Summary
Alcoholic hepatitis (AH) is a severe form of alcoholic liver disease with high mortality. The pathogenesis of AH is not fully understood, but it is generally believed that inflammation is a key factor leading to liver failure in AH. Steroids, which have broad immunosuppressive effects, have been used for the treatment of AH over the last forty years. Steroids elicit modest improvement in short-term survival rate in patients with severe AH, but also cause severe side effects. Several specific inflammatory targets (e.g., IL-1, LPS, and gut microbiota) are currently under investigation for the treatment of AH with the goal to obviate or reduce steroid administration. In addition to inflammation, impaired liver regeneration is another major cause of liver failure in AH, which deteriorates further after steroid treatment because inflammation plays a key role in promoting liver repair. Interleukin-22 (IL-22) is a promising drug for the treatment of AH because of its hepatoprotective and anti-fibrotic functions and relatively few known side effects. In addition, IL-22 treatment also ameliorates bacterial infection and kidney injury, two major complications associated with severe AH. IL-22 is currently under investigation in preclinical and clinical studies and may hold great promise for AH by providing more beneficial effects and fewer side effects than current therapies.
Introduction
More than 95% of people with excessive alcohol intake develop fatty liver (steatosis). Among them, approximately 20–40% also develop liver inflammation and fibrosis (also called steatohepatitis), and 5–10% may progress to liver cirrhosis and hepatocellular carcinoma [1]. Alcoholic hepatitis (AH) is a severe form of alcoholic liver disease (ALD), which often occurs in active alcohol drinkers with underlying ALD and can happen at any stage of ALD [2,3]. In severe cases and in patients with cirrhosis, AH has high short-term mortality due to severe complications related to liver failure, bacterial infection, portal hypertension, and renal failure among others [2–5]. Despite extensive research on ALD over the last forty years, the pathogenesis of AH is still not fully understood, and no new effective drugs for ALD have been identified [1]. Use of corticosteroids, which was proposed for the treatment of severe AH in early 1970s [6], is still currently the only drug treatment for this disease; however, they have a modest benefit for short-term survival and significant side effects. In this review, we briefly discuss the current understanding of pathogenesis and treatment of AH with a focus on combination therapy of anti-inflammatory and hepatoprotective drugs.
Pathogenesis of AH: inflammation and impaired liver repair
AH was initially proposed in 1961 as an acute disease that frequently occurs in chronic alcoholics with recent heavy drinking and is characterized by jaundice with severe clinical syndromes, such as anorexia, nausea, upper abdominal pain, hepatomegaly and fever [6]. Liver histology shows that the hallmark of AH is associated with hepatic infiltration of neutrophils [7]. Infiltration of many other types of immune cells in the liver is also observed in AH patients [8], but has not been carefully characterized. Microarray analyses of liver biopsy samples revealed that AH results in elevated levels of a wide variety of cytokines, chemokines, and other inflammatory mediators in the liver [9]. Collectively, inflammation is generally accepted as a key factor contributing to hepatocellular damage and pathogenesis of AH.
Emerging evidence suggests that multiple mechanisms may cause liver inflammation in AH patients [10]. First, excessive alcohol consumption leads to hepatocyte death and oxidative stress, which can activate Kupffer cells/macrophages. Damaged hepatocytes also release a large amount of cellular components, including damage-associated molecular patterns (DAMPs), that enter the circulation and activate neutrophils [10,11]. Second, chronic alcohol consumption results in gut bacterial overgrowth and dysbiosis along with an increase in gut permeability, followed by elevation of bacterial products (e.g., LPS) in the liver and subsequent liver inflammation [12,13]. The fact that AH often occurs in alcoholics with a recent history of excessive drinking suggests that excessive binge drinking is an important factor triggering liver inflammation in AH patients. This is consistent with recent studies in animal models demonstrating that acute ethanol gavage (binge) induces marked increases in neutrophil infiltration and liver injury in mice chronically fed an ethanol diet [14–16]. Future studies revealing the underlying mechanisms by which acute binge drinking activates hepatic inflammation in alcoholics may help identify new therapeutic strategies for the treatment of AH.
The liver is a highly regenerative tissue and is capable of rapid regeneration after injury or loss of tissue. Early studies in a rat model of partial hepatectomy have shown that chronic ethanol feeding inhibits liver regeneration via multiple mechanisms [17]. However, it is difficult to precisely determine liver regeneration in patients because the liver injury that triggers liver regeneration varies significantly between patients. We have previously demonstrated that alcoholic or alcoholic plus HCV cirrhotic livers have significantly fewer Ki67+ and phospho-STAT3+ hepatocytes and bile duct cells than HCV cirrhotic livers, suggesting that hepatocyte proliferation is suppressed in alcoholic cirrhosis compared with HCV cirrhosis [18]. In addition, measurement of ductular reaction and liver progenitor cell expansion, which often occur when hepatocyte proliferation is blocked, is used to indirectly determine liver regeneration in patients. Several recent studies demonstrated that expression of liver progenitor cell markers is highly elevated in AH patients, and this elevation is correlated with short-term mortality [19,20]. This suggests that hepatocyte proliferation is blocked in AH patients, which likely contributes to liver failure and short-term mortality in these patients.
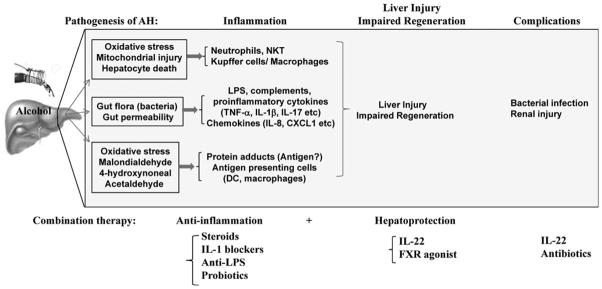
Collectively, inflammation and impaired liver repair are two major factors that lead to liver failure in patients with severe AH (Fig. 1), both of these factors should be targeted for the treatment of this devastating disease.
Figure 1.
Pathogenesis of AH and potential combination therapy for AH. Pathogenesis: excessive alcohol consumption causes liver inflammation via three major mechanisms. First, alcohol damages hepatocytes, leading to the release of damage-associated molecular patterns that activate inflammatory cells. Second, chronic alcohol consumption results in gut bacterial overgrowth and dysbiosis, an increase in gut permeability, followed by elevation of bacterial products (e.g., LPS) in the liver and subsequent liver inflammation. Third, alcohol metabolites interact with proteins to form protein adducts, which can activate adaptive immunity and trigger liver inflammation. In addition, liver regeneration is impaired in severe AH, but the underlying mechanisms are still not known. Finally, severe AH is accompanied by many complications. Combination therapy: severe AH, which is associated with multiple problems, requires combination therapy. Several types of potential combination therapy are listed.
Inflammatory targets of AH (e.g., steroids, IL-1, LPS, gut microbiota)
Because inflammation is commonly believed to play a key role in the pathogenesis of AH, anti-inflammatory therapy for this disorder has been extensively explored over the last forty years [2,3,10]. Treatment of AH with steroids, which are potent immunosuppressive drug, was first proposed in the early 1970s [6] and steroids are still widely used today to treat patients with severe AH. Results from several clinical trials suggest that steroid treatment improves short-term survival but has no beneficial effects on the long-term survival of severe AH patients [21,22]. The improvement of short-term survival by steroids is probably due to the suppression of systemic inflammatory responses. As inflammation plays an important role in promoting liver repair, the broad anti-inflammatory effects of steroids likely block liver regeneration and repair in AH patients. This notion is supported by data from studies in animal models demonstrating that treatment of mice with steroids markedly delays liver repair after hepatotoxin carbon tetrachloride-induced liver injury [23]. Another side effect of steroid treatment is the increased risk of bacterial infection [5]. Taken together, these detrimental effects of steroid treatment could exacerbate AH progression and lead to no beneficial effect for long-term survival in patients with severe AH.
To circumvent the use of broad-spectrum immunosuppressive drugs, such as steroids, researchers have been actively exploring more specific inflammatory targets and have identified a wide variety of inflammatory cytokines, chemokines, and their receptors that are elevated in AH patients and are involved in the pathogenesis of AH [9,10]. Antagonists to block several of these mediators (e.g., IL-1, LPS, and microbiota) are currently being investigated in clinical trials for the treatment of AH patients [3]. Because of the redundancy of many inflammatory factors, targeting a single inflammatory mediator may not be enough to control inflammation in AH patients. However, it is possible to use these inflammatory mediator inhibitors in combination with a reduced dose of steroids for the treatment of AH, which may reduce the side effects of steroid treatment.
Hepatoprotective functions of IL-22
Over the last thirty years, many factors have been identified as having the ability to protect against hepatocellular damage and promote liver regeneration, including IL-6, IL-6 family cytokines, and hepatocyte growth factor. However, the therapeutic application of these factors was limited by their potential side effects, which likely result from the ubiquitous expression of their receptors. The recent discovery of IL-22 and its therapeutic application have received considerable attention in various diseases areas [24], including AH, primarily due to two reasons. First, IL-22 is produced by a limited number of immune cells, such as Th17, Th22, and activated NK cells. Consistent with this observation, it has been shown that hepatic and serum IL-22 levels are not elevated in AH patients [25,26]. Second, IL-22R expression is restricted to hepatocytes and hepatic stellate cells (HSCs). However, immune cells do not express IL-22R [24]. Thus, IL-22 specifically targets hepatocytes and HSCs without affecting immune cells, and therefore, the side effects from IL-22 treatment are expected to be relatively low.
In 2004, we demonstrated for the first time that IL-22 is a potent survival factor for hepatocytes and protects against T cell hepatitis in 2004 [27]. Since then, the protective functions of IL-22 have been demonstrated in a variety of liver injury models (see review [28], references therein) and in many in vivo models using damaged epithelial cells of other organs, including the lung, pancreas, and kidneys [29]. The protective effect of IL-22 is mediated via binding to IL-22R1 and IL-10R2, followed by activation of signal transducer and activator of transcription 3 (STAT3) and subsequent induction of anti-apoptotic and proliferative genes [27]. Interestingly, HSCs, which are not cells of epithelial origin, also express high levels of IL-22R1 and IL-10R2. IL-22 treatment induces HSC senescence and consequently ameliorates liver fibrosis in mouse models [30,31].
Although IL-22-mediated hepatoprotective effects are well documented in animal models, its role in pathogenesis of human liver diseases are less clear. It has been reported that serum or hepatic levels of IL-22 are elevated and correlate positively with the severity of liver diseases and the number of liver progenitor cells in patients with viral hepatitis infection [32–34]. Production of IL-22 has also been implicated in ameliorating liver fibrosis in patients infected with Schistosomes [35]. In vitro treatment with IL-22 promotes liver progenitor cell survival and proliferation [34]. Collectively, these data suggest that elevated IL-22 contributes to disease progression by promoting liver inflammation or plays a compensatory role in promoting liver repair and ameliorating liver fibrosis in patients with hepatitis viral infection or schistosomiasis. In contrast to viral hepatitis, AH patients do not have elevated hepatic and serum IL-22 levels [25,26], although the frequency of IL-22-producing T helper cells was higher in AH patients compared with healthy controls [26]. The high frequency of IL-22 producing T helper cells is associated with a favorable short-term course in AH patients [26], suggesting that low levels of endogenous IL-22 play a protective role in these patients.
Additional benefits of IL-22 treatment: amelioration of bacterial infection and kidney injury
Severe AH is often associated with bacterial infection, which is a major cause of mortality in patients [4,5,36]. Moreover, bacterial infection is further aggravated by steroid treatment [5]. It has been well documented that IL-22 plays an important role in host defence against several invading pathogens by promoting anti-microbial protein production in epithelial cells [37]. Thus, treatment with IL-22 may have an additional benefit in ameliorating bacterial infection in AH.
Acute kidney injury (AKI) is another major cause of death in AH patients [4]. Pentoxifylline, a TNF-α blocker, was unexpectedly found to have the ability to prevent hepatorenal syndrome in AH and may be beneficial for treatment of AH [38]. Recently, others and we have demonstrated that IL-22 plays a role in preventing AKI, and treatment with IL-22 markedly ameliorates AKI in a mouse model of ischemia/reperfusion by promoting tubular epithelial cell survival and regeneration [39,40]. Thus, we speculate that IL-22 therapy also benefits the treatment of AH by ameliorating kidney injury.
Combination therapy: anti-inflammatory plus hepatoprotection
AH is a severe form of liver disease associated with liver inflammation and impaired liver repair, along with other complications, such as bacterial infection, renal failure, etc. This suggests that combination therapy is required for AH. Indeed, several types of combination therapy, including prednisolone plus N-acetylcysteine (anti-oxidant) [41] and prednisolone plus pentoxifylline, have been tested [42]. However, none of these combinations resulted in a better outcome than the prednisolone alone treatment. In addition, several anti-inflammatory targets (e.g., IL-1 blocker, anti-LPS, probiotics) are currently under investigation either alone or in combination with reduced doses of steroids for AH treatment [3]. Such combinations may reduce the side effects from steroid treatment. Another hepatoprotective compound that is current under consideration is the farnesoid X receptor (FXR) agonist. It has been shown that activation of FXR results in some beneficial effects in various types of liver diseases, including non-alcoholic steatohepatitis, by reducing oxidative stress and/or improving bile salt metabolism [3,43]. Treatment with FXR agonist plus or minus steroids may be beneficial for AH patients.
Steroid plus IL-22 could be a good combination for the treatment of severe AH. This combination most likely has more beneficial effects and relatively fewer side effects in AH patients compared with treatment with a single drug. First, steroid treatment attenuates systemic and liver inflammation, which ameliorates liver injury, but also results in inhibition of liver regeneration and promotion of bacterial infection. These two side effects can be ameliorated by additional IL-22 treatment because IL-22 promotes liver regeneration and suppresses bacterial infection. Second, AH is associated with the elevation of many inflammatory mediators, such as IL-6, which may reduce the sensitivity of hepatocytes to IL-22 stimulation because IL-22 shares signalling pathways with IL-6 and IL-6 family cytokines. However, steroid treatment blocks inflammation, thereby restoring hepatocyte response to IL-22 stimulation. Third, AH patients have low basal levels of endogenous IL-22, which likely makes them sensitive to IL-22 therapy [22,23]. Fourth, IL-22 treatment may also result in additional benefits, such as amelioration of bacterial infection and renal injury. Finally, IL-22R1 expression is restricted to hepatocytes, HSCs, and other types of epithelial cells, and it is not expressed in immune cells. Thus, IL-22 treatment is likely to have relatively few side effects. Previous studies have reported that IL-22 stimulates hepatocytes to produce acute phase responses and subsequently induces mild liver inflammation during chronic viral hepatitis [32,33]. But short-term treatment with IL-22 is unlikely to cause significant liver inflammation. Even if IL-22 causes mild inflammation, the steroid treatment could therapeutically control it. Another side effect is that IL-22 promotes liver cancer proliferation [44]. However, the fact transgenic mice with very high serum levels of IL-22 (~6000 pg/mL) have no obvious adverse phenotypes (e.g., inflammation) and do not spontaneously develop any cancers indicates that IL-22 is not toxic and does not initiate cancer development [44]. Therefore, short-term treatment with IL-22 should be safe for AH patients without liver cancer but probably should not be used for those with liver cancer. Indeed, early phase studies on safety of IL-22 in AH patients will soon commence.
In summary, AH is a severe disease with high short-term mortality and requires combination therapy. Several types of combination therapy are currently under consideration for the treatment of severe AH. It is anticipated that combination therapy will prove to be the effective treatment for this devastating disease in the near future.
Footnotes
This article is part of the special issue “Alcohol, Virus and Steatosis evolving to cancer” featuring the conference papers of the 10th International Symposium organized by the Brazilian Society of Hepatology in São Paulo, Brazil, September 30th–October 1st, 2015.
Disclosure of interest
BG declares that he has no conflict of interest concerning this article. Generon Inc (Shanghai, China) will provide recombinant IL-22Fc for an upcoming NIAAA funded study to test safety of IL-22 in AH patients. VJ is PI of this study.
References
- 1.Gao B, Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology. 2011;141:1572–85. doi: 10.1053/j.gastro.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lucey MR, Mathurin P, Morgan TR. Alcoholic hepatitis. N Engl J Med. 2009;360:2758–69. doi: 10.1056/NEJMra0805786. [DOI] [PubMed] [Google Scholar]
- 3.Singal AK, Kamath PS, Gores GJ, et al. Alcoholic hepatitis: current challenges and future directions. Clin Gastroenterol Hepatol. 2014;12:555–64. doi: 10.1016/j.cgh.2013.06.013. quiz e31–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Altamirano J, Fagundes C, Dominguez M, et al. Acute kidney injury is an early predictor of mortality for patients with alcoholic hepatitis. Clin Gastroenterol Hepatol. 2012;10:65e3–71e3. doi: 10.1016/j.cgh.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 5.Louvet A, Wartel F, Castel H, et al. Infection in patients with severe alcoholic hepatitis treated with steroids: early response to therapy is the key factor. Gastroenterology. 2009;137:541–8. doi: 10.1053/j.gastro.2009.04.062. [DOI] [PubMed] [Google Scholar]
- 6.Beckett AG, Livingstone AV, Hill KR. Acute alcoholic hepatitis. Br Med JS. 1961;2:1113–9. doi: 10.1136/bmj.2.5260.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Altamirano J, Miquel R, Katoonizadeh A, et al. A histologic scoring system for prognosis of patients with alcoholic hepatitis. Gastroenterology. 2014;146:1231–9. e1–6. doi: 10.1053/j.gastro.2014.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leevy CB, Elbeshbeshy HA. Immunology of alcoholic liver disease. Clin Liver Dis. 2005;9:55–66. doi: 10.1016/j.cld.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 9.Dominguez M, Miquel R, Colmenero J, et al. Hepatic expression of CXC chemokines predicts portal hypertension and survival in patients with alcoholic hepatitis. Gastroenterology. 2009;136:1639–50. doi: 10.1053/j.gastro.2009.01.056. [DOI] [PubMed] [Google Scholar]
- 10.Wang HJ, Gao B, Zakhari S, et al. Inflammation in alcoholic liver disease. Annu Rev Nutr. 2012;32:343–68. doi: 10.1146/annurev-nutr-072610-145138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kubes P, Mehal WZ. Sterile inflammation in the liver. Gastroenterology. 2012;143:1158–72. doi: 10.1053/j.gastro.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 12.Schnabl B, Brenner DA. Interactions between the intestinal microbiome and liver diseases. Gastroenterology. 2014;146:1513–24. doi: 10.1053/j.gastro.2014.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Szabo G. Gut–liver axis in alcoholic liver disease. Gastroenterology. 2015;148:30–6. doi: 10.1053/j.gastro.2014.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bertola A, Park O, Gao B. Chronic plus binge ethanol feeding synergistically induces neutrophil infiltration and liver injury in mice: a critical role for E-selectin. Hepatology. 2013;58:1814–23. doi: 10.1002/hep.26419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lazaro R, Wu R, Lee S, et al. Osteopontin deficiency does not prevent but promotes alcoholic neutrophilic hepatitis in mice. Hepatology. 2015;61:129–40. doi: 10.1002/hep.27383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bertola A, Mathews S, Ki SH, et al. Mouse model of chronic and binge ethanol feeding (the NIAAA model) Nat Protoc. 2013;8:627–37. doi: 10.1038/nprot.2013.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wands JR, Carter EA, Bucher NL, et al. Inhibition of hepatic regeneration in rats by acute and chronic ethanol intoxication. Gastroenterology. 1979;77:528–31. [PubMed] [Google Scholar]
- 18.Horiguchi N, Ishac EJ, Gao B. Liver regeneration is suppressed in alcoholic cirrhosis: correlation with decreased STAT3 activation. Alcohol. 2007;41:271–80. doi: 10.1016/j.alcohol.2007.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sancho-Bru P, Altamirano J, Rodrigo-Torres D, et al. Liver progenitor cell markers correlate with liver damage and predict short-term mortality in patients with alcoholic hepatitis. Hepatology. 2012;55:1931–41. doi: 10.1002/hep.25614. [DOI] [PubMed] [Google Scholar]
- 20.Dubuquoy L, Louvet A, Lassailly G, et al. Progenitor cell expansion and impaired hepatocyte regeneration in explanted livers from alcoholic hepatitis. Gut. 2015 doi: 10.1136/gutjnl-2014-308410. http://dx.doi.org/10.1136/gutjnl-2014-308410Epub ahead of print. [DOI] [PMC free article] [PubMed]
- 21.Mathurin P, O’Grady J, Carithers RL, et al. Corticosteroids improve short-term survival in patients with severe alcoholic hepatitis: meta-analysis of individual patient data. Gut. 2011;60:255–60. doi: 10.1136/gut.2010.224097. [DOI] [PubMed] [Google Scholar]
- 22.Christensen E. Glucocorticosteroids in acute alcoholic hepatitis: the evidence of a beneficial effect is getting even weaker. J Hepatol. 2010;53:390–1. doi: 10.1016/j.jhep.2009.12.045. [DOI] [PubMed] [Google Scholar]
- 23.Kwon HJ, Won YS, Park O, et al. Opposing effects of prednisolone treatment on T/NKT cell- and hepatotoxin-mediated hepatitis in mice. Hepatology. 2014;39:1094–106. doi: 10.1002/hep.26748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sabat R, Ouyang W, Wolk K. Therapeutic opportunities of the IL-22-IL-22R1 system. Nat Rev Drug Discov. 2014;13:21–38. doi: 10.1038/nrd4176. [DOI] [PubMed] [Google Scholar]
- 25.Ki SH, Park O, Zheng M, et al. Interleukin-22 treatment ameliorates alcoholic liver injury in a murine model of chronic-binge ethanol feeding: role of signal transducer and activator of transcription 3. Hepatology. 2010;52:1291–300. doi: 10.1002/hep.23837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stoy S, Sandahl TD, Dige AK, et al. Highest frequencies of interleukin-22-producing T helper cells in alcoholic hepatitis patients with a favourable short-term course. PLoS One. 2013;8:5510, e1. doi: 10.1371/journal.pone.0055101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Radaeva S, Sun R, Pan HN, et al. Interleukin 22 (IL-22) plays a protective role in T cell-mediated murine hepatitis: IL-22 is a survival factor for hepatocytes via STAT3 activation. Hepatology. 2004;39:1332–42. doi: 10.1002/hep.20184. [DOI] [PubMed] [Google Scholar]
- 28.Pan CX, Tang J, Wang XY, et al. Role of interleukin-22 in liver diseases. Inflamm Res. 2014;63:519–25. doi: 10.1007/s00011-014-0727-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muhl H, Scheiermann P, Bachmann M, et al. IL-22 in tissue-protective therapy. Br J Pharmacol. 2013;169:761–71. doi: 10.1111/bph.12196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kong X, Feng D, Wang H, et al. Interleukin-22 induces hepatic stellate cell senescence and restricts liver fibrosis in mice. Hepatology. 2012;56:1150–9. doi: 10.1002/hep.25744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meng F, Wang K, Aoyama T, et al. Interleukin-17 signaling in inflammatory Kupffer cells, and hepatic stellate cells exacerbates liver fibrosis in mice. Gastroenterology. 2012;143:765e1–76e3. doi: 10.1053/j.gastro.2012.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao J, Zhang Z, Luan Y, et al. Pathological functions of interleukin-22 in chronic liver inflammation and fibrosis with hepatitis B virus infection by promoting T helper 17 cell recruitment. Hepatology. 2014;59:1331–42. doi: 10.1002/hep.26916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Y, Cobleigh MA, Lian JQ, et al. A proinflammatory role for interleukin-22 in the immune response to hepatitis B virus. Gastroenterology. 2011;141:1897–906. doi: 10.1053/j.gastro.2011.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feng D, Kong X, Weng H, et al. Interleukin-22 promotes proliferation of liver stem/progenitor cells in mice and patients with chronic hepatitis B virus infection. Gastroenterology. 2012;143:188–98. doi: 10.1053/j.gastro.2012.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sertorio M, Hou X, Carmo RF, et al. Interleukin-22 and IL-22 binding protein (IL-22BP) regulate fibrosis and cirrhosis in hepatitis C virus and schistosome infections. Hepatology. 2015;61(4):1321–31. doi: 10.1002/hep.27629. [DOI] [PubMed] [Google Scholar]
- 36.Markwick LJ, Riva A, Ryan JM, et al. Blockade of PD1 and TIM3 restores innate and adaptive immunity in patients with acute alcoholic hepatitis. Gastroenterology. 2014 doi: 10.1053/j.gastro.2014.11.041. [DOI] [PubMed] [Google Scholar]
- 37.Eidenschenk C, Rutz S, Liesenfeld O, et al. Role of IL-22 in microbial host defense. Curr Top Microbiol Immunol. 2014;380:213–36. doi: 10.1007/978-3-662-43492-5_10. [DOI] [PubMed] [Google Scholar]
- 38.Akriviadis E, Botla R, Briggs W, et al. Pentoxifylline improves short-term survival in severe acute alcoholic hepatitis: a double-blind, placebo-controlled trial. Gastroenterology. 2000;119:1637–48. doi: 10.1053/gast.2000.20189. [DOI] [PubMed] [Google Scholar]
- 39.Xu MJ, Feng D, Wang H, et al. IL-22 ameliorates renal ischemia-reperfusion injury by targeting proximal tubule epithelium. J Am Soc Nephrol. 2014;25:967–77. doi: 10.1681/ASN.2013060611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kulkarni OP, Hartter I, Mulay SR, et al. Toll-like receptor 4-induced IL-22 accelerates kidney regeneration. J Am Soc Nephrol. 2014;25:978–89. doi: 10.1681/ASN.2013050528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nguyen-Khac E, Thevenot T, Piquet MA, et al. Glucocorticoids plus N-acetylcysteine in severe alcoholic hepatitis. N Engl J Med. 2011;365:1781–9. doi: 10.1056/NEJMoa1101214. [DOI] [PubMed] [Google Scholar]
- 42.Mathurin P, Louvet A, Duhamel A, et al. Prednisolone with vs without pentoxifylline and survival of patients with severe alcoholic hepatitis: a randomized clinical trial. JAMA. 2013;310:1033–41. doi: 10.1001/jama.2013.276300. [DOI] [PubMed] [Google Scholar]
- 43.Mudaliar S, Henry RR, Sanyal AJ, et al. Efficacy and safety of the farnesoid X receptor agonist obeticholic acid in patients with type 2 diabetes and nonalcoholic fatty liver disease. Gastroenterology. 2013;145:574e1–82e1. doi: 10.1053/j.gastro.2013.05.042. [DOI] [PubMed] [Google Scholar]
- 44.Park O, Wang H, Weng H, et al. In vivo consequences of liver-specific interleukin-22 expression in mice: implications for human liver disease progression. Hepatology. 2011;54:252–61. doi: 10.1002/hep.24339. [DOI] [PMC free article] [PubMed] [Google Scholar]