Abstract
Background
Prostate cancer is very common and many localized tumours are non-aggressive. Determining which cancers are aggressive is important for choosing the most appropriate treatment (e.g., surgery, radiation, active surveillance). Current clinical risk stratification is reliable in forecasting the prognosis of groups of men with similar clinical and pathologic characteristics, but there is residual uncertainty at the individual level. The Prolaris cell cycle progression (CCP) test, a genomic test that estimates how fast tumour cells are proliferating, could potentially be used to improve the accuracy of individual risk assessment. This health technology assessment sought to determine the clinical utility, economic impact, and patients' perceptions of the value of the CCP test in low- and intermediate-risk localized prostate cancer.
Methods
We conducted a systematic review of the clinical and economic evidence of the CCP test in low-and intermediate-risk, localized prostate cancer. Medical and health economic databases were searched from 2010 to June or July 2016. The critical appraisal of the clinical evidence included risk of bias and the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) Working Group criteria. We also analyzed the potential budget impact of adding the CCP test into current practice, from the perspective the Ontario Ministry of Health and Long-Term Care. Finally, we conducted qualitative interviews with men with prostate cancer, on the factors that influenced their treatment decision-making.
Results
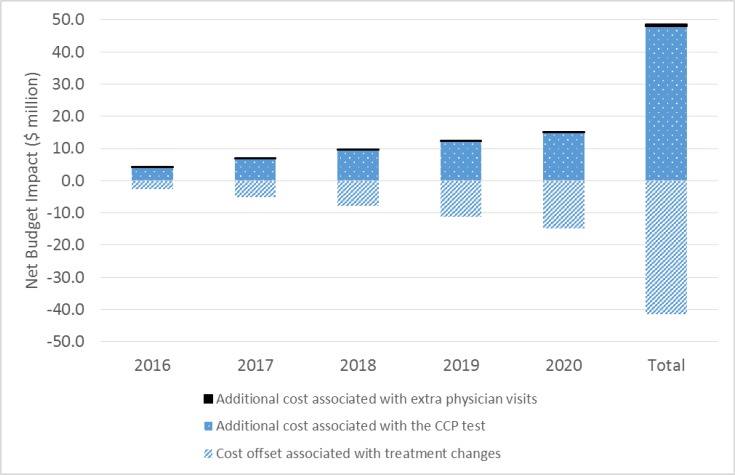
For the review of clinical effectiveness, we screened 3,021 citations, and two before–after studies met our inclusion criteria. In one study, the results of the CCP test appeared to change the treatment plan (from initial to final plan) in 64.9% of cases overall (GRADE rating of the quality of evidence: Very low). In the other study, the CCP test changed the treatment received in nearly half of cases overall, compared with the initial plan (GRADE: Very low). No evidence was available on clinical outcomes of patients whose treatment was informed by CCP results. For the review of cost-effectiveness, 100 citations were identified and screened. No studies met the inclusion criteria. In our economic evaluation, we estimated that publicly funding the CCP test would result in a total net budget impact of $41.3 million in the first 5 years, mostly due to the cost of the CCP test. In our model, the relatively small cost savings ($7.3 million) due to treatment change (increased use of active surveillance and decreased use of interventional treatment) was not large enough to offset the high cost of the test. Patients viewed the test as potentially helpful but, due to the complexity of treatment decision-making, were unsure the test would ultimately change their treatment choices.
Conclusions
We found no evidence to demonstrate the impact of the Prolaris CCP test on patient-important clinical outcomes. The limited evidence available shows that the test appears to provide information that, when considered in addition to clinical risk stratification, may change the treatment plan or actual treatment for some low- and intermediate-risk prostate cancer patients. As a result, there is insufficient data to inform the cost-effectiveness of the CCP test. Publicly funding the CCP test would result in a large incremental cost to the provincial budget.
BACKGROUND
Prostate Cancer
About 1 in 8 Canadian men will be diagnosed with prostate cancer, the most commonly diagnosed cancer in men.1 With the current screening guidelines,2 24,000 men in Canada are newly diagnosed with prostate cancer each year, translating to approximately 8,500 diagnoses per year in Ontario.3 Older age, black ethnicity, obesity, and a family history of the disease are the primary risk factors for developing prostate cancer.4 About 90% of newly diagnosed patients have cancer that is clinically localized (limited to the prostate) and these cancers are often asymptomatic.4 Only about 1 in 40 people with prostate cancer will experience symptoms,5 and 1 in 27 with this cancer are likely to die from it.2
At the initial diagnostic stage, patients may receive a blood test for prostate-specific antigen (PSA) and a digital rectal examination of the prostate to look for abnormalities that could indicate they have a higher risk for prostate cancer.4 The only way to diagnose prostate cancer is with a biopsy of the prostate. Tissue from the prostate biopsy is also used to assess tumour grade (description of how abnormal the cells are), which is classified using the Gleason score.2 The Gleason score is a value ranging from 2 to 10 and is the sum of two numbers each rated from 1 to 5: the primary (most common) tissue pattern and the secondary (highest grade or second most common) tissue pattern seen from the biopsy.6 A higher Gleason score means higher risk of extraprostatic extension and metastases (spreading of the cancer beyond the prostate).7 The tumour is also classified by T-stage, according to a system known as TNM, to reflect the extent of cancer in the prostate and elsewhere in the body (T = primary tumour, N = any spread to lymph nodes, and M = metastases).2,6
Clinical Risk Assessment
Importance of Risk Assessment
Risk assessment in prostate cancer is necessary to determine the treatment options appropriate for a given patient. Precise risk stratification is key to avoiding under- and overtreatment of the disease and the potential for poorer survival on the one hand and treatment-related side effects on the other. Prostate cancer is classified as high risk based on any one of the following: evidence of metastasis (formation of another tumour); a Gleason score of 8 to 10 representing poorly differentiated or undifferentiated (immature) cells which often grow and spread quickly; a clinical stage where the tumour is large or spread beyond the prostate; or a very high PSA level.6 Each of these factors indicate real potential for the cancer to develop into a fatal type. Thus, definitive treatment of high-risk prostate cancer is a central tenet of best practice for tumour control and prevention of spread.8 However, it is among patients with low- or intermediate-risk prostate cancer that the concern about choosing the most appropriate treatment is greatest. Low-risk patients represent approximately 40% to 50% of incident cases in Canada,9 and intermediate-risk patients comprise about one-third.10 High-risk patients comprise only up to 15% of new diagnoses.11
With residual uncertainty in some cases about which man's cancer is aggressive, overtreatment of low- and intermediate-risk cancers is a considerable concern. Low-risk prostate cancers that are actively monitored with tests (as opposed to being immediately treated) are associated with 97% survival after 5 years and 99% survival after 10 years; however, in Canada an estimated 30% to 40% of patients with low-risk cancers undergo definitive treatment.12,13 Conversely, there is some indication from time-trend analyses that undertreatment is also of growing concern.11
Accurate information on the aggressiveness of an individual's cancer allows treatment to be tailored to the unique needs and preferences of each patient.
Risk Grouping
When patients are newly diagnosed with prostate cancer, they are classified into risk groups based on their clinical and pathological (clinicopathologic) characteristics. These characteristics are signs and symptoms directly observed by the physician and from laboratory tests such as PSA levels, the tumour's clinical stage, and Gleason score. The risk groups relate to the likelihood of future events (e.g., disease progression) and are based on one or more well-established prognostic factors (clinical or biological features that can be used to estimate the chance of recovery or recurrence).7 The Gleason score is one of the most powerful prognostic factors for men with prostate cancer.2 Table 1 shows two common systems for risk grouping used in clinical practice.
Table 1:
Common Prostate Cancer Risk Stratification Systems Based on Clinicopathologic Features
| System | Criteria | ||
|---|---|---|---|
| Low Risk | Intermediate Risk | High Risk | |
| D'Amico / AUA | All of:
|
All of:
|
One or more of:
|
| NCCN | All of:
|
All of:
|
One or more of:
|
Very low risk
|
|
Very high risk
|
|
Abbreviations: AUA, American Urological Association; NCCN, National Comprehensive Cancer Network; PSA, prostate-specific antigen.
Source: Rodrigues et al, 2012.14
It is important to remember that the initial classification of risk based on biopsy findings is not perfect because the biopsy takes only a sample of cells to provide a snapshot of the prostate tissue.15 As many as 25% to 30% of patients have their risk group upgraded after a repeat biopsy, and this change is thought to be due to more comprehensive, accurate sampling in the subsequent biopsy.9 As a result, some Ontario doctors include an initial period with repeat PSA tests or biopsies to augment initial risk stratification. Disease risk is much more accurately classified after the prostate is removed (radical prostatectomy surgery), as the true pathologic grade is only determined by the analysis of the entire prostate.16
Individualized Risk Assessment
In contrast to risk grouping, there are more complex, individualized methods to forecast the likely outcome of a patient's prostate cancer. Instead of estimating risk based on a group of people with similar clinicopathologic characteristics, the process computes a risk estimate for a single patient and is therefore generally more accurate.17 The most accurate method of individualized risk assessment for prostate cancer management is the Kattan nomogram, a mathematical algorithm that predicts the risk of recurrence or treatment failure (defined as a rise in PSA level) after radical prostatectomy for individual patients with clinically localized cancer.18,19 Another method for individual risk prediction, used mainly in research, is the Cancer of the Prostate Risk Assessment (CAPRA) score, which is calculated from the factors included in the Kattan nomogram in addition to the percentage of cancer-positive biopsy cores and age at diagnosis.20,21 Both methods predict the likelihood of cancer recurrence and death after radical prostatectomy.18–21
Treatment
Definitive treatment of localized prostate cancer can consist of radiation therapy, hormone therapy, surgery, or combinations of treatments.4 All of these treatments have associated potential complications and harm, such as anxiety, pain, infection, bleeding, bowel dysfunction, urinary incontinence, and sexual dysfunction, all of which can considerably impact a man's quality of life.22–25 Some patients, such as men with other significant health conditions or a limited life expectancy (e.g., less than 10 years), are not candidates for curative treatment. These patients may pursue a strategy known as watchful waiting, where they wait until they have symptoms of disease progression before they start treatment either to manage the symptoms or as palliative care.26
To address concerns of potential overtreatment and side effects among otherwise healthy men with slow-growing prostate cancer, active surveillance has been promoted as a safe and appropriate management approach.4 During active surveillance, the cancer is closely monitored via regularly scheduled tests and examinations, and curative treatment (radical prostatectomy, commonly) begins only if there is evidence of cancer progression.7 Triggers for curative treatment include a repeat biopsy showing higher grade disease (e.g., Gleason pattern 4 or 5) or increased volume of cancer (increase in percentage of cores involved), or changes in PSA levels (doubling time or velocity), though the latter may be less reliable.9
A recent landmark study in Canada estimating the proportion of men undergoing active surveillance as initial treatment reported that among men with low-grade prostate cancer (Gleason 3+3), one-third to two-thirds of patients decide to pursue active surveillance, and that this management strategy has become increasingly common in the past decade.12 Nearly 30% of men on active surveillance eventually received definitive treatment.12 Another seminal Canadian study reported long-term outcomes of men with low- or favourable-risk localized prostate cancer who were on active surveillance. At 5, 10, and 15 years after diagnosis, 75.7%, 63.5%, and 55.0% of patients, respectively, continued on active surveillance and remained untreated.27
While active surveillance for low-risk prostate cancer is generally the primary management strategy, this approach is more controversial for patients with intermediate risk. This is because the patterns of prostate cancer–specific mortality, overall mortality, and biochemical recurrence after treatment have been observed to vary widely among intermediate-risk patients.28 In an attempt to address this heterogeneity and improve risk prediction, some classification systems now further categorize intermediate-risk prostate cancers as favourable or unfavourable risk.28 The distinction between the two is made mainly in the Gleason grade classification where a Gleason score of 7 can denote two different patterns of disease: either low-volume cancer (i.e., primary pattern 3 with secondary pattern 4, referred to as 3+4) or high-volume cancer (the reverse disease pattern, referred to as 4+3).29–31 Although not yet supported by randomized data, a body of observational data suggests that Gleason 3+4 tumours may carry similar risk of prostate cancer–specific mortality, overall mortality, and biochemical recurrence as low-risk cancer.28 A recent Ontario clinical practice guideline on active surveillance for localized prostate cancer recommends that active surveillance is appropriate for most low-risk patients and for selected intermediate-risk patients with low-volume cancer (Gleason score 3+4).32 Recently, the International Society of Urologic Pathologists recommended a five-tier Grade Grouping scheme for prostate cancer to more accurately reflect prognosis.33 In this system, Group 1 includes all Gleason score 6 or less, Group 2 includes Gleason 3+4, Group 3 is Gleason 4+3, Group 4 is Gleason score 8, and Group 5 is Gleason scores 9 and 10.33
Treatment Decision-Making
Deciding how to treat early or localized prostate cancer (tumours that present a low or intermediate risk) is complex and may involve not only physicians and patients but also the patient's family members. In a qualitative study of 128 men in the United States with newly diagnosed localized prostate cancer, the main factors reported as influencing treatment decision were their physician's recommendation and their perception of the evidence for a treatment's likelihood of success (cure or preventing tumour spread).34 Other considerations included preference for a non-invasive treatment and for a treatment other than surgery. A minority of men in this study (13%) reported weighing the risks and benefits of each treatment to reach their choice. When specifically considering active surveillance, men reported a number of reasons for deciding against this option (Table 2).34
Table 2:
Reasons Reported by Patients for Rejecting Active Surveillance to Manage Localized Prostate Cancer
| Type of Reason | Examples | % of Men Citinga |
|---|---|---|
| Fear of consequences | • Need to “combat” tumour | 64 |
| • Age (relative youth) | 14 | |
| • Fear of tumour spread | 13 | |
| Perception of elevated risk | • Elevated PSA or Gleason score | 12 |
| • Family history of cancer | 1 | |
| • Coexisting condition | 2 | |
| External persuasion against | • Physician recommendation | 12 |
| • Family advice | 4 |
Abbreviations: PSA, prostate-specific antigen.
Percentages add up to > 100% as men could select multiple reasons.
Source: Holmboe et al, 2000.34
This survey-based study shows that both internal and external factors influence men's thinking around their choice of treatment for localized prostate cancer. Anxiety is not uncommon after a diagnosis of localized prostate cancer.35 Patients may wish to have a more active or passive role,36 but it's essential to consider their values and preferences in treatment decision-making. Patient choice may change over time, and those initially adopting active surveillance may choose to pursue active treatment, even in the absence of cancer progression, because of anxiety or concerns about future changes. To better understand whether men with prostate cancer would value and use the information provided by the Prolaris cell cycle progression test, the Centre for Health Economics and Policy Research at McMaster University conducted a review of qualitative studies that examined the types of information men seek to help inform their treatment decisions.37
Current Ontario Practice
In Ontario, patients are risk stratified according to the D'Amico risk scheme,38 also called the American Urological Association criteria (Table 1). Once patients have been diagnosed with prostate cancer via core needle biopsy, they proceed to risk assessment based on PSA level, Gleason score, and tumour stage.26
For low- and intermediate-risk patients, their eligibility for curative treatment is then assessed, by considering comorbidities, life expectancy, and patient preferences; if ineligible, they proceed to watchful waiting.26,39 If they are a candidate for curative treatment, the decision to pursue one management strategy or another is made collaboratively between patients and health care providers (e.g., urologist, radiation oncologist) and is fundamentally considered in the context of a patient's risk level.39 It is at this stage that active surveillance or definitive treatment are considered. Cancer Care Ontario recommends an active surveillance protocol consisting of a yearly digital rectal examination, PSA test every 3 to 6 months, a confirmatory transrectal ultrasound-guided (TRUS) biopsy within 6 to 12 months, and then serial biopsies at least 3 to 5 years thereafter.32 Monitoring using multiparametric magnetic resonance imaging (MRI) can also be done as part of the active surveillance protocol, if cancer progression is suspected or there is discordance between the clinical and pathological findings.26
If the patient choses immediate definitive treatment, they will likely be treated by a urologist and receive radical prostatectomy with or without lymph node dissection (optional for low-risk patients, recommended for those with intermediate risk).26 If the surgery is not completely successful (i.e., the surgical margins are positive, meaning not all of the cancer was removed, or PSA levels remain detectable and persist), radiation therapy can then be administered.26 Alternatively, a patient can be treated by a radiation oncologist and receive external beam radiation therapy or brachytherapy (for low-risk tumours), or radiation therapy with or without androgen-deprivation therapy and brachytherapy (for intermediate-risk tumours).39 Men receiving any of these treatments are subsequently monitored and receive routine follow-up.
Technology
The Prolaris cell cycle progression (CCP) test is a prognostic test designed to help provide an individualized assessment of the risk of disease progression in patients with low- and intermediate-risk prostate cancer. It is a genomic test, meaning it measures the expression of certain genes in the tumour, and is intended to directly measure the growth characteristics of the prostate cancer.40 The test reflects changes in 31 cell cycle progression genes and 15 housekeeping genes to generate a score, providing information about prostate tumour cell proliferation (how fast the cells are dividing).40 The score ranges from 0 to 10, and each unit increase represents a doubling of risk of disease progression.40
The CCP test is performed in a laboratory and analyzes a sample of the same biopsy tissue that is collected for routine diagnosis. Once the sample has been analyzed, a report is generated that includes the patient's clinicopathologic features (including age, pre-biopsy PSA level, tumour stage, percent of positive biopsy cores, Gleason score, and clinical risk group) and an assessment based on the CCP score. The assessment states whether the tumour is less aggressive, more aggressive, or consistent with the average risk of the relevant clinical risk group (based on the American Urological Association system). The CCP report also provides an individualized estimate of a patient's 10-year prostate cancer–specific mortality risk (their risk of dying from prostate cancer within the next decade), reflecting the combined prediction of the clinicopathological variables and the CCP score.
Analytical and Clinical Validity
The CCP test has demonstrated reproducibility and robustness (analytical validity) in measuring the 31 cell cycle proliferation genes and 15 housekeeping genes when performed on both formalin-fixed paraffin-embedded (FFPE) needle biopsy and FFPE radical prostatectomy tissue samples.41,42 (See Table 1 in Cuzick et al41 for a list of all genes.) As well, clinical validation studies (to confirm an association between the test and a clinical endpoint) demonstrate that CCP test does offer additional prognostic information over and above the clinicopathologic characteristics used in current practice. Specifically, studies have established that the CCP test provides additional information, beyond standard clinical variables, to forecast prostate cancer–specific mortality.41,43,44 Studies have also demonstrated the test's ability to forecast cancer recurrence after surgery or other treatment.43,45–47 Table 3 shows an overview of the added prognostic information of the CCP score from some of the published clinical validation studies.
Table 3:
Prognostic Clinical Validation Studies of the Prolaris Cell Cycle Progression Test
| Author, Year | Cohort | Tissue Sample | Outcome | Clinical Characteristics Adjusted Fora | CCP HRb (95% CI) | P |
|---|---|---|---|---|---|---|
| Cuzick et al, 201243,c | Men diagnosed with localized PCa via TURP and treated conservatively | Biopsy | Prostate cancer–specific mortality |
|
2.57 (1.93–3.43) | < .0001 |
| Cuzick et al, 201544 | Men diagnosed with localized PCa via needle biopsy and managed conservatively | Biopsy | Prostate cancer–specific mortality |
|
1.76d (1.47–2.14) | < .0001 |
| Cuzick et al, 201243 | Men with prostate cancer treated with radical prostatectomy | Prostatectomy | Biochemical recurrence after RP |
|
1.74 (1.39–2.17) | < .0001 |
| Freedland et al, 201347 | Men diagnosed with prostate cancer and treated with EBRT +/- ADT | Biopsy | Biochemical recurrence after EBRT +/− ADT |
|
2.11 (1.05–4.25) | .034 |
| Cooperberg et al, 201346 | Men who underwent radical prostatectomy +/− adjuvant or neoadjuvant therapy | Prostatectomy | Biochemical recurrence after RP |
|
2.0e (1.4–2.8) | < .001 |
| Bishoff et al, 201445 | Men with prostate cancer treated with radical prostatectomy | Biopsyf | Biochemical recurrence after RP |
|
1.43 (1.23–1.76) | < .001 |
Abbreviations: ADT, androgen-deprivation therapy; CAPRA, Cancer of the Prostate Risk Assessment score; CCP, cell cycle progression; EBRT, external beam radiation therapy; HR, hazard ratio; PCa, prostate cancer; PSA, prostate-specific antigen; RP, radical prostatectomy; TURP, transurethral resection of the prostate.
Adjusted for in multivariable Cox proportional hazards analysis.
Per 1-unit increase in CCP score.
Earlier analysis of this cohort (Cuzick et al, 201141) adjusted for the same covariates (reported HR 1.65, 95% CI 1.31–2.09, P < .0001).
An analysis adjusting for CAPRA risk group yielded similar estimates (HR 1.76, 95% CI 1.44–2.15, P < .0001).
An analysis adjusting for CAPRA risk group yielded similar estimates (HR 1.7, 95% CI 1.3–2.3, P < .001).
Includes a subset of patients for whom CCP score was generated on a simulated biopsy specimen.
Clinical Utility
However, the core question in the consideration of medical tests is whether they improve patient-important clinical outcomes. As outlined by the Agency for Healthcare Research and Quality's framework for assessing prognostic tests, sequential pieces of evidence are needed to answer this core question: analytical validity, clinical validity, and ultimately, clinical utility.48 Clinical utility can be demonstrated ideally by the impact of the test on health outcomes, or by biological surrogates that precede health outcomes, or (only in the absence of the former two outcomes) by treatment strategies.48 The intended clinical application for the CCP test is to use it to assist with stratification of newly diagnosed, localized prostate cancers according to patients' individual risk, more accurately than is currently done, and thus determine the most appropriate treatment for each patient. This speaks to the clinical utility of the test—that is, whether the CCP test affects patient-important clinical outcomes and treatment decisions in a meaningful way.49
Regulatory Information
The Prolaris CCP test was released by Myriad Genetics, Inc. in 2010 and became available in Canada in 2013. The tissue analysis for the CCP test is performed by only one laboratory, in the United States, where all biopsy samples are sent. Thus, the test is classified as a service by Health Canada and is not subject to the Medical Device Regulations (Health Canada, email communication, February 3, 2016). The delivery and administration of Prolaris falls within the jurisdiction of the provinces and territories. The list price of the test is $3,400 USD per patient.50,51 At the time of writing, access to the CCP test in Ontario would be considered on request through the Exceptional Access Program (no reported claims at the time of writing; Ministry of Health and Long-Term Care, email communication, February 29, 2016).
Research Questions
What is the clinical utility (impact on patient-important outcomes or treatment decisions) of the Prolaris cell cycle progression (CCP) test for treatment selection in men with newly diagnosed, low- or intermediate-risk, localized prostate cancer, compared with current practice of clinical risk stratification alone?
What is the cost-effectiveness of the Prolaris CCP test compared with current practice using clinical risk stratification in men with newly diagnosed, low- or intermediate-risk, localized prostate cancer?
What is the 5-year budget impact of publicly funding the Prolaris CCP test for men with newly diagnosed, low- or intermediate-risk, localized prostate cancer, within the context of the Ontario Ministry of Health and Long-Term Care?
What is the lived experience of men with low- or intermediate-risk, localized prostate cancer; what factors influence their decision-making about treatment options; and how might the Prolaris CCP test affect that decision-making?
CLINICAL EVIDENCE REVIEW
Objective
The objective of this evidence review was to assess the clinical utility of the Prolaris cell cycle progression (CCP) score for treatment selection in men with newly diagnosed, low- or intermediate-risk, localized prostate cancer, compared with clinical risk stratification alone.
Methods
Sources
We performed a literature search on June 9, 2016, using the Ovid interface to search the following databases: MEDLINE, Embase, Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, Health Technology Assessment, National Health Service Economic Evaluation Database (NHSEED), and Database of Abstracts of Reviews of Effects (DARE), for studies published since January 1, 2010. Bi-weekly updates of new publications from MEDLINE and Embase were set up until September 30, 2016. The search start date was selected because the technology was released in 2011 and no studies published before 2010 were identified during scoping.
Search strategies were developed by medical librarians using controlled vocabulary (e.g., Medical Subject Headings) and relevant keywords. The final search strategy was peer-reviewed using the PRESS Checklist.52 See Appendix 1 for full details, including all search terms.
Inclusion Criteria
English-language full-text publications
Randomized controlled trials and comparative observational studies
-
Studies of men with newly diagnosed, low- or intermediate-risk, localized prostate cancer defined by clinical risk systems used in clinical practice, ideally the D'Amico risk criteria1:
-
–
Low risk: all of PSA < 10 ng/mL, Gleason score ≤ 6, clinical stage T1-T2a, and asymptomatic for metastases
-
–
Intermediate risk: all of PSA 10–20 ng/mL, Gleason score 7, clinical stage T2b, and asymptomatic for metastases (i.e., not meeting the criteria for high or low risk)
-
–
Prolaris cell cycle progression test performed on diagnostic biopsy sample and analyzed by authorized laboratory
-
Compared with clinical risk stratification according to one of the following validated risk stratification criteria:
-
–
DAmico/American Urological Association classification
-
–
University of California, San Francisco-Cancer of the Prostate Risk Assessment (UCSF-CAPRA) score
-
–
National Comprehensive Cancer Network criteria
-
–
Kattan preoperative nomogram
-
–
Exclusion Criteria
Editorials, conference proceedings, abstracts, case reports, or commentaries
Studies on gene discovery, analytical validation, or prognostic value (univariable or multivariable) or clinical validation
Studies of only high-risk prostate cancer patients with one or more of the following: PSA > 20 ng/mL, Gleason score ≥ 8 or clinical stage ∼T2c–3A, symptomatic for metastases
Patients who received prior treatment for prostate cancer
Patients with unconfirmed cancer or other causes of prostate abnormality (e.g., benign prostatic hyperplasia)
Other developmental or commercially available molecular tests that aspire to augment initial risk stratification in localized prostate cancer but that are not based solely on cell cycle proliferation gene expression profiling, or CCP tests performed on prostatectomy sample (rather than biopsy sample)
Outcomes of Interest
-
Clinical outcomes
-
–
Progression-free survival
-
–
Prostate cancer-specific mortality
-
–
Overall survival
-
–
Treatment-related complications
-
–
-
Treatment decision-making outcomes
-
–
Concordance between treatment decisions based only on clinical information and those including CCP
-
–
Concordance between true pathologic stage and true pathologic grade (i.e., post-prostatectomy) as determined by clinical predictor (i.e., Kattan nomogram) and CCP
-
–
Change in treatment decisions after CCP (e.g., proportion of cases)
-
–
Duration of time a patient remains on active surveillance
-
–
Proportion of cases that went to definitive treatment after CCP
-
–
Impact on patient or providers (e.g., quality of life, satisfaction measured by a validated method)
-
–
Screening
A single reviewer reviewed the abstracts and, for those studies meeting the eligibility criteria, we obtained full-text articles. We also examined reference lists for any additional relevant studies meeting the inclusion criteria.
Data Extraction
A single reviewer extracted relevant information on study context, research methods, PICOT (population, intervention, comparators, outcomes, and timing), results, and risk of bias items into a data form, based on the information available in the published articles. Eligibility criteria and population characteristics (age, PSA level at biopsy, Gleason score, tumour stage, family history, medical history, comorbidities, socioeconomic characteristics), information on management strategy (planned, received), and information related to all defined outcomes were also abstracted.
Statistical Analysis
We report the results from each included study. We did not perform a meta-analysis of the included studies as had been planned a priori, because of the heterogeneity in study design, interventions, comparators, and outcomes across the studies.
Quality of Evidence
We assessed the risk of bias of each study using the Quality Assessment Tool for Before–After (Pre–Post) Studies with No Control Group.53 The quality of the body of evidence for each outcome was examined according to the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) Working Group criteria.54 The overall quality (our confidence in the results) was determined to be high, moderate, low, or very low.
Expert Consultation
We solicited local expert consultation on the use of the Prolaris CCP test for low- and intermediate-risk localized prostate cancer. Experts consulted included physicians in the specialty areas of urology, oncology, and genetic testing. The role of the expert advisors was to provide important contextual information on the use of the CCP test, including expertise on the health condition, patients, diffusion of the technology, or clinical issues that contextualize the research question to Ontario. However, the statements, conclusions, and views expressed in this report do not necessarily represent the views of the consulted experts.
Results
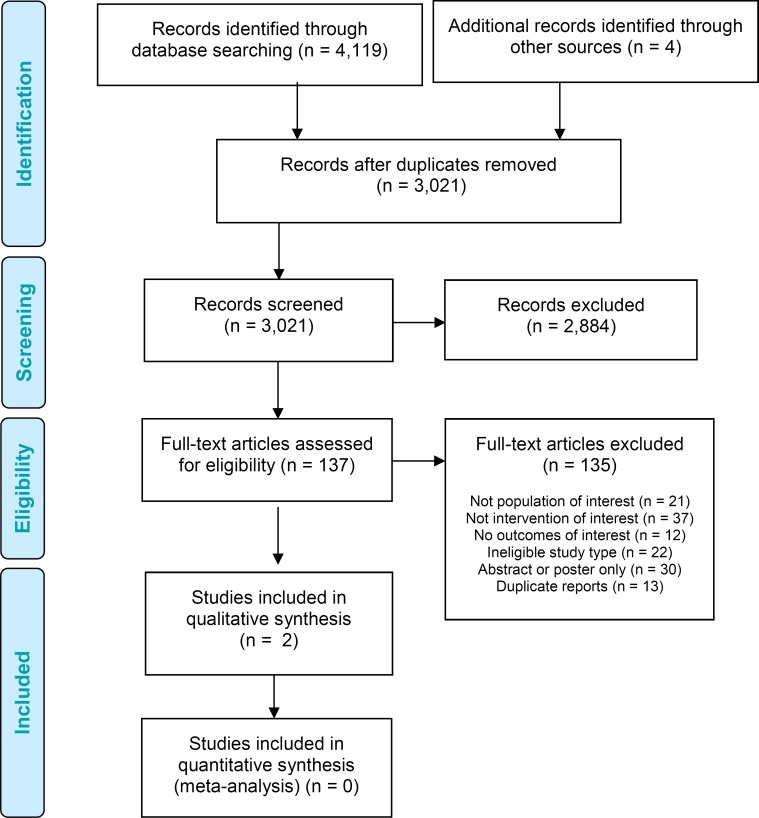
We identified 3,021 citations (after duplicates were removed) published between January 1, 2010, and June 9, 2016. We reviewed titles and abstracts to identify potentially relevant articles. We obtained the full texts of these articles for further assessment. Two studies met the inclusion criteria.55,56 We reviewed the reference lists of the included studies, along with health technology assessment websites and other sources, to identify additional relevant studies. Bi-weekly updates of new publications in MEDLINE and Embase were reviewed for relevant articles until September 26, 2016. Figure 1 depicts the flow diagram for the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). Appendix 3 lists the studies we excluded after full-text review, with the primary reason for exclusion.
Figure 1: PRISMA Flow Diagram for the Clinical Evidence Review.
Source: Adapted from Moher et al, 2010.57
Clinical Outcomes
No studies reported on patient-important clinical outcomes of patients whose treatment was informed by CCP, including progression-free survival, prostate cancer-specific mortality, overall survival, or treatment-related complications. The two included studies reported on surrogate outcomes—the test's influence on treatment decisions.
Influence on Treatment Decisions
Both studies reported the change in treatment decisions, from initial recommendation, with the addition of the CCP report.55,56 Crawford et al55 analyzed a registry of 331 patients with a documented diagnosis of prostate cancer to determine changes in treatment plan after clinical risk assessment alone, compared with after the CCP test. The registry included patients classified as low, intermediate, and high risk according to the American Urological Association (AUA) risk criteria. Similarly, the PROCEDE-1000 study reports on a prospective registry cohort of 1,596 prostate cancer patients from all AUA risk groups.56 As its primary outcome, the PROCEDE-1000 trial compared the initial treatment plan and actual treatment received, which was informed by the CCP. Table 4 shows the population characteristics of the patients who could be evaluated in each study.
Table 4:
Registry Cohort Characteristics of Evaluable Prostate Cancer Patients
| Author, Year | n, Country | Age, Years | Ethnicity, % | AUA Risk Group, n (%) | Gleason Score, n (%) | Tumour Stage, n (%) | PSA, ng/mL |
|---|---|---|---|---|---|---|---|
| Crawford et al, 201455,a | 305 US | M 67.4 (SD 7.43) Range: 43–93 | NR | Low: 135 (44.0) Intermediate: 131 (42.9) High: 39 (12.8) | ≤ 6: 157 (51.5) 7: 123b (40.3) 8–10: 25 (8.2) | T1a: 4 (1.3) T1b: 1 (0.3) T1c: 252 (82.6) T2a: 24 (7.9) T2b: 12 (4) T2c: 11 (3.6) T3b: 1 (0.3) | M 7.7 (SD 8.32) Range: 0.98–93 |
| Shore et al, 201656,c (PROCEDE-1000) | 1,206 US | M 65.9 (SD 8.36) Range: 40–89 | Caucasian 77.0 Latino/Hispanic 9.1 African 8.9 Asian 2 Unknown 1 Alaska Native/Pacific Islander < 1 Mixed race < 1 Other < 1 Native American 0 | Low: 486 (40.3) Intermediate: 506 (42.0) High: 214 (17.7) | 6: 577 (47.8) 7: 480d (39.8) 8: 100 (8.3) ≥ 9: 49 (4.1) | T1a: 15 (1.2) T1b: 7 (0.6) T1c: 870 (72.1) T2a: 167 (13.9) T2b: 77 (6.4) T2c: 57 (4.7) T3a: 12 (1.0) T3b: 1 (0.1) | M 7.8 (SD 8.15) Range: 0.4–99 |
Abbreviations: AUA, American Urological Association; M, mean; NR, not reported; PSA, prostate-specific antigen; SD, standard deviation; US, United States.
Primary outcome for this study was change from initial treatment plan to final treatment plan.
Includes 87 patients with Gleason 3+4 and 36 patients with Gleason 4+3.
Primary outcome for this study was change from initial treatment plan to actual treatment received.
Includes 337 patients with Gleason 3+4 and 143 patients with Gleason 4+3.
The two studies were comprised of similar groups of men in terms of age, clinicopathologic characteristics, and distribution of clinical risk, with more than 80% of patients falling into low- or intermediate-risk groups. Although high-risk men were also included in the registries, they represented 12% to 17% of the study population, approximately the expected proportion of new cases.11 Only one study reported the ethnic composition of the registry cohort, which was predominantly Caucasian.56
Change in Planned Treatment
Crawford and colleagues55 compared the recommended treatment recorded by each patient's urologist at the time the CCP test was ordered and the intended treatment chosen after the test results were reviewed. This study was the first to be conducted in a clinical practice context, surpassing prior studies that were based on retrospective, hypothetical decision-making in a research setting (see Appendix 3). The authors considered changes in planned therapy in a hierarchy of therapeutic burden, in decreasing order:
Interventional
-
1.
Radical prostatectomy
-
2.
Radiation
-
3.
Other therapy (cryotherapy, brachytherapy, etc)
-
4.
Androgen-deprivation therapy
Non-interventional
-
5.
Active surveillance
-
6.
Watchful waiting
The change was classified as a reduction if the treatment recommendation moved down one level (e.g., from 2 to 3) or changed from an interventional to a non-interventional treatment (i.e., from any of 1, 2, 3, or 4 to either 5 or 6).55 An increase was defined as a change where the treatment recommendation progressed up the hierarchy to any treatment above it. In addition, the authors examined changes between the dichotomous categories of interventional and non-interventional treatment.
Across all risk groups, final treatment plans for 64.9% of patients had some sort of change after CCP test results were available (95% confidence interval [CI] 59.4%–70.1%).55 Using the authors' therapeutic burden hierarchy, 24.9% of the changed treatment plans reflected an increase in therapy and 40% reflected a therapy reduction. No change of plans occurred after reviewing CCP test results for 34.1% of patients overall. Results by risk group for changes according to the treatment burden hierarchy were not reported.
This study also analyzed changes in treatment plan in the dichotomous categories: interventional versus non-interventional (Table 5). By clinical risk group, 24.4% of the low-risk patients changed their plan to non-interventional treatment, 7.4% of low-risk patients changed to interventional, and 68.1% had no change.55 Among intermediate-risk patients, 16.8% changed to a non-interventional treatment, 12.2% to interventional, and 71.0% no change. Among high-risk patients, 15.4% had a treatment plan change to non-interventional, 17.9% to interventional, and there were no changes to the treatment plans of 66.7%.55
Table 5:
Localized Prostate Cancer Treatment Plan Changes (Dichotomous Categories), By Risk Group, in a Study of Impact of Prolaris Cycle Cell Progression Test on Treatment Plans
| AUA Risk Group | Original Recommendation, n | Change to Non-Interventional, n (%) | Change to Interventional, n (%) | No Change, n (%) |
|---|---|---|---|---|
| Low | All, 135 | 33 (24.4) | 10 (7.4) | 92 (68.1) |
| Interventional,a 50 | 33 (66) | – | 17 (34) | |
| Non-interventional,b 85 | – | 10 (12) | 75 (88) | |
| Intermediate | All, 131 | 22 (16.8) | 16 (12.2) | 93 (71.0) |
| Interventional, 86 | 22 (26) | – | 64 (74) | |
| Non-interventional, 45 | – | 16 (36) | 29 (64) | |
| High | All, 39 | 6 (15.4) | 7 (17.9) | 26 (66.7) |
| Interventional, 28 | 6 (21) | – | 22 (79) | |
| Non-interventional, 11 | – | 7 (64) | 4 (36) | |
| All | All, 305 | 61 (20.0) | 33 (10.8) | 211 (69.2) |
| Interventional, 164 | 61 (37.2) | – | 103 (62.8) | |
| Non-interventional, 141 | – | 33 (23.4) | 108 (76.6) |
Abbreviations: AUA, American Urological Association.
Interventional treatments included radical prostatectomy, radiation, other therapy (cryotherapy, brachytherapy, etc.), and androgen-deprivation therapy.
Non-interventional treatments included active surveillance and watchful waiting.
Source: Crawford et al, 2014.55
Change in Actual Treatment
Shore et al56 investigated the effect of the CCP test on actual treatment by comparing the initial recommended treatment based on clinical information only (PSA, Gleason score, stage, age) with the actual treatment patients received. Actual treatment was assessed 3 to 6 months following the initial consultation at which the original treatment recommendation was made. Similarly to Crawford and colleagues,55 they examined changes overall across the study population, as well as according to dichotomous categories of interventional treatment (all relevant treatments chosen were included) and non-interventional treatment (either active surveillance or watchful waiting).56 The treatment options and categories for the analysis are in Table 6.
Table 6:
Localized Prostate Cancer Treatment Options and Dichotomous Categories in a Study of Impact of Prolaris Cycle Cell Progression Test on Treatment Selection
| Interventionala | Non-interventionalb |
|---|---|
| Radical prostatectomy | Active surveillance |
| EBRT primary | Watchful waiting |
| EBRT adjuvant | |
| CyberKnife robotic surgery | |
| Proton beam radiation | |
| Brachytherapy interstitial | |
| Brachytherapy high dose rate | |
| ADT primary | |
| ADT neoadjuvant | |
| ADT concurrent | |
| Pelvic lymph node dissection | |
| Cryosurgery | |
| High-intensity focused ultrasound | |
| Other |
Abbreviations: ADT, androgen-deprivation therapy; EBRT, external beam radiation therapy.
All relevant interventions recommended were recorded.
Only one non-interventional treatment strategy could be selected.
Source: Shore et al, 2016.56
Overall, 47.8% of patients (576 of 1,206) had a change from their initial treatment plan to actual treatment, with the addition of CPP test results. Of these changes, 72.1% were considered reductions in treatment intensity, 26.9% were increases (1% could not be determined).56 However, this study did not explicitly define which of the interventional options were considered higher or lower intensity relative to one another. The authors reported a general trend toward an increase in single-modality treatment recommendations (87.2%, up from 68.3%) along with a significant decrease in treatment plans that recommended 2 or more modalities (12.9%, down from 31.6%, P < .0001).56
Only 17.6% of this study population had a change between the dichotomous categories of interventional and non-interventional treatment. Informed by both the CCP test and all other information, treatment differed from the planned approach for 14.2% (95% CI 11.9%–16.8%) of patients originally recommended definitive intervention, and 24.2% (95% CI 20.4%–28.6%) of patients originally recommended non-interventional treatment.56 No change occurred from planned treatment in 993 of the 1,206 patients (Table 7). These data are limited as they are not analyzed separately for each clinical risk group.
Table 7:
Localized Prostate Cancer Treatment Category Changes From Initial Plan to Actual Treatment in a Study of Impact of Prolaris Cycle Cell Progression Test on Treatment Selection
| Original Recommendation | n | Change to Non-interventional, n (%) | Change to Interventional, n (%) | No Change, n (%) |
|---|---|---|---|---|
| Non-interventional | 417 | – | 101 (24.2) | 316 (75.8) |
| Interventional | 789 | 112 (14.2) | – | 677 (85.8) |
Source: Shore et al, 2016.56
Of the 101 patients whose treatment changed from non-interventional to interventional, 19 (13.3%) had a CCP score suggesting the cancer was less aggressive than suggested by clinical features, 56 (26.2%) had a test score consistent with clinical assessment, and 26 (43.3%) of the cancers were more aggressive.56 For the 112 patients who changed from interventional to non-interventional treatment, CCP scores were less aggressive for 39 (14.6%), consistent for 53 (16.0%), and more aggressive for 20 (10.5%).56 The authors found a net increase in non-interventional options of 2.6% across all patients. Table 8 shows the changes in the types of interventional treatment administered (compared with planned), by AUA risk group.
Table 8:
Changes in Interventional Treatments, by AUA Risk Group, in a Study of Impact of Prolaris Cycle Cell Progression Test on Treatment Selection
| Treatment | Δ Low Risk, % (n = 486) | Δ Intermediate Risk, % (n = 506) | Δ High Risk, % (n = 214) |
|---|---|---|---|
| Radical prostatectomy | −32.4 | −37.4 | −27.7 |
| EBRT primary | −48.8 | −35.0 | −37.6 |
| EBRT adjuvant | −60.0 | −63.3 | −44.0 |
| CyberKnife robotic surgery | −50.0 | −60.0 | −66.7 |
| Proton beam radiation | −83.3 | −71.4 | −100 |
| Brachytherapy interstitial | −46.0 | −43.1 | −55.2 |
| Brachytherapy high dose rate | −69.2 | −63.3 | −53.8 |
| ADT primary | NAa | −33.3 | +10.5 |
| ADT neoadjuvant | +25.0 | +32.5 | −32.4 |
| ADT adjuvant | 0.0 | −19.2 | +27.3 |
| ADT concurrent | NAb | −42.3 | −57.1 |
| Pelvic lymph node dissection | −50.0 | −29.4 | −50.0 |
| Cryosurgery | −56.5 | −64.6 | −73.9 |
| High-intensity focused ultrasound | −93.3 | −100.0 | −100.0 |
| Otherc | +200.0 | −25.0 | −25.0 |
Abbreviations: ADT, androgen-deprivation therapy; AUA, American Urological Association; EBRT, external beam radiation therapy.
An increase from zero low-risk patients to 2 was observed.
No low-risk patients were recommended or administered this treatment.
Other recommended treatments that did not fall into another category (no details reported).
Source: Shore et al, 2016.56
Although participating physicians rated the CCP test as influential,56 the relationship between the CCP results and decision-making on treatment are not well understood. As shown in Table 7, the concordance between treatment decisions based on clinical factors alone (initial treatment plan) and with the addition of the CCP test (actual treatment) was overall very high in this study: 76% for patients with a non-interventional approach and 86% for those for whom an interventional approach was deemed appropriate.56 More than 80% of patients did not have a change in treatment category, but rather a refinement in modalities, such as a change from several planned procedures to a single one. However, a reduction in number of treatments may or may not be clinically meaningful.
While an apparently substantial proportion of men had a change in their actual treatment (compared to their initial plan) after the CCP results were available, it is unclear if or how the CCP results directed this change. It is unclear whether there was a distinct pattern such that, for example, those with a CCP result indicating less aggressive cancer necessarily had a decrease in treatment and those with a CCP result indicating more aggressive cancer had an increase in treatment. This study is limited in its short duration of follow-up (3 months); thus, the clinical outcomes of these patients, overall or within their respective clinical risk groups, are unknown.
Quality of Evidence
Using the GRADE criteria, we assessed the quality of both included studies as very low (Table 9).
Table 9:
GRADE Evidence Profile for Clinical Utility of Prolaris Cell Cycle Progression Test
| Number of Studies (Design) | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Upgrade Considerations | Quality |
|---|---|---|---|---|---|---|---|
| Change in planned treatment | |||||||
| 1 (observationala,b) | Serious limitations (−1)c | No serious limitations | No serious limitations | No serious limitations | Undetected | NA | ⊕ Very low |
| Change in actual treatment | |||||||
| 1 (observationala,b) | No serious limitations | No serious limitations | Serious limitations (−1)d | No serious limitations | Undetected | NA | ⊕ Very low |
Abbreviations: NA, not applicable.
Evidence for this outcome begins at low quality as it is comprised of observational studies.
Change in treatment is a surrogate for patient-important outcomes as it remains unknown how or if change in treatment influences patient-important outcomes.
Very few of the best practices for reducing bias in this study design were reported in the article. See Appendix 2, Table A1, for full risk of bias assessment.
In Canada, treatment options and combinations differ from those studied, and treatment patterns are much more conservative overall, especially for low-risk patients.
Appendix 2, Table A1, provides our assessment of the risk of bias in these studies. The Crawford study was rated as poor due to a lack of reporting of several best practices for reducing bias, and the Shore study was rated as fair overall. The limitations of the included studies are further described in the Discussion, below.
Discussion
The two included studies found that information from the Prolaris cell cycle progression (CCP) test either changed patients' treatment plans or the actual treatment they received. However, the clinical outcomes of the patients whose treatment decision-making was informed by their CCP results are unknown.
The fundamental question about medical tests is whether they improve patient-important clinical outcomes. Given the currently available evidence, it is not clear how or if treatment selection based on the Prolaris CCP test influences progression-free survival, overall survival, or quality of life. None of the primary analyses of actual treatment were conducted on the risk groups separately, so there is residual confounding. This is a very active field of research and future studies may aid in understanding the clinical impact of CCP test. A randomized-controlled trial with a decade of follow-up would be ideal to address this evidence gap. These robust studies are seldom conducted because of the natural history of prostate cancer, specifically the long latency to survival outcomes. However, numerous patient-important and surrogate clinical outcomes aside from mortality can provide evidence of clinical utility and can be feasibly studied. Our findings are consistent with previous systematic reviews and technology assessments that have found limited evidence on the CCP test's clinical utility and a lack of evidence demonstrating impact on clinical outcomes.58–61 This lack of information leaves considerable uncertainty as to the test's true effect.
In considering the available evidence, the interpretation of change in treatment plan is challenging because it is a surrogate outcome for many clinical endpoints and might misrepresent the impact of the CCP test. The influence attributable to the CCP test is unclear as decision-making in prostate cancer does not follow an algorithm. Many factors weigh into the decision-making process, including characteristics of the patient and the tumour, treatment preferences of the patient and provider (which can evolve over time), and the dynamics of the patient-provider relationship. For low-risk patients, a change from interventional treatment to non-interventional is likely beneficial in terms of reducing system costs and avoiding treatment-related harms. But determining a clinically meaningful proportion of cases with a change in treatment category is challenging, and is not a figure likely to be definitively established.
Another key issue is the generalizability of the evidence. It is not clear that using the Prolaris CCP test would change treatment plans in Ontario as often as in other jurisdictions. In Ontario, some of the treatment options in the Shore et al study56 are not available (e.g., proton beam radiation) or are only in few or private clinics (e.g., CyberKnife robotic surgery, high-intensity focused ultrasound). Local standard practice often combines treatments that are listed separately in the studies (e.g., most radical prostatectomies include a pelvic lymph node dissection; androgen-deprivation therapy is often administered in conjunction with external beam radiation therapy or brachytherapy26). Thus, if the study results were translated to Ontario, the impact of the CCP test could potentially be more conservative. As well, neither study distinguishes between active surveillance and watchful waiting, which have important differences in patient characteristics and curative or non-curative management goals.
The proportion of low- and intermediate-risk prostate cancer among patients in the included studies is generally similar in Ontario. Local clinical experts advise that the distribution of T-stages in these studies is similar to that seen in practice in this province, but slightly more Gleason 7 grade (intermediate-risk) cancers are seen locally. High-risk patients would not be eligible for the CCP test in Ontario because their high risk—determined by one or more factors, unrelated to tumour genetics—necessitates definitive treatment to achieve favourable patient outcomes.8 Some other patients (e.g., those with limited life expectancy or significant comorbidities) would not also not be candidates for CCP because genomic information will not alter their overall clinical situation. In Ontario, most men of advanced age (e.g., 70 to 80 years old) who are otherwise stable proceed with active surveillance.
Prognostic tests for predicting recurrence after treatment (e.g., radical prostatectomy or radiation) were beyond the scope of this review. Owing to feasibility, we focused the review on the CCP test and did not evaluate the clinical utility of all commercially available genomic tests (e.g., OncotypeDX Genomic Prostate Score by Genomic Health, Decipher Genomic Classifier by GenomeDX) that might assist in risk estimation for localized prostate cancer. Similarly, we did not examine the clinical utility of augmenting risk stratification with imaging modalities such as magnetic resonance imaging that are not a current standard of care. While the D'Amico risk stratification scheme is used in Ontario, clinicians implicitly weigh other factors, such as the risk difference between Gleason grade of intermediate-risk patients. Local experts suggest that they would hesitate to depend on CCP score in treatment decisions with intermediate-risk patients with Gleason 4+3. There is no widely accepted consensus about which, if any, of the methods of augmenting risk stratification are effective or ready for implementation into clinical practice to improve patient outcomes.
Ongoing Studies
During our scoping and conduct of this review, two ongoing studies with potential relevance to our research question were identified on ClinicalTrials.gov (Table 10). As reported on that website, the primary outcome for both of these studies is related to treatment selection.
Table 10:
Potentially Relevant Ongoing Trials Identified of the Prolaris Cell Cycle Progression Test
| Trial Name | Trial Identifier | Estimated Primary Completion Datea |
|---|---|---|
| Open Registry Measuring Impact of Genomic Testing on Treatment Decision After Biopsy in Newly Diagnosed Prostate Cancer Patients (PROCEDE-2000) | NCT02209584 | September 2015 |
| Registry to Measure the Impact of Adding Genomic Testing (URO-006) | NCT02454595 | November 2016 |
Trial status information as reported on October 6, 2016.
Source: US National Institutes of Health, ClinicalTrials.gov.
Conclusions
We found no evidence demonstrating the impact on patient-important clinical outcomes of treatment decision-making informed by the Prolaris cell cycle progression test. Based on the limited evidence currently available, the test appears to provide information that, when considered in addition to clinical risk stratification, may change the treatment plan (GRADE: Very low) or actual treatment (GRADE: Very low) for some patients with low- or intermediate-risk localized prostate cancer.
ECONOMIC EVIDENCE REVIEW
Objectives
The objective of this study was to review the published literature on the cost-effectiveness of the Prolaris cell cycle progression (CCP) test compared with clinical risk stratification in men with newly diagnosed, low- or intermediate-risk, localized prostate cancer.
Methods
Sources
A literature search was performed on July 12, 2016, using the Ovid interface to search the following databases: MEDLINE, Embase, Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, Health Technology Assessment, National Health Service Economic Evaluation Database (NHSEED), and Database of Abstracts of Reviews of Effects (DARE). To retrieve relevant studies, the search was developed using the clinical search strategy with an economic filter applied. Database auto-alerts were created in MEDLINE and Embase and monitored for the duration of the HTA review. The reference lists of included economic literature were also reviewed to identify additional studies. The final search strategy was peer-reviewed using the PRESS Checklist.52 See Appendix 1 for full details, including all search terms.
Literature Screening
We based our search terms on those used in the clinical evidence review of this report and applied economic filters to the search results. A single reviewer reviewed titles and abstracts and, for those studies meeting the inclusion and exclusion criteria, we obtained full-text articles.
Inclusion Criteria
English-language full-text publications
Studies published between 2010 and July 12, 2016
Studies in men with newly diagnosed, low- or intermediate-risk, localized prostate cancer
Studies reporting on the addition of the Prolaris CCP test to clinical risk stratification compared with clinical risk stratification alone
Cost-utility, cost-effectiveness, cost-benefit, or cost analyses
Exclusion Criteria
Narrative reviews, letters/editorials, abstracts, posters, unpublished studies
Outcomes of Interest
Full economic evaluations: cost-utility analyses, cost-effectiveness analyses, cost-benefit analyses
Data Extraction
We extracted relevant data on the following:
Source (i.e., name, location year)
Population and comparator
Interventions
Outcomes (i.e., health outcomes, costs, and cost effectiveness)
Results
Literature Search
After removing duplicates (n = 26), the database search yielded 100 citations published between 2010 and July 12, 2016. We excluded 98 studies based on information in the title and abstract. The remaining two studies while relevant were excluded since no full text was available for further assessment (only abstracts have been published).62,63 Figure 2 presents the flow diagram for the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA).
Figure 2: PRISMA Flow Diagram for the Economic Evidence Review.
Source: Adapted from Moher et al, 2010.57
Discussion
Two abstracts provided relevant economic information on the CCP test but did not have full text publication available.62,63 Crawford et al62 calculated the economic impact of the CCP test on a US commercial health plan using a hypothetical cohort of patients with localized prostate cancer over a 10-year time horizon. The study found that the CCP test led to cost reductions due to increased use of active surveillance in low- and intermediate-risk patients, as well as reduced progression rates in high-risk patients. In France, de Pouvourville63 evaluated the cost-effectiveness of the CCP test using a Markov model. The CCP test was a dominant strategy (cost less and more effective) compared with making a decision without information from the Prolaris CCP test. However, the generalizability of these two studies to the Ontario context is limited due to the lack of detail about the model structure, the model inputs, and the different settings.
Conclusions
We did not find any cost-effectiveness studies of the CCP test in men with newly diagnosed, low- or intermediate-risk, localized prostate cancer.
PRIMARY ECONOMIC EVALUATION
We do not have sufficient data to support the development of a primary economic evaluation of the Prolaris cell cycle progression (CCP) test for localized prostate cancer. Based on the results of the clinical evidence review, the effect of the CCP test on patient-important clinical outcomes (e.g., survival or biochemical recurrence) is currently unknown. No prospective studies have been conducted to evaluate these outcomes. In addition, we did not find any published, full-text economic evaluation studies on the CCP test.
BUDGET IMPACT ANALYSIS
We conducted a budget impact analysis from the perspective of the Ontario Ministry of Health and Long-Term Care to estimate the cost burden of funding the Prolaris CCP test for men with newly diagnosed, low- or intermediate-risk, localized prostate cancer over the next 5 years. All costs were reported in 2016 Canadian dollars.
Objectives
The objective of this study was to estimate the 5-year budget impact of publicly funding the CCP test for men with newly diagnosed, low- or intermediate-risk, localized prostate cancer, within the context of the Ontario Ministry of Health and Long-Term Care.
Methods
The budget impact of the CCP test was estimated as the cost difference between two scenarios: the reference scenario, the current clinical practice without the CCP test, and the new scenario, the anticipated clinical practice with the CCP test. The model schematic is shown in Figure 3.
Figure 3: Budget Impact Model Schematic.
Abbreviations: CCP, Prolaris cell cycle progression test.
The key assumption of this analysis was that the CCP test can change only the distribution of the initial treatments, but not the downstream clinical outcomes such as recurrence, progression, or survival. We made this assumption based on the results of the clinical evidence review in this report. Two clinical utility studies showed that the CCP test may help to more accurately estimate a patient's risk level and thus lead to changes in treatment for some patients.55,56 However, there is no evidence to show how those treatment changes might impact clinical outcomes. Therefore, we assumed for this model that the CCP test does not delay progression of the disease or prolong a patient's survival; in other words, all treatment strategies were set to have equivalent clinical outcomes.
Expert Consultation
Throughout the development of this analysis, we solicited expert consultation from local physicians in the specialty areas of urology, surgical oncology, and radiation oncology. The role of the expert advisors was to review the assumptions and inputs used in the analysis and confirm that they reasonably reflect the current clinical practice in Canada. However, the statements, conclusions, and views expressed in this report do not necessarily represent the views of the consulted experts.
Target Population
The target population was men with newly diagnosed, low- or intermediate-risk, localized prostate cancer in Ontario. The size of the target population was estimated based on the published literature (Table 11). The most recent prostate cancer incidence (number of new diagnoses) available in Ontario is for the year 2012 (N = 8,500).3 To estimate the number of incident cases in 2016, we included an annual decline of 2.3%, based on reporting by Cancer Care Ontario showing that the incidence of prostate cancer fell by 4.9% per year from 2007 to 2012 and by 2.3% per year over 10 years (2003–2012).3 The report suggested that the recent drop in incidence rate was likely due to the US Preventive Services Task Force recommendations in 2012 against PSA-based screening for prostate cancer. In 2014, the Canadian Task Force on Preventive Health Care also recommended against PSA-based screening for prostate cancer (including men with lower urinary tract symptoms or with benign prostatic hyperplasia).3,64,65 We used the more moderate 10-year trend (−2.3% annually) for our base case, and the 5-year trend (−4.9% annually) and no change (0%) for the sensitivity analysis. We also assumed that prostate cancer incidence would stabilize after 2016 and stay constant for the next 5 years.
Table 11:
Epidemiological Inputs Used to Derive the Target Population
| Parameter | Value | Source |
|---|---|---|
| Prostate cancer incidence in Ontario (2012), N | 8,500 | CCO, 20163 |
| Under 65 years | 34.7% | |
| 65+ years | 65.3% | |
| Annual change in prostate cancer incidence | −2.3% | CCO, 20163 |
| Newly diagnosed with low- or intermediate-risk localized disease | 80% | Guy et al, 201666 |
| Low riska | 34% | |
| Intermediate riskb | 46% |
Abbreviations: CCO, Cancer Care Ontario.
Low risk: clinical stage ≤ T2b, Gleason score ≤ 6, and PSA ≤ 10 ng/mL
Intermediate risk includes low-intermediate risk (PSA ≤ 10 ng/mL and [Gleason score = 7 or clinical stage = T2c]) and high-intermediate risk (Gleason score = 7 and one or both of PSA 10–20 ng/mL and/or clinical stage = T2c).
Among all newly diagnosed patients, we estimated that approximately 80% will have low- or intermediate-risk localized disease.56,66 Guy et al66 reported that in Canada, approximately 34% of newly diagnosed prostate cancer patients have low-risk disease and 46% have intermediate-risk disease. This is consistent with the proportions observed by Shore et al52 and Crawford et al51 in the United States: roughly 40% with low risk and 40% with intermediate risk.55,56 We used lower (70%10) and higher (90%9) estimates found in the literature for additional sensitivity analysis. Therefore, the size of our target population for 2016 to 2020 was estimated to be 6,196 annually (= 8,500 × [1–2.3%]4 × 80%) (Tables 11 and 12).
Table 12:
Target Population
| 2016 | 2017 | 2018 | 2019 | 2020 | |
|---|---|---|---|---|---|
| Patients with newly diagnosed, low- or intermediate-risk, localized prostate cancer, N | 6,196 | 6,196 | 6,196 | 6,196 | 6,196 |
| Age: 40–64 years | 2,150 | 2,150 | 2,150 | 2,150 | 2,150 |
| 65+ years | 4,046 | 4,046 | 4,046 | 4,046 | 4,046 |
Current Treatment Pattern
For the reference scenario, we obtained the current treatment pattern for our target population from a 2016 Canadian publication by Guy et al.66 The study reported the diagnostic and treatment results of a large cohort of newly diagnosed prostate cancer patients at a prostate cancer centre in Toronto. A total of 1,277 patients were identified between June 2007 and April 2012, and divided into five risk groups based on the Prostate Cancer Risk Stratification (ProCaRS) database: low risk, low-intermediate risk, high-intermediate risk, high risk, and very high risk (Appendix 4, Table A2). ProCaRS is similar to the DAmico risk classification scheme except the intermediate and high risk groups are further divided into low and high tiers.
Based on data from Guy et al,66 we excluded treatments where the proportion of patients receiving them was unknown or very small (i.e., high-intensity focused ultrasound, 0.3%; primary androgen-deprivation therapy, 0.2%), and grouped similar treatments into one category (i.e., brachytherapy, external beam radiation therapy with or without brachytherapy, and stereotactic ablative radiotherapy were grouped as radiation therapy). We then recalculated the treatment proportions (Table 13). Among low-risk patients, 60.7% were initially managed by active surveillance or watchful waiting, and 86% of intermediate-risk patients were initially managed by radiation therapy or radical prostatectomy.
Table 13:
Initial Treatment by Prostate Cancer Risk Category
| Initial Treatment | Low Risk, % | Intermediate Risk, % | Overall, % |
|---|---|---|---|
| Active surveillance or watchful waiting | 60.7 | 14.0 | 33.7 |
| Watchful waiting | 15.2 | 3.5 | 8.4 |
| Active surveillance | 45.5 | 10.5 | 25.3 |
| Radiation therapy | 22.1 | 50.2 | 38.3 |
| Radical prostatectomy | 17.2 | 35.8 | 27.9 |
| Total | 100 | 100 | 100 |
Source: Guy et al, 2016.66
Guy et al66 reported active surveillance and watchful waiting as one group. Although both strategies are non-interventional, they are used for patients with different clinical characteristics. Patients managed by watchful waiting are usually older and have more comorbidities than those on active surveillance, and the follow-up regimen is also different.67,68 Therefore, we used an estimate of 25%, provided by clinical experts, for the proportion of patients treated by watchful waiting among all patients managed by non-interventional treatment (email communication, September 15 and 26, 2016). We also conducted sensitivity analyses by varying this proportion from 0% to 50%. We included 0% on the assumption that patients on watchful waiting could also be affected by the CCP test, since the clinical utility studies by Shore et al56 and Crawford et al55 did not exclude these patients.
Uptake of the Cell Cycle Progression Test
We estimated the expected uptake of the CCP test for our base case (Table 14) based on historical uptake of the CCP test in the US market, as provided by the manufacturer (Myriad Genetics, Inc., Toronto, Ontario, email communication, August 12, 2016). The lower and upper bounds were based on clinical expert opinion (email communication, September 15 and 26, 2016).
Table 14:
Forecasted Uptake Rates of the Prolaris Cell Cycle Progression Test, 2016–2020
| Low- or Intermediate-Risk Patients, % | |||
|---|---|---|---|
| Base Case | Lower Bound | Upper Bound | |
| Year 1 | 15 | 5 | 30 |
| Year 2 | 25 | 10 | 50 |
| Year 3 | 35 | 15 | 70 |
| Year 4 | 45 | 20 | 90 |
| Year 5 | 55 | 25 | 100 |
Effect of the Cell Cycle Progression Test on Treatment Allocation
The CCP test could provide additional information about the aggressiveness of the cancer and therefore may lead to a change in the treatment decision. We allocated proportions of patients to various changes in treatment after CCP testing, based on the two prospective clinical utility studies described in the clinical evidence review of this report.55,56 We used results from Shore et al56 for the base case analysis because that study reported the change from recommendation to actual treatments, after patients and their physicians had the additional information from the CCP test. Crawford et al55 studied only the change in treatment plans, not actual treatment received, and found that the CCP test had a greater impact on treatment change. We used those findings as a best-case scenario in our sensitivity analyses.
It must be noted that, since the studies by Shore et al56 and Crawford et al55 were both conducted in the United States where prostate cancer is often treated more aggressively than in Canada,12,66,69,70 the generalizability of these results to the Canadian population may be limited. Therefore, we also created a worst-case scenario for our sensitivity analyses, assuming that the CCP test would result in no change in treatment pattern.
In addition, clinical experts suggested that the CCP test result would not change the proportion of patients on watchful waiting (email communication, September 15 and 26, 2016). This is because, unlike active surveillance which has a curative intent, watchful waiting is the decision to forgo definitive treatment and to instead provide palliative treatment if the disease progresses.71 Additional information about cancer risk is unlikely to alter this treatment decision. Therefore, we assumed the test would only affect the proportion of patients on active surveillance.
Based on these decisions and assumptions, we calculated the expected treatment distribution in the new scenario, in which patients had information from CCP test results (Table 15). Active surveillance had a net increase of 3.3% in the base case and 18.7% in the best case. For the use of radiation therapy and radical prostatectomy, there were net reductions of 1.9% and 1.4%, respectively, in the base case and 10.8% and 7.9% in the best case.
Table 15:
Initial Treatment Distribution, With and Without Prolaris Cycle Cell Progression Test
| Treatment | Reference Scenario: Without CCP, % | New Scenario: With CCP | |||
|---|---|---|---|---|---|
| Base Case (Shore et al 201656), % | Change From Reference Scenario, % | Best Case Scenario (Crawford et al 201455), % | Change From Reference Scenario, % | ||
| Active surveillance or watchful waiting | 33.7 | 37.0 | 52.4 | ||
| Watchful waiting | 8.4 | 8.4 | 0 | 8.4 | 0 |
| Active surveillance | 25.3 | 28.6a | 3.3 | 44.0b | 18.7 |
| Radiation therapy | 38.3 | 36.4c | –1.9 | 27.5d | –10.8 |
| Radical prostatectomy | 27.9 | 26.6e | –1.4 | 20.0f | –7.9 |
| Total | 100 | 100 | 100 | ||
Abbreviations: CCP, cycle cell progression test.
28.6% = 25.3% * (1–24.2%) + (38.3% + 27.9%) * 14.2%
44.0% = 25.3% * (1–23.4%) + (38.3% + 27.9%) * 37.2%
36.4% = 38.3% * (1–14.2%) + 25.3% * 24.2% * 38.3% / (38.3% + 27.9%)
27.5% = 38.3% * (1–37.2%) + 25.3% * 23.4% * 38.3% / (38.3% + 27.9%)
26.6% = 27.9% * (1–14.2%) + 25.3% * 24.2% * 27.9% / (38.3% + 27.9%)
20.0% = 27.9% * (1–37.2%) + 25.3% * 23.4% * 27.9% / (38.3% + 27.9%)
Resource and Costs
We included the cost of the CCP test (after cancer diagnosis, at the time when clinical risk is being assessed), as well as direct health care costs related to the various initial treatments and the downstream costs such as post-treatment surveillance and treatment for disease that has recurred or progressed. Cost inputs were obtained from standard Ontario sources and published literature (Table 16). When 2016 costs were not available, the health care component of the Statistics Canada Consumer Price Index (CPI) was used to adjust all costs to 2016 Canadian dollars (2008 CPI = 112.80; 2016 CPI = 124.90).72
Table 16:
Cost Inputs
| Parameter | Value, $, 2016 CAD | Source |
|---|---|---|
| Unit cost | ||
| CCP test | 4,420 (3,400 USD) | Online sources50,51 |
| Urologist visit | 26 | Schedule of Benefits (A354: urology partial assessment)78 |
| Counselling visit to discuss treatment decision | 62.75 | Schedule of Benefits (K015 or K013: counselling or counselling of relatives)78 |
| PSA test | 30 | Tawfik, 201579 |
| Digital rectal examination | 0 | Assumed this cost is included in the urologist visit |
| TRUS biopsy | 1,156 | Ontario Case Costing Initiative 2010/201180; Schedule of Benefits (C353, J149)78 |
| Annual cost | ||
| Active surveillance (year 1) | 1,324 | Calculated based on CCO AS protocol32 |
| AS (years 2–5) | 457 | Calculated based on CCO AS protocol32 |
| Radiation therapy | 15,648 | Yong et al, 201276 |
| Radical prostatectomy | 18,898 | Krahn et al, 201474 |
| Post-RT care | 6,289 | Krahn et al, 201474 |
| Post-RP care | 2,958 | Krahn et al, 201474 |
| Recurrence | 7,756 | Krahn et al, 201474 |
Abbreviations: AS, active surveillance; CCO, Cancer Care Ontario; CCP, Prolaris cell cycle progression; PSA, prostate-specific antigen; RP, radical prostatectomy; RT, radiation therapy; TRUS, transrectal ultrasound.
The cost of the CCP test ($3,400 USD) was obtained from published online sources.50,51 We converted the US prices to Canadian dollars ($4,420) using an exchange rate of 1.30.73 We also conducted a sensitivity analysis by reducing the cost by 20%.
Costs of active interventions (radical prostatectomy and radiation therapy) and subsequent costs were obtained from a 2014 micro-costing study by Krahn et al,74 which used detailed chart reviews and provincial administrative data to determine the total health care costs associated with different health states experienced by prostate cancer patients in Ontario. All patients began in a non-treated, non-metastatic state (active surveillance or watchful waiting) and could experience treatment, post-treatment surveillance, recurrence or progression, metastasis, and eventually death. The active-treatment states included up to 182 days before the start of treatment to capture costs related to treatment planning and preparations, and one year after treatment to include costs related to post-treatment procedures. That study reported mean costs per 100 days, and we converted these to annual costs for our analyses.
The cost of radical prostatectomy was estimated to be $17,067 per year (2008 CAD), similar to other estimates in the literature. In a health technology assessment of robotic-assisted versus open and laparoscopic radical prostatectomy, the Canadian Agency for Drugs and Technology in Health estimated the costs per patient (2011 CAD) to be $11,822 for an open procedure and $15,862 for the robotic-assisted procedure (including the capital and operating cost of the robotic system).75 These estimates are lower because they included only the medical costs of the procedure but not the follow-up care after discharge from hospital.
We obtained the cost of radiation therapy from a more recent study since more advanced and expensive types of radiation therapy are being used now compared to those received by patients included in the Krahn et al study.74 In 2012, Yong et al76 reported the annual costs of intensity-modulated radiotherapy (IMRT) and 3-dimensional conformal radiotherapy (3D-CRT) for the treatment of prostate cancer in Ontario as $14,520 and $13,501 per patient, respectively (2009 CAD). We used the cost of IMRT for our analysis because it is the most commonly used technique (clinical expert, email communication, September 26, 2016). The costs estimated by Yong et al76 are similar to estimates in another Canadian study which estimated the per-patient cost of radiation therapy to be $12,262 (2012 CAD).77 The costs of post-treatment and recurrence health states reported by Yong et al76 are also similar to those estimated by Krahn et al.74
We calculated the annual per-patient costs of active surveillance based on the current Cancer Care Ontario protocol.32 The cost estimated by Krahn et al74 ($12,556, 2008 CAD) was substantially higher than in other studies, due to the inclusion of total health care costs; that study probably captured the costs of background comorbidities rather than only prostate cancer. In contrast, Dragomir et al,77 who included only costs related to regular PSA tests and biopsy, estimated the costs of active surveillance to be $1,224 in the first year and $1,767 over 5 years of follow-up (2012 CAD). This is closer to the costs we arrived at using the current protocol in Ontario (Table 16).
We included the following resources in the annual costs of active surveillance: PSA test every 3 to 6 months, followed by an urologist visit after each test; digital rectal examination every year; and 12- to 14-core confirmatory transrectal ultrasound (TRUS) biopsy within 6 to 12 months then serial biopsy a minimum of every 3 to 5 years thereafter.32 The cost of watchful waiting was not included since, as noted, the proportion of patients on watchful waiting is not expected to be affected by the CCP test (see page 36).
According to clinical expert opinion, the use of the CCP test would likely result in at least one additional counselling visit to review the test result and discuss treatment options (telephone communication, August 19, 2016; email communication, September 26, 2016).
Disease Progression
Our analysis also took into account the downstream costs due to post-treatment surveillance and disease recurrence or progression. We assumed that clinical outcomes for all treatment strategies are equivalent (i.e., percentages of patients who progress or die within 5 years are the same for those managed initially either by active surveillance or by active treatment). This is because we do not have enough information to predict how the CCP test would affect clinical outcomes. Predicted 5-year outcomes by initial treatment strategy for low- and intermediate-risk patients were available in a published Markov model.81 We chose the outcomes of radical prostatectomy to represent the outcomes of all treatments as it is a commonly used initial treatment.81 We weighted the 5-year outcomes from that model according to the proportions of Ontario patients with low- or intermediate-risk disease and converted this to annual outcomes assuming a fixed rate with respect to time (Table 17).
Table 17:
Clinical Outcomes for Low- and Intermediate-Risk Patients, by Treatment Strategy
| Active Surveillance | % on AS | Progressed on AS | % Died, All Causes | |
|---|---|---|---|---|
| % Received RP/RT | % Post-RP/RT | |||
| Year 1 | 96.6 | 2.1 | – | 1.3 |
| Year 2 | 93.3 | 2.7 | 1.5 | 2.6 |
| Year 3 | 90.0 | 2.7 | 3.6 | 3.8 |
| Year 4 | 86.7 | 2.6 | 5.6 | 5.1 |
| Year 5 | 83.6 | 2.6 | 7.5 | 6.3 |
| Radical Prostatectomy/ Radiation Therapy | % Received RP/RT | % Post-RP/RT Care | % Prostate Cancer Recurred | % Died, All Causes |
|---|---|---|---|---|
| Year 1 | 96.6 | – | 2.1 | 1.3 |
| Year 2 | – | 93.3 | 4.2 | 2.6 |
| Year 3 | – | 90.0 | 6.2 | 3.8 |
| Year 4 | – | 86.7 | 8.2 | 5.1 |
| Year 5 | – | 83.6 | 10.1 | 6.3 |
Abbreviations: AS, active surveillance; RP, radical prostatectomy; RT, radiation therapy.
Source: Calculated based on Sanyal et al, 2014.81
If their prostate cancer does not progress, patients on active surveillance continue with that approach. For those who progress while on active surveillance, we assumed they would receive either radical prostatectomy or radiation therapy (as recommended by Cancer Care Ontario guidelines32 and also as observed in clinical trials82) in the first year, and then post-surgery or post-radiation care in subsequent years while they are recurrence-free. If their disease progresses, patients would then receive treatment for the recurrent cancer.
We considered deaths due to prostate cancer and other causes by tracking the number of deaths each year, based on results from the published Markov model81 (Table 17).
Analysis
The net budget impact of publicly funding the CCP test was calculated as the difference in costs between the reference scenario (based on treatments chosen without the CCP test) and the new scenario (with the CCP test). The budget impact has three cost components: 1) incremental costs associated with the CCP test, 2) incremental costs associated with the additional physician visits to review the test result, and 3) the cost difference associated with changes in treatment due to information from the test. The sum of these three cost components equals the total net budget impact.
We calculated the incremental costs associated with the CCP tests by multiplying the unit cost of the test by the number of patients expected to receive the test. The incremental costs associated with extra physician visits were estimated by multiplying the physician visit cost per person by the number of patients expected to receive the test. The cost difference associated with treatment change (i.e., from active surveillance to active treatment, and vice versa) were determined by multiplying the change in the number of patients receiving each initial treatment strategy and the annual cost per patient. Different annual costs (for initial treatment and treatment in subsequent years) were applied to patients in different health states (e.g., recurrence-free, recurrence).
To fully understand the variability in budget impact resulting from different parameter assumptions, we calculated several budget impact scenarios. The parameters varied are shown in Table 18.
Table 18:
Parameters Varied in the Sensitivity Analyses
| Parameter | Base Case | Sensitivity Analysis 1 | Sensitivity Analysis 2 |
|---|---|---|---|
| Annual change in prostate cancer incidence | −2.3% | 0 | −4.9% |
| % low- to intermediate-risk localized disease | 80% | 70% | 90% |
| Change in prostate cancer incidence | No change after 2016 | Continue to change after 2016 | |
| Expected uptake of the CCP test | 15% in year 1 | 5% | 30% |
| 25% in year 2 | 10% | 50% | |
| 35% in year 3 | 15% | 70% | |
| 45% in year 4 | 20% | 90% | |
| 55% in year 5 | 25% | 100% | |
| Assumptions regarding the extent that CCP can change treatment | Shore et al 201656 | Crawford et al 201455 | No change |
| Proportion of WW in non-intervention group | 25% | 0% | 50% |
| Unit cost of the CCP test | $4,420 | −20% | |
| Annual cost of AS in year 1 | $1,324 | −50% | +50% |
| Annual cost of AS in year 2 to 5 | $457 | −50% | +50% |
| Annual cost of RP | $18,898 | −50% | +50% |
| Annual cost of RT | $15,648 | −50% | +50% |
| Annual cost of post RP | $2,958 | −50% | +50% |
| Annual cost of post RT | $6,289 | −50% | +50% |
| Annual cost of recurrence | $7,756 | −50% | +50% |
| Number of extra physician visits associated with the CCP test | 1 | 0 | 2 |
Abbreviations: AS, active surveillance; CCP, Prolaris cell cycle progression; RP, radical prostatectomy; RT, radiation therapy; WW, watchful waiting.
Results
Base Case
The base case results of our analysis are presented in Table 19 and graphically in Figure 4. We estimated the costs associated with the CCP test itself to be $47.9 million in the first 5 years, the costs associated with additional physician visits required to interpret the test result to be $0.7 million, and the savings due to treatment changes (increased use of active surveillance and decreased use of interventional treatment) to be $7.3 million. As a result, publicly funding the CCP test would result in a net budget impact of $41.3 million in the first 5 years.
Table 19:
Results of Budget Impact Analysis of Publicly Funding the Prolaris Cell Cycle Progression Test for Low- and Intermediate-Risk Localized Prostate Cancer
| Net Budget Impact, $ Million | ||||||
|---|---|---|---|---|---|---|
| 2016 | 2017 | 2018 | 2019 | 2020 | 5-Year Total | |
| Additional cost associated with the CCP test | 4.1 | 6.8 | 9.6 | 12.3 | 15.1 | 47.9 |
| Additional cost associated with extra physician visits | 0.1 | 0.1 | 0.1 | 0.2 | 0.2 | 0.7 |
| Cost offset associated with treatment changes | −0.5 | −0.9 | −1.4 | −2.0 | −2.6 | −7.3 |
| Total costs | 3.7 | 6.1 | 8.3 | 10.5 | 12.7 | 41.3 |
Abbreviation: CCP, Prolaris cell cycle progression.
Figure 4: Budget Impact of Funding the Prolaris Cell Cycle Progression Test.
Abbreviation: CCP, Prolaris cell cycle progression.
Sensitivity Analysis
Results of the sensitivity analyses are presented in the tornado diagram (Figure 5). The net budget impact was most sensitive to assumptions regarding the uptake of CCP and the extent to which the CCP test altered the distribution of treatment from current practice. Three factors had a moderate impact on the results by affecting the size of the target population: the percentage of low- and intermediate-risk tumours among all patients newly diagnosed with prostate cancer, the proportion of patients on watchful waiting among those on non-interventional treatment, and the annual change in prostate cancer incidence. In terms of costs, the budget impact was sensitive to the unit cost of the CCP test but not to the annual costs of prostate cancer treatments or the number of extra physician visits associated with the CCP test. This is because the cost of the test is much higher than either the extra physician visits or the savings associated with treatment change.
Figure 5: Tornado Diagram of the Influence of Key Parameters on the Net Budget Impact.
Abbreviations: AS, active surveillance; CCP, Prolaris cell cycle progression test; RP, radical prostatectomy; RT, radiation therapy; WW, watchful waiting.
Discussion
The base case results showed that publicly funding the Prolaris cell cycle progression (CCP) test in Ontario would have a large impact on the provincial budget, given the unit price of the test and the size of the target population. Sensitivity analyses showed that the net budget impact was relatively stable when we varied the annual costs of treatments. This is because the net budget impact was driven by the cost of the CCP test, so the cost offset due to treatment change was relatively small. However, the result was very sensitive to assumptions regarding the uptake of CCP and the extent to which CCP can change treatment decisions.
It is difficult to predict how quickly the CCP test would be adopted if publicly funded in Ontario. The base case assumed that the uptake would most likely be similar to historical uptake in the US market. However, it is possible that uptake may be slower in Canada than the US, as it has been with some other genomic tests.83,84 When we assumed uptake would be slower (5% in the first year, rising by 5% per year), the net budget impact decreased by about half, compared to the base case ($17.8 million vs. $41.3 million). However, clinical experts suggested that, once the test is publicly funded and physicians and patients learn about it, adoption could be much faster. When we assumed uptake would be higher (30% in the first year, rising by 20% per year and reaching 100%), the net budget impact almost doubled, to $80.2 million over 5 years.
It is also challenging to predict how much the CCP test would alter treatment practice in Ontario. When we used the findings on treatment change from the US study by Crawford et al55 (with 37.2% of patients changing from interventional to non-interventional treatment, vs. only 14.2% in the study by Shore et al56), the net budget impact decreased to $7 million (Figure 6). This significant shift is due to the large cost savings from decreased use of radical prostatectomy and radiation therapy (−$41.6 million) and increased use of active surveillance. However, this scenario is an unlikely one for Ontario, where treatment practice is already conservative, with greater use of active surveillance compared to the United States and to other provinces.12,66,69 Cancer Care Ontario guidelines on prostate cancer recommend active surveillance for low-risk patients,32 and Guy et al66 showed that in clinical practice in Ontario more than 60% of patients with low-risk prostate cancer are managed conservatively. In contrast, a US study using data from the National Cancer Database showed that among men with low-risk prostate cancer (based on the D'Amico criteria), approximately half were treated with radical prostatectomy and only 7.4% received active surveillance.70 In addition, as noted in our clinical evidence review, the estimates from the study by Crawford et al55 should be considered with caution as they captured only the change in physician's treatment recommendations after the test, not the change in actual treatments received by patients.
Figure 6: Sensitivity Analysis Results Using Larger Change from Interventional to Non-interventional Treatment.
Abbreviation: CCP, Prolaris cell cycle progression.
Note: Based on findings from Crawford et al.55
Our analysis has several strengths. The cost and treatment pattern inputs were based on Ontario sources and assumptions were validated by local clinical experts. We considered both the cost of the test and potential cost offsets associated with treatment change. Since the long-term effect of the CCP test on survival and disease progression is unknown, we made the neutral assumption that the test would neither positively nor negatively affect clinical outcomes. We also conducted extensive sensitivity analyses exploring the impact of different parameters and assumptions on the estimated budget impact.
Our analysis also had several limitations. Firstly, the effect of the CCP test on treatment change was estimated based on two studies in the United States, where treatment patterns for prostate cancer are quite different than in Canada. We addressed this limitation by conducting a sensitivity analysis assuming that the CCP test would result in no change in treatment pattern. Secondly, our analysis did not consider treatment change within the intervention category, such as a change from radical prostatectomy to radiation therapy, or from a single modality to two or more. These changes are considered a refinement of treatment modality, and the results from relevant studies are difficult to use for economic analysis. Thirdly, we assumed that the clinical outcomes are equivalent for all treatment strategies. Further investigations are necessary to show if the test affects patient important clinical outcomes. Lastly, our analysis did not include the resources needed to prepare the tissue sample for the CCP test. The sample is currently shipped to a US laboratory to be analyzed. The net budget impact would be higher if these costs were taken into account.
Conclusions
We found that the Prolaris cell cycle progression (CCP) test would result in a large increase in cost to the provincial budget—our best estimate was $41.3 million over 5 years—if the test were publicly funded for men with low- and intermediate-risk localized prostate cancer. The majority of the cost is associated with the CCP test itself because of the high unit cost and the large target population. The estimated cost savings due to increased use of active surveillance and decreased use of active treatment are relatively small. The results of this analysis were most sensitive to assumptions regarding how many tests will be performed annually and the extent to which the test could alter current treatment practice in Ontario.
PUBLIC AND PATIENT ENGAGEMENT
Background
Public and patient engagement is the process of exploring the personal experience of people who have a particular health condition, including how the condition and its treatment affects them, their families or other caregivers, and their personal environment. Public and patient engagement is intended to increase awareness and build appreciation for the needs, priorities, and preferences of the people at the centre of a health care program. Insights gained through this process provide an in-depth picture of lived experience, through an intimate look at the values that underpin the experience.
Input from patients and caregivers is a unique source of evidence about the personal impact of a health condition and how that condition is managed, including what it is like to navigate the health care system with that condition and how technologies (tests and treatments) might or might not make a difference in people's lives. Their perspectives can also provide information on the ethical and social-value implications of technologies and treatments. In addition, information shared from lived experience can identify gaps or limitations in published research (for example, outcome measures that do not reflect what is important to patients).85–87 Because the needs, priorities, preferences, and values of those with lived experience in Ontario are not often adequately explored in published studies, Health Quality Ontario makes an effort to reach out to, and directly speak with, people who live with the health condition in question, including those who have experience with a particular test or treatment.
This project began with the perception that a diagnosis of prostate cancer has significant bearing on quality of life for patients and their families. To understand this impact and the factors that influence treatment decision-making for these patients, we spoke directly with patients with prostate cancer at various stages of treatment. Appreciating their decision-making about treatment helps us understand the potential value of the Prolaris cell cycle progression (CCP) test from a lived-experience perspective.
Methods
Engagement Plan
Engagement as a concept captures a range of efforts used to involve the public and patients in various domains and stages of assessing a health technology.88 The engagement plan for this health technology assessment was consultation—specifically, interviews to examine the lived experience of patients with prostate cancer and their decision-making on treatment.
We chose qualitative interviews as an appropriate method because they allowed us to deeply explore the central themes in the lived experience of participants. The main task in interviewing is to understand the meaning of what participants say.89 Interviews are particularly useful for getting the story behind a participant's experiences, which was the objective for this part of the health technology assessment. The sensitive nature of exploring quality-of-life issues is another reason for using interviews for this project.
Recruitment of Participants
Our strategy for recruiting participants for this project was an approach called purposive sampling90–93 in which we actively invited individuals with direct lived experience. Staff of the Patient, Caregiver, and Public Engagement office of Health Quality Ontario contacted patients with prostate cancer through a variety of partner organizations, provincial prostate cancer associations, and word of mouth as participants reached out to other families after completing their own interviews.
Inclusion Criteria
We sought participants at various stages of the prostate cancer treatment journey. We also wanted to include people with a range of severity of prostate cancer, as we assumed that the values, needs, preferences, and decision-making priorities of patients and their families could evolve based on the severity of the disease. We sought a broad geographic representation, as we further assumed that access to treatment options could vary across the province.
Exclusion Criteria
No exclusion criteria were set.
Participants
Patient, Caregiver, and Public Engagement staff at Health Quality Ontario spoke with six patients with prostate cancer from across Ontario. Four of the six had made their treatment decision (two pursued surgery, one pursued brachytherapy, one chose watchful waiting) while two were still considering their treatment options. One patient who opted for surgery subsequently had radiation treatment as well.
Interview Approach
At the outset of the interview, we explained the purpose of the health technology assessment (including the role and mandate of Health Quality Ontario and the Ontario Health Technology Advisory Committee), risks to participation, and protection of personal health information. We provided this background verbally and through a letter of information, and then obtained participants' consent before commencing the interview. The letter of information and consent form are provided in Appendix 5. Interviews were recorded and transcribed.
The interview was semi-structured, consisting of a series of open-ended questions. The questions were initially based on a list developed by the Patient and Citizen Involvement Group of Health Technology Assessment International and designed to elicit lived experience specific to how a health technology or intervention affects people and their quality of life.94 Interviews lasted from approximately 25 to 60 minutes.
Due to the nature of the Prolaris CCP test as a source of information for treatment decision-making, interview questions focussed on how patients and their families weighed various factors and explored their insights into the factors and values that shaped their decisions. We also described the Prolaris test and asked patients about its potential value or impact on their decision-making. The interview guide is attached as Appendix 6.
Data Extraction and Analysis
We selected a modified version of a method called grounded theory to analyze the interview transcripts because it captured themes and allowed elements of the lived experience to be compared among other participants. The inductive nature of grounded theory follows an iterative process of eliciting, documenting, and analyzing responses while simultaneously collecting and analyzing data using a constant comparative approach.95,96 Through this approach, staff coded transcripts and compared themes by using NVivo, a qualitative software program. The software helped us identify and interpret patterns in the interview data about the meaning and implications of participants' experience, particularly from the perspective of what was important to them in deciding on treatment for their prostate cancer.
Results
Patients consistently reported and commented on three general areas that factored into their decision-making on prostate cancer treatment: the information they received from physicians and others, their perceptions of the risks associated with the different treatments, and the emotional component of the diagnosis. The three areas influenced each person's decision-making to differing degrees. Emotion was generally reported to have the least impact, while information was reported to have the greatest.
Information About Treatment Options for Prostate Cancer
Patients interviewed universally reported being presented with clear options following their diagnosis of prostate cancer. The diagnosis resulted from a prostate biopsy and necessitated close interaction with urologists and family doctors; these medical professionals were the primary conveyors of information on treatment options. Patients generally reported feeling confident about the information they received from these health care professionals.
“I had a lot of confidence in the information I was getting, particularly from
[doctor's name]. This was his recommended approach, and so I agreed with him.”
However, information surrounding these treatment options did not solely come from health care professionals. Several patients reported seeking out additional information from other sources, including family members, friends, colleagues, websites, and social media. Patients said these sources had varying degrees of influence on their treatment decisions. Patients often cited anecdotes from family members, friends, or colleagues who had been diagnosed with prostate cancer and how this information influenced their own decision-making.
“Well, as much as I can say that prostate cancer has a good support group, it's nothing in terms of what I think is needed. I've had to do a lot of the research on my own.”
“I actually did reach out to a couple of people I know that went through this a few years earlier than I did.”
“And I went online quite a bit. There was a good Mayo site—the Mayo Clinic on prostate cancer surgery and all the different options—and in the end … with talking to everybody, my family and that, I opted for the surgery.”
Patients whose treatment occurred several years ago reported feeling they had enough information to make an informed decision, but they also said they now wished that much of the information currently available about prostate cancer and treatment outcomes had been available at the time. Several patients spoke of the rapidly changing landscape of prostate cancer treatment and its effect on treatment choices and options.
“There probably wasn't as much information at that time out there as there is now.”
“So … it was additional information that has become available that I wish I'd had at the time and, you know, changes in diet, getting regular exercise, maybe that would have stemmed things a little bit.”
Perception of Risk in Treatment Options for Prostate Cancer
The patients interviewed reported receiving clear information about the potential risks and side effects of the different types of prostate cancer treatment. Patients reported learning that symptoms such as pain, incontinence, bowel dysfunction, and loss of sexual function are potential side effects for surgery or radiation treatments. In addition, several patients were informed that, even with the surgical removal of the prostate gland, there is still a risk of developing cancer in the surrounding area at a later time.
“The side effects of the treatment are probably the major concern to me.”
“That's really worrisome: that you go through this drastic procedure and it's still not a cure, it's a temporary relief.”
These risks were counterbalanced by patients' perceptions of the potential risk of choosing a more conservative treatment. All patients acknowledged being informed by their physicians that prostate cancer can often be a slow-moving cancer and that active surveillance was an option. For some patients, however, this was seen as too passive and too much of a risk.
“So I knew my option was to do nothing but I mean it wouldn't go away on that basis, it was just a matter of time. So I chose the active approach rather than the passive.”
Other patients, however, reported that the lack of symptoms of prostate cancer made the conservative treatment options more attractive, rather than facing the risks associated with surgery or radiation.
“And the problem is when you've got no symptoms, it makes the decision of going through drastic surgical results of having a prostate taken out or radiation, where you're going to burn it out, and having those drastic side effects … It's like ‘wow, I don't have any symptoms at this stage, maybe I can keep on prolonging it a big longer.”'
All patients reported being able to discuss these various risks with their physicians and the value they placed on these discussions. The physician's opinion and interpretation of risks was reported to be of great value by patients in their treatment decision-making.
“So [the doctor] thought that surgery was, I won't say drastic, but a severe action and at the time didn't think it necessary, so we kind of agreed together that we'll just monitor this situation and see how it goes.”
Emotion in Decision-Making for Treatment of Prostate Cancer
A number of patients commented on the emotional impact of their diagnosis of prostate cancer. Patients spoke of the positive and healthy lifestyle they led and their subsequent surprise at the diagnosis of cancer. Fear, anxiety, anger, and depression were all emotions reported by patients. This emotional impact extended to patients' friends and family members.
“So for all of a sudden to get cancer is, boy, it's a bit of a shock for all of us.”
“I'm waiting in Sunnybrook in the cancer ward there feeling somewhat traumatic and having almost an anxiety attack. Surrounded by that environment.”
Some patients also admitted that their treatment decision-making was influenced by their emotional state and by their perception of the cancer and its potential to spread. The fear of the cancer spreading was universally mentioned, and some patients reported that the anxiety of not knowing weighed heavily on their minds.
“I mean there's always the thought that this is going to progress, it's not something that's going to go away. And it can spread to other organs, etc. So I don't want it to reach that level. I want to deal with it before it gets to that.”
Patients also reported a strong emotional desire to be rid of the cancer, to carry forward with their lives and not allow the cancer to remain. These emotions contributed to patients choosing an active treatment option, rather than a conservative one, though patients reported that the information they received from their physicians was the bigger influence in such decisions.
“Yeah, I kind of wanted to deal with it, get it over with. And not always wonder.”
“I just wanted to carry on with my life.”
Being well-informed about treatment options and their potential side effects, several patients also reported anxiety and fear associated with the after-effects of their treatment and the long-term consequences of their choice.
“I mean, I'm having this treatment tomorrow. I haven't really felt that anxious about the treatment itself but I have had some anxiety about what will happen after the fact.”
“My anxiety is solely on the after-effects.”
Patients who had already undergone treatment said they did not regret their treatment decisions. Despite wishing that current information and technology had been available when their treatment decision was made, they did not second-guess that decision.
“Do I have regrets? I don't think so. Do I have the feeling that we took the best course? I kind of do.”
“That's the decision we made and, looking back, I'm not sure it was wrong. Maybe it was right, I don't know.”
Prolaris Cell Cycle Progression Test
We were unable to recruit patients who had been tested with the Prolaris CCP test. Instead, we gave participants basic information about the test, both in writing and verbally during the interview. This information included the logistics of the test and the type of information it can provide, as well as a summary of the broader discussion currently surrounding prostate cancer testing and treatment in Ontario's health care system. Each patient reported being aware of this current discussion and the importance of accurate risk stratification. Patients were then asked for their thoughts on the CCP test and whether such a test would be of value to them or if it would have, if applicable, changed their decision-making on treatment of their prostate cancer.
Patients reported a mostly positive response to the prospect of the CCP test. They all valued the information they had received from their physicians on treatment options, risks, and potential outcomes for their prostate cancer. Patients saw the test as another piece of information in that decision-making process and therefore valuable. However, its value was predicated on the ability to provide a definitive result, and patients were unsure whether this single test would have ultimately changed their own complex decisions.
“So when you talk about Prolaris having definitive result based on genetics and, if that's the case where it is a definitive result where they can say, you know, it's black or white, there's no grey zone, here's where you are, that would be in my opinion extremely valuable information in the sense that you've got some definitive results.”
“And for me again I wish at the initial onslaught that the test would have been available because, if it was definitive, it would have made the decision process a lot easier to go one route or the other.”
If the test did not provide a definitive result, several patients reported being unsure whether they would want it done. Patients also reported wishing to defer the decision on the test to their physicians, as the subject experts.
“Well, I mean the answer to that would be, what about the experts? The medical boys? How much emphasis do they put on that? Do they think this is valuable? And I would think that would be, in most cases, what the patient would think. I mean I don't know the science of it but, if my doctor said this is very important, that we know this, then I would probably consider doing it.”
“What it is, it's not a cure. It's not a procedure that cures you. It's just a process that gives us a more accurate assessment.”
Conclusions
From our interviews with patients with prostate cancer, it is clear that they face difficult and challenging decisions about their treatment. Each patient described the emotional impact of a cancer diagnosis. Quickly following the diagnosis, they were faced with various treatment options, each with its own potential risks and side effects.
Each patient interviewed greatly valued the medical advice and guidance they had received from health care professionals, who provided clear and thorough information about treatment options. In considering their treatment decision-making process, patients said the information received from physicians had the most impact. However, they also reported that their own perception of risks as well as their emotional state played a role in their ultimate decision on whether or not to pursue active treatment.
Patients viewed the additional information from a Prolaris cell cycle progression test as potentially valuable to help in that decision-making, so long as it provided a definitive result. However, due to the complex factors involved in treatment decision-making, patients were unsure whether the CCP test would ultimately change their own treatment pathway. They also wanted to know what value their physicians would place on the test, trusting their professional judgment and interpretation of the results.
Acknowledgments
The medical editor was Amy Zierler. Others involved in the development and production of this report were Merissa Mohammed, Claude Soulodre, Ana Laing, Kellee Kaulback, Andrée Mitchell, Anil Thota, Vivian Ng, Nancy Sikich, and Irfan Dhalla.
We are grateful to Dr. Glenn Bauman,a Dr. Rodney H. Breau,b Dr. Suzanne Kamel-Reid,c Dr. Alexander V. Louie,d and Dr. Stephen Pautlere for contributing their expertise through consultations in the development of this health technology assessment.
aProfessor and Chair/Chief, Department of Oncology, London Regional Cancer Program
bSurgical Oncologist in the Division of Urology, Department of Surgery; Research Chair in Urologic Oncology; and Associate Scientist, Ottawa Hospital Research Institute, The Ottawa Hospital/University of Ottawa
cChief, Clinical Laboratory Genetics; Director, Genome Diagnostics, the University Health Network
dRadiation Oncologist, London Regional Cancer Program; Clinician Scientist, Western University
eAssociate Professor, Departments of Surgery and Oncology, Western University; Consultant Surgeon, St. Joseph's Health Care, London, and London Health Sciences Centre
ABBREVIATIONS
- AUA
American Urological Association
- CI
Confidence interval
- CCP
Cell cycle progression
- GRADE
Grading of Recommendations Assessment, Development, and Evaluation
- PSA
Prostate-specific antigen
GLOSSARY
- Budget impact analysis
A technique to estimate the financial impact of a planned action over a specified time period by calculating the costs and savings of different options.
- Clinical risk stratification
Classifies people according to their likelihood of suffering a particular health effect, typically based on the average risk of people with similar characteristics, to assist in providing appropriate monitoring and care.
- Health technology assessment
A process that systematically assesses the clinical benefit, value for money, and patient preference and values of a health technology, usually to inform decision-making about the technology.
- Qualitative study
Some research topics are not well suited for objective data-driven (quantitative) studies. The qualitative study is a more subjective alternative where a researcher gathers and analyzes information about individuals or groups through observation, historical record search, and/or other non-statistical research approaches.
- Sensitivity analysis
Every evaluation contains some degree of uncertainty. Study results can vary depending on the values taken by key parameters. Sensitivity analysis is a method that allows estimates for each parameter to be varied to show the impact on study results. There are various types of sensitivity analyses. Examples include deterministic, probabilistic, and scenario.
- Systematic review
A process to answer a research question by methodically identifying and assessing all available studies that evaluate the specified research question. The systematic review process is designed to be transparent and objective and is aimed at reducing bias in determining the answers to research questions.
- Tumour genomic test
Analyzes a group of genes in a cancer tumour to see how active they are. This is done to evaluate the likelihood that the tumour will grow and spread. A genomic test is different from a genetic test, which focuses on the presence or absence of a specific gene or genetic abnormality.
APPENDICES
Appendix 1: Literature Search Strategies
Clinical Evidence Search
Databases searched: All Ovid MEDLINE, Embase, Cochrane Database of Systematic Reviews, Database of Abstracts of Reviews of Effects, CRD Health Technology Assessment Database, Cochrane Central Register of Controlled Trials, and NHS Economic Evaluation Database
Database: EBM Reviews - Cochrane Central Register of Controlled Trials <May 2016>, EBM Reviews - Cochrane Database of Systematic Reviews <2005 to June 08, 2016>, EBM Reviews - Database of Abstracts of Reviews of Effects <1st Quarter 2016>, EBM Reviews - Health Technology Assessment <2nd Quarter 2016>, EBM Reviews - NHS Economic Evaluation Database <1st Quarter 2016>, Embase <1980 to 2016 Week 23>, All Ovid MEDLINE(R) <1946 to Present>
Search Strategy:
| 1 | exp Prostatic Neoplasms/ (288643) |
| 2 | Prostate/ (73925) |
| 3 | exp Neoplasms/ (6452580) |
| 4 | 2 and 3 (37577) |
| 5 | (prostat* adj3 (cancer* or carcinoma* or neoplas* or tumo?r* or adenoma* or adenocarcinoma* or malignan* or metasta*)).tw. (274223) |
| 6 | Prostatectomy/ (69296) |
| 7 | prostatectom*.tw. (62616) |
| 8 | or/1,4–7 (370265) |
| 9 | Genetic Testing/ (59269) |
| 10 | ((genetic* or gene or genes or genome or genomes or genomic* or 17-gene) adj2 (test or tests or testing or panel* or assess* or screen* or profil* or algorithm* or combinatorial or prognosis or prognostic*)).tw. (232571) |
| 11 | exp Gene Expression Profiling/ (169928) |
| 12 | ((gene expression adj (profiling* or monitoring* or analys?s or test or tests or testing)) or transcript expression analys?s or (transcriptome adj (analys?s or profiling*)) or (mrna adj differential display*)).tw. (68730) |
| 13 | ((cell cycle progression adj (score or scores or test or tests or testing or assay* or signature* or instrument*)) or (CCP adj (score or scores or test or tests or testing)) or cell cycle gene expression assay* or cell cycle proliferation gene* or RNA profiling test* or molecular assay* or prostate cancer assay* or genomic prostate score* or molecular diagnostic assay* or multigene expression assay*).tw. (5741) |
| 14 | or/9–13 (424104) |
| 15 | 8 and 14 (6876) |
| 16 | prolaris*.tw. (30) |
| 17 | or/15–16 (6885) |
| 18 | limit 17 to english language [Limit not valid in CDSR, DARE; records were retained] (6749) |
| 19 | limit 18 to yr=“2010 -Current” [Limit not valid in DARE; records were retained] (4044) |
| 20 | 19 use pmoz,cctr,coch,dare,clhta,cleed (1495) |
| 21 | exp prostate tumor/ (179720) |
| 22 | prostate/ (73925) |
| 23 | exp neoplasm/ (6447430) |
| 24 | and/22–23 (37561) |
| 25 | (prostat* adj3 (cancer* or carcinoma* or neoplas* or tumo?r* or adenoma* or adenocarcinoma* or malignan* or metasta*)).tw. (274223) |
| 26 | prostatectomy/ (69296) |
| 27 | prostatectom*.tw. (62616) |
| 28 | or/21,24–27 (356159) |
| 29 | genetic screening/ (87257) |
| 30 | ((genetic* or gene or genes or genome or genomes or genomic* or 17-gene) adj2 (test or tests or testing or panel* or assess* or screen* or profil* or algorithm* or combinatorial or prognosis or prognostic*)).tw. (232571) |
| 31 | gene expression profiling/ (169891) |
| 32 | ((gene expression adj (profiling* or monitoring* or analys?s or test or tests or testing)) or transcript expression analys?s or (transcriptome adj (analys?s or profiling*)) or (mrna adj differential display*)).tw. (68730) |
| 33 | ((cell cycle progression adj (score or scores or test or tests or testing or assay* or signature* or instrument*)) or (CCP adj (score or scores or test or tests or testing)) or cell cycle gene expression assay* or cell cycle proliferation gene* or RNA profiling test* or molecular assay* or prostate cancer assay* or genomic prostate score* or molecular diagnostic assay* or multigene expression assay*).tw. (5741) |
| 34 | or/29–33 (439574) |
| 35 | 28 and 34 (6809) |
| 36 | prolaris*.tw. (30) |
| 37 | or/35–36 (6817) |
| 38 | limit 37 to english language [Limit not valid in CDSR,DARE; records were retained] (6675) |
| 39 | limit 38 to y=“2010 -Current” [Limit not valid in DARE; records were retained] (4030) |
| 40 | 39 use emez (2624) |
| 41 | 20 or 40 (4119) |
| 42 | 41 use pmoz (1453) |
| 43 | 41 use emez (2624) |
| 44 | 41 use coch (9) |
| 45 | 41 use cctr (24) |
| 46 | 41 use clhta (6) |
| 47 | 41 use cleed (1) |
| 48 | 41 use dare (2) |
| 49 | remove duplicates from 41 (3195) |
Economic Evidence Search
Databases searched: All Ovid MEDLINE, Embase, Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, Database of Abstracts of Reviews of Effects (DARE), Centre for Reviews and Dissemination (CRD) Health Technology Assessment Database, and National Health Service (NHS) Economic Evaluation Database
Database: EBM Reviews - Cochrane Central Register of Controlled Trials <June 2016>, EBM Reviews - Cochrane Database of Systematic Reviews <2005 to July 08, 2016>, EBM Reviews - Database of Abstracts of Reviews of Effects <1st Quarter 2016>, EBM Reviews - Health Technology Assessment <2nd Quarter 2016>, EBM Reviews - NHS Economic Evaluation Database <1st Quarter 2016>, Embase <1980 to 2016 Week 28>, All Ovid MEDLINE(R) <1946 to Present>
Search Strategy:
| 1 | exp Prostatic Neoplasms/ (290920) |
| 2 | Prostate/ (74575) |
| 3 | exp Neoplasms/ (6500732) |
| 4 | 2 and 3 (38049) |
| 5 | (prostat* adj3 (cancer* or carcinoma* or neoplas* or tumo?r* or adenoma* or adenocarcinoma* or malignan* or metasta*)).tw. (277087) |
| 6 | Prostatectomy/ (69779) |
| 7 | prostatectom*.tw. (63158) |
| 8 | or/1,4–7 (373736) |
| 9 | Genetic Testing/ (60447) |
| 10 | ((genetic* or gene or genes or genome or genomes or genomic* or 17-gene) adj2 (test or tests or testing or panel* or assess* or screen* or profil* or algorithm* or combinatorial or prognosis or prognostic*)).tw. (235791) |
| 11 | exp Gene Expression Profiling/ (171739) |
| 12 | ((gene expression adj (profiling* or monitoring* or analys?s or test or tests or testing)) or transcript expression analys?s or (transcriptome adj (analys?s or profiling*)) or (mrna adj differential display*)).tw. (69757) |
| 13 | ((cell cycle progression adj (score or scores or test or tests or testing or assay* or signature* or instrument*)) or (CCP adj (score or scores or test or tests or testing)) or cell cycle gene expression assay* or cell cycle proliferation gene* or RNA profiling test* or molecular assay* or prostate cancer assay* or genomic prostate score* or molecular diagnostic assay* or multigene expression assay*).tw. (5822) |
| 14 | or/9–13 (429710) |
| 15 | 8 and 14 (6949) |
| 16 | prolaris*.tw. (31) |
| 17 | or/15–16 (6958) |
| 18 | economics/ (251267) |
| 19 | economics, medical/ or economics, pharmaceutical/ or exp economics, hospital/ or economics, nursing/ or economics, dental/ (735077) |
| 20 | economics.fs. (381957) |
| 21 | (econom* or price or prices or pricing or priced or discount* or expenditure* or budget* or pharmacoeconomic* or pharmaco-economic*).tw. (686871) |
| 22 | exp “costs and cost analysis”/ (510897) |
| 23 | cost*.ti. (234727) |
| 24 | cost effective*.tw. (250095) |
| 25 | (cost* adj2 (util* or efficacy* or benefit* or minimi* or analy* or saving* or estimate* or allocation or control or sharing or instrument* or technolog*)).ab. (156523) |
| 26 | models, economic/ (134522) |
| 27 | markov chains/ or monte carlo method/ (119887) |
| 28 | (decision adj1 (tree* or analy* or model*)).tw. (33792) |
| 29 | (markov or markow or monte carlo).tw. (99281) |
| 30 | quality-adjusted life years/ (26577) |
| 31 | (QOLY or QOLYs or HRQOL or HRQOLs or QALY or QALYs or QALE or QALEs).tw. (50975) |
| 32 | ((adjusted adj (quality or life)) or (willing* adj2 pay) or sensitivity analys*s).tw. (98325) |
| 33 | or/18–32 (2279891) |
| 34 | 17 and 33 (242) |
| 35 | 34 use pmoz,cctr,coch,dare,clhta (57) |
| 36 | 17 use cleed (1) |
| 37 | or/35–36 (58) |
| 38 | limit 37 to english language [Limit not valid in CDSR,DARE; records were retained] (56) |
| 39 | limit 38 to yr=“2010 -Current” [Limit not valid in DARE; records were retained] (37) |
| 40 | exp prostate tumor/ (181348) |
| 41 | prostate/ (74575) |
| 42 | exp neoplasm/ (6495582) |
| 43 | and/41–42 (38033) |
| 44 | (prostat* adj3 (cancer* or carcinoma* or neoplas* or tumo?r* or adenoma* or adenocarcinoma* or malignan* or metasta*)).tw. (277087) |
| 45 | prostatectomy/ (69779) |
| 46 | prostatectom*.tw. (63158) |
| 47 | or/40,43–46 (359573) |
| 48 | genetic screening/ (88435) |
| 49 | ((genetic* or gene or genes or genome or genomes or genomic* or 17-gene) adj2 (test or tests or testing or panel* or assess* or screen* or profil* or algorithm* or combinatorial or prognosis or prognostic*)).tw. (235791) |
| 50 | gene expression profiling/ (171701) |
| 51 | ((gene expression adj (profiling* or monitoring* or analys?s or test or tests or testing)) or transcript expression analys?s or (transcriptome adj (analys?s or profiling*)) or (mrna adj differential display*)).tw. (69757) |
| 52 | ((cell cycle progression adj (score or scores or test or tests or testing or assay* or signature* or instrument*)) or (CCP adj (score or scores or test or tests or testing)) or cell cycle gene expression assay* or cell cycle proliferation gene* or RNA profiling test* or molecular assay* or prostate cancer assay* or genomic prostate score* or molecular diagnostic assay* or multigene expression assay*).tw. (5822) |
| 53 | or/48–52 (445179) |
| 54 | 47 and 53 (6881) |
| 55 | prolaris*.tw. (31) |
| 56 | or/54–55 (6889) |
| 57 | Economics/ (251267) |
| 58 | Health Economics/ or exp Pharmacoeconomics/ (214674) |
| 59 | Economic Aspect/ or exp Economic Evaluation/ (394850) |
| 60 | (econom* or price or prices or pricing or priced or discount* or expenditure* or budget* or pharmacoeconomic* or pharmaco-economic*).tw. (686871) |
| 61 | exp “Cost”/ (510897) |
| 62 | cost*.ti. (234727) |
| 63 | cost effective*.tw. (250095) |
| 64 | (cost* adj2 (util* or efficacy* or benefit* or minimi* or analy* or saving* or estimate* or allocation or control or sharing or instrument* or technolog*)).ab. (156523) |
| 65 | Monte Carlo Method/ (50881) |
| 66 | (decision adj1 (tree* or analy* or model*)).tw. (33792) |
| 67 | (markov or markow or monte carlo).tw. (99281) |
| 68 | Quality-Adjusted Life Years/ (26577) |
| 69 | (QOLY or QOLYs or HRQOL or HRQOLs or QALY or QALYs or QALE or QALEs).tw. (50975) |
| 70 | ((adjusted adj (quality or life)) or (willing* adj2 pay) or sensitivity analys*s).tw. (98325) |
| 71 | or/57–70 (1871276) |
| 72 | 56 and 71 (183) |
| 73 | limit 72 to english language [Limit not valid in CDSR,DARE; records were retained] (175) |
| 74 | limit 73 to yr=“2010 -Current” [Limit not valid in DARE; records were retained] (124) |
| 75 | 74 use emez (89) |
| 76 | 39 or 75 (126) |
| 77 | 76 use pmoz (26) |
| 78 | 76 use emez (89) |
| 79 | 76 use coch (8) |
| 80 | 76 use cctr (1) |
| 81 | 76 use clhta (1) |
| 82 | 76 use cleed (1) |
| 83 | 76 use dare (0) |
| 84 | remove duplicates from 76 (104) |
Appendix 2: Evidence Quality Assessment for the Clinical Evidence Review
Table A1:
Risk of Bias for Uncontrolled Before–After Studies
| Author, Year | Clearly Stated Objective | Pre-specified Eligibility Criteria | Representative Patients | All Eligible Patients Enrolled | Adequate Sample Size | Intervention Described and Delivered | Pre-specified, Valid Outcome Measure | Blind Outcome Assessment | Loss to Follow-Up Accounted for | Statistical Methods Appropriate | Multiple Outcome Measurement Times | Appropriate Group-Level Analysis |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crawford et al, 201455 | Y | N | CD | Y | Y | NR | N | N | N | N | N | NA |
| Shore et al, 201656 | Y | Y | Y | Y | N | Y | Y | N | Y | Y | N | NA |
Abbreviations: CD, cannot determine; N, no; NA, not applicable; NR, nor reported; Y, yes.
Note: The criteria for quality assessment of risk of bias in before-after (pre-post) studies with no control group are adapted from National Heart, Lung, and Blood Institute, 2014.53
The GRADE evidence profile for the two included studies is presented in Table 9.
Appendix 3: Selected Excluded Studies
For transparency, we provide this list of related studies that readers might expect to see but that did not meet the inclusion criteria, along with the primary reason for exclusion.
| Citation | Primary Reason for Exclusion |
|---|---|
| Alshalalfa M, Tsai H, Haddad Z, Ross A, Karnes RJ, Davicioni E, et al. Deciphering the genomic fingerprint of small cell prostate cancer with potential clinical utility. Journal of Clinical Oncology Conference. 2016;34(2 Suppl. 1). | Poster/abstract only |
| Bianconi M, Faloppi L, Mazzucchelli R, Giampieri R, Bittoni A, Del Prete M, et al. Multigene profiling in incidentally-and clinically detected prostate cancer. Ann Oncol. 2015;26:vi60. | Poster/abstract only |
| Bianconi M, Faloppi L, Zizzi A, Mazzucchelli R, Giampieri R, Bittoni A, et al. Multigene profiling in incidentally-and clinically detected prostate cancer. Eur J Cancer. 2015;51:S504–S5. | Poster/abstract only |
| Bianconi M, Faloppi L, Zizzi A, Mazzucchelli R, Scartozzi M, Montironi R, et al. Multigene profiling in incidentally-and clinically detected prostate cancer. Journal of Clinical Oncology Conference. 2015;33(15 Suppl. 1). | Poster/abstract only |
| Bishoff J, Freedland S, Schlomm T, Reid J, Brawer M, Stone S, et al. The CCP score provides significant prognostic information in Gleason score <7 patients. J Urol. 2016;195(4, Suppl):e18. | Poster/abstract only |
| Bishoff JT, Freedland SJ, Gerber L, Tennstedt P, Reid J, Welbourn W, et al. Prognostic utility of the cell cycle progression score generated from biopsy in men treated with prostatectomy. J Urol. 2014;192(2):409–14 | Population (prostatectomy) |
| Bishoff JT, Freedland SJ, Gerber L, Tennstedt P, Welbourn W, Reid JE, et al. Prognostic utility of the cell cycle progression (CCP) score generated from needle biopsy in men treated with prostatectomy. Journal of Clinical Oncology Conference. 2014;32(4 Suppl. 1). | Poster/abstract only |
| Bollito E, Manfredi M, Duregon E, Freschi M, Scattoni V, Papotti M, et al. The prognostic role of molecular testing in patients with prostate cancer: a preliminary study. Anticancer Res. 2013;33 (5):2301. | Poster/abstract only |
| Clar F, Bernet L, Morell L, Monserrat A, Lopez J, Gonzalvo V, et al. Retrospective study of gene prolaris (myriad genetics) signature in prostate biopsy from 29 patients with low and intermediate risk adenocarcinoma. European Urology, Supplements. 2014;13 (5):121. | Poster/abstract only |
| Cooperberg M, Freedland S, Schlomm T, Reid J, Stone S, Brawer M. Predicting radical prostatectomy outcome: CCP-CR score outperforms primary gleason grade among men with clinical Gleason <7 who are upgraded to Gleason 7. J Urol. 2014;191(4, Suppl):e937. | Poster/abstract only |
| Cooperberg MR, Simko J, Cowan JE, Reid JE, Bhatnagar S, Gutin A, et al. Validation of a panel of cell-cycle progression genes for improved risk stratification in a contemporary radical prostatectomy cohort. Journal of Clinical Oncology Conference. 2012;30(5 Suppl. 1). | Poster/abstract only |
| Cooperberg MR, Simko JP, Cowan JE, Reid JE, Djalilvand A, Bhatnagar S, et al. Validation of a cell-cycle progression gene panel to improve risk stratification in a contemporary prostatectomy cohort. J Clin Oncol. 2013;31(11):1428–34. | Validation study |
| Crawford E, Shore N, Scardino P, Davis J, Tward JD, Moyes K, et al. CCP score stratifies risk for prostate cancer patients at biopsy: Initial commercial results. International Journal of Radiation Oncology Biology Physics. 2014;(1):S433. | Poster/abstract only |
| Crawford ED, Cole D, Lewine N, Gustavsen G. Evaluation of the economic impact of the CCP assay in localized prostate cancer. Journal of Clinical Oncology Conference. 2015;33(15 Suppl. 1) Poster/abstract only | |
| Crawford ED, Kar AJ, Scholz MC, Fegan JE, Kaldate RR, Brawer MK. Cell cycle progression score to modify treatment decisions in prostate cancer: Results of an ongoing registry trial. Journal of Clinical Oncology Conference. 2014;32(15 Suppl. 1). | Poster/abstract only |
| Crawford ED, Shore N, Scardino PT, Davis JW, Tward JD, Harrison L, et al. CCP score and risk stratification for prostate cancer patients at biopsy. Journal of Clinical Oncology Conference. 2014;32(4 Suppl. 1). | Poster/abstract only |
| Crawford ED, Shore N, Scardino PT, Davis JW, Tward JD, Harrison L, et al. Performance of CCP assay in an updated series of biopsy samples obtained from commercial testing. Journal of Clinical Oncology Conference. 2015;33(7 Suppl. 1). | Poster/abstract only |
| Cuzick J, Berney DM, Fisher G, Mesher D, Moller H, Reid JE, et al. Prognostic value of a cell cycle progression signature for prostate cancer death in a conservatively managed needle biopsy cohort. Br J Cancer. 2012;106(6):1095–9. | Validation study |
| Cuzick J, Stone S, Fisher G, Yang ZH, North BV, Berney DM, et al. Validation of an RNA cell cycle progression score for predicting death from prostate cancer in a conservatively managed needle biopsy cohort. Br J Cancer. 2015;113(3):382–9. | Validation study |
| Cuzick J, Stone S, Reid J, Fisher G, Moller H, Brawer M, et al. Patient AUA risk classification based on combined clinical cell cycle risk (CCR) score. J Urol. 2015;1):e3. | Poster/abstract only |
| Cuzick J, Swanson GP, Fisher G, Brothman AR, Berney DM, Reid JE, et al. Prognostic value of an RNA expression signature derived from cell cycle proliferation genes in patients with prostate cancer: a retrospective study. Lancet Oncol. 2011;12(3):245–55. | Validation study |
| Cuzick JM, Fisher G, Berney D, Mesher D, Moller H, Lanchbury J, et al. Prognostic value of a cell cycle expression profile score among men with conservatively treated localized prostate cancer. Ann Oncol. 2010;21:viii63. | Poster/abstract only |
| Cuzick JM, Stone S, Fisher G, Hua Yang Z, North B, Berney D, et al. Validation of an RNA cell cycle progression (CCP) score for predicting prostate cancer death in a conservatively managed needle biopsy cohort. Journal of Clinical Oncology Conference. 2014;32(15 Suppl. 1). | Poster/abstract only |
| Cuzick JM, Stone S, Fisher G, North B, Berney DM, Beltran L, et al. Validation of an active surveillance threshold for the CCP score in conservatively managed men with localized prostate cancer. Journal of Clinical Oncology Conference. 2015;33(15 Suppl. 1). | Poster/abstract only |
| Davis JW, Crawford ED, Shore N, Scardino PT, Tward JD, Harrison L, et al. Performance of CCP assay in an updated series of biopsy samples obtained from commercial testing. Journal of Clinical Oncology Conference. 2015;33(15 Suppl. 1). | Poster/abstract only |
| Delouya G, Krishnan V, Bahary JP, Larrivee S, Taussky D. The cancer of the prostate risk assessment score predicts biochemical recurrence in intermediate-risk prostate cancer treated with EBRT dose escalation or LDR brachytherapy. Brachytherapy. 2014;13:S115–S6. | Poster/abstract only |
| Freedland SJ, Gerber L, Reid J, Welbourn W, Tikishvili E, Park J, et al. Prognostic utility of cell cycle progression score in men with prostate cancer after primary external beam radiation therapy. International Journal of Radiation Oncology Biology Physics. 2013;86(5):848–53. | Population (external beam radiation therapy) |
| Kar AJ, Scholz MC, Fegan JE, Crawford ED, Scardino PT, Kaldate RR, et al. The effect of cell cycle progression (CCP) score on treatment decisions in prostate cancer: Results of an ongoing registry trial. Journal of Clinical Oncology Conference. 2014;32(4 Suppl. 1). | Poster/abstract only |
| Kolquist KA, Cooperberg M, Freedland SJ, Schlomm T, Reid JE, Stone S, et al. Predicting radical prostatectomy outcome: Cell cycle progression (CCP) score outperforms primary gleason grade among men with clinical Gleason <7 who are upgraded to Gleason 7. Lab Invest. 2014;94:241A. | Poster/abstract only |
| Kwiatkowski M, Daniele L, Albers P, Correa Generoso R, Angeles Cabeza M, McNeill A, et al. Potential reduction of over treatment of localized prostate cancer using a cell cycle gene expression assay (Prolaris) in biopsy specimens: results from the European multi-center EMPATHY-P study. Eur J Cancer. 2015;51:S505. | Poster/abstract only |
| Oderda M, Cozzi G, Barale M, Garelli G, Gurioli A, Daniele L, et al. CCP-score improves the current risk assessment in newly diagnosed prostate cancer patients. European Urology, Supplements. 2016;15 (3):e732. | Poster/abstract only |
| Porpiglia F, Sapino A, Daniele L, Albers P, Arsov C, Correa R, et al. European multi-centre study to assess the aggressiveness of prostate adenocarcinoma in newly-diagnosed patients using a cell-cycle gene expression assay (ProlarisTM) in biopsy specimens (EMPATHY-P Study). European Urology, Supplements. 2015;14 (2):e321–ea. | Poster/abstract only |
| Scardino PT, Cuzick JM, Stone S, Evans B, Jorgensen MR, Eastham JA, et al. Application of active surveillance threshold to series of samples submitted for commercial testing. Journal of Clinical Oncology Conference. 2016;34(2 Suppl. 1). | Poster/abstract only |
| Schlomm T, Sangale Z, Lanchbury J, Gutin A, Reid J, Graefen M, et al. Value of cell cycle progression (CCP) score to predict biochemical recurrence and definitive post-surgical pathology. Urology. 2013;1):S156. | Poster/abstract only |
| Shore N, Boczko J, Kella N, Moran BJ, Bianco FJ, Crawford ED, et al. Impact of CCP test on personalizing treatment decisions: results from a prospective registry of newly diagnosed prostate cancer patients. Journal of Clinical Oncology Conference. 2015;33(15 Suppl. 1). | Poster/abstract only |
| Shore N, Boczko J, Kella N, Moran BJ, Bianco FJ, Crawford ED, et al. Significant reduction in therapeutic burden from use of CCP test in treatment decisions among newly diagnosed prostate cancer patients in a large prospective registry. J Urol. 2015;1):e708. | Poster/abstract only |
| Shore N, Concepcion R, Saltzstein D, Lucia MS, Van Breda A, Welbourn W, et al. Clinical utility of a biopsy-based cell cycle gene expression assay in localized prostate cancer. Curr Med Res Opin. 2014;30(4):547–53. | Validation study |
| Warf MB, Reid JE, Brown KL, Kolquist KA, Stone S, Roa B. Analytical validation of a cell cycle progression signature used as a prognostic marker in prostate cancer. J Mol Biomark Diagn. 2015;6(4):1–5. | Validation study |
Appendix 4: Parameters for Initial Treatment Used in the Budget Impact Analysis
Table A2:
Initial Treatment for Low- or Intermediate-Risk Prostate Cancer Patients in Ontario
| Treatment | Low Risk | Low-Intermediate Risk | High-Intermediate Risk | Total, N | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| AS/WW | 222 | 59.0 | 58 | 15.5 | 12 | 8.8 | 292 |
| BT | 52 | 13.8 | 33 | 8.8 | 9 | 6.6 | 94 |
| EBRT | 20 | 5.3 | 95 | 25.5 | 49 | 35.8 | 164 |
| EBRT + BT | 1 | 0.3 | 31 | 8.3 | 4 | 2.9 | 36 |
| EBRT + ADT | 0 | 0.0 | 8 | 2.1 | 12 | 8.8 | 20 |
| HIFUa | 2 | 0.5 | 1 | 0.3 | 0 | 0.0 | 3 |
| Primary ADTa | 0 | 0.0 | 0 | 0.0 | 2 | 1.5 | 2 |
| SABR | 8 | 2.1 | 6 | 1.6 | 4 | 2.9 | 18 |
| RP | 63 | 16.8 | 136 | 36.5 | 43 | 31.4 | 242 |
| Unknowna | 8 | 2.1 | 5 | 1.3 | 2 | 1.5 | 15 |
| Total | 376 | 100.0 | 373 | 100.0 | 137 | 100.0 | 886 |
Abbreviations: ADT, androgen-deprivation therapy; AS, active surveillance; BT, brachytherapy; EBRT, external beam radiation therapy; HIFU, high-intensity focused ultrasound; RP, radical prostatectomy; SABR, stereotactic ablative radiotherapy; WW, watchful waiting.
Treatments with small (HIFU, primary ADT) or unknown proportion of usage were excluded.
Appendix 5: Letter of Information and Consent Form for Patient Consultation
LETTER OF INFORMATION
SUMMARY
Health Quality Ontario (HQO) is conducting a formal assessment of the Prolaris cell cycle progression test for prostate cancer, to better understand whether this test should be funded by the health care system. An important part of this assessment involves speaking to families of patients with newly diagnosed prostate cancer, who may or may not be considering further treatment. Our goal is to illuminate the lived experience of patients and families with prostate cancer, their existing treatment options, and the context around the risk-stratification test such as Prolaris - what families view as its possibilities, implications, and value.
WHAT DO YOU NEED FROM ME?
-
✓
Willingness to share your story
-
✓
15 to 30 minutes of your time for a phone or in-person interview
-
✓
Permission to audio- (not video-) record the interview
WHY DO YOU NEED THIS INFORMATION?
Health Quality Ontario (HQO) is conducting a health technology assessment of the Prolaris cell cycle progression test for prostate cancer treatment selection. As part of HQO's core function to promote health care supported by the best evidence available, established scientific methods are used to analyze the evidence for a wide range of health interventions, including diagnostic tests, medical devices, interventional and surgical procedures, health care programs and models of care. These analyses may be informed and complemented by input from a range of individuals, including patients and clinical experts, and serve as the basis for recommendations about whether health care interventions should be publicly funded or not.
The perspective that you share will be useful to help provide context to the day-to-day realities of patients diagnosed with prostate cancer and the decisions they face in terms of therapies. The ultimate goal of the project is to provide recommendations to the Ontario Health Technology Advisory Committee which advises the Ontario Ministry of Health and Long-Term Care on the appropriateness of funding.
WHAT YOUR PARTICIPATION INVOLVES
If you agree to enroll, you will be asked to participate in an interview or focus group conducted by HQO staff. The interview or focus group will likely last 15 to 30 minutes. The session will be conducted in a private location and will be audio-taped. The interviewer will ask you questions about your lived experience with prostate cancer, existing therapies, and values when it comes to risk, knowledge, decision-making.
Participation is voluntary. You may refuse to participate, refuse to answer any questions or withdraw before your interview. Withdrawal will in no way affect care you receive.
CONFIDENTIALITY
All information collected for the review will be kept confidential and privacy will be protected except as required by law. The results of this review will be published, however no identifying information will be released or published. Any records containing information from your interview will be stored securely.
RISKS TO PARTICIPATION
There are no known physical risks to participating. Some participants may experience discomfort or anxiety after speaking about their lived experience. If this is the case, please contact any staff.
HEALTH QUALITY ONTARIO STAFF
Mark Weir
Senior Program Analyst, Patient, Family and Public Engagement
Tel: (416) 323-6868 ×. 653, Email: Mark.Weir@hqontario.ca
David Wells
Program Analyst, Patient, Family and Public Engagement
Tel: (416) 323-6868 ×. 710 Email: David.Wells@hqontario.ca
Consent and Release Form
This form is to be read and completed in accordance with the following instructions before it can be signed.
-
1.
I, _____ allow Health Quality Ontario (Ontario Health Quality Council) to use to inform the development of an evidence based review:
Check off all appropriate boxes:
-
a)
___ a recording of my voice
-
b)
___ a quotation or summary of my opinion that I expressed during an interview
-
c)
___ name & contact information
-
a)
-
2.
Please read the following paragraphs before affixing your signature under section 3.
-
a)
Personal information collected pursuant to, and on this form, will be used for purposes described on this form and for no other purpose. Health Quality Ontario (Ontario Health Quality Council) acknowledges that you have provided this personal information freely and voluntarily. If you have any questions about this collection of this personal information, contact:
Suzanne Dugard
Director, Communications
Tel: (416) 323-6868, ext. 223, E-mail: suzanne.dugard@hqontario.ca
-
b)
By signing this form as indicated below, you agree to hereby release and forever discharge the Health Quality Ontario (Ontario Health Quality Council), its officers, employees, agents and representatives from any and all claims, demands, expenses, actions, causes of action and for any and all liability howsoever caused, arising out of, or in any way related to the collection, use and disclosure of information, recordings and images authorized to be collected pursuant to, or on this form.
-
c)
By signing this form as indicated below, you agree to forever waive any and all rights that you may have to the use of information and recordings that are authorized to be collected pursuant to, or on this form; and you acknowledge that all information, recordings and images shall hereafter remain the exclusive property of the Health Quality Ontario (Ontario Health Quality Council).
-
a)
-
3.
Signature is to be affixed in the appropriate space provided below.
I have read this form after it was completed, I understand and agree to be bound by its contents, and I am eighteen (18) years of age or over.
Signature____________________
Print name____________________
Date____________________
Appendix 6: Patient Consultation Interview Guide
Interview for Prolaris HTA
Intro
History of prostate cancer diagnosis and treatment (if applicable)
Lived- Experience
Changes to day-to-day routine with diagnosis?
What is the impact on partner/spouse/family?
Decision-Making
What treatment options were presented?
(any equity issues in regards to treatment options? Cost/inconveniences?)
Role of family in decision-making? Physician? Other sources of information (Internet)?
Contrast emotion (anxiety, worry) vs logic? As this applies to risk and side-effects?
Value placed on having information in decision-making (vs trusting physician, for example?)
Was it difficult to weigh up potential benefits and risks when deciding on which therapies to go with?
Prolaris
Explain Prolaris to interviewee. Further stratification of low/intermediate risk prostate cancer patients. Provides information about aggressiveness (or lack thereof) of cancer.
Would this information be (or have been) of value? What if the information was neutral? Would cost be a factor?
Author contributions
This report was developed by a multidisciplinary team from Health Quality Ontario. The lead clinical epidemiologist was Alexis Schaink, the lead health economist was Chunmei Li, the patient engagement lead was David Wells, and the medical librarian was Corinne Holubowich.
KEY MESSAGES
One in 8 Canadian men will be diagnosed with prostate cancer. However, prostate cancers vary in terms of how aggressive they are. Many are slow growing, localized (have not spread), and do not present an immediate risk to overall health. Others grow rapidly and can eventually spread or be fatal. Active treatment options for localized prostate cancer include surgery and radiation therapy, which have side effects that can affect the patient's quality of life. Many patients choose a conservative approach called active surveillance. This means they get regular tests to see if the cancer is growing faster or spreading. If so, they switch to definitive treatment.
Knowing which prostate cancers are aggressive is important for choosing each patient's most appropriate treatment. Physicians currently use information about a man's age, health, and a biopsy of the tumour to determine whether a patient falls into a low-, intermediate-, or high-risk group. A relatively new genomic test, called the Prolaris cell cycle progression (CCP) test, looks at a group of genes in the tumour and tries to measure how quickly the cells are multiplying. This added information may provide a more individualized estimate of the patient's risk of dying from prostate cancer. This test could therefore allow patients to pursue the best possible treatment for them.
We looked at whether the Prolaris CCP test leads to better outcomes for men with low-risk or intermediate-risk prostate cancer. We found two studies showing the test could change some patients' planned or actual treatment, but there is no evidence yet that the test reduces important patient outcomes such as deaths from prostate cancer. We estimated the cost for Ontario to publicly fund the CCP test, considering the changes in treatment that could result. Because the cost of the test is high and prostate cancer is so common, we estimated the test would add about $41 million to provincial health costs over the next 5 years. We also interviewed patients with prostate cancer. They said the test could be helpful but weren't sure it would change their treatment decisions because many other factors come into play, such as how they perceive the risks of the disease and the risks and benefits of the treatment options.
Contributor Information
Health Quality Ontario:
Alexis Schaink, Chunmei Li, David Wells, and Corinne Holubowich
About Health Quality Ontario
Health Quality Ontario is the provincial advisor on the quality of health care. We are motivated by a single-minded purpose: Better health for all Ontarians.
Who We Are
We are a scientifically rigorous group with diverse areas of expertise. We strive for complete objectivity, and look at things from a vantage point that allows us to see the forest and the trees. We work in partnership with health care providers and organizations across the system, and engage with patients themselves, to help initiate substantial and sustainable change to the province's complex health system.
What We Do
We define the meaning of quality as it pertains to health care, and provide strategic advice so all the parts of the system can improve. We also analyze virtually all aspects of Ontario's health care. This includes looking at the overall health of Ontarians, how well different areas of the system are working together, and most importantly, patient experience. We then produce comprehensive, objective reports based on data, facts and the voice of patients, caregivers and those who work each day in the health system. As well, we make recommendations on how to improve care using the best evidence. Finally, we support large scale quality improvements by working with our partners to facilitate ways for health care providers to learn from each other and share innovative approaches.
Why It Matters
We recognize that, as a system, we have much to be proud of, but also that it often falls short of being the best it can be. Plus certain vulnerable segments of the population are not receiving acceptable levels of attention. Our intent at Health Quality Ontario is to continuously improve the quality of health care in this province regardless of who you are or where you live. We are driven by the desire to make the system better, and by the inarguable fact that better has no limit.
REFERENCES
- (1).Canadian Cancer Society's Advisory Committee on Cancer Statistics. Canadian cancer statistics 2015. Toronto (ON): Canadian Cancer Society; 2015. [Google Scholar]
- (2).Prostate cancer [Internet]. Toronto (ON): Canadian Cancer Society; c2016. [updated 2015; cited 2016 Feb 8]. Available from: http://www.cancer.ca/en/cancer-information/cancer-type/prostate/prostate-cancer/?region=on [Google Scholar]
- (3).Cancer Care Ontario. Ontario cancer statistics 2016 [Internet]. Toronto (ON): Cancer Care Ontario; 2016. Available from: https://www.cancercare.on.ca/common/pages/UserFile.aspx?fileId=360956 [Google Scholar]
- (4).Sun F, Oyesanmi O, Fontanarosa J, Reston J, Guzzo T, Schoelles K. Therapies for clinically localized prostate cancer: update of a 2008 systematic review. Rockville (MD): Agency for Healthcare Research and Quality; 2014. [PubMed] [Google Scholar]
- (5).Ganz PA, Barry JM, Burke W, Col NF, Corso PS, Dodson E, et al. NIH State-of-the-Science Conference Statement: role of active surveillance in the management of men with localized prostate cancer. NIH Consens State Sci Statements. 2011; 28 (1): 1–27. [PubMed] [Google Scholar]
- (6).National Cancer Institute. PDQ® prostate cancer treatment. [Internet]. Bethesda (MD): National Cancer Institute; 2016. Mar [cited 2016 Apr 8]. Available from: http://www.cancer.gov/types/prostate/hp/prostate-treatment-pdq. [Google Scholar]
- (7).NCI dictionary of cancer terms [Internet]. Bethesda (MD): National Institutes of Health; c2016. [cited 2016 Feb 8]. Available from: http://www.cancer.gov/publications/dictionaries/cancer-terms [Google Scholar]
- (8).Chang AJ, Autio KA, Roach M, 3rd, Scher HI. High-risk prostate cancer-classification and therapy. Nat Rev Clin Oncol. 2014; 11 (6): 308–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Klotz L. Active surveillance for prostate cancer: overview and update. Curr Treat Options Oncol. 2013; 14 (1): 97–108. [DOI] [PubMed] [Google Scholar]
- (10).Ishkanian AS, Mallof CA, Ho J, Meng A, Albert M, Syed A, et al. High-resolution array CGH identifies novel regions of genomic alteration in intermediate-risk prostate cancer. Prostate. 2009; 69 (10): 1091–100. [DOI] [PubMed] [Google Scholar]
- (11).Cooperberg MR, Broering JM, Carroll PR. Time trends and local variation in primary treatment of localized prostate cancer. J Clin Oncol. 2010; 28 (7): 1117–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Cristea O, Lavallee LT, Montroy J, Stokl A, Cnossen S, Mallick R, et al. Active surveillance in Canadian men with low-grade prostate cancer. CMAJ. 2016; 188 (8): E141–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Esserman LJ, Thompson IM, Reid B, Nelson P, Ransohoff DF, Welch HG, et al. Addressing overdiagnosis and overtreatment in cancer: a prescription for change. Lancet Oncol. 2014; 15 (6): e234–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Rodrigues G, Warde P, Pickles T, Crook J, Brundage M, Souhami L, et al. Pre-treatment risk stratification of prostate cancer patients: a critical review. Can Urol Assoc J. 2012; 6 (2): 121–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Eichler K, Wilby J, Hempel S, Myers L, Kleijnen J. Diagnostic value of systematic prostate biopsy methods in the investigation for prostate cancer: a systematic review. York (UK): Centre for Reviews and Dissemination; 2005. [Google Scholar]
- (16).Sved PD, Gomez P, Manoharan M, Kim SS, Soloway MS. Limitations of biopsy Gleason grade: implications for counseling patients with biopsy Gleason score 6 prostate cancer. J Urol. 2004; 172 (1): 98–102. [DOI] [PubMed] [Google Scholar]
- (17).Shariat SF, Karakiewicz PI, Suardi N, Kattan MW. Comparison of nomograms with other methods for predicting outcomes in prostate cancer: a critical analysis of the literature. Clin Cancer Res. 2008; 14 (14): 4400–7. [DOI] [PubMed] [Google Scholar]
- (18).Kattan MW, Eastham JA, Stapleton AM, Wheeler TM, Scardino PT. A preoperative nomogram for disease recurrence following radical prostatectomy for prostate cancer. J Natl Cancer Inst. 1998; 90 (10): 766–71. [DOI] [PubMed] [Google Scholar]
- (19).Stephenson AJ, Scardino PT, Eastham JA, Bianco FJ, Jr., Dotan ZA, Fearn PA, et al. Preoperative nomogram predicting the 10-year probability of prostate cancer recurrence after radical prostatectomy. J Natl Cancer Inst. 2006; 98 (10): 715–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Cooperberg MR, Broering JM, Carroll PR. Risk assessment for prostate cancer metastasis and mortality at the time of diagnosis. J Natl Cancer Inst. 2009; 101 (12): 878–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Cooperberg MR, Pasta DJ, Elkin EP, Litwin MS, Latini DM, Du Chane J, et al. The University of California, San Francisco Cancer of the Prostate Risk Assessment score: a straightforward and reliable preoperative predictor of disease recurrence after radical prostatectomy. J Urol. 2005; 173 (6): 1938–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Brett AS, Ablin RJ. Prostate-cancer screening–what the U.S. Preventive Services Task Force left out. N Engl J Med. 2011; 365 (21): 1949–51. [DOI] [PubMed] [Google Scholar]
- (23).Fowler FJ, Jr., Barry MJ, Walker-Corkery B, Caubet JF, Bates DW, Lee JM, et al. The impact of a suspicious prostate biopsy on patients' psychological, socio-behavioral, and medical care outcomes. J Gen Intern Med. 2006; 21 (7): 715–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).McNaughton-Collins M, Fowler FJ, Jr., Caubet JF, Bates DW, Lee JM, Hauser A, et al. Psychological effects of a suspicious prostate cancer screening test followed by a benign biopsy result. Am J Med. 2004; 117 (10): 719–25. [DOI] [PubMed] [Google Scholar]
- (25).Ransohoff DF, McNaughton-Collins M, Fowler FJ. Why is prostate cancer screening so common when the evidence is so uncertain? A system without negative feedback. Am J Med. 2002; 113 (8): 663–7. [DOI] [PubMed] [Google Scholar]
- (26).Cancer Care Ontario. Prostate cancer treatment pathway [Internet]. Toronto, ON: Cancer Care Ontario; 2015. [cited 2016 Jul 25]. Available from: https://www.cancercare.on.ca/common/pages/UserFile.aspx?fileId=349932 [Google Scholar]
- (27).Klotz L, Vesprini D, Sethukavalan P, Jethava V, Zhang L, Jain S, et al. Long-term follow-up of a large active surveillance cohort of patients with prostate cancer. J Clin Oncol. 2015; 33 (3): 272–7. [DOI] [PubMed] [Google Scholar]
- (28).Serrano NA, Anscher MS. Favorable vs unfavorable intermediate-risk prostate cancer: a review of the new classification system and its impact on treatment recommendations. Oncology (Williston Park). 2016; 30 (3): 229–36. [PubMed] [Google Scholar]
- (29).Albertsen PC. What is the risk posed by prostate cancer? J Natl Cancer Inst Monogr. 2012; 2012 (45): 169–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Gallina A, Chun FK, Suardi N, Eastham JA, Perrotte P, Graefen M, et al. Comparison of stage migration patterns between Europe and the USA: an analysis of 11 350 men treated with radical prostatectomy for prostate cancer. BJU Int. 2008; 101 (12): 1513–8. [DOI] [PubMed] [Google Scholar]
- (31).Helpap B, Egevad L. The significance of modified Gleason grading of prostatic carcinoma in biopsy and radical prostatectomy specimens. Virchows Arch. 2006; 449 (6): 622–7. [DOI] [PubMed] [Google Scholar]
- (32).Morash C, Tey R, Agbassi C, Klotz L, McGowan T, Srigley J, et al. Active surveillance for the management of localized prostate cancer: guideline recommendations. Can Urol Assoc J. 2015; 9 (5–6): 171–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Humphrey PA, Moch H, Cubilla AL, Ulbright TM, Reuter VE. The 2016 WHO classification of tumours of the urinary system and male genital organs–part B: prostate and bladder tumours. Eur Urol. 2016; 70 (1): 106–19. [DOI] [PubMed] [Google Scholar]
- (34).Holmboe ES, Concato J. Treatment decisions for localized prostate cancer: asking men what's important. J Gen Intern Med. 2000; 15 (10): 694–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Bisson JI, Chubb HL, Bennett S, Mason M, Jones D, Kynaston H. The prevalence and predictors of psychological distress in patients with early localized prostate cancer. BJU Int. 2002; 90 (1): 56–61. [DOI] [PubMed] [Google Scholar]
- (36).Davison BJ, Breckon E. Factors influencing treatment decision making and information preferences of prostate cancer patients on active surveillance. Patient Educ Couns. 2012; 87 (3): 369–74. [DOI] [PubMed] [Google Scholar]
- (37).Kandasamy S, Khalid AF, Majid U, Vanstone M. Prostate cancer patient perspectives on the use of information in treatment decision-making: a systematic review and qualitative meta-synthesis [Internet]. Ont Health Technol Assess Ser. Forthcoming 2017. [PMC free article] [PubMed]
- (38).D'Amico AV, Whittington R, Malkowicz SB, Schultz D, Blank K, Broderick GA, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998; 280 (11): 969–74. [DOI] [PubMed] [Google Scholar]
- (39).Cancer Care Ontario. Prostate cancer diagnosis pathway [Internet]. Toronto (ON): Cancer Care Ontario; 2015. [cited 2016 Feb 3]. Available from: https://www.cancercare.on.ca/common/pages/UserFile.aspx?fileId=349941 [Google Scholar]
- (40).Myriad Genetic Laboratories Inc. Prolaris® clinical summary. Salt Lake City (UT): Myriad Genetics, Inc.; 2014. [Google Scholar]
- (41).Cuzick J, Swanson GP, Fisher G, Brothman AR, Berney DM, Reid JE, et al. Prognostic value of an RNA expression signature derived from cell cycle proliferation genes in patients with prostate cancer: a retrospective study. Lancet Oncol. 2011; 12 (3): 245–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Warf MB, Fosso PG, Hughes E, Perry M, Brown KL, Reid JE, et al. Analytical validation of a proliferation-based molecular signature used as a prognostic marker in early stage lung adenocarcinoma. Biomark Med. 2015; 9 (9): 901–10. [DOI] [PubMed] [Google Scholar]
- (43).Cuzick J, Berney DM, Fisher G, Mesher D, Moller H, Reid JE, et al. Prognostic value of a cell cycle progression signature for prostate cancer death in a conservatively managed needle biopsy cohort. Br J Cancer. 2012; 106 (6): 1095–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Cuzick J, Stone S, Fisher G, Yang ZH, North BV, Berney DM, et al. Validation of an RNA cell cycle progression score for predicting death from prostate cancer in a conservatively managed needle biopsy cohort. Br J Cancer. 2015; 113 (3): 382–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Bishoff JT, Freedland SJ, Gerber L, Tennstedt P, Reid J, Welbourn W, et al. Prognostic utility of the cell cycle progression score generated from biopsy in men treated with prostatectomy. J Urol. 2014; 192 (2): 409–14. [DOI] [PubMed] [Google Scholar]
- (46).Cooperberg MR, Simko JP, Cowan JE, Reid JE, Djalilvand A, Bhatnagar S, et al. Validation of a cell-cycle progression gene panel to improve risk stratification in a contemporary prostatectomy cohort. J Clin Oncol. 2013; 31 (11): 1428–34. [DOI] [PubMed] [Google Scholar]
- (47).Freedland SJ, Gerber L, Reid J, Welbourn W, Tikishvili E, Park J, et al. Prognostic utility of cell cycle progression score in men with prostate cancer after primary external beam radiation therapy. Int J Radiat Oncol Biol Phys. 2013; 86 (5): 848–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Sun F, Bruening W, Erinoff E, Schoelles KM. Addressing challenges in genetic test evaluation. evaluation frameworks and assessment of analytic validity. Rockville (MD): Agency for Healthcare Research and Quality; 2011. [PubMed] [Google Scholar]
- (49).Canfield SE, Kibel AS, Kemeter MJ, Febbo PG, Lawrence HJ, Moul JW. A guide for clinicians in the evaluation of emerging molecular diagnostics for newly diagnosed prostate cancer. Rev Urol. 2014; 16 (4): 172–80. [PMC free article] [PubMed] [Google Scholar]
- (50).GenomeWeb. Myriad reports abstracts on cost-effectiveness, clinical utility of Prolaris [Internet]. New York: GenomeWeb LLC; 2014. [updated 2014 Dec 03; cited 2016 Dec 9]. Available from: https://www.genomeweb.com/molecular-diagnostics/myriad-reports-abstracts-cost-effectiveness-clinical-utility-prolaris [Google Scholar]
- (51).Mulcahy N. Gene test for prostate cancer validated: triumph or worry? [Internet]. WebMD LLC; c1994–2016. [updated 2014 May 22; cited 2016 Dec 9]. Available from: http://www.medscape.com/viewarticle/825578
- (52).McGowan J, Sampson M, Salzwedel D, Cogo E, Foerster V, Lefebvre C. PRESS peer review of electronic search strategies: 2015 guideline statement. J Clin Epidemiol. 2016; 75: 40–6. [DOI] [PubMed] [Google Scholar]
- (53).National Heart, Lung, and Blood Institute. Quality assessment tool for before-after (pre-post) studies with no control group [Internet]. Bethesda (MD): The Institute; 2014. [cited 2016 July 1]. Available from: http://www.nhlbi.nih.gov/health-pro/guidelines/in-develop/cardiovascular-risk-reduction/tools/before-after. [Google Scholar]
- (54).Guyatt GH, Oxman AD, Schunemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol. 2011; 64 (4): 380–2. [DOI] [PubMed] [Google Scholar]
- (55).Crawford ED, Scholz MC, Kar AJ, Fegan JE, Haregewoin A, Kaldate RR, et al. Cell cycle progression score and treatment decisions in prostate cancer: results from an ongoing registry. Curr Med Res Opin. 2014; 30 (6): 1025–31. [DOI] [PubMed] [Google Scholar]
- (56).Shore ND, Kella N, Moran B, Boczko J, Bianco FJ, Crawford ED, et al. Impact of the cell cycle progression test on physician and patient treatment selection for localized prostate cancer. J Urol. 2016; 195 (3): 612–8. [DOI] [PubMed] [Google Scholar]
- (57).Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010; 8 (5): 336–41. [DOI] [PubMed] [Google Scholar]
- (58).Little J, Wilson B, Carter R, Walker K, Santaguida P, Tomiak E, Beyene J, Raina P. Multigene panels in prostate cancer risk assessment. Evidence report no. 209. (Prepared by the McMaster University Evidence-based Practice Center under Contract No. 290-2007-10060-1.) AHRQ Publication No.12-E020-EF. Rockville (MD): Agency for Healthcare Research and Quality; July 2012. www.effectivehealthcare.ahrq.gov/reports/final.cfm. [Google Scholar]
- (59).BlueCross BlueShield Association. TEC assessment: gene expression analysis for prostate canacer management. Chicago (IL): The Association; 2015. [Google Scholar]
- (60).Health Policy Advisory Committee on Technology. Molecular testing for prostate cancer prognosis. Queensland (Australia): 2014. [Google Scholar]
- (61).National Institute for Health and Care Excellence. Prolaris gene expression assay for assessing long-term risk of prostate cancer progression. London (UK): The Institute; 2016. [Google Scholar]
- (62).Crawford ED, Cole D, Lewine N, Gustavsen G, editors. Evaluation of the economic impact of the CCP assay in localized prostate cancer. Proceedings of the 2015 ASCO annual meeting; 2015. May 29 – Jun 2; Chicago (IL): ASCO; 2015. [Google Scholar]
- (63).de Pouvourville G. Cost-effectiveness analysis for the use of the CCP score in the management of early low-risk prostate cancer in the French context. Value Health. 2015; 18 (7): A358. [Google Scholar]
- (64).Bell N, Connor Gorber S, Shane A, Joffres M, Singh H, Dickinson J, et al. Recommendations on screening for prostate cancer with the prostate-specific antigen test. CMAJ. 2014; 186 (16): 1225–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).Canadian Task Force for Preventive Health Care. Screening for prostate cancer (2014) [Internet]. Public Health Agency of Canada; 2014. [cited 2016 Aug 18]. Available from: http://canadiantaskforce.ca/ctfphc-guidelines/2014-prostate-cancer/
- (66).Guy D, Ghanem G, Loblaw A, Buckley R, Persaud B, Cheung P, et al. Diagnosis, referral, and primary treatment decisions in newly diagnosed prostate cancer patients in a multidisciplinary diagnostic assessment program. Can Urol Assoc J. 2016; 10 (3–4): 120–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).Stangelberger A, Waldert M, Djavan B. Prostate cancer in elderly men. Rev Urol. 2008; 10 (2): 111–9. [PMC free article] [PubMed] [Google Scholar]
- (68).Taussky D, Liu A, Abrahamowicz M, Leger-Belanger E, Bahary JP, Beauchemin MC, et al. Factors influencing treatment decisions in patients with low risk prostate cancer referred to a brachytherapy clinic. Can J Urol. 2008; 15 (6): 4415–20. [PubMed] [Google Scholar]
- (69).Canadian Partnership Against Cancer. Prostate cancer control in Canada: a system performance spotlight report [Internet]. Toronto (ON): The Partnership; 2015. [cited 2016 Jul 27]. Available from: http://www.systemperformance.ca/reports/ [Google Scholar]
- (70).Maurice MJ, Abouassaly R, Kim SP, Zhu H. Contemporary nationwide patterns of active surveillance use for prostate cancer. JAMA Intern Med. 2015; 175 (9): 1569–71. [DOI] [PubMed] [Google Scholar]
- (71).Thompson I, Thrasher JB, Aus G, Burnett AL, Canby-Hagino ED, Cookson MS, et al. Guideline for the management of clinically localized prostate cancer: 2007 update. J Urol. 2007; 177 (6): 2106–31. [DOI] [PubMed] [Google Scholar]
- (72).Statistics Canada. Consumer price index, health and personal care, by province (Canada) [Internet]. Ottawa (ON): Statistics Canada; 2016. [updated 2016 Jan 22; cited 2016 Jul 22]. Available from: http://www.statcan.gc.ca/tables-tableaux/sum-som/l01/cst01/econ161a-eng.htm [Google Scholar]
- (73).XE currency converter [Internet]. XE.com; 2016. [cited 2016 Jul 26]. Available from: http://www.xe.com/currencyconverter/convert/?Amount=1&From=USD&To=CAD [Google Scholar]
- (74).Krahn MD, Bremner KE, Zagorski B, Alibhai SM, Chen W, Tomlinson G, et al. Health care costs for state transition models in prostate cancer. Med Decis Making. 2014; 34 (3): 366–78. [DOI] [PubMed] [Google Scholar]
- (75).Ho C, Tsakonas E, Tran K, Cimon K, Severn M, Mierzwinski-Urban M, et al. CADTH health technology assessments. Robot-assisted surgery compared with open surgery and laparoscopic surgery: clinical effectiveness and economic analyses. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2011. [PubMed] [Google Scholar]
- (76).Yong JH, Beca J, McGowan T, Bremner KE, Warde P, Hoch JS. Cost-effectiveness of intensity-modulated radiotherapy in prostate cancer. Clin Oncol (R Coll Radiol). 2012; 24 (7): 521–31. [DOI] [PubMed] [Google Scholar]
- (77).Dragomir A, Cury FL, Aprikian AG. Active surveillance for low-risk prostate cancer compared with immediate treatment: a Canadian cost comparison. CMAJ Open. 2014; 2 (2): E60–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (78).Ontario Ministry of Health and Long-Term Care. Schedule of benefits: physician services under the Health Insurance Act [Internet]. Toronto (ON): The Ministry; 2015. [cited 2016 Aug 3]. Available from: http://www.health.gov.on.ca/english/providers/program/ohip/sob/physserv/sob_master20160406.pdf [Google Scholar]
- (79).Tawfik A. Prostate-specific antigen (PSA)-based population screening for prostate cancer: an economic analysis. Ont Health Technol Assess Ser. 2015; 15 (11): 1–37. [PMC free article] [PubMed] [Google Scholar]
- (80).Ontario Case Costing Initiative. Health data branch web portal [Internet]. Toronto (ON): Ministry of Health and Long-Term Care; [cited 2016 Aug 23]. Available from: https://hsimi.on.ca/hdbportal/ [Google Scholar]
- (81).Sanyal C, Aprikian A, Cury F, Chevalier S, Dragomir A. Clinical management and burden of prostate cancer: a Markov Monte Carlo model. PloS one. 2014; 9 (12): e113432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (82).Klotz L, Zhang L, Lam A, Nam R, Mamedov A, Loblaw A. Clinical results of long-term follow-up of a large, active surveillance cohort with localized prostate cancer. J Clin Oncol. 2010; 28 (1): 126–31. [DOI] [PubMed] [Google Scholar]
- (83).Deverka PA, Schully SD, Ishibe N, Carlson JJ, Freedman A, Goddard KA, et al. Stakeholder assessment of the evidence for cancer genomic tests: insights from three case studies. Genet Med. 2012; 14 (7): 656–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (84).Hannouf MB, Xie B, Brackstone M, Zaric GS. Cost-effectiveness of a 21-gene recurrence score assay versus Canadian clinical practice in women with early-stage estrogen- or progesterone-receptor-positive, axillary lymph-node negative breast cancer. BMC Cancer. 2012; 12: 447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (85).Barham L. Public and patient involvement at the UK National Institute for Health and Clinical Excellence. Patient. 2011; 4 (1): 1–10. [DOI] [PubMed] [Google Scholar]
- (86).Messina J, Grainger DL. A pilot study to identify areas for further improvements in patient and public involvement in health technology assessments for medicines. Patient. 2012; 5 (3): 199–211. [DOI] [PubMed] [Google Scholar]
- (87).OHTAC Public Engagement Subcommittee. Public engagement for health technology assessment at Health Quality Ontario—final report from the Ontario Health Technology Advisory Committee Public Engagement Subcommittee. Toronto (ON): Queen's Printer for Ontario; 2015. [Google Scholar]
- (88).Tjornhoj-Thomsen T, Hansen HP. Knowledge in health technology assessment: who, what, how? Int J Technol Assess Health Care. 2011; 27 (4): 324–9. [DOI] [PubMed] [Google Scholar]
- (89).Kvale S. Interviews: an introduction to qualitative research interviewing. Thousand Oaks (CA): Sage Publications; 1996. [Google Scholar]
- (90).Kuzel AJ. Sampling in qualitative inquiry. In: Miller WL, Crabtree BF, editors. Doing qualitative research. Thousand Oaks (CA): Sage Publications; 1999. p. 33–45. [Google Scholar]
- (91).Morse J. Emerging from the data: cognitive processes of analysis in qualitative research. In: Morse J, editor. Critical issues in qualitative research methods. Thousand Oaks (CA): Sage Publications; 1994. p. 23–41. [Google Scholar]
- (92).Patton MQ. Qualitative research and evaluation methods. 3rd ed. Thousand Oaks (CA): Sage Publications; 2002. [Google Scholar]
- (93).Strauss AL, Corbin JM. Basics of qualitative research: techniques and procedures of developing a grounded theory. 2nd ed. Thousand Oaks (CA): Sage Publications; 1998. [Google Scholar]
- (94).Health Technology Assessment International Interest Group on Patient and Citizen Involvement in HTA. Introduction to health technology assessment [Internet]. 2015. [cited 2015 Aug 5]. Available from: http://www.htai.org/fileadmin/HTAi_Files/ISG/PatientInvolvement/v2_files/Resource/PCISG-Resource-Intro_to_HTA__KFacey__Jun13.pdf
- (95).Strauss AL, Corbin JM. Grounded theory research: procedures, canons, and evaluative criteria. Qual Sociol. 1990; 13 (1): 3–21. [Google Scholar]
- (96).Strauss AL, Corbin JM. Grounded theory methodology: an overview. In: Denzin NK, Lincoln YS, editors. Handbook of qualitative research. Thousand Oaks (CA): Sage Publications; 1994. p. 273–85. [Google Scholar]