Abstract
Rationale
Emerging evidence suggests the potential utility of combining opioids with imidazoline I2 receptor agonists for chronic pain. However, chronic pain management requires prolonged pharmacotherapy and the consequence of such combination therapy remains unclear.
Objective
This study examined the anti-hyperalgesic effect of the opioid oxycodone, the selective I2 receptor agonist phenyzoline, alone and in combination, during prolonged treatment.
Methods
Von Frey filament test was used to examine the anti-hyperalgesic effect of drugs in complete Freund’s adjuvant (CFA)-induced inflammatory pain or chronic constriction injury (CCI)-induced neuropathic pain in rats. Twice daily treatment with oxycodone and phenyzoline, alone or in combination, was continued until the development of significant tolerance (oxycodone) or as long as 19 days passed (phenyzoline).
Results
In rats receiving CFA or CCI manipulation, mechanical hyperalgesia was dose-dependently reversed by oxycodone and phenyzoline. Twice daily treatment with 2 × ED50 dose of oxycodone for 7 days led to significant antinociceptive tolerance to oxycodone but not cross-tolerance to phenyzoline. Similarly, twice daily treatment with 2 × ED50 dose of phenyzoline for 19 days led to significant antinociceptive tolerance to phenyzoline but not cross-tolerance to oxycodone. Twice daily treatment with the combined oxycodone and phenyzoline using different ratios (1:3, 1:1 and 3: 1) at the doses that were functionally equivalent to the treatment doses of oxycodone and phenyzoline for 13–19 days generally led to delayed antinociceptive tolerance.
Conclusions
Combination therapy with oxycodone and I2 receptor agonists maintains prolonged antinociceptive effectiveness with reduced propensity to develop tolerance.
Keywords: Imidazoline I2 receptor, Oxycodone, Phenyzoline, Pain, Tolerance, Rats
Introduction
Pain affects more Americans than diabetes, heart disease, and cancer combined. In addition to being the most common reason patients access the health care system, pain is also the leading cause of long-term disability (NIH 2013). Currently available analgesics are insufficient for clinical needs. Indeed, all currently available treatments can only achieve roughly 30% pain reduction in about 50% of treated chronic pain patients (Turk et al. 2011). In addition, there lacks true breakthrough analgesic drug in the past half century’s drug discovery effort (Kissin 2010). Therefore, there is a dire clinical need to develop novel and effective pharmacotherapies.
Emerging evidence suggests that imidazoline I2 receptor agonists could be a novel class of analgesics, particularly for chronic pain (Ferrari et al. 2011a; Lanza et al. 2014; Li et al. 2014; Li and Zhang 2011; Meregalli et al. 2012). In addition, studies show that the combination of I2 receptor agonists and opioids could produce additive to synergic antinociceptive interactions in rat models of chronic pain (Li et al. 2014; Siemian et al. 2016a; Siemian et al. 2016b). For example, in a rat model of chronic inflammatory pain, a simple additive interaction was observed between the selective I2 receptor agonist 2-BFI and morphine (Li et al. 2014). Using the same rat model of chronic inflammatory pain, it was found that the selective I2 receptor agonists 2-BFI and phenyzoline produced additive and synergic interactions with the opioid oxycodone, respectively (Thorn et al. 2015). More recently, it was found that when studied with opioids with varying efficacy at μ-opioid receptors, 2-BFI produced additive antinociceptive interactions with opioids with high efficacy while synergic interactions with opioids with lower efficacy were found (Siemian et al. 2016b). Combined, it seems that I2 receptor agonists and opioids may be a useful combination therapy strategy, which may achieve similar antinociception at smaller doses and produce reduced adverse effects for the management of chronic pain.
Chronic pain is usually long lasting and pharmacotherapies for pain control necessitates prolonged dosing. Repeated treatment with many drugs including opioids may lead to tolerance such that increasing doses are needed to achieve similar therapeutic effects. Tolerance to opioids is a widely-recognized adverse effect. Relatively little is known of the consequences of prolonged treatment with I2 receptor agonists. In one study, daily treatment with an effective dose of two selective I2 receptor agonists, 2-BFI and CR4056, for one week did not lead to reduced antinociceptive effect in complete Freund’s adjuvant (CFA)-induced inflammatory pain and chronic constriction injury (CCI)-induced neuropathic pain, suggesting that I2 receptor agonists do not produce apparent tolerance under this dosing regimen (Li et al. 2014). This study was designed to examine the development of antinociceptive tolerance to the opioid oxycodone, the I2 receptor agonist phenyzoline, when used alone or in combination, during prolonged treatment under a dosing regimen that better mimics potential clinical use in rats.
METHODS
Subjects
Adult male Sprague-Dawley rats (n = 60) (Envigo, Indianapolis, IN) (12 weeks with initial body weights of 250–280 grams) were housed individually on a 12/12-h light/dark cycle (behavioral experiments were conducted during the light period) with free access to water and standard rodent chow except during experimental sessions. Animals were maintained and experiments were conducted in accordance with guidelines of the International Association for the Study of Pain (Zimmermann, 1983) and were approved by the Institutional Animal Care and Use Committee, University at Buffalo, the State University of New York, and with the 2011 Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources on Life Sciences, National Research Council, National Academy of Sciences, Washington DC).
Induction of inflammatory and neuropathic pain
Inflammatory pain was induced by CFA inoculation as previously described (Li et al., 2014). Briefly, 0.1 mL of CFA (Difco, Detroit, MI, USA) that contains 0.05 mg Mycobacterium butyricum dissolved in paraffin oil was injected in the right foot pad (hind paw) of the rats under isoflurane anesthesia (2% isoflurane mixed with 100% oxygen at a flow rate of 5 L/min). The level of anesthesia was assessed by loss of righting reflex.
Neuropathic pain was induced by CCI procedure (Bennett and Xie 1988; Li et al. 2014). Briefly, rats were anesthetized with a mixture of ketamine (60 mg/kg) and xylazine (15 mg/kg) intraperitoneally (i.p.) prior to surgery. The right sciatic nerve was exposed and four ligatures (4.0 chromic gut, Roboz Surgical Instrument Co. Inc., Rockville, MD) were placed around the nerve (around 1 mm apart) proximal to the trifurcation. Ligatures were loosely tied so that circulation through the epineural vasculature was uninterrupted. The incisions were closed with surgical clips.
Mechanical hyperalgesia
Mechanical hyperalgesia was measured using the von Frey filament test consisting of calibrated filaments (North Coast Medical, Morgan Hill, CA) (1.4g–26g). Rats (n=6 per group) were placed in elevated plastic boxes with a wire mesh floor (IITC Life Science Inc., Woodland Hills, CA, USA) immediately before the test. The filaments were then applied perpendicularly to the medial plantar surface of the hind paw from below the mesh floor in an ascending order beginning with the lowest filament (1.4g). A particular filament was applied until buckling of the filament occurred and maintained for approximately 2s (by the experimenter’s estimation). Mechanical thresholds (expressed in g) corresponded to the lowest force that elicits a behavioral response (withdrawal of the hind paw) with at least two out of three applications. All tests started beginning 1 day after CFA treatment or 2 days after CCI surgery as described previously (Li et al., 2014). For all rats, a vehicle control test session was conducted prior to the drug test during which a vehicle injection was administered and the paw withdrawal threshold (PWT) was measured thereafter every 15 min for two hours. For the test of duration of drug action, measurements were taken immediately prior to the drug administration and every 15 min thereafter until the effect of the drug dissipated. For the test of dose-effect curves, the cumulative dosing procedure was used with a 20 min inter-injection interval as described previously (Siemian et al. 2016b). When drug treatments were studied in combination, they were prepared in a mixture and administered as one injection. The experimenters were not blind to the treatments; however, they received extensive training with this procedure before the initiation of this study to ensure accurate judgment of paw withdrawal responses and minimize experimenter bias.
Experimental design
Five groups of rats (n=6 per group) were used for CFA-induced inflammatory pain studies and another 5 groups were used for CCI-induced neuropathic pain studies. For each pain condition, the 5 groups were used to study the treatment of oxycodone alone, phenyzoline alone, and the mixture of oxycodone and phenyzoline (1:3, 1:1, 3:1 ratios), respectively. For oxycodone alone group, cumulative dose-effect curves of the antinociceptive effects of oxycodone and phenyzoline were first determined, respectively. Rats were then treated with 2 × ED50 values of oxycodone twice daily, separated for 10 hours, in their home cages or in the experimental room (every 3rd day, see below). The duration of action of the daily treatment dose of oxycodone was determined every 3 days (i.e, days 1, 4, 7, …) and stopped when a near complete tolerance to the antinociceptive effect of the treatment dose of oxycodone was seen through visual inspection of the duration of action data. Oxycodone treatment was stopped after 7 days and a new dose-effect curve for oxycodone and phenyzoline was re-determined on days 8 and 9, respectively. For phenyzoline alone group, the same approach was used and the phenyzoline treatment was terminated after 19 days despite the fact that only a partial tolerance was seen. Because oxycodone treatment was terminated after 7 days, probe tests were performed on days 8 and 9 for oxycodone and phenyzoline dose-effect curves, respectively, in phenyzoline alone group. Both tests were conducted 4 hours after the morning treatment dose to minimize the impact of residual drug from the previous treatment. The antinociceptive effects of oxycodone and phenyzoline at similar treatment doses last less than 2.5 hours (Thorn et al. 2015). Three separate groups of rats were used to study the combination treatments using the following dose proportions: 1:3, 1:1, and 3:1. The treatment approach and drug dose-effect curve determinations were identical with the phenyzoline alone group. The dose-effect curves of oxycodone and phenyzoline were also determined prior to, during and after the combination drug treatments.
Because oxycodone and phenyzoline produce synergic antinociceptive interactions when studied as a combination (Thorn et al. 2015), the functionally equivalent doses of both drugs were used for the twice daily treatments in this study using information from the previous study (Thorn et al. 2015). Therefore, the treatment doses for all the 5 groups were as follows: 1.00 mg/kg oxycodone (ED50 value of oxycodone was 0.50 mg/kg, oxycodone alone group), 65.46 mg/kg phenyzoline (ED50 value of phenyzoline was 32.73 mg/kg, phenyzoline alone group), 0.06 mg/kg oxycodone + 13.02 mg/kg phenyzoline (2 × ED50 values of oxycodone and phenyzoline when both drugs were studied as a combination under a proportion ratio of 1:3, respectively), 0.28 mg/kg oxycodone + 19.2 mg/kg phenyzoline (2 × ED50 values of oxycodone and phenyzoline when both drugs were studied as a combination under a proportion ratio of 1:1, respectively), and 0.25 mg/kg oxycodone + 5.14 mg/kg phenyzoline (2 × ED50 values of oxycodone and phenyzoline when both drugs were studied as a combination under a proportion ratio of 3:1, respectively).
Data analyses
Measurements of CFA treatment and CCI surgery induced mechanical hyperalgesia were quantified in each animal as % maximal possible effect (MPE) at each drug dose. The following formula was used to quantify % MPE: % MPE = [(Post-drug value for a behavioral response (gram) — Pre-drug value for a behavioral response/(Pre-manipulation [CFA or CCI] value — Pre-drug value for a behavioral response) × 100. The ED50 values and 95% confidence limits (CLs) were estimated using interpolation or linear regression using the portion of the dose-effect curve spanning 50% as previously described (Li et al. 2009). Dose ratios of the drugs in individual rats were calculated by dividing the ED50 values of the drugs during or after drug treatments by the ED50 values of the drugs before treatments and then averaged across subjects. The drug potencies were considered significantly different when the 95% CLs of the dose ratio did not include 1.
For the duration of antinociceptive action data, the area under curves (AUC) between times 0–120 min of the treatment drugs were calculated and analyzed using one-way repeated measures ANOVA followed by Bonferroni’s post hoc test. P < 0.05 was considered statistically significant.
Drugs
Phenyzoline hydrochloride was synthesized by the co-author, Dr. Yanan Zhang, at Research Triangle Institute. Oxycodone hydrochloride was provided by Research Technology Branch, National Institute on Drug Abuse, National Institutes of Health (Rockville, MD, USA). Both drugs were dissolved in physiological saline and administered i.p. Doses are expressed as mg of the form indicated earlier per kg body weight. Injection volumes were 1 ml/kg.
Results
Under control conditions, rats lifted their right hind paws when the force of the filament applied to the hind paw increased to 26g. However, CFA injection and CCI surgery both markedly decreased the PWT (e.g., oxycodone alone group: 2.9 ± 1.6 g in rats receiving CFA and 6.3 ± 2.1 g in rats receiving CCI surgery). Both oxycodone and phenyzoline dose-dependently increased the PWT in rats receiving CFA (Fig. 1). Before repeated drug treatment, the ED50 values (95% CLs) of oxycodone and phenyzoline were similar in rats receiving oxycodone alone or phenyzoline alone [oxycodone: 0.28 (0.22, 0.37) and 0.22 (0.15, 0.30) mg/kg, respectively; phenyzoline: 29.70 (24.05, 36.67) and 26.21 (20.84, 32.96), respectively] (Table 1). For the oxycodone alone group, significant tolerance was developed to oxycodone after 7 days of oxycodone treatment, increasing the ED50 values to 3.39 (2.24, 5.13) mg/kg, a 16.40-fold rightward shift. In contrast, no significant cross-tolerance to phenyzoline was observed [33.37 (26.28, 42.37) mg/kg]. Similarly, for the phenyzoline alone group, significant tolerance was developed to phenyzoline after 19 days but not 7 days of phenyzoline treatment, increasing the ED50 values to 30.84 (21.91, 43.42) and 56.86 (39.34, 82.16) mg/kg after 7 and 19 days of phenyzoline treatment, respectively. This represents a maximum of 2.30-fold rightward shift. In contrast, no significant cross-tolerance to oxycodone was observed [0.29 (0.20, 0.42) and 0.36 (0.21, 0.62) mg/kg after 7 and 19 days of phenyzoline treatment, respectively]. These data suggest that prolonged treatment with oxycodone and phenyzoline developed significant tolerance but did not develop cross-tolerance to their antinociceptive effects in CFA-treated rats.
Figure 1.
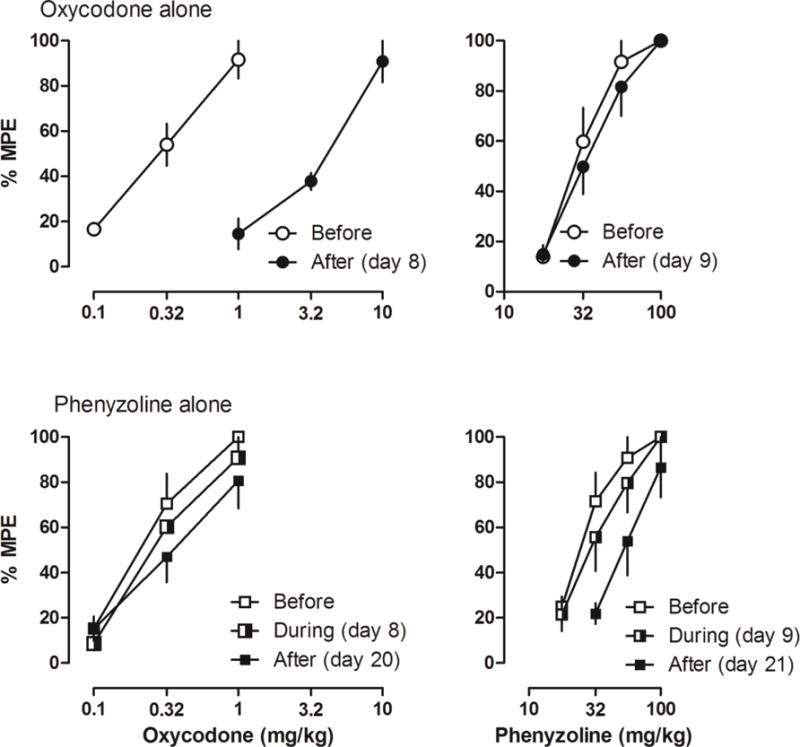
Antinociceptive effects of oxycodone (left) and I2 receptor agonist phenyzoline (right) on CFA-induced mechanical hyperalgesia before, during and after twice-daily oxycodone (top) and phenyzoline (bottom) treatment. Tests during the treatment were performed on days 8 and 9. Tests after the treatment were performed on days 8 and 9 in oxycodone alone group and on days 20 and 21 in phenyzoline alone group. Ordinates, percentage of maximal possible effects; Abscissa, drug doses (mg/kg).
Table 1.
ED50 values (95% CL) of oxycodone- and phenyzoline-induced antinociceptive effects in rat models of CFA-induced inflammatory pain and CCI-induced neuropathic pain.
| Pain model | Treatment | Test drug | Before | During | After | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| ED50 (95% CL) | ED50 (95% CL) | DR | ED50 (95% CL) | DR | |||
| CFA | Oxy alone | Oxy | 0.28 (0.22, 0.37) | – | – | 3.39 (2.24, 5.13) | 16.40(2.29, 30.51)* |
| Pheny | 29.70 (24.05, 36.67) | – | – | 33.37 (26.28, 42.37) | 1.24(0.74, 1.74) | ||
| Pheny alone | Oxy | 0.22 (0.15, 0.30) | 0.29 (0.20, 0.42) | 1.77(0.55, 2.99) | 0.36 (0.21, 0.62) | 2.46(0.71, 4.21) | |
| Pheny | 26.21 (20.84, 32.96) | 30.84 (21.91, 43.42) | 1.43(0.66, 2.21) | 56.86 (39.34, 82.16) | 2.30(1.64, 2.96)* | ||
| 1:3 | Oxy | 0.28 (0.15, 0.53) | 0.34 (0.17, 0.66) | 1.60(0.40, 2.80) | 0.56 (0.20, 1.63) | 1.35(−0.30, 2.99) | |
| Pheny | 28.84 (22.81, 36.47) | 35.48 (29.17, 43.15) | 1.27(0.97, 1.58) | 51.49 (30.46, 87.03) | 2.47(0.72, 4.23) | ||
| 1:1 | Oxy | 0.27 (0.17, 0.42) | 0.41 (0.16. 1.10) | 4.97(−2.79, 12.72 | 0.64 (0.24. 1.72) | 6.21(−0.34, 12.76 | |
| Pheny | 27.63 (21.77, 35.07) | 33.66 (24.55, 46.14) | 1.40(0.76, 2.05) | 34.91 (27.94, 43.63) | 1.33(0.99, 1.67) | ||
| 3:1 | Oxy | 0.31 (0.51, 1.90) | 0.64 (0.24, 1.72) | 2.68(0.92, 4.44) | 1.55 (0.65, 3.68) | 7.43(0.21, 14.64) | |
| Pheny | 24.70 (21.17, 28.83) | 31.86 (26.23, 38.69) | 1.32(1.09, 1.55)* | 35.58 (25.58, 49.49) | 1.49(1.14, 1.85)* | ||
|
| |||||||
| CCI | Oxy alone | Oxy | 0.22 (0.14, 0.35) | – | – | 2.82 (1.76, 4.49) | 15.03 (6.54, 23.52)* |
| Pheny | 26.29 (20.47, 33.75) | – | – | 30.21 (22.56, 40.46) | 1.21(0.80, 1.62) | ||
| Pheny alone | Oxy | 0.28 (0.15, 0.51) | 0.37 (0.21, 0.68) | 2.28(0.53, 4.04) | 0.35 (0.21, 0.57) | 2.24(0.46, 4.02) | |
| Pheny | 26.04 (20.58, 32.94) | 34.52 (25.62, 46.51) | 1.39(1.07, 1.70)* | 65.16 (43.75, 97.05) | 2.77(1.74, 3.81)* | ||
| 1:3 | Oxy | 0.28 (0.14, 0.56) | 0.34 (0.20, 0.60) | 1.69(0.74, 2.64) | 0.49 (0.32, 0.76) | 2.77(1.01, 4.52)* | |
| Pheny | 28.01 (21.40, 36.65) | 35.41 (26.27, 47.74) | 1.51(0.72, 2.30) | 42.41 (31.92, 56.35) | 1.80(0.86, 2.74) | ||
| 1:1 | Oxy | 0.24 (0.13, 0.45) | 0.33 (0.19, 0.55) | 1.48(0.91, 2.04) | 0.51 (0.28, 0.95) | 3.03(0.53, 5.53) | |
| Pheny | 26.43 (21.09, 33.11) | 34.38 (26.92, 43.92) | 1.32(1.11, 1.53)* | 38.97 (30.68, 49.49) | 1.57(1.10, 2.04)* | ||
| 3:1 | Oxy | 0.27 (0.16, 0.44) | 0.38 (0.21, 0.68) | 1.47(1.14, 1.80)* | 1.03 (0.46, 2.33) | 5.48(2.02, 8.95)* | |
| Pheny | 25.28 (20.24, 31.57) | 31.52 (24.10, 41.22) | 1.26(1.08, 1.45)* | 33.28 (30.10, 36.79) | 1.39(1.00, 1.78) | ||
95% confidence limits (CL) of the dose ratios [ED50 (during or after treatment)/ED50 (before treatment)] did not include 1, suggesting statistical significance. Oxy, oxycodone; Pheny, phenyzoline.
When the rats with CFA-induced inflammatory pain were repeatedly treated with the combined doses of oxycodone and phenyzoline that produced similar antinociceptive effects to those doses used in the drug alone treatment groups, only phenyzoline developed significant tolerance in rats that received a proportion ratio of 3:1 treatment with oxycodone and phenyzoline and no significant tolerance was observed under other treatment conditions (Fig. 2). In rats that received repeated treatment with a combined dose of oxycodone and phenyzoline at a proportion ratio of 1:3, neither oxycodone nor phenyzoline developed significant tolerance during 19 days of treatment (Table 1). Thus, after 19 days of treatment, the ED50 values of oxycodone [0.56 (0.20, 1.63) mg/kg] and phenyzoline [51.49 (30.46, 87.03) mg/kg] overlapped with those prior to drug treatments and the potency ratios were not significantly different from 1 as compared to before drug treatment (left, Fig. 2). Similar results were found for CFA-treated rats that received the proportion ratio of 1:1 oxycodone-phenyzoline combinations (Table 1, Fig. 2).
Figure 2.
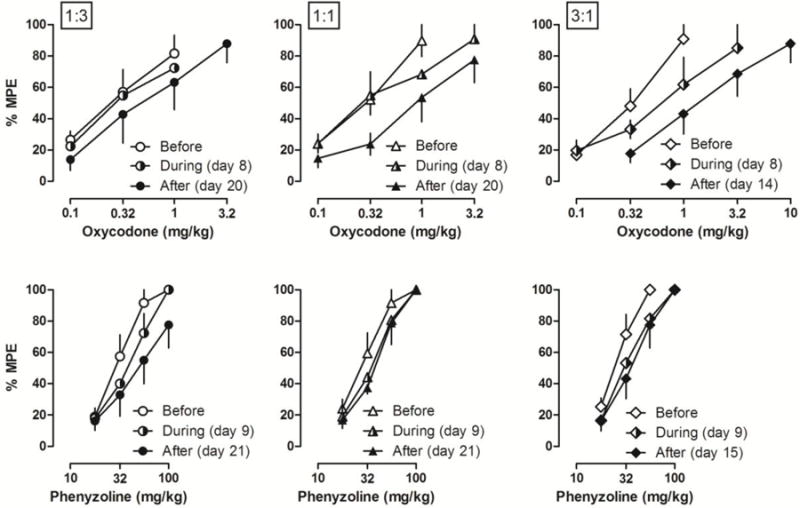
Antinociceptive effects of oxycodone (top) and I2 receptor agonist phenyzoline (bottom) on CFA-induced mechanical hyperalgesia before, during and after twice-daily treatment with the combination of oxycodone and phenyzoline at different proportion ratios: 1:3 (left), 1:1 (middle) and 3:1 (right). Tests during the treatment were performed on days 8 and 9. Tests after the treatment were performed on days 20 and 21 in 1:3 and 1:1 ratio groups and on days 14 and 15 in 3:1 ratio group. Ordinates, percentage of maximal possible effects; Abscissa, drug doses (mg/kg).
In the rats that received CCI surgery, both oxycodone and phenyzoline dose-dependently increased the PWT (Fig. 3). Before repeated drug treatment, the ED50 values (95% CLs) of oxycodone and phenyzoline were similar in rats receiving oxycodone alone or phenyzoline alone [oxycodone: 0.22 (0.14, 0.35) and 0.28 (0.15, 0.51) mg/kg, respectively; phenyzoline: 26.29 (20.47, 33.75) and 26.04 (20.58, 32.94), respectively] (Table 1). For the oxycodone alone group, significant tolerance was developed to oxycodone after 7 days of oxycodone treatment, increasing the ED50 values to 2.82 (1.76, 4.49) mg/kg, a 15.03-fold rightward shift. In contrast, no significant cross-tolerance to phenyzoline was observed [30.21 (22.56, 40.46) mg/kg]. Similarly, for the phenyzoline alone group, significant tolerance was developed to phenyzoline after 7 to 19 days of phenyzoline treatment, increasing the ED50 values to 34.52 (25.62, 46.51) and 65.16 (43.75, 97.05) mg/kg after 7 and 19 days of phenyzoline treatment, respectively, shifting the phenyzoline dose-effect curve 1.39- and 2.77-fold rightward, respectively. In contrast, no significant cross-tolerance to oxycodone was observed [0.37 (0.21, 0.68) and 0.35 (0.21, 0.57) mg/kg after 7 and 19 days of phenyzoline treatment, respectively]. These data suggest that prolonged treatment with oxycodone and phenyzoline developed significant tolerance to the respective treatment drug but did not develop cross-tolerance to the other drug for their antinociceptive effects in rats with CCI-induced neuropathic pain.
Figure 3.
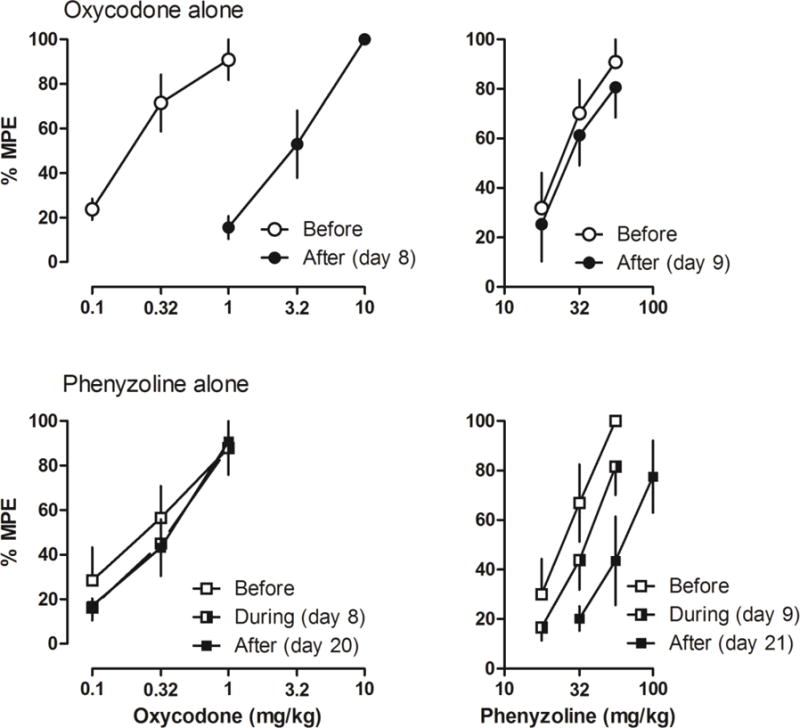
Antinociceptive effects of oxycodone (left) and I2 receptor agonist phenyzoline (right) on CCI surgery-induced mechanical hyperalgesia before, during and after twice daily oxycodone (top) and phenyzoline (bottom) treatment. Tests during the treatment were performed on days 8 and 9. Ordinates, percentage of maximal possible effects; Abscissa, drug doses (mg/kg). See Figure 1 for other details.
When the rats with CCI-induced neuropathic pain were repeatedly treated with the combined doses of oxycodone and phenyzoline that produced similar antinociceptive effects to those doses used in the drug alone treatment groups, a modest but significant antinociceptive tolerance to oxycodone or phenyzoline was observed under some conditions (Fig. 4, Table 1). In rats that received repeated treatment with a combined dose of oxycodone and phenyzoline at a proportion ratio of 1:3, only oxycodone developed significant tolerance during 19 days of treatment (Table 1). Thus, after 19 days of treatment, the ED50 values of oxycodone were increased 2.77-fold to 0.49 (0.32, 0.76) mg/kg (left, Fig. 4). At the proportion ratio of 1:1, significant tolerance was seen to phenyzoline after 7 and 19 days of the treatment while at the proportion ratio of 3:1 significant tolerance was seen to both oxycodone and phenyzoline (Table 1).
Figure 4.
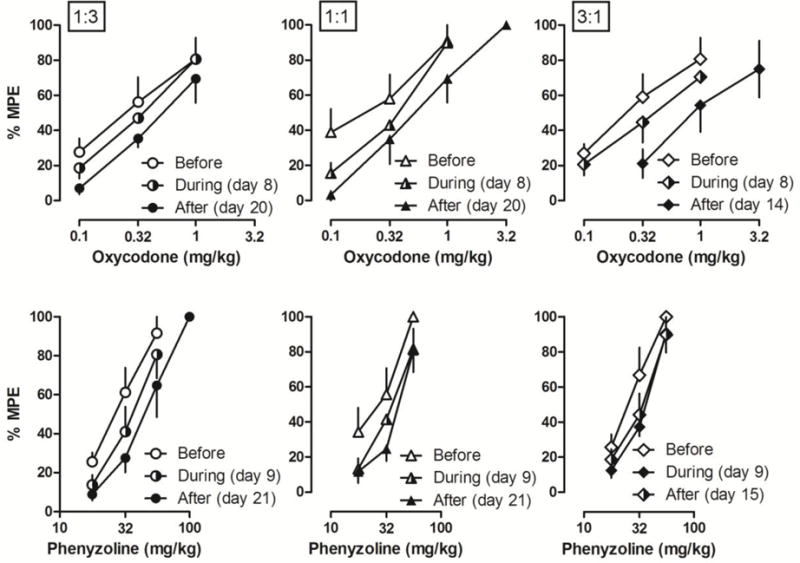
Antinociceptive effects of oxycodone (top) and I2 receptor agonist phenyzoline (bottom) on CCI surgery-induced mechanical hyperalgesia before, during and after twice daily treatment with the combination of oxycodone and phenyzoline at different proportion ratios: 1:3 (left), 1:1 (middle) and 3:1 (right). Tests during the treatment were performed on days 8 and 9. Ordinates, percentage of maximal possible effects; Abscissa, drug doses (mg/kg). See Figure 2 for other details.
The AUC data of the antinociceptive effects of the treatment doses from all the interspersed testing days were shown in Fig. 5. The trend of the development of antinociceptive tolerance was similar between rats that received CFA treatment and CCI surgery. The AUC among the 5 groups were not significantly different on the first day of test [CFA condition: F (4, 29) = 0.61, P > 0.05; CCI condition: F (4, 29) = 0.22, P > 0.05], confirming the functional equivalence of the different treatment regimens under both pain conditions. Visual inspection of the maximal effect and duration of actions of the treatment doses also showed no apparent differences (data not shown). Post hoc analysis revealed no significant difference among the groups (P > 0.05). Under both conditions, oxycodone quickly developed significant tolerance [CFA condition: F (3, 23) = 1.40, P > 0.05; CCI condition: F (3, 23) = 2.98, P < 0.05). Post hoc analysis revealed that the AUC at days 4 and 7 were not significantly different from day 1 (P > 0.05). Phenyzoline developed tolerance much slower than oxycodone [CFA condition: F (7, 47) = 5.42, P < 0.001; CCI condition: F (3, 23) = 5.48, P < 0.001]. Post hoc analysis revealed that the AUC from days 16 and 19 were not significantly different from day 1 for both CFA-treated and CCI rats. Overall, tolerance developed slower in rats that received the combination treatments than those that only received oxycodone treatment and faster than those that only received phenyzoline treatment under both pain conditions (Fig. 5). For rats that received 1:3 proportion of combination treatment, the AUC was not significantly different from day 10 onward in CFA-treated rats [F (7, 47) = 8.51, P < 0.001] and from day 7 onward in rats that receive CCI surgery [F (7, 47) = 6.63, P < 0.001]. For rats that received 1:1 proportion of combination treatment, the AUC was not significantly different from day 10 onward in CFA-treated rats [F (7, 47) = 3.69, P < 0.01] and from day 13 onward in rats that receive CCI surgery [F (7, 47) = 19.41, P < 0.0001]. For rats that received 3:1 proportion of combination treatment, the AUC was not significantly different from day 7 onward in CFA-treated rats [F (5, 35) = 9.00, P < 0.0001] and day 10 onward in rats that received CCI surgery [F (6, 41) = 5.12, P < 0.01].
Figure 5.
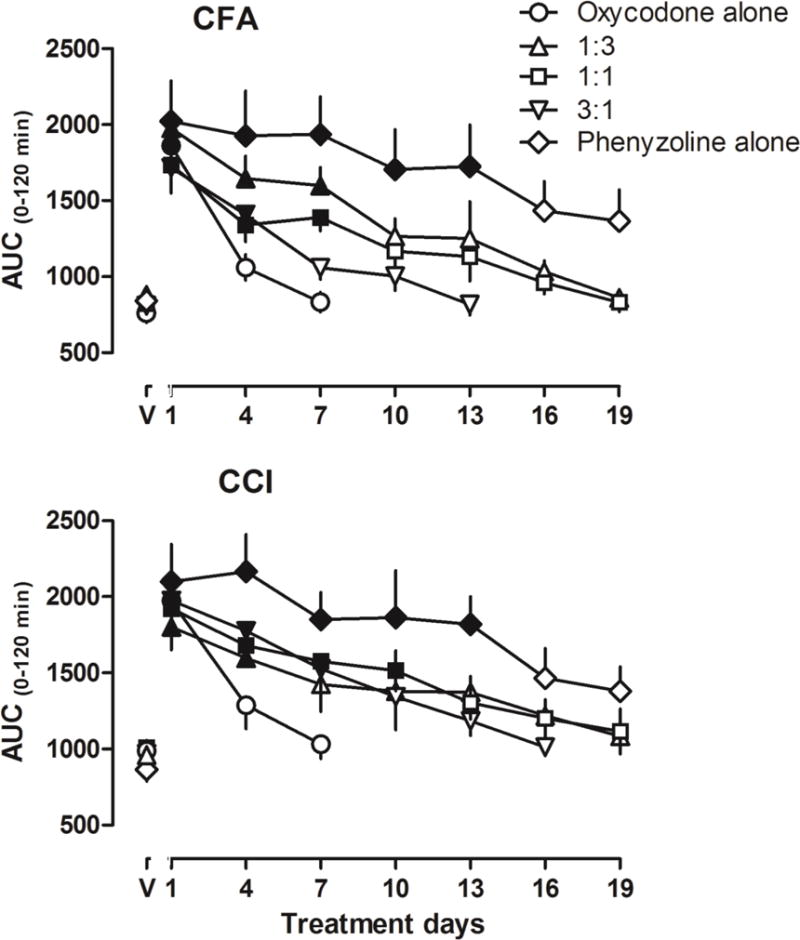
Area under curve (AUC) of the antinociceptive effects induced by the treatment drugs determined every 3rd day during the twice daily drug treatment in rats receiving CFA (top) and CCI surgery (bottom). Ordinates, AUC during 0–120 min; Abscissa, treatment days. Filled symbols represent data significantly different from the vehicle control conditions (data above “V”) as analyzed by one-way ANOVA followed by Bonferroni’s post hoc test.
Discussion
The primary findings of the current study were that prolonged treatment with the I2 receptor agonist phenyzoline at a dose that produced near maximal antinociceptive effect developed a small but significant tolerance in two rat models of chronic pain, and the development of tolerance was much slower than oxycodone. In addition, prolonged treatment with the combination of oxycodone and phenyzoline at functionally equivalent antinociceptive doses with oxycodone produced delayed tolerance than the condition that was only treated with oxycodone. Importantly, although tolerance to the combination treatment doses was developed, the antinociceptive effects of oxycodone and phenyzoline was not significantly altered.
Opioids remain the most effective analgesics for the control of many pain conditions. However, clinical opioid use for the treatment of chronic pain is limited because: (1) opioids are not effective for some chronic pain conditions such as certain neuropathic pain (Smith 2012); (2) prolonged opioid use can lead to various clinically significant adverse effects such as tolerance and physical dependence, which at least partially contribute to the recent opioid epidemic (Birke et al. 2016; Manchikanti et al. 2016; Rudd et al. 2016). Therefore, the development of new combination therapy that reduces opioid use and tolerance may have substantial clinical significance. Built on earlier findings that when used alone I2 receptor agonists are effective in several rodent models of chronic pain (Lanza et al. 2014; Li et al. 2014; Meregalli et al. 2012), that I2 receptor agonists produce additive to synergic antinociceptive interactions with opioids when used acutely (Li et al. 2014; Siemian et al. 2016b; Thorn et al. 2015), and that I2 receptor agonists reduce prolonged morphine treatment-induced physical dependence (Thorn et al. 2016), these results are critical complementary evidence to supporting the notion that I2 receptor agonists may be useful for the management of some chronic painful conditions, either used alone or as a combination therapy with opioids.
According to the definition of International Association of the Study of Pain, chronic pain is the pain that persists beyond normal tissue healing time, which is assumed to be 3 months (No author listed 1986). Due to the long-lasting nature, pharmacotherapeutic treatment of chronic pain requires prolonged dosing. Currently the major classes of analgesics that are clinically used for various chronic painful conditions include opioids, non-steroid anti-inflammatory drugs, certain antidepressants and anticonvulsants, and local anesthetics. Unfortunately, despite so many choices, existing analgesics only work in about half of the pain patients and can only achieve 30% pain relief (Turk et al. 2011). Opioids remain the most important analgesics for many chronic painful conditions. However, prolonged opioid use for chronic pain comes with many well-recognized adverse effects, including tolerance, physical dependence, propensity to addiction, and overdose (Agarin et al. 2015; Jones 2013). In order to overcome the development of tolerance during prolonged opioid use, opioid switching strategy is often used to obtain a balance between pain control and adverse effects with limited success (Mercadante and Bruera 2016). Another viable strategy for pain control is combination therapy, the practice of using two or more drugs to achieve improved pain relief and/or reduced adverse effects (Ramiro et al. 2011).
Imidazoline I2 receptors have been emerging as a novel target for the development of analgesics (Li and Zhang 2011; 2012). Sometimes referred to as I2 binding sites, I2 receptors probably is not a homogenous receptor but include multiple components instead (Escriba et al. 1999). The current consensus is that one component of the I2 receptors is an allosteric modulating site on monoamine oxidases A and B, which is different from the catalytic site (Basile et al. 2014; McDonald et al. 2010). Neurochemical studies seem to support this notion as brain microdialysis showed that selective I2 receptor agonists increase monoamine release in the frontal cortex (Hudson et al. 1999). A recent study confirmed these findings by showing that a novel I2 receptor agonist CR4056 increases the norepinephrine level in the rat cortex and spinal cord (Ferrari et al. 2011b). Given the critical role of monoamines in pain modulation and processing (Bannister and Dickenson 2016), it should come as no surprise that I2 receptor agonists demonstrate antinociceptive effects in preclinical studies. Indeed, studies show that several selective I2 receptor agonists are effective in various rat models of persistent pain (Ferrari et al. 2011b; Lanza et al. 2014; Li et al. 2014; Meregalli et al. 2012; Thorn et al. 2015). In addition, one compound, CR4056, has been moved to Phase II clinical trial for the treatment of osteoarthritis (Lanza et al. 2014). Because chronic painful conditions require long term analgesic use, it is important to understand the potential consequences of prolonged drug treatment such as tolerance. In this regard, relatively little is known with I2 receptor agonists. In one study, once daily treatment with two I2 receptor agonists 2-BFI and CR4056 for a week failed to demonstrate apparent tolerance to their antinociceptive effects in CFA-treated and CCI-induced chronic pain models (Li et al. 2014).
This study extended previous findings to showing that more frequent treatment (twice daily) with longer period of time (19 days) with the I2 receptor agonist phenyzoline can lead to a small but significant antinociceptive tolerance. Importantly, no cross-tolerance was observed between oxycodone and phenyzoline. The dosing regimen is clinically relevant, using an effective (but not too large) treatment dose for antinociception and treating twice daily. This information is important if I2 receptor agonists are eventually used in humans for pain management. It is important to note that such a magnitude of tolerance is markedly smaller than opioids and should be considered as an advantage. Because the opioid oxycodone has a synergic antinociceptive interaction with phenyzoline in a rat model of chronic pain (Thorn et al. 2015), the combination with the two drugs may be able to maintain similar antinociception but produce less adverse effects. This study examined the effects of prolonged treatment with this combination on the development of antinociceptive tolerance. Indeed, when treated with the combination of oxycodone and phenyzoline at different proportions using functionally equivalent doses, the development of tolerance to the drug combination was slower than oxycodone alone treatment condition but faster than phenyzoline alone treatment condition. There are two possible reasons why treatment with the drug combination leads to little tolerance. First, because oxycodone and phenyzoline work in a synergic manner to produce combined antinociception, the functionally equivalent doses of the combinations include smaller doses of each drug as compared to the individual drugs alone. Repeated treatment with smaller doses of the drug is expected to produce less tolerance. Second, I2 receptor agonists can reduce the development of tolerance to opioids under certain conditions (Thorn et al. 2016). Thus, when oxycodone and phenyzoline are co-administered to rats, phenyzoline may reduce the development of tolerance to oxycodone which further contributes to the observed delayed antinociceptive tolerance as seen in this study. Despite the tolerance development to the oxycodone-phenyzoline combination treatment doses, the antinociceptive potencies to oxycodone and phenyzoline were not significantly altered, suggesting that a drug switching strategy could be used even after the drug combination therapy becomes less effective due to tolerance.
In summary, this study found that using functionally equivalent doses for prolonged treatment, the combination therapy of oxycodone and phenyzoline developed delayed antinociceptive tolerance in two rat models of chronic pain than oxycodone. This information is an important addition to previous findings that I2 receptor agonists are effective against several preclinical pain conditions. Because preclinical studies have demonstrated that I2 receptor agonists 1) are effective when used alone, 2) can enhance the effects of opioids, 3) produce less tolerance when used together with opioids, and 4) mitigate opioid tolerance, these results strongly suggest that I2 receptor agonists may be a class of novel analgesics that can be used against chronic painful conditions either as a monotherapy or combination therapy.
Acknowledgments
This work was supported by the National Institute on Drug Abuse of the National Institutes of Health (Award no. R01DA034806) and by a grant from National Natural Science Foundation of China (81373390). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Agarin T, Trescot AM, Agarin A, Lesanics D, Decastro C. Reducing opioid analgesic deaths in America: what health providers can do. Pain Physician. 2015;18:E307–22. [PubMed] [Google Scholar]
- Bannister K, Dickenson AH. What do monoamines do in pain modulation? Curr Opin Support Palliat Care. 2016;10:143–8. doi: 10.1097/SPC.0000000000000207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basile L, Pappalardo M, Guccione S, Milardi D, Ramsay RR. Computational comparison of imidazoline association with the I2 binding site in human monoamine oxidases. J Chem Inf Model. 2014;54:1200–7. doi: 10.1021/ci400346k. [DOI] [PubMed] [Google Scholar]
- Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- Birke H, Kurita GP, Sjogren P, Hojsted J, Simonsen MK, Juel K, Ekholm O. Chronic non-cancer pain and the epidemic prescription of opioids in the Danish population: trends from 2000 to 2013. Acta Anaesthesiol Scand. 2016;60:623–33. doi: 10.1111/aas.12700. [DOI] [PubMed] [Google Scholar]
- Escriba PV, Ozaita A, Garcia-Sevilla JA. Pharmacologic characterization of imidazoline receptor proteins identified by immunologic techniques and other methods. Ann N Y Acad Sci. 1999;881:8–25. doi: 10.1111/j.1749-6632.1999.tb09336.x. [DOI] [PubMed] [Google Scholar]
- Ferrari F, Fiorentino S, Mennuni L, Garofalo P, Letari O, Mandelli S, Giordani A, Lanza M, Caselli G. Analgesic efficacy of CR4056, a novel imidazoline-2 receptor ligand, in rat models of inflammatory and neuropathic pain. J Pain Res. 2011a;4:111–125. doi: 10.2147/JPR.S18353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari F, Fiorentino S, Mennuni L, Garofalo P, Letari O, Mandelli S, Giordani A, Lanza M, Caselli G. Analgesic efficacy of CR4056, a novel imidazoline-2 receptor ligand, in rat models of inflammatory and neuropathic pain. J Pain Res. 2011b;4:111–25. doi: 10.2147/JPR.S18353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson AL, Gough R, Tyacke R, Lione L, Lalies M, Lewis J, Husbands S, Knight P, Murray F, Hutson P, Nutt DJ. Novel selective compounds for the investigation of imidazoline receptors. Ann N Y Acad Sci. 1999;881:81–91. doi: 10.1111/j.1749-6632.1999.tb09344.x. [DOI] [PubMed] [Google Scholar]
- Jones CM. Heroin use and heroin use risk behaviors among nonmedical users of prescription opioid pain relievers - United States, 2002–2004 and 2008–2010. Drug Alcohol Depend. 2013;132:95–100. doi: 10.1016/j.drugalcdep.2013.01.007. [DOI] [PubMed] [Google Scholar]
- Kissin I. The development of new analgesics over the past 50 years: a lack of real breakthrough drugs. Anesth Analg. 2010;110:780–9. doi: 10.1213/ANE.0b013e3181cde882. [DOI] [PubMed] [Google Scholar]
- Lanza M, Ferrari F, Menghetti I, Tremolada D, Caselli G. Modulation of imidazoline I2 binding sites by CR4056 relieves postoperative hyperalgesia in male and female rats. Br J Pharmacol. 2014;171:3693–701. doi: 10.1111/bph.12728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JX, Thorn DA, Qiu Y, Peng BW, Zhang Y. Antihyperalgesic effects of imidazoline I(2) receptor ligands in rat models of inflammatory and neuropathic pain. Br J Pharmacol. 2014;171:1580–90. doi: 10.1111/bph.12555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JX, Unzeitig A, Javors MA, Rice KC, Koek W, France CP. Discriminative stimulus effects of 1-(2,5-dimethoxy-4-methylphenyl)-2-aminopropane (DOM), ketanserin, and (R)-(+)-{alpha}-(2,3-dimethoxyphenyl)-1-[2-(4-fluorophenyl)ethyl]-4-pipidinemetha nol (MDL100907) in rats. J Pharmacol Exp Ther. 2009;331:671–9. doi: 10.1124/jpet.109.157560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JX, Zhang Y. Imidazoline I2 receptors: target for new analgesics? Eur J Pharmacol. 2011;658:49–56. doi: 10.1016/j.ejphar.2011.02.038. [DOI] [PubMed] [Google Scholar]
- Li JX, Zhang Y. Emerging drug targets for pain treatment. Eur J Pharmacol. 2012;681:1–5. doi: 10.1016/j.ejphar.2012.01.017. [DOI] [PubMed] [Google Scholar]
- Manchikanti L, Kaye AM, Kaye AD. Current State of Opioid Therapy and Abuse. Curr Pain Headache Rep. 2016;20:34. doi: 10.1007/s11916-016-0564-x. [DOI] [PubMed] [Google Scholar]
- McDonald GR, Olivieri A, Ramsay RR, Holt A. On the formation and nature of the imidazoline I2 binding site on human monoamine oxidase-B. Pharmacol Res. 2010;62:475–88. doi: 10.1016/j.phrs.2010.09.001. [DOI] [PubMed] [Google Scholar]
- Mercadante S, Bruera E. Opioid switching in cancer pain: From the beginning to nowadays. Crit Rev Oncol Hematol. 2016;99:241–8. doi: 10.1016/j.critrevonc.2015.12.011. [DOI] [PubMed] [Google Scholar]
- Meregalli C, Ceresa C, Canta A, Carozzi VA, Chiorazzi A, Sala B, Oggioni N, Lanza M, Letari O, Ferrari F, Avezza F, Marmiroli P, Caselli G, Cavaletti G. CR4056, a new analgesic I2 ligand, is highly effective against bortezomib-induced painful neuropathy in rats. J Pain Res. 2012;5:151–67. doi: 10.2147/JPR.S32122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIH. Pain Management Research Portfolio Online Reporting Tools. NIH; 2013. [Google Scholar]
- Classification of chronic pain. Descriptions of chronic pain syndromes and definitions of pain terms. Prepared by the International Association for the Study of Pain, Subcommittee on Taxonomy. Pain Suppl. 1986;3:S1–226. No author listed. [PubMed] [Google Scholar]
- Ramiro S, Radner H, van der Heijde D, van Tubergen A, Buchbinder R, Aletaha D, Landewe RB. Combination therapy for pain management in inflammatory arthritis (rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, other spondyloarthritis) Cochrane Database Syst Rev: CD008886. 2011 doi: 10.1002/14651858.CD008886.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudd RA, Seth P, David F, Scholl L. Increases in Drug and Opioid-Involved Overdose Deaths - United States, 2010–2015. MMWR Morb Mortal Wkly Rep. 2016;65:1445–1452. doi: 10.15585/mmwr.mm655051e1. [DOI] [PubMed] [Google Scholar]
- Siemian JN, Li J, Zhang Y, Li JX. Interactions between imidazoline I2 receptor ligands and acetaminophen in adult male rats: antinociception and schedule-controlled responding. Psychopharmacology (Berl) 2016a;233:873–82. doi: 10.1007/s00213-015-4166-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siemian JN, Obeng S, Zhang Y, Zhang Y, Li JX. Antinociceptive Interactions between the Imidazoline I2 Receptor Agonist 2-BFI and Opioids in Rats: Role of Efficacy at the mu-Opioid Receptor. J Pharmacol Exp Ther. 2016b;357:509–19. doi: 10.1124/jpet.116.232421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith HS. Opioids and neuropathic pain. Pain Physician. 2012;15:ES93–110. [PubMed] [Google Scholar]
- Thorn DA, Siemian JN, Zhang Y, Li JX. Anti-hyperalgesic effects of imidazoline I2 receptor ligands in a rat model of inflammatory pain: interactions with oxycodone. Psychopharmacology (Berl) 2015;232:3309–18. doi: 10.1007/s00213-015-3983-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorn DA, Zhang Y, Li JX. Effects of the imidazoline I2 receptor agonist 2-BFI on the development of tolerance to and behavioural/physical dependence on morphine in rats. Br J Pharmacol. 2016;173:1363–72. doi: 10.1111/bph.13435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk DC, Wilson HD, Cahana A. Treatment of chronic non-cancer pain. Lancet. 2011;377:2226–35. doi: 10.1016/S0140-6736(11)60402-9. [DOI] [PubMed] [Google Scholar]


