of 3%, so that the value of 1 lost QALY in year k of simulation (where treatment occurs in year 0) is
-
Individuals each undergo a check-up to see if they progress to TB disease during the current simulation year, with the risk of progression to TB disease as described above. Individuals who develop TB disease have their TB-related costs and QALYs lost updated as described in the Model Inputs and Assumptions section (discounted at an annual rate of 3%) and experience a one-time 5.8% chance of dying from TB disease. For those individuals who die from TB disease, the lost QALYs due to TB-related death are calculated in the same way as the lost QALYs due to toxicity-related death, as described above. Those individuals who develop TB disease lose 0.05 QALYs for each expected future year of life (discounted at an annual rate of 3%) based on the assumption that individuals have reduced quality of life for the duration of their lives after experiencing TB disease (see Model Inputs and Assumptions section). As with individuals who die from drug toxicity, the number of expected future years of life for each individual surviving TB disease is based on the 2007 US Life Tables.36 For each individual who develops TB, 0.46 additional cases are assumed to occur due to transmission. For the purpose of discounting (costs and number of TB cases), one third of these secondary cases are assumed to occur immediately in the current simulation year, a third are assumed to occur after 5 more years and a third are assumed to occur after 25 years. That is, each primary case is assumed to lead to:secondary cases, after discounting has been applied, where k is the current simulation year. Each secondary case is assumed to lead to costs and lost QALYs equal to those of an average TB case, discounted as above. The average cost of a TB case is taken to be:The average QALY loss of a TB case is:
See the Model Inputs and Assumptions section for information on costs and QALY values.
The year is incremented by one, and each individual’s age is incremented by one year.
-
The health system and societal costs to date for each individual are updated (individual cost = individual LTBI treatment cost + individual toxicity cost + individual cost of TB disease + costs arising from secondary TB cases). The QALYs lost to date are updated for each individual (individual QALYs lost = QALYs lost due to time on LTBI treatment + QALYs lost due to toxicity + QALYs lost due to TB disease + QALYs lost in secondary cases due to transmission). The current incremental cost per TB case prevented is calculated as:Where 3HP denotes the set of all individuals receiving 3HP and 9H denotes the set of all individuals receiving 9H, ci is the discounted cumulative cost to individual i during the simulation so far, and di is the discounted number of TB cases (including secondary cases) involving individual i during the simulation so far. The current incremental cost per QALY gained by 3HP compared to 9H is calculated as:
where qi is the discounted cumulative QALYs lost by individual i so far in the simulation.
The next simulation time step begins.
The simulation runs for 20 simulated years (20 simulation time steps). At the end it reports the total costs, health outcomes, incremental cost per TB case prevented by 3HP compared to 9H, and incremental cost per QALY gained by 3HP compared to 9H. Individual-level costs and health outcomes can also be queried after simulation.
The model was created and implemented using NetLogo version 5.0. NetLogo is free and publicly available, and can be downloaded from http://ccl.northwestern.edu/netlogo/. The file containing the model source code can be downloaded from the authors of this report at http://www.mtholyoke.edu/~dshepard/LTBI/LTBI-cost-effectiveness.zip. The model can be run (and modified) by users who download and install NetLogo.
Sensitivity analysis
Univariate sensitivity analysis
The results of univariate sensitivity analyses, where model parameters were each varied independently while holding other parameters constant at their base case values, are presented in Table A.10 (an abbreviated version appears in Table 3 in the main article). The same random number generator seed was used for each sensitivity run to reduce the amount of variability in the results. Individual parameter sensitivity ranges (Table A.10) were chosen in an attempt to cover all reasonable values for each parameter.
Multivariate sensitivity analysis
In multivariate sensitivity analysis, groups of parameters were simultaneously varied from their base case values. For one representative multivariate sensitivity analysis, the input parameters varied, and the values taken by each parameter are presented in Table A.11. Assuming that each parameter can take on its base case value or one of its alternate values listed in Table A.11, there are 324 combinations of these parameter values. The results of this multivariate sensitivity analysis are presented in Figures A.2 and A.3, which show acceptability curves comparing the incremental cost per QALY gained by 3HP compared to 9H from the health system (Figure A.2) and societal (Figure A.3) perspectives. The variable on the horizontal axis represents a threshold for the incremental cost per QALY gained by 3HP compared to 9H, and the vertical axis gives the fraction of the 324 scenarios from this multivariate sensitivity analysis (Table A.11) for which the incremental cost per QALY gained falls below this threshold. For more information on acceptability curves in cost-effectiveness analysis, see, for example, Briggs et al.38
Figure A.2.
Health system perspective acceptability curve for the multivariate sensitivity analysis from Table A.11. Curve shows the fraction of sensitivity runs that were cost-effective from the health system perspective for each threshold value λ (the fraction of simulations for which the incremental cost per QALY gained by 3HP compared to 9H was ≤λ). Each run represents one of the 324 combinations of parameters from Table A.11 and was simulated using 50 000 individuals for each regimen. QALY = quality-adjusted life years; 3HP = 12-dose regimen of weekly rifapentine plus isoniazid; 9H = 9 months of daily, self-administered isoniazid.
From the health system perspective, 3HP was found to be cost-saving relative to 9H for 23% of the simulated scenarios from Table A.11 (i.e., the incremental cost per QALY gained by 3HP compared to 9H was found to be negative for 23% of the simulations from Table A.11). For 50% of these simulations, the incremental cost per QALY gained by 3HP compared to 9H was found to fall below a threshold of $6250. For 98% of the simulations, the incremental cost per QALY gained by 3HP compared to 9H was found to fall below a threshold of $50 000, a value often used as a threshold for cost-effectiveness of public health interventions in the United States in the past (Figure A.2).39
Figure A.3.
Societal perspective acceptability curve for the multivariate sensitivity analysis from Table A.11. Curve shows the fraction of sensitivity runs that were cost-effective from the societal perspective for each threshold value λ (the fraction of simulations for which the incremental cost per QALY gained by 3HP compared to 9H was ≤λ). Each run represents one of the 324 combinations of parameters from Table A.11 and was simulated using 50 000 individuals for each regimen. QALY = quality-adjusted life years; 3HP = 12-dose regimen of weekly rifapentine plus isoniazid; 9H = 9 months of daily, self-administered isoniazid.
Table A.11.
Parameters adjusted in one multivariate sensitivity analysis*
| Input parameter | Parameter values explored
|
|
|---|---|---|
| Base case value US$ | Alternative values US$ | |
| 3HP medication cost per dose | 12.31 | 5, 25 |
| Directly observed therapy cost per dose | 17.76 | 0, 35 |
| TB hospitalization probability, % | 49 | 25 |
| Proportional change in risk of progression to tuberculosis disease, % | 100 | 50, 150 |
| Annual QALY loss from LTBI treatment | 0.03 | 0 |
| Cost of follow-up clinic visit | 27.92 | 20, 35 |
Each parameter was assigned to each of the values given below (324 total simulations, one for each combination of parameters). All other parameter values were maintained at their base case levels. Each simulation included 50 000 individuals for each regimen.
3HP = 12-dose regimen of weekly rifapentine plus isoniazid; QALY = quality-adjusted life years; LTBI = latent tuberculous infection.
From the societal perspective, 3HP was found to be cost-saving relative to 9H for 40% of the simulated scenarios from Table A.11. For 50% of these simulations, the incremental cost per QALY gained by 3HP compared to 9H was found to fall below a threshold of $2250. For 99.7% of the simulations, the incremental cost per QALY gained by 3HP compared to 9H was found to fall below a threshold of $50000 (Figure A.3).
We undertook other multivariate sensitivity analyses similar to the one outlined in Table A.11, where different input parameters and values were chosen for analysis. The results were broadly similar, i.e., our model predicts that the incremental cost per QALY gained by 3HP compared to 9H remains below $50 000 from both the health system and societal perspectives over a wide range of input parameter values.
Scenario sensitivity analysis
In addition to the sensitivity analyses described above, several specific scenarios were explored. In the clinical trial, after adjustment for HIV infection, smoking, and low body mass index patients receiving 3HP were found to be at lower risk for TB than patients receiving 9H, with the rate of TB in the 3HP population approximately half that in the 9H population, and our base case analysis assumes this is the case. The clinical trial investigators concluded that 3HP is at least as effective as 9H.1 In sensitivity analysis, we tested the case that 3HP and 9H are equivalent in two different ways:
The baseline TB risk for simulated individuals completing nine or more 3HP doses was set to the same level as the TB risk for those completing 241 or more 9H doses (i.e., the annual risk of progression to TB disease for simulated individuals taking nine or more doses of 3HP was raised from the base case value of 0.05% to 0.08%). Under this scenario, 3HP and 9H are assumed to be equally effective for patients who complete treatment, but 3HP retains an advantage due to greater adherence. In this case, the average number of TB cases per 1000 LTBI treatment starts rises from 3.9 to 5.1 for individuals receiving 3HP, and the cost per individual treated increases from $623 to $644 (health system) or from $728 to $751 (societal). The incremental cost per TB case prevented by 3HP compared to 9H increases from $21 525 to $38 775 (health system) or from $4294 to $13 223 (societal), and the incremental cost per QALY gained by 3HP compared to 9H rises from $4565 to $6361 (health system) or from $911 to $2169 (societal).
The baseline TB risk for all simulated individuals receiving 3HP and 9H was set to the level observed in the modified intention-to-treat population in the clinical trial (i.e., all individuals starting LTBI treatment had a baseline annual risk of progression to TB disease of 0.16%).1 In this case, 3HP and 9H are assumed to be equivalent in terms of their effectiveness in preventing TB. As both populations experience the same average number of TB cases, the cost per TB case prevented is not a valid measure of cost-effectiveness under this scenario. The average cost per person treated increases from the base case value of $623 to $710 (health system) or from $728 to $825 (societal), and the incremental cost per QALY gained by 3HP compared to 9H rises from $4565 to $14 292 (health system) or from $911 to $8559 (societal) with the difference in QALYs between the regimens due entirely to the different durations of LTBI treatment and risks of drug toxicity.
The gains in QALYs from 3HP are due in part to the assumptions that individuals on LTBI treatment experience reduced quality of life (0.03 lost QALY per year) for the duration of treatment and patients with previous active TB experience reduced quality of life (0.05 lost QALY per year) for their remaining years of life.25 When individuals are assumed to experience no loss in quality of life due to LTBI treatment or due to previous active TB, as in a recent cost-effectiveness analysis of screening strategies for LTBI,33 the cost per QALY gained for 3HP vs. 9H increases to $17 186 (health system) or $3429 (societal).
Due to computational limitations and the number of parameters involved, an exhaustive exploration of the space of possible parameter values is not possible. Readers who would like to explore other combinations of alternative input parameter values are encouraged to download the NetLogo source code for the simulation model which will allow them to set the input parameters to their desired values and conduct their own analyses using our simulation model.
Analysis of stochastic variability
Because of the stochastic nature of the individual-based model created here, there is randomness built into the model results. We analysed the stochastic variability in model results by using bootstrap re-sampling.40 Simulation data were used to populate 1000 different sets of 100 000 individuals for each regimen by resampling with replacement from the original simulation population of 100 000 individuals for each regimen. For each set of individuals created by bootstrap resampling, the number of TB cases and QALYs lost, as well as the mean health system and societal costs, were computed for each regimen. The health system and societal costs per TB case prevented and per QALY gained by 3HP compared to 9H were also calculated for each population created by bootstrap resampling. The distributions of these measures in the 1000 hypothetical populations created by bootstrap resampling of simulation data were used to estimate the 2.5 and 97.5 centiles and the 95% confidence interval for each measure.
Model availability
Interested users should first download and install NetLogo from http://ccl.northwestern.edu/netlogo/. Our simulation model is available from http://www.mtholyoke.edu/~dshepard/LTBI-cost-effectiveness.zip. Many model parameters (analytic horizon, price of RTP, secondary transmission rate, adherence rates for 3HP and 9H, and many others) can be modified through the model’s graphic user interface; other model parameters and the model structure can be modified by editing the source code (also provided) directly. Our hope is that by making the model publicly available, we will enable interested users to modify it to better suit local conditions.
- 1.Sterling TR, Villarino ME, Borisov AS, et al. Three months of rifapentine and isoniazid for latent tuberculosis infection. N Engl J Med. 2011;365:2155–2166. doi: 10.1056/NEJMoa1104875. [DOI] [PubMed] [Google Scholar]
- 2.Cain K, Benoit SR, Winston CA, Mac Kenzie WR. Tuberculosis among foreign-born persons in the United States. JAMA. 2008;300:405–412. doi: 10.1001/jama.300.4.405. [DOI] [PubMed] [Google Scholar]
- 3.Grosse SD, Krueger KV, Mvundura M. Economic productivity by age and sex: 2007 estimates for the United States. Med Care. 2009;47(Suppl 1):S94–S103. doi: 10.1097/MLR.0b013e31819c9571. [DOI] [PubMed] [Google Scholar]
- 4.Ricks M, Cain KP, Oeltmann JE, Kammerer JS, Moonan PK. Estimating the burden of tuberculosis among foreign-born persons acquired prior to entering the US, 2005–2009. PLOS ONE. 2011;6:e27405. doi: 10.1371/journal.pone.0027405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moonan K, Ghosh S, Oeltmann JE, Kammerer JS, Cowan LS, Navin TR. Using genotyping and geospatial scanning to estimate recent Mycobacterium tuberculosis transmission, United States. Emerg Infect Dis. 2012;18:458–465. doi: 10.3201/eid1803.111107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dye C. Breaking a law: tuberculosis disobeys Styblo’s rule. Bull World Health Organ. 2008;86:4. doi: 10.2471/BLT.07.049510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borgdorff MW, van den Hof S, Kremer K, et al. Progress towards tuberculosis elimination: secular trend, immigration and transmission. Eur Respir J. 2010;36:339–347. doi: 10.1183/09031936.00155409. [DOI] [PubMed] [Google Scholar]
- 8.Behr MA, Warren SA, Salamon H, et al. Transmission of Mycobacterium tuberculosis from patients smear-negative for acid-fast bacilli. Lancet. 1999;353:444–449. doi: 10.1016/s0140-6736(98)03406-0. [DOI] [PubMed] [Google Scholar]
- 9.Reichler MR, Reves R, Bur S, et al. Evaluation of investigations conducted to detect and prevent transmission of tuberculosis. JAMA. 2002;287:991–995. doi: 10.1001/jama.287.8.991. [DOI] [PubMed] [Google Scholar]
- 10.Marks SM, Taylor Z, Qualls NL, Shrestha-Kuwahara RJ, Wilce MA, Nguyen CH. Outcomes of contact investigations of infectious tuberculosis patients. Am J Respir Crit Care Med. 2000;162:2033–2038. doi: 10.1164/ajrccm.162.6.2004022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hernandez-Garduno E, Cook V, Kunimoto D, Elwood RK, Black WA, FitzGerald JM. Transmission of tuberculosis from smear negative patients: a molecular epidemiology study. Thorax. 2004;59:286–290. doi: 10.1136/thx.2003.011759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tostmann A, Kik SV, Kalisvaart NA, et al. Tuberculosis transmission by patients with smear-negative pulmonary tuberculosis in a large cohort in The Netherlands. Clin Infect Dis. 2008;47:1135–1142. doi: 10.1086/591974. [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization. Tuberculosis country profiles 2011. Geneva, Switzerland: WHO; 2013. [Accessed October 2013]. http://www.who.int/tb/data. [Google Scholar]
- 14.Salpeter EE, Salpeter SR. Mathematical model for the epidemiology of tuberculosis, with estimates of the reproductive number and infection-delay function. Am J Epidemiol. 1998;147:398–406. doi: 10.1093/oxfordjournals.aje.a009463. [DOI] [PubMed] [Google Scholar]
- 15.US Department of Commerce, United States Census Bureau. Total CPS population and per capita income. Washington DC, USA: US Census Bureau; 2013. [Accessed October 2013]. Census Bureau Table P-1. http://www.census.gov/hhes/www/income/data/historical/people/ [Google Scholar]
- 16.Bureau of Labor Statistics, United States Department of Labor. Current Population Survey. Washington DC, USA: BLS; 2013. [Accessed October 2013]. http://www.bls.gov/cps. [Google Scholar]
- 17.Center for Medicare and Medicaid Services. 2010 Clinical laboratory fee schedule. Baltimore, MD, USA: CMS; 2013. [Accessed October 2013]. http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ClinicalLabFeeSched/clinlab.html. [Google Scholar]
- 18.Bureau of Labor Statistics, United States Department of Labor. Occupational Employment Statistics Survey. Washington DC, USA: BLS; 2013. [Accessed October 2013]. http://www.bls.gov/oes. [Google Scholar]
- 19.Haddix AC, Teutsch SM, Corso S. Prevention effectiveness: a guide to decision analysis and economic evaluation. 2. Oxford, UK: Oxford University Press; 2003. [Google Scholar]
- 20.Burman WJ, Dalton CB, Cohn DL, Butler JR, Reves RR. A cost-effectiveness analysis of directly observed therapy vs self-administered therapy for treatment of tuberculosis. Chest. 1997;112:63–70. doi: 10.1378/chest.112.1.63. [DOI] [PubMed] [Google Scholar]
- 21.American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America. Treatment of tuberculosis. MMWR Recomm Rep. 2003;52(RR-11):1–77. [PubMed] [Google Scholar]
- 22.Bureau of Labor Statistics, United States Department of Labor. Consumer Price Index. Washington DC, USA: BLS; 2013. [Accessed October 2013]. http://www.bls.gov/cpi/ [Google Scholar]
- 23.Taylor Z, Marks SM, Ríos Burrows NM, Weis SE, Stricof RL, Miller B. Causes and costs of hospitalization of tuberculosis patients in the United States. Int J Tuberc Lung Dis. 2000;4:931–939. [PMC free article] [PubMed] [Google Scholar]
- 24.Brown RE, Miller B, Taylor WR. Health-care expenditures for tuberculosis in the United States. Arch Intern Med. 1995;155:1595–1600. [PubMed] [Google Scholar]
- 25.Holland D, Sanders GD, Hamilton CD, Stout JE. Costs and cost-effectiveness of four treatment regimens for latent tuberculosis infection. Am J Respir Crit Care Med. 2009;179:1055–1060. doi: 10.1164/rccm.200901-0153OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guo N, Marra F, Marra CA. Measuring health-related quality of life in tuberculosis: a systematic review. Health Qual Life Outcomes. 2009;7:14. doi: 10.1186/1477-7525-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guo N, Marra F, FitzGerald JM, Elwood RK, Marra CA. Impact of adverse drug reaction and predictivity of quality of life status in tuberculosis. Eur Respir J. 2010;36:206–208. doi: 10.1183/09031936.00159409. [DOI] [PubMed] [Google Scholar]
- 28.Marra CA, Marra F, Colley L, Moadebi S, Elwood RK, FitzGerald JM. Health-related quality of life trajectories among adults with tuberculosis: differences between latent and active infection. Chest. 2008;133:396–403. doi: 10.1378/chest.07-1494. [DOI] [PubMed] [Google Scholar]
- 29.Miller TL, McNabb SJ, Hilsenrath P, Pasipanodya J, Weis SE. Personal and societal health quality lost to tuberculosis. PLOS ONE. 2009;4:e5080. doi: 10.1371/journal.pone.0005080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dion MJ, Tousignant P, Bourbeau J, Menzies D, Schwartzman K. Measurement of health preferences among patients with tuberculous infection and disease. Med Decis Making. 2002;22(Suppl):S102–S114. doi: 10.1177/027298902237706. [DOI] [PubMed] [Google Scholar]
- 31.Dion MJ, Tousignant P, Bourbeau J, Menzies D, Schwartzman K. Feasibility and reliability of health-related quality of life measurements among tuberculosis patients. Qual Life Res. 2004;13:653–665. doi: 10.1023/B:QURE.0000021320.89524.64. [DOI] [PubMed] [Google Scholar]
- 32.Guo N, Marra CA, Marra F, Moadebi S, Elwood RK, Fitz-Gerald JM. Health state utilities in latent and active tuberculosis. Value Health. 2008;11:1154–1161. doi: 10.1111/j.1524-4733.2008.00355.x. [DOI] [PubMed] [Google Scholar]
- 33.Linas B, Wong AY, Freedberg KA, Horsburgh CR., Jr Priorities for screening and treatment of latent tuberculosis infection in the United States. Am J Respir Crit Care Med. 2011;184:590–601. doi: 10.1164/rccm.201101-0181OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saukkonen JJ, Cohn DL, Jasmer RM, et al. An official ATS statement: hepatotoxicity of antituberculosis therapy. Am J Respir Crit Care Med. 2006;174:935–952. doi: 10.1164/rccm.200510-1666ST. [DOI] [PubMed] [Google Scholar]
- 35.International Union Against Tuberculosis Committee on Prophylaxis. Efficacy of various durations of isoniazid preventive therapy for tuberculosis: five years of follow-up in the IUAT trial. Bull World Health Organ. 1982;60:555–564. [PMC free article] [PubMed] [Google Scholar]
- 36.Arias E. National Vital Statistics Reports 2011. Hyattsville, MD, USA: National Center for Health Statistics; 2011. United States Life Tables, 2007. [PubMed] [Google Scholar]
- 37.Wilensky U. NetLogo. Evanston, IL, USA: Center for Connected Learning and Computer-Based Modeling, Northwestern University; 1999. [Accessed October 2013]. http://ccl.northwestern.edu/netlogo/ [Google Scholar]
- 38.Briggs A, O’Brien BJ, Blackhouse G. Thinking outside the box: recent advances in the analysis and presentation of uncertainty in cost-effectiveness studies. Annu Rev Public Health. 2002;23:377–401. doi: 10.1146/annurev.publhealth.23.100901.140534. [DOI] [PubMed] [Google Scholar]
- 39.Grosse SD. Assessing cost-effectiveness in healthcare: history of the $50,000 per QALY threshold. Expert Rev Pharmacoecon Outcomes Res. 2008;8:165–178. doi: 10.1586/14737167.8.2.165. [DOI] [PubMed] [Google Scholar]
- 40.Efron B, Tibshirani R. An introduction to the bootstrap. New York, NY, USA: Chapman and Hall; 1993. [Google Scholar]
Footnotes
The Appendix is available in the online version of this article at http://www.ingentaconnect.com/content/iuatld/ijtld/2013/00000017/00000012/art00006
The Bureau of Labor Statistics calculates annual wages by multiplying the hourly mean wage by 2080 hours; we have used the same estimate of 2080 annual employment hours.
CPT Code 87206.
CPT Code 87116.
CPT Code 71015.
CPT Code 99232.
Data on hospitalization for treatment-related adverse events has not previously been published and was provided by the Tuberculosis Trials Consortium Data Management Center.
Assumed duration of treatment for TB disease (see Model Inputs and Assumptions).
Mean length of hospitalization for patients hospitalized for TB disease (see Model Inputs and Assumptions).
Calculated based on the number of expected future years of life for an individual aged 35 years and 1 lost QALY per year of lost life, based on the 2007 US Life Tables.
Calculated based on the number of expected future years of life for an individual aged 35 years, based on the 2007 US Life Tables and the 0.05 lost QALYs per year for individuals with previous TB disease.
References
- 1.Sterling TR, Bethel J, Goldberg S, et al. The scope and impact of treatment of latent tuberculosis infection in the United States and Canada. Am J Respir Crit Care Med. 2006;173:927–931. doi: 10.1164/rccm.200510-1563OC. [DOI] [PubMed] [Google Scholar]
- 2.Taylor Z, Nolan CM, Blumberg HM, et al. Controlling tuberculosis in the United States. Recommendations from the American Thoracic Society, CDC, and the Infectious Diseases Society of America. MMWR Morb Mortal Wkly Rep. 2005;54(RR-12):1–81. [PubMed] [Google Scholar]
- 3.Sterling TR, Villarino ME, Borisov AS, et al. Three months of rifapentine and isoniazid for latent tuberculosis infection. N Engl J Med. 2011;365:2155–2166. doi: 10.1056/NEJMoa1104875. [DOI] [PubMed] [Google Scholar]
- 4.Martinson NA, Barnes GL, Moulton LH, et al. New regimens to prevent tuberculosis in adults with HIV infection. N Engl J Med. 2011;365:11–20. doi: 10.1056/NEJMoa1005136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schechter M, Zajdenverg R, Falco G, et al. Weekly rifapentine/isoniazid or daily rifampin/pyrazinamide for latent tuberculosis in household contacts. Am J Respir Crit Care Med. 2006;173:922–926. doi: 10.1164/rccm.200512-1953OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. Recommendations for use of an isoniazid-rifapentine regimen with direct observation to treat latent Mycobacterium tuberculosis infection. MMWR Morb Mortal Wkly Rep. 2011;60:1650–1653. [PubMed] [Google Scholar]
- 7.NetLogo. Center for Connected Learning and Computer-Based Modeling, Northwestern University; Evanston, IL, USA: NetLogo; 1999. http://ccl.northwestern.edu/netlogo/ [computer program] [Google Scholar]
- 8.Holland DP, Sanders GD, Hamilton CD, Stout JE. Costs and cost-effectiveness of four treatment regimens for latent tuberculosis infection. Am J Respir Crit Care Med. 2009;179:1055–1060. doi: 10.1164/rccm.200901-0153OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taylor Z, Marks SM, Ríos Burrows NM, Weis SE, Stricof RL, Miller B. Causes and costs of hospitalization of tuberculosis patients in the United States. Int J Tuberc Lung Dis. 2000;4:931–939. [PMC free article] [PubMed] [Google Scholar]
- 10.American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America. Treatment of tuberculosis. MMWR Morb Mortal Wkly Rep. 2003;52(RR-11):1–77. [Google Scholar]
- 11.Center for Medicare and Medicaid Services. 2010 Clinical laboratory fee schedule. Woodlawn, MD, USA: CMS; 2010. [Accessed October 2013]. http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ClinicalLabFeeSched/clinlab.html. [Google Scholar]
- 12.Saukkonen JJ, Cohn DL, Jasmer RM, et al. An official ATS statement: hepatotoxicity of anti-tuberculosis therapy. Am J Respir Crit Care Med. 2006;174:935–952. doi: 10.1164/rccm.200510-1666ST. [DOI] [PubMed] [Google Scholar]
- 13.International Union Against Tuberculosis Committee on Prophylaxis. Efficacy of various durations of isoniazid preventive therapy for tuberculosis: five years of follow-up in the IUAT trial. Bull World Health Organ. 1982;60:555–564. [PMC free article] [PubMed] [Google Scholar]
- 14.Arias E. United States life tables, 2007. Hyattsville, MD, USA: National Center for Health Statistics; 2011. [PubMed] [Google Scholar]
- 15.United States Census Bureau. Total CPS population and per capita income. Washington DC, USA: Census Bureau; 2010. [Accessed September 2013]. Census Bureau Table P-1. http://www.census.gov/hhes/www/income/data/historical/people/ [Google Scholar]
- 16.Grosse SD, Krueger KV, Mvundura M. Economic productivity by age and sex: 2007 estimates for the United States. Med Care. 2009;47(Suppl 1):S94–103. doi: 10.1097/MLR.0b013e31819c9571. [DOI] [PubMed] [Google Scholar]
- 17.Ricks PM, Cain KP, Oeltmann JE, Kammerer JS, Moonan PK. Estimating the burden of tuberculosis among foreign-born persons acquired prior to entering the US, 2005–2009. PLOS ONE. 2011;6:e27405. doi: 10.1371/journal.pone.0027405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moonan PK, Ghosh S, Oeltmann JE, Kammerer JS, Cowan LS, Navin TR. Using genotyping and geospatial scanning to estimate recent Mycobacterium tuberculosis transmission, United States. Emerg Infect Dis. 2012;18:458–465. doi: 10.3201/eid1803.111107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Borgdorff MW, van den Hof S, Kremer K, et al. Progress towards tuberculosis elimination: secular trend, immigration and transmission. Eur Respir J. 2010;36:339–347. doi: 10.1183/09031936.00155409. [DOI] [PubMed] [Google Scholar]
- 20.Reichler MR, Reves R, Bur S, et al. Evaluation of investigations conducted to detect and prevent transmission of tuberculosis. JAMA. 2002;287:991–995. doi: 10.1001/jama.287.8.991. [DOI] [PubMed] [Google Scholar]
- 21.Cain KP, Benoit SR, Winston CA, Mac Kenzie WR. Tuberculosis among foreign-born persons in the United States. JAMA. 2008;300:405–412. doi: 10.1001/jama.300.4.405. [DOI] [PubMed] [Google Scholar]
- 22.Grosse SD. Assessing cost-effectiveness in healthcare: history of the $50,000 per QALY threshold. Expert Rev Pharmacoecon Outcomes Res. 2008;8:165–178. doi: 10.1586/14737167.8.2.165. [DOI] [PubMed] [Google Scholar]
- 23.Reuters Thomson. Thomson Reuters Micromedex 2.0. New York, NY, USA: Thomson Reuters; 2011. Red Book online. [Google Scholar]