Abstract
We continuously need to adapt to changing conditions within our surrounding environment, and our brain needs to quickly shift between resting and working activity states in order to allow appropriate behaviors. These global state shifts are intimately linked to the brain-wide release of the neuromodulators, noradrenaline and acetylcholine. Astrocytes have emerged as a new player participating in the regulation of brain activity, and have recently been implicated in brain state shifts. Astrocytes display global Ca2+ signaling in response to activation of the noradrenergic system, but whether astrocytic Ca2+ signaling is causative or correlative for shifts in brain state and neural activity patterns is not known. Here we review the current available literature on astrocytic Ca2+ signaling in awake animals in order to explore the role of astrocytic signaling in brain state shifts. Furthermore, we look at the development and availability of innovative new methodological tools that are opening up for new ways of visualizing and perturbing astrocyte activity in awake behaving animals. With these new tools at hand, the field of astrocyte research will likely be able to elucidate the causal and mechanistic roles of astrocytes in complex behaviors within a very near future.
Keywords: Astrocytes, Calcium, Brain state, Neuronal circuit, Wakefulness
Introduction
Every day, we continuously shift between different behavioral contexts, such as zoning out while waiting for the bus or getting a kick from high intensity workout at the gym. During these shifts in brain state, the internal dynamics of the brain and its responsiveness to incoming external sensory stimuli vary greatly [1–6] (Figure 1). The internal brain state can oscillate and shift even in the absence of marked behavioral changes of which the transition between different phases of sleep and from sleep to awake are the most well-recognized [7–11].
Fig. 1. Neuronal cortical state changes.
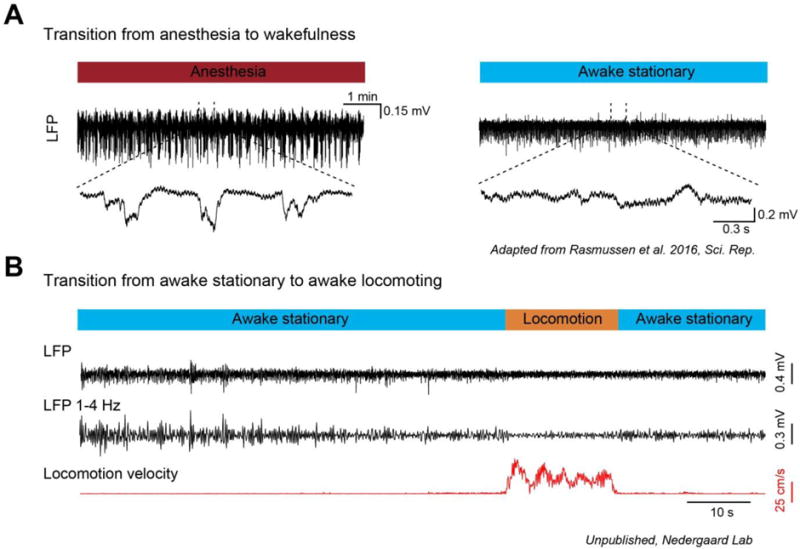
Local field potential (LFP) characteristics of cortical state changes in mice. (A) LFP recording from layer 2/3 of mouse sensori-motor cortex during the transition from anesthesia-induced slow-wave patterns to waking. During anesthesia and sleep, slow wave fluctuations are prominent in the LFP signal and appear as synchronized summed population up and down states. The transition from anesthesia, or sleep, to waking is associated with a suppression of slow fluctuations in the LFP and a loss of synchronized down states. (B) LFP recording (raw broadband and band-pass filtered 1–4 Hz) from layer 2/3 of mouse visual cortex during the transition from stationary (quiet wakefulness) to voluntary locomotion (active behavior) on a running wheel. Voluntary locomotion is associated with a substantial suppression of LFP low-frequency 1–4 Hz fluctuations. Panel A is from Rasmussen et al., 2016 [73] and panel B is from unpublished experiments from the Nedergaard laboratory.
The neuronal activity patterns during sleep and anesthesia have been interrogated in detail and much is known about both intrinsic and extrinsic properties governing these patterns. By comparison, neuronal activity pattern transitions in the awake brain are subtler, and the mechanisms orchestrating these transitions are less studied. However, an increasing body of literature are starting to emerge [3, 4, 12–16].
Different stages of sleep and wakefulness are characterized by specific patterns of electroencephalographic (EEG) activity: during non-rapid-eye-movement (NREM) sleep, slow 1–4 Hz oscillations are dominant, whereas REM sleep is characterized by desynchronized high-oscillatory activity. The awake brain state has been categorized into quiet wakefulness and active behavior with active behavior most often being associated with periods of locomotion or active whisking [13, 17]. Seminal studies have shown that quiet wakefulness is characterized by synchronized large-amplitude low-frequency fluctuations in the local field potential or membrane potential [3, 4, 12]. During the active state, the brain activity desynchronizes and low-frequency fluctuations are suppressed, while higher-frequency oscillations become more apparent [3, 4, 12]. Even though the shifts in brain activity patterns that occur during sleep (NREM to REM) and wakefulness (quiet wakefulness to active state) are quite similar, there are large differences in muscle tone between sleep and awake states.
In this review, we will focus on brain state shifts in the awake behaving brain. Functionally, the switch from quiet wakefulness to an active state have been associated with changes in sensory information processing and representations, such as an increased gain of neurons in primary visual cortex and modulated sound-evoked responses in primary auditory cortex [3, 4, 16, 18], but almost nothing is known about the role of astrocytes in these transitions.
Glial cells are the most abundant cell type in the human brain [19]. In the human forebrain, ~20% of all cells are astrocytes [20], and in the mammalian cerebral cortex the number of astrocytes exceeds that of neurons [21]. Despite this striking high cell number, we are just starting to comprehend and investigate the diversity and importance of astrocyte functions (reviewed in Khakh and Sofroniew, 2015). It is now accepted that astrocytes are involved in homeostatic functions such as ion regulation, transmitter clearance as well as neurovascular coupling. In sleep, evidence suggests that astrocytes might play a role in inducing and maintaining the different sleep phases. A study found that astrocytes oscillated in phase with slow-wave activity during sleep [22] through redistributing of extracellular K+ thus maintaining the depolarization (UP state) [23]. Another group found that by electrically stimulating single astrocytes thereby inducing Ca2+ fluctuations, they could induce cortical UP state synchronization [24, 25]. Several studies have also implicated astrocytes in sensory information processing in both anesthetized [26] and awake behaving animal [27] as well as in cortical state transitions in anesthetized animals [25], suggesting that astrocytes might participate in controlling the ongoing brain state and the shift between states.
If we are to understand how brain state transitions occur during wakefulness, and how this influences the representation and processing of the external world in cortical circuits, we need to start including astrocytes in our conceptualizations and hypotheses. Here we review recent work, largely in awake behaving animals, investigating the role of large global Ca2+ responses of astrocytes in brain state changes and arousal. Determining the functional role of these brain-wide astrocytic fluctuations in shaping neuronal circuits, sensory input, and ultimately decision making are important for deciphering the behavioral circuits in normal and pathological conditions.
Neuromodulator Systems Involved in Cortical State Transitions
The causal mechanisms underlying awake cortical state transitions are still debated and not fully understood, but an increasing amount of work has implicated central neuromodulator systems including norepinephrine (NE) and acetylcholine (ACh) (Eggermann et al., 2014; Fu et al., 2014b; Polack et al., 2013; Schiemann et al., 2015; reviewed in McGinley et al., 2015b) (Figure 2). NE is produced in the brain stem nuclei, locus coereleus (LC), which projects widely throughout the brain and provides the sole source of NE. ACh on the other hand originates from several brain stem clusters, including the pedunculopontine tegmental and the lateral dorsal tegmental nuclei, as well as from basal forebrain nuclei, including nucleus basalis. Both LC and cholinergic basal forebrain neurons ramp up their firing rate in response to behavioral states associated with desynchronized cortical neuronal activity, such as attention, arousal, and spontaneous locomotion, pointing to their involvement in state transitions [28–30].
Fig. 2. Diagram displaying selected pathways involved in brain state changes.
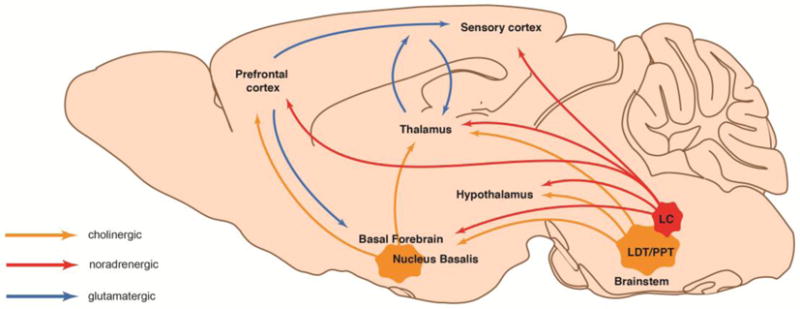
Sagittal view of a mouse brain showing glutamatergic (blue), noradrenergic (red), and cholinergic (orange) nuclei and projection pathways involved in shifts between resting and working activity states. LC: locus coereleus; LDT: laterodorsal tegmental nucleus; PPT: pedunculopontine tegmental nucleus. Adapted from Lee and Dan, 2012 [8].
A large body of literature suggests that NE and ACh modulate neuronal activity via several mechanisms. Earlier work have established that NE both excite and inhibit neurons [31–34]. Though, it appears that NE is mostly excitatory on cortical pyramidal neurons via its activation of metabotropic Gq-coupled noradrenergic α1-receptors [35, 36]. This effect is mostly ascribed to inhibition of the Ca2+-activated K+ current IAHP [37, 38], and a reduction in other K+ currents [36]. Experiments investigating the functional mechanisms of ACh on neuronal properties have demonstrated that ACh most often triggers prolonged and marked excitation of cortical pyramidal neurons via combined activation of cholinergic metabotropic muscarinic and ionotropic nicotinic receptors [39–41]. This is thought to be due to a general decrease in neuronal K+ conductance [42]. Thus, recordings from pyramidal neurons have shown that ACh block the voltage-dependent K+ current IM, the IAHP, and with and to some extend a leak K+ current [43–46]. Further evidence for key roles of both NE and ACh for the transition between awake states is based on elegant in vivo experiments using pharmacological blockage of NE and ACh receptors locally in the cortex [12, 14, 15, 28]. This has demonstrated that both NE and ACh are strongly involved in mediating the cortical state transition and neuronal activity modulation associated with locomotion, leading to the hypothesis that NE and ACh reduce low-frequency and facilitate higher-frequency oscillations during states of increased arousal [17]. Furthermore, NE and ACh can change the composition of extracellular ions and in this way control the electrocorticographic patterns [7].
Taken together, substantial evidence supports a prominent role for NE and ACh in mediating the state transition between quiet wakefulness and the active state. Given their widespread projection targets and ability to globally shift the levels of NE and ACh throughout the cerebral cortex, they are perfectly situated to regulate cortical state changes in the awake brain. However, several other systems, such as thalamo-cortical and cortico-cortical pathways, have also been linked to awake state shifts, and suggesting that brain state transitions and stabilization likely requires both global, as well as local signaling (reviewed in Lee et al., 2013; Lee and Dan, 2012; Zagha and McCormick, 2014).
Astrocytes Respond to Neuromodulators
Astrocytes respond to transmitter release with increases in their intracellular Ca2+ concentration via several mechanisms [21, 49]. This astrocytic Ca2+ signaling has attracted tremendous attention within the last 20 years, because it likely represents a form of signaling that astrocytes may employ for communication in the brain. A large variety of astrocytic Ca2+ responses have been uncovered that may play a role in regulating local neuronal activity (reviewed by Bazargani and Attwell [50]). However, many of these experiments have been conducted in preparations from immature animals, proving to be problematic due to developmental regulation of astrocytic receptor expression [51]. Furthermore, anesthesia significantly blunts astrocytic Ca2+ signaling in vivo [52, 53]. The most intriguing experiments are thus performed in vivo in awake animals. Initially, a clear link between peripheral sensory stimulations and astrocytic Ca2+ responses was reported: in anesthetized animals, Ca2+ transients within cortical astrocytes could be elicited by whisker stimulation, foot shock, and mechanical stimulation [26, 54, 55]. Early work in cultured astrocytes revealed that NE is a potent activator of astrocytic Ca2+ signaling [56–58]. A link between LC and NE-mediated activation of astrocytes was confirmed by Bekar et al. (2008) that demonstrated that in anesthetized animals, when LC is electrically stimulated, cortical astrocytes strongly respond with Ca2+ increases, which could be blocked by noradrenergic antagonists. LC activation is clearly connected to startle responses, which can be viewed as states of hyperarousal. Ding et al. (2013) confirmed and extended the findings from Bekar et al. (2008) in awake animals and showed that startle-induced LC activation caused long-ranging astrocytic cortical Ca2+ signals that were triggered by endogenous NE release from noradrenergic fibers acting on cortical astrocytic α1-adrenergic receptors. This has led to a novel concept within the field that astrocytes in the awake animal respond to arousing stimuli by elevating their Ca2+ levels globally via NE-mediated α1-receptor activation, potentially making astrocyte signaling an inherent player in global state transitions [27, 59, 60]. Thus, we need to consider astrocytes as NE mediators, when interpreting downstream-effects of LC activation. There are several phenomena that are necessary to consider and discuss with regards to this. First, neurons in LC and astrocytes display very different activity time courses: sensory stimuli elicits short-lasting LC bursting activity in vivo (less than a couple of 100 msec) followed by intervals of silence [61], whereas astrocytic Ca2+ responses rise and peak slowly (over the course of seconds). Therefore, one could speculate that NE is not solely responsible for astrocyte activation, but may permit the involvement of other sources of astrocyte activation. Second, it is a possibility that certain of the neuronal responses to NE might occur indirectly through astrocytes. Due to the slow time course of astrocytes, any immediate impact on neuronal responses would be expected to follow that time scale, or possibly create more long-term neuronal changes. This is interesting in context to a newly published study showing that astrocytic Ca2+ signals, mediated by fly analog to neuromodulators, are directly involved in the regulation of neuronal activity and sensory-driven behaviors in drosophila flies [62]. However, direct comparisons to rodent studies should be undertaken with caution, since there are large differences in the species-specific cell and transmitter characteristics.
In addition to NE-mediated astrocytic Ca2+ signaling, evidence for ACh-mediated astrocytic signaling also exists [63–67]. In anesthetized animals, stimulating the nucleus basalis of Meynert, a main source of cholinergic innervation to the cortex, triggered pronounced astrocytic Ca2+ signaling in sensory cortex, which was blocked by a muscarinic cholinergic receptor antagonist [63]. Complementing work was done in the visual cortex in vivo, where stimulation of the nucleus basalis of Meynert in anesthetized mice resulted in astrocytic Ca2+ signals that were mediated by direct activation of cholinergic muscarinic receptors on astrocytes [64]. Further work implicating ACh in astrocytic Ca2+ signaling has been performed in hippocampal slices showing that astrocytes respond to cholinergic muscarinic receptor stimulation with Ca2+ signaling [65, 67]. Most recently, experiments performed in vivo and ex vivo showed that hippocampal hillar astrocytes respond to optogenetical stimulation of septohippocampal fibers with Ca2+ signaling, and this was blocked with nicotonic ACh receptor antagonists [66]. Interestingly, stimulation of the nucleus basalis of Meynert elicited astrocytic Ca2+ responses that were persistent throughout the stimulation [63], whereas astrocytic Ca2+ responses induced by whisker stimulation returned to baseline even during stimulation [26] indicating that the origin of stimulation elicit responses mediated by different transmitter systems.
It is important to note, that the noradrenergic and cholinergic neuromodulators are not two separated signaling systems. They both play a significant role in arousal and vigilance and the signaling systems in the awake state are closely linked through reciprocally presynaptic modulation of transmitter release: NE inhibits the release of ACh through expression of inhibitory Gi-coupled α2-noradrenergic receptors on cholinergic axon terminals [68, 69], and ACh facilitates Ca2+-dependent NE release through activation of Gq-coupled mAChRs [70]. Taken together, a substantial amount of work points to astrocytes as a target of noradrenergic and cholinergic transmission, leading to broad global astrocytic Ca2+ signaling positioning them optimally for contributing to global brain state changes in the awake brain.
Are Astrocytes involved in Brain State Changes?
Whether astrocytes causally participate in the transition between quiet wakefulness and the active behaving state, associated with improved sensory processing [4, 12, 15, 18], is currently unresolved. Their strong and faithful activation by NE and ACh might indicate that they very well could be, but conflicting data also exist. The few studies in the awake behaving mouse that have investigated the involvement of astrocytes in brain state shifts have focused on the transition between still to a moving state [27, 71, 72], a behavioral transition that is also associated with cortical neuronal state changes [3, 4, 12, 15, 17, 18]. Dombeck et al. (2007) [71] and Nimmerjahn et al. (2009) [72] used a chemical Ca2+ indicator in combination with the astrocyte-specific fluorescent indicator, sulforhodamine 101, to distinguish between neuronal and astrocytic Ca2+ signals, whereas Paukert et al. (2014) [27] used a genetically encoded Ca2+ indicator specifically expressed in astrocytes. The use of genetically encoded Ca2+ indicators might be preferred over chemical indicators because it has been shown that sulforhodamine 101 in higher dosages can induce abnormal cortical neuronal activity in both anesthetized and awake mice [73]. Dombeck et al. (2007) reported that in layer 2/3 of sensory cortex, only 11% of astrocytes show strong Ca2+ signaling correlation to spontaneous locomotion activity, whereas 60% showed little correlation. In contrast, in cerebellar Bergmann glia, Nimmerjahn et al. (2009) found consistent increases in astrocytic Ca2+ levels with every spontaneous locomotor episode. However, a later study by Paukert et al. (2014) found that only around 30% of Bergmann glia responded to spontaneous locomotor episodes, confirming the observation from sensory cortex. Thus, it seems like spontaneous locomotion in mice does not trigger reliable activation of Ca2+ signaling in astrocytes. However, neither of these experiment did concomitantly record pupil dilations, often used as an “online” correlative readout of LC-mediated noradrenergic activity [3, 6, 17]. Thus, whether LC firing and noradrenergic signaling was co-occurring consistently together with locomotion onset in these experiments is unknown. The lack of correlation between astrocytic Ca2+ signaling and locomotion onset might potentially be explained be a lag of correlation between LC firing and locomotion onset in these behavioral contexts.
These three studies all show that astrocytic Ca2+ signals develop ~1–2 seconds following spontaneous locomotion onset [27, 71, 72], whereas neuronal activity pattern changes precede locomotion onset [3, 6, 12]. Hence, these data suggests that global astrocytic Ca2+ signaling is not required for initiation of awake cortical state shifts, but instead mainly depends on neuronal activity. Evidence suggests that neuronal activity also plays a role in the activation of locomotor-induced astrocyte Ca2+ activity: Nimmerjahn et al. (2009) showed that the locomotor-induced astrocytic Ca2+ transients in Bergmann glia were abolished after blocking neural activity [72], whereas Paukert et al. (2014) showed that AMPA and NMDA receptor antagonists attenuated, but did not completely block the locomotor-induced astrocytic Ca2+ responses [27]. This might suggest that glutamatergic and noradrenergic act synergistically to promote astrocytic Ca2+ activity.
Paukert et al. (2014) found that enforced locomotion on a treadmill, in contrast to spontaneous locomotion, elicited reliable astrocytic Ca2+ responses [27], which illustrates one potential caveat and concern regarding the behavioral contexts often used to trigger the global astrocytic noradrenergic-mediated Ca2+ signaling. Enforced locomotion is likely perceived as a startle-stimulation by the animal, similar to the astrocytic Ca2+ signals elicited by other types of startle-stimulation [59, 60]. Since spontaneous locomotor shifts do not reliable activate astrocytic Ca2+ fluctuations, one could speculate that the episodes leading to increased Ca2+ signaling involved some degree of startle, since initiating movement when head fixed might involve some discomfort and stress for the animal. One way to avoid this problem could be monitoring locomotor patterns employing a setup allowing real spontaneous state shifts, such as a floating ball [4, 71, 74].
Startle-stimulation is linked to NE release, and Paukert et al. (2014) showed that by pairing enforced locomotion, and thus global astrocytic NE-mediated signaling, with visual stimuli presentation, the astrocytes markedly enhanced their Ca2+ signaling in response to visual stimulation. In contrast, when mice were stationary, astrocytes did not seem to respond much to visual stimuli presentation in primary visual cortex. These results may suggest that startle-induced widespread noradrenergic signaling shifts the gain of neocortical astrocytic networks, allowing the astrocytes to respond to local sensory-evoked neuronal activity [27]. However, the study did not provide data documenting that visual stimulus alone in the absence of forced locomotion induced astrocytic Ca2+ signaling. Most importantly, it remains to be defined whether astrocytic Ca2+ signaling is required for enhancing the gain of primary visual cortex neurons during spontaneous locomotion as has been observed [4, 12, 15, 18].
Recent work in primary auditory cortex, and earlier concepts in humans, has shown that states of hyper-arousal are associated with reduced sensory discrimination and diminished cognitive abilities [3, 29, 75]. Thus, one might speculate if the global astrocytic startle-driven Ca2+ response is associated with improved or decreased (fight-or-flight response) cortical cognitive functions. Just as beneficial as short-lasting LC burst activation is for attention and arousal, just as harmful is chronic activation; stress is strongly linked to altered LC functionality [76–81] and can lead to depression or posttraumatic stress disorder [82, 83]. Thus, alterations in LC firing and NE release may cause these pathophysiological changes through astrocyte activation. In an animal model of epilepsy, hyper-synchronous neuronal activity depended on astrocyte activity, and treatment with antiepileptic drugs reduced astrocytic Ca2+ signaling, indicating that astrocyte activation might underlie dysfunctional neuronal circuit activity [84]. It is known that during states of attention, LC-mediated NE amplifies behavioral relevant information [85–87]. Thus, it could be interesting to evaluate the role of astrocytic Ca2+ signals in brain state shifts involving a transfer from passive to active attention performance. In order to delineate the function of astrocytes in more subtle cognitive brain state changes, it would be essential to have an experimental set-up that does not involve startle or stress-triggering components and that depends on active decision-making of the animal. An interesting recently accepted publication showed that in brain slices, wash-in of subthreshold NE sensitized α1-adrenergic receptor-dependent astrocytic Ca2+ responses to local short-lasting exposure to concentrated NE [88]. The increased responsiveness of astrocytes to low concentration of NE was mediated by activation of β-adrenergic receptors. Thus, tonic LC firing, resulting in low NE levels, may prime astrocytes to LC burst activity, leading to shorter-lasting but higher peak NE levels [89].
Future experiments should aim to answer the causal and mechanistic association between astrocytic noradrenergic Ca2+ signaling and changes in awake brain states and the consequent effect on cortical sensory information processing. Currently, the up- and downstream roles of astrocytes in brain state shifts remains unsolved. Downstream effects of astrocyte activation could involve maintaining and stabilizing the ongoing current brain state, as well as involvement in mediating down-stream priming of the neural networks during the active behaving state (Figure 3).
Fig. 3. Graphical summary of hypothesis.
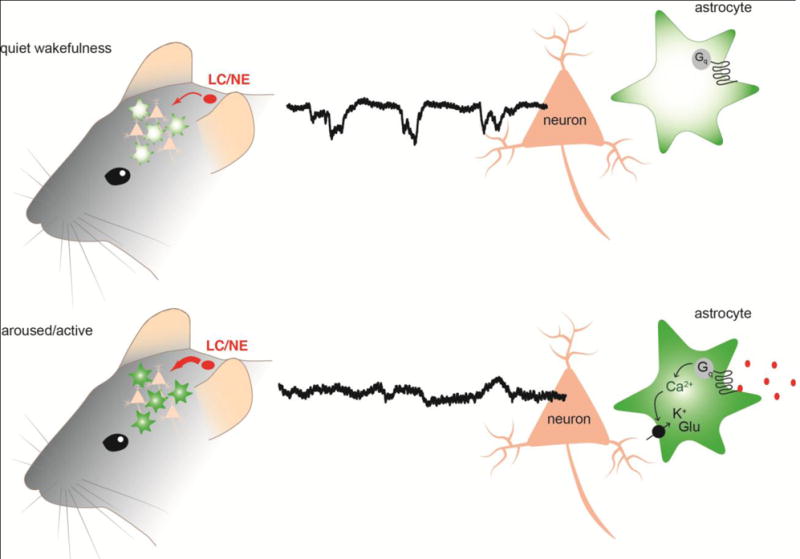
Top panel: during quiet wakefulness, the cortical noradrenergic and cholinergic pathways release low levels of neuromodulators, and astrocytes display transient asynchronous Ca2+ fluctuations within their processes. Bottom panel: during active brain states, there is increased cortical noradrenaline (NE) and acetylcholine (ACh) release. This activates Gq-coupled receptors located on astrocytes and leads to global astrocytic Ca2+ signaling. These Ca2+ level increases in astrocytes could lead to increased uptake of K+ and glutamate (Glu) from the extracellular space into astrocytes thereby possibly regulating the excitability of neural circuits.
Down-stream effects of astrocyte activation
One of the central questions in the field of astrocyte research is, what are the down-stream effects of astrocytic Ca2+ signaling? And how does this, if it does, contribute to cortical circuit processing? Astrocytes are globally distributed within the brain and in areas containing a high density of dendrites and axons, like the cerebral cortex, the number of astrocytes exceeds that of neurons [20, 21] providing a near-complete cortical coverage [90–92]. Thus, astrocytes are perfectly situated to mediate brain-wide activity changes, but at present, we still do not understand the down-stream consequences of astrocyte activation on neuronal synapses, circuits, and behaviors.
A wealth of down-stream functions of astrocytic Ca2+ activation have been presented during the past two decades including modulation of synaptic strength through release of so-called gliotransmitters such as glutamate, ATP, adenosine, or D-serine, which are mainly based on slice preparations from immature animals. These results are extensively covered in other reviews such as Haydon and Nedergaard, 2014 [93].
Studies suggest that interactions between astrocytic processes and synapses correlate with synaptic strength; synapses closely enwrapped with astrocytic processes appear larger compared to those without [94] and the motility of astrocytic processes mediated by intracellular Ca2+ fluctuations increases in response to sensory and synaptic activity, and primes the astrocytic coverage and general structural organization of synapses [95–97]. Furthermore, stimuli that elicits long-term potentiation of neuronal responses also increased motility of astrocytic processes [96, 98]. One study explored the consequences of increasing the proximity of astrocytic processes to synapses, and found that the reduced distance between hippocampal synapses and astrocytic glutamate transporters reduced the availability of glutamate thereby compromising AMPA-mediated long term potentiation and thereby generation of fear-memory [99]. Several other studies have previously established this link between astrocytic glutamate transporter expression and regulation of neuronal function [100, 101].
In contrast to glutamate, the extracellular level of GABA neurotransmitter was enhanced following astrocytic Ca2+ signaling due to reduced functional expression of GABA transporters on astrocytes [102]. Thus astrocytes seem to perform differential regulation of extracellular excitatory and inhibitory neurotransmitters indicating a role for astrocytes in the regulation of neuronal network state.
Another possible way for astrocytes to modulate neuronal networks is through modulation of extracellular K+ levels. Increased extracellular K+ increases the membrane potential of neurons enhancing the neuronal network excitation [103, 104]. Astrocytes have ben shown to be responsible for K+ buffering: astrocytic Ca2+ signaling activates the astrocytic Na+/K+ ATPase resulting in uptake of K+ and lowering of extracellular K+ concentration [105–108] (Figure 3). This astrocytic Ca2+-regulated K+ uptake reduced the frequency of excitatory input to neurons, while reducing synaptic failures, indicating that Ca2+ signaling in astrocytes might increase the signal-to-noise ratio of synaptic processing [104]. Another way K+ is cleared from the extracellular environment is via a co-transport system transporting Na+ and K+ ions in conjugation with Cl− ions into the astrocytes, and occurs in astrocytes following astrocytic Ca2+ influx caused by K+-mediated depolarization [109, 110].
Glycogen is important for energy production, and decomposition of glycogen has been shown to deliver energy for astrocytic K+ uptake [111, 112]. In brain, glycogen is stored mainly in astrocytes [113]. Astrocyte Ca2+ increases activates enzymes necessary for glycogenolysis, and Ca2+ influx into astrocytes may be driven by astrocytic K+ uptake [114]. In addition, astrocytic morphological remodeling has been shown to depend on activation of noradrenergic β-receptors [115–117] likely through activation of astrocytic cAMP-pathways, which initiates rapid degradation of glycogen for energy metabolism [118, 119]. In addition, intracellular increases in Ca2+ levels lead to mitochondrial ATP production across all cell types (reviewed in [120, 121]). Thus, NE-mediated astrocytic Ca2+ responses might prepare for an increased demand for K+ and glutamate buffering, which would be expected when shifting to an active brain state associated with desynchronization.
Tools for delineating down-stream effects of astrocyte activation
In order to assess the functional role of astrocyte activation on brain state shifts and the involved neuronal network activity, it is critical to be able to interfere with astrocyte Ca2+ activity in awake animals without affecting neuronal activity. However, specific modulation of astrocyte activity has proven difficult due to the fact that astrocytic Ca2+ fluctuations are mediated almost exclusively by G-protein coupled receptors that are not selectively expressed on astrocytes limiting the use of pharmacological approaches. Here we propose certain approaches that could prove useful for modulating astrocytic Ca2+ levels or G-protein coupled receptor activity.
For direct modulation of the intracellular concentration of Ca2+ in astrocytes, several groups has employed ultra-violet light flash photolysis, in which astrocytes are specifically loaded with caged Ca2+ or IP3 [122, 123]. However, for interrogation of circuit changes underlying brain state changes, a large number of astrocytes need to be modulated simultaneously making this approach insufficient. In addition, photolysis of caged Ca2+, but not physiological receptor-mediated activation of the Ca2+ signal, has been shown to trigger glutamate release, suggesting that Ca2+ uncaging triggers non-physiological effects [123].
A possible solution to modulating astrocyte activity on a global scale came with the development of genetically encoded modulators [124]. Designer receptors exclusively activated by designer drugs (DREADDs) are engineered G-protein coupled receptors that respond to a biological inert molecule, clozapine-N-oxide (CNO) [125, 126] and specific genetic targeting allows expression in selective cell populations such as astrocytes. Since neuromodulators enhance astrocytic Ca2+ activity through Gq-protein coupled receptors, this approach resembles the endogenous physiological response of astrocytes to aroused brain states. However, since DREADDs are activated by the systemically administered drug, CNO, there is limited spatial and temporal control over DREADD activation, which may lead to non-physiological high amplitudes, long-lasting Ca2+ increases within the stimulated cells. Activation of Gq-coupled DREADDs expressed brain-wide in astrocytes elicited pronounced phenotypic effects such as increases in blood pressure, heart rate, and saliva production, while reducing body temperature and increasing sedation in the presence of a GABA receptor agonist [124]. It remains to be determined whether these observations are physiological effects of peripheral or brain-wide astrocyte activity or might reflect some degree of pathological Ca2+ signaling in astrocytes.
Optogenetics is based on light-induced activation of ion fluxes with high temporal control, which is a very powerful tool for modulating neuronal activity, but since changes in voltage across the membrane does not reflect the primary G-protein coupled receptor mediated activation of astrocytes, the physiological relevance of this approach in astrocytes can be discussed. However, a number of studies have used optogenetic modulation of astrocytes and was able to elicit light-induced increases in the concentration of astrocytic Ca2+ following expression of channelrhodopsin in astrocytes [127–129]. Interestingly, a study also reported increased astrocytic Ca2+ fluctuations following light-induced activation of the proton pump, Archaerhodopsin (Arch) that mediate an hyperpolarizing outward current of H+ (hyperpolarization) [25].
The field of chemo- and optogenetics target genetically defined cell populations by transgenic approaches. The transgene often employ a cell-specific promoter and within astrocyte research, the GFAP promoter has been commonly used to obtain specific expression in astrocytes. However, a recent study has shown that a construct placed under control of the GFAP promoter (dominant-negative domain of vesicular SNARE) resulted in neural as well astrocytic expression [130]. Thus, when employing transgenic approaches, careful assessment and control needs to be undertaken.
Gq-coupled receptor activation of astrocytes is believed to initiate Ca2+ fluctuations through activation of IP3 receptor regulated intracellular Ca2+ stores. Astrocytes express only one of three subtypes of the receptor, the IP3 receptor type 2 (IP3R2), and germline IP3R2 knockout mice display reduced Gq-mediated astrocytic Ca2+ fluctuations, however, with no apparent behavioral alterations or impact on synaptic plasticity (Agulhon et al., 2010; reviewed in Xie et al., 2015). This same lack of behavioral changes was observed in a conditional knockout of IP3R2 restricted to GFAP positive cells within the CNS [133] indicating that astrocytic Ca2+ fluctuations may not be important for behavioral function. However, studies have found that Ca2+ signaling remains in the IP3R2 knockout mice indicating that astrocytic Ca2+ signaling can occur independently of IP3R-mediated internal release [134, 135] possibly through transient receptor potential (TRP) channels located in the membrane [136]
Conditional knockout approaches can prove highly useful in the evaluation of astrocyte responses in brain state changes. Conditional knockouts are most commonly based on the Cre-lox system, and for temporal control of the conditional knockout, inducible Cre recombinase can be employed. Interestingly, an inducible and conditional G-protein (Gαq/Gα11) conditional knockout mouse strain [137] are available, and could be crossed with astrocyte-specific Cre recombinase mouse lines to selectively knockout Gq-coupled signaling pathways in astrocytes.
Imaging astrocyte Ca2+ activity during brain state shifts
Genetically encoded Ca2+ indicators (GECIs) have recently become a strong tool for visualizing astrocytic Ca2+ fluctuations with targeted delivery to both the membrane or cytosol of astrocytes [136] using viral delivery or the use of transgenic mice. This approach has been widely used for in vivo studies, where Ca2+ fluctuations in awake head-fixed mice are visualized through cranial windows using two-photon microscopy. In addition, the use of transgenic mouse strains having a global expression of GECIs allow for macroscopic low-resolution imaging of large brain regions perfect for monitoring global Ca2+ signals [138]. However, these approaches are largely limited to imaging of upper cortical layer, and in order to determine the global brain changes underlying brain state shifts, access to deeper brain regions involved in the circuit of interest is needed as well. Recent developments of more sophisticated red-shifted GECIs opens up for imaging in deeper layers due to the tissue-penetrating effect of infrared light [139]. In addition, red-shifted GECIs may allow less invasive imaging approaches such as transcranial imaging, which would be very valuable for longer-lasting behavioral studies of astrocytic Ca2+ signals. Other approaches to gain access to deeper brain regions through implanted imaging cannulas have emerged due to recent development of head-mounted miniaturized microscopes as well as fiber photometry [140–143]. This also allows investigation of freely moving animals in contrast to head-fixed animals skipping the need for unnatural fixation and subsequent increased stress levels of the animal that will likely affect the behavior of the animals.
Conclusion
In this review, we have strived to present and discuss the current literature on awake cortical state transitions in light of the potential involvement of astrocytes and their Ca2+ signaling. The awake brain state is transitioning between periods of quiet wakefulness and states of active behavior governed by neuromodulators such as NE and ACh. Astrocytes are ubiquitously in the brain and display widespread global Ca2+ signals in the awake behaving animal, when activated by NE or ACh. This links them as an inherent and important player for brain-wide neuronal state transitions, but we still do not understand their role in these circuitries. An exciting new literature have started to investigate astrocytic Ca2+ signaling patterns, when mice spontaneously transition into the active brain state, but the tools for determining the causative role of astrocytes have been lacking. Several new transgenic modulating and imaging tools have within the last years have pioneered the field of neuronal circuits and their causality for behavior. We propose that the astrocyte research field starts to develop and implement these techniques in order to delineate the role of astrocytes in brain state transitions and ultimately their causality in behavior.
Acknowledgments
Funding:
This study was funded by the Novo Nordisk Foundation, the Office of Naval Research/Department of the Navy, and NIH.
Footnotes
Conflict of Interest:
The authors declare that they have no conflict of interest.
References
- 1.Harris KD, Thiele A. Cortical state and attention. Nat Rev Neurosci. 2011;12:509–523. doi: 10.1038/nrn3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee S-H, Dan Y. Neuromodulation of Brain States. Neuron. 2012;76:209–222. doi: 10.1016/j.neuron.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McGinley MJ, David SV, McCormick DA. Cortical Membrane Potential Signature of Optimal States for Sensory Signal Detection. Neuron. 2015;87:179–192. doi: 10.1016/j.neuron.2015.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Niell CM, Stryker MP. Modulation of Visual Responses by Behavioral State in Mouse Visual Cortex. Neuron. 2010;65:472–479. doi: 10.1016/j.neuron.2010.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schneider DM, Nelson A, Mooney R. A synaptic and circuit basis for corollary discharge in the auditory cortex. Nature. 2014;513:189–194. doi: 10.1038/nature13724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vinck M, Batista-Brito R, Knoblich U, Cardin JA. Arousal and Locomotion Make Distinct Contributions to Cortical Activity Patterns and Visual Encoding. Neuron. 2015;86:740–754. doi: 10.1016/j.neuron.2015.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ding F, ODonnell J, Xu Q, et al. Changes in the composition of brain interstitial ions control the sleep-wake cycle. Science (80−) 2016;352:550–555. doi: 10.1126/science.aad4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee SH, Dan Y. Neuromodulation of Brain States. Neuron. 2012;76:109–222. doi: 10.1016/j.neuron.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCormick Da, Bal T. Sleep and arousal: thalamocortical mechanisms. Annu Rev Neurosci. 1997;20:185–215. doi: 10.1146/annurev.neuro.20.1.185. [DOI] [PubMed] [Google Scholar]
- 10.Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993;262:679–85. doi: 10.1126/science.8235588. [DOI] [PubMed] [Google Scholar]
- 11.Steriade M, Amzica F, Nuñez A. Cholinergic and noradrenergic modulation of the slow (approximately 0.3 Hz) oscillation in neocortical cells. J Neurophysiol. 1993;70:1385–400. doi: 10.1152/jn.1993.70.4.1385. [DOI] [PubMed] [Google Scholar]
- 12.Polack P-O, Friedman J, Golshani P. Cellular mechanisms of brain state–dependent gain modulation in visual cortex. Nat Neurosci. 2013;16:1331–1339. doi: 10.1038/nn.3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamashita T, Pala A, Pedrido L, et al. Membrane potential dynamics of neocortical projection neurons driving target-specific signals. Neuron. 2013;80:1477–90. doi: 10.1016/j.neuron.2013.10.059. [DOI] [PubMed] [Google Scholar]
- 14.Schiemann J, Puggioni P, Dacre J, et al. Cellular mechanisms underlying behavioral state-dependent bidirectional modulation of motor cortex output. Cell Rep. 2015;11:1319–30. doi: 10.1016/j.celrep.2015.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fu Y, Tucciarone JM, Espinosa JS, et al. A Cortical Circuit for Gain Control by Behavioral State. Cell. 2014;156:1139–1152. doi: 10.1016/j.cell.2014.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mineault PJ, Tring E, Trachtenberg JT, Ringach DL. Enhanced Spatial Resolution During Locomotion and Heightened Attention in Mouse Primary Visual Cortex. J Neurosci. 2016;36:6382–92. doi: 10.1523/JNEUROSCI.0430-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McGinley MJ, Vinck M, Reimer J, et al. Waking State: Rapid Variations Modulate Neural and Behavioral Responses. Neuron. 2015;87:1143–61. doi: 10.1016/j.neuron.2015.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bennett C, Arroyo S, Hestrin S. Subthreshold mechanisms underlying state-dependent modulation of visual responses. Neuron. 2013;80:350–357. doi: 10.1016/j.neuron.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hawkins A, Olszewski J. Glia/nerve cell index for cortex of the whale. Science. 1957;126:76–7. doi: 10.1126/science.126.3263.76. [DOI] [PubMed] [Google Scholar]
- 20.Herculano-Houzel S. The glia/neuron ratio: how it varies uniformly across brain structures and species and what that means for brain physiology and evolution. Glia. 2014;62:1377–91. doi: 10.1002/glia.22683. [DOI] [PubMed] [Google Scholar]
- 21.Khakh BS, Sofroniew MV. Diversity of astrocyte functions and phenotypes in neural circuits. Nat Neurosci. 2015;18:942–952. doi: 10.1038/nn.4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amzica F, Neckelmann DAG. Membrane Capacitance of Cortical Neurons and Glia During Sleep Oscillations and Spike-Wave Seizures. J Neurophysiol. 1999;82:2731–2746. doi: 10.1152/jn.1999.82.5.2731. [DOI] [PubMed] [Google Scholar]
- 23.Amzica F. In vivo electrophysiological evidences for cortical neuron – glia interactions during slow (< 1 Hz) and paroxysmal sleep oscillations. J Physiol. 2002;96:209–219. doi: 10.1016/s0928-4257(02)00008-6. [DOI] [PubMed] [Google Scholar]
- 24.Poskanzer KE, Yuste R. Astrocytic regulation of cortical UP states. Proc Natl Acad Sci. 2011;108:18453–18458. doi: 10.1073/pnas.1112378108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poskanzer KE, Yuste R. Astrocytes regulate cortical state switching in vivo. Proc Natl Acad Sci U S A. 2016;113:E2675–84. doi: 10.1073/pnas.1520759113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang X, Lou N, Xu Q, et al. Astrocytic Ca2+ signaling evoked by sensory stimulation in vivo. Nat Neurosci. 2006;9:816–823. doi: 10.1038/nn1703. [DOI] [PubMed] [Google Scholar]
- 27.Paukert M, Agarwal A, Cha J, et al. Norepinephrine controls astroglial responsiveness to local circuit activity. Neuron. 2014;82:1263–70. doi: 10.1016/j.neuron.2014.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eggermann E, Kremer Y, Crochet S, Petersen CCH. Cholinergic Signals in Mouse Barrel Cortex during Active Whisker Sensing. Cell Rep. 2014;9:1654–1661. doi: 10.1016/j.celrep.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 29.Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci. 2005;28:403–50. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- 30.Mircea S, Robert MW. Brain control of Wakefulness and sleep. Vasa. 2005 doi: 10.1007/b102230. [DOI] [Google Scholar]
- 31.Armstrong-James M, Fox K. Effects of ionophoresed noradrenaline on the spontaneous activity of neurones in rat primary somatosensory cortex. J Physiol. 1983;335:427–447. doi: 10.1113/jphysiol.1983.sp014542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Videen TO, Daw NW, Rader RK. The effect of norepinephrine on visual cortical neurons in kittens and adult cats. J Neurosci. 1984;4:1607–17. doi: 10.1523/JNEUROSCI.04-06-01607.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bevan P, Bradshaw CM, Szabadi E. The pharmacology of adrenergic neuronal responses in the cerebral cortex: evidence for excitatory alpha- and inhibitory beta-receptors. Br J Pharmacol. 1977;59:635–641. doi: 10.1111/j.1476-5381.1977.tb07732.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bergles DE, Doze Va, Madison DV, Smith SJ. Excitatory actions of norepinephrine on multiple classes of hippocampal CA1 interneurons. J Neurosci. 1996;16:572–585. doi: 10.1523/JNEUROSCI.16-02-00572.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCormick DA. Neurotransmitter actions in the thalamus and cerebral cortex and their role in neuromodulation of thalamocortical activity. Prog Neurobiol. 1992;39:337–388. doi: 10.1016/0301-0082(92)90012-4. [DOI] [PubMed] [Google Scholar]
- 36.McCormick DA. Cellular mechanisms underlying cholinergic and noradrenergic modulation of neuronal firing mode in the cat and guinea pig dorsal lateral geniculate nucleus. J Neurosci. 1992;12:278–89. doi: 10.1523/JNEUROSCI.12-01-00278.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Madison DV, Nicoll RA. Cyclic adenosine 3′,5′-monophosphate mediates beta-receptor actions of noradrenaline in rat hippocampal pyramidal cells. J Physiol. 1986;372:245–259. doi: 10.1113/jphysiol.1986.sp016007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Madison DV, Nicoll RA. Actions of noradrenaline recorded intracellularly in rat hippocampal CA1 pyramidal neurones, in vitro. J Physiol. 1986;372:221–44. doi: 10.1113/jphysiol.1986.sp016006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McCormick DA, Prince DA. Actions of acetylcholine in the guinea-pig and cat medial and lateral geniculate nuclei, in vitro. J Physiol. 1987;392:147–65. doi: 10.1113/jphysiol.1987.sp016774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McCormick DA. Cholinergic and noradrenergic modulation of thalamocortical processing. Trends Neurosci. 1989;12:215–221. doi: 10.1016/0166-2236(89)90125-2. [DOI] [PubMed] [Google Scholar]
- 41.Sillito AM, Kemp JA, Berardi N. The cholinergic influence on the function of the cat dorsal lateral geniculate nucleus (dLGN) Brain Res. 1983;280:299–307. doi: 10.1016/0006-8993(83)90059-8. [DOI] [PubMed] [Google Scholar]
- 42.Krnjevjic K, Pumain R, Renaudt L. The Mechanism of Excitation By Acetylcholine in the Cerebral Cortex. J Physiol. 1971;215:247–268. doi: 10.1113/jphysiol.1971.sp009467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Constanti A, Galvan M. M-current in voltage-clamped olfactory cortex neurones. Neurosci Lett. 1983;39:65–70. doi: 10.1016/0304-3940(83)90166-0. [DOI] [PubMed] [Google Scholar]
- 44.Constanti A, Sim JA. Calcium-dependent potassium conductance in guinea-pig olfactory cortex neurones in vitro. J Physiol. 1987;387:173–194. doi: 10.1113/jphysiol.1987.sp016569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Madison DV, Lancaster B, Nicoll RA. Voltage clamp analysis of cholinergic action in the hippocampus. J Neurosci. 1987;7:733–741. doi: 10.1523/JNEUROSCI.07-03-00733.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McCormick DA, Williamson A. Convergence and divergence of neurotransmitter action in human cerebral cortex. Proc Natl Acad Sci U S A. 1989;86:8098–102. doi: 10.1073/pnas.86.20.8098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee S, Kruglikov I, Huang ZJ, et al. A disinhibitory circuit mediates motor integration in the somatosensory cortex. Nat Neurosci. 2013;16:1662–70. doi: 10.1038/nn.3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zagha E, McCormick DA. Neural control of brain state. Curr Opin Neurobiol. 2014;29:178–186. doi: 10.1016/j.conb.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Duffy S, MacVicar BA. Adrenergic calcium signaling in astrocyte networks within the hippocampal slice. J Neurosci. 1995;15:5535–50. doi: 10.1523/JNEUROSCI.15-08-05535.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bazargani N, Attwell D. Astrocyte calcium signaling: the third wave. Nat Neurosci. 2016;19:182–189. doi: 10.1038/nn.4201. [DOI] [PubMed] [Google Scholar]
- 51.Sun W, McConnell E, Pare J-F, et al. Glutamate -Dependent Neuroglial Calcium Signaling Differs Between Young and Adult Brain. Science (80−) 2013;339:197–200. doi: 10.1126/science.1226740.Glutamate-Dependent. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thrane AS, Rangroo Thrane V, Zeppenfeld D, et al. General anesthesia selectively disrupts astrocyte calcium signaling in the awake mouse cortex. Proc Natl Acad Sci U S A. 2012;109:18974–9. doi: 10.1073/pnas.1209448109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schummers J, Yu H, Sur M. Tuned responses of astrocytes and their influence on hemodynamic signals in the visual cortex. Science. 2008;320:1638–43. doi: 10.1126/science.1156120. [DOI] [PubMed] [Google Scholar]
- 54.Winship IR, Plaa N, Murphy TH. Rapid Astrocyte Calcium Signals Correlate with Neuronal Activity and Onset of the Hemodynamic Response In Vivo. J Neurosci. 2007;27 doi: 10.1523/JNEUROSCI.4801-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bekar LK, He W, Nedergaard M. Locus coeruleus alpha-adrenergic-mediated activation of cortical astrocytes in vivo. Cereb Cortex. 2008;18:2789–95. doi: 10.1093/cercor/bhn040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Duffy S, MacVicar B. Adrenergic calcium signaling in astrocyte networks within the hippocampal slice. J Neurosci. 1995;15 doi: 10.1523/JNEUROSCI.15-08-05535.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Salm AK, McCarthy KD. Norepinephrine-evoked calcium transients in cultured cerebral type 1 astroglia. Glia. 1990;3:529–38. doi: 10.1002/glia.440030612. [DOI] [PubMed] [Google Scholar]
- 58.Shao Y, McCarthy KD. Responses of Bergmann glia and granule neurons in situ to N-methyl-D-aspartate, norepinephrine, and high potassium. J Neurochem. 1997;68:2405–11. doi: 10.1046/j.1471-4159.1997.68062405.x. [DOI] [PubMed] [Google Scholar]
- 59.Srinivasan R, Huang BS, Venugopal S, et al. Ca2+ signaling in astrocytes from Ip3r2−/− mice in brain slices and during startle responses in vivo. Nat Neurosci. 2015 doi: 10.1038/nn.4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ding F, O’Donnell J, Thrane AS, et al. α1-Adrenergic receptors mediate coordinated Ca2+ signaling of cortical astrocytes in awake, behaving mice. Cell Calcium. 2013;54:387–94. doi: 10.1016/j.ceca.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Foote SL, Aston-Jones G, Bloom FE. Impulse activity of locus coeruleus neurons in awake rats and monkeys is a function of sensory stimulation and arousal. Proc Natl Acad Sci U S A. 1980;77:3033–7. doi: 10.1073/pnas.77.5.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ma Z, Stork T, Bergles DE, Freeman MR. Neuromodulators signal through astrocytes to alter neural circuit activity and behaviour. Nature. 2016;539:428–432. doi: 10.1038/nature20145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Takata N, Mishima T, Hisatsune C, et al. Astrocyte Calcium Signaling Transforms Cholinergic Modulation to Cortical Plasticity In Vivo. J Neurosci. 2011;31:18155–18165. doi: 10.1523/JNEUROSCI.5289-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen N, Sugihara H, Sharma J, et al. Nucleus basalis-enabled stimulus-specific plasticity in the visual cortex is mediated by astrocytes. Proc Natl Acad Sci. 2012;109:E2832–E2841. doi: 10.1073/pnas.1206557109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shelton MK, McCarthy KD. Hippocampal astrocytes exhibit Ca2+-elevating muscarinic cholinergic and histaminergic receptors in situ. J Neurochem. 2000;74:555–63. doi: 10.1046/j.1471-4159.2000.740555.x. [DOI] [PubMed] [Google Scholar]
- 66.Pabst M, Braganza O, Dannenberg H, et al. Astrocyte Intermediaries of Septal Cholinergic Modulation in the Hippocampus. Neuron. 2016;90:853–865. doi: 10.1016/j.neuron.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 67.Araque A, Martín ED, Perea G, et al. Synaptically released acetylcholine evokes Ca2+ elevations in astrocytes in hippocampal slices. J Neurosci. 2002;22:2443–50. doi: 10.1523/JNEUROSCI.22-07-02443.2002. doi: 20026212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tellez S, Colpaert F, Marien M. The alpha 2-adrenoceptor antagonist, (+)-efaroxan, enhances acetylcholine release in the rat cortex in vivo. Eur J Pharmacol. 1995;277:113–6. doi: 10.1016/0014-2999(95)00110-7. [DOI] [PubMed] [Google Scholar]
- 69.Aoki C, Go CG, Venkatesan C, Kurose H. Perikaryal and synaptic localization of alpha 2A-adrenergic receptor-like immunoreactivity. Brain Res. 1994;650:181–204. doi: 10.1016/0006-8993(94)91782-5. [DOI] [PubMed] [Google Scholar]
- 70.Rao TS, Correa LD, Adams P, et al. Pharmacological characterization of dopamine, norepinephrine and serotonin release in the rat prefrontal cortex by neuronal nicotinic acetylcholine receptor agonists. Brain Res. 2003;990:203–8. doi: 10.1016/s0006-8993(03)03532-7. [DOI] [PubMed] [Google Scholar]
- 71.Dombeck DA, Khabbaz AN, Collman F, et al. Imaging Large-Scale Neural Activity with Cellular Resolution in Awake, Mobile Mice. Neuron. 2007;56:43–57. doi: 10.1016/j.neuron.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nimmerjahn A, Mukamel EA, Schnitzer MJ. Motor Behavior Activates Bergmann Glial Networks. doi: 10.1016/j.neuron.2009.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rasmussen R, Nedergaard M, Petersen NC. Sulforhodamine 101, a widely used astrocyte marker, can induce cortical seizure-like activity at concentrations commonly used. Sci Rep. 2016;6:30433. doi: 10.1038/srep30433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dombeck DA, Graziano MS, Tank DW. Functional Clustering of Neurons in Motor Cortex Determined by Cellular Resolution Imaging in Awake Behaving Mice. J Neurosci. 2009;29:13751–13760. doi: 10.1523/JNEUROSCI.2985-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yerkes RM, Dodson JD. The relation of strength of stimulus to rapidity of habit-formation. J Comp Neurol Psychol. 1908;18:459–482. doi: 10.1037/h0073415. [DOI] [Google Scholar]
- 76.Chen F-J, Sara SJ. Locus coeruleus activation by foot shock or electrical stimulation inhibits amygdala neurons. Neuroscience. 2007;144:472–81. doi: 10.1016/j.neuroscience.2006.09.037. [DOI] [PubMed] [Google Scholar]
- 77.Nestler EJ, Alreja M, Aghajanian GK. Molecular control of locus coeruleus neurotransmission. Biol Psychiatry. 1999;46:1131–9. doi: 10.1016/s0006-3223(99)00158-4. [DOI] [PubMed] [Google Scholar]
- 78.Melia KR, Rasmussen K, Terwilliger RZ, et al. Coordinate regulation of the cyclic AMP system with firing rate and expression of tyrosine hydroxylase in the rat locus coeruleus: effects of chronic stress and drug treatments. J Neurochem. 1992;58:494–502. doi: 10.1111/j.1471-4159.1992.tb09748.x. [DOI] [PubMed] [Google Scholar]
- 79.Miner LH, Jedema HP, Moore FW, et al. Chronic stress increases the plasmalemmal distribution of the norepinephrine transporter and the coexpression of tyrosine hydroxylase in norepinephrine axons in the prefrontal cortex. J Neurosci. 2006;26:1571–8. doi: 10.1523/JNEUROSCI.4450-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fan Y, Chen P, Li Y, Zhu M-Y. Effects of chronic social defeat on expression of dopamine β-hydroxylase in rat brains. Synapse. 2013;67:300–12. doi: 10.1002/syn.21641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mana MJ, Grace AA. Chronic cold stress alters the basal and evoked electrophysiological activity of rat locus coeruleus neurons. Neuroscience. 1997;81:1055–64. doi: 10.1016/s0306-4522(97)00225-x. [DOI] [PubMed] [Google Scholar]
- 82.Weissman MM, Bland RC, Canino GJ, et al. Cross-national epidemiology of major depression and bipolar disorder. JAMA. 1996;276:293–9. [PubMed] [Google Scholar]
- 83.Breslau N. The epidemiology of trauma, PTSD, and other posttrauma disorders. Trauma Violence Abuse. 2009;10:198–210. doi: 10.1177/1524838009334448. [DOI] [PubMed] [Google Scholar]
- 84.Tian G-F, Azmi H, Takano T, et al. An astrocytic basis of epilepsy. Nat Med. 2005;11:973–81. doi: 10.1038/nm1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chandler DJ, Gao W-J, Waterhouse BD. Heterogeneous organization of the locus coeruleus projections to prefrontal and motor cortices. Proc Natl Acad Sci U S A. 2014;111:6816–21. doi: 10.1073/pnas.1320827111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bellesi M, Tononi G, Cirelli C, Serra PA. Region-Specific Dissociation between Cortical Noradrenaline Levels and the Sleep/Wake Cycle. Sleep. 2016;39:143–54. doi: 10.5665/sleep.5336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chandler DJ. Evidence for a specialized role of the locus coeruleus noradrenergic system in cortical circuitries and behavioral operations. Brain Res. 2015 doi: 10.1016/j.brainres.2015.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nuriya M, Takeuchi M, Yasui M. Background norepinephrine primes astrocytic calcium responses to subsequent norepinephrine stimuli in the cerebral cortex. Biochem Biophys Res Commun. 2016 doi: 10.1016/j.bbrc.2016.12.073. [DOI] [PubMed] [Google Scholar]
- 89.Devilbiss DM, Waterhouse BD. Phasic and tonic patterns of locus coeruleus output differentially modulate sensory network function in the awake rat. J Neurophysiol. 2011;105:69–87. doi: 10.1152/jn.00445.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Halassa MM, Fellin T, Takano H, et al. Synaptic islands defined by the territory of a single astrocyte. J Neurosci. 2007;27:6473–7. doi: 10.1523/JNEUROSCI.1419-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bushong EA, Martone ME, Jones YZ, Ellisman MH. Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. J Neurosci. 2002;22:183–92. doi: 10.1523/JNEUROSCI.22-01-00183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Oberheim NA, Tian GF, Han X, et al. Loss of astrocytic domain organization in the epileptic brain. J Neurosci. 2008;28:3264–3276. doi: 10.1523/JNEUROSCI.4980-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Haydon PG, Nedergaard M. How do astrocytes participate in neural plasticity? Cold Spring Harb Perspect Biol. 2014;7:a020438. doi: 10.1101/cshperspect.a020438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Witcher MR, Kirov SA, Harris KM. Plasticity of perisynaptic astroglia during synaptogenesis in the mature rat hippocampus. Glia. 2007;55:13–23. doi: 10.1002/glia.20415. [DOI] [PubMed] [Google Scholar]
- 95.Bernardinelli Y, Randall J, Janett E, et al. Activity-dependent structural plasticity of perisynaptic astrocytic domains promotes excitatory synapse stability. Curr Biol. 2014;24:1679–88. doi: 10.1016/j.cub.2014.06.025. [DOI] [PubMed] [Google Scholar]
- 96.Perez-Alvarez A, Navarrete M, Covelo A, et al. Structural and functional plasticity of astrocyte processes and dendritic spine interactions. J Neurosci. 2014;34:12738–44. doi: 10.1523/JNEUROSCI.2401-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Genoud C, Quairiaux C, Steiner P, et al. Plasticity of astrocytic coverage and glutamate transporter expression in adult mouse cortex. PLoS Biol. 2006;4:e343. doi: 10.1371/journal.pbio.0040343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lushnikova I, Skibo G, Muller D, Nikonenko I. Synaptic potentiation induces increased glial coverage of excitatory synapses in CA1 hippocampus. Hippocampus. 2009;19:753–62. doi: 10.1002/hipo.20551. [DOI] [PubMed] [Google Scholar]
- 99.Pannasch U, Freche D, Dallérac G, et al. Connexin 30 sets synaptic strength by controlling astroglial synapse invasion. Nat Neurosci. 2014;17:549–558. doi: 10.1038/nn.3662. [DOI] [PubMed] [Google Scholar]
- 100.Zeng X-N, Sun X-L, Gao L, et al. Aquaporin-4 deficiency down-regulates glutamate uptake and GLT-1 expression in astrocytes. Mol Cell Neurosci. 2007;34:34–9. doi: 10.1016/j.mcn.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 101.Li Y-K, Wang F, Wang W, et al. Aquaporin-4 deficiency impairs synaptic plasticity and associative fear memory in the lateral amygdala: involvement of downregulation of glutamate transporter-1 expression. Neuropsychopharmacology. 2012;37:1867–78. doi: 10.1038/npp.2012.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shigetomi E, Tong X, Kwan KY, et al. TRPA1 channels regulate astrocyte resting calcium and inhibitory synapse efficacy through GAT-3. Nat Neurosci. 2011;15:70–80. doi: 10.1038/nn.3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rangroo Thrane V, Thrane AS, Wang F, et al. Ammonia triggers neuronal disinhibition and seizures by impairing astrocyte potassium buffering. Nat Med. 2013;19:1643–8. doi: 10.1038/nm.3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang F, Smith NA, Xu Q, et al. Astrocytes modulate neural network activity by Ca2+-dependent uptake of extracellular K+ Sci Signal. 2012;5:ra26. doi: 10.1126/scisignal.2002334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wallraff A, Köhling R, Heinemann U, et al. The impact of astrocytic gap junctional coupling on potassium buffering in the hippocampus. J Neurosci. 2006;26:5438–47. doi: 10.1523/JNEUROSCI.0037-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Seifert G, Hüttmann K, Binder DK, et al. Analysis of astroglial K+ channel expression in the developing hippocampus reveals a predominant role of the Kir4.1 subunit. J Neurosci. 2009;29:7474–88. doi: 10.1523/JNEUROSCI.3790-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Larsen BR, Assentoft M, Cotrina ML, et al. Contributions of the Na+/K+-ATPase, NKCC1, and Kir4.1 to hippocampal K+ clearance and volume responses. Glia. 2014;62:608–22. doi: 10.1002/glia.22629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang F, Smith NA, Xu Q, et al. Astrocytes Modulate Neural Network Activity by Ca2+-Dependent Uptake of Extracellular K+ Sci Signal. 2012;5 doi: 10.1126/scisignal.2002334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tas PW, Massa PT, Kress HG, Koschel K. Characterization of an Na+/K+/Cl- co-transport in primary cultures of rat astrocytes. Biochim Biophys Acta. 1987;903:411–6. doi: 10.1016/0005-2736(87)90047-2. [DOI] [PubMed] [Google Scholar]
- 110.Tas PW, Massa PT, Koschel K. Preliminary characterization of an Na+,K+,Cl- co-transport activity in cultured human astrocytes. Neurosci Lett. 1986;70:369–73. doi: 10.1016/0304-3940(86)90581-1. [DOI] [PubMed] [Google Scholar]
- 111.Xu J, Song D, Xue Z, et al. Requirement of glycogenolysis for uptake of increased extracellular K+ in astrocytes: potential implications for K+ homeostasis and glycogen usage in brain. Neurochem Res. 2013;38:472–85. doi: 10.1007/s11064-012-0938-3. [DOI] [PubMed] [Google Scholar]
- 112.Choi HB, Gordon GRJ, Zhou N, et al. Metabolic communication between astrocytes and neurons via bicarbonate-responsive soluble adenylyl cyclase. Neuron. 2012;75:1094–104. doi: 10.1016/j.neuron.2012.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cataldo AM, Broadwell RD. Cytochemical identification of cerebral glycogen and glucose-6-phosphatase activity under normal and experimental conditions. II. Choroid plexus and ependymal epithelia, endothelia and pericytes. J Neurocytol. 1986;15:511–24. doi: 10.1007/BF01611733. [DOI] [PubMed] [Google Scholar]
- 114.Subbarao KV, Stolzenburg JU, Hertz L. Pharmacological characteristics of potassium-induced, glycogenolysis in astrocytes. Neurosci Lett. 1995;196:45–8. doi: 10.1016/0304-3940(95)11834-j. [DOI] [PubMed] [Google Scholar]
- 115.Vardjan N, Gabrijel M, Potokar M, et al. IFN-γ-induced increase in the mobility of MHC class II compartments in astrocytes depends on intermediate filaments. J Neuroinflammation. 2012;9:144. doi: 10.1186/1742-2094-9-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gharami K, Das S. Delayed but sustained induction of mitogen-activated protein kinase activity is associated with beta-adrenergic receptor-mediated morphological differentiation of astrocytes. J Neurochem. 2004;88:12–22. doi: 10.1046/j.1471-4159.2003.02148.x. [DOI] [PubMed] [Google Scholar]
- 117.Won CL, Oh YS. cAMP-induced stellation in primary astrocyte cultures with regional heterogeneity. Brain Res. 2000;887:250–8. doi: 10.1016/s0006-8993(00)02922-x. [DOI] [PubMed] [Google Scholar]
- 118.Prebil M, Vardjan N, Jensen J, et al. Dynamic monitoring of cytosolic glucose in single astrocytes. Glia. 2011;59:903–13. doi: 10.1002/glia.21161. [DOI] [PubMed] [Google Scholar]
- 119.Kreft M, Bak LK, Waagepetersen HS, Schousboe A. Aspects of astrocyte energy metabolism, amino acid neurotransmitter homoeostasis and metabolic compartmentation. ASN Neuro. 2012;4:187–199. doi: 10.1042/AN20120007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.McCormack JG, Halestrap AP, Denton RM. Role of calcium ions in regulation of mammalian intramitochondrial metabolism. Physiol Rev. 1990;70:391–425. doi: 10.1152/physrev.1990.70.2.391. [DOI] [PubMed] [Google Scholar]
- 121.Griffiths EJ, Rutter GA. Mitochondrial calcium as a key regulator of mitochondrial ATP production in mammalian cells. Biochim Biophys Acta - Bioenerg. 2009;1787:1324–1333. doi: 10.1016/j.bbabio.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 122.Fellin T, Pascual O, Gobbo S, et al. Neuronal synchrony mediated by astrocytic glutamate through activation of extrasynaptic NMDA receptors. Neuron. 2004;43:729–43. doi: 10.1016/j.neuron.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 123.Wang F, Smith NA, Xu Q, et al. Photolysis of caged Ca2+ but not receptor-mediated Ca2+ signaling triggers astrocytic glutamate release. J Neurosci. 2013;33:17404–12. doi: 10.1523/JNEUROSCI.2178-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Agulhon C, Boyt KM, Xie AX, et al. Modulation of the autonomic nervous system and behaviour by acute glial cell Gq protein-coupled receptor activation in vivo. J Physiol. 2013;591:5599–609. doi: 10.1113/jphysiol.2013.261289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Armbruster BN, Li X, Pausch MH, et al. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc Natl Acad Sci U S A. 2007;104:5163–8. doi: 10.1073/pnas.0700293104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhu H, Roth BL. DREADD: a chemogenetic GPCR signaling platform. Int J Neuropsychopharmacol. 2014 doi: 10.1093/ijnp/pyu007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gourine AV, Kasymov V, Marina N, et al. Astrocytes control breathing through pH-dependent release of ATP. Science. 2010;329:571–5. doi: 10.1126/science.1190721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Perea G, Yang A, Boyden ES, Sur M. Optogenetic astrocyte activation modulates response selectivity of visual cortex neurons in vivo. Nat Commun. 2014;5:3262. doi: 10.1038/ncomms4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yamashita A, Hamada A, Suhara Y, et al. Astrocytic activation in the anterior cingulate cortex is critical for sleep disorder under neuropathic pain. Synapse. 2014;68:235–47. doi: 10.1002/syn.21733. [DOI] [PubMed] [Google Scholar]
- 130.Fujita T, Chen MJ, Baoman Li X, et al. Cellular/Molecular Neuronal Transgene Expression in Dominant-Negative SNARE Mice. doi: 10.1523/JNEUROSCI.2585-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Xie AX, Petravicz J, McCarthy KD. Molecular approaches for manipulating astrocytic signaling in vivo. Front Cell Neurosci. 2015;9:144. doi: 10.3389/fncel.2015.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Agulhon C, Fiacco TA, McCarthy KD. Hippocampal short- and long-term plasticity are not modulated by astrocyte Ca2+ signaling. Science. 2010;327:1250–4. doi: 10.1126/science.1184821. [DOI] [PubMed] [Google Scholar]
- 133.Petravicz J, Boyt KM, McCarthy KD. Astrocyte IP3R2-dependent Ca2+ signaling is not a major modulator of neuronal pathways governing behavior. Front Behav Neurosci. 2014;8:384. doi: 10.3389/fnbeh.2014.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Haustein MD, Kracun S, Lu X-H, et al. Conditions and constraints for astrocyte calcium signaling in the hippocampal mossy fiber pathway. Neuron. 2014;82:413–29. doi: 10.1016/j.neuron.2014.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kanemaru K, Sekiya H, Xu M, et al. In vivo visualization of subtle, transient, and local activity of astrocytes using an ultrasensitive Ca(2+) indicator. Cell Rep. 2014;8:311–8. doi: 10.1016/j.celrep.2014.05.056. [DOI] [PubMed] [Google Scholar]
- 136.Shigetomi E, Bushong EA, Haustein MD, et al. Imaging calcium microdomains within entire astrocyte territories and endfeet with GCaMPs expressed using adeno-associated viruses. J Gen Physiol. 2013;141:633–47. doi: 10.1085/jgp.201210949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wettschureck N, Rütten H, Zywietz A, et al. Absence of pressure overload induced myocardial hypertrophy after conditional inactivation of Galphaq/Galpha11 in cardiomyocytes. Nat Med. 2001;7:1236–1240. doi: 10.1038/nm1101-1236. [DOI] [PubMed] [Google Scholar]
- 138.Monai H, Ohkura M, Tanaka M, et al. Calcium imaging reveals glial involvement in transcranial direct current stimulation-induced plasticity in mouse brain. Nat Commun. 2016;7:11100. doi: 10.1038/ncomms11100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Akerboom J, Carreras Calderón N, Tian L, et al. Genetically encoded calcium indicators for multi-color neural activity imaging and combination with optogenetics. Front Mol Neurosci. 2013;6:2. doi: 10.3389/fnmol.2013.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ghosh KK, Burns LD, Cocker ED, et al. Miniaturized integration of a fluorescence microscope. Nat Methods. 2011;8:871–878. doi: 10.1038/nmeth.1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gunaydin LA, Grosenick L, Finkelstein JC, et al. Natural neural projection dynamics underlying social behavior. Cell. 2014;157:1535–51. doi: 10.1016/j.cell.2014.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lerner TN, Shilyansky C, Davidson TJ, et al. Intact-Brain Analyses Reveal Distinct Information Carried by SNc Dopamine Subcircuits. Cell. 2015;162:635–647. doi: 10.1016/j.cell.2015.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sekiguchi KJ, Shekhtmeyster P, Merten K, et al. Imaging large-scale cellular activity in spinal cord of freely behaving mice. Nat Commun. 2016;7:11450. doi: 10.1038/ncomms11450. [DOI] [PMC free article] [PubMed] [Google Scholar]


