Summary
Objective
To estimate the incremental cost-effectiveness of tuberculosis screening and isoniazid preventive therapy (IPT) among HIV-infected adults in Rio de Janeiro.
Design
We used decision analysis, populated by data from a cluster-randomized trial, to project costs (in 2010 US dollars) and effectiveness (in disability-adjusted life years, DALYs, averted) of training healthcare workers to implement tuberculin skin testing (TST) followed by IPT for TST-positive patients with no evidence of active TB. This intervention was compared to a baseline of usual care. We used time horizons of one year for the intervention and 20 years for disease outcomes, with all future DALYs and medical costs discounted at 3% per year.
Results
Providing this intervention to 100 people would avert 1.14 discounted DALYs (1.57 undiscounted DALYs). The median estimated incremental cost-effectiveness ratio was $2273 (IQR: $1779-$3135) per DALY averted, less than Brazil's 2010 per-capita gross domestic product (GDP) of $11,700. Results were most sensitive to the cost of providing the training.
Conclusion
Training healthcare workers to screen HIV-infected adults with TST and provide IPT to those with latent TB infection can be considered cost-effective relative to the Brazilian GDP per capita.
Keywords: Brazil, TB/HIV co-infection, economic analysis, IPT, skin tests
Introduction
Despite WHO recommendations for isoniazid preventive therapy (IPT) for HIV infected patients1 and recent evidence that IPT reduces tuberculosis incidence among persons living with HIV/AIDS 2-4, few countries have prioritized IPT in their HIV strategy 5. HIV and tuberculosis programs are rarely integrated, thus responsibility for implementation of isoniazid preventive therapy and associated costs is a complicated issue that must be considered prior to implementing preventive therapy.
In Brazil, TB remains an important public health problem and 14 percent of new adult TB patients in Brazil are also estimated to be infected with HIV 6. Tuberculin skin testing (TST) and IPT for those testing positive has been recommended for persons living with HIV/AIDS in Brazil since 1995, but few HIV-infected patients had been tested prior to 2005. The Tuberculosis/HIV in Rio de Janeiro (THRio) study was conducted between 2005 and 2009, providing TST and IPT to HIV-infected patients in 29 clinics. The study showed that markedly increasing the number of patients receiving TST and IPT is possible in this setting, and that this increase can reduce TB incidence and mortality 3, 7. THRio's impact on TB incidence may not generalize to other settings where there are higher rates of transmission such as South African gold mines where TST and IPT was not able to reduce TB infections 8.
If the THRio intervention is to be scaled up in Brazil, or implemented in a similar setting, a better understanding of the resources allocated and the cost effectiveness of the intervention is necessary. Costs of such a program include training staff, reorganizing clinic services as well as paying additional costs for the medical care associated with administering TST and IPT. This report analyzes the costs, impact on disability adjusted life years (DALY), and cost effectiveness of the THRio study.
Methods
Study Design, Intervention, and Population
We used decision analysis with Markov methods to compare costs and health outcomes (i.e., DALYs) at public clinics in Rio de Janeiro, Brazil. Our primary outcome was the incremental cost-effectiveness ratio (ICER), comparing facilities exposed to a program designed to increase the rate of TST and IPT delivery among HIV patients in Rio de Janeiro to a baseline of similar facilities unexposed to this program. We expressed the outcome as the cost in 2010 US dollars per disability-adjusted life year (DALY) averted among adults treated at exposed and unexposed facilities. Data to populate the model were drawn from the TB/HIV in Rio de Janeiro (THRio) study, the design and primary results of which have been described elsewhere 3. Briefly, the THRio study was a cluster-randomized trial in which 29 public HIV clinics were randomly assigned, in stepped-wedge fashion, dates on which to receive training in using TST and IPT. Healthcare workers receiving the intervention were trained on the proper use of TST, and to provide IPT to eligible individuals with a positive TST and no evidence of active TB. Eligible patients were those with no history of prior TB or receipt of IPT; active TB was ruled out with sputum smear and chest X-ray (CXR) according to Brazilian guidelines and all individuals with a positive TST (≥ 5 mm of induration) prior to receiving IPT (six months of isoniazid 300 mg and pyridoxine 25 mg daily) 9. Prescriptions were refilled every 30 or 90 days depending on the clinic 9. Patients were followed to primary endpoints of incident TB or death from any cause. The median size of training sessions in each clinic required 4 physicians, 5 nurses, and 4 health assistants, with a total average of 30 person-hours required per clinic.
We projected costs and outcomes for 100 eligible patients (i.e., no evidence of active TB on presentation) receiving the THRio intervention versus usual care. We considered all activities related to the intervention over a time horizon of one year and followed patients for outcomes (i.e., TB cases and deaths) for a time horizon of 20 years. The incidence of TB and the protective effect of IPT were taken from the THRio trial 3. We assumed that the effect of IPT would last at least 20 years in this population, the majority of which was on antiretroviral therapy and at low annual risk of TB re-infection. We conservatively assumed that the only effect of IPT would be through a reduction in TB incidence (i.e., no ancillary benefits on mortality beyond a reduction in strictly measured incident TB cases). Thus, we adopted as our primary measure of effect the reduction in TB incidence alone; this conservative assumption provides the estimate of cost-effectiveness that is least favorable to IPT. In a sensitivity analysis, we considered the more liberal assumption that IPT had wider benefits on both TB incidence and mortality as seen in the THRio trial.
Cost Calculation
Costs were calculated using an ingredients approach as shown in the top panel of Table 2. We adopted the perspective of the medical sector as the primary party responsible for financing this intervention. We used actual program costs reflecting local market prices and wages 10, 11. We surveyed the trainers responsible for implementing the program at each of the 29 clinics to determine the job titles and salaries of clinic attendees who had to cancel their clinical operations to attend training. The survey also included information on logistics required to conduct each training session. Unit cost estimates were derived from personnel payrolls, financial records, and consultations with staff. All costs are reported in 2010 US dollars. Cost data from other years in Brazilian Reals were inflated to 2010 using the Brazilian inflation rate and then converted to US dollars using the 2010 exchange rate. Medical care costs were confined to the costs of treating TB cases. Some would argue that the incremental medical costs of antiretrovirals for TB patients, whose lives are saved, should have been included as well. Including these costs would make the intervention more costly per DALY averted. We discounted future costs and effectiveness outcomes at 3% per year.
Table 2. Effectiveness outcomes of training to provide TST and IPT in 100 HIV infected patients.
| Items | TB Deaths | TB Cases | DALYs lost by 100 people over 20 years | Discounted Lost DALYs |
|---|---|---|---|---|
| Without Intervention | 1.3 | 11.5 | 822 | 582 |
| With Intervention | 1.1 | 10.0 | 821 | 581 |
| Incremental | 0.1 | 1.5 | 1.6 | 1.1 |
| Incremental with Discounting | 1.1 |
Effectiveness calculation
Mortality outcomes have been estimated and published based on study data collected from September 2005 through August 2009 3. We computed years of life lost per TB death relative to the average survival of HIV infected-TB uninfected patients in the THRio trial. To estimate the disability weight for TB/HIV-coinfected individuals in Brazil, we used data drawn from 98 of the patients in this trial who reported on their disability using a visual analog scale 12. This survey supported our estimate of disability weights of 72.78, 74.09, and 77.4 among TB/HIV-coinfected, TB infected, and HIV infected individuals respectively 12. We assumed that the state of TB/HIV disability lasted for one year before reverting to the disability state of chronic stable HIV.
Sensitivity and Uncertainty Analysis
We conducted both one-way sensitivity analyses on selected model parameters and a probabilistic Monte Carlo uncertainty analysis in which we varied the hazard ratio of TB death associated with the intervention among all eligible patients (i.e., regardless of TST or IPT status) from a low of 0.7 to a high of 0.97, using a point estimate (hazard ratio for TB disease in the THRio trial) of 0.87 and a normal distribution. The probabilistic sensitivity analysis also varied the cost using a log normal distribution with the minimum costs shown in Table 1. This is another conservative assumption that gives extra consideration to the possibility that future costs will be higher than those observed in the study. The probabilistic analysis was programmed in @Risk™ and generated distributions around costs, DALYs and the incremental cost effectiveness ratio. We summarize this uncertainty range using median and inter-quartile ranges.
Table 1. Static version of cost effectiveness modeling per 100 patients over 20 years. Costs as of 2010.
| Cost Model | Items | $ Price per unit 2010 | Number needed for 100 patients | Total cost | |
| PPD costs (includes patient waiting time) | $28.12 | 58 | $1,631 | ||
| IPT | Sputum AFB | $28.12 | 58 | $1,631 | |
| Chest X Ray | $39.43 | 7 | $276 | ||
| INH | $30.85 | 7 | $216 | ||
| Clinic Start up | $6,654.54 | 0.22 | $1,464 | ||
| Minimum cost per 100 patients followed for 20 years | Items | Price per unit | Intervention | Without Intervention | |
| Training cost (0.22 Trainings) | $6,655 | $1,464 | $0 | ||
| TST cost (58 TSTs) | $28 | $1,631 | $0 | ||
| IPT cost (7 IPTs) | $91 | $640 | $0 | ||
| TB treatment cost | $653 | $523 | $601 | ||
| Total | $10,248 | $7,477 | |||
| Total Discounted | $8,992 | $6,034 | |||
| Incremental Cost Effectiveness Ratio In 100 patients followed 20 years | Items | Discounted DALYS | Minimum Discounted Costs $ | ICER (Δ$/ΔDALY) | |
| Without Intervention | 582.02 | $6034 | |||
| With Intervention | 580.88 | $8992 | |||
| Difference | 1.14 | $2958 | |||
| Difference per patient | 0.0114 | $29.58 | |||
| Incremental Cost- Effectiveness Ratio | $ 2594 | ||||
Ethics
This study was approved by the institutional review boards of the Johns Hopkins Medical Institutions and the Municipal Health Secretariat of Rio de Janeiro.
Results
Cost and Cost-Effectiveness
To carry out the intervention for 100 patients, we estimated a requirement of 0.22 clinic trainings, 58 TSTs placed, and 7 courses of IPT delivered. The cost to carry out the training intervention was $6655 per clinic (454 patients). To calculate the cost per 100 patients we note that 100/454 is 0.22 and [(0.22) × $6655]= $1464 per 100 patients. The median discounted cost including intervention implementation, diagnosis, follow-up, and therapy in 100 patients followed 20 years was $9,748 (IQR: $9530-$10,078) with the intervention and $6,461 (IQR: $6278-$6783) in the baseline condition; the median of the cost differences across 10,000 iterations was $3208 (IQR: $3108-$3355) or $32.08 per patient.
Intervention Effects
The results of the impact evaluation of the trial are available elsewhere 3. Key values derived from the trial include a crude hazard ratio for incident active TB (comparing the intervention to no intervention) of 0.87 and 58% TST coverage among the eligible population, of whom 20% tested positive, with 82% of people with positive TST receiving IPT. Crude rates of TB and TB or death, respectively, were 1.31 and 3.64/100 person-years in the control population versus 1.10 and 3.04/100 person-years in the intervention population. We projected that the THRio intervention would avert 0.17 TB deaths and 1.48 cases of active TB over a 20-year horizon (Table 2). These averted deaths and TB cases are estimated to account for 1.14 discounted DALYS averted in 100 people over 20 years. We estimate that 96% of the DALY burden averted is due to disability, with the remainder due to premature death. The bottom of Table 2 lists one estimate of the ICER, obtained by dividing the minimum cost by the mean estimate of discounted DALYs, at $2594. The ICER estimate based on the median of 10,000 iterations of the Monte Carlo model was $2273 per DALY averted (IQR: $1779-$3135).
Sensitivity Analysis
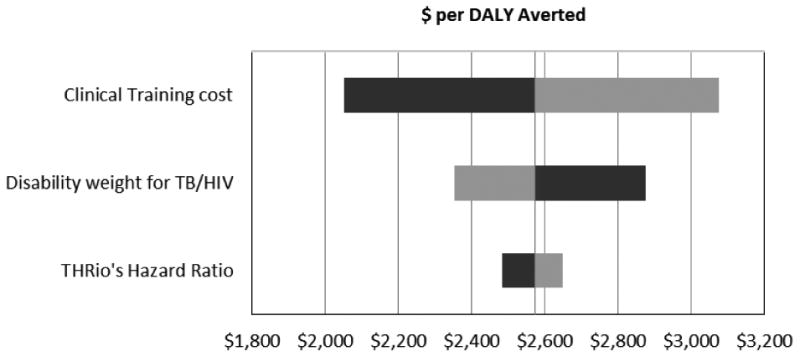
Among the parameters used in the model, the ones most likely to affect the results were chosen for univariate sensitivity analysis. We chose to examine the efficacy of the intervention because all estimates of benefit hinge on it. Similarly the disability weight on TB/HIV could have a large impact because 96% of the DALY burden is from disability. Among the cost parameters, training costs accounted for approximately 22% of the total cost of the intervention sowe varied that as well. Results of two-way sensitivity analysis (Table 3 and Figure 1) show that the cost-effectiveness of the intervention was robust to variation in the efficacy of IPT, cost of clinical training, and disability weight for TB/HIV. If the disability weight for TB/HIV was 10 points worse than HIV, it would make the intervention appear more cost-effective and it would lower the ICER to $2352 per DALY averted. Other factor associated with variation in the cost-effectiveness ratio included the cost of start up clinics.
Figure 1. Results of the sensitivity analysis on parameters used in the Cost-effectiveness model.
Discussion
This decision model based on data from a pragmatic cluster-randomized trial suggests that training to perform TST and deliver IPT to HIV-infected adults is likely to be cost-effective in medium-burden, middle-income settings. For every 100 individuals exposed to this intervention, we project that 1.5 cases and 0.2 TB deaths could be averted, at a median cost of $2273 (IQR: $1779-$3135) per DALY averted. Thus, although the gains from this feasible intervention may be relatively small in absolute terms, they are likely to present good “value for money” in comparison with other health interventions that may be available in middle-income settings such as urban Brazil. The incremental cost per DALY averted for this intervention was far less than Brazil's 2010 per-capita gross domestic product (GDP) of $11,700. Hence it meets criteria to be classified as highly cost-effective in the context of Brazil.
This is one of the first analyses to estimate the cost-effectiveness of preventive therapy for TB among HIV-infected individuals with an assumption of constant incidence of TB. Other analyses among non-HIV-infected low prevalence populations suggest that IPT may also be cost-effective in selected settings 13. Relative to these studies, the ICER of providing IPT to HIV-infected adults in Brazil is somewhat more cost-effective. This supports current WHO guidelines 14 to provide IPT to people living with HIV. Importantly, the THRio trial was designed to test a pragmatic intervention at the clinic level; this intervention remains cost-effective despite our effectiveness measures inherently accounting for programmatic realities, including incomplete coverage (e.g., only 58% of eligible individuals receiving TST). If the intervention could be delivered at even higher coverage (e.g., through provision of nurses or other personnel to administer TSTs), its cost-effectiveness might be enhanced further. However, if IPT efficacy or TB incidence were lower, then the cost per DALY averted would be higher.
As with any modeling analysis, our study has important limitations. While we found that this intervention was cost-effective against the threshold of Brazil's GDP, it would not meet this same threshold in other lower-income countries unless its cost was proportional to GDP. Compared to many other TB interventions – even some considered to be very expensive (e.g., treatment of MDR-TB 16) – this intervention is less cost-effective, though it may be more affordable due to its lower per-patient cost. Our findings – which assume long-term effectiveness of IPT – may not generalize to higher-burden settings where the long-term efficacy of six months of IPT is known to be lower15, 17.
The majority (67%) of participants were on ART at the time of IPT initiation. Of those who were not, many (35%) started ART at some point after IPT initiation. Thus, our findings may not generalize to populations that are largely ART-naive; however, many existing programs for IPT delivery are integrated with ART, as was the case here.
We took as our study population the population of THRio, which was largely ART-experienced at the time of IPT initiation. Providing IPT to a population with little ART experience may augment the impact of IPT (e.g., if TB risk is higher as a result), but more likely, ART and IPT have synergistic effects18. Epidemiological models of the Brazilian HIV-infected population estimated an annual risk of infection (ARI) of 0.8% per year which was consistent with observed TB incidence in this population19.
Finally, the costs and effectiveness of this intervention as measured in urban Brazil may not be reflective of those in rural settings, or in areas with lower ART coverage or general public health infrastructure; and also, our estimates of cost-effectiveness may overestimate the benefit of IPT, to the extent that individuals with a positive TST may have living conditions that put them at greater risk of reinfection. In summary, this decision analysis suggests that an intervention aimed at training healthcare workers to provide TST-guided IPT to people accessing HIV care is likely to be cost-effective in the setting of urban Brazil. Further study is required to see if these findings generalize to higher-burden or lower-income settings. In the interim, these findings support existing guidelines to provide IPT in this population, suggesting that preventive therapy is not only effective but likely to provide good value for money as well.
Acknowledgments
Funding was provided by the Bill & Melinda Gates Foundation, grant 19790.01 to the Consortium to Response Effectively to the AIDS Tuberculosis Epidemic (CREATE). Additional support was received from the National Institutes of Health Fogarty International Center grant U2RTW006885 and National Institutes of Health grants AI066994 and AI001637.
Contributor Information
Mojgan Azadi, Department of Population Family and Reproductive Health Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA.
David M. Bishai, Department of Population Family and Reproductive Health Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA.
Lawrence H. Moulton, Department of International Health Johns Hopkins Bloomberg School of Public Health, Center for Tuberculosis Research, Department of Medicine, Johns Hopkins University School of Medicine, Baltimore MD, USA.
Solange Cavalcante, Municipal Health Secretariat, Rio de Janeiro, Brazil, Scientific Computation Program, Oswaldo Cruz Foundation, Rio de Janeiro, Brazil.
Valeria Saraceni, Municipal Health Secretariat, Rio de Janeiro, Brazil.
Antonio Pacheco, Scientific Computation Program, Oswaldo Cruz Foundation, Rio de Janeiro, Brazil.
Silvia Cohn, Center for Tuberculosis Research, Department of Medicine, Johns Hopkins University School of Medicine, Baltimore MD, USA.
Richard E. Chaisson, Department of International Health Johns Hopkins Bloomberg School of Public Health, Center for Tuberculosis Research, Department of Medicine, Johns Hopkins University School of Medicine, Baltimore MD, USA.
Betina Durovni, Municipal Health Secretariat, Rio de Janeiro, Brazil, Federal University of Rio de Janeiro, Rio de Janeiro, Brazil.
Jonathan E. Golub, Center for Tuberculosis Research, Department of Medicine, Johns Hopkins University School of Medicine, Baltimore MD, USA.
References
- 1.World Health Organization. Global Tuberculosis Control. Geneva: WHO; 2011. [Google Scholar]
- 2.Samandari T, Agizew TB, Nyirenda S, et al. 6-month versus 36-month isoniazid preventive treatment for tuberculosis in adults with HIV infection in Botswana: a randomised, double-blind, placebo-controlled trial. Lancet. 2011;377:1588–98. doi: 10.1016/S0140-6736(11)60204-3. [DOI] [PubMed] [Google Scholar]
- 3.Durovni B, Saraceni V, Moulton LH, et al. Effect of improved tuberculosis screening and isoniazid preventive therapy on incidence of tuberculosis and death in patients with HIV in clinics in Rio de Janeiro, Brazil: a stepped wedge, cluster-randomised trial. The Lancet infectious diseases. 2013;13:852–8. doi: 10.1016/S1473-3099(13)70187-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martinson NA, Barnes GL, Moulton LH, et al. New regimens to prevent tuberculosis in adults with HIV infection. N Engl J Med. 2011;365:11–20. doi: 10.1056/NEJMoa1005136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization. Global Tuberculosis Report. Geneva: WHO; 2012. [Google Scholar]
- 6.United States Agency for International Development. Evaluation USAID/Brazil: Performance Evaluation of the Tuberculosis Portfolio, 2001-2012. Washington, DC: USAID; 2012. [Google Scholar]
- 7.Saraceni V, Pacheco AG, Golub JE, et al. Physician adherence to guidelines for tuberculosis and HIV care in Rio de Janeiro, Brazil. The Brazilian journal of infectious diseases : an official publication of the Brazilian Society of Infectious Diseases. 2011;15:249–52. doi: 10.1016/s1413-8670(11)70184-2. [DOI] [PubMed] [Google Scholar]
- 8.Churchyard GJ, Fielding KL, Lewis JJ, et al. A trial of mass isoniazid preventive therapy for tuberculosis control. N Engl J Med. 2014;370:301–10. doi: 10.1056/NEJMoa1214289. [DOI] [PubMed] [Google Scholar]
- 9.Durovni B, Cavalcante SC, Saraceni V, et al. The implementation of isoniazid preventive therapy in HIV clinics: the experience from the TB/HIV in Rio (THRio) study. AIDS. 2010;24(Suppl 5):S49–56. doi: 10.1097/01.aids.0000391022.95412.a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johns B, Baltussen R, Hutubessy R. Programme costs in the economic evaluation of health interventions. Cost Eff Resour Alloc. 2003;1:1. doi: 10.1186/1478-7547-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.UNAIDS. Costing Guidelines for HIV Prevention Strategies. Geneva: UNAIDS; 2000. [Google Scholar]
- 12.Dowdy DW, Israel G, Vellozo V, et al. Quality of life among people treated for tuberculosis and human immunodeficiency virus in Rio de Janeiro, Brazil. Int J Tuberc Lung Dis. 2013;17:345–7. doi: 10.5588/ijtld.12.0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mancuso JD, Niebuhr DW, Frick KD, Keep LW, Anderson KM. Cost-effectiveness analysis of targeted and sequential screening strategies for latent tuberculosis. Int J Tuberc Lung Dis. 2011;15:1223–30. doi: 10.5588/ijtld.10.0542. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization. Guidelines for intensified tuberculosis case-finding and isoniazid preventive therapy for people living with HIV in resource-constrained settings. Geneva: WHO; 2011. [Google Scholar]
- 15.Churchyard GJ, Fielding KL, Lewis JJ, et al. A Trial of Mass Isoniazid Preventive Therapy for Tuberculosis Control. New England Journal of Medicine. 2014;370:301–10. doi: 10.1056/NEJMoa1214289. [DOI] [PubMed] [Google Scholar]
- 16.Fitzpatrick C, Floyd K. A systematic review of the cost and cost effectiveness of treatment for multidrug-resistant tuberculosis. PharmacoEconomics. 2012;30:63–80. doi: 10.2165/11595340-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 17.Grant AD, Charalambous S, Fielding KL, et al. Effect of routine isoniazid preventive therapy on tuberculosis incidence among HIV-infected men in South Africa: a novel randomized incremental recruitment study. JAMA : the journal of the American Medical Association. 2005;293:2719–25. doi: 10.1001/jama.293.22.2719. [DOI] [PubMed] [Google Scholar]
- 18.Golub JE, Saraceni V, Cavalcante SC, et al. The impact of antiretroviral therapy and isoniazid preventive therapy on tuberculosis incidence in HIV-infected patients in Rio de Janeiro, Brazil. AIDS (London, England) 2007;21:1441. doi: 10.1097/QAD.0b013e328216f441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dowdy DW, Golub JE, Saraceni V, et al. Impact of Isoniazid Preventive Therapy for HIV-Infected Adults in Rio de Janeiro, Brazil: An Epidemiological Model. Journal of acquired immune deficiency syndromes (1999) 2014 doi: 10.1097/QAI.0000000000000219. [DOI] [PMC free article] [PubMed] [Google Scholar]


