Abstract
Objectives
Hypertension is a major risk factor for all cardiovascular disease, especially among African Americans. This study focuses on identifying specific BP genes using 15,914 individuals of African ancestry from eight cohorts (Africa America Diabetes Mellitus (AADM), Atherosclerosis Risk in Communities (ARIC) Study, Coronary Artery Risk Development in young Adults (CARDIA), Genetics Network (GenNet), Genetic Epidemiology Network of Arteriopathy (GENOA), Howard University Family Study (HUFS), Hypertension Genetic Epidemiology Network (HyperGEN), and Loyola University Chicago Cohort (LUC)) to further genetic findings in this population which has generally been underrepresented in blood pressure studies.
Methods
We genotyped and performed various single variant and gene-based exome-wide analyses on 15,914 individuals on the Illumina HumanExome Beadchip v1.0 or v1.1, to test association with systolic and diastolic blood pressure long-term average residuals which were adjusted for age, age-squared, sex and BMI.
Results
We identified rare variants affecting SBP and DBP in 10 genes: AFF1, GAPDHS, SLC28A3, COL6A1, CRYBA2, KRBA1, SEL1L3, YOD1, CCDC13, and QSOX1. Prior experimental evidence for six of these 10 candidate genes supports their involvement in cardiovascular mechanisms, corroborating their potential roles in blood pressure regulation.
Conclusions
While our results require replication or validation due to their low numbers of carriers, and an ethnicity-specific genotyping array may be more informative, this study, which has identified several candidate genes in this population most susceptible to hypertension, presents one of the largest African-ancestry blood pressure studies to date, and the largest including analysis of rare variants.
Keywords: Blood Pressure, Hypertension, Exome, African Americans, Epidemiologic Studies, Genome-Wide Association Study
Introduction
Hypertension is a leading risk factor for cardiovascular disease. Several genome-wide association studies (GWAS) have implicated common variants at approximately 166 loci [1–24] associated with systolic (SBP) and diastolic (DBP) blood pressure (BP) across multiple studies and populations, but the specific genes involved are unknown and are yet to be narrowed down within each locus. Further, these common variants collectively explain only a small fraction (<3%) of the total phenotypic variance of BP traits [25]. There is an emerging consensus that low frequency and rare variants may account for a significant fraction of the remaining variance [26]. If so, genotyping catalogs different from those that enable GWAS are required. The Illumina HumanExome Beadchip (“exome chip”) was designed as an array-based assay enriching for the lower frequency genetic variation that could be detected within exomes. The >240,000 variants on this chip were selected for their functional significance from ~12,000 human exome and genome sequences from individuals of varying ancestry (mainly European) and common disease states [27]. These variants include primarily non-synonymous, splice-site, and nonsense variants, with an enrichment of low frequency variants down to a frequency of occurrence at a minimum of two or three times in at least two datasets [28].
The present study focuses on identifying specific genes associated with SBP and DBP long-term average (LTA) residuals, similar to our previous efforts with common variants [7], adjusting for major BP confounders including body mass index (BMI), age, and sex. We analyzed individuals of African ancestry within each study and then combined the results by meta-analysis across eight cohorts, including Africa America Diabetes Mellitus (AADM), Atherosclerosis Risk in Communities (ARIC) Study, Coronary Artery Risk Development in young Adults (CARDIA), Genetics Network (GenNet), Genetic Epidemiology Network of Arteriopathy (GENOA), Howard University Family Study (HUFS), Hypertension Genetic Epidemiology Network (HyperGEN), and Loyola University Chicago Cohort (LUC). The prevalence of hypertension is increased in African Americans as compared to other populations in the United States [29] and, moreover, they are not highly represented in genetic epidemiological studies [6,23,24,30]; the only previous major GWAS with an exclusively African-ancestry discovery sample size larger than ours focused on common variant-level analyses [6,23]. Therefore, this study aims to expand the list of genes potentially involved in BP outcomes in these individuals, with a focus on single variants across the full frequency spectrum, as well as on the gene level. These analyses suggest several rare variant gene candidates for BP regulation, which are likely candidates based on published functional data. Additional analyses focusing on a subset of genes known to be involved in monogenic hypertension and hypotension syndromes, in which many rare variants of large effect have been implicated in BP [31–33], were also conducted.
Methods
Study Participants
Eight studies consisting of individuals (n=15,914 after quality control procedures) of African ancestry were analyzed in this study, including several individuals from the family-based studies AADM [34,35], GenNet [36,37], GENOA [36,37], HyperGEN [36,37], and HUFS [38,39] as well as from the population-based cohorts ARIC [40,41], LUC [42–45], and CARDIA [46,47]. The AADM and LUC studies consist of native Africans with no European admixture; the participants from the remaining cohorts all consist of African Americans. The AADM cohort was designed to study the genetic epidemiology of diabetes and related traits including hypertension, and the HUFS cohort was designed to study multiple cardiometabolic traits including hypertension. The ARIC and CARDIA studies focus overall on cardiovascular traits and events, including blood pressure. The remaining studies were recruited specifically to study blood pressure and associated traits. Brief descriptions and additional data collection methods for each study are provided in the Supplemental Digital Content, Description of Cohorts. Genotyping information and phenotypic characteristics from the initial examination or phase of samples analyzed from each cohort are described in Table 1. An extended table describing phenotypic characteristics of all physical examinations/phases for all studies is presented as Supplemental Digital Content Table S1. All studies obtained written informed consent from the participants as well as approval from their institutional review boards.
Table 1.
Genotypic and phenotypic characteristics for initial examinationa in the eight cohorts analyzed.
| Cohort | Chip Version | # analyzed | % Female | Mean Age (SD) | Mean BMI (SD) | Mean SBP (SD) | Mean DBP (SD) | % on anti-hypertensive medication |
|---|---|---|---|---|---|---|---|---|
| AADM | v1.1 | 2070 | 57 | 45.55 (16.02) | 25.96 (5.821) | 132.69 (22.65) | 81.01 (14.19) | 28 |
| ARIC | v1.0/v1.1 | 3095 | 63 | 53.61 (5.80) | 29.72 (6.26) | 128.63 (21.00) | 79.65 (12.15) | 41 |
| CARDIA | v1.0 | 1534 | 58 | 31.45 (3.80) | 28.24 (6.82) | 111.33 (13.01) | 70.92 (10.97) | 3.2 |
| GenNet | v1.1 | 782 | 60 | 40.42 (11.45) | 30.25 (8.12) | 127.09 (20.20) | 77.70 (13.60) | 100 |
| GENOA | v1.1 | 1373 | 69 | 56.67 (10.66) | 31.02 (6.57) | 131.69 (23.51) | 70.96 (11.53) | 100 |
| HUFS | v1.1 | 1784 | 61 | 43.41 (14.44) | 30.69 (8.672) | 127.38 (20.99) | 80.18 (12.87) | 27 |
| HyperGEN | v1.1 | 1798 | 65 | 48.56 (12.84) | 32.09 (7.61) | 130.10 (22.31) | 74.67 (12.00) | 100 |
| LUC | v1.1 | 2688 | 71 | 48.66 (11.72) | 26.12 (6.17) | 146.09 (29.98) | 91.92 (18.64) | 39 |
BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; SD, standard deviation
As this only illustrates the first visit or phase, there are many individuals in subsequent examinations that are included in these analyses but not in this table; the complete information may be found in Table S1.
Exome Chip Genotyping
Samples were genotyped either at the Center for Inherited Disease Research (CIDR) at Johns Hopkins University on the Illumina HumanExome BeadChip v1.0 (247,870 variants), or at the Human Genetics Center at the University of Texas Health Science Center at Houston (Houston) on either v1.0 or v1.1 (242,901 variants) (http://www.chargeconsortium.com/main/exomechip) [48]. Genotypes were called with Illumina’s GenTrain 1.0 (CIDR) or Gentrain 2.0 (Houston) clustering algorithm from the Illumina GenomeStudio v2011.1 software.
Quality control of Genotypes
Quality control (QC) of the samples and variants were carried out within each cohort, for this study, using the programs PLINK [49], R [50] and KING [51]. We began with 17,940 total individuals and either 247,870 or 242,901 variants, depending on the version of the chip used. Technical failure variants were removed prior to sample and variant QC. Blind duplicates, identified by CIDR’s QC reports where available, and unexpected duplicates, identified by PLINK’s IBS distance metric, DST>0.98, were then removed from the samples, and those remaining were filtered on call rate (>95% retained), X-chromosome heterozygosity and PLINK’s sex check F-statistic to identify sex discrepancies, as well as autosomal heterozygosity to identify individuals with unexpectedly high or low heterozygosity. Custom thresholds selected for the heterozygosity filters were a minimum of three standard deviations from the mean of each of the distributions. Finally, individuals with Mendelian errors, which may indicate genotyping errors or incorrect relationship assignment within their families, were excluded, as well as exhibiting too many first- or second-degree relationships within their cohort reflecting a data quality issue, as preliminary pedigree error correction; more extended pedigree error corrections were carried out after variant QC.
Quality control of variants include discarding those with >5% missing data, and those failing the Hardy-Weinberg Equilibrium (HWE) exact test in PLINK [24] (defined as p<1 × 10−6 on variants with minor allele frequency (MAF)>0.01 only, as HWE estimates on variants with lower MAF become less reliable) [23]. Duplicate SNPs with discordant genotypes were removed within each cohort for downstream analyses.
Pedigree errors and sample switches were subsequently identified and correctd using KING and PLINK by identity-by-state (IBS) information. For the ARIC and LUC studies, only samples related as third degree or beyond were retained for analysis. These procedures left 15,914 individuals with ~233K variants in each cohort genotyped on the v1.0 chip and ~238K variants in each cohort genotyped on the v1.1 chip. The vast majority of the variants removed were due to technical failure. The average call rate of the samples remaining after all quality control procedures is ~99.95%.
Variant annotation
Variants on the chip were annotated using ANNOVAR [52] and phyloP [53] scores. A subset of 42,018 variants was annotated as “deleterious”, defined as consensus intronic splice-site, nonsense-mediated decay (NMD)-compatible stop-gain, or conserved missense (phyloP > 4) variants. These variants were the ones considered in the Burden-T1-del analyses (refer to Statistical Analyses section).
Phenotypes
The traits SBP and DBP were analyzed in this study. For each sample, SBP and DBP measurements from all available visits were adjusted for medication use when applicable, by adding a fixed constant of 15 mmHg for SBP and 10 mmHg for DBP measurements [54]. These corrected measurements were then further adjusted in a linear regression using the known blood pressure measurement confounders age, age-squared, sex, BMI, the first 10 principal components of ancestry, and study center (where applicable for each cohort) per available visit per individual as covariates. Principal components were calculated for all cohorts using KING-mds, which accounts for pedigree structure, on a linkage disequilibrium (LD)-pruned set (r2<0.5 within window=50 SNPs, step=5 SNPs) of common variants (MAF>5%). The resulting adjusted BP values, or residuals, were then averaged across all available visits for each sample, and used as the phenotypes in the analyses described below. Selected individuals were dropped for issues including lower SBP than DBP, and implausible BMI values, but otherwise the full distribution was analyzed. The majority of individuals in some cohorts have data available for one examination only (AADM, GenNet, HUFS, HyperGEN, LUC), while in the others (ARIC, CARDIA, GENOA) the majority has data from more than one examination. The Pearson correlation coefficients for first examination residual versus average residual for individuals with multiple visits in each cohort are reported in Supplemental Digital Content Table S2.
Statistical Analyses
Study-level analyses were conducted, followed by fixed-effects meta-analyses to combine the results with the seqMeta [55] package in R, using SBP and DBP residuals as phenotypes. To account for correlations among relatives, a kinship matrix was used in seqMeta for family-based cohorts. For these meta-analyses, the union of non-monomorphic SNPs passing QC in each of the cohorts was analyzed (170,540 variants). Analyses were restricted to autosomal and X-chromosomal variants.
We performed both single-variant analyses assuming an additive model, as well as gene-based tests, using the included SNP information files in seqMeta for gene definitions. The gene-based tests are particularly useful for analysis of rare variants, which individually have low numbers of carriers in the population, by aggregating variants into functional units (genes) and testing for cumulative effects. The single variant analyses were grouped into three frequency classes (common: MAF≥0.05, low frequency: 0.01≤MAF<0.05 and rare: MAF<0.01). For gene-based analyses, we considered sequence-kernel association test (SKAT) [56], the T1 burden test [57] including all variants with MAF<0.01 (Burden-T1-all), and the T1 burden test including only those variants predicted to be deleterious (Burden-T1-del), which are defined as intronic splice, NMD-compatible stop-gain, or conserved missense (phyloP>4). The SKAT test is expected to be more powerful when the region contains variants that are largely neutral or have opposing directional effects, while burden tests are expected to be more powerful when the region contains variants largely in the same direction [56, 58]. Statistical significance was defined by the Bonferroni method of multiple test correction, considering the number of associations computed within each analysis. There was no additional correction for number of phenotypes (two) due to the high correlation between the phenotypes (Supplemental Digital Content Table S3: Pearson’s r, 0.75–0.87).
Power Calculations
Assuming an additive model, power was calculated as described previously [59]. Under an additive model, a quantitative trait can be represented by a two-component normal mixture distribution. One component represents the reference allele, weighted by its frequency, p, and the other the alternate allele, with a genetic effect shifted by s standard deviations with respect to the reference allele distribution, weighted by its frequency q (q=1-p). Both distributions are assumed to have variance of 1. Where n is sample size, Φ is the standard normal cumulative distribution function (CDF), and z1-α/2 is the standard normal distribution quantile at significance level α, the power to detect the difference in means of the two distributions is calculated as:
Results
We conducted single variant analyses by frequency class (common, low frequency and rare), as well as gene-based SKAT and T1 burden tests as defined above on 170,540 variants in 15,914 individuals of African ancestry. Singleton variants (n=18,217) were dropped in the single rare-variant analyses to prevent observing significance resulting from partiality due to phenotypic outliers. Further, in gene-based tests, genes with only a single variant (n=10,041) were excluded, as they were already included in single-variant analysis, allowing assessment of only those that may possibly reflect an aggregate effect of variants. Genomic inflation factors were calculated for all 170,540 single variants analyzed as well as the gene-based test results, for each of the cohorts and for the meta-analyses. The inflation factors for all of the meta-analyses are shown in Supplemental Digital Content Table, and quantile-quantile (QQ) plots for meta-analyses are displayed in Supplemental Digital Content Figures S1–S2. The inflation factors for these analyses for each cohort are provided in Supplemental Digital Content Table S5. The LUC cohort exhibits slight inflation for a number of the tests; however, no additional adjustment was made, as all inflation factors fall near or within the acceptable range for GWAS [60, 61]. Otherwise, minimal inflation was observed across most cohorts, even at the meta-analysis level, although the QQ plots for rare variants presents several variants deviated from the expected, despite well-controlled genomic inflation factors [62]. The inflation factor uses the median, which is robust to outliers and so may not be aligned with the QQ plot when a specific set of outliers is present.
A brief summary of the number of tests conducted, the Bonferroni correction threshold, and significant results for each analysis are shown in Table 2. A total of 152,323 (non-singleton) variants were used in the single variant analyses. Significant results from the single variant results are outlined in Table 3, and those from the gene-based tests are outlined in Table 4. Although the seqMeta program does not use approximated effect sizes (betas) in its score test, it provides estimates of the (non-standardized) betas and their standard errors. The beta estimates for all of these significant rare variants indicate a BP increasing effect.
Table 2.
Summary of number of significant results and tests per analysis.
| Test | # tests | Bonferroni Corrected Thresholda | # passing threshold (SBP) | # passing threshold (DBP) |
|---|---|---|---|---|
| Common | 28,851 | 1.73 × 10−6 | 0 | 0 |
| Low frequency | 17,985 | 2.78 × 10−6 | 0 | 0 |
| Rare | 105,487 | 4.74 × 10−7 | 7 | 2 |
| Gene-based | 15,554 | 3.21 × 10−6 | 0 | 0 |
| T1 (all variants) | 14,465 | 3.46 × 10−6 | 0 | 0 |
| T1 (deleterious variants) | 5,024 | 9.95 × 10−6 | 1 | 1 |
SBP, systolic blood pressure; DBP, diastolic blood pressure; # tests, number of variants or genes analyzed
The significance threshold as determined by the Bonferroni method corrects for the number of tests (0.05/# tests) in each analysis.
Table 3.
Rare Single Variant Hits for SBP and DBP (p<4.74 × 10−7).
| Variant | Gene | Site | Type | Trait | MAF | p-value | # copies | βsm (SE)a | βst (95%CI)b |
|---|---|---|---|---|---|---|---|---|---|
| rs11568416 | SLC28A3 | intronic | splice | SBP | 1.89 × 10−4 | 8.92 × 10−8 | 6 | 39.44 (7.38) | 1.96 (1.16 – 2.76) |
| rs536397959 | KRBA1 | exonic | synonymous | SBP | 6.28 × 10−5 | 2.35 × 10−8 | 2 | 73.65 (13.19) | 3.93 (2.55 – 5.32) |
| rs139989095 | SEL1L3 | exonic | missense | SBP | 6.28 × 10−5 | 3.23 × 10−8 | 2 | 49.02 (8.87) | 3.91 (2.53 – 5.30) |
| rs147110080 | YOD1 | exonic | missense | SBP | 6.28 × 10−5 | 1.18 × 10−9 | 2 | 64.41 (10.59) | 3.57 (2.18 – 4.96) |
| rs150432347 | COL6A1 | exonic | missense | SBP | 6.05 × 10−4 | 1.19 × 10−7 | 19 | 22.14 (4.18) | 1.16 (0.71 – 1.61) |
| rs138594727 | CRYBA2 | exonic | missense | SBP | 4.73 × 10−4 | 3.02 × 10−7 | 15 | 25.92 (5.06) | 1.27 (0.76 – 1.78) |
| rs148474705 | GAPDHS | exonic | missense | SBP | 6.28 × 10−5 | 4.60 × 10−7 | 2 | 53.85 (10.68) | 3.51 (2.12 – 4.89) |
| rs148474705 | GAPDHS | exonic | missense | DBP | 6.28 × 10−5 | 1.39 × 10−8 | 2 | 40.24 (7.09) | 3.97 (2.58-5.36) |
| rs142319329 | AFF1 | exonic | missense | DBP | 2.51 × 10−4 | 2.95 × 10−7 | 8 | 21.10 (4.12) | 1.77 (1.07 – 2.46) |
SBP, systolic blood pressure; DBP, diastolic blood pressure; MAF, minor allele frequency; βsm, seqMeta beta; βst, beta as standardized mean difference; SE, standard error; 95% CI, 95% confidence interval; # copies, allele count
The βsm (SE) values are reported from seqMeta output, in units of the phenotype residuals.
The βst (95% CI) values are calculated as the mean standardized difference between the LTA residuals for all carriers and all non-carriers for each variant.
Table 4.
Burden-T1-del significant genes for SBP and DBP (p<9.95 x 10−6).
| Gene | Test | Trait | p-value | # SNPs analyzed | Cumulative Carrier Count | βsm (SE)a |
|---|---|---|---|---|---|---|
| CCDC13 | Burden-T1-del | SBP | 3.54 × 10−7 | 2 | 2 | 54.38 (10.68) |
| QSOX1 | Burden-T1-del | DBP | 3.86 × 10−6 | 2 | 3 | 32.93 (7.13) |
Burden-T1-del, T1 burden test on deleterious variants with minor allele frequency (MAF)<0.001; # SNPs analyzed, number of SNPs included in test for gene; Cumulative Carrier Count, number of carriers (same as allele count) across all analyzed variants; βsm, seqMeta beta; SE, standard error
The βsm (SE) values are reported from seqMeta output, in units of the phenotype residuals.
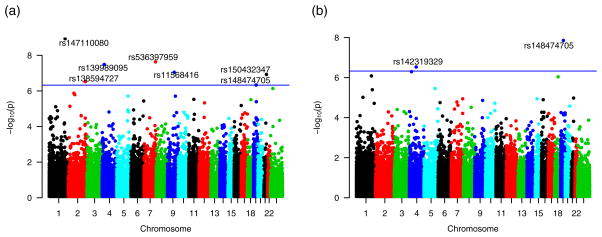
Single-variant analyses of 105,487 variants in the rare variant class revealed several associations with SBP and DBP (Bonferroni threshold p=4.74 × 10−7), summarized in Table 3, with supporting power calculations in Supplemental Digital Content Table S6, and depicted in the Manhattan plots in Figure 1. The vast majority of these variants are non-synonymous (missense). Significant associations of rs150432347 in COL6A1 (p=1.19 × 10−7), with 19 copies in four cohorts, as well as rs138594727 in CRYBA2 (p=3.02 × 10−7), with 15 copies in six cohorts, were identified with SBP. The intronic splice-site SNP rs11568416 in the gene SLC28A3 is additionally associated with SBP (p=8.92 × 10−8), with six copies present across unrelated individuals in four cohorts. Other single-variant analysis results include similar associations of rare variants in KRBA1, SEL1L3, and YOD1 with SBP, each with two to four carriers. The variant rs148474705 in GAPDHS is associated with both SBP and DBP, also with two carriers, and the SNP rs142319329 in the gene AFF1 is associated with DBP (p=2.95 × 10−7), with eight copies present across unrelated individuals in four cohorts. These variants are all present in heterozygous carriers. The mean standardized effect sizes generally indicate that more copies of the variant correlate with smaller effects, but results in this study are too sparse for this conclusion to be well supported.
Figure 1.
Manhattan Plots for 105,487 rare single variant analyses of SBP (a) and DBP (b) [78].
Table 4 contains the results for the two significant genes from the gene-based tests (Bonferroni threshold p=1.02 × 10−5), which are depicted in the Manhattan plots in Figure 2. The T1 test on deleterious variants identified significant associations of CCDC13 for SBP (p=3.54 × 10−7) and QSOX1 for DBP (p=3.86 × 10−6). The associations of CCDC13 with DBP (6.90 × 10−5) and QSOX1 with SBP (p=3.09 × 10−5) are also close to statistical significance. This analysis included two SNPs for CCDC13, each with one copy in different cohorts: rs182436192 (SBP single variant p=5.66 × 10−3) and rs143310118 (SBP single variant p=1.90 × 10−5). There were also two SNPs included in the analysis for QSOX1: rs202144688, with one copy (DBP single variant p=7.59 × 10−4), and rs201390473, with two copies (DBP single variant p=9.26 × 10−4) in different cohorts.
Figure 2.
Manhattan Plots for T1 analyses of deleterious variants at 5,024 genes for SBP (a) and DBP (b). Each gene is plotted at the position of its median SNP available on the chip.
The low frequency, the common single variant analyses, SKAT and Burden-T1-all analyses produced no significant results for either phenotype. Manhattan plots for these analyses are shown in Supplemental Digital Content Figures S3 and S4. However, it may be noted that the top result from the low frequency analysis for DBP was rs73828047 from ULK4 (p = 1.79 × 10−5); further, the ULK4 gene is second on the list of top results for the SKAT analysis in DBP (p=4.54 × 10−5), with rs73828047, rs2272007 (2.64 × 10−3), rs1052501 (p=2.75 × 10−3), rs1716975 (p=3.34 × 10−3), and rs192994614 (p=6.34 × 10−3) as the top five SNPs. These results are particularly of interest, as rs2272007 (MAF=0.30) and rs1716975 (MAF=0.30) are among the common variants previously associated (or in high LD with those associated) with DBP in multiple ethnicities [6, 15], including African Americans. We additionally examined a set of 212 variants representative of the 166 BP GWAS loci, of which 80 were represented in our final cleaned set. While our SBP single variant results fail to support any of these 80 variants, a few show evidence at p<0.01 in the DBP single variant results: rs6969780 in HOXA3 (p=5.37 × 10−4, MAF=0.32), rs6825911 in ENPEP (p=1.70 × 10−3, MAF=0.45), rs1925153 in COL21A1 (p=4.51 × 10−3, MAF=0.41), and rs926552 in SNORD32B (8.79 × 10−3, MAF=0.13). These results, though not significant in these analyses, may lend additional credence to the possibility of roles of these loci in BP regulation.
Assessment of gene-based test results for the known monogenic renal salt-handling BP genes surprisingly provided little evidence of their roles in BP regulation. The SKAT test for DBP produced the lowest p-value for the potassium channel gene, KCNJ5 (p=1.12 × 10−3). Four SNPs were analyzed in the SKAT test, but single-variant p-values suggest that this evidence is primarily driven by the rs115012103 SNP (p=9.95 × 10−4). The SBP SKAT test p-value (p=4.65 × 10−3) for this gene is similarly evidential. Results from variant-based tests for this gene are listed in Supplemental Digital Content Table S7. Overall, the lack of support for the monogenic genes may possibly be attributed to their explaining a small proportion of all essential hypertension cases.
Discussion
This meta-analyses of eight cohorts with individuals of African ancestry have identified several statistically significant associations of rare variants with SBP and DBP (AFF1, GAPDHS, SLC28A3, COL6A1, CRYBA2, KRBA1, SEL1L3, YOD1, CCDC13, QSOX1), and, further, provided additional evidence for previously identified BP loci. Beyond these statistical results, prior biological experiments offer compelling support for the plausibility of these candidates for BP regulation, presented in Table 5 [63–75].
Table 5.
Evidence of genes containing sentinel variants for roles in hypertension.
| Gene | Evidence of role in hypertension |
|---|---|
| COL6A1 | Encodes a collagen VI protein and provides structural support for a variety of tissues including the heart; has been shown previously, in addition to other VEGF-A pathway genes, to be associated with atrioventricular septal defect (AVSD) in patients with Down syndrome [63] |
| SLC28A3 | Encodes a sodium-dependent nucleoside transporter (NT); NTs have many physiological regulatory roles including that of mediating adenosine concentration, which in turn affects vascular tone [64, 65] |
| SEL1L3 | Differentially expressed between male familial combined hyperlipidemia and coronary heart disease (FCHL-CHD) patients and non-FCHL-non-CHD controls in a microarray study [66]. Also upregulated with a >6-fold expression change in a rabbit microarray study comparing simulating conditions before and after repair of coarctation of the aorta, a condition which may evolve into chronic hypertension [67]; as the authors stated, despite the surgically induced return to normal BP, the vasculature still retains its physical defects, and this gene may thus play a role in the continued residual effects. |
| YOD1 | A deubiquitinase, targeted by miR-21 in the distal small pulmonary arteries of mice with pulmonary arterial hypertension exposed to hypoxic conditions, as well as in human pulmonary artery smooth muscle cells with transfected miR-21 [68]. |
| CCDC13 | Encodes a centriolar satellite protein involved in primary ciliogenesis [44]; this supports a potential role in hypertension present in individuals with autosomal dominant polycystic kidney disease, in which impaired primary cilia affects vascular tone [70, 71] |
| QSOX1 | Recent experiments support cardiovascular function for QSOX1, encoding a sulfhydryl oxidase enzyme; one such function includes its induction of vascular smooth muscle cell migration and proliferation in vitro [72], a potential role in atherosclerosis [73], and its feasibility as a biomarker for acute decompensated heart failure [74, 75]. |
There were no significant results from either the common or low frequency single variant analyses, or from the SKAT and Burden-T1-all analyses. Given that the focus of the array is on rare, functional variants, the significant results are, as expected, from the rare single variant and Burden-T1-del analyses. Additionally, the majority of the significant rare variants have just two to three copies across the eight cohorts analyzed in this study; this is also expected as one criterion for variant selection, as stated before, is that the majority of these variants only be present in two to three copies in a minimum of two studies, across multiple populations. Supplemental Digital Content Table S8 details frequencies of these alleles in the African ancestry sample from the Exome Aggregation Consortium (ExAC), with similar numbers of copies and frequencies as in this study. Further, the consistency of measurements across multiple visits in a study where applicable for carriers augments the verity of the associations. Additionally, the power calculations in Table S6 support that there is sufficient statistical power (~55–80%+) to detect the majority of variants of these effects and frequencies in this sample size. Despite this, it still must be considered that the few numbers of copies, especially those with only two copies, of these significant results strongly indicate that they require replication and further study for additional support.
Though there is some evidence for the ~166 GWAS replicated loci as well as the Mendelian syndromic blood pressure genes, their lack of significance in this study is surprising. It is likely that the dearth of common variants available on the chip contributed to inadequate tagging of causal variants within these loci. Despite this, it should be noted that recent BP exome array studies by Liu et al. [20] and Surendran et al. [21] replicated several common, as well as low-frequency, variants. However, these studies consisted of mostly European ancestry participants, and as the sources of sequences that this chip was designed from were primarily European individuals, this likely made the array less informative for individuals of African and other ancestries. An ethnicity-specific chip may therefore be more revelatory, as the familial hypertension variants might be different in African ancestry patients. Additionally, these studies consisted of over 300,000 individuals combined across the discovery and replication stages, while our study consisted of just ~16,000 individuals, at which power is much lower to detect common variants at their typical effect sizes.
The Mendelian genes are contrastingly well known for containing rare causal variants with large effects, though we saw poor signal here as well. As stated above, an ethnicity-specific array may be more helpful here as well. Another possibility to explain the absence of a signal in these genes is that their effect size might be somewhat smaller than the one observed in the rare variants of this experiment, and therefore the statistical experimental power is insufficient to show an association signal.
Regardless, it is of interest that rare variants identified in this study may contribute to blood pressure variation as a polygenic trait. Studies of schizophrenia have identified rare variants from exome-based studies [76, 77], demonstrating an enrichment of rare variants in genes containing common variants implicated in previous schizophrenia GWAS, aiding in fine-mapping of those loci. In our study, though the results did not reach statistical significance, the statistical support for low-frequency variants in ULK4 provides evidence for this as the specific gene within its SBP/DBP GWAS locus.
In summary, we identified several rare variants in 10 genes (AFF1, GAPDHS, SLC28A3, COL6A1, CRYBA2, KRBA1, SEL1L3, YOD1, CCDC13, and QSOX1) that are significantly associated with SBP and DBP traits in 15,914 individuals of African ancestry. In contrast to the previously identified common variants of most BP GWAS, and the rare variants in monogenic blood pressure syndromes, this study has identified rare variants that are potentially contributory to blood pressure as a polygenic trait, particularly in individuals of African-ancestry. The prior experimental evidence of the involvement of these genes with related traits in animal models suggests that these genes are viable candidates for BP regulation, and will benefit from additional investigation. Additionally, the results of this study suggest that future studies relying on genotype arrays for analysis would offer more substantial findings using ethnicity-specific chips.
Supplementary Material
Supplemental Digital Content. Description of Cohorts; Supplementary Tables S1–S8 describing population demographics, phenotype correlations (LTA and SBP with DBP), genomic inflation factors for statistical tests, statistical power for the tests, KCNJ5 single variant test results, and ExAC frequencies for rare variants; Supplementary Figures S1–S4 depicting QQ and Manhattan plots for non-significant results in this study; Complete Acknowledgements. pdf
Acknowledgments
Sources of Support: FEHGAS2 collaboration (NIH R01 HL086694); LUC study (NIH R37-HL045508, R01-HL053353, R01-DK075787 and U01-HL054512); CARDIA (HHSN268201300025C, HHSN268201300026C, HHSN268201300027C, HHSN268201300028C, HHSN268201300029C, HHSN268200900041C); GENOA (NHLBI HL054464, HL054457, HL054481, HL087660, HL086694, HL119443); HyperGEN (U10 NHLBI HL54471, HL54472, HL54473, HL54495, HL54496, HL54497, HL54509, HL54515, and 2 R01 HL55673-12); AADM (NIH 3T37TW00041-03S2, NIDDK grant DK-54001); HUFS (NIGMS/MBRS/SCORE S06GM008016-320107 and S06GM008016-380111, 2M01RR010284, CRGGH—Z01HG200362); ARIC (NHLBI HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C), and funding support for “Building on GWAS for NHLBI-diseases: the U.S. CHARGE consortium” was provided by the NIH through the American Recovery and Reinvestment Act of 2009 (ARRA) (5RC2HL102419); GenNet (NIH HL086694).
Sources were received from National Institutes of Health (NIH)
We thank the participants of all of the studies involved and assistance of the research staff and all investigators that have made our analyses possible.
Footnotes
Conflicts of Interest: None
Previous Presentation of Work: This work has previously been presented as a poster at the American Society of Human Genetics meeting in 2015.
References
- 1.The International Consortium for Blood Pressure Genome-Wide Association Studies. Genetic Variants in Novel Pathways Influence Blood Pressure and Cardiovascular Disease Risk. Nature. 2011;478(7367):103–109. doi: 10.1038/nature10405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnson T, Gaunt TR, Newhouse SJ, Padmanabhan S, Tomaszewski M, Kumari M, et al. Blood Pressure Loci Identified with a Gene-Centric Array. Am J Hum Genet. 2011;89(6):688–700. doi: 10.1016/j.ajhg.2011.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Padmanabhan S, Melander O, Johnson T, Di Blasio AM, Lee WK, Gentilini D, et al. Genome-Wide Association Study of Blood Pressure Extremes Identifies Variant near UMOD Associated with Hypertension. In: Schork NJ, editor. PLoS Genet. 10. Vol. 6. 2010. p. e1001177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kato N, Takeuchi F, Tabara Y, Kelly TN, Go MJ, Sim X, et al. Meta-analysis of genome-wide association studies identifies common variants associated with blood pressure variation in East Asians. Nat Genet. 2011;43(6):531–538. doi: 10.1038/ng.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wain LV, Verwoert GC, O’Reilly PF, Shi G, Johnson T, Johnson AD, et al. Genome-wide association study identifies six new loci influencing pulse pressure and mean arterial pressure. Nat Genet. 2011;43(10):1005–1011. doi: 10.1038/ng.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Franceschini N, Fox E, Zhang Z, Edwards TL, Nalls MA, Sung YJ, et al. Genome-wide Association Analysis of Blood-Pressure Traits in African-Ancestry Individuals Reveals Common Associated Genes in African and Non-African Populations. Am J Hum Genet. 2013;93(3):545–554. doi: 10.1016/j.ajhg.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ganesh SK, Chasman DI, Larson MG, Guo X, Verwoert G, Bis JC, et al. Effects of Long-Term Averaging of Quantitative Blood Pressure Traits on the Detection of Genetic Associations. Am J Hum Genet. 2014;95(1):49–65. doi: 10.1016/j.ajhg.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tragante V, Barnes MR, Ganesh SK, Lanktree MB, Guo W, Franceschini N, et al. Gene-centric Meta-analysis in 87,736 Individuals of European Ancestry Identifies Multiple Blood-Pressure-Related Loci. Am J Hum Genet. 2014;94(3):349–360. doi: 10.1016/j.ajhg.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simino J, Shi G, Bis JC, Chasman DI, Ehret GB, Gu X, et al. Gene-Age Interactions in Blood Pressure Regulation: A Large-Scale Investigation with the CHARGE, Global BPgen, and ICBP Consortia. Am J Hum Genet. 2014;95(1):24–38. doi: 10.1016/j.ajhg.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Y, O’Connell JR, McArdle PF, Wade JB, Dorff SE, Shah SJ, et al. Whole-genome association study identifies STK39 as a hypertension susceptibility gene. Proc Natl Acad Sci U S A. 2009;106(1):226–231. doi: 10.1073/pnas.0808358106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ehret GB, Ferreira T, Chasman DI, Jackson AU, Schmidt EM, Johnson T, et al. The genetics of blood pressure regulation and its target organs from association studies in 342,415 individuals. Nat Genet. 2016;48(10):1171–84. doi: 10.1038/ng.3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson AD, Newton-Cheh C, Chasman DI, Ehret GB, Johnson T, Rose L, et al. Association of hypertension drug target genes with blood pressure and hypertension in 86,588 individuals. Hypertension. 2011;57:903–10. doi: 10.1161/HYPERTENSIONAHA.110.158667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ganesh SK, Tragante V, Guo W, Guo Y, Lanktree MB, Smith EN, et al. Loci influencing blood pressure identified using a cardiovascular gene-centric array. Hum Mol Genet. 2013;22:1663–78. doi: 10.1093/hmg/dds555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takeuchi F, Isono M, Katsuya T, Yamamoto K, Yokota M, Sugiyama T, et al. Blood pressure and hypertension are associated with 7 loci in the Japanese population. Circulation. 2010;121:2302–9. doi: 10.1161/CIRCULATIONAHA.109.904664. [DOI] [PubMed] [Google Scholar]
- 15.Levy D, Ehret GB, Rice K, Verwoert GC, Launer LJ, Dehghan A, et al. Genome-wide association study of blood pressure and hypertension. Nat genet. 2009;41(6):677–687. doi: 10.1038/ng.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Newton-Cheh C, Larson MG, Vasan RS, Levy D, Bloch KD, Surti A, et al. Association of common variants in NPPA and NPPB with circulating natriuretic peptides and blood pressure. Nat genet. 2009;41:348–53. doi: 10.1038/ng.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Newton-Cheh C, Johnson T, Gateva V, Tobin MD, Bochud M, Coin L, et al. Genome-wide association study identifies eight loci associated with blood pressure. Nat genet. 2009 Jun;41(6):666–76. doi: 10.1038/ng.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kato N, Loh M, Takeuchi F, Verweij N, Wang X, Zhang W, et al. Trans-ancestry genome-wide association study identifies 12 genetic loci influencing blood pressure and implicates a role for DNA methylation. Nat genet. 2015;47(11):1282–1293. doi: 10.1038/ng.3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parmar PG, Taal HR, Timpson NJ, Thiering E, Lehtimäki T, Marinelli M, et al. International GWAS Consortium Identifies Novel Loci Associated with Blood Pressure in Children and Adolescents. Circ Cardiovasc Genet. 2016;9(3):266–78. doi: 10.1161/CIRCGENETICS.115.001190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu C, Kraja AT, Smith JA, Brody JA, Franceschini N, Bis JC, et al. Meta-analysis identifies common and rare variants influencing blood pressure and overlapping with metabolic trait loci. Nat genet. 2016 Oct;48(10):1162–70. doi: 10.1038/ng.3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Surendran P, Drenos F, Young R, Warren H, Cook JP, Manning AK, et al. Trans-ancestry meta-analyses identify rare and common variants associated with blood pressure and hypertension. Nat genet. 2016 Oct;48(10):1151–61. doi: 10.1038/ng.3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoffmann TJ, Ehret GB, Nandakumar P, Ranatunga D, Schaefer C, Kwok PY, et al. Genome-wide association analyses using electronic health records identify new loci influencing blood pressure variation. Nat genet. 2016 Nov 14; doi: 10.1038/ng.3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu X, Feng T, Tayo BO, Liang J, Young JH, Franceschini N, et al. Meta-analysis of Correlated Traits via Summary Statistics from GWASs with an Application in Hypertension. Am J Hum Genet. 2015;96(1):21–36. doi: 10.1016/j.ajhg.2014.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fox ER, Young JH, Li Y, Dreisbach AW, Keating BJ, Musani SK, et al. Association of genetic variation with systolic and diastolic blood pressure among African Americans: the Candidate Gene Association Resource study. Hum Mol Genet. 2011;20(11):2273–2284. doi: 10.1093/hmg/ddr092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Munroe PB, Barnes MR, Caulfield MJ. Advances in Blood Pressure Genomics. Circ Res. 2013;112:1365–1379. doi: 10.1161/CIRCRESAHA.112.300387. [DOI] [PubMed] [Google Scholar]
- 26.Tomaszewski M, Debiec R, Braund PS, Nelson CP, Hardwick R, Christofidou P, et al. Genetic architecture of ambulatory blood pressure in the general population: insights from cardiovascular gene-centric array: Genetic architecture of blood pressure. Hypertension. 2010;56(6):1069–1076. doi: 10.1161/HYPERTENSIONAHA.110.155721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Illumina. [Accessed: 23 October 2015]; datasheet_humanexome_beadchips.pdf[Internet] Available at: http://products.illumina.com/content/dam/illumina-marketing/documents/products/datasheets/datasheet_humanexome_beadchips.pdf.
- 28. [Accessed: 23 October 2015];Exome Chip Design. [Internet] 2013 Available at: http://genome.sph.umich.edu/wiki/Exome_Chip_Design.
- 29.Fuchs Flávio D. Why Do Black Americans Have Higher Prevalence of Hypertension?: An Enigma Still Unsolved. Hypertension. 2011;57:379–380. doi: 10.1161/HYPERTENSIONAHA.110.163196. [DOI] [PubMed] [Google Scholar]
- 30.Zhu X, Young JH, Fox E, Keating BJ, Franceschini N, Kang S, et al. Combined admixture mapping and association analysis identifies a novel blood pressure genetic locus on 5p13: contributions from the CARe consortium. Hum Mol Genet. 2011;20(11):2285–2295. doi: 10.1093/hmg/ddr113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lifton RP. Molecular genetics of human blood pressure variation. Science. 1996;272:676–680. doi: 10.1126/science.272.5262.676. [DOI] [PubMed] [Google Scholar]
- 32.Lifton RP, Gharavi AG, Geller DS. Molecular mechanisms of human hypertension. Cell. 2001;104:545–556. doi: 10.1016/s0092-8674(01)00241-0. [DOI] [PubMed] [Google Scholar]
- 33.Ji W, Foo JN, O’Roak BJ, Zhao H, Larson MG, Simon DB, et al. Rare independent mutations in renal salt handling genes contribute to blood pressure variation. Nat Genet. 2008 May;40(5):592–599. doi: 10.1038/ng.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rotimi CN, Dunston GM, Berg K, Akinsete O, Amoah A, Owusu S, et al. In search of susceptibility genes for type 2 diabetes in West Africa: the design and results of the first phase of the AADM study. Ann Epidemiol. 2001;11(1):51–8. doi: 10.1016/s1047-2797(00)00180-0. [DOI] [PubMed] [Google Scholar]
- 35.Sumner AE, Zhou J, Doumatey A, Imoisili OE, Amoah A, Acheampong J, et al. Low HDL-Cholesterol with Normal Triglyceride Levels is the Most Common Lipid Pattern in West Africans and African Americans with Metabolic Syndrome: Implications for Cardiovascular Disease Prevention. CVD Prev Control. 2010;5(3):75–80. doi: 10.1016/j.cvdpc.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. [Accessed: 23 October 2015];Family Blood Pressure Program. [Internet] Available at: http://www.biostat.wustl.edu/fbpp/FBPP.shtml.
- 37.The FBPP Investigators. Multi-center genetic study of hypertension: the Family Blood Pressure Program (FBPP) Hypertension. 2002 Jan;39(1):3–9. doi: 10.1161/hy1201.100415. [DOI] [PubMed] [Google Scholar]
- 38.Howard University – National Human Genome Center. [Accessed: 23 October 2015]; [Internet] Available at: http://www.genomecenter.howard.edu/units/genetic_epidemiology/default.htm.
- 39.Adeyemo A, Gerry N, Chen G, Herbert A, Doumatey A, Huang H, et al. A Genome-Wide Association Study of Hypertension and Blood Pressure in African Americans. In: Dermitzakis ET, editor. PLoS Genet. 7. Vol. 5. 2009. p. e1000564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. [Accessed: 23 October 2015];Atherosclerosis Risk in Communities. [Internet] Available at: https://www2.cscc.unc.edu/aric.
- 41.Investigators The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 42.Cooper R, Rotimi C, Ataman S, McGee D, Osotimehin B, Kadiri S, et al. The prevalence of hypertension in seven populations of west African origin. Am J Public Health. 1997;87(2):160–168. doi: 10.2105/ajph.87.2.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kang SJ, Chiang CWK, Palmer CD, Tayo BO, Lettre G, Butler JL, et al. Genome-wide association of anthropometric traits in African- and African-derived populations. Hum Mol Genet. 2010;19(13):2725–2738. doi: 10.1093/hmg/ddq154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ataman SL, Cooper R, Rotimi C, McGee D, Osotimehin B, Kadiri S, et al. Standardization of blood pressure measurement in an international comparative study. J Clin Epidemiol. 1996;49(8):869–877. doi: 10.1016/0895-4356(96)00111-4. [DOI] [PubMed] [Google Scholar]
- 45.Cooper R, Puras A, Tracy J, Kaufman J, Asuzu M, Ordunez P, et al. Evaluation of an electronic blood pressure device for epidemiological studies. Blood Press Monit. 1997;2(1):35–40. [PubMed] [Google Scholar]
- 46. [Accessed: 23 October 2015];CARDIA Overview. [Internet] Available at: http://www.cardia.dopm.uab.edu/cardia-overview/overview-more.
- 47.Friedman GD, Cutter GR, Donahue RP, Hughes GH, Hulley SB, Jacobs DR, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41:1105–1116. doi: 10.1016/0895-4356(88)90080-7. [DOI] [PubMed] [Google Scholar]
- 48.Grove ML, Yu B, Cochran BJ, Haritunians T, Bis JC, Taylor KD, et al. Best Practices and Joint Calling of the HumanExome BeadChip: The CHARGE Consortium. In: Aulchenko YS, editor. PLoS One. 7. Vol. 8. 2013. p. e68095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, et al. PLINK: A Tool Set for Whole-Genome Association and Population-Based Linkage Analyses. Am J Hum Genet. 2007;81(3):559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2014. http://www.R-project.org/ [Google Scholar]
- 51.Manichaikul A, Mychaleckyj JC, Rich SS, Daly K, Sale M, Chen W-M. Robust relationship inference in genome-wide association studies. Bioinformatics. 2010;26(22):2867–2873. doi: 10.1093/bioinformatics/btq559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang K, Li M, Hakonarson H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010;38(16):e164. doi: 10.1093/nar/gkq603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pollard KS, Hubisz MJ, Rosenbloom KR, Siepel A. Detection of nonneutral substitution rates on mammalian phylogenies. Genome Res. 2010;20(1):110–121. doi: 10.1101/gr.097857.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tobin MD, Sheehan NA, Scurrah KJ, Burton PR. Adjusting for treatment effects in studies of quantitative traits: antihypertensive therapy and systolic blood pressure. Stat Med. 2005;24(19):2911–35. doi: 10.1002/sim.2165. [DOI] [PubMed] [Google Scholar]
- 55.Voorman A, Brody J, Chen H, Lumley T. seqMeta: An R package for meta-analyzing region-based tests of rare DNA variants. 2013 http://cran.r-project.org/web/packages/seqMeta/
- 56.Wu MC, Lee S, Cai T, Li Y, Boehnke M, Lin X. Rare-Variant Association Testing for Sequencing Data with the Sequence Kernel Association Test. Am J Hum Genet. 2011;89(1):82–93. doi: 10.1016/j.ajhg.2011.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li B, Leal SM. Methods for Detecting Associations with Rare Variants for Common Diseases: Application to Analysis of Sequence Data. Am J Hum Genet. 2008;83(3):311–321. doi: 10.1016/j.ajhg.2008.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee S, Emond MJ, Bamshad MJ, Barnes KC, Rieder MJ, Nickerson DA, et al. Optimal Unified Approach for Rare-Variant Association Testing with Application to Small-Sample Case-Control Whole-Exome Sequencing Studies. Am J Hum Genet. 2012;91(2):224–237. doi: 10.1016/j.ajhg.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nguyen KD, Pihur V, Ganesh SK, Rakha A, Cooper RS, Hunt SC, et al. Effects of Rare and Common Blood Pressure Gene Variants on Essential Hypertension: Results from the FBPP, CLUE and ARIC Studies. Circ Res. 2013;112(2):318–326. doi: 10.1161/CIRCRESAHA.112.276725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Price AL, Zaitlen NA, Reich D, Patterson N. New approaches to population stratification in genome-wide association studies. Nat Rev Genet. 2010;11(7):459–463. doi: 10.1038/nrg2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.The Wellcome Trust Case Control Consortium. Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447(7145):661–678. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pirie A, Wood A, Lush M, Tyrer J, Pharoah PD. The effect of rare variants on inflation of the test statistics in case–control analyses. BMC Bioinformatics. 2015;16(1):53. doi: 10.1186/s12859-015-0496-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ackerman C, Locke AE, Feingold E, Reshey B, Espana K, Thusberg J, et al. An Excess of Deleterious Variants in VEGF-A Pathway Genes in Down-Syndrome-Associated Atrioventricular Septal Defects. Am J Hum Genet. 2012;91(4):646–659. doi: 10.1016/j.ajhg.2012.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Löffler M, Morote-Garcia JC, Eltzschig SA, Coe IR, Eltzschig HK. Physiological roles of vascular nucleoside transporters. Arterioscler Thromb Vasc Biol. 2007;27:1004–1013. doi: 10.1161/ATVBAHA.106.126714. [DOI] [PubMed] [Google Scholar]
- 65.Ritzel MW, Ng AM, Yao SY, Graham K, Loewen SK, Smith KM, et al. Molecular identification and characterization of novel human and mouse concentrative Na-nucleoside cotransporter proteins (hCNT3 and mCNT3) broadly selective for purine and pyrimidine nucleosides (system cib) J Biol Chem. 2001;276:2914–2927. doi: 10.1074/jbc.M007746200. [DOI] [PubMed] [Google Scholar]
- 66.Horswell SD, Fryer LGD, Hutchison CE, Zindrou D, Speedy HE, Town MM, et al. CDKN2B expression in adipose tissue of familial combined hyperlipidemia patients. J Lipid Res. 2013;54(12):3491–3505. doi: 10.1194/jlr.M041814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.LaDisa JF, Bozdag S, Olson J, Ramchandran R, Kersten JR, Eddinger TJ. Gene Expression in Experimental Aortic Coarctation and Repair: Candidate Genes for Therapeutic Intervention? PLoS One. 2015;10(7):e0133356. doi: 10.1371/journal.pone.0133356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ye X, Zhang HM, Qiu Y, Hanson PJ, Hemida MG, Wei W, et al. Coxsackievirus-Induced miR-21 Disrupts Cardiomyocyte Interactions via the Downregulation of Intercalated Disk Components. In: Nelson JA, editor. PLoS Pathog. 4. Vol. 10. 2014. p. e1004070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Staples CJ, Myers KN, Beveridge RD, Patil AA, Howard AE, Barone G, et al. Ccdc13 is a novel human centriolar satellite protein required for ciliogenesis and genome stability. J Cell Sci. 2014;127(Pt 13):2910–9. doi: 10.1242/jcs.147785. [DOI] [PubMed] [Google Scholar]
- 70.Nauli SM, Jin X, Hierck BP. The Mechanosensory Role of Primary Cilia in Vascular Hypertension. Int J Vasc Med. 2011;2011:376281. doi: 10.1155/2011/376281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rahbari-Oskoui F, Williams O, Chapman A. Mechanisms and management of hypertension in autosomal dominant polycystic kidney disease. Nephrol Dial Transplant. 2014;29(12):2194–201. doi: 10.1093/ndt/gft513. [DOI] [PubMed] [Google Scholar]
- 72.Borges BE, Appel MH, Cofré AR, Prado ML, Steclan CA, Esnard F, et al. The flavor-oxidase QSOX1 supports vascular smooth muscle cell migration and proliferation: Evidence for a role in neointima growth. Biochim Biophys Acta. 2015;1852(7):1334–46. doi: 10.1016/j.bbadis.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 73.de Andrade CR, Stolf BS, Debbas V, Rosa DS, Kalil J, Coelho V, Laurindo FR. Quiescin sulfhydryl oxidase (QSOX) is expressed in the human atheroma core: possible role in apoptosis. In Vitro Cell Dev Biol Anim. 2011;47(10):716–727. doi: 10.1007/s11626-011-9461-0. [DOI] [PubMed] [Google Scholar]
- 74.Mebazaa A, Vanpoucke G, Thomas G, Verleysen K, Cohen-Solal A, Vanderheyden M, et al. Unbiased plasma proteomics for novel diagnostic biomarkers in cardiovascular disease: identification of quiescin Q6 as a candidate biomarker of acutely decompensated heart failure. Eur Heart J. 2012;33(18):2317–24. doi: 10.1093/eurheartj/ehs162. [DOI] [PubMed] [Google Scholar]
- 75.Doehner W. Diagnostic biomarkers in cardiovascular disease: the proteomics approach. Eur Heart J. 2012;33(18):2249–51. doi: 10.1093/eurheartj/ehs187. [DOI] [PubMed] [Google Scholar]
- 76.Purcell SM, Moran JL, Fromer M, Ruderfer D, Solovieff N, Roussos P, et al. A polygenic burden of rare disruptive mutations in schizophrenia. Nature. 2014;506(7487):185–190. doi: 10.1038/nature12975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Richards AL, Leonenko G, Walters JT, Kavanagh DH, Rees EG, Evans A, et al. Exome arrays capture polygenic rare variant contributions to schizophrenia. Hum Mol Genet. 2016;25(5):1001–1007. doi: 10.1093/hmg/ddv620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Turner SD. qqman: an R package for visualizing GWAS results using Q-Q and manhattan plots. 2014 doi: 10.1101/005165. biorXiv. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content. Description of Cohorts; Supplementary Tables S1–S8 describing population demographics, phenotype correlations (LTA and SBP with DBP), genomic inflation factors for statistical tests, statistical power for the tests, KCNJ5 single variant test results, and ExAC frequencies for rare variants; Supplementary Figures S1–S4 depicting QQ and Manhattan plots for non-significant results in this study; Complete Acknowledgements. pdf