Abstract
Background and study aims
Some guidelines recommend a minimum standard of 90 % cecal intubation rate (CIR) in routine clinics and 95 % in screening colonoscopy, while others have not made this distinction – both with limited evidence to support either view. This study questions the rationale for making such differentiation.
Patients and methods
We assessed cecum intubation rates amongst colonoscopies recorded in the Norwegian national quality register Gastronet by 35 endoscopists performing both clinical and screening colonoscopies. Colonoscopies were categorized into primary screening colonoscopy, work-up colonoscopy of screen-positives and clinical colonoscopy or surveillance. Cases with insufficient bowel preparation or mechanical obstruction were excluded. Endoscopists were categorized into “junior” and “senior” endoscopists depending on training and experience. Univariable and multivariable logistic regression analyses were applied.
Results
During a 2-year period, 10,267 colonoscopies were included (primary screening colonoscopy: 746; work-up colonoscopy of screen-positives: 2,604; clinical colonoscopy or surveillance: 6917). The crude CIR in clinical routine colonoscopy, primary screening colonoscopy and work-up colonoscopy was 97.1 %, 97.1 % and 98.6 %, respectively. In a multiple logistic regression analysis, there were no differences in CIR between the 3 groups. Poor bowel cleansing and female sex were independent predictors for intubation failure.
Conclusion
Cecal intubation rate in clinical colonoscopies and colonoscopy screening are similar. There is no reason to differentiate between screening and clinical colonoscopy with regard to CIR.
Introduction
Colonoscopy is a cornerstone procedure in the diagnosis of large bowel conditions and in colorectal cancer (CRC) screening programs. Several quality indicators have been proposed for colonoscopy, one of which is the cecum intubation rate. There is considerable variation in endoscopist performance and it has been shown that poor cecum intubation rate (CIR) is correlated to increased risk of post-colonoscopy CRC 1 .
Some guidelines differentiate between minimum standards for CIR, depending on whether it is in screening (95 % CIR) or clinics (90 %) and excluding cases with poor bowel cleansing, severe colitis and strictures requiring therapy 2 3 4 . Other guidelines do not differentiate and recommend 90 % CIR for all colonoscopies with no adjustments or exclusions 5 or with adjustment for cancer strictures requiring surgery 6 . These recommendations are largely based on historical data from routine clinics and screening 2 with limited consideration of self-selection of the target populations and performing endoscopists. The need to differentiate between screening and clinical standards may be a myth and simply due to the skills of the screening endoscopists who may be self-selected, specially trained or otherwise dedicated to perform screening colonoscopy.
Two studies have particularly addressed this issue, one showing similar CIR in colonoscopies whether due to screening, surveillance or symptoms in 129,549 colonoscopy reports from 507 endoscopists to the Clinical Outcomes Research Initiative (CORI) in Oregon, USA 7 . In this study, endoscopist characteristics were limited to level of experience and any organizational bias was not accounted for, e. g. the extent of allocating some endoscopists to certain groups of patients or screenees. The other was a smaller, single-center study comprising 1,056 colonoscopies from 5 endoscopists showing poorer CIR in routine clinics than screening colonoscopy 8 . In this study, all endoscopists were performing routine clinical and screening colonoscopies, but the study was restricted to 1 center and very few endoscopists. The present observational study from the Gastronet quality assurance register ( www.kreftregisteret.no/gastronet ) seeks to limit organizational bias by investigating cecum intubation rates among endoscopists who individually perform both clinical and screening colonoscopy in the same endoscopy unit. Given this background, the aim of the study was to investigate factors related to CIR in 3 different settings: routine clinical colonoscopy, primary screening colonoscopy and work-up colonoscopy of screen-positives after screening by other methods than colonoscopy (fecal occult blood testing or flexible sigmoidoscopy).
Patients and methods
The study includes all outpatients, non-emergency colonoscopies prospectively reported to the Gastronet quality register from January 1, 2013 to December 31, 2014 from 4 non-academic hospitals in Norway. These hospitals were hosting centers for 2 colorectal cancer screening trials and they provided clinical colonoscopy services for symptomatic patients (routine clinics). The screening methods used were immunochemical fecal occult blood tests (iFOBT), flexible sigmoidoscopy or primary colonoscopy screening. In all centers, Olympus colonoscopies were used with access to magnetic endoscope imaging (MEI – ScopeGuide).
In Norway, endoscopists are committed to reporting key performance quality indicators of their colonoscopies to the national quality register Gastronet. In the current study, endoscopists who had reported at least 30 colonoscopies for either screening or routine clinics in the same hospital in the study period, were included. Endoscopists were categorized into “seniors” (more than 2 years of experience from routine clinical colonoscopies), and “juniors”. Juniors were residents who completed colonoscopy training during the past 2 years before start of the study. They were primarily trained for performing flexible sigmoidoscopy screening and work-up colonoscopy when having reached a level of proficiency to permit running their own endoscopy lists. Seniors were not subjected to this systematic training. Other than having documented proficiency through Gastronet, there are no formal requirements to training to be allowed to perform screening colonoscopies in Norway.
Two of the hospitals were hosting units serving a randomized trial on primary colonoscopy screening – the NordICC study (Centers 1 and 2) 9 . Another 2 hospitals hosted a randomized trial comparing flexible sigmoidoscopy and iFOBT screening – the Bowel Cancer Screening in Norway (BCSN) (Center 3 and Center 4) ( www.kreftregisteret.no/en/screening/Screening-for-colorectal-cancer/ ). The endoscopists employed in the respective hosting hospitals were doing either a combination of clinical and primary screening colonoscopy (Centers 1 and 2) or a combination of clinical colonoscopy, flexible sigmoidoscopy screening and work-up colonoscopy of screen-positives from flexible sigmoidoscopy or iFOBT screening (Centers 3 and 4). Clinical colonoscopy was defined as outpatient colonoscopies performed due to symptoms or follow-up on these patients. Cases with missing registration of cecal intubation status (no information on having reached the cecum or not), examinations with no intent to reach the cecum (e. g. polypectomy site inspection after removal of distal polyps) and patients with previous CRC (resected bowel segments and therefore presumed easier to intubate) were also excluded from analyses. Further, cases with an explicitly stated reason for intubation failure either due to mechanical stricture or poor bowel preparation were also excluded, purely based on the subjective judgement of the endoscopist (not the authors). The Boston Bowel Prep Scale (BBPS) was used for assessment of the quality of bowel cleansing in a range from 1 to 9 collectively for all 3 segments (distal colon with rectum, transverse colon and proximal colon).
The primary outcome of the study was cecal intubation rate (CIR). Gastronet is approved by the Norwegian Social Science Services and the Norwegian data Inspectorate. Gastronet is granted a waiver from the ethics committee of South-East Norway to which this national register is affiliated.
Statistics
Independent Student’s t -test was used for analysis of continuous data and Chi-squared test for categorical data. Univariate and multivariate logistic regression analysis was performed for CIR with the following co-variables: colonoscopy category (patients in routine clinics, primary screening colonoscopy, work-up colonoscopy of screen-positives), patient gender, bowel cleansing (BBPS), indication for having a colonoscopy, endoscopist category (junior or senior endoscopist), patient age and endoscopy center (hospital location). Statistical significance was defined as P < 0.05 using two-sided tests. In the multivariable logistic regression analysis, only variables individually reaching statistical significance level in the univariate analyses were included. Multivariable analysis was performed both excluding and including cases declared by the endoscopist to be impossible to intubate due to mechanical obstruction or poor bowel cleansing. The statistical package IBM SPSS 19.0 was used.
Results
Thirty-five endoscopists were included. They were accountable for 11,577 study colonoscopies ( Fig. 1 ). 733 (9.1 %) of colonoscopies in routine clinics were excluded as they were defined by referral letter or performing endoscopist as opportunistic screening (n = 50 [0.6 %]) or screening due to CRC in relatives (n = 369 [4.6 %]). In addition, in both the screening and the routine clinical colonoscopy groups, patients who had undergone surgery for CRC (n = 314 [3.9 %]) with a colon resection were excluded. Individuals with no information on gender or cecal intubation status were excluded from all 3 groups, as well as cases defined pre-colonoscopy not to require cecal intubation and cases where stricture or fecal masses made intubation impossible as judged by the endoscopist ( Fig. 1 ).
Fig. 1.
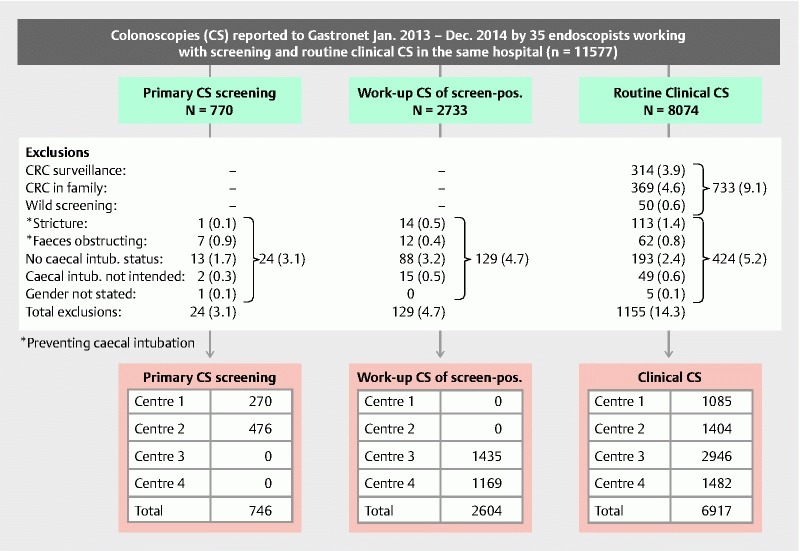
Flowchart for colonoscopies reported from 35 endoscopists performing colonoscopies in 4 hospital-integrated centers for organized colorectal cancer screening in addition to performing routine clinical colonoscopies in the same hospitals.
A total of 10,267 colonoscopies and 35 endoscopists were thus analyzed with a combination of primary colonoscopy screening and routine clinics in 2 centers (Centers 1 and 2) and a combination of work-up colonoscopy of screen-positives and of routine clinics in another 2 centers (Centers 3 and 4) ( Fig. 1 ).
Characteristics of clinical patients and screening participants, respectively, are presented in Table 1 . Older age and poorer bowel cleansing characterized patients for work-up colonoscopy compared to primary colonoscopy screening.
Table 1. Characteristics of individuals subjected to colonoscopy (CS).
|
Screening CS
(n = 746) |
Work-up CS of screen pos.
(n = 2604) |
Clinical CS
(n = 6917) |
|
| Gender | |||
|
381 (51.1) | 1547 (59.4) | 3294 (47.6) |
|
365 (48.9) | 1057 (40.6) | 3623 (52.4) |
| Age (mean, 95 %CI) | 60.9 (60.7 – 61.1) | 64.3 (64.0 – 64.6) | 60.6 (60.3 – 61.0) |
| BBPS 1 (mean, 95 %CI) | 8.0 (7.9 – 8.1) | 7.6 (7.5 – 7.7) | 7.5 (7.4 – 7.5) |
| BBPS 1 score ≥ 6 (%) | 703/733 (95.9) | 2096/2286 (91.7) | 5523/6177 (89.4) |
Boston Bowel Preparation Scale, summary score for all bowel segments
Among patients in routine clinics, the reasons for having a colonoscopy varied somewhat between centers with symptoms constituting 66.2 % to 72.5 % of referrals to colonoscopy and polyp surveillance requiring about 17 % of the colonoscopy capacity in routine clinics in all hospitals ( Table 2 ).
Table 2. Indications, n (%), for clinical colonoscopy (excluding colonoscopy due to opportunistic screening and family history) in four hospitals hosting units for clinical and screening colonoscopy.
| Symptoms | Polyp surveillance | IBD surveillance | Unspecified | Total | |
| Center 1 | 785 (72.4) | 198 (18.2) | 71 (6.5) | 31 (2.9) | 1085 |
| Center 2 | 1018 (72.5) | 252 (17.9) | 62 (4.4) | 72 (5.1) | 1404 |
| Center 3 | 2015 (68.4) | 428 (14.5) | 259 (8.8) | 244 (8.3) | 2946 |
| Center 4 | 981 (66.2) | 260 (17.5) | 90 (6.1) | 151 (10.2) | 1482 |
| Total | 4799 (69.4) | 1138 (16.5) | 482 (7.0) | 498 (7.2) | 6917 |
IBD, inflammatory bowel disease
Omission to report cecal intubation status was 1.7 %, 3.2 % and 2.4 % in primary screening, work-up of screen-positives and routine clinics, respectively ( Fig. 1 ). Cecal intubation rate in primary colonoscopy screening (724/746 [97.1 %]) was identical to routine clinical colonoscopy (6719/6917 [97.1 %]) ( P = 0.89). In work-up colonoscopy, the CIR (2567/2604 [98.6 %]) was slightly higher than the 97.1 % CIR observed in routine clinical colonoscopy ( P < 0.001). In the list of exclusions ( Fig. 1 ), “stricture” and “poor bowel preparation” were entirely dependent on the judgement of the endoscopist as photo or other documentation was not mandatory. Not excluding these variables from the analysis reduced the CIR slightly in all three colonoscopy categories to 6731/7092 (94.9 %) in routine clinics, 2568/2730(97.6 %) in work-up colonoscopy and 724/754 (96.0 %) in primary colonoscopy screening.
Senior and junior endoscopists had comparable results – all above 95 % CIR ( Table 3 ). Assistance of a second endoscopist was asked for in 494/3126 (15.5 %) of colonoscopies initiated by the junior group compared to 85/7141 (1.2 %) in the senior group ( P < 0.001). The reasons for the call for assistance were not specified, but presumed to be mainly for intubation and therapeutic procedures. Juniors contributed to 79 % of work-up CS (2052/2604) and 45 % of clinical CS (3126/6917).
Table 3. Cecal intubation according to endoscopist category based on experience and training.
| Endoscopist category | Cecal intubation (%) | P value | |||
| Screening | Work-up | Clinical | Total | ||
| Senior endoscopist 1 | 724/746 (97.1) |
542/552 (98.2) |
5670/5843 (97.0) |
6936/7141 (97.1) |
0.30 |
| Risk difference, percentage points, 95 % CI | 0.0 (-1.3 – 1.3) | 1.1 (-0.1 – 2.3) | Ref | ||
| Junior endoscopist 1 | 2025/2052 (98.7) |
1049/1074 (97.7) |
3074/3126 (98.3) |
0.04 | |
| Risk difference, percentage points, 95 % CI |
1.0 (0.0 – 2.0) | Ref | |||
End result after assistance when needed (1.2 % of colonoscopies by seniors and 15.5 % by juniors)
In the univariable model on CIR, patient age and endoscopy center did not reach statistical significance level and were not included in the multivariable model ( Table 4 ). This showed that categorization into routine clinical colonoscopy, work-up colonoscopy or primary colonoscopy screening was not a predictor for cecum intubation, while good bowel cleansing and examination by an endoscopist systematically trained for endoscopy screening, with procedural assistance when required, were predictors for higher cecum intubation rates. These results were not altered when including cases subjectively judged by the endoscopist to be impossible to intubate due to stricture or fecal masses ( Table 4 ).
Table 4. Odds ratios for cecal intubation in a logistic regression model adjusting for colonoscopy category, gender, Boston Bowel Preparation Score, indication for CS, endoscopist category.
| Unadjusted OR | Adjusted OR | ||||
| No. of CS | Mean (95 % CI) | P value | Mean (95 % CI) | P value | |
| CS category | |||||
| Clinical CS | 6917 [7092] |
1.0 (reference) | 1.0 (reference) | ||
| Work-up CS | 2604 [2630] |
2.0 (1.4 – 2.9) [2.2 (1.7 – 2.9)] |
< 0.001 [< 0.001] |
0.9 (0.5 – 1.7) [1.2 (0.7 – 2.0)] |
0.85 [0.51] |
| Screening CS | 746 [754] |
1.0 (0.62 – 1.52) [1.3 (0.9 – 1.9)] |
0.89 [0.18] |
0.8 (0.4 – 1.7) [0.8 (0.4 – 1.7)] |
0.60 [0.60] |
| Gender | |||||
| Men | 5222 [5324] |
1.0 (reference) | 1.0 (reference) | ||
| Women | 5045 [5152] |
0.5 (0.4 – 0.7) [0.6 (0.5 – 0.8)] |
< 0.001 [< 0.001] |
0.7 (0.5 – 1.0) [0.7 (0.5 – 0.9)] |
0.06 [0.01] |
| BBPS | 9196 [9272] |
1.9 (1.8 – 2.1) [2.1 (1.9 – 2.2)] |
< 0.001 [< 0.001] |
2.0 (1.8 – 2.1) [2.1 (1.9 – 2.3)] |
< 0.001 [< 0.001] |
| Indication for CS | |||||
| Symptoms | 4799 [4928] |
1.0 (reference) | 1.0 (reference) | ||
| Polyp surveillance | 1138 [1150] |
2.6 (1.5 – 4.5) [2.7 (1.8 – 4.2)] |
0.001 [< 0.001] |
2.8 (1.1 – 7.0) [2.9 (1.4 – 6.1)] |
0.03 [0.01] |
| IBD surveillance | 482 [492] |
1.1 (0.6 – 1.9) [1.1 (0.7 – 1.7)] |
0.77 [0.56] |
0.7 (0.3 – 1.4) [0.8 (0.4 – 1.6)] |
0.31 [0.56] |
| Unspecified | 498 [517] |
0.8 (0.5 – 1.3) [0.8 (0.5 – 1.1)] |
0.42 [0.12] |
1.5 (0.6 – 3.6) [1.8 (0.9 – 3.6)] |
0.35 [0.12] |
| Endoscopist group 1 | |||||
| Senior | 7141 [7295] |
1.0 (reference) | 1.0 (reference) | ||
| Junior | 3126 [3181] |
1.8 (1.3 – 2.4) [1.5 (1.2 – 1.8)] |
< 0.001 [0.001] |
2.0 (1.2 – 3.5) [2.3 (1.4 – 3.6)] |
0.01 [< 0.001] |
| Age (years) 2 | 10267 [10476] |
1.0 (1.0 – 1.0) [0.987 [0.980 – 0.995)] |
0.34 [0.001] |
– [0.997 (0.985 – 1.008)] |
[0.56] |
| Endoscopy center 2 | |||||
| Centre 1 | 1355 [1384] |
1.0 (reference) | – | ||
| Center 2 | 1880 [1909] |
0.9 (0.6 – 1.5) [1.1 (0.8 – 1.6)] |
0.80 [0.58] |
– | |
| Center 3 | 4381 [4493] |
1.0 (0.7 – 1.5) [0.9 (0.7 – 1.2)] |
0.90 [0.44] |
– | |
| Center 4 | 2651 [2690] |
0.9 (0.6 – 1.3) [1.1 (0.8 – 1.5)] |
0.51 [0.62] |
– | |
OR, odds ratio; CS, colonoscopy screening; BPPS, Boston Bowel Preparation Score; IBD, inflammatory bowel disease
End result of CIR after assistance when required (1.2 % of examinations by seniors and 15.5 % by juniors).
Patient age and endoscopy center did not reach statistical significance level in the univariate logistic regression and were not included in the adjusted model. Results including cases declared by the endoscopist to be impossible to intubate due to mechanical obstruction (stricture or fecal masses) are added in [brackets].
Discussion
This study suggests that there is no justification for recommending different thresholds for cecal intubation rate for colonoscopy in screening versus routine clinics. In the present Gastronet dataset, there was no difference in CIR between clinical routine colonoscopy, primary screening colonoscopy and work-up of screen-positives. This suggests that the endoscopist and his/her performance skills are more important for CIR than whether colonoscopy is for clinical or screening purposes. This is consistent with findings in a large retrospective study from Oregon, USA on 129,549 colonoscopies performed by 507 endoscopists for screening of average CRC-risk, symptoms or surveillance showing CIRs around 95 % in all three categories of patients 7 . Similar to several studies, they showed that older age, female gender and poor bowel cleansing were unfavourable for CIR – the two latter observations being similar to our findings.
We chose to keep the 3 categories of colonoscopy rather than merge the primary screening colonoscopy (presumptively healthy persons) and work-up colonoscopies (presumptively with a lesion in the colon) into a common “screening group” to be compared to routine clinical colonoscopies. Tolerance and motivation for a full colonoscopy may vary between these 3 categories, both for the patient and for the endoscopist. Both may be expected to endure more to reach the cecum knowing that there is a positive screening test compared to a primary CS screening with no relevant symptoms, signs or test results. With obvious differences in recruitment of patients and persons for routine clinical colonoscopy, screening or work-up of screen-positives, it is important to define inclusion and exclusion criteria for estimation of a recommended standard CIR. In the Oregon study with an overall 95 % CIR 7 , cases with a prior intent by the endoscopist not to reach the cecum were excluded (as in our study) in addition to cases with incomplete demographic data. The European Commission guidelines for CRC screening recommend a minimum CIR of 90 %, adjusted only for “obstruction leading to operative intervention” while more than 95 % is “desirable” 6 . The US Multi-Society Task force on Colorectal Cancer differentiates between an overall quality target of > 90 %, but > 95 % in screening, both when adjusting for poor bowel cleansing, but not for obstructing lesions 2 . Thus, the ease for comparing different guidelines and recommendations depends on which criteria may have been adjusted for, if any. As shown in Fig. 1 , obstructing strictures are more likely to prevent intubation in routine clinics than screening, but the difference does not account for a 5 % difference in recommended minimum CIR between screening and clinical colonoscopy, at least not in this material. Other differences in case-mix may justify a 5 % difference, such as age, gender, comorbidity with inactivity and poorer bowel cleansing. Using different minimum standards in a screening and a patient population may only be suggestive of a differentiated quality of services provided if potential mechanisms for making such a difference is not brought forward. Quality of performance like the CIR should be stated with and without adjusting for variables that may explain a differentiation in quality. Only then will sufficient information be available on which to act to improve sub-performance.
In the UK, accreditation is required to be allowed to perform colonoscopies in the national screening program. A series of 10,026 clinical colonoscopies performed by accredited and non-accredited colonoscopists showed that accreditation played an independent role for quality outcomes, including CIR, when adjusting for case mix 10 . With increasing pressure on endoscopy services to cope with implementation of CRC screening in several countries, different standards may develop formally or informally. Self-selection of endoscopists (informal recruitment) or setting higher standards for endoscopists allowed to perform colonoscopy screening (formal recruitment), may explain observed differences in CIR 8 , possibly giving a false impression that cecal intubation is harder to achieve in routine clinical colonoscopy of patients than in screening of presumptively healthy individuals. Therefore, it is of paramount importance in these times of screening implementation to follow time trends in the quality of clinical and screening endoscopy services and reduce the range of subjectivity in estimation of quality endpoints and of co-variables influencing on these endpoints.
Without photo or video documentation, cecal intubation is a subjective procedure endpoint as judged by the endoscopist. Also, some may consider “seeing the cecum at a distance” as being equivalent to “having reached the cecum”. Many efforts have been made to provide objective confirmation of complete intubation 11 . So far, the use of supportive photo documentation prevails although a photo of the cecum at distance may be erroneously accepted as a proof of reaching the cecum. The subjectivity of cecal intubation combined with reimbursement only for “complete colonoscopy” in some health insurance systems, may further encourage overestimating cecal intubation 12 .
In the Gastronet form, endoscopists may indicate mechanical reasons for intubation failure (split into “stricture” or “poor cleansing”) or “other reasons”. Even when excluding these mechanical reasons explaining non-intubation, bowel cleansing expressed by BBPS score did still play a role in the multivariable regression analysis on CIR in the present study, suggesting that poor bowel cleansing may have been underreported as a reason for intubation failure. This emphasizes the subjectivity also of co-variables important for CIR and not only the subjectivity of the endpoint itself: “Have I been to the cecum or not?” Also in Gastronet, the number of “strictures” causing intubation failure exceeds the number of CRC cases, suggesting that “sharp bends,” for example, may be misinterpreted as strictures (unpublished data). For these reasons, there should be no question of using either unadjusted or adjusted CIR, but rather both should be presented and both sets of information used to address sub-standard performance. Although differentiating between CIR recommendations in clinics and screening may be an understandable pragmatic approach, large series of colonoscopies now suggest that this may not be necessary and that a minimum CIR of about 95 % rather than 90 % should be the aim 7 . Our study supports this. When adjusting for “stricture” and “poor cleansing” as mechanical reasons for intubation failure in our study, this brought the crude (unadjusted) CIR from 94.9 % to 97.1 % in routine colonoscopy, from 97.6 % to 98.6 % in work-up colonoscopy and from 96.0 % to 97.1 % in primary colonoscopy screening, respectively.
The endoscopist category “junior endoscopists” consisted of young doctors typically recruited straight from junior resident posts to be the first in Norway to go through a formalized colonoscopy training program with some trainers having participated in Train the Colonoscopy Trainer courses 13 . In relation to screenees, they have so far only performed work-up colonoscopy and not primary screening colonoscopy. We registered their requirement for hands-on assistance by another endoscopist without specifying if it was for intubation, polypectomy or other procedures. This registration did, however, expose a much greater need to ask for help from a second endoscopist among junior (15.5 %) than senior (1.2 %) endoscopists, underlining their inexperience and junior status. The odds ratio for successful intubation suggested that senior endoscopists in our study might have improved their CIR if they had had called for assistance by a colleague more often. The adjusted OR for cecal intubation was 2.0 (95 % CI 1.2 – 3.5) for junior endoscopist (together with senior assistance when needed) compared to seniors. This is in line with a recent study from Canada showing that the presence of a trainee did not adversely affect quality outcomes in colonoscopy 14 .
A strength of the current study is that it is restricted to endoscopists engaged both in screening and clinical colonoscopy, thus reducing self-selection recruitment bias of endoscopists to screening. Further, it is restricted to communities with non-academic hospitals hosting endoscopy units for routine clinical colonoscopy and units for organized CRC screening, thus reducing the risk of organizational and cultural bias.
A weakness of this study is that the recently formally trained junior endoscopists were only involved in clinical and work-up colonoscopy, not in primary screening colonoscopy. Primary colonoscopy screening is not a service in Norway and endoscopists were already engaged in the primary colonoscopy screening trial subject to the current study when the formal training of juniors started. Another weakness is that photo documentation of cecal intubation was not mandatory. Also, we did not have information on comorbidity expected to be in excess and more severe than in the 2 groups of presumably healthy screenees. Whatever excess of comorbidity there may have been, that did not appear to affect the CIR since the CIRs were identical in the primary screening and the routine clinical CS groups.
Conclusion
Endoscopist performance is more important for CIR than whether an individual is referred for colonoscopy on clinical grounds or attends for CRC screening. Subjectivity of endpoint assessment (cecal intubation) and of important co-variables (e. g. bowel cleansing) requires both unadjusted (crude) and adjusted CIRs to be presented to address sub-performance. Unified threshold for CIR across different indications for colonoscopy may simplify quality assurance programs and improve acceptance and adherence to standards.
Acknowledgements
The Gastronet quality assurance register is funded by national govermental grants to the South-Eastern Norway Regional Health Authority.
Footnotes
Competing interests None
References
- 1.Baxter N N, Sutradhar R, Forbes S S et al. Analysis of administrative data finds endoscopist quality measures associated with postcolonoscopy colorectal cancer. Gastroenterology. 2011;1 40:65–72. doi: 10.1053/j.gastro.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 2.Rex D K, Bond J H, Winawer S et al. Quality in the technical performance of colonoscopy and the continuous quality improvement process for colonoscopy: recommendations of the U.S. Multi-Society Task Force on Colorectal Cancer. Am J Gastroenterol. 2002;97:1296–1308. doi: 10.1111/j.1572-0241.2002.05812.x. [DOI] [PubMed] [Google Scholar]
- 3.Rex D K, Petrini J L, Baron T H et al. Quality indicators for colonoscopy. Am J Gastroenterol. 2006;101:873–885. doi: 10.1111/j.1572-0241.2006.00673.x. [DOI] [PubMed] [Google Scholar]
- 4.Rex D K, Schoenfeld P S, Cohen J et al. Quality indicators for colonoscopy. Gastrointest Endosc. 2015;81:31–53. doi: 10.1016/j.gie.2014.07.058. [DOI] [PubMed] [Google Scholar]
- 5.Rembacken B, Hassan C, Riemann J F et al. Quality in screening colonoscopy: position statement of the European Society of Gastrointestinal Endoscopy (ESGE) Endoscopy. 2012;44:957–968. doi: 10.1055/s-0032-1325686. [DOI] [PubMed] [Google Scholar]
- 6.European Colorectal Cancer Screening Guidelines Working G, von Karsa L, Patnick J et al . European guidelines for quality assurance in colorectal cancer screening and diagnosis: overview and introduction to the full supplement publication. Endoscopy. 2013;45:51–59. doi: 10.1055/s-0032-1325997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gupta M, Holub J L, Eisen G. Do indication and demographics for colonoscopy affect completion? A large national database evaluation. Eur J Gastroenterol Hepatol. 2010;22:620–627. doi: 10.1097/MEG.0b013e3283352cd6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nagrath N, Phull P S. Variation in cecal intubation rates between screening and symptomatic patients. United European Gastroenterol J. 2014;2:295–300. doi: 10.1177/2050640614536898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaminski M F, Bretthauer M, Zauber A G et al. The NordICC Study: rationale and design of a randomized trial on colonoscopy screening for colorectal cancer. Endoscopy. 2012;44:695–702. doi: 10.1055/s-0032-1306895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhangu A, Bowley D M, Horner R et al. Volume and accreditation, but not specialty, affect quality standards in colonoscopy. Br J Surg. 2012;99:1436–1444. doi: 10.1002/bjs.8866. [DOI] [PubMed] [Google Scholar]
- 11.Baraza W, Brown S, Shorthouse A J et al. Direct photographic documentation of ileal mucosa in routine colonoscopy is not an independent valid or reliable proof of completion: quality assurance issues for the national colorectal cancer-screening programme. Colorectal Dis. 2009;11:89–93. doi: 10.1111/j.1463-1318.2008.01511.x. [DOI] [PubMed] [Google Scholar]
- 12.Pox C P, Altenhofen L, Brenner H et al. Efficacy of a nationwide screening colonoscopy program for colorectal cancer. Gastroenterology. 2012;142:1460–146700. doi: 10.1053/j.gastro.2012.03.022. [DOI] [PubMed] [Google Scholar]
- 13.Valori R, Sint Nicolaas J, de Jonge V. Quality assurance of endoscopy in colorectal cancer screening. Best Pract Res Clin Gastroenterol. 2010;24:451–464. doi: 10.1016/j.bpg.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 14.Pace D, Borgaonkar M, Hickey N et al. Does the hands-on, technical training of residents in colonoscopy affect quality outcomes? Surg Endosc. 2015;30:1352–1355. doi: 10.1007/s00464-015-4397-1. [DOI] [PubMed] [Google Scholar]


