Abstract
BACKGROUND
Long-term results from randomized, controlled trials that compare medical therapy with surgical therapy in patients with type 2 diabetes are limited.
METHODS
We assessed outcomes 5 years after 150 patients who had type 2 diabetes and a body-mass index (BMI; the weight in kilograms divided by the square of the height in meters) of 27 to 43 were randomly assigned to receive intensive medical therapy alone or intensive medical therapy plus Roux-en-Y gastric bypass or sleeve gastrectomy. The primary outcome was a glycated hemoglobin level of 6.0% or less with or without the use of diabetes medications.
RESULTS
Of the 150 patients who underwent randomization, 1 patient died during the 5-year follow-up period; 134 of the remaining 149 patients (90%) completed 5 years of follow-up. At baseline, the mean (±SD) age of the 134 patients was 49±8 years, 66% were women, the mean glycated hemoglobin level was 9.2±1.5%, and the mean BMI was 37±3.5. At 5 years, the criterion for the primary end point was met by 2 of 38 patients (5%) who received medical therapy alone, as compared with 14 of 49 patients (29%) who underwent gastric bypass (unadjusted P = 0.01, adjusted P = 0.03, P = 0.08 in the intention-to-treat analysis) and 11 of 47 patients (23%) who underwent sleeve gastrectomy (unadjusted P = 0.03, adjusted P = 0.07, P = 0.17 in the intention-to-treat analysis). Patients who underwent surgical procedures had a greater mean percentage reduction from baseline in glycated hemoglobin level than did patients who received medical therapy alone (2.1% vs. 0.3%, P = 0.003). At 5 years, changes from baseline observed in the gastric-bypass and sleeve-gastrectomy groups were superior to the changes seen in the medical-therapy group with respect to body weight (−23%, −19%, and −5% in the gastric-bypass, sleeve-gastrectomy, and medical-therapy groups, respectively), triglyceride level (−40%, −29%, and −8%), high-density lipoprotein cholesterol level (32%, 30%, and 7%), use of insulin (−35%, −34%, and −13%), and quality-of-life measures (general health score increases of 17, 16, and 0.3; scores on the RAND 36-Item Health Survey ranged from 0 to 100, with higher scores indicating better health) (P<0.05 for all comparisons). No major late surgical complications were reported except for one reoperation.
CONCLUSIONS
Five-year outcome data showed that, among patients with type 2 diabetes and a BMI of 27 to 43, bariatric surgery plus intensive medical therapy was more effective than intensive medical therapy alone in decreasing, or in some cases resolving, hyperglycemia.
Observational studies1–6 and randomized, controlled trials, which have generally been short-term studies,7–19 have shown that bariatric surgery, when used specifically to treat diabetes, significantly improves glycemic control and reduces cardiovascular risk factors. In the Surgical Treatment and Medications Potentially Eradicate Diabetes Efficiently (STAMPEDE) trial, we reported that, at 1 year and 3 years after randomization, both gastric bypass and sleeve gastrectomy were superior to intensive medical therapy alone in achieving excellent glycemic control (i.e., glycated hemoglobin ≤6.0%), reducing cardiovascular risk, improving quality of life, and decreasing medication use.8–10 The current article provides results of the final, 5-year follow-up analyses from that trial and attempts to address questions regarding the relative long-term efficacy and safety of bariatric surgery and its effects on diabetes-related end-organ disease.
METHODS
TRIAL DESIGN
The rationale, design, and methods of the trial have been reported previously.8,20 The complete protocol was approved by the institutional review board at the Cleveland Clinic and is available with the full text of this article at NEJM.org. Briefly, the trial was a three-group, randomized, controlled, nonblinded, single-center study involving 150 obese patients who had type 2 diabetes, in which the effects of intensive medical therapy alone were compared with those of intensive medical therapy plus either gastric bypass or sleeve gastrectomy. Patients were randomly assigned in a 1:1:1 ratio to one of the three study groups, with stratification according to baseline use of insulin. Eligibility criteria included an age of 20 to 60 years, a glycated hemoglobin level of more than 7.0%, and a body-mass index (BMI; the weight in kilograms divided by the square of the height in meters) of 27 to 43. All the patients provided written informed consent.
TRIAL OUTCOMES
The primary outcome was a glycated hemoglobin level of 6.0% or less with or without the use of diabetes medications.8,10,20 Prespecified secondary outcomes included measures of glycemic control, weight loss, blood pressure, lipid levels, renal function, ophthalmologic outcomes, medication use, adverse events, and quality of life (as evaluated with the use of the RAND 36-Item Health Survey).21 Methods for ophthalmologic evaluations are reported in the protocol.22 The strategy for all three groups was the adjustment of intensive medical therapy (every 3 months for 2 years and every 6 months thereafter) with the goal of achieving a glycated hemoglobin level of 6.0% or less, without unacceptable side effects associated with medical treatment. Patients in the surgical groups were instructed to take daily supplemental multivitamins, vitamin B12, vitamin D, calcium, and iron.
TRIAL OVERSIGHT
This investigator-initiated trial was financially supported by Ethicon, with additional support from LifeScan, the Cleveland Clinic, and the National Institutes of Health. The sponsors had no role in the accrual or analysis of the data or in the preparation of the manuscript. The first author wrote the initial draft of the manuscript. All the authors had independent access to the data and vouch for the completeness and accuracy of the data and for the fidelity of the trial to the protocol. Complete trial governance is outlined in the Supplementary Appendix, available at NEJM.org.
STATISTICAL ANALYSIS
We used Pearson’s chi-square test or Fisher’s exact test to evaluate the glycated hemoglobin level at clinical cutoff points of 6.0% or less (primary end point), 6.5% or less, and 7.0% or less. The primary population for analysis comprised patients who had undergone randomization and had glycated hemoglobin values at the 5-year visit; these patients were considered to have completed the trial. We also performed an imputed intention-to-treat analysis, which included all patients who underwent randomization (150 patients). An analysis of variance was used to analyze the change from baseline to year 5 for the secondary end points. We created graphs for glycemic measures and BMI over time by plotting the least-squares means and corresponding standard errors from a mixed model, using treatment assignment, visit, and the interaction between treatment assignment and visit as fixed factors. A stepwise multivariable logistic model was used to determine key baseline factors associated with achieving the primary end point. Finally, a logistic model examined the association between the percentage of weight loss at 1 year and the primary end point of a glycated hemoglobin level of 6.0% or less at 5 years. Analyses were performed with the use of SAS software, version 9.2 (SAS Institute). Additional details are available in the Supplementary Appendix and the protocol.
RESULTS
TRIAL PATIENTS
Of the 150 patients who underwent randomization from March 2007 through January 2011, a total of 9 patients never started the assigned treatment and withdrew from the trial immediately after randomization or during the initial 6 months after randomization (8 patients in the medical-therapy group and 1 patient in the sleeve-gastrectomy group); 6 patients were lost to follow-up. One patient in the medical-therapy group died from myocardial infarction during year 4. Overall, 134 of the 150 patients (89%) were included in the 5-year assessment. One patient in the medical-therapy group, in whom a glycated hemoglobin level of more than 9% had been reported, underwent gastric bypass during year 3, owing to failure of the medical treatment. One patient in the sleeve-gastrectomy group underwent gastric bypass during year 4 for the treatment of a gastric fistula.
The baseline characteristics of the 150 patients who underwent randomization were reported previously.8 In the current analysis of 134 patients, 66% of the patients were women. The mean (±SD) age was 49±8 years, and the mean BMI was 37±3.5; 49 patients (37%) had a BMI of less than 35. The mean glycated hemoglobin level was 9.2±1.5%, and the mean duration of diabetes was 8.4±5.2 years, with 44% of patients requiring insulin at baseline. There were no significant differences at baseline among the three groups (Table S1 in the Supplementary Appendix).
PRIMARY END POINT
Among the 134 patients who completed 5 years of follow-up, a glycated hemoglobin level of 6.0% or less at 5 years was achieved in 2 of 38 patients (5%) in the medical-therapy group, as compared with 14 of 49 patients (29%) in the gastric-bypass group (P = 0.01) and 11 of 47 patients (23%) in the sleeve-gastrectomy group (P = 0.03) (Table 1). After adjustment for multiple comparisons, the respective P values changed from 0.01 to 0.03 and from 0.03 to 0.07, respectively. When we performed an imputed intention-to-treat analysis that included all 150 patients who underwent randomization, including the 16 patients who had missing final values on glycated hemoglobin level, the respective P values changed to 0.08 and 0.17, respectively (with the use of the multiple imputation procedure in SAS software), 0.002 and 0.006 (with minimum value imputation), and 0.003 and 0.02 (with mean value imputation); further details are provided in Additional Statistical Methods and in Table S8, both in the Supplementary Appendix. A duration of diabetes of less than 8 years and random assignment to gastric bypass alone were the only significant predictors of achieving a glycated hemoglobin level of 6.0% or less (P = 0.007 and P = 0.03, respectively) (Table S2 in the Supplementary Appendix). The percentage of weight loss at 1 year was significantly associated with achieving the primary end point at 5 years (odds ratio, 1.10; 95% confidence interval, 1.04 to 1.16; P<0.001). Relapse of glycemic control (all groups), which was defined as having met the primary end point of a glycated hemoglobin level of 6% or less at 1 year but not at 5 years, was not associated with weight regain (Table S3 in the Supplementary Appendix).
Table 1.
Primary and Secondary End Points at 5 Years.*
| End Point | Study Group | P Value† | ||||
|---|---|---|---|---|---|---|
| Medical Therapy (N = 38) | Gastric Bypass (N = 49) | Sleeve Gastrectomy (N = 47) | Gastric Bypass vs. Medical Therapy | Sleeve Gastrectomy vs. Medical Therapy | Gastric Bypass vs. Sleeve Gastrectomy | |
| Primary end point | ||||||
|
| ||||||
| Glycated hemoglobin ≤6.0% | ||||||
|
| ||||||
| In analysis of patients who completed the trial — no. of patients (%) | 2 (5.3)‡ | 14 (28.6) | 11 (23.4) | 0.01 (unadjusted); 0.03 (adjusted) | 0.03 (unadjusted); 0.07 (adjusted) | 0.53 (unadjusted); 0.53 (adjusted) |
|
| ||||||
| Estimated rate from imputed analysis — %§ | 7.3 | 26.4 | 20.4 | 0.08 | 0.17 | 0.48 |
|
| ||||||
| Secondary end points | ||||||
|
| ||||||
| Glycated hemoglobin — no. of patients (%) | ||||||
|
| ||||||
| ≤6.0% without diabetes medications | 0 | 11 (22.4) | 7 (14.9) | 0.006¶ | 0.04¶ | 0.34 |
|
| ||||||
| ≤6.5% | 6 (15.8) | 19 (38.8) | 17 (36.2) | 0.06 | 0.06 | 0.79 |
|
| ||||||
| ≤6.5% without diabetes medications | 0 | 15 (30.6) | 11 (23.4) | 0.0033 | 0.0023 | 0.43 |
|
| ||||||
| ≤7.0% | 8 (21.1) | 25 (51.0) | 23 (48.9) | 0.012 | 0.016 | 0.84 |
|
| ||||||
| Glycated hemoglobin level — % | ||||||
|
| ||||||
| At baseline | 8.8±1.1 | 9.3±1.4 | 9.5±1.7 | |||
|
| ||||||
| At 5 yr | 8.5±2.2 | 7.3±1.5 | 7.4±1.6 | |||
|
| ||||||
| Change from baseline | −0.3±2.0 | −2.1±1.8 | −2.1±2.3 | 0.003 | 0.003 | 0.67 |
|
| ||||||
| Median fasting plasma glucose (IQR) — mg/dl | ||||||
|
| ||||||
| At baseline | 157 (120 to 193) | 196 (143 to 231) | 164 (129 to 229) | |||
|
| ||||||
| At 5 yr | 129 (97 to 172) | 110 (92 to 150) | 111 (93 to 141) | |||
|
| ||||||
| Change from baseline|| | −14 (−60 to 23) | −72 (−114 to −29) | −49 (−120 to −4) | 0.003 | 0.02 | 0.35 |
|
| ||||||
| Body weight — kg | ||||||
|
| ||||||
| At baseline | 105.0±14.4 | 106.8±14.9 | 100.4±16.8 | |||
|
| ||||||
| At 5 yr | 99.0±17.0 | 83.4±15.3 | 81.9±15.0 | |||
|
| ||||||
| Change from baseline | −5.3±10.8 | −23.2±9.6 | −18.6±7.5 | 0.003 | 0.003 | 0.01 |
|
| ||||||
| LDL cholesterol — mg/dl | ||||||
|
| ||||||
| At baseline | 100.9±36.8 | 91.4±28.9 | 105.7±40.2 | |||
|
| ||||||
| At 5 yr | 95.8±41.9 | 93.3±35.5 | 115.1±42.4 | |||
|
| ||||||
| % Change from baseline to 5 yr|| | 3.7±55.3 | 12.4±53.8 | 16.6±48.6 | 0.99 | 0.84 | 0.99 |
|
| ||||||
| HDL cholesterol — mg/dl | ||||||
|
| ||||||
| At baseline | 48.7±12.8 | 45.8±13.2 | 44.3±12.1 | |||
|
| ||||||
| At 5 yr | 50.4±12.4 | 60.0±20.2 | 57.0±16.6 | |||
|
| ||||||
| % Change from baseline to 5 yr|| | 7.0±44.5 | 31.9±29.1 | 29.6±29.5 | 0.012 | 0.016 | 0.75 |
|
| ||||||
| Median triglycerides (IQR) — mg/dl | ||||||
|
| ||||||
| At baseline | 166 (97 to 235) | 171 (125 to 257) | 160 (119 to 214) | |||
|
| ||||||
| At 5 yr | 118 (85 to 169) | 114 (81 to 165) | 108 (81 to 123) | |||
|
| ||||||
| % Change from baseline to 5 yr|| | −8.3 (−37.9 to 22.2) | −39.8 (−58.4 to 7.1) | −29.4 (−51.4 to −2.9) | 0.03 | 0.04 | 0.47 |
|
| ||||||
| Systolic blood pressure — mm Hg | ||||||
|
| ||||||
| At baseline | 135.6±17.7 | 134.7±18.9 | 136.7±17.9 | |||
|
| ||||||
| At 5 yr | 131.5±14.55 | 131.4±18.77 | 128.3±11.60 | |||
|
| ||||||
| Change from baseline to 5 yr | −4.0±20.1 | −3.3±22.8 | −8.3±20.4 | 0.88 | 0.78 | 0.78 |
|
| ||||||
| Diastolic blood pressure — mm Hg | ||||||
|
| ||||||
| At baseline | 82.0±11.4 | 81.8±10.2 | 82.2±11.7 | |||
|
| ||||||
| At 5 yr | 77.62±9.83 | 75.98±11.57 | 74.11±11.49 | |||
|
| ||||||
| Change from baseline to 5 yr | −4.2±11.4 | −5.8±12.6 | −8.1±14.7 | 0.86 | 0.57 | 0.86 |
Plus–minus values are means ±SD. To convert the values for glucose to millimoles per liter, multiply by 0.05551. To convert the values for cholesterol to millimoles per liter, multiply by 0.02586. To convert the values for triglycerides to millimoles per liter, multiply by 0.01129. HDL denotes high-density lipoprotein, IQR interquartile range, and LDL low-density lipoprotein.
P values for the primary end point in the analysis of patients who completed the trial are shown as unadjusted and as adjusted for multiple comparisons with the use of the Bonferroni step-down procedure. All P values for the secondary end points were adjusted for multiple comparisons.
One patient in the medical-therapy group crossed over to the gastric-bypass group during year 3 owing to the failure of the patient’s medical therapy and is counted in the denominator of the medical-therapy group.
The imputed intention-to-treat population comprised all 150 patients who underwent randomization (50 per group).
Values were compared with the use of Fisher’s exact test.
Because of the variability and skewness of the data, the change from baseline or the percent change from baseline is not the numerical difference between the group-level value at baseline and the value at 5 years.
GLYCEMIC CONTROL
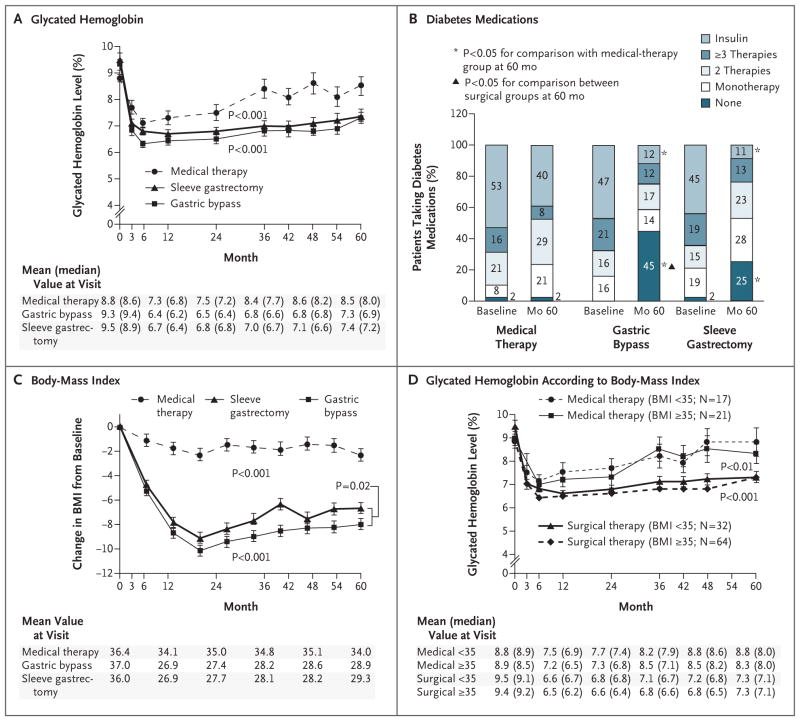
After 5 years, each of the two surgical procedures was superior to intensive medical therapy alone with respect to achievement of the exploratory targets for glycated hemoglobin of 6% or less without the use of diabetes medications (remission), 6.5% or less without the use of diabetes medications, and 7.0% or less with the use of diabetes medications (P<0.05 for all comparisons) (Table 1). The decreases from baseline in median fasting plasma glucose levels were greater in the two surgical groups than in the medical-therapy group (P<0.05 for both comparisons) (Table 1). There were more rapid, larger, and more sustained reductions in the levels of glycated hemoglobin and fasting plasma glucose, in BMI, and in the use of glucose-lowering medications in the two surgical groups than in the medical-therapy group (Fig. 1A, 1B, and 1C; and Table S3 and Figs. S1 through S5 in the Supplementary Appendix). The reductions in glycated hemoglobin levels and BMI in the surgical groups were similar among patients with a BMI of less than 35 and those with a BMI of 35 or more (Fig. 1D, and Fig. S4 in the Supplementary Appendix). Additional secondary end points are shown in Table S3 in the Supplementary Appendix.
Figure 1. Mean Changes in Measures of Diabetes Control from Baseline to 5 Years.
Shown are the mean glycated hemoglobin levels (Panel A), the percent change in diabetes medications during the study period (Panel B), the changes in body-mass index (BMI, the weight in kilograms divided by the square of the height in meters) (Panel C), and the mean glycated hemoglobin levels according to BMI (Panel D) over a 5-year period among patients receiving intensive medical therapy alone, those who underwent sleeve gastrectomy, and those who underwent a gastric bypass procedure. ⌶ bars indicate standard errors. Mean values in each group are provided below the graphs; in Panels A and D, median values are also provided in parentheses. P values for the comparison between each surgical group and the medical-therapy group in Panels A, C, and D were derived from overall treatment effect in the repeated measurements model. In Panel D, P<0.001 for the comparison between the surgical groups and the medical-therapy group for the subgroup of patients with a BMI of less than 35; P<0.01 for the comparison for the subgroup with a BMI of 35 or more.
MEDICATION USE
At 5 years, the use of cardiovascular and glucose-lowering medications, including insulin, was reduced from baseline in the two surgical groups, and patients in the surgical groups required significantly fewer such medications than did patients in the medical-therapy group at 5 years (Fig. 1B, and Table S4 and Fig. S5 in the Supplementary Appendix). The percentage of patients who were not taking any glucose-lowering medications was significantly higher in the gastric-bypass group than in the sleeve-gastrectomy group (P<0.05) (Fig. 1B, and Table S4 in the Supplementary Appendix). Approximately 89% of patients in the surgical groups were not taking insulin at 5 years and maintained an average glycated hemoglobin level of 7.0%, whereas only 61% of patients in the medical-therapy group were not taking insulin at 5 years, with an average glycated hemoglobin level of 8.5%.
WEIGHT LOSS
At 5 years, reductions in body weight, BMI, waist circumference, and waist-to-hip ratio were greater after gastric bypass and sleeve gastrectomy than after intensive medical therapy (P<0.05 for all comparisons) (Table 1 and Fig. 1C, and Table S3 in the Supplementary Appendix). The reduction in body weight was greater after gastric bypass than after sleeve gastrectomy (P = 0.01).
LIPID LEVELS AND BLOOD PRESSURE
The decrease from baseline in triglyceride levels and the increase from baseline in high-density lipoprotein (HDL) cholesterol levels were significantly greater at 5 years after the two surgical procedures than after intensive medical therapy (Table 1). No significant differences in blood pressure or low-density lipoprotein cholesterol levels were observed among the three study groups, although the number of medications needed to treat hyperlipidemia and hypertension was significantly lower in the surgical groups than in the medical-therapy group (Table 1 and Fig. 1B, and Table S4 in the Supplementary Appendix).
RENAL OUTCOMES
At 5 years, the urinary albumin-to-creatinine ratio (as measured in milligrams of albumin to grams of creatinine) had decreased significantly from baseline in the sleeve-gastrectomy group (P<0.001) only and was significantly lower in the sleeve-gastrectomy group than in the medical-therapy group (P<0.001) (Table S5 in the Supplementary Appendix). No significant changes from baseline in the rates of albuminuria were observed in any group at 5 years. Other measures of renal function, including serum creatinine level and glomerular filtration rate, are included in Table S5 in the Supplementary Appendix.
OPHTHALMOLOGIC OUTCOMES
No significant change from baseline in retinopathy scores, the incidence of macular edema, or visual acuity was observed in any study group. In addition, no significant differences in these ophthalmologic end points were seen among the three groups at 5 years (Tables S6a, S6b, and S6c in the Supplementary Appendix).
QUALITY OF LIFE
Scores on each domain of the RAND 36-Item Health Survey range from 0 to 100, with higher scores indicating better health. Among patients in the gastric-bypass and sleeve-gastrectomy groups as compared with the medical-therapy group, significant mean (±SD) changes from baseline in general health scores (17±20, 16±22, and 0.3±16, respectively; P<0.05 for both) and changes in bodily pain scores (−2.4±25, 0.5±21, and −17±25; P<0.05 for both) were observed, with results favoring the surgical groups at 5 years from randomization (Fig. S6 and Table S7 in the Supplementary Appendix). Among patients in the medical-therapy group, none of the quality-of-life components improved significantly from baseline, and bodily pain and emotional well-being significantly worsened. Patients in both surgical groups had significant improvements in the physical functioning, general health, and energy–fatigue components of the RAND-36, although emotional well-being worsened significantly among patients in the gastric-bypass group.
ADVERSE EVENTS
Adverse events reported through year 5 are shown in Table 2. Subsequent surgical interventions were required in four patients in the surgical groups during year 1.8 One late reoperation, a successful laparoscopic conversion of sleeve gastrectomy to gastric bypass owing to recurrent gastric fistula, occurred in year 4. One patient in the medical-therapy group had a fatal myocardial infarction and one patient in the sleeve-gastrectomy group had a stroke. Excessive weight gain was observed in 19% of the patients in the medical-therapy group and in no patients in either surgical group (P<0.001). Mild anemia (mean hemoglobin level, 11.9±1.5 g per deciliter) was more common in the two surgical groups than in the medical-therapy group (P<0.009).
Table 2.
Adverse Events through 5 Years.*
| Event | Medical Therapy (N = 43) | Gastric Bypass (N = 50) | Sleeve Gastrectomy (N = 49) |
|---|---|---|---|
| number of patients (percent) | |||
| Cardiovascular | |||
|
| |||
| Fatal myocardial infarction | 1 (2) | 0 | 0 |
|
| |||
| Stroke | 0 | 0 | 1 (2) |
|
| |||
| Gastrointestinal | |||
|
| |||
| Bowel obstruction | 1 (2) | 1 (2) | 1 (2) |
|
| |||
| Stricture | 0 | 1 (2) | 1 (2) |
|
| |||
| Ulcer | 1 (2) | 4 (8) | 1 (2) |
|
| |||
| Leak | 0 | 0 | 1 (2) |
|
| |||
| Bleeding | 0 | 2 (4) | 0 |
|
| |||
| Gastroesophageal reflux disease | 9 (21) | 5 (10) | 13 (27) |
|
| |||
| Dumping syndrome | 0 | 4 (8) | 1 (2) |
|
| |||
| Gallstone diseases | 0 | 1 (2) | 1 (2) |
|
| |||
| Urinary | |||
|
| |||
| Nephropathy† | 6 (14) | 11 (22) | 9 (18) |
|
| |||
| Calculus | 6 (14) | 6 (12) | 5 (10) |
|
| |||
| Incontinence | 2 (5) | 0 | 2 (4) |
|
| |||
| Neurologic and psychiatric | |||
|
| |||
| Memory loss | 1 (2) | 1 (2) | 1 (2) |
|
| |||
| Neuropathy | 4 (9) | 1 (2) | 5 (10) |
|
| |||
| Depression | 11 (26) | 7 (14) | 12 (24) |
|
| |||
| Soft tissue and musculoskeletal | |||
|
| |||
| Hernia | 1 (2) | 3 (6) | 1 (2) |
|
| |||
| Limb fracture | 4 (9) | 4 (8) | 3 (6) |
|
| |||
| Foot ulcer | 0 | 2 (4) | 2 (4) |
|
| |||
| Nutritional and metabolic | |||
|
| |||
| Intravenous treatment for dehydration | 3 (7) | 7 (14) | 4 (8) |
|
| |||
| Anemia | 7 (16) | 14 (28) | 24 (49)‡ |
|
| |||
| Hypoglycemic episode | 39 (91) | 32 (64)‡ | 40 (82) |
|
| |||
| Severe hypoglycemia requiring intervention | 0 | 2 (4) | 0 |
|
| |||
| Hypoalbuminemia | 0 | 0 | 0 |
|
| |||
| Hyperglycemia | 9 (21) | 3 (6) | 3 (6) |
|
| |||
| Ketoacidosis | 0 | 1 (2) | 0 |
|
| |||
| Excessive weight gain§ | 8 (19) | 0‡ | 0‡ |
|
| |||
| Excessive weight loss¶ | 0 | 0 | 0 |
|
| |||
| Infectious | |||
|
| |||
| Wound infection | 0 | 3 (6) | 3 (6) |
|
| |||
| Pneumonia | 0 | 2 (4) | 1 (2) |
|
| |||
| Sepsis | 0 | 0 | 1 (2) |
| Cancer | 2 (5) | 2 (4) | 3 (6) |
Not included in the safety analysis were seven patients in the medical-therapy group who withdrew immediately after randomization and one patient in the sleeve-gastrectomy group who had anemia before withdrawing from the trial before surgery. Other patients who started the trial but later withdrew or were lost to follow-up and patients who died were included in this analysis until their discontinuation or death.
Nephropathy was defined according to any one of the following criteria: doubling of the serum creatinine level or a decrease in the glomerular filtration rate of more than 20%; development of macroalbuminuria (urine albumin-to-creatinine ratio, >300 [as measured in milligrams of albumin to grams of creatinine]); or renal transplantation, initiation of dialysis, or an increase in the serum creatinine level of more than 3.3 mg per deciliter (290 μmol per liter) in the absence of an acute reversible cause.
P<0.05 for the comparison between the medical-therapy group and the surgical group.
Excessive weight gain was defined as a 5% increase in body weight over baseline.
Excessive weight loss was defined as attaining a body-mass index of less than 19 at 5 years.
DISCUSSION
The results of this 5-year follow-up analysis from the STAMPEDE trial showed that bariatric surgery was superior to intensive medical therapy in terms of glycemic control, weight reduction, medication reduction, improvement in lipid levels, and quality of life. Patients who underwent gastric bypass or sleeve gastrectomy were significantly more likely to achieve and maintain a glycated hemoglobin level of 6.0% or less, with or without medications, than were those who received intensive medical therapy alone (29% and 23%, respectively, vs. 5%; P<0.03 for both comparisons). In an analysis that included adjustment for multiple comparisons and in an intention-to-treat analysis (neither analysis was prespecified), the P values for gastric bypass and sleeve gastrectomy as compared with medical therapy alone remained directionally consistent with, and qualitatively similar to, the analyses that were prespecified in the protocol. The surgically treated patients had superior glycemic control throughout the 5-year period while also using fewer diabetes medications, including insulin. More than 88% of the surgical patients had glycemic control that was considered to be very good to acceptable (average glycated hemoglobin level of 7.0%), without the use of insulin. A majority of the surgical patients who achieved a glycated hemoglobin level of 6.0% or less reached that target without the use of diabetes medications, whereas none of the patients in the medical-therapy group reached that target without the use of diabetes medications. Surgical patients had a decrease of 2.1 percentage points in glycated hemoglobin levels at 5 years, as compared with a reduction of only 0.3 percentage points among the patients who received medical therapy alone. The results of surgery are striking in this population with long-standing, uncontrolled diabetes. A duration of diabetes of less than 8 years was the main predictor of achieving a glycated hemoglobin level of 6.0% or less; this finding, which was also seen in other studies,2–7,11,12,16 underscores the importance of early surgical intervention for maximal glycemic benefit. Weight loss by year 1 correlated with success in achieving the primary end point, but relapse of poor glycemic control was not associated with weight regain.
Analyses of the secondary end points, including BMI, body weight, waist circumference, levels of triglycerides and HDL cholesterol, and quality of life also showed results at 5 years that were more favorable in the surgical groups than in the medical-therapy group. A decrease from baseline in the urinary albumin-to-creatinine ratio was noted after sleeve gastrectomy but not after gastric bypass or after medical therapy alone. No significant differences from baseline in either the rates of albuminuria or retinopathy scores were observed after 5 years. The patients in the two surgical groups had a significant reduction in the use of antihypertensive and lipid-lowering agents. One late reoperation for fistula after sleeve gastrectomy occurred, and some adverse effects of surgical treatment were observed but were not debilitating and, with the exception of mild anemia, were relatively uncommon after the first year.
Until recently, most studies that evaluated the effect of bariatric surgery on glycemic control in patients with type 2 diabetes were observational, included only severely obese patients, and showed high rates of remission after surgery.1–6 The initial and 3-year analyses from the current trial8,10 showed that up to 3 years after randomization, patients who underwent bariatric surgery had better glycemic control than patients who received medical therapy alone, a finding consistent with that in other trials.7,12–17,19 In a 5-year randomized, controlled trial (involving 60 patients) that compared medical therapy (therapeutic goal of a glycated hemoglobin level of <7.0%) with gastric bypass and biliopancreatic diversion in severely obese patients (mean BMI, 44), Mingrone et al. found that 50% of surgical patients, as compared with none of the medically treated patients, maintained long-term diabetes remission (defined as a glycated hemoglobin level of <6.5% without diabetes medications) (P<0.001).11 Biliopancreatic diversion was associated with a higher rate of diabetes remission but also a higher rate of serious nutritional deficiencies than gastric bypass. Similarly, our current findings showed continued durability of glycemic improvement after gastric bypass and sleeve gastrectomy, persistent weight loss, and reductions in diabetes and cardiovascular medications at 5 years, with the gap in glycemic control between medical and surgical therapy appearing to widen over time (Fig. 1A). In contrast to the trial reported by Mingrone et al., our trial involving 150 patients with mild obesity (BMI, 27 to 34) assessed more intensive medical therapy and a more aggressive primary end point (glycated hemoglobin level of ≤6.0% with or without medications), and it included sleeve gastrectomy (the most common metabolic operation). Other randomized, controlled trials showed metabolic improvements after gastric bypass that were similar to those of our trial11–15,17,19 but showed less consistent improvements after gastric banding than did our trial.7,15,16,18 Lower rates of nutritional complications were observed after gastric bypass, sleeve gastrectomy, and gastric banding in randomized, controlled trials than the rates observed after biliopancreatic diversion.7–19
Data on changes in retinopathy scores and visual acuity from other randomized trials involving bariatric surgery are lacking. In our trial, no significant changes were observed at 2 years after randomization22 or in the current 5-year analysis (Table S6 in the Supplementary Appendix). In contrast, initial worsening of retinopathy was shown in the Diabetes Control and Complications Trial within the first year of intensive medical treatment.23 Our results should mitigate concerns that rapid glycemic improvement with surgery could worsen retinopathy.
Using a validated quality-of-life instrument, we found significant and durable decreases in bodily pain and improvements in general health in the surgical groups as compared with the medical-therapy group at 5 years. Despite some improvement in glycemic control and weight loss, intensive medical therapy resulted in no significant improvements from baseline in quality-of-life components, and bodily pain and emotional well-being actually worsened. Similarly, Mingrone et al. found that at 5 years, quality of life was superior among patients who had undergone bariatric surgery than among those who received medical therapy.11
Some advantages of gastric bypass over sleeve gastrectomy have emerged. At 5 years, gastric bypass was associated with greater weight loss than sleeve gastrectomy, with fewer diabetes medications. Our trial was not powered sufficiently to detect small but clinically significant differences between the two procedures. Further clarification will require larger trials with longer follow-up.
Our 3-year analysis10 and other short-term randomized, controlled trials7,12–17,19 showed that surgical patients who had a BMI of 27 to 34 (36% of the patients in our trial) and diabetes had improvement in glycemic control that was similar to that of surgical patients who had a BMI of 35 or more and that was superior to that of patients who received medical therapy alone. Nearly all financial coverage policies for bariatric surgery worldwide (public and private) exclude patients with a BMI of less than 35. Our 5-year follow-up analysis shows that improvement in glycemic control after bariatric surgery among patients with a BMI of 27 to 34 was durable and was superior to that with intensive medical therapy (Fig. 1D).
Limitations of our study include an inadequate sample size and duration to detect differences in the incidence of cardiovascular and end-organ complications and to detect some differences in outcomes between the two surgical procedures. Despite the intent to continue intensive medical treatment in the control group throughout the study, a reduction in diabetes medication use was observed after 3 years. Plausible explanations for nonadherence to medication use and lifestyle counseling include economic deterrents, unacceptable side effects from the drugs, and behavioral maladaptation. A higher degree of adherence may have decreased the efficacy gap between medical therapy and surgery.24
The current 5-year follow-up of patients in our trial showed that the beneficial effects of bariatric surgery on glycemic control were durable, even among patients with mild obesity (BMI of 27 to 34), which led to a sustained reduction in the use of diabetes and cardiovascular medications. Changes in body weight, lipid levels, and quality of life after surgery were superior to the changes observed after medical therapy alone. The potential benefits of bariatric surgery on clinical end points, such as myocardial infarction, stroke, renal failure, blindness, and death, as suggested in nonrandomized trials, can be adequately assessed only through larger, multicenter trials.
Supplementary Material
Acknowledgments
Supported by Ethicon Endo-Surgery (grant EES IIS 19900), LifeScan, the Cleveland Clinic, and the National Institutes of Health (grant R01 DK089547).
We thank Chytaine Hall and Beth Abood for recruitment and retention support; Craig Balog, Debbie Gladish, Susan Thomas, Andrew Pikus, and Randy Scott for statistical and data management support; Matthew Kroh, M.D., Tomasz Rogula, M.D., Bipan Chand, M.D., Derick Cetin, D.O., Archana Gorty, M.D., Bartolome Burguera, M.D., Ph.D., Betul Hatipoglu, M.D., Laurence Kennedy, M.D., Mario Skugor, M.D., Adi Mehta, M.D., Leslie Heinberg, Ph.D., Julie Merrell, Ph.D., Kathleen Ashton, Ph.D., Megan Lavery, Ph.D., Ellen Calogeras, Wendy Kirby, and Lauren Sullivan for medical-site support; Suzanne Turner for graphical support; and J. Michael Henderson, M.D. (chair), James B. Young, M.D., and Venu Menon, M.D., for serving on the data and safety monitoring board.
Funded by Ethicon Endo-Surgery and others; STAMPEDE ClinicalTrials.gov number, NCT00432809.
Footnotes
The contributions of the authors and committee members in the Surgical Treatment and Medications Potentially Eradicate Diabetes Efficiently (STAMPEDE) trial are listed in the Supplementary Appendix, available at NEJM.org.
Presented in part at the 65th Annual Scientific Session of the American College of Cardiology, Chicago, April 4, 2016.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Pories WJ, Swanson MS, MacDonald KG, et al. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg. 1995;222:339–52. doi: 10.1097/00000658-199509000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schauer PR, Burguera B, Ikramuddin S, et al. Effect of laparoscopic Roux-en Y gastric bypass on type 2 diabetes mellitus. Ann Surg. 2003;238:467–85. doi: 10.1097/01.sla.0000089851.41115.1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vest AR, Heneghan HM, Agarwal S, Schauer PR, Young JB. Bariatric surgery and cardiovascular outcomes: a systematic review. Heart. 2012;98:1763–77. doi: 10.1136/heartjnl-2012-301778. [DOI] [PubMed] [Google Scholar]
- 4.Sjöström L, Peltonen M, Jacobson P, et al. Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. JAMA. 2014;311:2297–304. doi: 10.1001/jama.2014.5988. [DOI] [PubMed] [Google Scholar]
- 5.Arterburn DE, Olsen MK, Smith VA, et al. Association between bariatric surgery and long-term survival. JAMA. 2015;313:62–70. doi: 10.1001/jama.2014.16968. [DOI] [PubMed] [Google Scholar]
- 6.Brethauer SA, Aminian A, Romero-Talamás H, et al. Can diabetes be surgically cured? Long-term metabolic effects of bariatric surgery in obese patients with type 2 diabetes mellitus. Ann Surg. 2013;258:628–37. doi: 10.1097/SLA.0b013e3182a5034b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dixon JB, O’Brien PE, Playfair J, et al. Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. JAMA. 2008;299:316–23. doi: 10.1001/jama.299.3.316. [DOI] [PubMed] [Google Scholar]
- 8.Schauer PR, Kashyap SR, Wolski K, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med. 2012;366:1567–76. doi: 10.1056/NEJMoa1200225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kashyap SR, Bhatt DL, Wolski K, et al. Metabolic effects of bariatric surgery in patients with moderate obesity and type 2 diabetes: analysis of a randomized control trial comparing surgery with intensive medical treatment. Diabetes Care. 2013;36:2175–82. doi: 10.2337/dc12-1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric surgery versus intensive medical therapy for diabetes — 3-year outcomes. N Engl J Med. 2014;370:2002–13. doi: 10.1056/NEJMoa1401329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric-metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 year follow-up of an open-label, single-centre, randomised controlled trial. Lancet. 2015;386:964–73. doi: 10.1016/S0140-6736(15)00075-6. [DOI] [PubMed] [Google Scholar]
- 12.Ikramuddin S, Billington CJ, Lee WJ, et al. Roux-en-Y gastric bypass for diabetes (the Diabetes Surgery Study): 2-year outcomes of a 5-year, randomised, controlled trial. Lancet Diabetes Endocrinol. 2015;3:413–22. doi: 10.1016/S2213-8587(15)00089-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liang Z, Wu Q, Chen B, Yu P, Zhao H, Ouyang X. Effect of laparoscopic Roux-en-Y gastric bypass surgery on type 2 diabetes mellitus with hypertension: a randomized controlled trial. Diabetes Res Clin Pract. 2013;101:50–6. doi: 10.1016/j.diabres.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 14.Halperin F, Ding SA, Simonson DC, et al. Roux-en-Y gastric bypass surgery or lifestyle with intensive medical management in patients with type 2 diabetes: feasibility and 1-year results of a randomized clinical trial. JAMA Surg. 2014;149:716–26. doi: 10.1001/jamasurg.2014.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Courcoulas AP, Belle SH, Neiberg RH, et al. Three-year outcomes of bariatric surgery vs lifestyle intervention for type 2 diabetes mellitus treatment: a randomized clinical trial. JAMA Surg. 2015;150:931–40. doi: 10.1001/jamasurg.2015.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wentworth JM, Playfair J, Laurie C, et al. Multidisciplinary diabetes care with and without bariatric surgery in overweight people: a randomised controlled trial. Lancet Diabetes Endocrinol. 2014;2:545–52. doi: 10.1016/S2213-8587(14)70066-X. [DOI] [PubMed] [Google Scholar]
- 17.Parikh M, Chung M, Sheth S, et al. Randomized pilot trial of bariatric surgery versus intensive medical weight management on diabetes remission in type 2 diabetic patients who do NOT meet NIH criteria for surgery and the role of soluble RAGE as a novel biomarker of success. Ann Surg. 2014;260:617–24. doi: 10.1097/SLA.0000000000000919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ding SA, Simonson DC, Wewalka M, et al. Adjustable gastric band surgery or medical management in patients with type 2 diabetes: a randomized clinical trial. J Clin Endocrinol Metab. 2015;100:2546–56. doi: 10.1210/jc.2015-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cummings DE, Arterburn DE, Westbrook EO, et al. Gastric bypass surgery vs intensive lifestyle and medical intervention for type 2 diabetes: the CROSSROADS randomised controlled trial. Diabetologia. 2016;59:945–53. doi: 10.1007/s00125-016-3903-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kashyap SR, Bhatt DL, Schauer PR STAMPEDE Investigators. Bariatric surgery vs. advanced practice medical management in the treatment of type 2 diabetes mellitus: rationale and design of the Surgical Therapy And Medications Potentially Eradicate Diabetes Efficiently trial (STAMPEDE) Diabetes Obes Metab. 2010;12:452–4. doi: 10.1111/j.1463-1326.2009.01172.x. [DOI] [PubMed] [Google Scholar]
- 21.Hays RD, Sherbourne CD, Mazel RM. The RAND 36-Item Health Survey 1. 0. Health Econ. 1993;2:217–27. doi: 10.1002/hec.4730020305. [DOI] [PubMed] [Google Scholar]
- 22.Singh RP, Gans R, Kashyap SR, et al. Effect of bariatric surgery versus intensive medical management on diabetic ophthalmic outcomes. Diabetes Care. 2015;38(3):e32–e33. doi: 10.2337/dc14-2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.The Diabetes Control and Complications Trial Research Group. Early worsening of diabetic retinopathy in the Diabetes Control and Complications Trial. Arch Ophthalmol. 1998;116:874–86. doi: 10.1001/archopht.116.7.874. [DOI] [PubMed] [Google Scholar]
- 24.Kolandaivelu K, Leiden BB, O’Gara PT, Bhatt DL. Non-adherence to cardiovascular medications. Eur Heart J. 2014;35:3267–76. doi: 10.1093/eurheartj/ehu364. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.