Abstract
Background:
Trans-synaptic degeneration (TSD) describes the propagation of neuronal injury through synaptic pathways in the human nervous system and may be linked to the accelerated retinal atrophy seen in multiple sclerosis (MS).
Results:
We report six cases where homonymous, hemi-macular ganglion cell + inner plexiform (GCIP) thickness reduction was seen in conjunction with posterior visual pathway lesions. Macular microcystoid changes of the inner nuclear layer (INL) were seen in a subset of three subjects.
Conclusion:
Our findings highlight the utility of assessing regional GCIP changes to identify potential retrograde TSD in MS and demonstrate that INL changes may be an accompaniment in such instances.
Keywords: Relapsing/remitting, T2 lesions, MRI, atrophy, retina, optical coherence tomography
Introduction
A putative mechanism of neuronal injury is trans-synaptic degeneration (TSD); a term referring to degeneration of neurons connected through a synaptic cleft to a distant primary degenerating neuron. Retrograde TSD of the visual axis has been described following occipital lobe damage due to stroke.1 In patients with multiple sclerosis (MS), prior studies have described a trend of association between retinal axonal degeneration and posterior visual pathway white matter (WM) lesions,2 as well as visual cortex gray matter (GM) volume.3 The integrity of the retinal ganglion cell layer, which harbors the neuronal bodies of optic nerve axons, can be estimated with optical coherence tomography (OCT) through the composite thickness of the ganglion cell + inner plexiform (GCIP) layers. We have previously described that homonymous hemi-macular thinning of the GCIP can be seen in relation to thalamic lesions involving the lateral geniculate nucleus (LGN).4 However, depictions of retrograde TSD in individual cases stemming from lesions beyond the LGN, involving the optic radiation or visual cortex, with objective retinal hemi-macular atrophy are limited. Furthermore, whether such possible patterns of TSD are limited to the innermost layers of the retina or may extend beyond the ganglion cell layer remains undetermined. Herein, we report six cases with evidence of neuroinflammatory injury to the posterior visual pathway on routine brain magnetic resonance imaging (MRI) demonstrating likely retrograde TSD characterized by a homonymous, hemi-macular pattern of GCIP thickness reduction on OCT, with evidence of macular microcystoid pathology (MMP) of the inner nuclear layer (INL) over a similar topographic distribution in a subset of eyes.
Cases
A total of six cases consistent with possible retrograde TSD of the visual pathway were identified. A diagnosis of relapsing–remitting multiple sclerosis (RRMS) was made in all six cases. The demographic and clinical characteristics are summarized in Table 1. No prior history of optic neuritis (ON) in either eye was present for any of the cases. The majority of patients reported visual symptomatology. Patients 1 and 2 described intermittent episodes of bilateral visual blurring that were insidious in nature and not categorized as an acute relapse. In contrast, patients 3–6 reported an acute onset of visual field difficulties consistent with homonymous hemianopia that was characterized as a relapse of inflammatory disease activity. This was confirmed by neuro-opthalmological assessment and automated perimetry testing.
Table 1.
Summary of demographic and clinical characteristics.
| Case no. | Sex/age, years | Clinical presentation | Disease duration, years | MRI lesion features | Site of GCIP thickness reduction | MMP | Lag timea | Time between MRI and OCT |
|---|---|---|---|---|---|---|---|---|
| 1 | M/37 | Intermittent blurred vision | 4 | Left occipital WM associated with focal volume loss | Left-temporal, right-nasal | Present | 6m | 6m |
| 2 | M/34 | Intermittent blurred vision | 8 | Right occipital WM involving the juxtacortical U-fibers, T1-hypointense | Right-temporal, left-nasal | Present | 2y 4m | Same day |
| 3 | F/39 | Left homonymous hemianopia | 7 | Right occipital WM | Right-temporal, left-nasal | Present | 7y 2m | 5y 9m |
| 4 | F/49 | Left homonymous hemianopia | 10 | Right occipital WM abutting the posterior horn of the lateral ventricle | Right-temporal, Left-nasal | Absent | 3y 10m | 3y 10m |
| 5 | M/33 | Left homonymous hemianopia | 3 | Right inferior thalamus | Right-temporal, left-nasal | Absent | 3y 7m | 1m |
| 6 | F/40 | Left homonymous hemianopia | 1 | Right thalamus | Right-temporal, left-nasal | Absent | 3m | 2m |
GCIP: ganglion cell + inner plexiform layer; m: month; MMP: macular microcystoid pathology; OCT: optical coherence tomography; RRMS: relapsing–remitting multiple sclerosis; GM: gray matter; WM: white matter; y: year.
Lag time denotes the time between symptom onset and the first OCT scan revealing hemi-macular GCIP thickness reduction.
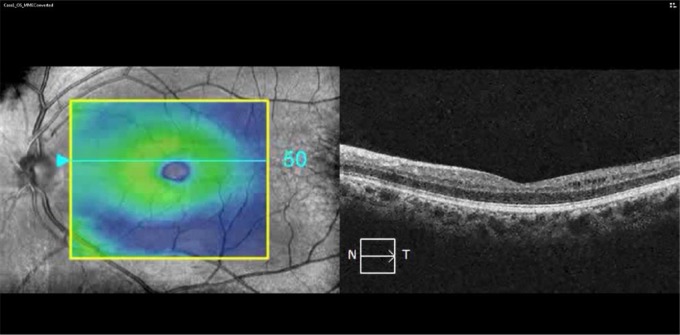
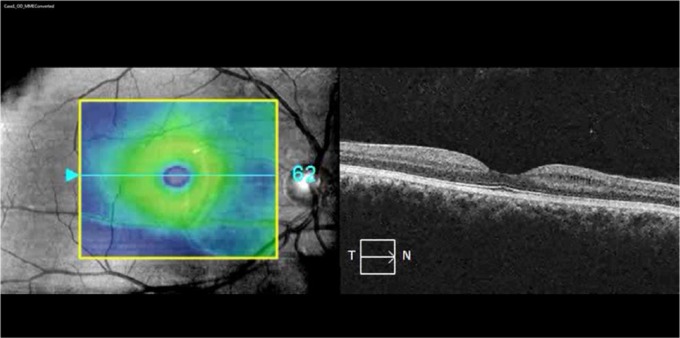
Brain MRI revealed multifocal, WM T2 hyperintensities (including variable involvement of the posterior visual pathways) with a distribution and morphology consistent with a clinical diagnosis of RRMS (Figure 1 and Table 1). OCT demonstrated topographically matching patterns of homonymous, hemi-macular GCIP thickness reduction. Patients 1–3 also exhibited lacunar areas of hyporeflectivity within the INL (characteristic of MMP) in a similar retinal distribution (Figure 1), which did not cross the vertical meridian of the macular cube scans (Videos 1 and 2).
Figure 1.
MRI and OCT findings: (a) case 1: FLAIR imaging showing a lesion in the left occipital white matter (red inset, arrow) associated with focal volume loss (red inset, arrowheads). OCT imaging revealed homonymous, hemi-macular GCIP thinning and MMP (red insets); (b) case 2: MPRAGE image showing a T1-hypointense lesion in the right occipital white matter extending into the adjacent striate cortex, which appears atrophic relative to the contralateral side (red inset). The lesion appeared T2 hyperintense on FLAIR imaging (green inset), while OCT showed homonymous, hemi-macular GCIP thinning and MMP; and (c) case 4: FLAIR image showing a right periventricular T2 hyperintensity extending into the occipital white matter in a patient who presented with left homonymous hemianopia. OCT images performed 4 years after presentation revealed hemi-macular GCIP thickness reduction without evidence of MMP.
FLAIR: fluid-attenuated inversion recovery; OCT: optical coherence tomography; GCIP: ganglion cell + inner plexiform layers; MMP: macular microcystoid pathology; MPRAGE: magnetization-prepared rapid-acquisition with gradient echo; N: nasal; T: temporal.
Video 1.
Homonymous, hemi-macular microcystoid changes: macular cube video of the left eye of case 1 showing the appearance of hemi-macular right-nasal and left temporal MMP. Of note, the MMP did not cross the vertical meridian.
MMP: macular microcystoid pathology.
Video 2.
Homonymous, hemi-macular microcystoid changes: macular cube video of the right eye of case 1 showing the appearance of hemi-macular right-nasal and left temporal MMP. Of note, the MMP did not cross the vertical meridian.
MMP: macular microcystoid pathology.
Discussion
We describe a cohort in which discrete areas of injury to the posterior visual pathway were related topographically to hemi-macular GCIP thickness reduction. Four of the patients exhibited evidence of central nervous system (CNS) inflammatory injury in the optic radiation and/or visual cortex, whereas in two cases, involvement of the LGN was suspected, although the possibility of terminal optic tract involvement cannot be excluded.
There is accumulating evidence from OCT and MRI studies to suggest the presence of TSD in patients with MS. Previous investigations have reported anterograde TSD of the optic radiations following ON.5 In contrast, our report highlights the capacity for neuroinflammatory lesions in the posterior visual pathway to result in changes suggestive of retrograde TSD at the level of the retina characterized by reduction in hemi-macular GCIP thicknesses. This sheds light on mechanisms underlying the accelerated retinal neuronal loss seen in MS in the absence of ON. We describe how examination of regional, hemi-macular GCIP thicknesses might serve utility in localizing presumably destructive neuroinflammatory pathology in the LGN or the posterior visual pathway, which is in contradistinction to peripapillary retinal nerve fiber layer thickness (RNFL) thickness where hemi-macular axonal segregation is less distinct. A few limitations do exist, however, and may explain why hemi-macular atrophy is not seen as frequently in MS even in the presence of lesions that may seemingly involve the posterior visual pathways. It is plausible that retrograde TSD may be related to the severity of axonal damage, primary neuronal loss, or anatomical location within the optic radiations or visual cortex. In our report, this was exemplified by case 1 in which the occipital lesion was associated with focal atrophy of the GM (implying neuronal loss) and case 2 in which the lesion was T1-hypointense (signifying axonal destruction). It is important to note that the presence of prior history of ON, especially if bilateral, subclinical optic nerve lesions, or local retinal inflammation would hinder the capability of OCT to detect co-existing retrograde TSD due to extensive, diffuse injury to the GCIP layer. Furthermore, the detection of retrograde TSD on OCT relies on differential and relative thinning of adjacent hemi-macular sectors which would be compromised if the optic radiations were bilaterally and symmetrically involved. It is also plausible that mild posterior visual pathway lesions may cause subclinical degeneration of retinal neurons that may not be accurately captured with gross visual screening of OCT images. However, it must be borne in mind that this study represents a cross-sectional case series suggesting the occurrence of TSD within the visual pathways, which does not encompass the timeline of TSD per se. As a result, longitudinal studies are required to more formally address this in the future.
A growing list of neurological disorders have been purported to result in MMP of the INL, including MS, neuromyelitis optica, and Leber’s hereditary optic neuropathy.6–9 In parallel, several theories have been proposed to explain MMP, including retrograde TSD, retinal traction, and/or Müller cell dysfunction.9 Importantly, the MMP observed in this case series (patients 1–3) was directly underlying areas of hemi-macular GCIP thinning without crossing the vertical meridian to the relatively unaffected side (Videos 1 and 2) suggesting a link between the two forms of pathology. Our findings raise the possibility that retinal ganglion cell dropout may instigate MMP development in the pathway undergoing TSD. Theoretically, this may represent a common denominator among a wide variety of neurological disorders that injure the visual system. Another etiology worth considering is that MMP may result from glial or Müller cell activation triggered by ganglion cell death.10 However, three out of six patients in this cohort demonstrated a manifest pattern of TSD of the GCIP layer without evidence of apparent, concurrent MMP. Therefore, it is conceivable that ganglion cell death solely may be insufficient to explain the occurrence of MMP in neurological conditions and that other temporal and susceptibility factors may play a role.
In summary, our findings illustrate that retrograde TSD stemming from posterior visual pathway lesions may be detected on OCT as hemi-macular GCIP thinning with or without MMP in a proportion of RRMS patients. The cascade of events separating GCIP pathology from MMP development remains unclear, and future research might help uncover cellular mediators behind this finding. Ultimately, a comprehensive understanding of the mechanisms of TSD and neuronal death in MS will help elucidate the neuronal circuitry’s response to axonal injury guiding novel targeted interventions and neuroprotective strategies.
Supplementary Material
Footnotes
Author contribution: Dr Al-Louzi: data collection, drafting, and concept of manuscript. J. Button: data collection, drafting, and revision of manuscript. Dr Newsome: data collection, drafting, and critical revision of manuscript for important intellectual content. Dr Calabresi: drafting and critical revision of manuscript for important intellectual content. Dr Saidha: concept of manuscript, drafting and critical revision of manuscript for important intellectual content.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr Al-Louzi and J. Button report no disclosures. Dr Newsome has received consultant fees for scientific advisory boards from Biogen, Genzyme, and Novartis and has received research funding from Biogen and Novartis. Dr Calabresi has received personal compensation for consulting and serving on scientific advisory boards from Vertex, Vaccinex, Merck, and AbbVie and has received research funding from Biogen-Idec, MedImmune, and Novartis and has also received National Institutes of Health grant 5R01NS082347-02. Dr Saidha has received consulting fees from Medical Logix for the development of CME programs in neurology, consulting fees from Axon Advisors LLC, Educational Grant Support from Novartis and Teva Neurosciences, speaking honoraria from the National Association of Managed Care Physicians, Family Medicine Foundation of West Virginia, and Advanced Studies in Medicine and served on scientific advisory boards for Biogen-Idec, Genzyme, and Novartis. He also received research funding from the Race to Erase MS and receives an unrestricted research grant from Genentech Corporation.
Funding: The author(s) declared receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by Race to Erase MS and Genentech Corporation grants to SS and a National Institutes of Health grant 5R01NS082347-02 to PAC.
Contributor Information
Omar Al-Louzi, The Division of Neuroimmunology and Neurological Infections, Department of Neurology, The Johns Hopkins hospital, Baltimore, MD, USA.
Julia Button, The Division of Neuroimmunology and Neurological Infections, Department of Neurology, The Johns Hopkins hospital, Baltimore, MD, USA.
Scott D Newsome, The Division of Neuroimmunology and Neurological Infections, Department of Neurology, The Johns Hopkins hospital, Baltimore, MD, USA.
Peter A Calabresi, The Division of Neuroimmunology and Neurological Infections, Department of Neurology, The Johns Hopkins hospital, Baltimore, MD, USA.
Shiv Saidha, The Division of Neuroimmunology and Neurological Infections, Department of Neurology, The Johns Hopkins hospital, Baltimore, MD, USA.
References
- 1. Jindahra P, Petrie A, Plant GT. The time course of retrograde trans-synaptic degeneration following occipital lobe damage in humans. Brain 2012; 135(Pt 2): 534–541. [DOI] [PubMed] [Google Scholar]
- 2. Klistorner A, Sriram P, Vootakuru N, et al. Axonal loss of retinal neurons in multiple sclerosis associated with optic radiation lesions. Neurology 2014; 82: 2165–2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gabilondo I, Martínez-Lapiscina EH, Martínez-Heras E, et al. Trans-synaptic axonal degeneration in the visual pathway in multiple sclerosis. Ann Neurol 2014; 75(1): 98–107. [DOI] [PubMed] [Google Scholar]
- 4. Oh J, Sotirchos ES, Saidha S, et al. In vivo demonstration of homonymous hemimacular loss of retinal ganglion cells due to a thalamic lesion using optical coherence tomography. JAMA Neurol 2013; 70(3): 410–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tur C, Goodkin O, Altmann DR, et al. Longitudinal evidence for anterograde trans-synaptic degeneration after optic neuritis. Brain 2016; 139(3): 816–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Saidha S, Sotirchos ES, Ibrahim MA, et al. Microcystic macular oedema, thickness of the inner nuclear layer of the retina, and disease characteristics in multiple sclerosis: A retrospective study. Lancet Neurol 2012; 11(11): 963–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sotirchos ES, Saidha S, Byraiah G, et al. In vivo identification of morphologic retinal abnormalities in neuromyelitis optica. Neurology 2013; 80(15): 1406–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Burggraaff MC, Trieu J, De Vries-Knoppert WAEJ, et al. The clinical spectrum of microcystic macular edema. Invest Ophthalmol Vis Sci 2014; 55(2): 952–961. [DOI] [PubMed] [Google Scholar]
- 9. Bhargava P, Calabresi PA. The expanding spectrum of aetiologies causing retinal microcystic macular change. Brain 2013; 136: 3212–3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kreutzberg GW. Microglia: A sensor for pathological events in the CNS. Trends Neurosci 1996; 19(8): 312–318. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.