Abstract
The data presented here are related to the research paper entitled “Transglycosylation reactions, a main mechanism of phenolics incorporation in coffee melanoidins: inhibition by Maillard reaction” (Moreira et al., 2017) [1]. Methanolysis was applied in coffee fractions to quantify glycosidically-linked phenolics in melanoidins. Moreover, model mixtures mimicking coffee beans composition were roasted and analyzed using mass spectrometry-based approaches to disclose the regulatory role of proteins in transglycosylation reactions extension. This article reports the detailed chemical composition of coffee beans and derived fractions. In addition, it provides gas chromatography–mass spectrometry (GC–MS) chromatograms and respective GC–MS spectra of silylated methanolysis products obtained from phenolic compounds standards, as well as the detailed identification of all compounds observed by electrospray mass spectrometry (ESI-MS) analysis of roasted model mixtures, paving the way for the identification of the same type of compounds in other samples.
Keywords: Coffee, Carbohydrates, Polysaccharides, Phenolics, Mass spectrometry, Melanoidins
Specifications Table
| Subject area | Chemistry |
| More specific subject area | Composition of coffee and mass spectrometry analyses of coffee related carbohydrates, phenolic compounds and peptides |
| Type of data | Tables and figures |
| How data was acquired | Methanolysis products were analyzed by GC–MS (Trace-GC with Polaris Q MS, Thermo-Finnnigan, San Jose, CA) |
| Content of chlorogenic acids, caffeine, adsorbed phenolics, and phenolics released by alkaline saponification and alkaline fusion was obtained by HPLC (Dionex Ultimate 3000, Thermo, Waltham, MA); | |
| Content of sucrose, glucose and fructose was determined by anion exchange chromatography (ICS 3000, Dionex); | |
| Total sugars were determined by GC-FID (Trace GC, Thermo-Finnigan); | |
| Protein content was determined using a carbon-nitrogen/protein analyzer (PRIMACS, Skalar Analytical B.V., Breda, The Netherlands); | |
| Roasted coffee powder colors were measured with a CR-300 Minolta chroma meter (Tokyo, Japan); | |
| HPLC-ESI-MS analysis used a Waters Alliance 2690 HPLC system (Milford, MA) coupled to the LXQ linear ion trap (LIT) mass spectrometer (Thermo Fisher Scientific Inc., Waltham, MA); | |
| Direct ESI-MS analyses were also performed using LIT mass spectrometer; | |
| High resolution and high mass accuracy measurements were performed on a LTQ-Orbitrap XL mass spectrometer (ThermoFisher Scientific, Germany). | |
| Data format | Analyzed |
| Experimental factors | Roasted model mixtures and coffee beans |
| Experimental features | Chemical characterization and identification of the changes induced by roasting |
| Data source location | Robusta coffee beans from India |
| Arabica coffee beans from Honduras | |
| Commercial standards of carbohydrates, phenolic compounds and peptides | |
| Data accessibility | Data is provided with this article |
Value of the data
-
•
Detailed chemical characterization of Arabica and Robusta coffee beans and derived fractions is able to be compared with data from other authors when profiling the phenolic compounds incorporated in melanoidins.
-
•
GC–MS data of methanolysis products of phenolic compounds standards provide information on the efficiency and linkages cleaved by methanolysis and are the basis for their identification in real samples.
-
•
Mass spectrometry data on the roasting-induced compounds formed from model mixtures mimicking coffee bean composition are valuable for the identification of the same type of compounds in roasted coffee, but also other complex roasted carbohydrate-rich matrices.
1. Data
The data presented in Section 1.1 include gas chromatography-mass spectrometry (GC–MS) chromatograms and respective GC–MS spectra of silylated methanolysis products obtained from phenolic compounds standards such as 4-hydroxycinnamic, ferulic and veratric acids (Fig. 1), 5-O-caffeoylquinic acid (Fig. 2), hesperidin (Fig. 3), naringin (Fig. 4), and ellagic acid (Fig. 5).
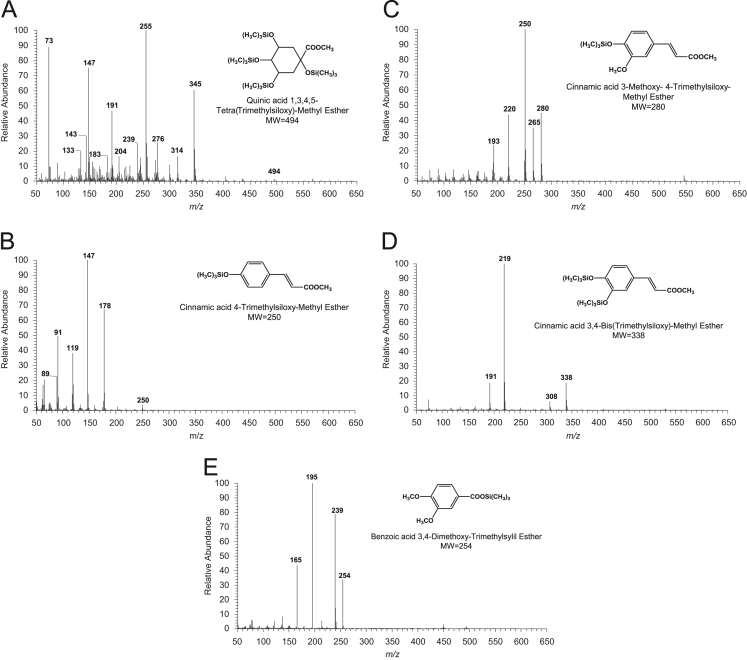
Fig. 1.
Structures and GC–MS spectra obtained from standards after silylation of the respective products released by methanolysis: A) quinic acid derivative; B) p-coumaric acid (4-hydroxycinnamic acid) derivative; and C) ferulic acid derivative. D) caffeic acid derivative; and E) veratric acid derivative.
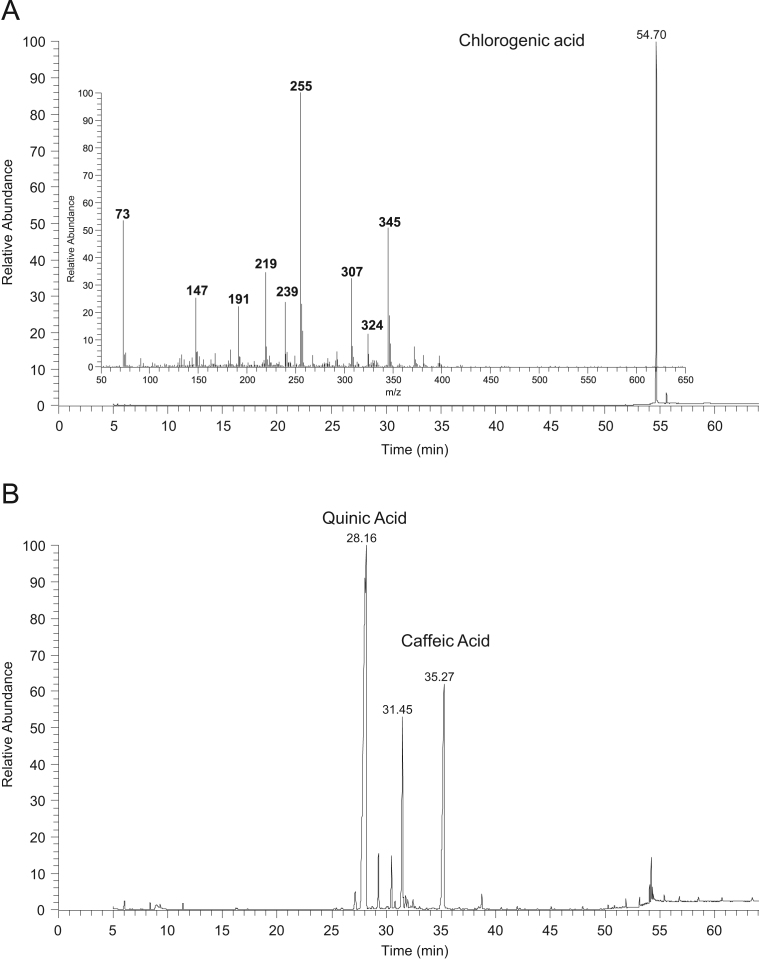
Fig. 2.
GC–MS chromatograms of A) 5-O-caffeoylquinic acid after silylation with the respective mass spectrum of the peak at the retention time 54.70 min, and B) products of 5-O-caffeoylquinic acid methanolysis after silylation.
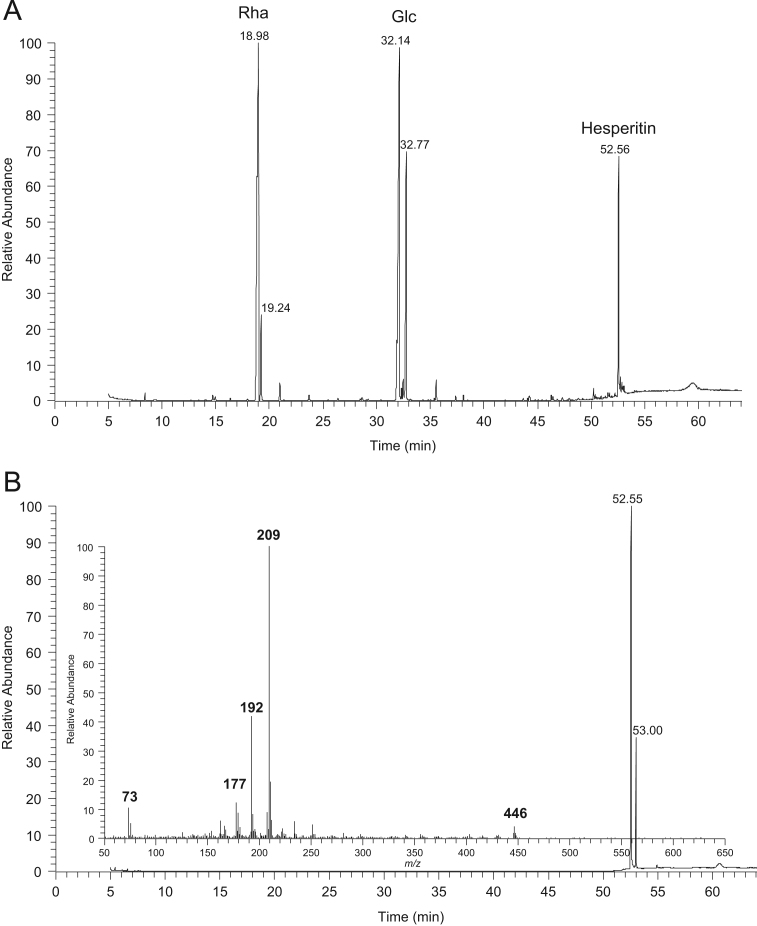
Fig. 3.
GC–MS chromatograms of A) products of hesperidin methanolysis after silylation and B) hesperetin after silylation with the respective mass spectrum of the peak at the retention time 52.55 min.
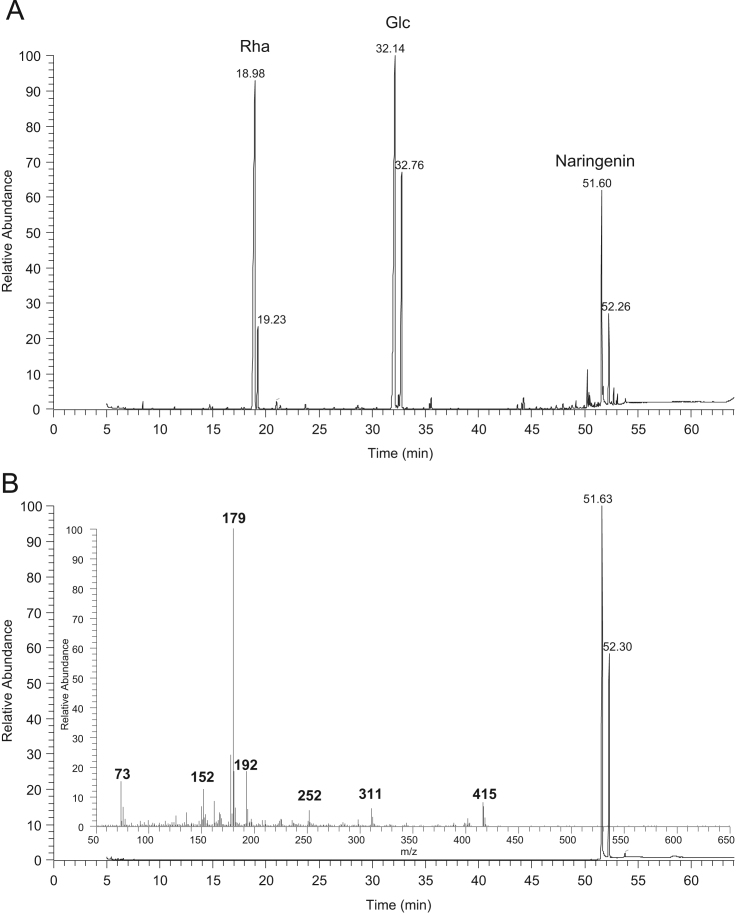
Fig. 4.
GC–MS chromatograms of A) products of naringin methanolysis after silylation and B) naringenin after silylation with the respective mass spectrum of the peak at the retention time 54.63 min.
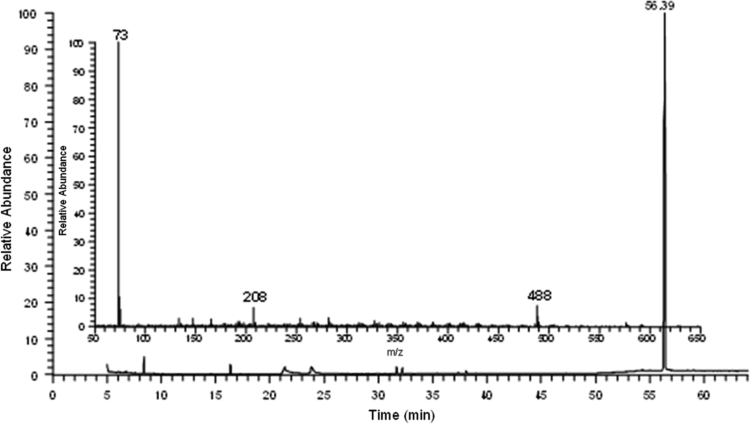
Fig. 5.
GC–MS chromatogram of products of ellagic acid methanolysis after silylation with the respective mass spectrum of the peak at the retention time 56.39 min.
In Section 1.2 are presented data on the chemical composition of Arabica and Robusta coffee beans, including chlorogenic acid composition (Table 1), simple sugars, caffeine, and protein contents (Table 2) and total sugar composition (Table 3), but also the chromatic properties of respective coffee powders (Table 4). Moreover, data on the chemical composition of high molecular weight materials (HMWMs) isolated from roasted Arabica and Robusta coffee infusions are presented in Subsection 1.2 in Table 5, Table 6. The latter includes the phenolic compounds and quinic acid released by methanolysis from coffee HMWMs.
Table 1.
Chlorogenic acid (CGA) composition (g/100 g of green or roasted coffee).
| CGA | Green coffees |
Roasted coffees |
||
|---|---|---|---|---|
| Arabica | Robusta | Arabica | Robusta | |
| 3-O-caffeoylquinic acid (3-CQA) | 0.501±0.034 | 0.666±0.025 | 0.079±0.007 | 0.090±0.000 |
| 1-O-feruloylquinic acid (1-FQA) | 0.059±0.000 | 0.059±0.000 | 0.018±0.000 | 0.043±0.000 |
| 3-O-coumaroylquinic acid (3-CoQA) | – | – | 0.073±0.000 | 0.080±0.000 |
| 5-O-caffeoylquinic acid (5-CQA) | 4.353±0.253 | 5.061±0.295 | 1.492±0.143 | 1.517±0.095 |
| 3-O-feruloylquinic acid (3-FQA) | 0.061±0.007 | 0.072±0.006 | 0.654±0.002 | 0.661±0.038 |
| 4-O-caffeoylquinic acid (4-CQA) | 0.657±0.001 | 0.932±0.031 | 0.029±0.005 | 0.014±0.001 |
| 5-O-coumaroylquinic acid (5-CoQA) | 0.098±0.004 | 0.070±0.004 | 0.182±0.000 | 0.185±0.000 |
| 5-O-feruloylquinic acid (5-FQA) | 0.369±0.000 | 0.843±0.000 | – | – |
| 4-O-feruloylquinic acid (4-FQA) | 0.060±0.008 | 0.059±0.008 | – | – |
| 4-O-coumaroylquinic acid (4-CoQA) | 0.069±0.005 | 0.070±0.005 | – | – |
| 3,4-di-O-caffeoylquinic acid (3,4-diCQA) | 0.284±0.036 | 0.760±0.025 | 0.054±0.000 | 0.060±0.000 |
| 3,5-di-O-caffeoylquinic acid (3,5-diCQA) | 0.459±0.088 | 0.798±0.020 | 0.201±0.029 | 0.379±0.005 |
| Total (CGA) | 6.972±0.140 | 9.397±0.346 | 2.781±0.209 | 3.029±0.192 |
Table 2.
Simple sugars, caffeine, and protein contents (g/100 g of green or roasted coffee).
| Coffee samples | Simple sugars |
Caffeine | Proteina | |||
|---|---|---|---|---|---|---|
| Sucrose | Glucose | Fructose | Total | |||
| Green coffees | ||||||
| Arabica | 7.14±0.53 | 0.02±0.00 | 0.03±0.01 | 7.19±0.53 | 1.05±0.05 | 11.68±0.92 |
| Robusta | 4.49±0.00 | 0.07±0.01 | 0.12±0.01 | 4.67±0.02 | 2.57±0.02 | 12.97±0.83 |
| Roasted coffees | ||||||
| Arabica | 0.14±0.00 | 0.05±0.00 | 0.01±0.00 | 0.20±0.00 | 0.88±0.05 | 13.34±0.98 |
| Robusta | 0.04±0.00 | 0.02±0.00 | 0.01±0.00 | 0.07±0.00 | 1.59±0.17 | 16.08±1.18 |
(Ntotal – Ncaffeine) × 6.25
Table 3.
Total sugar composition (g/100 g of green or roasted coffee).
| Coffee samples | Sugars |
Total | TotalPolymericb | |||||
|---|---|---|---|---|---|---|---|---|
| Rhamnose | Arabinose | Galactose | Mannose | Glucose | GlcPolymerica | |||
| Green coffees | ||||||||
| Arabica | 0.04±0.0 | 2.00±0.24 | 7.82±0.31 | 16.2±0.9 | 9.76±0.20 | 6.17 | 35.8±1.6 | 32.2 |
| Robusta | 0.06±0.0 | 1.98±0.06 | 9.96±0.30 | 15.0±1.2 | 9.00±1.1 | 6.69 | 36.0±2.7 | 33.7 |
| Roasted coffees | ||||||||
| Arabica | 0.02±0.0 | 1.15±0.16 | 6.25±1.07 | 16.9±2.5 | 6.11±1.01 | 5.99 | 30.5±4.7 | 30.4 |
| Robusta | 0.06±0.0 | 1.53±0.11 | 8.96±0.26 | 15.5±0.1 | 6.12±0.36 | 6.08 | 32.2±0.3 | 32.2 |
GlcPolymeric – polymeric glucose, determined by subtracting to the total glucose content the contribution of glucose present as free glucose and sucrose;
TotalPolymeric – polymeric sugars, determined by subtracting to the total sugar content the contribution of glucose present as free glucose and sucrose.
Table 4.
Chromatic properties of roasted coffee powders.a
| Coffee samples | L* | a* | b* | C* | h* |
|---|---|---|---|---|---|
| Arabica | 37.628±0.742 | 9.966±0.113a | 15.746±0.328 | 18.635±0.330 | 56.669 |
| Robusta | 40.406±0.210 | 9.864±0.112a | 18.984±0.571 | 21.394±0.554 | 62.544 |
Identical letters in the same column indicate no statistically significant differences.
Table 5.
Yield, chemical composition and spectroscopic properties of the high molecular weight material (HMWM) isolated from roasted Arabica and Robusta coffee infusions.a
| Arabica | Robusta | |
|---|---|---|
| Yield (g /100 g coffee) | 5.69 | 7.63 |
| Rhamnose (Rha) | 0.21±0.02 | 0.77±0.05 |
| Arabinose (Ara) | 3.47±0.07 | 4.67±0.25 |
| Galactose (Gal) | 28.2±1.3 | 32.7±3.8 |
| Mannose (Man) | 4.51±0.46 | 7.62±0.94 |
| Glucose (Glc) | 0.82±0.10 | 0.53±0.03 |
| Total sugars | 37.2±0.6a | 46.2±5.1b |
| Protein | 11.3±0.4a | 12.3±1.0a |
| Kmix 280 nm | 6.49±0.52a | 7.49±0.46a |
| Kmix 325 nm | 5.34±0.38a | 6.30±0.37b |
| Kmix 405 nm | 2.72±0.22a | 2.87±0.18a |
| Melanoidin brown index (MBI) | 5.28 | 6.93 |
For each chemical component, identical letters in the same row indicate no statistically significant differences (t-Student test, p<0.05); Kmix – specific extinction coefficient.
Table 6.
Phenolic compounds and quinic acid (mmol/100 g) released from HMWM isolated from roasted Arabica and Robusta coffee infusions.a
| Arabica | Robusta | |
|---|---|---|
| Adsorbed | ||
| 3-O-caffeoylquinic acid (3-CQA) | 0.011±0.002 | 0.010±0.004 |
| 3-O-coumaroylquinic acid (3-CoQA) | 0.005±0.001 | 0.011±0.006 |
| 5-O-caffeoylquinic acid (5-CQA) | 0.015±0.002 | 0.017±0.004 |
| 3-O-feruloylquinic acid (3-FQA) | 0.022±0.001 | 0.050±0.002 |
| 5-O-coumaroylquinic acid (5-CoQA) | 0.012±0.001 | 0.006±0.001 |
| 3,4-di-O-caffeoylquinic acid (3,4-diCQA) | 0.008±0.001 | 0.006±0.001 |
| 3,5-di-O-caffeoylquinic acid (3,5-diCQA) | 0.005±0.001 | 0.007±0.001 |
| Total (adsorbed phenolics) | 0.078±0.002§.a | 0.107±0.011§.a |
| Saponification | ||
| Caffeic acid | 1.74±0.02 | 2.89±0.078 |
| 4-Hydroxycinnamic acid | 0.030±0.001 | 0.029±0.000 |
| Ferulic acid | 0.161±0.000 | 0.407±0.029 |
| Total (phenolics released by saponification) | 1.93±0.11§.a | 3.33±0.02§.a |
| Methanolysis | ||
| Caffeic acid | 11.6±2.5 | 17.1±1.6 |
| 4-Hydroxycinnamic acid | n.d | n.d |
| Ferulic acid | 2.0±0.3 | 2.1±0.3 |
| Total (phenolics released by methanolysis) | 13.6±4.1§ | 19.2±1.3§ |
| Quinic acid | 4.8±0.3 | 11.8±1.5 |
| Alkaline fusion | ||
| Gallic acid | 0.200±0.006 | 0.305±0.056 |
| Hydroquinone | 1.80±0.27 | 3.23±0.37 |
| 3,4-Dihydroxybenzoic acid | 10.6±4.2 | 19.0±0.3 |
| 4-Hydroxybenzoic acid | 9.30±4.85 | 17.0±3.6 |
| 3,5-Dihydroxybenzoic acid | 5.46±0.88 | 3.86±0.20 |
| 2,3-Dihydroxybenzoic acid | 0.928±0.055 | 1.21±0.05 |
| Benzoic acid | 2.18±0.09 | 0.965±0.389 |
| Salicylic acid | 1.77±0.17 | 3.68±2.84 |
| Total (phenolics released by alkaline fusion) | 32.3±2.3 | 49.3±3.5 |
| ANOVA | %Variation | |
| Interaction | p<0.0001 (4) | 3.9 |
| Coffee variety | p<0.0001 (1) | 3.2 |
| Phenolic method | p<0.0001 (4) | 91.8 |
Rows with the same symbol (§) and columns with the same letter indicate no statistically significant differences (p<0.05). Tuckey post-hoc test.
The data presented in Section 1.3 include the mass losses observed after roasting of model mixtures prepared using commercial standards of coffee related carbohydrates, phenolic compounds and peptides (Table 7). The commercial standards used as models of coffee bean components were as follow: (β1→4)-d-mannotriose (Man3), an oligosaccharide structurally related to the backbone of coffee galactomannans; 5-O-caffeoylquinic acid (5-CQA), the most abundant phenolic compound in green coffee beans; and dipeptides composed by tyrosine (Y) and leucine (L), used as models of coffee proteins. Additionally, malic acid (MalA) and citric acid (CitA), the most abundant aliphatic acids present in green coffee beans, were also used. In Subsection 1.3 are also presented electrospray mass spectrometry (ESI-MS) data obtained from the model mixtures, either by direct infusion of the sample into the mass spectrometer, or online coupling to liquid chromatography (LC). Fig. 6 shows LC-MS reconstructed ion chromatograms (RICs) acquired from the roasted mixture Man3-CQA-YL. Table 8 contains the detailed identification of all the ions observed by LC-MS analysis of roasted Man3 and mixtures Man3-CQA-YL, Man3-CQA, Man3-YL, and Man3-LY. In Table 9 are presented the accurate masses obtained from high resolution and high mass accuracy measurements using a LTQ-Orbitrap mass spectrometer for the ions identified after roasting of the mixture Man3-CQA. Table 10, Table 11 summarize the ions identified by ESI-MS analysis of the roasted mixtures Man3-MalA and Man3-CitA, respectively. In Table 12 the accurate masses found by LTQ-Orbitrap for the ions identified after roasting of the mixture Man3-YL are presented. Table 13 provides data on the LC-MS2 fragmentation of roasting-induced compounds formed from the mixture Man3-YL.
Table 7.
Total mass loss (%) and mass loss between 150–175 °C (%) during thermal processing of Man3 and mixtures Man3-CQA-YL, Man3-CQA, Man3-MalA, Man3-CitA, Man3-YL, and Man3-LY, and, and color of the corresponding resulting material.
| Sample | Total mass loss | Mass loss between 150–175 °C | Color of the resulting material |
|---|---|---|---|
| Man3 | 7.1 | – | White |
| Man3-CQA-YL | 12.7 | 4.3 | Dark brown |
| Man3-CQA | 10.7 | 2.2 | Brown |
| Man3-MalA | 7.6 | 3.1 | Light brown |
| Man3-CitA | 12.4 | 3.6 | Light brown |
| Man3-YL | 14.9 | 5.2 | Dark brown |
| Man3-LY | 14.5 | 6.5 | Dark brown |
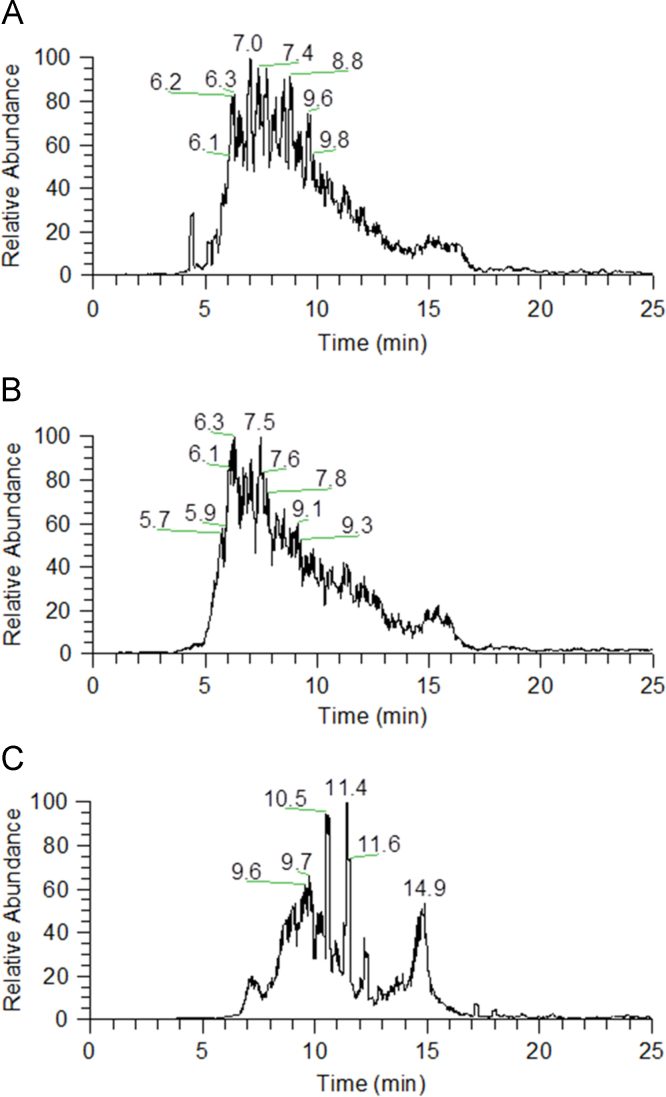
Fig. 6.
LC-MS reconstructed ion chromatograms (RICs) of the [M+H]+ ions of A) HexYL (m/z 457) and B) Hex2YL (m/z 619), acquired from the roasted Man3-CQA-YL mixture, and C) HexLY (m/z 457), acquired from the roasted Man3-LY mixture.
Table 8.
Summary of [M+Na]+ and [M+H]+ ions identified by LC-MS analysis after roasting (T1) of the Man3 and mixtures Man3-CQA-YL, Man3-CQA, Man3-YL, and Man3-LY, with the indication of the m/z values and the proposed assignments.
| Proposed assignment | Number of hexose (Hex) units (n) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| Roasted Man3 | |||||||||
| [Hexn+Na]+ | 365 | 527 | 689 | ||||||
| Roasted Man3-CQA-YLa | |||||||||
| [Hexn+Na]+ | 365 | 527 | 689 | ||||||
| [Hexn-H2O+Na]+ | 347 | 509 | |||||||
| [Hexn-2H2O+Na]+ | 329 | 491 | |||||||
| [Hexn-3H2O+Na]+ | 311 | 473 | |||||||
| [YLn+H]+ | 295 | ||||||||
| [YLn-H2O+H]+ | 277 | ||||||||
| [YLn-NH3+O+H]+ | 294 | ||||||||
| [(CQA)n+Na]+ | 377 | ||||||||
| [YLn(CQA)+H]+ | 631 | ||||||||
| [HexnYL+H]+ | 457 | 619 | 781 | 943 | 1105 | ||||
| [HexnYL-H2O+H]+ | 439 | 601 | 763 | 925 | 1087 | ||||
| [HexnYL-2H2O+H]+ | 421 | 583 | 745 | 907 | 1069 | ||||
| [HexnYL-3H2O+H]+ | 403 | 565 | 727 | 889 | |||||
| [HexnYL-4H2O+H]+ | 385 | 547 | 709 | 871 | |||||
| [Hexn(YL)2+H]+ | 733 | 895 | 1057 | ||||||
| [HexnCQAYL+H]+ | 793 | 955 | 1117 | ||||||
| Roasted Man3-CQAa | |||||||||
| [Hexn+Na]+ | 365 | 527 | 689 | 851 | 1013 | 1175 | 1337 | 1499 | |
| [Hexn-H2O+Na]+ | †347 | †509 | 671 | 833 | 995 | 1157 | 1319 | ||
| [Hexn-2H2O+Na]+ | 329 | 491 | 653 | ||||||
| [Hexn-3H2O+Na]+ | 311 | 473 | 635 | ||||||
| [(CQA)n+Na]+ | 377† | 713 | |||||||
| [(CQA)nCA+Na]+ | 539‡ | ||||||||
| [(CQA)nCA-H2O+Na]+ | 521‡ | ||||||||
| [HexnCQA+Na]+ | 539‡ | 701† | 863 | 1025 | 1187 | 1349 | |||
| [HexnCQA-H2O+Na]+ | 521‡ | 683† | |||||||
| [HexnCQA-3H2O+Na]+ | 485 | 647 | |||||||
| [Hexn(CQA)2+Na]+ | 875 | ||||||||
| [HexnQA+Na]+ | 377† | 539‡ | 701† | ||||||
| [HexnQA-H2O+Na]+ | 521‡ | 683† | |||||||
| [HexnCA-H2O+Na]+ | †347 | †509 | |||||||
| Roasted Man3-YLa | |||||||||
| [Hexn+Na]+ | 203 | 365 | 527 | 689 | 851 | 1013 | |||
| [Hexn-H2O+Na]+ | 347 | 509 | |||||||
| [Hexn-2H2O+Na]+ | 329 | 491 | |||||||
| [Hexn-3H2O+Na]+ | 311 | 473 | |||||||
| [(YL)n+H]+ | 295 | ||||||||
| [(YL)n-H2O+H]+ | 277 | ||||||||
| [(YL)n-NH3+O+H]+ | 294 | ||||||||
| [HexnYL+H]+ | 457 | 619 | 781 | 943 | 1105 | 1267 | |||
| [HexnYL-H2O+H]+ | 439 | 601 | 763 | 925 | 1087 | 1249 | |||
| [HexnYL-2H2O+H]+ | 421 | 583 | 745 | 907 | |||||
| [HexnYL-3H2O+H]+ | 403 | 565 | 727 | 889 | |||||
| [HexnYL-4H2O+H]+ | 385 | 547 | 709 | 871 | |||||
| [Hexn(YL)2+H]+ | 733 | 895 | 1057 | ||||||
| Roasted Man3-LY | |||||||||
| [Hexn+Na]+ | 203 | 365 | 527 | 689 | 851 | ||||
| [Hexn-H2O+Na]+ | 347 | 509 | 671 | 833 | |||||
| [Hexn-2H2O+Na]+ | 329 | 491 | |||||||
| [Hexn-3H2O+Na]+ | 311 | 473 | |||||||
| [(LY)n+H]+ | 295 | ||||||||
| [(LY)n-H2O+H]+ | 277 | ||||||||
| [(LY)n-NH3+O+H]+ | 294 | ||||||||
| [HexnLY+H]+ | 457 | 619 | 781 | 943 | 1105 | 1267 | |||
| [HexnLY-H2O+H]+ | 439 | 601 | 763 | 925 | 1087 | 1249 | |||
| [HexnLY-2H2O+H]+ | 421 | 583 | 745 | 907 | 1069 | ||||
| [HexnLY-3H2O+H]+ | 403 | 565 | 727 | 889 | |||||
| [HexnLY-4H2O+H]+ | 385 | 547 | 709 | 871 | |||||
| [Hexn(LY)2+H]+ | 733 | 895 | 1057 | ||||||
Ion assignment supported by accurate masses found by LTQ-Orbitrap for roasted mixtures Man3-CQA and Man3-YL. The ions marked with the symbol † or ‡ were attributed to different isobaric compounds: † for two and ‡ for three possible compounds. For roasted Man3-CQA-YL, the ion assignment was made considering the most abundant isobaric compounds identified in the roasted mixture Man3-CQA. However, the presence of isobars in roasted Man3-CQA-YL cannot be excluded.
Table 9.
Accurate masses found by LTQ-Orbitrap for the ions identified after roasting of the mixture Man3-CQA. The theoretical mass and the difference between the theoretical and experimental masses for each predicted formula were obtained from Xcalibur software.
| Experimental mass (m/z) | Theoretical mass (m/z) | Mass error (ppm) | RDB equiv. | Composition | Proposed assignment |
|---|---|---|---|---|---|
| 323.0757 | 323.0761 | −1.28 | 8.5 | C15H15O8 | [HexCA-H2O-H]− |
| 323.097 | 323.0973 | −0.94 | 3.5 | C12H19O10 | [Hex2-H2O-H]− |
| 341.1074 | 341.1078 | −1.17 | 2.5 | C12H21O11 | [Hex2-H]- |
| 353.0862 | 353.0867 | −1.41 | 8.5 | C16H17O9 | [CQA-H]− |
| 353.1072 | 353.1078 | −1.75 | 3.5 | C13H21O11 | [HexQA-H]− |
| 461.1071 | 461.1078 | −1.51 | 12.5 | C22H21O11 | [HexCQA-3H2O-H]− |
| 497.1071 | 497.1078 | −1.46 | 15.5 | C25H21O11 | [(CQA)CA-H2O-H]− |
| 497.1281 | 497.129 | −1.76 | 10.5 | C22H25O13 | [HexCQA-H2O-H]− |
| 497.1492 | 497.1501 | −1.86 | 5.5 | C19H29O15 | [Hex2QA-H2O-H]− |
| 503.1599 | 503.1607 | −1.61 | 3.5 | C18H31O16 | [Hex3-H]− |
| 515.1182 | 515.1184 | −0.37 | 14.5 | C25H23O12 | [(CQA)CA-H]− |
| 515.1385 | 515.1395 | −1.96 | 9.5 | C22H27O14 | [HexCQA-H]− |
| 515.1596 | 515.1607 | −2.02 | 4.5 | C19H31O16 | [Hex2QA-H]− |
| 623.1594 | 623.1607 | −2.02 | 13.5 | C28H31O16 | [Hex2CQA-3H2O-H]− |
| 659.1805 | 659.1818 | −1.97 | 11.5 | C28H35O18 | [Hex2CQA-H2O-H]− |
| 659.2029 | 659.2029 | −0.09 | 6.5 | C25H39O20 | [Hex3QA-H2O-H]− |
| 677.1908 | 677.1924 | −2.3 | 10.5 | C28H37O19 | [Hex2CQA-H]− |
| 677.2121 | 677.2135 | −1.97 | 5.5 | C25H41O21 | [Hex3QA-H]− |
| 689.1703 | 689.1712 | −1.4 | 16.5 | C32H33O17 | [(CQA)2-H]− |
| 839.2433 | 839.2452 | −2.29 | 11.5 | C34H47O24 | [Hex3CQA-H]− |
| 851.222 | 851.224 | −2.41 | 17.5 | C38H43O22 | [Hex(CQA)2-H]− |
| 1001.2961 | 1001.298 | −1.9 | 12.5 | C40H57O29 | [Hex4CQA-H]− |
| 1163.3479 | 1163.3508 | −2.51 | 13.5 | C46H67O34 | [Hex5CQA-H]− |
| 1325.3998 | 1325.4036 | −2.92 | 14.5 | C52H77O39 | [Hex6CQA-H]− |
Table 10.
Summary of [M+Na]+ ions identified by ESI-MS analysis after roasting of the mixture Man3-MalA, with the indication of the m/z values and the proposed assignments.
| Proposed assignment | Number of hexose (Hex) units (n) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| [Hexn+Na]+ | 203 | 365 | 527 | 689 | 851 | 1013 | 1175 | 1337 | 1499 |
| [Hexn-H2O+Na]+ | 185 | 347 | 509 | 671 | 833 | 995 | 1157 | 1319 | 1481 |
| [(MalA)n+Na]+ | 157 | ||||||||
| [HexnMalA+Na]+ | 319 | 481 | 643 | 805 | 967 | 1129 | 1291 | 1453 | |
| [HexnMalA-H2O+Na]+ | 301 | 463 | 625 | 787 | 949 | 1111 | 1273 | 1435 | |
| [Hexn(MalA)2+Na]+ | 435 | 597 | 759 | 921 | 1083 | 1245 | 1407 | ||
Table 11.
Summary of [M+Na]+ ions identified by ESI-MS analysis after roasting of the mixture Man3-CitA, with the indication of the m/z values and the proposed assignments.
| Proposed assignment | Number of hexose (Hex) units (n) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| [Hexn+Na]+ | 203 | 365 | 527 | 689 | 851 | 1013 | 1175 | 1337 | 1499 |
| [Hexn-H2O+Na]+ | 185 | 347 | 509 | 671 | 833 | 995 | 1157 | 1319 | 1481 |
| [(CitA)n+Na]+ | 215 | ||||||||
| [HexnCitA+Na]+ | 377 | 539 | 701 | 863 | 1025 | 1187 | 1349 | ||
| [HexnCitA-H2O+Na]+ | 359 | 521 | 683 | 845 | 1007 | 1169 | 1331 | ||
| [Hexn(CitA)2+Na]+ | 551 | 713 | 875 | 1037 | 1199 | 1361 | |||
Table 12.
Accurate masses found by LTQ-Orbitrap for the ions identified after roasting of the mixture Man3-YL. The theoretical mass and the difference between the theoretical and experimental masses for each predicted formula were obtained from Xcalibur software.
| Experimental mass (m/z) | Theoretical mass (m/z) | Mass error (ppm) | RDB equiv. | Composition | Proposed assignment |
|---|---|---|---|---|---|
| 275.1393 | 275.139 | 0.91 | 7.5 | C15H19O3N2 | [YL-H2O-H]− |
| 292.1184 | 292.1179 | 1.58 | 7.5 | C15H18O5N | [YL-NH3+O-H]− |
| 293.15 | 293.1496 | 1.32 | 6.5 | C15H21O4N2 | [YL-H]− |
| 341.1082 | 341.1078 | 1.18 | 2.5 | C12H21O11 | [Hex2-H]− |
| 383.1606 | 383.1601 | 1.15 | 11.5 | C21H23O5N2 | [HexYL-4H2O-H]− |
| 401.171 | 401.1707 | 0.69 | 10.5 | C21H25O6N2 | [HexYL-3H2O-H]− |
| 419.1815 | 419.1813 | 0.6 | 9.5 | C21H27O7N2 | [HexYL-2H2O-H]− |
| 437.1921 | 437.1918 | 0.59 | 8.5 | C21H29O8N2 | [HexYL-H2O-H]− |
| 455.2027 | 455.2024 | 0.67 | 7.5 | C21H31O9N2 | [HexYL-H]− |
| 503.1609 | 503.1607 | 0.55 | 3.5 | C18H31O16 | [Hex3-H]− |
| 545.2133 | 545.213 | 0.53 | 12.5 | C27H33O10N2 | [Hex2YL-4H2O-H]− |
| 563.2237 | 563.2235 | 0.34 | 11.5 | C27H35O11N2 | [Hex2YL-3H2O-H]− |
| 581.2342 | 581.2341 | 0.12 | 10.5 | C27H37O12N2 | [Hex2YL-2H2O-H]− |
| 599.2448 | 599.2447 | 0.24 | 9.5 | C27H39O13N2 | [Hex2YL-H2O-H]− |
| 617.255 | 617.2552 | −0.31 | 8.5 | C27H41O14N2 | [Hex2YL-H]− |
| 707.266 | 707.2658 | 0.25 | 13.5 | C33H43O15N2 | [Hex3YL-4H2O-H]− |
| 725.2764 | 725.2764 | 0.11 | 12.5 | C33H45O16N2 | [Hex3YL-3H2O-H]− |
| 731.3499 | 731.3498 | 0.16 | 13.5 | C36H51O12N4 | [Hex(YL)2-H]− |
| 743.287 | 743.2869 | 0.13 | 11.5 | C33H47O17N2 | [Hex3YL-2H2O-H]− |
| 761.2977 | 761.2975 | 0.25 | 10.5 | C33H49O18N2 | [Hex3YL-H2O-H]− |
| 779.3081 | 779.3081 | 0.03 | 9.5 | C33H51O19N2 | [Hex3YL-H]− |
| 869.3182 | 869.3186 | −0.49 | 14.5 | C39H53O20N2 | [Hex4YL-4H2O-H]− |
| 887.3288 | 887.3292 | −0.41 | 13.5 | C39H55O21N2 | [Hex4YL-3H2O-H]− |
| 905.3395 | 905.3397 | −0.31 | 12.5 | C39H57O22N2 | [Hex4YL-2H2O-H]− |
| 923.3493 | 923.3503 | −1.05 | 11.5 | C39H59O23N2 | [Hex4YL-H2O-H]− |
| 941.3579 | 941.3609 | −3.15 | 10.5 | C39H61O24N2 | [Hex4YL-H]− |
| 1085.4015 | 1085.4031 | −1.49 | 12.5 | C45H69O28N2 | [Hex5YL-H2O-H]− |
| 1247.454 | 1247.456 | −1.54 | 13.5 | C51H79O33N2 | [Hex6YL-H2O-H]− |
Table 13.
Compounds identified after roasting of the mixture Man3-YL: the m/z values of the ions identified, the proposed assignments, the retention time (RT), and the most abundant product ions observed in the respective LC-MS2 spectrum, with the indication of the m/z values, mass differences relative to the precursor ion, and the identification of the most informative product ions.
| m/z | Assignment | RT | LC-MS2 |
|---|---|---|---|
| 203 | [Hex+Na]+ | 4.0 | aNo LC-MS2 spectrum |
| 277 | [YL-H2O+H]+ | 10.4–15.6 | 249 (-28), 136 (-141, a1), 171 (-106) |
| 294 | [YL-NH3+O+H]+ | 17.7–29.9 | 248 (-46), 276 (-18), 132 (-162, -(Y-NH3+O)res, [L+H]+), 266 (-28), 220 (-74) |
| 295 | [YL+H]+ | 7.2–16.6 | 136 (-159, a1), 278 (-17, -NH3), 249 (-46, -HCO2H), 119 (-176, a1-NH3) |
| 311 | [Hex2-3H2O+Na]+ | 4.3 | 185 (-126, -(Hex-3H2O)), 149 (-162, -Hexres) |
| 329 | [Hex2-2H2O+Na]+ | 3.9 | 167 (-162, -Hexres), 185 (-144, -(Hex-2H2O)), 203 (-126, -(Hex-2H2O)res) |
| 347 | [Hex2-H2O+Na]+ | 4.1 | 329 (-18), 287 (-60), 185 (-162, -Hexres), 203 (-144, -(Hex-H2O)res) |
| 365 | [Hex2+Na]+ | 4.2 | 347 (-18), 305 (-60), 203 (-162, -Hexres), 185 (-180, -Hex) |
| 385 | [HexYL-4H2O+H]+ | 35.6 | 339 (-46), 367 (-18), 357 (-28), 226 (-159, (a1+(Hex-4H2O)res)), 311 (-74), |
| 254 (-131, -L) | |||
| 385 | [HexYL-4H2O+H]+ | 41.1 | 339 (-46), 367 (-18), 357 (-28), 226 (-159, (a1+(Hex-4H2O)res)), 311 (-74), |
| 254 (-131, -L) | |||
| 403 | [HexYL-3H2O+H]+ | 17.6 | 385 (-18), 244 (-159, (a1+(Hex-3H2O)res)), 126 (-277), 357 (-46), 278 (-125) |
| 403 | [HexYL-3H2O+H]+ | 25.1 | 272 (-131, -L), 244 (-159, (a1+(Hex-3H2O)res)), 385 (-18), 357 (-46), 279 (-124) |
| 421 | [HexYL-2H2O+H]+ | 18.1 | 290 (-131, -L), 262 (-159, (a1+(Hex-2H2O)res)), 403 (-18), 393 (-28), 375 (-46) |
| 421 | [HexYL-2H2O+H]+ | 20.2 | 290 (-131, -L), 262 (-159, (a1+(Hex-2H2O)res)), 403 (-18), 375 (-46), 391 (-30) |
| 421 | [HexYL-2H2O+H]+ | 29.9 | 262 (-159, (a1+(Hex-2H2O)res)), 403 (-18), 290 (-131, -L), 244 (-177) |
| 439 | [HexYL-H2O+H]+ | 18.0 | 280 (-159, (a1+(Hex-H2O)res)), 393 (-46), 295 (-144, -(Hex-H2O)res, [YL+H]+) |
| 457 | [HexYL+H]+ | 5.7–16.9 | 439 (-18), 373 (-84), 421 (-36), 307 (-150), 295 (-162, -Hexres, [YL+H]+), 403 (-54), 136 (-321, a1), 298 (-159, (a1+Hexres)) |
| 473 | [Hex3-3H2O+Na]+ | 4.2 | 347 (-126, -(Hex-3H2O)), 311 (-162, -Hexres) |
| 491 | [Hex3-2H2O+Na]+ | 4.1 | 329 (-162, -Hexres), 347 (-144, -(Hex-2H2O)), 365 (-126, -(Hex-2H2O)res) |
| 509 | [Hex3-H2O+Na]+ | 4.2 | 347 (-162, -Hexres), 491 (-18), 449 (-60), 365 (-144, -(Hex-H2O)res), 185 (-324, -2xHexres) |
| 527 | [Hex3+Na]+ | 4.2 | 365 (-162, -Hexres), 347 (-180, -Hex), 509 (-18), 467 (-60), 185 (-342, [Hexres+Na]+) |
| 547 | [Hex2YL-4H2O+H]+ | 18.4–24.9 | 529 (-18), 388 (-159, (a1+(Hex2-4H2O)res)), 501 (-46), 385 (-162, -(Hex-H2O)), |
| 416 (-131, -L), 511 (-36), 421 (-126, -(Hex-3H2O)), 403 (-144, -(Hex-3H2O)res) | |||
| 565 | [Hex2YL-3H2O+H]+ | 16.5 | 288 (-277), 547 (-18), 403 (-162, -Hexres), 406 (-159, a1+(Hex2-3H2O)res), 529 (-36) |
| 565 | [Hex2YL-3H2O+H]+ | 19.6–21.2 | 547 (-18), 406 (-159, a1+(Hex2-3H2O)res), 403 (-162, -Hexres), 529 (-36), |
| 439 (-126, -(Hex-3H2O)) | |||
| 583 | [Hex2YL-2H2O+H]+ | 24.1 | 421 (-162, -Hexres), 565 (-18), 262 (-321, a1+(Hex-2H2O)res)), 290 (-293, -(Hexres+L), 403 (-180, -Hex) |
| 601 | [Hex2YL-H2O+H]+ | 16.9 | 439 (-162, -Hexres), 280 (-321, a1+(Hex-H2O)res)), 295 (-306, [YL+H]+), |
| 393 (-208, -(Hexres+HCO2H)) | |||
| 619 | [Hex2YL+H]+ | 5.8–16.4 | 457 (-162, -Hexres), 601 (-18), 307 (-312, -(Hexres+150)), 373 (-246, -(Hexres+84)) |
| 689 | [Hex4+Na]+ | 3.9 | 527 (-162, -Hexres), 365 (-324, -2xHexres), 203 (-486, -3xHexres) |
| 709 | [Hex3YL-4H2O+H]+ | 15.7–21.1 | 547 (-162, -Hexres), 691 (-18), 635 (-74), 529 (-180, -Hex), 583 (-126, -(Hex-3H2O)), 550 (-159, (a1+(Hex3-4H2O)res)) |
| 727 | [Hex3YL-3H2O+H]+ | 16.6–20.6 | 565 (-162, -Hexres), 709 (-18), 403 (-324, -2xHexres), 547 (-180, -Hex), 295 (-270, [YL+H]+) |
| 733 | [Hex(YL)2+H]+ | 26.3 | 439 (-294, -YL), 280 (-453), 602 (-131, -L), 574 (-159), 393 (-340), 715 (-18), |
| 295 (-438, [YL+H]+), 571 (-162, -Hexres) | |||
| 733 | [Hex(YL)2+H]+ | 31.6 | 602 (-131, -L), 439 (-294, -YL), 280 (-453), 393 (-340), 715 (-18), 574 (-159), |
| 295 (-438, [YL+H]+), 571 (-162, -Hexres) | |||
| 745 | [Hex3YL-2H2O+H]+ | 22.3 | 421 (-324, -2xHexres), 583 (-162, -Hexres), 262 (-483, a1+(Hex-2H2O)res)), |
| 403 (-342, -(Hexres+Hex)), 290 (-455, -((2xHexres)+L)) | |||
| 745 | [Hex3YL-2H2O+H]+ | 23.2 | 421 (-324, -2xHexres), 583 (-162, -Hexres), 403 (-342, -(Hexres+Hex)), |
| 262 (-483, a1+(Hex-2H2O)res)), 290 (-455, -((2xHexres)+L)) | |||
| 763 | [Hex3YL-H2O+H]+ | 16.7 | 601 (-162, -Hexres), 439 (-324, -2xHexres), 280 (-483, (a1+(Hex-H2O)res)), 745 (-18), 295 (-468, [YL+H]+) |
| 763 | [Hex3YL-H2O+H]+ | 18.7 | 439 (-324, -2xHexres), 601 (-162, -Hexres), 280 (-483, (a1+(Hex-H2O)res)), 745 (-18), 393 (-370, -((2xHexres)+HCO2H)) |
| 781 | [Hex3YL+H]+ | 5.3–16.2 | 457 (-324, -2xHexres), 619 (-162, -Hexres), 373 (-408, -((2xHexres)+84), |
| 298 (-483, (a1+Hexres)) | |||
| 851 | [Hex5+Na]+ | 4.2 | 689 (-162, -Hexres), 527 (-324, -2xHexres), 671 (-180, -Hex), 365 (-486, -3xHexres) |
| 871 | [Hex4YL-4H2O+H]+ | 15.6–21.3 | 709 (-162, -Hexres), 547 (-324, -2xHexres), 745 (-126, -(Hex-3H2O)) |
| 889 | [Hex4YL-3H2O+H]+ | 18.6 | 727 (-162, -Hexres), 565 (-324, -2xHexres), 871 (-18), 709 (-180, -Hex), 373 (-516) |
| 889 | [Hex4YL-3H2O+H]+ | 19.8 | 565 (-324, -2xHexres), 727 (-162, -Hexres), 871 (-18), 272 (-455, -((2xHexres)+L)) |
| 889 | [Hex4YL-3H2O+H]+ | 22.1 | 565 (-324, -2xHexres), 403 (-486, -3xHexres), 727 (-162, -Hexres), 871 (-18), |
| 709 (-180, -Hex) | |||
| 895 | [Hex2(YL)2+H]+ | 22.8 | 733 (-162, -Hexres), 439 (-456, -(Hexres+YL)), 602 (-293, -(Hexres+L)), 764 (-131, -L), 280 (-615), 571 (-324, -2xHexres) |
| 895 | [Hex2(YL)2+H]+ | 27.3 | 733 (-162, -Hexres), 602 (-293, -(Hexres+L)), 439 (-456, -(Hexres+YL)), 764 (-131, -L), 280 (-615), 571 (-324, -2xHexres) |
| 907 | [Hex4YL-2H2O+H]+ | 16.8 | 745 (-162, -Hexres), 583 (-324, -2xHexres) |
| 907 | [Hex4YL-2H2O+H]+ | 27.4 | 745 (-162, -Hexres), 583 (-324, -2Hexres) |
| 925 | [Hex4YL-H2O+H]+ | 16.4 | 907 (-18), 601 (-324, -2xHexres), 763 (-162, -Hexres), 889 (-36), |
| 583 (-342, -(Hexres+Hex)) | |||
| 943 | [Hex4YL+H]+ | 4.3–15.3 | 619 (-324, -2xHexres), 925 (-18), 781 (-162, -Hexres), 457 (-486, -3xHexres), 373 (-570, -((3xHexres)+84)), 295 (-648, -4xHexres, [YL+H]+) |
| 1013 | [Hex6+Na]+ | 4.4 | 851 (-162, -Hexres), 527 (-486, -3xHexres), 689 (-324, -2xHexres), 953 (-60), 833 (-180, Hex) |
| 1057 | [Hex3(YL)2+H]+ | 21.7 | 733 (-324, -2xHexres), 439 (-618, -((2xHexres)+YL)), 895 (-162, -Hexres), |
| 602 (-455, -((2xHexres)+L)) | |||
| 1057 | [Hex3(YL)2+H]+ | 25.3 | 733 (-324, -2xHexres), 602 (-455, -((2xHexres)+L)), 439 (-618, -((2xHexres)+YL)), 895 (-162, -Hexres), 926 (-131, -L) |
| 1087 | [Hex5YL-H2O+H]+ | 16.2 | 925 (-162, -Hexres), 1069 (-18), 763 (-324, -2xHexres) |
| 1105 | [Hex5YL+H]+ | 4.2–16.7 | 943 (-162, -Hexres), 781 (-324, -2xHexres) |
| 1249 | [Hex6YL-H2O+H]+ | 15.9 | aNo LC-MS2 spectrum |
| 1267 | [Hex6YL+H]+ | 5.3–16.7 | aNo LC-MS2 spectrum |
No LC-MS2 spectrum, but the ion assignment is corroborated by the observation of other ions of the same series, eluting at a similar retention time (RT). Abbreviations: a1 – peptide fragment (Fig. 2A); Hexres – Hexose residue; L – Leucine; Lres – Leucine residue; Y – Tyrosine; Yres – Tyrosine residue.
1.1. GC–MS data of silylated methanolysis products of phenolic compounds standards
1.2. Chemical composition of coffee beans and derived fractions
1.3. Data on the model mixtures mimicking coffee composition
See Fig. 6 and Table 7, Table 8, Table 9, Table 10, Table 11, Table 12, Table 13
Some of the Hexn and dehydrated derivatives identified by HPLC-ESI-MS (Table 8) were not observed in the negative ESI-MS spectrum acquired on the LTQ-Orbitrap mass spectrometer (Table 9). This is due to the fact that neutral oligosaccharides ionize better in positive than in negative mode.
The analysis of the reconstructed ion chromatograms (RICs) corroborates the presence of isomeric compounds, i.e. compounds with the same elemental composition but different structures, eluting at different RTs. However, the exact structural differences were not possible to be inferred based on the respective LC-MSn spectra (n=2–3) because they were very similar, most probably due to the presence of positional isomers. In the case of the compounds bearing a sugar moiety, the structural differences of the isomers can be related to different structures of the sugar moiety, differing on glycosidic linkage positions, and anomeric configuration.
2. Experimental design, materials and methods
The methodologies that allowed the data here presented are described in [1] and in cited references. Here, only the protocol for glycosidic linkage analysis is provided, giving a large number of experimental details, usually omitted in research articles due to the words limit.
2.1. Glycosidic linkage analysis
A sample (0.5–1 mg) of each unroasted and roasted model (Man3 and mixtures) was dissolved with DMSO (1 mL), and then powdered NaOH (40 mg) was added to the solution. After 30 min at room temperature with continuous stirring, samples were methylated by adding of CH3I (80 µL), allowed to react 20 min under vigorous stirring. Distilled water (2 mL) was then added, and the solution was neutralized using HCl 1 M. Dichloromethane (3 mL) was then added and, upon vigorous manual shaking and centrifugation, the dichloromethane phase was recovered and washed two times by addition of distilled water (2–3 mL). The organic phase was evaporated to dryness and the resulting material was remethylated using the same procedure. The remethylated material was hydrolyzed with 500 µL of TFA 2 M at 121 °C for 1 h, and the acid was then evaporated to dryness. For carbonyl-reduction, the resulting material was then suspended in 300 µL of NH3 2 M and 20 mg of NaBD4 were added. The reaction mixture was incubated at 30 °C for 1 h. After cooling, the excess of borodeuteride was destroyed by the addition of glacial acetic acid (2×50 µL). The partially methylated alditol derivatives were acetylated with acetic anhydride (3 mL) in the presence of 1-methylimidazole (450 μL) during 30 min at 30 °C. To decompose the excess of acetic anhydride, distilled water (3 mL) was added while the tubes were in ice. Dichloromethane (2.5 mL) was then added and, upon vigorous manual shaking and centrifugation, the dichloromethane phase was recovered. The addition of water (3 mL) and dichloromethane (2.5 mL), and the recovery of the organic phase were performed once more. The dichloromethane phase was then washed two times by addition of distilled water (3 mL) and evaporated to dryness. The dried material was dissolved with anhydrous acetone (2 × 1 mL) followed by the evaporation of the acetone to dryness. The partially methylated alditol acetates (PMAAs) were redissolved with anhydrous acetone and identified by gas chromatography-mass spectrometry (GC–MS) on an Agilent Technologies 6890 N Network GC system (Santa Clara, CA) equipped with a DB-1ms column with 30 m of length, 0.25 mm of internal diameter, and 0.1 µm of film thickness (J&W Scientific, Folsom, CA). The GC was connected to an Agilent 5973 Network Mass Selective Detector operating with an electron impact mode at 70 eV, and scanning the m/z range 40–500 in a 1 s cycle in a full scan mode acquisition. The oven temperature program used was: initial temperature 50 °C, a linear increase of 8 °C/min up to 140 °C, standing at this temperature for 5 min, followed by linear increase of 0.5 °C/min up to 150 °C, ollowed by linear increase of 40 °C/min up to 250 °C, standing at this temperature for 1 min. The injector and detector temperatures were 220 and 230 °C, respectively. Helium was used as carrier gas at a flow rate of 1.7 mL/min. Relative abundance of each PMAA identified in both unroasted and roasted samples was determined upon integration of each peak using the equipment׳s software.
Acknowledgements
Thanks are due FCT/MEC for the financial support to QOPNA (UID/QUI/00062/2013), and CESAM (UID/AMB/50017/2013) at University of Aveiro and CQ-VR at UTAD Vila Real (PEst-OE/QUI/UI0616/2014) through national funds, and the co-funding by the FEDER, within the PT2020 Partnership Agreement. Thanks are also due to FCT for funding the Portuguese Mass Spectrometry Network (REDE/1504/REM/2005), and the grants of A.S.P. Moreira (SFRH/BD/80553/2011) and E. Maciel (SFRH/BPD/104165/2014).
Footnotes
Transparency data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.dib.2017.05.027.
Transparency document. Supplementary material
Supplementary material
.
Reference
- 1.Moreira A.S.P., Nunes F.M., Simões C., Maciel E., Domingues P., Domingues M.R.M., Coimbra M.A. Transglycosylation reactions, a main mechanism of phenolics incorporation in coffee melanoidins: inhibition by Maillard reaction. Food Chem. 2017;227:422–431. doi: 10.1016/j.foodchem.2017.01.107. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material