Abstract
Rationale
Hypothalamic-pituitary-adrenal (HPA) axis activity under different social settings in non-human primates is understudied.
Objective
Evaluate the response of pituitary-adrenal hormones (adrenocorticotropic hormone (ACTH) and cortisol) to pharmacological challenges of the HPA axis in male cynomolgus macaques under different social settings.
Methods
Male cynomolgus macaques (Macaca fascicularis, n=11) were individually (A) and socially housed (B) in alternation, over consecutive months, in an ABA design. During each experimental phase, plasma ACTH and cortisol were measured in response to low and mild intensity psychological stressors and following administration of saline, naloxone, ovine-corticotropin-releasing factor (oCRF) and dexamethasone.
Results
These data demonstrate that cortisol measured under low stress conditions is sensitive to social rank (dominance hierarchy) and distinguishes dominant from non-dominant animals during both individual and social settings. Administration of naloxone resulted in elevated circulating ACTH and cortisol, while oCRF only increased circulating cortisol. During social housing, the cortisol response to naloxone and oCRF was increased, whereas dexamethasone suppression of ACTH and cortisol remained consistent across all social settings.
Conclusions
Circulating ACTH and cortisol are differentially sensitive to changes in social settings in non-human primates. Cortisol response increased during social housing and could be stimulated by both naloxone and oCRF, whereas ACTH response was generally not influenced by social setting or oCRF but was increased by naloxone. These data show differential adrenal and pituitary response to changes in social settings and a small, but consistent, effect of social dominance.
Keywords: HPA axis, social rank, monkey, cortisol, ACTH
1. Introduction
Similar to humans (Foley & Gamble, 2009), non-human primates are highly social and form stable dominance hierarchies (de Waal, 1986, Abbott et al., 2003). These hierarchies regulate access to resources, which are unevenly distributed to benefit higher-ranking (dominant) individuals. Social rank within macaque hierarchies is inherited maternally, but depends on additional factors such as weight (Morgan et al., 2000) and dominance-related behaviors (Flack et al., 2004) in male monkeys. Dominance hierarchies in non-human primates provide an opportunity to study individual differences in hypothalamic-pituitary-adrenal (HPA) axis response as a function of social setting. Under normal homeostasis, HPA axis activation occurs in response to acute stress (injury, novelty, startle, etc.), resulting in release of corticotrophin-releasing factor (CRF) from the hypothalamic paraventricular nucleus (PVN). CRF then stimulates adrenocorticotrophic hormone (ACTH) release from the anterior pituitary, which acts on the adrenal cortex to stimulate cortisol release. The binding of cortisol to glucocorticoid receptors is a negative feedback signal at the hypothalamus and pituitary, which subsequently inhibits continued activation of the HPA axis. The integrity of the HPA axis in human and nonhuman primates can be evaluated by measuring sensitivity to the synthetic glucocorticoid dexamethasone to assess negative feedback, naloxone to assess opiate control of the PVN, and exogenous CRF to assess the pituitary responsiveness (Adinoff et al., 2005; Adinoff et al., 2010; Porcu et al., 2006).
Individual social rank has a strong impact on physiology and behavior. Socially subordinate monkeys receive limited resources and coping opportunities, and it has been suggested the subordinate status may be associated with greater stress than the dominant status (Shively et al., 1997; Henry & Stephens, 1977). This is supported by studies that have found social subordination in macaque monkeys are associated with heightened adrenocortical activity, with greater cortisol response to the exogenous administration of ACTH (females, Kaplan et al., 1986), and larger adrenal glands (Shively and Kaplan, 1984; Kaplan et al., 1986). However, the effect of social subordination on the HPA axis depends upon the stability of the social group and social setting (Sapolsky, 1992; Abbott et al., 2003). Recent studies in a natural setting suggest that the most dominant monkey may have the highest cortisol response in a stable troop, albeit in a changing environment (Gesquiere et al., 2011). In addition to social hierarchy effects on HPA activity, a number of early studies found greater adrenocortical activity during social housing of monkeys compared to when they were individually housed (Mason, 1959; Sassenrath, 1970; Leshner and Candland, 1972; Mendoza et al., 1978). Nevertheless, there are also reports of elevated cortisol amongst monkeys isolated from their social group (Lyons et al., 1999, Higley et al., 1992), suggesting that the relationship between HPA axis activity and social setting is dependent upon social rank and conditions to which the animal is acclimated (Suomi, 1976).
The present study evaluated the interaction between social rank and social setting on HPA axis response in male cynomolgus monkeys. To manipulate the social setting, we used an increasingly common method for forming social groups by changing the configuration of four individual housing units into a single social cage with four monkeys (Grant et al., 1998; Morgan et al. 2000, 2002; Czoty et al. 2009; Riddick et al., 2009). The current study expanded upon previous data by assessing HPA axis response to several pharmacological challenges within each social setting. We hypothesized that the HPA axis response would be greater among subordinate monkeys, particularly during social housing.
2. Materials and Methods
2.1 Animals
Experimentally naive male cynomolgus monkeys (n = 11, Macaca fascicularis, 50–62 months of age, 3.8–5.7 kg) served as subjects. After quarantine (2 months), they were relocated to the housing room where this study took place. Monkeys were housed in quadrant cages (0.8 m × 0.8 m × 0.9 m) with vertical and horizontal partitions that could be removed to form a single large cage for 4 monkeys (1.6 m × 0.8 m × 1.8 m). The room was maintained on a 12-hour light cycle (lights on at 700) at 20–22°C and 65% humidity. The monkeys were provided a nutritionally complete diet and were weighed weekly. The Wake Forest University IACUC approved all procedures involving animals, which were performed in accordance with the NIH and the Guide for the Care and Use of Laboratory Animals.
The monkeys were acclimated to the lab for 1 month then trained to participate in blood collection without the use of anesthesia (Porcu et al., 2006). All blood samples were collected from awake animals after an overnight fast. The study ran for 14 months, during which time the social setting was changed twice (Table 1), based on previous findings that primate adrenal activity changed from baseline after a minimum of two months in a new social environment (Goo and Sassenrath, 1980). During individual housing all monkeys had visual, auditory, and olfactory contact with conspecifics but the cage partitions were in place (see Morgan et al., 2000; Vivian et al. 2001). During social housing, the cage partitions were removed for 2 hours each morning allowing three groups of three or four monkeys full social contact. Previously, we reported that social housing had a lasting effect on elevated ACTH in the evening (i.e., nadir of the diurnal rhythm) (Helms et al. 2012b).
Table 1.
Housing conditions during challenges of the HPA axis.
| Housing | Cumulative months |
|---|---|
| Individual | 5 |
| Social | 10 |
| Individual | 14 |
We have published data collected from these subjects regarding different aspects of their experimental history, including an investigation of social rank, immune signaling molecules and ethanol self-administration (Grant et al., 2008; Helms et al., 2012a; Helms et al. 2012b). However, the ACTH and cortisol values reported here are distinct from the samples reported in earlier studies (i.e., samples were collected on different days, even when within the same experimental phase). Previously reported deoxycorticosterone response to naloxone and oCRF challenges are from the same plasma as the hormones reported here (Porcu et al., 2006). The social manipulations reported here occurred prior to ethanol self-administration studies.
2.2 Pharmacological challenges of the HPA
ACTH and cortisol were measured in response to psychological and pharmacological challenges of the HPA axis, as previously described (Porcu et al., 2006; Jimenez et al., 2015). All challenges were administered during each experimental phase (individual, social, and individual) (Table 1). As previously described (Porcu et al., 2006), animals were trained to participate in awake venipuncture under two conditions. In one condition the animals presented their leg through an opening in the front of the cage (low stress condition) and in a second condition the animals were removed from the home cage and seated in a primate restraint chair (mild stress condition). We have reported low levels of circulating stress hormones under the low stress condition (Helms et al., 2012b; Jimenez et al., 2015). Restraint in a primate chair has been shown to reliably increase circulating cortisol, even in the absence of behavioral signs of agitation (Ruys et al., 2004), consistent with its designation as a mild stressor. Exogenous ovine-CRF (1.0 μg/kg, intravenous) was administered to target the pituitary and adrenal glands. Naloxone, a nonspecific opioid receptor antagonist, was administered to target the hypothalamic paraventricular nucleus by blocking the opioid inhibition of CRF neurons (125 μg/kg or 375 μg/kg, intramuscular). Dexamethasone (0.13 mg/kg, intramuscular), a synthetic corticosteroid, was administered to assess negative feedback. Additionally, saline was administered (0.35ml, intramuscular) as a control condition. Blood (3 ml) was drawn from the femoral vein before and/or after drug administration according to Table 2.
Table 2.
Timing of blood samples for each endocrine challenge.
| Challenge | Blood sampling times (min) | |
|---|---|---|
| Pre-drug | Post-drug | |
| Saline | — | 15, 30, 60, 90, 120 |
| Naloxone (125 μg/kg) | — | 15, 30, 60, 90, 120 |
| Naloxone (375 μg/kg) | — | 15, 30, 60, 90, 120 |
| oCRF | −15 | 15, 30, 45, 60 |
| Dexamethasone | −840 | 600 |
—, none
2.3 Hormone assays
Blood samples were set on ice until centrifuged (3000 rpm, 15 min, 4°C) and stored at −80°C. Plasma samples were assayed for ACTH and cortisol at Yerkes Endocrine Core Laboratory as previously described (Helms et al. 2013).
2.4 Social Rank Classification
Social rank was determined as described by Helms et al. (2012b). Briefly, three trained observers rated social behaviors during the last week of the each housing condition (Table 1). Minutes spent initiating and receiving aggressions, submissions, grooming and sexual behaviors were recorded during 15-minute observations of each monkey, one observer per social group of 3–4 monkeys. Dyadic agonistic interactions were used to identify social rank, with the monkey the displayed the most aggressive conspecific behavior ranked as first-ranking (i.e. dominant). All monkeys except the first-ranking monkey submitted to the second-ranking monkey. Using this strategy, monkeys were ranked as dominant, submissive, or intermediate.
2.5 Statistical Analysis
All statistical tests were conducted as mixed effects models that varied by monkey, with fixed effects of housing condition (ABA design: individual, social, individual), dominance rank (dominant, intermediate and subordinate or dominant and non-dominant), stress condition (low, mild) and drug dose. All factors were checked for normal distribution using D’Agostino and Pearson omnibus normality test and log-transformed when necessary. The dependent variables were percent suppression by dexamethasone (calculated as a percentage of pre-dexamethasone hormone concentration), area under the curve (AUC, calculated using the trapezoid method) or peak (selected from post-drug timepoints) concentration of ACTH and cortisol over the time course of blood collection (Table 2). The saline challenge served as a control procedure for the naloxone and oCRF challenges and was entered into the model as an additional dose (0 μg/kg). Timepoints that were shared between the saline and the respective pharmacological challenge were used to calculate the AUC (i.e., 15-, 30-, 60-, 90- and 120-min samples were used for saline and naloxone comparisons while 15-, 30 -and 60-min samples were used for comparisons of saline and oCRF; Table 2). In general, the findings were highly consistent between AUC and peak, suggesting that these two variables provide similar information about the effect of housing condition and rank on circulating ACTH and cortisol. Results are reported for both peak and AUC while figures represent peak concentration. All interaction terms were initially included in all models and then dropped from the model if non-significant. Statistics were conducted using the NLME (Pinheiro et al., 2016) and multcomp (Hothorn et al., 2008) packages in R Statistical Computing software (version 3.1.2: R Core Team, 2015). Figures were created using Prism (GraphPad, version 6). Data are presented as mean ± standard deviation.
3. Results
3.1 ACTH and cortisol response to psychological stressors
Compared to the housing-cage (low stress) samples, both ACTH and cortisol were elevated in the primate chair, consistent with its designation as a mild stressor (main effect of stress condition: ACTH: F1,46 = 56.60, p < 0.0001; cortisol: F1,48 = 353.37, p < 0.0001; Figure 1). A main effect of social rank was found where cortisol was higher in dominant compared to intermediate and subordinate monkeys (F1,9 = 11.48, p = 0.008; Figure 1A), an effect that was only present under low stress conditions (rank by stress condition interaction: F1,48 = 34.94, p < 0.0001; p < 0.001). Cortisol was not different between intermediate and subordinate animals under these conditions (Figure 1A, right).
Fig 1. Low and mild stress.
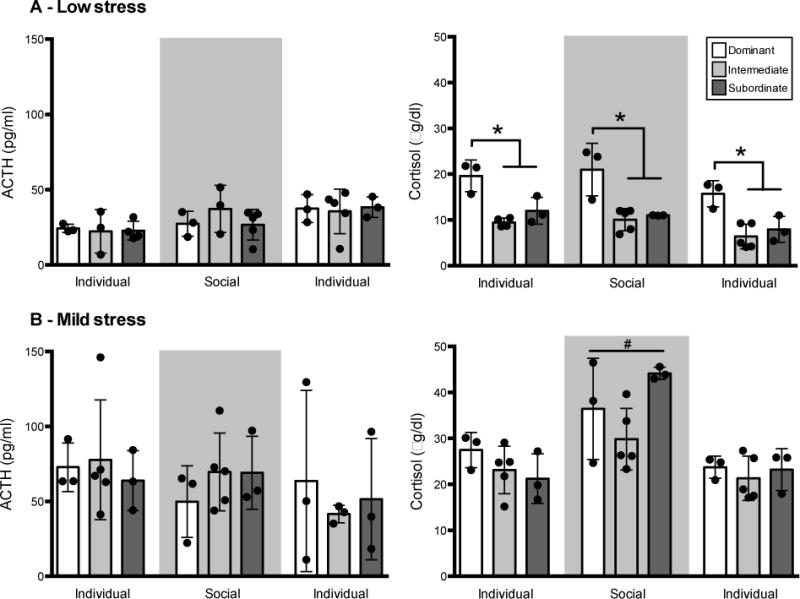
Peak (mean ± SD) ACTH (left) and cortisol (right) collected under low stress (home cage, A) and mild stress (primate chair, B) conditions. The mild stress condition reliably elevated both ACTH and cortisol for all animals across all social ranks and phases. Dominant animals had higher cortisol than intermediate and subordinate animals under the low stress condition (*: p < 0.05). Social housing potentiated the cortisol response to mild stress across all social ranks (#: p < 0.01).
Independent of social rank, there was a main effect of housing condition on cortisol (F2,48 = 26.90, p < 0.0001) and an interaction between housing and stress conditions (F1,48 = 6.11, p = 0.004), with the mild stress condition reliably associated with elevated circulating cortisol (p < 0.001). The effect of mild stress was amplified by the social housing compared to either individual housing phase (p < 0.01, Figure 1B, right). An interaction between housing and stress conditions was also found for ACTH (F2,46 = 6.98, p = 0.002) where the effect of the mild stress tended to decrease over the longitudinal design. This led to no difference in circulating ACTH between the two stress conditions during the final phase of individual housing. This analysis demonstrates a robust and stable distinction between dominant and non-dominant monkeys in basal circulating cortisol. Based on these data and support for differential regulation of the HPA in dominant animals (Kalin et al., 1981, Kaplan et al., 1986, Morgan et al., 2002), all subsequent analyses collapsed subordinate and intermediate animals into one non-dominant group (n = 8), and compared to dominant monkeys (n = 3).
3.2 Naloxone challenge
Two doses of naloxone (125 and 375 μg/kg, intramuscular) were tested on separate days, with 2–4 days between each dose. In general, naloxone robustly increased both ACTH and cortisol relative to saline (ACTH AUC: F2,74 = 32.79,p < 0.0001; ACTH peak: F2,79 = 29.04,p < 0.0001; cortisol AUC: F2,82 = 53.32,p < 0.0001; cortisol peak: F2,84 = 63.98,p < 0.0001). Post-hoc analyses revealed that peak ACTH was elevated after both doses of naloxone when compared to saline (125 μg/kg: p = 0.011; 375 μg/kg: p = 0.047; Figure 2), but there was no difference between the two doses of naloxone (p = 0.87). Similarly, both doses of naloxone increased peak cortisol relative to saline (p < 0.001), with a significantly higher peak cortisol measured following 375 μg/kg compared to 125 μg/kg naloxone (p = 0.030). The effect of naloxone on ACTH and cortisol was responsive to housing condition (ACTH AUC: F2,74 = 12.03,p < 0.0001; ACTH peak: F2,79 = 7.56,p = 0.001; cortisol AUC: F2,82 = 13.41,p < 0.0001; cortisol peak: F2,84 = 17.99, p < 0.0001; Figure 2B). Post-hoc analyses revealed that peak ACTH was lower in the final individual housing phase compared to social housing (p = 0.008) and the first individual phase (p = 0.001), however, this effect was largely due to an interaction between social settings and naloxone dose (0, 125 and 375 μg/kg) where the response to saline (0 μg/kg) decreased across the course of the experiment (ACTH AUC: F4,74 = 4.62, p = 0.002; ACTH peak: F4,79 = 2.37, p = 0.059). Peak cortisol was reliably higher during the social housing phase compared to either individual housing phases (p < 0.00001). No effect of social rank was found for peak or AUC response following administration of naloxone (p > 0.05).
Fig 2. Naloxone challenge.
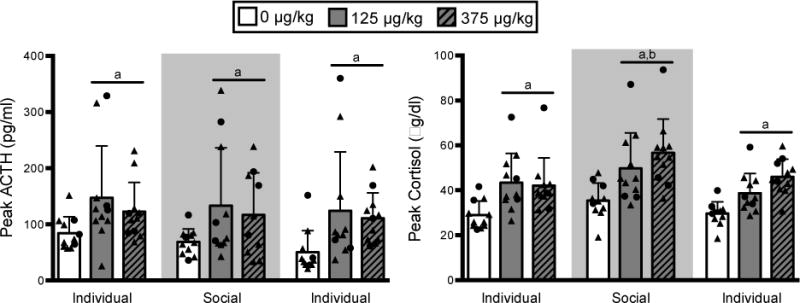
Peak (mean ± SD) ACTH (left) and cortisol (right) following saline (0 μg/kg) and naloxone (125 and 375 μg/kg) challenges. Both doses of naloxone increased ACTH and cortisol compared to saline (a: p < 0.05). The peak cortisol response to naloxone was greatest during the social housing phase (b: p < 0.05) when compared to both individual housed phases. Circles: dominant animals, triangles: non-dominant animals.
3.3 oCRF challenge
Exogenous oCRF (1.0 μg/kg, intravenous) produced elevations in cortisol (AUC: F1,52 = 39.00, p < 0.0001; peak: F1,52 = 33.01, p < 0.0001; Figure 3, right), and peak ACTH (F1,49 = 5.21, p = 0.03; Figure 3, left), but not ACTH AUC (p = 0.17). There was a main effect of housing condition on the cortisol response (AUC: F2,52 = 8.46, p = 0.0007; peak: F2,52 = 10.24, p = 0.0002), which was driven by an increased response during the social housing phase (p < 0.001, Figure 3). A main effect of housing condition on peak ACTH was also found (F2,49 = 14.38, p < 0.0001) where peak ACTH was lower in the final, compared to both the previous individual and social phases (p < 0.01). However, this effect was driven by a gradual decline in ACTH response to saline (0 μg/kg) over time as indicated by a dose by housing condition interaction (F2,49 = 3.68, p = 0.03). Social rank was not significantly related to ACTH or cortisol in response to oCRF (p > 0.05).
Fig 3. ovine-CRF challenge.
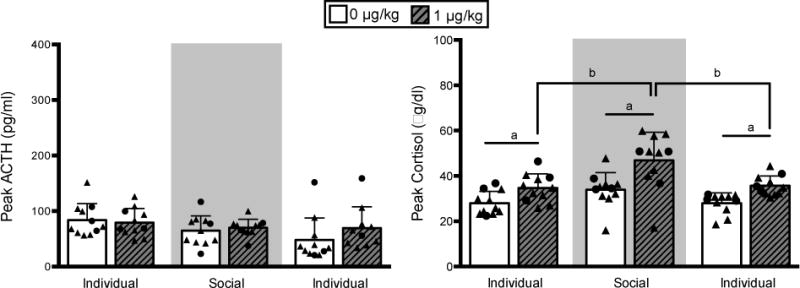
Peak (mean ± SD) ACTH (left) and cortisol (right) following saline (0 μg/kg) and ovine-CRF (1 μg/kg) challenge. Ovine-CRF increased cortisol compared to saline (a: p < 0.05). The peak cortisol response to ovine-CRF was greatest during the social housing phase (b: p < 0.001). Circles: dominant animals, triangles: non-dominant animals
3.4 Dexamethasone suppression
Overall, dexamethasone (0.13 mg/kg, intramuscular) reliably suppressed both ACTH and cortisol (Figure 4). There were no significant effects of housing condition or dominance rank on suppression of ACTH or cortisol following dexamethasone challenge (p > 0.05, Figure 4). However, during social housing there was an indication that dexamethasone was less effective in suppressing ACTH, although this did not reach statistical significance (p = 0.07).
Fig 4. Dexamethasone suppression.
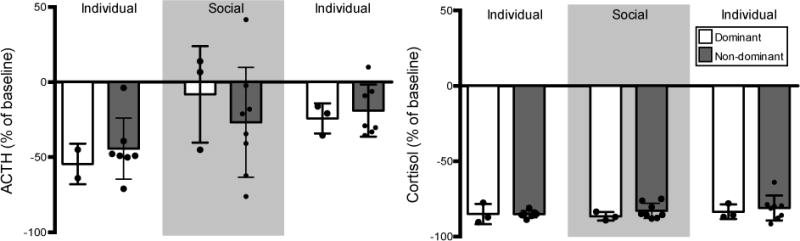
Percent of baseline (mean ± SD) ACTH (left) and cortisol (right) following dexamethasone administration (130 μg/kg) across housing conditions. Dexamethasone reliably suppressed both ACTH and cortisol across social ranks and during all housing conditions.
4. Discussion
Non-human primates have been used to investigate the relationship between dominance rank and the HPA axis for many years. Their complex social hierarchies and neuroendocrine physiology make them an ideal model system. However, regulation of the HPA axis is highly complex and reactive to changes in both the internal and external environmental changes. Thus, the experimental conditions of the current study (including the predictability of a nutritionally complete food supply, diurnal lighting, and limited variability of group and social structure) are critical to isolate the unique contribution of dominance hierarchy and social contact. Blood samples taken in the housing cage were significantly lower in ACTH and cortisol compared to samples collected while seated in the restraint chair (home cage averages below 20 μg/dl and 50 pg/ml, respectively). These data show the housing conditions in the laboratory reflect a low stress homeostatic condition. Additionally, the HPA axis response was evaluated without anesthesia in the current study, making the results comparable to only a small set of published data, but allowing us to highlight individual differences.
As expected, both cortisol and ACTH increased when the animals were temporarily removed from the housing cage and seated in a restraint chair (Figure 1). The magnitude of response was within the normal range and monkeys of all social ranks showed mildly elevated HPA axis activity consistent with increased arousal. The low-versus mild stress conditions (housing cage vs restraint chair) had a greater influence on cortisol response than either dominance rank or social setting, and interestingly, the combination of mild stress and social housing potentiated peak cortisol across all ranks. To further characterize this effect, pharmacological challenges were administered to target specific levels of the HPA axis.
As expected, both ACTH and cortisol concentrations increased in response to the naloxone and oCRF when compared to saline. In the absence of elevated pituitary secretion of ACTH, we observed amplification of the cortisol secretion during social housing in response to mild stress and pharmacological stimulation, independent of social rank (Figures 1B, 2 and 3). This suggests that 3–4 months of social housing resulted in increased sensitivity to ACTH. We hypothesize that the centrally-projecting autonomic parvocellular neurons in the PVN may contribute to this adaptation. Stressors that activate this distinct population of neurons will incorporate the sympathetic limb of the stress response, increasing epinephrine and norepinephrine from the adrenal medulla. Changes in social settings have been associated with higher circulating norepinephrine in cynomolgus males (Cohen et al., 1997) and male tree shrews (Fuchs et al., 1993). And in calves, splanchnic nerve stimulation, with physiologically relevant increases in adrenal-medullary release, can potentiate the glucocorticoid response to ACTH (Edwards and Jones, 1987). Future studies should incorporate measures of the sympathetic tone in order to better understand its role in regulating the stress response during social settings and as a function of dominance hierarchy.
It is important to consider the relatively high doses of naloxone used in the current study. In rhesus macaques, intravenous naltrexone increased cortisol and ACTH at doses higher than 32 and 100 μg/kg, respectively (Williams et al., 2003). The current results show the expected increases in peak and AUC following both 125 and 375 μg/kg naloxone with intramuscular administration. However, a significant adrenocortical dose response to naloxone was found in the absence of an ACTH dose response, which differs from Williams and colleagues (2003). It is difficult to directly compare these studies due to critical methodological differences including drug (naloxone/naltrexone), route of administration (intramuscular/intravenous), species (cynomolgus/rhesus macaques) and condition (seated in a primate chair or tethered in the home cage). Regardless, lower doses of naloxone may be necessary to reveal individual differences in naloxone sensitivity related to social rank or housing condition.
Our finding that dominant animals had higher cortisol under low stress conditions are in agreement with previous reports. In a comparable paradigm, dominant rhesus females had higher circulating cortisol during social housing, but the effect of social rank was lost when the animals were housed individually (Michopolous et al., 2012). Similarly, Czoty and colleagues (2009) found that upon entering a new social setting, subordinate male cynomolgus macaques had higher cortisol, but that after the hierarchy stabilized dominant animals had higher cortisol. In line with these earlier reports, we show that the effect of dominance rank was smaller than activation of the HPA axis due to restraint, changes in housing conditions and pharmacological challenge. In studies that report mixed effects of social rank on cortisol, it is important to note all the influences on cortisol concentration as a function of dominance hierarchy (reviewed in Abbott et al., 2003) including the use of ketamine sedation (Puri et al., 1981, Malaivijitnod et al., 1998, Hergovich et al., 2001, Bentson et al., 2003), sex, species and troop stability (Qin et al., 2013). Overall, it appears that in a laboratory environment unanesthetized dominant monkeys have higher morning cortisol. Because the subordinate monkeys respond to a similar degree to changing social settings, the lower basal state of the subordinate monkey reported here does not appear to be due to a hypo-functioning HPA axis.
The heightened HPA response in individually housed dominant monkeys (Figure 1A) may have functional significance for addiction models. We have reported in the same animals that dominant ranking individuals went on to self-administer less ethanol using an open-access paradigm (Helms et al., 2012b). This is in agreement with a large number of rodent studies showing that laboratory induced stress prior to the establishment of alcohol consumption may lower alcohol intake, whereas stress imposed after the establishment of alcohol consumption can heighten alcohol intake (Becker et al., 2011). Further, mifepristone, a glucocorticoid receptor (GR) antagonist has shown promising effects in treating alcohol use disorders and lowering alcohol consumption in rodents (Vendruscolo et al., 2015). The research presented here suggests that the efficacy of GR antagonists to decrease alcohol consumption in primates may be enhanced with knowledge of social rank as well as HPA response to social settings and reactivity to mild stress. Such an approach may allow improved personalized medication development for behavioral disorders that are comorbid with stress.
Overall, these data demonstrate that under stable laboratory conditions, the HPA axis reliably differs between dominant and non-dominant animals under low stress (housing cage). No differences were found between social ranks following activation of the HPA axis by a mild stressor (removal from housing into a restraint chair) or administration of pharmacological challenges. Social housing potentiated glucocorticoid secretion in response to mild stress and o-CRF challenge. The cortisol concentrations reported here, from awake animals trained to participate in blood collection, were within a healthy physiological range and are not suspected to have negative health consequences. Thus, the elevated cortisol response during social housing suggests a heightened state of vigilance in all animals that is maintained in dominant animals even when there is no direct physical contact (individual housing).
Acknowledgments
This work and preparation of the manuscript was supported by AA103510, the supplement to AA013510 and OD11092. The authors wish to thank Dr Christa Helms who provided initial analysis of the data.
References
- Abbott DH, Keverne EB, Bercovitch FB, Shively CA, Mendoza SP, Saltzman W, Snowdon CT, Ziegler TE, Banjevic M, Garland T, Sapolsky RM. Are subordinates always stressed? A comparative analysis of rank differences in cortisol levels among primates. Hormones & Behavior. 2003;43(1):67–82. doi: 10.1016/s0018-506x(02)00037-5. [DOI] [PubMed] [Google Scholar]
- Adinoff B, Krebaum SR, Chandler PA, Ye W, Brown MB, Williams MJ. Dissection of hypothalamic-pituitary-adrenal axis pathology in 1-month-abstinent alcohol-dependent men, part 2: response to ovine corticotropin-releasing factor and naloxone. ACER. 2005;29(4):528–537. doi: 10.1097/01.ALC.0000158939.25531.EE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adinoff B, Best SE, Ye W, Williams MJ, Iranmenesh A. Adrenocortical and pituitary glucocorticoid feedback in abstinent alcohol-dependent women. ACER. 2010;34(5):915–924. doi: 10.1111/j.1530-0277.2010.01164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker HC, Lopez MF, Doremus-Fitzwater TL. Effects of stress on alcohol drinking: a review of animal studies. Psychopharmacology (Berl) 2011;218(1):131–156. doi: 10.1007/s00213-011-2443-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentson KL, Capitanio JP, Mendoza SP. Cortisol response to immobilization with Telazol or ketamine in baboons (Papio cynocephalus/Anubis) and rhesus macaques (Macaca mulatta) J Med Primatol. 2003;32(3):148–160. doi: 10.1034/j.1600-0684.2003.00018.x. [DOI] [PubMed] [Google Scholar]
- Cohen S, Line S, Manuck SB, Rabin BS, Heise ER, Kaplan JR. Chronic social stress, social status and susceptibility to upper respiratory infections in nonhuman primates. Psychosomatic medicine. 1997;59(3):213–221. doi: 10.1097/00006842-199705000-00001. [DOI] [PubMed] [Google Scholar]
- Czoty PW, Gould RW, Nader MA. Relationship between social rank and cortisol and testosterone concentrations in male cynomolgus monkeys (Macaca fascicularis) J Neuroendocrinol. 2009;21(1):68–76. doi: 10.1111/j.1365-2826.2008.01800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Waal FB. The integration of dominance and social bonding in primates. Q Rev Biol. 1986;61(4):459–479. doi: 10.1086/415144. [DOI] [PubMed] [Google Scholar]
- Edwards AV, Jones CT. The effect of splanchnic nerve stimulation on adrenocortical activity in conscious calves. Journal of Physiology. 1987;382:385–396. doi: 10.1113/jphysiol.1987.sp016373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flack JC, Jeannotte LA, de Waal FB. Play signaling and the perception of social rules by juvenile chimpanzees (Pan troglodytes) J Comp Psychol. 2004;118(2):149–159. doi: 10.1037/0735-7036.118.2.149. [DOI] [PubMed] [Google Scholar]
- Foley R, Gamble C. The ecology of social transitions in human evolution. Philos Trans R Soc Lond B Biol Sci. 2009;364(1533):3267–3279. doi: 10.1098/rstb.2009.0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs E, Jöhren O, Flügge G. Psychosocial conflict in the tree shrew: effects on sympathoadrenal activity and blood pressure. Psychoneuroendocrinology. 1993;18(8):557–565. doi: 10.1016/0306-4530(93)90033-h. [DOI] [PubMed] [Google Scholar]
- Gesquiere LR, Onyango PO, Alberts SC, Altmann J. Endocrinology of year-round reproduction in a highly seasonal habitat: environmental variability in testosterone and glucocorticoids in baboon males. Am J Phys Anthropol. 2010;144(2):169–176. doi: 10.1002/ajpa.21374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goo GP, Sassenrath N. Persistent adrenocortical activation in female rhesus monkeys after new breeding groups formation. J Med Primatol. 1980;9(6):325–334. doi: 10.1159/000460162. [DOI] [PubMed] [Google Scholar]
- Grant KA, Shively CA, Nader MA, Ehrenkaufer RL, Line SW, Morton TE, Gage HD, Mach RH. Effect of social status on striatal dopamine D2 receptor binding characteristics in cynomolgus monkeys assessed with positron emission tomography. Synapse. 1998;29(1):80–83. doi: 10.1002/(SICI)1098-2396(199805)29:1<80::AID-SYN7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Grant KA, Leng X, Green HL, Szeliga KT, Rogers LSM, Gonzales SW. Drinking typography established by scheduled induction predicts chronic heavy drinking in a monkey model of ethanol self-administration. ACER. 2008;32(10):1824–1838. doi: 10.1111/j.1530-0277.2008.00765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms CM, Messaoudi I, Jeng S, Freeman WM, Vrana KE, Grant KA. A longitudinal analysis of circulating stress-related proteins and chronic ethanol self-administration in cynomolgus macaques. ACER. 2012a;36(6):995–1003. doi: 10.1111/j.1530-0277.2011.01685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms CM, McClintick MN, Grant KA. Social rank, chronic ethanol self-administration, and diurnal pituitary-adrenal activity in cynomolgus monkeys. Psychopharmacology. 2012b;224(1):133–143. doi: 10.1007/s00213-012-2707-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms CM, Gonzales SW, Green HL, Szeliga KT, Rogers LSM, Grant KA. Diurnal pituitary-adrenal activity during schedule-induced polydipsia of water and ethanol in cynomolgus monkeys (Macaca fascicularis) Psychopharmacology. 2013;228:541–549. doi: 10.1007/s00213-013-3052-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry JP, Stephens PM. Stress, health, and the social environment A sociobiological approach to medicine. New York: Springer Verlag; 1977. [Google Scholar]
- Hergovich N, Singer MD, Agneter E, Eichler GH, Graselli U, Simhandi C, Jilma B. Comparison of the effects of ketamine and memantine on prolactin and cortisol release in men: a randomized, double-blind, placebo-controlled trial. Neuropsychopharmacology. 2001;24:590–593. doi: 10.1016/S0893-133X(00)00194-9. [DOI] [PubMed] [Google Scholar]
- Higley JD, Suomi SJ, Linnoila M. A longitudinal assessment of CSF monoamine metabolite and plasma cortisol concentrations in young rhesus monkeys. Biol Psychiatry. 1992;32(2):127–145. doi: 10.1016/0006-3223(92)90016-s. [DOI] [PubMed] [Google Scholar]
- Hothorn T, Bretz F, Westfall P. Simultaneous Inference in General Parametric Models. Biometrical Journal. 2008;50(3):346–363. doi: 10.1002/bimj.200810425. [DOI] [PubMed] [Google Scholar]
- Jimenez VA, Helms CM, Cornea A, Meshul CK, Grant KA. An ultrastructural analysis of the effects of ethanol self-administration on the hypothalamic paraventricular nucleus in rhesus macaques. Frontiers Cellular Neuroscience. 2015;9:1–14. doi: 10.3389/fncel.2015.00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin NH, Cohen RM, Kraemer GW, Risch SC, Shelton S, Cohen M, McKinney WT, Murphy DL. The dexamethasone suppression test as a measure of hypothalamic-pituitary feedback sensitivity and its relationship to behavioral arousal. Neuroendocrinology. 1981;32(2):92–95. doi: 10.1159/000123137. [DOI] [PubMed] [Google Scholar]
- Kaplan JR, Adams MR, Koritnik DR, Rose JC, Manuck SB. Adrenal responsiveness and social status in intact and ovariectomized Macaca fascicularis. Am J Primatol. 1986;11:181–193. doi: 10.1002/ajp.1350110209. [DOI] [PubMed] [Google Scholar]
- Leshner AI, Candland DK. Endocrine effects of grouping and dominance rank in squirrel monkeys. Physiol Behav. 1972;8(3):441–445. doi: 10.1016/0031-9384(72)90326-5. [DOI] [PubMed] [Google Scholar]
- Lyons DM, Martel FL, Levine S, Risch NJ, Schatzberg AF. Postnatal experiences and genetic effects on squirrel monkey social affinities and emotional distress. Hormones & Behavior. 1999;36(3):266–275. doi: 10.1006/hbeh.1999.1547. [DOI] [PubMed] [Google Scholar]
- Malaivijitnod S, Takenaka O, Sankai T, Yoshida T, Cho F, Yoshikawa Y. Effects of single and multiple injections of ketamine hydrochloride on serum hormone concentration in male cynomolgus monkeys. Lab Anim Sci. 1998;48(3):270–274. [PubMed] [Google Scholar]
- Mason JW. Psychological influences on the pituitary-adrenal cortical system. Recent progress in hormone research. 1959;15:345–389. [Google Scholar]
- Mendoza SP, Coe CL, Lowe EL, Levine S. The physiological response to group formation in adult male squirrel monkeys. Psychoneuroendocrinology. 1978;3(3):221–229. doi: 10.1016/0306-4530(78)90012-4. [DOI] [PubMed] [Google Scholar]
- Michopoulos V, Reding KM, Wilson M, Toufexis D. Social subordination impairs hypothalamic-pituitary-adrenal function in female rhesus monkeys. Hormones & Behavior. 2012;62(4):389–399. doi: 10.1016/j.yhbeh.2012.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D, Grant KA, Prioleau OA, Nader SH, Kaplan JR, Nader MA. Predictors of social status in cynomolgus monkeys (Macaca fascicularis) after group formation. Am J Primatol. 2000;52(3):115–131. doi: 10.1002/1098-2345(200011)52:3<115::AID-AJP1>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Morgan D, Grant KA, Gage HD, Mach RH, Kaplan JR, Prioleau O, Nader SH, Buchheimer N, Ehrenkaufer RL, Nader MA. Social dominance in monkeys: dopamine D2 receptors and cocaine self-administration. Nat Neurosci. 2002;5(2):169–174. doi: 10.1038/nn798. [DOI] [PubMed] [Google Scholar]
- Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Team nlme: Linear and Nonlinear Mixed Effects Models. R package version 3.1-124. 2016 <URL: http://CRAN.R-project.org/package=nlme.
- Porcu P, Grant KA, Green HL, Rogers LSM, Morrow AL. Hypothalamic-pituitary-adrenal axis and ethanol modulation of deoxycorticosterone levels in cynomolgus monkeys. Psychopharmacology. 2006;186:293–301. doi: 10.1007/s00213-005-0132-2. [DOI] [PubMed] [Google Scholar]
- Puri CP, Puri V, Anand Kumar TC. Serum levels of testosterone, cortisol, prolactin and bioactive luteinizing hormone in adult male rhesus monkeys following cage-restraint or anaesthetizing with ketamine hydrochloride. Acta Endocrinol. 1981;97:118–124. doi: 10.1530/acta.0.0970118. [DOI] [PubMed] [Google Scholar]
- Qin DD, Rizak JD, Feng XL, Chu XX, Yang SC, Li CL, Lu LB, Ma Y, Y Hu. Social rank and cortisol among female rhesus macaques (Macaca mulatta) Zoological Research. 2013;34(2):42–49. doi: 10.3724/SP.J.1141.2013.E02E42. [DOI] [PubMed] [Google Scholar]
- R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2015. URL http://www.R-project.org/ [Google Scholar]
- Riddick NV, Czoty PW, Gage HD, Kaplan JR, Nader SH, Icenhower M, Pierre PJ, Bennett A, Garg PK, Garg S, Nader MA. Behavioral and neurobiological characteristics influencing social hierarchy formation in female cynomolgus monkeys. Neuroscience. 2009;158(4):1257–1265. doi: 10.1016/j.neuroscience.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruys JD, Mendoza SP, Capitanio JP, Mason WA. Behavioral and physiological adaptation to repeated chair restraint in rhesus macaques. Physiology & Behavior. 2004;82:205–213. doi: 10.1016/j.phybeh.2004.02.031. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Cortisol concentrations and the social significance of rank instability among wild baboons. Psychoneuroendocrinology. 1992;17(6):701–709. doi: 10.1016/0306-4530(92)90029-7. [DOI] [PubMed] [Google Scholar]
- Sassenrath EN. Increased adrenal responsiveness to social stress in rhesus monkeys. Hormones & Behavior. 1970;1:283–298. [Google Scholar]
- Shively CA. Social subordinate stress, behavior, and central monoaminergic function in female cynomolgus monkeys. Biological Psychiatry. 1997;44(9):882–891. doi: 10.1016/s0006-3223(97)00437-x. [DOI] [PubMed] [Google Scholar]
- Shively C, Kaplan J. Effects of social factors on adrenal weight and related physiology of Macaca fascicularis. Physiol Behav. 1984;33(5):777–782. doi: 10.1016/0031-9384(84)90047-7. [DOI] [PubMed] [Google Scholar]
- Shively CA, Grant KA, Ehrenkaufer RL, Mach RH, Nader MA. Social stress, depression, and brain dopamine in female cynomolgus monkeys. Ann N Y Acad Sci. 1997;15:574–577. doi: 10.1111/j.1749-6632.1997.tb51972.x. [DOI] [PubMed] [Google Scholar]
- Suomi SJ. Factors affecting responses to social separation in rhesus monkeys. Animal models in human psychobiology. 1976:9–26. [Google Scholar]
- Vendruscolo LF, Estey D, Goodell V, Macshane LG, Logrip ML, Schlosberg JE, McGinn MA, Zamora-Martinez ER, Belanoff JK, Hunt HJ, Sanna PP, George O, Koob GF, Edwards S, Mason BJ. Glucocorticoid receptor antagonism decreases alcohol seeking in alcohol-dependent individuals. J Clin Invest. 2015;3(125):3193–3197. doi: 10.1172/JCI79828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivian JA, Green HL, Young JE, Majerksy LS, Thomas BW, Shively CA, Tobin JR, Nader MA, Grant KA. Induction and maintenance of ethanol self-administration in cynomolgus monkeys (Macaca fascicularis): Long-term characterization of sex and individual differences. ACER. 2001;25(8):1087–1097. [PubMed] [Google Scholar]
- Williams KL, Holden Ko MC, Rice KC, Woods JH. Effect of opioid receptor antagonists on hypothalamic-pituitary-adrenal activity in rhesus monkeys. Psychoneuroendocrinology. 2003;28(4):513–528. doi: 10.1016/s0306-4530(02)00037-9. [DOI] [PubMed] [Google Scholar]


