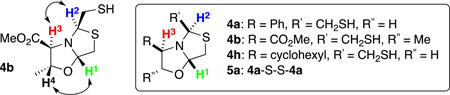
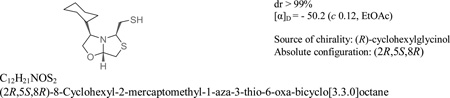Abstract
The synthesis of new oxazolidinylthiazolidines bicycles, oxygen analogues of bisthiazolidines, also known as metallo-β-lactamase inhibitors is described. The reaction of β-aminoalcohols and 2,5-dihydroxy-1,4-dithiane led to oxazolidinylthiazolidines and/or dithia-azabicycles as the main products. The distribution pattern depends mainly on the aminoalcohol substituents. In a one-pot reaction, four new bonds are formed in good yields and with high atom efficiency. When the oxazolidinylthiazolidines are formed, two stereogenic centres are generated with high enantiospecificity. The reaction mechanism is discussed based on crystallographic data and interconversion studies. Two oxazolidinylthiazolidines were evaluated as inhibitors of the potent lactamase NDM-1 and compound 4f displayed competitive inhibition with Ki = 1.6 ± 0.6 µM.
Graphical Abstract
Stereochemistry Abstract
1. Introduction
Novel fused heterocyclic systems are important scaffolds in medicinal chemistry due to their ability to generate complex structures.1,2 In particular, fused five-membered 1,3-heterocycles containing oxygen, nitrogen, or sulfur are interesting scaffolds in the preparation of complex chiral molecules such as natural products, pharmaceuticals or catalysts.3
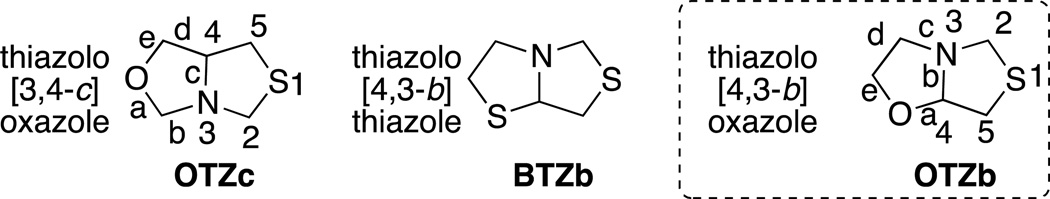
In this context, our group has explored the preparation of new bicycles by taking advantage of the ring-chain tautomerism, a process that involves the reversible movement of a proton followed by a change from an open structure to form a new heterocycle.4,5 By using this strategy, we have been able to prepare new heterocycles, including the synthesis of 5,5-fused heterocycles thiazolo[3,4-c]oxazoles4 and the sulfur analogues thiazolo[4,3-b]thiazoles,6 present different connectivity (Figure 1). The thiazolo[4,3-b]oxazole system is the oxygen analogue of thiazolo[4,3-b]thiazoles and attracted our interest (Figure 1).
Figure 1.
Previously prepared heterocycles thiazolo[3,4-c]oxazoles4 and thiazolo[4,3-b]thiazoles.6 Compound of synthetic interest: thiazolo[4,3-b]oxazole.
In an earlier approach, we prepared heterocycles thiazolo[3,4-c]oxazoles and thiazolo[4,3-b]thiazoles via simple synthetic pathways and with high diastereoselectivity, using low cost and readily available starting materials. The new bisthiazolidine scaffolds thiazolo[4,3-b]thiazoles showed diverse chemical and biological properties. We have recently constructed a dynamic combinatorial library suitable for disulfide exchange, using thiazolo[4,3-b]thiazole scaffolds. The dynamic library was responsive to the enzyme thioredoxin glutathione reductase, a flavoprotein from Echinococcus granulosus, and new inhibitors were identified.7 The prepared bisthiazolidines were also evaluated as metallo-β-lactamases inhibitors, enzymes that hydrolyze almost all β-lactam antibiotics and are unaffected by clinically available β-lactamase inhibitors. The thiol-bearing thiazolo[4,3-b]thiazole derivatives are competitive β-lactamase inhibitors of all metallo-β-lactamase subclasses, with Ki values in the low micromolar range against a broad range of metallo-β-lactamases enzymes such as NDM-1,8 VIM-2,9 Sfh-I, L1, IMP-1, GOB-13 and BcII.10
Due to our interest in the preparation and evaluation of new thiazolo[4,3-b]thiazole analogues, we were prompted to prepare fused oxazolidinylthiazolidines following the same methodology as used for thiazolo[4,3-b]thiazoles. There are few reports in the literature for the synthesis of the thiazolo[4,3-b]oxazole system (Figure 2). Different intermediates were used for thiazolo[4,3-b]oxazole preparation: azomethine ylides for (A)11,12,13 vinylogous N-acyliminium ions for (B),14 S-benzylthiocarbamates for (C)15 or CS2 for (D)16 (figure 2).
Figure 2.
Different synthetic methodologies for thiazolo[4,3-b]oxazole structures reported in literature.
Herein we focus our attention on the development of a new strategy for the preparation of new substituted thiazolo[4,3-b]oxazole, and report our findings on the synthesis of new thiazolo[4,3-b]oxazole and/or dithia-azabicycles through iminium ion formation by the condensation of β-aminoalcohols and mercaptoacetaldehyde.
2. Results and discussion
In order to synthesize thiazolo[4,3-b]oxazole via an efficient route, we used the same methodology we described before for the thiazolo[4,3-b]thiazole preparation.6 The strategy is based on a cascade reaction between mercaptoacetaldehyde and β-aminoalcohols. The preliminary results of heating linear β-aminoalcohols (X = O) and 2,5-dihydroxy-1,4-dithiane (mercaptoacetaldehyde dimer) in EtOH and catalytic p-TsOH acid led to dithia-azabicycles instead of the desired thiazolo[4,3-b]oxazole bicycle (Figure 3).
Figure 3.
Synthesis of bisthiazolidine or dithioazabicycle starting from linear aminothiols or aminoalcohols.
Crystallographic data allowed us to elucidate the correct structure of dithioazabicycle, previously misassigned as bisthiiranes.6 A reaction mechanism is proposed based on the results of a double iminium cyclization starting from an amine and two equivalents of mercaptoacetaldehyde, see Figure 4.
Figure 4.
Suggested mechanism for the synthesis of dithioazabicycle. ORTEP diagram of DTA-1 (R = p-F-Bn).
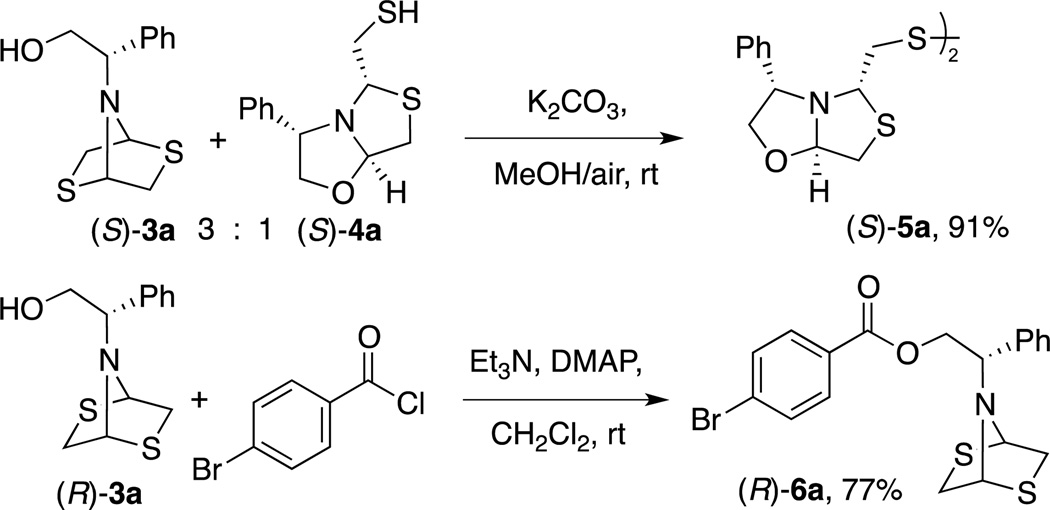
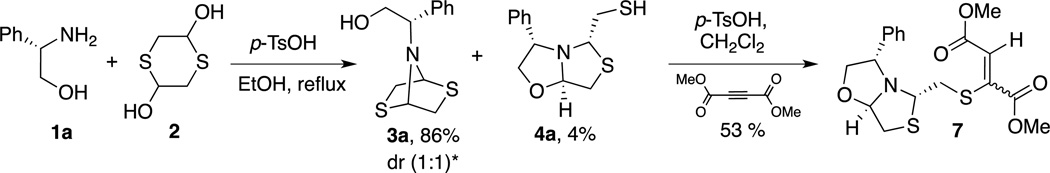
Intrigued by the structural requirements for thiazolo[4,3-b]oxazole formation, we used (S)-phenylglycinol 1a as the starting material, in order to study the influence of the substituent α to the nitrogen. The reaction of 1a and dithiane 2 catalyzed by p-TsOH acid in EtOH at reflux revealed the presence of 5% of the desired thiazolo[4,3-b]oxazole (S)-4a and dithioazabicycle 3a as the main product in 90% yield (entry 1, Table 1). We attributed the thiazolo[4,3-b]oxazole formation to the presence of a bulky substituent adjacent to the nitrogen in 1a.
Table 1.
Optimization of reaction conditions starting from glycinol 1a.
 | ||||
|---|---|---|---|---|
| Entry | Solvent | Time | Ratio 3a:4a* | Total yield (%) |
| 1 | EtOH, [1a] = 0.4 M | 2h | 9.5:0.5 | 90 |
| 2 | PhMe, [1a] = 0.1 M | 3h | 7:3 | 64 |
| 3 | PhMe, [1a] = 0.1 M | 7h | 7:3 | 51 |
| 4 | BA pH5, [1a] = 0.07 M | 30’ | 7:3 | 83 |
| 5 | BA pH5, [1a] = 0.07 M | 4h | 7:3 | 80 |
diastereomeric ratio 1:1. BA = acetic acid/acetate sodium, 1M.
In order to optimize the ratio of thiazolo[4,3-b]oxazole formation, we assayed different reaction conditions. Reflux in toluene gave dithia-azabicycle 3a/oxazolidine 4a in a 7:3 ratio (entry 2, Table 1). It is noteworthy that a higher temperature in toluene favoured the formation of the oxazolidine 4a, but longer reaction times increased decomposition of the products (entry 3, Table 1). The reaction was also performed in a buffer acetate at pH = 5, which led to the same distribution pattern, but higher yields than the reaction in toluene (entries 4 and 5, Table 1).
We isolated dithia-azabicycle (R)-3a by flash-column chromatography, but diastereomer (S)-3a co-eluted with oxazolidinythiazolidine (S)-4a. This result suggested that both compounds could be in tautomeric equilibrium. We performed different experiments to understand a possible interconversion mechanism. We first assigned the absolute configuration of the products after derivatization and crystallization. Fractions containing the mixture of diastereomer (S)-3a and bicycle (S)-4a in a 3:1 ratio were oxidized in basic media (K2CO3/MeOH/air) to give dimer (S)-5a as a unique product (91% yield) (Scheme 1). The formation of (S)-5a occurred at the expense of (S)-3a, through (S)-4a, triggered by blocking the reactivity of the thiol group to form the disulfide. This result supports our hypothesis about the tautomeric equilibria between 3a and 4a.
Scheme 1.
Derivatization of compounds (S)-4a and (R)-3a.
The crystal structure of (S)-5a was obtained after slow evaporation of CH2Cl2/n-hexanes (1:6) at room temperature and then determined by single-crystal X-ray diffraction methods. The molecular structure allowed us to confirm the absolute stereochemistry of the precursor oxazolidinylthiazolidine (S)-4a. The ORTEP view of compound is shown in Figure 5.
Figure 5.
ORTEP plots of compounds (S)-5a and (R)-6a.
On the other hand, esterification of pure compound (R)-3a was performed and single crystals of (R)-6a were obtained (Scheme 1). Compound (R)-6a was elucidated by X-ray experiments and the absolute configuration of this dithia-azabicycle was determined (Figure 5). Once the stereochemistry of the products was assigned, we studied the mechanism of the interconversion. We performed two parallel experiments: isolated diastereomers were dissolved in buffer solutions (pH 2, 5, 7 and 9) and stirred for 24h at room temperature. The product distribution was analyzed by 1H NMR (see Figure 6).
Figure 6.
Interconversion studies. (A): Starting material: mixture of (S)-3a and (S)-4a (2:1). (B): reaction mixture after stirring starting material (A) for 24 h in a buffer solution at pH 2. (C): Same procedure than (B) but at pH 5. (D): pH 7. (E): pH 9.
Interconversion of diastereomer (S)-3a to (R)-3a was observed, especially at pH 2. We also observed the inverse conversion from (R)-3a to tautomer (S)-4a and (S)-3a (data shown in Supplementary material).
These results indicate that a dynamic interconversion between diastereomers occurs mainly in acidic media. It is known that thiazolidines can undergo ring-chain tautomerism in solution.17 Our group has also reported this type of transformation for thiazolidines and oxazolidinylthiazolidines.4,5 The interconversion mechanism between dithiaazabicycles (R)-3a and (S)-3a could be explained by the formation of oxazolidine (S)-4a and (R)-4a intermediates (Scheme 2).
Scheme 2.
Suggested mechanism for the reversible interconversion of dithia-azabicycles (S)-3a into (R)-3a.
When (S)-3a equilibrates to (R)-3a, two stereogenic centers should be inverted. If we commence with dithia-azabicycle (S)-3a, the acetal bond could break, forming the iminium ion (S)-Ia with the loss of a stereogenic center. The intermediate could evolve to oxazolidinylthiazolidine (S)-4a, as observed by 1H-NMR. Compound (S)-4a could open and form the iminium ion Ib and then lead to the unstable oxazolidinylthiazolidine (R)-4a. This intermediate could then in turn open to form the iminium ion (R)-Ia followed by a ring closure to finally afford dithia-azabicycle (R)-3a (Scheme 2).
The interconversion between (S)-3a into (S)-4a was also observed in the Michael addition when a mixture of 3a:4a (95:5) in presence of DMAD led to the thiazolo[4,3-b]oxazole 7 in 53% yield (Scheme 3).
Scheme 3.
Diastereomeric resolution via 1,4-Michael addition of oxazolidinylthiazolidine 4a to DMAD. *dr = diasteromeric ratio.
This finding reinforces the concept of an interconversion between dithioazabicycle 3a and thiazolo[4,3-b]oxazole 4a as we started with a mixture of 4% of thiazolo[4,3-b]oxazole 4a and finally obtained the Michael adduct thiazolo[4,3-b]oxazole 7 in 53%.
On the other hand we explored the scope of the reaction using other β-aminoalcohols. Starting with methylthreonine.HCl 1b in PhMe we found erratic product distribution and yields. At this point, we suspected that the pH could be playing a crucial role in the reaction. We assayed different buffer solutions, from pH 2 to 6 (see Table 2). The best yield for the preparation of oxazolidinylthiazolidine 4b was obtained using buffer pH 5 (entry 4, Table 2). Lower and higher pH values led to decomposition products, probably due to lower reaction rates or dithiane decomposition.
Table 2.
Product distribution for 3b/4b formation at different pH.
 | |||
|---|---|---|---|
| Entry | Conditions* | Yield (%) | Ratio 3:4 |
| 1 | Buffer pH 2, 1 h | Decomp | - |
| 2 | Buffer pH 3, 1 h | Decomp | - |
| 3 | Buffer pH 4, 1 h | 40 % | 2:8 |
| 4 | Buffer pH 5, 1 h | 79 % | 1:9 |
| 5 | Buffer pH 6, 1 h | 53 % | 3:7 |
[1b] = 0.07M, Buffer pH 2 (HCl/KCl 1M), Buffer pH 3 (citric acid/citrate sodium 1M), Buffer pH 4 (BA: acetic acid/acetate sodium 1M), Buffer pH 5 (BA, 1M), Buffer pH 6 (NaH2PO4/NaOH 1M).
Having established the optimum pH for the thiazolo[4,3-b]oxazole formation, we explored the reaction using different β-aminoalcohols. We subjected aminoalcohols 1c–h to the same cyclization process in buffer acetic acid/acetate sodium pH 5 or an organic solvent, in order to study the influence of the solvent in the product distribution. Depending on the β-aminoalcohol substituents, we obtained dithia-azabicycle 3 and/or oxazolidinylthiazolidine 4 (see Table 3). The results showed that when the reaction was performed in an aqueous medium, it gave better yields than the same reaction in organic solvents. However the product distribution depended mainly on the aminoalcohol substituents R1 and R2. We observed that aminoalcohols with small or linear substituents at R1 only led to dithia-azabicycle 3 (entries 1–5, Table 3), but when R1 was bulky, an increased proportion of 4 was observed (entries 9 and 10, Table 3). The same result was observed for glycinol 1a and threonine 1b (see entry 4, Tables 1 and 2 respectively).
Table 3.
Reaction of aminothiols 1c-1h with dithiane 2.
 | ||||
|---|---|---|---|---|
| Entry | Starting material | Conditions | Ratio 3:4 | Yield (%) |
| 1 | BA pH5, 1h | 10:0 | 3c, 75% | |
| 2 | MeCN, p-TsOH ac, 2h | 10:0 | 3c, 65% | |
| 3 |  |
BA pH 5, 1 h | 10:0 | 3d*, 80% |
| 4 |  |
EtOH, p-TsOH ac, 2h | 10:0 | 3d* 100% |
| 5 |  |
BA pH5, 1 h | - | NR |
| 6 |  |
PhMe, p-TsOH ac., 3h | 10:0 | 3e, 97% |
| 7 |  |
BA pH5, 30min | 9:1 | 3f*+4f, 32% |
| 8 |  |
BA pH5, 30min | 10:0 | 3g, 74% |
| 9 |  |
PhMe, 3h | 8.5:1.5 | 3g+4g, 42% |
| 10 | BA pH5, 1h | 0:10 | 4h, 85% | |
| 11 | PhMe, 1h | 0:10 | 4h, 78% | |
diastereomeric ratio 1:1. NR = no reaction. BA = acetic acid/acetate sodium
The reaction with methylserine 1f mainly gave dithioazabicycle 3f and traces of OTZ 4f (entry 7, Table 3), but when we started with methylthreonine 1b, we observed thiazolo[4,3-b]oxazole 4b as the main product (entry 4, Table 2). This clearly indicates that the R2 substituents affect the product distribution. This could be explained by the Thorpe-Ingold effect, where the substituents increase the rate or equilibrium constant for ring forming reactions.18 In summary, both substituents at R1 and R2 are important: bulky substituents in R1 increase the formation of thiazolo[4,3-b]oxazole 4 vs dithioazabicycle 3 while the methyl group in R2 also shifts the equilibrium to the formation of thiazolo[4,3-b]oxazole.
In general, the absolute configuration of the oxazolidinylthiazolidines was assigned based on different experiments. The relative stereochemistry of compound 4b was established as syn by NMR using 1D gradient NOESY (Table 4). The configuration of compound 5a was determined by X-ray experiments; hence the same configuration for precursor 4a was proposed. Finally, the configuration of compound 4h was established as syn in analogy to 4b and 5a. This assignment was also supported by the analysis of the coupling constants observed for the oxazolidinylthiazolidines where the values for H1, H2 and H3 are in the same order of magnitude, see table 4.
Table 4.
NOE for 4b and coupling constants of oxazolidinylthiazolidines 4a, 4b,4h and dimer 5a.
Enzymatic activity
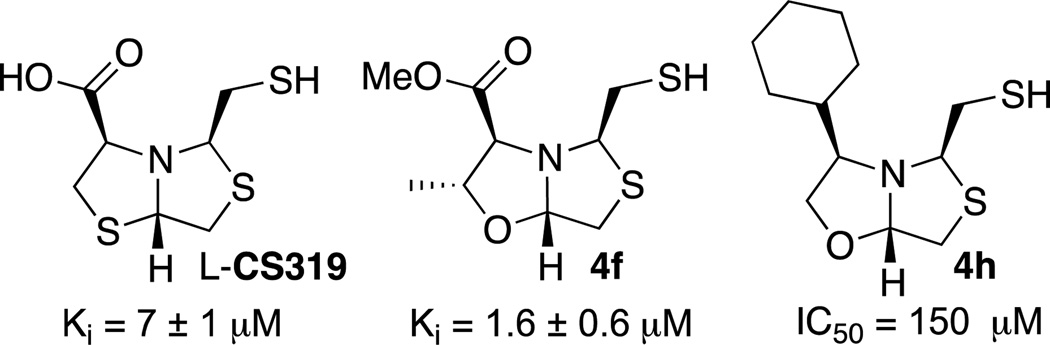
Recently we reported that bisthiazolidine compound, L-CS319 is able to inhibit metallo-β-lactamases from all subclasses,10 particularly the clinically relevant New Delhi metallo-β-Lactamase NDM-1.8 The molecular basis for the inhibition was based on the crystal structure of NDM-1:L-CS319. This complex indicated the importance of the thiol moiety that bridges the two Zn (II) ions, while the carboxylate interacts with Lys224 in the active site of the enzyme.
Two of the prepared compounds 4f and 4h are oxygen analogues of heterocycle L-CS319, which also bears a free thiol, but with different substituents at the carboxylate position.
We envisioned that thiazolo[4,3-b]oxazoles 4f and 4h could be evaluated as NDM-1 inhibitors and therefore we studied the effect on the initial rates of imipenem hydrolysis by NDM-1. Progress curves revealed a competitive inhibition model for 4f with an inhibition constant of Ki = 1.6 ± 0.6 µM, a good value for this type of enzymes. This compound is four times more potent than the sulfur analogue L-CS319, (see Figure 7). It is noteworthy that the isosteric replacement of the oxygen vs. sulphur is well tolerated. Neither the ester nor the methyl group present in 4f are deleterious to the inhibitor activity. Based on our previous results, we expected similar interactions at the active site with the thiol coordinating both Zn (II) ions and the ester interacting with a Lys residue of the active site. The methyl group probably has additional hydrophobic interactions leading to a lower Ki value than L-CS319. In contrast, compound 4h is less potent than 4f and L-CS319 and therefore was only characterized for IC50. The bulky cyclohexyl substitution present in 4h is counterproductive and probably responsible for the low inhibitory activity shown.
Figure 7.
Inhibition constant (Ki) of NDM-1 by L-CS319, 4f and half inhibitory concentration (IC50) of NDM-1 by 4h.
3. Conclusion
In conclusion we have enantiospecifically prepared oxazolidinylthiazolidine 4 bicycles with very high ee and good yields. The best yields were obtained when the reaction was performed in buffer acetate at pH 5 while the distribution pattern was independent of the solvent used. In general, the product distribution for this reaction depends on the aminoalcohol substituents (R1 and R2). Experimental data supported the idea that the products of the phenylglycinol cyclization 3a and 4a were in tautomeric equilibrium and an interconversion mechanism was proposed. Compound 4f was evaluated against the metallo-β-lactamase NDM-1, an oxygen analogue of known metallo-β-lactamase inhibitors bisthiazolidines and resulted in a good inhibitor.
4. Experimental
General
Reactions were monitored by analytical thin layer chromatography (TLC) 0.25 mm Silica gel plastic sheets (Macherey-Nagel, Polygram® SIL G/UV 254). Flash chromatography on Silica gel 60 (J. T. Baker, 40 µ m average particle diameter) was used to purify the crude reaction mixtures. NMR spectra were recorded at 400 MHz and 100 MHz (1H-NMR, 13C-NMR) using a Bruker AVANCE at 21 °C. Chemical shifts (δ) are reported as follows: chemical shift (multiplicity, coupling constant, integration). High-resolution mass spectrometry experiments were measured on a VG AutoSpect spectrometer (EIS mode). Crystallographic data was collected on a Bruker D8 Venture Single Crystal diffractometer. Melting points were determined using a Laboratory Devices Gallenkamp apparatus. Optical rotations were measured using a Kruss Optronic GmbH P8000 polarimeter with a 0.5-mL cell, [α]D values are given in 0.1 deg.cm2.g−1 (concentration c given as g/100 mL). All solvents were purified according to literature procedures. All reactions were carried out in dry, freshly distilled solvents under anhydrous conditions unless otherwise stated. All yields refer to isolated compounds after the final purification process, unless otherwise stated. Relative stereochemistry for 4b was determined using 1D gradient NOESY experiments, pulse sequence selnogp, D8 at 300 and 800 ms. Enantiomeric excess was determined using HPLC Chiral OD column 250 × 4.6 mm (L × I.D.). Diastereomeric excess was calculated based on 1H NMR spectra.
7-(4-Fluorobenzyl)-2,5-dithia-7-aza-bicyclo[2.2.1]heptane DTA-1
To a suspension of 1,4-dithiane-2,5-diol 2 (0.73g, 4.8 mmol) in ethanol (10 mL) were added p-F-benzylamine (0.5 g, 4.0 mmol) and catalytic p-TsOH acid (15 mg). The resulting mixture was heated at reflux for 2h. The solvent was removed under reduced pressure; the crude was poured into water and extracted with EtOAc (3 × 80 mL). The combined organic layers were dried over anhydrous Na2SO4 and filtered. The solvent was removed under reduced pressure and the crude was purified by column chromatography on SiO2 flash (1:5 EtOAc/hexanes) to afford pure DTA-1 as a white solid (0.9 g, 93% yield). The crude was recrystallized from a mixture of hexanes/CH2Cl2 to afford single crystals: mp 77–79 °C. 1H NMR (CDCl3) δ 3.24 (d, J = 9.5 Hz, 2H), 3.37 (dd, J = 9.5, 3.1 Hz, 2H), 3.56 (s, 2H), 4.77 (d, J = 3.1 Hz, 2H), 7.03 (t, J = 8.5 Hz, 2H), 7.37 (dd, J = 8.5, 5.5 Hz, 2H). 13C NMR (CDCl3) δ 44.0, 52.3, 69.7, 77.2, 115.4, 115.7, 130.5, 130.5, 133.3, 162.4 (C–F, J = 244.3 Hz); HRMS calcd for C11H14FNS2 [M+H]+ 242.0473, found: 242.0498.
General procedure for the synthesis of dithia-azabicycle 3 and oxazolidinylthiazolidine 4
Route A (organic solvent)
To a stirred solution of aminoalcohol 1 (3.6 mmol) in toluene (25 mL) were added 1,4-dithiane-2,5-dithiol 2 (4.3 mmol) and catalytic p-TsOH acid (0.025 g, 0.12 mmol). The mixture was heated at reflux for 3 h. The solvent was then removed under reduced pressure. The crude was poured into water (30 mL), extracted with EtOAc (3 × 50 mL), dried (Na2SO4) and filtered. The organic layer was evaporated under reduced pressure. The crude was purified by column chromatography (n-hexanes/EtOAc) to obtain compounds 3 and/or 4.
Route B (buffer acetate)
To a stirred suspension of aminoalcohol 1 (0.63 mmol) in acetic acid/acetate sodium [1M] pH = 5 (9 mL), was added 1,4-dithiane-2,5-dithiol 2 (0.750 mmol). The mixture was heated at reflux for 1h, cooled and extracted with EtOAc (4 × 30 mL), then dried (Na2SO4) and filtered. The organic layer was evaporated under reduced pressure. The crude was purified by column chromatography using (n-hexanes/EtOAc) to obtain compounds 3 and/or 4.
(2S)-2-((1S/R,4S/R)-2,5-dithia-7-azabicyclo[2.2.1]heptan-7’-yl)-2-phenylethanol (S,R)-3a and (2S,5S,8S)-8-phenyl-2-mercaptomethyl-1-aza-3-thio-6-oxabicyclo[3.3.0]octane (S)-4a
Prepared according to Route B, using (S)-phenylglycinol 1a as the starting aminoalcohol. The crude was purified by column chromatography on SiO2 flash (1:5 EtOAc/hexanes) to afford pure (R)-3a as a transparent oil and a mixture of (S)-3a and (S)-4a as an oil (0.585 g, total yield = 64%, (S/R)-3a/(S)-4a 7:3).
(2S)-2-((1R,4R)-2,5-Dithia-7-azabicyclo[2.2.1]heptan-7’-yl)-2-phenylethanol (R)-3a
[α]D = − 55.9 (c 0.1, EtOAc); 1H NMR (CDCl3) δ 3.19 (d, J = 9.5 Hz, 2H), 3.40 (dd, J = 9.5, 3.0 Hz, 2H), 3.46 (t, J = 5.2, 1H), 3.91 (d, J = 5.2 Hz, 2H), 5.00 (d, J = 3.0 Hz, 2H), 7.39 (m, 5 H); 13C NMR (CDCl3) δ 43.9, 63.8, 66.9, 68.4, 128.3, 128.6, 129.0, 138.7; HRMS Calcd for C6H12NS3 [M+H]+ 254.0698; found 254.0673.
Mixture of (S)-3a and (S)-4a. (S)-3a
1H NMR (CDCl3) δ 3.20 (d, J = 9.5 Hz, 2H), 3.40 (dd, J = 9.5, 3.1 Hz, 2H), 3.46 (t, J = 5.2 Hz, 1H), 3.90 (m, 2H), 4.96 (d, J = 3.1 Hz, 2H), 7.36 (m, 3H), 7.43 (m, 2H); 13C NMR (CDCl3) δ 43.4, 63.7, 66.1, 68.5, 127.8, 128.1, 128.8, 129.0, 139.7. (S)-4a: 1H NMR (CDCl3) δ 1.68 (dd, J = 8.8, 7.5 Hz, 1HSH), 2.55 (ddd, J = 13.6, 8.8, 6.7 Hz, 1H), 2.71 (dt, J = 13.6, 7.5 Hz, 1H), 3.12 (dd, J = 12.5, 1.8 Hz, 1H), 3.33 (dd, J = 12.5, 4.9 Hz, 1H), 3.62 (dd, J = 9.0, 8.2 Hz, 1H), 4.14 (dd, J = 9.2, 6.5 Hz, 1H), 4.28 (m, 2H), 5.40 (dd, J = 5.0, 1.8 Hz, 1H), 7.35 (m, 3H), 7.40 (m, 2H); 13C NMR (CDCl3) δ 32.8, 38.3, 69.2, 74.1, 100.1, 127.8, 128.2, 128.4, 129.0, 139.1.
(2R,5S,7R,8S)-2-Mercaptomethyl-7-methyl-8-carboxylate-1-aza-3-thio-6-oxa-bicyclo [3.3.0]octane 4b
Prepared following Route B, using l-threonine-OMe.HCl 1b as starting aminoalcohol. The crude was purified by chromatography (1:5, EtOAc:hexanes) to give 4b as oil (118 mg, 79 %, 99% ee): [α]D = − 87.0 (c 0.1, EtOAc); 1H NMR (CDCl3) δ 1.43 (d, J = 6.0 Hz, 1H), 2.04 (dd, J = 9.3, 7.0 Hz, 1H), 2.56 (ddd, J = 13.6, 9.3, 6.1 Hz, 1H), 2.77 (ddd, J = 13.6, 8.2, 7.0 Hz, 1H), 3.09 (d, J = 12.9 Hz, 1H), 3.26 (dd, J = 12.9, 4.4 Hz, 1H), 3.31 (d, J = 8.5, 1H), 3.79 (s, 3H), 4.08 (dd, J = 8.5, 6.0 Hz, 1H), 4.26 (dd, J = 8.2, 6.1 Hz, 1H), 5.22 (d, J = 4.4, 1H); 13C NMR (CDCl3) δ 18.1, 32.7, 37.3, 52.5, 73.9, 76.8, 78.5, 99.3, 171.4; HRMS calcd for C9H15NO3S2Na [M+Na]+ 272.0386, found 272.0491. The ee value was determined by Chiral HPLC analysis with a Chiralcel OD column [(hexane/2-propanol = 90/10, 1 mL/min, 220 nm); retention time: tR = 5.33 min (l), tR = 5.96 min].
Compounds 3c and 3d are described in literature.6
2-(2,5-Dithia-7-azabicyclo[2.2.1]heptan-7-yl)-2-(hydroxymethyl) propane-1,3-diol 3e
prepared according to Route A, using tris(hydroxyl-methyl)aminomethane 1e as starting aminoalcohol. The solvent was removed under reduced pressure, the residue was dissolved in EtOH (10 mL) and after the addition of Et2O (30 mL) the product precipitated as a white solid (0.906 g, 97%): mp = 129.8 – 130.0 °C (decomposition, from Et2O). 1H NMR (CDCl3) δ 2.83 (dd, J = 13.6, 7.2 Hz, 2H), 3.23 (dd, J = 13.6, 2.2 Hz, 2H), 3.47 (s, 6 H), 4.82 (m, 2H), 5.22 (s, 3H), 6.20 (s, 1H); 13C NMR (CDCl3) δ 35.1, 59.2, 61.1, 69.0.
(2S)-Methyl-2-(2,5-dithia-7-azabicyclo[2.2.1]heptan-7-yl)-3-hydroxypropanoate (S/R)-3f
Prepared according to Route B, using l-serine-OMe.HCl 1f as the starting aminoalcohol. The residue was purified by chromatography (EtOAc/hexanes 1:5) to give 3f (dr 1:1) as white solid and (S)-4f (3f/4f ratio 9:1, 45 mg, total yield = 32%). (S/R)-3f: 1H NMR (CDCl3) δ 3.25 (m, 3H), 3.37 (td, J = 10.0, 3.2 Hz, 2H), 3.80 (s, 3H), 3.97 (m, 2H), 5.15 (t, J = 2.7 Hz, 2H); 13C NMR (CDCl3) δ 43.5, 43.8, 52.7, 61.9, 62.1, 62.2, 62.8, 68.0, 68.2, 170.8, 170.9; HRMS calcd for C8H13NNaO3S2 [M+Na]+ 258.0019, found 258.0018.
2-(2,5-Dithia-7-azabicyclo[2.2.1]heptan-7-yl)phenol 3g
Prepared according to Route A, using o-aminophenol 1g as starting aminoalcohol. The residue was purified by column chromatography (EtOAc/hexanes 1:5) to give 3g as red foam and rac-4g (3g/4g ratio 8.5:1.5, 130 mg, total yield = 74%). 3g: 1H NMR (CDCl3) δ 3.34 (d, J = 9.4 Hz, 1H), 3.38 (dd, J = 9.4, 2.5 Hz, 1H), 5.26 (d, J = 2.5 Hz, 1H), 6.84 (m, 1H), 6.95 (dd, J = 8.0, 1.4 Hz, 1H), 7.09 (td, J = 8.0, 1.5 Hz, 1H), 7.62 (dd, J = 8.0, 1.5 Hz, 1H); 13C NMR (CDCl3) δ 45.2, 68.5, 76.8, 77.2, 77.5, 115.6, 120.6, 120.9, 127.0, 130.5, 150.6; HRMS calcd for C10H11NOS2 [M-nH]− 224.0198, found 224.0281. rac-4g: 1H NMR (CDCl3) δ 1.94 (dd, J = 10.3, 6.6 Hz, 1HSH), 2.70 (ddd, J = 13.7, 10.3, 4.9 Hz, 1H), 2.89 (ddd, J = 13.7, 9.0, 6.6 Hz, 1H), 3.19 (dd, J = 12.3, 3.7 Hz, 1H), 3.46 (dd, J = 12.3, 4.9 Hz, 1H), 4.74 (dd, J = 9.0, 4.9 Hz, 1H), 6.06 (m, 1H), 6.75 (m, 2H), 6.88 (m, 2H); 13C NMR (CDCl3) δ 33.5, 37.9, 75.6, 102.1, 108.7, 112.6, 121.9, 123.3, 135.5, 144.3, 144.9.
(2R,5S,8R)-8-Cyclohexyl-2-mercaptomethyl-1-aza-3-thio-6-oxa-bicyclo [3.3.0]octane 4h
Prepared following Route B, starting from (R)-cyclohexylglycinol 1h as the starting aminoalcohol. The residue was purified by column chromatography on flash SiO2 (EtOAc/hexanes 1:9) to afford 4h (0.114 g, 89%, 99% ee) as an oil: [α]D = − 50.2 (c 0.12, EtOAc); 1H NMR (CDCl3) δ 0.97 (m, 2H), 1.20 (m, 3H), 1.41 (m, 1H), 1.75 (m, 4H), 1.81 (dd, J = 8.8, 7.8 Hz, 1H), 1.90 (m, 1H), 2.62 (dt, J = 13.6, 7.8 Hz, 1H), 2.76 (ddd, J = 13.6, 8.8, 5.9 Hz, 1H), 2.91 (q, J = 7.1 Hz, 1H), 3.05 (dd, J = 12.5, 0.9, 1H), 3.24 (dd, J = 12.5, 4.2 Hz, 1H), 3.53 (dd, J = 8.4, 7.0 Hz, 1H), 4.00 (dd, J = 8.4, 7.0 Hz, 1H), 4.23 (dd, J = 7.8, 5.9 Hz, 1H), 5.04 (dd, J = 4.2, 0.9 Hz, 1H); 13C NMR (CDCl3) δ 26.1, 26.3, 26.6, 29.2, 30.7, 32.8, 37.1, 41.8, 68.4, 71.6, 79.0, 99.5; HRMS calcd for C12H21NOS2 [M+Na]+ 282.0957, found 282.1025.
(2S,5R,8S)-8-Phenyl-2-mercaptomethyl-1-aza-3-thio-6-oxa-bicyclo[3.3.0]octane disulfide (S)-5a
To a stirred solution of a mixture of (S)-3a/(S)-4a (0.1 g, 0.36 mmol) in MeOH (8 mL) and CH2Cl2 (2 mL) was added K2CO3 (0.1 g, 0.98 mmol). The mixture was stirred overnight at room temperature. The solvent was removed under reduced pressure and the residue was purified by column chromatography (1:8 EtOAc:hexanes) to afford compound (S)-5a as a white solid (82 mg, 91%, 99% ee). The product obtained was recrystallized from CH2Cl2/hexanes to afford single crystals: mp = 60.0 – 60.1 °C; [α]D = + 72.9 (c 0.09, EtOAc); 1H NMR (CDCl3) δ 2.70 (dd, J = 13.5, 6.1 Hz, 1H), 2.83 (dd, J = 13.5, 8.5 Hz, 1H), 3.09 (dd, J = 12.4, 2.0 Hz, 1H), 3.25 (dd, J = 12.4, 5.0 Hz, 1H), 3.58 (dd, J = 9.1, 8.4 Hz, 1H), 4.08 (dd, J = 9.2, 6.5 Hz, 1H), 4.25 (dd, J = 8.3, 6.5 Hz, 1H), 4.33 (dd, J = 8.4, 6.1 Hz, 1H), 5.32 (dd, J = 4.9, 1.9 Hz, 2H), 7.33 (m, 6H), 7.41 (m, 4H); 13C NMR (CDCl3) δ 38.6, 47.6, 68.6, 72.9, 74.1, 100.0, 127.9, 128.1, 128.6, 138.7; HRMS calcd for C24H28N2O2S4 [M+H]+ 505.1106, found 505.1112.
(S)-2-((1R,4R)-2,5-Dithia-7-azabicyclo[2.2.1]heptan-7-yl)-2-phenylethyl-4-bromo benzoate (R)-6a
To a stirred solution of (R)-3a (0.1 g, 0.4 mmol) in CH2Cl2 (5 mL) were added p-BrPhCOCl (0.1 g, 0.43 mmol), DIPEA (0.081 g, 0.63 mmol) and DMAP (0.002 g, 0.02 mmol). The mixture was stirred at room temperature for 3 h. After the addition of water (10 mL), the product was extracted with CH2Cl2 (3×15 mL), dried (Na2SO4) and filtered. The solvent was removed under reduced pressure and the residue was purified by chromatography (EtOAc/hexanes 1:5) to afford (R)-6a as a crystalline solid (135 mg, 77%). The product was recrystallized from CH2Cl2/hexanes to afford single crystals: mp 132.9 – 135.4 °C; [α]D = − 69.3 (c 0.1, EtOAc). 1H NMR (CDCl3) δ 3.21 (d, J = 9.6 Hz, 2H), 3.35 (dd, J = 9.6, 3.0 Hz, 2H), 3.70 (t, J = 5.1 Hz, 1H), 4.48 (dd, J = 11.5, 5.8 Hz, 1H), 4.63 (dd, J = 11.5, 4.4 Hz, 1H), 4.99 (d, J = 3.0 Hz, 2H), 7.38 (m, 3H), 7.46 (m, 2H), 7.59 (d, J = 8.6, 2H), 7.83 (d, J = 8.6 Hz, 2H); 13C NMR (CDCl3) δ 43.9, 61.4, 68.5, 68.4, 128.2, 128.5, 128.7, 128.8, 129.1, 131.2, 132.0, 138.4, 165.5; HRMS calcd for C19H18BrNO2S2 [M+Na]+ 457.9855, found 457.9916.
Dimethyl 2-(((((3S,5S,7aR)-3-phenyltetrahydro-2H-thiazolo[4,3-b]oxazol-5-yl))methyl) thio)but-2-enedioate 7
To a stirred solution of a mixture of 3a/4a (95:5) (0.098 g, 0.39 mmol) in CH2Cl2 (9 mL) was added dimethylacetylenedicarboxylate (0.085g, 0.5 mmol). The mixture was stirred overnight at room temperature. The crude was poured into water (20 mL), extracted with CH2Cl2 (3 × 20 mL), dried (Na2SO4) and filtered. The solvent was removed under reduced pressure and the residue was purified by column chromatography (EtOAc/hexanes 1:9) to afford 7 as a mixture of isomers 6:1 (81 mg, 53% total yield). Major isomer: [α]D = + 141.8 (c 0.1, EtOAc); 1H NMR (CDCl3) δ 2.92 (m, 2H), 3.16 (dd, J = 12.6, 1.8 Hz, 1H), 3.35 (dd, J = 12.6, 4.8 Hz, 1H), 3.60 (dd, J = 9.2, 8.5 Hz, 1H), 3.68 (s, 3H), 3.75 (s, 3H), 4.10 (dd, J = 9.2, 6.5 Hz, 1H), 4.26 (dd, J = 8.5, 6.5 Hz, 1H), 4.37 (dd, J = 8.3, 6.4 Hz, 1H), 5.41 (dd, J = 4.8, 1.8 Hz, 1H), 5.62 (s, 1H), 7.33 (m, 3H), 7.45 (m, 2H); 13C NMR (CDCl3) δ 38.6. 39.9, 52.0, 53.1, 69.2, 72.7, 74.3, 100.1, 114.7, 127.9, 128.2, 128.7, 138.5, 149.0, 164.1, 165.9. HRMS calcd for C18H21NO5S2 [M+H]+ 396.0934, found 396.1043. Minor isomer: 1H NMR (CDCl3) δ 2.93 (dd, J = 14.3, 6.0 Hz, 1H), 3.09 (dd, J = 12.4, 2.1 Hz, 1H), 3.29 (dd, J = 12.4, 5.0 Hz, 1H), 3.30 (dd, J = 14.3, 8.6 Hz, 1H), 3.62 (dd, J = 9.5, 8.4 Hz, 1H), 3.74 (s, 3H), 3.76 (s, 3H), 4.05 (dd, J = 9.5, 6.5 Hz, 1H), 4.24 (dd, J = 8.4, 6.5 Hz, 1H), 4.31 (dd, J = 8.6, 6.0 Hz, 1H), 5.33 (dd, J = 5.0, 2.1 Hz, 1H), 6.39 (s, 1H), 7.3 (m. 3 H), 7.41 (dd, J = 7.9, 1.3 Hz, 2H); 13C NMR (CDCl3) δ 38.6, 39.4, 51.8, 53.2, 68.7, 74.1, 74.2, 99.9, 120.6, 128.0, 128.2, 128.6, 138.0, 148.0, 164.5, 165.4.
Materials and methods
Interconversion studies
A mixture of (S)-3a/(S)-4a (3:2) (30 mg, 0.12 mmol) was stirred in an appropriated buffer solution [1M] (8 mL) and acetonitrile (2 mL) at room temperature for 24 h. The solution was then neutralized by the addition of NaHCO3 saturated solution and extracted with EtOAc (3 × 30 mL). The combined organic layers were dried (Na2SO4), filtered and the solvent was removed under reduced pressure. The mass balance was quantitative and the product distribution was determined by 1H NMR. The experiment was performed using different buffer solutions (pH 2, 5, 7 and 9). The same experiments were repeated starting from pure (R)-3a diasteromer.
Inhibition assays on NDM-1
Hydrolysis of imipenem by NDM-1 was monitored using a Jasco V-670 spectrophotometer by following the changes in absorbance at 300 nm using a De300 = − 9000 M−1 cm−1. The reaction medium employed was 10 mM HEPES pH 7.5, 200 mM NaCl, 50 µg/mL BSA and 20 µM ZnSO4 at 30 °C. Reactions were carried out in 0.1-cm path length quartz cuvettes in a final volume of 300 mL, with a final enzyme concentration of 1 nM. Oxazolidinylthiazolidines were dissolved in DMSO to a final concentration of 100 mM, and then diluted 10-fold (to 10 mM) in 10 mM HEPES pH 7.5, 200 mM NaCl. Appropriate volumes of the 10 mM stock solutions were used to achieve the desired final concentrations. The final DMSO concentration in the react ion mixture was then maintained between 0.01 to 0.07%, which did not alter the enzyme activity (data not shown). The assay was initiated by the addition of NDM-1 to the mixture of substrate and inhibitor. In the presence of inhibitor, the initial phase of the time courses was linear but showed a decreased rate of hydrolysis with respect to the reaction in the absence of inhibitor. The initial rate of reaction for each substrate or substrate-inhibitor concentration, under steady state conditions (< 5% of substrate consumed), was calculated from the slope of the initial linear phase of the respective time course. Inhibition constants (Ki) were evaluated by nonlinear fitting of the initial velocities, at various concentrations of the substrates and inhibitors, with the equations for different inhibition models as implemented in GraphPad 5.0. Best fits were obtained with the Competitive Inhibition Model.
Crystallography
Single-crystal X-ray diffraction experiments on DTA-1, (S)-5a and (R)-6a were performed with a Bruker D8 Venture diffractometer operating with a sealed-tube Mo Kα radiation (Δ=0.71069 Å) and a PHOTON100 CMOS area detector, at room temperature. Crystallographic data for compounds DTA-1, (S)-5a and (R)-6a have been deposited with the accession number CCDC 1451797, 1451798 and 1451799 respectively and can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/structures.
Supplementary Material
Acknowledgments
The work was supported by a grant from National Institutes of Health (R01AI100560 to G.M., A.J.V) and Agencia Nacional de Investigación e Innovación ANII FMV_1_2014_1_104234 and EQC_2012_7, CSIC-Grupos, CSIC equipamiento for X-ray. Authors would like to thank PhD. A. Rodríguez for HRMS, (Polo Tecnólogico-FQ, UdelaR).
Notes and references
- 1.Marson CM. Chem. Soc. Rev. 2011;40:5514–5533. doi: 10.1039/c1cs15119c. [DOI] [PubMed] [Google Scholar]
- 2.Walters WP, Green J, Weiss JR, Murcko MA. J. Med. Chem. 2011;54:6405–6416. doi: 10.1021/jm200504p. [DOI] [PubMed] [Google Scholar]
- 3.Royer J, Bonin M, Micouin L. Chem. Rev. 2004;104:2311–2352. doi: 10.1021/cr020083x. [DOI] [PubMed] [Google Scholar]
- 4.Saiz C, Wipf P, Mahler G. J. Org. Chem. 2011;76:5738. doi: 10.1021/jo2008498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saiz C, Manta E, Wipf P, Mahler G. Org. Lett. 2009;11:3170. doi: 10.1021/ol901104a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saiz C, Castillo V, Mahler G. Synlett. 2012;23:1090. [Google Scholar]
- 7.Saiz C, Castillo V, Fontán P, Bonilla M, Salinas G, Rodríguez A, Mahler G. Mol. div. 2014;18:1. doi: 10.1007/s11030-013-9485-3. [DOI] [PubMed] [Google Scholar]
- 8.González M, Kosmopoulou M, Mojica MF, Castillo V, Hinchliffe P, Pettinati I, Brem J, Schofield CJ, Mahler G, Bonomo RA, Llarrull LI, Spencer J, Vila AJ. ACS Infect. Dis. 2015;1:544. doi: 10.1021/acsinfecdis.5b00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mojica MF, Mahler G, Bethel CR, Taracila MA, Kosmopoulou M, Papp-Wallace KM, Llarrull LI, Wilson BM, Marshall SH, Wallace CJ, Villegas MV, Harris ME, Vila AJ, Spencer J, Bonomo RA. Biochem. 2015;54:3183–3196. doi: 10.1021/acs.biochem.5b00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hinchliffe P, González MM, Mojica MF, González JM, Castillo V, Saiz C, Kosmopoulou M, Tooke CL, Llarrull LI, Mahler G, Bonomo RA, Vila AJ, Spencer J. Proc. Natl. Acad. Sci. USA. 2016;113:E3745. doi: 10.1073/pnas.1601368113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kanemasa S, Doi K, Wada E. Bull. Chem. Soc. Jpn. 1990;63:2866. [Google Scholar]
- 12.Cardoso AL, Kaczor A, Silva A, Fausto R, Pinho e Melo T, Rocha Gonsalves A. Tetrahedron. 2006;62:9861. [Google Scholar]
- 13.Purushothaman S, Raghunathan R. Tetrahedron Lett. 2009;50:6848. [Google Scholar]
- 14.Stojanovic M, Markovic R, Kleinpeter E, Baranac-Stojanovic M. Tetrahedron. 2011;67:9541. [Google Scholar]
- 15.Stratmann O, Hoppe D, Fröhlich R. J. Prakt. Chem. 2000;8:342. [Google Scholar]
- 16.Stalling T, Pauly J, Schmidtmann M, Martens J. Eur. J. Org. Chem. 2014;4:833. [Google Scholar]
- 17.Fulöp F, Mattinenand J, Pihlaja K. Tetrahedron. 1990;46:6545. [Google Scholar]
- 18.Beesley R, Ingold C, Thorpe JJ. Chem. Soc. Trans. 1915;107:1080. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.