ABSTRACT
Across all kingdoms in the tree of life, calcium (Ca2+) is an essential element used by cells to respond and adapt to constantly changing environments. In multicellular organisms, it plays fundamental roles during fertilization, development and adulthood. The inability of cells to regulate Ca2+ can lead to pathological conditions that ultimately culminate in cell death. One such pathological condition is manifested in Parkinson's disease, the second most common neurological disorder in humans, which is characterized by the aggregation of the protein, α-synuclein. This Review discusses current evidence that implicates Ca2+ in the pathogenesis of Parkinson's disease. Understanding the mechanisms by which Ca2+ signaling contributes to the progression of this disease will be crucial for the development of effective therapies to combat this devastating neurological condition.
KEY WORDS: Calcium, α-synuclein, Parkinson's disease
Summary: This Review article discusses the important roles that calcium plays in the pathogenesis of Parkinson's disease.
Introduction
Parkinson's disease (PD) is the second most common, multifactorial, progressive neurodegenerative disorder in humans after Alzheimer's disease, affecting 6.3 million people worldwide (Marras and Tanner, 2004). Characterized by the aggregation of a small lipid-binding protein, α-synuclein, PD belongs to a larger group of neurodegenerative diseases, collectively known as synucleinopathies. This group includes dementia with Lewy bodies (DLB), neurodegeneration with brain iron accumulation and multiple system atrophy (MSA) (Martí et al., 2003; Teive et al., 2004). Although the common theme amongst these synucleinopathies is α-synuclein aggregation into structures called Lewy bodies, the pathological distinction between each disorder lies primarily in the cell type affected. In MSA and DLB, Lewy bodies are primarily found in oligodendrocytes and cortical neurons, respectively. In PD, Lewy bodies are detected primarily in dopaminergic (DA) neurons in a brain region called the substantia nigra pars compacta (SNc). Although it is true that the motor symptoms observed in PD, such as resting tremor, bradikinesia and postural rigidity, can be ascribed to the loss of DA neurons in the SNc, it is now very clear that there are many other brain regions with Lewy body pathology. In fact, many of these regions correspond to the non-motor symptoms that often precede the motor symptoms of PD, such as apathy, pain, sexual difficulties, constipation and sleep disorders, among others (Braak et al., 2004; Chaudhuri et al., 2006; Lees et al., 2009). The pathological overlap between different synucleinopathies suggests that these diseases might belong on a spectrum of the same disorder. Therefore, it is important to understand the consequences of α-synuclein aggregation in different cell types to fully understand the scope of PD pathology.
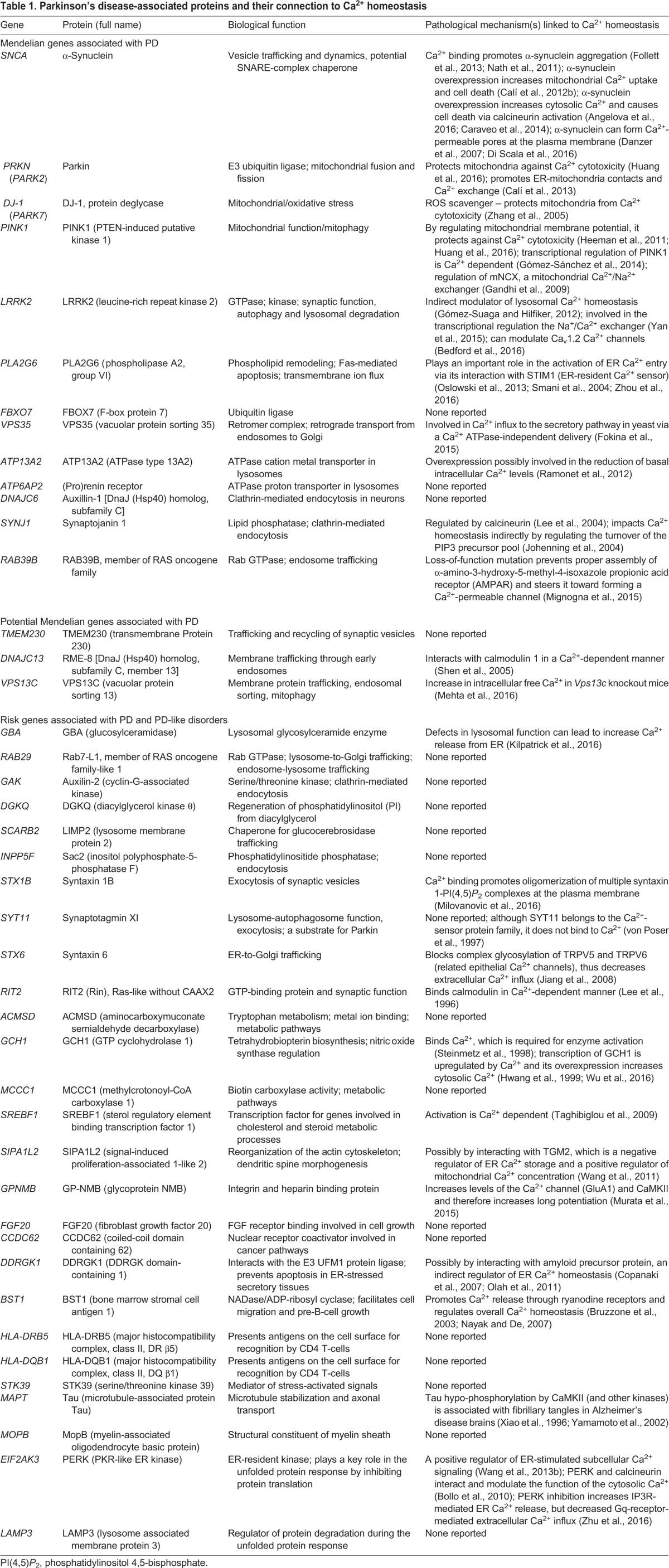
Over the past 10 years, an explosion of research has identified over 30 genetic loci and genes responsible for PD, and the list is still growing (Table 1) (Chen et al., 2013; Ghanbari et al., 2016; Höglinger et al., 2011; Kumar et al., 2011; Lin and Farrer, 2014; Martin et al., 2011; Nalls et al., 2014; Shulman et al., 2011; Wissemann et al., 2013). Although genetic cases represent only 10% of PD, genome-wide association (GWA) studies are increasingly being used to elucidate novel risk loci for PD. These studies provide new insights into the complex interplay between genetics, epigenetics and environmental factors that contribute to PD pathology. Whether the cause of PD is genetic, environmental and/or sporadic, α-synuclein aggregation is a key pathological hallmark of the disease. Point mutations, duplication and triplication of the α-synuclein locus are known to cause the early onset of PD (Polymeropoulos et al., 1997; Simón-Sánchez et al., 2009; Singleton et al., 2003). Moreover, GWA studies have revealed that the α-synuclein gene (SNCA) is a major risk factor that is linked to sporadic PD (Simón-Sánchez et al., 2009).
Table 1.
Parkinson's disease-associated proteins and their connection to Ca2+ homeostasis
An emerging, key pathological feature caused by α-synuclein aggregation is the disruption of calcium (Ca2+) homeostasis (Caraveo et al., 2014; Goldberg et al., 2012; Guzman et al., 2010; Hurley et al., 2013; Surmeier et al., 2010, 2016). Ca2+ is a universal and versatile second messenger that is present in all living organisms. Unlike Na+ and K+, which have ∼10- to 30-fold differences in ion concentration across the plasma membrane, Ca2+ ions have a 20,000-fold lower concentration in the cytoplasm compared to in the extracellular space (Surmeier and Schumacker, 2013). These gradients allow cells to use Ca2+ as a potent intracellular signal to respond and adapt to fast-changing extracellular and intracellular environments. By controlling the amplitude and frequency of Ca2+ dynamics, cells can temporarily or permanently change a wide variety of physiological functions by activating and/or inhibiting Ca2+-dependent signal transduction pathways (Berridge, 2005; Berridge et al., 2003, 2000; Bootman, 2012; Burgoyne, 2007; Carafoli, 2002; Clapham, 2007; Petersen et al., 2005; Rizzuto and Pozzan, 2006). In stimulated neurons, cytoplasmic Ca2+ can range from 100 nM up to 1-10 µM in selected microdomains, depending on the cell type. The polarized nature of neurons allows them to regulate specific processes that are generally not sensitive to bulk concentrations of Ca2+, such as neuronal development and synaptic plasticity (Augustine et al., 2003; Bootman et al., 2001; Carrasco and Hidalgo, 2006; Muller et al., 2005; Parekh, 2008). Because Ca2+ signaling affects all aspects of neuronal cell biology, cells must tightly regulate Ca2+ levels to avoid uncontrolled responses that could otherwise lead to pathological conditions and cell death (Rozkalne et al., 2011; White et al., 2000).
In this Review, we discuss the current evidence that implicates defective Ca2+ homeostasis in the pathogenesis of PD. Elucidating the role of α-synuclein, and of other PD-associated proteins, in Ca2+ homeostasis could provide new opportunities for developing novel therapeutics to treat synucleinopathies.
PD and Ca2+ signaling at the plasma membrane
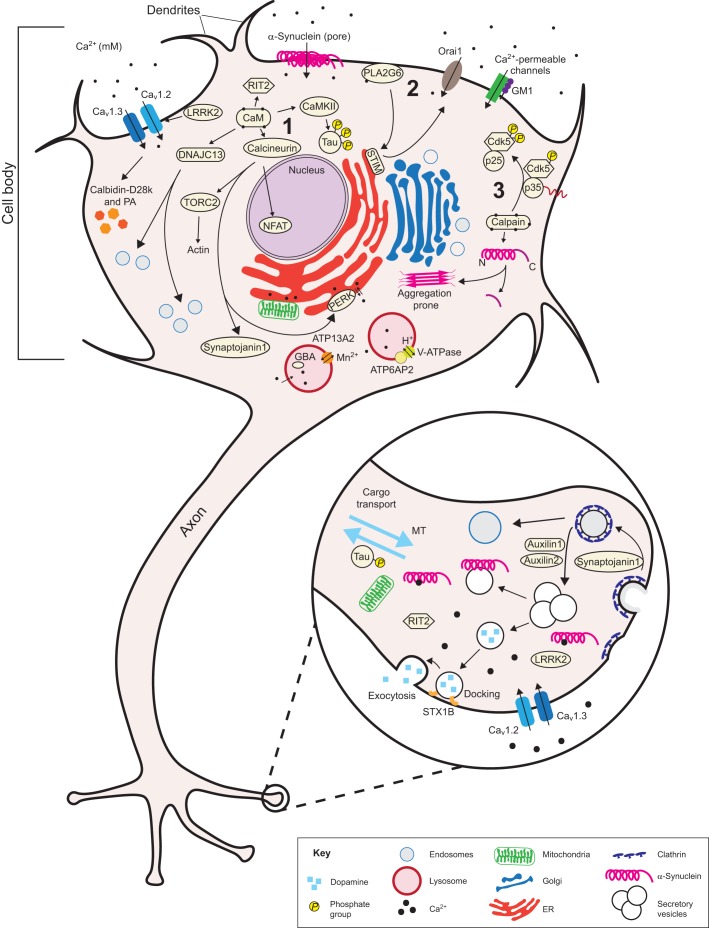
In neurons, the movement of Ca2+ can occur across the plasma membrane in response to electrical activity and/or through agonists. The electrical activity of neurons and other excitable cells relies on several different types of voltage- and ligand-gated ion channels that are permeable to inorganic ions, such as Na+, K+, Cl− and Ca2+. L-type (also known as Cav1 family) voltage-gated Ca2+ channels, Cav1.2 and Cav1.3, have been implicated in PD (Calí et al., 2014; Hurley and Dexter, 2012; Ortner and Striessnig, 2016; Schapira, 2013; Surmeier et al., 2016; Zamponi, 2016). Although Cav1.2 is prevalent in juvenile SNc DA neurons, in aging SNc DA neurons, Cav1.3 is preferentially used for Ca2+ influx and support of rhythmic pace-making activity (Fig. 1) (Bean, 2007; Chan et al., 2007; Dragicevic et al., 2014; Drion et al., 2011; Goldberg et al., 2012; Khaliq and Bean, 2010; Puopolo et al., 2007; Surmeier et al., 2012; Wilson and Callaway, 2000). Such pace-making is essential for maintaining basal dopamine levels in the striatum (Surmeier and Schumacker, 2013). Unlike Cav1.2, the Cav1.3 operating range does not allow the Cav1.3 channels to close fully during pace-making, which contributes to elevated intracellular Ca2+ levels (Puopolo et al., 2007; Wilson and Callaway, 2000). In adult mice, SNc DA neurons have an increased reliance on Cav1.3 channels, as well as a decreased ability to deal with high Ca2+ levels (Chan et al., 2007; Hurley et al., 2013). Interestingly, the expression of Cav1.3 is increased in the SNc DA neurons of deceased PD patients (Hurley et al., 2013). To test the importance of L-type channels in PD-like pathology, mice, midbrain slices or cultured neurons from mice were pretreated with Isradipine, an L-type Ca2+ channel blocker, and then exposed to α-synuclein pre-formed fibrils (PFF), or to the toxic effects of environmental factors known to cause PD by interfering with the mitochondrial complex I, namely rotenone or 1-methyl-4-phenyl-1,2,3,6 tetrahydropyridine (MPTP) (Brown et al., 2006; Chan et al., 2007; Dryanovski et al., 2013; Goldman, 2014; Ilijic et al., 2011; Van Maele-Fabry et al., 2012). In these experiments, Isradipine confers strong protection in SNc DA neurons, indicating that Ca2+ flux through L-type channels is an important contributor to neuronal cell death. The importance of this finding is also supported by the fact that the neighboring ventral tegmental midbrain DA neurons, which do not express the Cav1.3 channels, are less susceptible to cell death in PD (Hurley et al., 2013; Mouatt-Prigent et al., 1994; Neuhoff et al., 2002).
Fig. 1.
Ca2+ signaling and homeostasis in a dopaminergic neuron. A schematic of a dopamine (DA) neuron, illustrating several Ca2+-related proteins and pathways affected in Parkinson's disease (PD). The proteins shown directly or indirectly participate in Ca2+ homeostasis. Cav1.2, Cav1.3, Orai1, Ca2+-permeable channels (T-channels, NCX, TRPC5, PMCA) regulated by GM1, and α-synuclein Ca2+-permeable pores allow Ca2+ to enter the cell. Calbidin-D28k and parvalbumin (PA) are protective due to their capacity to buffer cytosolic Ca2+. Increases in cytosolic Ca2+ activate diverse pathways involved in PD, including: (1) calmodulin (CaM) and calcineurin to modify their respective downstream targets NFAT, TORC2 and synaptojanin1; (2) PLA2G6 (through SOCE); and (3) calpains. Increases in cytosolic Ca2+ also activate the lysosomal ion channels ATP13A2 and ATP6AP2. Lower right: A magnified pre-synaptic axonal terminal illustrates the role of RIT2, STX1B (syntaxin 1B), α-synuclein and synaptojanin1 in vesicle recycling. The role of LRRK2 in pre-synaptic vesicle recycling is not fully known. Hyperphosphorylation of Tau driven by CaMKII activation interferes with proper microtubule (MT) axonal transport. Abbreviations: ATP13A2, probable cation-transporting ATPase 13A2; ATP6AP2 (prorenin receptor), ATPase H+-transporting lysosomal accessory protein 2; CaMKII, calmodulin kinase II; Cav1.2 and Cav1.3, subunits of voltage-dependent L-type Ca2+ channels; Cdk5, cyclin-dependent kinase 5; DNAJC13, DnaJ homolog subfamily C member 13; ER, endoplasmic reticulum; GBA, glucocerebrosidase; GM1, monosialotetrahexosylganglioside; LRRK2, leucine-rich repeat kinase 2; NCX, Na+/Ca2+ exchanger; NFAT, nuclear factor of activated T cells; Orai1, Ca2+ release-activated Ca2+ channel protein 1; p35, cyclin-dependent kinase 5 activator encoded by CDK5R1; p25, a calpain cleavage product of p35; PERK, protein kinase RNA-like endoplasmic reticulum kinase; PLA2G6, phospholipase A2G6; PMCA, plasma membrane Ca2+ ATPase; RIT2, Ras-like without CAAX2; SOCE, store operated Ca2+ entry; STIM, stromal interaction molecule; TORC2, transducer of regulated CREB protein 2; TRPC5, short transient receptor potential channel 5.
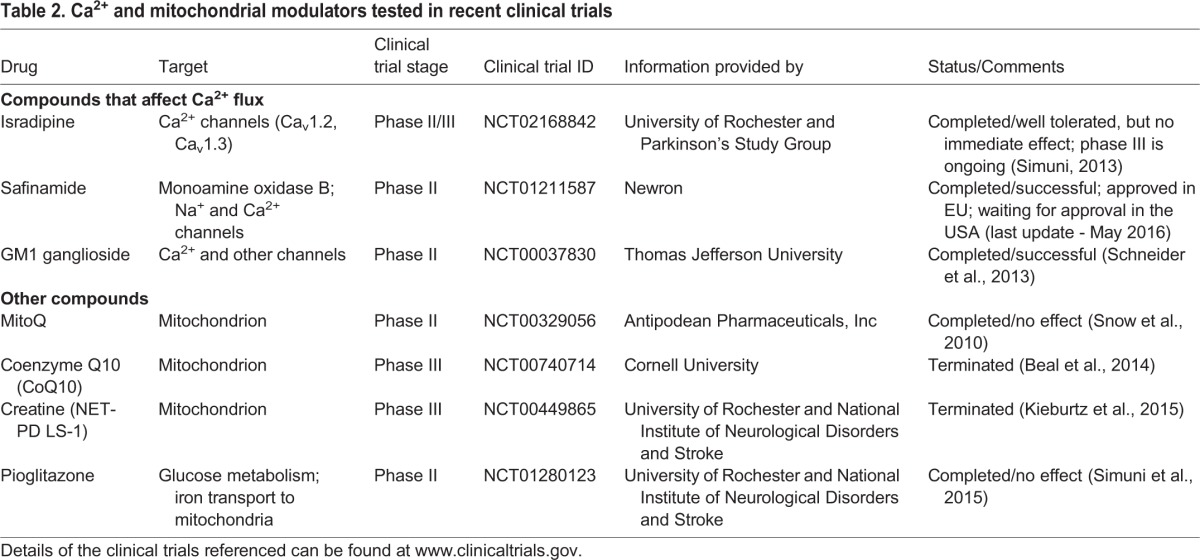
In the clinic, Isradipine and other L-type channel blockers have been widely used as anti-hypertensives to treat high blood pressure and other cardiovascular conditions. The proposed role of Ca2+ channels in neurodegeneration opens up the possibility of repurposing these drugs to treat PD. Several epidemiological studies suggest that there is indeed a reduced risk of developing PD in patients with long-term use of Isradipine (Becker et al., 2008; Lee et al., 2014; Pasternak et al., 2012; Ritz et al., 2010). A phase III clinical trial (NCT02168842; www.clinicaltrials.gov) to study the neuroprotective potential of Isradipine in early PD patients is currently ongoing and scheduled for completion in 2019 (Table 2). While the contribution of Cav1.3 channels to PD is undeniable, it is important to point out that Isradipine has, in fact, a higher affinity for Cav1.2 channels (Koschak et al., 2001; Lipscombe et al., 2004; Olson et al., 2005; Xu and Lipscombe, 2001). Cav1.2 channels are expressed throughout the brain and play important roles in regulating neurotransmitter release, predominantly at presynaptic terminals (Berger and Bartsch, 2014; Striessnig et al., 2006). Given that the exact roles of Cav1.2 channels in PD have not been fully elucidated, this should be an important consideration when interpreting the results of these clinical trials.
Table 2.
Ca2+ and mitochondrial modulators tested in recent clinical trials
Additional evidence also suggests a pathological role for α-synuclein in the increased influx of Ca2+ through the plasma membrane in PD. α-Synuclein can directly control the influx of Ca2+ through the plasma membrane by forming a specific type of oligomer, which can form Ca2+-permeable pores at the plasma membrane and induce cell death through Ca2+ (excitotoxicity) (Angelova et al., 2016; Danzer et al., 2007; Di Scala et al., 2016). Moreover, loss of function of Rab39B, a small GTPase that is involved in endosome trafficking and that is associated with early-onset PD, has recently been shown to alter the trafficking of an α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor (AMPAR) subunit and to steer AMPAR toward forming a Ca2+-permeable channel (Table 1) (Lesage et al., 2015; Mignogna et al., 2015).
Finally, monosialotetrahexosylganglioside (GM1), a member of the sialic acid-containing glycosphingolipids group that is highly expressed at the plasma membrane of neural cells, appears to have an important role in neuronal Ca2+ homeostasis. GM1 can modulate several receptors and membrane channels, including Ca2+-ATPase (PMCA), Na+/Ca2+ exchanger (NCX), T-type Ca2+ channels at the plasma membrane, and sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) pumps, to reduce excitotoxicity and oxidative stress (Hatzifilippou et al., 2008; Ledeen and Wu, 2015; Svennerholm et al., 1994). GM1 is also neuroprotective in rodent models of PD (Figs 1 and 2) (Schneider, 1998). In support of its protective role against PD pathogenesis, a completed phase II clinical trial in which PD patients were treated with GM1 (NCT00037830; www.clinicaltrials.gov) reported an overall improvement in the patients' motor symptoms and a delay in symptom progression during the two and a half-year trial period (Table 2) (Schneider et al., 2013, 2010).
Fig. 2.
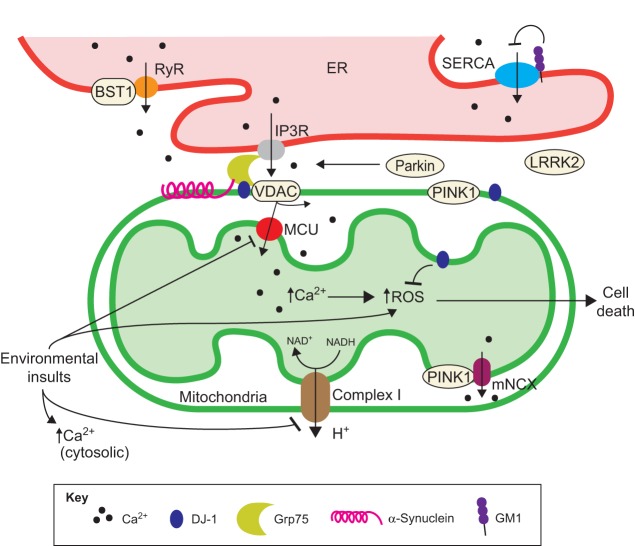
Ca2+ signaling and homeostasis at ER-mitochondria contact sites. A schematic of the mitochondria-associated membranes (MAMs) in the context of the PD-associated proteins (α-synuclein, PINK1, DJ-1 and BST1) involved in ER-mitochondria Ca2+ homeostasis. VDAC coupled with MCU mediates Ca2+ flow between the ER and mitochondria through its physical interaction with the IP3R via the Grp75 chaperone. ER Ca2+ homeostasis is also regulated by the RyR and SERCA pumps. BST1 activates RyR and depletes ER Ca2+, whereas GM1 inhibits SERCA-dependent ER Ca2+ uptake. PD-related environmental toxins (such as paraquat, MPTP and rotenone) lead to inhibition of MCU and Complex I, and to a concomitant increase in ROS and cytosolic Ca2+. Increased levels of mitochondrial Ca2+ can also lead to an increase in ROS and ultimately to cell death. Ca2+ is pumped out of mitochondria via Ca2+ exchange channels, such as the mitochondrial Na+/Ca2+ exchanger (mNCX), which is regulated by PINK1. DJ1 is a ROS scavenger that protects cells from ROS-induced cell death. DJ1, along with α-synuclein, interacts with Grp75 and promotes the formation of ER-mitochondria contact sites. Abbreviations: BST1, bone marrow stromal cell antigen-1; Complex 1, NADH coenzyme Q oxidoreductase; DJ1, protein deglycase; ER, endoplasmic reticulum; GM1, monosialotetrahexosylganglioside; Grp75, glucose-regulated protein 75; H+, hydrogen ion (protons); IP3R, inositol trisphosphate receptor; LRRK2, leucine-rich repeat kinase 2; MCU, mitochondrial Ca2+ uniporter; MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; NAD+, oxidized form of nicotinamide adenine dinucleotide; NADH, reduced form of nicotinamide adenine dinucleotide; Parkin, ligase encoded by the PRKN (PARK2) gene; PD, Parkinson’s disease; PINK1, PTEN-induced putative kinase 1; ROS, reactive oxygen species; RyR, Ryanodine receptor; SERCA, sarco/endoplasmic reticulum Ca2+-ATPase; VDAC, voltage-dependent anion channel type 1.
Intracellular Ca2+ stores and their deregulation in PD
Although the above evidence suggests that increased Ca2+ influx at the plasma membrane significantly contributes to the pathogenesis of PD, other findings implicate another form of Ca2+ deregulation in PD pathology. These findings report defects in the regulation of Ca2+ that comes from a cell's intracellular Ca2+ stores (Caraveo et al., 2014). The Ca2+ reservoir(s) responsible, as well as the mechanism behind this Ca2+ deregulation, have yet to be fully elucidated. Although the endoplasmic reticulum (ER), and to a lesser extent the mitochondria, are major intracellular Ca2+ stores, evidence suggests that other organelles, such as the lysosomes and Golgi, also act as important intracellular Ca2+ reservoirs (Kilpatrick et al., 2013; Patel and Docampo, 2010; Patel and Muallem, 2011). This is particularly relevant in the context of PD given that the malfunctioning of ER, mitochondria and, recently, lysosomes has been implicated in its etiology (Kilpatrick et al., 2016; Lloyd-Evans et al., 2008). Whether the deregulation of intracellular, store-derived Ca2+ plays a role in PD pathogenesis remains to be determined. One has to keep in mind that Ca2+-harboring organelles are not isolated static units but rather that they are highly dynamic and connected through a continuum of Ca2+ signaling. For example, the ER network is highly connected with many organelles through Ca2+-dependent pathways, including the plasma membrane, mitochondria, lysosomes and possibly other organelles (Bahar et al., 2016; Berridge et al., 2003; Bezprozvanny, 2010; Bootman, 2012; Calí et al., 2011; Carafoli, 2002; McBrayer and Nixon, 2013; Phillips and Voeltz, 2016; Rivero-Rios et al., 2014; Wojda et al., 2008). Connections also exist between lysosomes and peroxisomes, as well as between lysosomes and mitochondria. These interconnections are particularly relevant in the context of PD because it might support the argument for a ‘domino’ effect rather than an independent collection of defects, that is, if the ER is the first malfunctioning organelle, the other organelles that the ER is connected to, such as mitochondria and/or peroxisomes, will secondarily be affected. These organelles will, in turn, affect others that they are connected to and so on.
Ca2+ storage in the ER
The ER is a major Ca2+ storage organelle in the cell and is responsible for protein biosynthesis and N-linked glycosylation. ER-derived Ca2+ plays crucial roles in cell signaling and also serves as a protein quality control system in the ER lumen. For example, a drop in ER luminal Ca2+ caused by misfolded proteins, such as α-synuclein, can lead to ER stress by halting protein translation and initiating the unfolded protein response (Celardo et al., 2016; Lindholm et al., 2006; Omura et al., 2013; Tsujii et al., 2015). Although this is a part of the normal physiological response to stress, a chronic ER system overload – which is observed in PD – can lead to cell death due to severe problems in cytosolic Ca2+ homeostasis, in protein biosynthesis, in Ca2+-mediated signaling pathways and in other organelle functions that are highly dependent on ER contacts (as discussed later in this review). Interestingly, some PD-associated genes encode proteins that are involved in depleting Ca2+ from ER stores. For example, the gene BST1 (Bone marrow stromal cell antigen-1) is associated with sporadic PD in the European population (Table 1, Fig. 2) (Saad et al., 2011). BST1 is an adenosine diphosphate ribose (ADP) cyclase that can regulate Ca2+ release from the ER through the ryanodine receptors (RyR) by generation of cyclic ADP ribose (cADPR), a potent and universal Ca2+ mobilizer (Bruzzone et al., 2003; Nayak and De, 2007).
An additional link between ER Ca2+ homeostasis and PD is provided by phospholipase A2G6 (PLA2G6). Autosomal recessive mutations in PLA2G6 lead to early-onset dystonia-parkinsonism (Tomiyama et al., 2011). PLA2G6 is a Ca2+-dependent phospholipase A2 that is associated with the plasma membrane (Table 1, Fig. 1). It normally interacts with the ER-Ca2+ sensor stromal interaction molecule 1 (STIM1) and promotes refilling of the intracellular Ca2+ stores via activation of Ca2+ channels at the plasma membrane, a process called store operated Ca2+ entry (SOCE) (Oslowski et al., 2013). PLA2G6 loss of function impairs SOCE, thereby decreasing the appropriate refilling of the ER with Ca2+. Disruption of SOCE also leads to autophagic dysfunction, progressive loss of DA neurons in SNc and age-dependent L-3,4-dihydroxyphenylalanine (L-DOPA)-sensitive motor dysfunction in animal models (Oslowski et al., 2013; Smani et al., 2004; Zhou et al., 2016). In support of a role for SOCE in the normal physiology of DA neurons in the SNc, overexpression of a dominant-negative form of the SOCE channel in the Drosophila brain, Orai1 (see Fig. 1), decreases expression of both tyrosine hydroxylase (TH) and the dopamine transporter (DAT) (Pathak et al., 2015). These data indicate that SOCE is important for maintaining the appropriate levels of dopamine in a normal brain and that alterations in this pathway might lead to PD-like pathology.
Defects in ER Ca2+ homeostasis can also have profound effects on other organelles through their physical connections. A good example of such interconnections is the ER-mitochondria contact sites, which form via mitochondria-associated membranes (MAM). As we discuss in the following section, these membranes are involved in several key processes, such as phospholipid and Ca2+ transfer, mitochondrial fission, mitophagy, the ER-stress response, and the regulation of apoptosis and inflammatory/antiviral responses (Vance, 2014).
Ca2+ storage in the mitochondria
Mitochondria can temporally and spatially regulate cytosolic Ca2+ concentrations in distinct locations in a neuron. Aberrations in mitochondrial Ca2+ levels and in mitochondrial localization after organelle repositioning have been implicated in the pathogenesis of several neurodegenerative diseases, including PD (Fluegge et al., 2012; Rizzuto et al., 2012; Sheng and Cai, 2012). It is well established that mitochondrial Ca2+ overload can lead to oxidative stress – the increased production of reactive oxygen species (ROS) – and to changes in mitochondrial membrane permeability, both of which culminate in cell death (Krols et al., 2016; Lemasters et al., 2009; Marchi et al., 2014; McCormack and Denton, 1990). Indeed, defects in mitochondrial dynamics (fusion/fission and transport) and quality control are important contributors to PD pathology. Multiple PD-associated proteins [including α-synuclein, PINK1, DJ-1, Parkin and leucine-rich repeat kinase 2 (LRRK2)] are directly involved in regulating mitochondrial function, fusion/fission and oxidative stress (Table 1, Fig. 2), and are described in detail in many recent reviews (Bose and Beal, 2016; Calí et al., 2011, 2012a; Exner et al., 2012; Hu and Wang, 2016; Perier and Vila, 2012; Pickrell and Youle, 2015; Ryan et al., 2015). Importantly, treatment with Isradipine reduces mitochondrial oxidation and decreases the production of ROS in the SNc DA neuron in the DJ-1 knockout mouse (Guzman et al., 2010). This finding strongly supports the argument that Ca2+ has a causal role in controlling ROS production, a key pathological feature of PD. Additionally, exposure of isolated mitochondria or cultured neuroblastoma cells to environmental insults such as MPTP and rotenone lead to a drop in mitochondrial Ca2+ influx and to a consequent increase in cytosolic Ca2+ (Frei and Richter, 1986; Sousa et al., 2003; Wang and Xu, 2005). Whether mitochondrial damage is locally generated and/or a consequence of the connections between organelles (such as the ER or peroxisomes) remains to be determined. Regardless, the high energy levels required to maintain Ca2+ homeostasis can explain why mitochondrial abnormalities could result in defective Ca2+ handling, as observed in PD. Given the importance of mitochondria in PD, four clinical trials (NCT00329056, NCT00740714, NCT00449865, NCT01280123; www.clinicaltrials.gov) have been conducted in the last 10 years that have aimed at improving mitochondrial health during the course of the disease (Table 2). However, after promising early stages, none of the drugs tested in these trials has proven to be effective at improving motor symptoms in PD patients (Beal et al., 2014; Kieburtz et al., 2015; Simuni et al., 2015; Snow et al., 2010). Nevertheless, mitochondrial health and mitochondria-associated proteins remain an attractive target for developing future therapeutics for PD.
As mentioned earlier, mitochondria Ca2+ levels are tightly controlled by the ER via MAMs. MAMs are enriched with the mitochondrial Ca2+ uniporter (MCU) complex in the inner mitochondrial membrane and with the inositol trisphosphate receptor (IP3R) on the ER. MCU and IP3R are coupled via the chaperone protein Grp75, which connects IP3R to the voltage-dependent anion channel type 1 (VDAC1) on the outer mitochondrial membrane (Fig. 2) (Krols et al., 2016; Rizzuto et al., 2009). These connections allow for Ca2+ exchange between ER and mitochondria, and tight regulation of mitochondrial luminal Ca2+ concentration. Mitochondrial luminal Ca2+ is essential for the Krebs cycle and for driving the electron transport chain through complexes III and V (Gellerich et al., 2013; Glancy and Balaban, 2012). Both biochemical processes are vital for maintaining the mitochondrial membrane potential and ATP levels. A cell needs sufficient energy to regulate Ca2+ owing to the high-energy demands of Ca2+ homeostasis. Mitochondria export Ca2+ via the H+/Ca2+ exchanger (mHCX) and the Na+/Ca2+ exchanger (mNCX), which are located on the inner mitochondrial membrane. Although the exact mechanism of action has yet to be established, two PD-associated proteins affect these mitochondrial Ca2+ import pathways: PINK1, by triggering the mNCX, and Parkin by stimulating VDAC1 (Table 1, Fig. 2) (Calí et al., 2013; Gandhi et al., 2009; Rizzuto et al., 2012). Moreover, α-synuclein and DJ-1 have both been shown to interact with MAM via the chaperone Grp75 (Jin et al., 2007). These interactions promote MAM assembly and function by controlling ER-mitochondria Ca2+ and lipid homeostasis (Table 1, Fig. 2) (Calí et al., 2012b; Guardia-Laguarta et al., 2014; Ottolini et al., 2013). These data suggest that disruption of MAMs might also be an important contributor to the pathogenesis of PD.
Ca2+ storage in lysosomes and other acidic organelles
Lysosomes and autolysosomes are particularly important organelles for neuronal health given their long-lived nature and the high demand for constant nutrient turnover. Defects in autophagy and lysosomal function have both been observed in PD (Lynch-Day et al., 2012; Nixon, 2013; Xilouri et al., 2016). One of the strongest links between lysosomal function and PD is found with the enzyme β-glucocerebrosidase (GBA) (Table 1, Fig. 1). Autosomal recessive forms of the gene encoding this enzyme, GBA, cause the lysosomal storage disorder Gaucher's disease, which is characterized by the accumulation of glucosylceramide in hepatocytes. Individuals carrying a subset of these GBA mutations are 20 times more susceptible to developing PD (Beavan and Schapira, 2013; Dehay et al., 2013; Sidransky et al., 2009). This is likely to be due to the increased accumulation of α-synuclein aggregates in the lysosome due to the inability to degrade α-synuclein (Schapira et al., 2014). Interestingly, a defect in lysosomal trafficking caused by a PD-associated GBA mutation (L444P) was rescued in Gaucher patient-derived fibroblasts following treatment with the L-type Ca2+ channel blockers diltiazem or verapamil (Mu et al., 2008). Given that not all human carriers of GBA mutations develop PD, additional pathogenic mechanisms are likely to be at play.
Lysosomes and autosomes are especially important for the degradation of proteasome-insensitive protein aggregates, like those generated by α-synuclein; such degradation is dependent on lysosomal Ca2+ (Cuervo et al., 2004; Klionsky et al., 2010; Luzio et al., 2007, 2000). Lysosome-derived Ca2+ is thought to trigger Ca2+ release from the ER, possibly via lysosome-ER membrane contact sites (Kilpatrick et al., 2013; Phillips and Voeltz, 2016). Lysosomes mobilize their internal Ca2+ to signal in response to stimuli through a variety of channels, such as nicotinic acid adenine dinucleotide phosphate (NAADP)-dependent Ca2+ channels and members of the transient receptor potential channel superfamily, such as TPC (two-pore channels) and TRP (transient receptor potential channels) (Finbow and Harrison, 1997; Pitt et al., 2010). The Ca2+ content of these acidic compartments depends on the luminal pH, establishing a direct correlation between the efficiency of the proton pump V-ATPase and Ca2+ homeostasis (Christensen et al., 2002; Churchill et al., 2002; Guse and Lee, 2008; Lee et al., 2015). Such a link is evident in X-linked parkinsonism with spasticity (XPDS), which is associated with a mutation in ATP6AP2, which causes altered splicing of this prorenin receptor, a key regulator of V-ATPase function (Table 1, Fig. 1) (Jansen and Martens, 2012; Korvatska et al., 2013).
A mutation in ATP13A2 links Ca2+ homeostasis and lysosomal function to autosomal recessive juvenile onset of PD (Table 1) (Di Fonzo et al., 2007; Ramirez et al., 2006; Ramonet et al., 2012; Santoro et al., 2011). ATP13A2 is a P5-type ATPase cation Mn2+ transporter that localizes to lysosomal membranes (Fig. 1). This protein protects against α-synuclein and Mn2+ toxicity in a yeast model of α-synuclein toxicity (Gitler et al., 2009). Mutant forms of ATP13A2 that are associated with PD mislocalize to the ER, causing defects in protein degradation and leading to parkinsonism that is levodopa-responsive (Di Fonzo et al., 2009; Matsui et al., 2013; Park et al., 2011; Schröder et al., 2007). Interestingly, silencing the expression of ATP13A2 leads to a drop in cytosolic Ca2+ and to fragmented mitochondria in cortical neurons through an as yet unknown mechanism (Ramonet et al., 2012).
Three other proteins associated with autosomal-dominant forms of PD affect Ca2+ homeostasis both in the lysosome and in endosomal compartments. Two of these function in endosomal compartments: the vacuolar protein sorting 35 (VPS35), which is important for vesicular transport from the endosomes to the Golgi, and the vacuolar protein sorting 13 (VPS13C), which is important for vesicular transport from the Golgi to the endosomes. These proteins affect Ca2+ influx in the secretory pathway and protein recycling (Table 1) (Fokina et al., 2015; Mehta et al., 2016; Wang et al., 2016). The third is a lysosomal protein, LRRK2, that is linked through an unknown mechanism to the regulation of Ca2+ homeostasis in this organelle (Table 1) (Funayama et al., 2002; Gómez-Suaga et al., 2012; Gómez-Suaga and Hilfiker, 2012; Hockey et al., 2015).
Vesicles, another type of acidic organelle, are also highly affected in PD. Proper trafficking and priming of vesicles is crucial for synaptic function. Genes that are often mutated in PD encode proteins that have important functions in synaptic vesicle recycling, such as α-synuclein, LRRK2, TMEM230, SYNJ1, RIT2, SYT11, etc. (Table 1). So far, only a handful of these proteins are known to have a direct connection to Ca2+. α-Synuclein by itself can alter vesicle fusion by changing membrane curvature (Jensen et al., 2011; Nuscher et al., 2004), and it can also affect fusion by affecting the recruitment of several soluble NSF attachment (SNARE) proteins (Burre et al., 2010; Choi et al., 2013). Other evidence suggests that Ca2+ binding at the α-synuclein C-terminus can accelerate its aggregation, inhibiting its ability to bind to membranes and consequently promoting vesicle fusion (Table 1, Fig. 1) (Follett et al., 2013; Nath et al., 2011). Syntaxin 1B (STX1B) is an important member of the Ca2+-dependent proteins that mediate vesicle fusion at the plasma membrane (Südhof, 2013). A genome-wide association study identified the STX1B rs4889603 variant as a sporadic PD susceptibility locus in the Chinese population (Wang et al., 2015). Ca2+ binding to STX1B is necessary for the oligomerization of this protein and for the proper regulation of vesicle docking from the readily releasable pool at the synapse, although the underlying mechanism for this binding remains unknown (Table 1, Fig. 1) (Milovanovic et al., 2016; Mishima et al., 2014).
Ca2+ storage in the Golgi
All lysosomal and secreted proteins are trafficked through the Golgi, the organelle responsible for the O-linked glycosylation of proteins and for the generation of endosomes for the secretory pathway. Although glycosylation enzymes inside the Golgi are highly dependent on internal Ca2+, the contribution of cytosolic Ca2+ to the Golgi has yet to be fully elucidated. Nevertheless, increases in cytosolic Ca2+ in neurons can lead to Golgi fragmentation, a reversible process mediated by CaMKII and/or CaMKIV (Thayer et al., 2013). Golgi fragmentation has been observed in cellular and animal models of PD, as well as in post-mortem brain samples from PD patients (Fujita et al., 2006; Gosavi et al., 2002; Lin et al., 2012; Rendón et al., 2013). Interestingly, two related Ca2+ channels, TRPV5 and TRPV6, can increase Ca2+ influx into the cytoplasm when glycosylated in Xenopus laevis oocytes (Jiang et al., 2008). Syntaxin 6 (STX6) inhibits TRPV channel glycosylation to allow their activation (Table 1). Although the role of STX6 in DA neurons is not known, the STX6 rs1411478 variant is associated with progressive supranuclear palsy (PSP), a neurodegenerative disease that shares some characteristics with PD (Höglinger et al., 2011). Another connection between the Golgi and PD comes from the discovery that the yeast Ca2+/Mn2+ pump, PMR1 (homologous to the plasma membrane Ca2+-ATPase 1 in mammalian cells), is a modifier of cytosolic Ca2+ and α-synuclein toxicity in yeast (Büttner et al., 2013; Cooper et al., 2006). The role of Golgi as Ca2+ reservoirs in PD pathology remains unclear at present.
Cytosolic Ca2+ signaling hubs and PD
Regardless of whether Ca2+ derives from the extracellular environment or from intracellular stores, what makes it such a powerful second messenger is its ability to affect protein conformation and ultimately protein function (Berridge et al., 2003; Clapham, 2007). An essential transducer of Ca2+ gradients into cellular responses is the highly evolutionary conserved protein calmodulin (CaM) (Fig. 1) (Chin and Means, 2000; Meador et al., 1992, 1993). CaM binds to Ca2+ via helix-loop-helix Ca2+-binding motifs called EF-hands. Ca2+ binding causes a large conformation change in CaM that exposes a hydrophobic surface capable of binding a diverse array of proteins (Table 1) (Gifford et al., 2007; Grabarek, 2011; Kawasaki et al., 1998; Lewit-Bentley and Réty, 2000). Binding of CaM to α-synuclein accelerates α-synuclein fibril formation in vitro, potentially contributing to PD pathogenesis (Lee et al., 2002; Martinez et al., 2003). Interestingly, some of the proteins implicated in PD are directly regulated by CaM, although the functional significance of these interactions in the context of PD has not yet been established. These include: RIT2 (Rin), a GTP-binding protein that is involved in DA neuronal function by regulating DAT trafficking and consequently extracellular dopamine concentrations (Table 1, Fig. 1) (Lee et al., 1996; Navaroli et al., 2011; Pankratz et al., 2012; Shi et al., 2005; Zhou et al., 2011); and DnaJ heat shock protein family member C13 (DNAJC13), which is involved in endocytosis and membrane trafficking through early endosomes (Table 1, Fig. 1) (Fujibayashi et al., 2008; Shen et al., 2005; Vilarino-Guell et al., 2014; Zhang et al., 2001).
Many CaM actions rely on activating and/or inhibiting downstream effectors that will, in turn, contribute to the pathogenesis of PD. One of the CaM-Ca2+ effectors is CaMKII, one of the most predominant protein kinases in the brain that is particularly important for synaptic function, learning and memory (Fig. 1) (Coultrap and Bayer, 2012; Hudmon and Schulman, 2002). Although the presynaptic role of CaMKII in PD is not fully understood, it has a role in both the initiation and prevention of dopamine release (Hoover et al., 2014; Michael et al., 2006; Wang, 2008). CaMKII also phosphorylates several microtubule-associated proteins, such as Tau, leading to defects in cytoskeleton dynamics and intracellular trafficking (Table 1, Fig. 1) (Baratier et al., 2006; Hashimoto et al., 2000; Johnson and Foley, 1993; Wang et al., 2007; Wei et al., 2015; Yamauchi and Fujisawa, 1983, 1984). The hyperphosphorylation of Tau contributes to its aggregation and thus to the consequent formation of neurofibrillary tangles, the pathological hallmark of tauopathies, such as Alzheimer’s disease (Wang et al., 2013a). Moreover, CaMKII and phosphorylated Tau are found in Lewy bodies, suggesting an important role for these proteins in the etiology of PD (Iwatsubo et al., 1991; McKee et al., 1990; Moussaud et al., 2014). CaMKII also phosphorylates TH, the rate-limiting enzyme in the biosynthesis of catecholamines, such as dopamine, noradrenaline and adrenaline, and increases dopamine synthesis (Fitzpatrick, 1999; Albert et al., 1984; Lehmann et al., 2006). Abnormal increases in cytosolic dopamine are reportedly neurotoxic in cultured rat midbrain DA neurons. Importantly, reducing cytosolic Ca2+ significantly decreases cytosolic dopamine and prevents toxicity in DA neurons in the rat SNc (Mosharov et al., 2009).
Another essential transducer of Ca2+ signaling is the highly conserved Ca2+-CaM-dependent serine/threonine phosphatase calcineurin. Calcineurin is an essential enzyme, which in the adult brain plays a key role in neurite extension, synaptic plasticity, memory and learning (Zeng et al., 2001), and is implicated as a key mediator of α-synuclein toxicity (Table 1) (Caraveo et al., 2014; Martin et al., 2012). Most importantly, our group has found that persistent and excessively high levels of calcineurin activity caused by α-synuclein drive dephosphorylation of target proteins, such as the nuclear factor of activated T cells (NFAT), setting up a program that leads to cell death (Fig. 1) (Caraveo et al., 2014; Luo et al., 2014). However, low levels of calcineurin activity, achieved with low doses of the calcineurin specific inhibitor (FK506) or by genetic means, lead to the dephosphorylation of a distinct subset of proteins, such as the target of rapamycin complex 2 (TORC2), which protects cells from the toxic effects of α-synuclein (Fig. 1). The complete inhibition of calcineurin with high doses of FK506 or deletion of the calcineurin gene eliminates its ability to dephosphorylate any target proteins, which also leads to cell death (Caraveo et al., 2014). We named this the ‘Goldilocks’ effect, where too much or no activity leads to cell death, but an intermediate level of activity is neuroprotective.
In addition to NFAT and TORC2, there are other calcineurin substrates implicated in PD. These include the transcription factor cAMP-responsive element binding (CREB), which has important roles in synaptic plasticity and long-term memory formation, and which is activated by phosphorylation and repressed in a calcineurin-dependent manner (Marambaud et al., 2009; Sakamoto et al., 2011). As a surrogate for high calcineurin activity, CREB has been found to be repressed in both primary mouse DA neurons treated with the neurotoxin 6-hydroxydopamine (6-OHDA) and in human PD brain samples (Chalovich et al., 2006; Sakamoto et al., 2011). Another calcineurin substrate is Synaptojanin 1 (SYNJ1), a lipid phosphatase that, when dephosphorylated by calcineurin, enhances clathrin-mediated endocytosis (Table 1, Fig. 1). Mutations in SYNJ1 that affect its phosphatase function are associated with early-onset progressive parkinsonism with generalized seizures (EOP) (Krebs et al., 2013; Lee et al., 2004). EIF2AK3, also known as protein kinase RNA-like endoplasmic reticulum kinase (PERK), couples ER stress to translation inhibition during protein misfolding (Table 1, Fig. 1) (Mercado et al., 2015). EIF2AK3 is a risk gene associated with progressive supranuclear palsy (PSP) (Höglinger et al., 2011). Although the effect of the single nucleotide polymorphism (SNP) associated with PSP is unknown, it is noteworthy that the interaction of calcineurin with PERK promotes PERK auto-phosphorylation, leading to translation inhibition (Bollo et al., 2010). In support of the role of PERK in the pathology of PD, phosphorylated PERK is found in SNc DA neurons from deceased PD patients, as well as in Lewy bodies (Hoozemans et al., 2007). In addition, another substrate of calcineurin, calnexin (CNX, an ER-resident chaperone), when dephosphorylated, releases the block caused by SERCA pumps and restores Ca2+ homeostasis in the ER (Bollo et al., 2010; Wang et al., 2013b). Although the ‘Goldilocks’ property of calcineurin has been demonstrated on just a handful of substrates, many other targets are likely to be involved with α-synuclein toxicity that remain to be discovered.
Another interesting Ca2+-dependent group of enzymes implicated in PD are calpains. These cytosolic cysteine proteases are involved in the regulation of synaptic plasticity and long-term potentiation. Acute calpain activation is beneficial to neurons, whereas chronic activation induced by sustained cytosolic Ca2+ can lead to cell death. In support of a role for Ca2+ deregulation in PD, over-activated calpains have been detected in postmortem PD brains (Crocker et al., 2003; Mouatt-Prigent et al., 2000; Samantaray et al., 2008). Moreover, bioinformatic analysis has revealed two single SNPs in the gene encoding the only endogenous inhibitor of calpain, the calpastatin gene (CAST), which might predispose Caucasian individuals with European ancestry to idiopathic PD (Allen and Satten, 2009, 2010; Dauer and Przedborski, 2003). Some studies suggest that calpains have a protective role in PD through promotion of α-synuclein degradation via the modulation of the E3 ubiquitin ligase Parkin (Kim et al., 2003), whereas others point to their having two possible toxic roles. First, calpains can promote α-synuclein aggregation in vitro and in vivo by cleaving the α-synuclein C-terminal domain (Table 1, Fig. 1) (Diepenbroek et al., 2014; Dufty et al., 2007; Nuber and Selkoe, 2016; Xu et al., 2015). Second, calpains can cleave p35 (CDK5R1). The p35 activates Cdc5, a cyclin-dependent kinase that has a key role in neuronal development (Ko et al., 2001; Ohshima et al., 1996), axonal transport (Julien and Mushynski, 1998), synaptic activity (Rosales et al., 2000) and dopamine signaling (Chergui et al., 2004; Nishi et al., 2002). In MPTP-treated animals and in α-synuclein cell model systems, the activation of calpain leads to p35 being cleaved into its pathological form, p25, which results in the mislocalization and hyperactivation of Cdk5, and in DA neuronal loss in the mouse SNc (Czapski et al., 2013; Smith et al., 2006). p25 and overactive Cdk5 are detected in PD animal models (Qu et al., 2007; Smith et al., 2003) and in Lewy bodies from postmortem PD brains (Alvira et al., 2008; Takahashi et al., 2000). Importantly, the inhibition of calpains is effective at reducing overactive Cdk5 and p25, and is protective against toxicity in animal models of PD (Chagniel et al., 2012). In addition, the peptide TFP5, which is derived from p35, is reported to be neuroprotective in the MPTP-treated rat cortical neurons and a mouse model of PD (Binukumar and Pant, 2016; Binukumar et al., 2015; Zhang et al., 2012).
Cytosolic Ca2+ buffering and PD
As we mentioned earlier, an important contributor to the vulnerability of SNc DA neurons in PD is their inability to buffer Ca2+, caused by decreased expression of Ca2+-buffering proteins such as calbindins and parvalbumin. Calbindins, which include calbindin-D28k (encoded by CALB1) and calbindin-D9k (encoded by S100G), are vitamin D-dependent Ca2+-binding proteins. Calbindin-D9k is mostly known for buffering Ca2+ in erythrocytes, whereas calbindin-D28k buffers Ca2+ in the central nervous system where it participates in the blockade of multiple pro-apoptotic pathways (Baimbridge et al., 1992). Overexpression of calbindin-D28k in the midbrain ventral tegmental DA neurons is associated with reduced cell death in human PD samples and in mouse models of the disease, compared to the calbidin-D28k-negative more vulnerable SNc DA population (Damier et al., 1999; Lavoie and Parent, 1991). Reduced expression of Ca2+-buffering proteins, as well as a chronic increase in intracellular Ca2+ in aging SNc DA neurons, are likely to be mechanisms that contribute to the mitochondrial and ER stress observed in PD. Mice overexpressing calbindin-D28k are resistant to the toxic effects of MPTP and to α-synuclein aggregation (Rcom-H'cheo-Gauthier et al., 2016; Yuan et al., 2013), establishing a causal link between buffering Ca2+ and protection against cell death. Interestingly, calbindin-D28k is also a reported risk factor for sporadic forms of PD in a Japanese population (Mizuta et al., 2008; Soto-Ortolaza et al., 2010).
Parvalbumin (PA) is another Ca2+-binding protein that is selectively expressed in a class of GABAergic interneurons of the dorsolateral prefrontal cortex (Benes and Berretta, 2001; Kretsinger and Nockolds, 1973), a region also affected in PD patients (Kikuchi et al., 2001). Altered PA levels are likely to contribute to the altered cortical excitability and oscillatory activity previously documented in PD (Lanoue et al., 2013). Moreover, loss of PA-positive neurons is reported in animal models of PD and in human PD brain samples (Fernández-Suárez et al., 2012). This suggests that decreased PA expression is associated with defects in Ca2+ buffering and cell death.
Discussion
Given the universal nature of Ca2+ signaling in biology, its involvement in the etiology of PD and other neurodegenerative disorders (Box 1) might not come as a surprise. Although it is now increasingly recognized that gradual Ca2+ dysregulation might be a key contributor for aging, what distinguishes its contribution to PD from that to normal aging and/or other neurological diseases is that many of the genes that give rise to PD have a known causal role in Ca2+ homeostasis. As we have described in this review, compelling evidence implicates the deregulation of Ca2+ flux both from the plasma membrane (through mechanisms involving Cav1.3, α-synuclein pore formation, etc.) and from intracellular stores (through other mechanisms involving α-synuclein and GBA, among others). As such, understanding the mechanisms by which Ca2+ signaling contributes to the progression of PD is vitally important for developing effective therapies to treat this disease.
Box 1. Ca2+ signaling and other neurodegenerative diseases.
Defects in Ca2+ homeostasis might also play a causal role in neurodegenerative disorders other than Parkinson's disease, such as Alzheimer’s disease (AD). Oligomeric forms of the amyloid β (Aβ)-peptide, the major component of amyloid plaques (a pathological hallmark of AD), can create pores at the plasma membrane and trigger Ca2+-induced toxicity (Arispe et al., 1993), similar to α-synuclein oligomers. Presenilins are a family of related multi-pass ER transmembrane proteins that constitute the catalytic subunits of the γ-secretase intramembrane protease complex. Presenilins can modify lysosomal and ER Ca2+ channels (Kayala et al., 2012; Nelson et al., 2011) and have been implicated in familial forms of AD (Tolia and De Strooper, 2009). Mutations in the presenilins cause severe defects in ER Ca2+ homeostasis through a combination of mechanisms that involve an increase in SOCE, expression of RyR and IP3R, and inhibition of Ca2+ leakage from the ER, leading to ER Ca2+ overload and, consequently, to cell death (Bezprozvanny, 2009). Decreased expression of Ca2+-binding buffer proteins, such as calbindin, in the hippocampus has also been directly correlated with cognitive decline in the mouse AD model (Palop et al., 2003), reminiscent of the protective role of Ca2+ buffering in PD. The inhibition of calcineurin by FK506 reportedly restores memory, alters behavior and increases survival in mouse models of AD (Dineley et al., 2007; Mukherjee et al., 2010; Reese et al., 2008). Finally, the Ca2+ homeostasis modulator, CALHM1, is also reported to be a risk gene for AD (Dreses-Werringloer et al., 2008).
Amyotrophic lateral sclerosis (ALS) is characterized by selective degeneration of motor neurons. The most compelling evidence linking Ca2+ defects with cell death in ALS is excitotoxicity caused by glial cells (Sasabe et al., 2007). Huntington's disease is a genetic disorder characterized by an increased number (over 40) of glutamine amino acids at the N-terminus of the huntingtin protein (Htt), which affect the medium spiny neurons. Expanded Htt binds to IP3R, which increases its sensitivity to IP3, thereby stimulating Ca2+ efflux from the ER (Chan et al., 2003; Tang et al., 2003).
Most of the organelles affected in PD are major Ca2+ reservoirs. This suggests that Ca2+ could be a key player in coordinating complex organelle networks to ultimately achieve metabolic interactions, intracellular signaling, cellular maintenance and regulation of cell survival. Although neuronal cell culture models, in vivo rodent models and midbrain DA neurons derived from patient induced pluripotent stem cells (iPSCs) are vitally important tools for understanding the mechanisms underlying the pathology of PD, a significant investment of time and money is needed to make the most of these tools. Time is an issue when phenotypes are age dependent, as is the case for PD, and when the lifespan of rodents and/or primates is years. Even for iPSC-derived neurons, the time it takes in cell culture for any meaningful phenotype to appear can be up to a year. This, added to the high cost of performing mammalian-based experiments, makes these systems less than amenable for exploratory mechanistic research, despite the fact that they provide an essential validation tool for translation into the clinic. The use of model organisms such as yeast, flies and worms, can effectively circumvent these roadblocks. Indeed, these models have proven to be invaluable tools for uncovering conserved disease and cell biological processes that are affected in PD, which range from lipid biology, vesicular trafficking and function, lysosomal and peroxisomal function, autophagy, apoptosis, cell cycle, mitochondria and oxidative stress, Ca2+ signaling, ion channels and transporters, and the protein folding, quality control and degradation pathways. Model organisms can provide an excellent means to understand the mechanisms of Ca2+ deregulation in PD and could also shed light on how organelle networks operate to achieve cellular plasticity by using Ca2+ as a messenger, ultimately leading to novel therapeutic alternatives for combating PD.
Acknowledgements
We would like to thank Niccolo E. Mencacci and Steven J. Lubbe for helpful discussions.
Footnotes
This article is part of a special subject collection ‘Neurodegeneration: from Models to Mechanisms to Therapies’, which was launched in a dedicated issue guest edited by Aaron Gitler and James Shorter. See related articles in this collection at http://dmm.biologists.org/collection/neurodegenerative-disorders.
Competing interests
The authors declare no competing or financial interests.
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
References
- Albert K. A., Helmer-Matyjek E., Nairn A. C., Muller T. H., Haycock J. W., Greene L. A., Goldstein M. and Greengard P. (1984). Calcium/phospholipid-dependent protein kinase (protein kinase C) phosphorylates and activates tyrosine hydroxylase. Proc. Natl. Acad. Sci. USA 81, 7713-7717. 10.1073/pnas.81.24.7713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen A. S. and Satten G. A. (2009). A novel haplotype-sharing approach for genome-wide case-control association studies implicates the calpastatin gene in Parkinson's disease. Genet. Epidemiol. 33, 657-667. 10.1002/gepi.20417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen A. S. and Satten G. A. (2010). SNPs in CAST are associated with Parkinson disease: a confirmation study. Am. J. Med. Genet. B Neuropsychiatr. Genet. 153B, 973-979. 10.1002/ajmg.b.31061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvira D., Ferrer I., Gutierrez-Cuesta J., Garcia-Castro B., Pallàs M. and Camins A. (2008). Activation of the calpain/cdk5/p25 pathway in the girus cinguli in Parkinson's disease. Parkinsonism Relat. Disord. 14, 309-313. 10.1016/j.parkreldis.2007.09.005 [DOI] [PubMed] [Google Scholar]
- Angelova P. R., Ludtmann M. H. R., Horrocks M. H., Negoda A., Cremades N., Klenerman D., Dobson C. M., Wood N. W., Pavlov E. V., Gandhi S. et al. (2016). Ca2+ is a key factor in alpha-synuclein-induced neurotoxicity. J. Cell Sci. 129, 1792-1801. 10.1242/jcs.180737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arispe N., Pollard H. B. and Rojas E. (1993). Giant multilevel cation channels formed by Alzheimer disease amyloid beta-protein [A beta P-(1-40)] in bilayer membranes. Proc. Natl. Acad. Sci. USA 90, 10573-10577. 10.1073/pnas.90.22.10573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine G. J., Santamaria F. and Tanaka K. (2003). Local calcium signaling in neurons. Neuron 40, 331-346. 10.1016/S0896-6273(03)00639-1 [DOI] [PubMed] [Google Scholar]
- Bahar E., Kim H. and Yoon H. (2016). ER stress-mediated signaling: action potential and Ca(2+) as key players. Int. J. Mol. Sci. 17, 1558 10.3390/ijms17091558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baimbridge K. G., Celio M. R. and Rogers J. H. (1992). Calcium-binding proteins in the nervous system. Trends Neurosci. 15, 303-308. 10.1016/0166-2236(92)90081-I [DOI] [PubMed] [Google Scholar]
- Baratier J., Peris L., Brocard J., Gory-Faure S., Dufour F., Bosc C., Fourest-Lieuvin A., Blanchoin L., Salin P., Job D. et al. (2006). Phosphorylation of microtubule-associated protein STOP by calmodulin kinase II. J. Biol. Chem. 281, 19561-19569. 10.1074/jbc.M509602200 [DOI] [PubMed] [Google Scholar]
- Beal M. F., Oakes D., Shoulson I., Henchcliffe C., Galpern W. R., Haas R., Juncos J. L., Nutt J. G., Voss T. S., Ravina B., et al. (2014). A randomized clinical trial of high-dosage coenzyme Q10 in early Parkinson disease: no evidence of benefit. JAMA Neurol. 71, 543-552. 10.1001/jamaneurol.2014.131 [DOI] [PubMed] [Google Scholar]
- Bean B. P. (2007). Neurophysiology: stressful pacemaking. Nature 447, 1059-1060. 10.1038/4471059a [DOI] [PubMed] [Google Scholar]
- Beavan M. S. and Schapira A. H. V. (2013). Glucocerebrosidase mutations and the pathogenesis of Parkinson disease. Ann. Med. 45, 511-521. 10.3109/07853890.2013.849003 [DOI] [PubMed] [Google Scholar]
- Becker C., Jick S. S. and Meier C. R. (2008). Use of antihypertensives and the risk of Parkinson disease. Neurology 70, 1438-1444. 10.1212/01.wnl.0000303818.38960.44 [DOI] [PubMed] [Google Scholar]
- Bedford C., Sears C., Perez-Carrion M., Piccoli G. and Condliffe S. B. (2016). LRRK2 Regulates Voltage-Gated Calcium Channel Function. Front. Mol. Neurosci. 9, 35 10.3389/fnmol.2016.00035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes F. M. and Berretta S. (2001). GABAergic interneurons: implications for understanding schizophrenia and bipolar disorder. Neuropsychopharmacology 25, 1-27. 10.1016/S0893-133X(01)00225-1 [DOI] [PubMed] [Google Scholar]
- Berger S. M. and Bartsch D. (2014). The role of L-type voltage-gated calcium channels Cav1.2 and Cav1.3 in normal and pathological brain function. Cell Tissue Res. 357, 463-476. 10.1007/s00441-014-1936-3 [DOI] [PubMed] [Google Scholar]
- Berridge M. J. (2005). Unlocking the secrets of cell signaling. Annu. Rev. Physiol. 67, 1-21. 10.1146/annurev.physiol.67.040103.152647 [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Lipp P. and Bootman M. D. (2000). The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 1, 11-21. 10.1038/35036035 [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Bootman M. D. and Roderick H. L. (2003). Calcium signalling: dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 4, 517-529. 10.1038/nrm1155 [DOI] [PubMed] [Google Scholar]
- Bezprozvanny I. (2009). Calcium signaling and neurodegenerative diseases. Trends Mol. Med. 15, 89-100. 10.1016/j.molmed.2009.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezprozvanny I. B. (2010). Calcium signaling and neurodegeneration. Acta Naturae 2, 72-82. [PMC free article] [PubMed] [Google Scholar]
- Binukumar B. K. and Pant H. C. (2016). TFP5/TP5 peptide provides neuroprotection in the MPTP model of Parkinson's disease. Neural Regen. Res. 11, 698-701. 10.4103/1673-5374.182681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binukumar B. K., Shukla V., Amin N. D., Grant P., Bhaskar M., Skuntz S., Steiner J. and Pant H. C. (2015). Peptide TFP5/TP5 derived from Cdk5 activator P35 provides neuroprotection in the MPTP model of Parkinson's disease. Mol. Biol. Cell 26, 4478-4491. 10.1091/mbc.E15-06-0415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollo M., Paredes R. M., Holstein D., Zheleznova N., Camacho P. and Lechleiter J. D. (2010). Calcineurin interacts with PERK and dephosphorylates calnexin to relieve ER stress in mammals and frogs. PLoS ONE 5, e11925 10.1371/journal.pone.0011925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bootman M. D. (2012). Calcium signaling. Cold Spring Harb. Perspect. Biol. 4, a011171 10.1101/cshperspect.a011171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bootman M. D., Lipp P. and Berridge M. J. (2001). The organisation and functions of local Ca(2+) signals. J. Cell Sci. 114, 2213-2222. [DOI] [PubMed] [Google Scholar]
- Bose A. and Beal M. F. (2016). Mitochondrial dysfunction in Parkinson's disease. J. Neurochem. 139 Suppl. 1, 216-231. 10.1111/jnc.13731 [DOI] [PubMed] [Google Scholar]
- Braak H., Ghebremedhin E., Rüb U., Bratzke H. and Del Tredici K. (2004). Stages in the development of Parkinson's disease-related pathology. Cell Tissue Res. 318, 121-134. 10.1007/s00441-004-0956-9 [DOI] [PubMed] [Google Scholar]
- Brown T. P., Rumsby P. C., Capleton A. C., Rushton L. and Levy L. S. (2006). Pesticides and Parkinson's disease--is there a link? Environ. Health Perspect. 114, 156-164. 10.1289/ehp.8095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruzzone S., Kunerth S., Zocchi E., De Flora A. and Guse A. H. (2003). Spatio-temporal propagation of Ca2+ signals by cyclic ADP-ribose in 3T3 cells stimulated via purinergic P2Y receptors. J. Cell Biol. 163, 837-845. 10.1083/jcb.200307016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoyne R. D. (2007). Neuronal calcium sensor proteins: generating diversity in neuronal Ca2+ signalling. Nat. Rev. Neurosci. 8, 182-193. 10.1038/nrn2093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burre J., Sharma M., Tsetsenis T., Buchman V., Etherton M. R. and Sudhof T. C. (2010). Alpha-synuclein promotes SNARE-complex assembly in vivo and in vitro. Science 329, 1663-1667. 10.1126/science.1195227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büttner S., Faes L., Reichelt W. N., Broeskamp F., Habernig L., Benke S., Kourtis N., Ruli D., Carmona-Gutierrez D., Eisenberg T. et al. (2013). The Ca2+/Mn2+ ion-pump PMR1 links elevation of cytosolic Ca(2+) levels to alpha-synuclein toxicity in Parkinson's disease models. Cell Death Differ. 20, 465-477. 10.1038/cdd.2012.142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calì T., Ottolini D. and Brini M. (2011). Mitochondria, calcium, and endoplasmic reticulum stress in Parkinson's disease. Biofactors 37, 228-240. 10.1002/biof.159 [DOI] [PubMed] [Google Scholar]
- Calì T., Ottolini D. and Brini M. (2012a). Mitochondrial Ca(2+) and neurodegeneration. Cell Calcium 52, 73-85. 10.1016/j.ceca.2012.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calì T., Ottolini D., Negro A. and Brini M. (2012b). alpha-Synuclein controls mitochondrial calcium homeostasis by enhancing endoplasmic reticulum-mitochondria interactions. J. Biol. Chem. 287, 17914-17929. 10.1074/jbc.M111.302794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calì T., Ottolini D., Negro A. and Brini M. (2013). Enhanced parkin levels favor ER-mitochondria crosstalk and guarantee Ca(2+) transfer to sustain cell bioenergetics. Biochim. Biophys. Acta 1832, 495-508. 10.1016/j.bbadis.2013.01.004 [DOI] [PubMed] [Google Scholar]
- Calì T., Ottolini D. and Brini M. (2014). Calcium signaling in Parkinson's disease. Cell Tissue Res. 357, 439-454. 10.1007/s00441-014-1866-0 [DOI] [PubMed] [Google Scholar]
- Carafoli E. (2002). Calcium signaling: a tale for all seasons. Proc. Natl. Acad. Sci. USA 99, 1115-1122. 10.1073/pnas.032427999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caraveo G., Auluck P. K., Whitesell L., Chung C. Y., Baru V., Mosharov E. V., Yan X., Ben-Johny M., Soste M., Picotti P. et al. (2014). Calcineurin determines toxic versus beneficial responses to alpha-synuclein. Proc. Natl. Acad. Sci. USA 111, E3544-E3552. 10.1073/pnas.1413201111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco M. A. and Hidalgo C. (2006). Calcium microdomains and gene expression in neurons and skeletal muscle cells. Cell Calcium 40, 575-583. 10.1016/j.ceca.2006.08.021 [DOI] [PubMed] [Google Scholar]
- Celardo I., Costa A. C., Lehmann S., Jones C., Wood N., Mencacci N. E., Mallucci G. R., Loh S. H. Y. and Martins L. M. (2016). Mitofusin-mediated ER stress triggers neurodegeneration in pink1/parkin models of Parkinson's disease. Cell Death Dis. 7, e2271 10.1038/cddis.2016.173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chagniel L., Robitaille C., Lebel M. and Cyr M. (2012). Striatal inhibition of calpains prevents levodopa-induced neurochemical changes and abnormal involuntary movements in the hemiparkinsonian rat model. Neurobiol. Dis. 45, 645-655. 10.1016/j.nbd.2011.10.011 [DOI] [PubMed] [Google Scholar]
- Chalovich E. M., Zhu J.-H., Caltagarone J., Bowser R. and Chu C. T. (2006). Functional repression of cAMP response element in 6-hydroxydopamine-treated neuronal cells. J. Biol. Chem. 281, 17870-17881. 10.1074/jbc.M602632200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan E. Y., Tang T., Tu H., Maximov A., Wang Z., Wellington C. L., Hayden M. R. and Bezprozvanny I. (2003). Huntingtin and huntingtin-associated protein 1 influence neuronal calcium signaling mediated by inositol-(1,4,5) triphosphate receptor type 1. Am. J. Hum. Genet. 73, 561-561 10.1016/S0896-6273(03)00366-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan C. S., Guzman J. N., Ilijic E., Mercer J. N., Rick C., Tkatch T., Meredith G. E. and Surmeier D. J. (2007). ‘Rejuvenation’ protects neurons in mouse models of Parkinson's disease. Nature 447, 1081-1086. 10.1038/nature05865 [DOI] [PubMed] [Google Scholar]
- Chaudhuri K. R., Healy D. G. and Schapira A. H. V. (2006). Non-motor symptoms of Parkinson's disease: diagnosis and management. Lancet Neurol. 5, 235-245. 10.1016/S1474-4422(06)70373-8 [DOI] [PubMed] [Google Scholar]
- Chen Y. P., Song W., Huang R., Chen K., Zhao B., Li J., Yang Y. and Shang H.-F. (2013). GAK rs1564282 and DGKQ rs11248060 increase the risk for Parkinson's disease in a Chinese population. J. Clin. Neurosci. 20, 880-883. 10.1016/j.jocn.2012.07.011 [DOI] [PubMed] [Google Scholar]
- Chergui K., Svenningsson P. and Greengard P. (2004). Cyclin-dependent kinase 5 regulates dopaminergic and glutamatergic transmission in the striatum. Proc. Natl. Acad. Sci. USA 101, 2191-2196. 10.1073/pnas.0308652100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin D. and Means A. R. (2000). Calmodulin: a prototypical calcium sensor. Trends Cell Biol. 10, 322-328. 10.1016/S0962-8924(00)01800-6 [DOI] [PubMed] [Google Scholar]
- Choi B.-K., Choi M.-G., Kim J.-Y., Yang Y., Lai Y., Kweon D.-H., Lee N. K. and Shin Y.-K. (2013). Large alpha-synuclein oligomers inhibit neuronal SNARE-mediated vesicle docking. Proc. Natl. Acad. Sci. USA 110, 4087-4092. 10.1073/pnas.1218424110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen K. A., Myers J. T. and Swanson J. A. (2002). pH-dependent regulation of lysosomal calcium in macrophages. J. Cell Sci. 115, 599-607. [DOI] [PubMed] [Google Scholar]
- Churchill G. C., Okada Y., Thomas J. M., Genazzani A. A., Patel S. and Galione A. (2002). NAADP mobilizes Ca(2+) from reserve granules, lysosome-related organelles, in sea urchin eggs. Cell 111, 703-708. 10.1016/S0092-8674(02)01082-6 [DOI] [PubMed] [Google Scholar]
- Clapham D. E. (2007). Calcium signaling. Cell 131, 1047-1058. 10.1016/j.cell.2007.11.028 [DOI] [PubMed] [Google Scholar]
- Cooper A. A., Gitler A. D., Cashikar A., Haynes C. M., Hill K. J., Bhullar B., Liu K., Xu K., Strathearn K. E., Liu F. et al. (2006). Alpha-synuclein blocks ER-Golgi traffic and Rab1 rescues neuron loss in Parkinson's models. Science 313, 324-328. 10.1126/science.1129462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copanaki E., Schürmann T., Eckert A., Leuner K., Müller W. E., Prehn J. H. M. and Kögel D. (2007). The amyloid precursor protein potentiates CHOP induction and cell death in response to ER Ca2+ depletion. Biochim. Biophys. Acta 1773, 157-165. 10.1016/j.bbamcr.2006.10.002 [DOI] [PubMed] [Google Scholar]
- Coultrap S. J. and Bayer K. U. (2012). CaMKII regulation in information processing and storage. Trends Neurosci. 35, 607-618. 10.1016/j.tins.2012.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocker S. J., Smith P. D., Jackson-Lewis V., Lamba W. R., Hayley S. P., Grimm E., Callaghan S. M., Slack R. S., Melloni E., Przedborski S. et al. (2003). Inhibition of calpains prevents neuronal and behavioral deficits in an MPTP mouse model of Parkinson's disease. J. Neurosci. 23, 4081-4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuervo A. M., Stefanis L., Fredenburg R., Lansbury P. T. and Sulzer D. (2004). Impaired degradation of mutant alpha-synuclein by chaperone-mediated autophagy. Science 305, 1292-1295. 10.1126/science.1101738 [DOI] [PubMed] [Google Scholar]
- Czapski G. A., Gąssowska M., Wilkaniec A., Cieślik M. and Adamczyk A. (2013). Extracellular alpha-synuclein induces calpain-dependent overactivation of cyclin-dependent kinase 5 in vitro. FEBS Lett. 587, 3135-3141. 10.1016/j.febslet.2013.07.053 [DOI] [PubMed] [Google Scholar]
- Damier P., Hirsch E. C., Agid Y. and Graybiel A. M. (1999). The substantia nigra of the human brain. II. Patterns of loss of dopamine-containing neurons in Parkinson's disease. Brain 122, 1437-1448. 10.1093/brain/122.8.1437 [DOI] [PubMed] [Google Scholar]
- Danzer K. M., Haasen D., Karow A. R., Moussaud S., Habeck M., Giese A., Kretzschmar H., Hengerer B. and Kostka M. (2007). Different species of alpha-synuclein oligomers induce calcium influx and seeding. J. Neurosci. 27, 9220-9232. 10.1523/JNEUROSCI.2617-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauer W. and Przedborski S. (2003). Parkinson's disease: mechanisms and models. Neuron 39, 889-909. 10.1016/S0896-6273(03)00568-3 [DOI] [PubMed] [Google Scholar]
- Dehay B., Martinez-Vicente M., Caldwell G. A., Caldwell K. A., Yue Z., Cookson M. R., Klein C., Vila M. and Bezard E. (2013). Lysosomal impairment in Parkinson's disease. Mov. Disord. 28, 725-732. 10.1002/mds.25462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Fonzo A., Chien H. F., Socal M., Giraudo S., Tassorelli C., Iliceto G., Fabbrini G., Marconi R., Fincati E., Abbruzzese G. et al. (2007). ATP13A2 missense mutations in juvenile parkinsonism and young onset Parkinson disease. Neurology 68, 1557-1562. 10.1212/01.wnl.0000260963.08711.08 [DOI] [PubMed] [Google Scholar]
- Di Fonzo A., Dekker M. C. J., Montagna P., Baruzzi A., Yonova E. H., Correia Guedes L., Szczerbinska A., Zhao T., Dubbel-Hulsman L. O. M., Wouters C. H. et al. (2009). FBXO7 mutations cause autosomal recessive, early-onset parkinsonian-pyramidal syndrome. Neurology 72, 240-245. 10.1212/01.wnl.0000338144.10967.2b [DOI] [PubMed] [Google Scholar]
- Di Scala C., Yahi N., Boutemeur S., Flores A., Rodriguez L., Chahinian H. and Fantini J. (2016). Common molecular mechanism of amyloid pore formation by Alzheimer's beta-amyloid peptide and alpha-synuclein. Sci. Rep. 6, 28781 10.1038/srep28781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diepenbroek M., Casadei N., Esmer H., Saido T. C., Takano J., Kahle P. J., Nixon R. A., Rao M. V., Melki R., Pieri L. et al. (2014). Overexpression of the calpain-specific inhibitor calpastatin reduces human alpha-Synuclein processing, aggregation and synaptic impairment in [A30P]alphaSyn transgenic mice. Hum. Mol. Genet. 23, 3975-3989. 10.1093/hmg/ddu112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dineley K. T., Hogan D., Zhang W.-R. and Taglialatela G. (2007). Acute inhibition of calcineurin restores associative learning and memory in Tg2576 APP transgenic mice. Neurobiol. Learn. Mem. 88, 217-224. 10.1016/j.nlm.2007.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragicevic E., Poetschke C., Duda J., Schlaudraff F., Lammel S., Schiemann J., Fauler M., Hetzel A., Watanabe M., Lujan R. et al. (2014). Cav1.3 channels control D2-autoreceptor responses via NCS-1 in substantia nigra dopamine neurons. Brain 137, 2287-2302. 10.1093/brain/awu131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreses-Werringloer U., Lambert J.-C., Vingtdeux V., Zhao H., Vais H., Siebert A., Jain A., Koppel J., Rovelet-Lecrux A., Hannequin D. et al. (2008). A polymorphism in CALHM1 influences Ca2+ homeostasis, A beta levels, and Alzheimer's disease risk. Cell 133, 1149-1161. 10.1016/j.cell.2008.05.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drion G., Massotte L., Sepulchre R. and Seutin V. (2011). How modeling can reconcile apparently discrepant experimental results: the case of pacemaking in dopaminergic neurons. PLoS Comput. Biol. 7, e1002050 10.1371/journal.pcbi.1002050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dryanovski D. I., Guzman J. N., Xie Z., Galteri D. J., Volpicelli-Daley L. A., Lee V. M.-Y., Miller R. J., Schumacker P. T. and Surmeier D. J. (2013). Calcium entry and alpha-synuclein inclusions elevate dendritic mitochondrial oxidant stress in dopaminergic neurons. J. Neurosci. 33, 10154-10164. 10.1523/JNEUROSCI.5311-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufty B. M., Warner L. R., Hou S. T., Jiang S. X., Gomez-Isla T., Leenhouts K. M., Oxford J. T., Feany M. B., Masliah E. and Rohn T. T. (2007). Calpain-cleavage of alpha-synuclein: connecting proteolytic processing to disease-linked aggregation. Am. J. Pathol. 170, 1725-1738. 10.2353/ajpath.2007.061232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exner N., Lutz A. K., Haass C. and Winklhofer K. F. (2012). Mitochondrial dysfunction in Parkinson's disease: molecular mechanisms and pathophysiological consequences. EMBO J. 31, 3038-3062. 10.1038/emboj.2012.170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Suárez D., Celorrio M., Lanciego J. L., Franco R. and Aymerich M. S. (2012). Loss of parvalbumin-positive neurons from the globus pallidus in animal models of Parkinson disease. J. Neuropathol. Exp. Neurol. 71, 973-982. 10.1097/NEN.0b013e3182717cba [DOI] [PubMed] [Google Scholar]
- Finbow M. E. and Harrison M. A. (1997). The vacuolar H+-ATPase: a universal proton pump of eukaryotes. Biochem. J. 324, 697-712. 10.1042/bj3240697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick P. F. (1999). Tetrahydropterin-dependent amino acid hydroxylases. Annu. Rev. Biochem. 68, 355-381. 10.1146/annurev.biochem.68.1.355 [DOI] [PubMed] [Google Scholar]
- Fluegge D., Moeller L. M., Cichy A., Gorin M., Weth A., Veitinger S., Cainarca S., Lohmer S., Corazza S., Neuhaus E. M. et al. (2012). Mitochondrial Ca(2+) mobilization is a key element in olfactory signaling. Nat. Neurosci. 15, 754-762. 10.1038/nn.3074 [DOI] [PubMed] [Google Scholar]
- Fokina A. V., Chechenova M. B., Karginov A. V., Ter-Avanesyan M. D. and Agaphonov M. O. (2015). Genetic evidence for the role of the vacuole in supplying secretory organelles with Ca2+ in Hansenula polymorpha. PLoS ONE 10, e0145915 10.1371/journal.pone.0145915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follett J., Darlow B., Wong M. B., Goodwin J. and Pountney D. L. (2013). Potassium depolarization and raised calcium induces alpha-synuclein aggregates. Neurotox. Res. 23, 378-392. 10.1007/s12640-012-9366-z [DOI] [PubMed] [Google Scholar]
- Frei B. and Richter C. (1986). N-methyl-4-phenylpyridine (MMP+) together with 6-hydroxydopamine or dopamine stimulates Ca2+ release from mitochondria. FEBS Lett. 198, 99-102. 10.1016/0014-5793(86)81192-9 [DOI] [PubMed] [Google Scholar]
- Fujibayashi A., Taguchi T., Misaki R., Ohtani M., Dohmae N., Takio K., Yamada M., Gu J., Yamakami M., Fukuda M. et al. (2008). Human RME-8 is involved in membrane trafficking through early endosomes. Cell Struct. Funct. 33, 35-50. 10.1247/csf.07045 [DOI] [PubMed] [Google Scholar]
- Fujita Y., Ohama E., Takatama M., Al-Sarraj S. and Okamoto K. (2006). Fragmentation of Golgi apparatus of nigral neurons with alpha-synuclein-positive inclusions in patients with Parkinson's disease. Acta Neuropathol. 112, 261-265. 10.1007/s00401-006-0114-4 [DOI] [PubMed] [Google Scholar]
- Funayama M., Hasegawa K., Kowa H., Saito M., Tsuji S. and Obata F. (2002). A new locus for Parkinson's disease (PARK8) maps to chromosome 12p11.2-q13.1. Ann. Neurol. 51, 296-301. 10.1002/ana.10113 [DOI] [PubMed] [Google Scholar]
- Gandhi S., Wood-Kaczmar A., Yao Z., Plun-Favreau H., Deas E., Klupsch K., Downward J., Latchman D. S., Tabrizi S. J., Wood N. W. et al. (2009). PINK1-associated Parkinson's disease is caused by neuronal vulnerability to calcium-induced cell death. Mol. Cell 33, 627-638. 10.1016/j.molcel.2009.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellerich F. N., Gizatullina Z., Gainutdinov T., Muth K., Seppet E., Orynbayeva Z. and Vielhaber S. (2013). The control of brain mitochondrial energization by cytosolic calcium: the mitochondrial gas pedal. IUBMB Life 65, 180-190. 10.1002/iub.1131 [DOI] [PubMed] [Google Scholar]
- Ghanbari M., Darweesh S. K. L., de Looper H. W. J., van Luijn M. M., Hofman A., Ikram M. A., Franco O. H., Erkeland S. J. and Dehghan A. (2016). Genetic variants in MicroRNAs and their binding sites are associated with the risk of Parkinson disease. Hum. Mutat. 37, 292-300. 10.1002/humu.22943 [DOI] [PubMed] [Google Scholar]
- Gifford J. L., Walsh M. P. and Vogel H. J. (2007). Structures and metal-ion-binding properties of the Ca2+-binding helix-loop-helix EF-hand motifs. Biochem. J. 405, 199-221. 10.1042/BJ20070255 [DOI] [PubMed] [Google Scholar]
- Gitler A. D., Chesi A., Geddie M. L., Strathearn K. E., Hamamichi S., Hill K. J., Caldwell K. A., Caldwell G. A., Cooper A. A., Rochet J.-C. et al. (2009). Alpha-synuclein is part of a diverse and highly conserved interaction network that includes PARK9 and manganese toxicity. Nat. Genet. 41, 308-315. 10.1038/ng.300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glancy B. and Balaban R. S. (2012). Role of mitochondrial Ca2+ in the regulation of cellular energetics. Biochemistry 51, 2959-2973. 10.1021/bi2018909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg J. A., Guzman J. N., Estep C. M., Ilijic E., Kondapalli J., Sanchez-Padilla J. and Surmeier D. J. (2012). Calcium entry induces mitochondrial oxidant stress in vagal neurons at risk in Parkinson's disease. Nat. Neurosci. 15, 1414-1421. 10.1038/nn.3209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman S. M. (2014). Environmental toxins and Parkinson's disease. Annu. Rev. Pharmacol. Toxicol. 54, 141-164. 10.1146/annurev-pharmtox-011613-135937 [DOI] [PubMed] [Google Scholar]
- Gómez-Sánchez R., Gegg M. E., Bravo-San Pedro J. M., Niso-Santano M., Alvarez-Erviti L., Pizarro-Estrella E., Gutiérrez-Martín Y., Alvarez-Barrientos A., Fuentes J. M., González-Polo R. A. et al. (2014). Mitochondrial impairment increases FL-PINK1 levels by calcium-dependent gene expression. Neurobiol. Dis. 62, 426-440. 10.1016/j.nbd.2013.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Suaga P. and Hilfiker S. (2012). LRRK2 as a modulator of lysosomal calcium homeostasis with downstream effects on autophagy. Autophagy 8, 692-693. 10.4161/auto.19305 [DOI] [PubMed] [Google Scholar]
- Gómez-Suaga P., Churchill G. C., Patel S. and Hilfiker S. (2012). A link between LRRK2, autophagy and NAADP-mediated endolysosomal calcium signalling. Biochem. Soc. Trans. 40, 1140-1146. 10.1042/BST20120138 [DOI] [PubMed] [Google Scholar]
- Gosavi N., Lee H.-J., Lee J. S., Patel S. and Lee S.-J. (2002). Golgi fragmentation occurs in the cells with prefibrillar alpha-synuclein aggregates and precedes the formation of fibrillar inclusion. J. Biol. Chem. 277, 48984-48992. 10.1074/jbc.M208194200 [DOI] [PubMed] [Google Scholar]
- Grabarek Z. (2011). Insights into modulation of calcium signaling by magnesium in calmodulin, troponin C and related EF-hand proteins. Biochim. Biophys. Acta 1813, 913-921. 10.1016/j.bbamcr.2011.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guardia-Laguarta C., Area-Gomez E., Rub C., Liu Y., Magrane J., Becker D., Voos W., Schon E. A. and Przedborski S. (2014). alpha-Synuclein is localized to mitochondria-associated ER membranes. J. Neurosci. 34, 249-259. 10.1523/JNEUROSCI.2507-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guse A. H. and Lee H. C. (2008). NAADP: a universal Ca2+ trigger. Sci. Signal. 1, re10 10.1126/scisignal.144re10 [DOI] [PubMed] [Google Scholar]
- Guzman J. N., Sanchez-Padilla J., Wokosin D., Kondapalli J., Ilijic E., Schumacker P. T. and Surmeier D. J. (2010). Oxidant stress evoked by pacemaking in dopaminergic neurons is attenuated by DJ-1. Nature 468, 696-700. 10.1038/nature09536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto R., Nakamura Y., Komai S., Kashiwagi Y., Tamura K., Goto T., Aimoto S., Kaibuchi K., Shiosaka S. and Takeda M. (2000). Site-specific phosphorylation of neurofilament-L is mediated by calcium/calmodulin-dependent protein kinase II in the apical dendrites during long-term potentiation. J. Neurochem. 75, 373-382. 10.1046/j.1471-4159.2000.0750373.x [DOI] [PubMed] [Google Scholar]
- Hatzifilippou E., Koutsouraki E., Banaki T., Traka M., Costa V. G. and Baloyannis S. J. (2008). Antibodies against GM1 in demented patients. Am. J. Alzheimers Dis. Other Demen. 23, 274-279. 10.1177/1533317508317816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeman B., Van den Haute C., Aelvoet S.-A., Valsecchi F., Rodenburg R. J., Reumers V., Debyser Z., Callewaert G., Koopman W. J. H., Willems P. H. G. M. et al. (2011). Depletion of PINK1 affects mitochondrial metabolism, calcium homeostasis and energy maintenance. J. Cell Sci. 124, 1115-1125. 10.1242/jcs.078303 [DOI] [PubMed] [Google Scholar]
- Hockey L. N., Kilpatrick B. S., Eden E. R., Lin-Moshier Y., Brailoiu G. C., Brailoiu E., Futter C. E., Schapira A. H., Marchant J. S. and Patel S. (2015). Dysregulation of lysosomal morphology by pathogenic LRRK2 is corrected by TPC2 inhibition. J. Cell Sci. 128, 232-238. 10.1242/jcs.164152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höglinger G. U., Melhem N. M., Dickson D. W., Sleiman P. M. A., Wang L.-S., Klei L., Rademakers R., de Silva R., Litvan I., Riley D. E. et al. (2011). Identification of common variants influencing risk of the tauopathy progressive supranuclear palsy. Nat. Genet. 43, 699-705. 10.1038/ng.859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover C. M., Edwards S. L., Yu S.-C., Kittelmann M., Richmond J. E., Eimer S., Yorks R. M. and Miller K. G. (2014). A novel CaM kinase II pathway controls the location of neuropeptide release from Caenorhabditis elegans motor neurons. Genetics 196, 745-765. 10.1534/genetics.113.158568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoozemans J. J. M., van Haastert E. S., Eikelenboom P., de Vos R. A. I., Rozemuller J. M. and Scheper W. (2007). Activation of the unfolded protein response in Parkinson's disease. Biochem. Biophys. Res. Commun. 354, 707-711. 10.1016/j.bbrc.2007.01.043 [DOI] [PubMed] [Google Scholar]
- Hu Q. and Wang G. (2016). Mitochondrial dysfunction in Parkinson's disease. Transl. Neurodegener. 5, 14 10.1186/s40035-016-0060-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z., Ren S., Jiang Y. and Wang T. (2016). PINK1 and Parkin cooperatively protect neurons against constitutively active TRP channel-induced retinal degeneration in Drosophila. Cell Death Dis. 7, e2179 10.1038/cddis.2016.82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudmon A. and Schulman H. (2002). Neuronal Ca2+/calmodulin-dependent protein kinase II: the role of structure and autoregulation in cellular function. Annu. Rev. Biochem. 71, 473-510. 10.1146/annurev.biochem.71.110601.135410 [DOI] [PubMed] [Google Scholar]
- Hurley M. J. and Dexter D. T. (2012). Voltage-gated calcium channels and Parkinson's disease. Pharmacol. Ther. 133, 324-333. 10.1016/j.pharmthera.2011.11.006 [DOI] [PubMed] [Google Scholar]
- Hurley M. J., Brandon B., Gentleman S. M. and Dexter D. T. (2013). Parkinson's disease is associated with altered expression of CaV1 channels and calcium-binding proteins. Brain 136, 2077-2097. 10.1093/brain/awt134 [DOI] [PubMed] [Google Scholar]
- Hwang O., Choi H. J. and Park S. Y. (1999). Up-regulation of GTP cyclohydrolase I and tetrahydrobiopterin by calcium influx. Neuroreport 10, 3611-3614. 10.1097/00001756-199911260-00027 [DOI] [PubMed] [Google Scholar]
- Ilijic E., Guzman J. N. and Surmeier D. J. (2011). The L-type channel antagonist isradipine is neuroprotective in a mouse model of Parkinson's disease. Neurobiol. Dis. 43, 364-371. 10.1016/j.nbd.2011.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwatsubo T., Nakano I., Fukunaga K. and Miyamoto E. (1991). Ca2+/calmodulin-dependent protein kinase II immunoreactivity in Lewy bodies. Acta Neuropathol. 82, 159-163. 10.1007/BF00294440 [DOI] [PubMed] [Google Scholar]
- Jansen E. J. R. and Martens G. J. M. (2012). Novel insights into V-ATPase functioning: distinct roles for its accessory subunits ATP6AP1/Ac45 and ATP6AP2/(pro) renin receptor. Curr. Protein Pept. Sci. 13, 124-133. 10.2174/138920312800493160 [DOI] [PubMed] [Google Scholar]
- Jensen M. B., Bhatia V. K., Jao C. C., Rasmussen J. E., Pedersen S. L., Jensen K. J., Langen R. and Stamou D. (2011). Membrane curvature sensing by amphipathic helices: a single liposome study using alpha-synuclein and annexin B12. J. Biol. Chem. 286, 42603-42614. 10.1074/jbc.M111.271130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y., Cong P., Williams S. R., Zhang W., Na T., Ma H.-P. and Peng J.-B. (2008). WNK4 regulates the secretory pathway via which TRPV5 is targeted to the plasma membrane. Biochem. Biophys. Res. Commun. 375, 225-229. 10.1016/j.bbrc.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J., Li G. J., Davis J., Zhu D., Wang Y., Pan C. and Zhang J. (2007). Identification of novel proteins associated with both alpha-synuclein and DJ-1. Mol. Cell. Proteomics 6, 845-859. 10.1074/mcp.M600182-MCP200 [DOI] [PubMed] [Google Scholar]
- Johenning F. W., Wenk M. R., Uhlén P., Degray B., Lee E., De Camilli P. and Ehrlich B. E. (2004). InsP3-mediated intracellular calcium signalling is altered by expression of synaptojanin-1. Biochem. J. 382, 687-694. 10.1042/BJ20040418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson G. V. W. and Foley V. G. (1993). Calpain-mediated proteolysis of microtubule-associated protein 2 (MAP-2) is inhibited by phosphorylation by cAMP-dependent protein kinase, but not by Ca2+/calmodulin-dependent protein kinase II. J. Neurosci. Res. 34, 642-647. 10.1002/jnr.490340607 [DOI] [PubMed] [Google Scholar]
- Julien J.-P. and Mushynski W. E. (1998). Neurofilaments in health and disease. Prog. Nucleic Acid Res. Mol. Biol. 61, 1-23. 10.1016/S0079-6603(08)60823-5 [DOI] [PubMed] [Google Scholar]
- Kawasaki H., Nakayama S. and Kretsinger R. H. (1998). Classification and evolution of EF-hand proteins. Biometals 11, 277-295. 10.1023/A:1009282307967 [DOI] [PubMed] [Google Scholar]
- Kayala K. M. N., Dickinson G. D., Minassian A., Walls K. C., Green K. N. and LaFerla F. M. (2012). Presenilin-null cells have altered two-pore calcium channel expression and lysosomal calcium: implications for lysosomal function. Brain Res. 1489, 8-16. 10.1016/j.brainres.2012.10.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaliq Z. M. and Bean B. P. (2010). Pacemaking in dopaminergic ventral tegmental area neurons: depolarizing drive from background and voltage-dependent sodium conductances. J. Neurosci. 30, 7401-7413. 10.1523/JNEUROSCI.0143-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieburtz K., Tilley B. C., Elm J. J., Babcock D., Hauser R., Ross G. W., Augustine A. H., Augustine E. U., Aminoff M. J., Bodis-Wollner I. G. et al. (2015). Effect of creatine monohydrate on clinical progression in patients with Parkinson disease: a randomized clinical trial. JAMA 313, 584-593. 10.1001/jama.2015.120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi A., Takeda A., Kimpara T., Nakagawa M., Kawashima R., Sugiura M., Kinomura S., Fukuda H., Chida K., Okita N. et al. (2001). Hypoperfusion in the supplementary motor area, dorsolateral prefrontal cortex and insular cortex in Parkinson's disease. J. Neurol. Sci. 193, 29-36. 10.1097/01.WCB.0000093326.88757.49 [DOI] [PubMed] [Google Scholar]
- Kilpatrick B. S., Eden E. R., Schapira A. H., Futter C. E. and Patel S. (2013). Direct mobilisation of lysosomal Ca2+ triggers complex Ca2+ signals. J. Cell Sci. 126, 60-66. 10.1242/jcs.118836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick B. S., Magalhaes J., Beavan M. S., McNeill A., Gegg M. E., Cleeter M. W. J., Bloor-Young D., Churchill G. C., Duchen M. R., Schapira A. H. et al. (2016). Endoplasmic reticulum and lysosomal Ca(2)(+) stores are remodelled in GBA1-linked Parkinson disease patient fibroblasts. Cell Calcium 59, 12-20. 10.1016/j.ceca.2015.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C. H., Kim J. H., Lee J., Hsu C. Y. and Ahn Y. S. (2003). Thiol antioxidant reversal of pyrrolidine dithiocarbamate-induced reciprocal regulation of AP-1 and NF-kappaB. Biol. Chem. 384, 143-150. 10.1515/BC.2003.015 [DOI] [PubMed] [Google Scholar]
- Klionsky D. J., Codogno P., Cuervo A. M., Deretic V., Elazar Z., Fueyo-Margareto J., Gewirtz D. A., Kroemer G., Levine B., Mizushima N. et al. (2010). A comprehensive glossary of autophagy-related molecules and processes. Autophagy 6, 438-448. 10.4161/auto.6.4.12244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko J., Humbert S., Bronson R. T., Takahashi S., Kulkarni A. B., Li E. and Tsai L. H. (2001). p35 and p39 are essential for cyclin-dependent kinase 5 function during neurodevelopment. J. Neurosci. 21, 6758-6771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korvatska O., Strand N. S., Berndt J. D., Strovas T., Chen D.-H., Leverenz J. B., Kiianitsa K., Mata I. F., Karakoc E., Greenup J. L. et al. (2013). Altered splicing of ATP6AP2 causes X-linked parkinsonism with spasticity (XPDS). Hum. Mol. Genet. 22, 3259-3268. 10.1093/hmg/ddt180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koschak A., Reimer D., Huber I., Grabner M., Glossmann H., Engel J. and Striessnig J. (2001). alpha 1D (Cav1.3) subunits can form l-type Ca2+ channels activating at negative voltages. J. Biol. Chem. 276, 22100-22106. 10.1074/jbc.M101469200 [DOI] [PubMed] [Google Scholar]
- Krebs C. E., Karkheiran S., Powell J. C., Cao M., Makarov V., Darvish H., Di Paolo G., Walker R. H., Shahidi G. A., Buxbaum J. D. et al. (2013). The Sac1 domain of SYNJ1 identified mutated in a family with early-onset progressive Parkinsonism with generalized seizures. Hum. Mutat. 34, 1200-1207. 10.1002/humu.22372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretsinger R. H. and Nockolds C. E. (1973). Carp muscle calcium-binding protein. II. Structure determination and general description. J. Biol. Chem. 248, 3313-3326. [PubMed] [Google Scholar]
- Krols M., Bultynck G. and Janssens S. (2016). ER-Mitochondria contact sites: a new regulator of cellular calcium flux comes into play. J. Cell Biol. 214, 367-370. 10.1083/jcb.201607124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar K. R., Djarmati-Westenberger A. and Grünewald A. (2011). Genetics of Parkinson's disease. Semin. Neurol. 31, 433-440. 10.1055/s-0031-1299782 [DOI] [PubMed] [Google Scholar]
- Lanoue A. C., Blatt G. J. and Soghomonian J.-J. (2013). Decreased parvalbumin mRNA expression in dorsolateral prefrontal cortex in Parkinson's disease. Brain Res. 1531, 37-47. 10.1016/j.brainres.2013.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie B. and Parent A. (1991). Dopaminergic neurons expressing calbindin in normal and parkinsonian monkeys. Neuroreport 2, 601-604. 10.1097/00001756-199110000-00012 [DOI] [PubMed] [Google Scholar]
- Ledeen R. W. and Wu G. (2015). The multi-tasked life of GM1 ganglioside, a true factotum of nature. Trends Biochem. Sci. 40, 407-418. 10.1016/j.tibs.2015.04.005 [DOI] [PubMed] [Google Scholar]
- Lee C. H., Della N. G., Chew C. E. and Zack D. J. (1996). Rin, a neuron-specific and calmodulin-binding small G-protein, and Rit define a novel subfamily of ras proteins. J. Neurosci. 16, 6784-6794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D., Lee S.-Y., Lee E.-N., Chang C.-S. and Paik S. R. (2002). alpha-Synuclein exhibits competitive interaction between calmodulin and synthetic membranes. J. Neurochem. 82, 1007-1017. 10.1046/j.1471-4159.2002.01024.x [DOI] [PubMed] [Google Scholar]
- Lee S. Y., Wenk M. R., Kim Y., Nairn A. C. and De Camilli P. (2004). Regulation of synaptojanin 1 by cyclin-dependent kinase 5 at synapses. Proc. Natl. Acad. Sci. USA 101, 546-551. 10.1073/pnas.0307813100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y.-C., Lin C.-H., Wu R.-M., Lin J.-W., Chang C.-H. and Lai M.-S. (2014). Antihypertensive agents and risk of Parkinson's disease: a nationwide cohort study. PLoS ONE 9, e98961 10.1371/journal.pone.0098961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.-H., McBrayer M. K., Wolfe D. M., Haslett L. J., Kumar A., Sato Y., Lie P. P. Y., Mohan P., Coffey E. E., Kompella U. et al. (2015). Presenilin 1 maintains lysosomal Ca(2+) homeostasis via TRPML1 by regulating vATPase-mediated lysosome acidification. Cell Rep. 12, 1430-1444. 10.1016/j.celrep.2015.07.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees A. J., Hardy J. and Revesz T. (2009). Parkinson's disease. Lancet 373, 2055-2066. 10.1016/S0140-6736(09)60492-X [DOI] [PubMed] [Google Scholar]
- Lehmann I. T., Bobrovskaya L., Gordon S. L., Dunkley P. R. and Dickson P. W. (2006). Differential regulation of the human tyrosine hydroxylase isoforms via hierarchical phosphorylation. J. Biol. Chem. 281, 17644-17651. 10.1074/jbc.M512194200 [DOI] [PubMed] [Google Scholar]
- Lemasters J. J., Theruvath T. P., Zhong Z. and Nieminen A.-L. (2009). Mitochondrial calcium and the permeability transition in cell death. Biochim. Biophys. Acta 1787, 1395-1401. 10.1016/j.bbabio.2009.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesage S., Bras J., Cormier-Dequaire F., Condroyer C., Nicolas A., Darwent L., Guerreiro R., Majounie E., Federoff M., Heutink P. et al. (2015). Loss-of-function mutations in RAB39B are associated with typical early-onset Parkinson disease. Neurol Genet. 1, e9 10.1212/NXG.0000000000000009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewit-Bentley A. and Réty S. (2000). EF-hand calcium-binding proteins. Curr. Opin. Struct. Biol. 10, 637-643. 10.1016/S0959-440X(00)00142-1 [DOI] [PubMed] [Google Scholar]
- Lin M. K. and Farrer M. J. (2014). Genetics and genomics of Parkinson's disease. Genome Med. 6, 48 10.1186/gm566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X., Parisiadou L., Sgobio C., Liu G., Yu J., Sun L., Shim H., Gu X.-L., Luo J., Long C.-X. et al. (2012). Conditional expression of Parkinson's disease-related mutant alpha-synuclein in the midbrain dopaminergic neurons causes progressive neurodegeneration and degradation of transcription factor nuclear receptor related 1. J. Neurosci. 32, 9248-9264. 10.1523/JNEUROSCI.1731-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindholm D., Wootz H. and Korhonen L. (2006). ER stress and neurodegenerative diseases. Cell Death Differ. 13, 385-392. 10.1038/sj.cdd.4401778 [DOI] [PubMed] [Google Scholar]
- Lipscombe D., Helton T. D. and Xu W. (2004). L-type calcium channels: the low down. J. Neurophysiol. 92, 2633-2641. 10.1152/jn.00486.2004 [DOI] [PubMed] [Google Scholar]
- Lloyd-Evans E., Morgan A. J., He X., Smith D. A., Elliot-Smith E., Sillence D. J., Churchill G. C., Schuchman E. H., Galione A. and Platt F. M. (2008). Niemann-Pick disease type C1 is a sphingosine storage disease that causes deregulation of lysosomal calcium. Nat. Med. 14, 1247-1255. 10.1038/nm.1876 [DOI] [PubMed] [Google Scholar]
- Luo J., Sun L., Lin X., Liu G., Yu J., Parisiadou L., Xie C., Ding J. and Cai H. (2014). A calcineurin- and NFAT-dependent pathway is involved in alpha-synuclein-induced degeneration of midbrain dopaminergic neurons. Hum. Mol. Genet. 23, 6567-6574. 10.1093/hmg/ddu377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzio J. P., Rous B. A., Bright N. A., Pryor P. R., Mullock B. M. and Piper R. C. (2000). Lysosome-endosome fusion and lysosome biogenesis. J. Cell Sci. 113, 1515-1524. [DOI] [PubMed] [Google Scholar]
- Luzio J. P., Bright N. A. and Pryor P. R. (2007). The role of calcium and other ions in sorting and delivery in the late endocytic pathway. Biochem. Soc. Trans. 35, 1088-1091. 10.1042/BST0351088 [DOI] [PubMed] [Google Scholar]
- Lynch-Day M. A., Mao K., Wang K., Zhao M. and Klionsky D. J. (2012). The role of autophagy in Parkinson's disease. Cold Spring Harb. Perspect. Med. 2, a009357 10.1101/cshperspect.a009357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marambaud P., Dreses-Werringloer U. and Vingtdeux V. (2009). Calcium signaling in neurodegeneration. Mol. Neurodegener. 4, 20 10.1186/1750-1326-4-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchi S., Patergnani S. and Pinton P. (2014). The endoplasmic reticulum-mitochondria connection: one touch, multiple functions. Biochim. Biophys. Acta 1837, 461-469. 10.1016/j.bbabio.2013.10.015 [DOI] [PubMed] [Google Scholar]
- Marras C. and Tanner C. M. (2004). Epidemiology of Parkinson's Disease. New York, NY: McGraw Hill. [Google Scholar]
- Martí M. J., Tolosa E. and Campdelacreu J. (2003). Clinical overview of the synucleinopathies. Mov. Disord. 18 Suppl. 6, 21-27. 10.1002/mds.10559 [DOI] [PubMed] [Google Scholar]
- Martin I., Dawson V. L. and Dawson T. M. (2011). Recent advances in the genetics of Parkinson's disease. Annu. Rev. Genomics Hum. Genet. 12, 301-325. 10.1146/annurev-genom-082410-101440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin Z. S., Neugebauer V., Dineley K. T., Kayed R., Zhang W., Reese L. C. and Taglialatela G. (2012). alpha-Synuclein oligomers oppose long-term potentiation and impair memory through a calcineurin-dependent mechanism: relevance to human synucleopathic diseases. J. Neurochem. 120, 440-452. 10.1111/j.1471-4159.2011.07576.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez J., Moeller I., Erdjument-Bromage H., Tempst P. and Lauring B. (2003). Parkinson's disease-associated alpha-synuclein is a calmodulin substrate. J. Biol. Chem. 278, 17379-17387. 10.1074/jbc.M209020200 [DOI] [PubMed] [Google Scholar]
- Matsui H., Sato F., Sato S., Koike M., Taruno Y., Saiki S., Funayama M., Ito H., Taniguchi Y., Uemura N. et al. (2013). ATP13A2 deficiency induces a decrease in cathepsin D activity, fingerprint-like inclusion body formation, and selective degeneration of dopaminergic neurons. FEBS Lett. 587, 1316-1325. 10.1016/j.febslet.2013.02.046 [DOI] [PubMed] [Google Scholar]
- McBrayer M. K. and Nixon R. A. (2013). Lysosome and calcium dysregulation in Alzheimer's disease: partners in crime. Biochem. Soc. Trans. 41, 1495-1502. 10.1042/BST20130201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack J. G. and Denton R. M. (1990). The role of mitochondrial Ca2+ transport and matrix Ca2+ in signal transduction in mammalian tissues. Biochim. Biophys. Acta 1018, 287-291. 10.1016/0005-2728(90)90269-A [DOI] [PubMed] [Google Scholar]
- McKee A. C., Kosik K. S., Kennedy M. B. and Kowall N. W. (1990). Hippocampal neurons predisposed to neurofibrillary tangle formation are enriched in type II calcium/calmodulin-dependent protein kinase. J. Neuropathol. Exp. Neurol. 49, 49-63. 10.1097/00005072-199001000-00006 [DOI] [PubMed] [Google Scholar]
- Meador W. E., Means A. R. and Quiocho F. A. (1992). Target enzyme recognition by calmodulin: 2.4 A structure of a calmodulin-peptide complex. Science 257, 1251-1255. 10.1126/science.1519061 [DOI] [PubMed] [Google Scholar]
- Meador W. E., Means A. R. and Quiocho F. A. (1993). Modulation of calmodulin plasticity in molecular recognition on the basis of x-ray structures. Science 262, 1718-1721. 10.1126/science.8259515 [DOI] [PubMed] [Google Scholar]
- Mehta Z. B., Fine N., Pullen T. J., Cane M. C., Hu M., Chabosseau P., Meur G., Velayos-Baeza A., Monaco A. P., Marselli L. et al. (2016). Changes in the expression of the type 2 diabetes-associated gene VPS13C in the beta-cell are associated with glucose intolerance in humans and mice. Am. J. Physiol. Endocrinol. Metab. 311, E488-E507. 10.1152/ajpendo.00074.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercado G., Castillo V., Vidal R. and Hetz C. (2015). ER proteostasis disturbances in Parkinson's disease: novel insights. Front. Aging Neurosci. 7, 39 10.3389/fnagi.2015.00039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael D. J., Cai H., Xiong W., Ouyang J. and Chow R. H. (2006). Mechanisms of peptide hormone secretion. Trends Endocrinol. Metab. 17, 408-415. 10.1016/j.tem.2006.10.011 [DOI] [PubMed] [Google Scholar]
- Mignogna M. L., Giannandrea M., Gurgone A., Fanelli F., Raimondi F., Mapelli L., Bassani S., Fang H., Van Anken E., Alessio M. et al. (2015). The intellectual disability protein RAB39B selectively regulates GluA2 trafficking to determine synaptic AMPAR composition. Nat. Commun. 6, 6504 10.1038/ncomms7504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milovanovic D., Platen M., Junius M., Diederichsen U., Schaap I. A. T., Honigmann A., Jahn R. and van den Bogaart G. (2016). Calcium Promotes the Formation of Syntaxin 1 Mesoscale Domains through Phosphatidylinositol 4,5-Bisphosphate. J. Biol. Chem. 291, 7868-7876. 10.1074/jbc.M116.716225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishima T., Fujiwara T., Sanada M., Kofuji T., Kanai-Azuma M. and Akagawa K. (2014). Syntaxin 1B, but not Syntaxin 1A, is necessary for the regulation of synaptic vesicle exocytosis and of the readily releasable pool at central synapses. PLoS ONE 9, e90004 10.1371/journal.pone.0090004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuta I., Tsunoda T., Satake W., Nakabayashi Y., Watanabe M., Takeda A., Hasegawa K., Nakashima K., Yamamoto M., Hattori N. et al. (2008). Calbindin 1, fibroblast growth factor 20, and alpha-synuclein in sporadic Parkinson's disease. Hum. Genet. 124, 89-94. 10.1007/s00439-008-0525-5 [DOI] [PubMed] [Google Scholar]
- Mosharov E. V., Larsen K. E., Kanter E., Phillips K. A., Wilson K., Schmitz Y., Krantz D. E., Kobayashi K., Edwards R. H. and Sulzer D. (2009). Interplay between cytosolic dopamine, calcium, and alpha-synuclein causes selective death of substantia nigra neurons. Neuron 62, 218-229. 10.1016/j.neuron.2009.01.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouatt-Prigent A., Agid Y. and Hirsch E. C. (1994). Does the calcium binding protein calretinin protect dopaminergic neurons against degeneration in Parkinson's disease? Brain Res. 668, 62-70. 10.1016/0006-8993(94)90511-8 [DOI] [PubMed] [Google Scholar]
- Mouatt-Prigent A., Karlsson J.-O., Yelnik J., Agid Y. and Hirsch E. C. (2000). Calpastatin immunoreactivity in the monkey and human brain of control subjects and patients with Parkinson's disease. J. Comp. Neurol. 419, 175-192. [DOI] [PubMed] [Google Scholar]
- Moussaud S., Jones D. R., Moussaud-Lamodiere E. L., Delenclos M., Ross O. A. and McLean P. J. (2014). Alpha-synuclein and tau: teammates in neurodegeneration? Mol. Neurodegener. 9, 43 10.1186/1750-1326-9-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu T.-W., Fowler D. M. and Kelly J. W. (2008). Partial restoration of mutant enzyme homeostasis in three distinct lysosomal storage disease cell lines by altering calcium homeostasis. PLoS Biol. 6, e26 10.1371/journal.pbio.0060026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee A., Morales-Scheihing D., Gonzalez-Romero D., Green K., Taglialatela G. and Soto C. (2010). Calcineurin inhibition at the clinical phase of prion disease reduces neurodegeneration, improves behavioral alterations and increases animal survival. PLoS Pathog. 6, e1001138 10.1371/journal.ppat.1001138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller A., Kukley M., Stausberg P., Beck H., Muller W. and Dietrich D. (2005). Endogenous Ca2+ buffer concentration and Ca2+ microdomains in hippocampal neurons. J. Neurosci. 25, 558-565. 10.1523/JNEUROSCI.3799-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata K., Yoshino Y., Tsuruma K., Moriguchi S., Oyagi A., Tanaka H., Ishisaka M., Shimazawa M., Fukunaga K. and Hara H. (2015). The extracellular fragment of GPNMB (Glycoprotein nonmelanosoma protein B, osteoactivin) improves memory and increases hippocampal GluA1 levels in mice. J. Neurochem. 132, 583-594. 10.1111/jnc.13010 [DOI] [PubMed] [Google Scholar]
- Nalls M. A., Pankratz N., Lill C. M., Do C. B., Hernandez D. G., Saad M., DeStefano A. L., Kara E., Bras J., Sharma M. et al. (2014). Large-scale meta-analysis of genome-wide association data identifies six new risk loci for Parkinson's disease. Nat. Genet. 46, 989-993. 10.1038/ng.3043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath S., Goodwin J., Engelborghs Y. and Pountney D. L. (2011). Raised calcium promotes alpha-synuclein aggregate formation. Mol. Cell. Neurosci. 46, 516-526. 10.1016/j.mcn.2010.12.004 [DOI] [PubMed] [Google Scholar]
- Navaroli D. M., Stevens Z. H., Uzelac Z., Gabriel L., King M. J., Lifshitz L. M., Sitte H. H. and Melikian H. E. (2011). The plasma membrane-associated GTPase Rin interacts with the dopamine transporter and is required for protein kinase C-regulated dopamine transporter trafficking. J. Neurosci. 31, 13758-13770. 10.1523/JNEUROSCI.2649-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak L. and De R. K. (2007). An algorithm for modularization of MAPK and calcium signaling pathways: comparative analysis among different species. J. Biomed. Inform. 40, 726-749. 10.1016/j.jbi.2007.05.007 [DOI] [PubMed] [Google Scholar]
- Nelson O., Supnet C., Tolia A., Horre K., De Strooper B. and Bezprozvanny I. (2011). Mutagenesis mapping of the Presenilin 1 calcium leak conductance pore. J. Biol. Chem. 286, 22339-22347. 10.1074/jbc.M111.243063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhoff H., Neu A., Liss B. and Roeper J. (2002). I(h) channels contribute to the different functional properties of identified dopaminergic subpopulations in the midbrain. J. Neurosci. 22, 1290-1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi A., Bibb J. A., Matsuyama S., Hamada M., Higashi H., Nairn A. C. and Greengard P. (2002). Regulation of DARPP-32 dephosphorylation at PKA- and Cdk5-sites by NMDA and AMPA receptors: distinct roles of calcineurin and protein phosphatase-2A. J. Neurochem. 81, 832-841. 10.1046/j.1471-4159.2002.00876.x [DOI] [PubMed] [Google Scholar]
- Nixon R. A. (2013). The role of autophagy in neurodegenerative disease. Nat. Med. 19, 983-997. 10.1038/nm.3232 [DOI] [PubMed] [Google Scholar]
- Nuber S. and Selkoe D. J. (2016). Caspase-1 clipping causes complications for alpha-synuclein. Proc. Natl. Acad. Sci. USA 113, 9958-9960. 10.1073/pnas.1610993113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuscher B., Kamp F., Mehnert T., Odoy S., Haass C., Kahle P. J. and Beyer K. (2004). alpha-synuclein has a high affinity for packing defects in a bilayer membrane - a thermodynamics study. J. Biol. Chem. 279, 21966-21975. 10.1074/jbc.M401076200 [DOI] [PubMed] [Google Scholar]
- Ohshima T., Ward J. M., Huh C. G., Longenecker G., Veeranna G., Pant H. C., Brady R. O., Martin L. J. and Kulkarni A. B. (1996). Targeted disruption of the cyclin-dependent kinase 5 gene results in abnormal corticogenesis, neuronal pathology and perinatal death. Proc. Natl. Acad. Sci. USA 93, 11173-11178. 10.1073/pnas.93.20.11173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olah J., Vincze O., Virok D., Simon D., Bozso Z., Tokesi N., Horvath I., Hlavanda E., Kovacs J., Magyar A. et al. (2011). Interactions of pathological hallmark proteins: tubulin polymerization promoting protein/p25, beta-amyloid, and alpha-synuclein. J. Biol. Chem. 286, 34088-34100. 10.1074/jbc.M111.243907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson P. A., Tkatch T., Hernandez-Lopez S., Ulrich S., Ilijic E., Mugnaini E., Zhang H., Bezprozvanny I. and Surmeier D. J. (2005). G-protein-coupled receptor modulation of striatal CaV1.3 L-type Ca2+ channels is dependent on a Shank-binding domain. J. Neurosci. 25, 1050-1062. 10.1523/JNEUROSCI.3327-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omura T., Kaneko M., Okuma Y., Matsubara K. and Nomura Y. (2013). Endoplasmic reticulum stress and Parkinson's disease: the role of HRD1 in averting apoptosis in neurodegenerative disease. Oxid. Med. Cell Longev. 2013, 239854 10.1155/2013/239854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortner N. J. and Striessnig J. (2016). L-type calcium channels as drug targets in CNS disorders. Channels 10, 7-13. 10.1080/19336950.2015.1048936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oslowski C. M., Zhou Q., Schafer C., Kirber M. and Bolotina V. (2013). New causal relationship between PLA2g6, store-operated Ca2+ entry, refilling of Ca2+ stores and ER stress in mouse embryonic fibroblasts. FASEB J. 27 Suppl. 1198.2. [Google Scholar]
- Ottolini D., Cali T., Negro A. and Brini M. (2013). The Parkinson disease-related protein DJ-1 counteracts mitochondrial impairment induced by the tumour suppressor protein p53 by enhancing endoplasmic reticulum-mitochondria tethering. Hum. Mol. Genet. 22, 2152-2168. 10.1093/hmg/ddt068 [DOI] [PubMed] [Google Scholar]
- Palop J. J., Jones B., Kekonius L., Chin J., Yu G.-Q., Raber J., Masliah E. and Mucke L. (2003). Neuronal depletion of calcium-dependent proteins in the dentate gyrus is tightly linked to Alzheimer's disease-related cognitive deficits. Proc. Natl. Acad. Sci. USA 100, 9572-9577. 10.1073/pnas.1133381100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankratz N., Beecham G. W., DeStefano A. L., Dawson T. M., Doheny K. F., Factor S. A., Hamza T. H., Hung A. Y., Hyman B. T., Ivinson A. J. et al. (2012). Meta-analysis of Parkinson's disease: identification of a novel locus, RIT2. Ann. Neurol. 71, 370-384. 10.1002/ana.22687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh A. B. (2008). Ca2+ microdomains near plasma membrane Ca2+ channels: impact on cell function. J. Physiol. 586, 3043-3054. 10.1113/jphysiol.2008.153460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J.-S., Mehta P., Cooper A. A., Veivers D., Heimbach A., Stiller B., Kubisch C., Fung V. S., Krainc D., Mackay-Sim A. et al. (2011). Pathogenic effects of novel mutations in the P-type ATPase ATP13A2 (PARK9) causing Kufor-Rakeb syndrome, a form of early-onset parkinsonism. Hum. Mutat. 32, 956-964. 10.1002/humu.21527 [DOI] [PubMed] [Google Scholar]
- Pasternak B., Svanstrom H., Nielsen N. M., Fugger L., Melbye M. and Hviid A. (2012). Use of calcium channel blockers and Parkinson's disease. Am. J. Epidemiol. 175, 627-635. 10.1093/aje/kwr362 [DOI] [PubMed] [Google Scholar]
- Patel S. and Docampo R. (2010). Acidic calcium stores open for business: expanding the potential for intracellular Ca2+ signaling. Trends Cell Biol. 20, 277-286. 10.1016/j.tcb.2010.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S. and Muallem S. (2011). Acidic Ca(2+) stores come to the fore. Cell Calcium 50, 109-112. 10.1016/j.ceca.2011.03.009 [DOI] [PubMed] [Google Scholar]
- Pathak T., Agrawal T., Richhariya S., Sadaf S. and Hasan G. (2015). Store-operated calcium entry through orai is required for transcriptional maturation of the flight circuit in Drosophila. J. Neurosci. 35, 13784-13799. 10.1523/JNEUROSCI.1680-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perier C. and Vila M. (2012). Mitochondrial biology and Parkinson's disease. Cold Spring Harb. Perspect. Med. 2, a009332 10.1101/cshperspect.a009332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen O. H., Michalak M. and Verkhratsky A. (2005). Calcium signalling: past, present and future. Cell Calcium 38, 161-169. 10.1016/j.ceca.2005.06.023 [DOI] [PubMed] [Google Scholar]
- Phillips M. J. and Voeltz G. K. (2016). Structure and function of ER membrane contact sites with other organelles. Nat. Rev. Mol. Cell Biol. 17, 69-82. 10.1038/nrm.2015.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickrell A. M. and Youle R. J. (2015). The roles of PINK1, parkin, and mitochondrial fidelity in Parkinson's disease. Neuron 85, 257-273. 10.1016/j.neuron.2014.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitt S. J., Funnell T. M., Sitsapesan M., Venturi E., Rietdorf K., Ruas M., Ganesan A., Gosain R., Churchill G. C., Zhu M. X. et al. (2010). TPC2 is a novel NAADP-sensitive Ca2+ release channel, operating as a dual sensor of luminal pH and Ca2+. J. Biol. Chem. 285, 35039-35046. 10.1074/jbc.M110.156927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polymeropoulos M. H., Lavedan C., Leroy E., Ide S. E., Dehejia A., Dutra A., Pike B., Root H., Rubenstein J., Boyer R. et al. (1997). Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science 276, 2045-2047. 10.1126/science.276.5321.2045 [DOI] [PubMed] [Google Scholar]
- Puopolo M., Raviola E. and Bean B. P. (2007). Roles of subthreshold calcium current and sodium current in spontaneous firing of mouse midbrain dopamine neurons. J. Neurosci. 27, 645-656. 10.1523/JNEUROSCI.4341-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu D., Rashidian J., Mount M. P., Aleyasin H., Parsanejad M., Lira A., Haque E., Zhang Y., Callaghan S., Daigle M. et al. (2007). Role of Cdk5-mediated phosphorylation of Prx2 in MPTP toxicity and Parkinson's disease. Neuron 55, 37-52. 10.1016/j.neuron.2007.05.033 [DOI] [PubMed] [Google Scholar]
- Ramirez A., Heimbach A., Gründemann J., Stiller B., Hampshire D., Cid L. P., Goebel I., Mubaidin A. F., Wriekat A.-L., Roeper J. et al. (2006). Hereditary parkinsonism with dementia is caused by mutations in ATP13A2, encoding a lysosomal type 5 P-type ATPase. Nat. Genet. 38, 1184-1191. 10.1038/ng1884 [DOI] [PubMed] [Google Scholar]
- Ramonet D., Podhajska A., Stafa K., Sonnay S., Trancikova A., Tsika E., Pletnikova O., Troncoso J. C., Glauser L. and Moore D. J. (2012). PARK9-associated ATP13A2 localizes to intracellular acidic vesicles and regulates cation homeostasis and neuronal integrity. Hum. Mol. Genet. 21, 1725-1743. 10.1093/hmg/ddr606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rcom-H'cheo-Gauthier A. N., Davis A., Meedeniya A. C. B. and Pountney D. L. (2016). Alpha-synuclein aggregates are excluded from calbindin-D28k-positive neurons in dementia with Lewy bodies and a unilateral rotenone mouse model. Mol. Cell. Neurosci. 77, 65-75. 10.1016/j.mcn.2016.10.003 [DOI] [PubMed] [Google Scholar]
- Reese L. C., Zhang W. R., Dineley K. T., Kayed R. and Taglialatela G. (2008). Selective induction of calcineurin activity and signaling by oligomeric amyloid beta. Aging Cell 7, 824-835. 10.1111/j.1474-9726.2008.00434.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendón W. O., Martínez-Alonso E., Tomás M., Martínez-Martínez N. and Martínez-Menárguez J. A. (2013). Golgi fragmentation is Rab and SNARE dependent in cellular models of Parkinson's disease. Histochem. Cell Biol. 139, 671-684. 10.1007/s00418-012-1059-4 [DOI] [PubMed] [Google Scholar]
- Ritz B., Rhodes S. L., Qian L., Schernhammer E., Olsen J. H. and Friis S. (2010). L-type calcium channel blockers and Parkinson disease in Denmark. Ann. Neurol. 67, 600-606. 10.1002/ana.21937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivero-Rios P., Gomez-Suaga P., Fdez E. and Hilfiker S. (2014). Upstream deregulation of calcium signaling in Parkinson's disease. Front. Mol. Neurosci. 7, 53 10.3389/fnmol.2014.00053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzuto R. and Pozzan T. (2006). Microdomains of intracellular Ca2+: molecular determinants and functional consequences. Physiol. Rev. 86, 369-408. 10.1152/physrev.00004.2005 [DOI] [PubMed] [Google Scholar]
- Rizzuto R., Marchi S., Bonora M., Aguiari P., Bononi A., De Stefani D., Giorgi C., Leo S., Rimessi A., Siviero R. et al. (2009). Ca(2+) transfer from the ER to mitochondria: when, how and why. Biochim. Biophys. Acta 1787, 1342-1351. 10.1016/j.bbabio.2009.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzuto R., De Stefani D., Raffaello A. and Mammucari C. (2012). Mitochondria as sensors and regulators of calcium signalling. Nat. Rev. Mol. Cell Biol. 13, 566-578. 10.1038/nrm3412 [DOI] [PubMed] [Google Scholar]
- Rosales J. L., Nodwell M. J., Johnston R. N. and Lee K.-Y. (2000). Cdk5/p25(nck5a) interaction with synaptic proteins in bovine brain. J. Cell. Biochem. 78, 151-159. [DOI] [PubMed] [Google Scholar]
- Rozkalne A., Hyman B. T. and Spires-Jones T. L. (2011). Calcineurin inhibition with FK506 ameliorates dendritic spine density deficits in plaque-bearing Alzheimer model mice. Neurobiol. Dis. 41, 650-654. 10.1016/j.nbd.2010.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan B. J., Hoek S., Fon E. A. and Wade-Martins R. (2015). Mitochondrial dysfunction and mitophagy in Parkinson's: from familial to sporadic disease. Trends Biochem. Sci. 40, 200-210. 10.1016/j.tibs.2015.02.003 [DOI] [PubMed] [Google Scholar]
- Saad M., Lesage S., Saint-Pierre A., Corvol J.-C., Zelenika D., Lambert J.-C., Vidailhet M., Mellick G. D., Lohmann E., Durif F. et al. (2011). Genome-wide association study confirms BST1 and suggests a locus on 12q24 as the risk loci for Parkinson's disease in the European population. Hum. Mol. Genet. 20, 615-627. 10.1093/hmg/ddq497 [DOI] [PubMed] [Google Scholar]
- Sakamoto K., Karelina K. and Obrietan K. (2011). CREB: a multifaceted regulator of neuronal plasticity and protection. J. Neurochem. 116, 1-9. 10.1111/j.1471-4159.2010.07080.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samantaray S., Ray S. K. and Banik N. L. (2008). Calpain as a potential therapeutic target in Parkinson's disease. CNS Neurol. Disord. Drug Targets 7, 305-312. 10.2174/187152708784936680 [DOI] [PubMed] [Google Scholar]
- Santoro L., Breedveld G. J., Manganelli F., Iodice R., Pisciotta C., Nolano M., Punzo F., Quarantelli M., Pappatà S., Di Fonzo A. et al. (2011). Novel ATP13A2 (PARK9) homozygous mutation in a family with marked phenotype variability. Neurogenetics 12, 33-39. 10.1007/s10048-010-0259-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasabe J., Chiba T., Yamada M., Okamoto K., Nishimoto I., Matsuoka M. and Aiso S. (2007). D-Serine is a key determinant of glutamate toxicity in amyotrophic lateral sclerosis. EMBO J. 26, 4149-4159. 10.1038/sj.emboj.7601840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schapira A. H. V. (2013). Calcium dysregulation in Parkinson's disease. Brain 136, 2015-2016. 10.1093/brain/awt180 [DOI] [PubMed] [Google Scholar]
- Schapira A. H. V., Olanow C. W., Greenamyre J. T. and Bezard E. (2014). Slowing of neurodegeneration in Parkinson's disease and Huntington's disease: future therapeutic perspectives. Lancet 384, 545-555. 10.1016/S0140-6736(14)61010-2 [DOI] [PubMed] [Google Scholar]
- Schneider J. S. (1998). GM1 ganglioside in the treatment of Parkinson's disease. Ann. N. Y. Acad. Sci. 845, 363-373. 10.1111/j.1749-6632.1998.tb09688.x [DOI] [PubMed] [Google Scholar]
- Schneider J. S., Sendek S., Daskalakis C. and Cambi F. (2010). GM1 ganglioside in Parkinson's disease: results of a five year open study. J. Neurol. Sci. 292, 45-51. 10.1016/j.jns.2010.02.009 [DOI] [PubMed] [Google Scholar]
- Schneider J. S., Gollomp S. M., Sendek S., Colcher A., Cambi F. and Du W. (2013). A randomized, controlled, delayed start trial of GM1 ganglioside in treated Parkinson's disease patients. J. Neurol. Sci. 324, 140-148. 10.1016/j.jns.2012.10.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder B., Wrocklage C., Pan C., Jäger R., Kösters B., Schäfer H., Elsässer H.-P., Mann M. and Hasilik A. (2007). Integral and associated lysosomal membrane proteins. Traffic 8, 1676-1686. 10.1111/j.1600-0854.2007.00643.x [DOI] [PubMed] [Google Scholar]
- Shen X., Valencia C. A., Szostak J., Dong B. and Liu R. (2005). Scanning the human proteome for calmodulin-binding proteins. Proc. Natl. Acad. Sci. USA 102, 5969-5974. 10.1073/pnas.0407928102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng Z. H. and Cai Q. (2012). Mitochondrial transport in neurons: impact on synaptic homeostasis and neurodegeneration. Nat. Rev. Neurosci. 13, 77-93. 10.1038/nrn3156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi G.-X., Han J. and Andres D. A. (2005). Rin GTPase couples nerve growth factor signaling to p38 and b-Raf/ERK pathways to promote neuronal differentiation. J. Biol. Chem. 280, 37599-37609. 10.1074/jbc.M507364200 [DOI] [PubMed] [Google Scholar]
- Shulman J. M., De Jager P. L. and Feany M. B. (2011). Parkinson's disease: genetics and pathogenesis. Annu. Rev. Pathol. 6, 193-222. 10.1146/annurev-pathol-011110-130242 [DOI] [PubMed] [Google Scholar]
- Sidransky E., Nalls M. A., Aasly J. O., Aharon-Peretz J., Annesi G., Barbosa E. R., Bar-Shira A., Berg D., Bras J., Brice A. et al. (2009). Multicenter analysis of glucocerebrosidase mutations in Parkinson's disease. N. Engl. J. Med. 361, 1651-1661. 10.1056/NEJMoa0901281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simón-Sánchez J., Schulte C., Bras J. M., Sharma M., Gibbs J. R., Berg D., Paisan-Ruiz C., Lichtner P., Scholz S. W., Hernandez D. G. et al. (2009). Genome-wide association study reveals genetic risk underlying Parkinson's disease. Nat. Genet. 41, 1308-1312. 10.1038/ng.487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simuni T., and the Parkinson Study Group. (2013). Phase II safety, tolerability, and dose selection study of isradipine as a potential disease-modifying intervention in early Parkinson's disease (STEADY-PD). Mov. Disord. 28, 1823-1831. 10.1002/mds.25639 [DOI] [PubMed] [Google Scholar]
- Simuni T., Kieburtz K., Tilley B. J., Elm J., Ravina B., Babcock D., Emborg M., Hauser R., Kamp C., Morgan J. C. et al. (2015). Pioglitazone in early Parkinson's disease: a phase 2, multicentre, double-blind, randomised trial. Lancet Neurol. 14, 795-803. 10.1016/S1474-4422(15)00144-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton A. B., Farrer M., Johnson J., Singleton A., Hague S., Kachergus J., Hulihan M., Peuralinna T., Dutra A., Nussbaum R. et al. (2003). alpha-Synuclein locus triplication causes Parkinson's disease. Science 302, 841 10.1126/science.1090278 [DOI] [PubMed] [Google Scholar]
- Smani T., Zakharov S. I., Csutora P., Leno E., Trepakova E. S. and Bolotina V. M. (2004). A novel mechanism for the store-operated calcium influx pathway. Nat. Cell Biol. 6, 113-120. 10.1038/ncb1089 [DOI] [PubMed] [Google Scholar]
- Smith P. D., Crocker S. J., Jackson-Lewis V., Jordan-Sciutto K. L., Hayley S., Mount M. P., O'Hare M. J., Callaghan S., Slack R. S., Przedborski S. et al. (2003). Cyclin-dependent kinase 5 is a mediator of dopaminergic neuron loss in a mouse model of Parkinson's disease. Proc. Natl. Acad. Sci. USA 100, 13650-13655. 10.1073/pnas.2232515100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. D., Mount M. P., Shree R., Callaghan S., Slack R. S., Anisman H., Vincent I., Wang X., Mao Z. and Park D. S. (2006). Calpain-regulated p35/cdk5 plays a central role in dopaminergic neuron death through modulation of the transcription factor myocyte enhancer factor 2. J. Neurosci. 26, 440-447. 10.1523/JNEUROSCI.2875-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow B. J., Rolfe F. L., Lockhart M. M., Frampton C. M., O'Sullivan J. D., Fung V., Smith R. A. J., Murphy M. P. and Taylor K. M. (2010). A double-blind, placebo-controlled study to assess the mitochondria-targeted antioxidant MitoQ as a disease-modifying therapy in Parkinson's disease. Mov. Disord. 25, 1670-1674. 10.1002/mds.23148 [DOI] [PubMed] [Google Scholar]
- Soto-Ortolaza A. I., Behrouz B., Wider C., Vilariño-Güell C., Heckman M. G., Aasly J. O., Mark Gibson J., Lynch T., Jasinska-Myga B., Krygowska-Wajs A. et al. (2010). Calbindin-1 association and Parkinson's disease. Eur. J. Neurol. 17, 208-211. 10.1111/j.1468-1331.2009.02769.x [DOI] [PubMed] [Google Scholar]
- Sousa S. C., Maciel E. N., Vercesi A. E. and Castilho R. F. (2003). Ca2+-induced oxidative stress in brain mitochondria treated with the respiratory chain inhibitor rotenone. FEBS Lett. 543, 179-183. 10.1016/S0014-5793(03)00421-6 [DOI] [PubMed] [Google Scholar]
- Steinmetz M. O., Plüss C., Christen U., Wolpensinger B., Lustig A., Werner E. R., Wachter H., Engel A., Aebi U., Pfeilschifter J. et al. (1998). Rat GTP cyclohydrolase I is a homodecameric protein complex containing high-affinity calcium-binding sites. J. Mol. Biol. 279, 189-199. 10.1006/jmbi.1998.1649 [DOI] [PubMed] [Google Scholar]
- Striessnig J., Koschak A., Sinnegger-Brauns M. J., Hetzenauer A., Nguyen N. K., Busquet P., Pelster G. and Singewald N. (2006). Role of voltage-gated L-type Ca2+ channel isoforms for brain function. Biochem. Soc. Trans. 34, 903-909. 10.1042/BST0340903 [DOI] [PubMed] [Google Scholar]
- Südhof T. C. (2013). A molecular machine for neurotransmitter release: synaptotagmin and beyond. Nat. Med. 19, 1227-1231. 10.1038/nm.3338 [DOI] [PubMed] [Google Scholar]
- Surmeier D. J. and Schumacker P. T. (2013). Calcium, bioenergetics, and neuronal vulnerability in Parkinson's disease. J. Biol. Chem. 288, 10736-10741. 10.1074/jbc.R112.410530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier D. J., Guzman J. N., Sanchez-Padilla J. and Goldberg J. A. (2010). The origins of oxidant stress in Parkinson's disease and therapeutic strategies. Antioxid Redox Signal. 14, 1289-1301. 10.1089/ars.2010.3521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier D. J., Guzman J. N., Sanchez J. and Schumacker P. T. (2012). Physiological phenotype and vulnerability in Parkinson's disease. Cold Spring Harb. Perspect. Med. 2, a009290 10.1101/cshperspect.a009290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier D. J., Schumacker P. T., Guzman J. D., Ilijic E., Yang B. and Zampese E. (2016). Calcium and Parkinson's disease. Biochem. Biophys. Res. Commun. 483, 1013-1019. 10.1016/j.bbrc.2016.08.168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svennerholm L., Boström K., Jungbjer B. and Olsson L. (1994). Membrane lipids of adult human brain: lipid composition of frontal and temporal lobe in subjects of age 20 to 100 years. J. Neurochem. 63, 1802-1811. 10.1046/j.1471-4159.1994.63051802.x [DOI] [PubMed] [Google Scholar]
- Taghibiglou C., Martin H. G. S., Lai T. W., Cho T., Prasad S., Kojic L., Lu J., Liu Y., Lo E., Zhang S. et al. (2009). Role of NMDA receptor-dependent activation of SREBP1 in excitotoxic and ischemic neuronal injuries. Nat. Med. 15, 1399-1406. 10.1038/nm.2064 [DOI] [PubMed] [Google Scholar]
- Takahashi M., Iseki E. and Kosaka K. (2000). Cyclin-dependent kinase 5 (Cdk5) associated with Lewy bodies in diffuse Lewy body disease. Brain Res. 862, 253-256. 10.1016/S0006-8993(00)02086-2 [DOI] [PubMed] [Google Scholar]
- Tang T.-S., Tu H. P., Chan E. Y. W., Maximov A., Wang Z. N., Wellington C. L., Hayden M. R. and Bezprozvanny I. (2003). Huntingtin and Huntingtin-associated protein 1 influence neuronal calcium signaling mediated by inositol-(1,4,5) triphosphate receptor type 1. Neuron 39, 227-239. 10.1016/S0896-6273(03)00366-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teive H. A. G., Troiano A. R., Germiniani F. M. B. and Werneck L. C. (2004). Flunarizine and cinnarizine-induced parkinsonism: a historical and clinical analysis. Parkinsonism Relat. Disord. 10, 243-245. 10.1016/j.parkreldis.2003.12.004 [DOI] [PubMed] [Google Scholar]
- Thayer D. A., Jan Y. N. and Jan L. Y. (2013). Increased neuronal activity fragments the Golgi complex. Proc. Natl. Acad. Sci. USA 110, 1482-1487. 10.1073/pnas.1220978110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolia A. and De Strooper B. (2009). Structure and function of gamma-secretase. Semin. Cell Dev. Biol. 20, 211-218. 10.1016/j.semcdb.2008.10.007 [DOI] [PubMed] [Google Scholar]
- Tomiyama H., Yoshino H., Ogaki K., Li L., Yamashita C., Li Y., Funayama M., Sasaki R., Kokubo Y., Kuzuhara S. et al. (2011). PLA2G6 variant in Parkinson's disease. J. Hum. Genet. 56, 401-403. 10.1038/jhg.2011.22 [DOI] [PubMed] [Google Scholar]
- Tsujii S., Ishisaka M. and Hara H. (2015). Modulation of endoplasmic reticulum stress in Parkinson's disease. Eur. J. Pharmacol. 765, 154-156. 10.1016/j.ejphar.2015.08.033 [DOI] [PubMed] [Google Scholar]
- Van Maele-Fabry G., Hoet P., Vilain F. and Lison D. (2012). Occupational exposure to pesticides and Parkinson's disease: a systematic review and meta-analysis of cohort studies. Environ. Int. 46, 30-43. 10.1016/j.envint.2012.05.004 [DOI] [PubMed] [Google Scholar]
- Vance J. E. (2014). MAM (mitochondria-associated membranes) in mammalian cells: lipids and beyond. Biochim. Biophys. Acta 1841, 595-609. 10.1016/j.bbalip.2013.11.014 [DOI] [PubMed] [Google Scholar]
- Vilarino-Guell C., Rajput A., Milnerwood A. J., Shah B., Szu-Tu C., Trinh J., Yu I., Encarnacion M., Munsie L. N., Tapia L. et al. (2014). DNAJC13 mutations in Parkinson disease. Hum. Mol. Genet. 23, 1794-1801. 10.1093/hmg/ddt570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Poser C., Ichtchenko K., Shao X., Rizo J. and Sudhof T. C. (1997). The evolutionary pressure to inactivate. A subclass of synaptotagmins with an amino acid substitution that abolishes Ca2+ binding. J. Biol. Chem. 272, 14314-14319. [DOI] [PubMed] [Google Scholar]
- Wang Z.-W. (2008). Regulation of synaptic transmission by presynaptic CaMKII and BK channels. Mol. Neurobiol. 38, 153-166. 10.1007/s12035-008-8039-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.-J. and Xu J.-X. (2005). Possible involvement of Ca2+ signaling in rotenone-induced apoptosis in human neuroblastoma SH-SY5Y cells. Neurosci. Lett. 376, 127-132. 10.1016/j.neulet.2004.11.041 [DOI] [PubMed] [Google Scholar]
- Wang J.-Z., Grundke-Iqbal I. and Iqbal K. (2007). Kinases and phosphatases and tau sites involved in Alzheimer neurofibrillary degeneration. Eur. J. Neurosci. 25, 59-68. 10.1111/j.1460-9568.2006.05226.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Huo K., Ma L., Tang L., Li D., Huang X., Yuan Y., Li C., Wang W., Guan W. et al. (2011). Toward an understanding of the protein interaction network of the human liver. Mol. Syst. Biol. 7, 536 10.1038/msb.2011.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. Z., Xia Y. Y., Grundke-Iqbal I. and Iqbal K. (2013a). Abnormal hyperphosphorylation of tau: sites, regulation, and molecular mechanism of neurofibrillary degeneration. J. Alzheimers Dis. 33 Suppl. 1, S123-S139. 10.3233/JAD-2012-129031 [DOI] [PubMed] [Google Scholar]
- Wang R., McGrath B. C., Kopp R. F., Roe M. W., Tang X., Chen G. and Cavener D. R. (2013b). Insulin secretion and Ca2+ dynamics in beta-cells are regulated by PERK (EIF2AK3) in concert with calcineurin. J. Biol. Chem. 288, 33824-33836. 10.1074/jbc.M113.503664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J.-Y., Gong M.-Y., Ye Y.-L., Ye J.-M., Lin G.-L., Zhuang Q.-Q., Zhang X. and Zhu J.-H. (2015). The RIT2 and STX1B polymorphisms are associated with Parkinson's disease. Parkinsonism Relat. Disord. 21, 300-302. 10.1016/j.parkreldis.2014.12.006 [DOI] [PubMed] [Google Scholar]
- Wang W., Wang X., Fujioka H., Hoppel C., Whone A. L., Caldwell M. A., Cullen P. J., Liu J. and Zhu X. (2016). Parkinson's disease-associated mutant VPS35 causes mitochondrial dysfunction by recycling DLP1 complexes. Nat. Med. 22, 54-63. 10.1038/nm.3983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y., Han C., Wang Y., Wu B., Su T., Liu Y. and He R. (2015). Ribosylation triggering Alzheimer's disease-like Tau hyperphosphorylation via activation of CaMKII. Aging Cell 14, 754-763. 10.1111/acel.12355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White B. C., Sullivan J. M., DeGracia D. J., O'Neil B. J., Neumar R. W., Grossman L. I., Rafols J. A. and Krause G. S. (2000). Brain ischemia and reperfusion: molecular mechanisms of neuronal injury. J. Neurol. Sci. 179, 1-33. 10.1016/S0022-510X(00)00386-5 [DOI] [PubMed] [Google Scholar]
- Wilson C. J. and Callaway J. C. (2000). Coupled oscillator model of the dopaminergic neuron of the substantia nigra. J. Neurophysiol. 83, 3084-3100. [DOI] [PubMed] [Google Scholar]
- Wissemann W. T., Hill-Burns E. M., Zabetian C. P., Factor S. A., Patsopoulos N., Hoglund B., Holcomb C., Donahue R. J., Thomson G., Erlich H. et al. (2013). Association of Parkinson disease with structural and regulatory variants in the HLA region. Am. J. Hum. Genet. 93, 984-993. 10.1016/j.ajhg.2013.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojda U., Salinska E. and Kuznicki J. (2008). Calcium ions in neuronal degeneration. IUBMB Life 60, 575-590. 10.1002/iub.91 [DOI] [PubMed] [Google Scholar]
- Wu H.-E., Baumgardt S. L., Fang J., Paterson M., Liu Y., Du J., Shi Y., Qiao S., Bosnjak Z. J., Warltier D. C. et al. (2016). Cardiomyocyte GTP Cyclohydrolase 1 Protects the Heart Against Diabetic Cardiomyopathy. Sci. Rep. 6, 27925 10.1038/srep27925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao J., Perry G., Troncoso J. and Monteiro M. J. (1996). alpha-calcium-calmodulin-dependent kinase II is associated with paired helical filaments of Alzheimer's disease. J. Neuropathol. Exp. Neurol. 55, 954-963. 10.1097/00005072-199609000-00002 [DOI] [PubMed] [Google Scholar]
- Xilouri M., Brekk O. R. and Stefanis L. (2016). Autophagy and alpha-synuclein: relevance to Parkinson's disease and related synucleopathies. Mov. Disord. 31, 178-192. 10.1002/mds.26477 [DOI] [PubMed] [Google Scholar]
- Xu W. and Lipscombe D. (2001). Neuronal Ca(V)1.3alpha(1) L-type channels activate at relatively hyperpolarized membrane potentials and are incompletely inhibited by dihydropyridines. J. Neurosci. 21, 5944-5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B., Liu W., Deng Y., Yang T. Y., Feng S. and Xu Z. F. (2015). Inhibition of calpain prevents manganese-induced cell injury and alpha-synuclein oligomerization in organotypic brain slice cultures. PLoS ONE 10, e0119205 10.1371/journal.pone.0119205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H., Yamauchi E., Taniguchi H., Ono T. and Miyamoto E. (2002). Phosphorylation of microtubule-associated protein tau by Ca2+/calmodulin-dependent protein kinase II in its tubulin binding sites. Arch. Biochem. Biophys. 408, 255-262. 10.1016/S0003-9861(02)00556-8 [DOI] [PubMed] [Google Scholar]
- Yamauchi T. and Fujisawa H. (1983). Disassembly of microtubules by the action of calmodulin-dependent protein kinase (Kinase II) which occurs only in the brain tissues. Biochem. Biophys. Res. Commun. 110, 287-291. 10.1016/0006-291X(83)91293-7 [DOI] [PubMed] [Google Scholar]
- Yamauchi T. and Fujisawa H. (1984). Calmodulin-dependent protein kinase (kinase II) which is involved in the activation of tryptophan 5-monooxygenase catalyzes phosphorylation of tubulin. Arch. Biochem. Biophys. 234, 89-96. 10.1016/0003-9861(84)90327-8 [DOI] [PubMed] [Google Scholar]
- Yan J., Almilaji A., Schmid E., Elvira B., Shimshek D. R., van der Putten H., Wagner C. A., Shumilina E. and Lang F. (2015). Leucine-rich repeat kinase 2-sensitive Na+/Ca2+ exchanger activity in dendritic cells. FASEB J. 29, 1701-1710. 10.1096/fj.14-264028 [DOI] [PubMed] [Google Scholar]
- Yuan H.-H., Chen R.-J., Zhu Y.-H., Peng C.-L. and Zhu X.-R. (2013). The neuroprotective effect of overexpression of calbindin-D(28k) in an animal model of Parkinson's disease. Mol. Neurobiol. 47, 117-122. 10.1007/s12035-012-8332-3 [DOI] [PubMed] [Google Scholar]
- Zamponi G. W. (2016). Targeting voltage-gated calcium channels in neurological and psychiatric diseases. Nat. Rev. Drug Discov. 15, 19-34. 10.1038/nrd.2015.5 [DOI] [PubMed] [Google Scholar]
- Zeng H., Chattarji S., Barbarosie M., Rondi-Reig L., Philpot B. D., Miyakawa T., Bear M. F. and Tonegawa S. (2001). Forebrain-specific calcineurin knockout selectively impairs bidirectional synaptic plasticity and working/episodic-like memory. Cell 107, 617-629. 10.1016/S0092-8674(01)00585-2 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Nijbroek G., Sullivan M. L., McCracken A. A., Watkins S. C., Michaelis S. and Brodsky J. L. (2001). Hsp70 molecular chaperone facilitates endoplasmic reticulum-associated protein degradation of cystic fibrosis transmembrane conductance regulator in yeast. Mol. Biol. Cell 12, 1303-1314. 10.1091/mbc.12.5.1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Shimoji M., Thomas B., Moore D. J., Yu S. W., Marupudi N. I., Torp R., Torgner I. A., Ottersen O. P., Dawson T. M. et al. (2005). Mitochondrial localization of the Parkinson's disease related protein DJ-1: implications for pathogenesis. Hum. Mol. Genet. 14, 2063-2073. 10.1093/hmg/ddi211 [DOI] [PubMed] [Google Scholar]
- Zhang L., Liu W., Szumlinski K. K. and Lew J. (2012). p10, the N-terminal domain of p35, protects against CDK5/p25-induced neurotoxicity. Proc. Natl. Acad. Sci. USA 109, 20041-20046. 10.1073/pnas.1212914109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q., Li J., Wang H., Yin Y. and Zhou J. (2011). Identification of nigral dopaminergic neuron-enriched genes in adult rats. Neurobiol. Aging 32, 313-326. 10.1016/j.neurobiolaging.2009.02.009 [DOI] [PubMed] [Google Scholar]
- Zhou Q., Yen A., Rymarczyk G., Asai H., Trengrove C., Aziz N., Kirber M. T., Mostoslavsky G., Ikezu T., Wolozin B. et al. (2016). Impairment of PARK14-dependent Ca(2+) signalling is a novel determinant of Parkinson's disease. Nat. Commun. 7, 10332 10.1038/ncomms10332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S., McGrath B. C., Bai Y., Tang X. and Cavener D. R. (2016). PERK regulates Gq protein-coupled intracellular Ca2+ dynamics in primary cortical neurons. Mol. Brain 9, 87 10.1186/s13041-016-0268-5 [DOI] [PMC free article] [PubMed] [Google Scholar]