Abstract
Rational
Smoking typically begins during adolescence or early adulthood in a social context, yet the role of social context in animal models is poorly understood.
Objectives
The present study examined the effect of social context on acquisition of nicotine self-administration.
Methods
Sixty day-old male and female Sprague-Dawley rats were trained to press a lever for nicotine (0.015 mg/kg, IV) or saline infusions (males only) on a fixed-ratio (FR1) schedule of reinforcement across 9 sessions in duplex chambers that were conjoined with either a solid wall or a wall containing wire mesh creating a social context between rat dyads (social visual, auditory, and olfactory cues). In a subsequent experiment, sex differences and dose-dependent effects of nicotine [0 (saline), 0.015 or 0.03 mg/kg, IV] were directly compared in rats trained in the isolated or social context on a schedule progressing from FR1 to FR3. These rats were given 20 sessions followed by 3 extinction sessions.
Results
We consistently found transient social facilitation of low dose nicotine self-administration in males during the first session. However, across training overall we found social suppression of nicotine intake that was most prominent in females during later sessions.
Conclusions
Collectively, these findings suggest that at the age of transition from adolescence to adulthood, a social context enhances the initial reinforcing effects of nicotine in males, but protects against nicotine intake during later sessions especially in females. These findings highlight the importance of sex and social context in studying neural mechanisms involved in initiation of nicotine use.
Keywords: Adolescence, Drug self-administration, Social behavior. Dose-response, Sex differences, Extinction
Initiation of tobacco use in humans typically occurs in a social setting in which peer interaction serves to reinforce the behavior (West et al., 1999, Baker et al., 2004, Geckova et al., 2005, Sussman, 2005). Preclinical models of drug self-administration have also found that drug intake can be facilitated by the presence of a social partner that has prior experience performing the necessary response for drug reinforcement (see Neisewander et al., 2012, Bardo et al., 2013, and Strickland and Smith, 2015 for review). For instance, Chen and colleagues (2011) demonstrated that exposure to a social partner on the opposite side of perforated partition who is consuming a scented, flavored solution will enhance acquisition of intravenous nicotine self-administration in a partner rat for whom nicotine is delivered contingent upon this same response (i.e., licking a spout that delivers the scented, flavored solution). Similar results have been obtained regardless of whether the taste cue delivered upon licking is aversive or appetitive (Wang et al., 2016). These findings suggest that social learning plays a facilitative role in acquisition of nicotine self-administration in rats.
In addition to social learning, another factor that may contribute to facilitated acquisition of nicotine self-administration is the interaction between the rewarding effect of nicotine and the rewarding effect of a social companion. Consistent with this idea, we have shown that nicotine and social reward interact synergistically in male adolescent rats using the conditioned place preference (CPP) model. We found that CPP is observed after low dose nicotine injections when given to each rat of a pair that are together during conditioning whereas neither rewarding stimulus (i.e., nicotine injections or presence of another rat) supported CPP when given alone (Thiel et al., 2009, Bastle et al., 2016). We have also found that nicotine-induced activation of the hypothalamic-pituitary-adrenal (HPA) axis is blunted in male and female adolescent rats given nicotine immediately before being placed into a cage with a same-sex partner compared to controls that were placed into the cage alone (Pentkowski et al., 2011).
Two other factors known to contribute to nicotine reinforcement are age and sex. Rodents are more sensitive to the rewarding, and less sensitive to the aversive, effects of nicotine during adolescence compared to adulthood (Vastola et al., 2002, Belluzzi et al., 2004, O'Dell et al., 2006, Levin et al., 2007, but see Shram et al., 2008). In fact, earlier onset of nicotine self-administration in rats leads to higher levels of intake, which persist into adulthood (Levin et al., 2011). Sex differences involving drug abuse are well documented (see Carroll et al., 2004, and Roth et al., 2004 for review), but the involvement of sex and gonadal hormones on nicotine-related behaviors appears to be complicated by age as well as the drug paradigm utilized. Sex differences in which females are more sensitive than males have been reported for nicotine-CPP in both adolescent (Torres et al., 2009) and adult rodents (Isiegas et al., 2009, Pogun and Yararbas, 2009, Yararbas et al., 2010); however, neither sex nor estrous cycle phase appears to influence nicotine self-administration in adults (Donny et al., 2000, Chaudhri et al., 2005, Feltenstein et al., 2012). In adolescent rats, self-administration findings have been less consistent with either no sex difference (Chen et al., 2011), enhancement in males (Levin et al., 2011) or enhancement in females (Lynch, 2009), and inconsistent estrous cycle effects across these same studies. Other studies have failed to detect sex differences or estrous cycle effects on cue or stress-primed reinstatement of nicotine-seeking behavior (Feltenstein et al., 2012) or nicotine-induced hyperactivity (Kuo et al., 1999) in young adult rats.
The purpose of the present study was to directly test the effects of social context (i.e., presence of a conspecific) on acquisition of IV nicotine self-administration (0.015 and 0.03 mg/kg, IV) in drug-naïve male and female rats at the transition from adolescence to young adulthood. We custom-built dual-chambered apparatus that were conjoined by a removable partition that was either solid to isolate the rats from contact with each other or contained a mesh window that allowed for limited physical contact, but full olfactory, visual and auditory social cues, during self-administration. Such limited social contact is rewarding in CPP paradigms (Kummer et al., 2011, Peartree et al., 2012a). We avoided procedures used to facilitate acquisition of self-administration, such as food restriction, lever baiting, or response-contingent cues with intrinsic reinforcing value as these manipulations would confound interpretation of social influences on acquisition. We hypothesized that social context facilitates acquisition of nicotine self-administration through reward interaction.
Method
Animals and Experimental Designs
Male and female Sprague Dawley rats (Charles River, San Diego, CA) were housed in a climate-controlled colony room with a 12-h reverse light/dark cycle. Rats had ad libitum access to food and water in their home cage. All rats were handled for 2 min/day until the start of self-administration training. Housing, care and euthanasia were in accordance with the Guide for the Care and Use of Laboratory Animals (2011) and National Institutes of Health standards; all procedures were approved by the IACUC at Arizona State University.
Experiments 1 and 2 examined nicotine (Nic; 0.015 mg/kg, IV) self-administration in male and female rats, respectively. Experiment 3 examined saline (Sal; 0.00 mg/kg, IV) self-administration in male rats. The same procedures were used for all three of these experiments, beginning with arrival of the rats on post-natal day (PND) 27, with the exception of one cohort of males in Experiment 1 that arrived on PND 22. The rats were single-housed PNDs 27-46, and then on PNDs 47-51, they were pair-housed with a same sex partner to allow for rough-and-tumble play, which is important for social development (Panksepp, 1981, Vanderschuren et al., 1997). Experiment 4 was performed subsequently and included both male and female rats in order to directly examine sex differences, as well as separate dosage groups given access to saline (Sal; 0.00 mg/kg, IV) or 0.015 mg/kg and 0.03 mg/kg, IV nicotine (Nic) in order to examine dose-dependent effects. All doses are the free base concentration of (−)nicotine hydrogen tartrate (Sigma, St. Louis, MO), which was dissolved in saline, adjusted to a pH of 7.4±0.1, and filtered through a 0.2 μm filter. Several procedural changes were made in Experiment 4. First, rats arrived at a later age (PND 37) and were immediately pair-housed with a same sex partner in order to better foster social development and reduce stress. Second, we added an inactive control lever to assess inadvertent responses. Third, we examined extinction of self-administration. Finally, because the saline rats in Experiment 3 exhibited high response rates, we modified habituation and self-administration procedures to address this issue (detailed below). The experimental designs and final n/condition for each experiment are summarized in Table 1.
Table 1.
Summary of experimental designs and n/condition.
| Experiment | Sex | Dose (mg/kg) | Group | First Day N | Final Day N |
|---|---|---|---|---|---|
| 1 | Males | 0.015 | Isolated | 16 | 16 |
| Social | 20 | 20 | |||
| 2 | Females | 0.015 | Isolated | 16 | 15 |
| Social | 20 | 19 | |||
| 3 | Males | Saline | Isolated | 8 | 8 |
| Social | 10 | 10 | |||
| 4 | Males | Saline | Isolated | 14 | 14 |
| Social | 14 | 14 | |||
| 0.015 | Isolated | 10 | 10 | ||
| Social | 12 | 11 | |||
| 0.03 | Isolated | 14 | 11 | ||
| Social | 13 | 11 | |||
| Females | Saline | Isolated | 12 | 11 | |
| Social | 10 | 10 | |||
| 0.015 | Isolated | 12 | 9 | ||
| Social | 13 | 11 | |||
| 0.03 | Isolated | 14 | 8 | ||
| Social | 13 | 12 |
Surgery
On PND 51, catheters were implanted intravenously as described by Pockros et al. (2011) under isoflurane (2-4%) anesthesia. Rats were given subcutaneous (SC) injections of buprenorphine analgesic (0.05 mg/kg, SC) immediately prior to surgery and an anti-inflammatory agent, meloxicam (1 mg/kg, SC) immediately after surgery. To maintain catheter patency, a 0.1 ml IV solution of saline containing heparin sodium (70 USOU/ml; Baxter Healthcare Corporation, Deerfield, IL) and ticarcillin disodium (66.67 mg/ml: GlaxoSmithKline, Research Triangle Park, NC) was administered daily. Catheter patency was confirmed immediately after the first and last self-administration sessions and as needed by infusing 0.05 ml methohexital sodium (16.67 mg/ml IV; Sigma), which produces anesthetic effects only when administered IV.
Apparatus
The apparatus and dimensions are detailed in Figure 1. Briefly, duplex operant conditioning chambers had an adjoining wall that was either solid, black Plexiglas or black Plexiglas with a wire mesh window to create an isolated (Iso) or social (Soc) conditioning context between the 2 chambers, respectively. For Experiments 1-3, each chamber contained only 1, retractable lever that was used to control delivery of intravenous infusions of nicotine or saline (i.e., active lever). An additional, non-retractable lever was installed and present in each chamber throughout Experiment 4. Responses on this lever had no programmed consequences (i.e., inactive lever) and were used as a control for inadvertent lever presses.
Figure 1.
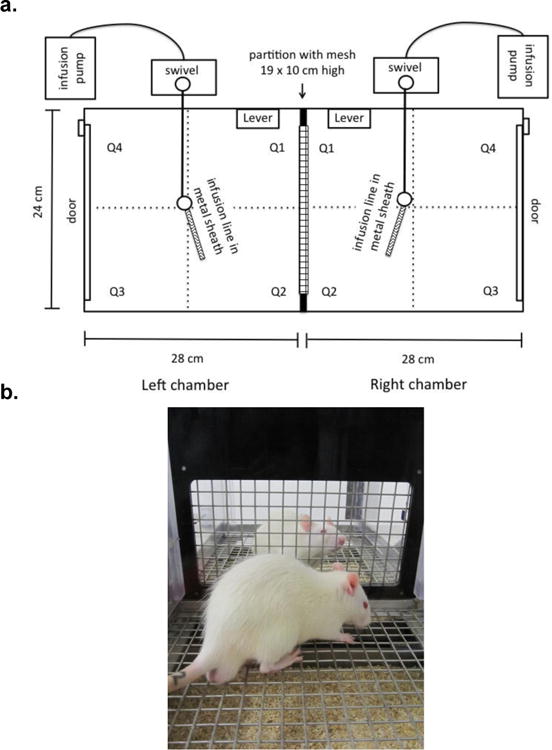
Arial configuration (a) and a side profile picture (b) of the self-administration apparatus with conjoined chambers that were separated by a partition. Rats were connected to infusion lines surrounded by a flexible metal sheath and then placed into the neighboring chambers either with a solid black Plexiglas partition in place isolating the rats during the session (Iso; not shown) or with a black Plexiglas partition containing a wire mesh section that allowed for visual and some tactile social cues, and stronger olfactory and auditory social cues during sessions (Soc; shown in b). Each chamber contained a retractable lever (active lever; i.e., reinforced lever) located 2.5 cm from the dividing partition wall and 7.5 cm above the floor. Experiment 4 included the addition of a non-retractable inactive lever (i.e., non-reinforced control lever; not pictured) on the wall opposite the active lever located 2.5 cm from the dividing partition wall and 7.5 cm above the floor. A camera sensitive to low levels of light (Panasonic WV-CP284, color CCTV, Suzhou, China) was used to record self-administration sessions and was mounted 60 cm above the center of the apparatus. A WinTV 350 personal video recorder (Hauppage, NJ, USA) captured live video and encoded it into MPEG streams for later analysis. Later videos were analyzed for entries into the 4 quadrants (Q1-Q4) demarcated by lines drawn on the display.
Habituation Procedures
All rats underwent habituation sessions on PNDs 57-58 during which they were allowed to explore their respective conditioning chambers while attached to their infusion line; however, no drug was available during these habituation sessions. For Experiments 1-3, rats received 2, 30-min exposure sessions/day over 2 consecutive days. For one of the daily sessions, the partition between the 2 self-administration chambers was solid black Plexiglas and for the other session the partition contained a mesh window allowing for social interaction. For Experiment 4, rats received a 1-hour exposure session/day over 2 consecutive days. For both of these habituation sessions, the partition between the 2 self-administration chambers was either solid black Plexiglas for the rats assigned to the Iso self-administration condition or contained a mesh window for the rats assigned to the Soc self-administration condition. For all experiments, the dyads of rats that were paired together during self-administration training were the same dyads that had been pair-housed together previously.
Self-Administration Procedures
Experiments 1-3 were conducted using the same procedures. On PND 59, the dyads were randomly assigned to training conditions with either the solid partition (Iso) or the mesh partition (Soc) in place throughout acquisition training. Nine self-administration sessions occurred daily for 2 h at the same time of day and were conducted 6-7 days/week. Sessions began by connecting the rats to their infusion line followed by a 1-min habituation period after which the retractable active levers were presented. Completion of a fixed ratio 1 (FR1) schedule of reinforcement resulted in retraction of the active lever, followed 0.5 s later by a 0.1 ml infusion of nicotine (0.015 mg/kg, IV) or saline (0.00 mg/kg) over 1.2 s. The levers remained retracted for a 20 s timeout. No other response-contingent cue lights/tones were used nor were the rats food-restricted or lever-baited in order to avoid potential confounding effects of these stimuli on acquisition (Peartree et al. 2012b).
Experiment 4 employed similar procedures as Experiments 1-3, with the exceptions that an inactive control lever was added and that rats progressed from an FR1 to an FR3 reinforcement schedule across 20 self-administration sessions. Also both male and female rats were included in Experiment 4 for analysis of sex differences. On PND 59, the same-sex dyads began self-administration sessions with either their assigned solid (Iso) or mesh (Soc) partition in place. Completion of a FR1 schedule of reinforcement resulted in retraction of the active lever followed 0.5 s later by a 0.1 ml infusion of either saline, 0.015 or 0.03 mg/kg nicotine, IV, delivered over 1.2 s. For sessions 4-20, rats began on a FR1schedule; however, the schedule progressed from a FR1 to FR2 then FR3 schedule of reinforcement depending on the rats' performance. The schedule increases were programmed to occur after 5 reinforcers had been delivered within 1 h on the current schedule. Responses on the inactive lever had no programmed consequences. After the last self-administration session, three daily nicotine extinction sessions occurred using identical procedures as sessions 4-20, except that saline was substituted for both nicotine doses.
Rats with catheter failure were eliminated from analyses but remained in the study to maintain contextual conditions for the partner with a patent catheter.
Time-Sampled Behavior Observations
Video recordings were made for one cohort of the male rats in Experiment 1 and the videos were later analyzed to determine whether there was a relationship between lever presses and locomotor activity for Soc male rats that displayed increased nicotine intake during the first self-administration session. A marked transparency was overlaid onto the computer screen that divided the chamber into 4 quadrants (Q1-Q4, see Fig. 1). A given rat's location and activity was measured using a time-sampling procedure of 4, 15-minute intervals with the first beginning once animals were placed into their chambers with levers presented, and subsequent intervals beginning 15 min after the end of each previous interval (i.e., alternating 15 min intervals of sampling vs. no sampling yielding 1 h total of observation distributed across the 2-h session). Horizontal locomotion was measured as the number forepaw/head entries into each quadrant. Vertical activity was measured as the number of rears within each quadrant, defined as raising forepaws off the ground in a vertical motion. The number of forepaw contacts with the adjoining wall and the number of rears over the lever were also counted. In Experiment 2, a different time-sampling procedure was used to determine whether there were changes across training days in the likelihood of a rat being near the adjoining wall of the conjoined self-administration chambers. While watching a video tape of the session, a tone sounded and at that instant the observer noted whether or not the rat was located in the side of the chamber with the adjoining wall. For a given rat, the tone sounded every 30 s throughout the first and ninth training sessions for a total of 240 observations/2 h.
Estrous Cycle Monitoring
Female rats were monitored daily after every self-administration session for estrous cycle phase beginning on PND 51 as detailed previously (Acosta et al. 2009; Becker et al. 2005; Goldman et al. 2007). Briefly, a sterile cotton applicator dipped in distilled water was gently inserted into the vaginal opening and removed after a circular motion along the vaginal walls to collect epithelial cells. Cells were transferred onto slides and assessed for vaginal cytology using bright-field microscopy at 10× and 40× magnification as described previously (see Becker et al. 2005; Caligioni 2009; Goldman et al. 2007; and Marcondes et al. 2002 for review). In Experiment 4, we controlled for genital stimulation by also gently swabbing the males around the anogenital region after each self-administration session.
Data Analysis
Reinforcers and lever presses were analyzed using mixed factor ANOVAs with session as a repeated measure and social condition (Iso vs. Soc) as a between-subjects factor in all experiments and sex and nicotine dose as between-subject factors in Experiment 4. ANOVAs were conducted with cycle phase and social condition as between-subject factors in Experiment 2 and dose as an additional factor in Experiment 4. We observed an increase in intake for Soc vs. Iso males self-administering 0.015 mg/kg Nic during the first session in Experiment 1, and therefore we predicted that Soc males would self-administer more Nic relative to Iso males at this dose during the first session in Experiment 4. This prediction was analyzed using an a priori independent samples t-test. Time-sampled behaviors in Experiment 1 during the first session were analyzed by quadrant using within-subject Wilcoxon signed-ranks tests and between groups for a given quadrant using independent sample t-tests, with Bonferroni correction for multiple comparison. Time-sampled observations in Experiment 2 were analyzed by a repeated measures ANOVA. All significant interactions were further analyzed using additional simpler ANOVAs and tests of simple effects (i.e., t-tests). These analyses were performed using SPSS 21 (IBM, Somers, NY) and descriptive data are expressed as mean ± SEM.
Results
Experiments 1-3: Effects of Social Context on Self-Administration
Figure 2a illustrates the timeline of Experiments 1-3 and Figure 2b-d illustrates the number of nicotine and saline reinforcers obtained across self-administration sessions in these experiments. In all three experiments, omnibus ANOVAs of reinforcers/session revealed a main effect of Session: Experiment 1 F(8,272) = 5.88, p <0.01, Experiment 2 F(8,256) = 2.78, p <0.01, and Experiment 3 F(1,8) = 9.88, p <0.0001. A Session × Social Condition interaction was also found for nicotine reinforcement in Experiment 1 [F(8,272) = 2.68, p <0.05] and Experiment 2 [F(8,256) = 2.36, p <0.05], but not for saline reinforcement in Experiment 3. Subsequent tests of simple effects revealed that in males given access to nicotine in Experiment 1, intake was higher in the Soc group compared to the Iso group during the first session [t(34) = 2.28, p <0.05], suggesting that social context enhances nicotine intake initially. In females given access to nicotine in Experiment 2, intake was similar in the Soc and Iso groups initially; however, intake was lower in the Soc group than in the Iso group during sessions 8 [t(32) = 2.09, p <0.05] and 9 [t(32) = 1.99, p=0.05], suggesting that social context protects against nicotine intake during later sessions in Soc females. In males given access to saline in Experiment 3, the lack of a Social Condition main effect or Session × Social Condition interaction indicates there was no difference between Iso and Soc groups, suggesting that the presence of a social partner failed to alter saline intake. Both saline groups exhibited a decrease in intake across sessions regardless of social condition.
Figure 2.
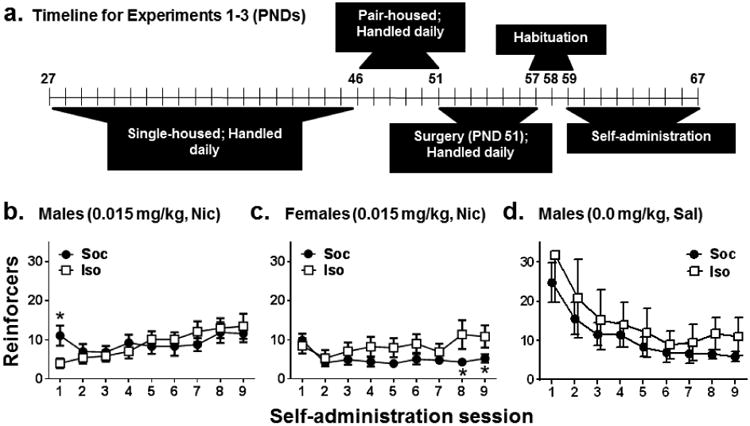
Timeline of experimental procedures across PNDs in Experiments 1-3 (a) and mean reinforcers obtained (±SEM) across acquisition sessions in (b) Experiment 1 with male rats given access to 0.015 mg/kg, IV nicotine, (c) Experiment 2 with female rats given access to 0.015 mg/kg, IV nicotine, and (d) Experiment 3 with male rats given access to saline (i.e., 0.00 mg/kg, IV). Sessions were conducted on a FR1 schedule of reinforcement while rats were isolated (Iso: open squares) or while allowed limited social contact through a mesh barrier (Soc: closed circles). See Table 1 for n/condition. Asterisk (*) represents a difference from Iso, test of simple effects, p <0.05.
Experiment 1: Analysis of Locomotor Activity during the First Session in Males
Several behaviors were measured in the male rats from Experiment 1 in order to assess confounding factors that may influence active levers presses, such as hyperactivity or increased proximity to the active lever. Figure 3 illustrates time-sampled observations of contacts with the adjoining wall and rears above the active lever, as well as rears and entries into each quadrant of the chamber as a measure of vertical and horizontal locomotion, respectively. Independent samples t-tests revealed that Soc males made more contacts with the adjoining wall (mesh partition) compared to the Iso males (solid partition) [t(16) = 8.72, p <0.001], suggesting motivation for social investigation and contact in the Soc group. There was no significant difference in number of rears directly over the lever between Iso and Soc rats, suggesting that increased nicotine intake in the Soc rats during this session was not merely due to inadvertent lever pressing as a result of increased proximity to the lever. There were no significant differences between Soc and Iso males for total number of quadrant entries or rears, suggesting that general activity did not differ between Iso and Soc males during the first session. Additionally, there were no differences between Soc and Iso rats' entries or rears by quadrant, although there were significantly more entries and rears in the adjoining side quadrants (Q1 and Q2) versus non-adjoining side quadrants (Q3 and Q4) regardless of social condition (Wilcoxon Signed-Ranks tests, p<0.05).
Figure 3.
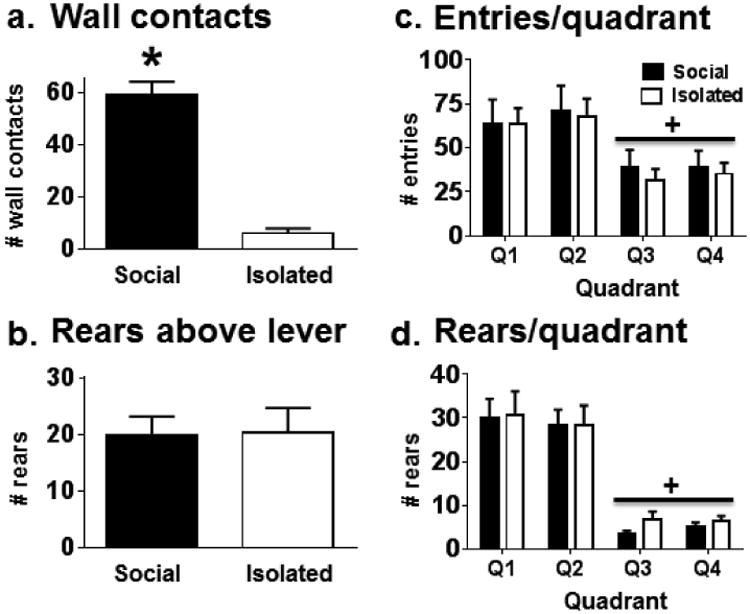
Mean (±SEM) time-sampled observations of (a) contacts with the adjoining wall, (b) rears above the active lever, (c) forepaw entries into each quadrant (Q1-Q4) of the chamber (Q1 and Q2 boarder the adjoining wall), and (d) rears observed in each quadrant in male rats during the first nicotine self-administration session in Experiment 1. Rats were either isolated (white bars) or given limited social contact through a mesh barrier (Social, black bars) during the session. See Table 1 for n/condition. Asterisk (*) represents an increase compared to the Isolated group, independent samples t-test, p <0.001. Plus sign (+) represents lower incidences in Q3/Q4 compared to Q1/Q2, Wilcoxon signed ranks tests, p <0.05.
Experiment 2: Proximity to Adjoining Wall across Sessions in Female Rats
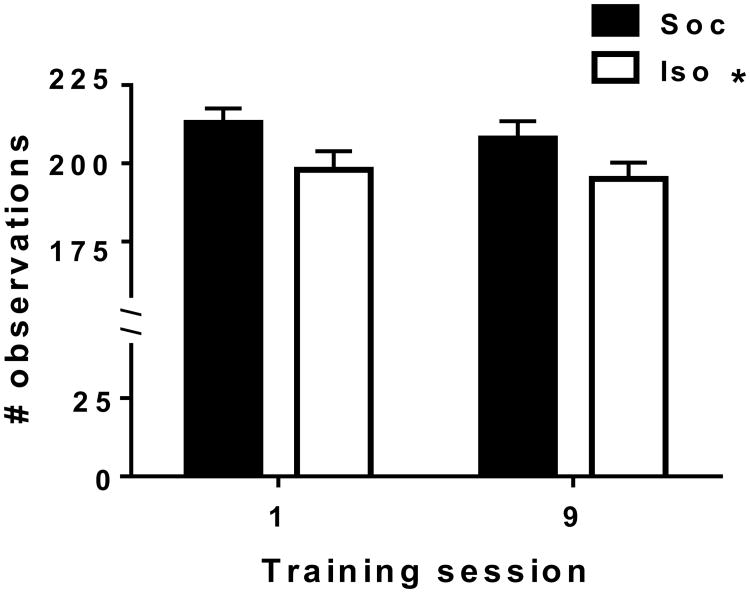
To assess whether the presence of another rat behind the adjoining wall of the chambers differentially influenced approach to that wall in the Iso (solid wall) versus Soc (mesh wall) conditions, we measured whether each rat was in the adjoining quadrants or not every 30 s for the duration of training sessions 1 and 9. The ANOVA of observations in the adjoining quadrants revealed an effect of Social Condition [F(1,32)=5.10, p <0.05; see Figure 4], but no effect of session nor interaction, indicating that female Soc rats showed more approach to the adjoining wall than Iso rats regardless of session.
Figure 4.
Mean (±SEM) time-sampled observations of location in quadrants Q1 and Q2 the contained the adjoining wall during training sessions 1 and 9 in female rats in Experiment 2. Rats were either isolated (white bars) or given limited social contact through a mesh barrier (Social, black bars) during the sessions. See Table 1 for n/condition. Asterisk (*) in legend denotes a main effect of Soc condition, p<0.05.
Experiment 4: Effects of Social Context, Sex and Dose on Self-Administration
The omnibus ANOVA of reinforcers obtained during the first session in Experiment 4, including all rats with patent catheters, revealed a Dose × Social Condition interaction [F(2,139) = 4.37, p <0.05; see Figure 5]. Subsequent post-hoc t-tests revealed that Sal intake was higher in the Soc rats relative to Iso rats during the first session [t(34.23) = 2.32, p <0.05; see Figure 5 insert]. A planned comparison revealed that male Soc rats self-administered more nicotine at the 0.015 mg/kg dose relative to their Iso counterparts [p<0.05], replicating the Social enhancement found in Experiment 1. Examination of the time course of infusions during the first session in this group did not reveal any consistent differences across social conditions (data not shown) and there was individual variation in nicotine intake within the Soc pairs.
Figure 5.
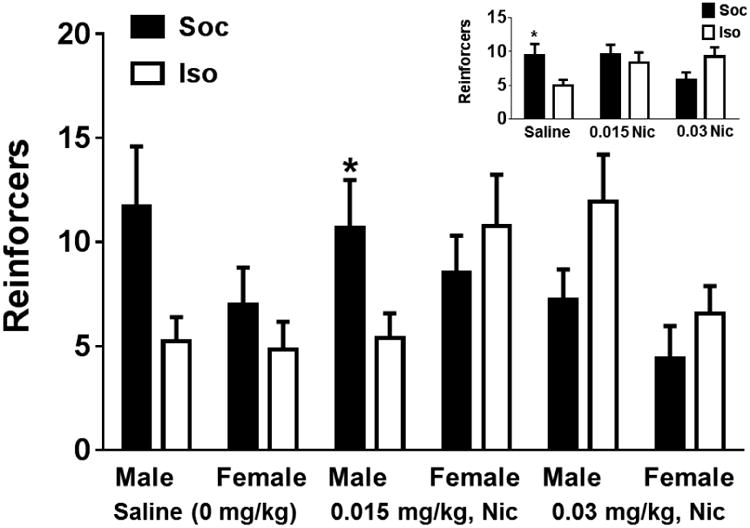
Mean reinforcers obtained (±SEM) during the first session of Experiment 4 conducted with male and female rats given saline (0.00 mg/kg, Sal), 0.015 mg/kg, IV nicotine (Nic), or 0.03 mg/kg, IV Nic on a FR1 schedule of reinforcement while allowed limited social contact through a mesh barrier (Soc: solid bars) or while isolated (Iso: open bars). Inset illustrates the social condition by dose interaction. N/condition ranged from 10-14. Asterisk (*) represents a difference from Iso, test of simple effects, p <0.05.
Figure 6 illustrates the number of nicotine and saline reinforcers obtained by rats that had patent catheters across all 20 self-administration sessions for Experiment 4 (see Table 1 for n/condition). The omnibus ANOVA of reinforcers obtained across sessions revealed main effects of Session [F(19,2280) = 11.17, p <0.001], Dose [F(2,120) = 4.07, p<0.05], and Social Condition [F(1,120) = 11.42, p<0.001], and Sex × Social Condition [F(1,120) = 8.13, p <0.01], Session × Dose [F(38,2280) = 2.61, p <0.001], Session × Social Condition [F(19,2280) = 2.05, p <0.01], Session × Sex × Dose [F(38,2280) = 2.41, p <0.001], and Session × Sex × Dose × Social Condition [F(38,2280) = 1.59, p<0.05] interactions. The 4-way interaction was subsequently analyzed using simpler ANOVAs that were systematically conducted to detect the source of the interaction.
Figure 6.
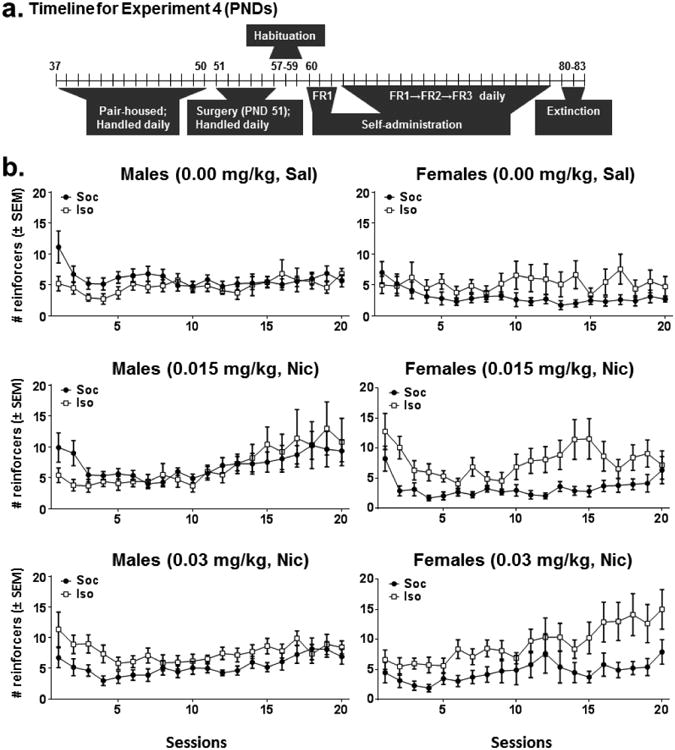
Timeline of experimental procedures across PNDs in Experiment 4 (a) and mean reinforcers obtained (±SEM) in Experiment 4 in male (b column) and female (c column) rats that had access to saline (0.00 mg/kg, IV, Sal) or a given dose of nicotine (0.015 or 0.03 mg/kg, IV, Nic) across acquisition on a FR1 schedule of reinforcement during sessions 1-3 and a progressive ratio schedule of reinforcement sessions 4-20 while allowed limited social contact through a mesh barrier (Soc: closed circles) or while isolated (Iso: open squares). The progressive schedule increased from an FR1 to FR2 to FR 3 depending on individual performance with the criterion for advancement of 5 reinforcers obtained within 60 min. See Table 1 for n/condition. A 4-way Session × Sex × Dose × Social Condition interaction was detected, ANOVA p <0.05. Further analyses of the interaction are shown in Figures 7 and 8.
Figure 7 illustrates the Session × Social Condition and the Sex × Social Condition interactions. Tests of simple effects of the Session × Social Condition interaction revealed that Iso rats obtained more reinforcers relative to Soc rats during sessions 4,7,11, 13-17 [t's(90.03-130) = 2.14-3.37, p's<0.05]. Tests of simple effects of the Sex × Social Condition interaction revealed that female Soc rats obtained fewer reinforcers than all other groups [t's(44.66-67) = 3.38-4.52, p's<0.01].
Figure 7.
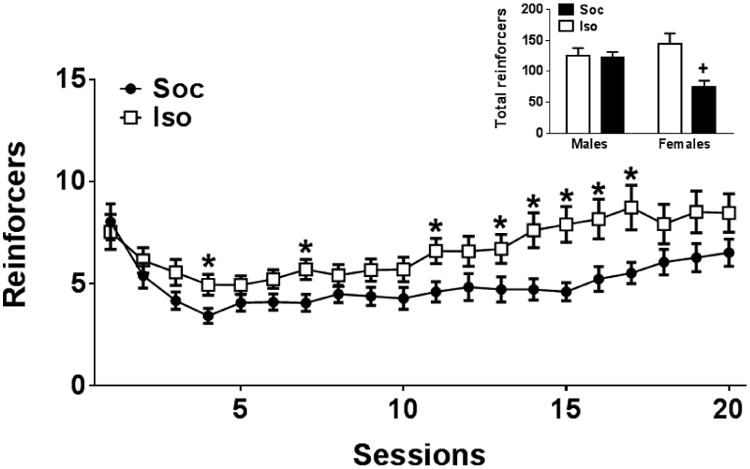
Mean reinforcers obtained (±SEM) in isolated (Iso: open squares/bars) and Social (Soc: closed circles/bars) groups from Experiment 4 across acquisition sessions collapsed across Sex and Dose and the total reinforcers obtained (±SEM) collapsed across Session and Dose (inset). An FR1 schedule of reinforcement was in effect during sessions 1-3 and a progressive ratio schedule was in effect during sessions 4-20 that increased from an FR1 to FR2 to FR 3 depending on individual performance with the criterion for advancement of 5 reinforcers obtained within 60 min. Asterisk (*) represents a difference from Soc, test of simple effects, ps<0.05. Plus sign (+) represent a difference from all other groups, test of simple effects, p <0.05.
Figure 8 illustrates the Session × Dose and the Session × Sex × Dose interactions. Subsequent tests of simple effects of the Session × Dose interaction (i.e., collapsed across sex and social condition) revealed that rats had lower Sal intake relative to Nic intake at both nicotine doses during later self-administration sessions: Sal vs. 0.015 mg/kg Nic on sessions 13, 15, 18-20 [t's(52.83-88) = 2.31-2.66, p's<0.05] and Sal vs. 0.03 mg/kg Nic on sessions 12, 13, 15-20 [t's(64.19-89) = 2.03-3.42, p's<0.05]. Subsequent Dose × Session ANOVAs conducted separately for males and females revealed a Session × Dose interaction for males [F(38,1235) = 1.92, p <0.01] and females [F(38,1045) = 3.06, p <0.001]. Tests of simple effects revealed that male rats displayed increased intake at 0.015 mg/kg and 0.03 mg/kg Nic relative to 0 mg/kg on multiple sessions: 0.015 mg/kg Nic vs. 0 mg/kg on sessions 13, 15, 19 [t's(23.91-47) = 2.00-2.38, p's<0.05] and 0.03 mg/kg Nic vs 0 mg/kg on sessions 3,19 [t's(48) = 2.21-2.52, p's<0.05]. Female rats displayed increased intake of 0.015 mg/kg relative to 0.03 mg/kg Nic [t(3330) = 2.29, p <0.05] and relative to Sal [t(32.03) = 2.06, p <0.05] on the first session as well as increased intake of 0.03 mg/kg Nic relative to Sal on sessions 15, 16, 18, 20 [t's(27.05-39) = 2.11-3.30, p's<0.05]. Though there was a significant Session × Sex interaction for 0.015 mg/kg Nic, there were no significant simple effects, although males showed some trends (ps = 0.059-0.10) toward more nicotine intake relative to females in later sessions.
Figure 8.
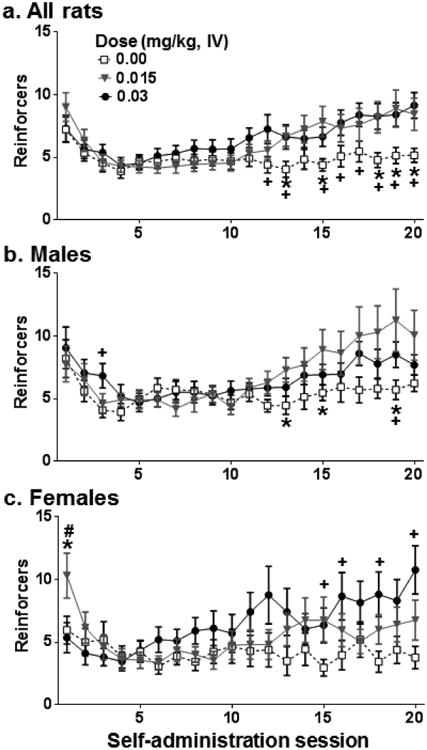
Dose-dependent differences in the mean reinforcers obtained (±SEM) across acquisition sessions in Experiment 4 collapsed across Sex and Social Condition (a; all rats), and collapsed across Social Condition for males (b) and females (c). Rats either had access to saline (0.00 mg/kg, Sal, open squares) or nicotine (0.015 mg/kg, Nic, triangles; 0.03 mg/kg, Nic, closed circles). An FR1 schedule of reinforcement was in effect during sessions 1-3 and a progressive ratio schedule was in effect during sessions 4-20 that increased from an FR1 to FR2 to FR 3 depending on individual performance with the criterion for advancement of 5 reinforcers obtained within 60 min. Asterisks (*) represent a difference between 0.015 and 0.00 mg/kg doses, test of simple effects, ps<0.05. Plus signs (+) represent a difference between 0.03 and 0.00 mg/kg doses, test of simple effects, ps<0.05. Pound sign (#) represents a difference between 0.015 and 0.03 mg/kg doses, test of simple effects, p <0.05.
Experiment 4: Effects of Social Context, Sex and Dose on Lever Responses
The omnibus ANOVA of active vs. inactive lever presses/session revealed main effects of Session [F(19,4560) = 8.45, p <0.0001], Dose [F(2,240) = 4.71, p <0.05], Social Condition [F(1,240) = 11.85, p <0.01], Lever [F(1,240) =21.88, p <0.0001], and Sex × Social Condition [F(1,240) = 6.22, p <0.05], Social Condition × Lever [F(1,240) = 6.42, p <0.05], Session × Dose [F(38,4560) = 3.02, p <0.0001], Session × Social Condition [F(19,4560) = 2.95, p <0.0001], Session × Lever [F(19,4560) = 10.83, p <0.0001], Session × Sex × Dose [F(38,4560) = 2.31, p <0.0001], Session × Dose × Lever [F(38,4560) = 1.95, p <0.0001], and Session × Social Condition × Lever [F(19,4560) = 2.14, p <0.01] interactions. To examine the Session × Dose × Lever interaction smaller ANOVAs for each dose revealed Session × Lever interactions for the 0.015 mg/kg Nic [F(19,1520) =5.57, p <0.0001] and 0.03 mg/kg Nic [F(19,1558) =3.90, p <0.0001], but not for 0.00 mg/kg Sal (see Figure 9 panels a-c). Subsequent post-hoc t-tests revealed that active lever presses were greater than inactive lever presses on sessions 11-20 for the 0.015 mg/kg Nic dose group [t's(42.10-63.56) = 2.03-3.16, p's<0.05] and on sessions 9 and 15-20 in the 0.03 mg/kg dose group [t's(54.30-73.64) = 1.99-3.48, p's<0.05] demonstrating that both doses served as a reinforcer.
Figure 9.
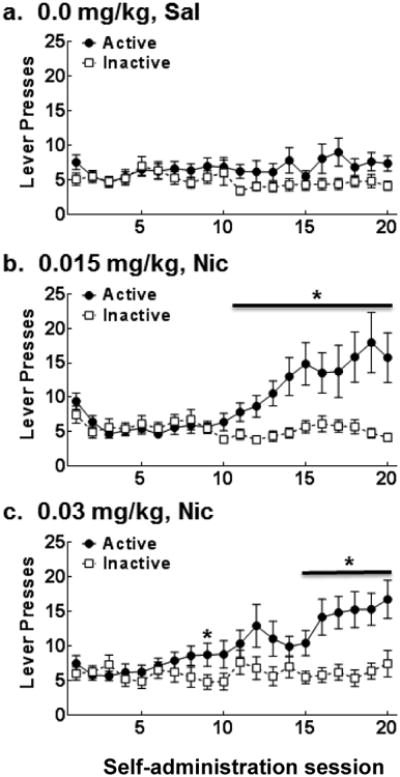
Mean lever presses (±SEM) on the active (closed circle) and inactive (open square) levers across sessions in Experiment 4 collapsed across Sex and Social Condition in rats with access to (a) saline (0.00 mg/kg, Sal) or nicotine at doses of 0.015 (b) or 0.03 (c) mg/kg, IV. An FR1 schedule of reinforcement was in effect during sessions 1-3 and a progressive ratio schedule was in effect during sessions 4-20 that increased from an FR1 to FR2 to FR 3 depending on individual performance with the criterion for advancement of 5 reinforcers obtained within 60 min. See Table 1 for n/condition. Asterisks (*) represent a difference between active and inactive lever, test of simple effects, ps<0.05.
Experiment 4: Effects of Social Context, Sex and Dose on Responses during Extinction
The omnibus ANOVA of reinforcers/session during extinction revealed main effects of Session [F(2,240) = 12.49, p <0.001], Sex [F(1,120) = 4.34, p <0.05], Dose [F(2,120) = 6.63, p <0.01], and Social Condition [F(1,120) = 4.32, p <0.05] as well as a Session × Dose [F(4,240) = 3.09, p <0.05] interaction. The main effect of Sex revealed that males exhibited higher response rates compared to females during extinction sessions. The mean saline reinforcers obtained (±SEM) across Extinction sessions was 21.77 ± 1.74 for males and 16.65 ± 1.94 for females. The main effect of Dose revealed that the 2 nicotine dosage groups exhibited higher response rates relative to Saline controls during extinction sessions. The mean total saline reinforcers obtained (±SEM) across Extinction sessions was 13.96 ± 1.74 for Sal, 23.29 ± 2.90 for 0.015 mg/kg Nic, and 21.98 ± 1.92 for 0.03 mg/kg Nic. The main effect of Social Condition revealed that Iso groups exhibited higher response rates compared to Soc groups during Extinction sessions. The mean saline reinforcers obtained (±SEM) across Extinction sessions was 21.73 ± 2.12 for Iso and 17.29 ± 1.56 for Soc. The Session × Dose interaction is illustrated in Figure 10. Tests of simple effects revealed that the 2 nicotine dosage groups exhibited higher response rates relative to saline controls on the first 2 days of extinction. No significant differences were found among groups on session 3, suggesting that Nic rats had extinguished their nicotine-seeking behavior by the third session.
Figure 10.
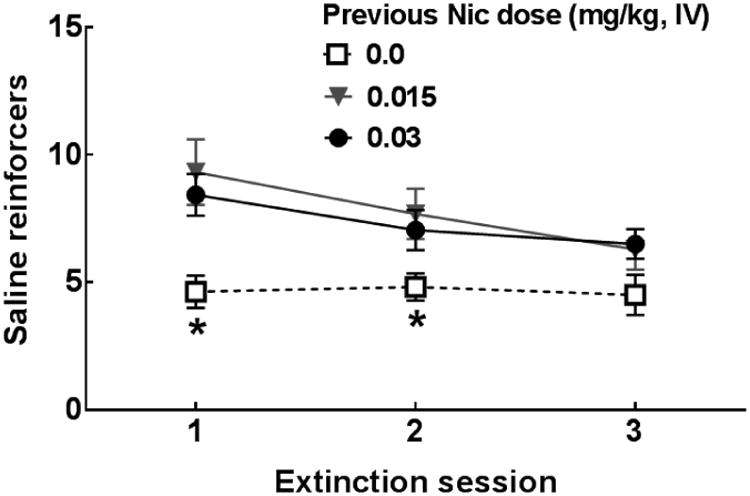
Mean saline reinforcers obtained (±SEM) in Experiment 4 across Extinction sessions collapsed across Sex and Social Condition for rats that had previously self-administered saline (0.00 mg/kg, Sal: squares) or nicotine at doses of 0.015 (triangles) or 0.03 (circles) mg/kg, IV. See Table 1 for n/condition. Asterisks (*) represent a decrease relative to both of the other groups, test of simple effects, ps<0.05.
Experiment 2 and 4: Effects of Estrous Cycle Phase on Self-administration
There were no significant effects of estrous cycle phase on reinforcers obtained during sessions 1 or 9 in Experiment 2 (data not shown), however, this experiment was not designed to address cycle phase influence. Indeed with the small number of naturally cycling rats used in this experiment and their uneven distribution across cycle phases, there was insufficient power to detect an effect (n's=2-12). In Experiment 4, the ANOVA of estrous cycle phase effects on reinforcers obtained revealed a significant Dose × Estrous Cycle Phase interaction on session 1 [F(4,45) =2.85, p <0.05] and a strong trend towards a Dose × Estrous Cycle Phase interaction on session 20 [p=0.051]. Despite the interaction observed for session 1, post-hoc simple effects t-tests failed to reveal any significant differences among the rats tested at different estrous cycle phases at any of the doses (data not shown). There were no effects of Estrous Cycle Phase for Extinction session 1. Overall the results failed to support an estrous cycle effect on nicotine or saline intake. To simplify presentation of the data, mean reinforcers/session (±SEM) are displayed in Table 2 for rats tested at different phases of the estrous cycle collapsed across dose.
Table 2.
Reinforcers obtained (±SEM) during self-administration sessions 1 and 20 and the first extinction session (Ext 1) by female rats (n in parentheses) at different phases of the estrous cycle in Experiment 4.*
| Cycle phase | Self-administration session | ||
|---|---|---|---|
| 1 | 20 | EXT1 | |
| Estrus | 7.5 ± 1.3 (22) | 7.1 ± 1.3 (33) | 6.4 ± 1.6 (23) |
| Diestrus/metestrus | 7.4 ± 1.3 (33) | 7.1 ± 1.4 (24) | 6.9 ± 1.0 (26) |
| Proestrus | 4.8 ± 1.7 (6) | 6.0 ± 4.0 (4) | 5.6 ± 1.6 (12) |
There were no significant effects of estrous cycle phase on reinforcers obtained.
Discussion
To model the age when humans usually initiate smoking (West et al., 1999, Baker et al., 2004, Geckova et al., 2005, Sussman, 2005), training began at the rats' transition from adolescence to adulthood. We predicted that nicotine self-administration would be enhanced in a social context regardless of sex and nicotine doses. Contrary to our predictions, females did not exhibit social facilitation of self-administration. As predicted, males reliably exhibited social facilitation at the low dose of nicotine (0.015 mg/kg) during the first session, however, they also exhibited social facilitation of saline self-administration and failed to exhibit the effect at a higher dose (0.03 mg/kg). We also found that isolated rats overall had higher nicotine intake than rats trained in a social context, and this effect appeared more robust in females than males.
We suggest that the social facilitation of nicotine self-administration in male rats resulted from the rewarding effects of social context (Kummer et al., 2011, Peartree et al., 2012a) interacting with the reinforcing effects of saline and low dose nicotine during the initial session. This explanation is consistent with our previous findings of enhanced nicotine-CPP when adolescent male rats experience nicotine in a social context versus in isolation (Thiel et al., 2009). In addition to a synergistic interaction between nicotine and social rewards, social context may blunt nicotine-induced stress. Consistent with this idea, nicotine-induced increases in corticosterone in adolescent male and female rats is attenuated in a social context versus isolation (Pentkowski et al., 2011).
The predicted social facilitation of operant responding during the first self-administration session appeared to be specific to males as females did not show this effect in either experiment 2 or 4. Females are more sensitive than males to some nicotine effects (Isiegas et al., 2009, Lynch, 2009, Pogun and Yararbas, 2009, Torres et al., 2009, Yararbas et al., 2010), and so it is possible that social facilitation may have been observed in females at a nicotine dose lower than the 0.015 mg/kg, IV dose used. We did not examine a lower dose because several male and female rats failed to acquire self-administration at the low dose and therefore an even higher attrition rate at a lower dose would make it difficult to detect an effect. The findings in males suggest that they are more prone to interact with environmental stimuli (i.e., the lever) when in a social context than when isolated, perhaps related to territorial behavior (Beatty, 1979). Alternatively, but not mutually exclusive, enhancement of the mildly reinforcing stimulus effects of saline and low dose nicotine self-administration by the social context is likely more robust in males than in females.
It was surprising that the social facilitation effect occurred in male rats self-administering saline in Experiment 4. This finding contrasts with those across Experiments 1 and 3 where social facilitation was only observed for nicotine and not saline self-administration, as well as with previous studies reporting social facilitation of nicotine and cocaine intake compared to saline intake (Chen et al., 2011, Smith, 2012) and d-amphetamine intake compared to sucrose intake (Gipson et al., 2011). In the latter studies, one rat of the dyad was experienced with operant responding, and therefore as suggested by these authors, the social facilitation was likely due to social learning. By contrast, both rats of the dyads in the present study were experimentally naïve. Thus, our findings suggest that mechanisms other than social learning may initially contribute to social context effects.
The lack of drug experience of the partner rats may have also contributed to our discrepant finding that overall isolated rats obtained more reinforcers than social rats regardless of nicotine dose. This effect primarily emerged during later sessions and appeared more robust in isolated females. The effect also contrasts with our hypothesis and with previous research demonstrating that the presence of another rat enhances responding for IV cocaine, d-amphetamine, and nicotine (Chen et al., 2011, Gipson et al., 2011, Smith, 2012). The reason we chose to use drug-naïve rats was to allow us to examine interactions between nicotine and social context reward apart from social learning influences. Social learning, however, contributes to tobacco use in teens as family members and friends who use tobacco increase risk of use (Jackson et al., 1997, Brandon et al., 2004). In general previous studies with alcohol or psychostimulants employing a demonstrator rat (i.e, a rat with prior drug and/or operant conditioning experience) have found social facilitation effects (Anacker et al., 2011a, Anacker et al., 2011b, Chen et al., 2011, Gipson et al., 2011, Smith, 2012). Also, pairing drug-experienced rats together sometimes alters the drinking patterns of the rats such that intake is similar in the dyads even if there were differences in intake prior to pairing (Anacker et al., 2011b). Thus, the degree of drug experience is an important variable to consider in assessing social context effects on drug self-administration.
The decreased reinforcement rate in social rats in the present study may be a result of protective effects of social interaction against nicotine reinforcement. Alternatively, engaging in social interaction could detract from the amount of time available to self-administer nicotine. However, the latter explanation seems unlikely given that initially social context enhances both reinforcement rate and contact with the mesh wall, demonstrating increases in seeking/consumption of both rewards simultaneously rather than an increase in one at the expense of the other. It seems more likely that the motivation for, or rewarding effects of, nicotine are diminished across time when rats are in a social context. Indeed, previous studies have shown that drug and social rewards compete (Hecht et al., 1999, Carroll et al., 2004, Seip and Morrell, 2007, Fritz et al., 2011b). For instance, opportunity for social interaction competes with, and decreases, expression of cocaine CPP in adult male rats (Fritz et al., 2011). More research is needed to better characterize the conditions under which social context enhances versus attenuates drug self-administration.
It is unlikely that general activity or location in the chamber accounts for the differences in self-administration between social and isolated rats. In Experiment 1, neither time-sampled observations of entries into the quadrant of the chamber containing the lever nor rears above the lever differed between Iso and Soc males, suggesting that the increase in lever presses in Soc males was not due to inadvertent presses made during exploration. There were also no differences between Soc and Iso males in entries into any other chamber quadrant, suggesting that their overall activity was similar. These rats did show an increase in contacts with the adjoining wall of the chamber, which was expected due to the opportunity to interact with a social partner. Similarly, Soc females in Experiment 2 were in the quadrants with the adjoining wall more often than Iso females. The finding that the Soc males in Experiment 1 had higher nicotine intake than the Iso males even though they also made more contacts with the adjoining wall indicates that increased nicotine self-administration is possible even when rats show increased social seeking and interaction. Furthermore, several previous experiments with nicotine and other stimulants have also found increases in drug self-administration in both male and female rats in a social context compared to isolated (Chen et al., 2011, Gipson et al., 2011, Smith, 2012, Smith et al., 2014, Wang et al., 2016), again suggesting that social interaction does not compete against operant behavior effectively enough to interfere with enhanced nicotine self-administration.
Nicotine served as a reinforcer in Experiment 4 with dose effects dependent on sex. Preferential responding on the reinforced (active) versus non-reinforced (inactive) lever was evident for both nicotine dosage groups, but not for saline controls. These effects were robust during later sessions due to the increased demand of the schedule of reinforcement used after session 4 (FR1 → FR2 → FR3). Response rates in the nicotine groups were also higher than the saline group during the first 2 extinction sessions, further supporting that nicotine served as a reinforcer. The nicotine extinction curve is similar to previous studies examining response rates when saline was substituted for nicotine (LeSage et al., 2004, Liu et al., 2006, O'Dell et al., 2007). Although it is less clear that nicotine served as a reinforcer in Experiments 1 and 2, several aspects of the data support this interpretation. Response rates without reinforcement should either not change or decline across time due as the rat habituates to the chamber, yet we observed drug-dependent differences across time. For instance, even though Experiments 1 and 3 used the same training procedures the nicotine reinforcement rate was surprisingly lower in Experiment 1 compared to the saline reinforcement rate in Experiment 3. We have shown previously that it takes about one week or longer to observe an increase in active lever response rates when using training procedures that omit response-contingent cue lights or lever baiting (Peartree et al., 2012b). The present findings suggest that this time lag in the acquisition curve is due to nicotine reinforcement initially suppressing operant responding during training rather than to difficulty in learning to acquire the reinforcement contingency. Further research directly comparing nicotine and saline self-administration rates in the same experiment is needed to confirm this idea.
We did not observe estrous cycle effects in this study, however, this study was not designed with the goal of examining this issue and therefore the unequal n across cycle phases impaired the power to detect cycle phase effects. Estrous cycle effects on nicotine self-administration are not well understood given that some studies have failed to find an effect in adolescent (Levin et al., 2011) or adult female rats (Donny et al., 2000), whereas Lynch and colleagues (2009) found enhanced nicotine intake on a progressive ratio schedule of reinforcement during estrus in female adolescent rats. The discrepancies may involve age and/or the schedule of reinforcement used. Research with other models has failed to find estrous cycle phase effects, including studies using nicotine-induced place preference (Torres et al., 2009), cue- or stress-primed reinstatement of nicotine-seeking behavior (Feltenstein et al., 2012) or nicotine-induced hyperlocomotion (Kuo et al., 1999). Taken together, it seems estrous cycle phase does not exert a strong effect on nicotine self-administration.
In summary, the present findings suggest that social factors exert reliable influences on nicotine self-administration that vary depending on sex in rats transitioning from adolescence to adulthood. Specifically, social context initially facilitates intake in male rats at a low nicotine dose, yet in later sessions attenuates nicotine intake particularly in female rats. Since initiation of nicotine use in humans typically occurs in a social setting (West et al., 1999, Baker et al., 2004, Geckova et al., 2005, Sussman, 2005), the use of social context during acquisition of nicotine self-administration is important and under-utilized in preclinical animal studies. Given that different neural circuits are activated in social versus isolated rodents (Insel, 1992, Young et al., 2001, Fritz et al., 2011a, El Rawas et al., 2012, Bastle et al., 2016), which may influence neural activity when exposed to drugs of abuse (Pentkowski et al., 2011, Bastle et al., 2016), future research aimed at understanding the neural mechanisms that underlie social influences on nicotine self-administration may have important implications for developing treatments for nicotine dependence.
Acknowledgments
The authors wish to thank Nathan Pentkowski, Kenneth Thiel, Lara Pockros, Ryan Bastle, Suzanne Weber, Lauren Hood, Jared Deunsing, Colter Whillock, Jonathan Griffin, Jose Alba, Matthew Adams, Lindsey Robertson-Hammerslag, Quintana Carter, Kenneth Leslie, and John Paul Bonadonna for assistance with surgery and data collection and Dr. Heather Bimonte-Nelson for her laboratory's assistance with analysis of cell cytology. The authors wish to dedicate this manuscript in memory of the late Suzanne Weber. Support was provided by the National Institute on Drug Abuse under award numbers R01DA11064, R21DA023123, F31DA033805 and by Arizona State University's More Graduate Education at Mountain States Alliance Program and Undergraduate Science Education Program. These funding sources had no further role in this study or manuscript.
Footnotes
Conflict of Interest: All authors, Natalie A. Peartree, Kayla N. Chandler, Julianna G. Goenaga, Nora R. Dado, Hanna Molla, Martin Dufwenberg, Allegra Campagna, Rachel Mendoza, Timothy H.C. Cheung, Joshua S. Talboom, and Janet L. Neisewander declare that they have no conflict of interest to disclose.
References
- Anacker AM, Loftis JM, Kaur S, Ryabinin AE. Prairie voles as a novel model of socially facilitated excessive drinking. Addict Biol. 2011a;16:92–107. doi: 10.1111/j.1369-1600.2010.00234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anacker AM, Loftis JM, Ryabinin AE. Alcohol intake in prairie voles is influenced by the drinking level of a peer. Alcohol Clin Exp Res. 2011b;35:1884–1890. doi: 10.1111/j.1530-0277.2011.01533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker TB, Brandon TH, Chassin L. Motivational influences on cigarette smoking. Annu Rev Psychol. 2004;55:463–491. doi: 10.1146/annurev.psych.55.090902.142054. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Neisewander JL, Kelly TH. Individual differences and social influences on the neurobehavioral pharmacology of abused drugs. Pharmacol Rev. 2013;65:255–290. doi: 10.1124/pr.111.005124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastle RM, Peartree NA, Goenaga J, Hatch KN, Henricks A, Scott S, Hood LE, Neisewander JL. Immediate early gene expression reveals interactions between social and nicotine rewards on brain activity in adolescent male rats. Behavioural Brain Research. 2016 doi: 10.1016/j.bbr.2016.07.024. Epub Ahead of Print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty WW. Gonadal hormones and sex differences in nonreproductive behaviors in rodents: organizational and activational influences. Horm Behav. 1979;12:112–163. doi: 10.1016/0018-506x(79)90017-5. [DOI] [PubMed] [Google Scholar]
- Belluzzi JD, Lee AG, Oliff HS, Leslie FM. Age-dependent effects of nicotine on locomotor activity and conditioned place preference in rats. Psychopharmacology (Berl) 2004;174:389–395. doi: 10.1007/s00213-003-1758-6. [DOI] [PubMed] [Google Scholar]
- Brandon TH, Herzog TA, Irvin JE, Gwaltney CJ. Cognitive and social learning models of drug dependence: implications for the assessment of tobacco dependence in adolescents. Addiction. 2004;99(1):51–77. doi: 10.1111/j.1360-0443.2004.00737.x. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Lynch WJ, Roth ME, Morgan AD, Cosgrove KP. Sex and estrogen influence drug abuse. Trends Pharmacol Sci. 2004;25:273–279. doi: 10.1016/j.tips.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Booth S, Gharib MA, Craven LA, Allen SS, Sved AF, Perkins KA. Sex differences in the contribution of nicotine and nonpharmacological stimuli to nicotine self-administration in rats. Psychopharmacology (Berl) 2005;180:258–266. doi: 10.1007/s00213-005-2152-3. [DOI] [PubMed] [Google Scholar]
- Chen H, Sharp BM, Matta SG, Wu Q. Social Interaction Promotes Nicotine Self-Administration with Olfactogustatory Cues in Adolescent Rats. Neuropsychopharmacology. 2011 doi: 10.1038/npp.2011.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Rowell PP, Gharib MA, Maldovan V, Booth S, Mielke MM, Hoffman A, McCallum S. Nicotine self-administration in rats: estrous cycle effects, sex differences and nicotinic receptor binding. Psychopharmacology (Berl) 2000;151:392–405. doi: 10.1007/s002130000497. [DOI] [PubMed] [Google Scholar]
- El Rawas R, Klement S, Salti A, Fritz M, Dechant G, Saria A, Zernig G. Preventive role of social interaction for cocaine conditioned place preference: correlation with FosB/DeltaFosB and pCREB expression in rat mesocorticolimbic areas. Front Behav Neurosci. 2012;6:8. doi: 10.3389/fnbeh.2012.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltenstein MW, Ghee SM, See RE. Nicotine self-administration and reinstatement of nicotine-seeking in male and female rats. Drug and alcohol dependence. 2012;121:240–246. doi: 10.1016/j.drugalcdep.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz M, El Rawas R, Klement S, Kummer K, Mayr MJ, Eggart V, Salti A, Bardo MT, Saria A, Zernig G. Differential effects of accumbens core vs. shell lesions in a rat concurrent conditioned place preference paradigm for cocaine vs. social interaction. PLoS One. 2011a;6:e26761. doi: 10.1371/journal.pone.0026761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz M, El Rawas R, Salti A, Klement S, Bardo MT, Kemmler G, Dechant G, Saria A, Zernig G. Reversal of cocaine-conditioned place preference and mesocorticolimbic Zif268 expression by social interaction in rats. Addict Biol. 2011b;16:273–284. doi: 10.1111/j.1369-1600.2010.00285.x. [DOI] [PubMed] [Google Scholar]
- Geckova A, Stewart R, van Dijk JP, Orosova O, Groothhoff VW, Post D. Influence of socio-economic status, parents and peers on smoking behavior in adolescents. European Addiction Research. 2005;11:204–209. doi: 10.1159/000086403. [DOI] [PubMed] [Google Scholar]
- Gipson CD, Yates JR, Beckmann JS, Marusich JA, Zentall TR, Bardo MT. Social facilitation of d-amphetamine self-administration in rats. Exp Clin Psychopharmacol. 2011 doi: 10.1037/a0024682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht GS, Spear NE, Spear LP. Changes in progressive ratio responding for intravenous cocaine throughout the reproductive process in female rats. Developmental psychobiology. 1999;35:136–145. [PubMed] [Google Scholar]
- Insel TR. Oxytocin--a neuropeptide for affiliation: evidence from behavioral, receptor autoradiographic, and comparative studies. Psychoneuroendocrinology. 1992;17:3–35. doi: 10.1016/0306-4530(92)90073-g. [DOI] [PubMed] [Google Scholar]
- Isiegas C, Mague SD, Blendy JA. Sex differences in response to nicotine in C57Bl/6:129SvEv mice. Nicotine Tob Res. 2009;11:851–858. doi: 10.1093/ntr/ntp076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson C, Henriksen L, Dickinson D, Levine DW. The early use of alcohol and tobacco: its relation to children's competence and parents' behavior. Am J Public Health. 1997;87:359–364. doi: 10.2105/ajph.87.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kummer K, Klement S, Eggart V, Mayr MJ, Saria A, Zernig G. Conditioned place preference for social interaction in rats: contribution of sensory components. Frontiers in behavioral neuroscience. 2011;5:80. doi: 10.3389/fnbeh.2011.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo DY, Lin TB, Huang CC, Duh SL, Liao JM, Cheng JT. Nicotine-induced hyperlocomotion is not modified by the estrous cycle, ovariectomy and estradiol replacement at physiological level. The Chinese journal of physiology. 1999;42:83–88. [PubMed] [Google Scholar]
- LeSage MG, Burroughs D, Dufek M, Keyler DE, Pentel PR. Reinstatement of nicotine self- administration in rats by presentation of nicotine-paired stimuli, but not nicotine priming. Pharmacol Biochem Behav. 2004;79:507–513. doi: 10.1016/j.pbb.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Levin ED, Lawrence SS, Petro A, Horton K, Rezvani AH, Seidler FJ, Slotkin TA. Adolescent vs. adult-onset nicotine self-administration in male rats: duration of effect and differential nicotinic receptor correlates. Neurotoxicol Teratol. 2007;29:458–465. doi: 10.1016/j.ntt.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Slade S, Wells C, Cauley M, Petro A, Vendittelli A, Johnson M, Williams P, Horton K, Rezvani AH. Threshold of adulthood for the onset of nicotine self-administration in male and female rats. Behavioural brain research. 2011;225:473–481. doi: 10.1016/j.bbr.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Caggiula AR, Yee SK, Nobuta H, Poland RE, Pechnick RN. Reinstatement of nicotine- seeking behavior by drug-associated stimuli after extinction in rats. Psychopharmacology (Berl) 2006;184:417–425. doi: 10.1007/s00213-005-0134-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ. Sex and ovarian hormones influence vulnerability and motivation for nicotine during adolescence in rats. Pharmacology, biochemistry, and behavior. 2009;94:43–50. doi: 10.1016/j.pbb.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National-Research-Council-Committee. Guide for the Care abd Use of Laboratory Animals. Washington, D.C: National Academies Press (US); 2011. [Google Scholar]
- Neisewander JL, Peartree NA, Pentkowski NS. Emotional valence and context of social influences on drug abuse-related behavior in animal models of social stress and prosocial interaction. Psychopharmacology. 2012;224:33–56. doi: 10.1007/s00213-012-2853-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Dell LE, Bruijnzeel AW, Smith RT, Parsons LH, Merves ML, Goldberger BA, Richardson HN, Koob GF, Markou A. Diminished nicotine withdrawal in adolescent rats: implications for vulnerability to addiction. Psychopharmacology (Berl) 2006;186:612–619. doi: 10.1007/s00213-006-0383-6. [DOI] [PubMed] [Google Scholar]
- O'Dell LE, Chen SA, Smith RT, Specio SE, Balster RL, Paterson NE, Markou A, Zorrilla EP, Koob GF. Extended access to nicotine self-administration leads to dependence: Circadian measures, withdrawal measures, and extinction behavior in rats. J Pharmacol Exp Ther. 2007;320:180–193. doi: 10.1124/jpet.106.105270. [DOI] [PubMed] [Google Scholar]
- Panksepp J. The ontogeny of play in rats. Dev Psychobiol. 1981;14:327–332. doi: 10.1002/dev.420140405. [DOI] [PubMed] [Google Scholar]
- Peartree NA, Hood LE, Thiel KJ, Sanabria F, Pentkowski NS, Chandler KN, Neisewander JL. Limited physical contact through a mesh barrier is sufficient for social reward-conditioned place preference in adolescent male rats. Physiol Behav. 2012a;105:749–756. doi: 10.1016/j.physbeh.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peartree NA, Sanabria F, Thiel KJ, Weber SM, Cheung TH, Neisewander JL. A new criterion for acquisition of nicotine self-administration in rats. Drug Alcohol Depend. 2012b doi: 10.1016/j.drugalcdep.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pentkowski NS, Painter MR, Thiel KJ, Peartree NA, Cheung TH, Deviche P, Adams M, Alba J, Neisewander JL. Nicotine-induced plasma corticosterone is attenuated by social interactions in male and female adolescent rats. Pharmacol Biochem Behav. 2011;100:1–7. doi: 10.1016/j.pbb.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pockros LA, Pentkowski NS, Swinford SE, Neisewander JL. Blockade of 5-HT2A receptors in the medial prefrontal cortex attenuates reinstatement of cue-elicited cocaine-seeking behavior in rats. Psychopharmacology (Berl) 2011;213:307–320. doi: 10.1007/s00213-010-2071-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogun S, Yararbas G. Sex differences in nicotine action. Handb Exp Pharmacol. 2009:261–291. doi: 10.1007/978-3-540-69248-5_10. [DOI] [PubMed] [Google Scholar]
- Roth ME, Cosgrove KP, Carroll ME. Sex differences in the vulnerability to drug abuse: a review of preclinical studies. Neurosci Biobehav Rev. 2004;28:533–546. doi: 10.1016/j.neubiorev.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Seip KM, Morrell JI. Increasing the incentive salience of cocaine challenges preference for pup-over cocaine-associated stimuli during early postpartum: place preference and locomotor analyses in the lactating female rat. Psychopharmacology. 2007;194:309–319. doi: 10.1007/s00213-007-0841-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shram MJ, Funk D, Li Z, Le AD. Nicotine self-administration, extinction responding and reinstatement in adolescent and adult male rats: evidence against a biological vulnerability to nicotine addiction during adolescence. Neuropsychopharmacology. 2008;33:739–748. doi: 10.1038/sj.npp.1301454. [DOI] [PubMed] [Google Scholar]
- Smith MA. Peer influences on drug self-administration: social facilitation and social inhibition of cocaine intake in male rats. Psychopharmacology. 2012;224:81–90. doi: 10.1007/s00213-012-2737-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Lacy RT, Strickland JC. The effects of social learning on the acquisition of cocaine self-administration. Drug Alcohol Depend. 2014;141:1–8. doi: 10.1016/j.drugalcdep.2014.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland JC, Smith MA. Animal models of social contact and drug self-administration. Pharmacol Biochem Behav. 2015;136:47–54. doi: 10.1016/j.pbb.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman S. Risk factors for and prevention of tobacco use. Pediatr Blood Cancer. 2005;44:614–619. doi: 10.1002/pbc.20350. [DOI] [PubMed] [Google Scholar]
- Thiel KJ, Sanabria F, Neisewander JL. Synergistic interaction between nicotine and social rewards in adolescent male rats. Psychopharmacology (Berl) 2009;204:391–402. doi: 10.1007/s00213-009-1470-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres OV, Natividad LA, Tejeda HA, Van Weelden SA, O'Dell LE. Female rats display dose-dependent differences to the rewarding and aversive effects of nicotine in an age-, hormone-, and sex-dependent manner. Psychopharmacology. 2009;206:303–312. doi: 10.1007/s00213-009-1607-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschuren LJ, Niesink RJ, Van Ree JM. The neurobiology of social play behavior in rats. Neurosci Biobehav Rev. 1997;21:309–326. doi: 10.1016/s0149-7634(96)00020-6. [DOI] [PubMed] [Google Scholar]
- Vastola BJ, Douglas LA, Varlinskaya EI, Spear LP. Nicotine-induced conditioned place preference in adolescent and adult rats. Physiol Behav. 2002;77:107–114. doi: 10.1016/s0031-9384(02)00818-1. [DOI] [PubMed] [Google Scholar]
- Wang T, Han W, Chen H. Socially acquired nicotine self-administration with an aversive flavor cue in adolescent female rats. Psychopharmacology (Berl) 2016;233:1837–1844. doi: 10.1007/s00213-016-4249-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West P, Sweeting H, Ecob R. Family and friends' influences on the uptake of regular smoking from mid-adolescence to early adulthood. Addiction. 1999;94:1397–1411. doi: 10.1046/j.1360-0443.1999.949139711.x. [DOI] [PubMed] [Google Scholar]
- Yararbas G, Keser A, Kanit L, Pogun S. Nicotine-induced conditioned place preference in rats: sex differences and the role of mGluR5 receptors. Neuropharmacology. 2010;58:374–382. doi: 10.1016/j.neuropharm.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Young LJ, Lim MM, Gingrich B, Insel TR. Cellular mechanisms of social attachment. Horm Behav. 2001;40:133–138. doi: 10.1006/hbeh.2001.1691. [DOI] [PubMed] [Google Scholar]