Abstract
Objective
Comorbidity of cancer with ALS has been studied previously. Detailed description of the temporal relationship between cancer and ALS is however lacking.
Methods
We conducted a nested case-control study of ALS in Sweden during 1987–2009, including 5481 cases of ALS identified from the Swedish Patient Register and 27,405 controls randomly selected from the general Swedish population. Odds ratios (ORs) for association of ALS with previous cancer diagnosis and incidence rate ratios (IRRs) of cancer after diagnosis were calculated to compare ALS patients to ALS-free individuals.
Results
Overall, a previous cancer diagnosis was not associated with subsequent risk of ALS (OR 1.00; 95% CI 0.91–1.10). No overall association was observed for any specific cancer type. An increased risk of ALS was observed during the first year after cancer diagnosis (OR 1.50; 95% CI 1.17–1.92). In contrast, a lower risk of cancer was observed in ALS patients after diagnosis compared to ALS-free individuals (IRR 0.84; 95% CI 0.69–1.02). The risk reduction was seen primarily two or more years after ALS diagnosis (IRR 0.64; 95% CI 0.45–0.88).
Conclusions
Our results provide no evidence for comorbidity of cancer and ALS overall. Surveillance biases seem the most likely explanation for the limited associations detected.
Keywords: amyotrophic lateral sclerosis, cancer, melanoma, odds ratio, relative risk
Introduction
Cancer comorbidity has been described for a variety of complex diseases (1). Neurodegenerative diseases may be of special interest in studying cancer comorbidity given the largely different biology of neurodegeneration and carcinogenesis, i.e., premature cell death in neurodegeneration but resistance to cell death in carcinogenesis. Understanding the relationship between neurodegeneration and cancer may therefore shed light on the underlying pathogenesis and provide insights into potential therapeutic options for both conditions (2).
With the exception of a high co-occurrence of Parkinson’s disease and melanoma (3, 4), Alzheimer’s and Parkinson’s diseases have both been shown in many epidemiological studies to be associated with reduced risks of smoking-related and non-smoking-related cancers (3, 5), although the results on non-smoking related cancers appeared to be less consistent (6, 7). Based on clinical case series or case reports, earlier studies suggested a positive association between amyotrophic lateral sclerosis (ALS) and cancer (8–10). However, more recent large-scale epidemiological studies do not support a link (11–14), with the possible exception of melanoma (12, 14, 15).
In addition to potentially shared biological mechanisms, it is possible that clinical investigation, therapies and lifestyle changes after diagnosis of ALS lead to a change in cancer risk or detection and vice versa. A clear description of the temporal relationship between ALS and cancer may therefore be vital. However, majority of the previous epidemiological studies used mortality data for ALS ascertainment (12, 14, 15), precluding the possibility of examining the modifying effect of such factors on the studied association. Instead of using the general population as the reference group, another study compared patients with a diagnosis of ALS to individuals with other diseases (13).
Given the existing methodological shortcomings in previous epidemiological studies on ALS and cancer, in the present study, we took advantage of the nationwide population and health registers in Sweden to study the association between ALS and cancer, focusing on incidence rather than mortality, and using a population-based comparison group. Special attention was given to the temporality of the possible associations.
Materials and Methods
Study population
The study population was defined as all individuals included in the Swedish Population and Housing Census in 1980 who were born in Sweden (N=7,692,281).
Swedish Patient Register
Since 1964/1965, the Swedish National Board of Health and Welfare has compiled data on individual hospital discharge diagnoses coded according to the International Classification of Diseases (ICD) (ICD-7 before 1969, ICD-8 for 1969–1986, ICD-9 for 1987–1996, and ICD-10 for 1997 onward); registration of hospital discharge data has been nationwide since 1987. From 2001 the Patient Register has also collected data on outpatient visits to specialist care. The Patient Register records both primary and secondary diagnoses for each clinic visit, along with information on the dates of contact and discharge. Although the vast majority of ALS patients were identifiable through the hospital discharge data, about 10% cases were only seen in outpatient care (16). Given the nationwide complete coverage of the Patient Register for hospital discharge record since 1987, we identified ALS patients from the entire Patient Register (hospital discharge data during 1987–2009 and outpatient specialist care during 2001–2009). Assuming that ALS patients often need to visit a specialist for a definitive diagnosis and treatment, we have previously found that in Sweden ALS patients were hospitalized on average 5 months after their first specialist care record for ALS (16), suggesting that even the hospital discharge data might have identified ALS cases in a timely manner.
Follow-up
Using the unique National Registration Numbers, the study population was followed from January 1st, 1987 to December 31st, 2009, through cross-linkages to the Swedish Patient, Causes of Death, and Migration Registers. Follow-up was censored at the time of a first diagnosis of ALS (i.e., date of hospital admission as noted on the first hospital discharge record for ALS or date of the first specialist care for ALS, whichever came first), death, or emigration out of Sweden, whichever occurred first. A total of 628,033 individuals (8.2%) were excluded from follow-up because they had died (N=544,708), or emigrated out of Sweden (N=83,325) before January 1st, 1987, leaving 7,064,248 individuals in the study cohort.
Nested case-control study of previous cancer in patients with ALS
A nested case-control study was conducted within the study base. During follow-up, we identified 5481 cases of ALS. Using the method of incidence density sampling (17), we randomly selected five controls per case that were individually matched to the cases by sex and year of birth (N=27,405). These were persons who had not yet died, emigrated out of Sweden, or been diagnosed with ALS at the time of index case diagnosis. The index date was defined as the date of ALS diagnosis for cases and selection date (i.e., diagnosis date of their matched ALS cases) for controls.
The exposure of interest was a diagnosis of first primary malignant cancer before the index date. Through the National Registration Numbers, all matched cases and controls were linked to the Swedish Cancer Register. The Cancer Register is based on mandatory reports from clinicians and pathologists, as required by Swedish law since cancer registration began in 1958, and has a very high overall completeness (18). Multiple malignant cancers are recorded in the Cancer Register for all cancer patients. We selected only the first malignant cancer for each individual.
Follow-up of ALS cases and controls for cancer after index date
To examine the relative risk of cancer after ALS diagnosis, we prospectively followed the cases and controls from the index date to a diagnosis of first primary malignant cancer, death, emigration out of Sweden, or December 31st, 2009, whichever came first. Cases and controls that had been diagnosed with cancer before the index date were excluded, leaving 4926 cases and 24,635 controls in this follow-up analysis.
Covariates
Information on the highest achieved educational level at the index date was obtained from the Swedish Education Register, which was established by Statistics Sweden in 1985 and includes information on the highest level of formal education for all individuals living in Sweden between the age of 16 and 74 years; the Register is updated annually. Education was categorized as high (≥9 years), low (<9 years), or unknown. Since the earliest cases and controls were identified in 1987, information on socio-economic status and region of residence was obtained primarily from the 1990 Swedish Population and Housing Census. Socio-economic status was categorized as blue collar, white collar, self-employed, or unclassified (e.g., due to retirement, sick leave, etc). Region of residence was classified as southern, central, or northern Sweden. If region of residence or socio-economic status was missing or unclassified in the 1990 Census, information from the 1980 Census was used (N=8370).
Statistical analysis
In the nested case-control study, odds ratios (ORs) for ALS and 95% confidence intervals (CIs) were estimated by conditional logistic regression models. Multivariable models included the covariates education, socio-economic status and region of residence. Missing values of these covariates were treated as separate groups in all models. We first conducted overall analysis for any cancer. Given the previous studies linking ALS and melanoma (12, 14, 15), we separately examined the risk of ALS after a diagnosis of melanoma. Since smoking is generally accepted as a risk factor for ALS (19), we further conducted analysis for smoking-related cancers, including oral, esophageal, gastric, colorectal, hepatic, pancreatic, sinonasal, laryngeal, tracheal, lung, cervical, prostate, and renal and urinary tract cancers (20, 21). To evaluate modification of the association between cancer and ALS by sex and age, we stratified the analyses of any cancer by sex or age (<65 or ≥65 years at the index date) separately. To evaluate potential temporal variation of the association between cancer and ALS, we specifically examined different times before ALS diagnosis (<1, ≥1 to <2, and ≥2 years).
In the follow-up analysis, we first calculated the incidence rates of cancer among cases and controls by dividing the number of cancers by the accumulated person-years. Incidence rate ratios (IRRs) and 95% CIs were derived from Poisson regression models, after adjustment for age, sex, education, socio-economic status and region of residence. To illustrate the importance of the immediate period after diagnosis, we separately examined the cancer IRRs during the first two years after ALS diagnosis and thereafter. We also plotted the cumulative incidence curves (CICs) of cancer for ALS cases and controls, controlling for other competing causes of death (22).
Analyses were performed using SAS software, version 9.1 (SAS Institute, Inc., Cary, North Carolina).
This study was approved by the Regional Ethical Vetting Board in Stockholm, Sweden.
Results
The basic characteristics of ALS cases are shown in Table I.
Table I.
Characteristics of amyotrophic lateral sclerosis patients, a nested case-control study in Sweden, 1987–2009
| Characteristics | No (%) |
|---|---|
| Total | 5481 |
| Sex | |
| Men | 3084 (56.3) |
| Women | 2397 (43.7) |
| Age at diagnosis, yrs | |
| <55 | 686 (12.5) |
| ≥55 and <65 | 1170 (21.4) |
| ≥65 and <75 | 1813 (33.1) |
| ≥75 and <85 | 1552 (28.3) |
| ≥85 | 260 (4.7) |
| Calendar period at diagnosis | |
| 1987–1992 | 1113 (20.3) |
| 1993–1998 | 1179 (21.5) |
| 1999–2004 | 1660 (30.3) |
| 2005–2009 | 1529 (27.9) |
In total, 555 out of 5481 ALS cases and 2770 out of 27,405 controls had been diagnosed with a cancer before the index date. The most common cancer was prostate cancer followed by breast cancer (Table II). Overall, ALS cases and controls did not differ concerning previous cancer history (OR 1.00; 95% CI 0.91–1.10). No difference was seen when stratifying the analysis by sex or age (men: 302 cancer patients among 3084 cases and 1481 cancer patients among 15,420 controls; OR 1.02; 95% CI 0.89–1.16; women: 253 cancer patients among 2397 cases and 1289 cancer patients among 11,985 controls; OR 0.98; 95% CI 0.85–1.13; <65 year at the index date: 80 cancer patients among 1856 cases and 403 cancer patients among 9284 controls; OR 0.98; 95% CI 0.76–1.26; and ≥65 year at the index date: 475 cancer patients among 3625 cases and 2367 cancer patients among 18,121 controls; OR 1.00; 95% CI 0.90–1.11). ALS cases did not, overall, have more preceding melanomas (OR 1.21; 95% CI 0.84–1.76) or smoking-related cancers (OR 1.04; 95% CI 0.91–1.20), than controls.
Table II.
Previous cancer among amyotrophic lateral sclerosis (ALS) patients and their birth year and sex matched controls, a nested case-control study in Sweden, 1987–2009a
| Controls (n=27405) | ALS cases (n=5481) | |
|---|---|---|
| No cancer, n (%) | 24635 (89.9) | 4926 (89.9) |
| Any cancer, n (%) | 2770 (10.1) | 555 (10.1) |
| Oral, n | 52 | 16 |
| Anal, n | 126 | 28 |
| Lung/trachea, n | 43 | 12 |
| Renal, n | 262 | 47 |
| Breast, n | 451 | 81 |
| Prostate, n | 539 | 117 |
| Melanoma, n | 143 | 35 |
| Other skin, n | 139 | 31 |
| Endocrine, n | 81 | 17 |
| Brain, n | 69 | 15 |
| Colon, n | 189 | 39 |
| Lymphoma, n | 103 | 12 |
| Cervix, n | 76 | 16 |
| Corpus, n | 131 | 26 |
| Ovarian, n | 64 | 8 |
| Thyroid, n | 34 | 8 |
| Leukemia, n | 30 | 5 |
| Other, n | 238 | 42 |
Only cancers with more than 5 cases among ALS cases presented.
However, compared to controls, cases were more likely to have a cancer diagnosis during the year preceding the index date (OR 1.50; 95% CI 1.17–1.92), but not one or more years before the index date (Table III). During the year preceding the index date, ALS was diagnosed after a median of 138 days after cancer diagnosis (range = 0 to 340 days). This association was mainly due to a higher probability of diagnosis of melanoma (OR 5.13; 95% CI 1.65–15.9), prostate cancer (OR 1.97; 95% CI 1.27–3.06) or brain tumor (OR 4.25; 95% CI 1.14–15.9) (Table III). No statistically significant association was found for other cancer sites (data not shown).
Table III.
Previous cancer and subsequent risk of amyotrophic lateral sclerosis (ALS), a nested case-control study in Sweden, 1987–2009
| Controls (n=27405) | ALS (n=5481) | OR (95% CI) a | |
|---|---|---|---|
| No cancer | 24635 | 4926 | Reference |
| Any cancer | |||
| <1 yr before index date | 270 | 81 | 1.50 (1.17–1.92) |
| ≥1 to <2 yrs before index date | 243 | 57 | 1.17 (0.88–1.57) |
| ≥2 yrs before index date | 2257 | 417 | 0.92 (0.82–1.03) |
| Melanoma | |||
| <1 yr before index date | 6 | 6 | 5.13 (1.65–15.9) |
| ≥1 to <2 yrs before index date | 8 | 2 | 1.24 (0.26–5.82) |
| ≥2 yrs before index date | 129 | 27 | 1.03 (0.68–1.56) |
| Prostate cancer | |||
| <1 yr before index date | 71 | 28 | 1.97 (1.27–3.06) |
| ≥1 to <2 yrs before index date | 66 | 18 | 1.37 (0.81–2.33) |
| ≥2 yrs before index date | 402 | 71 | 0.88 (0.68–1.14) |
| Brain tumor | |||
| <1 yr before index date | 5 | 4 | 4.25 (1.14–15.9) |
| ≥1 to <2 yrs before index date | 2 | 0 | - |
| ≥2 yrs before index date | 62 | 11 | 0.88 (0.46–1.68) |
OR, odds ratio;
CI, confidence interval; ORs were adjusted for age, sex, education level, region of area and socio-economic status
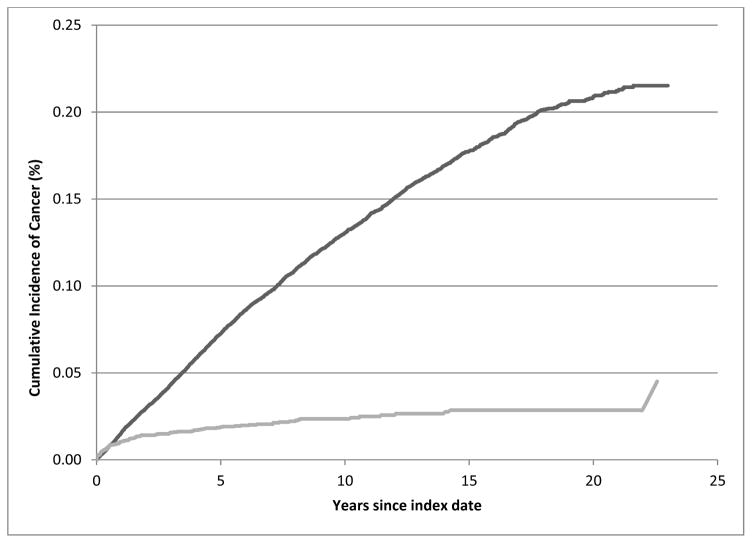
After excluding cases and controls that had been diagnosed with cancer before the index date, 4926 cases and 24,635 controls remained in the follow-up analysis. A total of 103 ALS cases and 2948 controls were diagnosed with a first primary malignant cancer after the index date (Table IV). Overall, ALS cases tended to have a lower risk of subsequent cancer diagnosis after the index date, compared to their controls (IRR 0.84; 95% CI 0.69–1.02). This was also shown by a higher cumulative incidence of cancer among the controls than cases (Figure 1). The inverse association was most evident two or more years after the index date (Table IV).
Table IV.
Risk of first primary cancer after diagnosis of amyotrophic lateral sclerosis (ALS), a cohort study in Sweden, 1987–2009
| No. of cancers | Person-years | IRR (95% CI) a | |
|---|---|---|---|
| Overall | |||
| ALS-free (n=24635) | 2948 | 179,443 | Reference |
| ALS (n=4926) | 103 | 8688 | 0.84 (0.69–1.02) |
| First year after index date | |||
| ALS-free | 371 | 23,427 | Reference |
| ALS | 50 | 3184 | 1.09 (0.80–1.45) |
| Second year after index date | |||
| ALS-free | 319 | 21,098 | Reference |
| ALS | 17 | 1615 | 0.82 (0.48–1.29) |
| >2 years after index date | |||
| ALS-free | 2258 | 134,919 | Reference |
| ALS | 36 | 3889 | 0.64 (0.45–0.88) |
IRR, incidence rate ratio;
CI, confidence interval; IRRs were adjusted for age, sex, education level, region of area and socio-economic status
Figure 1.
Cumulative incidence curves of cancer by amyotrophic lateral sclerosis status, a cohort study in Sweden, 1987–2009.
Discussion
In this large population-based study, we found no overall association of previous cancer with subsequent risk of developing ALS. No statistically significantly altered risk of ALS was observed after a diagnosis of either melanoma or smoking-related cancers. However, compared to controls, more ALS cases were diagnosed with cancer, especially melanoma, prostate cancer and brain tumors, during the year before ALS diagnosis. On the other hand, ALS patients were significantly less likely to be diagnosed with cancer two or more years after the diagnosis of ALS.
Following a few case series suggesting a higher than chance co-occurrence of cancer and ALS (8–10), four epidemiological studies reported a lack of overall association between cancer and ALS (11–14). Given that ALS is relatively rare, and the intersection with cancer (especially when restricted to site-specific cancers) will be rarer still, register-based studies appear to be the most realistic setting to investigate the comorbidity of cancer and ALS (12–14). Two studies used the US Surveillance, Epidemiology and End Results Program and examined the risk of ALS death among cancer survivors (12, 14). Because of the design, these studies were not able to assess the risk of cancer after ALS diagnosis. A third study was similar to ours in its focus on ALS incidence rather than mortality, using records of hospital admission and outpatient care, and its evaluation of cancer both before and after ALS diagnosis (13). However, this study used patients with other minor health conditions as controls, rather than randomly selected individuals from the general population, leaving potentially altered risk of cancer among these control patients a potential concern (13).
In the present analysis we were also able to examine the temporal relationship between cancer and ALS, especially the time periods immediately before and after ALS diagnosis. Whilst paraneoplastic neuromuscular disorders are described (23), the general view is that ALS-like disorders and cancer represent an unfortunate coincidence. We believe the more plausible explanation for the temporal association of cancer and ALS shortly before ALS diagnosis is surveillance bias, whereby the frequency of clinical examination and investigation associated with either ALS or cancer increases the likelihood of incidental diagnosis of the other. The earliest pathological changes in ALS seem likely to occur long before the onset of symptoms, as recognized in Alzheimer’s and Parkinson’s diseases. It is therefore also a possibility that neurotoxic chemotherapy given for the treatment of cancer might precipitate the onset of ALS in vulnerable individuals, but this remains speculative at present.
On the other hand, our finding of a reduced risk of cancer diagnosis among ALS patients, especially later in disease progression (i.e., more than two years after diagnosis) might suggest under-diagnosis of cancer among those with established ALS. With a median survival of 1–3 years from diagnosis, ALS patients often succumb rapidly after diagnosis without surviving long enough for cancer development. Reduced surveillance might be one of the reasons for such under-diagnosis. Possibilities include a lack of normal screening in the context of the focus on palliative care after a diagnosis of ALS, automatic attribution of weight loss to ALS rather than cancer, and, less plausibly, lack of communication in those “locked in” or with severe cognitive impairment (both relatively unusual in ALS).
Pooling all cancers together as a group might mask real associations between specific cancer sites and ALS, given the varying biology of different cancers. Previous studies have attempted to study separate cancer sites in addition to an overall estimate. For example, a higher than expected co-occurrence of breast cancer and ALS was reported in case series (8, 10, 24), although not confirmed in any of the epidemiological studies (12–14). Another cancer of interest is melanoma. Patients with melanoma were shown in three studies to have a higher risk of ALS (12, 14, 15), but this was not reported in a fourth study (13). In the present study, although melanoma was not associated with an altered risk of ALS overall, a recent melanoma diagnosis was associated with a five-fold increase in ALS risk. The lack of association for melanoma diagnosed earlier in time before ALS contrasts with two previous studies which showed a consistent positive association for melanoma diagnosed at different times (i.e., 1–5, 5–10, and >10 years) before ALS death (14, 15). It should be noted that because of the relative rarity of the combined conditions, all studies are based on small numbers of cases.
Similar to melanoma, we found that during the year before diagnosis, ALS patients had also higher risk of brain tumor and prostate cancer. Although a total of 15 cases had concurrent brain tumor, only four were diagnosed with brain tumor in the year before ALS diagnosis including three cases of meningioma and one case of glioma. Detection of an incidental brain tumor (e.g., benign meningioma) as part of the ALS work-up seems the most plausible explanation. The positive association between prostate cancer and ALS has not been reported previously. The widespread availability and use of serum prostate specific antigen measurement by non-specialists may be contributory but unsubstantiated at present. Although not statistically significant, similar associations were suggested for other skin cancer as well as cancer in the cervix or corpus uteri (data not shown). At onset, ALS may be associated with unspecific symptoms such as fatigue and weight loss which could likely elicit a search for malignancy by non-neurologists and this may in turn cause an increased detection rate with respect to common cancer types despite an absence of a pathophysiological association with ALS. Overall, intensified medical surveillance, in addition to chance association, seem the most possible explanations for the observed associations between ALS and individual cancers. Similar possibilities for Parkinson’s disease have also been raised previously (25, 26).
The strengths of the present study included the large sample size, the independently and prospectively collected data on cancer and ALS, and the complete and long follow-up. As in all other register-based studies, a major limitation is the lack of control for confounding factors that might be associated with both ALS and cancer, such as smoking (19). We did however adjust for socio-economic status, education and region of residence in all the analyses and the relative risk estimates with these adjustments did not differ greatly from the estimates that were unadjusted for these factors (data not shown). Another limitation is that ALS cases were identified from the Patient Register and, before 2001, solely via the hospital discharge records. It is likely that we missed cases that were never hospitalized during the study period. However, through comparison of the outpatient specialist care and hospital discharge records, we found that about 90% of all ALS patients could be identified through hospital discharge records (16). Finally, as cases were identified from the Patient Register, we did not have detailed data on ALS diagnosis and clinical features, such as the date and site of onset.
In conclusion, in this large population-based study, we did not observe an overall association between ALS and cancer. The higher risk of ALS immediately after cancer diagnosis is assumed to be due to surveillance bias and the lower risk of cancer two years or more after ALS diagnosis likely due to a combination of reduced survival and a predominantly palliative care approach to overall management in the ALS patients.
Acknowledgments
This study was supported by Swedish Research Council (Grant No. 521-2011-2742 to Dr Fang and SIMSAM Grant No. 80748301 to Dr Ye) and the Swedish Society for Medical Research (Dr Fang).
Footnotes
Disclosure of interests
All authors declared no financial interest.
References
- 1.Tabares-Seisdedos R, Dumont N, Baudot A, Valderas JM, Climent J, Valencia A, et al. No paradox, no progress: inverse cancer comorbidity in people with other complex diseases. Lancet Oncol. 2011;12:604–8. doi: 10.1016/S1470-2045(11)70041-9. [DOI] [PubMed] [Google Scholar]
- 2.Plun-Favreau H, Lewis PA, Hardy J, Martins LM, Wood NW. Cancer and neurodegeneration: between the devil and the deep blue sea. PLoS Genet. 2010;6:e1001257. doi: 10.1371/journal.pgen.1001257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bajaj A, Driver JA, Schernhammer ES. Parkinson’s disease and cancer risk: a systematic review and meta-analysis. Cancer Causes Control. 2010;21:697–707. doi: 10.1007/s10552-009-9497-6. [DOI] [PubMed] [Google Scholar]
- 4.Liu R, Gao X, Lu Y, Chen H. Meta-analysis of the relationship between Parkinson disease and melanoma. Neurology. 2011;76:2002–9. doi: 10.1212/WNL.0b013e31821e554e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Driver JA, Beiser A, Au R, Kreger BE, Splansky GL, Kurth T, et al. Inverse association between cancer and Alzheimer’s disease: results from the Framingham Heart Study. BMJ. 2012;344:e1442. doi: 10.1136/bmj.e1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elbaz A, Peterson BJ, Bower JH, Yang P, Maraganore DM, McDonnell SK, et al. Risk of cancer after the diagnosis of Parkinson’s disease: a historical cohort study. Mov Disord. 2005;20:719–25. doi: 10.1002/mds.20401. [DOI] [PubMed] [Google Scholar]
- 7.Lo RY, Tanner CM, Van Den Eeden SK, Albers KB, Leimpeter AD, Nelson LM. Comorbid cancer in Parkinson’s disease. Mov Disord. 2010;25:1809–17. doi: 10.1002/mds.23246. [DOI] [PubMed] [Google Scholar]
- 8.Brain L, Croft PB, Wilkinson M. Motor neurone disease as a manifestation of neoplasm (with a note on the course of classical motor neurone disease) Brain. 1965;88:479–500. doi: 10.1093/brain/88.3.479. [DOI] [PubMed] [Google Scholar]
- 9.Mitchell DM, Olczak SA. Remission of a syndrome indistinguishable from motor neurone disease after resection of bronchial carcinoma. BMJ. 1979;2:176–7. doi: 10.1136/bmj.2.6183.176-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sadot E, Carluer L, Corcia P, Delozier Y, Levy C, Viader F. Breast cancer and motor neuron disease: clinical study of seven cases. Amyotroph Lateral Scler. 2007;8:288–91. doi: 10.1080/17482960701419505. [DOI] [PubMed] [Google Scholar]
- 11.Chio A, Brignolio F, Meineri P, Rosso MG, Tribolo A, Schiffer D. Motor neuron disease and malignancies: results of a population-based study. J Neurol. 1988;235:374–5. doi: 10.1007/BF00314238. [DOI] [PubMed] [Google Scholar]
- 12.Freedman DM, Travis LB, Gridley G, Kuncl RW. Amyotrophic lateral sclerosis mortality in 1.9 million US cancer survivors. Neuroepidemiology. 2005;25:176–80. doi: 10.1159/000087447. [DOI] [PubMed] [Google Scholar]
- 13.Fois AF, Wotton CJ, Yeates D, Turner MR, Goldacre MJ. Cancer in patients with motor neuron disease, multiple sclerosis and Parkinson’s disease: record linkage studies. J Neurol Neurosurg Psychiatry. 2010;81:215–21. doi: 10.1136/jnnp.2009.175463. [DOI] [PubMed] [Google Scholar]
- 14.Freedman DM, Curtis RE, Daugherty SE, Goedert JJ, Kuncl RW, Tucker MA. The association between cancer and amyotrophic lateral sclerosis. Cancer Causes Control. 2013;24:55–60. doi: 10.1007/s10552-012-0089-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baade PD, Fritschi L, Freedman DM. Mortality due to amyotrophic lateral sclerosis and Parkinson’s disease among melanoma patients. Neuroepidemiology. 2007;28:16–20. doi: 10.1159/000097851. [DOI] [PubMed] [Google Scholar]
- 16.Fang F, Chen H, Wirdefeldt K, Ronnevi LO, Al-Chalabi A, Peters TL, et al. Infection of the central nervous system, sepsis and amyotrophic lateral sclerosis. PLoS One. 2011;6:e29749. doi: 10.1371/journal.pone.0029749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Richardson DB. An incidence density sampling program for nested case-control analyses. Occup Environ Med. 2004;61:e59. doi: 10.1136/oem.2004.014472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barlow L, Westergren K, Holmberg L, Talback M. The completeness of the Swedish Cancer Register: a sample survey for year 1998. Acta Oncol. 2009;48:27–33. doi: 10.1080/02841860802247664. [DOI] [PubMed] [Google Scholar]
- 19.Armon C. Smoking may be considered an established risk factor for sporadic ALS. Neurology. 2009;73:1693–8. doi: 10.1212/WNL.0b013e3181c1df48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huncharek M, Haddock KS, Reid R, Kupelnick B. Smoking as a risk factor for prostate cancer: a meta-analysis of 24 prospective cohort studies. Am J Public Health. 2010;100:693–701. doi: 10.2105/AJPH.2008.150508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Secretan B, Straif K, Baan R, Grosse Y, El Ghissassi F, Bouvard V, et al. A review of human carcinogens--Part E: tobacco, areca nut, alcohol, coal smoke, and salted fish. Lancet Oncol. 2009;10:1033–4. doi: 10.1016/s1470-2045(09)70326-2. [DOI] [PubMed] [Google Scholar]
- 22.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 23.Sharp L, Vernino S. Paraneoplastic neuromuscular disorders. Muscle Nerve. 2012;46:839–40. doi: 10.1002/mus.23502. [DOI] [PubMed] [Google Scholar]
- 24.Forsyth PA, Dalmau J, Graus F, Cwik V, Rosenblum MK, Posner JB. Motor neuron syndromes in cancer patients. Ann Neurol. 1997;41:722–30. doi: 10.1002/ana.410410608. [DOI] [PubMed] [Google Scholar]
- 25.Driver JA, Kurth T, Buring JE, Gaziano JM, Logroscino G. Prospective case-control study of nonfatal cancer preceding the diagnosis of Parkinson’s disease. Cancer Causes Control. 2007;18:705–11. doi: 10.1007/s10552-007-9005-9. [DOI] [PubMed] [Google Scholar]
- 26.Driver JA, Logroscino G, Buring JE, Gaziano JM, Kurth T. A prospective cohort study of cancer incidence following the diagnosis of Parkinson’s disease. Cancer Epidemiol Biomarkers Prev. 2007;16:1260–5. doi: 10.1158/1055-9965.EPI-07-0038. [DOI] [PubMed] [Google Scholar]