Abstract
Despite the tropical climate which is characterized by generally high temperatures and persistent mild temperatures during the winter season, Bangladesh, along with many other tropical countries, experiences strong winter and cold-related excess mortality. The objective of this paper was to analyse the nature of these cold effects and understand the role of season vs. temperature and diurnal changes in temperature. For approaching these questions, we applied different Poisson regression models. Temperature as well as diurnal temperature range (DTR) were considered as predictor variables. Different approaches to seasonality adjustment were evaluated and special consideration was given to seasonal differences in atmospheric effects. Our findings show that while seasonality adjustment affected the magnitude of cold effects, cold-related mortality persisted regardless the adjustment approach. Strongest effects of low temperatures were observed at the same day (lag 1) with an increase of 1.7% (95% CI = 0.86–2.54%) per 1 °C decrease in temperature during the winter season. Diurnal temperature affected mortality with increasing levels at higher ranges. Mortality increased with 0.97% (95% CI = 0.17–1.75%) when looking at the entire season, but effects of DTR were not significant during winter when running a seasonal model. Different from effects observed in the mid-latitudes, cold effects in Bangladesh occurred on a very short time scale highlighting the role of temperature versus season. Insufficient adaptation with regard to housing and clothing might lead to such cold-related increases in mortality despite rather moderate temperature values. Although the study did not demonstrate an effect of DTR during the cold season, the strong correlation with (minimum) temperature might cause a multicollinearity problem and effects are difficult to attribute to one driver.
Keywords: Mortality, Cold, Seasonality adjustment, Lag effects, Tropical monsoon climate
Introduction
A multitude of studies have shown seasonal variations in mortality in various areas of the world (Burkart et al. 2014b; Rau 2006). While the seasonal mortality pattern has shifted over time, today, mortality generally peaks during the cold season in most regions and population groups (Rau 2006; Sakamoto-Momiyama 1978). To some degree, these variations are believed to be a consequence of seasonally varying weather conditions, especially temperature. Several studies have established a relationship with low temperatures and associated risk factors such as vasoconstriction and high blood pressure, increased platelet counts and viscosity, cholesterol levels, heart rate, plasma fibrinogen and inflammatory markers (Donaldson and Keatinge 1997; Halonen et al. 2010 ; Hong et al. 2012; Keatinge et al. 1984; Mercer et al. 1999; Pell and Cobbe 1999; Schäuble et al. 2012). In addition to biochemical factors, behavioural aspects such as time spend indoors and crowding are often advanced as risk factors for influenza, and some recent studies implied that influenza infections may underlie winter mortality risk (Kinney et al. 2015; Rau 2006; Reichert et al. 2004; Von Klot et al. 2012). Moreover, there is evidence that respiratory infections might induce plasma fibrinogen and other coagulation factors via activation of the active phase response thereby leading to increased mortality risk during winter (Woodhouse et al. 1994). Another pathway points at low specific humidity levels being a risk factor for the respiratory virus infection (Shaman and Kohn 2009).
What drives winter excess and cold-related mortality is a research question that has received increased attention during past years. Particularly considering the impact of climate change, the pending question is whether cold effects will diminish in a warming climate and whether a potential reduction might compensate for increased heat effects. In tropical regions, the excess in mortality during the low temperature season is particularly counterintuitive as temperatures remain rather high during the so-called winter season and are often perceived as rather agreeable. Nevertheless, several studies have highlighted the association between low temperatures and increased mortality in areas such as Bangladesh, Burkina Faso, Kuwait or Brazil (Burkart et al. 2011a; Douglas et al. 1991; McMichael et al. 2008). Even though monthly mean temperatures do not fall below 18 °C in December and January, the terms winter and cold season are usually applied. For practical reasons, we are following this terminology throughout this paper. Moreover, we refer to cold effects or low temperatures when speaking of temperatures that are relatively low, i.e. lower and colder in comparison to temperatures during other times of the year.
So far, several studies have assessed bioclimatological and temperature-related effects on mortality in Bangladesh (Burkart et al. 2011b; Burkart et al. 2011a). Special attention in these studies was given to heat effects and summer excess mortality, while excess mortality due to low temperatures and during the cold season is largely understudied. Given the pronounced annual temperature amplitude, rather uncharacteristic for a tropical low-latitude climate, the low temperature season is often referred to as winter, whereas, the pre-monsoon and monsoon season are referred to as summer. A recent study highlighted strong seasonal variations in mortality with a pronounced excess mortality during the winter season (Burkart et al. 2011a). Other studies revealed a J-shaped temperature-mortality relationship in Bangladesh with an increase in mortality with decreasing temperature over a wide range of temperature values, i.e. a cold effect. Only at the upper end of the temperature distribution, approximately the 90th percentile, the temperature-mortality relationship changes, and mortality increases with increasing temperature values, i.e. a heat effect occurs (Burkart et al. 2014a). The observed short-term mortality increase of 2–3% mortality per decrease in 1 °C exceeds cold effects observed in higher latitudes, e.g. in Berlin where an approximate increase of 0.4% increase in mortality per 1° decrease in temperature was found (Burkart et al. 2013; Burkart et al. 2011b).
The major objective of this study was to understand the drivers behind winter- and cold-related excess mortality in Bangladesh. In doing so, we aimed at separating seasonal from temperature effects and particularly looked into the temporal displacement of cold effects. We investigated the role of mean and extreme temperatures and diurnal changes in temperature (i.e. difference between daily maximum and minimum) in order to understand the significance of each predictor and conducted a season-specific analysis.
Material and methods
Meteorological data
Meteorological data was collected from the Bangladesh Meteorological Department (BMD) for 26 stations located across Bangladesh. Daily mean, maximum and minimum values were calculated from three-hourly values for each station if at least 75% of the measurements for a day were available. Following, the correlation between stations was calculated and the Kaiser–Guttmann criterion determined. As stations were strongly correlated and as the Kaiser-Guttmann criterion indicated that one principal component would be sufficient to reflect 99% of the variance, we calculated nationwide spatial averages of daily temperature values. Spatial temperature maps were generated for each day using inverse distance interpolation (R, Version 2.11.0, package ‘gstat’). Following, a mean value was calculated from the map grid values. This spatial aggregation helped to maintain a high statistical power by accumulating a high number of daily deaths. Diurnal temperature ranges (DTRs), which are defined as the difference between daily maximum and minimum temperature were calculated from spatially averaged temperature values.
Mortality data
Mortality data used within this study was collected within the Sample Vital Registration System (SVRS)—a core activity of the Bangladesh Bureau of Statistics (BBS). Within this registration system, vital information, such as birth and death events of a sample population, are collected. This sample population, of approximately one million people, is organised within 1000 Primary Sample Units (PSUs) distributed across Bangladesh. Data is collected under a dual recording system with events being recorded by a local registrar when they occur as well as retrospectively by officials from the Upazila division on a quarterly basis. After being compared by quality control personnel of the BBS, partially matched and non-matched events are subject to further verification through field visits. A cause of death is attributed which however, is not medically certified (Bangladesh Bureau of Statistics 2007). For the purpose of this study, external-causes and maternity-related deaths were excluded. In total, a number of 25,226 deaths occurring over a time frame from 2003 to 2007 were analysed. Time series were complete over the whole period. For further information on the SVRS, we refer to Burkart et al. (Burkart et al. 2013; Burkart et al. 2011b).
Statistical modelling
In our analysis, we used two types of Poisson regression models: Generalized Additive Models (GAMs) and Distributed Lag non-Linear Models (DLNMs). While GAMs are a standard tool in mortality time-series analysis, more recently developed DLNMs allow a sophisticated assessment of the temporal displacement and the lag structure of effects (Gasparrini 2011; Gasparrini et al. 2010). For both modelling approaches, we fitted confounder models based on the minimization of the GCV-score. The models were adjusted for long-term trend and seasonal variations by including a counter variable for each day of the time series. We then fitted a spline with one to 11 df per year. One of the objectives of this research was to explore the sensitivity of the fitted temperature-mortality functions to different dfs used for seasonality adjustment. Based on the partial autocorrelation function of the model residuals, we decided to use five dfs per year for the subsequent analysis (cf. Figure S1, Supplementary Material). In addition to trend, day of the month was included as a numeric variable into the model and a penalized spline was fitted. As predictor variables, we considered mean, maximum and minimum temperature and DTR. We included predictor variables of the same day into GAMs (lag 1) and calculated higher lags as averages over the considered time period (e.g. a lag of 7 days would be the lag over the current and the previous 6 days). Models assessing the effect of DTR were also adjusted for temperature.
In addition to global models, i.e. models assessing the atmosphere-mortality relationship over the whole year, we fitted seasonal models assessing the atmosphere-mortality relationship for individual seasons. The months from November to February were considered as winter and the months from June to September as summer; all remaining months were considered as transition months. GAMs with interaction terms, allowing for interaction between the predictor and season, were fitted. In order to provide quantitative estimates of temperature and DTR effect, we included linear terms into the regression models.
In order to assess the sensitivity of the regression models, we re-conducted all statistical analyses, using data from selected divisions. Outcomes were mostly the same, but in general significance, levels are lower or results are not significant.
Results
Distribution, time-course, and correlation of meteorological and mortality data
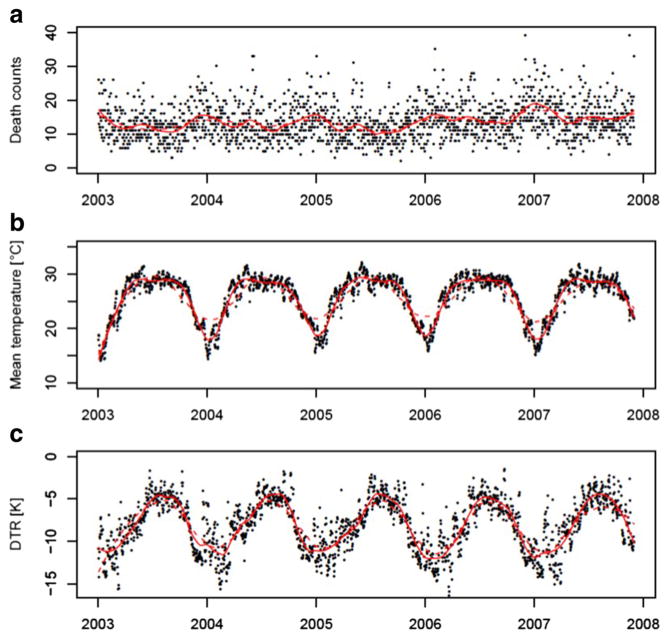
Bangladesh is in most parts classified as Aw tropical wet and dry climate with a pronounced rainy and a dry season. The annual monthly mean temperature amounts to 25.5 °C with monthly maximum temperatures of 29.1 °C and minimum temperatures of 17.5 °C. The monthly mean DTR was 8.2 K, monthly minimum and maximum DTRs ranged between 4.5 and 11.4 K respectively (Table 1). On average, 13.7 people died per day in the sample population; the lowest death count per day was 2 and the highest 52 (Table 1). Death counts as well as meteorological parameters show a marked seasonal pattern and are correlated among each other (cf. Figure 1, Table 2). Figure 1 shows time series of death counts (aggregated over all primary sample units) and spatial means of daily mean temperature as well as DTR. Death counts and DTR peaks during the winter season while temperature is highest during the summer and monsoon season.
Table 1.
Mean, minimum and maximum statistics of death counts, temperature and DTR
| Variable | Mean (min to max) |
|---|---|
| Death counts | 13.7 (2 to 52)a |
| Mean temperature (°C) | 25.5 (17.5 to 29.1)b |
| DTR (K) | −8.2 (−11.4 to −4.5)b |
Monthly mean, minimum and maximum
Daily mean, minimum and maximum
Fig. 1.
Time-series plots of daily death counts (a), daily mean temperatures (b), and diurnal temperature range (c), from January 2003 to 2007. Death counts aggregated over all Primary Sample Units (PSUs), temperature values and ranges depict nationwide spatial means. Red solid lines are derived through loess smoothers using an alpha of 0.1, red dotted lines from loess smoothers using an alpha of 0.25
Table 2.
Correlation coefficients between death counts and atmospheric predictors
| Death counts | Tmean | Tmax | Tmin | DTR | |
|---|---|---|---|---|---|
| Death counts | 1 | 0.22* | −0.20* | −0.26* | 0.22* |
| Tmean | 1 | 0.90* | 0.95* | −0.61* | |
| Tmax | 1 | 0.80* | −0.27* | ||
| Tmin | 1 | −0.79* | |||
| DTR | 1 |
p value < 0.0001
Table 2 depicts correlation coefficients between death counts and meteorological predictors. While mean and extreme temperatures are highly correlated among each other, only a weak correlation is observed with death counts. DTR is strongly correlated with mean and minimum temperature but not with maximum temperature (Table 2). The Supplementary Material provides a Table containing season-specific correlation coefficients (Supplementary Material, Table S1). For the season-specific analysis, we found similarly strong associations among variables during winter as observed for the whole year; the relationship weakened during summer and the transition months.
Temperature effects
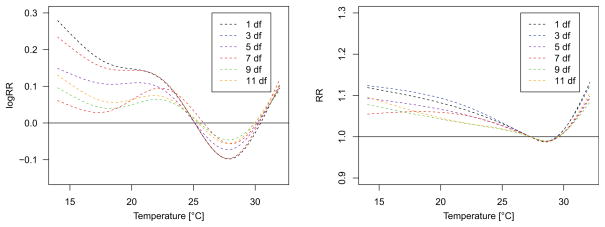
In order to understand the role of seasonality adjustment, we fitted GAMs and DLNMs with different degrees of freedom for long-term trend and seasonality adjustment. Figure 2a, b show the temperature response curves using 1 to 11 df per year. Cold effects decrease when using higher degrees of freedom in the GAM, as well as the DLNM but persist in both modelling approaches, regardless the seasonality adjustment approach. In a Supplementary file, we present exposure-response curves for 1, 5 and 10 df per year displaying confidence bands. The partial autocorrelation function of the model residuals suggested strong overfitting when using more than 5 df per year (cf. Figure S2, Supplementary Material). For this reason, 5 df were used for all further analyses.
Fig. 2.
Exposure-response curves fit to the relation between same day (lag 1) temperature and mortality in Bangladesh. Curves are adjusted for trend and day of the month. The black dashed line shows the relationship when season is left uncontrolled. The coloured lines represent exposure-response curves when season is controlled for using 3 to 11°df per year
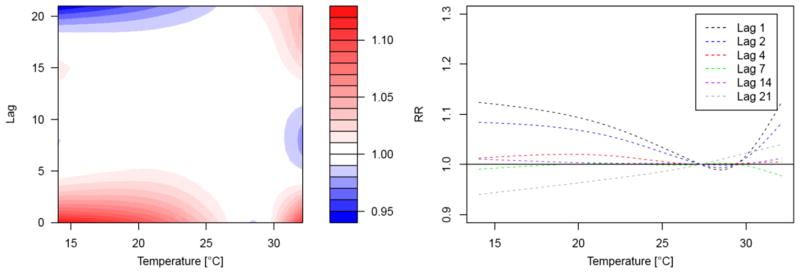
When looking at the temporal structure and the effect displacement of cold effects, we found that cold-related mortality occurred at a very short-term lag (Fig. 3). Figure 3a displays the DLNM output with a relative risk surface along temperature and different lags (up to 21 days). The highest relative risks were observed at low temperatures at the same day. After a few days, cold-related mortality increase quickly subsided with no effect observed at a lag of 4 days (Fig. 3b). Figure S3 included in the Supplementary file shows exposure-response curves for one, three and seven lag days with confidence bands derived from GAMs as well as DLNMs. While we observed significant cold-related excess mortality for a lag of one and 2 days and no effect at a lag of 7 days for the DLNM outputs, this decrease in cold effects was less pronounced for the GAM analysis (cf. Figure S3, Supplementary Material).
Fig. 3.
Lag structure of temperature effects in Bangladesh. Contour plot on the left hand-side depicts the relative risk of mortality along temperature and lag day. Splines on the right hand-side display the relative risk of mortality along temperature for different lags. Outputs are adjusted for long-term and seasonal variations and day of the month
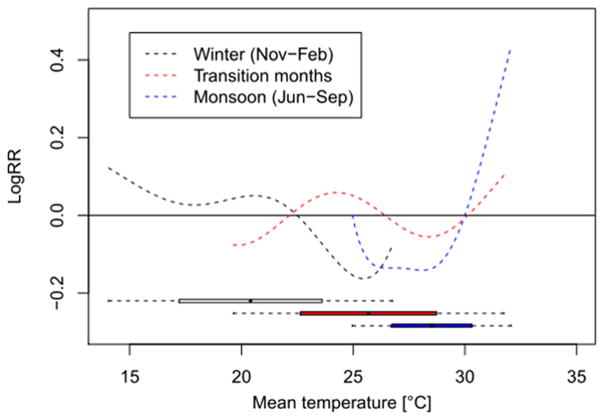
For further assessing the role of season (as compared to temperature), we stratified our data by season, distinguishing between winter (Nov–Feb) and monsoon season (Jun–Sep) as well as transition months (Mar–May and Oct). Significant relationships between temperature and mortality were found for the winter and the monsoon season but not for the months in-between. During the winter season, we observed an increase in mortality with decreasing temperature, comparable to the increase for the global model. During the summer season, even stronger effects were observed in the seasonal model as compared to the global model underlining the relevance of heat effects (Fig. 4). Figure S4 in the Supplementary file shows exposure-response curves and confidence bands for all three seasons. Outcomes were mostly unaffected by including minimum or maximum temperature as predictors instead of mean temperature (data not shown).
Fig. 4.
Exposure response curves fit to the relationship between same day (lag 1) and mortality during the winter and monsoon season, and for transition months. Curves are adjusted for trend and day of month
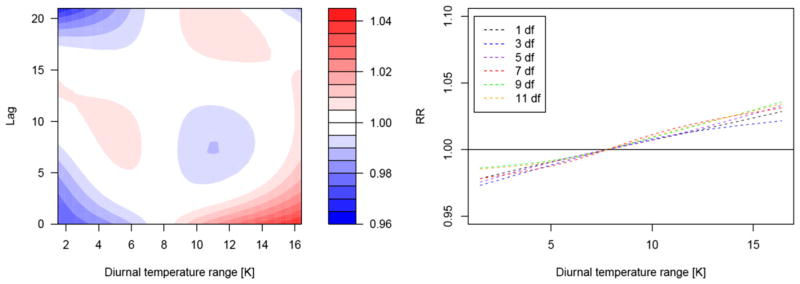
The effect of diurnal temperature range
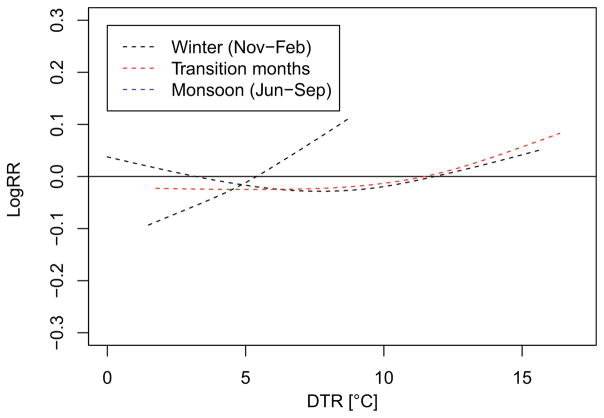
Figure 5 shows the relative risk surface of mortality along the DTR and lag days. A moderate but significant increase in mortality was observed with increasing DTR at a short lag with the strongest effect observed at the same day (Fig. 5). The increase in mortality was estimated to be 0.97% (95% CI = 0.17–1.75%) per 1 K increase in DTR. For the seasonal model, i.e. a GAM allowing for interaction between season and DTR, we found increases in mortality with growing DTR during all seasons but effects were not significant (Fig. 6; Supplementary Material, Figure S6). The strongest increase in DTR was observed during the summer season despite rather small DTRs. For exposure-response curves including confidence bands, we refer to the Supplementary Material, Figures S5 and S6.
Fig. 5.
Lag structure of diurnal temperature range (DTR) effects in Bangladesh. Contour plot on the left hand-side depicts the relative risk of mortality along DTR and lag day. Splines on the right hand-side
Fig. 6.
Exposure response curves fit to the relationship between diurnal temperature range (DTR) and mortality during the winter and monsoon season, and for transition months. Curves are adjusted for trend, day of month and daily mean temperature
Discussion
The overarching goal of this study was to understand the nature and drivers of cold-related excess mortality in Bangladesh, a tropical South Asian monsoon climate characterized by high to moderate temperatures throughout the year. We assessed the role of season and took several approaches to account for seasonal influences. Further, we assessed the temporal displacement and lag structure of temperature effects and investigated the influence of diurnal changes in temperature.
In general, our analysis demonstrated the persistence of cold effects regardless the approach of seasonality adjustment. Fitting GAMs with different df for long-term and seasonal adjustment revealed decreasing cold effects with increasing df but cold effects persisted for high df. The number of df had little influence when fitting DLNMs. As a second approach to account for the seasonal influence, we analysed the association between temperature and mortality by season. Analysing the relationship of interest, within small strata of the confounder in our case season, is a standard approach in epidemiology. Outcomes of this study demonstrated persistent cold effects, similar to these observed for the global whole year’s analysis. Interestingly, when stratifying by season, we observed an increase in heat effects, demonstrating an even greater relevance of high temperatures. Investigating temperature effects by season has rarely been done in previous studies: a recent study investigating temperature effects by month could not find increases in mortality at low values in North American and French cities (Kinney et al. 2015).
In addition to the assessment of seasonality, we focused on the lag structure of cold effects. For this purpose, we fitted DLNM over 21 days. The outcomes demonstrated increased mortality at a very short lag of a few days. The strongest effect was observed at the immediate day and then quickly subsided. At a lag of 4 days, no cold effect was found anymore. The analysis demonstrated that cold effects in Bangladesh occurred on a very short-term time scale, different from cold effects observed in mid-latitude countries. In North America, Anderson and Bell (2009) demonstrated short-term heat effects and longer-term cold effects with an average of the current day up to 25 previous days in the USA. Similar observations were made by other authors demonstrating cold effects occurring at a time scale of 2 to 4 weeks in Europe and North America (Ballester et al. 1997; Braga et al. 2001; Burkart et al. 2013; Pattenden et al. 2003). While the prolonged effect of low temperatures observed in the USA and Europe might have a seasonal component, the very immediate effect observed in Bangladesh is rather indicative of a true effect of low temperature. So far, only few studies have systematically assessed the lag structure of cold effects in tropical countries. Similarly, Ingole et al. (2012) found a rather short-term cold effect at a lag of 2 to 6 days in India. McMichael et al. (2008) observed increased mortality at low temperatures in Monterrey (Mexico) and Chiangmai (Thailand) for a lag period of 2 days, while in Bangkok or Cape Town, cold effects were only observed for a 2 weeks lag period.
In order to better understand the drivers of cold-related mortality, we assessed the impact of minimum and maximum temperatures on mortality. Daily mean and extreme temperatures were strongly correlated throughout the years as well as during the winter season, while this association weakened during the summer season. In this analysis, mean temperature showed a slight predictive advantage when integrated into the seasonal interaction models. When fitting models including display the cumulative relative risk of mortality along DTR for different lags. Outputs are adjusted for long-term and seasonal variations, day of the month and daily mean temperature only data for the winter season, a significant temperature effect was only found for mean temperature.
The strong effect of rather moderate low temperatures raises questions and suggests that other factors apart from temperature (or season) might underlie the observed effect. We hypothesised that diurnal changes in temperature are causing increases in mortality. Several recent studies have established a relationship between temperature and DTR in Asia and North America (Lim et al. 2013; Lim et al. 2012; Lim et al. 2014; Ding et al. 2015; Kan et al. 2007). In Bangladesh, DTR shows a strong seasonal pattern with the highest ranges observed during the cold season and is highly correlated with temperature. Large ranges of diurnal temperatures might put stress on the human organism as it might be difficult to adjust to a wider range of temperatures. Lim et al. (Lim et al. 2013; Lim et al. 2014) argues that DTR is likely to increase heart rate and blood pressure and furthermore suggests that respiratory symptoms may be exacerbated by immune system depression (Bull 1980). Furthermore, the authors suggest that an inflammatory nasal responses (Graudenz et al. 2006) may result from sudden temperature changes. In this study, we found a small negative effect of DTR with increasing mortality at higher ranges (models adjusted for temperature) which correspond well with findings from other studies (Lim et al. 2013; Lim et al. 2014).
However, when looking into the DTR effect by season, we observed a very pronounced effect during the monsoon season (despite smaller DTRs), while effects during winter and the transition months were rather little pronounced. Daily mean and minimum temperatures and DTR correlate strongly over the whole year and during the winter season. During the summer season, DTR is strongly correlated with mean temperature and particularly with maximum temperature while the correlation with minimum temperature is rather weak. Due to the strong relationship between DTR and temperature, disentangling the contribution of each particular predictor to increased mortality is difficult and final conclusions about their influence cannot be drawn. Nevertheless, we argue that at least part of the cold-related increase in mortality might be due to larger ranges in diurnal temperatures.
Concluding, the question why the observed cold effects occur, despite mild winter temperatures, remains open. One reasonable explanation might relate to immediate exposure and insufficient adaptation regarding clothing as well as housing and general behaviour. While the overall effort aims at adaptation to high temperatures, warm clothes, closed shoes, socks, heavy blankets or heating are rather uncommon in Bangladesh. Similar to conclusions gained from the EuroWinter project (which found high winter excess mortality in European regions with rather moderate winter temperatures), the adaptation to low temperatures might be insufficient (The Eurowinter Group 1997, 2000).
Conclusions
Bangladesh shows a pronounced seasonal mortality pattern with distinct excess mortality during the winter season, despite rather moderate temperatures. Furthermore, mortality increases with decreasing temperatures during the winter season. Different from mid-latitude regions, where this cold effect occurs on a more mid-term scale, in Bangladesh, a very short-term effect occurring at the same day was observed. This short-term nature of cold effects underlines the relevance of temperature over season. DTR affected mortality with increasing risk at higher ranges. Given the strong correlation of DTR with temperature and the large ranges during the cold season, separating the effect of both predictors was not possible. Although DTR did not reveal a significant influence during winter, its influence cannot entirely be ruled out.
Supplementary Material
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00484-016-1287-8) contains supplementary material, which is available to authorized users.
References
- Anderson BG, Bell ML. Weather-related mortality: how heat, cold, and heat waves affect mortality in the United States. Epidemiology (Cambridge, Mass) 2009;20(2):205–213. doi: 10.1097/EDE.0b013e318190ee08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballester F, Corella D, Pérez-Hoyos S, Sáez M, Hervás A. Mortality as a function of temperature. A study in Valencia, Spain, 1991–1993. Int J Epidemiol. 1997;26(3):551–561. doi: 10.1093/ije/26.3.551. [DOI] [PubMed] [Google Scholar]
- Bangladesh Bureau of Statistics. Report on the Sample Vital Registration System Bangladesh Bureau of Statistics. Dhaka, Bangladesh: 2007. 2008. [Google Scholar]
- Braga ALF, Zanobetti A, Schwartz J. The time course of weather-related deaths. Epidemiology. 2001;12(6):662–667. doi: 10.2307/3703185. [DOI] [PubMed] [Google Scholar]
- Bull GM. The weather and deaths from pneumonia. Lancet. 1980;315(8183):1405–1408. doi: 10.1016/S0140-6736(80)92666-5. [DOI] [PubMed] [Google Scholar]
- Burkart K, Khan M, Kramer A, Breitner S, Schneider A, Endlicher W. Seasonal variations of all-cause and cause-specific mortality by age, gender, and socioeconomic condition in urban and rural areas of Bangladesh. Int J Equity Health. 2011a:10. doi: 10.1186/1475-9276-10-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkart K, Schneider A, Breitner S, Khan MH, Krämer A, Endlicher W. The effect of atmospheric thermal conditions and urban thermal pollution on all-cause and cardiovascular mortality in Bangladesh. Environ Pollut. 2011b;159(8–9):2035–2043. doi: 10.1016/j.envpol.2011.02.005. [DOI] [PubMed] [Google Scholar]
- Burkart K, Canário P, Breitner S, Schneider A, Scherber K, Andrade H, Alcoforado MJ, Endlicher W. Interactive short-term effects of equivalent temperature and air pollution on human mortality in Berlin and Lisbon. Environ Pollut. 2013;183:54–63. doi: 10.1016/j.envpol.2013.06.002. [DOI] [PubMed] [Google Scholar]
- Burkart K, Breitner S, Schneider A, Khan MMH, Krämer A, Endlicher W. An analysis of heat effects in different subpopulations of Bangladesh. Int J Biometeorol. 2014a;58(2):227–237. doi: 10.1007/s00484-013-0668-5. [DOI] [PubMed] [Google Scholar]
- Burkart K, Khan MMH, Schneider A, Breitner S, Langner M, Krämer A, Endlicher W. The effects of season and meteorology on human mortality in tropical climates: a systematic review. Trans R Soc Trop Med Hyg. 2014b doi: 10.1093/trstmh/tru055. [DOI] [PubMed] [Google Scholar]
- Ding Z, Guo P, Xie F, Chu H, Li K, Pu J, Pang S, Dong H, Liu Y, Pi F, Zhang Q. Impact of diurnal temperature range on mortality in a high plateau area in Southwest China: a time series analysis. Sci Total Environ. 2015;526:358–365. doi: 10.1016/j.scitotenv.2015.05.012. [DOI] [PubMed] [Google Scholar]
- Donaldson GC, Keatinge WR. Early increases in ischaemic heart disease mortality dissociated from and later changes associated with respiratory mortality after cold weather in South East England. J Epidemiol Community Health. 1997;51(6):643–648. doi: 10.1136/jech.51.6.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas AS, Rawles JM, Al-Sayer H, Allan TM. Seasonality of disease in Kuwait. Lancet. 1991;337:1393–1397. doi: 10.1016/0140-6736(91)93069-l. [DOI] [PubMed] [Google Scholar]
- Gasparrini A. Distributed lag linear and non-linear models in R: the package dlnm. J Stat Softw. 2011;43(8):1–20. [PMC free article] [PubMed] [Google Scholar]
- Gasparrini A, Armstrong B, Kenward M. Distributed lag nonlinear models. Stat Med. 2010;29(21):2224–2234. doi: 10.1002/sim.3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graudenz GS, Landgraf RG, Jancar S, Tribess A, Fonseca SG, Faé KC, Kalil J. The role of allergic rhinitis in nasal responses to sudden temperature changes. J Allergy Clin Immunol. 2006;118(5):1126–1132. doi: 10.1016/j.jaci.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Halonen J, Zanobetti A, Sparrow D, Vokonas P, Schwartz J. Associations between outdoor temperature and markers of in flammation: a cohort study. Environ Health. 2010;9(42) doi: 10.1186/1476-069X-9-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong Y-C, Kim H, Oh S-Y, Lim Y-H, Kim S-Y, Yoon H-J, Park M. Association of cold ambient temperature and cardiovascular markers. Sci Total Environ. 2012;435–436:74–79. doi: 10.1016/j.scitotenv.2012.02.070. [DOI] [PubMed] [Google Scholar]
- Ingole V, Juvekar S, Muralidharan V, Sambhudas S, Rocklöv J. The short-term association of temperature and rainfall with mortality in Vadu Health and Demographic Surveillance System: a population level time series analysis. Global Health Action. 2012;5:10. doi: 10.3402/gha.v5i0.19118. 3402/gha.v3405i3400.19118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan H, London SJ, Chen H, Song G, Chen G, Jiang L, Zhao N, Zhang Y, Chen B. Diurnal temperature range and daily mortality in Shanghai, China. Environ Res. 2007;103(3):424–431. doi: 10.1016/j.envres.2006.11.009. [DOI] [PubMed] [Google Scholar]
- Keatinge WR, Coleshaw SR, Cotter F, Mattock M, Murphy M, Chelliah R. Increases in platelet and red cell counts, blood viscosity, and arterial pressure during mild surface cooling: factors in mortality from coronary and cerebral thrombosis in winter. Br Med J (Clin Res Ed) 1984;289(6456):1405–1408. doi: 10.1136/bmj.289.6456.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney P, Schwartz J, Pascal M, Petkova E, Tertre AL, Medina S, Vautard R. Winter season mortality: will climate warming bring benefits? Environ Res Lett. 2015;10(6) doi: 10.1088/1748-9326/10/6/064016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim Y-H, Park A, Kim H. Modifiers of diurnal temperature range and mortality association in six Korean cities. Int J Biometeorol. 2012;56(1):33–42. doi: 10.1007/s00484-010-0395-0. [DOI] [PubMed] [Google Scholar]
- Lim Y-H, Kim H, Kim J, Bae S, Hong Y-C. Effect of diurnal temperature range on cardiovascular markers in the elderly in Seoul, Korea. Int J Biometeorol. 2013;57(4):597–603. doi: 10.1007/s00484-012-0587-x. [DOI] [PubMed] [Google Scholar]
- Lim Y-H, Reid C, Mann J, Jerrett M, Kim H. Diurnal temperature range and short-term mortality in large US communities. International Journal of Biometeorology. 2014:1–9. doi: 10.1007/s00484-014-0941-2. [DOI] [PubMed] [Google Scholar]
- McMichael AJ, Wilkinson P, Kovats RS, Pattenden S, Hajat S, Armstrong B, Vajanapoom N, Niciu EM, Mahomed H, Kingkeow C, Kosnik M, O’Neill MS, Romieu I, Ramirez-Aguilar M, Barreto ML, Gouveia N, Nikiforov B. International study of temperature, heat and urban mortality: the ‘ISOTHURM’ project. Int J Epidemiol. 2008;37(5):1121–1131. doi: 10.1093/ije/dyn086. [DOI] [PubMed] [Google Scholar]
- Mercer JB, Østerud B, Tveita T. The effect of short-term cold exposure on risk factors for cardiovascular disease. Thromb Res. 1999;95(2):93–104. doi: 10.1016/S0049-3848(99)00028-6. [DOI] [PubMed] [Google Scholar]
- Pattenden S, Nikiforov B, Armstrong BG. Mortality and temperature in Sofia and London. J Epidemiol Community Health. 2003;57(8):628–633. doi: 10.1136/jech.57.8.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pell JP, Cobbe SM. Seasonal variations in coronary heart disease. QJM. 1999;92(12):689–696. doi: 10.1093/qjmed/92.12.689. [DOI] [PubMed] [Google Scholar]
- Rau R. A Demographic Approach. Berlin: 2006. Seasonality in human mortality. [Google Scholar]
- Reichert TA, Simonsen L, Sharma A, Pardo SA, Fedson DS, Miller MA. Influenza and the winter increase in mortality in the United States, 1959–1999. Am J Epidemiol. 2004;160(5):492–502. doi: 10.1093/aje/kwh227. [DOI] [PubMed] [Google Scholar]
- Sakamoto-Momiyama M. Changes in the seasonality of human mortality: a medico–geographical study. Soc Sci Med. 1978;12:29–42. [PubMed] [Google Scholar]
- Schäuble CL, Hampel R, Breitner S, Rückerl R, Phipps R, Diaz-Sanchez D, Devlin RB, Carter JD, Soukup J, Silbajoris R, Dailey L, Koenig W, Cyrys J, Geruschkat U, Belcredi P, Kraus U, Peters A, Schneider AE. Short-term effects of air temperature on blood markers of coagulation and inflammation in potentially susceptible individuals. Occup Environ Med. 2012;69(9):670–678. doi: 10.1136/oemed-2011-100469. [DOI] [PubMed] [Google Scholar]
- Shaman J, Kohn M. Absolute humidity modulates influenza survival, transmission, and seasonality. Proc Natl Acad Sci. 2009;106(9):3243–3248. doi: 10.1073/pnas.0806852106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Eurowinter Group. Cold exposure and winter mortality from ischaemic heart disease, cerebrovascular disease, respiratory disease, and all causes in warm and cold regions of Europe. Lancet. 1997;349(9062):1341–1346. doi: 10.1016/S0140-6736(96)12338-2. [DOI] [PubMed] [Google Scholar]
- The Eurowinter Group. Winter mortality in relation to climate. Int J Circumpolar Health. 2000;59:154–159. [PubMed] [Google Scholar]
- Von Klot S, Zanobetti A, Schwartz J. Influenza epidemics, seasonality, and the effects of cold weather on cardiac mortality. Environ Health. 2012;11(74) doi: 10.1186/1476-069X-11-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodhouse PR, Khaw K, Plummer M, Meade TW, Foley A. Seasonal variations of plasma fibrinogen and factor VII activity in the elderly: winter infections and death from cardiovascular disease. Lancet. 1994;343(8895):435–439. doi: 10.1016/S0140-6736(94)92689-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.